Abstract
Introduction
Use of labor induction has increased rapidly in most middle‐ and high‐income countries over the past decade. The reasons for the stark rise in labor induction are largely unknown. We aimed to assess the extent to which the rising rate of labor induction is explained by changes in rates of underlying indications over time.
Material and methods
The study was based on nationwide data from the Icelandic Medical Birth Register on 85 620 singleton births from 1997 to 2018. The rate of labor induction and indications for induction was calculated for all singleton births in 1997–2018. Change over time was expressed as relative risk (RR), using Poisson regression with 95% confidence intervals (CI) adjusted for maternal characteristics and indications for labor induction.
Results
The crude rate of labor induction rose from 12.5% in 1997–2001 to 23.9% in 2014–2018 (crude RR = 1.91, 95% CI 1.81–2.01). While adjusting for maternal characteristics had little impact, adjusting additionally for labor induction indications lowered the RR to 1.43 (95% CI 1.35–1.51). Induction was increasingly indicated from 1997–2001 to 2014–2018 by gestational diabetes (2.4%–16.5%), hypertensive disorders (7.0%–11.1%), prolonged pregnancy (16.2%–23.7%), concerns for maternal wellbeing (3.2%–6.9%) and maternal age (0.5%–1.2%). No indication was registered for 9.2% of inductions in 2014–2018 compared with 16.3% in 1997–2001.
Conclusions
Our results show that the increase in labor induction over the study period is largely explained by an increase in various underlying conditions indicating labor induction. However, indications for 9.2% of labor inductions remain unexplained and warrant further investigation.
Keywords: elective, epidemiology, indication, labor induction, maternal request
The increase in induction was largely explained by an increase in conditions indicating labor induction (i.e hypertensive disorders, gestational diabetes and post‐term pregnancy). About one in ten inductions were not explained by a medical diagnosis.

Abbreviations
- CI
confidence interval
- HYPITAT
Hypertension and Pre‐eclampsia Intervention Trial at Near Term
- ICD‐10
International Classification of Diseases, 10th revision
- IMBR
Icelandic medical birth register
- NCSP
Nordic Medico‐Statistical Committee, the Classification of Surgical Procedures
- NICE
National Institute for Health and Care Excellence
- RR
relative risk
1. INTRODUCTION
Labor induction is a common obstetric intervention and its use has increased rapidly in most middle‐ and high‐income countries over the past decade. 1 In Iceland, the induction rate almost doubled, from 15.0% in 2002–2007 to 28.1% in 2014–2018. 2 , 3 The reason for the stark rise in labor induction is largely unknown but may be explained by an increase in conditions indicating an induction or an increase in induction by maternal request. 4 , 5 , 6 Interestingly, rates of adverse maternal and neonatal outcomes decreased over the same study period in Iceland. 3 However, there was no evidence that these improvements over time were mediated by the increased rate of labor induction. 3
Common indicators for labor induction include post‐term pregnancy, 7 hypertension/preeclampsia, 8 , 9 premature rupture of membranes, diabetes, 10 , 11 twin pregnancy, suspected fetal compromise, intrahepatic cholestasis of pregnancy, suspected macrosomia, maternal age over 35 or 40 years, and maternal cardiac disease. 12 However, the level of evidence supporting these common indications varies widely, ranging from low to high. 13 For example, the quality of evidence for post‐term pregnancy and hypertension/preeclampsia is high in terms of improved neonatal or maternal outcomes, but the evidence to support labor induction in women with term rupture of membranes is weak. 13
Following publications of major impact in 2008 11 , 14 and 2009, 9 obstetric guidelines changed considerably in Iceland, which likely contributed to the rise in labor induction. 2 In 2008, the National Institute for Health and Care Excellence (NICE) changed its guidelines to recommend induction earlier for post‐term pregnancy (41 weeks). 14 Pregnancies complicated by preeclampsia were induced earlier, instead of waiting until 39 weeks if possible, as a result of the Hypertension and Pre‐eclampsia Intervention Trial at Near Term (HYPITAT) study in 2009. 9 Beyond this, little is known about contributing factors to the rise in induced labor since early 2000s. Therefore, the aim of our study is (1) to provide a comprehensive description of factors contributing to the increased rate of labor induction in Iceland and (2) to assess whether the rising rate of labor induction may be explained by changes in rates of underlying indications for induction over time in the population.
2. MATERIAL AND METHODS
2.1. Study setting, data sources and population
We conducted a nationwide population‐based study to assess the underlying reasons for the increased induction rate in Iceland from January 1, 1997 to December 31, 2018. In Iceland, healthcare is publicly funded, maternity care is accessible and free of charge, and all births are attended by midwives in collaboration with obstetricians when problems arise.
Our study is based on data from the Icelandic Medical Birth Register (IMBR), a nationwide centralized registry with complete coverage of all live births and stillbirths in Iceland for infants weighing >500 g or having gestational age >22 weeks. The high quality and compulsory notification of the Nordic Medical Registers has been described previously. 15 There were a total of 95 733 births in Iceland during the study period. About 75% of births in Iceland occur at Landspitali University Hospital, which is the only tertiary hospital in Iceland.
