Abstract
Neutrophils play a critical role in the clearance of bacteria from the lung and other organs by their capacity for phagocytosis and killing. Previously, we identified an important role for the leukotrienes in rat alveolar macrophage phagocytosis of Klebsiella pneumoniae. In this report, we explored the possibility that the leukotrienes play an important role in phagocytosis by neutrophils as well. Inhibition of endogenous leukotriene synthesis by 5-lipoxygenase knockout in mice or by pharmacologic means in human peripheral blood neutrophils attenuated phagocytosis of opsonized K. pneumoniae. Reduced phagocytosis was also observed in human neutrophils pretreated with a leukotriene B4 receptor but not a cysteinyl-leukotriene receptor antagonist. While leukotriene B4 reconstituted defective phagocytosis in leukotriene-deficient neutrophils and enhanced phagocytosis in neutrophils capable of leukotriene synthesis, leukotriene C4, leukotriene D4, 5-hydroperoxyeicosatetraenoic acid, and 5-oxo-eicosatetraenoic acid were ineffective. To determine the opsonin dependence of the leukotriene B4 augmentation of phagocytosis, we assessed the ability of leukotriene B4 to modulate neutrophil phagocytosis and the adherence of sheep erythrocytes opsonized with immunoglobulin G or the complement fragment C3bi. While leukotriene B4 augmented both Fc receptor- and complement receptor-mediated phagocytosis, increased adherence to leukotriene B4-treated neutrophils was limited to complement opsonized targets. In conclusion, we have identified a novel role for leukotriene B4 in the augmentation of neutrophil phagocytosis mediated by either the Fc or complement receptor.
The alveolar epithelial surface represents the body's largest interface with the external environment, and it is continually challenged with bacteria aspirated from the oropharynx that evade the mechanical clearance mechanisms of the upper respiratory tract. The alveolar macrophage (AM) is the primary means of innate host defense against bacterial pathogens that attempt to reach the alveolar space. When the bacterial burden in the alveolar space is great enough to activate a sufficient number of AMs to secrete chemotactic substances such as chemokines, leukotriene B4 (LTB4), and complement fragments, polymorphonuclear leukocytes (PMNs) are recruited from a marginated pool in the pulmonary vasculature to the alveolar epithelial surface (19). PMNs play a critical role in clearing encapsulated bacteria, such as Klebsiella pneumoniae, from the lung and in preventing bacterial dissemination to the systemic circulation (26). The ability of these cells to perform this function can be enhanced by inflammatory mediators that activate the PMN for increased phagocytosis and killing of ingested bacteria.
The LTs are potent lipid mediators of inflammation derived from the 5-lipoxygenase (5-LO)-catalyzed oxygenation of arachidonic acid (AA). The enzyme 5-LO in concert with the 5-LO activating protein can oxygenate AA to form the intermediate metabolite, 5-hydroperoxyeicosatetraenoic acid. This 5-LO product can either be dehydrated to LTA4 or reduced to 5-hydroxyeicosatetraenoic acid (5-HETE). LTA4 can be hydrolyzed to form the potent PMN chemoattractant LTB4 or conjugated with glutathione to form the cysteinyl-LTs (LTC4, LTD4, and LTE4), well known to elicit smooth muscle contraction and microvascular permeability (15).
LTs are readily acknowledged as important mediators of pathologic states of inflammation such as asthma. To ascertain whether LTs participate in the homeostatic form of inflammation, i.e., antimicrobial host defense, we subjected 5-LO knockout (KO) mice to an intrapulmonary challenge with K. pneumoniae (2). Compared to their wild-type (WT) counterparts, the LT-deficient mice exhibited enhanced lethality and reduced bacterial clearance from the lung in this model. This defect in 5-LO KO mice in vivo was associated with reduced AM phagocytosis and killing of K. pneumoniae in vitro. Further in vitro studies indicated that the inhibition of LT synthesis by pharmacologic means also reduced AM phagocytosis of bacteria. Moreover, exogenous LTs restored phagocytosis when added to LT-deficient AMs and also augmented phagocytosis above baseline capacity when added to LT-competent AMs (17) (2). Finally, mechanistic studies revealed that the LT augmentation of phagocytosis in AMs was largely Fc receptor (FcR) mediated and protein kinase C dependent in AMs (16).
