PURPOSE
Infections are a significant cause of morbidity and mortality in patients with multiple myeloma (MM). In Latin America, data on infectious complications in this patient population are lacking.
METHODS
We conducted a prospective cohort study of patients with newly diagnosed MM (NDMM) in seven Latin American countries between June 2019 and May 2020. Patients with active disease, on active therapy, and with a follow-up of 6 months from the time of diagnosis were included. Our primary end point was the number of infectious events that required hospitalization for ≥ 24 hours.
RESULTS
Of 248 patients with NDMM, 89 (35.9%) had infectious complications (113 infectious events), the majority (67.3%) within the first 3 months from diagnosis. The most common sites of infection were respiratory (38%) and urinary tract (31%). The microbial agent was identified in 57.5% of patients with gram-negative bacteria (73.5%) as the most common pathogen. Viral infections were infrequent, and no patients with fungal infection were reported. In the multivariable analysis, diabetes mellitus (odds ratio [OR], 2.71; 95% CI, 1.23 to 6.00; P = .014), creatinine ≥ 2 mg/dL (OR, 4.87; 95% CI, 2.29 to 10.35; P < .001), no use of trimethoprim-sulfamethoxazole prophylaxis (OR, 6.66; 95% CI, 3.43 to 12.92; P < .001), and treatment with immunomodulatory drugs (OR, 3.02; 95% CI, 1.24 to 6.29; P = .003) were independent factors associated with bacterial infections. At 6 months, 21 patients (8.5%) had died, 47.6% related to infectious complications.
CONCLUSION
Bacterial infections are a substantial cause of hospital admissions and early death in patients with NDMM. Antibiotic prophylaxis should be considered to reduce infectious complications in patients with MM.
INTRODUCTION
The advances in the management of multiple myeloma (MM) have yielded improved outcomes.1,2 As a result, complications are detected in long-term survivors. Infections are an important cause of morbidity and the leading cause of death in patients with MM, responsible for approximately 50% of early MM deaths.3 A study showed a 7- and 10-fold increased risk for the development of bacterial and viral infections, respectively.4 Pneumonia and sepsis are the most common infections, typically caused by Streptococcus pneumoniae, Haemophilus influenzae, and other gram-negative bacteria.4-8 An impaired cellular and humoral immunity coupled with demographic features in these patients (ie, older age, frailty, and coexisting comorbid conditions) play a role in the increased susceptibility to infections.9
CONTEXT
Key Objective
What clinical features and risk factors are associated with early infectious complications in patients with newly diagnosed myeloma multiple (NDMM) in Latin America?
Knowledge Generated
Bacterial infections, particularly gram-negative agents, are a substantial cause of morbidity and early mortality in NDMM. Diabetes, renal impairment, no antibacterial prophylaxis, and use of immunomodulatory drugs were associated with higher risk of infections. The prevailing site of infections was the respiratory tract.
Relevance
To our knowledge, this is the first study investigating the spectrum of infections in Latin American patients with NDMM. Preventing bacterial infections, particularly those with risk factors, may decrease early morbidity and mortality.
In the recent years, the addition of novel agents (eg, proteasome inhibitors [PIs] and immunomodulatory drugs [IMiDs]) during induction treatment has shifted the epidemiology of infections to an increased number of events happening earlier during therapy.10 The use of PIs has been associated with an increased risk for varicella-zoster virus (VZV) reactivation.11 IMiDs-based therapies have shown infection rates up to 20%, mainly during the first months of therapy and in patients with high disease burden.12-14 Although the use of prophylactic measures (ie, immunization and prophylactic antimicrobials) may reduce this risk, they have not been standardized.5,15 Current guidelines on the prevention of infectious complications are based on expert opinion and panel consensus.16,17
To date, data on the epidemiology of infectious complications in patients with MM treated in Latin America are lacking. Therefore, we aimed to prospectively study the epidemiology of infections and to investigate risk factors associated with the development of infections in patients with newly diagnosed MM (NDMM) within the first 6 months from diagnosis. Identifying clinical and epidemiological characteristics associated with infections may help define the appropriate prophylactic approach to reduce this complication.
METHODS
Patients
We conducted an international, multicenter, prospective cohort study of all consecutive patients with NDMM between June 2019 and May 2020. All centers of the Grupo de Estudio Latinoamericano de Mieloma Múltiple (GELAMM) were invited to participate. Inclusion criteria were active disease, be on therapy, and have a follow-up of at least 6 months from diagnosis and before proceeding to autologous stem-cell transplantation or until death, whatever occurred first. Patients with monoclonal gammopathy of undetermined significance, smoldering MM, plasma cell leukemia, amyloidosis, and HIV infection were excluded. Patient demographics, comorbidities, laboratory data, and myeloma-specific features were obtained from medical records. Institutional Review Boards approved this study at each participating institution. All the patients provided written informed consent, and the study was conducted in accordance with the principles of the Declaration of Helsinki.
