Abstract
Streptococcus iniae causes meningoencephalitis and death in commercial fish species and has recently been identified as an emerging human pathogen producing fulminant soft tissue infection. As identified by pulsed-field gel electrophoresis (PFGE), strains causing disease in either fish or humans belong to a single clone, whereas isolates from nondiseased fish are genetically diverse. In this study, we used in vivo and in vitro models to examine the pathogenicity of disease-associated isolates. Strains with the clonal (disease-associated) PFGE profile were found to cause significant weight loss and bacteremia in a mouse model of subcutaneous infection. As little as 102 CFU of a disease-associated strain was sufficient to establish bacteremia, with higher inocula (107) resulting in increased mortality. In contrast, non-disease-associated (commensal) strains failed to cause bacteremia and weight loss, even at inocula of 108 CFU. In addition, disease-associated strains were more resistant to phagocytic clearance in a human whole blood killing assay compared to commensal strains, which were almost entirely eradicated. Disease-associated strains were also cytotoxic to human endothelial cells as measured by lactate dehydrogenase release from host cells. However, both disease-associated and commensal strains adhered to and invaded cultured human epithelial and endothelial cells equally well. While cellular invasion may still contribute to the pathogenesis of invasive S. iniae disease, resistance to phagocytic clearance and direct cytotoxicity appear to be discriminating virulence attributes of the disease-associated clone.
Streptococcus iniae is a hemolytic, gram-positive coccus first isolated in 1976 from a subcutaneous abscess of a captive freshwater dolphin (27, 28). It causes meningoencephalitis in tilapia, yellowtail, rainbow trout, and coho salmon (7–9, 18, 19, 29, 34) and has been associated with disease outbreaks in aquaculture farms, with mortality rates of up to 50% (8). S. iniae has more recently been reported to cause fulminant soft tissue infection in humans (35). Since 1995, 11 cases in Canada of upper limb cellulitis have been associated with S. iniae infection following percutaneous injury while handling fish. Analysis by pulsed-field gel electrophoresis (PFGE) demonstrated only two, virtually identical, clones (differing by a single band) among strains capable of causing disease in both humans and fish (21 isolates). In contrast, isolates from nondiseased fish (32 isolates), as well as the American Type Culture Collection (ATCC) type strain 29178, were genetically diverse (35). This suggests that specific, chromosomally encoded virulence determinants may account for the pathogenicity of the clonal (disease-associated) versus non-disease-associated (commensal) strains.
S. iniae has been well characterized biochemically (27). However, with regard to potential virulence factors, no phenotypic differences between disease-associated and commensal strains have been reported. Data on S. iniae infection in experimental animals are limited. Early studies reported that rabbits, guinea pigs, and mice were resistant to subcutaneous, intravenous, and intraperitoneal injection of 108 CFU of S. iniae type strain, ATCC 29178 (28). Conversely, disease commonly observed in fish was reproduced in tilapia following fish-fish passage of a diseased-fish isolate (8). However, a mammalian model relevant to human infection had not been established. Since human disease is characterized by soft tissue infection following skin puncture, we examined the pathogenicity of representative disease-associated and commensal S. iniae strains in a murine model of subcutaneous infection. We also used established tissue culture methods to explore potential mechanisms of S. iniae pathogenicity including resistance to phagocytic clearance, direct cytotoxicity, and intracellular invasion. This work represents the first study demonstrating that the unique genetic profile of S. iniae disease-associated isolates correlates with virulence in experimental model systems.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Strains used in this study are listed in Table 1. S. iniae and other gram-positive bacterial strains were grown in Todd-Hewitt broth (THB) or on Todd-Hewitt agar (THA) or Columbia base agar (Difco, Detroit, Mich.) supplemented with 5% defibrinated sheep erythrocytes (Quelab). Overnight cultures were diluted 1/25 in THB and incubated at 37°C with low agitation. Optical density at 620 nm was measured over an 8-h period using a Beckman spectrophotometer (Beckman Instruments, Fullerton, Calif.), and viable counts were determined. Mid-log phase (108 CFU) was found to correspond to an optical density at 620 nm of 0.35 to 0.40 for all strains.
TABLE 1.
Bacterial strains and associated phenotypes
| Strain | Species | Isolate site | Disease | PFGE profile | Reference |
|---|---|---|---|---|---|
| 9116 | S. iniae | Patient, blood | Cellulitis | A | 35a |
| 9117 | S. iniae | Patient, blood | Cellulitis | A | 35a |
| 9033 | S. iniae | Fish, diseased | Meningoencephalitis | A′b | 35a |
| 9041 | S. iniae | Fish, swab | B | This work | |
| 9059 | S. iniae | Fish, swab | C | This work | |
| 9066 | S. iniae | Fish, swab | D | This work | |
| 9085 | S. iniae | Tilapia, swab | E | This work | |
| 9098 | S. iniae | Tilapia, swab | F | This work | |
| COH1 | GBS; type III | NAc | Sepsis | NA | 23 |
| A909 | GBS; type Ia | NA | NA | NA | 36 |
| NZ131 | GAS; T14/M49 | Patient, blood | Glomerulonephritis | NA | 33 |
| 0507 | S. epidermidis | Clinical isolate | NA | NA | This work |
| 2207 | S. sanguis | Patient, blood | Bacteremia | NA | This work |
Mouse model.
