PURPOSE
The WHO essential medicines list (EML) guides selection of drugs for national formularies. Here, we evaluate which medicines are considered highest priority by Indian oncologists and the extent to which they are available in routine practice.
METHODS
This is a secondary analysis of an electronic survey developed by the WHO EML Cancer Medicine Working Group. The survey was distributed globally using a hierarchical snowball method to physicians who prescribe systemic anticancer therapy. The survey captured the 10 medicines oncologists considered highest priority for population health and their availability in routine practice.
RESULTS
The global study cohort included 948 respondents from 82 countries; 98 were from India and 67 were from other low- and middle-income countries. Compared with other low- and middle-income countries, the Indian cohort was more likely to be medical oncologist (70% v 31%, P < .001) and work exclusively in the private health system (52% v 17%, P < .001). 14/20 most commonly selected medicines were conventional cytotoxic drugs. Universal access to these medicines was reported by a minority of oncologists; risks of significant out-of-pocket expenditures for each medicine were reported by 19%-58% of oncologists. Risk of catastrophic expenditure was reported by 58%-67% of oncologists for rituximab and trastuzumab. Risks of financial toxicity were substantially higher within the private health system compared with the public system.
CONCLUSION
Most high-priority cancer medicines identified by Indian oncologists are generic chemotherapy agents that provide substantial improvements in survival and are already included in WHO EML. Access to these treatments remains limited by major financial burdens experienced by patients. This is particularly acute within the private health system. Strategies are urgently needed to ensure that high-quality cancer care is affordable and accessible to all patients in India.
BACKGROUND
Advances in systemic therapy have led to improved survival in many cancers. However, there is a wide range in the magnitude of benefit associated with these advances.1,2 Translating these gains from clinical trials into improved outcomes in the real world is contingent on the medicines being accessible and affordable. These newer therapies are very expensive, often disproportionate to the gains in survival.3-5 Procurement of these medicines strain budgets in all health systems but most acutely in low- and middle-income countries (LMICs) such as India. Most low-resource health systems do not include expensive cancer medicines in their public reimbursement schemes, thereby putting patients at risk of significant out-of-pocket (OOP) or catastrophic expenditure. This calls for a careful assessment of anticancer systemic therapy before their inclusion in national formularies. The WHO essential medicines list (EML) is developed through a systematic appraisal of evidence on the magnitude of benefit, safety, and feasibility of delivery across diverse health systems.6 The EML provides much-needed guidance for policymakers tasked with the prioritization and procurement of medicines.7 Over the years, the WHO EML has grown from seven cancer medicines in 1977 to 59 in 2021, reflecting the development of new highly effective therapeutic options in the field, increasing burden of cancer and WHO's response to cancer prevention and control.6,8,9
CONTEXT
Key Objective
To understand which cancer medicines are considered most important by Indian oncologists and the extent to which they are available in routine clinical practice.
Knowledge Generated
The majority of high-priority medicines selected by Indian oncologists are older generic chemotherapy and hormone medicines. All the medicines selected by the Indian oncologists are already included in the WHO-essential medicines list and India's National List of Essential Medicine. Access to these high-priority off-patent drugs continues to result in significant out-of-pocket or catastrophic expenditure; this is most pressing in the private sector.
Relevance
The findings from this study highlight the need to strengthen existing systems for pricing, quality monitoring, and procurement to promote the universal access for essential medicines.
The public health burden of cancer in India is growing steadily, with a projected cancer incidence of 1.4 million cases in the year 2020.10 More than 80% of these patients present with advanced-stage disease because of awareness, access, and affordability challenges.11 This translates into a higher need for effective and affordable systemic anticancer therapy options and palliative care. The National List of Essential Medicine (NLEM) in India was established in 1996 and has undergone periodic revisions since then.12 The NLEM is based on the same principles as WHO EML complemented by relevant local information such as country-specific disease patterns and locally approved drugs. Inclusion of a drug in NLEM ensures price control as per the Drug Price Control Order Act and thus can improve affordability and access.13
Our group has recently reported the results of an international survey among oncologists from 82 countries to understand the extent to which the EML reflects high-priority medicines of clinicians and whether these drugs are accessible on the frontlines of care.14 The study showed substantial convergence between the EML and medicines that clinicians prioritize. However, the study illustrated striking financial barriers to accessing these medicines in routine care, especially in LMICs and upper-middle-income countries (UMICs).