Study Variables
Data on all infectious events that required hospitalization for ≥ 24 hours were recorded. The variables analyzed were site of infection, type of isolated microbial agent, severity of infection, time to occurrence, and outcome from the infection. Patients with SARS-CoV-2 infection were excluded from the analysis. Catheter-related infections, but no exit site of port-a-cath infections, were included in the analysis. The antimyeloma treatments were defined as IMiDs-based (ie, thalidomide or lenalidomide), PIs-based (ie, bortezomib), and IMiDs plus PIs-based. The choice of therapy and antimicrobial prophylaxis was decided by the treating physician. Comorbidities included were diabetes mellitus, chronic pulmonary disease, asthma, and heart failure.
Definitions
The diagnosis of MM was defined according to the International Myeloma Working Group (IMWG) 2014 criteria, and staging was performed in adherence to the International Staging System (ISS) recommendations.18,19 We defined infectious event as the presence of body temperature ≥ 38°C and/or the presence of clinical symptoms or signs of infection. Events were classified as clinically defined when there was clinical evidence but microbial isolation was negative; microbiologically defined (MD) when the microbial agent was identified from blood test and/or other body sources; and fever of unknown origin when the only clinical sign was fever without microbial isolation. The type of infection (ie, bacterial, viral, or fungal) was defined on the basis of combined clinical, imaging, and microbiological findings. Bacterial infections were identified by conventional culture methods, and enzyme immunoassay in stools was used to identify Clostridium difficile infection. Culture-independent methods to identify viral and fungal infections (eg, respiratory viral panel, serum galactomannan, and urine histoplasma antigen) were recorded when available. When the infectious agent was not identified, if the response to empiric antibiotic, antifungal, or antiviral therapy was documented, they were classified as bacterial, fungal, or viral infection, respectively. Early death was defined as death within the first 6 months from diagnosis. Cause of death (classified as either infectious or noninfectious) was determined by the treating physicians.
Statistical Analyses
Demographics, clinical features, and therapies received were summarized using descriptive statistics. The primary study outcome was the number of infectious events that required hospitalization for ≥ 24 hours within the first 6 months of follow-up. Secondary outcomes were mortality rate at 6 months and its cause. Quantitative variables were described in terms of median; qualitative variables were described as absolute percentage. Patients were divided on the basis of the presence or absence of infectious events. Comparisons between subgroups were analyzed using the chi-square test and Student's t test, as appropriate. Univariable analysis was performed using the chi-square test to identify possible risk factors for infection; those with a P < .05 were selected and included in the multivariable analysis, which was performed using a