Virulence of S. iniae strains was assessed using a murine model of subcutaneous infection as previously described for Streptococcus pyogenes (group A streptococcus [GAS]) (3). The GAS strain NZ131 was used as a positive control. Mid-log-phase cultures were washed twice in phosphate-buffered saline (PBS) and diluted to the required inoculum (102 to 108 CFU). Bacterial suspensions were mixed with an equal volume of sterilized Cytodex beads (Sigma Laboratories, St. Louis, Mo.) that were suspended in PBS to a concentration of 20 μg/ml. This mixture (200 μl) was injected into the right flanks of five to seven hairless, outbred female SKH1 mice (Charles River Laboratories, Wilmington, Mass.) aged 4 to 5 weeks and weighing 15 to 20 g. Mice were weighed prior to injection and every 24 h for 3 days. Blood and tissue from the injection site were removed from a single mouse at 24 h and from the remaining mice at the end of each trial (72 h). Blood was collected by cardiac puncture and mixed with citrate-buffered saline, and viable counts were determined. The injection site was excised, halved, and weighed following cardiac puncture and euthanization of each animal. For determination of bacterial count, one half of the tissue specimen was placed in 1 ml of PBS and homogenized in a PowerGen 700D homogenizer (Fisher Scientific, Ottawa, Ontario, Canada), and CFU per milligram of tissue was determined. The remaining tissue was immersed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histologic examination. The experimental procedures performed on the mice were conducted according to the principles of the Animal Care Committee of Mount Sinai Hospital, Toronto, Ontario, Canada.
Phagocytosis assay.
Resistance to phagocytosis in whole blood was examined using a modification of the Lancefield bactericidal assay for GAS (20). Mid-log-phase bacteria (108 CFU) were washed and serially diluted in PBS. Bacterial suspensions (100 μl) containing 102 CFU were added to 1 ml of fresh, heparinized human blood in sterile glass tubes and incubated on an orbital shaker for 1.5 h at 37°C. The survival index was calculated as the CFU recovered after the 1.5-h incubation divided by the initial inoculum added prior to incubation. A clinical strain of Staphylococcus epidermidis, 0507, was chosen as a negative control based on the reported susceptibility of this bacterium to whole blood phagocytic killing (6).
LDH release.
Lactate dehydrogenase (LDH) release was measured using a Sigma LDH detection kit as previously described (25), with several modifications. Briefly, 10 ml of bacteria (108 CFU/ml) was washed in PBS and resuspended in 1 ml of RPMI 1640 without fetal calf serum (Becton Dickinson, Bedford, Mass.). Human brain microvascular endothelial cell (BMEC) monolayers, grown in a 24-well tissue culture plate (Corning Glass works, Corning, N.Y.), were washed twice in PBS, and 0.5 ml of the above suspension (5.0 × 108 CFU) of S. iniae was added. The group B streptococcus (GBS) strain A909 (5.0 × 107 CFU) was used as a positive control based on its reported cytotoxicity to BMEC monolayers (25) and the association of GBS with meningitis in humans and infrequently with fish (2). The higher inoculum used for S. iniae was required to obtain equivalent results. Plates were incubated for 3 h and then centrifuged to pellet bacteria. Twofold dilutions of each sample were prepared across a 96-well microtiter plate in sterile water, and 20-μl aliquots were transferred to a replica plate. A 100-μl aliquot of 0.1% NADH in standardized pyruvate substrate was added to each well, and the plate was incubated for 30 min at 37°C. Sigma color reagent (100 μl) was then added to each well for 20 min at room temperature. The A450 was used to calculate the residual pyruvic acid activity, which is inversely proportional to the LDH activity. The reciprocal A450 value of the dilution producing 50% LDH release was calculated as a percentage of the total LDH released from complete monolayer lysis.
Adherence and invasion assay.
HEp-2 cells were incubated in minimal essential medium (BioWhittaker) supplemented with 6% heat-inactivated fetal calf serum, 200 mM glutamine, and amphotericin B (4 μg/ml) at 37°C in 5% CO2. BMEC were cultured in RPMI 1640 supplemented with 10% fetal calf serum, 10% NuSerum (Becton Dickinson), modified Eagle's medium nonessential amino acids, l-glutamine, and penicillin-streptomycin (26). Twenty-four-well tissue culture plates (Corning) were precoated with rat tail collagen to support BMEC monolayers. Cultures were incubated at 37°C in 5% CO2.
