Abstract
Introduction
While there is growing interest in applying patient‐reported measures (PRMs) in clinical routine, limited collective evidence of the impact of PRMs hinder their widespread use in specific contexts, such as maternity care. Our objective was to synthesize existing emperical evidence on the impact of implementing PRMs in routine maternity care.
Material and methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta‐analyses guidelines (version 2020). We electronically searched six databases for the literature on the implementation of PRMs in maternity care. A multi‐level (woman, clinical, organizational, national and societal) analytic framework for analyzing and synthesizing emperically proven impacts of PRMs was developed. Quality was assessed using the Mixed Method Appraisal Tool. The GRADE‐CERQual approach was used to assess the confidence in the review findings and arguments. The protocol was registered in PROSPERO (CRD42021234501).
Results
Overall, 4971 articles were screened. The emperical evidence, collected from 11 relevant studies, showed that the use of PRMs in routine maternity care could produce positive effects on clinical process (assessment and detection of health problems, clinical visit preparation, resource use, woman–professional communication, decision‐making, woman–professional relationship, and care quality), and health behavior and outcomes (women's health and wellbeing, quality of life, health behavior, experiences and satisfaction with healthcare services), awareness, engagement and self‐management of own health, and disclosure of health issues. The confidence in the review findings was low to moderate due to a limited number of studies, inadequate data and methodological limitations of included studies.
Conclusions
The limited emperical evidence available suggested that the use of PRMs may have positive effects at the individual health level and clinical process level. However, the evidence was not strong enough to provide policy recommendations on the use of PRMs in routine maternity care. This review revealed limitations of currently available research, such as lack of generalizability and narrow scopes in investigating impact. Efforts are needed to improve the quality of research on the use of PRMs in routine maternity care by widening the study population, including different types of PRMs, and considering the effects of PRMs at different levels and domains of healthcare.
Keywords: healthcare quality, impact, implementation, maternity care, patient reported measure, pregnancy and childbirth
The limited emperical evidence tentatively suggested that the routine use of patient‐reported measures in maternity care could have positive effects at individual health level and clinical level, but currently available evidence was far from convincing and consistent, and the confidence on the review findings was low to moderate.

Abbreviations
- GRADE‐CERQual
confidence in the evidence from reviews of qualitative research
- PRM
patient‐reported measure
- SRM
self‐reported measures
Key message.
Limited emperical evidence suggests that the routine use of patient‐reported measures in maternity care may have positive effects on individual health and clinical process, but current evidence is insufficient, and the confidence in the review findings was low to moderate.
1. INTRODUCTION
Healthcare processes, outcomes and quality are currently measured, but most measurements are performed for the professional side, at the clinical process level (eg preoperative antibiotic coverage before cesarean section), clinical outcomes level (eg glycemic index and blood pressure) or at the public health level (eg disease‐specific mortality rates). They are insufficient to fully capture the effects of care on a patient's health status or quality of life. For a greater positive impact on patient satisfaction, safety and wellbeing, performance measurement of healthcare services should include experiences and outcomes as viewed by patients. Patient‐reported measures (PRMs) or self‐reported measures (SRMs) mainly include patient‐reported outcome measures (PROMs) and patient‐reported experience measures (PREMs) and are commonly administrated as standardized, multi‐item, self‐completed questionnaires. These measures help collect information directly from patients about their health status, health‐related quality of life and health service experiences. 1 , 2 , 3 , 4 They are expected to play a more crucial role in improving healthcare quality by promoting patient‐centered care and value‐based care. 5 , 6 , 7 Over the past decades, there has been growing interest in the developing and using PRMs not only in research but also in routine clinical practice. The routine use of PRMs seems particularly important in maternity care, as it has the critical responsibility of monitoring women's health during the course of pregnancy and postnatal period to optimize their physical and mental wellbeing, understanding women's views, perceptions and experiences, maximizing favorable maternal and perinatal outcomes and improving quality of life for both women and their families. 2
Some potential impacts of the use of PRMs in clinical routines have long been identified. 8 , 9 , 10 , 11 , 12 They include empowering patients, informing clinicians' decisions, and improving the processes and outcomes of care that contribute to healthcare quality. 8 , 9 , 10 , 11 , 12 However, widespread use of PRMs in healthcare systems, particularly in the field of pregnancy and childbirth, is still limited. 5 , 13 This is partially due to inadequate and inconsistent emperically proven evidence showing the impact of the routine use of PRMs. Previous studies and reviews within specific clinical settings, such as cancer care, management of chronic diseases, and palliative care, indicated that PRMs may have complex and heterogeneous effects on care process and outcomes, influencing patient engagement, patient satisfaction, physician–patient communication, patient health behavior, clinical decision making, length of clinical encounter, health outcomes, etc. 8 , 11 , 12 , 14 , 15 , 16 , 17 , 18
To our knowledge, none of the previously collective evidence on the impact of PRMs was specific to maternity care. The evidence and knowledge from other clinical settings in terms of favoring or opposing the routine use of PRMs is fragmented and may not be generalizable across study populations of pregnant and postpartum women. Without clear and convincing emperical evidence, it is premature to make definitive policy or practice recommendations for the use of PRMs in routine maternity care. Thus, there is a need to synthesize existing evidence on the implementation of PRMs in routine maternity care before promoting their use. In this study, we systematically reviewed the literature on the implementation of PRMs in routine maternity care and qualitatively synthesized emperical evidence specifically regarding their impact on maternity care process and outcomes.
