Abstract
The morphology of gastrulation driving the internalization of the mesoderm and endoderm differs markedly among vertebrate species. It ranges from involution of epithelial sheets of cells through a circular blastopore in amphibians to ingression of mesenchymal cells through a primitive streak in amniotes. By targeting signaling pathways controlling critical cell behaviors in the chick embryo, we generated crescent- and ring-shaped mesendoderm territories in which cells can or cannot ingress. These alterations subvert the formation of the chick primitive streak into the gastrulation modes seen in amphibians, reptiles, and teleost fish. Our experimental manipulations are supported by a theoretical framework linking cellular behaviors to self-organized multicellular flows outlined in detail in the accompanying paper. Together, this suggests that the evolution of gastrulation movements is largely determined by changes in a few critical cell behaviors in the mesendoderm territory across different species and controlled by a relatively small number of signaling pathways.
Manipulation of mesendoderm territory and cell behavior recreates distinct vertebrate gastrulation morphologies in the chick.
INTRODUCTION
During gastrulation, embryos transform from a simple epithelial layer into a three-layer structure. In vertebrate embryos, this process requires large morphogenetic movements that locate the three primary germ layers—the ectoderm, mesoderm, and endoderm—in their topologically correct positions (1, 2). However, the detailed tissue movements and morphology of the embryo during gastrulation vary greatly among vertebrate animals (3). For example, Xenopus forms a blastopore through which the mesoderm and endoderm precursors internalize as epithelial sheets, while, at the other end of the spectrum, amniote embryos such as chick, mouse, and human embryos internalize these precursors as mesenchymal cells through a structure known as the primitive streak (4, 5).
The morphogenetic tissue flows in all organisms result from the integration of a few critical cell behaviors, including cell division, differentiation, shape changes, and movement. These cell behaviors are patterned and coordinated through a combination of short- and long-range chemical and mechanical signals (6–8). Different spatiotemporal organizations of these cell behaviors, in conjunction with geometrical and mechanical constraints imposed by the size and shape of the embryo and extraembryonic tissues, are thought to determine the diverse morphogenetic programs in vertebrate gastrulation (9, 10). However, how the large-scale cell movements are coordinated and coupled via feedback to the signaling processes as part of robust developmental control mechanisms remains to be resolved (11–14).
The formation of the primitive streak in the chick embryo involves intercalation of cells in the sickle-shaped mesendoderm precursor domain initially located in the posterior epiblast of the embryo followed by ingression of these cells in the primitive streak (15, 16). Previously, we established quantitative light sheet microscopy and computational data analysis methods to characterize both the embryo-wide tissue flows and critical cell behaviors driving them during streak formation (15). This showed quantitatively how the tissue flows underlying streak formation are driven by directional cell intercalations and ingression of the mesendoderm precursor cells. Furthermore, the directional cell intercalations were shown to be associated with and dependent on myosin cables in cellular junctions aligned in the direction of intercalation (15). We here confirm and expand these findings and undertake a study to investigate how the modulation of a limited number of critical cell behaviors during gastrulation in the chick embryo alters the spatiotemporal dynamics and morphology of gastrulation.
RESULTS
Using the chick embryo as a model organism, we explore how the patterning of cell behaviors determines primitive streak morphogenesis during gastrulation (Fig. 1, A to C and G). To study the tissue-wide coordination of the cell behaviors in the epiblast, we image, as before, gastrulation in a chick line expressing a membrane-targeted green fluorescent protein (GFP) using a custom-built light sheet microscope (15) and image analysis (see Materials and Methods and movie S1).
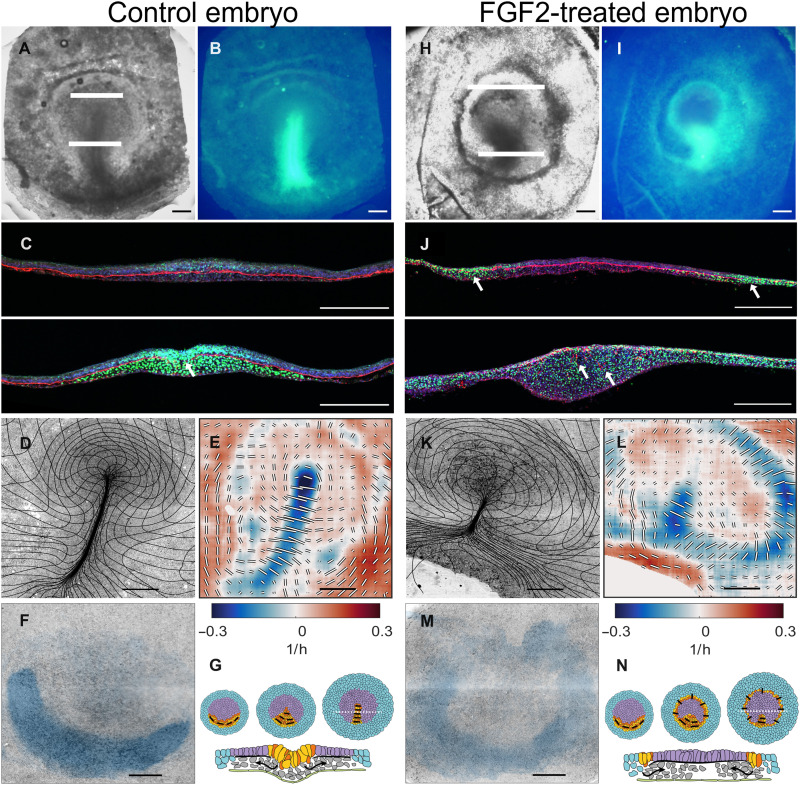
Fig. 1. Characterization of gastrulation for control embryo and after FGF2 treatment.
(A) Control, bright-field image of embryo at HH3+. (B) SNAI expression in embryo shown in (A). (C) Two sections taken at the white lines in (A) showing SNAI (green), FN1 (red), and actin (blue). Note that in the streak, the FN1 layer is fragmented as indicated by the white arrow. (D) Overview images of embryo from light sheet microscope at the end (HH3+) of the experiment overlaid with a deformation mesh. (E) Strain rate tensor for stages shown in (D). Isotropic strain rate component shown as color blue for contraction and red for expansion. The anisotropic part of the strain rate tensor is shown as black and white bars indicating the magnitude and direction of contraction. (F) Cells that will ingress through the streak [domain of attraction (DOA)] during the experiment calculated from the dynamic morphoskeleton (DM). (G) Schematic summary of development (stages HH1, HH2, and HH3), extraembryonic area in blue, mesendoderm precursors in yellow/red, and embryonic area in purple; black bars show the direction of cell intercalation, section taken at the broken white line in HH3 embryo schematic in (E). Scale bars, 500 μm except in (C), which is 250 μm. For (E) and (L), the anisotropic scale bar length represents 0.5/hour for the anisotropic component of the strain rate. (H to N) Same layout as (A) to (F) for FGF2-treated embryo for the same time as the control embryo. FGF (50 μg/ml on the hypoblast; see Materials and Methods)–treated embryos generate a circular primitive streak surrounding the epiblast (eight of eight treated embryos in the light sheet microscope).
