Abstract
Introduction
The detection of a fetal anomaly during routine obstetric ultrasound is a potentially traumatic experience. The aim of this study is to examine longitudinally the impact of diagnosis of fetal anomaly on symptoms of depression and traumatic stress among mothers and fathers, and to examine how variations in psychological adjustment relate to diagnostic severity and prognostic ambiguity.
Material and methods
In this prospective observational study conducted at a tertiary perinatal referral center, 81 mothers and 69 fathers with ultrasound findings of fetal anomaly completed the Edinburgh Postnatal Depression Scale (EPDS) and Impact of Events Scale (IES) at four time points in pregnancy (T1–T4) and 6 weeks after birth (T5). We compared this with depression and traumatic stress in a sample of non‐affected parents (n = 110 mothers, 98 fathers).
Results
Linear mixed effects models indicated that parents who received a diagnosis of fetal anomaly experienced higher levels of depression and traumatic stress over time, compared with non‐affected parents. Depression: mean difference mothers = 4.46 ± 0.47, fathers = 2.80 ± 0.42. Traumatic stress: mean difference mothers = 20.04 ± 2.13, fathers = 12.66 ± 1.74. Parents with a more severe diagnosis experienced elevated symptoms compared with parents with a less severe diagnosis. Among mothers, prognostic ambiguity and changes in the anticipated diagnosis after birth were also associated with increased distress, regardless of whether the change was for the better or worse.
Conclusions
Diagnosis of fetal anomaly increases risk of depression and traumatic stress in expectant mothers and fathers, both acutely and over time.
Keywords: congenital malformation, depression, postpartum, pregnancy, prenatal diagnosis, psychological distress, traumatic stress
This study examines the impact of diagnosis of fetal anomaly on parental depression and traumatic stress through pregnancy and after birth. We found that diagnosis of fetal anomaly increases risk of depression and traumatic stress acutely and over time. In particular, diagnostic severity, prognostic ambiguity and changes in diagnosis may increase the risk of persistent elevated distress.

Abbreviations
- EPDS
Edinburgh Postnatal Depression Scale
- IES
Impact of Events Scale
Key message.
The diagnosis of fetal anomaly is associated with higher levels of parental depression and traumatic stress through pregnancy and after birth. In particular, diagnostic severity, prognostic ambiguity and changes in diagnosis may increase the risk of persistent elevated distress.
1. INTRODUCTION
Fetal anomaly is a genetic or physical condition that occurs in around 4% of births. 1 Learning that one's expectant child has a serious or potentially life‐threatening condition can be a traumatic experience. 2 A majority of previous research on parental psychological stress reactions have been conducted among parents that terminate the pregnancy, suggesting potential long‐term impact on parental distress. 3 , 4 , 5 In pregnancies with a diagnosis of fetal anomaly not resulting in termination, a few studies suggest that parental stress levels remain elevated through pregnancy 2 , 6 , 7 and after birth. 8 , 9 , 10 , 11 However, a limitation in these previous studies is the use of cross‐sectional designs. 2 , 7 , 8 , 9 , 10 To our knowledge, none of the few longitudinal studies that exist, assessed distress at several time points during pregnancy and continuing after birth. 6 , 11 , 12 , 13
In addition, little attention has been given to how the severity of the fetal anomaly influences the development and maintenance of parental psychological stress symptoms. Studies that have looked at the impact of diagnostic severity on parental stress have found conflicting results. 2 , 14 Furthermore, in addition to variations in diagnostic severity there may also be significant prognostic ambiguity associated with the diagnosis of fetal anomaly. Uncertainty is a major component in the experience of detecting an anomaly and can dramatically affect psychosocial adaptation to the diagnosis. 15 To advance our understanding of the parental stress experience it is therefore important to consider how variations in diagnostic severity and uncertainty affect parental depression and traumatic stress.
To date, most previous studies have focused on maternal stress, yet the expectant father is often present at the prenatal ultrasound appointment, at delivery, and in the neonatal intensive care unit. 16 With the current study, we will include expectant fathers as well as mothers, thus filling this important gap in the literature.
The overall aim of this study is to examine longitudinally the impact of diagnosis of fetal anomaly on symptoms of depression and traumatic stress among mothers and fathers. We will do this by (1) describing symptoms of depression and traumatic stress among parents with ultrasound findings of fetal anomaly at four time points in pregnancy and 6 weeks after birth; (2) comparing that group to a group of parents with normal ultrasound findings; and (3) examining how variations in psychological adjustment relate to diagnostic severity, prognostic ambiguity and changes in diagnosis.