2. MATERIAL AND METHODS
In this present work we synthesize and present the emperical evidence on the impact of implementing PRMs in routine maternity care as a part of a larger systematic literature review project that explores existing evidence, knowledge and experience of implementing and using PRMs in routine maternity care. The protocol is registered in the Prospective Register of Systematic Reviews (PROSPERO) database (CRD42021234501). This review is reported following the Preferred Reporting Items for Systematic Reviews and Meta‐analyses guidelines 2020 (PRISMA 2020) where applicable. 19 , 20 , 21
2.1. Searches
The literature search followed the PRESS (Peer Review of Electronic Search Strategies) 2015 guideline. 22 Two researchers (AC and KV) developed the primary search strategy, which was reviewed by researcher PT. One librarian provided technical support. The search terms and strategies were informed by previous reviews on the use of PRMs in clinical routines. 2 , 9 , 23 The initial searches, starting in January 2021, were conducted in the following important and popular electronic databases in healthcare and medicine, which were accessible to the researchers: PsycARTICLES, PubMed (NCBI), Scopus, Web of Science, Cochrane Database of Systematic Reviews and Cumulative Index to Nursing and Allied Health Literature (CINAHL). The search terms were derived from three overarching concepts: patient‐reported measure, maternity care and implementation. The full strategy of the initial search conducted in different databases is provided in Table S1. In additional searches, a snowballing strategy was applied by going through references in the articles already included in the study, as well as articles citing them. We also searched the studies included in previous reviews that identified PRMs used in pregnancy and childbirth. 2 , 24 , 25 , 26 , 27 , 28 , 29 , 30 Additional searches were continued until no other relevant studies were found.
2.2. Eligibility criteria
In this review, we defined PRMs as self‐administrated questionnaires, instruments or tools that help to collect information directly from patients, which measure (1) patients' health status, (2) patients' perceived effects of treatments and interventions on their health and (3) patient experiences and satisfaction with health services. 9 , 31 , 32 A PRM was considered to be a standardized or validated measure if the study itself or another published study reported the measure's validity, reliability, sensitivity or responsiveness, as described by the Consensus‐Based Standards for the Selection of Health Measurement Instruments (COSMIN) group. 29 We only included studies that applied at least one validated PRM to collect data. In Table S6, validated PRMs used in included studies are presented.
We built a review database with studies that provided evidence‐based knowledge or practical experience of using PRMs in routine maternity care. We focused on the routine use of PRMs for assessing pregnant or postpartum women's health status across care process, monitoring women's health progress, measuring the results of treatment, and evaluating the service quality. Studies reporting the use of PRM in other clinical fields rather than pregnancy and childbirth or studying the implementation of PRM in healthcare in general were excluded. We also excluded studies that were concerned solely with the devolvement and/or validation of PRMs rather than their implementation in routine clinical practice. Table S2 provides a full list of the inclusion and exclusion criteria used for building the review database.
For the analysis of the impact of using PRMs in routine maternity care, we only selected from our review database the post‐implementation studies that provided emperical evidence (based on observation, measurement or actual experience, rather than belief, expectation or theoretical formulation) of the impact of the use of PRMs in routine maternity care.
2.3. Study screening and selection
Studies retrieved from the identified databases were imported to Endnote 20 for screening. After duplicates were eliminated, a 2‐step screening process was performed to form our review database. First, “title screening” was performed. Researcher AC screened the studies using predetermined exclusion criteria and categorized them into “removed after title screening” and “remaining after title screening” groups for researcher KV to check. Consensus was reached through discussion or consultation with a third researcher (PT). Subsequently, “abstract screening” was conducted. Two researchers (AC and KV) independently read the remaining abstracts after title screening and categorized the studies (1—included, 2—excluded and 3—not sure). Cohen's kappa coefficient was used to measure the inter‐rater reliability of the abstract screening. Table S3 shows that the level of agreement between the researchers (98.48%) was high, and the inter‐rater reliability (0.81) was almost perfect. Disagreements between the researchers (AC and KV) in this step were resolved through discussion or by involving a third researcher (PT). The exclusion criteria were refined during the discussions. Title and abstract screening produced a list of potentially eligible studies. The full texts of these studies were retrieved and assessed by researcher AC against inclusion and exclusion criteria and checked by KV. After several rounds of full‐text reading and discussions between the primary researchers, a review database was generated, from which researcher AC made a further selection and identified post‐implementation studies that provided emperical evidence of the impact of the use of PRMs in routine maternity care. Researcher KV double‐checked the selection.
2.4. Data extraction
Based on this review's purpose, informed by earlier reviews on the implementation of PRMs, and applying standard instruments developed by the Cochrane Collaboration 33 , 34 and Joanna Briggs Institute, 35 a data extraction form was created in Microsoft EXCEL. The data extraction form was piloted in two articles and improved based on the pilot by two researchers (AC and KV). The information extracted from each study included study characteristics (eg title, author, country, study type/design/methods), implementation details (eg implementation context/setting, purpose of administrating PRMs, validated instruments in use), key findings, author's interpretation of results, author‐proposed recommendations and suggestions for PRM implementation, and author‐identified limitations and future research opportunities. Table S4 lists all the items defined in the data extraction form. In the formal extraction process, data were extracted by researcher AC and checked for accuracy by researcher KV.