Quantitation of cell flows and behaviors
The dynamics of tissue deformation (Fig. 1D) can be directly quantified by measuring changes in the velocity field associated with tissue shape changes and movement using particle image velocimetry (PIV; fig. S1, A and B). We previously showed that the decomposed strain rates calculated from the velocity fields are a robust indicator of individual cell behaviors driving the tissue flows and allow the identification and quantification of cell behaviors resulting from different perturbations (15, 17). Negative values of the isotropic part of the strain rate, associated with a decrease in tissue area, denote regions where cells are undergoing apical contraction and cell ingression. Areas characterized by larger values in the anisotropic components of the strain rate, associated with shear deformation, indicate the convergence and extension of the tissue produced by directed cell intercalations (fig. S2 and movie S2). Our current observations on whole embryos confirm our earlier observations on embryo slices (15) that the primitive streak forms in a region undergoing convergent extension produced by apical contraction and cell ingression (negative values of the isotropic strain rate tensor) and cell intercalation (contracting anisotropic component of the strain rate tensor oriented perpendicular to the streak) (Fig. 1E).
Quantifying the scale and nature of these movements requires an effective description of the relative motions of cells. While Eulerian quantities (decomposed strain rates) correlate with distinct cell behaviors, morphogenetic features arise from different processes integrated over space and time along cell trajectories. To account for this, we compute the dynamic morphoskeleton (DM) (18), which consists of Lagrangian attractors, their domain of attraction (DOA), and repellers that together organize tissue flows [figure 2 and movie 3 of the accompanying paper (19)]. The DOA consists of the back projection of the attractor on the initial stage of development and reveals the extent of the sickle-shaped posterior-located mesendoderm precursors fated to ingress through the streak (Fig. 1F). See Materials and Methods for details on DM computation.
Tissue flows are driven by mesoderm cells
Previous work has established how cells in the sickle-shaped posterior territory form the primitive streak. Initially, cell-cell signaling between cells in the extra- and embryonic areas results in the induction of mesendoderm precursors in a sickle-shaped domain in the posterior embryonic epiblast (20). These cells then start a myosin II–mediated directional cell-cell intercalation process that drives the convergence and extension of the sickle-shaped mesendoderm in the extending primitive streak (15, 21, 22). This developmental phase is characterized by large counterrotating vortical flows in the epiblast (fig. S1B), known as polonaise movements (23, 24). During this process, the mesendodermal precursor cells begin to contract apically in preparation for their ingression in the streak (16, 25). Following an epithelial-to-mesenchymal transition (EMT) and ingression, the cells migrate away as cohorts of loosely associated cells (26, 27) to form the various endodermal and mesodermal structures in the embryo (Fig. 1C) (28–30).
These findings suggest that directed cell intercalation and cell ingression in the mesendoderm territory drive the morphogenetic flows during avian gastrulation. To test this idea, we inhibited mesoderm differentiation by blocking fibroblast growth factor (FGF) signaling using the pan-FGF receptor inhibitor LY2874455 (31). The addition of LY2874455 resulted in the complete inhibition of mesoderm differentiation and the loss of the characteristic tissue flows associated with streak formation (movie S3). Analysis of the strain rate shows that there is little organized intercalation, confirming that the presence of mesendoderm tissue is a prerequisite for streak formation (fig. S3).
Mesoderm expansion produces a circular streak
While inhibiting FGF signaling blocks the mesoderm differentiation, the addition of an excess of FGF results in the circular expansion of the SNAI2 (snail family transcriptional repressor 2) expression territory along the marginal zone (Fig. 1, H to J), confirming previous observations that SNAI2, brachyury, and cChordin expression is activated by FGF (20). During normal development of SNAI2+, mesendoderm cells travel from the sickle-shaped territory where they are specified toward the primitive streak, where they undergo EMT and cell ingression (25, 32). This can be seen via the presence of SNAI2-expressing cells in the inner layers and the breakdown of the Fibronectin 1 (FN1) layer in the streak (Fig. 1, B and C, arrow). Embryos treated with excess FGF show that SNAI2+ cells have extended along a ring-shaped region along the marginal zone (Fig. 1, H to J), while the FN1 basal layer is degraded (Fig. 1J, arrows). In vivo imaging shows that FGF-treated embryos exhibit a ring of tissue (movie S4) that presents the two characteristic signatures of the late primitive streak, isotropic contraction along the ring, indicating that cells in the ring are undergoing apical contraction and ingression. Furthermore, shear contraction across the ring shows that cells intercalate in a direction perpendicular to the circular streak (Fig. 1, K to M, and movie S5). These directional intercalations generate convergent-extending flows along the ring (fig. S4).
The resulting circular streak structure resembles the germ ring of teleost fish gastrulation, where cells undergo ingression and migrate toward the animal pole (33–36) [see also figure 3F and movie 9 of (19) for the corresponding DM]. Similarly, in FGF-treated embryos, cells that ingress in the circular streak migrate toward the center of the epiblast, the corresponding animal pole (movie S6).
Blocking ingression results in epiblast folding
Bone morphogenetic protein (BMP) signaling has been shown to occur in the anterior epiblast before the onset of gastrulation (37–39). Furthermore, blocking BMP signaling can result in the induction of mesoderm and the generation of ectopic primitive streaks (37, 38, 40). In line with these previous observations, we induced the expansion of the mesendoderm territory along the anterior marginal zone by blocking BMP signaling using the pan BMP type I receptor (ALK1, ALK2, ALK3, ALK6) inhibitor LDN-193189 (41) (LDN; fig. S5, A to D), as seen by the expression of SNAI2+ cells in a circular domain (fig. S5, A to D). Therefore, blocking BMP signaling is an alternative to FGF activation to generate SNAI2-expressing mesendoderm rings. The SNAI2-positive ectopic domains induced by LDN form rapidly and are more circular than those formed after FGF addition, suggesting that relieving inhibition of mesoderm differentiation by LDN acts faster than the induction of mesendoderm by FGF. Blocking Nodal receptors ALK4, ALK5 by the inhibitor SB50124 does not block mesoderm formation (fig. S6). In LDN-treated embryos, concomitant with the formation of the mesoderm ring, we note the appearance of a circular fold at the boundary of the domain, which contracted over time, engulfing the internal epiblast (fig. S5, A to F). The observed tissue buckling after blocking BMP signaling results from the inhibition of EMT and cell ingression, even in the presence of exogenous FGF (figs. S5, G to I, and S7). Closer inspection of the development of these LDN-treated embryos showed that they are noticeably smaller due to substantial inhibition of cell proliferation (fig. S8A). This prevented longer-term observation of the embryos in the light sheet microscope to follow the details of the closure of the ingression rings, because, in the absence of tissue growth but in the presence of extensive tissue buckling and involution, the embryo shrinks and detaches from the vitelline membrane, which is fixed in the light sheet imaging chamber (42).
We observed that the combination of the BMP signaling inhibitor LDN and the glycogen synthase kinase 3 (GSK3) inhibitor CHIR-99021 (CHIR), conditions that have been used to produce self-renewing mesendoderm precursors in vitro from induced pluripotent stem and embryonic stem cells (43), successfully overcame the LDN-induced shrinking and detachment. Treated embryos still showed the formation of mesodermal precursor rings and inhibition of EMT, resulting in buckling and involution of the central tissue (Fig. 2, A to G, and movie S7), but without significant inhibition of cell division (fig. S8B). Sectioning revealed that tissue in this internal domain had folded inside the embryo, and the mesendoderm cells that internalized through the circular fold failed to undergo EMT, as shown by the cells staying connected in a continuous epithelial sheet and the presence of a continuous nondegraded layer of FN1 underlying the involuted epithelial cells (Fig. 2G).