binary logistic regression model (forward likelihood ratio). The degree of collinearity between variables was evaluated using the variance inflation factor statistic. Clinical and treatment factors evaluated were age, Eastern Cooperative Oncology Group (ECOG) performance status, smoking habit, comorbidities, myeloma subtype, ISS score, Durie-Salmon stage, anemia (hemoglobin level < 10 g/dL), renal impairment (serum creatinine level ≥ 2 mg/dL), hypercalcemia (serum calcium > 11 mg/dL), presence of osteolytic lesions (one or more on skeletal imaging), lymphopenia (blood lymphocyte count ≤ 1 × 109/L), hypoalbuminemia (serum albumin < 3.5 g/dL), elevated serum lactate dehydrogenase (above the upper limit of normal), immunoparesis (decreased serum concentration of any polyclonal immunoglobulin class in serum), and type of therapy. In all patients, P < .05 was considered significant. The statistical analysis was performed using IBM SPSS version 25.0 (Armonk, NY).
RESULTS
Epidemiological and Clinical Features
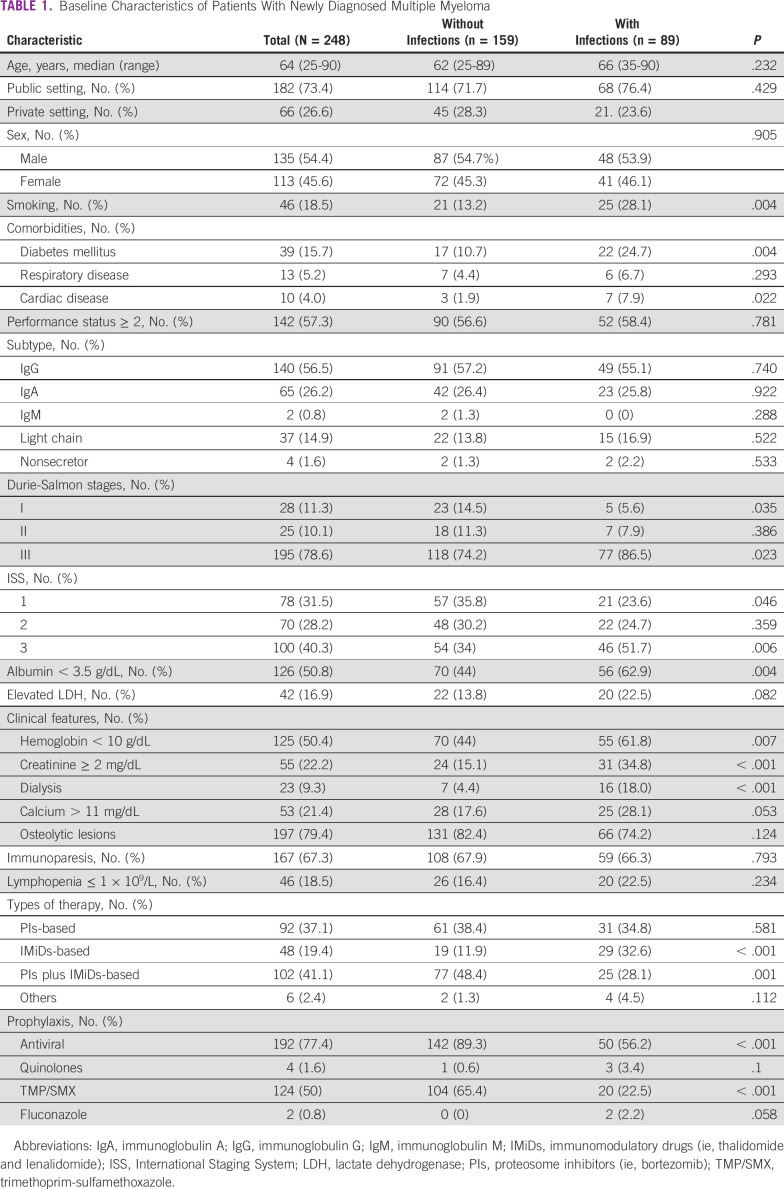
A total of 248 patients with NDMM were included. Seventy-five (30.2%) patients were from Uruguay, 64 (25.8%) from Peru, 47 (19%) from Chile, 22 (8.9%) from Cuba, 17 (6.9%) from Argentina, 16 (6.5%) from Panama, and 7 (2.8%) from Venezuela. The clinical features of patients with NDMM are summarized in Table 1.
TABLE 1.
Baseline Characteristics of Patients With Newly Diagnosed Multiple Myeloma
Infection Rates and Outcomes
Infections were found in 89 patients (35.9%) with a median time to first infection of 2 months from diagnosis (range, 1-6 months). A total of 113 infectious events were identified in the 89 patients; 23.6% (n = 21) had ≥ 2 infectious events. The majority of infectious events (n = 76 of 113, 67.3%) occurred in the first 3 months from diagnosis, particularly within the first month (n = 53 of 113, 46.9%).
Patients experiencing infections had more advanced Durie-Salmon stage (stage III 86.5% v 74.2%, P = .023), ISS 3 (51.7% v 34%, P = .006), anemia (61.8% v 44%, P = .007), renal impairment (34.4% v 15.1%, P < .001), and hypoalbuminemia (62.9% v 44%, P = .004) at diagnosis. A history of smoking (28.1% v 13.1%, P = .004) and diabetes mellitus (24.7% v 10.7%, P = .004) were more frequent in patients who developed infectious complications. Median age at diagnosis, sex, ECOG performance status ≥ 2, MM subtype, and presence of hypercalcemia, osteolytic lesions, immunoparesis, and lymphopenia were not different between those developing infections versus those who did not. Most patients (n = 182, 73.4%) were managed at public institutions (Table 1).
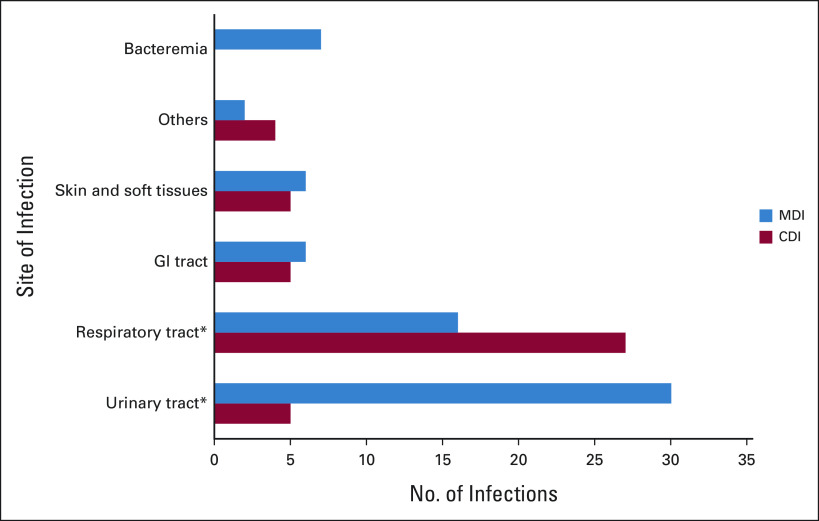
The most common site of infection was the respiratory tract (n = 43, 38%), followed by urinary tract (n = 35 patients, 31%), skin and soft tissue (n = 11, 9.7%), gastrointestinal tract (n = 11, 9.7%), blood stream (n = 8, 7.1%), and central nervous system (n = 3, 2.7%). In two patients (1.8%), the site of infection was not identified and were classified as fever of unknown origin. Respiratory infections were predominantly clinically defined (P < .001), whereas urinary tract infections were MD (P < .001; Fig 1).