2.5. Data analysis and synthesis
In this review, we descriptively and qualitatively synthesized evidence on how the use of PRMs in routine maternity care would change maternity care process, outcomes and even the service system, which could be observed by researchers or perceived and reported by women and other stakeholders. Thematic analysis combined with narrative synthesis was performed. 36 Informed by concepts, constructs and frameworks used in previous research 8 , 11 , 12 , 16 , 17 , 18 , 31 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 for assessing the impact of using PRMs in clinical practice and based on discussions and workshops within the research team, we developed a multi‐level (patient, clinical, organizational, national and level) analytic framework for analyzing and synthesizing the “PRMs impact”‐related emperical evidence presented in included studies. Under each level, there were different domains of impact. Table S5 shows the analytic framework. All the quantitative and qualitative evidence about the impact of using PRMs presented in each study was identified and interpreted by two researchers (AC and KV), placed at appropriate levels of a predefined framework, and grouped into certain domains. The evidence (identified in Results, Findings and Conclusions) reflecting similar effects was descriptively gathered, and the original texts showing the evidence were extracted and kept. Quantitative data was converted into qualitative description or interpretation. After aggregating the evidence from included studies, we identified the patterns across the studies and made a summary for each domain.
2.6. Quality and confidence assessment
As the studies selected for this review used a range of study designs, and evidence generated by the studies was presented in various forms, the Mixed Method Appraisal Tool (Version 2018) 45 that enables researchers to separately score the quality of different types of studies and deliver an integrative assessment of the literature base was applied to assess the quality and risk of bias of the included studies. First, the studies were assessed using two general criteria: (1) Are there clear research questions? (2) Do the collected data address the research questions? The studies that passed the first‐step assessment were grouped into different categories and scored for quality against five appraisal criteria specific to study types (study is given one point if meeting one criterion; 5 is the full score). Table S7 shows the use of Mixed Method Appraisal Tool and the specific appraisal questions for different types of studies. Studies with a score of 1/5 or 2/5 from the appraisal were deemed to be of low quality, studies with 3/5 or 4/5 moderate quality, and studies with a full score of 5 were deemed to have a high quality. Two researchers (AC and KV) independently assessed the quality of each study, cross‐checked the results of the appraisals, and reached a consensus after discussions. No studies were excluded if they passed the first step of assessment by Mixed Method Appraisal Tool, because this review purposely collected all relevant evidence, knowledge and experience on the implementation of maternity care‐related PRMs.
The GRADE‐CERQual (confidence in the evidence from reviews of qualitative research) approach was applied to evaluate the reliability of the evidence gathered by this review and assess the confidence in the findings and arguments generated by this review. 42 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 The findings in each domain were assessed separately. Table S8 shows the use of the GRADE‐CERQual tool in this review.
3. RESULTS
3.1. Selection and inclusion of studies
Overall, 4971 records were retrieved from electronic searches in PsycARTICLES (249), PubMed (1318), Scopus (876), Web of Science (1435), Cochrane Database of Systematic Reviews (187) and CINAHL (906). After eliminating duplicates, abstract browsing and full‐text reading, five studies from the initial search were added to our review database. Starting with these five studies, we conducted an extensive additional search that helped identify another 21 studies. Consequently, a total of 26 studies were included in our database for the systematic review on the implementation of PRMs in routine maternity care. Of these, 11 studies were considered eligible for this review that collected emperical evidence of the impact of the use of PRMs in maternity care. The search, screening and selection processes are described in Figure 1.
FIGURE 1.
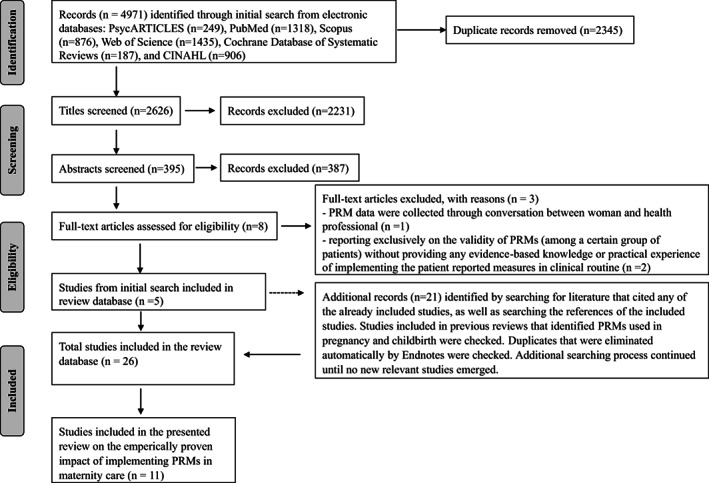
Flow diagram of search, screening and selection process of studies included in the systematic review. PRM, patient‐reported measure.
3.2. Characteristics of studies included in the review on the impact of PRMs in maternity care
Eleven studies, 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 published between 2004 and 2021, were included in this review. An overview of these studies with selected basic information and the evidence of the impact of PRMs is provided in Table S6. Table 1 summarizes the characteristics of the studies in terms of countries, geographic areas, publication types, PRM data collection approaches, health or healthcare issues addressed by PRMs, study designs and study participants.
TABLE 1.
