Abstract
Context
Active surveillance of small renal masses highlights the need for accurate prognostication of biopsies.
Objective
To comprehensively evaluate the accuracy of biopsies in assessing known prognostic parameters including histologic subtype by comparison with subsequent nephrectomy samples.
Design
We retrospectively identified patients at UT Southwestern Medical Center, Dallas, Texas, who had a biopsy for a renal mass between 2004–2018. Biopsy samples were evaluated for known prognostic factors such as tumor grade, necrosis, sarcomatoid/rhabdoid change, and BAP1 status, which we previously showed is an independent prognostic factor for clear cell renal cell carcinoma. Accuracy was determined by comparison with subsequent analyses of nephrectomy specimens. Statistical analyses were performed to assess biopsy accuracy and correlation with tumor size and pathologic stage.
Results
From 805 biopsies with a diagnosis of renal neoplasm, 178 had subsequent resection of the biopsied tumor. Concordance rate for histologic subtype was 96.9% (kappa [w] 0.90; 95% CI 0.82–0.99) and excellent for small renal masses (98.8%; kappa [w] 0.97; 95% CI 0.90–1). Amongst the prognostic variables evaluated, BAP1 immunohistochemistry in clear cell RCC had the highest agreement (94.8%; kappa [w] 0.83; 95% CI 0.66–0.99). The presence of one or more aggressive features (grade 3–4, tumor necrosis, BAP1 loss, sarcomatoid/rhabdoid change) in a biopsy significantly correlated with pT stage (P=.004).
Conclusions
Biopsy analyses showed high accuracy for subtyping renal tumors, but it underestimated several poor prognostic features. Addition of BAP1 for clear cell RCC may increase prognostic accuracy. If validated, routine incorporation of BAP1 immunohistochemistry in clear cell RCC biopsies may refine prognosis and aid in the selection of patients for active surveillance.
Keywords: RCC, Renal cell carcinoma, Biopsy, concordance, nephrectomy, histologic subtypes, BAP1
INTRODUCTION
The incidence of kidney cancer continues to increase1. Many of these tumors are small masses discovered incidentally due to increasing use of cross-sectional imaging2–4. Renal cell carcinoma (RCC) is the most common type of kidney cancer, with clear cell RCC (CCRCC) being the most frequent subtype (75–85%)5. Although the incidence of RCC, particularly of small renal masses (SRMs; <4 cm), is increasing, mortality trends have remained stable6. Conservative management with active surveillance is increasingly being considered7,8. The current European Association of Urology (EAU) guidelines recommend renal mass biopsy for active surveillance9. Biopsies are generally safe, have a high diagnostic yield, and may inform patient management by reducing unnecessary surgeries10,11. The National Comprehensive Cancer Network (NCCN) similarly recommends needle biopsies for SRMs to establish a diagnosis of RCC and guide management including active surveillance and ablation strategies12.
Several histopathological characteristics are associated with outcomes in patients with RCC, including histologic subtype, tumor size, pathologic stage, nucleolar grade, tumor necrosis, as well as rhabdoid and sarcomatoid change13–16. These features have also been shown to correlate with worse prognosis in SRMs17. However, these studies are largely based on histologic analyses of resection specimens. Prognostic parameters on biopsies could help in risk stratification and management especially for SRMs. However, significant intratumoral heterogeneity in CCRCC may pose a challenge18,19. While the subtypes of RCC can be accurately determined on renal mass biopsy20–22, the detection of the other prognostic parameters by core biopsy is not well understood.
Herein, we sought to determine the accuracy of renal mass biopsy for the evaluation of prognostic factors by comparison to subsequent nephrectomies. We evaluated histologic subtype, grade, tumor necrosis, as well as sarcomatoid and rhabdoid changes. In addition, we assessed BRCA1-associated protein-1 (BAP1) status, a tumor suppressor protein of prognostic significance in CCRCC18,23. We and others have shown that BAP1 is mutated in ~15% of CCRCCs, where it results in loss of the protein24. BAP1-deficient tumors tend to be of high grade and are associated with 3-fold lower survival rates24–27. Moreover, recent analyses from the IMmotion151 phase 3 trial show that BAP1-mutated RCCs are enriched in CCRCCs with preferential responsiveness to immune checkpoint inhibitor containing regimen28,29. As mutations of BAP1 typically result in loss of the protein24, we developed an immunohistochemical (IHC) assay and showed that it was highly concordant with the mutation status24. Using this assay, we previously showed that BAP1 predicts outcome independently of known prognostic factors (including UCLA Integrated Staging System variables) and may have implications in the management of patients with SRMs26. Recognizing that intratumoral heterogeneity may undermine prognostication accuracy of traditional parameters such as tumor grade, necrosis and sarcomatoid/ rhabdoid changes, which may be present focally in more advanced subclones, we considered whether BAP1, which is a relatively proximal mutational truncal event in CCRCC19, could be useful18,23. In this study we explore, for the first time, the utility of BAP1 status on biopsies of CCRCC.
MATERIALS AND METHODS
After approval from the institutional review board, we retrospectively identified patients who had a renal mass cytology and subsequent nephrectomy of the biopsied tumor at the University of Texas Southwestern Medical Center, Dallas, Texas (UTSW), Kidney Cancer Program between January 2004 and Dec 2018. Renal mass biopsy (RMB) based on our institution guidelines, are routinely performed under computed tomography or ultrasound guidance with at least 2 passes taken per lesion depending upon the adequacy assessment by an onsite cytopathologist. This rapid onsite adequacy assessment is done using a touch imprint smear that is air-dried and stained with Diff-Quick (Romanowsky) stain and assessed for adequacy based on cellularity and presence of lesional material compatible with the clinical/radiologic findings. On rare events when a biopsy is not possible, fine needle aspirates (FNAs) are performed and they were included in our study. Cases with non-RCC diagnoses, apart from oncocytoma, were excluded from this analysis. In cases with multiple tumor subtypes in nephrectomy specimens, we focused on tumors that were biopsied based on the radiological location of the needle.
Pathologic parameters were recorded from the original pathology reports for RMB and nephrectomy. During the span of 15 years, consensus guidelines for histologic subtypes, availability and use of immunohistochemical (IHC) assays to assist in tumor characterization, and grading guidelines have evolved. For this study, tumors were subtyped according to World Health Organization (WHO) classification in vigor at the time30,31, and they were graded using the Fuhrman or WHO grading systems31,32. This study includes also cases prior to surgical pathology subspecialization in 2012 at our institution. Subspecialized practice led to increased attempt at rendering a subtype diagnosis and reporting prognostic variables on RMB. The current diagnostic and IHC approach for histologic subclassification of renal masses is shown in Figure 1–2. Oncocytic tumors on biopsies are diagnosed as “low grade renal oncocytic neoplasm” and may be favored to be oncocytoma or eosinophilic variant of chromophobe RCC. For the purpose of this study, the favored diagnosis was considered when stated.
Figure 1.
