Background
Low- and middle-income countries (LMICs) now have increasing access to cancer medicines, which are being integrated into national cancer control programs. This development is, in part, due to the WHO's expansion of the list of essential medicines (EMLs), and the growth of multisectoral partnerships that have made cancer medicines more affordable in emerging markets.1 However, limited access to cancer diagnostics remains a critical bottleneck to efficiently tailoring available medicines. There have been increased calls to couple investments in cancer diagnostics to those in cancer medicines—including harmonization of the EML with the List of Essential In Vitro Diagnostics (EDL) and List of Priority Medical Devices for Cancer Management (PMDL).2-4
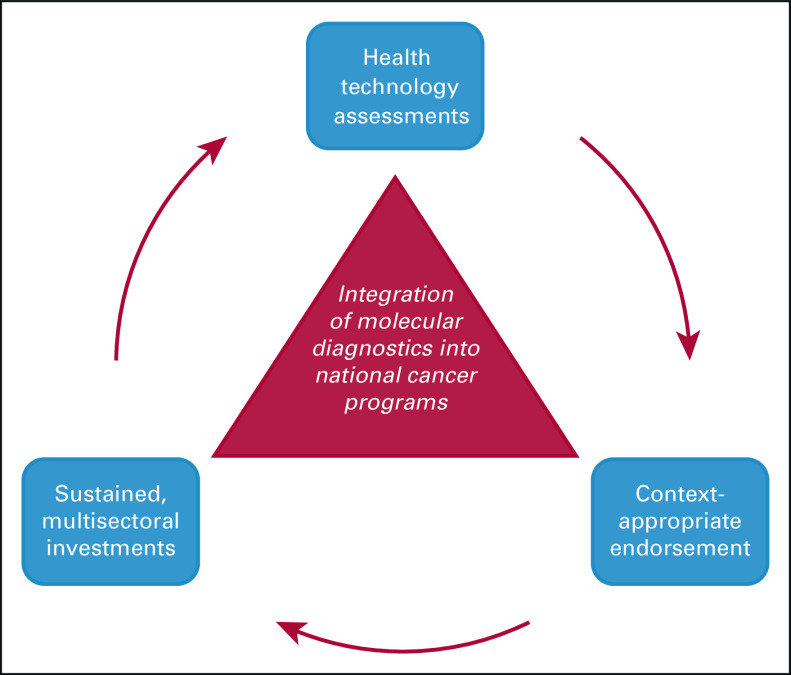
To date, the limited efforts toward advancing cancer diagnostics in LMICs have focused on basic pathology services. Molecular diagnostics—now a standard part of cancer management in high-income countries—remain underutilized. This lag in uptake has been primarily due to concerns regarding affordability, technical capacity, and limited diagnostic and clinical infrastructure. However, the landscape of molecular diagnostics is rapidly evolving in health care, with development and deployment of smaller and more efficient point-of care instruments, as well as a greater appreciation for molecular technologies in the context of the COVID-19 pandemic. In this article, we draw on lessons from global infectious disease control and our experience with the GeneXpert (GX) platform to argue that innovations in molecular diagnostics can be used to narrow the global cancer pathology gap. We provide a framework for how stakeholders can leverage molecular diagnostics to advance cancer care in LMICs moving forward—including in-country health technology assessments, context-appropriate endorsement of relevant technologies by international governing bodies, and sustained, multisectoral investments (Fig 1).
FIG 1.
Framework to integrate context-appropriate molecular diagnostic technologies into national cancer programs.