Cancer care delivery in India is unique in terms of mix of private and public sector facilities.15 Cancer care in the public system is supported through government-funded schemes to a certain extent, whereas care in the private sector is largely financed through OOP expenditure, with trivial proportion being covered by private insurances or reimbursement from employers. Approximately 60%-75% of patients with cancer in India receive care in private health care facilities and are thus subject to the risk of significant OOP expenditure and distressed financing.15 Moreover, even within the public system, payment schemes may not cover the full cost of medicines and their availability can be erratic, leading to OOP expenditures for patients who have to obtain medicines elsewhere. This model of health care coupled with wide income inequalities in the country brings considerable challenges for access and affordability of anticancer drugs. Given the unique features of India's health system, we undertook a secondary analysis of the primary global cohort to understand which medicines are considered most relevant by physicians in India and the extent to which they are accessible and affordable on the frontlines of care.
METHODS
Study Population
The current report is a secondary analysis of the global study recently reported elsewhere.14 The study concept was developed by investigators with expertise in research methodology, health policy, global health, and clinical cancer care in close conjunction with the activities of the WHO Essential Medicines Cancer Working Group, which supports the identification and evaluation of cancer medicines for inclusion on the WHO Model List. The study population for this cross-sectional survey included fully qualified physicians who deliver systemic anticancer therapy. Physicians who treat only children were excluded as they were part of a similar survey being done for pediatric cancers.
Survey Design and Distribution
The original survey was developed using Qualtrics online survey platform.16 Pilot testing was performed by the study team and 10 additional oncologists from diverse global settings. The final survey consisted of 28 questions and took 8-10 minutes to complete (Data Supplement). The survey was only available in English and responses remained anonymous. The study was approved by the Research Ethics Board at Queen's University.
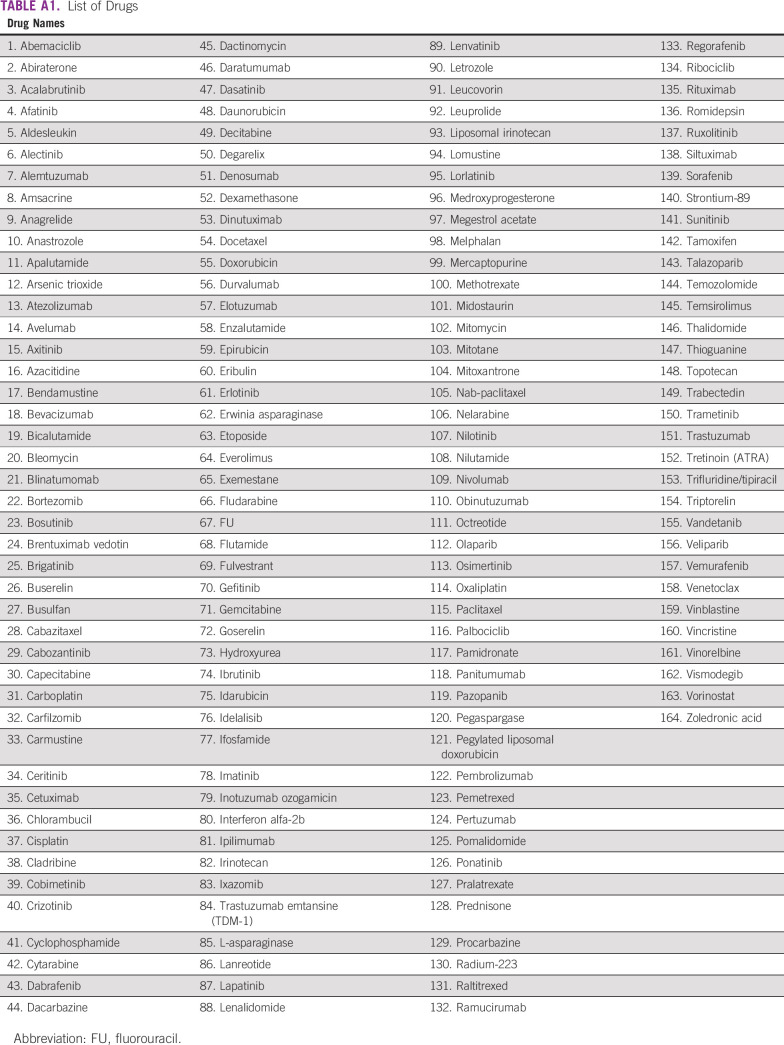
The primary survey question asked “Imagine your government has put you in charge of selecting anticancer medicines for your country. You are only allowed to select a maximum of 10 medicines that will be available to treat all cancers in your country. Which drugs would you recommend to the government to achieve the greatest benefit for the most patients? Assume that cost (system and patient) is not an issue and that you have access to the necessary supportive care medicines, diagnostic, and laboratory services.” The survey allowed respondents to select up to 10 drugs from an expansive list of 164 anticancer drugs approved by Health Canada as of September 2020.17 Out of these 164 anticancer drugs, 138 are approved in India (Appendix Table A1).
