Abstract
The roles of CXC chemokine-mediated host responses were examined with an A/J mouse model of Legionella pneumophila pneumonia. After intratracheal inoculation of 106 CFU of L. pneumophila, the bacterial numbers in the lungs increased 10-fold by day 2; this increase was accompanied by the massive accumulation of neutrophils. Reverse transcription-PCR data demonstrated the up-regulation of CXC chemokines, such as keratinocyte-derived chemokine, macrophage inflammatory protein 2 (MIP-2), and lipopolysaccharide-induced CXC chemokine (LIX). Consistent with these data, increased levels of KC, MIP-2, and LIX proteins were observed in the lungs and peaked at days 1, 2, and 2, respectively. Although the administration of anti-KC or anti–MIP-2 antibody resulted in an approximately 20% decrease in neutrophil recruitment on day 2, no increase in mortality was observed. In contrast, the blockade of CXC chemokine receptor 2 (CXCR2), a receptor for CXC chemokines, including KC and MIP-2, strikingly enhanced mortality; this effect coincided with a 67% decrease in neutrophil recruitment. Interestingly, anti-CXCR2 antibody did not affect bacterial burden by day 2, even in the presence of a lethal challenge of bacteria. Moreover, a significant decrease in interleukin-12 (IL-12) levels, in contrast to the increases in KC, MIP-2, and LIX levels, was demonstrated for CXCR2-blocked mice. These data indicated that CXCR2-mediated neutrophil accumulation may play a crucial role in host defense against L. pneumophila pneumonia in mice. The increase in lethality without a change in early bacterial clearance suggested that neutrophils may exert their protective effect not through direct killing but through more immunomodulatory actions in L. pneumophila pneumonia. We speculate that a decrease in the levels of the protective cytokine IL-12 may explain, at least in part, the high mortality in the setting of reduced neutrophil recruitment.
Legionella pneumophila is an important pathogen that frequently causes a life-threatening pneumonia, especially in immunocompromised individuals (25, 33). This organism is a gram-negative facultative intracellular bacillus and is quite ubiquitous in nature, being present in soil and water. Legionella organisms usually infect humans via inhalation of contaminated aerosols from waterborne environmental sources. In the lungs, bacteria infect cells and multiply predominantly in monocytes and macrophages (20, 27, 29). Unfortunately, high mortality rates, reaching 10 to 50%, have been reported, illustrating the fact that Legionella pneumonia remains a challenging infectious disease (13, 30, 39).
Recently, a replicative A/J mouse model of L. pneumophila pneumonia has been reported; this model is believed to be based on the fact that macrophages in this strain are specifically permissive for the growth of Legionella organisms (6). Inoculation of L. pneumophila into the lungs of A/J mice induced pneumonia characterized by specific pathological findings and cytokine responses, accompanied by multiplication of bacteria in the lungs. Important roles of T1-type cytokines, such as tumor necrosis factor alpha (TNF-α) (7), gamma interferon (IFN-γ) (14), and interleukin-12 (IL-12) (8), have been elucidated with the A/J mouse model of L. pneumophila pneumonia. Since intracellular growth is a critical characteristic of this organism, cellular immunity in concert with cytokine or chemokine responses is believed to be essential for the resolution of a primary infection. On the other hand, it is generally believed that neutrophils play a minor role because previous reports have demonstrated that Legionella organisms resist killing by neutrophils, even under conditions of good opsonization (19, 42). However, the early accumulation of neutrophils at infection sites is a consistent observation for Legionella pneumonia in animals and humans (6, 43, 44), suggesting that these cells may have certain protective roles in Legionella infection. To our knowledge, there are no reports investigating how neutrophils contribute to host defense against L. pneumophila in A/J mice.
Chemokines are a family of small chemotactic proteins, which are divided into four families based on their structural differences (34). The CXC chemokine family is further distinguished by the presence or absence of an amino acid sequence, glutamic acid-leucine-arginine (the ELR motif), that precedes the CXC sequence. ELR-positive (ELR+) CXC chemokines have been shown to induce neutrophil chemotaxis and stimulate neutrophil activation in inflammatory responses (1, 15, 45). Several ELR+ CXC chemokines exist in humans, including IL-8 and the growth-related oncogene family (GRO-α,β). Murine ELR+ CXC chemokines have also been identified, including macrophage inflammatory protein 2 (MIP-2), keratinocyte-derived chemokine (KC), lipopolysaccharide-induced CXC chemokine (LIX), and lungkine (36, 37). Macrophages are reported to be the main sources for MIP-2 and KC, whereas LIX and lungkine are predominantly produced by fibroblasts and lung epithelial cells, respectively (21, 36, 38, 46). Two receptors for ELR+ CXC chemokines, CXC chemokine receptors 1 and 2 (CXCR1 and CXCR2, respectively), have been identified for humans (24); mice have only CXCR2 which, like its human counterpart, binds all ELR+ CXC chemokines (5, 17, 23). In CXCR2 knockout mice, neutrophils were not recruited in vivo in response to MIP-2 or KC but did respond to other chemoattractants, suggesting that the binding of ELR+ CXC chemokines to CXCR2 is essentially for neutrophil recruitment and that CXCR2 is the exclusive receptor for these ligands (23).
Recently, McColl and Clark-Lewis demonstrated the inhibition of murine neutrophil recruitment in vivo by the administration of CXC chemokine receptor antagonists, such as GROα(8–73) and PF4(9–70) (26). Previously, we reported a critical role for CXCR2-mediated neutrophil accumulation in murine pulmonary infection models, such as Pseudomonas aeruginosa (41) and Aspergillus fumigatus (28). In these studies, we observed increased lethality for CXCR2-blocked mice; this finding was well correlated with a decrease in neutrophil recruitment and increases in bacterial and fungal burdens in the lungs. The roles of CXC chemokines and CXCR2-mediated neutrophil accumulation in infection due to the intracellular organism Legionella, which is resistant to killing effects by neutrophils, are less clear.