FIG 1.
Distribution of sites of infection. There was a significant difference in the proportion of MDI and in the proportion of CDI in patients with respiratory and urinary tract infections (*P < .001). CDI, clinically defined infections; MDI, microbiologically defined infections.
Overall, 77.4% of patients received antiviral prophylaxis, the majority during bortezomib treatment (n = 181 of 194, 93.3%). Prophylaxis with trimethoprim-sulfamethoxazole was performed in 50% of the patients, and its use was associated with less infectious events (22.5% v 65.4%, P < .001). Fluoroquinolones and fluconazole prophylaxis were used in a low number of patients (1.6% and 0.8%, respectively; Table 1). Immunization against Haemophilus influenza and Streptococcus pneumoniae was documented in 28.2% (n = 70) and 18.1% (n = 45) of patients, respectively.
Distribution of the Pathogens
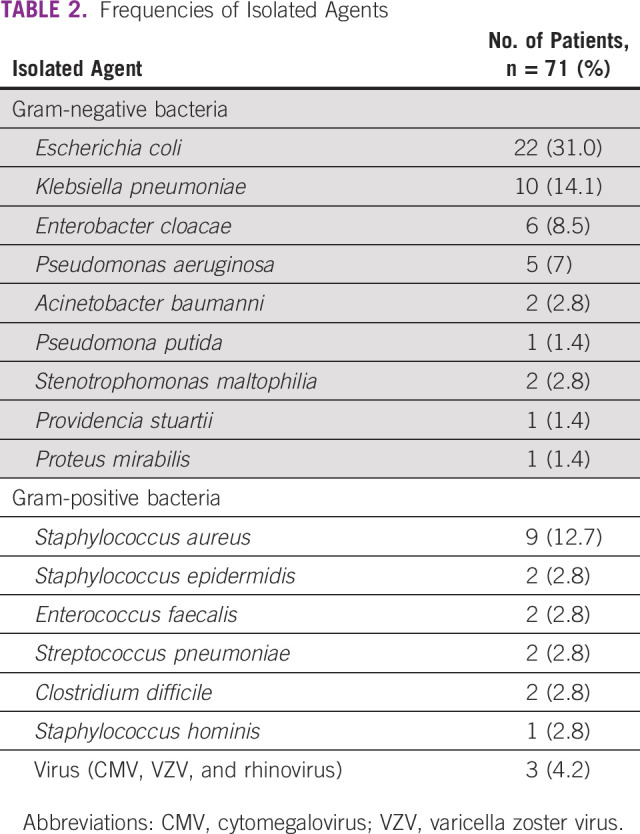
In the 113 infectious events, the microbial agent was isolated in 65 (57.5%) patients; six (9.2%) had more than one microorganism isolated. Bacterial infections represented 97.3% of the episodes. Viral infections were rare (three patients), and no patients with fungal infections were reported. Gram-negative bacteria represented 73.5% (n = 50 of 68) and gram-positive bacteria 26.5% (n = 18 of 68) of MD patients. The most frequent pathogen was Escherichia coli (31%), followed by Klebsiella pneumoniae (14.1%) and Staphylococcus aureus (12.7%; Table 2). The most frequently isolated bacteria causing respiratory tract infection was Klebsiella pneumoniae (n = 4, 28.6%) and Escherichia coli for urinary tract infection (n = 18, 60%). The major sources for microorganism isolation were urine (46.2%), blood culture (27.7%), bronchoalveolar lavage (12.3%), and stool (6.2%).
TABLE 2.
Frequencies of Isolated Agents
Rates of Infection According to MM Treatment
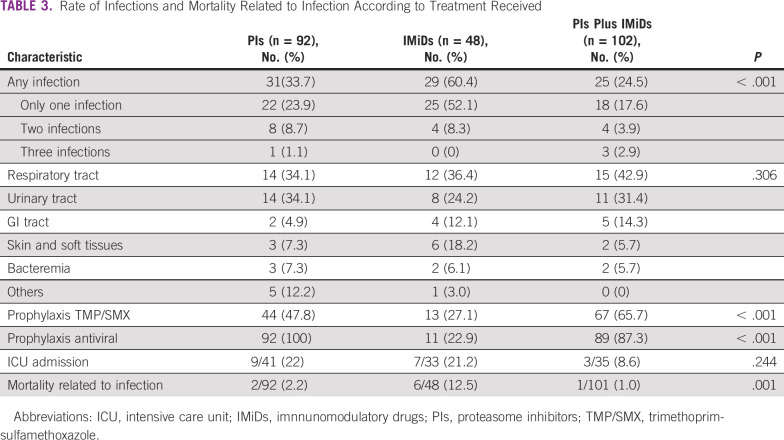
To analyze the effect of MM therapy on the rate of infection, we categorized patients according to the drug for which the regimen was based (ie, PIs-based, IMiDs-based, or combination of both; Table 3). PIs-based therapy was administered in 92 patients (37.1%), IMiDs-based therapy in 48 patients (19.4%), and a combination of both in 102 patients (41.1%). The remaining patients were treated with conventional chemotherapy. Infections were reported in 60.4% of patients treated with IMiDs, 33.7% treated with PIs, and 24.5% receiving PIs plus IMiDs (P < .001). Antimicrobial prophylaxis with trimethoprim-sulfamethoxazole was significantly less used in the IMiDs group compared with PIs and PIs plus IMiDs group (27.1% v 47.8% and 65.7%, respectively; P < .001). The highest mortality rate related to infections were seen in patients treated with IMiDs (12.5%, P = .001) compared with PIs (2.2%) and PIs plus IMiDs (1%). Across all treatment modalities, the most frequent causes of infection were respiratory (PIs 34.1%, IMiDs 36.4%, and PIs plus IMiDs 42.9%) and urinary (PIs 34.1%, IMiDs 24.2%, and PIs plus IMiDs 31.4%) tract infections.
