Abstract
Introduction
Despite the high prevalence of miscarriages, they are not systematically registered and few epidemiological studies have been done. As Finnish health registries are comprehensive and widely used in research, we validated the Finnish register data concerning diagnostics and treatment of miscarriage, and treatment‐related adverse events.
Material and methods
We conducted a validation study regarding miscarriage‐related codes of diagnoses and surgical procedures in a Finnish National Hospital Discharge Registry (NHDR) by comparing the information from the NHDR with that of the hospital records. We selected a random sample of 4 months during 1998–2016 from three hospitals, comprising 687 women aged 15–49 experiencing a first miscarriage during follow‐up. Women with diagnoses unrelated to miscarriage, or proven to be other than miscarriage, were excluded. The final sample consisted of 643 women with confirmed miscarriage, which was used for analyses regarding the diagnosis, treatment and adverse events of miscarriage treatment.
Results
The majority of miscarriages registered in the NHDR were confirmed by the hospital records (positive predictive value [PPV] = 93.6% [95% confidence interval [CI] 91.8%–95.4%]). Different types of miscarriage were also reliably identified; spontaneous abortion with PPV = 85.6% (95% CI 80.9%–89.2%), missed abortion with PPV = 92.7% (95% CI 88.8%–95.3%) and blighted ovum with PPV = 91.1% (95% CI 84.3%–95.1%). The PPV of surgical treatment (62.2% [95% CI 55.7%–68.3%]) was lower than the PPV of non‐surgical treatment (93.3% [95% CI 90.5%–95.3%]). The diagnoses regarding adverse events of miscarriage treatment could be reliably identified. The PPV for clinical infections was 76.0% (95% CI 56.6%–88.5%) and for retained products of conception or/and vaginal bleeding 96.8% (95% CI 83.8%–99.4%).
Conclusions
The coverage of the NHDR was good concerning identification of miscarriages, different types of miscarriages and non‐surgical treatment. Nevertheless, there is a need for clearly defined procedural codes concerning to medical treatment of miscarriage. The register‐based data are reliable and practicable for both clinical evaluation and research concerning miscarriage.
Keywords: blighted ovum, coverage, miscarriage, missed abortion, spontaneous abortion, treatment of miscarriage, validation study
Despite the high prevalence of miscarriages, they are not systematically registered and few epidemiological studies have been done. Finnish health registries are comprehensive and widely used in research, thus we validated the Finnish register data concerning diagnostics and treatment of miscarriage, and treatment‐related adverse events.The quality coverage of the NHDR was good concerning the miscarriage diagnoses, the different types of miscarriage and non‐surgical treatment of miscarriage.
Abbreviations
- ICD‐10 codes
International Statistical Classification of Diseases and Related Health Problems, 10th Revision
- NCSP
Nordic Medico‐Statistical Committee Classification of Surgical Procedures
- NHDR
Finnish National Hospital Discharge Registry
- PPV
positive predictive value
Key message.
The quality coverage of the Finnish National Hospital Discharge Registry was good concerning the miscarriage diagnoses, the different types of miscarriage and non‐surgical treatment of miscarriage.
1. INTRODUCTION
Miscarriage in early pregnancy is very common, impacting 30% of all pregnancies. Approximately 25% of women will experience an early pregnancy loss in their lifetime. 1 , 2 The mean age of all parturients in the Nordic countries has increased from 26 to 30 years between 1978 and 2018, and that of primiparous women from 24 to 29 years. 3 Increasing maternal age is the most important recognized risk factor for miscarriage 4 , 5 , 6 , 7 and, as women are postponing their first pregnancy, miscarriage is an increasing healthcare issue.