In the present study, we examined the roles and significance of CXCR2 ligand-mediated neutrophil recrutment in an A/J mouse model of L. pneumophila pneumonia. Our data clearly demonstrated critical roles of CXCR2 in neutrophil accumulation and lethality. On the other hand, blocking of each CXC chemokine, KC or MIP-2, had a minor effect, supporting the concept of CXC chemokine redundancy. Interestingly, the augmentation of lethality was not accompanied by an early increase in the pulmonary bacterial burden in CXCR2-blocked mice, suggesting that neutrophils may exert their protective effect not through direct killing but through more immunomodulatory actions in L. pneumophila pneumonia.
MATERIALS AND METHODS
Reagents.
Murine recombinant KC, MIP-2, and LIX and monoclonal anti-murine KC antibody were purchased from R & D Systems (Minneapolis, Minn.). Polyclonal anti-murine KC, MIP-2, and LIX antibodies for enzyme-linked immunosorbent assays (ELISAs) were produced by immunization of rabbits at multiple intradermal sites with these murine recombinant cytokines in complete Freund's adjuvant. Purified polyclonal goat antimurine CXCR2 antibody was produced by intradermal immunization of goats with a 17-amino-acid peptide segment comprising a portion of the seven-transmembrane receptor that resides on the cell surface of CXCR2 and that has previously been shown to be the binding site for ligands (18). This antibody has been shown to detect CXCR2 by Western blotting and fluorescence-activated cell sorter analysis of neutrophils (data not shown). We have demonstrated that this antibody is neutralizing both in vitro and in vivo and that binding of this antibody to CXCR2 on neutrophils does not alter peripheral blood neutrophil counts (28).
Animals.
Female specific-pathogen-free 6- to 8-week-old A/J mice were purchased from Jackson Laboratory (Bar Harbor, Maine). All mice were housed in specific-pathogen-free conditions within the animal care facility at the University of Michigan.
L. pneumophila inoculation.
We used a clinical isolate of L. pneumophila serogroup 1 for animal experiments; this isolate was obtained from the sputum of a patient with L. pneumophila pneumonia. In preliminary experiments, we observed that intratracheal inoculation of this isolate (105 to 106 CFU per mouse) consistently induced pneumonia in A/J mice, characterized by increases in bacterial numbers, cytokine responses, and pathological changes in the lungs (data not shown). N-(2-Acetamido)-2-aminoethanesulfonic acid (ACES; Sigma)-buffered yeast extract broth supplemented with l-cysteine (0.4 mg/ml) and ferric nitrate (0.135 mg/ml) was used as a liquid medium (BYE broth) (9). To prepare solid medium, activated charcoal (2 mg/ml) and agar (15 mg/ml) were added to liquid medium (BCYE agar). Bacteria were incubated on BCYE agar for 14 days at 37°C. A single colony was transferred to 3 ml of BYE broth and then incubated overnight at 37°C with constant shaking. The bacterial suspension was again transferred to fresh BYE broth and incubated overnight under the same conditions. After confirmation of bacterial motility by microscopic observation, the concentration of bacteria in the broth was determined by measuring the absorbance at 600 nm. Postexponential-phase bacteria (optical density 600 nm, 1.7 to 1.8; motility, > 30%) were used as challenge organisms because the expression of virulence in L. pneumophila is dependent on growth phase (9). According to a standard of absorbancies based on known CFU, the bacterial suspension was diluted to the desired concentration in saline. Each animal was anesthetized intraperitoneally (i.p.) with approximately 1.8 to 2 mg of pentobarbital. The trachea was exposed, and 30 μl of inoculum or saline was administered via a sterile 26-gauge needle. The skin incision was closed with surgical staples.
Lung harvesting for bacterial number, MPO, and cytokine analyses.
At designated time, the mice were sacrificed by CO2 asphyxia. Prior to lung removal, the pulmonary vasculature was perfused with 1 ml of phosphate-buffered saline (PBS) containing 5 mM EDTA via the right ventricle. Whole lungs were then harvested for assessment of bacterial numbers, myeloperoxidase (MPO) levels, and cytokine protein expression. After removal, whole lungs were homogenized in 1.0 ml of PBS with protease inhibitor (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) using a tissue homogenizer (Biospec Products, Inc.) under a vented hood. Portions of homogenates (10 μl) were inoculated on BCYE agar after serial 1:10 dilutions with PBS. Lung MPO activity, as an indirect measurement of total neutrophil number, was quantitated by a method described previously (16). Briefly, 100 μl of lung homogenate was mixed with 100 μl of homogenization buffer (0.5% hexadecyltrimethylammonium bromide–5 mM EDTA) and vortexed. The mixture was sonicated and centrifuged at 12,000 × g for 15 min. The supernatant was then mixed 1:15 with assay buffer, and the absorbance was read at 490 nm. MPO levels were calculated as the change in absorbance over time. The remaining homogenates were incubated on ice for 30 min and then centrifuged at 1,100 × g for 10 min. Supernatants were collected, passed through a 0.45-μm-pore-size filter (Gelman Sciences, Ann Arbor, Mich.), and stored at −20°C for assessment of cytokine levels.
Murine cytokine ELISAs.