Summary of the characteristics of included studies
| Characteristics of included studies | Overall (n = 11) |
|---|---|
| Countries | |
| Australia | 4 (36.36%) |
| Canada | 2 (18.18%) |
| United Kingdom | 1 (9.10%) |
| Netherlands | 1 (9.10%) |
| Denmark | 1 (9.10%) |
| Spain | 1 (9.10%) |
| Japan | 1 (9.10%) |
| Geographic areas | |
| Europe | 4 (36.36%) |
| Australia | 4 (36.36%) |
| North America | 2 (18.18%) |
| Asia | 1 (9.10%) |
| Publication types | |
| Journal article | 9 (81.82%) |
| Conference paper | 2 (18.18%) |
| PRM data collection approach | |
| Digital | 9 (81.82%) |
| Paper‐based | 3 (27.27%) |
| Issues addressed by PRMs | |
| Mental health related issues | 9 (81.82%) |
| Multiple issues | 2 (18.18%) |
| Methodology | |
| Quantitative studies | 8 (72.73%) |
| Qualitative studies | 3 (27.27%) |
| Study participants (n = 4971) | |
| Women (n = 4965, 99.88%) | 11 (100%) |
| Health professionals and other stakeholders (n = 6, 0.12%) | 1 (9.10%) |
3.3. Findings about the impact of implementing PRMs in routine maternity care
From the collected evidence, this review identified the impact of using PRMs in maternity care routine at two levels: woman level (ie “patient level” of the predeveloped analytic structure) and clinical level. The three other levels from the predefined analytic framework (organizational, national and societal) were not addressed in the studies.
Nine studies 54 , 55 , 56 , 57 , 58 , 59 , 61 , 62 , 64 provided evidence of the impact of using PRMs in maternity care routine at the woman level. The collected evidence showed that the use of PRMs could help to improve women's health, quality of life and well‐being, change women's health behavior, improve women's experiences and satisfaction with healthcare services, and increase women's awareness, engagement and self‐management of their health; it can also help women in disclosing information they may have not otherwise been able or comfortable to disclose.
Six studies 54 , 57 , 58 , 60 , 61 , 63 provided evidence of the impact of using PRMs in routine maternity care at the clinical level. The collected evidence showed that the use of PRMs could help to detect and assess health problems, help in preparing clinical visits and in better using clinical resources, support communication during visits, facilitate shared decision‐making, support the woman–health professional relationship, and help in delivering appropriate, personalized care. Tables 2 and 3 summarize the emperical evidence that shows the impact of using PRMs in maternity care routine at the woman level and the clinical level, respectively.
TABLE 2.
Synthesized emperical evidence of the impact of implementing self‐reported measures in routine maternity care—woman level
| Domains | Review of findings with evidence extracted from studies |
|---|---|
|
Women's health, quality of life, and well‐being (study n = 1) |
The use of self‐reported measures can improve women's well‐being. Evidence from survey studies
|
|
women's health behavior (study n = 1) |
The use of self‐reported measures can change health behaviors to support wellness. Evidence from survey studies
|
|
Women's experiences and satisfaction with healthcare services (study n = 3) |
The use of self‐reported measures can make women feel heard, cared for and supported, and feel more comfortable in seeking support for their health. Evidence from survey studies
|
|
Women's awareness, engagement and self‐management (study n = 6) |
The use of self‐reported measures can help women reflect on their health behavior and lifestyle, pay closer attention to their health, increase awareness of their health status and risks, learn about and understand both normal and abnormal aspects of pregnancy and childbirth, such as risk factors, concerning symptoms and other health issues, and manage their own health. Evidence from survey studies
Evidence from interview studies
|
|
Disclosure (study = 7) |
The use of self‐reported measures that had structured inquiries, offered enough time for women to think, and were delivered with a sense of anonymity, support disclosure. Evidence from survey studies
Evidence from interview studies
Evidence from observation studies
|
Abbreviation: PREM, patient reported experience measure.
TABLE 3.
Synthesized emperical evidence of the impact of implementing self‐reported measures in routine maternity care—clinical level
| Domains | Review findings with evidence extracted from studies |
|---|---|
|
Health problem detection, assessment and diagnosis (study = 6) |
The use of self‐reported measures can help to detect and assess health problems and identify important issues. Evidence from survey studies
Evidence from interview studies
Evidence from observation studies
|
|
Woman–health professional communication (study = 2) |
The use of self‐reported measures can support clinical visits. Evidence from survey studies
Evidence from interview studies
|
|
Resources utilization (study = 2) |
The use of self‐reported measures can help to prepare clinical visits, properly use visit time, and save time for health professionals. Evidence from survey studies
Evidence from interview studies
Evidence from observation studies
|
|
Shared decision making (study = 1) |
The use of self‐reported measures can support shared decision‐making. Evidence from survey studies Over half (58%) of women agreed self‐reported measures supported shared decision making. (Depla et al. 61 ) |
|
Woman–health professional relationship (study = 2) |
The use of self‐reported measures can support the woman–health professional relationship. Evidence from survey studies
Evidence from interview studies
|
|
Personalized care (study = 2) |
The use of self‐reported measures can support appropriate, personalized care. Evidence from survey studies
Evidence from interview studies
|
3.4. Assessment of included studies and synthesized evidence
The overall quality of these 11 studies included in this review was acceptable. Studies varied in methodologic quality, from moderate to high. Five 55 , 56 , 57 , 62 , 64 (45.5%, three qualitative studies 55 , 57 , 62 and two quantitative studies 56 , 64 ) were rated as high quality and six (54.5%, all quantitative studies) 54 , 58 , 59 , 60 , 61 , 63 as medium quality. Main methodologic limitations identified across quantitative studies included insufficient information about the representativeness of samples to the target population (n = 4), 58 , 59 , 60 , 61 and the obscurity in the risk of bias caused by nonresponse and missing data (n = 3). 58 , 61 , 63 The confidence in the review findings was low to moderate, mainly because of methodologic limitations and data inadequacy of original studies. Generally, data and findings were reasonably consistent within and across all studies. However, there was one conflicting observation: whereas the evidence from Austin et al., 64 Doherty et al., 58 and Nishizono‐Maher et al. 54 indicated that self‐reported measures could help to better detect health problems and identify important issues when compared to other modes of inquiry (eg interviewer‐administrated measures), Reilly et al. 60 showed that there were no significant differences in the detection of women with current major depression between the interviewer‐administered and self‐administered versions. Reilly et al. 60 also showed that a greater proportion of women in the interviewer‐administered phone group as compared with women in the self‐completed online group met criteria for current minor depression and reported a past depressive or a past anxiety disorder. The quality assessment of the studies included in the review and the confidence in review findings are shown in Tables S7 and S8.