Since PMNs are professional phagocytes known to synthesize LTs and express plasma membrane receptors for LTs, we explored the possibility that these mediators modulate PMN phagocytosis of K. pneumoniae as they do in AMs. In this study, we demonstrate that PMN phagocytosis of K. pneumoniae is indeed augmented by LTB4 and that the enhancement in PMN phagocytosis can occur through FcR- or complement receptor (CR)-mediated phagocytosis.
MATERIALS AND METHODS
Cell isolation and culture.
Human PMNs, isolated from venous blood from healthy volunteers, were purified by centrifugation through Polymorphprep (Nycomed Pharma, Oslo, Norway), followed by hypotonic lysis of erythrocytes (3). Elicited PMNs were obtained from 129/SvEv WT and 5-LO KO mice by peritoneal lavage 4 h after an intraperitoneal injection of 1% glycogen solution in saline. Ninety percent of the cells obtained by peritoneal lavage were identified as PMNs by a modified Wright-Giemsa stain (Diff-Quik; American Scientific Products, McGaw Park, Ill.). Following PMN isolation, the cells were enumerated using a hemocytometer and suspended in RPMI 1640 (Gibco, Grand Island, N.Y.) to a final concentration of 5 × 105 cells/ml. For phagocytosis experiments, 105 PMNs were adhered to glass eight-well Falcon culture slides (Becton Dickinson, Franklin Lakes, N.J.) for 1 h in RPMI 1640.
K. pneumoniae preparation.
K. pneumoniae strain 43816 (serotype 2; American Type Culture Collection) was grown in tryptic soy broth (Difco, Detroit, Mich.) for 18 h at 37°C. The concentration of bacteria in culture was determined spectrophotometrically (A600) (10).
Pharmacologic modulation of PMNs.
In some experiments, PMNs were pretreated for 15 min with the 5-LO enzyme inhibitor zileuton (10 μM; Abbott Laboratories, Chicago, Ill.), the 5-LO activating protein inhibitor MK-886 (1 μM; Merck-Frosst, Montreal, Quebec, Canada), the cysteinyl-LT receptor antagonist LY171883 (1 μM), or the LTB4 receptor antagonist LY292476 (1 μM) (the latter two from Eli Lilly, Indianapolis, Ind.), each diluted in Hanks balanced salt solution. The use of these pharmacologic agents at these doses was previously shown to inhibit LT synthesis and block the LT receptors (4, 7) and reduce phagocytosis of K. pneumoniae in AMs (17).
Phagocytosis of K. pneumoniae.
Following the addition of 106 K. pneumoniae opsonized with 1% immune serum (17), PMN cultures were incubated for 30 min at 37°C. Following the incubation period, the extracellular bacteria were removed by three washes with Hanks balanced salt solution. The monolayers containing bacteria were stained with Diff-Quik and enumerated. For each slide, a standard pattern of high-powered fields was examined by light microscopy (1,000 × objective) to enumerate 100 cells. By comparing the phagocytic index in the presence and absence of cytochalasin D (20), we determined that 90% of the cell-associated bacteria using this method were actually internalized.
Preparation of E opsonized with IgG or C3bi.
Sheep erythrocytes (E; ICN Biomedicals, Costa Mesa, Calif.) were washed and opsonized with either rabbit anti-E immunoglobulin G (IgG) (ICN Biomedicals, Inc.) or IgM (ICN Biomedicals) and C3bi (Sigma) as described previously (1, 29). Opsonized E were enumerated using a hemocytometer and diluted to a final concentration of 20 × 107 E/ml.
Phagocytosis of opsonized E.
E-IgG or E-C3bi (107) was added to the PMN monolayers following pretreatment with medium alone (control), LTs, or LT modulators for 5 to 10 min; 15 min later, internalization of E-C3bi was initiated by the addition of 15 nM phorbol myristate acetate (PMA). After a 30-min incubation at 37°C, water was added to each well for 10 s followed by 3× saline to lyse the surface-bound E and to restore isotonic conditions. A phagocytic index was calculated as described previously (17).
Adherence of opsonized E.
To assess adherence of E-IgG or E-C3bi to the surface of PMNs, monolayers were incubated with cytochalasin D (5 μg/ml) at 37°C in 5% CO2, which would allow surface binding of E's, but not phagocytosis (13). Thirty minutes after the addition of opsonin-coated E's, the total number of adherent targets was enumerated for 100 cells, using the microscopic procedure described above for phagocytosis. The adherence index was calculated by determining the total number of adhered E-IgG or E-C3bi per 100 PMNs.
