Abstract
Introduction
Transabdominal electrocardiographic (TAfECG) acquisition of fetal heart rate (FHR) signals has recently been introduced into leading commercial cardiotocographic (CTG) monitors. Continuous wireless transmission of signals has raised the possibility of the technology being used during maternal mobilization in labor. This study aims to evaluate signal quality and accuracy of TAfECG acquisition of FHR signals during static and active maternal positions in labor when compared with Doppler signals and with the gold‐standard method of fetal scalp electrode (FSE).
Material and Methods
A total of 76 women with singleton term pregnancies in the active first stage of labor had simultaneously acquired FHR with TAfECG, Doppler, and FSE. Participants were asked to complete a supervised mobilization scheme, comprising five sequential 10‐min periods of lying down, standing, sitting, walking, and rocking on the birthing ball. The three FHR signals were compared, defining signal loss as the percentage of signals under 20 bpm or exceeding 250 bpm and accuracy as the difference with FSE values. Computer analysis was used to quantify variability, accelerations, and decelerations. Static labor positions (lying down, standing, and sitting) were compared with active labor positions (walking and rocking on the birthing ball).
Results
Average signal loss was 5.3% with TAfECG (3.2% in static and 7.4% in active positions) and 15.5% with Doppler (8.3% in static and 30.7% in active positions). Average accuracy was 3.5 bpm with TAfECG (1.9 bpm in static and 5.04 bpm in active positions) and 13.9 bpm with Doppler (3.2 bpm in static and 24.7 bpm in active positions). Average variability was similar with TAfECG and FSE in static positions but significantly higher with TAfECG in active positions (23.6 vs. 13.5 bpm, p < 0.001).
Conclusions
In static labor positions, TAfECG provides a low signal loss, similar to that obtained with FSE, and a good signal accuracy, so the technique can be considered reliable when the mother is lying down, standing, or sitting. During maternal movement, TAfECG causes an artificial increase in FHR variability, which can cause false reassurance regarding fetal oxygenation. Doppler signals are unreliable during maternal movements.
Keywords: cardiotocography, fetal electrocardiogram, fetal heart rate, intrapartum, monitoring, transabdominal ECG
Transabdominal electrocardiographic acquisition is a nouvelle technique that allows continuous fetal monitoring during maternal mobilization. It has better quality than Doppler during the first stage of labor, but overestimates variability during maternal movements. This study reinforces the potential of TAfECG device in intrapartum monitoring, in particular due to its high overall accuracy and high reliability.
Abbreviations
- BMI
body mass index
- bpm
beats per minute
- CI
confidence interval
- CTG
cardiotocographic
- ECG
electrocardiogram
- FHR
fetal heart rate
- FSE
fetal scalp electrode
- MHR
maternal heart rate
- NICU
neonatal intensive care unit
- SD
standard deviation,
- TAfECG
transabdominal fetal electrocardiography
Key message.
Transabdominal fetal electrocardiography provides good‐quality signals during the first stage of labor when women are lying down, standing, or sitting. During maternal movements, the technique provides lower signal quality, lower signal accuracy, and an artificial increase in fetal heart rate variability.
1. INTRODUCTION
Restriction of maternal movements is considered a major limitation for the generalized use of continuous cardiotocographic (CTG) monitoring in labor. For many decades, the technique required cable connections between sensors and the fetal monitor, thus limiting the extent of maternal movement. Several randomized clinical trials have shown that maternal mobilization during the first stage of labor decreases the duration of labor and the incidence of cesarean birth. 1 In addition, freedom of movement has a positive impact on women's birth experiences. 2
Over the last two decades, wireless transmission of signals from sensors to the fetal monitor has been incorporated into several commercial monitors, avoiding the need for cables and thus allowing greater freedom of movement. More recently, non‐invasive transabdominal electrocardiographic (TAfECG) acquisition of fetal heart rate (FHR) signals has been introduced into leading commercial monitors. This technique uses electrodes applied to the maternal abdominal skin to detect electrical signals originating from the maternal and fetal hearts and applies complex mathematical algorithms to extract both FHR and maternal heart rate (MHR). 3 , 4 It is usually used together with electrohysterography, a technique that captures the electrical signals originating from myometrial cells. These recent technological developments have raised the possibility that FHR signals may continue to be accurately monitored non‐invasively while allowing the mother freedom of movements during labor.