Molecular Diagnostics in Infectious Disease
In the past 2 decades, progress in global infectious disease control has partially been from strategic deployment of molecular diagnostics. In the case of tuberculosis (TB), utilization of the GX platform—a fully automated cartridge-based reverse transcriptase polymerase chain reaction (PCR) system from Cepheid that requires minimal hands-on sample preparation and produces results in < 2 hours—was a game changer. The GX platform and the Xpert assay for detection of Mycobacterium tuberculosis and rifampin resistance (Xpert MTB/RIF) simplified TB diagnosis in people living with HIV and those with multidrug-resistant TB; critical results that previously took several weeks to obtain were now available within a single clinic visit. After multicountry assessments of the assay's effectiveness, the WHO endorsed the technology in 2010, and the assay continues to be included in the EDL.3,5,6 Although the cost of GX and Xpert MTB/RIF assay initially raised concerns regarding its scalability and feasibility in LMICs, multisectoral investments made its widespread impact possible. In 2011, Cepheid launched the High-Burden Developing Country access program to make GX machines and cartridges available to certain LMICs at a reduced price. And in 2012, a prepurchasing collaboration between the Bill and Melinda Gates Foundation, United States President's Emergency Plan for AIDS Relief, United States Agency for International Development, and Unitaid helped reduce the cost of the MTB/RIF assay by 40% from approximately $17 US dollars (USD) to $10 USD in 145 countries.7
Before the severe acute respiratory syndrome coronavirus 2 pandemic, more than 10,000 GX machines and tens of millions of TB cartridges were procured by LMICs through the High-Burden Developing Country access program.8 The platform's widespread deployment helped improve global TB outcomes—especially in rural clinical settings, where historically, patients were not started on appropriate TB treatment because of long turn-around-times for diagnosis and the need for patients to return to clinic.9-12 Similar advances in HIV, Hepatitis C, Chlamydia trachomatis, and Neisseria gonorrhoeae control have been accomplished globally through utilization of GX and other molecular diagnostic technologies; between 2014 and 2016, of 21 high-TB-burden countries, 37% were using the GX platform for diseases beyond TB.13
Molecular Diagnostics in Cancer: The Case of Chronic Myelogenous Leukemia
Similar to their impact in infectious disease control, novel molecular diagnostics can also be leveraged to strengthen cancer programs in LMICs. We share the example of an innovative cancer medicine access program through The Max Foundation to highlight the potential impact of molecular diagnostics in cancer care. CMLPath to Care, a collaboration between Novartis and The Max Foundation (previously named Glivec International Patient Assistance Program) has helped make imatinib for chronic myelogenous leukemia (CML), GI stromal tumor, and other select cancers available at no cost to patients in many LMICs.14,15 Since 2001, The Max Foundation has provided tyrosine kinase inhibitor treatment to over 90,000 patients, with 30,267 patients with CML on treatment as of December 31, 2021 (source The Max Foundation database). Patients enrolled in the program have had a 5-year survival rate of 89%, which compares favorably with survival in high-income countries.14
At the onset of the imatinib access program, BCR-ABL1 testing for CML diagnosis and management (which involves PCR or fluorescence in situ hybridization) was not available in many LMICs. Testing was performed by shipping blood or bone marrow samples to other countries—an approach that was costly, time-consuming, and difficult to scale.16 In 2006, the first version of a semiautomated Xpert BCR-ABL cartridge-based reverse transcriptase PCR assay designed for CML monitoring became commercially available. A more sensitive version, Xpert BCR-ABL Ultra, followed several years later. The Max Foundation partnered with Cepheid to make GX technologies available at a preferential price in more than 60 LMICs and facilitate in-country BCR-ABL monitoring.16,17 The expansion of on-site point-of-care BCR-ABL testing has streamlined appropriate tyrosine kinase inhibitor selection for patients in the Max Access Solutions program.16
Despite investments and efforts to scale GX technology for CML management, BCR-ABL testing continues to be a primary barrier for optimal CML outcomes in many LMICs. Logistical challenges of importation and shelf life, limited access to technical support, and lack of local registration of the test have contributed to persistent gaps in molecular testing for CML. Moreover, the test's price and maintenance costs have also limited its adoption. A recent study estimated that over a 5-year period, the gap in PCR monitoring capacity for patients with CML in countries covered by the Max Access Solutions program was approximately $29 million USD, 86% of which was driven by cartridge costs.16 Although the Xpert BCR-ABL Ultra cartridge is approved for CML monitoring, there remains a lack of accessible molecular technologies for primary CML diagnosis. Overcoming the PCR gap for CML diagnosis and monitoring will require large investments but doing so could improve survival for thousands of patients and potentially reduce treatment costs through treatment optimization and discontinuation for select patients.
Ultimately, investments in molecular monitoring with GX may have health dividends for cancers beyond CML. GX is a cross-cutting platform that can be leveraged for molecular diagnostics across several cancers, such as human papillomavirus (HPV) testing for cervical cancer (Xpert HPV) and biomarker classification for breast cancer (Xpert Breast Cancer STRAT4).18,19 Both Xpert HPV and Xpert Breast Cancer STRAT4 tests are CE-IVD In Vitro Diagnostic Medical Devices but are not available in the United States. Similarly, there are other molecular diagnostic technologies proving to have value for management of other cancers in LMICs, including the diagnosis and classification of lymphoma.20-23
The Path Forward for Molecular Diagnostics and Global Oncology
Our experience with on-site diagnostics for the management of CML has shown that molecular technologies can be leveraged to advance cancer care in LMICs. The question that remains is how and to what extent. Leaning on the lessons learned in infectious disease, we present a three-pronged approach to leverage molecular diagnostics in global oncology: implementation evaluation, context-appropriate endorsement, and multisectoral investment in relevant technologies (Fig 1).