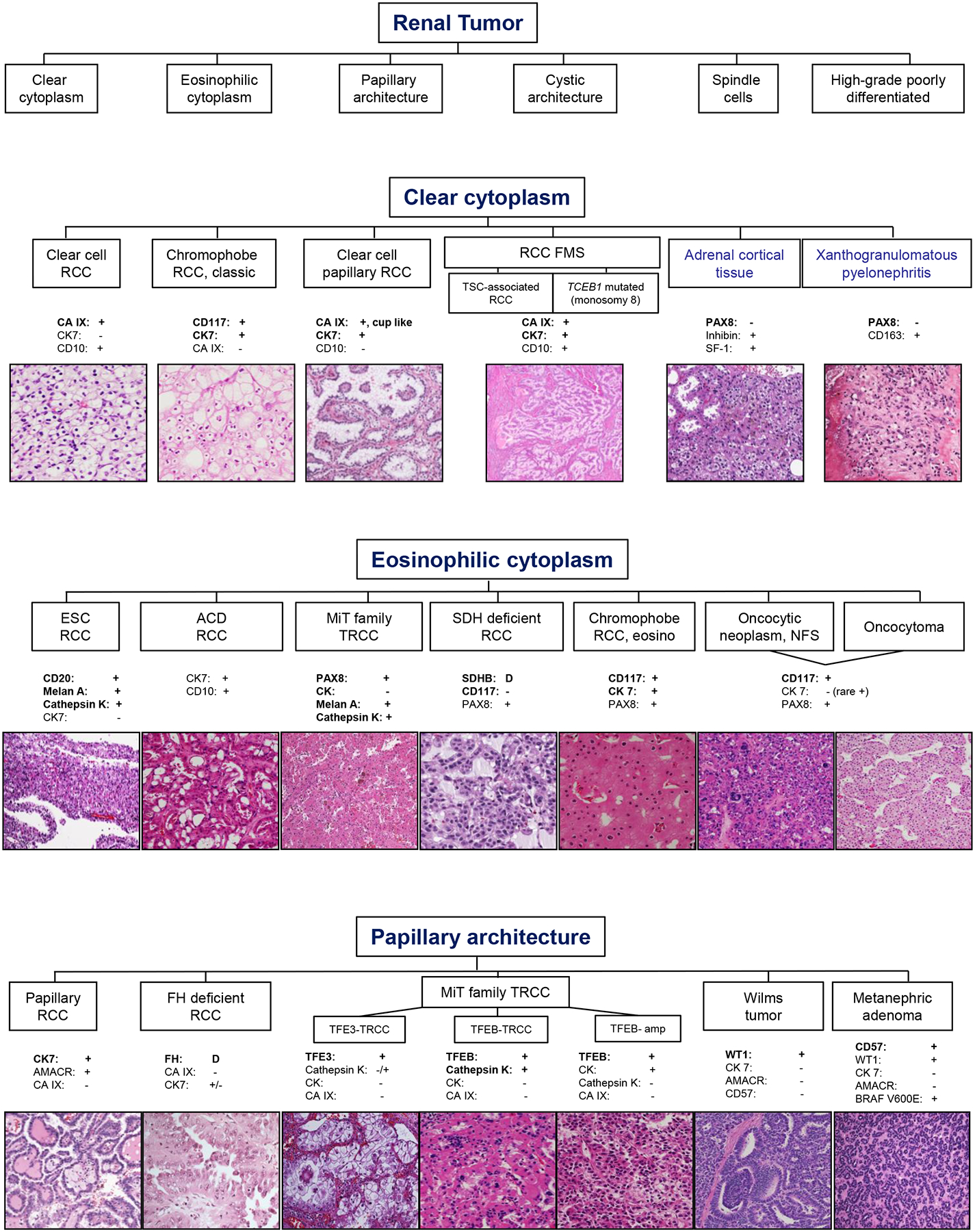
Diagnostic algorithm based on morphological features of renal tumors and supportive immunohistochemical (IHC) stains. Representative hematoxylin & eosin (H&E) images are shown (magnification range from 40x-200x). Photomicrographs for SDH-deficient RCC and ALK translocation RCC are provided by Liwei Jia, MD1. Abbreviations used: RCC=Renal cell carcinoma; FMS=Fibromyomatous stroma; TSC=Tuberous sclerosis complex; TCEB1=Transcription elongation factor B polypeptide 1 gene; CA IX=Carbonic anhydrase IX; CK=Cytokeratin; PAX8=Paired box gene 8; SF-1=Steroidogenic factor 1; ESC=Eosinophilic, solid and cystic; ACD=Acquired cystic disease; MiT= Microphthalmia Transcription Factor; TRCC=Translocation RCC; SDH=Succinate dehydrogenase; D=deficient/loss; eosino=eosinophilic variant; NFS=not further subtyped; FH=Fumarate hydratase; amp=amplification.
Figure 2.
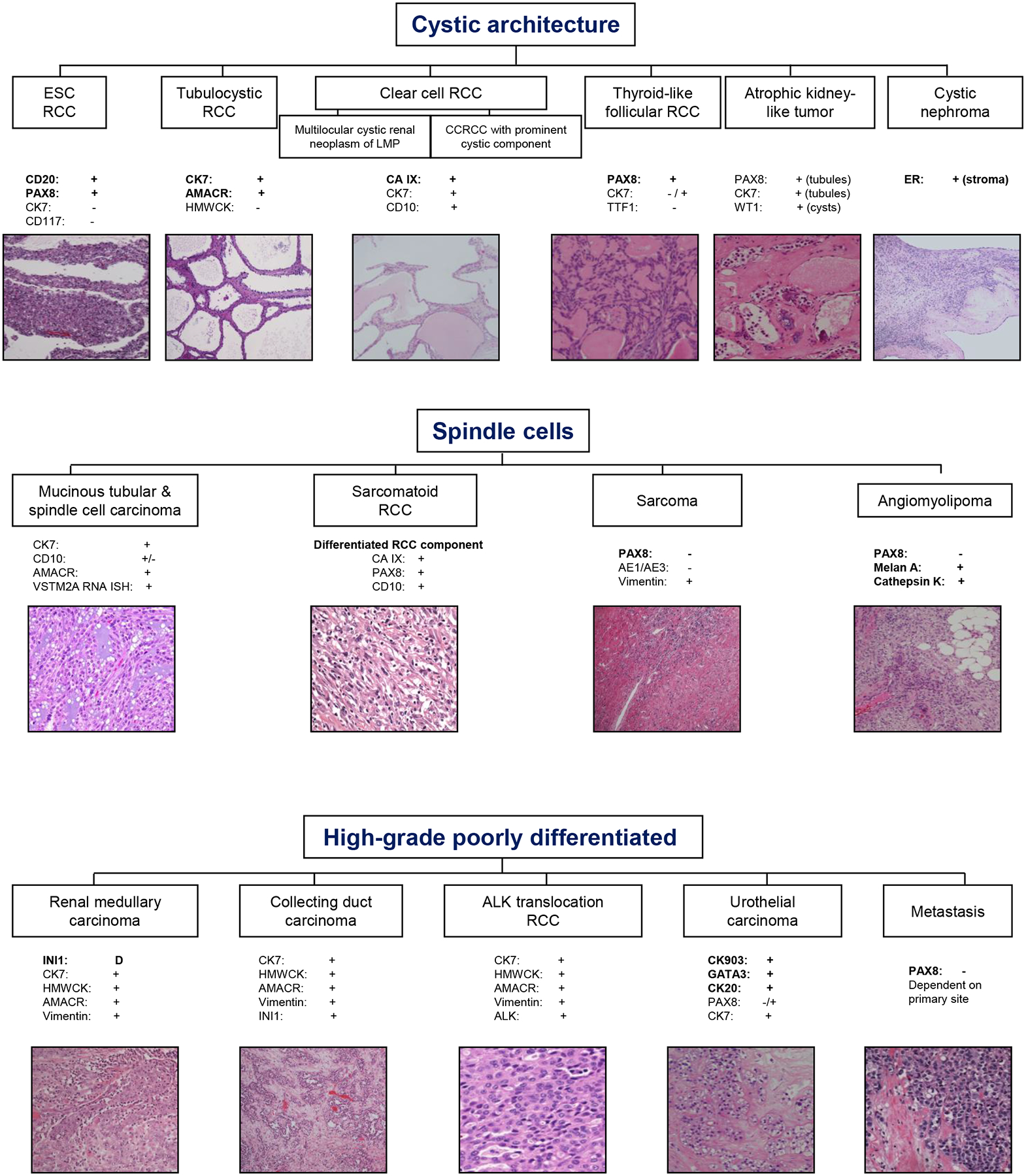
Continuation of diagnostic algorithm based on morphological features of renal tumors and supportive immunohistochemical (IHC) stains. Representative hematoxylin & eosin (H&E) images are shown (magnification range from 40x-200x). Abbreviations used: ESC=Eosinophilic, solid and cystic; RCC=Renal cell carcinoma; LMP=Low malignant potential; CC=Clear cell; CK=Cytokeratin; PAX8=Paired box gene 8; HMW=High molecular weight; AMACR=alpha-methylacyl-CoA racemase; CA IX=Carbonic anhydrase IX; TTF1=Thyroid transcription factor 1; D=deficient/loss of expression; RNA-ISH=RNA in situ hybridization; INI1=Integrase interactor 1 (or BAF47); ALK=Anaplastic lymphoma kinase; ER=estrogen receptor.
BAP1 IHC (C-4, sc-28383; 1:90 dilution; heat induced Tris-based epitope retrieval) is routinely performed at our Clinical Laboratory Improvement Amendment (CLIA)-certified clinical laboratory as described previously using Benchmark XT automated stainer (Ventana)24. For CCRCC cases where BAP1 was not originally reported, BAP1 IHC was performed and evaluated on available samples. BAP1 was positive (retained, wild-type) when there was nuclear reactivity and in each tumor section endothelial cells, lymphocytes, stromal fibroblasts and normal renal parenchyma served as internal positive control.
