Abstract
The sensory experience of transcranial magnetic stimulation (TMS) evokes cortical responses measured in electroencephalography (EEG) that confound interpretation of TMS‐evoked potentials (TEPs). Methods for sensory masking have been proposed to minimize sensory contributions to the TEP, but the most effective combination for suprathreshold TMS to dorsolateral prefrontal cortex (dlPFC) is unknown. We applied sensory suppression techniques and quantified electrophysiology and perception from suprathreshold dlPFC TMS to identify the best combination to minimize the sensory TEP. In 21 healthy adults, we applied single pulse TMS at 120% resting motor threshold (rMT) to the left dlPFC and compared EEG vertex N100‐P200 and perception. Conditions included three protocols: No masking (no auditory masking, no foam, and jittered interstimulus interval [ISI]), Standard masking (auditory noise, foam, and jittered ISI), and our ATTENUATE protocol (auditory noise, foam, over‐the‐ear protection, and unjittered ISI). ATTENUATE reduced vertex N100‐P200 by 56%, “click” loudness perception by 50%, and scalp sensation by 36%. We show that sensory prediction, induced with predictable ISI, has a suppressive effect on vertex N100‐P200, and that combining standard suppression protocols with sensory prediction provides the best N100‐P200 suppression. ATTENUATE was more effective than Standard masking, which only reduced vertex N100‐P200 by 22%, loudness by 27%, and scalp sensation by 24%. We introduce a sensory suppression protocol superior to Standard masking and demonstrate that using an unjittered ISI can contribute to minimizing sensory confounds. ATTENUATE provides superior sensory suppression to increase TEP signal‐to‐noise and contributes to a growing understanding of TMS‐EEG sensory neuroscience.
Keywords: auditory‐evoked potential, electroencephalogram, evoked potentials, transcranial magnetic stimulation, vertex potential
We introduce a sensory suppression protocol superior to Standard masking and demonstrate that using an unjittered interstimulus interval can contribute to minimizing sensory confounds. ATTENUATE provides superior sensory suppression to increase transcranial magnetic stimulation (TMS)‐evoked potential signal‐to‐noise and contributes to a growing understanding of TMS‐electroencephalography sensory neuroscience.

1. INTRODUCTION
Transcranial magnetic stimulation (TMS) is a powerful noninvasive tool for stimulating brain networks (Barker et al., 1985; Ferrarelli et al., 2010; Massimini et al., 2005) and has proven useful for the neurophysiological characterization and treatment of neurological and neuropsychiatric disorders (Casarotto et al., 2011; Fischer et al., 2016; Pascual‐Leone et al., 2011; Rogasch, Daskalakis, & Fitzgerald, 2014; Shafi et al., 2015). Neural changes caused by TMS are measurable and quantifiable using electroencephalography (EEG; Bortoletto et al., 2015; Ilmoniemi et al., 1997; Kerwin et al., 2018; Rosanova et al., 2009; Wu et al., 2018). For instance, an averaged single pulse TMS‐evoked EEG potential (TEP) can be used to characterize local and network excitability as well as plasticity following repetitive TMS protocols (Eshel et al., 2020; Ilmoniemi et al., 1997; Ozdemir et al., 2021; Rogasch & Fitzgerald, 2013). Gaining a better understanding of and utilizing TMS‐induced EEG changes is critical for targeted and personalized circuit manipulation for robust clinical use.
Although TEPs are a promising measure of TMS‐evoked neural activity, it has become evident that off‐target sensory effects of single TMS pulses can severely confound the interpretation of the TEP (Belardinelli et al., 2019; Biabani et al., 2019; Conde et al., 2019; Freedberg et al., 2020; Rocchi et al., 2021; Siebner et al., 2019). These off‐target effects include sensory potentials that are peripherally evoked due to the multisensory nature of TMS (Nikouline et al., 1999). Although the TEP is reproducible (Kerwin et al., 2018; Lioumis et al., 2009) and has been shown to reflect localized TMS‐evoked activity at the earliest latencies after the pulse (up to ~60–80 ms; Freedberg et al., 2020; Gordon, Desideri, et al., 2018; Gosseries et al., 2015; Harquel et al., 2016; Nikouline et al., 1999; Ozdemir et al., 2021; Rosanova et al., 2009), there is accumulating evidence that the later TEP (>80 ms) is contaminated by off‐target sensory potentials (Biabani et al., 2019; Freedberg et al., 2020; Rocchi et al., 2021). One such component of the later TEP is an evoked response (Mouraux et al., 2011) induced from the sound of TMS (referred to as the auditory‐evoked potential [AEP]) and not specific to the site of stimulation (Conde et al., 2019; Nikouline et al., 1999). The greatest amplitude and most robustly measured subcomponents of this sensory potential occur at the vertex at ~100 and 200 ms with an accompanying smaller potential at ~50 ms (Belardinelli et al., 2019; Čeponien et al., 2002; Eggermont et al., 1997; Gordon, Desideri, et al., 2018; Knight et al., 1980; Näätänen & Picton, 1987; Sharma et al., 1997). These vertex potentials are described as an N100‐P200 complex, which overlaps with all but the earliest TEP components. In summary, sensory potentials in the TEP remain a significant confound to the direct effects of TMS and minimization or removal is necessary to improve interpretability.
Experimental modifications have been proposed to suppress the sensory vertex N100‐P200, but the most effective combination for suprathreshold TMS is unknown. This is particularly true for targeting the dlPFC, the primary treatment location for many neuropsychiatric disorders (Fox et al., 2012; George et al., 1995; O et al., 2007; Padberg & George, 2009; Pascual‐Leone et al., 1996; Siddiqi et al., 2021). Here, we focus on the following experimental modifications: auditory masking, a foam separator between the coil and the scalp, and spacing TMS pulses predictably. Earplugs and/or auditory noise masking are standard in the field (Massimini et al., 2005; Rocchi et al., 2021), and a very common sensory masking protocol is to pair these with a foam separator—this masking combination used with a jittered interstimulus interval (ISI) is hereafter called Standard masking. Recent evidence for effective masking appears promising for subthreshold TMS to primary motor cortex (90% of the resting motor threshold [rMT]: Massimini et al., 2005; Rocchi et al., 2021). However, it has also been shown that these methods often do not fully suppress the sensory vertex N100‐P200 (Biabani et al., 2019; Conde et al., 2019; Herring et al., 2015; Ilmoniemi & Kičić, 2010; Ross et al., 2021; Tchumatchenko & Reichenbach, 2014; ter Braack et al., 2015) and this is particularly true for higher intensity protocols (Conde et al., 2019; Ross et al., 2021). Rocchi et al. (2021) used over‐the‐ear protection in addition to noise masking to further minimize vertex N100‐P200, with positive results for subthreshold M1 stimulation. However, how over‐the‐ear protection performs for higher stimulation intensities and non‐M1 targets is unknown. Using foam padding between the coil and scalp is thought to suppress the vertex N100‐P200 by reducing bone conduction of the sound (ter Braack et al., 2015). However, it is unclear what type or thickness of foam should be used. In addition, there is no consensus regarding how to adjust stimulation intensity to account for higher coil to cortex distance when foam is added. Modifying the ISI timing changes the predictability of TMS pulses, which can have an effect on motor evoked potential (MEP) amplitude (Stupacher et al., 2013; Tran et al., 2021). However, whether more predictable TMS timing results in a similar attenuation of the TEP is unknown. In summary, a thorough investigation into the optimal experimental methodology to suppress sensory vertex N100‐P200 following suprathreshold TMS to the clinically significant dlPFC is necessary.
In this study, we develop an optimal combination of experimental modifications that maximally reduce the vertex N100‐P200 complex and sensory perception following suprathreshold single pulse TMS to the dlPFC. In a sample of 21 typically healthy adults, we compared the effects of three masking protocols on the vertex N100‐P200 and on perception of the TMS loudness, scalp sensation, and pain: No masking, Standard masking, and a novel procedure. We hypothesized that our novel combination of experimental procedures, with the addition of further sound dampening and modification of TMS timing, would best suppress the nonspecific sensory component of the TEP. This work contributes to a growing understanding of TMS‐EEG sensory neuroscience, and the novel protocol has the potential to enhance interpretability of TMS‐EEG findings.
2. METHODS
2.1. Participants and study design
All data were collected at Stanford University under an approved institutional review board protocol after participants gave their written informed consent. Participants (N = 21) were 19–64 years old (44.0 mean ± 14.58 SD) and without current psychiatric or neurological diagnoses. A wide age range was chosen so as not to constrain findings to any a priori group. Table S1 includes demographic information for all subjects. For each participant, the experiment was conducted on a single day. The experiment was split into six single pulse TMS‐EEG blocks, described below, and presented in a randomized order. Each block consisted of 80 individual single pulse TMS trials applied to the left dlPFC. Eighty trials were chosen as they provided high test–retest reliability of the N100 and P200 (Kerwin et al., 2018). TMS‐evoked potentials (TEPs) and perceptual scores were quantified, as described below and in schematic in Figure 1a.
FIGURE 1.