Statistical analysis.
A minimum of three replicate wells per condition was studied in each experiment, and the number of individual experiments is indicated in the figure legends. Data are expressed as the mean ± standard error (SE). Where appropriate, mean values were compared using a paired t test, a one-way analysis of variance, or a Kruskal-Wallis test on ranks for nonparametric data. The Dunnett's test or the Student-Newman-Keuls tests were used for mean separation. In all cases, a P value of <0.05 was considered significant.
RESULTS
Inhibition of endogenous LT synthesis reduces PMN phagocytosis of K. pneumoniae.
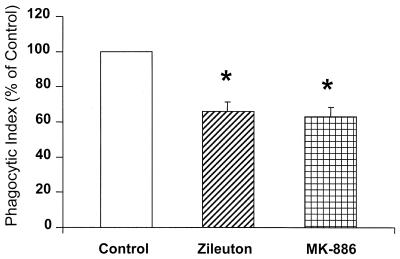
To determine if endogenously produced LTs have a functional role in PMN phagocytosis, human PMN monolayers were pretreated with either zileuton (10 μM) or MK-886 (1 μM) at concentrations known to inhibit LT synthesis. They were then incubated with K. pneumoniae opsonized with immune serum. Both agents, which block LT synthesis by distinctly different mechanisms of action, reduced phagocytosis of opsonized K. pneumoniae by approximately 35% (P < 0.05) (Fig. 1). The results of this experiment indicate that endogenously produced LTs are necessary for maximal bacterial phagocytosis in human PMNs.
FIG. 1.
Effect of endogenous LT synthesis inhibition on PMN phagocytosis of opsonized K. pneumoniae. Human PMNs were pretreated in the absence (control) or presence of zileuton (10 μM) or MK-886 (1 μM) for 15 min prior to the addition of K. pneumoniae opsonized with 1% immune serum. The phagocytic index of the control was 93 ± 23. Data are presented as the mean ± SE (n = 5). ∗, P < 0.05 with respect to the control, using a paired t test.
Decreased phagocytosis of opsonized K. pneumoniae by elicited PMNs from 5-LO KO mice.
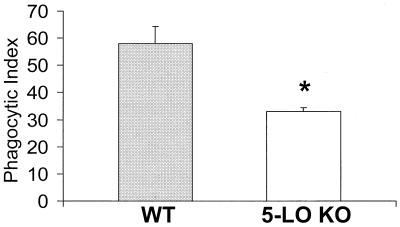
During bacterial infection of the lung and other organs, PMNs are recruited to tissue from the peripheral circulation in response to a chemoattractant gradient. During the recruitment process, the PMN encounters a number of mediators which prime the cells for enhanced antimicrobial defense. These include tumor necrosis factor alpha, gamma interferon, interleukin-12, and LTs. To assess the importance of endogenously generated LTs in phagocytosis by PMNs recruited to a site of inflammation, we assessed phagocytosis of opsonized K. pneumoniae in PMNs recruited in response to glycogen instillation into the peritoneal cavity of 5-LO KO and WT mice. Phagocytosis by elicited peritoneal PMNs obtained from 5-LO KO mice was reduced by approximately 50% compared with cells from WT animals (P < 0.05) (Fig. 2). This reduction in phagocytosis underscores the importance of LTs in the antimicrobial function of PMNs at a site of inflammation.
FIG. 2.
Effect of 5-LO gene knockout on phagocytosis of opsonized K. pneumonia by elicited peritoneal PMNs. Data are presented as the mean ± SE (n = 3). ∗, P < 0.05 with respect to the control, using a paired t test.
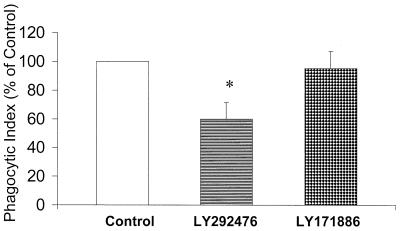
LTB4 receptor antagonist attenuates phagocytosis.
In a previous study (17), we determined that receptor antagonists to both LTB4 and cysteinyl-LTs in rat AMs reduced phagocytosis. This indicated that endogenous production of both classes of LTs augmented phagocytosis. We used this same approach to determine the contribution of endogenously produced LTs of each class to phagocytosis by human PMNs. The LTB4 receptor antagonist LY292476 reduced bacterial phagocytosis by approximately 40% (P < 0.05) (Fig. 3), indicating that endogenously produced LTB4 was produced during phagocytosis, exited the cell, and activated the plasma membrane receptor for LTB4. In contrast, the cysteinyl-LT receptor antagonist LY171886 had a minimal effect on PMN phagocytosis.