Monolayers of HEp-2 and BMEC were grown to ∼80% confluency (∼105 cells) in 24-well tissue culture plates and washed three times with PBS, and 500 μl of tissue culture medium was added prior to each assay. Bacterial cultures were washed three times in PBS and resuspended in tissue culture medium without antibiotics. Bacterial inocula of 107 or 105 CFU were added to HEp-2 and BMEC, respectively. HEp-2 cells were incubated for 3 h to allow bacterial adherence and invasion. BMEC plates were centrifuged at 2,000 rpm for 10 min to place bacteria at the surface of the monolayer and incubated for 2 h. To kill extracellular and surface-adherent bacteria, monolayers were washed three times with PBS, followed by the addition of 1 ml of tissue culture medium containing 100 μg of gentamicin and 5 μg of penicillin G to each well and incubation for 2 to 3 h at 37°C. Monolayers were gently washed six times with PBS and, following the addition of 100 μl of trypsin-EDTA, incubated for 10 min at 37°C. A 0.025% solution of Triton X-100 (400 μl) was added, and cells were disrupted by repeated pipetting. Serial dilutions of the resultant lysate were plated onto THA and incubated overnight, and viable counts were determined. To calculate the number of host cell-associated bacteria (total invaded and surface adherent), monolayers were infected as described above, and viable counts were performed without prior exposure to antibiotics. The invasive GBS strain COH1 and the Streptococcus sanguis clinical isolate 2207 served as positive and negative controls, respectively. Invasion or host cell association was determined by the number of the adherent and/or invasive bacteria as a percentage (mean ± standard deviation [SD]) of the original inoculum.
Cytochalasin D, an actin microfilament aggregation inhibitor, was used in internalization assays to determine the participation of cytoskeletal elements in bacterial adherence and invasion. HEp-2 monolayers were preincubated with 12.5 to 50.0 ng of cytochalasin D for 30 min before addition of bacteria.
RESULTS
Mouse model.
Based on our finding that doses greater than 106 CFU of disease-associated strains caused significant mortality in mice within 24 h, we selected 106 CFU as the standard infectious dose for this model. Bacteremia developed only in mice infected with the disease-associated isolates 9116, 9117, and 9033, and as little as 102 CFU (9117) was sufficient to cause bacteremia within 24 h; lower doses were not tested. Twenty-four hours following injection of 102 CFU, the bacterial count in the blood increased to 104 CFU/ml, indicating significant in vivo replication of disease-associated organisms. Whether replication occurred at the injection site or in the bloodstream was not examined. In contrast, commensal strains 9041, 9059, 9066, 9085, and 9098 were not present in the blood of infected mice after 24 or 72 h at a dose of 106 CFU, and even at 108 CFU, 9066 was not recovered from blood. Viable organisms (≤103 CFU/mg of tissue) were recovered from the subcutaneous injection site of mice exposed to both disease-associated and commensal isolates 24 h following injection. Few (≤2.0 × 102 CFU/mg of tissue) or no viable organisms were recovered from injection sites examined 72 h postinjection. Collectively, these results indicate that bacteremia is associated with S. iniae strain type (PFGE pattern A/A′ [Table 1]) and not the magnitude of the bacterial inoculum.
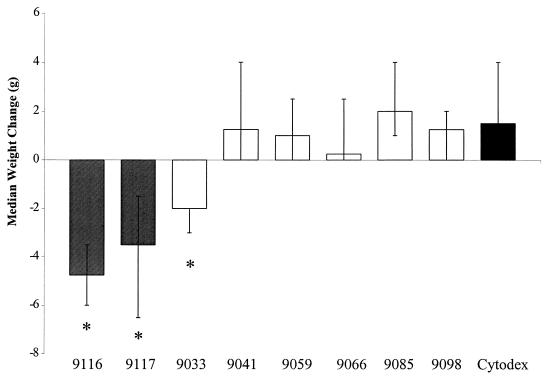
Mice injected with 106 CFU of the disease-associated strains 9116, 9117, and 9033 showed median weight losses of 4.75 g (range, −3.5 to −6.0 g), 3.5 g (range, −1.5 to −6.5 g), and 2.0 g (range −2.0 to −3.0 g), respectively. In contrast, a weight gain was observed for those mice injected with sterile Cytodex and no bacteria (1.5 g; range, 0 to 4.0 g) (P < 0.005; Wilcoxon signed-rank test) (Fig. 1). Similarly, weight gain was observed in mice that received 106 CFU of the commensal strains 9041 (1.25 g; range, 0 to 4.0 g), 9059 (1.0 g; range, 0 to 2.5 g), 9066 (0.25 g; range, 0 to 2.5 g), 9085 (2.0 g; range, 1.0 to 4.0 g), and 9098 (1.25 g; range, 0 to 2.0 g). Morbidity, marked by severe lethargy and wasting, correlated with weight loss in disease-associated strain-infected animals. In contrast, mice infected with commensal isolates remained asymptomatic. Examination of hematoxylin-and-eosin-stained tissue sections from mice injected with disease-associated or commensal strains did not reveal local tissue damage or increased polymorphonuclear leukocyte infiltration compared to tissue sections from control mice that received Cytodex and no bacteria.