Detection and reporting of prognostic parameters, including histologic subtype, grade, tumor necrosis, rhabdoid change, sarcomatoid change, and BAP1 immunoexpression were compared between preoperative biopsies and resection specimens. The final pathology at nephrectomy was considered to represent the ground-truth diagnosis in all cases. Cases with missing data were not included for concordance assessment. Cases with discordant histologic subtypes were reviewed to identify factors contributing to misclassification. Additional ancillary studies were performed on these cases as needed during review to further characterize the tumors. Considering that sarcomatoid/rhabdoid features are not consistently reported on RMB, we evaluated the corresponding available biopsy specimens for cases with reported sarcomatoid/rhabdoid change on nephrectomy.
Data were summarized using frequencies and percentages for categorical variables and standard deviations, and ranges for continuous variables. Chi-square tests were used to test for associations between categorical parameters, while Student’s t-test (for two groups) or ANOVA (for three or more groups) were used to test for differences in continuous measures of clinicopathologic features. Kappa and weighted kappa were used for concordance assessment, rank biserial coefficient and Phi coefficient were calculated for correlation analyses, and the method of receiver operating characteristic (ROC) curve was used for biomarker studies. All P values were 2-sided, and P values <.05 were considered statistically significant. Statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
During a period of 15 years, 875 RMBs/FNAs were performed of which, 805 (92.0%) yielded a diagnosis of a neoplasm and 70 (8.0%) were non-diagnostic. Six hundred and forty-three were diagnosed as RCC or oncocytic neoplasm. Of those, 178 cases (27.7%) had a subsequent nephrectomy, including 92SRMs (169 RMBs and 9 FNAs) for the same mass. All 9 FNAs had adequate tissue and in 8 samples a histologic subtype was rendered (hereafter referred together with RMB). The current diagnostic approach for histologic subclassification of renal masses is shown in Figure 1–2.
Table 1 describes the 178 patient and tumor characteristics at nephrectomy. As expected, the most common histologic subtype was CCRCC followed by papillary RCC (PRCC), and chromophobe ChRCC. Other tumor subtypes included renal oncocytoma (RO), clear cell papillary RCC (CCPRCC), MiT family translocation RCC (MiTF TRCC), RCC associated with Tuberous Sclerosis Complex (RCC-TSC), collecting duct carcinoma (CDC) and RCCs that were left unclassified (UnRCC). One case was diagnosed as hybrid oncocytic-chromophobe tumor (HOCT), which for the purpose of this study is considered under ChRCC. More than one renal tumor subtype was found in 4 nephrectomy specimens.
Table 1.
Tumor characteristics on nephrectomy
| Small renal masses (%) (n = 92) | Total (%) (n = 178) | |
|---|---|---|
| Histologic subtype | ||
| CCRCC | 58 (63.0%) | 111 (62.4%) |
| PRCC | 20 (21.7%) | 32 (18.0%) |
| ChRCC* | 8 (8.7%) | 16 (9.0%) |
| RO | 2 (2.2%) | 8 (4.5%) |
| CCPRCC | 2 (2.2%) | 2 (1.1%) |
| MiTF TRCC | 1 (1.1%) | 2 (1.1%) |
| RCC-TSC | 1 (1.1%) | 1 (0.6%) |
| UnRCC | 0 (0%) | 5 (2.8%) |
| CDC | 0 (0%) | 1 (0.6%) |
| pT stage** | (n = 90) | (n = 170) |
| pT1a | 76 (84.4%) | 76 (44.7%) |
| pT1b | - | 30 (17.7%) |
| pT2a | - | 4 (2.4%) |
| pT2b | - | - |
| pT3a | 13 (14.4%) | 53 (31.2%) |
| pT3b | 1 (1.1%) | 3 (1.8%) |
| pT3c | 0 (0%) | 1 (0.6%) |
| pT4 | 0 (0%) | 3 (1.8%) |
| pN stage** | ||
| pN0 | 2 (2.2%) | 16 (9.4%) |
| pN1 | 1 (1.1%) | 13 (7.7%) |
| pNX | 87 (96.7%) | 141 (82.9%) |
| M stage** | ||
| M0 | 89 (98.9%) | 154 (90.6%) |
| M1 | 1 (1.1%) | 16 (9.4%) |
| Stage** | ||
| 1 | 75 (83.3%) | 103 (60.6%) |
| 2 | - | 4 (2.3%) |
| 3 | 14 (15.6%) | 46 (27.1%) |
| 4 | 1 (1.1%) | 17 (10.0%) |
| Grade*** | (n= 82) | (n = 154) |
| 1 | 4 (4.9%) | 4 (2.6%) |
| 2 | 48 (58.5%) | 66 (42.9%) |
| 3 | 28 (34.2%) | 64 (41.6%) |
| 4 | 2 (2.4%) | 20 (13.0%) |
| Sarcomatoid change | (n= 90) | (n = 170) |
| Identified | 1 (1.1%) | 7 (4.1%) |
| Not identified | 89 (98.9%) | 163 (95.9%) |
| Rhabdoid change | (n= 32) | (n = 68) |
| Identified | 1 (3.1%) | 7 (10.3%) |
| Not identified | 31 (96.9%) | 61 (89.7%) |
| Tumor necrosis | (n= 74) | (n = 151) |
| Identified | 23 (31.1%) | 68 (45.0%) |
| Not identified | 51 (68.9%) | 83 (55.0%) |
| CCRCC with BAP1 IHC | (n= 55) | (n = 104) |
| BAP1 loss | 8 (14.6%) | 17 (16.4%) |
| BAP1 retained | 47 (85.4%) | 87 (83.6%) |
n= total number of cases with available data on nephrectomy for each parameter;
including 1 hybrid oncocytic-chromophobe tumor;
excluding RO;
excluding RO and ChRCC.
Abbreviations used: RCC=renal cell carcinoma, CCRCC=clear cell RCC, PRCC=papillary RCC, ChRCC=chromophobe RCC, RO=renal oncocytoma, CCPRCC=clear cell papillary RCC, MiTF TRCC=MiT family translocation RCC, RCC-TSC=RCC associated with Tuberous Sclerosis Complex, UnRCC=unclassified RCC, CDC=collecting duct carcinoma, BAP1=BRCA1-associated protein-1, IHC=immunohistochemistry.
Eight (4.5%) nephrectomy specimens were diagnosed as RO and all were favored to be RO on corresponding RMB (Table 2). One hundred seventy nephrectomies were diagnosed with RCC and included 167 RMBs with RCC and 3 others where a diagnosis of RO was favored; 2 were classified as eosinophilic variant of ChRCC and 1 as HOCT on resection. All 3 biopsies were reported as low-grade renal oncocytic neoplasm of uncertain malignant potential with a comment that RO was favored but that eosinophilic variant of ChRCC could not be excluded based on the limited biopsy material. The biopsy slides were no longer available for review (2 biopsies were performed at outside institutions and slides returned). Review of the nephrectomy slides did not result in change of diagnosis. One of these tumors was SRM.
Table 2.
Concordance of histology and prognostic factors all renal masses
| All renal masses | ||||
|---|---|---|---|---|
| Nephrectomy | Biopsy in agreement (%) | Total agreement (%) | Cohen’s Kappa (95% CI) | |
| RO vs RCC | ||||
| RO | 8 | 8 (100%) | 175 (98.3%) | 0.83 (0.65, 1) |
| RCC | 170 | 167 (98.2%) | ||
| Histologic subtype* | ||||
| CCRCC | 106 | 106 (100%) | 157 (96.9%) | 0.90 (0.82, 0.99)§ |
| PRCC | 27 | 27 (100%) | ||
| ChRCC | 15 | 12 (80.0%) | ||
| RO | 8 | 8 (100%) | ||
| CCPRCC | 1 | 1 (100%) | ||
| CDC | 1 | 1 (100%) | ||
| MiTF TRCC | 1 | 1 (100%) | ||
| UnRCC | 3 | 1 (33.3%) | ||
| Grade** | ||||
| 1 | 4 | 3 (75.0%) | 78 (55.7%) | 0.33 (0.22, 0.44)§ |
| 2 | 63 | 53 (84.1%) | ||
| 3 | 58 | 18 (31.0%) | ||
| 4 | 15 | 4 (26.7%) | ||
| Low grade (1–2) | 67 | 65 (97.0%) | 93 (66.4%) | 0.34 (0.22, 0.47) |
| High grade (3–4) | 73 | 28 (38.4%) | ||
| Sarcomatoid change | ||||
| Identified | 5 | 2 (40.0%) | 73 (96.1%) | 0.55 (0.11, 1) |
| Not identified | 71 | 70 (100%) | ||
| Rhabdoid change | ||||
| Identified | 6 | 2 (33.3%) | 39 (90.7%) | 0.46 (0.04, 0.89) |
| Not identified | 37 | 37 (100%) | ||
| Tumor necrosis | ||||
| Identified | 38 | 16 (42.1%) | 52 (68.4%) | 0.37 (0.19, 0.55) |
| Not identified | 38 | 36 (94.7%) | ||
| CCRCC with BAP1 IHC | ||||
| BAP1 loss | 16 | 12 (75.0%) | 73 (94.8%) | 0.83 (0.66, 0.99) |
| BAP1 retained | 61 | 61 (100%) | ||
Cases with missing data in either nephrectomy or biopsy are not included.
excluding RCC not further classified;
excluding RO and ChRCC;
Weighted kappa.