Our final study sample included 85 620 singleton births in Iceland from 1997 to 2018. We excluded births with fetal demise before the onset of labor using the variable “fetal demise before onset of labor” in the IMBR, as standard care for women with fetal demise is labor induction. Over the study period there were inconsistencies with how care for women with premature rupture of labor without spontaneous contractions was recorded (augmentation vs. induction). We therefore excluded births with premature rupture of labor from the analysis using ICD‐code O42 (n = 6897 births).
2.2. Induction of labor
Information on obstetric interventions during labor and delivery is recorded in the Icelandic Medical Birth Register according to the recommendations of the Nordic Medico‐Statistical Committee, the Classification of Surgical Procedures (NCSP) and the International Classification of Diseases, 10th revision (ICD‐10). 16 We captured induction of labor by an onset of labor variable as recorded in the IMBR, ICD‐10 code O83.8 (induction of labor) and NCSP codes MASC00 (induction by rupture of amniotic membrane), MAXC02 (prostaglandin induction of labor) and MAXC09 (other induction of labor).
2.3. Sociodemographic and pregnancy‐related characteristics
We obtained information on the following maternal sociodemographic characteristics from the IMBR: age at delivery (continuous years; ≤25, 26–30, 31–34, 35–39, ≥40 years), citizenship (Icelandic, other), residence (urban, rural), marital status (single/widowed/divorced, married/cohabiting), employment (employed, student, unemployed, homemaker/on disability), parity (primipara, multipara), gestational age (continuous week; pre‐term [<37 weeks], early‐term [37+0 to 38+6 weeks], full‐term [39+0 to 40+6 weeks], late‐term [41+0 to 41+6 weeks], post‐term [>42+0 weeks]).
2.4. Indications associated with labor induction
Indications for labor induction are not recorded in the IMBR. Therefore, for the purposes of this study an expert panel of obstetricians and midwives identified pregnancy complications that were considered common indications for induction of labor in Iceland and grouped them together, using ICD‐10 codes (Table S1). We designated an order of importance to the labor indication groups, with the first group being the most likely to explain a labor induction and thus overriding all others. The groups are preeclampsia/eclampsia, pregestational diabetes, suspected placental insufficiency, hypertensive disorders other than preeclampsia and eclampsia, gestational diabetes, prolonged pregnancy, obstetric cholestasis, Rhesus immunization, maternal wellbeing, other (subluxation of symphysis pubis in pregnancy, problem related to unspecific psychosocial circumstances, exhaustion and fatigue, supervision of other high‐risk pregnancy, fetal wellbeing, other [polyhydramnios, macrosomia, maternal care of unstable lie of fetus]), gestational week >41 and maternal age >40 years. These will hereafter be referred to as indications for labor induction. The hierarchy of associated indications is shown in Table S1. For example, if a woman had gestational diabetes and preeclampsia, the indication for labor induction was recorded as preeclampsia. If none of the identified indications was recorded in the data, we defined the indication as missing.
2.5. Statistical analyses
First, we described the distribution of sociodemographic and pregnancy‐related characteristics for all singleton births in Iceland across calendar time. Statistical significance was assessed with Chi‐square tests for categorical variables and Student's t‐test for continuous variables. A p‐level of 0.05 was considered statistically significant. We categorized calendar time as two periods before 2008 (1997–2001, 2002–2007) and after 2008 (2008–2013, 2014–2018), as the induction rate was relatively stable until 2008; however, following publications of major impact in 2008 11 , 14 and 2009 9 there were considerable changes in obstetric guidelines in Iceland that likely resulted in the increased rate of labor induction. 2
We then calculated the rate of indications for induction per 100 (%) induction of labor births across time periods. To assess whether there was a difference between induced labors with and without an indication in terms of sociodemographic and pregnancy‐related characteristics, we further described the distribution of age, gestational length, marital status and citizenship among births where the indication for labor induction was missing.
To assess whether the rising rate of labor induction could be explained by changes in rates of underlying indications for induction over time in the population, we conducted a Poisson regression analysis and estimated the relative risks (RRs) of labor induction and corresponding 95% confidence intervals (CI) by calendar period using 1997–2001 as a reference period. We adjusted the regression model in steps; first adding parity, age, citizenship, residency, marital and employment status into the model, then hypertension and gestational diabetes, and finally other labor induction indications. We stratified the fully adjusted model by gestational age.
We also conducted a stratified analysis by maternal age to assess the potential impact of maternal age on the rising induction rate, adjusting for all sociodemographic factors (except maternal age), gestational age and labor induction indications.
2.6. Ethics statement
This study was approved by the National Bioethics Committee in Iceland on February 26, 2019 (reference VSNb2019020007/03.01).