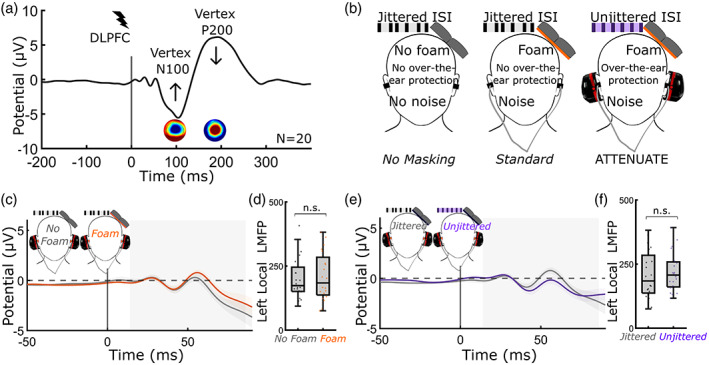
Experimental design. (a) Single pulse TMS‐EEG to the left dlPFC. TMS was applied at 120% rMT (80 trials). Perceptual reports of loudness of the TMS “click,” intensity of scalp sensation, and pain followed each condition. Vertex N100‐P200 was quantified using LMFP. Arrows denote that the goal of the study was to minimize the N100‐P200 and sensory perception while preserving the early TEP. (b) Three experimental conditions were compared: No masking (jittered ISI/no foam/no noise/no over‐the‐ear protection), Standard masking (jittered ISI/foam/noise/no over‐the‐ear protection), and ATTENUATE (unjittered ISI/foam/noise/over‐the‐ear protection). (c–f) neither foam (c,d) nor unjittered ISI (e,f) altered the early local TEP (14–86 ms). (c,d) Effect of foam on early (14–86 ms) local TEP. Foam did not modify the early local TEP (T = −0.35, DF = 19, p = .73). (e,f) effect of using an unjittered ISI on early local TEP. Modifying the timing of TMS pulses did not change the early local TEP (T = −0.58, DF = 19, p = .57). All error bars denote standard error. dlPFC, dorsolateral prefrontal cortex; EEG, electroencephalography; ISI, interstimulus interval; LMFP, local mean field power; N.S., not significant; rMT, resting motor threshold; TMS, transcranial magnetic stimulation.
Conditions included the three sensory suppression protocols and all individual modifications (three auditory conditions, two foam conditions, and two jitter conditions) with all other factors matched. Total number of conditions collected is six.
No masking/No noiseS jittered ISI, no foam, no over‐the‐ear protection, and no noise.
Noise: jittered ISI, no foam, no over‐the‐ear protection, and noise.
Noise plus over‐the‐ear protection/No foam: jittered ISI, no foam, over‐the‐ear protection, and noise.
Foam/Jittered: jittered ISI, foam, over‐the‐ear protection, and noise.
Standard masking: jittered ISI, foam, no over‐the‐ear protection, and noise.
ATTENUATE/Unjittered: unjittered ISI, foam, over‐the‐ear protection, and noise.
2.2. Transcranial magnetic stimulation
TMS was performed with a MagPro X100 stimulator (MagVenture, Denmark) and a MagVenture Cool B65 figure‐of‐eight coil (MagVenture, Denmark). The motor hotspot for the right first dorsal interosseous (FDI) was determined by delivering single TMS pulses to the left motor cortex. rMT was obtained once at the beginning of the experiment, after placing and gelling the EEG cap, and defined as the intensity that produced a visible twitch in relaxed FDI in ≥5/10 stimulations (Pridmore et al., 1998; Stokes et al., 2005). Neuronavigation (Localite TMS Navigator MR‐less system, Alpharetta, GA) was utilized to determine the left dlPFC location on a standard Montreal Neurological Institute (MNI) brain map, fitted to individual participants' heads based on scalp measurements. The left dlPFC site (MNI‐38, MNI‐22, MNI‐38) was used to target the fronto‐parietal control network (Chen et al., 2013). TMS coil angle was placed at the angle between 0° and 90° (Brasil‐Neto et al., 1992; Janssen et al., 2015; Laakso et al., 2014; Mills et al., 1992; Tervo et al., 2021) that most minimized discomfort and pain for each subject (M = 52°, SD = 27). Table S1 includes the optimal angle for each subject.
To identify the set of procedures that maximally reduces the vertex N100‐P200, we tested our novel combination of experimental procedures, which we refer to as ATTENUATE (Auditory: noise masking, Timing: unjittered ISI, Tactile: foam, and over‐the‐Ear protection to Negate Unwanted Artifacts in TMS‐EEG). We tested ATTENUATE against a common Standard masking procedure (auditory noise and foam, jittered ISI) and No masking (no auditory noise, no foam, and jittered ISI). See Figure 1b for schematics of the three masking procedures. As noted in the Introduction, it is becoming increasingly common in TMS‐EEG studies to employ the Standard masking protocol that uses foam and a “click” frequency auditory masking noise (Conde et al., 2019; Rocchi et al., 2021).
To examine the effectiveness of auditory masking (auditory noise with and without over‐the‐ear protection), foam, and ISI timing modifications alone (i.e., when each of the other factors is held constant). Figure 3a,h,o depict auditory, foam, and ISI timing conditions, respectively. Conditions were presented in a pseudorandomized order. All conditions were collected in each subject unless the experiment ended early due to time constraints. Tables S2 and S3 reflect for each subject the conditions performed.
FIGURE 3.
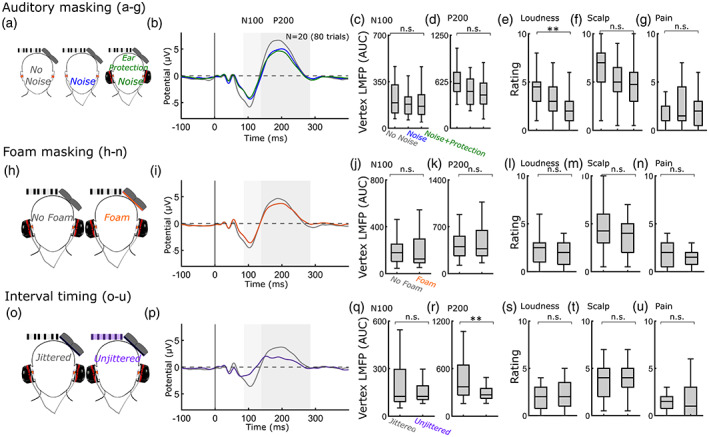
Individual auditory, foam, or timing modifications are only minimally effective for reducing vertex N100‐P200 or sensory perception. (a) Auditory masking conditions: No noise, Noise, and Noise with over‐the‐ear protection (b) TEPs from the vertex ROI (c,d) LMFP for N100 (c) and P200 (d) time windows. ANOVAs revealed that auditory masking protocols only had a marginal but insignificant effect on vertex P200. (e–g) perceptual ratings of “click” loudness (e), scalp sensation (f), and pain (g). ANOVAs revealed that auditory masking protocols had an effect on loudness rating, driven by Noise with over‐the‐ear protection change from No masking, an insignificant reduction in scalp sensation, and no effect on pain. (h) No foam and Foam conditions (i) TEPs from the vertex ROI (j,k) LMFP. t‐Tests revealed that Foam had no effect on vertex N100 or P200. (l,n) Perceptual ratings of loudness (l), scalp sensation (m), and pain (n). t‐Tests revealed that Foam had no effect on any perceptual ratings. (o) Jittered and Unjittered ISI conditions (p) TEPs from the vertex ROI (q,r) LMFP. t‐Tests revealed that using an Unjittered ISI had a nonsignificant effect in vertex N100, and a significant effect on vertex P200. (s–u) perceptual ratings of loudness (s), scalp sensation (t), and pain (u). t‐Tests revealed that using an Unjittered ISI had no effect on any perceptual ratings. p < .05, **p < .01, ***p < .001. All error bars denote standard error. Shaded areas indicate time windows used for analysis. ANOVAs, analysis of variances; ISI, interstimulus interval; LMFP, local mean field power; ROI, region of interest; TEPs, transcranial magnetic stimulations.
Each TMS‐EEG condition consisted of 80 single pulses (biphasic pulses at 280 μs pulse width) at an intensity of 120% rMT. Stimulator recharge was delayed to 500 ms to prevent recharge artifact from affecting EEG in the time period of interest (Siebner et al., 2009). Participants were instructed to keep their eyes open and gaze relaxed throughout each run. For conditions using auditory noise, the noise sound matched the frequency of the TMS click (Rosanova et al., 2009) and was delivered with earplug earbuds (Elgin USA Ruckus Earplug Earbuds, NRR 25 dB, Arlington, Texas) at the maximum volume comfortable for each participant. In conditions using over‐the‐ear protection, to further dampen the TMS “click” sound before reaching the ear canal, we used over‐the‐ear noise‐reducing foam‐filled earmuffs (3 M Ear Peltor Optime 105 behind‐the‐head earmuffs, NRR 29 dB, Maplewood, Minnesota). In conditions without auditory noise, earplug earbuds were still kept in the ear canals but no noise was played. In conditions requiring foam, a thin (0.5 cm) foam pad was attached to the TMS coil, and rMT was redetermined using this foam to accurately deliver a TMS intensity at 120% rMT while accounting for the increase in coil to scalp distance (see Nikouline et al., 1999) for effects of separator and of distance to scalp on amplitude of vertex N100‐P200). Table S1 includes all rMTs, with and without foam, for all subjects. To determine whether predictability of TMS pulses can attenuate sensory components in the TEP, we compared jittered ISI (2 ± 1 s jitter) and unjittered ISI (2 s) protocols.
2.3. Electroencephalography
Sixty‐four‐channel EEG data were obtained using a BrainVision actiCHamp Plus amplifiers (5 kHz sampling rate), with ActiCAP slim active electrodes in an extended 10–20 system montage (actiCHamp, Brain Products GmbH, Munich, Germany). EEG data were online referenced to Afz, recorded using BrainVision Recorder software v1.24.0001 (Brain Products GmbH, Germany). Impedances were maintained below 5 kΩ.