Some studies have evaluated the signal loss and accuracy of different TAfECG acquisition sensors and FHR extraction algorithms, both before and during labor. 3 , 4 , 5 , 6 However, to the best of our knowledge, the clinical performance of TAfECG during maternal movement has not been previously evaluated. The aim of this study was to evaluate the signal loss and accuracy of FHR signals acquired with TAfECG compared with Doppler and the gold‐standard method of fetal scalp electrode (FSE) during the first stage of labor, when the mother is adopting different positions or moving.
2. MATERIAL AND METHODS
This prospective observational study was conducted between May and December 2020 in the labor ward of a tertiary care university hospital. Pregnant women were informed of the study by patient information leaflets distributed in the outpatient clinic and in labor admission rooms. Women were approached for enrolment in the labor ward if they met the following inclusion criteria: aged >18 years, able to provide written informed consent, gestational age between 37 and 42 weeks, singleton pregnancy, cephalic presentation, in the active first stage of labor (i.e. 4–9 cm of cervical dilatation), no contraindications to internal FHR monitoring, no abdominal skin diseases, and normal CTG at the time of enrolment.
After providing written informed consent, participants were simultaneously monitored with TAfECG, Doppler, and FSE sensors using a triple‐channel fetal monitor (Philips Avalon FM30 with CL wireless transducer system; Philips Healthcare). A single trained operated placed the TAfECG patch on the maternal abdomen following the manufacturer's instructions. The skin was previously prepared by washing with soap and water and then gently exfoliated at the sites of patch attachment using medical abrasive paper. Skin impedance was automatically evaluated by the fetal monitor, and skin preparation was repeated if signal quality was inadequate. This occurred in 14 participants but always before the mobilization scheme was started, and no skin preparation was repeated at later stages. The disposable patch (Philips Avalon CL Fetal and Maternal Patch; Philips Healthcare) provides both FHR and MHR signals using the height and width of the QRS complex to differentiate the different ECG signals obtained. A wireless transmitter was connected to the patch for signal transmission to the fetal monitor. The same operator subsequently applied a Doppler ultrasound sensor on the maternal abdomen and an FSE on the fetal scalp, according to standard clinical practice. Wireless transmission of signals was assured for all three acquisition methods. The position of the Doppler sensor was corrected between segments of mobilization, whenever clinically required. There was no need to reposition the TAfECG or the FSE in any of the women during the time period evaluated.
After all sensors had been placed, women were asked to follow a supervised mobilization scheme comprising five sequential 10‐min periods of lying down, sitting, standing, walking, and rocking on a birthing ball (the total time period evaluated in the study was 50 min). Each type of movement was performed for 10 min, and all signals acquired during these periods were evaluated. Suboptimal signals were not rejected. FHR and MHR signals were recorded and stored at 0.25 s intervals, in beats per minute (bpm), rounded off to the nearest 0.25 bpm, as automatically provided by the fetal monitor. Acquired signals were analyzed offline using the SisPorto 4.0 program for computer analysis of CTGs. 7 , 8
Signal loss was defined as the percentage of FHR signals below 20 bpm (including periods with no signal) or exceeding 250 bpm. In addition to automatically evaluating signal loss, SisPorto 4.0 was used to quantify variability, number of accelerations, and number of decelerations in each 10‐min segment. Stored signals were also exported to Microsoft Excel for detailed analysis of signal accuracy and evaluation of possible MHR contaminations of the FHR signal. Analysis was performed by an investigator who was blinded to the signal source. Signal accuracy was calculated as the average absolute difference between each TAfECG and Doppler signal and its corresponding FSE value. Possible MHR contamination of the FHR signal was identified when differences between these two signals were under 5 bpm.
Comparisons were performed for each of the five maternal labor positions and between static positions (lying down, standing, and sitting) and active positions (walking and rocking on the birthing ball).