FIG. 3.
Effect of LT receptor antagonists on phagocytosis of opsonized K. pneumoniae by human PMNs. The phagocytic index of the control was 93 ± 23. Data are presented as the mean ± SE (n = 4). ∗, P < 0.05 with respect to the control, using a paired t test.
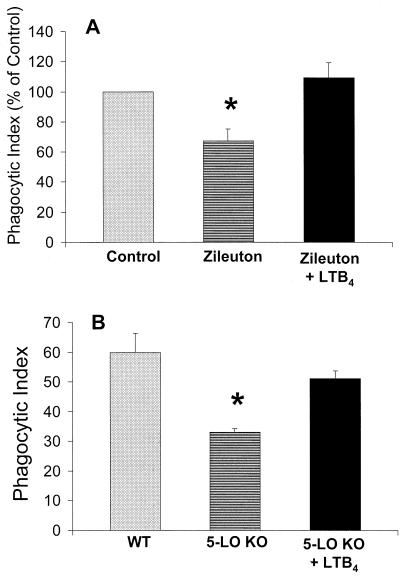
Exogenous LTB4 restores phagocytosis in LT-deficient human and murine PMNs.
To determine the specificity of the relationship between various LTs and phagocytosis, we examined whether exogenous LTs could restore the impairment of phagocytosis observed in human PMNs pretreated with zileuton and elicited PMNs from 5-LO KO mice. Prior to the addition of opsonized K. pneumoniae, exogenous LTB4 or LTC4 (1 nM) was added to human PMNs pretreated for 15 min with zileuton (10 μM) or to murine elicited PMNs. The addition of exogenous LTB4 completely restored phagocytosis in human PMNs (Fig. 4A) and nearly restored phagocytosis in murine elicited PMNs (Fig. 4B). In contrast, the addition of exogenous LTC4 did not enhance phagocytosis in either instance (data not shown).
FIG. 4.
Ability of exogenous LTB4 to restore phagocytosis in human PMNs pretreated with zileuton (10 μM) for 15 min (A) or in elicited murine PMNs from 5-LO KO mice (B). The phagocytic index of the control (A) was 50 ± 2. Data are expressed as the mean ± SE (n = 3). ∗, P < 0.05 with respect to the control, using a paired t test.
Exogenous LTB4 dose dependently augments phagocytosis of opsonized K. pneumoniae in PMNs capable of LT synthesis.
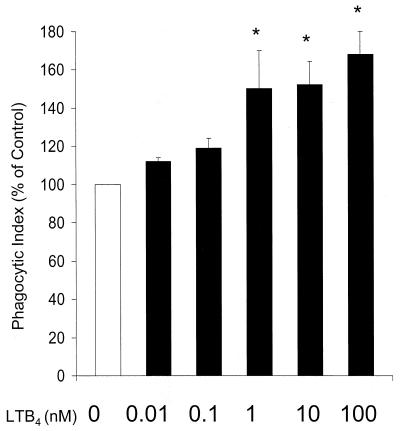
Having established the importance of endogenously produced LTB4 for full phagocytic capacity of PMNs, we considered the possibility that exogenously added LTs may augment PMN phagocytosis even in cells competent for LT synthesis. Human PMNs were incubated with various doses (0.01 to 100 nM) of exogenous LTB4, LTD4, LTC4, 5-oxo-eicosatetraenoic acid (5-oxo-ETE), or 5-HETE for 5 min prior to the addition of opsonized K. pneumoniae. As shown in Fig. 5, phagocytosis was enhanced above the basal level with LTB4 in a dose-dependent fashion, reaching statistical significance at 1 to 100 nM. In contrast, none of the other lipids were able to significantly enhance phagocytosis at any of the doses tested (data not shown).
FIG. 5.
Effect of exogenous LTB4 dose on human PMN phagocytosis of K. pneumoniae opsonized with 1% immune serum. Human PMNs were incubated with LTB4 in the dose indicated for 5 to 10 min prior to the addition of opsonized K. pneumoniae. The phagocytic index of the control was 40 ± 3. Data are expressed as the mean ± SE (n = 3). ∗, P < 0.05 with respect to the control by analysis of variance.