The second set of questions related to the access of each selected drug by eligible patients in the routine clinical practice setting of the participant. A scale was developed on the basis of prior work by the European Society for Medical Oncology with modifications to explicitly identify the risks of significant and catastrophic expenditure.18-20 The scale included four categories: (1) universally accessible (no significant OOP expenses for > 90% of patients); (2) accessible with significant OOP expenses (mixed/partial reimbursement model and not universal health coverage); (3) accessible with high-risk of catastrophic expenditure (significant OOP for > 50% of patients with substantial risk of catastrophic health expenditure defined as spending that absorbs more than 40% of total consumption, net of food expenditures); and (4) unavailable for other reasons (ie, procurement and regulatory).
The primary study population was enrolled from a global network of oncologists. This sampling frame was derived from two sources: (1) membership of national/regional oncology organizations and (2) personal networks of a single oncologist contact in countries where national organizations did not exist or were unable to distribute the survey.
Distribution of the web survey used a hierarchical snowball method through a primary global network composed of oncologist contacts in 89 distinct countries/regions. This method was used earlier for a global study addressing oncologists' workload.21 The survey was open during October 15-December 7, 2020. After the initial distribution, one reminder was sent to each country/regional contact. In India, the survey was distributed through the mailing list of the National Cancer Grid, which is a network of more than 250 cancer centers, research organizations, and patient groups,22 and through the personal network of medical oncologists using WhatsApp and e-mails.
Statistical Analysis
Survey responses were downloaded into IBM SPSS (version 27.0 for Windows, Armonk, NY, 2020). Participants were classified into three groups on the basis of World Bank income status of their country of practice: LMICs, UMICs, and high-income countries (HICs).23 Respondents who did not complete the primary survey question (listing of top 10 medicines) were deemed incomplete and excluded. Missing data for demographic variables and access to medicines were identified in the Appendix Table A2 footnotes; percentages were calculated on the basis of those who did provide a response. Frequency tables were derived for rank order of medications that respondents listed as most essential. The demographic data and clinical practice setting details of the respondents from India were compared with 18 other LMICs (excluding India). A comparison for access to these drugs was done between oncologists working in private health systems and those who work in public health systems or both.