2.3.1. Preprocessing of TEPs
All EEG preprocessing and analyses were performed in MATLAB R2021a (Mathworks, Natick, Massachusetts) using the EEGLAB v2021.1 toolbox (Delorme & Makeig, 2004) and custom scripts. Removal of artifactual EEG data was performed using a custom preprocessing pipeline, as is most common (Bertazzoli et al., 2021), but followed most closely with Ross et al. (2021) (steps prior to sensory removal), TMSEEG (Atluri et al., 2016), and TESA (Rogasch et al., 2017). Due to a marked impact of preprocessing pipelines on the TEP, as reported in (Bertazzoli et al., 2021), we took a conservative approach in all steps that required human judgement (with minimal data deletion) and describe all preprocessing steps used in detail with justification for each choice and supporting literature.
All details of EEG data cleaning can be found in Section S1.1.
2.3.2. Quantification of TEPs
For time window and region of interest (ROI) selection, and calculation of global mean field power and local mean field power (LMFP), see Section S1.2, and Figures S2–5. To compare vertex N100‐P200 across experimental conditions, TEPs were generated as averages over the vertex ROI: FC1, FCz, FC2, C1, Cz, and C2 (Figure S5C for ROI). LMFP was calculated for the ROI and the area under the curve (AUC) of the LMFP was quantified for the appropriate time windows. Supporting that our time windows and ROI capture the vertex N100‐P200 complex, we observed a strong correlation between vertex N100 and P200 (Figure S7A,B; AUC of LMFP; r(19) = 0.91, p = .00000004; regression: F[1, 19] = 81.68, p = .00000004; R 2 = 0.82).
To verify that sensory suppression techniques did not alter the early local TEP, we compared LMFP of the early window (14–86 ms) in electrodes local to the site of stimulation. For each condition, the AUC of the LMFP was utilized (and referred to simply as LMFP in the article). These comparisons included No foam versus Foam conditions (with other factors matched) and Jittered versus Unjittered conditions (with other factors matched). To identify an ROI for examining local response to TMS, electrodes maximally different from baseline in the early window were chosen: AF3, AFz, F3, F1, FC3, and FC1 (Section S1.2 and Figure S6C for ROI). We found no significant effect on the early TEP response (LMFP) of using Foam (T = −0.3534, DF = 19, p = .7277) or an Unjittered protocol (T = −0.5773, DF = 19, p = .5705; Figure 1c–f).
To ensure that an unjittered ISI did not induce changes in the early local TEP over time, we compared the first half of trials to the second half in the Unjittered condition (Huang et al., 2019; Keller et al., 2018). We observed no significant difference in the early LMFP between the first half and second half of trials in the Unjittered (2 s ISI) condition (Figure S6; T = 1.2542, DF = 19, p = .2250, CI = −11.4509, 45.6910).
2.3.3. Statistical analyses of TEPs
To compare single pulse TEP responses across the three masking protocols, we computed the LMFP for the N100 and P200 time windows in the central ROI. We performed an analysis of variance (ANOVA, repeated measures) with three levels (No masking, Standard masking, and ATTENUATE), followed by post hoc pairwise comparisons using Tukey's HSD procedure where appropriate.
2.4. Perceptual ratings
To assess perceptual experience during each stimulation condition, participants were asked to respond verbally immediately following each condition to rate loudness, scalp sensation, and pain perception on scales ranging from 0 to 10. These scores were inputted into the research electronic data capture system (REDCAP, Vanderbilt, Nashville, TN). To ensure consistency in how these questions were phrased across conditions and subjects, the following scripts were used:
With 0 being you could not hear it, and 10 being as loud as a fire alarm, how loud did you perceive the “click” sound to be?
With 0 being you could not feel it, and 10 being it felt as intense as a hard flick, how much did you feel the tapping sensation?
With 0 being no pain at all, and 10 being unbearable pain, how much pain did you feel?
2.4.1. Statistical analyses of perceptual ratings
Raw perceptual ratings were compared across the three conditions using a rm ANOVA with three levels (No masking, Standard masking, and ATTENUATE), followed by post hoc pairwise comparisons using Tukey's HSD procedure where appropriate.
2.5. Relationship between perceptual ratings and vertex N100‐P200
To further understand the relationship between perceptual ratings of loudness, scalp sensation, pain, and the vertex N100‐P200, an exploratory analysis compared perceptual ratings and vertex N100‐P200 LMFP values across subjects for the No masking condition only. The goal of this analysis was to better understand the relationship between unsuppressed sensory contributions. For this analysis, a Pearson's correlation matrix was generated with correlation coefficients (Figure S7), and follow‐up linear univariate regression analyses were performed for significantly correlated factors using the regress function in MATLAB R2021a (Mathworks, Natick, MA) (Chatterjee & Hadi, 1986; Draper & Smith, 1981).
3. RESULTS
3.1. The ATTENUATE protocol is superior to Standard masking at reducing vertex N100‐P200
The vertex N100 and P200 LMFPs (see Section 2.3.2 above for ROI and window selection) were compared across the three sensory suppression protocols (No masking, Standard masking, and ATTENUATE). Vertex LMFP was significantly different across conditions in the N100 (F[2,57] = 3.64, p = .03) and P200 (F[2,57] = 9.40, p = .0003) time windows (Figure 2b–d). Post hoc pairwise comparisons revealed that ATTENUATE reduced the LMFP vertex N100 (M = 145.84, SD = 62.13) compared with No masking (M = 304.28, SD = 288.54; Tukey's HSD, p = .03). Standard masking did not show statistical differences from No masking (M = 236.38, SD = 167.59; p = .52) or from ATTENUATE (p = .26). ATTENUATE reduced the LMFP vertex P200 (M = 311.98, SD = 130.51) compared with No masking (M = 727.68, SD = 401.13; p = .0002) and compared with Standard masking (M = 563.90, SD = 309.63; p = .03). Standard masking did not show statistical differences from No masking (p = .21). These results reflect that ATTENUATE reduced vertex N100 by 54.41% and vertex P200 by 56.58% from No masking (average of 55.94% reduction across the vertex N100‐P200 complex). In comparison, Standard masking reduced vertex N100 by 22.31% and vertex P200 by 22.51% (average of 22.45% across the vertex N100‐P200 complex). In summary, we observed a significant group effect across sensory suppression procedures and ATTENUATE reduced the vertex N100 and P200 more than Standard masking.
FIGURE 2.
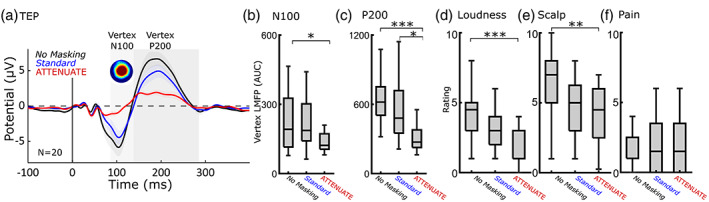
The ATTENUATE protocol is superior to standard masking at reducing vertex N100‐P200, “click” loudness perception, and scalp sensation. (a) Group mean TEPs of vertex ROI (N = 20). Shaded areas indicate time windows used for analysis. (b,c) ATTENUATE reduces the vertex N100‐P200. Vertex LMFP is reduced in both N100 (F[2,57] = 3.64, p = .03) and P200 (F[2,57] = 9.40, p = .0003) time windows across the three conditions. Pairwise comparisons revealed that ATTENUATE reduced vertex N100‐P200 compared with No masking (N100: p = .03; P200: p = .0002), and that ATTENUATE reduced vertex P200 compared with Standard masking (p = .03). Comparisons between No masking and Standard masking were nonsignificant (N100 p = .52; P200 p = .21). (d,e) sensory suppression protocols reduced perception of loudness (F[2,57] = 8.53, p = .0006) and scalp sensation (F[2,57] = 5.47, p = .007). Pairwise comparisons demonstrate that ATTENUATE reduced both loudness (p = .0004) and scalp sensation (p = .006) from No masking, but did not reduce compared with Standard masking in loudness rating (p = .15) or scalp sensation (p = .56). Comparisons between No masking and Standard masking were nonsignificant (loudness p = .07, scalp sensation p = .08). (f) Pain was not effected by protocol (F[2,57] = 0.06, p = .9461). All significant pairwise comparisons are indicated with brackets and asterisks mark level of significance. *p < .05, **p < .01, ***p < .001. All error bars denote standard error. LMFP, local mean field power; ROI, region of interest; TEPs, transcranial magnetic stimulations.
3.2. ATTENUATE is more effective than Standard masking at reducing loudness perception and scalp sensation
Raw perceptual ratings for loudness of “click,” sensation on the scalp, and pain were compared across the three masking protocols (No masking, Standard masking, and ATTENUATE). See Figure 2a for perceptual ratings following Standard masking and ATTENUATE conditions. We found a significant difference in loudness perception (F[2,57] = 8.53, p = .0006) and scalp sensation (F[2,57] = 5.47, p = .0067) across the conditions (Figure 2a). Perception of pain did not change between the conditions (F[2,57] = .06, p = .9461). Post hoc pairwise comparisons revealed that ATTENUATE reduced loudness rating (M = 2.24, SD = 1.51; p = .0004) and scalp sensation (M = 4.15, SD = 2.10; p = .006) compared with No masking (Loudness: M = 4.23, SD = 1.92; Scalp: M = 6.30, SD = 2.36). Standard masking (Loudness: M = 3.08, SD = 1.51; Scalp: M = 4.78, SD = 2.07) did not show a statistical difference from No masking (Loudness: p = .07; Scalp: p = .08) or from ATTENUATE (Loudness: p = .15; Scalp: p = .56). These results reflect that ATTENUATE reduced loudness rating by 50.30% and scalp sensation by 35.52% from No masking. In comparison, Standard masking reduced loudness rating by 27.22% and scalp sensation by 24.21%. In summary, we observed a significant group effect across sensory suppression procedures with ATTENUATE reducing the perception of “click” loudness and scalp sensation compared with No masking.