4. DISCUSSION
This review, which qualitatively synthesizes emperical evidence specifically regarding the impact of the use of PRMs in routine maternity care, tentatively suggests that the systematic use of PRMs may have positive effects on maternity care processes and health outcomes. More specifically, it suggests that PRMs may positively influence multiple aspects of routine maternity care, such as women's childbearing‐related health behavior, women's experiences and satisfaction with maternity care services, women's awareness and engagement in managing their own health, disclosure of health and general life issues to health professionals, general detection and assessment of health problems, preparation for clinical visits, utilization of clinical resources, communication between women and health professionals, shared decision‐making, the woman–professional relationship, and overall quality of care. The evidence collected from the literature was generally consistent. Our observations support the findings of some previous systematic reviews on the impact of the routine use of PRMs in different medical specialties and contexts. Focusing on treatment for non‐malignant pain, Holmes et al. 42 found that PRMs impacted the patient outcomes, helped in assessment, had an effect on patient engagement, facilitated shared decision‐making, improved communication between patients and clinicians, and influenced the therapeutic relationship. In an oncologic setting, Chen et al. 11 also identified convincing evidence of the impact of PRMs in improving patient–provider communication, patient satisfaction and the detection of unrecognized problems. A review by Marshall et al. 12 found consistent evidence showing that PRMs have a fairly substantial positive impact on the detection of mental health conditions. However, the heterogeneity of research designs and measurements of “impact” applied in included studies prevented the performance of meta‐analyses.
Our review found mixed evidence regarding the impact of PRMs on the detection of health problems: while three studies 54 , 58 , 63 reported quantitative evidence showing that PRMs could increase the detection rate of health problems, one study, 60 which also reported quantitative evidence, indicated that PRMs may not significantly influence detection and that any influence may depend on the severity of health issues. The weakness and inconsistency of evidence about the impact of PRMs in detection add to concerns about whether higher detection by PRMs could prevent women from being left unsupported or lead to unnecessary referral for additional assessment at the expense of scarce health resources. 60
We observed that a high portion of studies included in this review (9 of 11) used PRMs that specifically addressed mental health‐related issues. This observation was consistent with the finding of Dickinson et al. 2 that five of six studies included in their review used mental health‐specific PRMs or PRMs including mental health‐related questions, and that 12 of 14 PRMs used in reviewed studies were concerned with mental health issues during pregnancy and childbirth. This might be due to the phenomenon that mental health‐related PRMs are more commonly and widely used in maternity care than PRMs that address other health issues. Thus, the findings about the impact of using PRMs in routine maternity care may not be generalizable to all maternity care‐related PRMs.
Due to the primary concerns related to the low volume of participants, limited diversity of population, narrow scopes, methodologic limitations and lack of generalizability of primary studies identified and included in this review, our confidence in the review findings was low to moderate. Similar to many other reviews conducted on the impact of PRMs, 11 , 16 , 18 , 42 , 43 due to insufficient evidence we were unable to build a systematic and comprehensive understanding of how PRMs might impact clinical practices, health outcomes and care quality. Thus, the full potential of PRMs remains unknown.
The insufficiency of current evidence requires more research including various measures, diverse outcomes, wider populations and better quality data. Different validated and standardized maternity related measures (general or specific) should be considered in future studies. Further research is clearly needed to provide specific evidence addressing whether PRMs have any effects on domains at the organizational level (eg resource arrangement and allocation, frequency of resource use, operational efficiency and managerial decision‐making), the regional and national level (eg benchmarking and learning across institutions and health sectors) and the society level (eg family and population wellbeing). In the included studies, implementation of PRMs was evaluated mainly based on women's perceptions and experiences. More frontline professionals working in maternity care should be consulted and included in study population, and the changes in healthcare process and outcomes should also be quantitatively measured. More attention needs to be paid to middle‐ and low‐income countries and regions. Methodologically stronger studies, such as well‐planned and properly executed process and outcome evaluation using appropriate, standard methods, are warranted to evaluate the impact of using PRMs in maternity care routine and exploring associated mechanisms.