Demographics and clinical practice setting were compared with Pearson chi-square or the Fisher's exact test for categorical data, and the one-way analysis of variance adjusted with Tukey's post hoc tests for multiple comparisons for age and years in practice. A P of < .05 was used as the cutpoint for statistical significance, and no additional adjustment for multiple comparisons was made.
RESULTS
The global study cohort included 948 respondents from 82 countries (after excluding 423 who did not answer the primary study question and 326 who were ineligible as they were trainees or did not prescribe chemotherapy). Median survey response rate for the global population was 6%; the response rate for India was unknown as the number of oncologists who received the survey was not measurable.
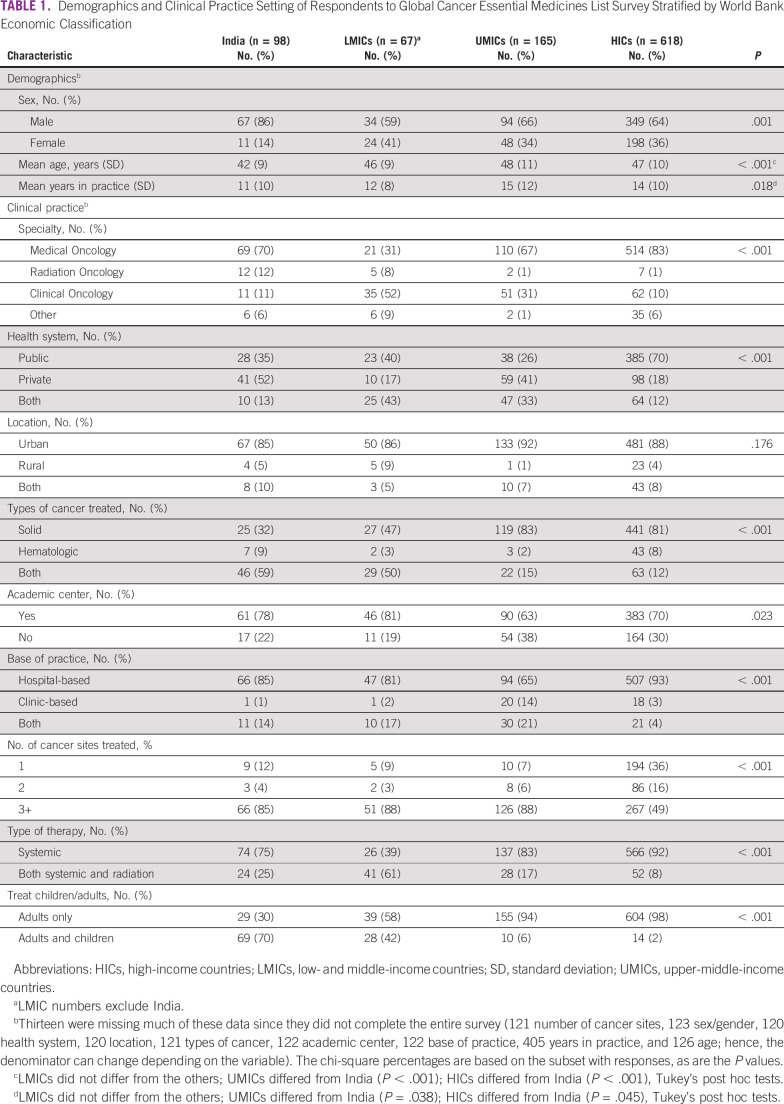
Among the 948 respondents, 618 (65%) were from HICs, 165 (17%) from UMICs, 67 (7%) from other LMICs, and 98 (10%) from India (Table 1). Eighty-six percent of respondents from India were males; this was substantially higher than other LMICs (59%), UMICs (66%), and HICs (64%; P < .001). Medical oncologists constituted 70% of the respondents from India but only 31% in other LMICs (where there were a higher proportion of clinical oncologists [P < .001]). Fifty-two percent of the respondents from India worked exclusively in private health systems. This was substantially higher than other LMICs (17%), UMICs (41%), and HICs (18%; P < .001).