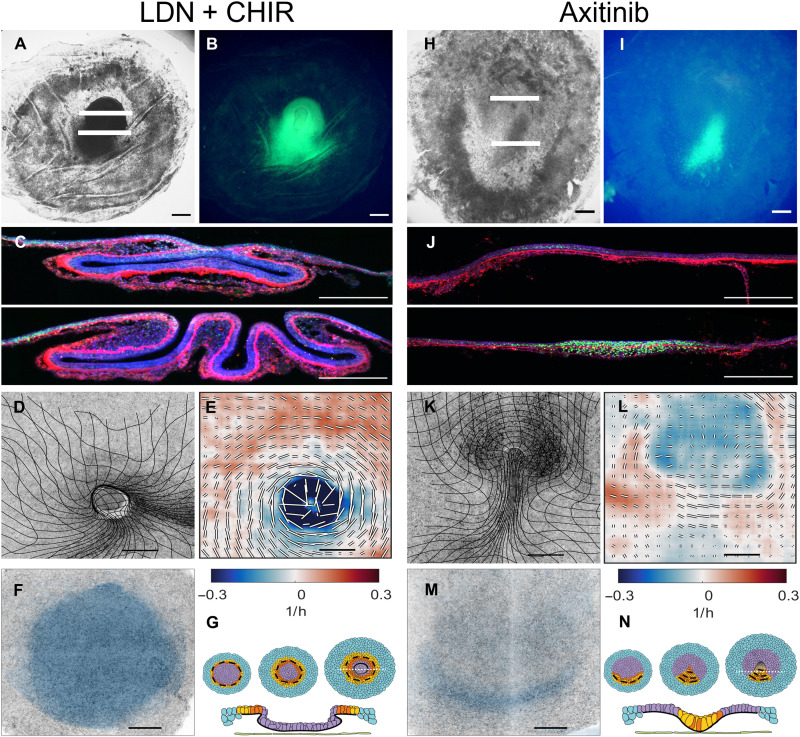
Fig. 2. Characterization of gastrulation in embryos after perturbation of the BMP/Wnt and Vegf signalling pathways.
(A) Bright-field image of embryo treated with the BMP receptor inhibitor LDN (100 nM) and the GSK3 inhibitor CHIR (3 μM) for 16 hrs from stage HH1 onward (time after which control embryos reach stage HH3+). The embryos generate a large circular invagination that engulfs the central epiblast. (B) SNAI2 expression in the embryo shown in (A). (C) Two sections taken at the white lines in (A) showing SNAI (green), FN1 (red), and actin (blue). Note that the FN1 layer is not fragmented at the site of invagination. (D) Lightsheet microscope overview image of an embryo at the end of the experiment overlaid with a deformation mesh (typical for four of five embryos in the light sheet microscope). (E) Strain rate tensor for the embryo shown in (D). Isotropic strain rate component shown as color blue for contraction and red for expansion. The anisotropic part of the strain rate tensor is shown as black and white bars indicating the magnitude and direction of contraction. (F) Cells that will ingress through the streak [domain of attraction (DOA)] during the experiment calculated from the dynamic morphoskeleton (DM). (G) Schematic of the development of a contractile ring which in the absence of EMT results in the invagination of the central epiblast. (H to N) Same layout as (A) to (F) for embryos treated for 16 hrs with the vascular endothelial growth factor (VEGF) receptor inhibitor axitinib (100 nM). (H) Bright field image of axinitib treated embryo. (I) SNAI2 expression in embryo shown in (H). Axinitib treated embryos show limited breakdown of FN1 in the posterior streak (J), an inhibition of the isotropic contraction in the region of the primitive streak (L), a pronounced folding in the anterior streak (K) and a reduced ingression of mesendoderm cells in the streak (M) (four of five in the light sheet microscope). (N) Schematic of the formation of the primitive streak, which in the absence of EMT buckles into the embryo.
Analysis of the anisotropic strain rate (Fig. 2E) in these embryos showed a pronounced contraction oriented tangentially to the ectopic mesendoderm ring produced by cell intercalation (movie S8). The tangential intercalation drives the contraction of the ring, and because these cells do not undergo EMT (Fig. 2C) supported by the absence of apical contraction (movie S8), this contraction does not result in ingression but instead in buckling. The measured negative isotropic strain rate at these stages of buckling indicates the disappearance of cells into the inside of the embryo rather than ingression. This situation reproduces some aspects of the tissue organization and flow during the closing blastopore in Xenopus, where tissue is invaginated as a continuous epithelial sheet (44) [see also figure 3H and movie 12 of (19) for the corresponding DM]. However, while Xenopus embryos invaginate their vegetal side during gastrulation, in LDN-treated chick embryos, the system resolves to engulf the central epiblast, corresponding to the animal side. This is most likely caused by the attachment of the extraembryonic edge of the embryo to the vitelline membrane functioning as an important mechanical constraint that prevents the invagination of the vegetal side (see also HNF3B expression in the next section).
Last, we explored the effects of blocking growth factors that have been implicated in EMT and the migration of mesoderm cells of the platelet-derived growth factor and vascular endothelial growth factor (VEGF) families (45, 46). Cells in the posterior portion of the primitive streak express VEGFR2 and manifest a chemotactic response to several VEGF isoforms after ingression. We observed that the use of the VEGF signaling inhibitor axitinib (Fig. 2, H to N, and movie S9) (47) or competitive VEGFR2-Fc fragments (fig. S9) results in the formation of a short fat streak when added at early stages (2 to 3 hours of incubation). Light sheet microscopy of developing embryos showed that cell intercalation was not significantly inhibited (Fig. 2L), and the vortical flow of tissue into the ventral midline of the embryo continued (movie S10). However, there was a distinct absence of ingression of cells in the streak evidenced by the absence of tissue contraction in the midline (Fig. 2L and fig. S9, A and B) and reduction of FN1 degradation (Fig. 3, axitinib).
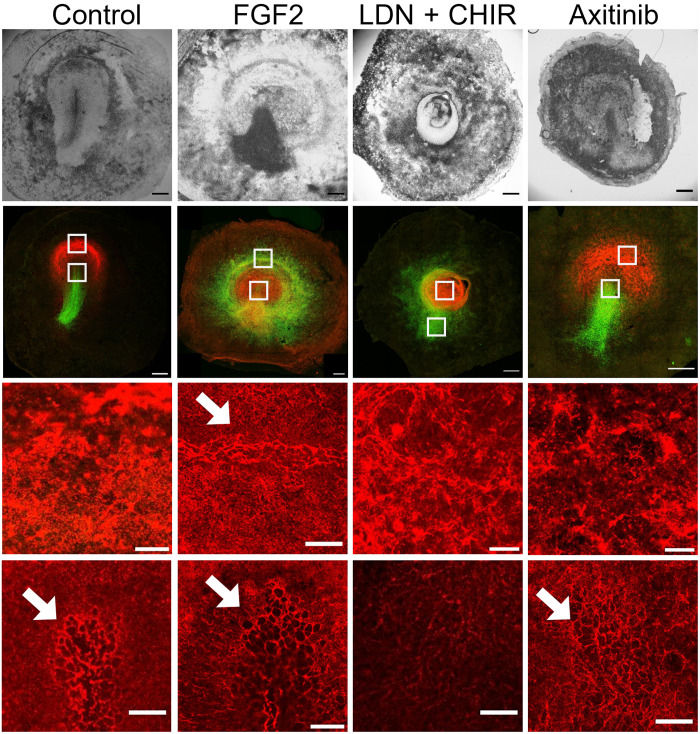
Fig. 3. Comparison of SNAI and FN1 expression after various perturbations.
Top row: Bright-field images of embryos after 16 hours in EC culture after various treatments. Second row: Confocal images of the embryos in the top row showing the expression of SNAI (green) and FN1 (red). Third and fourth rows: Higher-magnification images taken in front of the streak (top white square) and at the tip of the streak (bottom white square). It can be seen in the control (first row) that the region anterior to the streak expresses high levels of FN1, forming a densely packed meshwork of fibrils. In the streak, FN1 shows a larger pore meshwork (arrows) probably associated with cell ingressions. Note that in the ring-shaped streak after FGF treatment (second column), FN1 shows the same large porous structure as in the streak, indicating that this is also a region where the cells ingress. After LDN and axitinib treatment, the anterior region shows a dense fibrillar network, while in the streak or ring cases, the porous network is less well developed as in the control or after FGF treatment. The data show that the presence or absence of ingression is reflected in the FN1 network structure. Scale bars, 500 μm (two top rows) and 100 μm (two bottom rows).