Several Nordic studies concerning miscarriage have been published in recent years. 4 , 6 , 7 , 8 , 9 Nevertheless, epidemiological studies on miscarriage are limited for several reasons. Even in countries with reliable health registers, miscarriages are not systematically recorded, and only few countries have a specific miscarriage data, eg Norway for all cases from 12 weeks onwards since 2008. 6 The clinical presentation of miscarriage varies, as do the diagnostic codes used at different phases of miscarriage. Thus, the true incidence of miscarriage, treatment of miscarriage and treatment outcomes at a population level are difficult to assess. A nationwide study from Denmark concluded that 9.6% of all register‐identified pregnancies ended in miscarriage but their proportion decreased from 10.7% in 2000 to 9.1% in 2015–2017. 7 Similarly, we reported that the proportion of miscarriages among register‐identified pregnancies declined from 11.2% to 8.3% during 1998–2016. 10 One possible explanation for this could be that the treatment of the early miscarriage has significantly evolved since 2000. Miscarriages are no longer routinely surgically evacuated and, due to this, not all recognized early miscarriages are necessarily referred to the hospital and are therefore not included in the hospital register data.
Finland has extensive population‐based health registries which are widely used in medical research, including the Finnish National Hospital Discharge Register (NHDR). According to previous studies, the quality of the NHDR is good, and the coverage exceeds 80% for most diagnoses and treatments. 11 , 12 However, the information in NHDR concerning miscarriage has not been validated previously.
The primary aim of the present study was to evaluate the accuracy of the national health register (NHDR) in recognition of diagnoses and treatments of miscarriages. Secondarily, we aimed to define and differentiate primary and secondary treatment based on timing, and to validate adverse events of miscarriage treatment by using the NHDR.
2. MATERIAL AND METHODS
2.1. The national hospital discharge register
The NHDR is maintained by the National Institute for Health and Welfare and covers information on all public hospitalization in Finland since 1967. After 1998, outpatient visits have also been registered. 11 This register contains personal identification numbers, the hospital ID, dates of admission and discharge, together with diagnoses and surgical procedures performed. The diagnoses and surgical procedures are recorded by physicians using the International Statistical Classification of Diseases and Related Health Problems (ICD‐diagnostic codes, ICD‐10 [10th Revision since 1996]) and NOMESCO (Nordic Medico‐Statistical Committee) Classification of Surgical Procedures (NCSP codes, The Finnish version since 1997, updated yearly).
2.2. Study sample and data collection
We utilized data derived from NHDR. The study population (n = 128 381) consisted of women of fertile age (15–49 years) with at least one miscarriage managed during the years 1998–2016 in public hospitals in Finland and reported to the NHDR. The data included both inpatient and outpatient visits. For each woman, only the first recorded diagnosis of miscarriage in NHDR was included to avoid selection bias. Exclusion criteria were register information suggestive of ectopic pregnancy, molar pregnancy, induced abortion or continuing pregnancy. To study the validity of the NHDR information on miscarriage, we restricted evaluation to a feasible sample of cases from three hospitals (South Karelia Central Hospital, Oulu University Hospital, Helsinki University Hospital). Further, to obtain a representative sample regarding the study period 1998–2016, we selected four different years, the first year, 1998, and each sixth year thereafter, ie 2004, 2010 and 2016. From this subset, by sampling randomly 1 month for each year, we gathered 687 (0.5% of the initial cases) women with register‐based first miscarriage and compared the information from the NHDR with that in hospital records (Figure 1).
FIGURE 1.
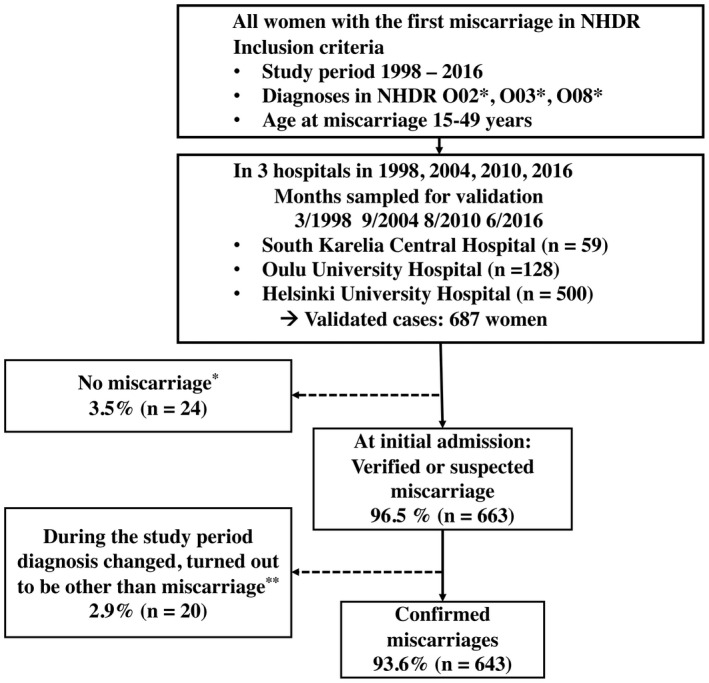
Flow diagram of the study. NHDR, Finnish National Hospital Discharge Registry. *Molar pregnancy, continuing pregnancy, induced abortion or its complication, ectopic pregnancy, twin pregnancy, hospital records not available. **Molar pregnancy, continuing pregnancy, ectopic pregnancy
The register data to validate consisted of ICD‐10 diagnostic codes and NCSP codes of the initial admission (inpatient or outpatient). The following admissions to hospital within 42 days and related to the initial one were also analyzed (Table S1). Identification of these women in NHDR and hospital records was based on the personal identification number assigned to every Finnish citizen or permanent resident at birth or immigration. The data from hospital records and NHDR were linked using the personal identification numbers. The data collection form for the hospital records was independently completed for each individual woman.