3. RESULTS
Comparing 2014–2018 with 1997–2001, women were more likely to be older than 35 years when they gave birth (25.2% vs. 19.4%), more likely to have foreign citizenship (13.1% vs. 2.6%) and less likely to have post‐term pregnancies (2.0% vs. 6.1%; Table 1). The proportion of pre‐term and early‐term pregnancies remained similar over the study period. The rate of hypertensive disorders (other than preeclampsia) and gestational diabetes increased over the study period; from 2.9% to 5.8%, and from 0.7% to 11.6%, respectively (Table 1). Similarly, there was an increase in diagnoses of suspected placental insufficiency and diagnoses that relate to other concerns for maternal and fetal wellbeing (subluxation of symphysis pubis in pregnancy, exhaustion and fatigue, problem related to unspecific psychosocial circumstances, supervision of other high risk pregnancy, fetal macrosomia, polyhydramnios and maternal care for unstable lie of fetus). Overall, the rate of births without any medical diagnosis decreased from 60.3% to 49.2% over the study period.
TABLE 1.
Socio‐demographic and pregnancy‐related characteristics of singleton births in Iceland in 1997–2018 (N = 85 620)
| 1997–2001 | 2002–2007 | 2008–2013 | 2014–2018 | |||||
|---|---|---|---|---|---|---|---|---|
| 19 501 | 23 592 | 24 996 | 17 531 | |||||
| n | % | n | % | n | % | n | % | |
| Age | ||||||||
| ≤25 | 5544 | 28.4 | 5573 | 23.6 | 5031 | 20.1 | 3066 | 17.5 |
| 26–30 | 6063 | 31.1 | 7661 | 32.5 | 8144 | 32.6 | 5723 | 32.6 |
| 31–34 | 4111 | 21.1 | 5395 | 22.9 | 6061 | 24.2 | 4321 | 24.6 |
| 35–39 | 3044 | 15.6 | 3881 | 16.5 | 4465 | 17.9 | 3365 | 19.2 |
| ≥40 | 739 | 3.8 | 1082 | 4.6 | 1295 | 5.2 | 1056 | 6.0 |
| Missing | 16 | 0.1 | 4 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Citizenship | ||||||||
| Icelandic | 18 986 | 97.4 | 22 121 | 93.8 | 22 023 | 88.1 | 15 239 | 86.9 |
| Other | 515 | 2.6 | 1471 | 6.2 | 2973 | 11.9 | 2292 | 13.1 |
| Missing | 15 | 0.1 | 4 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Residency | ||||||||
| Urban | 11 013 | 56.5 | 14 466 | 61.3 | 16 480 | 65.9 | 11 358 | 64.8 |
| Rural | 1155 | 5.9 | 8249 | 35.0 | 8433 | 33.7 | 6043 | 34.5 |
| Missing | 7333 | 37.6 | 877 | 3.7 | 83 | 0.3 | 130 | 0.7 |
| Marital status | ||||||||
| Married/cohabiting | 16 827 | 86.3 | 19 965 | 15.0 | 20 689 | 82.8 | 14 416 | 82.2 |
| Single | 2660 | 13.6 | 3539 | 0.4 | 3660 | 14.6 | 2454 | 14.0 |
| Missing | 14 | 0.1 | 88 | 0.4 | 647 | 2.6 | 661 | 3.8 |
| Occupation | ||||||||
| Employed | 16 777 | 86.0 | 20 634 | 87.5 | 19 450 | 77.8 | 13 275 | 75.7 |
| Student | 1420 | 7.3 | 1614 | 6.8 | 1769 | 7.1 | 1005 | 5.7 |
| Unemployed | 0 | 0.0 | 28 | 0.1 | 686 | 2.7 | 177 | 1.0 |
| Disability/pension | 139 | 0.7 | 212 | 0.9 | 127 | 0.5 | 123 | 0.7 |
| Other | 1157 | 5.9 | 700 | 3.0 | 27 | 0.1 | 30 | 0.2 |
| Missing | 8 | 0.0 | 404 | 1.7 | 2937 | 11.7 | 2921 | 16.7 |
| Parity | ||||||||
| Primipara | 7832 | 40.2 | 9351 | 39.6 | 9687 | 38.8 | 7025 | 40.1 |
| Multipara | 11 669 | 59.8 | 14 241 | 60.4 | 15 309 | 61.2 | 10 506 | 59.9 |
| Gestational age | ||||||||
| Preterm | 588 | 3.0 | 715 | 3.0 | 763 | 3.1 | 617 | 3.5 |
| Early‐term | 2548 | 13.1 | 3052 | 12.9 | 3515 | 14.1 | 2480 | 14.1 |
| Full‐term | 10 602 | 54.4 | 12 320 | 52.2 | 13 440 | 53.8 | 10 192 | 58.1 |
| Late‐term | 4502 | 23.1 | 4927 | 20.9 | 5501 | 22.0 | 3841 | 21.9 |
| Post‐term | 1180 | 6.1 | 1153 | 4.9 | 596 | 2.4 | 351 | 2.0 |
| Missing | 80 | 0.4 | 1425 | 6.0 | 1181 | 4.7 | 50 | 0.3 |
| Complications in pregnancy indicating induction | ||||||||
| Pre‐eclampsia and eclampsia | 497 | 2.5 | 899 | 3.8 | 938 | 3.8 | 624 | 3.6 |