Murine cytokines were quantitated using a modification of a double-ligand method as previously described (16). Briefly, wells of flat-bottom 96-well microtiter plates (Immuno-Plate I 96-F; Nune, Roskilde, Denmark) were each coated with 50 μl of rabbit anticytokine antibodies (1 μg/ml in 0.6 M NaCl–0.26 M H3BO4–0.08 N NaOH [pH 9.6]) for 16 h at 4°C; the plates were then washed with PBS (pH 7.5) containing 0.05% Tween 20 (wash buffer). Nonspecific binding sites were blocked with 2% bovine serum albumin in PBS, and the plates were incubated for 90 min at 37°C. Plates were rinsed four times with wash buffer, and diluted (neat and 1:10) cell-free supernatants (50 μl) in duplicate were added, followed by incubation for 1 h at 37°C. Plates were washed four times, followed by the addition (per well) of 50 μl of biotinylated rabbit anticytokine antibodies (R & D Systems; 3.5 μg/ml in PBS [pH 7.5]–0.05% Tween 20–2% fetal calf serum); the plates were incubated for 30 min at 37°C. Plates were washed four times, streptavidin-peroxidase conjugate (Bio-Rad Laboratories, Richmond, Calif.) was added, and the plates were incubated for 30 min at 37°C. Plates were washed again four times, and chromogen substrate (Bio-Rad) was added. Plates were incubated at room temperature to the desired extinction, and the reaction was terminated with 50 μl of 3 M H2SO4 solution/well. The absorbance was read at 490 nm in an ELISA reader. Standards were twofold dilutions of murine recombinant cytokine from 1 pg/ml to 100 ng/ml. This ELISA method consistently detected murine KC and MIP-2 concentrations above 25 and 50 pg/ml, respectively. The ELISA did not cross-react with other cytokines, such as IL-1, IL-2, IL-6, or TNF-α. In addition, the ELISA did not cross-react with other members of the murine chemokine family, including murine JE/MCP-1, MIP-1α, or RANTES (16). Levels of IL-12 p70 in the lungs were determined using a commercially available ELISA kit (DuoSet ELISA development system; R & D Systems) according to the manufacturer's directions. p70 was measured because this heterodimer represents the biologically active form of IL-12.
Neutralization of KC, MIP-2, or CXCR2.
In blockage experiments with endogenous MIP-2 and CXCR2, mice were injected i.p. with 0.5 ml of specific antiserum 2 h before L. pneumophila infection. For studies extending beyond 1 day postinfection, mice received another 0.2-ml dose of antiserum 36 h after infection. For neutralization of endogenous KC, 50 μg of purified antibody was injected i.p. 2 h before infection. Fifteen to 30 μg of anti-murine KC antibody/ml has been demonstrated to neutralize the bioactivity of 1 μg of murine KC/ml by measurement of MPO release from human neutrophils as the bioassay (41). Control mice in which CXCR2 or MIP-2 was blocked received normal goat serum or normal rabbit serum, respectively. Infected mice receiving control reagents instead of anti-CXCR2 and anti–MIP-2 antibodies had no detrimental effects compared with animals that were only infected.
BAL.
Bronchoalveolar lavage (BAL) was performed to obtain BAL cells for enumeration. The trachea was exposed and intubated using a 1.7-mm-outer-diameter polyethylene catheter. BAL was performed by instilling PBS containing 5 mM EDTA in 1-ml aliquots. Approximately 5 ml of lavage fluid was retrieved per mouse. Cytospin samples were subsequently prepared from BAL cells and stained with Diff-Quick (Baxter, McGaw Park, Ill.). Differential cell counts were determined by direct counting.
Isolation and RT-PCR amplification of whole-lung mRNA.
Whole lungs were harvested, immediately snap-frozen in liquid nitrogen, and stored at −70°C; reverse transcription (RT)-PCR was performed as previously described (16). Briefly, total cellular RNA from frozen lungs was isolated, reversed transcribed into cDNA, and then amplified as previously described using specific primers for KC, MIP-2, and LIX, with β-actin serving as a control. The primers had the sequences 5′-TGA-GCT-GCG-CTG-TCA-GTG-CCT-3′ and 5′-AGA-AGC-CAG-CGT-TCA-CCA-GGA-3′ for KC, 5′-TGC-CTG-AAG-ACC-CTG-CCA-AGG-3′ and 5′-GTT-AGC-CTT-GCC-TTT-GTT-CAG-3′ for MIP-2, 5′-CTC-AGT-CAT-AGC-CGC-AAC-CGA-GC-3′ and 5′-CCG-TTC-TTT-CCA-CTG-CGA-GTG-C-3′ for LIX, and 5′-ATG-GAT-GAC-GAT-ATC-GCT-C-3′ and 5′-GAT-TCC-ATA-CCC-AGG-AAG-G-3′ for β-actin. After amplification, the samples (20 μl) were separated on a 2% agarose gel containing 0.3 mg of ethidium bromide per ml (0.003%), and bands were visualized and photographed using UV transillumination.
Statistical analysis.
Statistical significance was determined using the unpaired two-tailed alternate Welsh t test and the nonparametric Mann-Whitney test. Calculations were performed using InStat for Macintosh (GraphPad Software, San Diego, Calif.).
RESULTS
Bacterial numbers, MPO levels, and leukocyte accumulation in the lungs of mice infected with L. pneumophila.