2.3. The validation procedure
The validation study was conducted by comparing the information from the NHDR with that of the hospital records. We (authors NH, RL, MM, MN) identified the study patient population with NHDR and sought the hospital records of these women using the personal identification numbers. We studied all hospital records relevant for miscarriage up to 42 days after the register‐based index date (the initial admission to hospital). Information concerning background characteristics of the woman, duration of gestation (as defined by both duration of amenorrhea and ultrasonography), type of miscarriage, its treatment and miscarriage‐related adverse events were collected. The NHDR data included four women for whom hospital records were unavailable.
2.4. Diagnosis and treatment of miscarriage and adverse events based on clinical assessment
The miscarriages were defined as occurring before 23 gestational weeks (<22+0) and categorized into three subcategories (spontaneous abortion, missed abortion and blighted ovum) defined by clinical and ultrasonographic findings stated in the hospital records. We compared the diagnostic criteria used with those defined in the literature. 13 , 14 , 15 , 16 , 17 , 18 , 19 The definition of spontaneous abortion was ongoing vaginal bleeding with visible products of conception without heart activity via ultrasonography. Missed abortion was defined by the absence of cardiac activity in a visible embryo (crown–rump length ≥5 mm) and blighted ovum was defined by the absence of an embryo within a gestational sac with diameter ≥25 mm via ultrasonography. 1 , 13 , 16 , 20 If the type of miscarriage could not be determined using the above‐mentioned definitions, we acknowledged the overall situation individually, including duration of gestation, changes in serum levels of human chorionic gonadotrophin and ultrasound findings. In these situations, absence of fetal heartbeat or embryo was used as diagnostic criteria irrespective of crown–rump length or gestational sac diameter measurement.
The primary treatment of miscarriage was classified into three groups: surgical and medical treatment, and expectant management.
The adverse events of miscarriage treatment were divided into two groups: clinical infections and retained products of conception and/or vaginal bleeding. The definition of clinical infection included clinical findings indicative of infection: fever, lower abdominal pain, tenderness of the uterus at the bimanual examination, increased levels of blood infection parameters, and use of antibiotics. The group of retained products of conception and/or vaginal bleeding was defined by admissions or procedures in relation to vaginal bleeding or verified or suspected retained products of conception.
2.5. Definition of register‐based miscarriage diagnosis, treatment and treatment‐related adverse events of miscarriage
Women with the relevant main ICD‐10 codes at the NHDR for the initial admission were defined as having a register‐identified miscarriage. Miscarriages were categorized into three groups using the ICD‐10 diagnostic codes.
The treatment of miscarriage was classified into two groups using the NCSP codes indicative of surgical treatment (surgical treatment) or absence of the above‐mentioned codes (non‐surgical treatment) within 3 days after initial admission. Non‐surgical treatment includes both medical treatment and expectant management because there are no reliable ICD‐10 or NCSP codes to separate these treatment options based on the register records.