| Pregestational diabetes | 76 | 0.4 | 82 | 0.3 | 112 | 0.4 | 127 | 0.7 |
| Suspected placental insufficiency | 416 | 2.1 | 736 | 3.1 | 895 | 3.6 | 817 | 4.7 |
| Other hypertensive disorders | 571 | 2.9 | 829 | 3.5 | 1194 | 4.8 | 1015 | 5.8 |
| Gestational diabetes | 145 | 0.7 | 626 | 2.7 | 1027 | 4.1 | 2028 | 11.6 |
| Prolonged pregnancy | 429 | 2.2 | 901 | 3.8 | 647 | 2.6 | 1182 | 6.7 |
| Obstetric cholestasis | 27 | 0.1 | 115 | 0.5 | 321 | 1.3 | 167 | 1.0 |
| Rhesus isoimmunization | 49 | 0.3 | 60 | 0.3 | 86 | 0.3 | 54 | 0.3 |
| Maternal wellbeing, other | 522 | 2.7 | 724 | 3.1 | 620 | 2.5 | 615 | 3.5 |
| Fetal wellbeing, other | 118 | 0.6 | 315 | 1.3 | 454 | 1.8 | 390 | 2.2 |
The rate of labor induction nearly doubled, from 12.5% in 1997–2001 to 23.9% in 2014–2018. Comparing indications for labor induction in 2014–2018 to 1997–2001, there was a rise in hypertensive disorders other than preeclampsia and eclampsia (7.0% to 11.1%), but no change was observed in preeclampsia and eclampsia (11.6% in 2014–2018). Gestational diabetes was increasingly an indication for induction (2.4% to 16.5%) as well as maternal wellbeing, other (3.2% to 6.9%) and prolonged pregnancy (16.2% to 23.7%). In 2014–2018, 9.2% of induced births were missing an indication compared with 16.3% in 1997–2001 (Table 2).
TABLE 2.
Rate of indications associated with induced labor among inductions of singleton births in Iceland 1997–2018 (n = 14 985) by calendar time
| 1997–2001 | 2002–2007 | 2008–2013 | 2014–2018 | p‐value ** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| 1 a | Pre‐eclampsia and eclampsia | 270 | 11.1 | 480 | 14.7 | 640 | 12.6 | 487 | 11.6 | <0.001 |
| 2 | Pregestational diabetes | 35 | 1.4 | 31 | 0.9 | 51 | 1.0 | 55 | 1.3 | 0.182 |
| 3 | Suspected placental insufficiency | 155 | 6.4 | 242 | 7.4 | 408 | 8.0 | 399 | 9.5 | <0.001 |
| 4 | Other hypertensive disorders | 170 | 7.0 | 232 | 7.1 | 578 | 11.4 | 465 | 11.1 | <0.001 |
| 5 | Gestational diabetes | 58 | 2.4 | 216 | 6.6 | 384 | 7.5 | 693 | 16.5 | <0.001 |
| 6 | Prolonged pregnancy | 396 | 16.2 | 737 | 22.6 | 558 | 11.0 | 993 | 23.7 | <0.001 |
| 7 | Obstetric cholestasis | 14 | 0.6 | 60 | 1.8 | 157 | 3.1 | 75 | 1.8 | <0.001 |
| 8 | Rh isoimmunization | 19 | 0.8 | 24 | 0.7 | 37 | 0.7 | 19 | 0.5 | 0.274 |
| 9 | Maternal wellbeing, other | 79 | 3.2 | 99 | 3.0 | 334 | 6.6 | 291 | 6.9 | <0.001 |
| 10 | Fetal wellbeing, other | 23 | 0.9 | 65 | 2.0 | 92 | 1.8 | 56 | 1.3 | 0.004 |
| 11 | Gestational age >41 weeks | 818 | 33.5 | 609 | 18.7 | 1085 | 21.3 | 233 | 5.6 | <0.001 |
| 12 | Maternal age ≥40 years | 13 | 0.5 | 19 | 0.6 | 39 | 0.8 | 49 | 1.2 | 0.158 |
| Missing | 398 | 16.3 | 451 | 13.8 | 727 | 14.3 | 385 | 9.2 | <0.001 | |
| Total labor inductions | 2439 | 100.0 | 3265 | 100.0 | 5090 | 100.0 | 4191 | 100.0 | ||
The designated order of importance is shown by numbers 1–12, with category number one being the most likely cause for induction and thus overriding all other indications.
p‐values were calculated with Chi‐Square test, testing for differences across time periods.
Comparing the rate of labor induction across time periods (2014–2018 vs. 1997–2001) yielded a crude RR = 1.91 (95% CI 1.81–2.01; Table 3). Adjusting for maternal sociodemographic factors (Model A) had little effect on the RR. However, adjusting for hypertensive disorders and gestational diabetes lowered the RR to 1.85 (95% CI 1.76–1.95) and 1.66 (95% CI 1.54–1.72), respectively. Adjusting the model additionally for other labor induction indications (Model D) lowered the RR considerably (RR = 1.43, 95% CI 1.35–1.51; Table 3).
TABLE 3.