2. MATERIAL AND METHODS
2.1. Study design and participants
The present study is part of a larger longitudinal study examining parental stress reactions following the detection of fetal anomalies (the SOFUS study). Recruitment occurred among pregnant women and their partners receiving obstetric care at Oslo University Hospital, Rikshospitalet. Participants in the study group were recruited following the identification of a suspected structural fetal anomaly during second trimester routine ultrasound scan. In the comparison group, we recruited participants following normal ultrasound findings. The initial sample consisted of 180 expectant mothers and 150 of their partners with a detected malformation (study group), and 110 expectant mothers and 98 of their partners with normal ultrasound findings and an uncomplicated pregnancy history (comparison group). In both groups, all the women's partners were male. In the study group, 87 of the 180 women terminated the pregnancy following diagnosis, and these parents are not included in the current study. An additional 12 women were excluded because the fetal anomaly was diagnosed after gestational week 28, which was deemed too late to participate in the present study. Women with multiple pregnancies, who were under the age of 18 years, not fluent in Norwegian, or who were not legally competent to provide informed consent were not eligible to participate.
2.2. Procedure
Expectant mothers and fathers answered questionnaires about their symptoms of depression and traumatic stress at four time points during pregnancy. The first questionnaire was completed within 72 hours of either a diagnosis of fetal anomaly or normal ultrasound findings (T1; 18–28 weeks’ gestation). Data was gathered three more times during pregnancy: 2–3 weeks after inclusion (T2), at 30 weeks’ gestation (T3) and at 36 weeks’ gestation (T4). Postnatal data were completed 6 weeks after birth (T5). Data collection occurred between May 2006 and February 2009.
2.2.1. Ultrasound examination and diagnosis
The ultrasound examinations were performed by trained midwives and specialists in fetal medicine. Among women in the study group, further consultations with specialists were conducted as needed. All the fathers were present at the initial ultrasound examination and were invited to attend all consultations. Diagnostic severity and prognostic ambiguity of the diagnoses were classified according to a modified version of Kaasen et al. 17 Three of the authors performed the classification, with strong interrater agreement (κ = 0.86). This led to the following four categories of fetal anomaly:
Lethal or serious, with or without prognostic ambiguity (eg holoprosencephaly, myelomeningocele with hydrocephalus, hypoplastic left heart syndrome)
Mild to moderate severity, but with prognostic ambiguity (eg bilateral clubfoot or cleft lip with no other markers, conditions known to be associated with syndromes not apparent prenatally)
Mild to moderate severity, and without prognostic ambiguity (eg gastroschisis, unilateral clubfoot)
Severity not classified, awaiting clarification. Prognosis highly dependent on the results of an invasive test (eg omphalocele, bilateral clubfoot with chromosomal soft markers) or a reliable diagnosis was not available at inclusion because of an incomplete ultrasound examination (eg maternal obesity).
Within 2 weeks after birth there were 12 changes in diagnosis/prognosis compared with what had been diagnosed at inclusion. Of these, three were an improvement in diagnosis/prognosis and nine were a worsening. In three of these 12 cases the parents had been informed of a change during pregnancy, following further prenatal testing, but were also informed of additional changes in diagnosis/prognosis postpartum. In the remaining nine cases, the change was only detected postpartum.
2.3. Measures
Depression was measured using the Edinburgh Postnatal Depression Scale (EPDS). 18 The scale was originally developed to detect postpartum depression, but has since been validated for use during pregnancy, 19 with men 20 and with Norwegian populations. 21 The scale consists of 10 items: five measure dysphoric mood, two measure anxiety and one item each measures guilt, suicidal ideas and incidence of “not coping” in the past week. Items were summarized based on answers to a Likert scale from 0 “not at all” to 3 “most of the time” (total range 0–30). A total score of 11 or higher is considered clinically significant. 22 Cronbach's alpha for the measure was 0.90.
Traumatic stress was measured using the Impact of Event Scale (IES). The IES is a 22‐item questionnaire measuring emotional and behavioral symptoms over the past week in response to a defined stressful or traumatic event. In this case, the questions were asked with reference to “your child's condition”. The original IES contains two subscales with seven items measuring intrusion (disturbing affects and thoughts about the traumatic event) and eight items measuring avoidance (effortful attempts at avoiding thoughts and images related to the event). The IES version used in this study includes six additional items measuring arousal (ie irritability, difficulty concentrating, hypervigilance) and one additional item measuring intrusion, as published by Weiss. 23 Items were scored from 0–5, with 0 meaning “not at all” and 5 meaning “often”, and the totals were summarized for each subscale. Scores ≥20 on any subscale are considered a clinically significant response. A score of Cronbach's alpha for the measure ranged from 0.84 to 0.90 for the three subscales.
Sociodemographic factors were assessed at inclusion using self‐report questionnaires. Medical and obstetric history was collected using self‐report questionnaires and electronic charts at baseline (T1).
