Abstract
Objective:
Central (abdominal) obesity is associated with elevated adrenergic activity and arterial blood pressure (BP). Therefore, we tested the hypothesis that transduction of spontaneous muscle sympathetic nerve activity (MSNA) to BP, i.e., sympathetic transduction, is augmented in abdominal obesity (increased waist circumference) and positively related to prevailing BP.
Methods:
Young/middle-age obese (32±7years; BMI:36±5kg/m2, n=14) and non-obese (29±10years; BMI:23±4kg/m2, n=14) without hypertension (24-hr ambulatory average BP<130/80mmHg) were included. MSNA (microneurography) and beat-to-beat BP (finger cuff) were measured continuously and the increase in mean arterial pressure (MAP) during 15 cardiac cycles following MSNA bursts of different patterns (single, multiples) and amplitude (quartiles) was signal-averaged over a 10 min baseline period.
Results:
MSNA burst frequency was not significantly higher in obese vs. non-obese (21±3vs.17±3 bursts/min, P=0.34). However, resting supine BP was significantly higher in obese compared with non-obese (systolic:127±3vs.114±3; diastolic:76±2vs.64±1 mmHg, both P<0.01). Importantly, obese showed greater increases in MAP following multiple MSNA bursts (P=0.02) and MSNA bursts of higher amplitude (P=0.02), but not single MSNA bursts (P=0.24), compared with non-obese when adjusting for MSNA burst frequency. The increase in MAP following higher amplitude bursts among all participants was associated with higher resting supine systolic (R=0.48; P=0.01) and diastolic (R=0.48; P=0.01) BP when controlling for MSNA burst frequency, but not when also controlling for waist circumference (P>0.05). In contrast, sympathetic transduction was not correlated with 24-hour ambulatory average BP.
Conclusions:
Sympathetic transduction to BP is augmented in abdominal obesity and positively related to higher resting supine BP but not 24-hr ambulatory average BP.
Keywords: Muscle sympathetic nerve activity, hypertension, obesity, blood pressure, adrenergic receptor, sympathetic transduction
Introduction
The prevalence of obesity has increased to over 42% of adults in the United States [1]. Obesity, particularly elevations in central adiposity, is associated with the development of hypertension [2,3], which is a prominent cause of cardiovascular diseases (CVD), such as stroke [4], myocardial infarction [5,6], heart failure [7], and chronic kidney disease [8]. Pathophysiology of obesity hypertension includes several different categories of mechanisms, such as sympathetic activation, inflammation, and renal dysfunction [9]. However, the relative importance and contribution of these mechanisms to the initiation of obesity hypertension remains uncertain.
Obesity is characterized by elevated peripheral vascular tone [10,11]. Specifically, larger decreases in arterial blood pressure (BP) were observed following ganglionic blockade (trimethaphan) in obese individuals compared with non-obese controls, suggesting greater autonomic support of BP in obesity [10]. Similarly, 4 weeks of combined α- and β-adrenergic receptor blockade produced larger reductions in BP in obese participants with hypertension compared with non-obese controls with hypertension [11]. These data are consistent with the large body of evidence suggesting that obesity elevates muscle sympathetic nerve activity (MSNA) [12–18]. However, MSNA may not be elevated in obesity if development of hypertension is absent [19,20]. Therefore, the extent to which MSNA contributes to the initial development of BP dysregulation in obese men and women without hypertension remains unclear.
Obesity-related increases in vascular tone may be, in part, a result of increased vascular responsiveness to MSNA. In fact, elevated vascular responsiveness to MSNA has been reported in obesity-related conditions such as type 2 diabetes [21]. However, to our knowledge, only one study has directly examined sympathetic vascular tone in obese participants without hypertension [22], reporting similar passive increases in forearm blood flow following α-adrenergic receptor blockade when compared to age- and sex-matched non-obese participants. These data suggest that obesity alone does not alter passive dilation of the forearm resulting from α-adrenergic receptor blockade. However, an extrapolation to systemic BP regulation in obesity from an examination of forearm dilation is challenging for several reasons. First, passive dilation following α-adrenergic receptor blockade may not reflect the blood flow response to α-adrenergic receptor activation, i.e., endogenous sympathetic activation. Second, in normal adults, vascular responsiveness to sympathetic innervation is heterogenous across vascular regions. For example, the lower limbs exhibit greater vascular sensitivity to sympathetic stimulation compared with the forearm vasculature as a result of greater α-adrenergic receptor density and/or sensitivity in the lower limbs [23,24]. Third, obese individuals exhibit regional differences in endogenous norepinephrine kinetics compared with non-obese individuals [25]. Thus, although regional sympathetic vascular tone has been assessed in obesity, there are limited data available regarding potential alterations in systemic BP responsiveness to endogenous activation of adrenergic receptors in this population who are highly prone to development of hypertension.
Therefore, we employed a technique that quantifies the systemic pressor response to spontaneous bursts of MSNA with high temporal resolution (i.e., sympathetic transduction) [21,26,27]. We hypothesized that sympathetic transduction would be augmented in young/middle-aged men and women with abdominal obesity (increased waist circumference) compared with age- and sex-matched non-obese controls. We further hypothesized that augmented sympathetic transduction in obesity would be positively related to higher prevailing BP.
Methods
All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the Institutional Review Board at the University of Iowa (ID#201701762) and the University of Kansas Medical Center (STUDY00146744). Each subject received a verbal and written explanation of the study objectives, measurement techniques, and risks and benefits associated with the investigation prior to providing written informed consent on the initial visit. To match obese and non-obese groups for age and sex, data were pooled from an ongoing study at the University of Kansas Medical Center (STUDY00146744) and previously published studies from our group with unrelated hypotheses [28,29]. Therefore, this study was not prospectively registered in a public database.
Subjects:
Twenty-eight young and middle-aged men and women (14 obese and 14 age- and sex-matched controls) that were nonsmokers and free of metabolic or neurological disease were included. The sample size is in line with previous studies (average group sizes: n=13 vs. n=13) detecting a significant difference in sympathetic transduction between different populations [21,30–33]. Study participants were recruited though mass email at the University of Iowa and a registry at the University of Kansas Medical Center. Visceral adiposity was estimated by waist circumference [34,35] and defined as waist circumference > 102 cm for men and > 88 cm for women [36]. Criteria for normal waist circumference was < 94 cm for men and < 80 cm for women [36]. Age < 45 years was considered young and age 45–65 years was considered middle-age, as previously defined [29,37]. Exclusion criteria included use of anti-hypertensive medications, history of diabetes, and history of smoking within 3 months prior to study participation. Although all participants reported no history of hypertension, status of hypertension was determined by 24-hour ambulatory average BP (<130/80 mmHg). No pregnant women were studied as confirmed by a urine pregnancy test. No postmenopausal women were included, and the phase of the menstrual cycle was not controlled as previous work indicates that sympathetic transduction is not altered by the menstrual cycle in healthy women [38].
Experimental Measurements
24-hr ambulatory BP monitoring:
24-hr ambulatory brachial artery BP, which is regarded as the gold standard for the prediction of risk related to BP [39,40], was obtained using upper arm cuff oscillometric monitors (SpaceLabs Healthcare, Snoqualmie, WA) [41]. Monitors were programmed to obtain BP readings at intervals of 30 min during the day from 0600 to 2200 hours and at night every 60 min from 2200 to 0600 hours. Nocturnal BP “dipping” was calculated as the difference between mean daytime systolic and mean nocturnal (nighttime) systolic BP expressed as a percentage of the daytime value. Daytime and nocturnal BP was adjusted to the nearest hour based on each participant’s written record of their activities and sleep periods for the 24-hr monitoring period. At least 10 daytime readings and 5 nighttime readings and at least 80% successful readings of planned measurements over the 24 hours were required [28].
Resting cardiovascular variables:
Heart rate (HR) was determined from lead II of a three-lead ECG and BP was monitored via auscultatory BP at the brachial artery and beat-to-beat via finger photoplethysmography. Multiunit postganglionic MSNA was recorded using standard microneurographic techniques as previously described [20,28,42,43]. A tungsten microelectrode was placed into the peroneal nerve near the left fibular head. Signals were amplified, filtered (bandwidth 0.7–2.0 kHz), rectified and integrated (0.1 s time constant) to obtain mean voltage neurograms (Nerve Traffic Analyzer; University of Iowa Bioengineering, Iowa City, IA). MSNA was identified by the presence of spontaneous bursts with characteristic pulse synchronicity and by its responsiveness to end-expiratory breath holds, but not to arousal or skin stimulation. Data were acquired using a Powerlab data acquisition system (ADInstruments, Colorado Springs, CO).