TABLE 1.
Demographics and Clinical Practice Setting of Respondents to Global Cancer Essential Medicines List Survey Stratified by World Bank Economic Classification
Compared with oncologists in UMICs and HICs, those from India and other LMICs were more likely to treat both solid and hematologic tumors (UMICs 15%, HICs 12%, India 59%, and other LMICs 50%; P < .001). Seventy percent of oncologists from India treated both adults and children, which was higher than those from other LMICs (42%), UMICs (6%), and HICs (2%; P < .001).
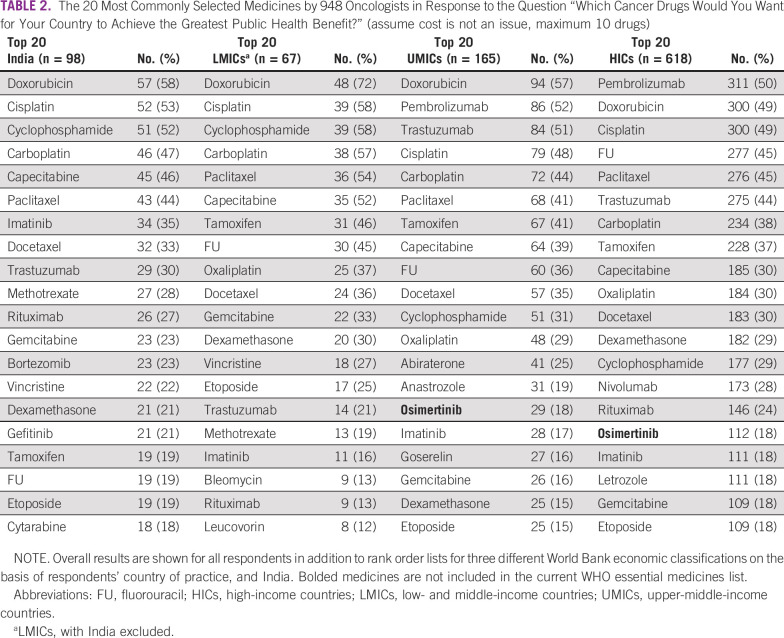
Table 2 shows the top 20 anticancer drugs selected by the respondents. All the top 20 medicines selected by Indian physicians are currently included in the WHO EML. The majority of highest-priority medicines were conventional cytotoxic therapy (14/20); 5/20 were targeted therapy, and 1/20 was hormonal therapy. There was substantial convergence between the top 20 list from India and other LMICs (17/20 medicines are identical). Lack of convergence was seen for bortezomib, gefitinib, and cytarabine (on India's list), and oxaliplatin, bleomycin, and leucovorin (on other LMICs list). It is notable that the top 20 lists from India and other LMICs did not include immunotherapy drugs or newer-generation hormones; pembrolizumab, nivolumab, letrozole, and anastrozole were included in lists from higher-income countries.
TABLE 2.