3.3. Individual auditory, foam, or ISI timing modifications are not sufficient for reducing vertex N100‐P200 or sensory perception
To determine if components of these sensory suppression modifications in isolation reduce the vertex N100‐P200 or sensory perception, we compared vertex N100‐P200 LMFP and perceptual ratings across auditory (No noise, Noise, and Noise and over‐the‐ear protection; Figure 3a–d), foam (No foam and Foam; Figure 3e–h), and ISI timing (Jittered and Unjittered; Figure 3i–l) conditions. For each comparison, all other modifications were matched.
3.3.1. Auditory suppression
An ANOVA across the three auditory conditions (No noise, Noise, and Noise and over‐the‐ear protection) revealed no effect on vertex N100 (Figure 3c; F[2,57] = 1.27, p = .29) with an insignificant but marginal effect on vertex P200 (Figure 3d; F[2,57] = 3.10, p = .05). Auditory suppression had an effect on loudness rating across the three conditions (Figure 3e; F[2,57] = 6.12, p = .0039). Post hoc pairwise comparisons revealed that Noise with over‐the‐ear protection reduced loudness (M = 2.25, SD = 1.59) compared with No noise (M = 4.23, SD = 1.92; Tukey's HSD; p = .003). Noise alone did not reduce loudness rating (M = 3.10, SD = 1.84) from No noise (p = .12) but was also not different from Noise with over‐the‐ear protection (p = .30). Auditory suppression protocols did not reduce scalp sensation (Figure 3f; F[2,57] = 3.08, p = .05) or pain rating (Figure 3g; F[2,57] = 0.56, p = .5730).
3.3.2. Foam
The use of Foam had no effect on vertex N100 (Figure 3j; T = −0.2886, DF = 19, p = .7760, CI = −46.9654, 35.5838) or vertex P200 (Figure 3k; T = −0.8277, DF = 19, p = .4181, CI = −122.5488, 53.0926). Foam also had no effect on loudness rating (Figure 3l; T = 1.0918, DF = 19, p = .2886; CI = −0.3210, 1.0210), scalp sensation (Figure 3m; T = 1.4690, DF = 19, p = .1582; CI = −0.3398, 1.9398), or pain rating (Figure 3n; T = 1.5305, DF = 19, p = .1424; CI = −0.2114, 1.3614).
3.3.3. ISI timing
Using an Unjittered ISI had a nonsignificant suppressive effect on vertex N100 (Figure 3q; T = 1.8574, DF = 19, p = .0788, CI = −7.5349, 126.3170) and a significant suppressive effect on vertex P200 (Figure 3r; T = 3.8362, DF = 19, p = .0011, CI = 78.0444, 265.4598). Unjittered ISI had no effect on loudness rating (Figure 3s; T = −0.8193, DF = 19, p = .4228; CI = −0.8443, 0.3693), scalp sensation (Figure 3t; T = −1.1981, DF = 19, p = .2456; CI = −1.6482, 0.4482), or pain rating (Figure 3u; T = −0.0901, DF = 19, p = .9291; CI = −0.6056, 0.5556).
In summary, auditory, foam, or ISI timing modifications alone are only minimally effective strategies for reducing vertex N100‐P200 LMFP and perceptual ratings of “click” loudness, scalp sensation, or pain.
3.4. Relationship between electrophysiology and perception
Finally, to better understand the relationship between electrophysiology and perception, we compared the vertex N100, vertex P200, and perceptual ratings in the No masking condition (Figure S7) using a correlation matrix of all measures. All relationships were insignificant except for between N100 and P200 as well as between Pain and the P200 (Figure S7A and Section 2.3.2). Pain rating had a positive correlation with vertex P200 (Figure S7C; correlation: r[19] = 0.45, p = .04; regression: F[1,19] = 4.67, p = .04; R 2 = 0.20), but may be spurious due to one high leverage observation.
4. DISCUSSION
In this study, in an effort to reduce the sensory effects of TMS, we sought to experimentally minimize the vertex N100‐P200 and sensory perception arising from suprathreshold TMS to the dlFPC. We developed a novel combination of experimental sensory suppression techniques, termed ATTENUATE, which consisted of using auditory noise masking, foam, over‐the‐ear protection, and unjittered pulse timing. To the best of our knowledge, this is the first study to present the ATTENUATE protocol. We find the following: (1) The ATTENUATE protocol significantly reduced the vertex N100‐P200 by 56%, outperforming other standard masking procedures, with no effect on the early TEP; (2) The ATTENUATE protocol reduced “click” loudness rating by 50% and scalp sensation by 36%, outperforming standard approaches; and (3) Single sensory experimental modifications alone are not sufficient to significantly reduce vertex N100‐P200 or sensory perception.
We show that additional experimental modifications above noise masking alone are needed to reduce the N100‐P200 after suprathreshold TMS to dlPFC (Figure 3a–g). When compared with prior studies that suggest that noise masking alone can minimize the sensory TEP (Massimini et al., 2005; Rocchi et al., 2021), our study differs by intensity and brain target. Regarding intensity, compared with previous work that focused on subthreshold intensities (90% rMT: Massimini et al., 2005; Rocchi et al., 2021), our suprathreshold stimulation protocol (120% rMT) better mimicked clinical stimulation parameters (McClintock et al., 2018), but is more difficult to mask (Biabani et al., 2019; Fuggetta et al., 2005; Ross et al., 2021; ter Braack et al., 2015). In regards to brain target, whereas other studies have explored sensory suppression after TMS to the primary motor (Rocchi et al., 2021) or premotor (Massimini et al., 2005) targets, here we focus on the dlPFC, which may have different sensory contributions to the vertex N100‐P200 compared with motor targets (Herring et al., 2015; Lioumis et al., 2018).
Although these differences in intensity (80% vs. 120% rMT) or brain target (premotor/M1 vs. dlPFC) may partially explain the inability to fully suppress the vertex N100‐P200, other factors may be contributing. The link between sensory potentials, brain target, and intensity is not yet clear (Herring et al., 2015). TEPs from M1 stimulation highly correlate with those from nonbrain regions (shoulder), regardless of stimulation intensity (120% vs. 80% rMT; Biabani et al., 2019), suggesting that peripherally evoked contributions to the TEP may be considerable regardless of stimulation intensity or target. Multiple studies have demonstrated that N100‐P200 components can persist, both for subthreshold and suprathreshold M1 stimulation, even after suppression of TMS click perception (Fuggetta et al., 2005; Paus et al., 2001). Therefore, we cannot conclude that auditory suppression protocols will be effective for all designs, nor that auditory perception of the “click” will be an effective indication of suppression of sensory components in the TEP. We may be yet to find the most effective sensory suppression protocol for all designs and populations. However, our proposed novel combination of sensory reduction procedures (ATTENUATE), which includes extra‐auditory reduction (with the use of over‐the‐ear protection), foam, and predictable timing of TMS pulses, is superior at reducing vertex N100‐P200 and sensory perception (loudness and scalp feeling) compared with standard experimental procedures.
Another explanation for the inability to fully suppress the vertex N100‐P200 is that there could be nonsensory processes occurring in the same time window. Intracranial stimulation supports the presence of nonsensory TEPs occurring in this time range (Parmigiani et al., 2022), although scalp topography or source estimations of this activity should be explored in more detail. TEP remaining after independent component analysis (ICA)‐based removal of vertex N100‐P200 suggests there may be TMS‐induced oscillatory changes (Ross et al., 2021). It is generally recognized that the TEP in this time window may be the summation of sensory and nonsensory contributions, which is precisely why it is critical to build a toolbox of techniques for suppressing or removing the sensory contributions to the TEP to better understand all other contributors. However, due to the persistent participant perception of loudness and scalp sensation, coupled with a vertex‐localized topography and similar time course of the TEP resulting from ATTENUATE (Figure S8), it is likely that there are residual sensory contributions to the TEP. Future work should explore the conditions in which ATTENUATE can fully suppress the vertex N100‐P200. These conditions include intensities, types of noise, ISIs, sham TMS, or use of a solid spacer underneath the coil that allows for skull conductance but not magnetic field induced cortical activation.
4.1. Effect of a foam spacer on the sensory TEP
We find that a foam spacer attached to the bottom of the coil had no effect on vertex N100‐P200, “click” loudness perception, scalp feeling, or pain. Although foam could be contributing to the combined effectiveness of ATTENUATE, there was no impact when other modifications were matched (Figure 3h–n). Foam has been suggested to reduce bone conduction of the TMS “click” sound (Nikouline et al., 1999), and shown to be effective when used in combination with auditory suppression methods (ter Braack et al., 2015). As such, foam padding between the coil and scalp has become standard procedure to help suppress the vertex N100‐P200. However, foam increases coil to cortex distance and there are no guidelines for adjusting stimulation intensity to account for this increased distance, and no guidelines for reporting whether foam was used in determining motor thresholds. Coil to cortex distance has a strong influence on induced electric field in cortex (Mantell et al., 2021)—enough to significantly increase MT determination (ter Braack et al., 2015), as also observed in this study (Table S1). The lack of reduction in vertex N100‐P200 with foam in our conditions when other factors were matched could be due to this increased intensity of TMS with compared with without foam. Interestingly, we also observed no difference in early localized TEP, supporting that the adjusted rMT with foam likely resulted in a matched induced electrical field, potentially diminishing the argument that the adjusted rMT accounts for our lack of suppression. Overall, our results suggest that foam may not be alone effective for reducing sensory confounds. If used it is important that stimulation intensity is adjusted to account for the increased distance from coil to cortex, and this adjusted rMT is reported in future studies to allow further analysis into this critical question.