Experimental protocol:
On the first visit to the laboratory, subjects received verbal explanation of the study and provided written informed consent. Subjects completed a health history survey and were instrumented with a 24-hr ambulatory BP monitor. On the experimental day (within 2 weeks of the initial visit), participants were instructed to refrain from medication use and fast overnight prior to arriving at the laboratory between 0700 and 0900 hr. Subjects were also instructed to abstain from caffeinated beverages the morning of the study and strenuous physical activity and alcohol for at least 24 hours before experimental sessions. All experiments were performed in a dimly lit room at an ambient temperature of 22–24°C. First, participants underwent blood draw for a comprehensive metabolic panel and lipid panel, followed by a 20 min rest period. Participants were then instrumented for heart rate, finger photoplethysmography (beat-to-beat BP), and microneurography (MSNA). Once the MSNA signal was acquired, data were collected under normal resting conditions in the supine position for at least a 10-min duration.
Data analysis
Muscle sympathetic nerve activity:
Resting MSNA was calculated as a mean value over the 10-min baseline period and quantified as burst frequency (bursts∙min−1) and as burst incidence (bursts∙100 heartbeats−1) to account for interindividual differences in heart rate. Relative MSNA burst amplitude was calculated by attributing the value of 100 to the average of the 3 largest bursts during the baseline MSNA recording and expressing the amplitude of all MSNA bursts as a percentage [20,29,44–46].
Sympathetic transduction:
The transduction analysis of MSNA to BP was performed as previously described [27,33,43,45,47]. Briefly, signal averaging was performed in which bursts of MSNA act as a trigger and beat-to-beat BP was tracked for 15 subsequent cardiac cycles thereafter (Figure 1). The 15 cardiac cycle window is sufficient to fully characterize the BP response because peak BP response latency following MSNA bursts is consistently within 5–8 heart beats in humans [33,47,48]. All detected bursts of MSNA are included regardless of proximity to other bursts. The change in BP is defined as the instantaneous MAP at each cardiac cycle subtracted by MAP at the cardiac cycle in which the burst occurred. The MAP response was signal averaged in response to single MSNA bursts that occur in isolation or multiple successive bursts that are adjacent to at least one other burst. The amplitude of all bursts of MSNA were divided into quartiles to quantify the contribution of burst amplitude to the ensuing MAP response.
Figure 1.
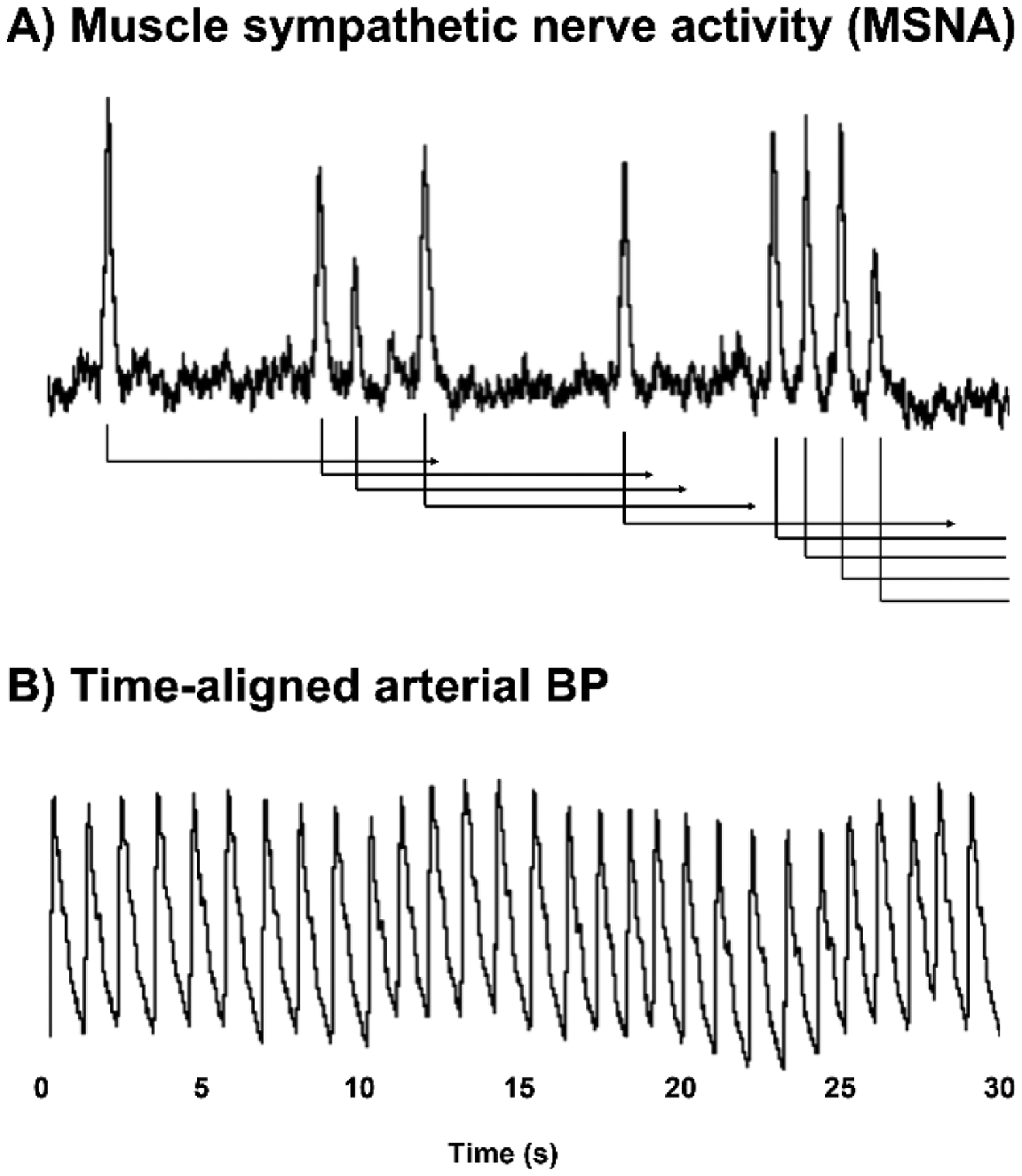
Methodology for determining sympathetic transduction in humans. Muscle sympathetic nerve activity (MSNA) is measured via microneurography at the peroneal nerve (A). Arterial blood pressure (BP) via finger photoplethysmography is time-aligned with MSNA, and the change in BP during 15 sec following each burst of MSNA (triggering event) is signal averaged over the entire resting baseline period of at least 10 min.
Statistical Analysis:
The primary endpoint was the peak MAP response to all bursts of MSNA, regardless of amplitude or pattern, and adjusted for resting MSNA burst frequency. Testing for equal variance was performed using Levene’s Test of Equality of Variances. Group differences in demographics were examined using one-way ANOVA (Table 1), and when normality failed, Kruskal-Wallis one-way ANOVA (ranks) tests was used. Group differences were also examined using analysis of co-variance (ANCOVA) to adjust for resting MSNA, waist circumference, and BMI as indicated in Fig. 2–4. Sex was not a significant covariate in any of the ANCOVA models and therefore group means were not adjusted for this variable. Linear mixed models were used to make group comparisons in the BP response curves (15 cardiac cycles) following different MSNA burst patterns and amplitude (Fig. 3–4). Pearson bivariate regressions were used to evaluate the relation between sympathetic transduction and prevailing BP, and partial regression analysis was used to determine the relation between sympathetic transduction and prevailing BP while adjusting for MSNA burst frequency, waist circumference, and BMI as indicated (Table 2, Fig. 5). Data are reported as mean ± standard deviation and as box plots (median, 25th and 75th percentiles, and 10th and 90th percentiles). Statistical significance was set at P < 0.05.
Table 1.
