Abstract
Background:
Providing personalized genetic risk feedback of a child’s susceptibility to adult-onset health conditions is a topic of considerable debate. Family health history (FHH), specifically parental overweight/obesity status, is a useful assessment for evaluating a child’s genetic and environmental risk of becoming obese. It is unclear whether such risk information may influence parents’ efforts to reduce their child’s risk of obesity.
Purpose:
To evaluate whether telling mothers the magnitude of their child’s risk of becoming obese based on personal FHH influenced food choices for their young child from a virtual reality-based buffet restaurant.
Methods:
Overweight/obese mothers of a child aged 4-5 who met eligibility criteria (N=221) were randomly assigned to one of three experimental arms which emphasized different health information: Arm 1, Food safety control (Control); Arm 2, Behavioral risk information alone (BRI); or Arm 3, Behavioral risk information plus personal FHH-based risk assessment (BRI+FHH). Mothers donned a head-mounted display to be immersed in a virtual restaurant buffet where they selected virtual food and beverages as a lunch for their child.
Results:
Mothers who were randomized to BRI+FHH filled the index child’s plate with an average of 45 fewer calories than those in the Control arm (p<.05); those in the BRI arm filled the plate with 35 fewer calories than the Control arm, a non-significant difference. Calorie restriction was greatest among mothers in the BRI+FHH arm who received the weaker risk message (i.e., only one overweight parent).
Conclusions:
The influence of communicating a child’s inherited risk of obesity on mothers’ feeding practices may vary by the risk level conveyed. High risk messages may best be coupled with strategies to increase mother’s perceptions that efforts can be undertaken to reduce risk and build requisite behavioral skills to reduce risk.
Keywords: genomic risk, parents, communication, family health history
INTRODUCTION
The occurrence of overweight and obesity is increasing among children, beginning at an earlier age and with significant long term public health impact. An estimated 17% of children are now classified as obese.1 Furthermore, children who are overweight in early childhood are also more likely to be overweight or obese in later life. For example, evidence from the National Child Health and Human Development Study shows that those who were in the top 20th percentile of body mass index (BMI) at age 6 were six times more likely to be overweight or obese by age 12 than children in lower BMI categories.2 A troubling result of these trends is the growing number of children diagnosed with increased lipid and blood pressure profiles and Type 2 diabetes, disorders typically characterized by adult onset. There is widespread agreement that prevention holds the greatest promise for redressing the obesity epidemic. Thus, interventions targeted to early childhood when eating and activity patterns are being established will be essential.3
Heritability of Obesity and the Family Environment
There is consistent and growing evidence that genetic4 and environmental factors contribute to overweight and obesity.5 Twin studies have shown heritability of body weight to be between 50 and 80%.4 Ongoing research in animal models and most recently, genome wide association studies, have identified more than 100 candidate genes associated with obesity.6 “Obesigenic” family environments that encourage calorie dense diets and low levels of physical activity also significantly increase the risk of overweight and obesity among children.7
Potential Impact of Genomic-Based Risk Information for Childhood Obesity Prevention
One forecast advantage of emerging genomic discovery is the future capability to provide individuals with personalized genomic-based risk information.8 Conceptual models seeking to explain adoption and change of health behaviors suggest that such personalization of risk messages could increase the perceived salience of risk information and in so doing motivate efforts to reduce risk more so than generic risk messages.9, 10
For example, stress and coping theory holds that compared to standard risk information, more personalized genomic-based risk information would be expected to present a stronger threat and prompt a greater stress response that in turn, should increase efforts to cope.11 Likewise, communal coping theory suggests that what may make genomic-based risk information especially influential is the inherent interpersonal nature of the information, as it could prompt coping strategies undertaken on behalf of other family members.12 This may be especially true when parents’ are aware that preventive actions taken could benefit their children’s health. Risk information processing theories9 suggest that such personalized risk information could engage parents in “systematic processing”, that is, greater attention to and increased effort to understand information. This “systematic processing” is posited to prompt greater commitment to act on the information.
Family health history (FHH) information based on parents’ weight status could be a useful risk assessment that reflects both genetic and environmental contributors to child’s risk of overweight and obesity.13 Evidence consistently shows that a child’s lifetime risk of becoming overweight or obese increases incrementally with the number of biological parents who are overweight or obese.14, 15 Moreover, maternal weight appears to be especially important in children’s weight trajectories due in part to mothers’ primary role in child care and feeding.16, 17 There is theoretical support for the notion that FHH-based risk messages about a child’s risk for obesity will be particularly motivating to parents. However, to our knowledge the only research that has evaluated obesity-related risk information, based on one’s own genetics18 or a child’s BMI19 did not directly test the above assertion or include behavioral outcome measures.