The 20 Most Commonly Selected Medicines by 948 Oncologists in Response to the Question “Which Cancer Drugs Would You Want for Your Country to Achieve the Greatest Public Health Benefit?” (assume cost is not an issue, maximum 10 drugs)
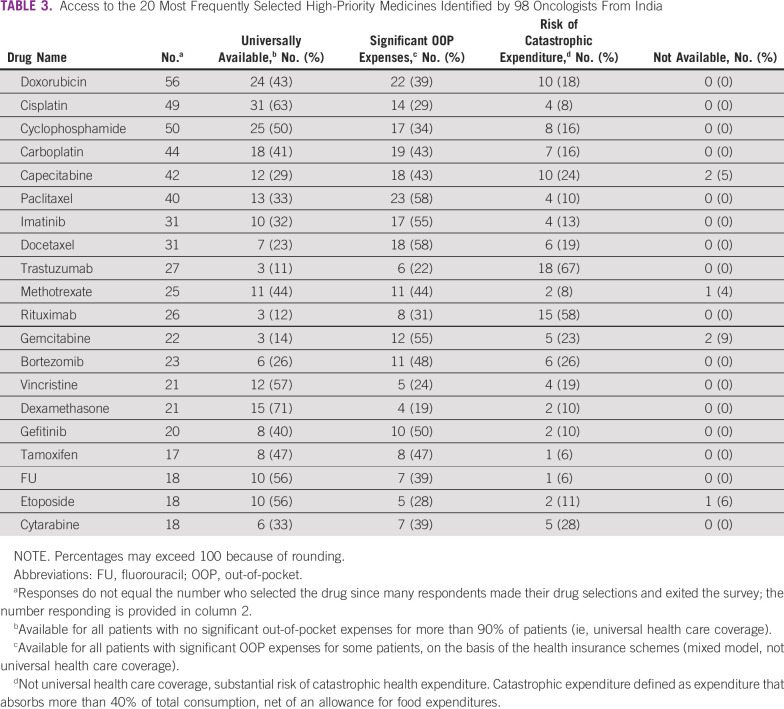
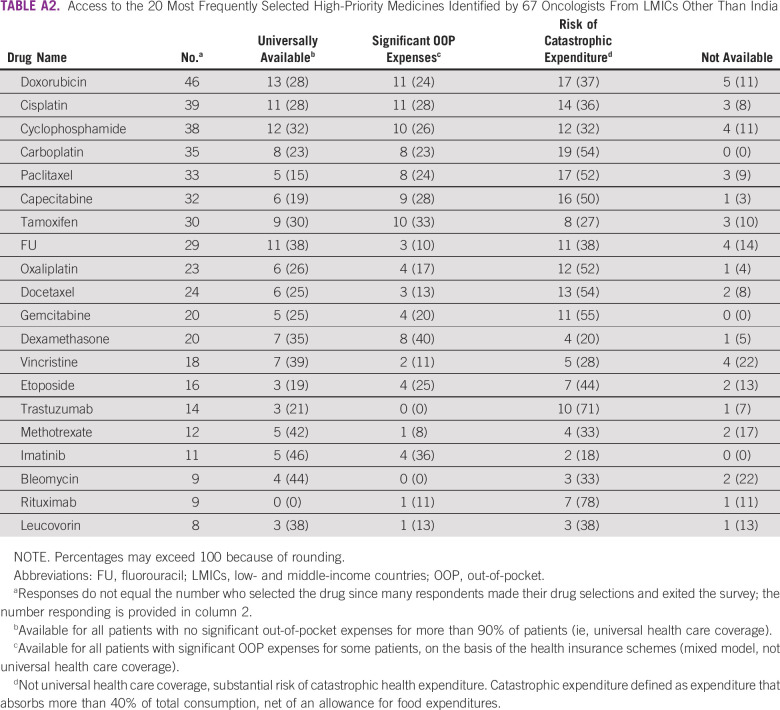
Access to the 20 highest-priority medicines in India is shown in Table 3. Significant OOP expenditure for conventional cytotoxic therapy was reported by 19%-58% of oncologists from India. Catastrophic expenditure was reported by more than 20% of oncologists for six drugs, three of which are well-established cytotoxic drugs (capecitabine, gemcitabine, and cytarabine). Access to rituximab and trastuzumab was most challenging, with 58% and 67% of oncologists reporting risks of catastrophic expenditure, respectively. These results are comparable with access reported by oncologists in other LMICs (Appendix Table A2).
TABLE 3.