Demographics
|
t-test P-value |
|||
|---|---|---|---|
| Men / women, n | 8 / 6 | 8 / 6 | -- |
| Age, years | 29 ± 10 | 32 ± 7 | 0.13 |
| Age range, years | 19–52 | 25–49 | -- |
| Waist circumference, cm | 79 ± 10 | 110 ± 11 | <0.01 |
| Hip circumference, cm | 99 ± 10 | 122 ± 17 | <0.01 |
| Waist/hip ratio | 0.8 ± 0.1 | 0.9 ± 0.1 | <0.01 |
| BMI, kg∙m2 (−1) | 23.5 ± 3.6 | 36.0 ± 5.1 | <0.01 |
| Glucose, mg∙dL−1 | 90 ± 7 | 91 ± 11 | 0.64 |
| Insulin, μIU·mL−1 | 8.5 ± 6.1 | 16.9 ± 13.1 | 0.06 |
| HOMA-IR | 2.0 ± 1.6 | 4.1 ± 4.1 | 0.12 |
| Triglycerides, mg∙dL−1 | 73 ± 33 | 142 ± 82 | <0.01 |
| LDL cholesterol, mg∙dL−1 | 96 ± 30 | 118 ± 20 | 0.03 |
| HDL cholesterol, mg∙dL−1 | 50 ± 18 | 43 ± 7 | 0.16 |
| Total cholesterol, mg∙dL−1 | 171 ± 43 | 187 ± 25 | 0.07 |
| Metabolic syndrome, n (m/w) | 0 | 4 / 2 | -- |
| 24-hour ambulatory BP | |||
| Day systolic BP, mmHg | 122 ± 7 | 127 ± 5 | 0.06 |
| Day diastolic BP, mmHg | 72 ± 6 | 75 ± 6 | 0.35 |
| Nocturnal systolic BP, mmHg | 108 ± 6 | 116 ± 6 | <0.01 |
| Nocturnal diastolic BP, mmHg | 60 ± 4 | 64 ± 6 | 0.02 |
| Systolic BP “dipping”, % | −11.4 ± 3.8 | −7.9 ± 5.1 | 0.05 |
| Diastolic BP “dipping”, % | −16.9 ± 4 | −13.4 ± 6.6 | 0.23 |
Values are means ± SD. P-values are obese vs. lean controls. BMI, body mass index; LDL and HDL, low/high density lipoprotein; BP, arterial blood pressure. Comparisons were made using independent t-tests.
Figure 2.
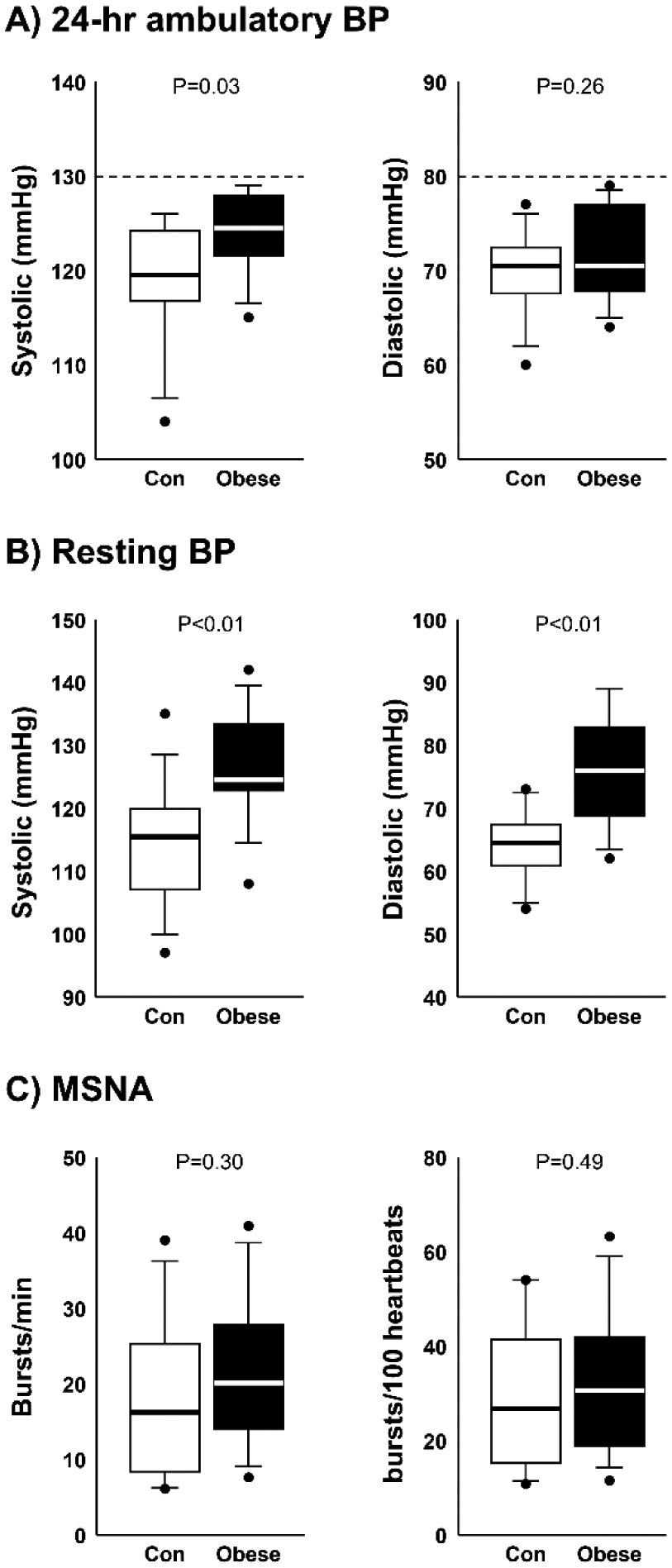
Box plots of average 24-hour ambulatory systolic and diastolic blood pressure (BP) (A), resting supine systolic and diastolic BP (B), and muscle sympathetic nerve activity (MSNA) burst frequency (burst/min) and burst incidence (bursts/100 heartbeats) (C) in non-obese controls (n=14) and obese participants (n=14). Group differences determined by analysis of variance (t-tests). Horizontal lines in boxes show the median, ends of boxes define the 25th and 75th percentiles, whiskers define the 10th and 90th percentiles, and individual data points indicate values outside the 10th and 90th percentiles.
Figure 4.