Crude and adjusted relative risk for labor induction among singleton births in Iceland in 1997–2018 by calendar time stratified by gestational and maternal age (N = 85 620)
| Crude model | n | Ref | 2002–2007 | 2008–2013 | 2014–2018 | |||
|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |||
| 85 620 | 1.12 | 1.05–1.17 | 1.63 | 1.55–1.71 | 1.91 | 1.81–2.01 | ||
| Adjusted stepwise | ||||||||
| + Sociodemographic a | 85 620 | Ref | 1.10 | 1.05–1.16 | 1.66 | 1.58–1.75 | 1.95 | 1.85–2.05 |
| + Hypertension b | 85 620 | Ref | 1.10 | 1.04–1.16 | 1.61 | 1.53–1.69 | 1.85 | 1.76–1.95 |
| + Gestational diabetes c | 85 620 | Ref | 1.07 | 1.02–1.13 | 1.55 | 1.47–1.63 | 1.66 | 1.54–1.72 |
| Fully adjusted model d | 85 620 | Ref | 1.02 | 0.97–1.08 | 1.40 | 1.33–1.47 | 1.43 | 1.35–1.51 |
| Stratified by gestational age d | ||||||||
| Early‐term (37+0–38+6 weeks) | 11 595 | Ref | 1.07 | 0.92–1.24 | 1.68 | 1.46–1.93 | 1.62 | 1.40–1.88 |
| Full‐term (39+0–40+6 weeks) | 46 554 | Ref | 1.03 | 0.93–1.14 | 1.54 | 1.40–1.69 | 1.39 | 1.26–1.53 |
| Late‐term (41+0–41+6 weeks) | 18 771 | Ref | 0.95 | 0.85–1.05 | 1.80 | 1.64–1.98 | 2.06 | 1.86–2.27 |
| Post‐term (>42+0 weeks) | 3280 | Ref | 1.09 | 0.98–1.21 | 1.15 | 1.01–1.31 | 1.33 | 1.15–1.54 |
| Stratified by maternal age e | ||||||||
| ≤25 years | 19 214 | Ref | 1.00 | 0.90–1.12 | 1.49 | 1.34–1.67 | 1.58 | 1.41–1.78 |
| 26–30 years | 27 591 | Ref | 1.02 | 0.92–1.12 | 1.43 | 1.30–1.58 | 1.49 | 1.35–1.65 |
| 31–34 years | 19 888 | Ref | 0.95 | 0.85–1.03 | 1.41 | 1.27–1.57 | 1.27 | 1.13–1.42 |
| 35–39 years | 14 755 | Ref | 0.94 | 0.83–1.07 | 1.31 | 1.17–1.48 | 1.27 | 1.12–1.44 |
| ≥40 years | 4172 | Ref | 0.88 | 0.71–1.11 | 1.35 | 1.09–1.67 | 1.53 | 1.23–1.91 |
Model adjusted for sociodemographic covariates: parity, age, citizenship, residency, marital and employment status.
Model adjusted for sociodemographic covariates and hypertension.
Model adjusted for sociodemographic covariates, gestational diabetes and gestational diabetes.
Model adjusted for sociodemographic covariates and the following labor indications: preeclampsia/eclampsia, hypertension, diabetes (gestational and pre‐existing), fetal distress, cholestasis, Rh immunization, maternal wellbeing, other and fetal wellbeing, other.
Model adjusted for parity, citizenship, residency, marital/employment status and gestational age; and the following labor indications: preeclampsia/eclampsia, hypertension, diabetes (gestational and pre‐existing), fetal distress, cholestasis, Rh immunization, maternal wellbeing, other and fetal wellbeing, other.
There was an increased risk of labor induction across time periods among all gestational age groups (Figure 1, Table 3). The increased risk was most evident among late‐term pregnancies (RR = 2.06, 95% CI 1.86–2.27; Table 3). Overall, the prevalence of late‐ and post‐term pregnancies decreased from 23.1% to 21.9% and from 6.1% to 2.0%, respectively. By the end of the study period, 16.1% of full‐term labors, 34.7% of late‐term labors and 83.2% of post‐term labors were induced (Table S2). Among pre‐term and early‐term births, the rate of induction was about 29%, and increased considerably over the study period (Table S2). Hypertensive disorders including preeclampsia and eclampsia explained about one in every three inductions among early‐term births (Table S3). Suspected placental insufficiency accounted for 18% and diabetes (pre‐gestational and gestational) for about 16% of early‐term inductions towards the end of the study period. Indications such as those included in the group maternal wellbeing, other accounted for 9.4% of inductions among early‐term births. An indication was missing in 11.7% of inductions.
FIGURE 1.
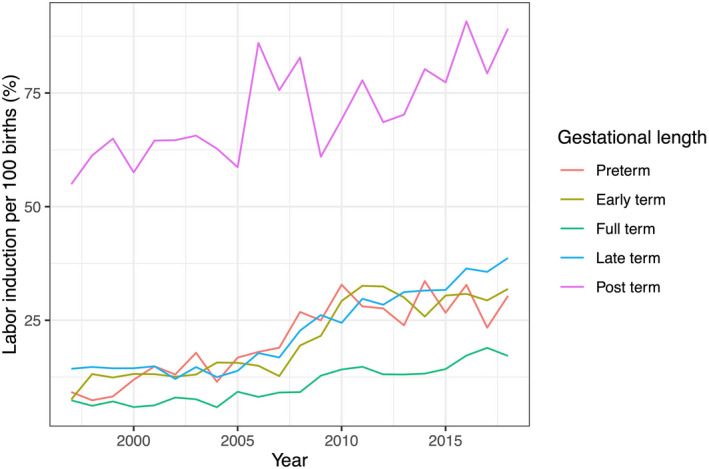
Labor induction rate per 100 singleton births among women in Iceland by gestational age (N = 85 620)
There was an increased risk of labor induction across time periods among all maternal age groups (Figure 2, Table 3). The risk was greatest among births with maternal age under 25 years or over 40 years (RR = 1.58, 95% CI 1.41–1.78 and RR = 1.53, 95% CI 1.23–1.91, respectively).