FIG. 1.
Weight change observed in hairless, female mice 72 h after subcutaneous injection with disease-associated (shaded bars) and commensal (open bars) strains of S. iniae. Bars represent median weight gain ± range. ∗, significantly different (P < 0.005) from weight change in the Cytodex control group as calculated by the Wilcoxon signed-rank test.
Resistance to whole blood killing.
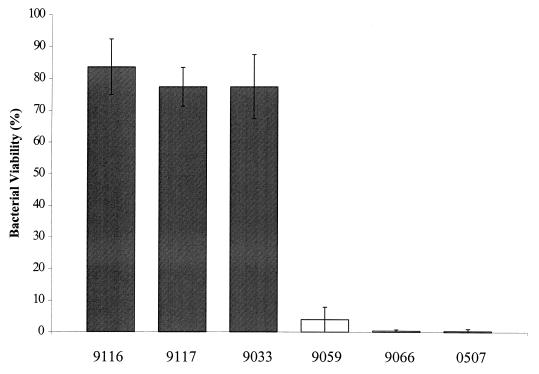
Following exposure of disease-associated S. iniae strains to human whole blood, the viable CFU recovered, expressed as percent survival, was slightly less than the initial inoculum of each strain. As shown in Fig. 2, 84 and 77% of human isolates 9116 and 9117, respectively, and 77% of the fish isolate 9033 were recovered from blood after 1.5 h of exposure. In contrast, commensal S. iniae strains (9059 and 9066) were rapidly killed by human whole blood. Survival of isolate 9059 was markedly reduced to 4% of the initial inoculum, while isolate 9066 was nearly eradicated (0.4%) (Fig. 2).
FIG. 2.
Resistance to phagocytosis of S. iniae strains in whole blood. Bars indicate the percentage of viable organisms relative to initial inoculum (100%) remaining after 1.5 h of rotation in fresh human blood. The results represent the mean ± SD for disease-associated (shaded bars) and commensal (open bars) strains.
Cellular injury.
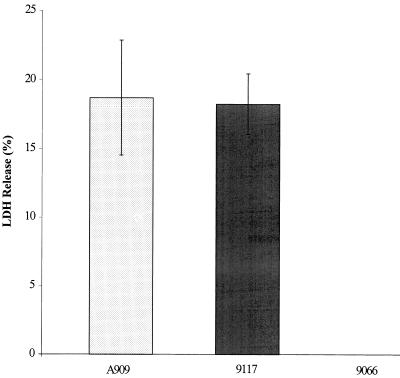
To examine potential cytotoxic effects of S. iniae on host cells, release of a eukaryotic cytoplasmic enzyme, LDH, from BMEC monolayers exposed to a high inoculum of bacteria was quantified using a microtiter plate assay. The level of LDH released was reported as a percentage of the total LDH calculated from complete lysis of BMEC monolayers. The disease-associated S. iniae strain 9117 (108 CFU) induced LDH release from BMEC cells (18.26%) similar to a 10-fold-lower inoculum of the cytolytic GBS strain A909 (18.71%) (Fig. 3). Conversely, the commensal strain 9066 (108 CFU) did not cause a release of LDH above background levels (medium alone) (Fig. 3).
FIG. 3.
Injury to BMEC monolayers exposed to disease-associated (9117) and commensal (9066) strains of S. iniae (5.0 × 108 CFU) for 3 h in comparison to the cytolytic GBS strain A909 (5.0 × 107 CFU). The higher inoculum used for S. iniae was required in order to obtain equivalent results. Bars indicate the mean LDH released, ± SD, relative to total LDH released from lysed monolayers.
Adherence and invasion.