The lack of cell ingression at the primitive streak in combination with an unaltered rate of cell division (fig. S9, C and D) results in more cells flowing into the midline than flowing out of it. When the epiblast cannot accommodate more tissue, the anterior portion of the streak can buckle inside (movie S11). Furthermore, the rate of cell proliferation is not affected by blocking VEGF signaling (fig. S9, C and D), suggesting that the buckling is not the result of reduced tissue fluidity produced by a reduced number of dividing cells (48). The resulting fold structure might resemble the blastoporal canal observed in chameleon gastrulation (49) [see also figure 3D and movie 7 of (19) for the corresponding DM]. Chameleon gastrulation is bimodal, they internalize part of the anterior mesoderm by involution through the blastoporal canal, and the posterior mesoderm is internalized by a limited amount by individual cell ingressions through the blastoporal plate (50). Intriguingly, the reduction of the individual cell ingression in the primitive streak of the chick embryo can originate a similar anterior invagination.
Mode of ingression and cell type identities after perturbation
The breakdown of the basement membrane is necessary for EMT to occur and cells to ingress through the primitive streak (5, 25, 51). To further characterize the mode of ingression associated with each chemical perturbation, we analyzed the breakdown of the basement membrane by inspecting the meshwork of FN1 (Fig. 3) and tenascin C (TNC; fig. S10) (25, 52, 53). During normal development, the anterior epiblast shows a very dense FN1 layer (Fig. 3, control). The region of SNAI2 expression in the primitive streak, where cells undergo EMT and ingress, is associated with the formation of large pores in the FN1 network (Fig. 3, control), as has been observed in mice (54). Furthermore, this region is characterized by the assembly of oriented fibers of TNC (fig. S10, control). This situation is fully reproduced in the ectopic ring of SNAI2 expression generated in FGF2-treated embryos (Fig. 3, FGF2, and fig. S10, FGF2), further supporting the idea that this ring functions as a circular primitive streak (Fig. 1, H to N).
Instead, the ectopic ring of SNAI2 expression generated in LDN-treated embryos is not associated with large pores in the FN1 network (Fig. 3, LDN + CHIR) or the TNC fiber assembly (fig. S10, LDN), suggesting that cell ingression associated with EMT is blocked in LDN-treated embryos. Combined LDN and FGF treatment, which results in the formation of a large invagination, indicating that LDN dominates at the morphogenetical level (fig. S5, G to K), shows FN1 breakdown (fig. S5I, arrow) and assembly of TNC fibers (fig. S10, FGF2 + LDN), indicating that there could be a partial EMT and possibly some cell ingression. Last, axitinib-treated embryos show a less defined FN1 meshwork along the primitive streak (Fig. 3, axitinib), associated with a reduction of cell ingression.
In addition, we studied whether the ectopic rings of SNAI2+ cells generated with different treatments have a more endodermal or mesodermal character. The tip of the primitive streak is a source of endoderm precursors, and it is characterized by the expression of the transcription factor HNF3B, a marker of the definitive endoderm (55–57). We noted that cells in the tip of the streak showed reduced levels of SNAI2 expression concomitant with elevated HNF3B expression (figs. S11 and S12, control). It is noteworthy that scattered HNF3B-expressing cells are also found in the epiblast anterior to the streak (fig. S12, control). Among the ectopic ring of SNAI2-expressing cells in FGF-treated embryos, some scattered HNF3B+ cells can also be observed; however, their expression levels and density are not the same as in the tip of the streak remnants, suggesting that FGF treatment especially induces mesoderm precursors (fig. S12, FGF). In LDN-treated embryos, a large population of the SNAI2-expressing cells in the ring coexpresses HNF3B (fig. S12, LDN), which might indicate that these cells are shifted toward an endoderm precursor fate. Furthermore, LDN-treated embryos show elevated HNF3B expression across the central epiblast (fig. S12, LDN), which will be engulfed by the contracting ring. Intriguingly, this makes this process more similar to Xenopus gastrulation, where the endoderm is also engulfed by the contracting blastopore.
Aligned myosin cables direct intercalations
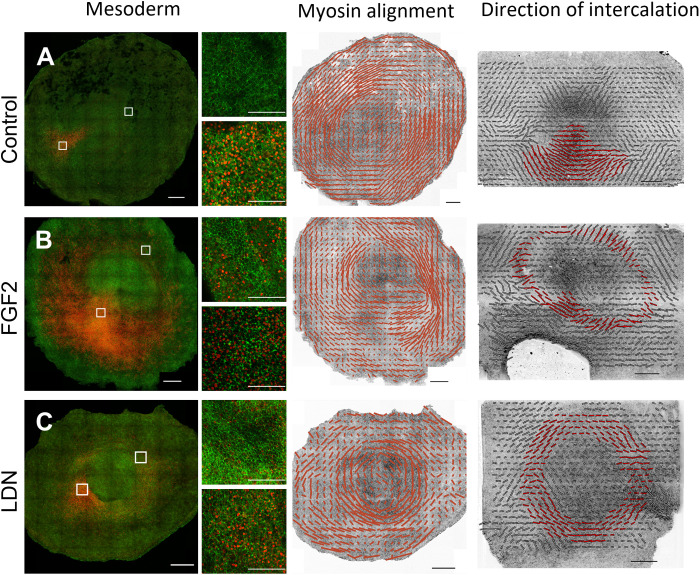
Our experiments show that the shape of the mesendoderm, together with cell intercalation and ingression behaviors, determines the morphogenetic outcome of gastrulation. While cell intercalations drive the main in-plane tissue flows, the presence or absence of individual cell ingressions determines whether the tissue will remain flat or will buckle. During normal development, the direction of intercalations of mesendoderm cells is strongly correlated with the direction of cables of phosphorylated myosin II that span several cells (15). At early gastrulation stages, these myosin cables extend from posterior to anterior, creating a semicircle between the embryonic and extraembryonic territories, and they correlate with the direction of intercalation, as shown by the intercalation (P) tensor (58) computed from cell tracking data (Fig. 4A), as described in detail in our previous work (15). We show that this strong correlation between mesendoderm expression domain and myosin planar cell polarity persists in the cases of circular mesendoderm domains, as verified with the FGF2 and LDN treatments (Fig. 4, B and C). As in normal early development, the myosin planar cell polarity is initially oriented along the long axis of the mesendoderm expression domain, in the same direction of cell intercalations, which explains the contraction of the ring in the LDN + CHIR case. However, during normal streak extension at late stages (HH3 to HH4), cell intercalations orient perpendicular to the long axis of the mesendoderm (the primitive streak) driven by cell ingression in the streak. The same occurs in embryos treated with FGF, where many cells ingress through the circular streak (negative values in the isotropic part of the strain rate), which results in the reorientation of the direction of intercalation of nearby cells perpendicular to the ring (direction of the shear). Presumably, in the LDN case, the cell intercalations continue to occur along the long axis of the mesendoderm (tangential to the ring) because cell ingression and EMT are never activated.
Fig. 4. Comparison of the patterns of mesendoderm differentiation, myosin alignment, and the direction of cell intercalation.