Challenges of Conveying Personalized Genomic Risk Information about Children
Providing personalized genetic risk feedback via genetic susceptibility testing to children for adult onset conditions is a topic of considerable debate. Pediatric guidelines are consistent in recommending against such approaches.20 Critics assert that increasing parents’ awareness of a child’s genetic vulnerability to traits or health conditions such as obesity might increase their concerns about child health in ways that prompt ineffective or problematic parenting practices. In the case of eating behaviors, these negative practices might include being overly restrictive of their child’s food choices.21 Such controlling behaviors (e.g., overly restricting desired foods or using them as rewards) could override the child’s own internal signals of satiety in ways that unintentionally reinforce the child’s tendency to eat in the absence of hunger.22 In this way, parents’ efforts to reduce their child’s obesity risk, could, if taken too far, backfire and actually increase the child’s risk of overeating and becoming obese.
To date studies evaluating genetic risk feedback provided to parents and children have focused on families at high risk for conditions such as heart disease23 and cancer24. These studies have focused solely on psychological responses to such feedback generally showing no association with enduring negative psychological outcomes such as anxiety or depression for parents or children.25
Current clinical guidelines do recommend that pediatricians routinely assess FHH of obesity-related health conditions.26 In addition, initial research suggests that hypothetical FHH information may prompt the same level of concern about children’s health as risk information based on genetic susceptibility testing.27 However, no studies have explored the impact that this information has on parenting behaviors related to children’s health habits.
To this end, we conducted a randomized experimental trial in which we evaluated whether telling mothers about the magnitude of their child’s risk of becoming obese based on personal FHH of overweight/obesity was associated with food choices mothers’ made for their young child using a virtual reality-based buffet restaurant. We hypothesized that mothers who received FHH-based risk assessment and behavioral risk information would fill a plate with fewer total calories than those who received no obesity risk information; and those who received behavioral risk information alone would fill a plate with fewer total calories than mothers who received no obesity information.
METHODS
Study Population
The target population for the study was overweight and obese mothers of 4-5 year old children. Specifically, eligible mothers: (1) were ≥ age 18; (2) had a self-reported height and weight that indicated a body mass index of ≥ 25; (3) had a biological child between the ages of 4 and 5 inclusive living in the same household at least 1 day out of the past 30 days, without major food allergies or diet-related health conditions, developmental delays, or disabilities; (4) could report that child’s FHH of overweight/obesity; and (5) had the ability to read and write in English. Women were excluded if they reported a past or current history of eating or seizure disorders, strong propensity for motion sickness, were pregnant, had uncorrected poor vision or hearing, or were employees of the National Human Genome Research Institute.
Mothers were selected because of the influence they exert over their children’s eating. Overweight and obese mothers were targeted to ensure that the index child had at least one biological parent on which to base a high risk message. The selection of a narrow age range for the index child was to enhance the credibility of the experimental scenario in which mothers would be instructed to fill a plate for a young child who presumably would be unable to serve his or her own food.
Mothers were recruited from the local Washington DC metropolitan area, using flyers posted or distributed in schools, day cares, and related locations, postings on web forums, listservs, and through word-of-mouth. Mothers were compensated $100 for their participation
Design
Mothers were randomly assigned to one of three experimental arms: Arm 1, Food safety (Control); Arm 2, Behavioral risk information alone (BRI); or Arm 3, Behavioral risk information plus personal FHH-based obesity risk assessment (BRI+FHH). Random assignment was blocked on the index child’s BMI status (underweight or average weight, versus overweight, or obese). The index child did not directly take part in any research activities.
Procedures
As depicted in Figure 1, prospective participants were screened for eligibility over the telephone by a member of the study team. During the screening call, participants who were deemed eligible were asked to identify an index son or daughter (using inclusion criteria described above), and to refer to this child in answering questions and completing study tasks.