First, implementation of molecular diagnostics should be rigorously evaluated in-country beyond their clinical validation. Stakeholders can use existing implementation science paradigms, such as the Implementation Outcomes Framework, to study how contextual factors influence technology effectiveness.24,25 For example, the Implementation Outcomes Framework focuses on evaluation of eight implementation outcomes, including (1) acceptability, (2) adoption, (3) appropriateness, (4) cost, (5) feasibility, (6) fidelity, (7) penetration, and (8) sustainability. Evaluation of these outcomes in one country can promote the systematic uptake of evidence-based practices that improve effectiveness of technologies across similar contexts. For example, the sample handling and preparation requirements for certain Xpert assays may be a challenge in some settings; the Xpert STRAT4 assay for breast cancer biomarker classification currently requires formalin-fixed, paraffin-embedded tissue, which requires functioning histology laboratory procedures. In pursuing clinical validation of the assay and ensuring production of high-quality tissue samples, laboratories will likely face implementation barriers. As they design solutions to overcome these barriers, their implementation knowledge should be shared widely to guide adoption across similar contexts. In 2014, the National Cancer Institute's Center for Global Health launched the Affordable Cancer Technologies program to support the adaptation, application, and validation of cancer technologies in LMICs, including molecular diagnostics.26 We need similar, multilateral funding initiatives to support the implementation and evaluation of cancer technologies across LMICs.
Second, the WHO should consider endorsement of novel cancer molecular diagnostic technologies, especially as diagnostic and implementation studies continue to underscore their value.27 The affordability of cancer molecular diagnostics may be a barrier to WHO endorsement in LMICs. However, endorsements and inclusion of relevant technologies in the EDL or PMDL can catalyze differential pricing programs and multisectoral partnerships that make these technologies more affordable, as was the case for Xpert MTB/RIF.3 There are currently several cancer-related diagnostic tests—such as immunohistochemical testing for relevant markers of solid and liquid tumors and BCR-ABL transcript, HPV, and epidermal growth factor receptor testing—included in the EDL. However, no molecular diagnostic technologies are recommended in either the EDL or PMDL to operationalize these tests.3,4 Several molecular diagnostic technologies for HPV testing (including Xpert HPV) have been included on the WHO list of prequalified in vitro diagnostics, but none are yet included as recommended assays.28 As the evidence behind the value of molecular diagnostic technologies in LMICs grows, WHO endorsement of validated technologies should be heavily considered. Alongside endorsements, governing bodies should recommend prioritization of these technologies to guide public procurement by LMICs. Prioritization of cancer diagnostic technologies can be based on their influence on treatment selection, degree of clinical benefit, and estimated prevalence of the relevant cancer types—factors similar to those considered for selection of medicines for EML.29
Third, multisectoral, sustained investments will be vital to maximizing the impact of cancer molecular diagnostics in LMICs. A buy-down approach including up-front, high-volume prepurchases of endorsed diagnostic technologies can be used to reduce prices and expand access. Organizations such as the Foundation of Innovative New Diagnostics can work with industry partners (through initiatives such as Access Accelerated) to negotiate purchasing strategies for molecular diagnostics.30,31 Although Foundation of Innovative New Diagnostics has previously focused on infectious disease diagnostics, the organization has more recently entered the global oncology space.32
Although investments in molecular diagnostics are necessary, they will only influence patient outcomes if matched molecular therapeutics are available and affordable. Similarly, administration of molecularly targeted therapies without confirmation of the specific molecular aberration is not responsible and possibly dangerous. Therefore, investments in molecular diagnostics must be coupled with those in therapeutics. Access initiatives, such as the Cancer Access Partnership—a collaboration between African Cancer Coalition, Clinton Health Access Initiative, and the American Cancer Society—could match their investments and negotiating leverage for cancer diagnostics to those for molecular therapeutics.33 To date, this potential has been largely unrealized. Moreover, to sustain financing for cancer diagnostics, these investments from nonprofit organizations should be matched with long-term commitments from biotechnology companies, government agencies, and regional and international governing bodies such as the African Union, African Development Bank, and World Bank. Investments should also prioritize building of local research and development capacities in LMICs.