Subsequent clinical management of labor was left to the attending healthcare professionals, who had access to both Doppler and FSE signals. Maternal demographic and obstetrical data were collected at the time of study enrolment, and maternal and neonatal outcomes were collected retrospectively from the hospital's electronic patient records.
2.1. Statistical analyses
Normal distributions were assessed using the Kolmogorov–Smirnov test. Differences in characteristics were sought using one‐way analysis of variance and Student's t test. Means, standard deviations (SD), and 95% confidence intervals (95% CIs) were calculated for signal accuracy.
Bland–Altman plots were created to evaluate agreement between TAfECG–FSE and Doppler–FSE values. Mean differences between two methods of measurement were calculated, as well as 95% limits of agreement (LoA) for these differences. 7 LoA were determined as the mean absolute differences ± 1.96 SD. 7 Correlation was also evaluated using the intraclass correlation coefficients (ICCs). Values >0.85 were considered as signifying an excellent correlation, 0.50–0.85 a moderate correlation, and <0.50 a poor correlation.
For all comparisons, p‐values <0.05 were considered statistically significant. The sample size of this study had a power of 100% to find differences between the accuracy of FSE and TAfECG. Statistical analysis was performed using SPSS version 25 (IBM Corp.) and Microsoft Excel 16.5.
3. RESULTS
A total of 101 laboring women gave their consent to participate. Of these, 76 were monitored simultaneously with the three FHR acquisition techniques and successfully completed the five mobilization periods. Acquisition failure was defined as the inability to obtain a reasonable FHR signal after 30 minutes of attempted connections. This occurred in two participants with the TAfECG signal (2%) and in two others with the FSE signal (2%). In a further two participants, triple‐channel monitoring failed to record the Doppler signal. All of these were excluded from subsequent analyses. A flow diagram of patient inclusions is provided in Figure 1. Table 1 shows the main demographic and obstetric characteristics of study participants.
FIGURE 1.
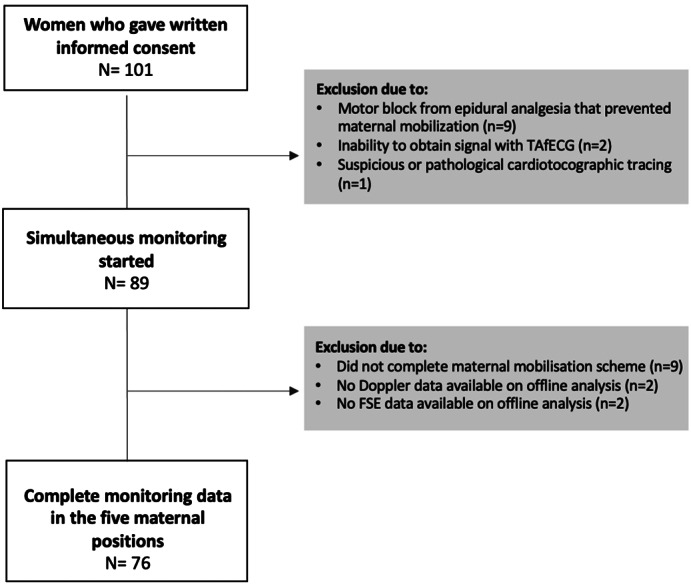
Flow diagram of patient inclusions and final study participants. FSE, fetal scalp electrode; TAfECG, transabdominal fetal electrocardiography.
TABLE 1.