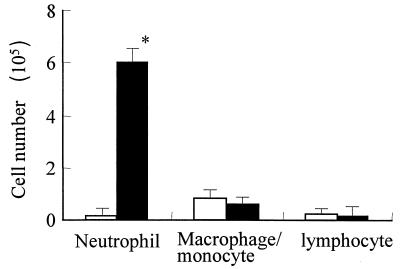
After intratracheal inoculation of 106 CFU of L. pneumophila, bacterial numbers in the lungs increased approximately 10-fold by day 2 and gradually decreased thereafter (Fig. 1a). A sharp increase in lung MPO levels was observed on days 1 and 2 after infection, indicative of massive accumulation of neutrophils at the site of infection (Fig. 1b). Figure 2 shows cell numbers and differentiation in BAL fluid of mice 2 days after infection. Total cell numbers increased approximately eight fold after L. pneumophila administration. Neutrophils, macrophages or monocytes, and lymphocytes constituted 91.6, 6.4, and 2.0% of the cells, respectively, in BAL fluid of infected mice. A greater-than-100-fold increase in BAL neutrophil numbers was noted in infected animals (P < 0.01), whereas no significant changes in the numbers of macrophages or monocytes or of lymphocytes were observed at this time.
FIG. 1.
Bacterial numbers and MPO levels in the lungs of five mice infected with L. pneumophila. Bacterial numbers (a) and MPO levels (b) in lung homogenates were determined 1, 2, 4, and 8 days after inoculation of 106 CFU of L. pneumophila. Data are means and standard deviations.
FIG. 2.
Leukocyte numbers and cell differentiation in BAL fluid of five mice before (open bars) and 2 days after (closed bars) inoculation of L. pneumophila. An asterisk indicates a P value of <0.01. Data are means and standard deviations.
Expression of CXC chemokine mRNAs in the lungs after infection.
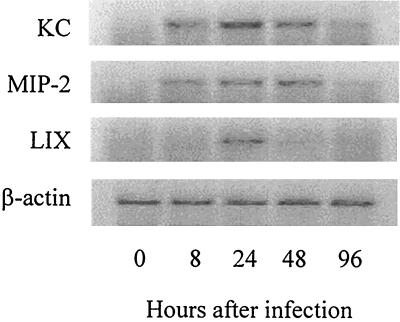
We next examined mRNA expression for several murine CXC chemokines, including KC, MIP-2, and LIX, in the lungs of mice infected with L. pneumophila. mRNA expression for these chemokines was examined with mixed samples from two mice at each time point because similar results were obtained among the mice. Before infection, no expression of KC, MIP-2, or LIX mRNA was detected. In contrast, intratracheal inoculation of L. pneumophila induced up-regulation of these CXC chemokine mRNAs in the lungs (Fig. 3). Induction of mRNA expression for KC, MIP-2, and LIX peaked at 24, 48, and 24 h after intratracheal challenge, respectively.
FIG. 3.
Expression of CXC chemokine mRNAs in the lungs after infection. The expression of CXC chemokine mRNAs, such as KC, MIP-2, and LIX, was examined 8, 24, 48, and 96 h after inoculation of L. pneumophila. All cDNAs were amplified by 35 cycles of PCR, with the exception of β-actin, which required 25 cycles. Each lane represents the lungs of two animals combined.
KC, MIP-2, and LIX production in the lungs after infection.
The production of KC, MIP-2, and LIX proteins in the lungs of mice 1, 2, 4, and 8 days after infection was examined. As shown in Fig. 4, all chemokines were induced in the lungs after L. pneumophila challenge, consistent with the results of RT-PCR experiments. Peak production of KC, MIP-2, and LIX was observed on days 1, 2, and 2 after infection, respectively (P < 0.05), and the levels of these chemokines decreased thereafter.
FIG. 4.
KC, MIP-2, and LIX production in the lungs after infection. The production of KC (a), MIP-2 (b), and LIX (c) proteins in the lungs of five mice was examined 1, 2, 4, and 8 days after inoculation of 106 CFU of L. pneumophila. An asterisk indicates a P value of < 0.05 for a comparison with the control. Data are means and standard deviations.
Effects of blockade of KC, MIP-2, or CXCR2 on survival.
Intratracheal challenge with 106 CFU of L. pneumophila did not induce death in control and anti–MIP-2 antibody-treated mice (Fig. 5). For KC-neutralized mice, death was observed with this challenge dose, but the overall mortality rate was not significantly different from that for control mice. In addition, the simultaneous neutralization of KC and MIP-2 did not result in synergistic effects on lethality, compared to blockade of each chemokine independently. In contrast, neutralization of CXCR2, a receptor for CXC chemokines, including KC and MIP-2, strikingly augmented the mortality of mice. By day 5 after infection, all CXCR2-blocked mice had died; the median day to death was 4.0 ± 0.6 days after challenge. To further characterize the increased susceptibility of CXCR2-blocked mice, we examined the lethality of L. pneumophila infection for control and anti-CXCR2 antibody-treated mice (Table 1). For control mice, the 50% lethal dose was considered to be between 1.8 × 107 and 3.6 × 106 CFU. Neutralization of CXCR2 strikingly sensitized mice to the lethal effects of this organism. A challenge dose of 7.2 × 105 CFU killed all mice, and the death of CXCR2-blocked mice was still observed at a dose as low as 1.4 × 105 CFU. These results demonstrated that CXCR2 blockade resulted in an approximately 100-fold increase in sensitivity to L. pneumophila-induced lethality.
FIG. 5.
Effects of blockade of KC, MIP-2, or CXCR2 on survival. Five or six mice were injected i.p. with specific anti–MIP-2 serum, anti-CXCR2 serum, or control serum (normal rabbit serum or normal goat serum) 2 h before (0.5 ml) and 2 days after (0.2 ml) L. pneumophila infection. For neutralization of endogenous KC, 50 μg of purified antibody was injected i.p. 2 h before infection. Survival was examined twice a day for 8 days after infection. No death was observed in mice treated with normal rabbit serum or normal goat serum.