2.4. Power analyses
Preliminary analyses were performed using IBM SPSS version 27 (Statistical Package for the Social Sciences, IBM, Armonk, NY, USA). The minimum number of participants needed to detect a difference in traumatic stress between the study and comparison group was calculated based on data from Nes et al. 6 We found that a total sample size of 68 mothers should be sufficient to detect a difference of one SD with power = 0.80 and α = 0.05.
2.5. Statistical analyses
Linear mixed effects models were calculated using R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria) with the package “lme4” 24 and α = 0.05. The models were fitted to test whether a diagnosis of fetal anomaly was significantly related to depression and traumatic stress scores over time, as well as the impact of diagnostic severity and prognostic ambiguity on parental symptom levels. Model parameters were estimated by means of maximum likelihood, an approach that makes use of all observed data. Competing models were compared using the Likelihood Ratio Test. 25 Since a group mean can conceal changes on an individual level, individual trajectories, as well as means and 95% CIs were included in one graph. The impact of change in diagnosis on parental postnatal distress was calculated using linear regression with depression and traumatic stress 6 weeks postpartum (T5) as the dependent variables. The difference between groups is presented as mean ± SD.
Attrition analyses comparing those with and those without missing data, indicated no significant differences in diagnostic severity (P = 0.360), prognostic ambiguity (P = 0.635), gestational age at inclusion (P = 0.265), education (P = 0.244), age (P = 0.738), initial depression (P = 0.391) or initial traumatic stress (P = 0.161).
2.6. Ethics statement
Written informed consent was obtained prior to participation. The study was approved by the Regional Ethics Committee of Southern Norway on December 21, 2005 (reference number S‐05281) and on May 10, 2016 (reference number 2016/776/REK Sør‐Øst). In accordance with the study protocol, any participant who indicated suicidal ideation on self‐report questionnaires was contacted for clinical evaluation and offered psychiatric assistance if necessary.
3. RESULTS
Women in the study group differed from the comparison group in terms of age, education and gestational age at inclusion (see Table 1 for details). Among expectant fathers, there was a significant difference in education between the groups. The mean traumatic stress scores for men and women in both groups at each time point are detailed in Table 2.
TABLE 1.
Demographic characteristics of mothers and fathers with and without a diagnosis of fetal anomaly
| Mothers | Fathers | |||||
|---|---|---|---|---|---|---|
| Study group n = 81 | Comparison group n = 110 | P‐value | Study group n = 69 | Comparison group n = 98 | P‐value | |
| Parental age, mean (SD) | 29.9 (4.78) | 31.6 (3.86) | 0.014 | 32.4 (4.09) | 33.9 (5.03) | 0.061 |
| Education, n (%) | <0.001 | <0.001 | ||||
| High school or less | 32 (39.5) | 16 (14.4) | 34 (47.9) | 15 (15.0) | ||
| Any university | 49 (60.5) | 95 (85.6) | 37 (52.1) | 85 (85.0) | ||
| Married or cohabiting, n (%) | 79 (97.5) | 110 (100) | 0.173 | 69 (100) | 98 (100) | 0.999 |
| Gestational weeks at inclusion, mean (SD) | 21.9 (4.8) | 18.8 (2.1) | <0.001 | |||
| Parity, n (%) | 0.645 | |||||
| 0 | 42 (51.8) | 61 (55.4) | ||||
| 1+ | 39 (48.1) | 49 (44.5) | ||||
| In vitro fertilization, n (%) | 5 (6.1) | 8 (7.2) | 0.898 | |||
| Previous miscarriage, n (%) | 14 (17.3) | 27 (24.5) | 0.338 | |||
| Previous termination of pregnancy, n (%) | 20 (24.6) | 17 (15.4) | 0.189 | |||
| Classification of severity, fetal anomaly, n (%) | ||||||
| 1 – Lethal or serious with or without prognostic ambiguity | 20 (24.7) | 18 (25.4) | ||||
| 2 – Mild to moderate severity and without prognostic ambiguity | 23 (28.4) | 18 (25.4) | ||||
| 3 – Mild to moderate severity and with prognostic ambiguity | 15 (18.5) | 15 (21.1) | ||||
| 4 – Severity not classified, awaiting clarification | 23 (28.4) | 20 (28.2) | ||||
Note: Significant group differences are highlighted in bold.
TABLE 2.