Main demographic and obstetric characteristics of study participants
| Demographic characteristics | (n = 76) |
|---|---|
| Maternal age | 30 ± 5.3 years |
| Gestational age | 39.4 ± 0.9 weeks |
| Ethnic background | |
| White Caucasian | 56 (73.6) |
| African origin | 10 (13.3) |
| East Asian origin | 2 (2.6) |
| South Asian origin | 8 (10.5) |
| BMI at preconception (kg/m2) | 25.8 ± 4.5 |
| BMI at delivery (kg/m2) | 29.5 ± 3.3 |
| BMI category at delivery | |
| ≤30 kg/m2 | 46 (60.5) |
| >30 kg/m2 | 30 (39.5) |
| Parity | |
| Nulliparous | 15 (19.7) |
| Multiparous | 61 (80.3) |
| Epidural analgesia | 76 |
| Abdominal hirsutism | 6 (7.9) |
| Abdominal stretch marks | 13 (17.1) |
| Obstetric characteristics | |
| Clinical chorioamnionitis | 2 (3) |
| Type of delivery | |
| Normal vaginal | 58 (76.4) |
| Instrumental vaginal | 15 (19.7) |
| Cesarean | 3 (3.9) |
| Birthweight | 3354 ± 379 g |
| Apgar <7 at 5 min | 0 |
| Umbilical artery pH | 7.21 ± 1.7 |
| NICU admission | 0 |
Note: Data are presented as average ± standard deviation or n (%).
Abbreviations: BMI, body mass index; NICU, neonatal intensive care unit.
Average signal loss for TAfECG was 5.3% ± 1.3 SD (3.2% in static positions and 7.4% in active positions). Average signal loss for Doppler was 15.5% ± 8.2 SD (8.3% in static and 30.7% in active positions). Signal loss was significantly lower with TAfECG than with Doppler (p < 0.0001) and significantly lower with static than with active positions (p < 0.0001).
The accuracy of TAfECG and Doppler signals compared with the gold standard FSE is depicted in Table 2. Figure 2 shows Bland–Altman plots for these analyses. Both techniques show excellent agreement with FSE in static positions but not in active positions. For TAfECG, the ICC was 0.95 (95% CI 0.91–0.98) in static positions and 0.68 (95% CI 0.65–0.71) in active positions. For Doppler, the ICC was 0.89 (95% CI 0.86–0.93) in static positions and 0.41 (95% CI 0.37–0.45) in active positions.
TABLE 2.
Accuracy of fetal heart rate measurements when comparing transabdominal fetal electrocardiography (TAfECG) and Doppler signal with the gold standard of fetal scalp electrode (FSE). The average difference was computed as an absolute number.
| TAfECG vs FSE | |||||
|---|---|---|---|---|---|
| Average difference (bpm) | SD | 95% CI | Limits of agreement | ||
| Lower | Upper | ||||
| Overall | 3.5 | 5.3 | 1.88–4.12 | −6.9 | 13.9 |
| Static positions | 1.9 | 3.7 | 0.34–1.67 | −5.4 | 9.2 |
| Lying down | 1.8 | 3.7 | 0.33–1.67 | −5.5 | 9.1 |
| Sitting | 1.7 | 4.1 | 0.1–1.9 | −6.3 | 9.7 |
| Standing | 2.2 | 3.4 | 1.33–2.67 | −4.5 | 8.9 |
| Active positions | 5.04 | 6.9 | 3.65–6.35 | −8.5 | 18.6 |
| Walking | 3.6 | 4.3 | 2.1–3.9 | −4.8 | 12 |
| Birthing ball | 6.5 | 9.5 | 3.98–8.02 | −12.2 | 25,1 |
| Doppler versus FSE | |||||
| Overall | 13.9 | 7.5 | 11.4–14.6 | −0.8 | 28.6 |
| Static positions | 3.2 | 2.5 | 2.55–3.45 | −1.7 | 8.1 |
| Lying down | 2.4 | 2.2 | 1.55–2.45 | −1.9 | 6.7 |
| Sitting | 2.8 | 2.2 | 1.55–2.45 | −1.5 | 7.1 |
| Standing | 4.4 | 3.1 | 3.33–4.67 | −1.7 | 10.4 |
| Active positions | 24.7 | 12.4 | 21.3–26.7 | 0.8 | 48.6 |
| Walking | 18.4 | 11.7 | 15.5–20.5 | −4.5 | 41.3 |
| Birthing ball | 31 | 13.1 | 28.1–34 | 5.3 | 56.7 |
Abbreviations: bpm, beats per minute; CI, confidence interval; SD, standard deviation.
FIGURE 2.