TABLE 1.
Lethality in control and CXCR2-blocked micea
| Challenge dose (CFU/mouse) | No. of mice surviving in the following group:
|
|
|---|---|---|
| Control | Anti-CXCR2 antibody treated | |
| 9.0 × 107 | 0 | ND |
| 1.8 × 107 | 0 | ND |
| 3.6 × 106 | 5 | 0 |
| 7.2 × 105 | ND | 0 |
| 1.4 × 105 | ND | 4 |
Mice (n = 5) were treated i.p. with control or anti-CXCR2 goat serum 2 h before (0.5 ml) and 2 days after (0.2 ml) infection. The indicated doses of L. pneumophila were injected intratracheally, and survival was observed twice a day for 7 days after infection. ND, not determined.
Effects of blockade of KC, MIP-2, or CXCR2 on neutrophil numbers in BAL fluid.
Neutrophil numbers in BAL fluid of mice were determined 2 days after infection (Fig. 6). In control mice, more than 6 × 105 neutrophils accumulated in the air space. Although the neutralization of KC, MIP-2, and KC plus MIP-2 reduced neutrophil numbers to 81.1, 80.6, and 62.1% those in control mice, respectively, these values were not statistically different from those for control animals. In contrast, blockade of CXCR2 induced a clear reduction in neutrophil numbers (33.5% those in control mice) by day 2 after infection. These data were in concert with survival data and demonstrated that CXCR2-mediated neutrophil accumulation is a critical event for resistance to L. pneumophila pneumonia.
FIG. 6.
Effects of blockade of KC, MIP-2, or CXCR2 on neutrophil numbers in BAL fluid. Five mice were injected i.p. with anti-KC antibody, anti–MIP-2 serum, anti-KC antibody plus anti–MIP-2 serum, anti-CXCR2 serum, or control serum (normal rabbit serum or normal goat serum) 2 h before infection. Neutrophil numbers in BAL fluid were determined 2 days after infection. An asterisk indicates a P value of < 0.05. Data are means and standard deviations.
Bacterial numbers in the lungs of mice treated with anti-CXCR2 antibody.
Interestingly, although the challenge dose of 106 CFU was lethal for CXCR2-blocked mice, this treatment did not significantly enhance pulmonary bacterial burden on days 1 and 2 after infection (Fig. 7). However, by day 3, bacterial numbers in control mice had decreased to 106 CFU per lung, while more than 107 CFU of bacteria were recovered from the lungs of anti-CXCR2 antibody-treated mice. These data suggested that neutrophils played a minor role in the direct killing of L. pneumophila during the acute phase but that the absence of these cells at the site of infection critically affected the later clearance of bacteria and the subsequent course of infection.
FIG. 7.
Bacterial numbers in the lungs of mice treated with anti-CXCR2 antibody. Five mice were injected i.p. with anti-CXCR2 serum or control goat serum 2 h before infection, and the bacterial numbers in the lungs were determined on days 1, 2, and 3. An asterisk indicates a P value of < 0.05. Data are means and standard deviations.
Chemokines or cytokines in the lungs of mice treated with anti-CXCR2 antibody.
Figure 8 shows the KC, MIP-2, LIX, and IL-12 p70 levels in the lungs of control and CXCR2-blocked mice on days 1 and 2. We observed significantly higher levels of KC (day 2), MIP-2 (day 1), and LIX (day 2) in CXCR2-blocked mice. Interestingly, we observed a significant reduction of IL-12 p70 levels in CXCR2-blocked mice on day 1. Importantly, IL-12 p70 is known to be a critical mediator of host defense against L. pneumophila pneumonia. The attenuation of the IL-12 p70 response therefore may explain, at least in part, the increase in lethality in the setting of reduced neutrophil recruitment.
FIG. 8.
KC, MIP-2, LIX, and IL-12 p70 levels in the lungs of mice treated with anti-CXCR2 antibody. Five mice were injected i.p. with anti-CXCR2 serum (closed bars) or control goat serum (open bars) 2 h before infection. KC (a), MIP-2 (b), LIX (c), and IL-12 p70 (d) levels in the lungs were examined on days 1 and 2 after infection. An asterisk indicates a P value of < 0.05. Data are means and standard deviations.
DISCUSSION
In the clinical setting, early accumulation of neutrophils is a common feature of Legionella infection (43, 44), and it is known that neutropenia is an important risk factor for this disease (10). In the present study, we observed neutrophil-dominant leukocyte accumulation during the early phases of infection, suggesting that neutrophils play certain protective roles in Legionella infection. However, few investigators have examined the roles of neutrophils in host defense against Legionella organisms. Horwitz and Silverstein have clearly shown that Legionella organisms are resistant to killing by polymorphonuclear leukocytes in vitro, even under conditions of good opsonization (19). Additional studies have demonstrated that the virulent L. micdadei, one of the other pathogenic Legionella species, is phagocytized but not killed by human neutrophils (42). In contrast, Blanchard and colleagues have reported augmentation of human neutrophil bactericidal activity against L. pneumophila when the cells are stimulated with IFN-γ and TNF in vitro (2). In the present study, we have shown that the early accumulation of neutrophils is a critical event for resistance to L. pneumophila pneumonia in A/J mice. Our data suggested that neutrophils may play an important role, not as phagocytic cells in Legionella infection but rather as immunomodulatory cells. Furthermore, this study demonstrated the crucial roles of CXC chemokines and their functional receptor CXCR2 in L. pneumophila pneumonia.