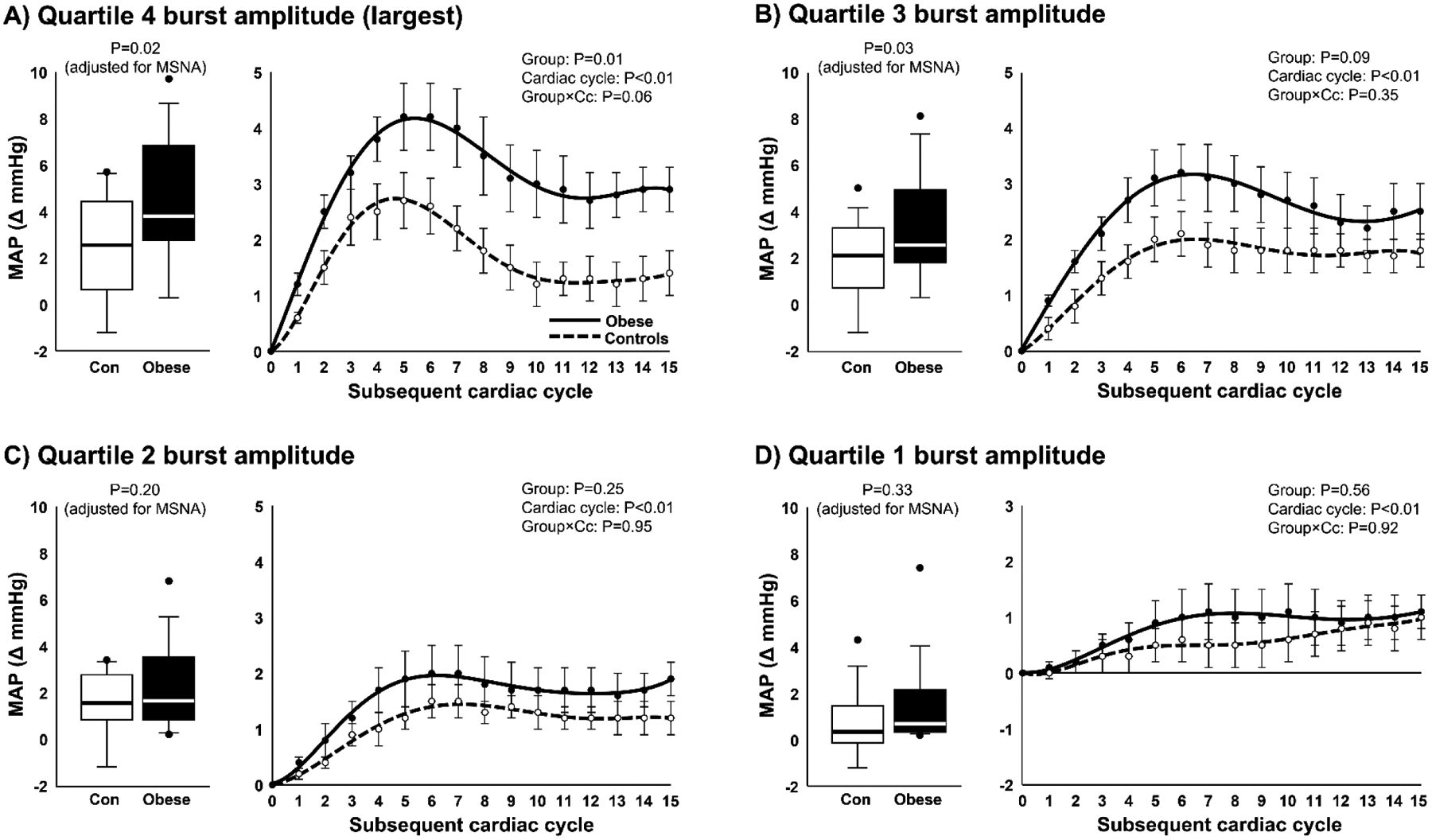
Peak mean arterial pressure (MAP) responses (box plots) and MAP curves following MSNA bursts group within the 4th quartile of burst amplitude (A), 3rd quartile of burst amplitude (B), 2nd quartile of burst amplitude (C), and the 1st quartile of burst amplitude (D) in non-obese controls (n=14) and obese participants (n=14). Peak MAP responses were adjusted for resting MSNA burst frequency using one-way analysis of covariance (ANCOVA), and group comparisons of MAP curves following bursts of MSNA were assessed by linear mixed models. MAP curves are displayed as polynomial trendlines (line of best fit). Horizontal lines in boxes show the median, ends of boxes define the 25th and 75th percentiles, whiskers define the 10th and 90th percentiles, and individual data points indicate values outside the 10th and 90th percentiles.
Figure 3.
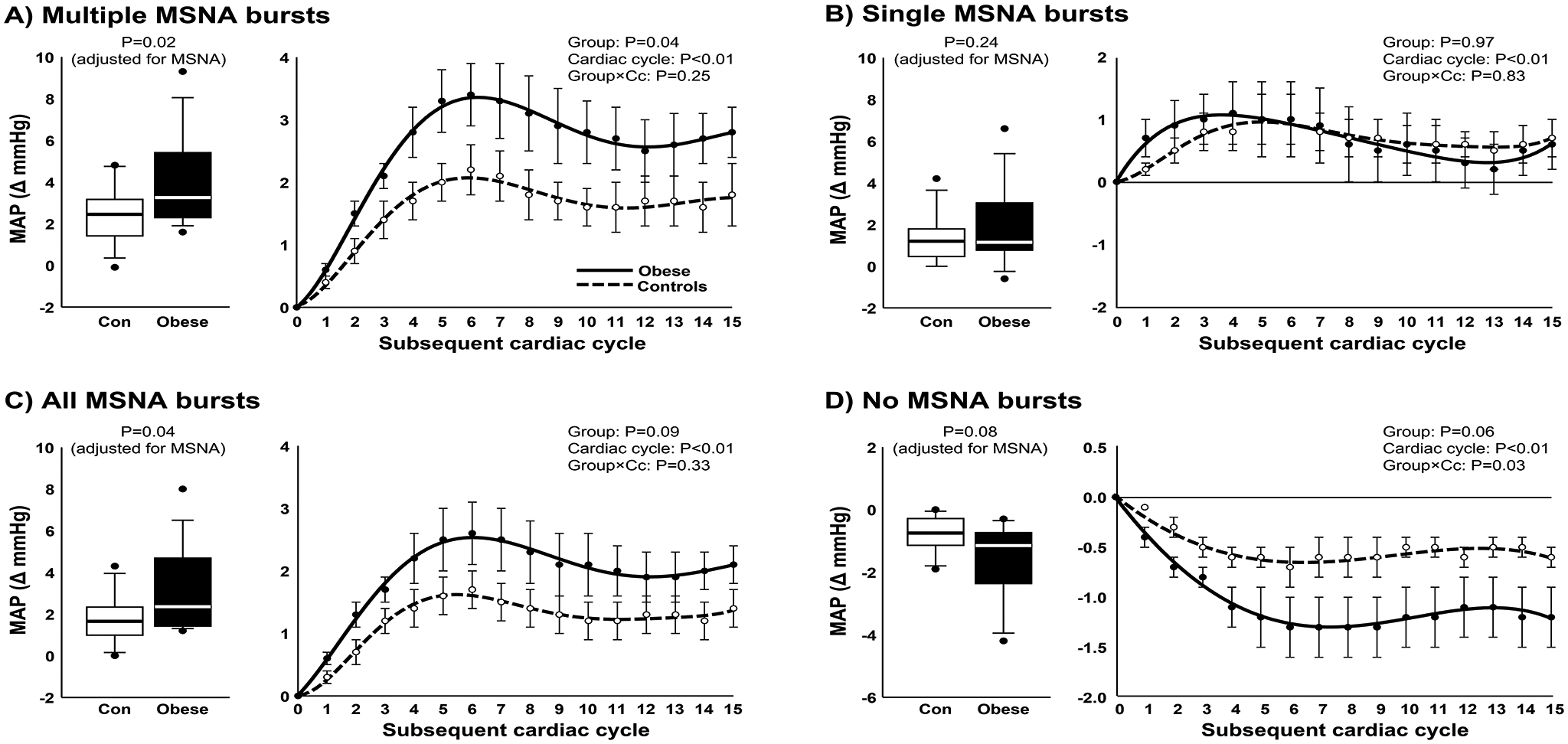
Peak mean arterial pressure (MAP) responses (box plots) and MAP curves following multiple consecutive bursts of MSNA (A), single bursts of MSNA (B), all detected bursts of MSNA (C), and cardiac cycles without bursts of MSNA (D) in non-obese controls (n=14) and obese participants (n=14). Peak MAP responses were adjusted for resting MSNA burst frequency using one-way analysis of covariance (ANCOVA), and group comparisons of MAP curves following bursts of MSNA were assessed by linear mixed models. MAP curves are displayed as polynomial trendlines (line of best fit). Horizontal lines in boxes show the median, ends of boxes define the 25th and 75th percentiles, whiskers define the 10th and 90th percentiles, and individual data points indicate values outside the 10th and 90th percentiles.
Table 2.
Partial correlation analysis
| Partial correlation | |||||||
|---|---|---|---|---|---|---|---|
| Bivariate correlation | Model 1 | Model 2 | Model 3 | ||||
| P-value | P-value | P-value | P-value | ||||
| 0.03 | 0.01 | 0.14 | 0.11 | ||||
| 0.49 | 0.25 | 0.76 | 0.94 | ||||
| 0.21 | 0.10 | 0.57 | 0.40 | ||||
| 0.04 | 0.01 | 0.12 | 0.13 | ||||
| 0.33 | 0.11 | 0.67 | 0.60 | ||||
| 0.21 | 0.05 | 0.30 | 0.26 | ||||
| Avg. 24-hr ambulatory SBP | |||||||
| 0.33 | 0.27 | 0.68 | 0.63 | ||||
| 0.61 | 0.47 | 0.95 | 0.91 | ||||
| 0.51 | 0.43 | 0.87 | 0.76 | ||||
| Avg. 24-hr ambulatory DBP | |||||||
| 0.54 | 0.44 | 0.30 | 0.36 | ||||
| 0.69 | 0.51 | 0.35 | 0.45 | ||||
| 0.51 | 0.40 | 0.29 | 0.36 | ||||
Data shown include largest quartile (quartile 4) of MSNA burst cluster and amplitude (control: n=14, obese: n=14). Model 1: adjusting for MSNA burst frequency, Model 2: adjusting for MSNA burst frequency and waist circumference; Model 3: adjusting for MSNA burst frequency and body mass index.