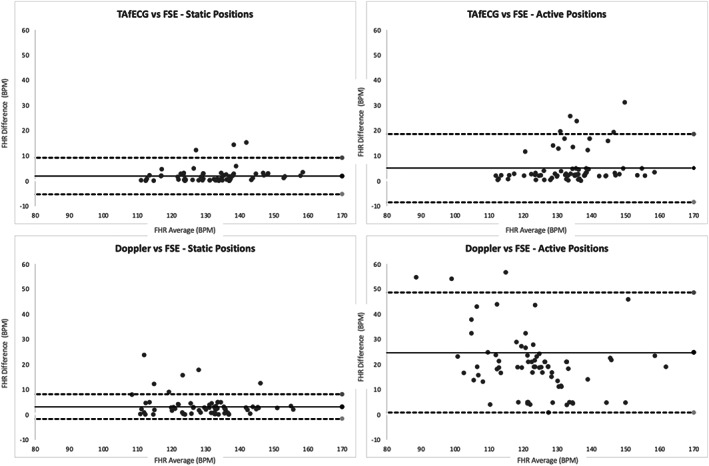
Bland–Altman plots and limits of agreement between transabdominal fetal electrocardiography (TAfECG) and fetal scalp electrode (FSE) (top) and between Doppler and FSE (bottom). Static positions on the left graphs and active positions on the right graphs. The average of the absolute values of each difference per participant (represented by a datapoint) was considered. FHR, fetal heart rate.
Considering 5 bpm as an acceptable difference in accuracy between FHR acquisition techniques, TAfECG signals were inside this margin 89.3% of the time (95% CI 88.2–91.6). This value was 93.6% (95% CI 92.9–94.3) for static positions and 84.9% (95% CI 83.9–85.9) for active positions. Doppler signals were inside the 5 bpm margin 78.2% of the time (74.1–83.1). This value was 92.1% (95% CI 90.8–93.3) for static positions and 64.3% (95% CI 56.9–72.9) for active positions.
Possible MHR contaminations of the FHR signal occurred in 0.8% of TAfECG signals (0.3% in static and 1.3% in active positions) and in 9.2% of Doppler signals (2.6% in static and 15.8% in active positions).
Computer‐evaluated overall average FHR variability with TAfECG was 18.1 bpm ± 5.2 SD (12.6 bpm in static positions and 23.6 bpm in active positions). This parameter was 11.9 bpm ± 3.5 with Doppler (11.5 bpm in active positions and 12.3 bpm in static positions). For FSE, it was 12.9 bpm ± 4.2 SD (12.3 bpm in static positions and 13.5 bpm in active positions). Differences were statistically significant only between TAfECG and FSE acquisition in active positions (p < 0.001).
There were no differences between the three acquisition modes in the overall average number of computer‐detected accelerations (TAfECG 2.3, Doppler 2.2, FSE 2.8). There were also no differences in the overall number of computer‐detected decelerations (TAfECG 0.3, Doppler 0.4, FSE 0.3).
4. DISCUSSION
During the overall 50‐min evaluation period, TAfECG recordings displayed a low signal loss and good accuracy. Doppler signals performed significantly worse than TAfECG regarding these parameters. During maternal movement, signal quality and accuracy were significantly worse with both TAfECG and Doppler. With the latter, signal loss occurred 30.7% of the time, signals differed an average of 24.7 bpm to those of FSE, and possible MHR contamination occurred 15.8% of the time. With TAfECG, signal quality and accuracy also worsened during maternal movements, and a higher FHR variability was detected.