Access to the 20 Most Frequently Selected High-Priority Medicines Identified by 98 Oncologists From India
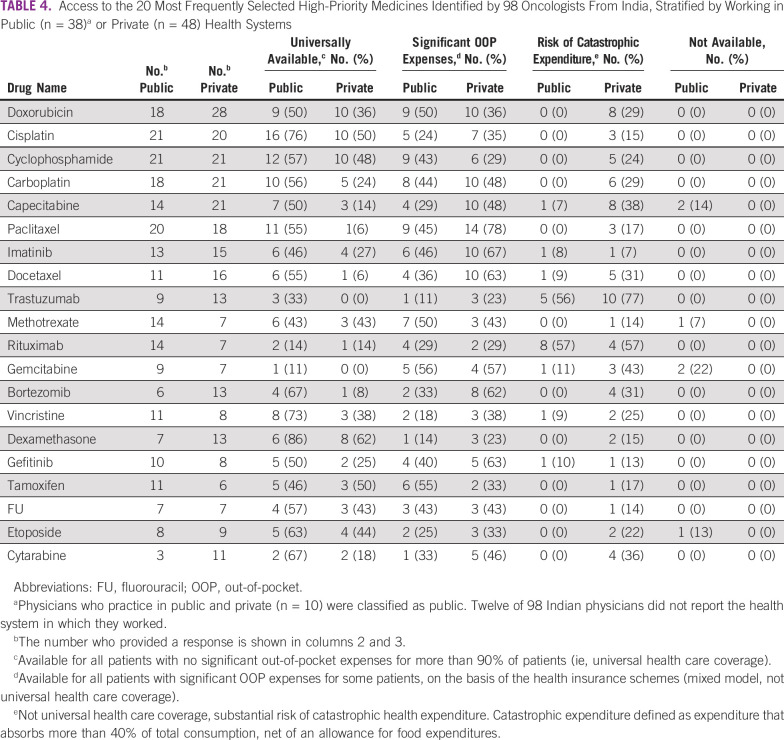
The results stratified by practice setting of oncologists in India (exclusively private v public ± private health sector) are shown in Table 4. The proportion of oncologists who report universal access to high-priority medicines is much higher in the public system. The risk of financial toxicity is substantially higher in the private system, where 23%-78% of oncologists reported each drug being associated with significant OOP expenditure. Catastrophic expenditure was reported by more than 20% of oncologists from private health care systems for 13 drugs, including 10 conventional cytotoxic drugs. Access to trastuzumab, rituximab, and bortezomib was limited by risks of catastrophic expenditure as reported by 77%, 57%, and 31% of private sector oncologists, respectively.
TABLE 4.
Access to the 20 Most Frequently Selected High-Priority Medicines Identified by 98 Oncologists From India, Stratified by Working in Public (n = 38)a or Private (n = 48) Health Systems
DISCUSSION
Our study offers important insight into the cancer medicines considered highest priority in the Indian context and the extent to which they are affordable. The most critical finding of the current study is the lack of universal access to these essential medicines in India. This places considerable economic burden on patients while undergoing cancer treatment; this is felt most acutely by the most vulnerable populations. This has important implications for Indian health policy, particularly for progressive universalism for social coverage of systemic therapies.
The results of this study also highlight the fact that the majority of recent blockbuster medicines in oncology are not considered high priority by oncologists in India. This is likely because of the small incremental gains offered by most new medicines and the fact that many are only effective in small subgroups of patients.3 Our study identifies those medicines that offer the greatest patient- and population-level benefit in terms of survival gains; these agents should be the target of policy actions, including being prioritized for reimbursement by health care systems.