The adverse events of miscarriage treatment were classified into two groups and were defined with relevant NCSP‐ and ICD‐10 diagnostic codes occurring more than 3 days after the initial admission (Table S1).
2.6. Statistical analyses
Variables were summarized with mean and standard deviation (SD) (continuous variables) or with counts (n) and percentages (categorical variables). The positive predictive value (PPV) presents the accuracy of NHDR and was defined as the proportion of the women with certain codes in the NHDR who had that diagnosis verified by hospital record review. PPVs were calculated according to month/year, miscarriage type, treatment of miscarriage and adverse events of miscarriage treatment. The 95% confidence interval (95% CI) for (binomial) PPV was calculated using the Wilson Score method, which is based on inverting the z‐test for a single proportion and provides more reliable coverage than the alternatives.
2.7. Ethics statement
We received approval from the Finnish Institute for Health and Welfare (THL/841/5.05.00/2017) for the use of NHDR. We also received permission from The Hospital District of Helsinki and Uusimaa (HUS/42/2017), South Karelia Social and Health Care District (EKS/2495/13.01.05/2018), and Northern Ostrobothnia Hospital district (254/2018) to access the relevant hospital records. According to Finnish legislation, approval from an ethics committee is not needed for a register‐based study.
3. RESULTS
3.1. Description of study population
According to the NHDR, 687 women received diagnostic code(s) indicative of miscarriage at the initial admission in the three hospitals during the four periods of 1‐month studied. In 24 (3.5%) cases, the diagnosis registered in the NHDR was incorrect, ie the diagnosis at the hospital admission was unrelated to miscarriage. Based on the hospital records, 663 women had either suspected or verified miscarriage at the initial admission (PPV = 96.5% [95% CI 95.1%–97.9%]). Further, we excluded 20 (2.9%) women, as based on the hospital records the diagnosis during further evaluation proved to be other than miscarriage. The final sample consisting of 643 women with verified miscarriage (PPV 93.6% [95% CI 91.8%–95.4%]) was used for analyses concerning the diagnosis, treatment and adverse events of miscarriage treatment (Figure 1).
At initial admission the mean age of 643 women was 32.0 years (range 16–47 years) and the mean gestational age was 70.1 days (range 28–153 days). The majority (68.8%) of the 643 women with miscarriage had previous pregnancies; 16.6% had a history of induced abortion and 13.7% of previous miscarriage. Demographic characteristics of the women according to study year are described in Table 1.
TABLE 1.
Characteristics of the women
| 1998 | 2004 | 2010 | 2016 | |
|---|---|---|---|---|
| (n = 122) | (n = 163) | (n = 189) | (n = 179) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Age at the time of miscarriage (years) | 31.8 ± 6.9 | 32.2 ± 6.1 | 31.1 ± 6.5 | 32.8 ± 5.9 |
| Mean gestational age (days) a | 70.3 ± 20.8 | 69.6 ± 18.7 | 69.9 ± 16.4 | 70.5 ± 18.6 |
| Parity | n (%) | n (%) | n (%) | n (%) |
| Primigravid | 32 (28.6) | 54 (33.1) | 61 (32.3) | 55 (30.7) |
| Multigravid | 80 (71.4) | 109 (66.9) | 128 (67.7) | 124 (69.3) |
| History of | ||||
| induced abortions | 18 (16.1) | 32 (19.6) | 33 (17.5) | 24 (13.4) |
| miscarriages | 25 (22.3) | 18 (11.0) | 22 (11.6) | 23 (12.8) |
Abbreviation: SD, standard deviation.
Among the 620 women with recorded duration of amenorrhea in the hospital records.
3.2. Types of miscarriage
In a review of the hospital records, the type of miscarriage was spontaneous abortion in 252 (39.2%) cases, missed abortion in 269 (41.8%) cases and blighted ovum in 122 (19.0%) cases.
In a validation analysis, we found that of the confirmed miscarriages, the register‐based type of miscarriage matched with hospital records in 568 cases (PPV = 88.3% [95% CI 85.6%–90.6%]). The highest PPV of 92.7% [95% CI 88.8%–95.3%] for missed abortion was followed by PPV of 91.1% [95% CI 84.3%–95.1%] for blighted ovum and PPV of 85.6% [95% CI 80.9%–89.2%] for spontaneous abortion (Figure 2). The PPV of different miscarriage diagnostic codes varied during the study period, but there were no certain detectable trends concerning these values (Table S2).