Other studies have evaluated the signal quality and accuracy of TAfECG signals during the first stage of labor. 3 , 4 , 5 , 6 Using a different acquisition sensor, Lempersz et al. reported that TAfECG signals were recordable 91.3% of the time during the first stage of labor and that overall accuracy was 1.46 bpm. 3 With a previous version of the sensor used in the present study, Cohen et al. found that TAfECG was more accurate than Doppler (5.3 bpm ± 2.4 bpm vs 10.9 bpm ± 5.8 bpm) and less likely to contain MHR contamination. 4 Also with a previous version of the sensor used in the present study, Reinhard et al. reported better signal quality with TAfECG than with Doppler during the first stage of labor (95.7%) and similar values for both techniques during the second stage of labor. 5 Monson et. al found that TAfECG performed similarly to Doppler during the whole of labor but was superior to Doppler in obese patients (body mass index [BMI] >30 kg/m2) regarding the duration of interpretable FHR tracings. 6 An additional benefit of TAfECG is the continuous acquisition of MHR, allowing simultaneous display of the two signals and an easier pickup of MHR contamination. 10 , 11 , 12 TAfECG has well‐known advantages over FSE in the early stages of labor, as it can be started before cervical dilatation and avoids the need for artificial rupture of the membranes, thereby reducing the possibility of infection and other complications associated with this procedure. It has no contraindications and may also be more acceptable to women.
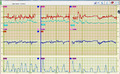
A novel finding of the present study is the higher FHR variability of TAfECG signals during maternal movements. This suggests persistent contamination of the signal by random noise, as depicted in Figure 3. Therefore, this technology should be used with caution during maternal mobilization, as it may give false reassurance regarding fetal oxygenation. The most likely origin of this contamination is the contraction of maternal striated abdominal muscles. Other studies have alluded to a possible contamination of electrical signals arising from maternal muscles in the acquisition of TAfECG signals during the second stage of labour. 13 , 14 In particular, TAfECG signals have also been reported to be of lesser quality during maternal pushing. 3
FIGURE 3.
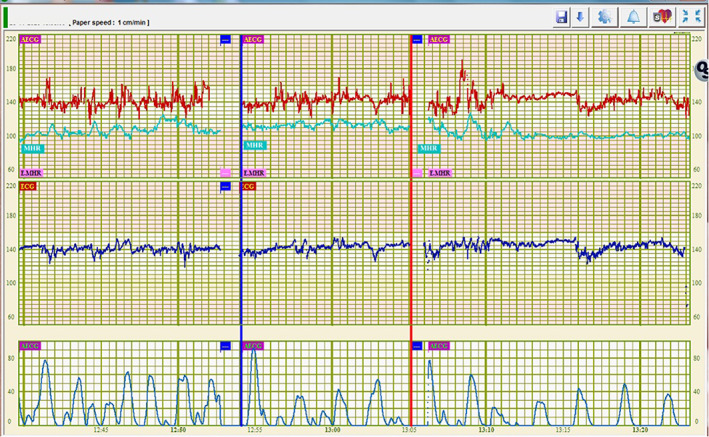
Cardiotocographic signals simultaneously acquired from the same fetus by transabdominal fetal electrocardiography ([TAfECG] top tracing in red) and fetal scalp electrode (bottom tracing in blue). Maternal heart rate signals are also displayed in light green on the top tracing. The blue and red vertical bars delimit a 10‐min (minute) period of maternal walking. Higher fetal heart rate variability can be seen in signals acquired by TAfECG. ECG, electrocardiogram; MHR, maternal heart rate; min, minute.
The lower FHR variability detected by Doppler signals than by TAfECG during maternal movements can be partly explained by the fact that computer systems use filters to clean short‐lasting FHR signals distant from the baseline as well as MHR contaminations.
Another original finding of the present study was the alarmingly high signal loss (30.7%) and reduced accuracy of Doppler signals (average difference 24.7 bpm) acquired during maternal movements, mostly caused by MHR contamination (affecting 15.8% of signals). Signal loss is well above the acceptable threshold of 20% proposed by the International Federation of Obstetrics and Gynecology. 15 Overall signal loss with Doppler in labor has been reported to range between 10% and 40%, but previous studies did not explicitly describe the type of maternal movements that occurred during acquisition. 15 , 16 , 17
Failure of TAfECG signal acquisition was similar to that with FSE (2%). However, further studies are needed to evaluate whether results are similar when multiple operators are involved in signal acquisition and whether it is influenced by maternal characteristics.