Figure 1:
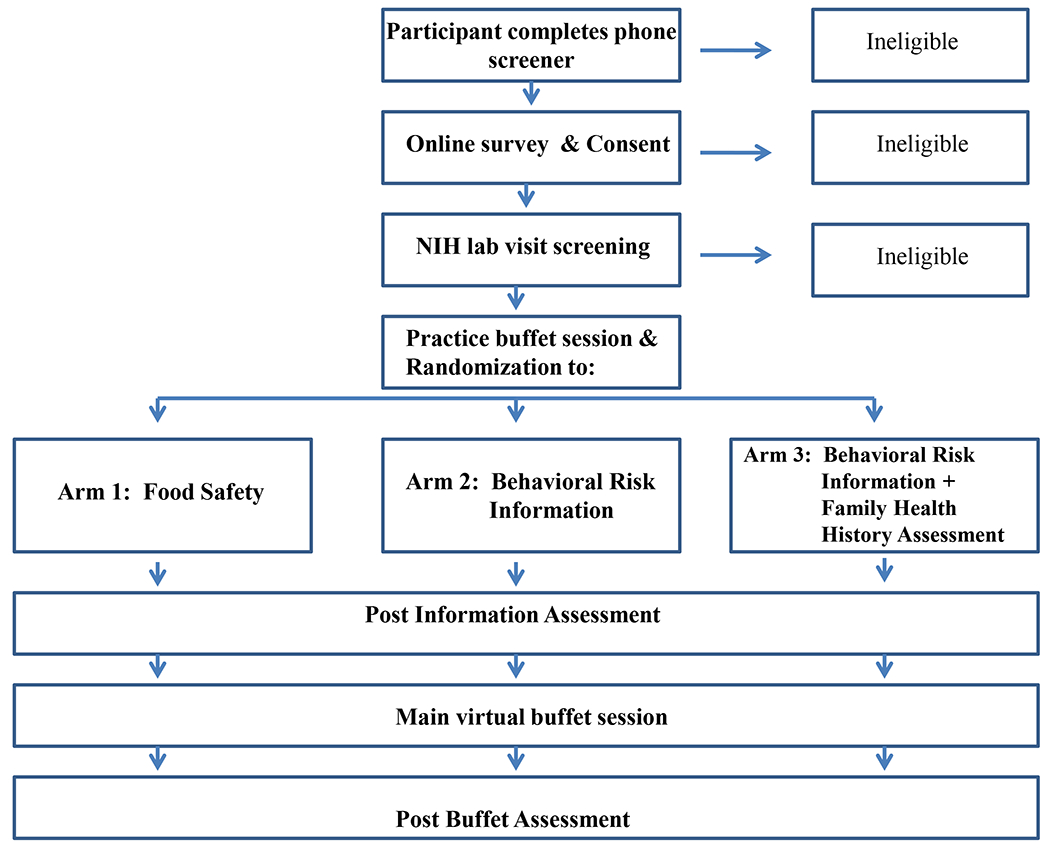
Study flow diagram
Eligible mothers were sent login information via email and asked to visit an encrypted study website to give consent and complete pre-test questionnaires. The pretest questionnaires assessed the child’s height and weight, perceptions of the index child’s risk for becoming obese, and mothers’ beliefs about causes of obesity. Following completion of the online questionnaires, participants were scheduled to come to the NIH Clinical Center to complete the experimental phase of the study.
The in-person portion of the study took approximately 90 minutes. Mothers were told that the study was exploring the impact of health information on choices that mothers make about food for their children, and that using a virtual reality model of a buffet enabled a naturalistic research setting.
Mothers were first introduced to the virtual buffet scenario for a practice session. In this session, mothers were given only one food and beverage option (i.e., Spaghetti-os in tomato sauce and fruit punch). Mothers were instructed to choose as much virtual food and drink during one trip to the buffet as they would normally choose for a lunch for their child at a buffet-style restaurant. This practice session provided a baseline assessment to evaluate individuals’ inclinations towards portion sizes prior to provision of risk information. After completing the practice session, mothers viewed an arm-specific computer-based informational module and completed a post-information questionnaire.
Mothers then entered the virtual buffet environment a second time to complete the main task. Mothers were instructed that the new task was to create a lunch meal for their index child filling a plate with choices from a new and larger set of food options (described below). Mothers could choose as many and as much of the virtual food and beverages as they wanted during one trip to the buffet. They were instructed to choose food they thought their child would eat. Mothers also were able to choose from several possible beverages and condiments. Mothers used a pointing device to select the food for the plate (i.e., each click selected one spoonful, or one piece), the condiments, and beverage. After completing the virtual buffet scenario, mothers completed post-test questionnaires and were debriefed about the purpose of the study. They also had the option to take resources for further information about childhood obesity risk and the importance of FHH.
Information Modules
The information modules provided in each of the experimental arms were developed at a 5th grade reading level. An overview of the content of the sessions and corresponding number of informational slides is provided in Table 1. Briefly, the Control module included causes of food borne illness and provided specific tips for enhancing food safety for children. The BRI module focused on the role of lifestyle in the development of obesity, the health risks associated with obesity, and specific tips on how to develop healthy eating and exercise patterns. The BRI+FHH duplicated the BRI module content and included additional information about the role heredity plays in obesity risk and the mechanisms through which genes might influence obesity. Mothers in the BRI+FHH arm were provided with a population level absolute and relative risk of the index child’s likelihood of becoming an obese adult based on whether the child had one or two overweight/obese parents. Information about parents’ weight status was reported by mothers and assessed in the study screening and the online pre-test questionnaire. Mothers assigned to this condition were presented with icon arrays28 showing the numbers of children who would become obese based on the level of risk indicated by the index child’s FHH of obesity. The FHH risk estimate was drawn from figures reported by Magarey and colleagues15 indicating that with: 0 overweight/obese parents yielded 13% chance, 1 parent – 28% chance, both parents - 58% chance of being obese. Based on these estimates, an icon array (see Figure 2) was constructed to illustrate that a child who had one overweight/obese parent was at approximately double the risk of becoming overweight/obese compared to a child with no overweight/obese parents; a child with two overweight/obese parents was at quadruple the risk compared to a child with no overweight/obese parents.