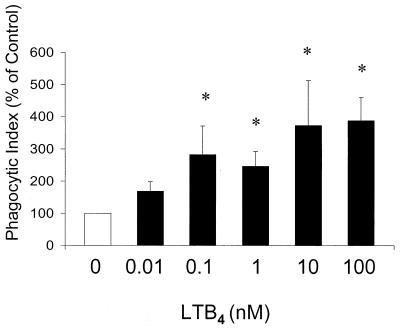
LTB4 increases human PMN phagocytosis of E-IgG.
The phagocytic target used in all of the previously described experiments was K. pneumoniae opsonized with 1% immune serum. Immune serum would be expected to contain two distinct opsonins, IgG and complement. To determine whether FcR-dependent phagocytosis in human PMNs is modulated by LTB4, cells were pretreated with increasing doses of LTB4 for 10 min prior to a 30-min incubation with E-IgG. After incubation, extracellular Es were removed by hypotonic lysis and a phagocytic index was calculated to determine the number of ingested E-IgG per 100 PMNs. In a dose-dependent fashion, LTB4 enhanced phagocytosis of E-IgG, with the maximum effect (∼3.5-fold the control level) observed at 10 to 100 nM (Fig. 6). We next explored the possibility that LTB4 enhanced phagocytosis of E-IgG by increasing target adherence. Following pretreatment with LTB4, PMN monolayers were cultured at 37°C and cooled to 15°C prior to the addition of E-IgG, in order to allow adherence but not phagocytosis (9). In contrast to the effects on phagocytosis demonstrated in Fig. 6, E-IgG adherence to human PMNs was not significantly affected by LTB4 pretreatment at doses up to 100 nM (control phagocytic index of 100 versus phagocytic index with 100 nM LTB4 of 99 ± 11). These results indicate that LTB4 augments the ability of the PMN to internalize, rather than to bind, E-IgG.
FIG. 6.
Effect of exogenous LTB4 dose on human PMN phagocytosis of E-IgG. Human PMNs were incubated with LTB4 for 5 to 10 min prior to the addition of E-IgG. The phagocytic index of the control was 20 ± 5. Data are expressed as the mean ± SE (n = 3). ∗, P < 0.05 with respect to the control by Kruskal-Wallis test on ranks, using Dunnett's test for means separation.
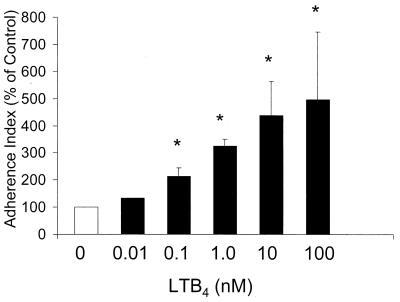
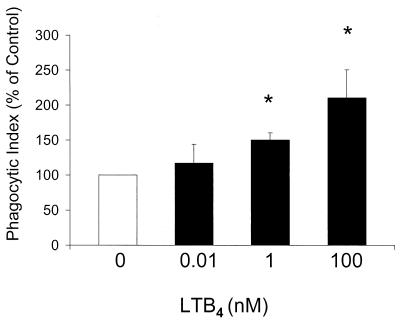
LTB4 enhances human PMN adherence and PMA-stimulated phagocytosis of E-C3bi.
Since LTB4 is known to cause mobilization of CR to the cell surface (18), we next evaluated whether LTB4 increased the ability of the PMN to bind complement-opsonized targets. PMNs were pretreated with increasing doses of LTB4 (0.01 to 100 nM) prior to the addition of E-C3bi. LTB4 at 0.1 to 100 nM significantly increased E-C3bi adherence to human PMNs (Fig. 7). To determine if LTB4-mediated enhancement of E-C3bi adherence is associated with increased phagocytosis, human PMNs were pretreated with various concentrations of LTB4 prior to the addition of E-C3bi. Phagocytosis was initiated 15 min later with the addition of 15 nM PMA, since phagocytic cells must be activated by an additional stimulus in order to phagocytose complement-opsonized targets (9). LTB4 dose dependently augmented phagocytosis of E-C3bi following treatment with PMA (Fig. 8). These results indicate that LTB4 enhances CR-dependent phagocytosis, and that this may occur, at least in part, via an effect on increasing target adherence.
FIG. 7.