Top row: Control embryo after 16 hours of incubation in EC culture as described in Materials and Methods. Second row: Embryo treated with FGF2 for 16 hours. Third row: Embryo treated with LDN (100 nM) for 16 hours (100 nM LDN + 3 μM CHIR for the P tensor). First column: SNAI2+ expression (red) and pMLC (green). The regions in the top and bottom white squares in (A) to (C) are shown at higher magnification on the right of these images as top and bottom images, respectively. Second main column: The quantitation of myosin cable alignment. Scale bars represent ∼30% anisotropy. Third main column: The direction of intercalation calculated as the anisotropic component of the intercalation (P) tensor (58). The scale bar represents a magnitude of ∼0.4 1/h. Regions of intercalating mesendoderm cells are highlighted in red. Scale bars, 500 μm (A to C) and 100 μm (small regions).
A mechanochemical model describes tissue flows: Predictions and implications
To quantify these observations, we propose and explore a theoretical model for gastrulation as a mechanosensitive self-organizing process in the accompanying paper (19). Our continuum model couples the myosin-driven tension-sensitive active stress to tissue flow and cable ordering and leads to an interpretation of gastrulation as the result of an instability in the early embryo (fig. S13) (19). Our model can recapitulate these different gastrulation modes by varying the extension of the mesendoderm (an initial condition of our model) and a nondimensional parameter modulating the amount of active cell ingression [see figures 2 to 4 and movies 2 to 12 of (19)]. Notably, providing only the initial experimental distribution of actomyosin cables’ orientation and active myosin intensity, our model predicts the observed orientation of the cable, the myosin intensity, cell velocities, and velocity divergence at later times without data fitting. The model predicts a strong correlation between the mesendoderm expression domain and myosin planar cell polarity, which persists in the cases of circular mesendoderm domains, as verified with the FGF2 and LDN treatments (fig. S14). By varying the initial mesendoderm domain shape in combination with the degree of myosin-dependent ingression (p0, parameter), this model can also generate the cell flows and DOAs after the various perturbations described here and thus provide a mechanistic understanding of the biophysical mechanisms and role of tissue stresses and strains involved. For further details on the model and results, see (19).
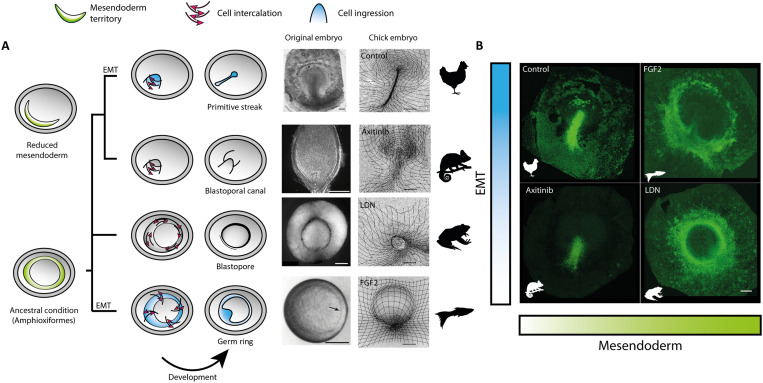
The manipulations described here are sufficient to induce large morphogenetic changes that recapitulate the hallmark dynamics and morphologies of different vertebrate gastrulation modes in a single organism, the chick embryo (Fig. 5). These results suggest that the modulation of the extension of the mesendoderm territory and the presence or absence of EMT controlling cell ingression largely determined the different morphologies observed during the evolution of vertebrate gastrulation.
Fig. 5. Changes in the extent of the mesendoderm territory and the degree of EMT could underlie the evolution of vertebrate gastrulation.
(A) Evolutionary relationships of representative vertebrate morphologies recapitulated in the chick embryo via modification of key cell behaviors. Images of gastrulation showing the blastopoal canal in chameleon (28 days), the blastopore in Xenopus (12 hours), and the germ ring in zebrafish (5.7 hours) are adapted from (33, 44, 49). Scale bars, 250 μm. (B) SNAI2+ cell distributions in HH3+ chick embryos after different treatments show that manipulating the extent of the mesendoderm territory and the level of EMT controlling ingression in the chick embryo reproduce critical morphological structures of gastrulation in other vertebrates. Scale bars, 500 μm (chick embryos).
DISCUSSION
Here, we perturbed the mechanisms that control directed cell intercalations and cell ingressions within the mesendoderm, which generate the forces that drive the tissue flows that generate the primitive streak during chick gastrulation. By targeting FGF, WNT, BMP, and VEGF developmental signaling pathways, known critical regulators of early development, we modulated the size and shape of the mesendoderm territory and uncoupled cell intercalation and ingression.
Our results suggest that FGF may predominantly induce mesoderm precursors, while inhibition of BMP signaling could result in the formation of excess endoderm precursors. These findings could suggest that the differentiation status of the cells affects how these cells internalize. However, it remains unclear whether signaling pathways that control differentiation can modulate cell behaviors, such as cell ingression, independently. Another open question is what is the fate of the cells that are internalized through these ectopic structures and how different internalization modes might affect the cell fate.
The experiments have also shown that directed intercalations along the long axis of the mesendoderm territory are a major part of the mechanism driving the formation of the internalization structure, which is a linear, a circular streak, or a partial or circular blastopore. These cell intercalations are driven by long myosin cables oriented along the long axis of the evolving mesendoderm territory (15). We have developed a mechanochemical model that, starting from an initial sickle- or ring-shaped mesendoderm precursor region containing a tension-dependent myosin recruitment mechanism, can explain the emergence of the tissue flows that drive the formation of the primitive streak and predict the dynamics of the domains of ingressing cells, which are in excellent agreement with those experimentally observed, both under normal and experimentally perturbed conditions (19). The model has highlighted and elucidated the importance of the mechanochemical feedback of the flow on myosin recruitment and organization of the epiblast tissue flow. However, the mechanisms that set the initial directionality of the myosin cable alignment during normal development and after the experimental perturbations described here resulting in the observed different mesendoderm territories morphologies, sickle or ring, remain to be elucidated.
The purpose of gastrulation is to internalize the mesoderm and endoderm precursors. Unexpectedly, despite being an essential phase of early development, the exact morphology of gastrulation shows considerable variation across the evolutionary tree. Mesoderm and endoderm precursors are typically formed on the surface of the developing embryo from epithelial tissues. Besides differences in the shape of the early embryos, spheres (frogs and fish) or sheets (amniotes), and size, there is a great diversity in the mode and geometry of the structures through which the cells internalize (1, 2). In spherical vertebrate embryos, the cells typically internalize through spherical structures such as the germ ring in fish and blastopores in amphibians, while in amniotes, cells internalize a structure known as the primitive streak. Furthermore, cells internalize either through ingression of individual cells, such as in amniotes, or through involution of cells in epithelial sheets, such as in Xenopus (44), or in a mixed mode as observed in reptiles (49).
Our experiments suggest that adjusting the extension of the mesendoderm territory, from a ring to a crescent, and regulating the capacity of these cells to undergo cell ingression are enough to invoke large morphogenetic changes reminiscent of other gastrulation modes. Similarly, it is possible to induce an invaginating gastrulation mode in a fly that normally gastrulates by stochastic individual cell ingression (Chironomus riparius) by expressing two genes (folded gastrulation and t48) that are known to control blastoderm invagination during gastrulation in another fly species (Drosophila melanogaster) (59). In yet another example, it is possible to switch the gastrulation mode of the sea anemone Nematostella vectensis from invagination to individual cell ingression by dissociating and reaggregating the cells in the embryo (60). Together, these experiments suggest that different gastrulation modes could easily evolve because relatively small quantitative changes in signaling, gene expression, or the developmental context can drive major morphogenetic transitions through their effects on the modulation of individual cell behaviors.