To our knowledge, this is the first systematic review on the impact of using PRMs in routine maternity care. This review followed the updated version of the PRISMA. For this review, we carried out a comprehensive search for eligible studies, using multiple electronic databases, followed by thorough manual searching. Although we did not search Embase separately, as the Scopus database includes almost all Embase content as well as the Embase index terms, it is unlikely that we have missed any relevant literature. Our analysis was guided by a well‐developed framework, ensuring that this topic was systematically examined. Although thorough and comprehensive searches were performed to identify potential studies for inclusion, the searches might still be inefficient. We assessed the search using Peer Review of Electronic Search Strategies (PRESS) 2015 Evidence‐Based Checklist 22 (please see Table S9). The fact that our initial search identified five studies while 21 were obtained through additional searching was probably due to the limitations in the search terms. Some terms appearing in studies obtained by additional search, such as “self‐administrated” and “self‐completed”, were commonly used for research‐oriented surveys rather than routine care practice‐related studies, so they were not included in the initial search. The use of term “screening” resulted in a large volume of irrelevant literature and therefore this term was not included in the initial search. During search, we realized that there was no standard definition of PRM and that the shortages in terminology development and standardization of this concept made the search challenging. To make up for those shortages in the initial search, we applied a very extensive additional search. In addition, we have to acknowledge that this systematic review only included articles written in certain languages, which may limit its international scope and generalizability. Furthermore, there is a possibility that some implementation projects may have taken place but were not reported, as we did not include unpublished data in this systematic review.
5. CONCLUSION
We systematically reviewed studies that have assessed the impact of implementing PRMs in routine maternity care. Tentative and limited evidence suggests that the use of PRMs may have positive effects at the individual health level and the clinical practice level. Although the findings were subject to considerable uncertainty and provided little support for policy recommendations on the use of PRMs in routine maternity care, this review provided insights into the current status of evidence available in this area that may inform future research and implementation work related to the use of PRMs in maternity care as well as in other clinical settings.
AUTHOR CONTRIBUTIONS
AC: conceptualization, methodology, formal analysis, investigation, data curation, writing ‐ original draft, writing ‐ review & editing, visualization, funding acquisition. KV: conceptualization, methodology, formal analysis, investigation, writing ‐ review & editing, funding acquisition. AS: formal analysis, investigation, writing ‐ review & editing. RL, PL, SH: conceptualization, methodology, investigation, writing ‐ review & editing, funding acquisition. AT: conceptualization, methodology, investigation, writing ‐ review & editing, funding acquisition, resources, project administration. GA: conceptualization, methodology, investigation, writing ‐ review & editing.
FUNDING INFORMATION
This study is financially supported by National Research Foundation for University‐Level Research in Finland (HUS/358/2020‐TYH2021127) and Finland PoDoCo Foundation of Economic Education.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Supporting information
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Table S8
Table S9
ACKNOWLEDGMENTS
Special gratitude is owed to information specialists and librarians at Aalto University who supported this review work.
Chen A, Väyrynen K, Schmidt A, et al. The impact of implementing patient‐reported measures in routine maternity care: a systematic review. Acta Obstet Gynecol Scand. 2022;101:1184‐1196. doi: 10.1111/aogs.14446
Aydin Tekay and Ganesh Acharya contributed equally to this study.
DATA AVAILABILITY STATEMENT
Please contact the corresponding author to request original database, codes and other materials.
REFERENCES
- 1. Barr PJ, Elwyn G. Measurement challenges in shared decision making: putting the “patient” in patient‐reported measures. Health Expect. 2016;19:993‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dickinson F, McCauley M, Smith H, van den Broek N. Patient reported outcome measures for use in pregnancy and childbirth: a systematic review. BMC Pregnancy Childbirth. 2019;19:155‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krawczyk M, Sawatzky R, Schick‐Makaroff K, et al. Micro‐Meso‐macro practice tensions in using patient‐reported outcome and experience measures in hospital palliative care. Qual Health Res. 2019;29:510‐521. [DOI] [PubMed] [Google Scholar]
- 4. Kingsley C, Patel S. Patient‐reported outcome measures and patient‐reported experience measures. BJA Educ. 2017;17:137‐144. [Google Scholar]
- 5. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167. [DOI] [PubMed] [Google Scholar]
- 6. Miller D, Steele Gray C, Kuluski K, Cott C. Patient‐centered care and patient‐reported measures: let's look before we leap. Patient. 2015;8:293‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jenkinson C, Coulter A, Bruster S. The Picker Patient Experience Questionnaire: development and validation using data from in‐patient surveys in five countries. Int J Qual Health Care. 2002;14:353‐358. [DOI] [PubMed] [Google Scholar]
- 8. Valderas J, Kotzeva A, Espallargues M, et al. The impact of measuring patient‐reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17:179‐193. [DOI] [PubMed] [Google Scholar]