In the coming decades, as cancer incidence rates rise in LMICs, improving cancer care delivery will be an increasing public health priority. To narrow the existing global cancer outcomes gap, efforts to expand access to cancer therapeutics should be coupled with those to strengthen health care infrastructure, including the ability to diagnose and monitor cancers. Although innovations in cancer molecular technologies can facilitate this process, they have been an underutilized tool. Moving forward, robust implementation research, context-appropriate technology endorsements, and sustained multisectoral investments in cancer molecular diagnostics will be key to achieving universal health coverage and equity in cancer care.
Michael Bates
Employment: Cepheid/Danaher
Stock and Other Ownership Interests: Danaher
Dan A. Milner
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Employment: Libragem Consulting LLC
Timothy R. Rebbeck
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Lawrence N. Shulman
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Genentech
Research Funding: Celgene (Inst), Independence Blue Cross (Inst)
Temidayo Fadelu
Research Funding: Celgene (Inst), Cepheid (Inst)
No other potential conflicts of interest were reported.
SUPPORT
P.E. is supported by the Fogarty International Center of the National Institutes of Health under Award No. D43 TW010543. T.F. is supported by a 2021 Conquer Cancer Breast Cancer Research Foundation Career Development Award for Diversity, Inclusion and Breast Cancer Disparities in honor of Susan Hirschhorn and in memory of her mother, supported by Breast Cancer Research Foundation. He is also supported by an Early Career Faculty Innovation Grant from Dana-Farber Cancer Institute.
DISCLIAMER
Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the funders.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Provision of study materials or patients: Michael Bates, Pat Garcia-Gonzalez
Collection and assembly of data: Parsa Erfani
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Michael Bates
Employment: Cepheid/Danaher
Stock and Other Ownership Interests: Danaher
Dan A. Milner
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Employment: Libragem Consulting LLC
Timothy R. Rebbeck
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Lawrence N. Shulman
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Genentech
Research Funding: Celgene (Inst), Independence Blue Cross (Inst)
Temidayo Fadelu
Research Funding: Celgene (Inst), Cepheid (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.World Health Organization Model List of Essential Medicines—22nd List, 2021. World Health Organization, 2021. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 [Google Scholar]
- 2.LeJeunem A, Brock J, Morgan E, et al. : Harmonization of the essentials: Matching diagnostics to treatments for global oncology. JCO Glob Oncol 6:1352-1356, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Selection and Use of Essential In Vitro Diagnostics: Report of the Third Meeting of the WHO Strategic Advisory Group of Experts on In Vitro Diagnostics, 2020. World Health Organization, 2021. https://www.who.int/publications/i/item/9789240019102 [Google Scholar]
- 4.WHO List of Priority Medical Devices for Cancer Management. World Health Organization, 2017. https://www.who.int/publications/i/item/9789241565462 [Google Scholar]
- 5.Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System: Policy Statement. World Health Organization, 2011. https://apps.who.int/iris/handle/10665/44586 [PubMed] [Google Scholar]
- 6.Small PM, Pai M: Tuberculosis diagnosis—Time for a game change. N Engl J Med 363:1070-1071, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Public-Private Partnership Announces Immediate 40 Percent Cost Reduction for Rapid TB Test. The Bill & Melinda Gates Foundation, 2012. https://www.gatesfoundation.org/Ideas/Media-Center/Press-Releases/2012/08/PublicPrivate-Partnership-Announces-Immediate-40-Percent-Cost-Reduction-for-Rapid-TB-Test [Google Scholar]
- 8.de Dieu Iragena J: Decade of Field Experience with TB GeneXpert. World Health Organization, 2019. http://programme.ias2019.org/PAGMaterial/PPT/1822_4690/Decade of field experience with TB GeneXpert_ IAS Mexico by JI 22July2019.pptx [Google Scholar]
- 9.Cox HS, Mbhele S, Mohess N, et al. : Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: A pragmatic randomised trial. PLoS Med 11:e1001760, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Kampen SC, Susanto NH, Simon S, et al. : Effects of introducing Xpert MTB/RIF on diagnosis and treatment of drug-resistant tuberculosis patients in Indonesia: A pre-post intervention study. PLoS One 10:e0123536, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iruedo J, O'Mahony D, Mabunda S, et al. : The effect of the Xpert MTB/RIF test on the time to MDR-TB treatment initiation in a rural setting: A cohort study in South Africa's Eastern Cape Province. BMC Infect Dis 17:1-9, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Tuberculosis Report 2018. World Health Organization, 2018. https://apps.who.int/iris/handle/10665/274453 [Google Scholar]