4.2. Nonmodal or multimodal component contributions to the TEP
The vertex N100‐P200 complex has been described as an auditory component (see AEP; Ilmoniemi & Kičić, 2010; Nikouline et al., 1999), but it is likely to have multimodal sensory contributions. Although observed in the TEP, vertex N100‐P200 complexes with similar/matching time course of peak latencies and similar source activations have been more rigorously examined and described in response to sensory stimuli other than the TMS “click” sound. Many of these studies describe multisensory or cross‐modal impacts on the vertex N100‐P200 (Shahin, 2019; Shahin et al., 2018; Shen et al., 2020), suggesting that it is not modality specific and instead largely determined by the intrinsic saliency of the stimulus and its task relevance (Mouraux et al., 2011; Novembre et al., 2019).
In TMS‐EEG experiments, it is difficult to distinguish between unimodal auditory and nonmodal or multimodal sensory contributions to the vertex N100‐P200. In addition, it is unclear if sensory contributions are likely to sum linearly. In light of this, sensory suppression protocols for TMS‐EEG may be more effective if the vertex N100‐P200 is assumed to be multimodal. Our proposed ATTENUATE procedure may show superiority to a Standard masking procedure due to additional over‐the‐ear auditory masking or saliency reduction through the predictable ISI of the TMS pulses. Of note is that neither auditory masking (even with over‐the‐ear protection) nor predictable ISI timing was more than borderline or minimally effective at suppressing vertex N100‐P200 when used alone. Maximal suppression was achieved when combining auditory masking and predictable ISI timing, suggesting that a combined sensory masking and sensory attenuation protocol is most effective.
4.3. Repetition suppression, salience, and habituation
Repetition suppression (RS), a reduction of neural activation following repeated presentation of a stimulus, may play a role here and requires further investigation. RS has been observed in response to auditory tones alone and to TMS, both in the TMS‐EEG and in the MEP, and has been observed as early as the second stimulus in a train (Löfberg et al., 2013). Its underlying mechanism has not been fully elucidated but include habituation, perceptual sharpening, or suppression of motor excitability when no movements are intended (Grill‐Spector et al., 2006). Further work is needed to distinguish whether RS is due to a reduction in salience in the saliency network, reduction in habituation in auditory–motor excitability, or a result of the interactions between salience and sensorimotor excitability. However, although there does not appear to be a trend toward recovery within short trains (Fruhstorfer, 1971; Löfberg et al., 2013), the dynamics of RS and recovery should be studied in more detail in longer trains such as the 80 TMS pulses in sequence studied here. This is particularly relevant to TMS brain targets in sensorimotor networks, such as M1 and S1. Further, although we do not observe a direct impact on prefrontal excitability in these data, this possibility does need to be investigated in a focused study tracking the dynamics of modulation across long TMS trains.
4.4. Is sensory suppression the most effective strategy for reducing vertex N100‐P200?
One clear limitation of our results is that neither perceptual ratings nor vertex N100‐P200 were fully eliminated. It should be noted that our design was intended to evoke a large vertex N100‐P200 by using suprathreshold stimulation (120% rMT) and with a stimulation target that is known to induce significant sensory artifact (Lioumis et al., 2018). Future work should examine the efficacy of the ATTENUATE protocol across stimulation intensities and targets. ATTENUATE may fully eliminate vertex N100‐P200 at lower stimulation intensities or other stimulation targets, but this will need to be investigated experimentally.
Furthermore, when the study design allows, sensory suppression techniques should be considered after other options. For instance, if the experimental question allows for conditions with matched intensity, matched sensory suppressive protocols, and target locations with active TMS, then perception and cortical sensory components in the TEP should also be matched. Although this design is optimal, it is not feasible for many studies. Alternatively, one can isolate the sensory contributions to the TEP using sensory‐matched sham protocols. Although it is difficult to match the sensory experience of active TMS with sham TMS, the topography and time course of evoked sensory potentials may be similar (Biabani et al., 2019) enough to employ an ICA‐based technique for removal (Rogasch, Thomson, et al., 2014; Ross et al., 2021). Of course, a combination of sensory suppression, such as ATTENUATE, coupled with sensory‐matched sham TMS may be most effective for reducing the impact of sensory confounds while ensuring that residual sensory contributions to TEP can be more easily identified.
4.5. Sensory potentials and pain perception
Our results suggest that perception of TMS pain may be relevant to the vertex N100‐P200 complex. This result is perhaps unsurprising as previous work has demonstrated substantial overlap between auditory/somatosensory responses and activity in a “pain matrix” network with nociceptive stimuli applied to the skin (Mouraux et al., 2011). Due to a high correlation between the response to sensory and nociceptive stimulation as well as sensory/nociceptive responses and saliency ratings, the authors suggest that sensory responses and pain matrix activity may be best characterized as stimulus saliency‐related network activity. Although our correlation and regression analyses were exploratory, this work suggests that reducing the saliency of TMS, including minimization of pain, should be investigated to minimize the vertex N100‐P200.
5. FUTURE DIRECTIONS
Although this work provides critical improvements in sensory suppression during TMS studies, several important questions remain. Given the wide variety of acoustic and somatosensory differences, the ATTENUATE protocol should be tested with a range of stimulation intensities, coils, and brain targets to establish its efficacy for different stimulation environments. ATTENUATE should also be tested in combination with different noise masking properties. A recent tool, TMS Adaptable Auditory Control (TAAC), shows promise for customizing auditory noise properties for individual participants (Russo et al., 2022). For TMS‐click tracks recorded at 70% maximum stimulator output, the noise properties can be adjusted in real‐time for optimizing perceptual masking. Although TAAC needs to be tested for higher intensity TMS‐click sounds and for masking TMS pulses applied to the scalp (rather than just auditory tracks), it is possible that ATTENUATE used with TAAC instead of the click frequency noise used in this study may provide additional auditory suppression.
It is also important to explore how ATTENUATE performs in patients with sensory deficits such as hearing loss and sensory processing disorders. Additionally, our data suggest that predictability of TMS pulse timing can contribute to amplitude suppression in sensory TEP, building on prior work showing MEP attenuation with predictable M1 stimulation (Stupacher et al., 2013; Tran et al., 2021). Although we did not observe a cumulative effect on the TEP using 80 unjittered single pulses of TMS, the sensory predictive suppressive effect should be examined with more single pulses and with a range of unjittered ISI lengths to ensure that the unjittered protocol does not induce a build‐up effect on plasticity. Sensorimotor prediction for the timing of sensory events is well documented (See Ross et al., 2016; Ross & Balasubramaniam, 2014 for reviews), and may account for motor and sensory attenuation with predictable TMS pulse timing. However, the suggestion that the principles of sensorimotor timing can be used to optimize nonmotor and nonsensory TEP is novel to the best of our knowledge. Future work should compare the effects on the TEP of interval and phase timing in rhythmically predictable TMS sequences (Grube, Cooper, et al., 2010; Grube, Lee, et al., 2010; Iversen & Balasubramaniam, 2016; Patel & Iversen, 2014; Ross et al., 2016; Ross et al., 2018; Teki et al., 2011; Teki et al., 2012), the role of task‐relevant sensorimotor experience (Stupacher et al., 2013; Wu et al., 2017), and readiness‐to‐act (Gordon et al., 2017; Gordon, Iacoboni, & Balasubramaniam, 2018) as variables in the TMS sensory predictive suppressive effect.
6. CONCLUSIONS
We investigated the electrophysiological and perceptual consequences of applying different sensory suppression protocols with suprathreshold TMS to dlPFC. We find that ATTENUATE outperforms the Standard masking protocol for reducing both the vertex N100‐P200 and sensory perception. Further, our data support that auditory suppression, foam spacing, or pulse timing alone are not sufficient to reduce the vertex N100‐P200, likely due to the nonmodal or multimodal contributions of the sensory experience of the TMS pulse.
FUNDING INFORMATION
This research was supported by the National Institute of Mental Health under award number R01MH126639 and a Burroughs Wellcome Fund Career Award for Medical Scientists (Correy J. Keller). Jessica M. Ross was supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the Veterans Affairs Palo Alto Health Care System, and the Department of Veterans Affairs Sierra‐Pacific Data Science Fellowship.
CONFLICT OF INTEREST
Corey J. Keller holds equity in Alto Neuroscience, Inc.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGMENTS
We extend gratitude to all of our research participants. We would also like to acknowledge the generous contributions of the members of the Personalized Neurotherapeutics Laboratory for helpful feedback on the article and throughout the course of the study.
Ross, J. M. , Sarkar, M. , & Keller, C. J. (2022). Experimental suppression of transcranial magnetic stimulation‐electroencephalography sensory potentials. Human Brain Mapping, 43(17), 5141–5153. 10.1002/hbm.25990
Funding information Burroughs Wellcome Fund, Grant/Award Number: Career Award for Medical Scientists; National Institute of Mental Health, Grant/Award Number: R01MH129018
DATA AVAILABILITY STATEMENT
Data availability upon request.