Table 1:
Overview of educational content of modules by experimental arm
| Experimental Arm | Number of slides | Module Content |
|---|---|---|
|
Food safety (Control) |
40 |
Prevalence of food borne illnesses in children Steps to reduce risk of food contamination Interactions with health care providers for food poisoning in children |
|
Behavioral risk information (BRI) |
19 |
Trends in obesity and importance of prevention* Defining healthy BMI* Health behaviors associated with obesity and risk reduction* Mothers as role models and for promoting healthy food choices and activity* |
|
Behavioral risk information + family health history risk assessment (BRI+FHH) |
39 |
Hereditary nature of obesity (evidence from twin studies) Traits affected by genes (e.g., metabolism, eating in the absence of hunger) High risk is not destiny -- lifestyle changes can lower risk |
Content also included in the Family History Risk Assessment Arm
Figure 2:

Family History Risk Information
Immersive Virtual Environment Technology and the Virtual Buffet
The virtual buffet scenario was staged in a digital immersive virtual environment (IVE). Mothers donned a head-mounted display to be immersed in a three-dimensional digital restaurant buffet environment (see Figure 3). While in the virtual environment, mothers could interact with the elements of a buffet restaurant using natural physical body movements (e.g., walking, and pointing). Previous research has shown IVE simulations to be very compelling and to elicit realistic psychological responses.29 Using the virtual buffet scenario offers several advantages over commonly used hypothetical vignettes or food photographs. IVE use enabled direct observation of mothers’ behaviors as well as detailed assessments of food portioning.
Figure 3:
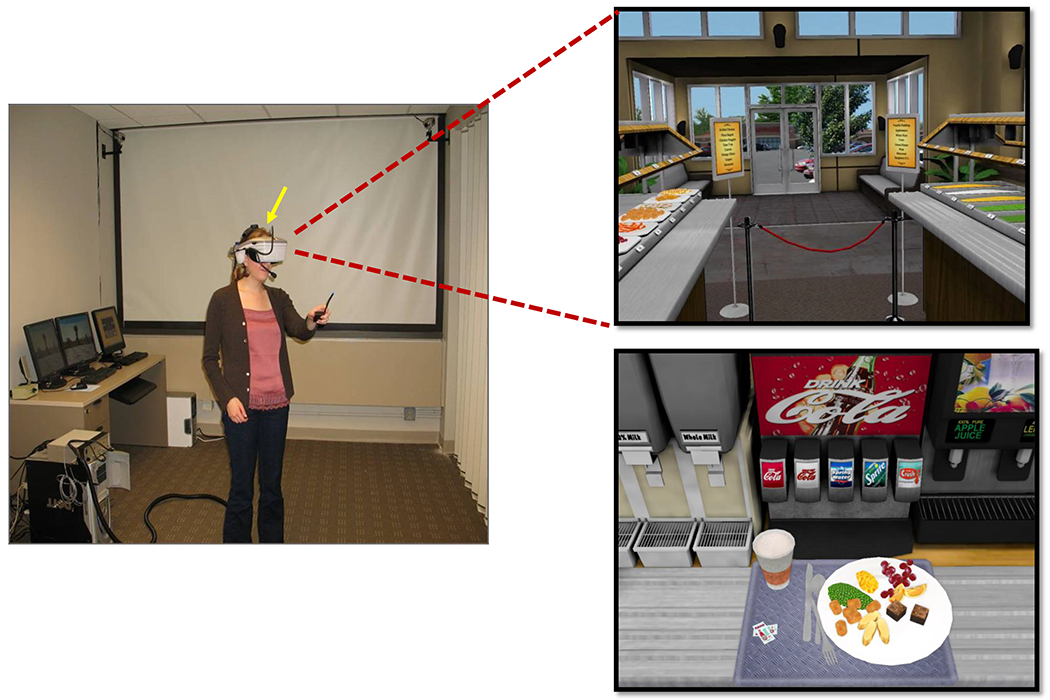
Virtual Restaurant Buffet
Foods included in the buffet were selected to meet the following criteria: (1) palatable to children; (2) range of nutrient and calorie density; and (3) readily translated into calories by volume or weight. The buffet contained at least two options for each food category that would typically be present at lunch (main dish, vegetable, fruit, starch, dessert, condiments, and beverages). To ensure a range of nutrient and calorie density, we based food choices on NHLBI’s evidence-based heuristic developed for the Coordinated Approach to Child Health study. This heuristic defines three categories of foods: “Go” foods, “Slow” foods, and “Whoa” foods using estimated calorie requirements to recommend how much of these foods to eat to maintain energy balance.30 Foods included in the buffet were: grilled chicken strips, macaroni and cheese, cheese pizza bagels, breaded chicken nuggets, peas, steamed baby carrots, green beans, corn, white rice, tater tots, orange slices, grapes, apple sauce, brownies, and vanilla pudding. Drinks included were: water, diet and regular soda, milk (skim, 2% and whole), lemonade, 100% apple juice, and 100% orange juice. Condiments included were: ketchup, BBQ sauce, and butter.