Psychological distress among mothers and fathers with and without a diagnosis of fetal anomaly over time
| Mothers | Fathers | |||||
|---|---|---|---|---|---|---|
| Study group Mean (SD) | Comparison group Mean (SD) | P‐value | Study group Mean (SD) | Comparison group Mean (SD) | P‐value | |
| T1 | n = 81 | n = 110 | n = 69 | n = 98 | ||
| EPDS total | 11.26 (6.18) | 3.18 (3.15) | <0.001 | 5.94 (5.15) | 1.39 (1.86) | <0.001 |
| IES intrusion | 22.93 (10.29) | 9.49 (6.60) | <0.001 | 15.51 (9.54) | 7.07 (6.22) | <0.001 |
| IES avoidance | 10.34 (8.36) | 2.45 (4.05) | <0.001 | 7.13 (7.07) | 1.68 (2.92) | <0.001 |
| IES arousal | 12.09 (9.95) | 3.68 (4.25) | <0.001 | 6.49 (5.98) | 2.21 (2.46) | <0.001 |
| T2 | n = 60 | n = 104 | n = 46 | n = 95 | ||
| EPDS total | 6.85 (5.72) | 2.54 (3.08) | <0.001 | 3.38 (3.53) | 1.13 (2.01) | <0.001 |
| IES intrusion | 17.00 (10.61) | 7.13 (6.41) | <0.001 | 11.00 (7.61) | 5.09 (5.45) | <0.001 |
| IES avoidance | 7.42 (7.96) | 1.32 (2.51) | <0.001 | 4.50 (5.49) | 1.03 (1.81) | <0.001 |
| IES arousal | 8.27 (7.66) | 2.96 (3.79) | <0.001 | 4.93 (4.84) | 1.59 (1.89) | <0.001 |
| T3 | n = 51 | n = 108 | n = 39 | n = 95 | ||
| EPDS total | 5.02 (3.64) | 3.13 (3.46) | 0.002 | 2.03 (3.36) | 1.13 (1.89) | 0.122 |
| IES intrusion | 11.63 (8.76) | 6.91 (6.81) | 0.001 | 6.10 (5.99) | 4.43 (4.91) | 0.096 |
| IES avoidance | 5.08 (7.08) | 1.38 (3.28) | 0.001 | 3.21 (4.91) | 0.83 (2.15) | <0.001 |
| IES arousal | 6.06 (5.78) | 3.53 (3.72) | 0.006 | 2.97 (3.67) | 1.65 (1.97) | 0.008 |
| T4 | n = 59 | n = 103 | n = 45 | n = 83 | ||
| EPDS total | 5.21 (4.69) | 2.82 (2.75) | 0.001 | 2.47 (2.84) | 0.86 (1.45) | 0.001 |
| IES intrusion | 11.64 (8.74) | 8.09 (7.51) | 0.007 | 6.82 (6.59) | 4.43 (4.95) | 0.022 |
| IES avoidance | 5.44 (7.77) | 1.04 (2.48) | <0.001 | 2.76 (3.39) | 0.75 (1.90) | <0.001 |
| IES arousal | 5.98 (6.22) | 4.17 (4.17) | 0.050 | 2.60 (3.51) | 2.07 (2.87) | 0.361 |
| T5 | n = 68 | n = 103 | n = 53 | n = 88 | ||
| EPDS total | 4.87 (4.98) | 2.90 (3.09) | 0.004 | 2.67 (3.83) | 1.49 (2.03) | 0.044 |
| IES intrusion | 10.76 (9.42) | 6.39 (7.97) | 0.001 | 7.98 (7.60) | 4.67 (6.31) | 0.009 |
| IES avoidance | 5.44 (7.01) | 0.70 (2.23) | <0.001 | 3.13 (5.20) | 0.83 (2.25) | 0.003 |
| IES arousal | 5.24 (5.93) | 3.14 (3.68) | 0.005 | 4.23 (5.62) | 2.19 (2.44) | 0.015 |
Note: T1 = gestational age 18–28 weeks; T2 = 2–3 weeks after T1; T3 = gestational age 30 weeks; T4 = gestational age 36 weeks; T5 = 6 weeks postpartum.
Significant group differences are highlighted in bold.
Abbreviations: EPDS, Edinburgh Postnatal Depression Scale; IES, Impact of Event Scale.
There was a significant difference in symptoms of depression among mothers with and without a diagnosis of fetal anomaly across time (T1–T5) such that mean depression was higher in the study group than in the comparison group: χ2(1) = 73.89, P < 0.001, mean difference 4.46 ± 0.47. Similarly, traumatic stress was higher across time among mothers in the study group than in the control group: IES intrusion χ2(1) = 63.75, P < 0.001, mean difference 8.73 ± 1.02 (see Figure 1); IES avoidance: χ2(1) = 65.15, P < 0.001, mean difference 6.29 ± 0.72; and IES arousal: χ2(1) = 46.79, P < 0.001, mean difference 4.95 ± 0.68.
FIGURE 1.