Effect of exogenous LTB4 dose on human PMN adherence of E-C3bi. Human PMNs were incubated with LTB4 for 5 to 10 min prior to the addition of E-C3bi. The adherence index of the control was 96.7 ± 51. Data are expressed as the mean ± SE (n = 3). ∗, P < 0.05 with respect to the control by Kruskal-Wallis test on ranks, using Dunnett's test for means separation.
FIG. 8.
Effect of exogenous LTB4 dose on human PMN phagocytosis of E-C3bi. Human PMNs were incubated with LTB4 for 5 to 10 min prior to the addition of E-C3bi. E-C3bi were allowed to adhere to the PMNs for 15 min, and phagocytosis was stimulated by a 15-min incubation with PMA (15 nM). The phagocytic index of the control was 22 ± 5. Data are expressed as the mean ± SE (n = 4). ∗, P < 0.05 with respect to the control by Kruskal-Wallis test on ranks, using Dunnett's test for means separation.
DISCUSSION
In this study, we used both genetic and pharmacologic approaches to demonstrate that endogenously produced LTs promote PMN phagocytosis of bacteria opsonized with immune serum. This conclusion was based on the observations that PMNs elicited from the peritoneum of 5-LO KO mice and human PMNs pretreated with zileuton and MK-886, two drugs that block LT synthesis by distinctly different mechanisms (5, 23), exhibited reduced phagocytosis. It is very likely that LTB4 is the endogenous 5-LO product responsible for this effect on phagocytosis, since (i) it is the principal 5-LO product in PMNs and (ii) LTB4 receptor antagonist, but not cysteinyl-LT receptor antagonist, also reduced phagocytosis. Although they do not synthesize LTC4, some PMN effector functions have been shown to respond to cysteinyl-LTs (11, 14). Taken together, the results of these experiments suggest that endogenously produced LTB4 acts as an autocrine stimulus for enhanced PMN phagocytosis of opsonized K. pneumonia.
Experiments demonstrating the ability of exogenous LTB4 to restore phagocytosis in elicited PMNs from 5-LO KO mice and human PMNs pretreated with the LT synthesis inhibitor zileuton supported the conclusion that reduced phagocytosis of opsonized bacteria was indeed due to the lack of LT synthesis rather than other potential phenotypic abnormalities. Based on our previous studies with AMs (17), we considered the possibility that LTs might have pharmacologic effects on PMN phagocytosis of bacteria. While LTB4, LTC4, 5-HETE, and 5-oxo-ETE all enhanced phagocytosis of opsonized K. pneumonia by normal AMs in our previous study (17), only LTB4 stimulated increased PMN phagocytosis in the present study. It is also noteworthy that the concentration (1 to 100 nM) of exogenously added LTB4 that restored phagocytosis in LT-deficient PMNs and enhanced phagocytosis in LT-competent cells was within the physiologic range. Nanomolar quantities of LTB4 have been recovered from the bronchoalveolar lavage fluid of pneumonia patients (12). Moreover, concentrations of LTB4 at which binding to the two types of PMN LTB4 receptors (BLT) are half-maximal are 1.1 nM (BLT1) and 20 nM (BLT2) (30). These results indicate that only LTB4, the principal 5-LO product of PMNs (8), is capable of enhancing phagocytosis in these cells at physiologic levels.
The positive effect of LTB4 on PMN phagocytosis is not surprising since there is ample evidence demonstrating that LTB4 activates many PMN functions, including upregulation of the CR (18), superoxide generation (28), and increased calcium mobilization (22). PMNs recruited to a site of inflammation have been exposed to various inflammatory mediators and are representative of the activated cells called upon for antimicrobial effector functions (24). The fact that the phagocytic capacity of elicited PMNs from 5-LO KO mice was compromised suggests that LTs play an important role in PMN activation for enhanced bacterial phagocytosis.
It is well known that optimal recognition of phagocytic targets is mediated by surface receptors for specific opsonins (e.g., complement and IgG) (13). Initial experiments showing LTB4 enhancement of phagocytosis utilized bacteria opsonized with complete immune serum that would contain complement and IgG. Whether both or either of these opsonins was required for the LTB4 augmentation of phagocytosis was addressed by coating inert targets (E) with only a single opsonin, either IgG or C3bi. These experiments also served to exclude the possibilities that other opsonins that may be present in the rat serum or nonopsonic recognition of a moiety that may be present on the surface of K. pneumoniae were responsible for the LTB4 modulation of phagocytosis. They revealed that the effects of LTB4 on phagocytosis could be mediated through either the FcR or the CR. This result was in contrast to our previous study that concluded that the effects of exogenous LTs on rat AM phagocytosis were limited to IgG-opsonized targets. The fact that rat AMs express low levels of the CR would explain this disparity (21, 27).