TABLE 3.
Rate of Infections and Mortality Related to Infection According to Treatment Received
Risk Factors for Bacterial Infection Development
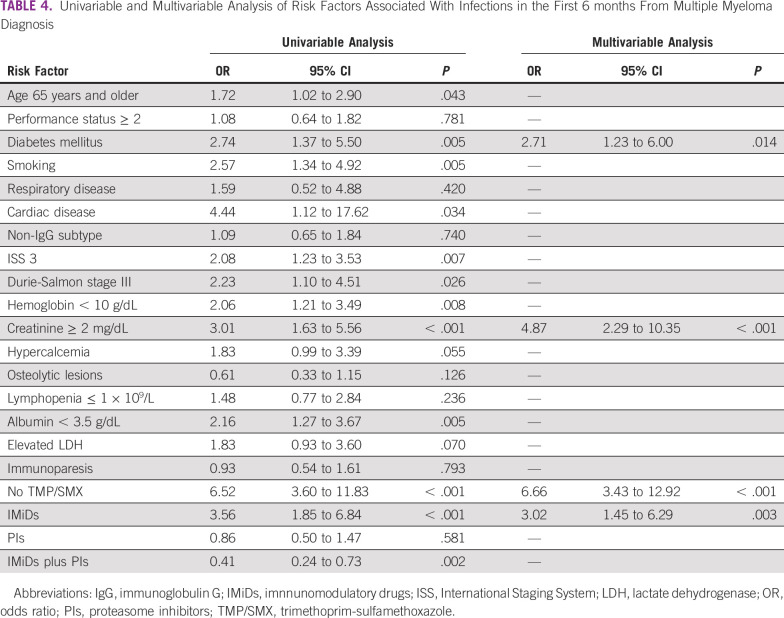
In the univariable analysis, the variables associated with a higher risk for bacterial infection in the first 6 months from diagnosis were age 65 years and older (P = .043), smoking (P = .005), presence of coexisting comorbidities such as diabetes mellitus (P = .005) and cardiac disease (P = .034), ISS 3 (P = .007), Durie-Salmon stage III (P = .026), hemoglobin < 10 g/dL (P = .008), creatinine ≥ 2 mg/dL (P < .001), serum albumin < 3.5 g/dL (P = .005), no trimethoprim-sulfamethoxazole prophylaxis (P < .001), and therapies with IMiDs-based regimen (P < .001) and combined IMiDs and PIs regimen (P = .002). In the multivariable analysis, the factors with an independent prognostic value for the development of infections were diabetes mellitus (odds ratio [OR], 2.71; 95% CI, 1.23 to 6.00; P = .014), creatinine ≥ 2 mg/dL (OR, 4.87; 95% CI, 2.29 to 10.35; P < .001), no trimethoprim-sulfamethoxazole prophylaxis (OR, 6.66; 95% CI, 3.43 to 12.92; P < .001), and IMiDs-based regimen (OR, 3.02; 95% CI, 1.45 to 6.29; P = .003; Table 4). There was no collinearity among the factors (variance inflation factor < 2 in all patients). Analysis of risk factors associated with fungal and viral infections was not performed given the small sample size.
TABLE 4.
Univariable and Multivariable Analysis of Risk Factors Associated With Infections in the First 6 months From Multiple Myeloma Diagnosis
Intensive Care Unit Admission and Mortality Rate
Overall, 18.6% (n = 21 of 113) of infectious events resulted on admission to the intensive care unit. A total of 21 (8.5%) patients died within 6 months from diagnosis; 10 (47.6%) of these deaths were due to infectious complications.
DISCUSSION
To our knowledge, this study is the first multi-institutional study evaluating the epidemiology, clinical features, and outcomes associated with infectious complications in nontransplanted patients with NDMM in Latin America. Around 36% of patients experienced infectious complications early on their treatment, particularly in the first 3 months from diagnosis. With a median follow-up of 6 months, the overall mortality rate was 8.5%, and almost half of these deaths were due to infections. This study confirms infections as a major cause of morbidity and early mortality in this patient population. This highlights the importance of preventing infectious complications early during MM management.