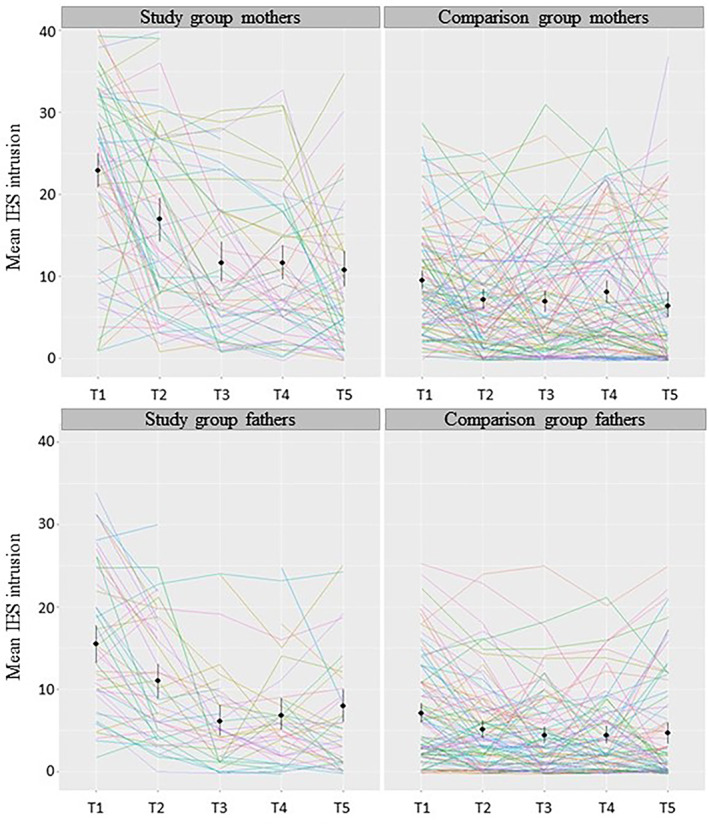
Impact of Events Scale (IES) subscale intrusion among parents in the study and comparison group over time (T1 = gestational age 18–28 weeks; T2 = 2–3 weeks after T1; T3 = gestational age 30 weeks; T4 = gestational age 36 weeks; T5 = 6 weeks postpartum). Individual participants’ trajectories are presented as colored lines and group means and 95% confidence intervals are marked in black
Among fathers, depression was higher across time among those with a diagnosis of fetal anomaly than those without a diagnosis: χ2(1) = 40.75, P < 0.001, mean difference 2.80 ± 0.42. There was also a significant difference on all subscales of traumatic stress between fathers with and without a diagnosis of fetal anomaly: IES intrusion χ2(1) = 36.59, P < 0.001, mean difference 5.66 ± 0.90 (see Figure 1); IES avoidance χ2(1) = 44.67, P < 0.001, mean difference 3.81 ± 0.54; and IES arousal χ2(1) = 32.92, P < 0.001, mean difference 3.08 ± 0.52.
Among mothers with a diagnosis of fetal anomaly, those who had a diagnosis classified as serious to lethal, experienced higher levels of intrusion and avoidance over time than those with a diagnosis classified as mild to moderate (see Figure 2): IES intrusion, χ2(1) = 3.90, P < 0.01, mean difference 4.72 ± 2.39; IES avoidance, χ2(1) = 2.15, P < 0.05, mean difference 2.79 ± 1.91. There was no difference in IES arousal or depression among mothers depending on severity of the diagnosis. Among fathers, those with a diagnosis categorized as severe to lethal also experienced more intrusion and arousal than those with a less severe diagnosis: IES intrusion, χ2(1) = 7.26, P < 0.001, mean difference 5.98 ± 2.18; IES arousal, χ2(1) = 5.03, P < 0.01, mean difference 3.50 ± 1.56. There was no difference in IES avoidance or depression among fathers with a more or a less serious diagnosis.
FIGURE 2.
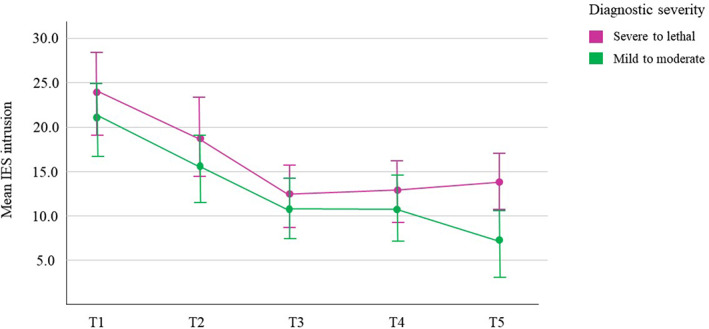
Mean and 95% confidence interval of Impact of Events Scale (IES) subscale intrusion among women by category of diagnostic severity: severe to lethal (n = 20) vs mild to moderate (n = 38). T1 = gestational age 18–28 weeks (n = 58); T2 = 2–3 weeks after T1 (n = 45); T3 = gestational age 30 weeks (n = 31); T4 = gestational age 36 weeks (n = 42); T5 = 6 weeks postpartum (n = 54)
Mothers with a diagnosis that had prognostic ambiguity experienced higher levels of traumatic stress over time compared with those with a diagnosis without prognostic ambiguity (see Figure 3): IES intrusion, χ2(1) = 3.90, P < 0.01, mean difference 4.63 ± 1.98; IES avoidance, χ2(1) = 4.41, P < 0.01, mean difference 3.61 ± 1.72; IES arousal, χ2(1) = 2.55, P < 0.05, mean difference 2.36 ± 1.48. There was no significant difference in depression among mothers depending on prognostic ambiguity. Fathers did not significantly differ in traumatic stress or depression depending on prognostic ambiguity.