One of the limitations of this study is that its population consisted mainly of white women with moderate BMI. Increasing BMI is known to affect the Doppler signal 15 , 16 , 17 but not the TAfECG signal, 6 so higher differences in signal quality between the two methods would be expected in a high‐BMI population. On the other hand, increased skin impedance has been reported in women of African and Asian descent, 18 , 19 suggesting a lower quality of TAfECG signals in these populations. Also, the majority of the study population were multiparous, had an epidural analgesia, and had a vaginal delivery, which may not represent most laboring women in some settings. The results are also limited to term pregnancies, as it is known that a poorly conductive layer (vernix caseosa) forms on the fetal body surface between the 28th and the 34th week of gestation, affecting the quality of TAfECG signals. 8 , 9
A further limitation was the lack of a standardized approach for readjustment of the Doppler sensor during maternal mobilization. The quality of Doppler signals depends on the commitment of healthcare professionals to readjusting the sensor, particularly during long periods in labor. This is less practical during maternal walking or rocking on the birthing ball. The hypothesis of self‐adjustment of the sensor by the mother was not evaluated. All TAfECG signals were acquired by the same experienced operator, allowing for a more homogenous experience, but this is not possible in everyday clinical practice, and there appears to be a small learning curve with this technique. Our results apply only to the TAfECG acquisition equipment evaluated (Philips Avalon CL Fetal and Maternal Patch). Cardiotocography also incorporates monitoring of uterine activity by tocodynamometer or electromyography, the latter usually used in conjunction with TAfECG. This aspect was not evaluated in the present study and needs to be the subject of future research.
5. CONCLUSION
When laboring women are lying down, sitting, or standing, TAfECG provides an FHR signal similar to that obtained with FSE, with low signal loss and good signal accuracy. However, during maternal movement, TAfECG causes an artificial increase in FHR variability, probably related to external contamination of the signal. The present study also reinforces previous reports that Doppler signals are unreliable when women are moving during labor.
AUTHOR CONTRIBUTIONS
CRC: Conception of the work, design and drafting of the work, and data acquisition, analysis, interpretation and final approval of the version to be published. PN: Data analysis and final approval of the version to be published. DAC: Conception of the work, critical revision for important intellectual content, and final approval of the version to be published.
6. ETHICS STATEMENT
The study was approved by the hospital ethics committee (Centro Académico de Medicina de Lisboa, reference number 256/17 on November 23, 2017), and all participants signed a written informed consent form to participate.
FUNDING INFORMATION
The fetal ECG acquisition patches were provided by Philips Healthcare (Boeblingen, Germany).
CONFLICT OF INTEREST
The TAfECG patches used in this study were kindly provided by Philips Healthcare (Boeblingen, Germany). D. Ayres‐de‐Campos is a consultant for Philips Healthcare, Boeblingen. The authors report no other conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the SisPorto Research Group (Department of Obstetrics and Gynaecology, Faculty of Medicine, University of Porto, Porto, Portugal) project for the scientific support.
Reis‐de‐Carvalho C, Nogueira P, Ayres‐de‐Campos D. Quality of fetal heart rate monitoring with transabdominal fetal ECG during maternal movement in labor: A prospective study. Acta Obstet Gynecol Scand. 2022;101:1269‐1275. doi: 10.1111/aogs.14434
REFERENCES
- 1. Lawrence A, Lewis L, Hofmeyr GJ, Styles C. Maternal positions and mobility during first stage labour. Cochrane Database Syst Rev. 2013;10:CD003934. [DOI] [PubMed] [Google Scholar]