Patients with MM experience a higher rate of infection compared with the general population,3,4 particularly in the first 2 months of induction therapy.20-23 This may be explained by the immunosuppressive nature of active disease added to the immunosuppressive effect of antimyeloma agents.3,24 In this study of nontransplanted patients undergoing early phase of MM treatment, the majority of infectious complications were bacterial, particularly in the respiratory tract and caused by gram-negative bacteria. These findings are concordant with previously published data.5,23,25 Historically, a high risk of infection with encapsulated bacteria has been reported in patients with MM.26-28 In recent studies, infections due to Streptococcus pneumoniae and Haemophilus influenzae represented only 5%-9% and 2%, respectively.5,8,25,29 In line with these results, our study found Streptococcus pneumoniae in 2.9% of all isolations, suggesting that in patients treated in the era of PIs and IMIDs, infection with encapsulated bacteria is relatively low, even in a population where pneumococcal vaccination is not routinely performed. Although response to immunizations is frequently impaired in patients with MM, pneumococcal vaccines are effective in reducing the risk of pneumonia; therefore, routine vaccination against Streptococcus pneumoniae and Haemophilus influenzae is recommended.16,30,31
Blimark et al4 found that viral infections were ten times higher in patients with MM compared with matched controls. The APEX study described an increased incidence of VZV reactivation in bortezomib-treated patients.11 In our study, viral infections were infrequent, with only one case of VZV reactivation in a patient receiving bortezomib but not on antiviral prophylaxis. A high adherence (93.3%) to antiviral prophylaxis in PIs-treated patients may explain the low incidence of VZV reactivation in our cohort. Studies have reported a low incidence of fungal infections in patients with MM, with invasive fungal disease documented in < 2.4% of patients, mostly during disease progression.29,32 Consistent with this, in our study, no patients with fungal infection were found after 6 months of follow-up.
Data on the risk for infection with the use of IMiDs and PIs are conflicting.4,5,15,33 Brioli et al15 reported that use of IMiDs and PIs was not associated with a significantly increased risk of infection. Recently, a study reported that use of PIs-based therapy and increasing lines of therapy (> 4) were independently associated with an increased risk of infection. However, IMiDs-based therapy was not associated with an increased risk.33 In our study, the use of IMiDs was associated with an increased risk for infections (P = .003). Although we cannot entirely explain this finding, a lower use of antimicrobial prophylaxis in patients who received IMiDs might explain the observed outcome (trimethoprim-sulfamethoxazole prophylaxis was used in 27.1%, 47.8%, and 65.7% of patients on IMiDs, PIs, and PIs plus IMiDs, respectively, P < .001). Some prospective studies have evaluated the role of prophylactic antimicrobials in patients with MM. A randomized study on 212 patients with NDMM evaluated prophylactic antibiotics during the first 2 months of treatment and found no significant differences in the incidence of severe bacterial infections in patients receiving ciprofloxacin or trimethoprim-sulfamethoxazole or under observation.34 A phase III study performed on 977 patients with NDMM showed that levofloxacin was associated with a significantly reduction of febrile episodes and deaths compared with placebo.35 In our cohort, we found that no use of trimethoprim-sulfamethoxazole prophylaxis was associated with an increased risk for bacterial infections (P < .001). This finding is in line with that of Teh et al25 who reported a decreased risk for infection in patients receiving trimethoprim-sulfamethoxazole prophylaxis.
Patients who developed infections had significantly more advanced disease supporting that tumor burden is an important risk factor.8,15,29 Smoking, diabetes mellitus, and cardiac disease were also more frequent in patients developing infections. In the multivariable analysis, diabetes mellitus (P = .014) and renal impairment (P < .001) were independently associated with an increased risk for bacterial infections. Unlike other studies, ECOG performance status of ≥ 2, immunoparesis, elevated lactate dehydrogenase, and lymphopenia were not significantly associated with an increased risk of infection.8,22,36,37 Although immunoparesis seemed the most logical risk for infection, a study in NDMM showed that infection does not appear to be the main mechanism through which immunoparesis affects survival in patients with NDMM.38 On the basis of all the above, we suggest antibacterial prophylaxis in NDMM that have one or more of the above described risk factors and during at least the first 6 months of induction therapy. A similar recommendation has already been suggested by other researchers, who have recommended antibacterial prophylaxis in patients receiving IMiDs or bortezomib, those with a high tumor burden, and those with a history of frequent infections or comorbidities.16,35,39
Our study has limitations. First, the voluntary nature of recruiting participating centers may have unintentionally biased patient selection (most patients came from public than private institutions), the absence of centralized laboratory review, the lack of standardized workflow protocols in patients with suspected infections as well as the heterogeneity of methods used for microbiological characterization could have led to underestimate the frequency of infectious events, the causative microorganism, and/or an incomplete characterization of the spectrum of infections in our study population. In addition, the heterogeneity on the management of infectious episodes may have influenced the outcomes. Data regarding MM response status at the time of death would have been of great interest to document. Nonetheless, the main strengths of this analysis are its prospective nature and the inclusion of patients treated only at specialized cancer centers from Latin America. Moreover, the outcomes of this study are consistent with those reported internationally. To our knowledge, this is the first study investigating the spectrum of infections in Latin American patients with NDMM.