MIP-2 and KC are the best-studied murine ELR+ CXC chemokines and are functional homologues of the human CXC chemokines IL-8 and GRO-α/β. The potential of these chemokines to contribute to neutrophil influx and activation during pulmonary inflammation is suggested by a number of observations (16, 22). In the Legionella pneumonia model, we observed induction of MIP-2 and KC in mRNA and protein levels. These were well correlated with neutrophil influx at the site of infection, although the peak for KC (day 1) was earlier than that for MIP-2 (day 2), suggesting different roles of these chemokines in neutrophil recruitment. In addition, the administration of anti-KC or anti–MIP-2 antibody caused some reduction of neutrophil influx. These data suggested that MIP-2 and KC are involved in neutrophil chemotactic responses elaborated by Legionella organisms. However, the contribution of each chemokine, KC or MIP-2, to neutrophil recruitment in Legionella infection was modest given that the reduction of neutrophil influx was small (approximately 20%) in KC- or MIP-2-blocked mice. Simultaneous blockade of KC and MIP-2 caused a greater reduction in neutrophil numbers, although this reduction did not achieve statistical significance. In contrast, the blockade of CXCR2, a receptor for CXC chemokines, including KC and MIP-2, induced significant suppression of neutrophil recruitment and markedly sensitized mice to Legionella infection. Taken together, these results demonstrated a critical role of CXCR2-mediated neutrophil influx in Legionella infection.
A variety of neutrophil chemotaxins other than CXC chemokines, such as complement, leukotrienes, and platelet-activating factor, have the potential to initiate and amplify neutrophil recruitment in inflammatory, infectious, and immunological process. The relative contributions of these factors to neutrophil influx may be different in each pathological situation and may depend on various experimental conditions, such as the stimulus used, the organ examined, and the timing of observations. Previously we have examined the effects of the blockade of CXCR2 on murine neutrophil recruitment in several pulmonary infection models, such as P. aeruginosa (41) and A. fumigatus (28). More than a 50% decrease in the levels of total lung neutrophils on day 1 was demonstrated in the P. aeruginosa infection model. In the A. fumigatus infection model a 63% reduction of MPO activity on day 2 was observed for CXCR2-blocked mice. The present data from the Legionella model were quite similar to those previous data, and in all infection models examined, the suppression of neutrophil influx by CXCR2 blockade strikingly enhanced mortality in mice. These data suggested that CXCR2-mediated neutrophil accumulation is a major part of the host biological response, at least in pulmonary infection models.
Importantly, we observed a discrepancy between anti-CXCR2 antibody effects and combination blockade of KC and MIP-2. These facts suggested the involvement of additional CXCR2-binding chemokines in Legionella-induced neutrophil influx. A newly described murine chemokine, LIX, is a possible candidate and has been shown to share structural homology with the human chemokines ENA-78 and GCP-2 (37). Although LIX was reported to be prominently expressed in the heart, we observed the production of LIX in infected lungs. Another recently described murine ELR+ CXC chemokine, lungkine, has also been shown to be chemotactic for neutrophils in vitro, is constitutively expressed by lung epithelial cells, but is not expressed in other organs (36). The preferential expression of chemokines in specific organs and in response to specific stimuli is of interest and suggests that various ELR+ CXC chemokines may have distinct biological roles. Since each factor is involved in neutrophil chemotactic responses not only through direct action but also through cross-amplifying effects, systemic analysis including blockade of LIX, lungkine, or both simultaneously may be necessary in future investigations. In addition, how differently each chemokine activates neutrophils or how CXCR2 blockade affects the neutrophil activation process in the infection model remains to be investigated.
Neutralization of CXCR2 dramatically sensitized mice to several respiratory pathogens, including Legionella organisms. Because the sensitization was associated with marked increases in the pulmonary microbial burden in the P. aeruginosa (41) and A. fumigatus (28) models, the effects of CXCR2 blockade may be attributed to the attenuation of neutrophil-dependent microbial killing. In contrast, we observed substantially different results in the Legionella model. Although augmentation of the lethality of L. pneumophila was observed for CXCR2-blocked mice, we could not demonstrate a significant increase in the bacterial burden in the lungs on days 1 and 2. These data supported previous reports that neutrophils could not kill Legionella organisms (19, 42). Furthermore, the present data demonstrated that the reduction of neutrophil influx did not affect bacterial numbers during the acute phase but dramatically influenced later survival. We speculate that neutrophils may exert their protective effect not through direct killing but through more immunomodulatory actions in L. pneumophila pneumonia.
In this regard, we observed a significant decrease in the levels of IL-12 p70 in the lungs of CXCR2-blocked mice. IL-12 is a pivotal cytokine for the development of Th1 cells and the initiation of cell-mediated immune responses to several pathogens (40). Recently, IL-12 has been shown to play a critical role in controlling the pulmonary growth of L. pneumophila in the A/J mouse model of pneumonia (8). In the past few years, several investigators have reported immunomodulatory roles of neutrophils in several infection models, such as Mycobacterium tuberculosis (31), M. avium (32), Candida albicans (35), and Listeria monocytogenes (12). In particular, accumulating data suggest that neutrophils may produce several chemokines and cytokines, including IL-12, under certain conditions (3, 4, 11, 32, 35). Although the cellular sources of IL-12 in the A/J mouse model of L. pneumophila pneumonia have not yet been determined, the present data strongly suggest that neutrophils may be a potential candidate. To this end, we observed a significant reduction in the levels of IL-12 in the lungs when mice were kept neutropenic by administration of neutrophil-depleting antibody (anti-Gr1 antibody). Importantly, in these experiments, neutrophil depletion was accompanied by a drastic increase in lethality but not by an increase in the bacterial burden during the acute phase (data not shown). Studies are ongoing to evaluate the contribution and significance of neutrophils as immunomodulatory cells, especially in IL-12 responses in L. pneumophila pneumonia.