Behavioral Outcomes: Calculation of Calories from Virtual Food
Calorie content of the food selected for the plate was calculated based on the cubic volume of food chosen. The number of calories associated with food volume was determined using information contained in USDA food nutrient databases and on food packaging for brand-name items (e.g., tater tots). Additionally, we created variables for: grams of fat in foods chosen; number of processed foods selected, selecting dessert (yes versus no), or selecting a sweetened versus unsweetened beverage.
Self-Report Measures
We assessed three subscales of the Child Feeding Questionnaire, a validated instrument created by Birch and colleagues31 prior to the buffet experience. The subscales assessed mothers’ perceived responsibility for feeding the index child (e.g., How often are you responsible for deciding what your child’s portion sizes are?), her attitudes about restricting the index child’s food intake (e.g., I have to be sure that my child does not eat too many sweets) and her concern about the child’s weight (e.g., How concerned are you about your child eating too much when you are not around?). Each subscale had good reliability in this sample (respectively, Cronbach’s α=.88, .73, .75). As part of the post-buffet survey, questions were included to assess mothers’ experience of the virtual buffet including the extent to which she felt involved in the world (rated on 5-point scale – not at all to extremely), how realistic the buffet seemed, how difficult it was to use the pointing device to select serving sizes, and whether the food on the buffet was the same kind of food their child typically ate (all rated on a 7-point scale – not at all to completely).
Data Analysis
Socio-demographics and attitudes towards food restriction were cross-tabulated by experimental arm to verify that randomization balanced the groups on factors associated with study outcomes. Differences between experimental arms were tested using analysis of covariance for continuous variables and logistic regression for categorical variables.
We compared plated calories, fat content, processed foods, dessert choice and sweetened beverage choice for each of the three experimental arms to the others using planned contrasts. These analyses controlled for the index child’s current weight status, the variable on which randomization was blocked, and the plated calories from the practice session to reduce variability in the dependent variable. We also included mother’s BMI, and child’s gender, as additional covariates in the models.
RESULTS
Characteristics of Mothers and Index Children by Experimental Arm
A total of 281 mothers completed the pre-test survey. Of these, 232 completed the virtual buffet activity. Eleven mothers who participated in piloting the BRI+FHH arm were omitted from the analyses. The final analyses included 221 mothers. There were no differences in mothers’ demographic characteristics by arm. Mothers were on average 38 years old with a BMI of 30; 75% were college educated. Forty-five percent were white and 76% had more than one child; 62% reported that the index child had two overweight/obese parents. Mothers tended to report moderate restriction of their child’s eating habits (Mean = 3.55; SD = 0.76 on a 5 point scale). Mothers also perceived FHH to be an important contributor to obesity (Mean = 6.3; SD = 1.07 on a 7 point scale). Similarly, there were no differences by arm in the characteristics of the index child. The index child had an average BMI of 17, 38% were overweight/obese and 45% were male.
Immersion in the Virtual Buffet
Mothers rated themselves to be highly involved in the virtual buffet scenario (Mean 4.12 on a 5-point scale, SD = 0.88), and viewed the virtual buffet as highly realistic (Mean = 5.5 on a 7-point scale; SD = 1.41). These mothers also reported they were able to select the amount of food they intended (Mean = 6.3 on a 7 point scale, SD = 1.08). There were no differences by arm in the level of perceived realism of the world or the ease of portioning foods for the plate.
Food Choices by Experimental Arm
As shown in Table 2, multivariate analyses partially support our initial hypotheses for plated calories. Mothers in the BRI+FHH arm filled the index child’s plate with 45 fewer calories than those in the Control arm (p<0.05). Mothers who were randomized to the BRI arm filled their child’s plate with an average of 35 fewer calories than mothers randomized to the Control arm. However, this difference was not statistically significant. There were no differences by experimental arm in grams of fat, selection of processed foods, desserts or sweetened beverages (results not shown).