FIGURE 2.
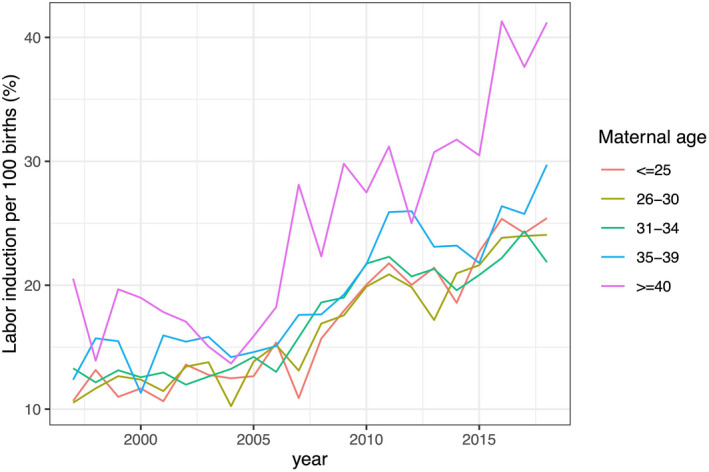
Labor induction rate per 100 singleton births among women in Iceland by maternal age (N = 85 620 singleton births)
4. DISCUSSION
In this population‐based study, we observed that an increase in labor induction was in part explained by an increase in underlying conditions indicating labor induction. The most common indications for induction in Iceland were prolonged pregnancy, hypertensive disorders and gestational diabetes. Overall, indications accounting for about half of all inductions (ie prolonged pregnancy and hypertensive disorders) are based on high‐quality evidence. However, at the end of the study period, a significant increase in induction was due to gestational diabetes, although there is little quality evidence to inform management between induction of labor at term or expectant management for women with gestational diabetes. At the end of the study period, we observed that about 9% of inductions were missing a medical diagnosis and we also observed an overall increase in early‐term induction.
According to a newly published systematic scoping review 13 and a recent Swedish multicenter randomized trial (SWEPIS), 7 the evidence to support induction for prolonged pregnancy is high. Labor induction beyond 41–42 weeks was associated with fewer perinatal deaths and reduced cesarean section rates, even though the number needed to treat to prevent perinatal mortality was relatively high (approximately 230–450). 7 , 13 Interestingly, all stillborn infants in the study were born to primiparous women, raising questions about whether the results also apply to multiparous women. A more recent population‐based register study from Sweden including almost 900 000 births supports this, as the results indicate only an association between gestational age and an increased risk of stillbirth among primiparous women. 17 An Icelandic study furthermore concluded that although the rates of adverse maternal and neonatal outcomes had decreased over a 20‐year period, there was no evidence that these improvements were mediated by the evident increased rate of labor induction. Keeping in mind that this study excluded stillbirth, both the Icelandic 3 and Swedish 17 studies raise important questions about whether the rise in induction due to prolonged pregnancy is truly based on sound evidence.
The prevalence of hypertensive disorders, other than preeclampsia/eclampsia, nearly doubled in the population over the study period and hypertensive disorders were increasingly an indication for induction. Globally, the prevalence of hypertension is rising among women of reproductive age; likely explanations include an increase in lifestyle‐related risk factors such as an unhealthy diet, obesity and physical inactivity. 18 There is little agreement on the timing of birth for women with chronic or gestational hypertension at term, but evidence indicates that planned birth between 37 and 39 weeks is associated with the lowest maternal and neonatal morbidity/mortality. 8 , 13
There was a considerable increase in numbers of women diagnosed with gestational diabetes over the study period. These changes are likely explained by two factors: first, a significant change in diagnostic criteria for gestational diabetes resulting in a larger group of women diagnosed with gestational diabetes, 19 and secondly, an overall increase in women with high body mass index in Iceland 20 and therefore lifestyle‐related illness. This has sparked a debate on the appropriate level for the diagnosis of gestational diabetes, especially considering the consequences that the diagnosis may have on labor and delivery, that is, induction of labor. In addition, there is little quality evidence to inform management of induction of labor at term or expectant management for women with gestational diabetes. 13 Studies comparing induction at term and expectant management among women with gestational diabetes have found limited differences between groups in terms of maternal and neonatal outcomes. 16 , 21 , 22 , 23 However, induction prior to 39 weeks increases the risk of Neonatal Intensive Care Unit admission and adverse neonatal outcomes among women with gestational diabetes. 16
In our study we were unable to determine whether inductions were elective, as this variable is not captured in the Icelandic Medical Birth Register. However, about 9% of births towards the end of the study period did not have a recorded diagnosis considered a likely indication. This is similar to findings in a recent Norwegian study, where 10% of all inductions were recorded as elective. 24 For a more complete understanding of induction in the absence of medical indication, we recommend that the IMBR be amended to include the registration of labor induction indication, as well as the option to record induction specifically by maternal request.