- 9. Briggs MS, Rethman KK, Crookes J, et al. Implementing patient‐reported outcome measures in outpatient rehabilitation settings: a systematic review of facilitators and barriers using the consolidated framework for implementation research. Arch Phys Med Rehabil. 2020;101:1796‐1812. [DOI] [PubMed] [Google Scholar]
- 10. Freel J, Bellon J, Hanmer J. Better physician ratings from discussing PROs with patients. NEJM Catalyst. 2018. Accessed April 20, 2022. Available from: https://catalyst.nejm.org/ratings‐patients‐discussing‐pros/ [Google Scholar]
- 11. Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13:1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marshall S, Haywood K, Fitzpatrick R. Impact of patient‐reported outcome measures on routine practice: a structured review. J Eval Clin Pract. 2006;12:559‐568. [DOI] [PubMed] [Google Scholar]
- 13. Black N, Burke L, Forrest CB, et al. Patient‐reported outcomes: pathways to better health, better services, and better societies. Qual Life Res. 2016;25:1103‐1112. [DOI] [PubMed] [Google Scholar]
- 14. Espallargues M, Valderas JM, Alonso J. Provision of feedback on perceived health status to health care professionals: a systematic review of its impact. Med Care. 2000;38:175‐186. [DOI] [PubMed] [Google Scholar]
- 15. Antunes B, Harding R, Higginson IJ. Implementing patient‐reported outcome measures in palliative care clinical practice: a systematic review of facilitators and barriers. Palliat Med. 2014;28:158‐175. [DOI] [PubMed] [Google Scholar]
- 16. Howell D, Molloy S, Wilkinson K, et al. Patient‐reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol. 2015;26:1846‐1858. [DOI] [PubMed] [Google Scholar]
- 17. Kotronoulas G, Kearney N, Maguire R, et al. What is the value of the routine use of patient‐reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480‐1510. [DOI] [PubMed] [Google Scholar]
- 18. Boyce MB, Browne JP. Does providing feedback on patient‐reported outcomes to healthcare professionals result in better outcomes for patients? A systematic review. Qual Life Res. 2013;22:2265‐2278. [DOI] [PubMed] [Google Scholar]
- 19. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:1‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103‐112. [DOI] [PubMed] [Google Scholar]
- 21. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40‐46. [DOI] [PubMed] [Google Scholar]
- 23. van Egdom LSE, Oemrawsingh A, Verweij LM, et al. Implementing patient‐reported outcome measures in clinical breast cancer care: a systematic review. Value Health. 2019;22:1197‐1226. [DOI] [PubMed] [Google Scholar]
- 24. Mogos MF, August EM, Salinas‐Miranda AA, Sultan DH, Salihu HM. A systematic review of quality of life measures in pregnant and postpartum mothers. Appl Res Qual Life. 2013;8:219‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Evans K, Spiby H, Morrell CJ. A psychometric systematic review of self‐report instruments to identify anxiety in pregnancy. J Adv Nurs. 2015;71:1986‐2001. [DOI] [PubMed] [Google Scholar]
- 26. Nilvér H, Begley C, Berg M. Measuring women's childbirth experiences: a systematic review for identification and analysis of validated instruments. BMC Pregnancy Childbirth. 2017;17:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharawi N, Klima L, Shah R, Blake L, Carvalho B, Sultan P. Evaluation of patient‐reported outcome measures of functional recovery following caesarean section: a systematic review using the consensus‐based standards for the selection of health measurement instruments (COSMIN) checklist. Anaesthesia. 2019;74:1439‐1455. [DOI] [PubMed] [Google Scholar]
- 28. Beecher C, Greene R, O'Dwyer L, et al. Measuring women's experiences of maternity care: a systematic review of self‐report survey instruments. Women Birth. 2020;24:231‐241. [DOI] [PubMed] [Google Scholar]
- 29. Sultan P, Sadana N, Sharawi N, et al. Evaluation of domains of patient‐reported outcome measures for recovery after childbirth: a scoping and systematic review. JAMA Netw Open. 2020;3:e205540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doumouchtsis SK, Loganathan J, Fahmy J, et al. Patient‐reported outcomes and outcome measures in childbirth perineal trauma research: a systematic review. Int Urogynecol J. 2021;32:1695‐1706. [DOI] [PubMed] [Google Scholar]
- 31. Yang LY, Manhas DS, Howard AF, Olson RA. Patient‐reported outcome use in oncology: a systematic review of the impact on patient‐clinician communication. Support Care Cancer. 2018;26:41‐60. [DOI] [PubMed] [Google Scholar]
- 32. Laureij LT, Been JV, Lugtenberg M, et al. Exploring the applicability of the pregnancy and childbirth outcome set: a mixed methods study. Patient Educ Couns. 2019;103:642‐651. [DOI] [PubMed] [Google Scholar]
- 33.Cochrane Data collection form for intervention reviews: RCTs and non‐RCTs 2014. Accessed December 19, 2021. Available from: https://dplp.cochrane.org/data‐extraction‐forms
- 34. Cochrane Data collection form for intervention reviews: RCTs only. Accessed December 19, 2021. Available from: https://dplp.cochrane.org/data‐extraction‐forms
- 35. JBI . JBI Manual for Evidence Synthesis 2020. Accessed December 19 2021. Available from: https://synthesismanualjbiglobal
- 36. Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme Version 2006. Accessed December 20, 2021. Available from: https://www.lancaster.ac.uk/media/lancaster‐university/content‐assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1‐April2006.pdf
- 37. Santana M‐J, Feeny D. Framework to assess the effects of using patient‐reported outcome measures in chronic care management. Qual Life Res. 2014;23:1505‐1513. [DOI] [PubMed] [Google Scholar]
- 38. Greenhalgh J, Long AF, Flynn R. The use of patient reported outcome measures in routine clinical practice: lack of impact or lack of theory? Soc Sci Med. 2005;60:833‐843. [DOI] [PubMed] [Google Scholar]
- 39. Abernethy AP, Ahmad A, Zafar SY, Wheeler JL, Reese JB, Lyerly HK. Electronic paient‐reported data capture as a foundation of rapid learning cancer care. Med Care. 2010;48:S32‐S38. [DOI] [PubMed] [Google Scholar]