Table 2:
Total calories on the plate by experimental arm adjusting for key moderators
| Independent Variables | Estimate | SE |
|---|---|---|
| Intercept | 248.73 ** | 88.83 |
| Practice session calories | 0.67 *** | 0.20 |
| Mother’s age | −2.68 | 1.61 |
| Mother is white | −34.65 | 18.22 |
| Mother’s BMI | 2.75 | 1.80 |
| Behavioral Risk Information Arm | −35.48 | 21.10 |
| Behavioral Risk Information + Family Health History Assessment Arm | −45.26 * | 21.19 |
| Index child is overweight | 15.19 | 17.80 |
| Index child is male | 35.72 * | 17.32 |
| Family has one child only | 0.48 | 20.13 |
p≤0.05,
p≤0.01,
p≤0.001
Effect of Risk Message Dose on Plated Calories
Multivariate analyses adjusting for covariates showed that among the BRI+FHH arm, restriction of calories was most pronounced among mothers who received the weaker risk message (based on having one biological parent who was overweight/obese) as compared to those receiving the stronger risk message (based on two overweight/obese parents). Mothers receiving the risk message based on one versus two overweight/obese parents averaged 78 and 35 fewer calories fewer than the Control arm, respectively. None of the other covariates in the model were significant predictors of plated calories among mothers in the BRI+FHH arm.
We also explored whether this calorie differential was specific to the BRI+FHH arm or might be an artifact of having two overweight/obese biological parents. The arm by number of overweight parents interaction was not significant (p=0.27). We considered whether this effect was specific to plated calories or present for the selection of a sweetened beverage (yes versus no). The significant association of increased plated calories and sweetened beverages with having two overweight/obese biological parents was only observed in the BRI+FHH arm (See Table 3).
Table 3:
Association of number of overweight parents with total calories and choice of a sweetened beverage by experimental arm
|
Outcome |
Food Safety Control | Behavioral Risk Information BRI | Beh Risk Information + Family Health Hx Assessment BRI+FHH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of overweight biological parents | Number of overweight biological parents | Number of overweight biological parents | |||||||
| One | Two |
p-value |
One | Two |
p-value |
One | Two |
p-value |
|
| n=31 | n=42 | n=29 | n=43 | n=22 | N=53 | ||||
| Mean total calories | 372.20 | 406.52 | 0.275 | 359.50 | 368.49 | 0.784 | 286.9 | 360.9 | 0.051 |
| Percent chose a sweetened beverage |
45.2 |
47.6 |
0.835 |
48.3 |
51.2 |
0.810 |
13.6 |
37.7 |
0.039 |
DISCUSSION
Providing FHH-based risk assessment to mothers about their young child was associated with greater calorie restriction, but only when the risk information was based on only the mothers’ overweight status. Mothers who were told that their child was at four-fold increased risk based on having two overweight/obese parents did not engage in calorie restriction. Thus, our hypotheses were only partially confirmed. According to our conceptual rationale, the stronger risk message associated with having two overweight/obese parents should have engaged more systematic processing by mothers and induced greater communal coping, thereby increasing mothers’ effort to reduce the child’s risk. It is possible that receiving feedback of such a substantial increase in risk associated with having two overweight/obese parents overwhelmed mothers’ perceptions that her actions would reduce the child’s risk. Mothers may have felt that any efforts they took to restrict eating would either be insufficient to reduce their child’s risk or would be undermined by the actions of the biological father. Frosch and colleagues.18 found that undergraduates who were asked to imagine receiving feedback of being at high risk of becoming overweight based on a genetic test perceived significantly less behavioral control than those who were told they were at average risk.18 Unfortunately, we cannot evaluate the extent to which this occurred as we did not assess pre-to post-test changes in self- or response efficacy.
In contrast, mothers who were solely responsible for their child’s increased obesity risk did behave as we hypothesized. It appears that framing the child’s risk as a direct result of mother’s weight status and the implicit responsibility conveyed in this information prompted communal coping efforts in the form of calorie restriction. This outcome also may relate to the effects of the intervention message on mothers’ feelings of guilt or self-blame that will be explored in a future report.