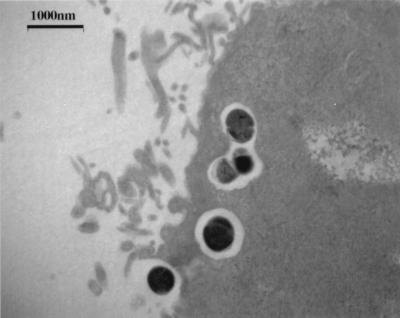
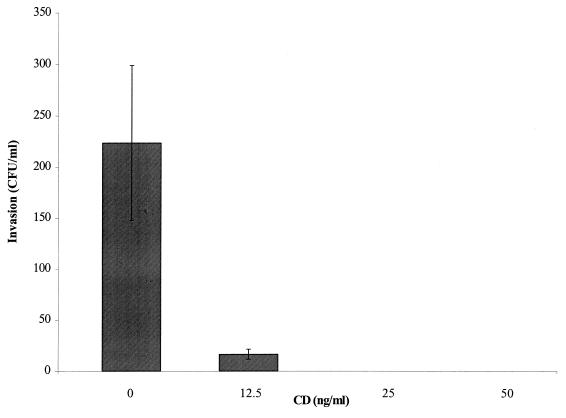
Representative disease-associated and commensal strains of S. iniae (9117 and 9085, respectively) adhered equally to the surface of HEp-2 cells (∼105 CFU/ml) from an initial inoculum of 106 cells. Moreover, each strain invaded HEp-2 cells with an efficiency of 0.1% (102 CFU/ml) of total cell-associated bacteria. The negative control, S. sanguis strain 2207, remained completely noninvasive to HEp-2 cells as expected. The interaction of S. iniae 9117 with HEp-2 cells was examined by electron microscopy. Monolayers infected for 2 h with 105 CFU revealed internalized bacteria clearly enclosed within membrane-bound vesicles (Fig. 4). Dividing forms of streptococci were also observed. To determine the fate of intracellular bacteria, viability was assessed 2, 4, 8, 24, and 48 h following addition of antibiotics to the cell cultures. The number of internalized bacteria (9117) remained relatively unchanged for 24 h but then declined significantly over the subsequent 24-h period. Cytochalasin D was used to determine the participation of cytoskeletal elements in bacterial adherence and invasion. Although bacterial adherence to HEp-2 cells was not affected, increasing doses of cytochalasin D quickly abolished invasion of S. iniae (Fig. 5), suggesting that actin microfilaments of the host cytoskeleton were required for internalization of S. iniae.
FIG. 4.
Electron micrograph of HEp-2 epithelial cells exposed to disease-associated S. iniae strain 9117 illustrating streptococci internalized within a membrane-bound vesicle.
FIG. 5.
Invasion of HEp-2 epithelial cells by S. iniae disease-associated strain 9117 in the presence of the actin polymerization inhibitor cytochalasin D (CD). Bars indicate the mean level of invasion ± SD at increasing concentrations of inhibitor.
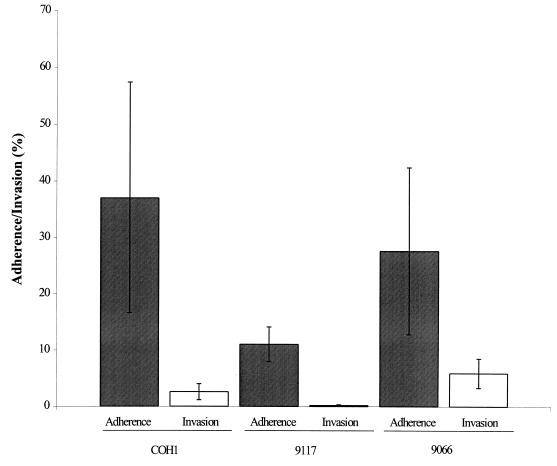
Disease-associated and commensal strains of S. iniae (105 CFU of strains 9117 and 9066, respectively) both adhered to and invaded BMEC cells (Fig. 6). The total number of cell-associated bacteria of strain 9066 was 28% of the initial inoculum, with 6.0% intracellular invasion. In comparison to the commensal strain, the disease-associated strain 9117 demonstrated fewer total BMEC-associated bacteria (11%) as well as a diminished level of invasion (0.18%). Thus, the overall efficiencies of BMEC invasion by commensal and disease-associated strains were 4.7- and 61-fold lower than the level of total cell-associated bacteria, respectively (Fig. 6).
FIG. 6.
BMEC adherence (shaded bars) and invasion (open bars) by disease-associated (9117) and commensal (9066) strains of S. iniae in comparison to the invasive GBS strain COH1. Bars indicate the mean adherence/invasion, ± SD, relative to the initial inoculum.
DISCUSSION
This is the first report demonstrating differences in virulence between disease-associated and non-disease-associated (commensal) isolates of S. iniae. Our in vivo studies revealed that only disease-associated strains of S. iniae were able to induce weight loss, visible signs of morbidity, and sustained bacteremia in infected mice. In particular, bacteremia was induced at infectious doses as low as 102 CFU. In contrast to human disease, cellulitis at the site of injection was not discernible macroscopically in mice infected with either disease-associated or commensal strains. However, as reported for the majority of human cases (35), bacteremia was evident in mice. Thus, as indicated by bacteremia and weight loss in mice, both of which are restricted to disease-associated strain infections, this model has proven to be useful for studying the virulence of S. iniae. It is interesting that previously reported animal models studying the virulence of the type strain (ATCC 29178), isolated from a dermal lesion of a dolphin, could not establish disease even with high inocula (27). Given the genetic unrelatedness of this strain from the clonal type (35), it would seem that the original S. iniae isolate is not virulent, as defined by the experimental systems used here.