P<0.05.
Figure 5.
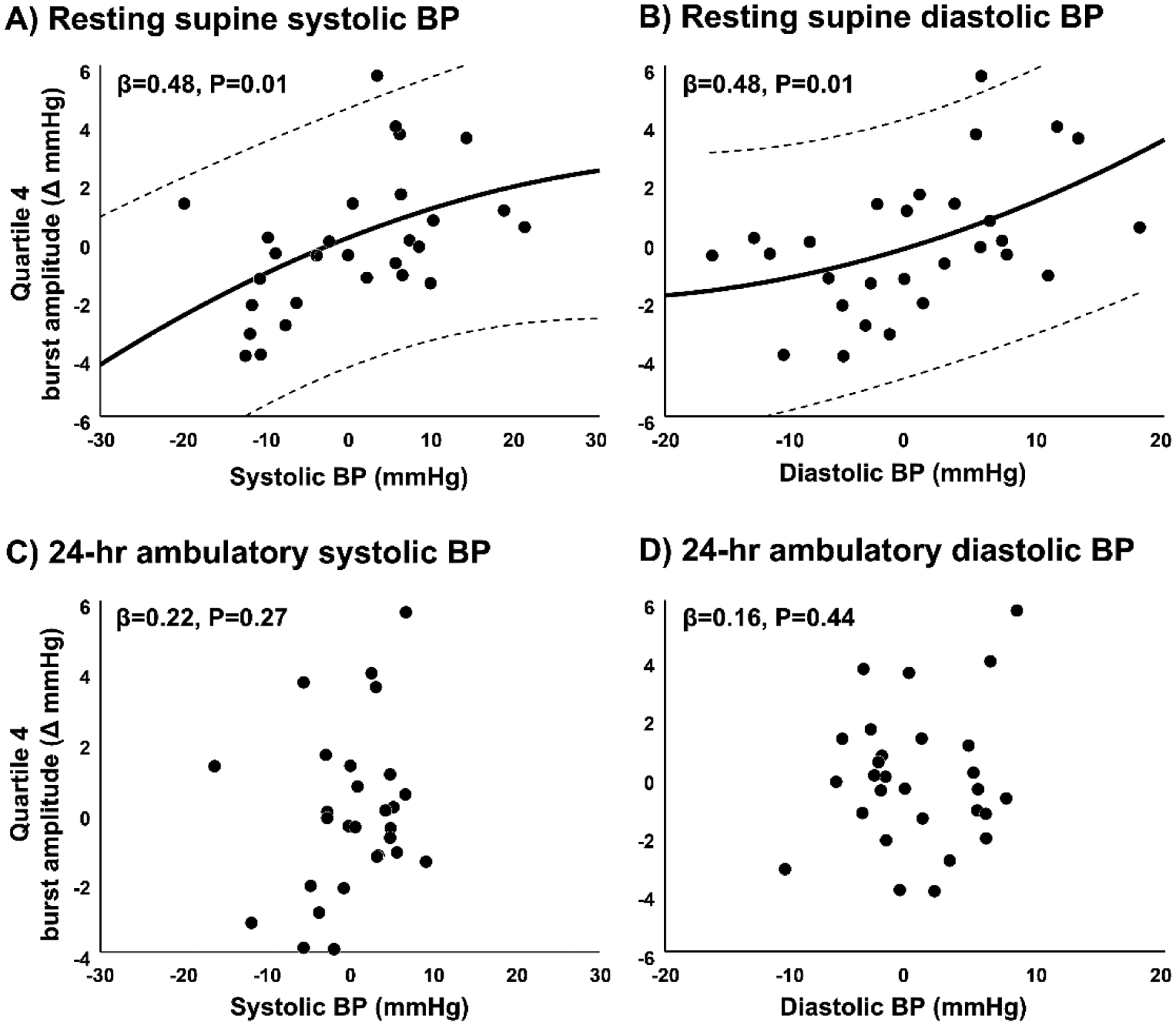
Partial regression plots (controlling for MSNA burst frequency) between the peak arterial blood pressure (BP) response following the largest amplitude bursts of MSNA (4th quartile) and resting supine systolic BP (A), resting supine diastolic BP (B), 24-hr ambulatory systolic BP (C), and 24-hr ambulatory diastolic BP (D). Plots include all study participants. Variables on the horizontal axis and vertical axis are adjusted for the independent variable (MSNA burst frequency) in the partial regression plot procedure, which creates standardized values centered around zero. Regression lines (solid) are quadratic with 95% confidence intervals (dashed lines).
Results
Blood chemistries:
Obese participants had significantly higher fasting plasma concentrations of triglycerides (P<0.01) and LDL cholesterol (P=0.03) (Table 1). Six of the 14 obese participants were considered to have metabolic syndrome (meeting 3 or more of the following criteria: Waist circumference of ≥102 cm for men and ≥89 cm for women, BP ≥ 130/85 mmHg or taking an anti-hypertensive medication, triglycerides > 150 mg/dL, fasting plasma glucose > 100 mg/dL or taking glucose-lowering medications, and high-density lipoprotein level (HDL) < 40 mg/dL for men and 50 mg/dL for women.
Ambulatory BP:
Although all participants showed 24-hour ambulatory average BP <130/80 mmHg, obese participants exhibited significantly higher 24-hour ambulatory average systolic BP compared with controls (P=0.03) (Fig. 2A). Similarly, obese participants showed significantly higher nocturnal systolic BP (P<0.01) and a smaller dip in systolic BP (P=0.05) from daytime to nighttime compared with non-obese controls (Table 1).
Resting cardiovascular variables:
Resting heart rate was significantly higher in obese vs. control participants (68 ± 9 vs. 58 ± 9 bpm, P=0.01). Similarly, resting supine systolic (P<0.01) and diastolic (P<0.01) BP via arm cuff were significantly elevated in obese participants compared with controls (Fig. 2B). However, no significant difference in MSNA burst frequency (P=0.30) or burst incidence (P=0.49) were observed between obese and control participants (Fig. 2C).
MSNA burst pattern:
The peak MAP response following multiple MSNA bursts (2 or more consecutive bursts) was significantly greater in obese compared with controls (P=0.02) (Fig. 3A). A post hoc analysis on group averages and variance (Obese: 3.9 ± 2.2 vs. Control: 2.4 ± 1.4 mmHg) revealed a large effect size of 0.81 and power of 0.66. Means are adjusted for resting MSNA burst frequency (ANCOVA) with similar results when adjusting for burst incidence (P=0.02). In accordance, the temporal pattern of the MAP response following multiple MSNA bursts was significantly greater in obese participants compared with controls (P=0.04). In contrast, the peak MAP response following single isolated MSNA bursts was similar between obese and controls (P=0.24) (Fig. 3B). Overall, obese participants demonstrated a greater MAP response following MSNA bursts regardless of pattern (all bursts, P=0.04) (Fig. 3C). Means are adjusted for resting MSNA burst frequency (ANCOVA) with similar results when adjusting for burst incidence (P=0.04). When considering MAP following cardiac cycles without bursts of MSNA, no statistically significant difference was observed between groups while adjusting for MSNA burst frequency (P=0.08) (Fig. 3D) or MSNA burst incidence (P=0.05). Sex was not a significant covariate in any of the ANCOVA models; therefore, mean values were not adjusted for this independent variable.