FIGURE 2.
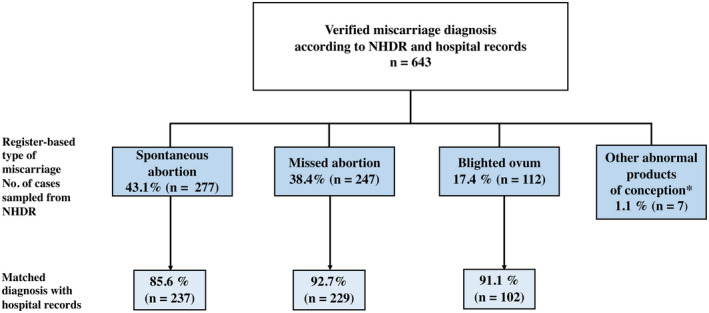
Validity of different miscarriage diagnosis codes. NHDR, Finnish National Hospital Discharge Registry. *Confirmed diagnosis of group of other abnormal products of conception: missed abortion (3 women) spontaneous abortion (4 women)
3.3. The treatment of miscarriage
In a review of the hospital records, the treatment of miscarriage was carried out in an inpatient setting in 34.1% and in an outpatient setting in 65.9% of 643 women. In all, 168 women (26.1%) women underwent primary surgical and 475 (73.9%) non‐surgical treatment of miscarriage. We detected that a majority of women with primary surgical treatment (87.0%) had received the NCSP code indicative of surgical treatment within 3 days after the initial admission. A majority of women with surgical treatment as a secondary treatment (67.6%) had received the valid code more than 3 days after the initial admission. Consequently, we defined primary treatment as one occurring within 3 days (ie next working day).
In a validation analysis, we detected 293 women (45.6%) with NCSP codes indicative of surgical treatment in the register data during 42 days after the initial admission and this was confirmed in 161 women in the hospital record review (PPV 54.9% [95% CI 49.2%–60.5%]). The surgical treatment was recorded as a primary treatment, within 3 days, in the NHDR in 225 women. This was verified in 140 cases (PPV of 62.2% [95% CI 55.7%–68.3%]). The PPV of surgical treatment declined during the study period from 92.0% (95% CI 84.5%–96.1%) in 1998 to 62.5% (95% CI 30.6%–86.3%) in 2016.
Conversely, 350 women (54.4%) received no NCSP codes indicative of surgical treatment in the register data during the 42 days after the initial admission, and the primary conservative treatment was confirmed in 343 women (PPV 98.0% [95% CI 95.9%–99.0%]). The majority of the women (n = 418, 65.0%) were primarily treated conservatively (eg without surgical treatment, within 3 days) according to the NHDR data. In 390 of these women, non‐surgical treatment was confirmed (PPV = 93.3% [95% CI 90.5%–95.3%]). The PPV of non‐surgical treatment improved during the study period from 75.0% (95% CI 55.1%–88.0%) in 1998 to 97.1% (95% CI 93.3%–98.7%) in 2016 (Figure 3, Table S3).
FIGURE 3.
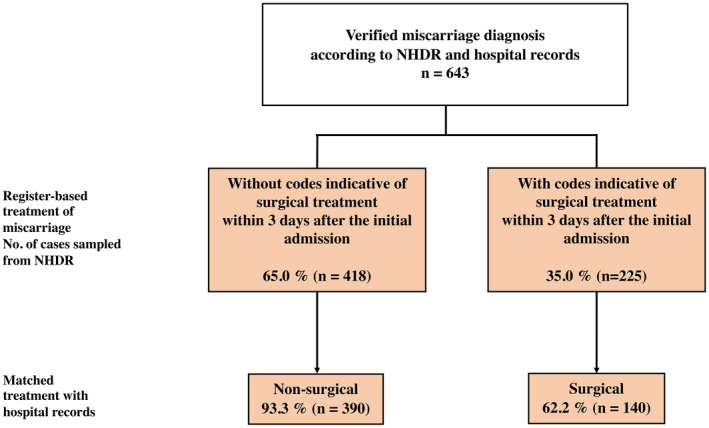
Validity of surgical and non‐surgical treatment of miscarriage. NHDR, Finnish National Hospital Discharge Registry
The definitions used concerning timing of primary and secondary treatment turned out to be competent, also considering the PPV of both surgical and non‐surgical treatment (Table S4).