- 13.Cazabon D, Pande T, Kik S, et al. : Market penetration of Xpert MTB/RIF in high tuberculosis burden countries: A trend analysis from 2014-2016. Gates Open Res 2:35, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umeh CA, Garcia-Gonzalez P, Tremblay D, et al. : The survival of patients enrolled in a global direct-to-patient cancer medicine donation program: The Glivec International Patient Assistance Program (GIPAP). EClinicalMedicine 19:100257, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Gonzalez P, Boultbee P, Epstein D: Novel Humanitarian Aid Program: The Glivec International Patient Assistance Program—Lessons learned from providing access to breakthrough targeted oncology treatment in low- and middle-income countries. J Glob Oncol 1:37-45, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowley S, Garcia-Gonzalez P, Radich JP, et al. : Analysis of the gap in PCR monitoring availability for patients with chronic myeloid leukemia in 60 low- and middle-income countries. Cost Eff Resour Alloc 19:18, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ukoma CK, Ogbe OP, David E, et al. : Providing molecular diagnosis and monitoring of patients with chronic myeloid leukemia in Abuja, Nigeria. Blood Adv 1:29-31, 2017. (suppl) [Google Scholar]
- 18.Cubie HA, Campbell C: Cervical cancer screening—The challenges of complete pathways of care in low-income countries: Focus on Malawi. Womens Health (Lond) 16, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mugabe M, Ho KE, Ruhangaza D, et al. : Use of the Xpert breast cancer STRAT4 for biomarker evaluation in tissue processed in a developing country. Am J Clin Pathol 156:766-776, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogt SL, Patel M, Omar T, et al. : Molecular diagnostics for AIDS lymphoma diagnosis in South Africa and the potential for other low- and middle-income countries. J Glob Oncol 4:1-6, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogt SL, Patel M, Lakha A, et al. : Feasibility of cell-free DNA collection and clonal immunoglobulin sequencing in South African patients with HIV-associated lymphoma. JCO Glob Oncol 7:611-621, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valvert F, Silva O, Solórzano-Ortiz E, et al. : Low-cost transcriptional diagnostic to accurately categorize lymphomas in low- and middle-income countries. Blood Adv 5:2447-2455, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radich JP, Briercheck E, Chiu DT, et al. : Precision medicine in low- and middle-income countries. Annu Rev Pathol 17:387-402, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Equity-Oriented Health Technology Assessment Toolkit. WHO Collaborating Centre for Knowledge Translation and Health Technology Assessment in Health Equity. 2008. http://www.cgh.uottawa.ca/whocc/projects/eo_toolkit/index.htm [Google Scholar]
- 25.Proctor E, Silmere H, Raghavan R, et al. : Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 38:65-76, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Affordable Cancer Technologies (ACTs) Program. National Cancer Institute, 2021. https://www.cancer.gov/about-nci/organization/cgh/research-training-programs/affordable-cancer-technology [Google Scholar]
- 27.Kuhn L, Saidu R, Boa R, et al. : Clinical evaluation of modifications to a human papillomavirus assay to optimise its utility for cervical cancer screening in low-resource settings: A diagnostic accuracy study. Lancet Glob Health 8:e296-e304, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Public Reports for In Vitro Diagnostics. World Health Organization, 2022. https://extranet.who.int/pqweb/vitro-diagnostics/prequalification-reports/whopr [Google Scholar]
- 29.Shulman LN, Wagner CM, Barr R, et al. : Proposing essential medicines to treat cancer: Methodologies, processes, and outcomes. J Clin Oncol 34:69-75, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Accessible Pricing—FIND. The Foundation for Innovative New Diagnostics. 2022. https://www.finddx.org/pricing/ [Google Scholar]
- 31.Our Mission. Access Accelerated. 2022. https://accessaccelerated.org/about-us/our-mission/ [Google Scholar]
- 32.Milner D, Vetter B, Zurn SJ: The role of in vitro diagnostics in early detection and treatment of cancer. Union for International Cancer Control, 2021. https://www.uicc.org/resources/role-vitro-diagnostics-early-detection-and-treatment-cancer [Google Scholar]
- 33.Cancer Access Partnership. Allied Against Cancer, 2022. https://www.alliedagainstcancer.org/access-partnership [Google Scholar]