MSNA burst amplitude:
The peak MAP response was significantly greater in obese compared with controls when considering the largest (4th) quartile of MSNA burst amplitude (P=0.02) (Fig. 2A). A post hoc analysis on group averages and variance (Obese: 5.0 ± 2.4 vs. Control: 3.1 ± 2.0 mmHg) revealed a large effect size of 0.86 and power of 0.70. Means are adjusted for resting MSNA (ANCOVA) with similar results when adjusting for burst incidence (P=0.02). Additionally, the temporal pattern of the MAP response following the 4th quartile of MSNA burst amplitude was significantly greater in obese participants compared with controls (P=0.01). While the peak MAP response following the 3rd quartile of MSNA burst amplitude was also significantly greater among obese participants compared with controls while adjusting for MSNA burst frequency (P=0.03) (Fig. 4B) or burst incidence (P=0.04), no significant group differences were observed for the 2nd quartile (P=0.20) (Fig. 4C) and 1st quartile (P=0.33) (Fig. 4D) while adjusting for MSNA burst frequency. Similar results were observed when adjusting for MSNA burst incidence (all P>0.05). Sex was not a significant covariate in any of the ANCOVA models; therefore, mean values were not adjusted for this independent variable.
Relation between sympathetic transduction and prevailing BP:
When considering the largest (4th) quartile of MSNA burst amplitude, a significant bivariate correlation was observed between sympathetic transduction and resting supine systolic BP (R=0.40, P=0.03) and diastolic BP (R=0.38, P=0.04) (Table 2). Importantly, adjusting for resting MSNA (model 1) did not change these results (supine systolic BP: β=0.48, P=0.01; supine diastolic BP: β=0.48, P=0.01) (Fig. 5A and Table 2). However, these correlations were no longer statistically significant when adjusting for MSNA and waist circumference (model 2) and MSNA and BMI (model 3), suggesting obesity is an important determinant in the relation between sympathetic transduction and resting supine BP. In contrast, when considering MSNA burst pattern (multiples, ≥2 MSNA bursts), no relation was observed between sympathetic transduction and resting supine systolic BP (R=0.13, P=0.49) and diastolic BP (R=0.19, P=0.33) in any of the statistical models. Surprisingly, no models were statistically significant when examining 24-hour ambulatory average systolic BP (β=0.22, P=0.27) (Fig. 5C), average diastolic BP (β=0.16, P=0.44) (Fig. 5D), or other parameters of 24-hr ambulatory BP (e.g., day/night BP, “dipping”). Significant correlations were noted between higher 24-hr ambulatory systolic BP variability (standard deviation) and sympathetic transduction among all participants; however, these correlations were not specific to obesity because they remained statistically significant after adjusting for MSNA and waist circumference (multiple bursts: β=0.43, P=0.03; higher burst amplitude: β=0.72, P<0.01; all bursts: β=0.60, P<0.01). Thus, sympathetic transduction was positively related to prevailing BP in obesity when sympathetic transduction was being assessed under resting conditions but not BP across a 24-hour period.
Discussion
The present study examined whether central obesity augments transduction of MSNA to BP in humans without hypertension and the extent to which it is associated with prevailing BP. Two novel findings were noted. First, transduction of MSNA to BP was significantly greater in obese participants compared with controls with no significant difference in resting MSNA. More specifically, BP responses following two or more consecutive bursts (multiples) and larger amplitude MSNA bursts were significantly greater in obese compared with controls, whereas no group difference was noted in the BP response following single isolated MSNA bursts. Second, prevailing BP in the resting supine position was significantly correlated with higher transduction of large amplitude MSNA bursts, but not when statistically adjusting for waist circumference or BMI. Indeed, resting supine BP was significantly higher in obese individuals compared with controls, despite all participants showing clinically normal 24-hour ambulatory BP. Taken together, these findings demonstrate that the pressor response to spontaneous bursts of MSNA of larger magnitude is selectively augmented in individuals with abdominal obesity and is positively related to prevailing BP at the time of the sympathetic transduction assessment. This increase in sympathetic transduction despite no increase in MSNA burst frequency may describe an early stage of BP dysregulation in obesity.
To our knowledge, only one previous study has directly examined sympathetic vascular tone in obese subjects without hypertension [22] and reported similar changes in forearm blood flow during α1-adrenergic receptor blockade when compared to control participants. However, only the forearm vasculature was examined without addressing regional differences in α-adrenergic receptor density and/or sensitivity, such as the upper and lower limbs [23,24]. In contrast, the present study employed the sympathetic transduction technique, which characterizes the effector organ response to endogenous norepinephrine and the aggregate end point from the perspective of overall BP regulation. Thus, the augmented BP response following bursts of MSNA in obese participants observed in the present study represents global sympathetic vascular constriction, thereby overcoming the limitation of regional differences in α-adrenergic receptor density and/or sensitivity.
Although the specific mechanism(s) that account for the obesity-related rise in sympathetic transduction were not tested, there are two points worth speculating. First, norepinephrine release and/or turnover in the sympathetic nerve terminal may be enhanced in obesity to the extent that bursts of MSNA cause augmented vasoconstrictor responses, particularly when MSNA increases with higher amplitude bursts or multiple bursts in succession. In support of this, there is evidence of enhanced renal norepinephrine spillover in obesity [25], although human studies focused on obesity-related changes in norepinephrine spillover in the vasculature of skeletal muscle remain scarce. Second, vascular α-adrenergic receptor sensitivity may be enhanced by the circulating milieu in obesity (e.g., hyperlipidemia, oxidative stress). For example, elevations in plasma free fatty acids can increase reactive oxygen species [49–52], which can enhance α1-adrenergic receptor control of vascular smooth muscle contraction [53]. However, direct evidence in humans demonstrating enhanced α-adrenergic receptor sensitivity via plasma free fatty acids is needed. Nevertheless, our findings support our hypothesis that abdominal obesity increases transduction of MSNA to BP and may inform upcoming studies aiming to address the development hypertension in this population.
Resting MSNA is elevated in obesity-related hypertension [13,18]. However, compared with controls, we did not observe a significant elevation in MSNA among obese participants in the present study, attributed primarily to the exclusion of individuals with 24-hr ambulatory average BP ≥130/80. Our findings are in line with previous reports of similar resting MSNA in obese and control subjects without established hypertension [17,19] and support the notion of heightened sympathetic transduction in obesity despite normal resting MSNA. In fact, there is substantial evidence that higher sympathetic transduction in normal adults may be requisite for normal BP regulation when resting MSNA is low [32,54–58]. However, no association was observed between sympathetic transduction and resting MSNA burst frequency in the present study (data not shown). Therefore, it remains unclear whether the commonly observed inverse relation between sympathetic transduction and resting MSNA is modified by obesity.
It is important to note that the sympathetic transduction analysis in the present study was performed under resting steady-state conditions and may not translate to BP responses to sympathoexcitatory stimuli for several reasons. First, the influence of peripheral sympathetic vasoconstriction on blood pressure, which the sympathetic transduction analysis captures, cannot reliably be isolated if other hemodynamic variables, such as cardiac output, are increasing simultaneously. Sympathoexcitatory stimuli, such as the cold pressor test and handgrip, typically cause major elevations in cardiac output and MSNA, and also do not provide an adequate duration of data needed for the signal-averaging technique (≥10 min). Although previous studies have examined changes in blood pressure for a given increase in sympathetic nerve activity during the cold pressor test or handgrip, these studies were not performed with the signal-averaging technique and therefore were not isolating the influence of peripheral sympathetic vasoconstriction on BP. Second, there is considerable interindividual variability in MSNA responses to the cold pressor test and handgrip with less than optimal reproducibility [59]. For these reasons, it would not be surprising if results from sympathoexcitatory maneuvers were not parallel with the sympathetic transduction analysis.
Strengths and limitations
The strength of the present study can be appreciated in several ways. First and foremost is the method of assessing sympathetic transduction. Methods of assessing sympathetic transduction following spontaneous bursts of MSNA can be categorized as either the signal-averaging technique, as used the present study, or the linear regression approach (discussed in a recent review, [60]). The signal-averaging technique is the most established technique for assessing sympathetic transduction [48] and results have been validated as an almost entirely α-adrenergic receptor mechanism using intra-arterial infusion of an α-adrenergic receptor antagonist with proper control conditions [47]. However, the linear regression approach has not been validated with α-adrenergic receptor antagonists or with measures of blood flow and conductance. Indeed, recent work has demonstrated important differences in results when comparing these methodologies [61]. Secondly, the obese and control groups were matched by age and sex, thereby minimizing the influence of these variables in the comparison between groups. Third, a comprehensive assessment of BP was performed using 24-hour ambulatory BP, thereby providing high confidence in the negative status of hypertension for all participants.