In conclusion, this study shows that bacterial infections are a substantial cause of morbidity and early mortality in patients with NDMM. The choice of the optimal infection prevention strategy is highly needed while considering the emergence of antimicrobial-resistant because of the indiscriminate use of antibiotics. This document raises a concern regarding the impact of infectious complications in NDMM in our region.
Eloísa Riva
Honoraria: Sanofi
Travel, Accommodations, Expenses: ROEMMERS
Jule Vásquez
Honoraria: Janssen, Roche
Consulting or Advisory Role: Janssen, AbbVie
Research Funding: Roche (Inst)
Travel, Accommodations, Expenses: Janssen, AbbVie
Camila Peña
Honoraria: Janssen, Bristol Myers Squibb/Medarex
Consulting or Advisory Role: Janssen
Travel, Accommodations, Expenses: Tecnofarma
César Samanez
Honoraria: Janssen Oncology, Roche, Merck
Dorotea Fantl
Speakers' Bureau: Janssen, Takeda, Sanofi, Amgen
Research Funding: Janssen, Takeda, Helsinn Therapeutics, Sanofi, Roche
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Virginia Bove, Eloísa Riva, Camila Peña, Cristian Seehaus, Julio Fernández, Oliday Ríos, Yusaima Rodríguez, Irving Figueredo, Dorotea Fantl, Luis Malpica
Provision of study materials or patients: Eloísa Riva, Jule Vásquez, Camila Peña, Justina Bustos, Marcos Hernández, Dorotea Fantl
Collection and assembly of data: Virginia Bove, Eloísa Riva, Jule Vásquez, Camila Peña, Cristian Seehaus, César Samanez, Justina Bustos, Marcos Hernández, Julio Fernández, Oliday Ríos, Yusaima Rodríguez, Irving Figueredo
Data analysis and interpretation: Virginia Bove, Eloísa Riva, Luis Malpica
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eloísa Riva
Honoraria: Sanofi
Travel, Accommodations, Expenses: ROEMMERS
Jule Vásquez
Honoraria: Janssen, Roche
Consulting or Advisory Role: Janssen, AbbVie
Research Funding: Roche (Inst)
Travel, Accommodations, Expenses: Janssen, AbbVie
Camila Peña
Honoraria: Janssen, Bristol Myers Squibb/Medarex
Consulting or Advisory Role: Janssen
Travel, Accommodations, Expenses: Tecnofarma
César Samanez
Honoraria: Janssen Oncology, Roche, Merck
Dorotea Fantl
Speakers' Bureau: Janssen, Takeda, Sanofi, Amgen
Research Funding: Janssen, Takeda, Helsinn Therapeutics, Sanofi, Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Mateos MV, San Miguel JF: Management of multiple myeloma in the newly diagnosed patient. Hematol Am Soc Hematol Educ Program 2017:498-507, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajkumar SV: Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol 95:548-567, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Augustson BM, Begum G, Dunn JA, et al. : Early mortality after diagnosis of multiple myeloma: Analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002—Medical Research Council Adult Leukaemia Working Party. J Clin Oncol 23:9219-9226, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Blimark C, Holmberg E, Mellqvist UH, et al. : Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica 100:107-113, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teh BW, Harrison SJ, Worth LJ, et al. : Risks, severity and timing of infections in patients with multiple myeloma: A longitudinal cohort study in the era of immunomodulatory drug therapy. Br J Haematol 171:100-108, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Savage DG, Lindenbaum J, Garrett TJ: Biphasic pattern of bacterial infection in multiple myeloma. Ann Intern Med 96:47-50, 1982 [DOI] [PubMed] [Google Scholar]
- 7.Huang CT, Liu CJ, Ko PS, et al. : Risk factors and characteristics of blood stream infections in patients with newly diagnosed multiple myeloma. BMC Infect Dis 17:33, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sørrig R, Klausen TW, Salomo M, et al. : Risk factors for blood stream infections in multiple myeloma: A population-based study of 1154 patients in Denmark. Eur J Haematol 101:21-27, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Teh BW, Harrison SJ, Pellegrini M, et al. : Changing treatment paradigms for patients with plasma cell myeloma: Impact upon immune determinants of infection. Blood Rev 28:75-86, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Teh BW, Harrison SJ, Worth LJ, et al. : Infection risk with immunomodulatory and proteasome inhibitor–based therapies across treatment phases for multiple myeloma: A systematic review and meta-analysis. Eur J Cancer 67:21-37, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Chanan-Khan A, Sonneveld P, Schuster MW, et al. : Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol 26:4784-4790, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Caravita T, Offidani M, Siniscalchi A, et al. : Infection complications in an unselected cohort of patients with multiple myeloma treated with lenalidomide combinations. Eur J Haematol 89:276-277, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Offidani M, Corvatta L, Polloni C, et al. : Infectious complications in patients with multiple myeloma treated with new drug combinations containing thalidomide. Leuk Lymphoma 52:776-785, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Chen N, Lau H, Kong L, et al. : Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol 47:1466-1475, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Brioli A, Klaus