Taken together, these results suggest that some caution should be exercised in providing parents with children’s risk feedback based on FHH assessments. For many common health conditions such as heart disease and Type 2 diabetes, having a related FHH will convey sizable increases in risk.32, 33 Thus, these risk communications may be perceived to hold high threat.10 As such, FHH-based risk communications likely will best be coupled with efforts to increase parent’s perceptions that efforts can be undertaken to reduce risk, and that these efforts will be effective. Additionally, parents will also need to have the requisite behavioral skills to reduce their child’s risk. Consideration of risk communication approaches also will be important for conveying FHH generally, that is, even when the child is not the focus. FHH risk is unique in that it conveys risk information that relates to more than one family member and as such likely will need to consider broader communication within the family system to effectively reduce risk.12
Compared to those in the Control group, the calorie restriction observed among mothers who received the weaker FHH-risk message for the child, where the intervention was most influential, was on average about 78 calories. This level of restriction could be significant in lowering a child’s risk of overweight and obesity. Indeed, among young children, the ‘energy gap’ (i.e., imbalance between energy intake and expenditure) that promotes childhood obesity may be as small as ~30 calories/day.34 It is noteworthy that the average BMI of 17 for the index children was at the 85th percentile of age-recommended BMI, and thus associated with moderate risk of becoming obese.35 Therefore, calorie restriction for these children in particular could have been advantageous. Whether, alternatively, this level of restriction could be harmful is unclear. However, mothers did not limit fat content, avoid desserts or avoid processed foods. These behaviors would be indicative of labeling some foods to be ‘off limits’ which could backfire and make these foods more attractive to children.
As in any study, there were limitations that should be considered in interpreting these results. First, the sample was relatively well-educated. The sample size for the subgroup analyses was limited. However, results of this study suggest that FHH-based risk messages should be evaluated for their potential to add value to weight gain prevention interventions for young families.
REFERENCES
- 1.Ogden C, Carroll M, Curtin L, McDowell M, Tabak C, Flegal K. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA: The Journal of the American Medical Association 2006; 295(13): 1549–1555. [DOI] [PubMed] [Google Scholar]
- 2.Nader P, O’Brien M, Houts R, Bradley R, Belsky J, Crosnoe R et al. Identifying risk for obesity in early childhood. Pediatrics 2006; 118(3): e594–601. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Bann C, Das A, Lester B, Bada H, Bauer C et al. Risk for obesity in adolescence starts in early childhood. Journal of Perinatology 2011; 31(11): 711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kral T, Faith M. Influences on child eating and weight development from a behavioral genetics perspective. Journal of Pediatric Psychology 2009; 34(6): 596–605. [DOI] [PubMed] [Google Scholar]
- 5.Davison K, Francis L, Birch L. Links between parents’ and girls’ television viewing behaviors: a longitudinal examination. Journal of Pediatrics. 2005; 147(4): 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marti A, Martinez-Gonzalez M, Martinez J. Interaction between genes and lifestyle factors on obesity. 2008; 67(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 7.Krahnstoever Davison K, Francis LA, Birch LL. Reexamining obesigenic families: parents’ obesity-related behaviors predict girls’ change in BMI. Obesity Research 2005; 13(11): 1980–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green E, Guyer M. Charting a course for genomic medicine from base pairs to bedside. Nature 2011; 470(7333): 204–13. [DOI] [PubMed] [Google Scholar]
- 9.Griffin R, Dunwoody S, Neuwirth K. Proposed model of the relationship of risk information seeking and processing to the development of preventive behaviors. Environmental Research 1999; 80(2 Pt 2): S230–S245. [DOI] [PubMed] [Google Scholar]
- 10.Etchegary H, Perrier C. Information processing in the context of genetic risk: implications for genetic-risk communication. Journal of Genetic Counseling 2007; 16(4): 419–432. [DOI] [PubMed] [Google Scholar]
- 11.Gooding H, Organista K, Burack J, Biesecker B. Genetic susceptibility testing from a stress and coping perspective. Social Science & Medicine 2006; 62(8): 1880–1890. [DOI] [PubMed] [Google Scholar]
- 12.Koehly L, Peters J, Kenen R, Hoskins L, Ersig A, Kuhn N et al. Characteristics of health information gatherers, disseminators, and blockers within families at risk of hereditary cancer: implications for family health communication interventions. American Journal of Public Health 2009; 99(12): 2203–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valdez R, Greenlund K, Khoury M, Yoon P. Is family history a useful tool for detecting children at risk for diabetes and cardiovascular diseases? A public health perspective. Pediatrics 2007; 120(2): S78–86. [DOI] [PubMed] [Google Scholar]