Abbreviations used: RO=renal oncocytoma, RCC=renal cell carcinoma, CCRCC=clear cell RCC, PRCC=papillary RCC, ChRCC=chromophobe RCC, CCPRCC=clear cell papillary RCC, CDC=collecting duct carcinoma, MiTF TRCC=MiT family translocation RCC, UnRCC=unclassified RCC, BAP1=BRCA1-associated protein-1, IHC=immunohistochemistry.
Sixteen biopsies were diagnosed as RCCs without further subtype. The subtype classification was assigned on the nephrectomy specimen in 14 of these cases (5 CCRCCs, 5 PRCCs, 1 ChRCC, 1 CCPRCC, 1 MiTF TRCC, and 1 RCC-TSC) and 2 were left unclassified. Only 2 cases diagnosed with a specific RCC subtype on biopsy showed histologic subtype discordance in nephrectomy. Both were diagnosed as CCRCC on biopsy. At nephrectomy, both cases were left unclassified (UnRCC) after performing multiple IHCs. In order to obtain further insight, we reviewed the diagnostic material for these two cases. Both biopsies showed a small tumor fragment (<2mm). However, both nephrectomies revealed large RCCs (>pT1a) with marked morphologic heterogeneity and diverse IHC staining patterns, including focal areas mimicking CCRCC. Findings from the review of these two cases are illustrated in Figures 3–4. The biopsy from case 1 was diagnosed as RCC and favored as CCRCC. As illustrated in Figure 3A, hematoxylin & eosin (H&E) stained slides showed a high grade RCC without the characteristic vascular network diagnostic of CCRCC. On IHC, the tumor cells were positive for PAX8, pankeratin, racemase, vimentin, CD10 (focal), and were negative for cytokeratin 7 and CD117. CA IX immunostaining (Figure 3B) on the biopsy showed strong cytoplasmic staining with only focal membranous staining that may have led to the diagnosis of CCRCC. Sections from the resection specimen showed a heterogenous tumor with tubular, nested and papillary architectures (Figure 3C,3D). Interestingly, the areas resembling CCRCC in nephrectomy (Figure 3C) had strong membranous CA IX IHC staining (Figure 3E) that could potentially be misleading on a limited biopsy sample. During our current review, we noticed prominent nucleoli with focal perinucleolar clearing (Figure 3D) and obtained IHC for fumarate hydratase (FH) that showed loss of FH staining in tumor cells supporting the diagnosis to FH-deficient RCC (Figure 3F). The case was diagnosed prior to subspecialized sign-out and FH IHC was not obtained at the time of diagnosis. Review of case 2 revealed a morphologically heterogenous RCC with focal nested/tubular architecture and tumor cells exhibiting clear cytoplasm that on biopsy were misdiagnosed as CCRCC (Figure 4A,4B). IHCs were not obtained on the biopsy. Similar morphology was present focally in the nephrectomy specimen (Figure 4C), but also included high grade areas with papillary architecture (Figure 4D) and low-grade areas with nested, oncocytic tumor-like architecture (Figure 4E). IHCs on the nephrectomy specimen showed a PAX8 positive renal tumor that was otherwise negative for TFE3, TFEB, CK20, p63, high molecular weight cytokeratin, INI1, cathepsin K and HMB-45 and retained FH and SDHB. The oncocytic tumor-like areas were diffusely positive for CD117 and focally for cytokeratin (CK) 7 (Figure 4F–H), while the papillary areas were focally positive for CA-IX, and negative for CD117 and CK7 (Figure 4F,4H). Overall, the tumor did not fit well with currently recognized entities and was left unclassified.
Figure 3.
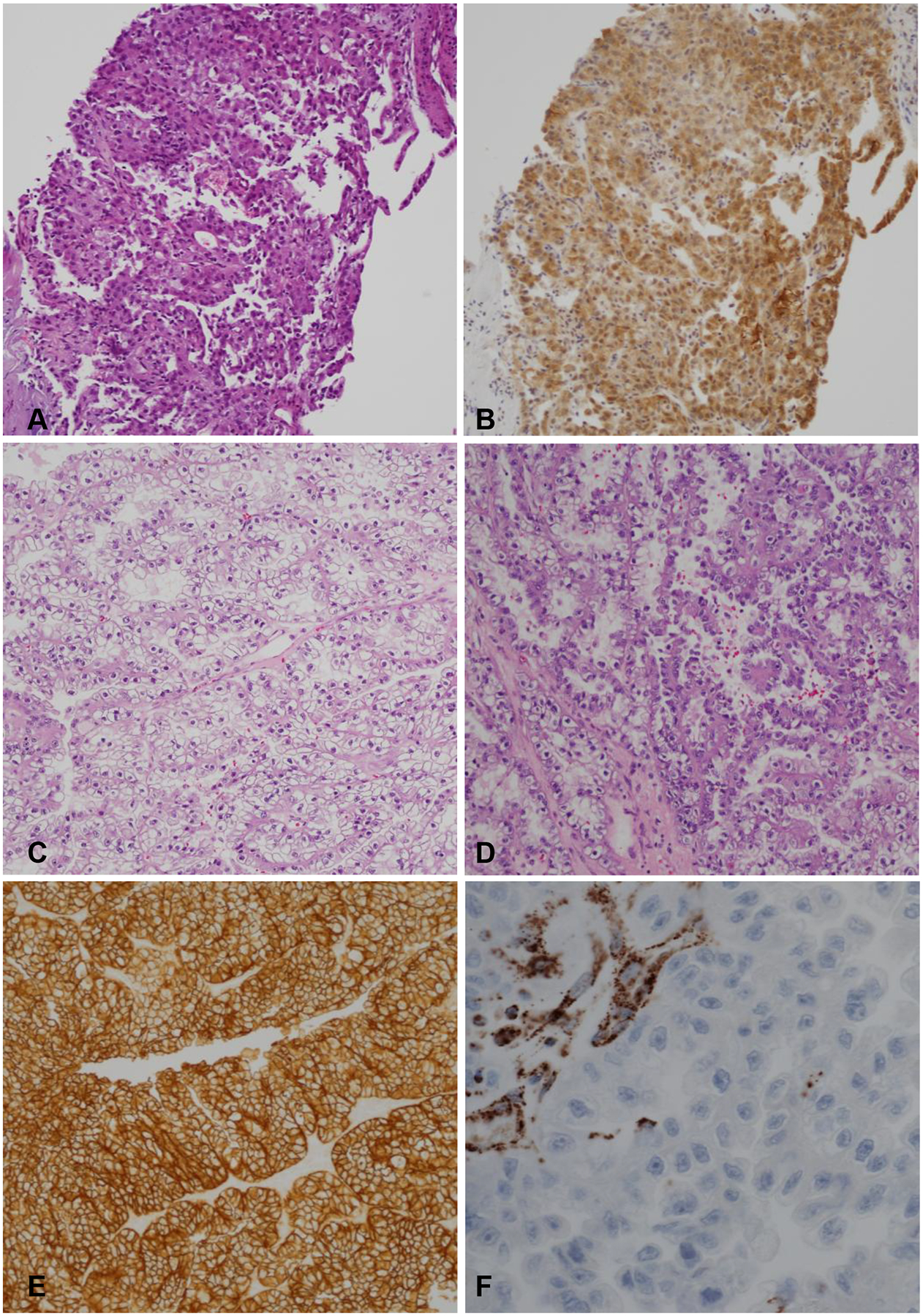
Representative images from case 1. (A) H&E stained sections from the biopsy shows neoplastic cells with eosinophilic to clear cytoplasm. (B) IHC stain for CA IX with predominantly cytoplasmic staining that may have led to misclassification as CCRCC. (C-D) H&E stained sections from the nephrectomy specimen showing heterogeneous morphology with focal areas exhibiting tubular and nested architecture with clear cytoplasm and others with papillary architecture and eosinophilic cytoplasm. (E) CA IX IHC with strong membranous staining in focal areas (F). Fumarate Hydratase (FH) IHC with loss of staining in the tumor cells and retained staining in endothelial cells. (A-E: 100x magnification; F: 400x magnification)
Figure 4.