FIGURE 3.
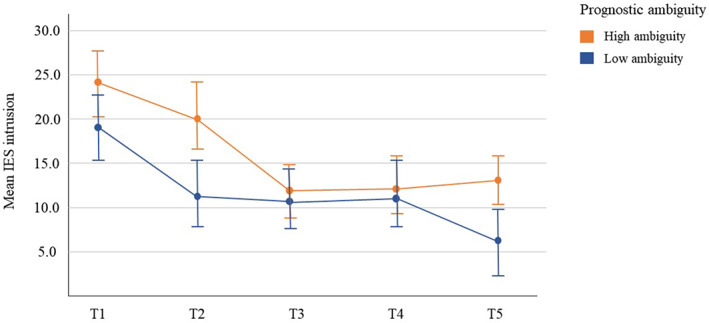
Mean and 95% confidence interval of Impact of Events Scale (IES) subscale intrusion among women by category of prognostic ambiguity: high prognostic ambiguity (n = 46) vs low prognostic ambiguity (n = 35). T1 = gestational age 18–28 weeks (n = 81); T2 = 2–3 weeks after T1 (n = 69); T3 = gestational age 30 weeks (n = 53); T4 = gestational age 36 weeks (n = 63); T5 = 6 weeks postpartum (n = 68)
Among mothers, a postnatal change in diagnosis from what had been diagnosed at the second trimester ultrasound examination predicted greater traumatic stress 6 weeks after birth: intrusion standardized beta = 0.484, P < 0.001; avoidance standardized beta = 0.383, P < 0.01; arousal standardized beta = 0.425, P < 0.001. This was independent of whether the change was for the better or for the worse: t (11) = 0.43, P = 0.674. There was a similar but slightly weaker association between diagnostic change and depression: standardized beta = 0.270, P < 0.05. Among fathers, postnatal change in diagnosis did not predict traumatic stress or depression at 6 weeks postpartum. Excluding the three of the 12 couples who experienced a change of diagnosis/prognosis during pregnancy from the analyses, did not change the association (results not shown).
4. DISCUSSION
We found that prenatal knowledge about fetal pathology represents both an acute and potentially long‐lasting psychological stressor, and that depression, intrusion, avoidance and arousal may characterize the remainder of the pregnancy and postnatal period for many parents. This is in line with previous studies that have documented elevated depression and traumatic stress among parents to children with congenital malformations in the weeks after diagnosis 7 and after birth. 8 , 10
Furthermore, we found that level of traumatic stress depended on diagnostic severity. Acute reactions were similar but, after birth, traumatic stress increased among mothers with a more severe diagnosis, whereas it decreased among those with a less severe diagnosis. This may mean that the impact of parenting a child with a severe congenital malformation reflects a persistent challenge that may be evident as early as 6 weeks after birth. Nes et al. 6 found that during pregnancy, mothers of children with cleft lip/palate and mothers of children with Down's syndrome both experienced increased anxiety compared with mothers of healthy babies. After 6 months, only mothers of children with Down's syndrome showed persistent distress. This may indicate that in cases of less severe and curable conditions, such as cleft lip/palate, the effect of diagnosis on parental distress is only temporary, whereas more severe cases are associated with long‐term distress.
Among mothers, diagnostic ambiguity predicted elevated traumatic stress trajectories. Previous studies have hypothesized that uncertainty about an illness prognosis may be preferential to knowing about a poor prognosis. 26 However, we found that uncertainty predicted greater traumatic stress independent of diagnostic severity. Thus, it seems that ambiguity may be perceived as a unique stressor in itself. Consequently, healthcare providers must be able to recognize and facilitate management of uncertainty among parents with a diagnosis of fetal anomaly.
Furthermore, a postpartum change in the diagnosis from what had been anticipated based on the initial ultrasound examination was associated with increased traumatic stress and depression at 6‐week follow‐up among mothers. This was irrespective of whether the change was for the better or worse. This may be due to the perceived reintroduction of ambiguity. It could also suggest that parents’ psychological coping capacity has been depleted by the distress of the initial diagnosis. In that case, when parents are informed about a change in diagnosis, processing and adapting to this new knowledge can be experienced as so demanding that distress increases even when the information is for the better. Among mothers who experienced a postnatal change in diagnosis, traumatic stress levels at 6 weeks postpartum were comparable to those observed at 2–3 weeks after the initial diagnosis. Thus, it seems that the traumatic stress response to a changed diagnosis may be similar to the acute reaction following the initial diagnosis. The relation between diagnostic change and depression was weaker, and there was no significant association between variations in ambiguity and depression. This suggests that traumatic stress responses may be more sensitive than depressive symptoms to diagnosis‐specific factors.