REFERENCES
- Atluri, S. , Frehlich, M. , Mei, Y. , Garcia Dominguez, L. , Rogasch, N. C. , Wong, W. , Daskalakis, Z. J. , & Farzan, F. (2016). TMSEEG: A MATLAB‐based graphical user Interface for processing electrophysiological signals during transcranial magnetic stimulation. Frontiers in Neural Circuits, 10, 78. 10.3389/fncir.2016.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, A. T. , Jalinous, R. , & Freeston, I. L. (1985). Non‐invasive magnetic stimulation of human motor cortex. The Lancet, 325, 1106–1107. 10.1016/S0140-6736(85)92413-4 [DOI] [PubMed] [Google Scholar]
- Belardinelli, P. , Biabani, M. , Blumberger, D. M. , Bortoletto, M. , Casarotto, S. , David, O. , Desideri, D. , Etkin, A. , Ferrarelli, F. , Fitzgerald, P. B. , Fornito, A. , Gordon, P. C. , Gosseries, O. , Harquel, S. , Julkunen, P. , Keller, C. J. , Kimiskidis, V. K. , Lioumis, P. , Miniussi, C. , … Ilmoniemi, R. J. (2019). Reproducibility in TMS–EEG studies: A call for data sharing, standard procedures and effective experimental control. Brain Stimulation, 12, 787–790. 10.1016/j.brs.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Bertazzoli, G. , Esposito, R. , Mutanen, T. P. , Ferrari, C. , Ilmoniemi, R. J. , Miniussi, C. , & Bortoletto, M. (2021). The impact of artifact removal approaches on TMS–EEG signal. NeuroImage, 239, 118272. 10.1016/j.neuroimage.2021.118272 [DOI] [PubMed] [Google Scholar]
- Biabani, M. , Fornito, A. , Mutanen, T. P. , Morrow, J. , & Rogasch, N. C. (2019). Characterizing and minimizing the contribution of sensory inputs to TMS‐evoked potentials. Brain Stimulation, 12, 1537–1552. 10.1016/j.brs.2019.07.009 [DOI] [PubMed] [Google Scholar]
- Bortoletto, M. , Veniero, D. , Thut, G. , & Miniussi, C. (2015). The contribution of TMS–EEG coregistration in the exploration of the human cortical connectome. Neuroscience & Biobehavioral Reviews, 49, 114–124. 10.1016/j.neubiorev.2014.12.014 [DOI] [PubMed] [Google Scholar]
- Brasil‐Neto, J. P. , Cohen, L. G. , Panizza, M. , Nilsson, J. , Roth, B. J. , & Hallett, M. (1992). Optimal focal transcranial magnetic activation of the human motor cortex: Effects of coil orientation, shape of the induced current pulse, and stimulus intensity. Journal of Clinical Neurophysiology, 9, 132–136. [PubMed] [Google Scholar]
- Casarotto, S. , Määttä, S. , Herukka, S.‐K. , Pigorini, A. , Napolitani, M. , Gosseries, O. , Niskanen, E. , Könönen, M. , Mervaala, E. , Rosanova, M. , Soininen, H. , & Massimini, M. (2011). Transcranial magnetic stimulation‐evoked EEG/cortical potentials in physiological and pathological aging. Neuroreport, 22, 592–597. 10.1097/WNR.0b013e328349433a [DOI] [PubMed] [Google Scholar]
- Čeponien, R. , Rinne, T. , & Näätänen, R. (2002). Maturation of cortical sound processing as indexed by event‐related potentials. Clinical Neurophysiology, 113, 870–882. 10.1016/S1388-2457(02)00078-0 [DOI] [PubMed] [Google Scholar]
- Chatterjee, S. , & Hadi, A. S. (1986). Influential observations, high leverage points, and outliers in linear regression. Statistical Science, 1, 379–393. 10.1214/ss/1177013622 [DOI] [Google Scholar]
- Chen, A. C. , Oathes, D. J. , Chang, C. , Bradley, T. , Zhou, Z.‐W. , Williams, L. M. , Glover, G. H. , Deisseroth, K. , & Etkin, A. (2013). Causal interactions between fronto‐parietal central executive and default‐mode networks in humans. Proceedings of the National Academy of Sciences of the United States of America, 110, 19944–19949. 10.1073/pnas.1311772110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde, V. , Tomasevic, L. , Akopian, I. , Stanek, K. , Saturnino, G. B. , Thielscher, A. , Bergmann, T. O. , & Siebner, H. R. (2019). The non‐transcranial TMS‐evoked potential is an inherent source of ambiguity in TMS‐EEG studies. NeuroImage, 185, 300–312. 10.1016/j.neuroimage.2018.10.052 [DOI] [PubMed] [Google Scholar]
- Delorme, A. , & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Draper, N. , & Smith, H. (1981). Applied regression analysis (2nd ed.). Wiley. [Google Scholar]
- Eggermont, J. J. , Ponton, C. W. , Don, M. , Waring, M. D. , & Kwong, B. (1997). Maturational delays in cortical evoked potentials in Cochlear implant users. Acta Oto‐Laryngologica, 117, 161–163. 10.3109/00016489709117760 [DOI] [PubMed] [Google Scholar]
- Eshel, N. , Keller, C. J. , Wu, W. , Jiang, J. , Mills‐Finnerty, C. , Huemer, J. , Wright, R. , Fonzo, G. A. , Ichikawa, N. , Carreon, D. , Wong, M. , Yee, A. , Shpigel, E. , Guo, Y. , McTeague, L. , Maron‐Katz, A. , & Etkin, A. (2020). Global connectivity and local excitability changes underlie antidepressant effects of repetitive transcranial magnetic stimulation. Neuropsychopharmacology, 45, 1018–1025. 10.1038/s41386-020-0633-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli, F. , Massimini, M. , Sarasso, S. , Casali, A. , Riedner, B. A. , Angelini, G. , Tononi, G. , & Pearce, R. A. (2010). Breakdown in cortical effective connectivity during midazolam‐induced loss of consciousness. Proceedings of the National Academy of Sciences of the United States of America, 107, 2681–2686. 10.1073/pnas.0913008107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, A. S. , Keller, C. J. , & Etkin, A. (2016). The clinical applicability of functional connectivity in depression: Pathways toward more targeted intervention. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1, 262–270. 10.1016/j.bpsc.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , Buckner, R. L. , White, M. P. , Greicius, M. D. , & Pascual‐Leone, A. (2012). Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the Subgenual cingulate. Biological Psychiatry, 72, 595–603. 10.1016/j.biopsych.2012.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg, M. , Reeves, J. A. , Hussain, S. J. , Zaghloul, K. A. , & Wassermann, E. M. (2020). Identifying site‐ and stimulation‐specific TMS‐evoked EEG potentials using a quantitative cosine similarity metric. PLoS One, 15, e0216185. 10.1371/journal.pone.0216185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhstorfer, H. (1971). Habituation and dishabituation of the human vertex response. Electroencephalography and Clinical Neurophysiology, 30, 306–312. 10.1016/0013-4694(71)90113-1 [DOI] [PubMed] [Google Scholar]
- Fuggetta, G. , Fiaschi, A. , & Manganotti, P. (2005). Modulation of cortical oscillatory activities induced by varying single‐pulse transcranial magnetic stimulation intensity over the left primary motor area: A combined EEG and TMS study. NeuroImage, 27, 896–908. 10.1016/j.neuroimage.2005.05.013 [DOI] [PubMed] [Google Scholar]
- George, M. S. , Wassermann, E. M. , Williams, W. A. , Callahan, A. , Ketter, T. A. , Basser, P. , Hallett, M. , & Post, R. M. (1995). Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport, 6, 1853–1856. 10.1097/00001756-199510020-00008 [DOI] [PubMed] [Google Scholar]
- Gordon, C. L. , Iacoboni, M. , & Balasubramaniam, R. (2018). Multimodal music perception engages motor prediction: A TMS study. Frontiers in Neuroscience, 12, 736. 10.3389/fnins.2018.00736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, C. L. , Spivey, M. J. , & Balasubramaniam, R. (2017). Corticospinal excitability during the processing of handwritten and typed words and non‐words. Neuroscience Letters, 651, 232–236. 10.1016/j.neulet.2017.05.021 [DOI] [PubMed] [Google Scholar]
- Gordon, P. C. , Desideri, D. , Belardinelli, P. , Zrenner, C. , & Ziemann, U. (2018). Comparison of cortical EEG responses to realistic sham versus real TMS of human motor cortex. Brain Stimulation, 11, 1322–1330. 10.1016/j.brs.2018.08.003 [DOI] [PubMed] [Google Scholar]
- Gosseries, O. , Sarasso, S. , Casarotto, S. , Boly, M. , Schnakers, C. , Napolitani, M. , Bruno, M. A. , Ledoux, D. , Tshibanda, J. F. , Massimini, M. , Laureys, S. , & Rosanova, M. (2015). On the cerebral origin of EEG responses to TMS: Insights from severe cortical lesions. Brain Stimulation, 8, 142–149. 10.1016/j.brs.2014.10.008 [DOI] [PubMed] [Google Scholar]