3.4. Adverse events of miscarriage treatment
In a review of the hospital records, 124 (19.3%) women had adverse event(s) related to miscarriage or its treatment.
We detected analogous results as with the secondary treatment concerning the timing of the codes in the register and therefore adverse events of miscarriage treatment were defined as those occurring more than 3 days after the initial admission.
In the validation analysis we found that, according to NHDR, 39 women (6.1%) received the codes indicative of clinical infection in the register data in the 42 days after the initial admission with confirmation of the diagnosis in 26 women (PPV 66.7% [95% CI 51.0%–79.4%]). The PPV of women with codes indicative of retained products of conception or/and vaginal bleeding was 106/370 = 28.6% [95% CI 24.3%–33.5%].
Altogether 25 (3.9%) women had received codes indicative of clinical infection more than 3 days after the initial admission and the diagnosis was confirmed in 19 women (PPV = 76.0% [95% CI 56.6%–88.5%]). In 31 (4.8%) women, the codes were indicative of retained products of conception or/and vaginal bleeding more than 3 days after the initial admission, and the codes indicative of surgical treatment within 3 days after the above‐mentioned secondary admission. The diagnosis was confirmed in 30 women (PPV = 96.8% [95% CI 83.8%–99.4%]).
4. DISCUSSION
We found that the data quality of the NHDR on miscarriage diagnoses was good. The vast majority (93.6%) of registry‐identified miscarriages could be confirmed from the hospital records. Moreover, the coverage of the NHDR was judged good concerning the different types of miscarriage (PPV 85.6%–92.7%) as well as non‐surgical treatment of miscarriage (PPV 93.3%).
A key finding of our study is that practically all miscarriages registered in the NHDR represent true miscarriages. To our knowledge, the present study is the first comprehensive validation concerning miscarriage‐related codes of diagnoses and surgical procedures in a national register. Similarly, a recent Danish study validated the diagnosis of spontaneous abortion recorded in the Danish National Registry of Patients, where the PPV was 97.4% (95% CI 92.7%–99.5%). 8 The hospitals used in the validation study represent different parts of Finland (eastern, northern and southern) and both central and university hospitals. We believe that our results can be generalized to countries with easy access to modern miscarriage treatment and high‐quality health registers.
Different types of miscarriages could also be reliably recognized from the NHDR. This indicates that the diagnostic criteria of the different types of miscarriages are well established among Finnish physicians. The frequently updated guidelines and recommendations concerning diagnosis of miscarriage causes difficulties defining different types of miscarriage. For example, during the follow‐up in 1998–2016, the recommendations concerning crown–rump length measurements above which cardiac activity should be visible in a normal pregnancy, have varied from 5 to 7 mm. 1 , 14 , 15 , 16 , 17 , 18 , 20 , 21 , 22 , 23 , 24
In the validation analysis, the register data concerning surgical treatment of miscarriage were not satisfactory. However, it is noteworthy that the PPV of surgical treatment declined during the follow‐up. Over the past two decades, there has been a significant change in the treatment of miscarriage. Earlier, most women underwent surgical evacuation of the uterus, whereas nowadays, and at the end of the follow‐up, non‐surgical treatment is the standard treatment of miscarriage. 1 , 25 In contrast, non‐surgical treatment could be reliably identified from the NHDR. The natural explanation could be that the codes clinicians use regularly are reported more precisely. Adverse events of miscarriage treatment could also be reliably identified from the NHDR data.
Based on the analysis of hospital records, the primary surgical treatment could be defined as one occurring within 3 days of the initial admission. In contrast, secondary treatment and adverse events of miscarriage treatment could be defined using relevant codes assigned more than 3 days after the initial admission.