However, there were also several limitations. For example, our study does not reveal any causal nature of the associations described. Also, although the obese and control groups were age- and sex-matched, the sample size was relatively small, thereby limiting comparisons between- and within-sex. However, it should be noted that sex was not a significant covariate in any of the ANCOVA models. Indeed, most studies comparing men and women do not report a sex difference in sympathetic transduction [43,56,62,63], despite a sex difference in the relation between sympathetic transduction and resting MSNA [62]. Nonetheless, we cannot rule out the possibility of an interaction between sex and obesity because the study was designed only to address the question of an obesity-related change in sympathetic transduction. Second, our study did not include an additional group of obese individuals based on BMI but without “central adiposity.” Comparing obese participants with and without significant central adiposity would provide further information on the role of central adiposity in altered sympathetic transduction. Along these lines, the present study was limited by utilizing only waist circumference as an index of visceral fat because there are exceptions, such as reciprocal changes in subcutaneous and visceral abdominal fat that do not affect waist circumference but may have metabolic implications. Indeed, subcutaneous abdominal obesity has been considered “metabolically healthy obesity” characterized by normal glucose and lipid metabolism and absence of hypertension [64]. Thus, highly accurate and detailed methods characterizing body composition, such as dual-energy X-ray absorptiometry (DEXA), magnetic resonance imaging (MRI), or computed tomography (CT), would aid in future studies examining obesity-related increases in sympathetic transduction. Finally, cardiac output responses following bursts of MSNA were not examined. However, it is important to note that when examining the temporal response, the peak increase in MAP following bursts of MSNA coincides with the increase in peripheral resistance but not the relatively small and brief increase in cardiac output [45,47,65]. In contrast to the present study, cardiac output does become important when examining the MAP response to MSNA following acute stressors, such as handgrip, cold pressor test, etc., which can generate substantial increases in heart rate and stroke volume in addition to an increase in peripheral resistance.
Summary
The primary novel findings were an obesity-related increase in sympathetic transduction, including the BP response following larger MSNA burst amplitude and multiple MSNA bursts, despite resting MSNA burst frequency that was not significantly higher in obese participants. As a result, these data suggest that α-adrenergic receptor sensitivity to MSNA may potentially be elevated by moderate central obesity. Importantly, prevailing BP in the resting supine position was significantly correlated with higher transduction of large amplitude MSNA bursts. These findings advance our knowledge by demonstrating an alteration in sympathetic control of BP in the setting of abdominal obesity and may inform future studies focused on understanding the prevention of hypertension in this population. This is also the first study to demonstrate an association between elevated resting BP and sympathetic transduction.
Acknowledgements
We acknowledge the research staff at the University of Iowa Institute for Clinical and Translational Science Clinical Research Unit and the Frontiers: University of Kansas Clinical and Translational Science Institute. We also acknowledge Amy Stroud, Ryan Ward, and Allene Gremaud for their dedicated assistance with studies.
Sources of funding
R01HL159370-01 (S.W. H.) and R01AG063790 (G.L.P.). This work was also supported by a CTSA grant from NCATS awarded to the Frontiers: University of Kansas Clinical and Translational Science Institute (#UL1TR002366) and to the University of Iowa Institute for Clinical and Translational Science (#UL1TR002537). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS. This work was also supported in part by a National Institutes of Health Iowa Cardiovascular Interdisciplinary Research Fellowship T32HL007121 (S.W.H.), American Heart Association Grants 17POST33440101 (S.W. H.) and 13SDG143400012 (G.L.P.) and NIH P01 HL-014388-48 (G.L.P). G.L.P is supported by the Russell B. Day and Florence D. Day Chair in Liberal Arts and Sciences at the University of Iowa.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no 360. National Center for Health Statistics. 2020. Feb(360). [PubMed] [Google Scholar]
- 2.Kanai H, Tokunaga K, Fujioka S, Yamashita S, Kameda-Takemura KK, Matsuzawa Y. Decrease in intra-abdominal visceral fat may reduce blood pressure in obese hypertensive women. Hypertension 1996; 27:125–129. [DOI] [PubMed] [Google Scholar]
- 3.Chandra A, Neeland IJ, Berry JD, Ayers CR, Rohatgi A, Das SR, et al. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J Am Coll Cardiol 2014; 64:997–1002. [DOI] [PubMed] [Google Scholar]
- 4.Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension 2013; 62:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990; 335:827–838. [DOI] [PubMed] [Google Scholar]
- 6.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335:765–774. [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA 1996; 275:1557–1562. [PubMed] [Google Scholar]
- 8.Hall JE, Kuo JJ, da Silva AA, de Paula RB, Liu J, Tallam L. Obesity-associated hypertension and kidney disease. Curr Opin Nephrol Hypertens 2003; 12:195–200. [DOI] [PubMed] [Google Scholar]
- 9.Hall ME, Cohen JB, Ard JD, Egan BM, Hall JE, Lavie CJ, et al. Weight-Loss Strategies for Prevention and Treatment of Hypertension: A Scientific Statement From the American Heart Association. Hypertension 2021; 78:e38–e50. [DOI] [PubMed] [Google Scholar]
- 10.Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, et al. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension 2007; 49:27–33. [DOI] [PubMed] [Google Scholar]
- 11.Wofford MR, Anderson DC Jr., Brown CA, Jones DW, Miller ME, Hall JE. Antihypertensive effect of alpha- and beta-adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens 2001; 14:694–698. [DOI] [PubMed] [Google Scholar]
- 12.Grassi G, Biffi A, Seravalle G, Trevano FQ, Dell’Oro R, Corrao G, et al. Sympathetic Neural Overdrive in the Obese and Overweight State. Hypertension 2019; 74:349–358. [DOI] [PubMed] [Google Scholar]
- 13.Grassi G, Seravalle G, Dell’Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension 2000; 36:538–542. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 2002; 106:2533–2536. [DOI] [PubMed] [Google Scholar]
- 15.Kuniyoshi FH, Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Gowdak MM, et al. Abnormal neurovascular control during sympathoexcitation in obesity. Obes Res 2003; 11:1411–1419. [DOI] [PubMed] [Google Scholar]
- 16.Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation 1994; 89:2634–2640. [DOI] [PubMed] [Google Scholar]
- 17.Lambert E, Straznicky N, Eikelis N, Esler M, Dawood T, Masuo K, et al. Gender differences in sympathetic nervous activity: influence of body mass and blood pressure. J Hypertens 2007; 25:1411–1419. [DOI] [PubMed] [Google Scholar]
- 18.Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, et al. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension 2007; 50:862–868. [DOI] [PubMed] [Google Scholar]
- 19.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 1998; 98:772–776. [DOI] [PubMed] [Google Scholar]
- 20.Holwerda SW, Vianna LC, Restaino RM, Chaudhary K, Young CN, Fadel PJ. Arterial baroreflex control of sympathetic nerve activity and heart rate in patients with type 2 diabetes. Am J Physiol Heart Circ Physiol 2016; 311:H1170–H1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young BE, Holwerda SW, Vranish JR, Keller DM, Fadel PJ. Sympathetic Transduction in Type 2 Diabetes Mellitus. Hypertension 2019; 74:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agapitov AV, Correia ML, Sinkey CA, Haynes WG. Dissociation between sympathetic nerve traffic and sympathetically mediated vascular tone in normotensive human obesity. Hypertension 2008; 52:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol (1985) 2002; 92:2105–2113. [DOI] [PubMed] [Google Scholar]
- 24.Jacob G, Costa F, Shannon J, Robertson D, Biaggioni I. Dissociation between neural and vascular responses to sympathetic stimulation : contribution of local adrenergic receptor function. Hypertension 2000; 35:76–81. [DOI] [PubMed] [Google Scholar]