- 2. Walsh D. Part five: why we should reject the bed birth myth. Br J Midwifery. 2000;8:554‐558. [Google Scholar]
- 3. Lempersz C, Noben L, van Osta G, et al. Intrapartum non‐invasive electrophysiological monitoring: a prospective observational study. Acta Obstet Gynecol Scand. 2020;99:1387‐1395. [DOI] [PubMed] [Google Scholar]
- 4. Cohen WR, Ommani S, Hassan S, et al. Accuracy and reliability of fetal heart rate monitoring using maternal abdominal surface electrodes. Acta Obstet Gynecol Scand. 2012;91:1306‐1313. [DOI] [PubMed] [Google Scholar]
- 5. Reinhard J, Hayes‐Gill BR, Schiermeier S, et al. Intrapartum signal quality with external fetal heart rate monitoring: a two way trial of external doppler CTG ultrasound and the abdominal fetal electrocardiogram. Arch Gynecol Obstet. 2012;286:1103‐1107. [DOI] [PubMed] [Google Scholar]
- 6. Monson M, Heuser C, Einerson BD, et al. Evaluation of an external fetal electrocardiogram monitoring system: a randomized controlled trial. Am J Obstet Gynecol. 2020;223:244.e1‐244.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Myles PS, Cui JI. Using the bland‐Altman method to measure agreement with repeated measures. Br J Anaesth. 2007;99:309‐311. [DOI] [PubMed] [Google Scholar]
- 8. Stinstra JG, Peters MJ. The influence of fetoabdominal tissues on fetal ECGs and MCGs. Arch Physiol Biochem. 2002;110:165‐176. [DOI] [PubMed] [Google Scholar]
- 9. Keenan E, Karmakar CKPM. The effects of asymmetric volume conductor modeling on non‐invasive fetal ECG extraction. Physiol Meas. 2018;1(39):1. [DOI] [PubMed] [Google Scholar]
- 10. Cohen WR, Hayes‐Gill B. Influence of maternal body mass index on accuracy and reliability of external fetal monitoring techniques. Acta Obstet Gynecol Scand. 2014;93:590‐595. [DOI] [PubMed] [Google Scholar]
- 11. Graatsma EM, Miller J, Mulder EJH, Harman C, Baschat AA, Visser GHA. Maternal body mass index does not affect performance of fetal electrocardiography. Am J Perinatol. 2010;27:573‐577. [DOI] [PubMed] [Google Scholar]
- 12. Euliano TY, Darmanjian S, Nguyen MT, Busowski JD, Euliano N, Gregg AR. Monitoring fetal heart rate during labor: a comparison of three methods. J Pregnancy. 2017;5:8529816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fotiadou E, van Laar JOEH, Oei SG, Vullings R. Enhancement of low‐quality fetal electrocardiogram based on time‐sequenced adaptive filtering. Med Biol Eng Comput. 2018;56:2313‐2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joseph J, Gini JR, Ramachandran KI. Removal of BW and respiration noise in abdECG for fECG extraction. In: Thampi, S. , Krishnan, S. , Corchado Rodriguez, J. , Das, S. , Wozniak, M. , Al‐Jumeily, D. (eds) Advances in Signal Processing and Intelligent Recognition Systems. SIRS 2017. Advances in Intelligent Systems and Computing, vol 678. Springer. 10.1007/978-3-319-67934-1_1 [DOI] [Google Scholar]
- 15. Spencer JAD, Belcher R, Dawes GS. The influence of signal loss on the comparison between computer analyses of the fetal heart rate in labour using pulsed doppler ultrasound (with autocorrelation) and simultaneous scalp electrocardiogram. Eur J Obstet Gynecol Reprod Biol. 1987;25:29‐34. [DOI] [PubMed] [Google Scholar]
- 16. Bakker PCAM, Colenbrander GJ, Verstraeten AA, Van Geijn HP. The quality of intrapartum fetal heart rate monitoring. Eur J Obstet Gynecol Reprod Biol. 2004;116:22‐27. [DOI] [PubMed] [Google Scholar]
- 17. Dawes GS, Visser GHA, Goodman JDS, Redman CWG. Numerical analysis of the human fetal heart rate: the quality of ultrasound records. Am J Obstet Gynecol. 1981;141:43‐52. [DOI] [PubMed] [Google Scholar]
- 18. Wesley NO, Maibach HI. Racial (ethnic) differences in skin properties: the objective data. Am J Clin Dermatol. 2003;4:843‐860. [DOI] [PubMed] [Google Scholar]
- 19. Macfarlane PW, Katibi IA, Hamde ST, et al. Racial differences in the ECG ‐ selected aspects. J Electrocardiol. 2014;47:809‐814. [DOI] [PubMed] [Google Scholar]