The neonatal risks of early‐term births and the potential neonatal complications associated with elective delivery prior to 39 weeks are well described. 25 , 26 Therefore, it is an interesting finding that early‐term inductions increased over the study period in our study and that indications such as subluxation of symphysis pubis, exhaustion and fatigue, and psychosocial problems as well as missing medical indication accounted for about 20% of induced early‐term births in 2014–2018. In addition, evidence for the benefits of early‐term induction for gestational diabetes in the absence of hypertension is weak and therefore questionable. This increase in early‐term induction may therefore suggest a shift in perspective of what is considered normal pregnancy length and has been demonstrated in previous studies as a recent distributional shift towards lower gestational ages in singleton pregnancies. 27 , 28 It is unclear, however, how much of this shift is related to elective inductions, although an association was observed between early‐term birth rates and clinician‐initiated obstetric interventions in a study of pre‐term and early‐term births in six high‐income countries in Europe and North America. 29
The main strength of our study is the use of data from a nationwide centralized Medical Birth Register with complete coverage of all births in Iceland over a 20‐year study period. The Nordic Registers are of high quality, with compulsory notification, and offer unique opportunities for clinical research with a collection of data spanning decades. 15
Limitations of our study mainly pertain to the reliability of the variables used in this study, as they have not been specifically validated in the IMBR. However, the use of internationally standardized diagnostic and surgical codes ensures that statistics are comparable between countries. Another limitation might be that the ICD‐10 diagnoses used to identify medical indications for the labor induction may not have been the direct indications for the induction. Even though the ICD‐10 diagnoses were registered, it was not clear whether they were the main indication for inducing labor. However, after an analysis of the ICD‐10 diagnoses, the most likely indication for the induction was chosen by an expert panel of consulting obstetricians and midwives. Finally, the analysis excluded births with premature rupture of membranes. Therefore, we were unable to assess induction trends for births with premature rupture of membranes.
5. CONCLUSION
Our data reveal an increase in labor induction in Iceland from 1997 to 2018, which is in part explained by an increase in obstetric indications for induction. Throughout the study period the most common indications for induction were hypertensive disorders and prolonged pregnancy. In 2014–2018, gestational diabetes emerged as one of the most common indications for labor induction. This is interesting despite the lack of evidence to support the benefits of induction for women with gestational diabetes. Induced labor was increasingly associated with the diagnosis of subluxation of symphysis pubis, exhaustion and fatigue, problems related to unspecific psychosocial circumstances, or supervision of other high‐risk pregnancies, even when labor was induced before 39 weeks of gestation. This may suggest an overall shift towards a lower threshold for labor induction. Our results highlight the need for international discussion and consensus around acceptable medical indications for labor induction as well as a discussion about elective induction.
AUTHOR CONTRIBUTIONS
Kristjana Einarsdottir, Johanna Gunnarsdottir and Emma Marie Swift designed the study. Emma Marie Swift performed the analysis, drafted the report and designed the figures and tables. All authors discussed the results and commented on the report, and approved the final version.
FUNDING INFORMATION
This study was funded by the University of Iceland Research Fund (Rannís). Helga Zoega was supported by a UNSW Scientia Program Award.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Supporting information
Table S1
Table S2
Table S3
Swift EM, Gunnarsdottir J, Zoega H, Bjarnadottir RI, Steingrimsdottir T, Einarsdottir K. Trends in labor induction indications: A 20‐year population‐based study. Acta Obstet Gynecol Scand. 2022;101:1422‐1430. doi: 10.1111/aogs.14447
REFERENCES
- 1. Hedegaard M, Lidegaard Ø, Skovlund C, Mørch L. Perinatal outcomes following an earlier post‐term labour induction policy: a historical cohort study. BJOG. 2015;122:1377‐1385. [DOI] [PubMed] [Google Scholar]
- 2. Swift EM, Tomasson G, Gottfreðsdóttir H, Einarsdóttir K, Zoega H. Obstetric interventions, trends, and drivers of change: a 20‐year population‐based study from Iceland. Birth. 2018;45:368‐376. [DOI] [PubMed] [Google Scholar]
- 3. Gunnarsdottir J, Swift E, Jakobsdottir J, Smárason A, Thorkelsson T, Einarsdóttir K. Cesarean birth, obstetric emergencies, and adverse neonatal outcomes in Iceland during a period of increasing labor induction. Birth. 2021;48:493‐500. [DOI] [PubMed] [Google Scholar]
- 4. Davies‐Tuck M, Wallace EM, Homer CS. Why ARRIVE should not thrive in Australia. Women Birth. 2018;31:339‐340. [DOI] [PubMed] [Google Scholar]