- 40. Bele S, Mohamed B, Chugh A, Haverman L, Santana MJ. Impact of using patient‐reported outcome measures in routine clinical care of paediatric patients with chronic conditions: a systematic review protocol. BMJ Open. 2019;9:e027354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Greenhalgh J, Meadows K. The effectiveness of the use of patient‐based measures of health in routine practice in improving the process and outcomes of patient care: a literature review. J Eval Clin Pract. 1999;5:401‐416. [DOI] [PubMed] [Google Scholar]
- 42. Holmes MM, Lewith G, Newell D, Field J, Bishop FL. The impact of patient‐reported outcome measures in clinical practice for pain: a systematic review. Qual Life Res. 2017;26:245‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kendrick T, El‐Gohary M, Stuart B, et al. Routine use of patient reported outcome measures (PROMs) for improving treatment of common mental health disorders in adults. Cochrane Database Syst Rev. 2016;7(7):CD011119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lizée T, Basch E, Trémolières P, et al. Cost‐effectiveness of web‐based patient‐reported outcome surveillance in patients with lung cancer. J Thorac Oncol. 2019;14:1012‐1020. [DOI] [PubMed] [Google Scholar]
- 45. Hong QN, Fàbregues S, Bartlett G, et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Edu Inform. 2018;34:285‐291. [Google Scholar]
- 46. Noyes J, Booth A, Lewin S, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings–paper 6: how to assess relevance of the data. Implement Sci. 2018;13:51‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Munthe‐Kaas H, Bohren MA, Glenton C, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings—paper 3: how to assess methodological limitations. Implement Sci. 2018;13:25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lewin S, Booth A, Glenton C, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings: introduction to the series. Implement Sci. 2018;13:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lewin S, Bohren M, Rashidian A, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings—paper 2: how to make an overall CERQual assessment of confidence and create a summary of qualitative findings table. Implement Sci. 2018;13:11‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Glenton C, Carlsen B, Lewin S, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings—paper 5: how to assess adequacy of data. Implement Sci. 2018;13:43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Colvin CJ, Garside R, Wainwright M, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings—paper 4: how to assess coherence. Implement Sci. 2018;13:33‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Booth A, Lewin S, Glenton C, et al. Applying GRADE‐CERQual to qualitative evidence synthesis findings–paper 7: understanding the potential impacts of dissemination bias. Implement Sci. 2018;13:63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Edwards DJ, Sakellariou D, Anstey S. Barriers to, and facilitators of, access to cancer services and experiences of cancer care for adults with a physical disability: a mixed methods systematic review. Disabil Health J. 2020;13:1‐13. [DOI] [PubMed] [Google Scholar]
- 54. Nishizono‐Maher A, Kishimoto J, Yoshida H, et al. The role of self‐report questionnaire in the screening of postnatal depression. Soc Psychiatry Psychiatr Epidemiol. 2004;39:185‐190. [DOI] [PubMed] [Google Scholar]
- 55. Bayrampour H, McNeil DA, Benzies K, et al. A qualitative inquiry on pregnant women's preferences for mental health screening. BMC Pregnancy Childbirth. 2017;17:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kingston D, Austin M‐P, van Zanten SV, et al. Pregnant women's views on the feasibility and acceptability of web‐based mental health e‐screening vs paper‐based screening: a randomized controlled trial. J Med Internet Res. 2017;19:e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Johnsen H, Clausen JA, Hvidtjørn D, Juhl M, Hegaard HK. Women's experiences of self‐reporting health online prior to their first midwifery visit: a qualitative study. Women Birth. 2018;31:e105‐e114. [DOI] [PubMed] [Google Scholar]
- 58. Doherty K, Marcano‐Belisario J, Cohn M, et al. Engagement with mental health screening on mobile devices: results from an antenatal feasibility study. Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems. 2019;1–15. doi: 10.1145/3290605.3300416 [DOI]
- 59. Martínez‐Borba V, Suso‐Ribera C, Osma J. Usability, acceptability, and feasibility of two technology‐based devices for mental health screening in perinatal care: a comparison of web vs app. International Symposium on Pervasive Computing Paradigms for Mental Health. ICST Institute for Computer Sciences, Social Informatics and Telecommunications Engineering, 2019, 176–189. Springer. [Google Scholar]
- 60. Reilly N, Talcevska K, Black E, Matthey S, Austin MP. A comparison of the interviewer‐administered phone and self‐complete online versions of the computerized eMINI 6.0 in a sample of pregnant women. J Affect Disord. 2019;242:265‐269. [DOI] [PubMed] [Google Scholar]
- 61. Depla AL, Ernst‐Smelt HE, Poels M, Crombag NM, Franx A, Bekker MN. A feasibility study of implementing a patient‐centered outcome set for pregnancy and childbirth. Health Sci Rep. 2020;3:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Willey SM, Blackmore RP, Gibson‐Helm ME, et al. “If you don't ask… you don't tell”: refugee women's perspectives on perinatal mental health screening. Women Birth. 2020;33:e429‐e437. [DOI] [PubMed] [Google Scholar]
- 63. Austin M‐PV, Reilly N, Mule V, Kingston D, Black E, Hadzi‐Pavlovic D. Disclosure of sensitive material at routine antenatal psychosocial assessment: the role of psychosocial risk and mode of assessment. Women Birth. 2022;35:e125‐e132. [DOI] [PubMed] [Google Scholar]
- 64. Reilly N, Austin M‐P. Attitudes and engagement of pregnant and postnatal women with a web‐based emotional health tool (Mummatters): cross‐sectional study. J Med Internet Res. 2021;23:e18517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Table S8
Table S9
Data Availability Statement
Please contact the corresponding author to request original database, codes and other materials.