As described, histopathology of mouse tissue sections, following subcutaneous injection of disease-associated strains, did not reveal gross cell damage, yet mice developed persistent bacteremia. This suggested that S. iniae could potentially traverse endothelial cell barriers, gaining direct access to the bloodstream. Our observations clearly indicate that disease-associated strains, whether isolated from humans or diseased fish, are capable of resisting phagocytic activity of human whole blood and that commensal strains are severely limited in this respect. Disease-associated strain survival was marginally reduced, yet commensal strains were nearly abolished. The growth rates of strains tested in bacterial culture media were similar and thus not pertinent to the observed differences in bacterial survival. For several streptococcal pathogens, extracellular polysaccharide capsule (GAS, GBS, Streptococcus pneumoniae, and S. suis) (4, 17, 24, 37) and surface-associated proteins (GAS and type 3 pneumococci) (1, 12) deter phagocytosis and prevent opsonization of bacteria by complement. We have noted that disease-associated strains of S. iniae display a high buoyancy (turbid) in broth culture, whereas commensal strains have a low buoyancy, forming a granular precipitate (unpublished data) as described for the type strain (ATCC 29178) (27). For GBS, buoyancy in broth culture is directly related to the level of capsular polysaccharide expression (15, 16). Furthermore, in an in vivo study, low-buoyancy strains with small amounts of capsule became highly encapsulated and established bacteremia after 5 days of inoculation. Our studies with S. iniae indicate that commensal strains do not cause bacteremia up to 5 days following injection and capsule expression is not evident even in virulent strains (data not shown) (15). Although we have not observed mucoidy in our studies, S. iniae was originally described as an encapsulated organism (27), and mucoid colonies, characteristic of encapsulation, were reported in a fish infection model (8). Furthermore, although M-type surface proteins, which render certain groups of streptococci resistant to phagocytosis, have not been identified in S. iniae, the existence of an uncharacterized surface component or even a secreted factor that might interfere with phagocytosis, as described for SpeB of GAS (22), cannot be excluded.
The ability to induce host cell injury, which is considered a primary step in pathogenesis, has been recognized for several streptococcal pathogens, such as GBS (13, 25) and S. suis type 2 (5). Injury to BMEC monolayers, as determined by the release of LDH, was evident following exposure to the disease-associated S. iniae strain 9117 but not with exposure to the commensal isolate 9066. Damage to endothelial layers could aid bacterial access to the bloodstream and systemic spread, as observed in the murine model. Furthermore, the meningoencephalitis reported in fish disease may be attributed in part to the ability of S. iniae to promote cell injury and disruption of the blood-brain barrier.
Another distinct feature of many streptococcal species, such as GAS, GBS, S. pneumoniae and S. suis (5, 21, 30, 31), is the ability to adhere to host tissue and invade intracellularly where invasion is defined as bacterial internalization within nonphagocytic cells in an in vitro system. Adherence and invasion may bestow resistance to host clearance mechanisms or facilitate systemic dissemination by breaching cell barriers. In this study, we found that both disease-associated and commensal strains could adhere to and invade epithelial and endothelial cells. Internalized bacteria were enclosed within membrane-bound vesicles and were able to survive for considerable lengths of time. GAS and GBS have also been shown to survive intracellularly for long durations without causing damage to the host (14, 31). In our studies, the actin microfilament inhibitor cytochalasin D abrogated invasion, indicating that this process is dependent on host cell cytoskeletal microfilaments as described for pathogens such as GBS, Yersinia enterocolitica, Shigella flexneri, and Escherichia coli (10, 11, 26).
Surprisingly, a reduced adherence and invasion of the disease-associated isolate to BMEC was observed in comparison to the commensal strain, suggesting that the latter was more proficient than the disease-associated strain at invading these cells. However, it should be noted that cellular invasion is not a prerequisite for systemic infections. Highly encapsulated strains of GAS do not invade epithelial cells in vitro, likely due to physical separation of receptor-ligand pairs, yet exhibit an enhanced virulence in vivo (32). The presence of an unidentified surface component in disease-associated strains of S. iniae that could interfere with receptor binding, and perhaps recognition by host phagocytic cells, might explain the lower level of invasion observed.