- Grill‐Spector, K. , Henson, R. , & Martin, A. (2006). Repetition and the brain: Neural models of stimulus‐specific effects. Trends in Cognitive Sciences, 10, 14–23. 10.1016/j.tics.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Grube, M. , Cooper, F. E. , Chinnery, P. F. , & Griffiths, T. D. (2010). Dissociation of duration‐based and beat‐based auditory timing in cerebellar degeneration. Proceedings of the National Academy of Sciences of the United States of America, 107, 11597–11601. 10.1073/pnas.0910473107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube, M. , Lee, K.‐H. , Griffiths, T. D. , Barker, A. T. , & Woodruff, P. W. (2010). Transcranial magnetic theta‐burst stimulation of the human cerebellum distinguishes absolute, duration‐based from relative, beat‐based perception of subsecond time intervals. Frontiers in Psychology, 1, 171. 10.3389/fpsyg.2010.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harquel, S. , Bacle, T. , Beynel, L. , Marendaz, C. , Chauvin, A. , & David, O. (2016). Mapping dynamical properties of cortical microcircuits using robotized TMS and EEG: Towards functional cytoarchitectonics. NeuroImage, 135, 115–124. 10.1016/j.neuroimage.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Herring, J. D. , Thut, G. , Jensen, O. , & Bergmann, T. O. (2015). Attention modulates TMS‐locked alpha oscillations in the visual cortex. Journal of Neuroscience, 35, 14435–14447. 10.1523/JNEUROSCI.1833-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Hajnal, B. , Entz, L. , Fabó, D. , Herrero, J. L. , Mehta, A. D. , & Keller, C. J. (2019). Intracortical dynamics underlying repetitive stimulation predicts changes in network connectivity. The Journal of Neuroscience, 39, 6122–6135. 10.1523/JNEUROSCI.0535-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi, R. J. , & Kičić, D. (2010). Methodology for combined TMS and EEG. Brain Topography, 22, 233–248. 10.1007/s10548-009-0123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi, R. J. , Virtanen, J. , Ruohonen, J. , Karhu, J. , Aronen, H. J. , Näätänen, R. , & Katila, T. (1997). Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport, 8, 3537–3540. 10.1097/00001756-199711100-00024 [DOI] [PubMed] [Google Scholar]
- Iversen, J. R. , & Balasubramaniam, R. (2016). Synchronization and temporal processing. Current Opinion in Behavioral Sciences, 8, 175–180. 10.1016/j.cobeha.2016.02.027 [DOI] [Google Scholar]
- Janssen, A. M. , Oostendorp, T. F. , & Stegeman, D. F. (2015). The coil orientation dependency of the electric field induced by TMS for M1 and other brain areas. Journal of NeuroEngineering and Rehabilitation, 12, 47. 10.1186/s12984-015-0036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, C. J. , Huang, Y. , Herrero, J. L. , Fini, M. E. , Du, V. , Lado, F. A. , Honey, C. J. , & Mehta, A. D. (2018). Induction and quantification of excitability changes in human cortical networks. The Journal of Neuroscience, 38, 5384–5398. 10.1523/JNEUROSCI.1088-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin, L. J. , Keller, C. J. , Wu, W. , Narayan, M. , & Etkin, A. (2018). Test‐retest reliability of transcranial magnetic stimulation EEG evoked potentials. Brain Stimulation, 11, 536–544. 10.1016/j.brs.2017.12.010 [DOI] [PubMed] [Google Scholar]
- Knight, R. T. , Hillyard, S. A. , Woods, D. L. , & Neville, H. J. (1980). The effects of frontal and temporal‐parietal lesions on the auditory evoked potential in man. Electroencephalography and Clinical Neurophysiology, 50, 112–124. 10.1016/0013-4694(80)90328-4 [DOI] [PubMed] [Google Scholar]
- Laakso, I. , Hirata, A. , & Ugawa, Y. (2014). Effects of coil orientation on the electric field induced by TMS over the hand motor area. Physics in Medicine and Biology, 59, 203–218. 10.1088/0031-9155/59/1/203 [DOI] [PubMed] [Google Scholar]
- Lioumis, P. , Kičić, D. , Savolainen, P. , Mäkelä, J. P. , & Kähkönen, S. (2009). Reproducibility of TMS‐evoked EEG responses. Human Brain Mapping, 30, 1387–1396. 10.1002/hbm.20608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioumis, P. , Zomorrodi, R. , Hadas, I. , Daskalakis, Z. J. , & Blumberger, D. M. (2018). Combined transcranial magnetic stimulation and electroencephalography of the dorsolateral prefrontal cortex. Journal of Visualized Experiments, 138, 57983. 10.3791/57983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfberg, O. , Julkunen, P. , Tiihonen, P. , Pääkkönen, A. , & Karhu, J. (2013). Repetition suppression in the cortical motor and auditory systems resemble each other – A combined TMS and evoked potential study. Neuroscience, 243, 40–45. 10.1016/j.neuroscience.2013.03.060 [DOI] [PubMed] [Google Scholar]
- Mantell, K. E. , Sutter, E. N. , Shirinpour, S. , Nemanich, S. T. , Lench, D. H. , Gillick, B. T. , & Opitz, A. (2021). Evaluating transcranial magnetic stimulation (TMS) induced electric fields in pediatric stroke. NeuroImage: Clinical, 29, 102563. 10.1016/j.nicl.2021.102563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini, M. , Ferrarelli, F. , Huber, R. , Esser, S. K. , Singh, H. , & Tononi, G. (2005). Breakdown of cortical effective connectivity during sleep. Science, 309, 2228–2232. 10.1126/science.1117256 [DOI] [PubMed] [Google Scholar]
- McClintock, S. M. , Reti, I. M. , Carpenter, L. L. , McDonald, W. M. , Dubin, M. , Taylor, S. F. , Cook, I. A. , O'Reardon, J. , Husain, M. M. , Wall, C. , Krystal, A. D. , Sampson, S. M. , Morales, O. , Nelson, B. G. , Latoussakis, V. , George, M. S. , Lisanby, S. H. , National Network of Depression Centers rTMS Task Group , & American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments . (2018). Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression: (consensus statement). The Journal of Clinical Psychiatry, 79, 35–48. 10.4088/JCP.16cs10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, K. R. , Boniface, S. J. , & Schubert, M. (1992). Magnetic brain stimulation with a double coil: The importance of coil orientation. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section, 85, 17–21. 10.1016/0168-5597(92)90096-T [DOI] [PubMed] [Google Scholar]
- Mouraux, A. , Diukova, A. , Lee, M. C. , Wise, R. G. , & Iannetti, G. D. (2011). A multisensory investigation of the functional significance of the “pain matrix”. NeuroImage, 54, 2237–2249. 10.1016/j.neuroimage.2010.09.084 [DOI] [PubMed] [Google Scholar]
- Näätänen, R. , & Picton, T. (1987). The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology, 24, 375–425. 10.1111/j.1469-8986.1987.tb00311.x [DOI] [PubMed] [Google Scholar]
- Nikouline, V. , Ruohonen, J. , & Ilmoniemi, R. J. (1999). The role of the coil click in TMS assessed with simultaneous EEG. Clinical Neurophysiology, 110, 1325–1328. 10.1016/S1388-2457(99)00070-X [DOI] [PubMed] [Google Scholar]
- Novembre, G. , Pawar, V. M. , Kilintari, M. , Bufacchi, R. J. , Guo, Y. , Rothwell, J. C. , & Iannetti, G. D. (2019). The effect of salient stimuli on neural oscillations, isometric force, and their coupling. NeuroImage, 198, 221–230. 10.1016/j.neuroimage.2019.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reardon, J. P. , Solvason, H. B. , Janicak, P. G. , Sampson, S. , Isenberg, K. E. , Nahas, Z. , McDonald, W. M. , Avery, D. , Fitzgerald, P. B. , Loo, C. , Demitrack, M. A. , George, M. S. , & Sackeim, H. A. (2007). Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62, 1208–1216. 10.1016/j.biopsych.2007.01.018 [DOI] [PubMed] [Google Scholar]
- Ozdemir, R. A. , Tadayon, E. , Boucher, P. , Sun, H. , Momi, D. , Ganglberger, W. , Westover, M. B. , Pascual‐Leone, A. , Santarnecchi, E. , & Shafi, M. M. (2021). Cortical responses to noninvasive perturbations enable individual brain fingerprinting. Brain Stimulation, 14, 391–403. 10.1016/j.brs.2021.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg, F. , & George, M. S. (2009). Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Experimental Neurology, 219, 2–13. 10.1016/j.expneurol.2009.04.020 [DOI] [PubMed] [Google Scholar]
- Parmigiani, S. , Mikulan, E. , Russo, S. , Sarasso, S. , Zauli, F. M. , Rubino, A. , Cattani, A. , Fecchio, M. , Giampiccolo, D. , Lanzone, J. , D'Orio, P. , del Vecchio, M. , Avanzini, P. , Nobili, L. , Sartori, I. , Massimini, M. , & Pigorini, A. (2022). Simultaneous stereo‐EEG and high‐density scalp EEG recordings to study the effects of intracerebral stimulation parameters. Brain Stimulation, 15, 664–675. 10.1016/j.brs.2022.04.007 [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone, A. , Freitas, C. , Oberman, L. , Horvath, J. C. , Halko, M. , Eldaief, M. , Bashir, S. , Vernet, M. , Shafi, M. , Westover, B. , Vahabzadeh‐Hagh, A. M. , & Rotenberg, A. (2011). Characterizing brain cortical plasticity and network dynamics across the age‐span in health and disease with TMS‐EEG and TMS‐fMRI. Brain Topography, 24, 302–315. 10.1007/s10548-011-0196-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual‐Leone, A. , Rubio, B. , Pallardó, F. , & Catalá, M. D. (1996). Rapid‐rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug‐resistant depression. The Lancet, 348, 233–237. 10.1016/s0140-6736(96)01219-6 [DOI] [PubMed] [Google Scholar]
- Patel, A. D. , & Iversen, J. R. (2014). The evolutionary neuroscience of musical beat perception: The action simulation for auditory prediction (ASAP) hypothesis. Frontiers in Systems Neuroscience, 8, 57. 10.3389/fnsys.2014.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus, T. , Sipila, P. K. , & Strafella, A. P. (2001). Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: An EEG study. Journal of Neurophysiology, 86, 1983–1990. 10.1152/jn.2001.86.4.1983 [DOI] [PubMed] [Google Scholar]
- Pridmore, S. , Fernandes Filho, J. A. , Nahas, Z. , Liberatos, C. , & George, M. S. (1998). Motor threshold in transcranial magnetic stimulation: A comparison of a neurophysiological method and a visualization of movement method. The Journal of ECT, 14, 25–27. [PubMed] [Google Scholar]