Our study has several strengths. The follow‐up between 1998 and 2016 represents a period of modern diagnostics of miscarriage during which ultrasonography has been widely available for examining women with suspected miscarriage. The representative sample regarding the study period, coverage of different hospitals in Finland and availability of high‐quality, detailed hospital records emphasized the value of the study and reduced the risk of selection bias in the study.
Register studies also have limitations. In this setting, it is impossible to identify women who experienced a miscarriage but who had received a false diagnosis registered in the NHDR. Also, only women treated in hospital are registered in the NHDR. For example, very early spontaneous miscarriages are not captured by the NHDR because in such cases there is either no need for medical treatment or women are managed without hospital admission and thus not included in the register data. It is not possible to determine the proportion of miscarriages managed in primary care during our follow‐up between 1998 and 2016 because the Finnish primary healthcare register only started in 2011 and was not complete before 2013. Information on self‐reported miscarriages is not collected in the registers. Also, some women might have had miscarriage(s) prior to 1998. Nevertheless, it is likely that the great majority of the miscarriages included, truly represents the first miscarriage during the follow‐up time. Moreover, due to the lack of generally used procedural codes for medical treatment of miscarriage, it is impossible reliably to differentiate medical treatment within the non‐surgical treatment of miscarriage. The evolving treatment of miscarriage, as well as changes of diagnostic and procedural codes within the time period, creates challenges over such a long follow‐up. Finally, there is no registration of gestational age in the NHDR.
5. CONCLUSION
Both the cases and types of miscarriage, as well as non‐surgical treatment of miscarriage and the treatment‐related adverse events can be reliably identified in the Finnish National Hospital Discharge Register. However, clearly defined procedural codes are urgently needed for the increasingly used medical treatment of miscarriage. Based on our results, the use of register‐based data is justified for research on miscarriage to improve the treatment and follow‐up of this common event in women's reproductive lifespan.
AUTHOR CONTRIBUTIONS
All authors have contributed to planning the study protocol and to writing of the article. NH, RL, MM and MN collected the validation data. The statistical analysis was performed by NH with the assistance of AB. NH wrote the first draft of the report with the assistance of MN, MM and OH. All authors participated the final drafting of the work and approved this submission for publication. MN, MM and OH were responsible for the overall study, and OH obtained funding.
FUNDING INFORMATION
Helsinki and Uusimaa Hospital System (EVO grant, O.H.)
CONFLICT OF INTEREST
None.
Supporting information
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
The professional help of Aini Bloigu concerning the data collection and processing is gratefully acknowledged.
Helle N, Niinimäki M, Linnakaari R, et al. National register data are of value in studies on miscarriage—Validation of the healthcare register data in Finland. Acta Obstet Gynecol Scand. 2022;101:1245‐1252. doi: 10.1111/aogs.14445
REFERENCES
- 1. Jurkovic D, Overton C, Bender‐Atik R. Diagnosis and management of first trimester miscarriage. BMJ. 2013;346:f3676. [DOI] [PubMed] [Google Scholar]
- 2. Warburton D, Fraser FC. Spontaneous abortion risks in man: data from reproductive histories collected in a medical genetics unit. Am J Hum Genet. 1964;16:1‐25. [PMC free article] [PubMed] [Google Scholar]
- 3. THL (National Institute for health and welfare) . Statistical Report 48/2020 ‐ Perinatal statistics – parturients, delivers and newborns 2019. 2019.