In summary, we have demonstrated that only S. iniae strains that display the distinct genetic profile associated with clinical disease are virulent in a murine model of subcutaneous infection. This suggests that these clonally related strains express one or more virulence determinants, not present in commensal isolates, which are responsible for systemic infections caused by S. iniae. Both disease-associated and commensal strains adhered to and invaded human epithelial and endothelial cells, indicating that enhanced cellular attachment and intracellular invasion were not discriminating features of disease-associated isolates. In contrast, only disease-associated isolates were resistant to phagocytic clearance and promoted host cell injury, each a phenotypic property that could contribute to virulence of S. iniae. However, in consideration of the direct route of transmission preceding human disease, the antiphagocytic properties of disease-associated strains reported here most likely play a critical role in the pathogenesis of S. iniae infection. Ongoing studies in our laboratory will focus on identifying virulence determinants responsible for the antiphagocytic potential of the disease-associated, clonally related strains of S. iniae.
ACKNOWLEDGMENTS
This work was supported by a grant from the Canadian Bacterial Diseases Network. J.D.F. is a recipient of a scholarship from the Ontario Graduate Scholarship Programme of the Ministry of Education and Training of Ontario.
We thank Jackie Pittman for assistance with the electron microscopy.
REFERENCES
- 1.Angel C S, Ruzek M, Hostetter M K. Degradation of C3 by Streptococcus pneumoniae. J Infect Dis. 1994;170:600–608. doi: 10.1093/infdis/170.3.600. [DOI] [PubMed] [Google Scholar]
- 2.Bercovier H, Ghittino C, Eldar A. Immunization with bacterial antigens: infections with streptococci and related organisms. Dev Biol Stand. 1997;90:153–160. [PubMed] [Google Scholar]
- 3.Betschel S D, Borgia S M, Barg N L, Low D E, De Azavedo J C. Reduced virulence of group A streptococcal Tn916 mutants that do not produce streptolysin S. Infect Immun. 1998;66:1671–1679. doi: 10.1128/iai.66.4.1671-1679.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brazeau C, Gottschalk M, Vincelette S, Martineau-Doize B. In vitro phagocytosis and survival of Streptococcus suis capsular type 2 inside murine macrophages. Microbiology. 1996;142:1231–1237. doi: 10.1099/13500872-142-5-1231. [DOI] [PubMed] [Google Scholar]
- 5.Charland N, Nizet V, Rubens C E, Kim K S, Lacouture S, Gottschalk M. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect Immun. 2000;68:637–643. doi: 10.1128/iai.68.2.637-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeVoe I W, Storm D W, Gilchrist J E. A study of phagocytosis of radio-labeled Staphylococcus epidermidis and on structural events during intracellular degradation. Can J Microbiol. 1973;19:525–530. doi: 10.1139/m73-084. [DOI] [PubMed] [Google Scholar]
- 7.Eldar A, Bejerano Y, Bercovier H. Streptococcus shiloi and Streptococcus difficile: two new streptococcal species causing a meningoencephalitis in fish. Curr Microbiol. 1994;28:193–143. [Google Scholar]
- 8.Eldar A, Bejerano Y, Livoff A, Horovitcz A, Bercovier H. Experimental streptococcal meningo-encephalitis in cultured fish. Vet Microbiol. 1995;43:33–40. doi: 10.1016/0378-1135(94)00052-x. [DOI] [PubMed] [Google Scholar]
- 9.Eldar A, Frelier P F, Assenta L, Varner P W, Lawhon S, Bercovier H. Streptococcus shiloi, the name for an agent causing septicemic infection in fish, is a junior synonym of Streptococcus iniae. Int J Syst Bacteriol. 1995;45:840–842. doi: 10.1099/00207713-45-4-840. [DOI] [PubMed] [Google Scholar]
- 10.Finlay B B, Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988;70:1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- 11.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischetti V A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson R L, Nizet V, Rubens C E. Group B streptococcal beta-hemolysin promotes injury of lung microvascular endothelial cells. Pediatr Res. 1999;45:626–634. doi: 10.1203/00006450-199905010-00003. [DOI] [PubMed] [Google Scholar]
- 14.Greco R, De Martino L, Donnarumma G, Conte M P, Seganti L, Valenti P. Invasion of cultured human cells by Streptococcus pyogenes. Res Microbiol. 1995;146:551–560. doi: 10.1016/0923-2508(96)80561-4. [DOI] [PubMed] [Google Scholar]
- 15.Hakansson S, Bergholm A M, Holm S E, Wagner B, Wagner M. Properties of high and low density subpopulations of group B streptococci: enhanced virulence of the low density variant. Microb Pathog. 1988;5:345–355. doi: 10.1016/0882-4010(88)90035-6. [DOI] [PubMed] [Google Scholar]