A strength of the study is the use of well‐known assessment methods applied in numerous other studies that have measured psychological responses to other traumatic and medical events. 11 To our knowledge, this is the first prospective longitudinal study to assess parental distress in response to diagnosis of fetal anomaly at several time points during pregnancy and after birth. Furthermore, in most previous studies, different categories of fetal anomalies were pooled, assuming that the main difference is between having a baby with or a baby without a congenital malformation. We add to this by examining the impact of diagnostic severity and ambiguity on parental traumatic stress and depression. Also contrary to most of previous research, we included both mothers and fathers and attained high response rates throughout the study.
Our study design is observational, and the study group and control group were not equal in terms of several baseline variables. Due to the relative rarity and unpredictability of fetal anomalies, it was not feasible to collect data prior to the diagnosis or to match the groups. However, the observed differences in sociodemographic factors may not necessarily affect associations between study variables. 27 , 28
5. CONCLUSION
The present study provides new insight into the consequences of prenatal diagnosis of congenital malformations. Our findings strongly indicate that careful support of parents is important following prenatal diagnosis. Specifically, these findings highlight the importance of bearing in mind variations in diagnostic severity and ambiguity when considering parental distress in response to the detection of fetal anomaly. Particular attention should be given to parents with more severe and ambiguous diagnoses, as well as cases where the diagnosis may change. A change in diagnosis from what was expected may be perceived as distressing, even when the change is for the better. This study also highlights the importance of a longitudinal design, as our research suggests that persistent stress responses following prenatal diagnosis of fetal anomaly are common. It appears many parents experience long‐lasting distress and may be in need of additional psychological support during pregnancy and in the postnatal period.
AUTHORS’ CONTRIBUTIONS
AK and GNH contributed to the design and implementation of the study. AO wrote the article with support from MB, GNH, NOC and AK. AO and NOC conducted the statistical analyses with contributions from all authors. MB, GNH and AK contributed with funding acquisition.
ACKNOWLEDGMENT
We acknowledge Arvid Heiberg and Egil Haug for their contribution to the design of the study.
FUNDING INFORMATION
This work was supported by the Research Council of Norway (RCN; grant number 288083 and 301004), Norwegian Women's Public Health Association, the Norwegian Association for Children with Congenital Heart Disease, University of Oslo, and Oslo University Hospital.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Oftedal A, Bekkhus M, Haugen GN, Czajkowski NO, Kaasen A. The impact of diagnosed fetal anomaly, diagnostic severity and prognostic ambiguity on parental depression and traumatic stress: a prospective longitudinal cohort study. Acta Obstet Gynecol Scand. 2022;101:1291‐1299. doi: 10.1111/aogs.14453
REFERENCES
- 1. Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Adv Exp Med Biol. 2010;686:349‐364. [DOI] [PubMed] [Google Scholar]
- 2. Aite L, Zaccara A, Mirante N, et al. Antenatal diagnosis of congenital anomaly: a really traumatic experience? J Perinatol. 2011;31:760‐763. [DOI] [PubMed] [Google Scholar]
- 3. Davies V, Gledhill J, McFadyen A, Whitlow B, Economides D. Psychological outcome in women undergoing termination of pregnancy for ultrasound‐detected fetal anomaly in the first and second trimesters: a pilot study. Ultrasound Obstet Gynecol. 2005;25:389‐392. [DOI] [PubMed] [Google Scholar]
- 4. Kersting A, Kroker K, Steinhard J, et al. Psychological impact on women after second and third trimester termination of pregnancy due to fetal anomalies versus women after preterm birth—a 14‐month follow up study. Arch Womens Ment Health. 2009;12:193‐201. [DOI] [PubMed] [Google Scholar]
- 5. Korenromp MJ, Page‐Christiaens GC, van den Bout J, Mulder EJ, Visser GH. Adjustment to termination of pregnancy for fetal anomaly: a longitudinal study in women at 4, 8, and 16 months. Am J Obstet Gynecol. 2009;201:160.e1‐e7. [DOI] [PubMed] [Google Scholar]
- 6. Nes RB, Røysamb E, Hauge LJ, et al. Adaptation to the birth of a child with a congenital anomaly: A prospective longitudinal study of maternal well‐being and psychological distress. Dev Psychol. 2014;50:1827‐1839. [DOI] [PubMed] [Google Scholar]