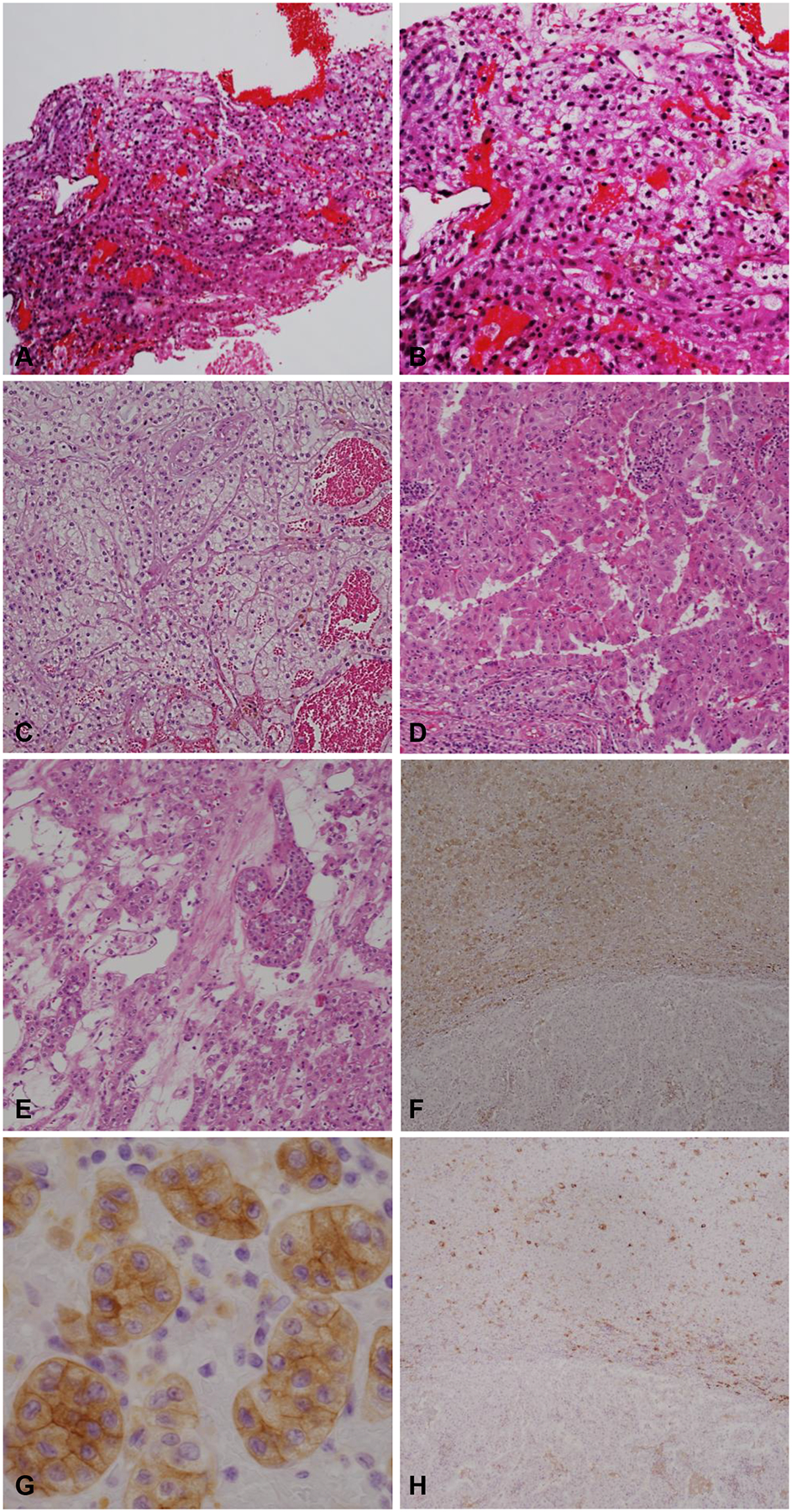
Representative images from case 2. (A-B) Sections from the biopsy with nests of tumor cells and capillary network resembling CCRCC. (C-E) Representative H&E stained sections from the nephrectomy specimen illustrating diverse morphologies including CCRCC-like, papillary, and oncocytic. (F-G) CD117 with diffuse cytoplasmic membranous positive staining in the nested oncocytic area and negative in high grade papillary areas. (H) Cytokeratin 7 stained focally in the oncocytic area and was negative in the high-grade papillary areas. (A, C-E: 100x magnification; B: 200x magnification; F, H: 40x magnification; G: 400x magnification).
The total percentage agreement and the more robust calculated weighted Cohen’s kappa coefficient that considers the possibility of the agreement occurring by chance are shown in Tables 2 and 3 for all parameters considered. The overall concordance rate of RCC histologic subtyping was 96.9%. After applying a weighted Cohen’s kappa coefficient, the values remained high for all renal tumors (kappa [w] 0.90; 95% CI 0.82–0.99) as well as the subset of SRMs (98.8%; kappa [w] 0.97; 95% CI 0.90–1). We wondered whether the histologic subtype agreement changed with size of the tumor. As shown in Table 4, discrepant histologic subtype diagnosis increased with size.
Table 3.
Concordance of histology and prognostic factors in small renal masses (SRM)
| Small renal masses | ||||
|---|---|---|---|---|
| Nephrectomy | Biopsy in agreement (%) | Total agreement (%) | Cohen’s Kappa (95% CI) | |
| RO vs RCC | ||||
| RO | 2 | 2 (100%) | 91 (98.9%) | 0.79 (0.40, 1) |
| RCC | 90 | 89 (98.9%) | ||
| Histologic subtype* | ||||
| CCRCC | 55 | 55 (100%) | 83 (98.8%) | 0.97 (0.90, 1)§ |
| PRCC | 17 | 17 (100%) | ||
| ChRCC | 8 | 7 (87.5%) | ||
| RO | 2 | 2 (100%) | ||
| CCPRCC | 1 | 1 (100%) | ||
| MiTF TRCC | 1 | 1 (100%) | ||
| Grade** | ||||
| 1 | 4 | 3 (75.0%) | 50 (67.6%) | 0.36 (0.17, 0.54)§ |
| 2 | 46 | 39 (84.8%) | ||
| 3 | 24 | 8 (33.3%) | ||
| 4 | 0 | |||
| Low grade (1–2) | 50 | 48 (96.0%) | 56 (75.7%) | 0.35 (0.13, 0.56) |
| High grade (3–4) | 24 | 8 (33.3%) | ||
| Sarcomatoid change | ||||
| Identified | 0 | 0 (0%) | 37 (100%) | - |
| Not identified | 37 | 37 (100%) | ||
| Rhabdoid change | ||||
| Identified | 0 | - | 16 (100%) | - |
| Not identified | 16 | 16 (100%) | ||
| Tumor necrosis | ||||
| Identified | 10 | 2 (20.0%) | 25 (73.5%) | 0.20 (−0.12, 0.51) |
| Not identified | 24 | 23 (95.8%) | ||
| CCRCC with BAP1 IHC | ||||
| BAP1 loss | 8 | 6 (75.0%) | 38 (95.0%) | 0.83 (0.60, 1) |
| BAP1 retained | 32 | 32 (100%) | ||
Abbreviations used: RO=renal oncocytoma, RCC=renal cell carcinoma, CCRCC=clear cell RCC, PRCC=papillary RCC, ChRCC=chromophobe RCC, CCPRCC=clear cell papillary RCC, MiTF TRCC=MiT family translocation RCC, BAP1=BRCA1-associated protein-1, IHC=immunohistochemistry
Weighted kappa.
excluding RO and ChRCC;
excluding RCC not further classified;
Cases with missing data in either nephrectomy or biopsy are not included.