M, Sayer H, et al. : The risk of infections in multiple myeloma before and after the advent of novel agents: A 12-year survey. Ann Hematol 98:713-722, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Girmenia C, Cavo M, Offidani M, et al. : Management of infectious complications in multiple myeloma patients: Expert panel consensus-based recommendations. Blood Rev 34:84-94, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Terpos E, Kleber M, Engelhardt M, et al. : European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica 100:1254-1266, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. : International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15:e538-e548, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Greipp PR, San Miguel J, Durie BGM, et al. : International staging system for multiple myeloma. J Clin Oncol 23:3412-3420, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Perri RT, Hebbel RP, Oken MM: Influence of treatment and response status on infection risk in multiple myeloma. Am J Med 71:935-940, 1981 [DOI] [PubMed] [Google Scholar]
- 21.Rayner HC, Haynes AP, Thompson JR, et al. : Perspectives in multiple myeloma: Survival, prognostic factors and disease complications in a single centre between 1975 and 1988. Q J Med 79:517-525, 1991 [PubMed] [Google Scholar]
- 22.Dumontet C, Hulin C, Dimopoulos MA, et al. : A predictive model for risk of early grade ≥3 infection in patients with multiple myeloma not eligible for transplant: Analysis of the FIRST trial. Leukemia 32:1404-1413, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bladé J, Rosiñol L: Renal, hematologic and infectious complications in multiple myeloma. Best Pract Res Clin Haematol 18:635-652, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Pratt G, Goodyear O, Moss P: Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol 138:563-579, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Teh BW, Harrison SJ, Slavin MA, et al. : Epidemiology of bloodstream infections in patients with myeloma receiving current era therapy. Eur J Haematol 98:149-153, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Hargreaves RM, Lea JR, Griffiths H, et al. : Immunological factors and risk of infection in plateau phase myeloma. J Clin Pathol 48:260-266, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nørgaard M, Larsson H, Pedersen G, et al. : Risk of bacteraemia and mortality in patients with haematological malignancies. Clin Microbiol Infect 12:217-223, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Twomey JJ: Infections complicating multiple myeloma and chronic lymphocytic leukemia. Arch Intern Med 132:562-565, 1973 [PubMed] [Google Scholar]
- 29.Valkovic T, Gacic V, Ivandic J, et al. : Infections in hospitalised patients with multiple myeloma: Main characteristics and risk factors. Turk J Haematol 32:234-242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoma I, Karpov I, Iskrov I, et al. : Clinical efficacy of pneumococcal vaccination in multiple myeloma patients on novel agents: Results of a prospective clinical study. Vaccine 38:4713-4716, 2020 [DOI] [PubMed] [Google Scholar]
- 31.Robertson JD, Nagesh K, Jowitt SN, et al. : Immunogenicity of vaccination against influenza, Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma. Br J Cancer 82:1261-1265, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teh BW, Teng JC, Urbancic K, et al. : Invasive fungal infections in patients with multiple myeloma: A multi-center study in the era of novel myeloma therapies. Haematologica 100:e28-e31, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim C, Sinha P, Harrison SJ, et al. : Epidemiology and risks of infections in patients with multiple myeloma managed with new generation therapies. Clin Lymphoma Myeloma Leuk 21:444-450.e3, 2021 [DOI] [PubMed] [Google Scholar]
- 34.Vesole DH, Oken MM, Heckler C, et al. : Oral antibiotic prophylaxis of early infection in multiple myeloma: A URCC/ECOG randomized phase III study. Leukemia 26:2517-2520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drayson MT, Bowcock S, Planche T, et al. : Levofloxacin prophylaxis in patients with newly diagnosed myeloma (TEAMM): A multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. Lancet Oncol 20:1760-1772, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nucci M, Anaissie E: Infections in patients with multiple myeloma. Semin Hematol 46:277-288, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Hyun SY, Han SH, Kim SJ, et al. : Pretreatment lymphopenia, poor performance status, and early courses of therapy are risk factors for severe bacterial infection in patients with multiple myeloma during treatment with bortezomib-based regimens. J Korean Med Sci 31:510-518, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heaney JLJ, Campbell JP, Iqbal G, et al. : Characterisation of immunoparesis in newly diagnosed myeloma and its impact on progression-free and overall survival in both old and recent myeloma trials. Leukemia 32:1727-1738, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung SH, Kang SJ, Jang HC, et al. : Effect of levofloxacin prophylaxis for prevention of severe infections in multiple myeloma patients receiving bortezomib-containing regimens. Int J Hematol 100:473-477, 2014 [DOI] [PubMed] [Google Scholar]