- 16.Hakansson S, Holm S. Influence of polysaccaride capsule and ionic strength on buoyant density of group B streptococci. Acta Pathol Microbiol Immunol Scand Sect B. 1986;94:139–143. doi: 10.1111/j.1699-0463.1986.tb03033.x. [DOI] [PubMed] [Google Scholar]
- 17.Hostetter M K. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J Infect Dis. 1986;153:682–693. doi: 10.1093/infdis/153.4.682. [DOI] [PubMed] [Google Scholar]
- 18.Kitao T, Aoki T, Sakoh R. Epizootic caused by beta-hemolytic Streptococcus species in cultured freshwater fish. Fish Pathol. 1981;19:173–180. [Google Scholar]
- 19.Kusuda R. Bacterial fish diseases in marineculture in Japan with special emphasis on streptococcosis. Isr J Aquacult. 1992;44:140. [Google Scholar]
- 20.Lancefield R C. Current knowledge of the type-specifec M antigens of group A streptococci. J Immunol. 1962;89:307–313. [PubMed] [Google Scholar]
- 21.LaPenta D, Rubens C, Chi E, Cleary P P. Group A streptococci efficiently invade human respiratory epithelial cells. Proc Natl Acad Sci USA. 1994;91:12115–12119. doi: 10.1073/pnas.91.25.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukomski S, Burns E H, Jr, Wyde P R, Podbielski A, Rurangirwa J, Moore-Poveda D K, Musser J M. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect Immun. 1998;66:771–776. doi: 10.1128/iai.66.2.771-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madoff L C, Michel J L, Kasper D L. A monoclonal antibody identifies a protective C-protein alpha-antigen epitope in group B streptococci. Infect Immun. 1991;59:204–210. doi: 10.1128/iai.59.1.204-210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin T R, Ruzinski J T, Rubens C E, Chi E Y, Wilson C B. The effect of type-specific polysaccharide capsule on the clearance of group B streptococci from the lungs of infant and adult rats. J Infect Dis. 1992;165:306–314. doi: 10.1093/infdis/165.2.306. [DOI] [PubMed] [Google Scholar]
- 25.Nizet V, Gibson R L, Chi E Y, Framson P E, Hulse M, Rubens C E. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect Immun. 1996;64:3818–3826. doi: 10.1128/iai.64.9.3818-3826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nizet V, Kim K S, Stins M, Jonas M, Chi E Y, Nguyen D, Rubens C E. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peir G B, Madin S H. Streptococcus iniae sp. nov., a beta-hemolytic streptococcus isolated from an Amazon freshwater dolphin, Inia geoffrensis. Int J Syst Bacteriol. 1976;26:545–553. [Google Scholar]
- 28.Peir G B, Madin S H. Isolation and characterization of a second isolate of Streptococcus iniae. Int J Syst Bacteriol. 1978;28:311–314. [Google Scholar]
- 29.Perera R P, Johnson S K, Collins M D, Lewis D H. Streptococcus iniae associated with mortality of Tilapia nilotica × T. aurea hybrids. J Aquat Anim Health. 1994;6:335–340. [Google Scholar]
- 30.Ring A, Weiser J N, Tuomanen E I. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Investig. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubens C E, Smith S, Hulse M, Chi E Y, van Belle G. Respiratory epithelial cell invasion by group B streptococci. Infect Immun. 1992;60:5157–5163. doi: 10.1128/iai.60.12.5157-5163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrager H M, Rheinwald J G, Wessels M R. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Investig. 1996;98:1954–1958. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon D, Ferretti J J. Electrotransformation of Streptococcus pyogenes with plasmid and linear DNA. FEMS Microbiol Lett. 1991;66:219–224. doi: 10.1016/0378-1097(91)90336-9. [DOI] [PubMed] [Google Scholar]
- 34.Teixeira L M, Merquior V L, Vianni M C, Carvalho M G, Fracalanzza S E, Steigerwalt A G, Brenner D J, Facklam R R. Phenotypic and genotypic characterization of atypical Lactococcus garvieae strains isolated from water buffalos with subclinical mastitis and confirmation of L. garvieae as a senior subjective synonym of Enterococcus seriolicida. Int J Syst Bacteriol. 1996;46:664–668. doi: 10.1099/00207713-46-3-664. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein M R, Litt M, Kertesz D A, Wyper P, Rose D, Coulter M, McGeer A, Facklam R, Ostach C, Willey B M, Borczyk A, Low D E. Invasive infections due to a fish pathogen, Streptococcus iniae. S. iniae Study Group. N Engl J Med. 1997;337:589–594. doi: 10.1056/NEJM199708283370902. [DOI] [PubMed] [Google Scholar]
- 36.Wessels M R, Benedi V J, Kasper D L, Heggen L M, Rubens C E. Type III capsule and virulence of group B streptococci. In: Dunny G M, Cleary P P, McKay L L, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C.: American Society for Microbiology; 1991. pp. 219–223. [Google Scholar]
- 37.Whitnack E, Bisno A L, Beachey E H. Hyaluronate capsule prevents attachment of group A streptococci to mouse peritoneal macrophages. Infect Immun. 1981;31:985–991. doi: 10.1128/iai.31.3.985-991.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]