MATERIALS AND METHODS
Chemicals and other reagents
LDN, CHIR, axitinib, and SB50124 were acquired from Sigma-Aldrich. Hydromount was obtained from National Diagnostics. Ultrapure grade was acquired from Merck/VWR. Recombinant human FGF2 (233-FB) and recombinant human VEGFR2/KDR-Fc (357-KD-050) were obtained from (R&D Systems). Primary antibodies phospho-myosin light chain 2 (S19) mouse monoclonal antibody (mAb) (3675), phospho-myosin light chain 2 (T18, S19) rabbit mAb (3674), and SNAI2 (C19G7) rabbit mAb (9585) were from Cell Signaling Technology. The TNC mouse mAb M1-B4 and the Hnf3β/FoxA2 mAb 4C7 were from the Developmental Studies Hybridoma Bank. Alexa fluorophore-conjugated secondary antibodies were obtained from Invitrogen.
Chick lines and embryo culture
Fertile eggs from white Leghorn chicks were obtained from Henry Stewart & Co., Lincolnshire, UK. Membrane GFP embryos were obtained from the National Avian Research facility at Edinburgh University. Embryos were cultured using the Embryo Culture method according to published procedures (61).
Imaging
To image the complete chick embryo during gastrulation, we use a chick line expressing GFP in the cell membranes (myr-GFP) and a dedicated light sheet microscope as described in detail in (15). To cover the entire embryo (~4 mm in diameter), we acquire images at intervals of 1.84 μm at 45° with respect to the surface in two overlapping sequential scans (~5000 images of 2560 × 400 pixels), covering roughly an area of 3.2 mm by 4.7 mm every 3 min. Embryos are cultured during imaging as described in (42). Under these conditions, chick embryos can develop for more than 24 hours at 37°C (480 time points, >2 terabytes).
Image processing
Surface focusing
First, we transform the image volumes from the original 45° geometry to a 90° geometry. Once the image volumes have been transformed, we find the surface using a method based on the square gradient focusing algorithm (62, 63). Briefly, we tile the volume in 25 × 25 pixel columns and use the algorithm to find the position of the surface in each column, which constitutes the height map. Last, we apply a smoothing filter to the height map and use this to section the image volume to produce a two-dimensional image of the surface of the embryo (fig. S1A).
Velocity fields and strain rates
The velocity fields at the surface of the embryo are computed by digital PIV (fig. S1B) using PIVLab vs1.32 for MATLAB with two passes of 64 × 64 pixels and 32 × 32 pixels with a 50% overlap as in (15). The gradient of the velocity can be used to compute the strain rate at the surface of the embryo. They can be decomposed into two parts: the isotropic term, which describes the local rate of change in the area, and the anisotropic term, which describes the local rate of shear deformation. Together, they can be used as an indirect but robust measure of cell behaviors. In a confluent epithelial sheet, as the epiblast, the isotropic term is the product of the balance of changes in the cell area, cell divisions, and cell ingressions. In contrast, the anisotropic part is the result of directed cell intercalation or coordinated asymmetric changes in cell shape (fig. S1C). The implementation details can be found in (15).
Division detection
We developed a robust algorithm to detect and track individual cell divisions independently of image segmentation (63). During cytokinesis, epithelial cells round up on the surface, acquiring a very typical circular shape. We used the circular Hough transform implementation in MATLAB to find the characteristic circular shapes associated with dividing cells. The algorithm uses PIV to follow the dividing cells to avoid counting the same division event repeatedly between consecutive frames.
Myosin quantification
To quantify the myosin concentration and alignment, the position of the apical epiblast surface needs to be identified. To achieve this more reliably, we used the stronger actin signal to find the precise location of the apical surface using the above-described method (fig. S1, E and F). This surface projection was analyzed for myosin anisotropy using a Fourier transform–based algorithm (fig. S1G) (64) to find cellular anisotropy and size using a subtiling of the surface in 256 × 256 pixels and a 50% overlap between tiles.
Chemical manipulations
For in vivo imaging, the embryos were later transferred to imaging chambers as described in (65), and the chemical inhibitors were dissolved in the 10-ml albumen that covers the embryos during imaging. For immunocytochemistry experiments, chemical inhibitors were dissolved in the EC culture substrate. Recombinant human FGF2 and VEGFR2-Fc were added as a 1-μl drop (50 μg/ml) and carefully deposited on the hypoblast side of embryos in EC culture. For life imaging, the embryos were later transferred to imaging chambers maintaining the added protein on the hypoblast side of the embryo. All experiments were repeated two to three times, and each experiment typically contained six to eight embryos per treatment and six to eight internal control embryos to score variations in development success and timing between different batches of fertilized eggs obtained at different periods during the year.
Immunocytochemistry
After the chemical manipulations, the embryos were fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS) containing 0.1% Tween 20 on ice for 3 hours and later washed three times in PBS. Later, antibody detection was performed as described (15) followed by staining with 4′,6-diamidino-2-phenylindole and Alexa 405 Phalloidin. Embryos were mounted in Hydromount and imaged using a wide-field microscope or a Leica TCS SP8 confocal microscope at ×10 to ×20 magnifications. Selected embryos were processed for sectioning; first, embryos were embedded in 7.5% gelatin/15% sucrose, followed by freezing in a dry ice/isopentane mixture, and sectioned on a Leica cryostat. Sections were mounted on lysine-coated microscope slides and mounted with Hydromount.
Confocal microscopy
To image the embryos, confocal tile scans were taken at ×10 and ×20 magnifications using sequential line scanning on a Leica SP8 confocal microscope. To cover the thickness of the embryo, stacks of 20 to 40 slices were taken at 2- to 4-μm intervals along the z axis.
Dynamic morphoskeletons
Given a planar velocity field v(x, t), we compute the DM from the backward and forward finite-time Lyapunov exponents (FTLEs) (18). We compute the FTLE as
| (1) |
where λ2(x0) denotes the highest singular value of the Jacobian of the flow map and the flow map describing the trajectories from their initial x0 to final xf positions. To compute the FTLE, we first calculate by integrating the cell velocity field v(x, t) using the MATLAB built-in Runge-Kutta solver ODE45 with an absolute and relative tolerance of 10−6, linear interpolation in space and time, and a uniform dense grid of the initial conditions. Then, denoting the ith component of the flow map , we compute the deformation gradient using the finite-difference approximation
| (2) |
where δ is the initial conditions’ grid spacing. After computing , we use Eq. 1 for computing the FTLE field.
Acknowledgments
Funding: This work was supported by the EASTBIO BBSRC PhD student training grant 1785593 (to G.S.N.); Swiss National Foundation, Schmidt Science Fellowship, and the Postdoc Mobility Fellowship (to M.S.); NSF-Simons Center for Mathematical and Statistical Analysis of Biology Award 1764269 (to L.M.); NIH 1R01HD097068 (to L.M.); Simons Foundation (to L.M.); Henri Seydoux Fund (to L.M.); BBSRC grants BB/N009789/1, BB/K00204X/1, BB/R000441/1, BB/T006781/1 (to C.J.W.); and Wellcome Trust: Imaging equipment award 101468/Z/13/Z (to C.J.W.).