- Rocchi, L. , Di Santo, A. , Brown, K. , Ibáñez, J. , Casula, E. , Rawji, V. , Di Lazzaro, V. , Koch, G. , & Rothwell, J. (2021). Disentangling EEG responses to TMS due to cortical and peripheral activations. Brain Stimulation, 14, 4–18. 10.1016/j.brs.2020.10.011 [DOI] [PubMed] [Google Scholar]
- Rogasch, N. C. , Daskalakis, Z. J. , & Fitzgerald, P. B. (2014). Cortical inhibition, excitation, and connectivity in schizophrenia: A review of insights from transcranial magnetic stimulation. Schizophrenia Bulletin, 40, 685–696. 10.1093/schbul/sbt078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch, N. C. , & Fitzgerald, P. B. (2013). Assessing cortical network properties using TMS‐EEG. Human Brain Mapping, 34, 1652–1669. 10.1002/hbm.22016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch, N. C. , Sullivan, C. , Thomson, R. H. , Rose, N. S. , Bailey, N. W. , Fitzgerald, P. B. , Farzan, F. , & Hernandez‐Pavon, J. C. (2017). Analysing concurrent transcranial magnetic stimulation and electroencephalographic data: A review and introduction to the open‐source TESA software. NeuroImage, 147, 934–951. 10.1016/j.neuroimage.2016.10.031 [DOI] [PubMed] [Google Scholar]
- Rogasch, N. C. , Thomson, R. H. , Farzan, F. , Fitzgibbon, B. M. , Bailey, N. W. , Hernandez‐Pavon, J. C. , Daskalakis, Z. J. , & Fitzgerald, P. B. (2014). Removing artefacts from TMS‐EEG recordings using independent component analysis: Importance for assessing prefrontal and motor cortex network properties. NeuroImage, 101, 425–439. 10.1016/j.neuroimage.2014.07.037 [DOI] [PubMed] [Google Scholar]
- Rosanova, M. , Casali, A. , Bellina, V. , Resta, F. , Mariotti, M. , & Massimini, M. (2009). Natural frequencies of human Corticothalamic circuits. Journal of Neuroscience, 29, 7679–7685. 10.1523/JNEUROSCI.0445-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, J. M. , & Balasubramaniam, R. (2014). Physical and neural entrainment to rhythm: Human sensorimotor coordination across tasks and effector systems. Frontiers in Human Neuroscience, 8, 576. 10.3389/fnhum.2014.00576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, J. M. , Iversen, J. R. , & Balasubramaniam, R. (2016). Motor simulation theories of musical beat perception. Neurocase, 22, 558–565. 10.1080/13554794.2016.1242756 [DOI] [PubMed] [Google Scholar]
- Ross, J. M. , Iversen, J. R. , & Balasubramaniam, R. (2018). The role of posterior parietal cortex in beat‐based timing perception: A continuous theta burst stimulation study. Journal of Cognitive Neuroscience, 30, 634–643. 10.1162/jocn_a_01237 [DOI] [PubMed] [Google Scholar]
- Ross, J. M. , Ozdemir, R. A. , Lian, S. J. , Fried, P. J. , Schmitt, E. M. , Inouye, S. K. , et al. (2021). A structured ICA‐based process for removing auditory evoked potentials. Scientific Reports, 12, 1391. 10.1038/s41598-022-05397-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, S. , Sarasso, S. , Puglisi, G. E. , Dal Palù, D. , Pigorini, A. , Casarotto, S. , D'Ambrosio, S. , Astolfi, A. , Massimini, M. , Rosanova, M. , & Fecchio, M. (2022). TAAC ‐ TMS adaptable auditory control: A universal tool to mask TMS clicks. Journal of Neuroscience Methods, 370, 109491. 10.1016/j.jneumeth.2022.109491 [DOI] [PubMed] [Google Scholar]
- Shafi, M. M. , Vernet, M. , Klooster, D. , Chu, C. J. , Boric, K. , Barnard, M. E. , Romatoski, K. , Westover, M. B. , Christodoulou, J. A. , Gabrieli, J. D. E. , Whitfield‐Gabrieli, S. , Pascual‐Leone, A. , & Chang, B. S. (2015). Physiological consequences of abnormal connectivity in a developmental epilepsy: Cortical connectivity. Annals of Neurology, 77, 487–503. 10.1002/ana.24343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin, A. J. (2019). Neural evidence accounting for interindividual variability of the McGurk illusion. Neuroscience Letters, 707, 134322. 10.1016/j.neulet.2019.134322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin, A. J. , Backer, K. C. , Rosenblum, L. D. , & Kerlin, J. R. (2018). Neural mechanisms underlying cross‐modal phonetic encoding. The Journal of Neuroscience, 38, 1835–1849. 10.1523/JNEUROSCI.1566-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A. , Kraus, N. , TJ, M. G. , & Nicol, T. G. (1997). Developmental changes in P1 and N1 central auditory responses elicited by consonant‐vowel syllables. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section, 104, 540–545. 10.1016/S0168-5597(97)00050-6 [DOI] [PubMed] [Google Scholar]
- Shen, S. , Kerlin, J. R. , Bortfeld, H. , & Shahin, A. J. (2020). The cross‐modal suppressive role of visual context on speech intelligibility: An ERP study. Brain Sciences, 10, 810. 10.3390/brainsci10110810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi, S. H. , Schaper, F. L. W. V. J. , Horn, A. , Hsu, J. , Padmanabhan, J. L. , Brodtmann, A. , Cash, R. F. H. , Corbetta, M. , Choi, K. S. , Dougherty, D. D. , Egorova, N. , Fitzgerald, P. B. , George, M. S. , Gozzi, S. A. , Irmen, F. , Kuhn, A. A. , Johnson, K. A. , Naidech, A. M. , Pascual‐Leone, A. , … Fox, M. D. (2021). Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nature Human Behavior, 5, 1707–1716. 10.1038/s41562-021-01161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner, H. R. , Bergmann, T. O. , Bestmann, S. , Massimini, M. , Johansen‐Berg, H. , Mochizuki, H. , Bohning, D. E. , Boorman, E. D. , Groppa, S. , Miniussi, C. , Pascual‐Leone, A. , Huber, R. , Taylor, P. C. J. , Ilmoniemi, R. J. , de Gennaro, L. , Strafella, A. P. , Kähkönen, S. , Klöppel, S. , Frisoni, G. B. , … Rossini, P. M. (2009). Consensus paper: Combining transcranial stimulation with neuroimaging. Brain Stimulation, 2, 58–80. 10.1016/j.brs.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Siebner, H. R. , Conde, V. , Tomasevic, L. , Thielscher, A. , & Bergmann, T. O. (2019). Distilling the essence of TMS‐evoked EEG potentials (TEPs): A call for securing mechanistic specificity and experimental rigor. Brain Stimulation, 12, 1051–1054. 10.1016/j.brs.2019.03.076 [DOI] [PubMed] [Google Scholar]
- Stokes, M. G. , Chambers, C. D. , Gould, I. C. , Henderson, T. R. , Janko, N. E. , Allen, N. B. , & Mattingley, J. B. (2005). Simple metric for scaling motor threshold based on scalp‐cortex distance: Application to studies using transcranial magnetic stimulation. Journal of Neurophysiology, 94, 4520–4527. 10.1152/jn.00067.2005 [DOI] [PubMed] [Google Scholar]
- Stupacher, J. , Hove, M. J. , Novembre, G. , Schütz‐Bosbach, S. , & Keller, P. E. (2013). Musical groove modulates motor cortex excitability: A TMS investigation. Brain and Cognition, 82, 127–136. 10.1016/j.bandc.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Tchumatchenko, T. , & Reichenbach, T. (2014). A cochlear‐bone wave can yield a hearing sensation as well as otoacoustic emission. Nature Communications, 5, 4160. 10.1038/ncomms5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teki, S. , Grube, M. , & Griffiths, T. D. (2012). A unified model of time perception accounts for duration‐based and beat‐based timing mechanisms. Frontiers in Integrative Neuroscience, 5, 90. doi: 10.3389/fnint.2011.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teki, S. , Grube, M. , Kumar, S. , & Griffiths, T. D. (2011). Distinct neural substrates of duration‐based and beat‐based auditory timing. Journal of Neuroscience, 31, 3805–3812. 10.1523/JNEUROSCI.5561-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Braack, E. M. , de Vos, C. C. , & van Putten, M. J. A. M. (2015). Masking the auditory evoked potential in TMS–EEG: A comparison of various methods. Brain Topography, 28, 520–528. 10.1007/s10548-013-0312-z [DOI] [PubMed] [Google Scholar]
- Tervo, A. E. , Nieminen, J. O. , Lioumis, P. , Metsomaa, J. , Souza, V. H. , Sinisalo, H. , et al. (2021). Closed‐loop optimization of transcranial magnetic stimulation with electroencephalography feedback. Brain Stimulation, 15(2), 523–531. 10.1016/j.brs.2022.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, D. M. D. , McNair, N. A. , Harris, J. A. , & Livesey, E. J. (2021). Expected TMS excites the motor system less effectively than unexpected stimulation. NeuroImage, 226, 117541. 10.1016/j.neuroimage.2020.117541 [DOI] [PubMed] [Google Scholar]
- Wu, C. C. , Hamm, J. P. , Lim, V. K. , & Kirk, I. J. (2017). Musical training increases functional connectivity, but does not enhance mu suppression. Neuropsychologia, 104, 223–233. 10.1016/j.neuropsychologia.2017.08.029 [DOI] [PubMed] [Google Scholar]
- Wu, W. , Keller, C. J. , Rogasch, N. C. , Longwell, P. , Shpigel, E. , Rolle, C. E. , & Etkin, A. (2018). ARTIST: A fully automated artifact rejection algorithm for single‐pulse TMS‐EEG data. Human Brain Mapping, 39, 1607–1625. 10.1002/hbm.23938 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
Data availability upon request.