Subsequent experiments addressed the possibility that LTs modulate FcR- and CR-dependent phagocytosis by increasing adherence of targets to PMNs. This could result from increases in receptor number or enhanced affinity of the receptor for its ligand. Indeed, LTB4 is recognized to enhance CR expression in PMNs (18). While exogenous LTB4 had no effect on the adherence of E-IgG, it induced an increase in the internalization of this target. In addition, LTB4 enhanced E-C3bi adherence in a dose-dependent fashion, indicating that its ability to augment PMN phagocytosis may involve enhanced adherence of complement-opsonized targets.
In summary, we have revealed an important role for endogenously produced LTB4 in the augmentation of PMN phagocytosis of opsonized bacteria. This enhancement of phagocytosis can be mediated through either the FcR or CR. In view of the role of endogenous LTB4 in promoting phagocytosis by PMNs, increased susceptibility to infection observed in conditions such as human immunodeficiciency virus infection (6) and malnutrition (25) may relate to their recognized LT deficiency.
ACKNOWLEDGMENTS
This work was supported by National Heart, Lung, and Blood Institute grant RO1 HL58897. Support for P.M. was provided by an American Lung Association of Michigan Research Fellowship Training Award.
We thank Joel A. Swanson, Thomas G. Brock, Michael Coffey, and Theodore J. Standiford for helpful advice and discussion, and we thank Maria Diakonova for technical assistance.
REFERENCES
- 1.Araki N, Johnson M T, Swanson J A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis in macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailie M, Standiford T, Laichalk L, Coffey M, Strieter R, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol. 1996;157:5221–5224. [PubMed] [Google Scholar]
- 3.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Investig. 1968;97(Suppl.):77–89. [PubMed] [Google Scholar]
- 4.Brock T, McNish R, Peters-Golden M. Capacity for repeatable leukotriene generation after transient stimulation of mast cells and macrophages. Biochem J. 1998;329:519–525. doi: 10.1042/bj3290519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter G, Young D, Albert J, Dyer R, Bell R, Summers J, Brooks D. 5-Lipoxygenase inhibitory activity of zileuton. Biochim Biophys Acta. 1991;836:361–367. [PubMed] [Google Scholar]
- 6.Coffey M, Phare S, Kazanjian P, Peters-Golden M. 5-Lipoxygenase metabolism in alveolar macrophages from subjects infected with the human immunodeficiency virus. J Immunol. 1996;157:393–399. [PubMed] [Google Scholar]
- 7.Fleisch J, Rinkema L, Haisch K, Swanson-Bean D, Goodson T, Ho P, Marshall W. LY171883, 1-less than 2-hydroxy-3-propyl-4-less than 4-(1H-tetrazol-5-yl) butoxy greater than phenyl greater than ethanone, an orally active leukotriene D4 antagonist. J Pharmacol Exp Ther. 1985;233:148–157. [PubMed] [Google Scholar]
- 8.Ford-Hutchinson A, Bray M, Doig M. Leukotriene B4, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286:264. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg S, Silverstein S C. Phagocytosis. In: Paul W, editor. Fundamental immunology. 3rd ed. New York, N.Y: Raven Press; 1993. pp. 941–964. [Google Scholar]
- 10.Greenberger M, Strieter R, Kunkel S, Danforth J, Goodman R, Standiford T. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 11.Heimburger M, Palmblad J E. Effects of leukotriene C4 and D4, histamine and bradykinin on cytosolic calcium concentrations and adhesiveness of endothelial cells and neutrophils. Clin Exp Immunol. 1996;103:454–460. doi: 10.1111/j.1365-2249.1996.tb08302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkins H, Stull T, Von Essen S, Robbins R, Rennard S. Neutrophil chemotactic factors in bacterial pneumonia. Chest. 1989;95:1021–1027. doi: 10.1378/chest.95.5.1021. [DOI] [PubMed] [Google Scholar]
- 13.Jones S, Lindberg F, Brown E. Phagocytosis. In: Paul W, editor. Fundamental immunology. 4th ed. Philadelphia, Pa: Lippincott-Raven; 1999. pp. 997–1020. [Google Scholar]