- 14.Whitaker R, Wright J, Pepe M, Seidel K, Dietz W. Predicting obesity in young adulthood from childhood and parental obesity. The New England Journal of Medicine 1997; 337(13): 869–873. [DOI] [PubMed] [Google Scholar]
- 15.Magarey A, Daniels L, Boulton T, Cockington R. Predicting obesity in early adulthood from childhood and parental obesity. International journal of obesity and related metabolic disorders 2003; 27(4): 505–513. [DOI] [PubMed] [Google Scholar]
- 16.Sonneville K, Rifas-Shiman S, Kleinman K, Gortmaker S, Gillman M, Taveras E. Associations of obesogenic behaviors in mothers and obese children participating in a randomized trial. Obesity 2012; 20(7): 1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davison K, Birch L. Childhood overweight: a contextual model and recommendations for future research. Obesity Reviews 2001; 2(3): 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frosch D, Mello P, Lerman C. Behavioral consequences of testing for obesity risk. Cancer Epidemiology, Biomarkers & Prevention 2005; 14(6): 1485–1489. [DOI] [PubMed] [Google Scholar]
- 19.Grimmett C, Croker H, Carnell S, Wardle J. Telling parents their child’s weight status: psychological impact of a weight-screening program. Pediatrics 2008; 122(3): e682–688. [DOI] [PubMed] [Google Scholar]
- 20.Segal M, Sankar P, Reed D. Research issues in genetic testing of adolescents for obesity. Nutrition Reviews 2004; 62(8): 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kral T, Faith M. Child eating patterns and weight regulation: a developmental behaviour genetics framework. Acta Paediatrica - International Journal of Paediatrics & Supplements 2007; 96(454): 29–34. [DOI] [PubMed] [Google Scholar]
- 22.Birch L, Fisher J, Davison K. Learning to overeat: maternal use of restrictive feeding practices promotes girls’ eating in the absence of hunger. The American Journal of Clinical Nutrition 2003; 78(2): 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson J, Case D, Andrews J, Allard S. Genomics--the perfect information-seeking research problem. Journal of Health Communication 2005; 10(4): 323–329. [DOI] [PubMed] [Google Scholar]
- 24.Patenaude A, Demarco T, Peshkin B, Valdimarsdottir H, Garber J, Schneider K et al. Talking to Children About Maternal BRCA1/2 Genetic Test Results: A Qualitative Study of Parental Perceptions and Advice. Journal of Genetic Counseling; e-pub ahead of print 2012. Oct 24 doi: 10.1007/s10897-012-9549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heshka J, Palleschi C, Howley H, Wilson B, Wells P. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genetics in Medicine 2008; 10(1): 19–32. [DOI] [PubMed] [Google Scholar]
- 26.Olney R, Yoon P. Role of family medical history information in pediatric primary care and public health: introduction. Pediatrics 2007; 120(2): S57–59. [DOI] [PubMed] [Google Scholar]
- 27.Tarini B, Singer D, Clark S, Davis M. Parents’ concern about their own and their children’s genetic disease risk: potential effects of family history vs genetic test results. Archives of Pediatrics & Adolescent Medicine 2008; 162(11): 1079–1083. [DOI] [PubMed] [Google Scholar]
- 28.Ancker J, Senathirajah Y, Kukafka R, Starren J. Design features of graphs in health risk communication: a systematic review. Journal of the American Medical Informatics Association 2006; 13(6): 608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailenson J, Blascovich J, Beall A, Loomis J. Interpersonal distance in immersive virtual environments. Personality and Social Psychology Bulletin 2003; 29(7): 819–833. [DOI] [PubMed] [Google Scholar]
- 30.National Heart, Lung and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Cancer Institute. We Can! Ways to Enhance Children’s Activity & Nutrition. Accessed November 6, 2012. (http://www.nhlbi.nih.gov/health/public/heart/obesity/wecan/downloads/go-slow-whoa.pdf)
- 31.Birch L, Fisher J, Grimm-Thomas K, Markey C, Sawyer R, Johnson S. Confirmatory factor analysis of the Child Feeding Questionnaire: a measure of parental attitudes, beliefs and practices about child feeding and obesity proneness. Appetite 2001; 36(3): 201–210. [DOI] [PubMed] [Google Scholar]
- 32.Shear C, Webber L, Freedman D, Srinivasan S, Berenson G. The relationship between parental history of vascular disease and cardiovascular disease risk factors in children: the Bogalusa Heart Study. American Journal of Epidemiology 1985; 122(5): 762–771. [DOI] [PubMed] [Google Scholar]
- 33.Muhonen L, Burns T, Nelson R, Lauer R. Coronary risk factors in adolescents related to their knowledge of familial coronary heart disease and hypercholesterolemia: the Muscatine Study. Pediatrics 1994; 93(3): 444–451. [PubMed] [Google Scholar]
- 34.Goran M. Metabolic precursors and effects of obesity in children: a decade of progress, 1990-1999. The American Journal of Clinical Nutrition 2001; 73(2): 158–171. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Division of Nutrition PA, and Obesity, National Center for Chronic Disease Prevention and Health Promotion. Healthy Weight - it’s not a diet, it’s a lifestyle! In. Atlanta, Georgia, 2011. [Google Scholar]