- 25.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 1997; 96:3423–3429. [DOI] [PubMed] [Google Scholar]
- 26.Vranish JR, Holwerda SW, Kaur J, Fadel PJ. Augmented pressor and sympathoexcitatory responses to the onset of isometric handgrip in patients with type 2 diabetes. Am J Physiol Regul Integr Comp Physiol 2020; 318:R311–R319. [DOI] [PubMed] [Google Scholar]
- 27.Fairfax ST, Padilla J, Vianna LC, Holwerda SH, Davis MJ, Fadel PJ. Influence of spontaneously occurring bursts of muscle sympathetic nerve activity on conduit artery diameter. Am J Physiol Heart Circ Physiol 2013; 305:H867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holwerda SW, Luehrs RE, Gremaud AL, Wooldridge NA, Stroud AK, Fiedorowicz JG, et al. Relative burst amplitude of muscle sympathetic nerve activity is an indicator of altered sympathetic outflow in chronic anxiety. J Neurophysiol 2018; 120:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holwerda SW, Luehrs RE, DuBose L, Collins MT, Wooldridge NA, Stroud AK, et al. Elevated Muscle Sympathetic Nerve Activity Contributes to Central Artery Stiffness in Young and Middle-Age/Older Adults. Hypertension 2019; 73:1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobetic MD, Burchell AE, Ratcliffe LEK, Neumann S, Adams ZH, Nolan R, et al. Sympathetic-transduction in untreated hypertension. J Hum Hypertens 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Notarius CF, Murai H, Morris BL, Floras JS. Effect of fitness on reflex sympathetic neurovascular transduction in middle-age men. Med Sci Sports Exerc 2012; 44:232–237. [DOI] [PubMed] [Google Scholar]
- 32.Steinback CD, Fraser GM, Usselman CW, Reyes LM, Julian CG, Stickland MK, et al. Blunted sympathetic neurovascular transduction during normotensive pregnancy. J Physiol 2019; 597:3687–3696. [DOI] [PubMed] [Google Scholar]
- 33.Vranish JR, Holwerda SW, Young BE, Credeur DP, Patik JC, Barbosa TC, et al. Exaggerated Vasoconstriction to Spontaneous Bursts of Muscle Sympathetic Nerve Activity in Healthy Young Black Men. Hypertension 2018; 71:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol 2006; 35:83–92. [DOI] [PubMed] [Google Scholar]
- 35.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020; 16:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ 1995; 311:158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howden Lindsay M., and Meyer Julie A.. Age and sex composition: 2010. (2011): 2–5. Retrieved from https://www.census.gov/library/publications/2011/dec/c2010br-03.html. Nov 10 2022 [Google Scholar]
- 38.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 2000; 101:862–868. [DOI] [PubMed] [Google Scholar]
- 39.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med 2006; 354:2368–2374. [DOI] [PubMed] [Google Scholar]
- 40.Verdecchia P Prognostic value of ambulatory blood pressure : current evidence and clinical implications. Hypertension 2000; 35:844–851. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien E, Mee F, Atkins N, O’Malley K. Accuracy of the SpaceLabs 90207 determined by the British Hypertension Society protocol. J Hypertens 1991; 9:573–574. [DOI] [PubMed] [Google Scholar]
- 42.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 1979; 59:919–957. [DOI] [PubMed] [Google Scholar]
- 43.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 2012; 302:H2419–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am J Physiol Heart Circ Physiol 2016; 310:H300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 2013; 304:H759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert E, Hotchkin E, Alvarenga M, Pier C, Richards J, Barton D, et al. Single-unit analysis of sympathetic nervous discharges in patients with panic disorder. J Physiol 2006; 570:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fairfax ST, Holwerda SW, Credeur DP, Zuidema MY, Medley JH, Dyke PC 2nd, et al. The role of alpha-adrenergic receptors in mediating beat-by-beat sympathetic vascular transduction in the forearm of resting man. J Physiol 2013; 591:3637–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallin BG, Nerhed C. Relationship between spontaneous variations of muscle sympathetic activity and succeeding changes of blood pressure in man. J Auton Nerv Syst 1982; 6:293–302. [DOI] [PubMed] [Google Scholar]
- 49.Paolisso G, Gambardella A, Tagliamonte MR, Saccomanno F, Salvatore T, Gualdiero P, et al. Does free fatty acid infusion impair insulin action also through an increase in oxidative stress? J Clin Endocrinol Metab 1996; 81:4244–4248. [DOI] [PubMed] [Google Scholar]
- 50.Umpierrez GE, Smiley D, Robalino G, Peng L, Kitabchi AE, Khan B, et al. Intravenous intralipid-induced blood pressure elevation and endothelial dysfunction in obese African-Americans with type 2 diabetes. J Clin Endocrinol Metab 2009; 94:609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopes HF, Morrow JD, Stojiljkovic MP, Goodfriend TL, Egan BM. Acute hyperlipidemia increases oxidative stress more in African Americans than in white Americans. Am J Hypertens 2003; 16:331–336. [DOI] [PubMed] [Google Scholar]
- 52.Gosmanov AR, Smiley DD, Robalino G, Siquiera J, Khan B, Le NA, et al. Effects of oral and intravenous fat load on blood pressure, endothelial function, sympathetic activity, and oxidative stress in obese healthy subjects. Am J Physiol Endocrinol Metab 2010; 299:E953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai MH, Jiang MJ. Reactive oxygen species are involved in regulating alpha1-adrenoceptor-activated vascular smooth muscle contraction. J Biomed Sci 2010; 17:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berthelsen LF, Fraser GM, Simpson LL, Vanden Berg ER, Busch SA, Steele AR, et al. Highs and lows of sympathetic neurocardiovascular transduction: influence of altitude acclimatization and adaptation. Am J Physiol Heart Circ Physiol 2020; 319:H1240–H1252. [DOI] [PubMed] [Google Scholar]
- 55.Briant LJ, Burchell AE, Ratcliffe LE, Charkoudian N, Nightingale AK, Paton JF, et al. Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: insights into sex, ageing and blood pressure control. J Physiol 2016; 594:4753–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hissen SL, Macefield VG, Brown R, Taylor CE. Sympathetic baroreflex sensitivity is inversely related to vascular transduction in men but not women. Am J Physiol Heart Circ Physiol 2019; 317:H1203–H1209. [DOI] [PubMed] [Google Scholar]
- 57.Steele AR, Berthelsen LF, Fraser GM, Phillips DB, Fuhr DP, Wong EYL, et al. Blunted sympathetic neurovascular transduction is associated to the severity of obstructive sleep apnea. Clin Auton Res 2021. [DOI] [PubMed] [Google Scholar]
- 58.Tan CO, Tamisier R, Hamner JW, Taylor JA. Characterizing sympathetic neurovascular transduction in humans. PLoS One 2013; 8:e53769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dillon GA, Lichter ZS, Alexander LM, Vianna LC, Wang J, Fadel PJ, et al. Reproducibility of the neurocardiovascular responses to common laboratory-based sympathoexcitatory stimuli in young adults. J Appl Physiol (1985) 2020; 129:1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young BE, Greaney JL, Keller DM, Fadel PJ. Sympathetic transduction in humans: recent advances and methodological considerations. Am J Physiol Heart Circ Physiol 2021; 320:H942–H953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Brien MW, Schwartz BD, Petterson JL, Kimmerly DS. Comparison of signal-averaging and regression approaches to analyzing sympathetic transduction. Clin Auton Res 2022; 32:299–302. [DOI] [PubMed] [Google Scholar]
- 62.Robinson AT, Babcock MC, Watso JC, Brian MS, Migdal KU, Wenner MM, et al. Relation between resting sympathetic outflow and vasoconstrictor responses to sympathetic nerve bursts: sex differences in healthy young adults. Am J Physiol Regul Integr Comp Physiol 2019; 316:R463–R471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coovadia Y, Adler TE, Steinback CD, Fraser GM, Usselman CW. Sex differences in dynamic blood pressure regulation: beat-by-beat responses to muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 2020; 319:H531–H538. [DOI] [PubMed] [Google Scholar]
- 64.Bluher M Metabolically Healthy Obesity. Endocr Rev 2020; 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Brien MW, Ramsay D, Johnston W, Kimmerly DS. Aerobic fitness and sympathetic responses to spontaneous muscle sympathetic nerve activity in young males. Clin Auton Res 2020. [DOI] [PubMed] [Google Scholar]