- 14.Larfars G L F, Devynck M A, Palmblad J, Gyllenhammer H. Activation of nitric oxide release and oxidative metabolism by leukotrienes B4, C4, and D4 in human polymorphonuclear leukocytes. Blood. 1999;93:1399–1405. [PubMed] [Google Scholar]
- 15.Lewis R A, Austen K F, Soberman R J. Leukotrienes and other products of the 5-lipoxygenase pathway: biochemistry and relation to pathobiology in human disease. N Engl J Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 16.Mancuso P, Peters-Golden M. Modulation of alveolar macrophage phagocytosis by leukotrienes is Fc receptor-mediated and protein kinase C-dependent. Am J Respir Cell Mol Biol. 2000;23:727–733. doi: 10.1165/ajrcmb.23.6.4246. [DOI] [PubMed] [Google Scholar]
- 17.Mancuso P, Marshall T, Standiford T, Peters-Golden M. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect Immun. 1998;66:5140–5146. doi: 10.1128/iai.66.11.5140-5146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marder P, Sawyer J, Froelich L, Mann L, Spaethe S. Blockade of human neutrophil activation by 2-[2-propyl-3-[3-[2-ethyl-4-(4-fluorophenyl)-5-hydroxphenoxy]propoxy]phenoxy]benzoic acid (LY29311), a novel leukotriene B4 receptor antagonist. Biochem Pharmocol. 1995;49:1683–1690. doi: 10.1016/0006-2952(95)00078-e. [DOI] [PubMed] [Google Scholar]
- 19.Nelson S, Mason C, Knolls J, Summer W. Pathophysiology of pneumonia. Clin Chest Med. 1995;16:1–12. [PubMed] [Google Scholar]
- 20.Newman S, Mikus L, Tucci M. Differential requirements for cellular cytoskeleton in human macrophage complement receptor- and Fc receptor-mediated phagocytosis. J Immunol. 1991;146:967–974. [PubMed] [Google Scholar]
- 21.Nguyen B-Y T, Peterson P, Verbrugh H, Quie P, Hoidal J. Differences in phagocytosis and killing by alveolar macrophages from humans, rabbits, rats, and hamsters. Infect Immun. 1982;36:504–509. doi: 10.1128/iai.36.2.504-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell W S, Rokach J, Khanapure S P, Manna S, Hahefi M, Gravel S, Macleod R J, Falck J R, Bhatt R K. Effects of metabolites of leukotriene B4 on human neutrophil migration and cytosolic calcium levels. Pharmacol Exp Ther. 1996;276:728–736. [PubMed] [Google Scholar]
- 23.Rouzer C A, Ford-Hutchinson A W, Morton H E, Gillard J W. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J Biol Chem. 1990;265:1436–1442. [PubMed] [Google Scholar]
- 24.Savige J A, Pinching A J. A functional comparison of Illindium-labeled elicited peripheral blood neutrophils and peritoneal neutrophils in the rat. Clin Exp Immunol. 1984;58:737–744. [PMC free article] [PubMed] [Google Scholar]
- 25.Skerrett S, Henderson W, Martin T. Alveolar macrophage function in rats with severe protein calorie malnutrition: arachidonic acid metabolism, cytokine release, and antimicrobial activity. J Immunol. 1990;144:1052–1061. [PubMed] [Google Scholar]
- 26.Standiford T. Cytokines and pulmonary host defenses. Curr Opin Pulm Med. 1997;3:81–88. doi: 10.1097/00063198-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Stokes R, Thorson L, Speert D. Nonopsonic and opsonic association of Mycobacterium tuberculosis with resident alveolar macrophages is inefficient. J Immunol. 1998;160:5514–5521. [PubMed] [Google Scholar]
- 28.Sumimoto H, Takeshige K, Minakami S. Superoxide production of human polymorphonuclear leukocytes by leukotriene B4. Biochim Biophys Acta. 1984;803:271–277. doi: 10.1016/0167-4889(84)90117-4. [DOI] [PubMed] [Google Scholar]
- 29.Wright S, Silverstein S. Tumor-promoting phorbol esters stimulate C3b and C3bi receptor-mediated phagocytosis in cultured human monocytes. J Exp Med. 1982;156:1149–1164. doi: 10.1084/jem.156.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokomizu T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B4 receptor, BLT2: a new therapeutic target in inflammation and immunological disorders. J Exp Med. 2000;192:421–431. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]