Author contributions: Conceptualization: M.C., G.S.N., M.S., L.M., and C.J.W. Methodology: M.C., G.S.N., M.S., and C.J.W. Investigation: M.C., G.S.N., and M.S. Supervision: L.M. and C.J.W. Writing—original draft: G.S.N. and C.J.W. Writing—review and editing: M.C., G.S.N., M.S., L.M., and C.J.W.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: High-resolution images of confocal data and the original high-resolution images from the light sheet microscopy (movies S1, S4, S7, and S9) have been deposited in the BioImage Archive under accession number S-BIAD553 (www.ebi.ac.uk/biostudies/BioImages/studies/S-BIAD553). Higher-resolution versions of movies S1 to S11 are available at https://doi.org/10.15132/10000190. All other data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S14
Other Supplementary Material for this : manuscript includes the following:
Movies S1 to S11
REFERENCES AND NOTES
- 1.C. D. Stern, Gastrulation: From Cells to Embryo (CSHL Press, 2004). [Google Scholar]
- 2.D. Arendt, K. Nübler-Jung,Rearranging gastrulation in the name of yolk: Evolution of gastrulation in yolk-rich amniote eggs. Mech. Dev. 81,3–22 (1999). [DOI] [PubMed] [Google Scholar]
- 3.L. Solnica-Krezel, Gastrulation: From Embryonic Pattern to Form (Academic Press, 2020). [Google Scholar]
- 4.R. Keller,Shaping the vertebrate body plan by polarized embryonic cell movements. Science 298,1950–1954 (2002). [DOI] [PubMed] [Google Scholar]
- 5.M. Williams, C. Burdsal, A. Periasamy, M. Lewandoski, A. Sutherland,Mouse primitive streak forms in situ by initiation of epithelial to mesenchymal transition without migration of a cell population. Dev. Dyn. 241,270–283 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D. Kimelman,Mesoderm induction: From caps to chips. Nat. Rev. Genet. 7,360–372 (2006). [DOI] [PubMed] [Google Scholar]
- 7.T. Merle, E. Farge,Trans-scale mechanotransductive cascade of biochemical and biomechanical patterning in embryonic development: The light side of the force. Curr. Opin. Cell Biol. 55,111–118 (2018). [DOI] [PubMed] [Google Scholar]
- 8.P.-F. Lenne, E. Munro, I. Heemskerk, A. Warmflash, L. Bocanegra-Moreno, K. Kishi, A. Kicheva, Y. Long, A. Fruleux, A. Boudaoud,Roadmap for the multiscale coupling of biochemical and mechanical signals during development. Phys. Biol. 18,041501 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.G. Sheng, A. M. Arias, A. Sutherland,The primitive streak and cellular principles of building an amniote body through gastrulation. Science 374,eabg1727 (2021). [DOI] [PubMed] [Google Scholar]
- 10.M. Valet, E. D. Siggia, A. H. Brivanlou,Mechanical regulation of early vertebrate embryogenesis. Nat. Rev. Mol. Cell Biol. 23,169–184 (2022). [DOI] [PubMed] [Google Scholar]
- 11.T. Brunet, A. Bouclet, P. Ahmadi, D. Mitrossilis, B. Driquez, A.-C. Brunet, L. Henry, F. Serman, G. Béalle, C. Ménager, F. Dumas-Bouchiat, D. Givord, C. Yanicostas, D. Le-Roy, N. M. Dempsey, A. Plessis, E. Farge,Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria. Nat. Commun. ,1–15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.E. Hannezo, C.-P. Heisenberg,Mechanochemical feedback loops in development and disease. Cell 178,12–25 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Y. Maroudas-Sacks, K. Keren,Mechanical patterning in animal morphogenesis. Annu. Rev. Cell Dev. Biol. 37,469–493 (2021). [DOI] [PubMed] [Google Scholar]
- 14.C. Collinet, T. Lecuit,Programmed and self-organized flow of information during morphogenesis. Nat. Rev. Mol. Cell Biol. 22,245–265 (2021). [DOI] [PubMed] [Google Scholar]
- 15.E. Rozbicki, M. Chuai, A. I. Karjalainen, F. Song, H. M. Sang, R. Martin, H. J. Knolker, M. P. MacDonald, C. J. Weijer,Myosin-II-mediated cell shape changes and cell intercalation contribute to primitive streak formation. Nat. Cell Biol. 17,397–408 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O. Voiculescu, L. Bodenstein, I. J. Lau, C. D. Stern,Local cell interactions and self-amplifying individual cell ingression drive amniote gastrulation. eLife 3,e01817 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.G. Serrano Nájera, C. J. Weijer,Cellular processes driving gastrulation in the avian embryo. Mech. Dev. 163,103624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.M. Serra, S. Streichan, M. Chuai, C. J. Weijer,Dynamic morphoskeletons in development. Proc. Natl. Acad. Sci. U.S.A. 117,11444–11449 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.M. Serra, G. Serrano Nájera, M. Chuai, V. Spandan, C. J. Weijer, L. Mahadevan, A mechanochemical model recapitulates distinct vertebrate gastrulation modes. bioRxiv 10.1101/2021.10.03.462928 (2021). 10.1101/2021.10.03.462928. [DOI] [PMC free article] [PubMed]
- 20.C. Alev, Y. Wu, Y. Nakaya, G. Sheng,Decoupling of amniote gastrulation and streak formation reveals a morphogenetic unity in vertebrate mesoderm induction. Development 140,2691–2696 (2013). [DOI] [PubMed] [Google Scholar]
- 21.O. Voiculescu, F. Bertocchini, L. Wolpert, R. E. Keller, C. D. Stern,The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature 449,1049–1052 (2007). [DOI] [PubMed] [Google Scholar]
- 22.M. Saadaoui, D. Rocancourt, J. Roussel, F. Corson, J. Gros,A tensile ring drives tissue flows to shape the gastrulating amniote embryo. Science 367,453–458 (2020). [DOI] [PubMed] [Google Scholar]
- 23.L. Gräper,Die Primitiventwicklung des Hühnchens nach stereokinematographischen Untersuchungen, kontrolliert durch vitale Farbmarkierung und verglichen mit der Entwicklung anderer Wirbeltiere. Dev. Genes Evol. 116,382–429 (1929). [DOI] [PubMed] [Google Scholar]
- 24.M. Chuai, W. Zeng, X. Yang, V. Boychenko, J. A. Glazier, C. J. Weijer,Cell movement during chick primitive streak formation. Dev. Biol. 296,137–149 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Y. Nakaya, E. W. Sukowati, Y. Wu, G. Sheng,RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat. Cell Biol. 10,765–775 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Y. Hatada, C. D. Stern,A fate map of the epiblast of the early chick embryo. Development 120,2879–2889 (1994). [DOI] [PubMed] [Google Scholar]
- 27.X. Yang, H. Chrisman, C. J. Weijer,PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development 135,3521–3530 (2008). [DOI] [PubMed] [Google Scholar]
- 28.M. A. Selleck, C. D. Stern,Fate mapping and cell lineage analysis of Hensen’s node in the chick embryo. Development 112,615–626 (1991). [DOI] [PubMed] [Google Scholar]
- 29.X. Yang, D. Dormann, A. E. Munsterberg, C. J. Weijer,Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev. Cell 3,425–437 (2002). [DOI] [PubMed] [Google Scholar]
- 30.A. Lawson, G. C. Schoenwolf,Epiblast and primitive-streak origins of the endoderm in the gastrulating chick embryo. Development 130,3491–3501 (2003). [DOI] [PubMed] [Google Scholar]
- 31.G. Zhao, W. Li, D. Chen, J. R. Henry, H.-Y. Li, Z. Chen, M. Zia-Ebrahimi, L. Bloem, Y. Zhai, K. Huss,A novel, selective inhibitor of fibroblast growth factor receptors that shows a potent broad spectrum of antitumor activity in several tumor xenograft models. Mol. Cancer Ther. 10,2200–2210 (2011). [DOI] [PubMed] [Google Scholar]