- 4. Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320:1708‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feodor Nilsson S, Andersen P, Strandberg‐Larsen K, Nybo Andersen A‐M. Risk factors for miscarriage from a prevention perspective: a nationwide follow‐up study. BJOG. 2014;121:1375‐1385. [DOI] [PubMed] [Google Scholar]
- 6. Magnus MC, Wilcox AJ, Morken N‐H, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ. 2019;364:I869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lidegaard Ø, Mikkelsen AP, Egerup P, Kolte AM, Rasmussen SC, Nielsen HS. Pregnancy loss: a 40‐year nationwide assessment. Acta Obstet Gynecol Scand. 2020;99:1492‐1496. [DOI] [PubMed] [Google Scholar]
- 8. Lohse SR, Farkas DK, Lohse N, et al. Validation of spontaneous abortion diagnoses in the Danish National Registry of patients. Clin Epidemiol. 2010;2:247‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Begtrup LM, Specht IO, Hammer PEC, et al. Night work and miscarriage: a Danish nationwide register‐based cohort study. Occup Environ Med. 2019;76:302‐308. [DOI] [PubMed] [Google Scholar]
- 10. Linnakaari R, Helle N, Mentula M, et al. Trends in the incidence, rate and treatment of miscarriage‐nationwide register‐study in Finland. Hum Reprod. 2019;34:2120‐2128. [DOI] [PubMed] [Google Scholar]
- 11. Sund R. Quality of the Finnish hospital discharge register: a systematic review. Scand J Public Health. 2012;40:505‐515. [DOI] [PubMed] [Google Scholar]
- 12. Gissler M, Haukka J. Finnish health and social welfare registers in epidemiological research. Norsk Epidemiologi. 2004;14:113‐120. [Google Scholar]
- 13. Campion EW, Doubilet PM, Benson CB, et al. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443‐1451. [DOI] [PubMed] [Google Scholar]
- 14. Webster K, Eadon H, Fishburn S, Kumar G. Summary of updated NICE guidance: ectopic pregnancy and miscarriage: diagnosis and initial management. BMJ. 2019;367:I6283. [DOI] [PubMed] [Google Scholar]
- 15. NICE Guideline . Ectopic pregnancy and miscarriage: diagnosis and initial management in early pregnancy of ectopic pregnancy and miscarriage. 2012 [cited 2021 Jun 27].
- 16. Jeve Y, Rana R, Bhide A, Thangaratinam S. Accuracy of first‐trimester ultrasound in the diagnosis of early embryonic demise: a systematic review. Ultrasound Obstet Gynecol. 2011;38:489‐496. [DOI] [PubMed] [Google Scholar]
- 17. Abdallah Y, Daemen A, Kirk E, et al. Limitations of current definitions of miscarriage using mean gestational sac diameter and crown–rump length measurements: a multicenter observational study. Ultrasound Obstet Gynecol. 2011;38:497‐502. [DOI] [PubMed] [Google Scholar]
- 18. Morin L, Van den Hof MC, Society of Obstetricians and Gynaecologists of Canada . SOGC clinical practice guidelines. Ultrasound evaluation of first trimester pregnancy complications. Number 161, June 2005. Int J Gynaecol Obstet. 2006;93:77‐81. [DOI] [PubMed] [Google Scholar]
- 19. Prine LW, MacNaughton H. Office management of early pregnancy loss. Am Fam Physician. 2011;84:75‐82. [PubMed] [Google Scholar]
- 20. Guideline NICE. Ectopic pregnancy and miscarriage: diagnosis and initial management. 2019. Available from: www.nice.org.uk/guidance/ng126
- 21. Guideline NICE. Management of Miscarriage. 2016.
- 22. Abdallah Y, Daemen A, Guha S, et al. Gestational sac and embryonic growth are not useful as criteria to define miscarriage: a multicenter observational study. Ultrasound Obstet Gynecol. 2011;38:503‐509. [DOI] [PubMed] [Google Scholar]
- 23. Ledger WL, Turner MJ. Implementation of the findings of a national enquiry into the misdiagnosis of miscarriage in the Republic of Ireland: impact on quality of clinical care. Fertil Steril. 2016;105:417‐422. [DOI] [PubMed] [Google Scholar]
- 24. Lane BF, Wong‐You‐Cheong JJ, Javitt MC, Glanc P, Brown DL, Dubinsky T, Harisinghani MG, Harris RD, Khati NJ, Mitchell DG, Pandharipande PV, Pannu HK, Podrasky AE, Shipp TD, Siegel CL, Simpson L, Wall DJ, Zelop CM, American College of Radiology ACR Appropriateness Criteria\First Trimester Bleeding. Ultrasound Q 2013; 29: 91–6, ACR appropriateness criteria® first trimester bleeding. [DOI] [PubMed] [Google Scholar]
- 25. Smith LFP, Ewings PD, Quinlan C. Incidence of pregnancy after expectant, medical, or surgical management of spontaneous first trimester miscarriage: long term follow‐up of miscarriage treatment (MIST) randomised controlled trial. BMJ. 2009;339:b3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4