ACKNOWLEDGMENTS
We thank Michele S. Swanson and Brenda Byrne for critical advice about the A/J mouse model of L. pneumophila pneumonia and Robert M. Strieter and Steven L. Kunkel for generous gifts of anti-CXCR2 antibody. We also thank Pamela M. Lincoln and Holly L. Evanoff for assistance with the ELISA and Galen B. Toews, Gary B. Huffnagle, and their laboratory members for helpful discussions.
This research was supported in part by National Institutes of Health grants HL57243, HL58200, and P50HL60289.
REFERENCES
- 1.Antony V B, Godbey S W, Kunkel S L, Hott J W, Hartman D L, Burdick M D, Strieter R M. Recruitment of inflammatory cells to the pleural space. Chemotactic cytokines, IL-8, and monocyte chemotactic peptide-1 in human pleural fluids. J Immunol. 1993;151:7216–7223. [PubMed] [Google Scholar]
- 2.Blanchard D K, Friedman H, Klein T W, Djeu J Y. Induction of interferon-gamma and tumor necrosis factor by Legionella pneumophila: augmentation of human neutrophil bactericidal activity. J Leukoc Biol. 1989;45:538–545. doi: 10.1002/jlb.45.6.538. [DOI] [PubMed] [Google Scholar]
- 3.Bliss S K, Marshall A J, Zhang Y, Denkers E Y. Human polymorphonuclear leukocytes produce IL-12, TNF-alpha, and the chemokines macrophage-inflammatory protein-1 alpha and -1 beta in response to Toxoplasma gondii antigens. J Immunol. 1999;162:7369–7375. [PubMed] [Google Scholar]
- 4.Bliss S K, Zhang Y, Denkers E Y. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-gamma-independent IL-12. J Immunol. 1999;163:2081–2088. [PubMed] [Google Scholar]
- 5.Bozic C R, Gerard N P, von Uexkull-Guldenband C, Kolakowski L F, Jr, Conklyn M J, Breslow R, Showell H J, Gerard C. The murine interleukin 8 type B receptor homologue and its ligands. Expression and biological characterization. J Biol Chem. 1994;269:29355–29358. [PubMed] [Google Scholar]
- 6.Brieland J, Freeman P, Kunkel R, Chrisp C, Hurley M, Fantone J, Engleberg N C. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires' disease. Am J Pathol. 1994;145:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 7.Brieland J K, Remick D G, Freeman P T, Hurley M C, Fantone J C, Engleberg N C. In vivo regulation of replicative Legionella pneumophila lung infection by endogenous tumor necrosis factor alpha and nitric oxide. Infect Immun. 1995;63:3253–3258. doi: 10.1128/iai.63.9.3253-3258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brieland J K, Remick D G, LeGendre M L, Engleberg N C, Fantone J C. In vivo regulation of replicative Legionella pneumophila lung infection by endogenous interleukin-12. Infect Immun. 1998;66:65–69. doi: 10.1128/iai.66.1.65-69.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrne B, Swanson M S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carratala J, Gudiol F, Pallares R, Dorca J, Verdaguer R, Ariza J, Manresa F. Risk factors for nosocomial Legionella pneumophila pneumonia. Am J Respir Crit Care Med. 1994;149:625–629. doi: 10.1164/ajrccm.149.3.8118629. [DOI] [PubMed] [Google Scholar]
- 11.Cassatella M A, Meda L, Gasperini S, D'Andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 12.Conlan J W, North R J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.el-Ebiary M, Sarmiento X, Torres A, Nogue S, Mesalles E, Bodi M, Almirall J. Prognostic factors of severe Legionella pneumonia requiring admission to ICU. Am J Respir Crit Care Med. 1997;156:1467–1472. doi: 10.1164/ajrccm.156.5.97-04039. [DOI] [PubMed] [Google Scholar]
- 14.Gebran S J, Yamamoto Y, Newton C, Klein T W, Friedman H. Inhibition of Legionella pneumophila growth by gamma interferon in permissive A/J mouse macrophages: role of reactive oxygen species, nitric oxide, tryptophan, and iron(III) Infect Immun. 1994;62:3197–3205. doi: 10.1128/iai.62.8.3197-3205.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman R B, Wood R G, Martin T R, Hanson-Painton O, Kinasewitz G T. Cytokine-stimulated human mesothelial cells produce chemotactic activity for neutrophils including NAP-1/IL-8. J Immunol. 1992;148:457–465. [PubMed] [Google Scholar]
- 16.Greenberger M J, Strieter R M, Kunkel S L, Danforth J M, Goodman R E, Standiford T J. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 17.Harada A, Kuno K, Nomura H, Mukaida N, Murakami S, Matsushima K. Cloning of a cDNA encoding a mouse homolog of the interleukin-8 receptor. Gene. 1994;142:297–300. doi: 10.1016/0378-1119(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 18.Hebert C A, Chuntharapai A, Smith M, Colby T, Kim J, Horuk R. Partial functional mapping of the human interleukin-8 type A receptor. Identification of a major ligand binding domain. J Biol Chem. 1993;268:18549–18553. . (Erratum, 269:16520, 1994.) [PubMed] [Google Scholar]
- 19.Horwitz M A, Silverstein S C. Interaction of the Legionnaires' disease bacterium (Legionella pneumophila) with human phagocytes. I. L. pneumophila resists killing by polymorphonuclear leukocytes, antibody, and complement. J Exp Med. 1981;153:386–397. doi: 10.1084/jem.153.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horwitz M A, Silverstein S C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Investig. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Introna M, Bast R C, Jr, Tannenbaum C S, Hamilton T A, Adams D O. The effect of LPS on expression of the early “competence” genes JE and KC in murine peritoneal macrophages. J Immunol. 1987;138:3891–3896. [PubMed] [Google Scholar]