Table 4.
Rank biserial correlation of prognostic variables and tumor size
| Biopsy histology concordant | Rank biserial coefficient (absolute value) | Estimated P-value | ||
|---|---|---|---|---|
| No | Yes | |||
| Tumor size (cm) | ||||
| < 2 | 0 | 10 | 0.413 | .10 |
| 2 – 4 | 1 | 64 | ||
| 4 – 6 | 1 | 46 | ||
| > 6 | 3 | 37 | ||
| Biopsy grade concordant | Rank biserial coefficient (absolute value) | Estimated P-value | ||
| No | Yes | |||
| Tumor size (cm) | ||||
| < 2 | 2 (25%) | 06 (75%) | 0.213 | .03 |
| 2 – 4 | 12 (21%) | 44 (79%) | ||
| 4 – 6 | 20 (47%) | 23 (53%) | ||
| > 6 | 13 (39%) | 20 (61%) | ||
Histologic grade was available in biopsy and resection specimens for 140 RCCs (excluding ChRCC) of which 78 cases (55.7%; kappa [w] 0.33; 95% CI 0.22–0.44) agreed (Table 2). As expected, in low-grade tumors and in SRMs (SRMs have fewer high grade tumors), concordance for histologic grade on biopsy and nephrectomy was higher (67.6%; kappa [w] 0.36; 95% CI 0.17–0.54). Using simplified low (1–2) and high (3–4) grade groups, the overall histologic grade concordance rate was higher for both the overall cohort as well as SRMs (66.4% and 75.7% respectively) (Table 3). As for histologic subtype, grade discrepancy on biopsy increased with size, and the result was statistically significant (P=.03; Table 4).
Presence of tumor necrosis, rhabdoid, and sarcomatoid changes were less consistently reported, and cases with missing data were not included for concordance assessment. Of the 7 RCCs with sarcomatoid change on nephrectomy, only 2 showed sarcomatoid features on biopsy (2 biopsies did not comment on sarcomatoid change and were not available for review). The nephrectomies of the two biopsies with sarcomatoid change, had >70% sarcomatoid differentiation. In contrast, the remaining 5 nephrectomies exhibit <30% sarcomatoid differentiation. Similarly, rhabdoid change was observed in 2 of the 6 available biopsies with rhabdoid change on corresponding nephrectomy (Table 2). Two SRMs showed small foci of sarcomatoid or rhabdoid change on resection, however, the corresponding biopsies were not available for review. Of 38 nephrectomy specimens showing tumor necrosis, only 16 biopsies (68.4% agreement) exhibited necrosis. The agreement was higher in SRMs (73.5%) (Table 3).
BAP1 IHC was performed on 77 paired CCRCCs. BAP1 was deficient in 17 CCRCCs and predominantly noted in high grade tumors (94.1% [16 of 17] vs 5.9% [1 of 17]) (Figure 5A–B). Of 16 CCRCCs that were BAP1 deficient, 12 showed loss of staining in the biopsy (94.8%; kappa [w] 0.83; 95% CI 0.66–0.99) (Table 2). Similar rates of concordance were observed in SRMs (95.0%; kappa [w] 0.83; 95% CI 0.60–1) (Table 3). We asked if BAP1 analysis on biopsies when considered along with other prognostic variables could increase prognostic accuracy of CCRCC. The ROC curve in Figure 5C illustrates that BAP1 IHC in combination with the grade on biopsy can better predict grade on resection in CCRCCs. Inclusion of other prognostic features such as presence of tumor necrosis and/or sarcomatoid/rhabdoid change, did not improve predictive value. However, as shown in Table 5, the presence of one or more aggressive features (grade 3–4, tumor necrosis, BAP1 loss, sarcomatoid/rhabdoid change) on biopsy correlated significantly with pT stage in resection (P=.004).
Figure 5.
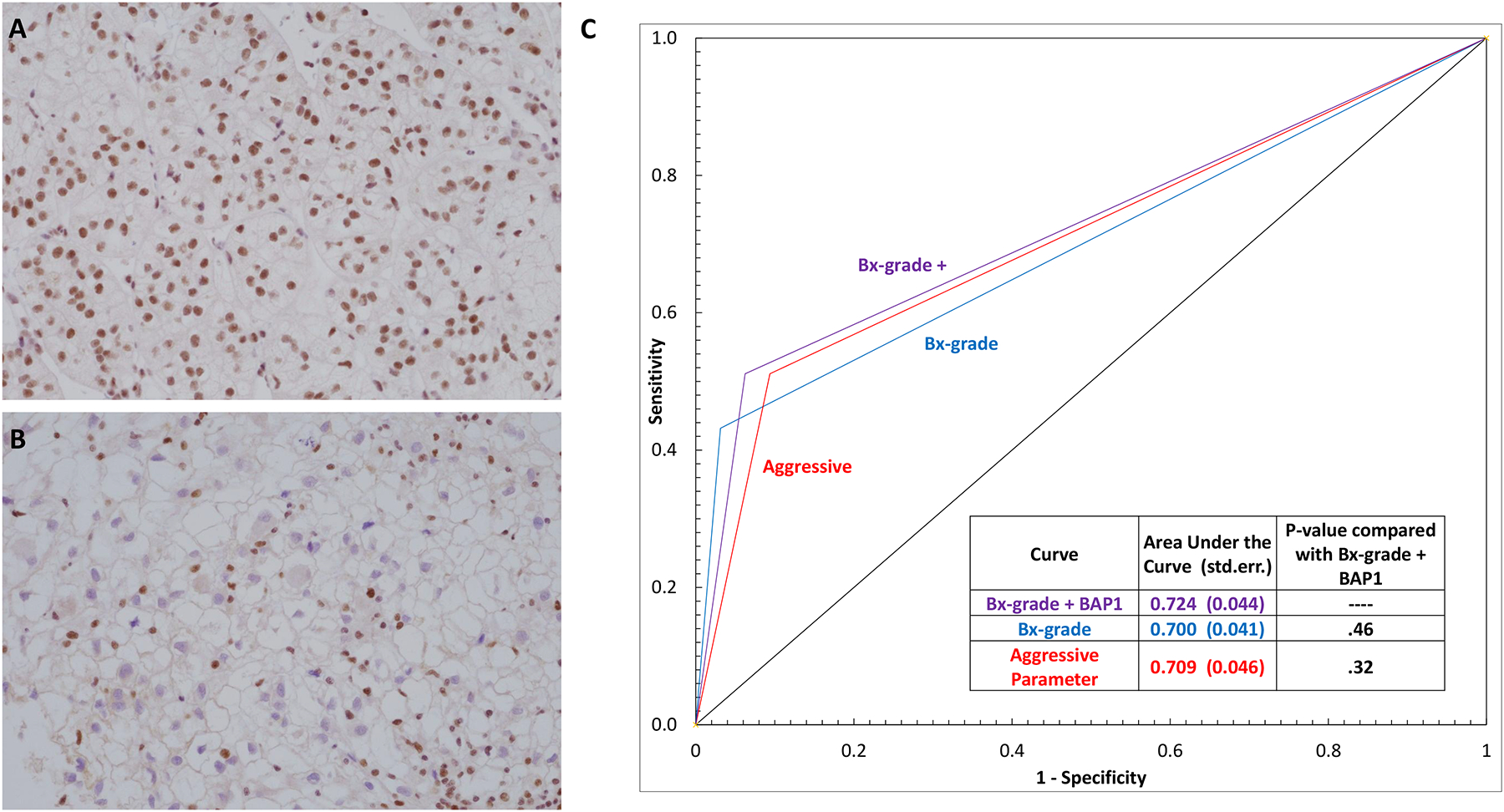
Representative BAP1 IHC images with (A) retained and (B) loss of nuclear BAP1 staining in the tumor cells. Retained nuclear expression in the background endothelial cells and lymphocytes serves as a positive control in each slide. (C) Receiver operating characteristic (ROC) curve showing correlation of presence of prognostic parameters on biopsy with tumor grade (grade 1–2 vs 3–4) on nephrectomy. The corresponding area under the curve and P-values are embedded in the table inset. Abbreviations used: Bx=biopsy; std. err.=standard error; aggressive parameter=presence of one or more of the following features: tumor grade 3 or 4, BAP1 loss, tumor necrosis, sarcomatoid and/or rhabdoid change. (A-B: 200x magnification)
Table 5.