- 7. Cole JC, Moldenhauer JS, Berger K, et al. Identifying expectant parents at risk for psychological distress in response to a confirmed fetal abnormality. Arch Womens Ment Health. 2016;19:443‐453. [DOI] [PubMed] [Google Scholar]
- 8. Hunfeld JA, Tempels A, Passchier J, Hazebroek F, Tibboel D. Brief report: parental burden and grief one year after the birth of a child with a congenital anomaly. J Pediatr Psychol. 1999;24:515‐520. [DOI] [PubMed] [Google Scholar]
- 9. Nagata S‐i, Funakosi S, Amae S, et al. Posttraumatic stress disorder in mothers of children who have undergone surgery for congenital disease at a pediatric surgery department. J Pediatr Surg. 2008;43:1480‐1486. [DOI] [PubMed] [Google Scholar]
- 10. Giuliani R, Tripani A, Pellizzoni S, et al. Pregnancy and postpartum following a prenatal diagnosis of fetal thoracoabdominal malformation: the parental perspective. J Pediatr Surg. 2014;49:353‐358. [DOI] [PubMed] [Google Scholar]
- 11. Skari H, Malt U, Bjornland K, et al. Prenatal diagnosis of congenital malformations and parental psychological distress—a prospective longitudinal cohort study. Prenat Diagn. 2006;26:1001‐1009. [DOI] [PubMed] [Google Scholar]
- 12. Kaasen A, Helbig A, Malt UF, Næs T, Skari H, Haugen G. Maternal psychological responses during pregnancy after ultrasonographic detection of structural fetal anomalies: a prospective longitudinal observational study. PloS One. 2017;12:e0174412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bekkhus M, Oftedal A, Braithwaite E, Haugen G, Kaasen A. Paternal psychological stress after detection of fetal anomaly during pregnancy. A prospective longitudinal observational study. Front Psychol. 2020;11:1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brosig CL, Whitstone B, Frommelt MA, Frisbee SJ, Leuthner SR. Psychological distress in parents of children with severe congenital heart disease: the impact of prenatal versus postnatal diagnosis. J Perinatol. 2007;27:687‐692. [DOI] [PubMed] [Google Scholar]
- 15. Neville KL. Uncertainty in illness: an integrative review. Orthop Nurs. 2003;22:206‐214. [DOI] [PubMed] [Google Scholar]
- 16. Redshaw M, Henderson J. Fathers’ engagement in pregnancy and childbirth: evidence from a national survey. BMC Pregnancy Childbirth. 2013;13:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaasen A, Helbig A, Malt U, Naes T, Skari H, Haugen G. Acute maternal social dysfunction, health perception and psychological distress after ultrasonographic detection of a fetal structural anomaly. BJOG. 2010;117:1127‐1138. [DOI] [PubMed] [Google Scholar]
- 18. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10‐item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782‐786. [DOI] [PubMed] [Google Scholar]
- 19. Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). J Reprod Infant Psychol. 1990;8:99‐107. [Google Scholar]
- 20. Matthey S, Barnett B, Kavanagh DJ, Howie P. Validation of the Edinburgh Postnatal Depression Scale for men, and comparison of item endorsement with their partners. J Affect Disord. 2001;64:175‐184. [DOI] [PubMed] [Google Scholar]
- 21. Eberhard‐Gran M, Eskild A, Tambs K, Schei B, Opjordsmoen S. The Edinburgh postnatal depression scale: validation in a Norwegian community sample. Nord J Psychiatry. 2001;55:113‐117. [DOI] [PubMed] [Google Scholar]
- 22. Levis B, Negeri Z, Sun Y, Benedetti A, Thombs BD. Accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for screening to detect major depression among pregnant and postpartum women: systematic review and meta‐analysis of individual participant data. BMJ. 2020;371:m4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weiss DS. The impact of event scale: revised. Cross‐cultural assessment of psychological trauma and PTSD. Springer; 2007:219‐238. [Google Scholar]
- 24. Bates D. lme4: Linear mixed‐effects models using S4 classes. R package version 0.999375–33. 2010. http://CRAN‐R‐projectorg/package=lme4/index.html
- 25. Crainiceanu CM, Ruppert D. Likelihood ratio tests in linear mixed models with one variance component. J R Stat Soc Series B Stat Methodology. 2004;66(1):165‐185. [Google Scholar]
- 26. Zhang Y. Uncertainty in Illness: Theory Review, Application, and Extension. Oncol Nurs Forum. 2017;44:645‐649. [DOI] [PubMed] [Google Scholar]
- 27. Nilsen RM, Vollset SE, Gjessing HK, et al. Self‐selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23:597‐608. [DOI] [PubMed] [Google Scholar]
- 28. Wolke D, Waylen A, Samara M, et al. Selective drop‐out in longitudinal studies and non‐biased prediction of behaviour disorders. Br J Psychiatry. 2009;195:249‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]