- 22.Kooguchi K, Hashimoto S, Kobayashi A, Kitamura Y, Kudoh I, Wiener-Kronish J, Sawa T. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect Immun. 1998;66:3164–3169. doi: 10.1128/iai.66.7.3164-3169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Cacalano G, Camerato T, Toy K, Moore M W, Wood W I. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- 24.Lee J, Horuk R, Rice G C, Bennett G L, Camerato T, Wood W I. Characterization of two high affinity human interleukin-8 receptors. J Biol Chem. 1992;267:16283–16287. [PubMed] [Google Scholar]
- 25.Marston B J, Lipman H B, Breiman R F. Surveillance for Legionnaires' disease. Risk factors for morbidity and mortality. Arch Intern Med. 1994;154:2417–2422. [PubMed] [Google Scholar]
- 26.McColl S R, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol. 1999;163:2829–2835. [PubMed] [Google Scholar]
- 27.McDade J E, Shepard C C, Fraser D W, Tsai T R, Redus M A, Dowdle W R. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 28.Mehrad B, Strieter R M, Moore T A, Tsai W C, Lira S A, Standiford T J. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J Immunol. 1999;163:6086–6094. [PubMed] [Google Scholar]
- 29.Nash T W, Libby D M, Horwitz M A. Interaction between the Legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J Clin Investig. 1984;74:771–782. doi: 10.1172/JCI111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedro-Botet M L, Sabria-Leal M, Sopena N, Manterola J M, Morera J, Blavia R, Padilla E, Matas L, Gimeno J M. Role of immunosuppression in the evolution of Legionnaires' disease. Clin Infect Dis. 1998;26:14–19. doi: 10.1086/516258. [DOI] [PubMed] [Google Scholar]
- 31.Pedrosa J, Saunders B M, Appelberg R, Orme I M, Silva M T, Cooper A M. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68:577–583. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrofsky M, Bermudez L E. Neutrophils from Mycobacterium avium-infected mice produce TNF-alpha, IL-12, and IL-1 beta and have a putative role in early host response. Clin Immunol. 1999;91:354–358. doi: 10.1006/clim.1999.4709. [DOI] [PubMed] [Google Scholar]
- 33.Reingold A L. Role of legionellae in acute infections of the lower respiratory tract. Rev Infect Dis. 1988;10:1018–1028. doi: 10.1093/clinids/10.5.1018. [DOI] [PubMed] [Google Scholar]
- 34.Rollins B J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 35.Romani L, Mencacci A, Cenci E, Spaccapelo R, Del Sero G, Nicoletti I, Trinchieri G, Bistoni F, Puccetti P. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–5356. [PubMed] [Google Scholar]
- 36.Rossi D L, Hurst S D, Xu Y, Wang W, Menon S, Coffman R L, Zlotnik A. Lungkine, a novel CXC chemokine, specifically expressed by lung bronchoepithelial cells. J Immunol. 1999;162:5490–5497. [PubMed] [Google Scholar]
- 37.Rovai L E, Herschman H R, Smith J B. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J Leukoc Biol. 1998;64:494–502. doi: 10.1002/jlb.64.4.494. [DOI] [PubMed] [Google Scholar]
- 38.Smith J B, Herschman H R. Glucocorticoid-attenuated response genes encode intercellular mediators, including a new C-X-C chemokine. J Biol Chem. 1995;270:16756–16765. doi: 10.1074/jbc.270.28.16756. [DOI] [PubMed] [Google Scholar]
- 39.Tkatch L S, Kusne S, Irish W D, Krystofiak S, Wing E. Epidemiology of legionella pneumonia and factors associated with legionella-related mortality at a tertiary care center. Clin Infect Dis. 1998;27:1479–1486. doi: 10.1086/515040. [DOI] [PubMed] [Google Scholar]
- 40.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 41.Tsai W C, Strieter R M, Mehrad B, Newstead M W, Zeng X, Standiford T J. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun. 2000;68:4289–4296. doi: 10.1128/iai.68.7.4289-4296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinbaum D L, Bailey J, Benner R R, Pasculle A W, Dowling J N. The contribution of human neutrophils and serum to host defense against Legionella micdadei. J Infect Dis. 1983;148:510–517. doi: 10.1093/infdis/148.3.510. [DOI] [PubMed] [Google Scholar]
- 43.Winn W C, Jr, Glavin F L, Perl D P, Keller J L, Andres T L, Brown T M, Coffin C M, Sensecqua J E, Roman L N, Craighead J E. The pathology of Legionnaires' disease. Fourteen fatal cases from the 1977 outbreak in Vermont. Arch Pathol Lab Med. 1978;102:344–350. [PubMed] [Google Scholar]
- 44.Winn W C, Jr, Myerowitz R L. The pathology of the Legionella pneumonias. A review of 74 cases and the literature. Hum Pathol. 1981;12:401–422. doi: 10.1016/s0046-8177(81)80021-4. [DOI] [PubMed] [Google Scholar]
- 45.Wolpe S D, Davatelis G, Sherry B, Beutler B, Hesse D G, Nguyen H T, Moldawer L L, Nathan C F, Lowry S F, Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988;167:570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolpe S D, Sherry B, Juers D, Davatelis G, Yurt R W, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]