Rank biserial correlation for the presence of aggressive features and pT stage
| Aggressive parameter present in biopsy | Rank biserial coefficient (absolute value) | Estimated P-value | ||
|---|---|---|---|---|
| No | Yes | |||
| pT stage | ||||
| pT1 | 76 | 20 (21%) | 0.255 | .004 |
| pT2 | 2 | 01 (33%) | ||
| pT3 | 31 | 19 (38%) | ||
| pT4 | 0 | 03 (100%) | ||
Aggressive parameters were defined as presence of one or more of the following features on biopsy: tumor grade 3 or 4, BAP1 loss, tumor necrosis, sarcomatoid and/or rhabdoid change.
DISCUSSION
Our study evaluates the prognostic accuracy of biopsies in renal tumors by comparing with subsequent nephrectomies. These parameters include histologic subtype, tumor grade, necrosis, sarcomatoid/rhabdoid change, and BAP1 status. To our knowledge, this is the first study to evaluate accuracy of prognostic parameters on RMB that includes BAP1.
Expanding previous studies, our study shows that image-guided biopsy/FNA of renal masses has high efficacy [805 of 875 (92%) RMBs performed were diagnostic]. This may in part be due to the onsite cytology assessment performed at our institution during these procedures. Although there are limited data reported in literatures regarding subtyping of renal tumors on FNA, we included FNA (there were only 9 in our cohort) in our analysis due to similar results as observed with the RMBs in our cohort. Similar to prior studies, our study shows that RMBs have excellent accuracy for the diagnosis of malignancy across renal masses (98.3%; kappa [w] 0.83; 98% CI 0.65–1) including SRMs (98.9%; kappa [w] 0.79; 98% CI 0.40–1). In the clinic, favored diagnoses in the pathology report are often considered as final and we included them in this study. We found a high RCC subtyping concordance rate of 96.9% in all tumors and this rate reached 98.8% in SRMs. Similar to our findings, prior studies show excellent concordance for tumor subtypes on RMB (78–100%). In a systematic review including meta-analysis of published studies on the diagnostic performance of RMB, Marconi et al. showed an overall high diagnostic yield of 92% (range: 81–97%) for the diagnosis of malignancy on biopsy with excellent sensitivity (99.1%) and specificity (99.7%)10. The same meta-analysis showed an accuracy of 90.3% (range: 84–94%) for tumor subtype that reached 96% (range: 90–100%) for SRMs. Most studies included were from large academic institutions with subspecialization and large volumes. However, the accuracy may not be as high at small practices without subspecialization, especially with the growing number of newly described rare RCC subtypes. We share in Figure 1–2 our approach to histologic subtyping of renal tumors.
Our study suggests that misclassification of renal tumors on RMB is more likely to occur in: 1) tumors with eosinophilic cytoplasm; 2) where there is limited tumor sampling in biopsy; 3) with infrequent use or misinterpretation of IHCs; 4) and where corresponding tumors are large and heterogenous. In our study, the two misclassified high-grade RCCs on biopsy showed marked histologic and IHC heterogeneity in the nephrectomy specimens. Both cases emphasize the liberal utility of IHCs in diagnosing histologic subtype and caution in interpreting them when limited sample is available. Diffuse membranous expression of CA IX can be a helpful marker for the diagnosis of CCRCC, with the caveat that nonrenal tumors (including urothelial carcinoma), hypoxic tumor tissue, and clear cell papillary RCC can also express CA IX.
Accurate diagnosis can be more challenging in the biopsy of low-grade oncocytic or eosinophilic tumors. At our institution, these cases are usually diagnosed as “low-grade renal oncocytic neoplasm of uncertain malignant potential”. Many institutions, including ours, only favor a diagnosis of RO on a biopsy and most of these tumors do not undergo nephrectomy. RO is disfavored when morphologic features such as solid architecture, nuclear pleomorphism and atypia, perinuclear clearing, prominent cell borders, atypical mitosis, or papillary architecture are present (even focally) or if CK7 IHC shows diffuse positivity33. In 3 cases, a diagnosis of RO was favored in our study that were subsequently diagnosed as ChRCC (2 cases eosinophilic variant and 1 HOCT) on resection. The biopsies were unfortunately no longer available to help learn from these discrepancies.
Similar to our data, the concordance rates of other prognostic parameters such as nucleolar grade are significantly lower in the literature20–22,34–42. In a meta-analysis, the degree of tumor grade concordance was only fair (median kappa=0.34) with median rate of 62.5% (range: 52–72%) across renal masses and 66.7% (range: 60–70%) for SRMs10. Our results show a concordance rate of 55.7% for histologic grade, and 68.4% for tumor necrosis, similar to that seen in previously published studies10. These discrepancies may be due to interobserver variability, intratumoral heterogeneity, and sampling bias. In our study, we analyzed the data as reported in the pathology reports over a span of 15 years. Given that nephrectomies are usually performed within a short time after the biopsy, criteria used for grading and prognostication would be similar and representative of routine practice in vigor at the time. Tumor heterogeneity is a well-recognized factor for histology, grade, and mutation status, especially in CCRCC43–45. Grade agreement decreased with size (Table 4), suggesting intratumoral heterogeneity to be a dominant factor. The higher concordance rates in SRMs may also be influenced by their tendency to be low grade and lack of intratumoral heterogeneity17,18. A contributing factor for poor concordance especially for necrosis may be the sampling bias and techniques for obtaining viable tissue during the biopsy procedure. Our study included only 7 nephrectomies that contained sarcomatoid and/or rhabdoid changes on final pathology with poor concordance rates on paired biopsies. A study assessing a larger number of cases with sarcomatoid/rhabdoid differentiation may be needed to evaluate concordance.
Sequencing and IHC studies by our group and others have shown that BAP1 mutant tumors are associated with worse clinical outcomes and aggressive clinicopathological features in CCRCC25–27. In our study loss of BAP1 immunoexpression showed a high concordance rate of 94.8% across renal masses. This rate was significantly higher than histologic grade, tumor necrosis, and sarcomatoid/rhabdoid change. The biopsy BAP1 IHC along with tumor grade predicted the grade on resection most accurately compared to other prognostic parameters either alone or in combination (Figure 5). Most importantly, we show that presence of any aggressive feature (grade 3–4, tumor necrosis, BAP1 loss, sarcomatoid/rhabdoid change) in biopsy correlated significantly with pT stage upon resection (Table 5). If validated, routine incorporation of BAP1 IHC in ccRCC biopsies may refine prognosis and aid in the selection of patients for active surveillance.
Limitations of this study include having a retrospective design, using a single institution database, being skewed in favor of higher-grade tumors as they undergo nephrectomy more frequently, and interventional radiology protocols regarding the tumor sampling preference of non-necrotic tumor tissue to obtain viable parts of tumors.
CONCLUSIONS
We show that renal mass biopsy has high accuracy for diagnosing and subtyping renal tumors especially SRMs. Histological subtyping may be more challenging when tumor cells show an eosinophilic cytoplasm and might be aided by IHC. RMBs often underestimate poor prognostic features such as high nucleolar grade, tumor necrosis, and sarcomatoid/rhabdoid changes and absence of these features cannot be determined conclusively in a biopsy. In contrast, BAP1 status could be more accurately predicted in biopsy material, and may serve as an additional reliable prognostic indicator of CCRCC aggressiveness.
Acknowledgments:
We thank the patients who made possible this analysis, the Kidney Cancer Program, and the Clinical Data Warehouse team at Simmons Comprehensive Cancer Center, Dallas, TX for the support and assistance.
Funding Sources:
This work was supported by the NIH sponsored Kidney Cancer SPORE grant (P50CA196516) and endowments from Brock Fund for Medical Science Chair in Pathology and the Jan and Bob Pickens Distinguished Professorship in Medical Science.