- 32.H. Acloque, O. H. Ocana, A. Matheu, K. Rizzoti, C. Wise, R. Lovell-Badge, M. A. Nieto,Reciprocal repression between Sox3 and snail transcription factors defines embryonic territories at gastrulation. Dev. Cell 21,546–558 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.C. B. Kimmel, W. W. Ballard, S. R. Kimmel, B. Ullmann, T. F. Schilling,Stages of embryonic development of the zebrafish. Dev. Dyn. 203,253–310 (1995). [DOI] [PubMed] [Google Scholar]
- 34.J. P. Trinkaus,Ingression during early gastrulation of Fundulus. Dev. Biol. 177,356–370 (1996). [DOI] [PubMed] [Google Scholar]
- 35.L. A. Rohde, C.-P. Heisenberg,Zebrafish gastrulation: Cell movements, signals, and mechanisms. Int. Rev. Cytol. 261,159–192 (2007). [DOI] [PubMed] [Google Scholar]
- 36.D. Pinheiro, C.-P. Heisenberg,Zebrafish gastrulation: Putting fate in motion. Curr. Top. Dev. Biol. 136,343–375 (2020). [DOI] [PubMed] [Google Scholar]
- 37.A. Streit, K. J. Lee, I. Woo, C. Roberts, T. M. Jessell, C. D. Stern,Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development 125,507–519 (1998). [DOI] [PubMed] [Google Scholar]
- 38.F. Bertocchini, C. D. Stern,Gata2 provides an early anterior bias and uncovers a global positioning system for polarity in the amniote embryo. Development 139,4232–4238 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.H. C. Lee, H.-C. Lu, M. Turmaine, N. M. M. Oliveira, Y. Yang, I. De Almeida, C. D. Stern,Molecular anatomy of the pre-primitive-streak chick embryo. Open Biol. 10,190299 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.C. F. Arias, M. A. Herrero, C. D. Stern, F. Bertocchini,A molecular mechanism of symmetry breaking in the early chick embryo. Sci. Rep. 7,15776 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.G. Sanchez-Duffhues, E. Williams, M. J. Goumans, C. H. Heldin, P. ten Dijke,Bone morphogenetic protein receptors: Structure, function and targeting by selective small molecule kinase inhibitors. Bone 138,115472 (2020). [DOI] [PubMed] [Google Scholar]
- 42.E. Rozbicki, M. Chuai, C. J. Weijer, Technique for liquid culture of early chick embryos suitable for long term live imaging (Research Square, 2015).
- 43.J. Chal, Z. Al Tanoury, M. Hestin, B. Gobert, S. Aivio, A. Hick, T. Cherrier, A. P. Nesmith, K. K. Parker, O. Pourquié,Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat. Protoc. 11,1833–1850 (2016). [DOI] [PubMed] [Google Scholar]
- 44.D. R. Shook, E. M. Kasprowicz, L. A. Davidson, R. Keller, Large, long range tensile forces drive convergence during Xenopus blastopore closure and body axis elongation. eLife 7, e26944 (2018). [DOI] [PMC free article] [PubMed]
- 45.A. Eichmann, C. Corbel, V. Nataf, P. Vaigot, C. Bréant, N. M. Le Douarin,Ligand-dependent development of the endothelial and hemopoietic lineages from embryonic mesodermal cells expressing vascular endothelial growth factor receptor 2. Proc. Natl. Acad. Sci. U.S.A. 94,5141–5146 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.M. Chuai, D. Hughes, C. J. Weijer,Collective epithelial and mesenchymal cell migration during gastrulation. Curr. Genomics 13,267–277 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.J. Ma, D. J. Waxman,Modulation of the antitumor activity of metronomic cyclophosphamide by the angiogenesis inhibitor axitinib. Mol. Cancer Ther. 7,79–89 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.J. Firmino, D. Rocancourt, M. Saadaoui, C. Moreau, J. Gros,Cell division drives epithelial cell rearrangements during gastrulation in chick. Dev. Cell 36,249–261 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.M. J. Stower, R. E. Diaz, L. C. Fernandez, M. W. Crother, B. Crother, A. Marco, P. A. Trainor, S. Srinivas, F. Bertocchini,Bi-modal strategy of gastrulation in reptiles. Dev. Dyn. 244,1144–1157 (2015). [DOI] [PubMed] [Google Scholar]
- 50.M. J. Stower, F. Bertocchini,The evolution of amniote gastrulation: The blastopore-primitive streak transition. Wiley Interdiscip. Rev. Dev. Biol. 6,1–17 (2017). [DOI] [PubMed] [Google Scholar]
- 51.K. Mogi, R. Toyoizumi,Invasion by matrix metalloproteinase-expressing cells is important for primitive streak formation in early chick blastoderm. Cells Tissues Organs 192,1–16 (2010). [DOI] [PubMed] [Google Scholar]
- 52.K. L. Crossin, S. Hoffman, M. Grumet, J. P. Thiery, G. M. Edelman,Site-restricted expression of cytotactin during development of the chicken embryo. J. Cell Biol. 102,1917–1930 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Y. Nakaya, G. Sheng,An amicable separation: Chick’s way of doing EMT. Cell Adh. Migr. 3,160–163 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.C. Kyprianou, N. Christodoulou, R. S. Hamilton, W. Nahaboo, D. S. Boomgaard, G. Amadei, I. Migeotte, M. Zernicka-Goetz,Basement membrane remodelling regulates mouse embryogenesis. Nature 582,253–258 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.S. C. Chapman, F. R. Schubert, G. C. Schoenwolf, A. Lumsden,Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev. Biol. 245,187–199 (2002). [DOI] [PubMed] [Google Scholar]
- 56.S. C. Chapman, K. Matsumoto, Q. Cai, G. C. Schoenwolf,Specification of germ layer identity in the chick gastrula. BMC Dev. Biol. 7,1–16 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.I. Burtscher, H. Lickert,Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development 136,1029–1038 (2009). [DOI] [PubMed] [Google Scholar]
- 58.F. Graner, B. Dollet, C. Raufaste, P. Marmottant,Discrete rearranging disordered patterns, part I: Robust statistical tools in two or three dimensions. Eur. Phys. J. E 25,349–369 (2008). [DOI] [PubMed] [Google Scholar]
- 59.S. Urbansky, P. G. Avalos, M. Wosch, S. Lemke,Folded gastrulation and T48 drive the evolution of coordinated mesoderm internalization in flies. eLife 5,e18318 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.K. Anastasia, G. Grigory, P. Ekaterina, D. Adrien, K. Yulia, T. Ulrich,Germ-layer commitment and axis formation in sea anemone embryonic cell aggregates. Proc. Natl. Acad. Sci. U.S.A. 115,1813–1818 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.S. C. Chapman, J. Collignon, G. C. Schoenwolf, A. Lumsden,Improved method for chick whole-embryo culture using a filter paper carrier. Dev. Dyn. 220,284–289 (2001). [DOI] [PubMed] [Google Scholar]
- 62.A. M. Eskicioglu, P. S. Fisher,Image quality measures and their performance. IEEE Trans. Commun. 43,2959–2965 (1995). [Google Scholar]
- 63.G. Serrano Nájera, thesis, University of Dundee (2021). [Google Scholar]
- 64.M. Durande, S. Tlili, T. Homan, B. Guirao, F. Graner, H. Delanoe-Ayari,Fast determination of coarse-grained cell anisotropy and size in epithelial tissue images using Fourier transform. Phys. Rev. E 99,62401 (2019). [DOI] [PubMed] [Google Scholar]
- 65.E. Rozbicki, M. Chuai, C. J. Weijer, Liquid culture technique for early chick embryos suitable for long term live imaging. Nat. Protoc. Exchange 2015). 10.1038/protex.2015.012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S14
Movies S1 to S11