- 5. Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low‐risk nulliparous women. N Engl J Med. 2018;379:513‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drevin J, Hansson M. Swepis har lett till överilade ändringar i förlossningsrutiner ‐ Befogat att avbryta studien i förtid, men forskningen bör granskas i sin helhet innan slutsatser dras och rutiner ändras [SWEPIS has led to rash changes in routines within Swedish obstetric care]. Lakartidningen. 2020;117:1‐2. [PubMed] [Google Scholar]
- 7. Wennerholm U‐B, Saltvedt S, Wessberg A, et al. Induction of labour at 41 weeks versus expectant management and induction of labour at 42 weeks (SWEdish Post‐term induction study, SWEPIS): multicentre, open label, randomised, superiority trial. BMJ. 2019;367:l6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Broekhuijsen K, van Baaren G‐J, Van Pampus MG, et al. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT‐II): an open‐label, randomised controlled trial. Lancet. 2015;385:2492‐2501. [DOI] [PubMed] [Google Scholar]
- 9. Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre‐eclampsia after 36 weeks' gestation (HYPITAT): a multicentre, open‐label randomised controlled trial. Lancet. 2009;374:979‐988. [DOI] [PubMed] [Google Scholar]
- 10. Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Metzger BE, Contreras M, Sacks D, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991‐2002. [DOI] [PubMed] [Google Scholar]
- 12. Oron G, Hirsch R, Ben‐Haroush A, et al. Pregnancy outcome in women with heart disease undergoing induction of labour. BJOG. 2004;111:669‐675. [DOI] [PubMed] [Google Scholar]
- 13. Coates D, Makris A, Catling C, et al. A systematic scoping review of clinical indications for induction of labour. PloS One. 2020;15:e0228196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Excellence NifC . Induction of labour. Clinical guideline. 2008. https://www.nice.org.UK/guidance/cg70
- 15. Langhoff‐Roos J, Krebs L, Klungsøyr K, et al. The Nordic medical birth registers–a potential goldmine for clinical research. Acta Obstet Gynecol Scand. 2014;93:132‐137. [DOI] [PubMed] [Google Scholar]
- 16. Vitner D, Hiersch L, Ashwal E, Shmueli A, Yogev Y, Aviram A. Induction of labor versus expectant management for gestational diabetes mellitus at term. Arch Gynecol Obstet. 2019;300:79‐86. [DOI] [PubMed] [Google Scholar]
- 17. Lindegren L, Stuart A, Herbst A, Källén K. Stillbirth or neonatal death before 45 post‐menstrual weeks in relation to gestational duration in pregnancies at 39 weeks of gestation or beyond: the impact of parity and body mass index. A National Cohort Study. BJOG. 2022;129:761‐768. [DOI] [PubMed] [Google Scholar]
- 18. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Landspitali University Hospital . Clinical guidelines for screening, diagnosis and treatment for gestational diabetes (Klínískar leiðbeiningar um skimun, greiningu og meðferð sykursýki á meðgöngu). 2012.
- 20. Thórsson B, Aspelund T, Harris TB, Launer LJ, Gudnason V. Thróun holdafars og sykursýki í 40 ár á Islandi [trends in body weight and diabetes in forty years in Iceland]. Laeknabladid. 2009;95:259‐266. [PubMed] [Google Scholar]
- 21. Bettikher OA, Zazerskaya IE, Popova PV, Kustarov VN. A comparison of the clinical outcomes of induced and spontaneous labour in patients with gestational diabetes. Diabetes Mellitus. 2016;19:158‐163. [Google Scholar]
- 22. Grabowska K, Stapińska‐Syniec A, Saletra A, Jarmużek P, Bomba‐Opoń D. Labour in women with gestational diabetes mellitus. Ginekol pol. 2017;88:81‐86. [DOI] [PubMed] [Google Scholar]
- 23. Melamed N, Ray JG, Geary M, et al. Induction of labor before 40 weeks is associated with lower rate of cesarean delivery in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2016;214(364):e1‐e8. [DOI] [PubMed] [Google Scholar]
- 24. Dögl M, Romundstad P, Berntzen LD, et al. Elective induction of labor: a prospective observational study. PloS One. 2018;13:e0208098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tita AT, Landon MB, Spong CY, et al. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360:111‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clark SL, Miller DD, Belfort MA, Dildy GA, Frye DK, Meyers JA. Neonatal and maternal outcomes associated with elective term delivery. Am J Obstet Gynecol. 2009;200(156):e1‐e4. [DOI] [PubMed] [Google Scholar]
- 27. Nassar N, Schiff M, Roberts CL. Trends in the distribution of gestational age and contribution of planned births in New South Wales, Australia. PLoS One. 2013;8:e56238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diniz CSG, de Miranda MJ, Reis‐Queiroz J, Queiroz MR, de Oliveira Salgado H. Why do women in the private sector have shorter pregnancies in Brazil? Left shift of gestational age, caesarean section and inversion of the expected disparity. J Hum Growth Dev. 2016;26:33‐40. [Google Scholar]
- 29. Richards JL, Kramer MS, Deb‐Rinker P, et al. Temporal trends in late preterm and early term birth rates in 6 high‐income countries in North America and Europe and association with clinician‐initiated obstetric interventions. JAMA. 2016;316:410‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3