Footnotes
Conflicts of interest disclosures: None
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Lee CT, Katz J, Fearn PA, Russo P. Mode of presentation of renal cell carcinoma provides prognostic information. Urol Oncol. 2002;7(4):135–140. [DOI] [PubMed] [Google Scholar]
- 3.Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol. 2000;163(3):730–736. [PubMed] [Google Scholar]
- 4.Nguyen MM, Gill IS, Ellison LM. The evolving presentation of renal carcinoma in the United States: trends from the Surveillance, Epidemiology, and End Results program. J Urol. 2006;176(6 Pt 1):2397–2400; discussion 2400. [DOI] [PubMed] [Google Scholar]
- 5.Ho TH, Kapur P, Joseph RW, et al. Loss of PBRM1 and BAP1 expression is less common in non-clear cell renal cell carcinoma than in clear cell renal cell carcinoma. Urol Oncol. 2015;33(1):23 e29–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67(3):519–530. [DOI] [PubMed] [Google Scholar]
- 7.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271–1279. [DOI] [PubMed] [Google Scholar]
- 8.Thomas AA, Campbell SC. Small renal masses: toward more rational treatment. Cleve Clin J Med. 2011;78(8):539–547. [DOI] [PubMed] [Google Scholar]
- 9.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924. [DOI] [PubMed] [Google Scholar]
- 10.Marconi L, Dabestani S, Lam TB, et al. Systematic Review and Meta-analysis of Diagnostic Accuracy of Percutaneous Renal Tumour Biopsy. Eur Urol. 2016;69(4):660–673. [DOI] [PubMed] [Google Scholar]
- 11.Richard PO, Jewett MA, Bhatt JR, et al. Renal Tumor Biopsy for Small Renal Masses: A Single-center 13-year Experience. Eur Urol. 2015;68(6):1007–1013. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(6):804–834. [DOI] [PubMed] [Google Scholar]
- 13.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–2400. [DOI] [PubMed] [Google Scholar]
- 14.Sorbellini M, Kattan MW, Snyder ME, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173(1):48–51. [DOI] [PubMed] [Google Scholar]
- 15.Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20(23):4559–4566. [DOI] [PubMed] [Google Scholar]
- 16.Przybycin CG, McKenney JK, Reynolds JP, et al. Rhabdoid differentiation is associated with aggressive behavior in renal cell carcinoma: a clinicopathologic analysis of 76 cases with clinical follow-up. Am J Surg Pathol. 2014;38(9):1260–1265. [DOI] [PubMed] [Google Scholar]
- 17.Yang C, Shuch B, Serrano M, et al. Adverse Histopathologic Characteristics in Small Clear Cell Renal Cell Carcinomas Have Negative Impact on Prognosis: A Study of 631 Cases With Clinical Follow-up. Am J Surg Pathol. 2019;43(10):1413–1420. [DOI] [PubMed] [Google Scholar]
- 18.Cai Q, Christie A, Rajaram S, et al. Ontological analyses reveal clinically-significant clear cell renal cell carcinoma subtypes with convergent evolutionary trajectories into an aggressive type. EBioMedicine. 2020;51:102526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turajlic S, Xu H, Litchfield K, et al. Deterministic Evolutionary Trajectories Influence Primary Tumor Growth: TRACERx Renal. Cell. 2018;173(3):595–610 e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gellert LL, Mehra R, Chen YB, et al. The diagnostic accuracy of percutaneous renal needle core biopsy and its potential impact on the clinical management of renal cortical neoplasms. Arch Pathol Lab Med. 2014;138(12):1673–1679. [DOI] [PubMed] [Google Scholar]
- 21.Hu R, Montemayor-Garcia C, Das K. Role of percutaneous needle core biopsy in diagnosis and clinical management of renal masses. Hum Pathol. 2015;46(4):570–576. [DOI] [PubMed] [Google Scholar]
- 22.Dave CN, Seifman B, Chennamsetty A, et al. Office-based Ultrasound-guided Renal Core Biopsy Is Safe and Efficacious in the Management of Small Renal Masses. Urology. 2017;102:26–30. [DOI] [PubMed] [Google Scholar]
- 23.Kapur P, Christie A, Rajaram S, Brugarolas J. What morphology can teach us about renal cell carcinoma clonal evolution Kidney Cancer Journal. 2020;18(3):68–75. [PMC free article] [PubMed] [Google Scholar]
- 24.Pena-Llopis S, Vega-Rubin-de-Celis S, Liao A, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44(7):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapur P, Pena-Llopis S, Christie A, et al. Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: a retrospective analysis with independent validation. Lancet Oncol. 2013;14(2):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph RW, Kapur P, Serie DJ, et al. Loss of BAP1 protein expression is an independent marker of poor prognosis in patients with low-risk clear cell renal cell carcinoma. Cancer. 2014;120(7):1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapur P, Christie A, Raman JD, et al. BAP1 immunohistochemistry predicts outcomes in a multi-institutional cohort with clear cell renal cell carcinoma. J Urol. 2014;191(3):603–610. [DOI] [PubMed] [Google Scholar]
- 28.Motzer RJ, Banchereau R, Hamidi H, et al. Molecular Subsets in Renal Cancer Determine Outcome to Checkpoint and Angiogenesis Blockade. Cancer cell. 2020. Nov 5:S1535–6108(20)30542-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brugarolas J, Rajaram S, Christie A, Kapur P. The Evolution of Angiogenic and Inflamed Tumors: The Renal Cancer Paradigm. Cancer cell. 2020; 2020 Nov 5:S1535–6108(20)30552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eble JN SG, Epstein JI, Sesterhenn IA. Pathology and Genetics. Tumors of the urinary system and male genital organs. IARC: Lyon: 2016. [Google Scholar]
- 31.Holger Moch PHA, Thomas M. Ulbright, Reuter Victor E.. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th edIARC: Lyon: 2016. [Google Scholar]
- 32.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6(7):655–663. [DOI] [PubMed] [Google Scholar]
- 33.Alderman MA, Daignault S, Wolf JS Jr., , et al. Categorizing renal oncocytic neoplasms on core needle biopsy: a morphologic and immunophenotypic study of 144 cases with clinical follow-up. Hum Pathol. 2016;55:1–10. [DOI] [PubMed] [Google Scholar]
- 34.Menogue SR, O’Brien BA, Brown AL, Cohen RJ. Percutaneous core biopsy of small renal mass lesions: a diagnostic tool to better stratify patients for surgical intervention. BJU Int. 2013;111(4 Pt B):E146–151. [DOI] [PubMed] [Google Scholar]
- 35.Leveridge MJ, Finelli A, Kachura JR, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol. 2011;60(3):578–584. [DOI] [PubMed] [Google Scholar]
- 36.Sofikerim M, Tatlisen A, Canoz O, Tokat F, Demirtas A, Mavili E. What is the role of percutaneous needle core biopsy in diagnosis of renal masses? Urology. 2010;76(3):614–618. [DOI] [PubMed] [Google Scholar]
- 37.Blumenfeld AJ, Guru K, Fuchs GJ, Kim HL. Percutaneous biopsy of renal cell carcinoma underestimates nuclear grade. Urology. 2010;76(3):610–613. [DOI] [PubMed] [Google Scholar]
- 38.Volpe A, Mattar K, Finelli A, et al. Contemporary results of percutaneous biopsy of 100 small renal masses: a single center experience. J Urol. 2008;180(6):2333–2337. [DOI] [PubMed] [Google Scholar]
- 39.Shannon BA, Cohen RJ, de Bruto H, Davies RJ. The value of preoperative needle core biopsy for diagnosing benign lesions among small, incidentally detected renal masses. J Urol. 2008;180(4):1257–1261; discussion 1261. [DOI] [PubMed] [Google Scholar]
- 40.Schmidbauer J, Remzi M, Memarsadeghi M, et al. Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. Eur Urol. 2008;53(5):1003–1011. [DOI] [PubMed] [Google Scholar]
- 41.Patel HD, Johnson MH, Pierorazio PM, et al. Diagnostic Accuracy and Risks of Biopsy in the Diagnosis of a Renal Mass Suspicious for Localized Renal Cell Carcinoma: Systematic Review of the Literature. J Urol. 2016;195(5):1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halverson SJ, Kunju LP, Bhalla R, et al. Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. J Urol. 2013;189(2):441–446. [DOI] [PubMed] [Google Scholar]
- 43.Ball MW, Bezerra SM, Gorin MA, et al. Grade heterogeneity in small renal masses: potential implications for renal mass biopsy. J Urol. 2015;193(1):36–40. [DOI] [PubMed] [Google Scholar]
- 44.Conti A, Santoni M, Sotte V, et al. Small renal masses in the era of personalized medicine: Tumor heterogeneity, growth kinetics, and risk of metastasis. Urol Oncol. 2015;33(7):303–309. [DOI] [PubMed] [Google Scholar]
- 45.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]