All of the top 20 highest-priority medicines selected by Indian oncologists are already included on the WHO EML and NLEM of India. Well-established cytotoxic drugs, mostly discovered in the 20th century and exposed to global generic competition in the past 10-20 years (eg, doxorubicin, cyclophosphamide, paclitaxel, carboplatin, methotrexate, cytarabine, capecitabine, fluorouracil, and gemcitabine), dominated the list. Universal access for these old and inexpensive conventional chemotherapy drugs was reported by < 50% of Indian oncologists. Significant OOP expenditure for some of these older drugs that are essential components of treatment regimens for common curable cancers in India can have a significant detrimental effect. The use of targeted therapies such as rituximab and trastuzumab, which have shown remarkable benefits in survival, would lead to catastrophic expenditure as reported by more than 50% of oncologists and therefore cannot be used for all eligible patients. These challenges were even more pronounced within the private sector. The risk of catastrophic expenditure was reported by 7%-77% of private-sector oncologists for all the medicines in the list. These barriers exist despite the fact that India has multiple generic and biosimilar options for the top 20 priority medicines that should promote competition and lower prices.24
The National Pharmaceutical Pricing Authority has set a ceiling price for the medicines listed in NLEM (drug pricing control order).13 Several government-funded programs including the Ayushman Bharat Pradhan Mantri Jan Arogya Yojana (a large government-funded insurance scheme covering the poorest 40% of India's population) were created to support treatment of the marginalized segment of society within the public health system.25,26 Notably, all the high-priority drugs identified by oncologists in our survey are covered by this scheme; however, frequent stockouts and concerns about drug quality often force patients to obtain their own supply. Although these measures likely reduce financial toxicity in the public system, major problems still exist in the private sector, where most Indian patients access health care.15 Price control has led to many innovators moving out of the market in India and limited suppliers with variable quality bidding for cancer medicine tenders within the public health system. Lack of systems to ensure stringent credential checks and inability to sustain production of drugs at the price-controlled costs lead to supply chain management failures, resulting in frequent stockouts and concerns about drug quality. This leads to patients buying these drugs from outside the public hospitals with significant expenditures. The process of procurement and sale of the drugs in the private sector remains largely unregulated and contributes to significant OOP and catastrophic expenditure.27,28 Further work is needed to better understand the medicine supply chain and identify the steps leading to prices becoming unaffordable for patients.
The priority list of oncologists from India had 85% concordance with that of other LMICs, highlighting the wide consensus on relevance of these drugs in similar settings. Notably, immunotherapy drugs such as pembrolizumab and nivolumab were not identified as high-priority medicine by Indian physicians (but were selected by oncologists in UMICs and HICs). Similarly, gefitinib was selected instead of osimertinib.29 Accordingly, although the survey explicitly asked respondents not to consider cost, it is possible that Indian oncologists remained mindful of the potential cost implications associated with these medicines, especially in light of the fact that many of the older and generic medicines remain unaffordable for patients in their day-to-day practice. The study highlights that oncologists on the frontline do prefer value-based care to optimize health-resource utilization. A concerted effort to perform an objective value assessment for each breakthrough therapy with measurement of willingness-to-pay threshold and budget impact calculation will be helpful to further assist oncologists in choosing the right drugs for patients without further burdening the limited health budget and resources.30
Our findings must be considered in the light methodologic limitations. First, the snowball methodology made it difficult to ascertain response rates in many countries, but the overall response was low including India. The methodology did not allow to have fixed proportion on the basis of the practice setting, country, or sex of oncologists. Accordingly, the survey respondents are not necessarily representative of all Indian oncologists. Our affordability evaluation was based on a pragmatic modification of a prior survey tool rather than a validated instrument.18 Because our conclusions on access to medicines within a country are based on oncologists' perception rather than actual utilization data, there is a potential that these estimates may be inaccurate.
The findings from this study should serve as a collective call to action to address barriers to delivering high-quality and affordable cancer care across India. Cancer care in India is associated with significant financial toxicity, which translates into poor health-related quality of life, worsening financial status in households, deprioritization of their other needs, and treatment abandonment.28,31 Reduction in OOP expenditure for accessing essential health care, including anticancer drugs, should be one of the top priorities for policymakers (Table 5). Inclusion of the drugs in WHO-EML, NLEM, and the clinical guidelines has important implications but access needs further action to address the barriers because of quality, differential pricing, and stockouts. Novel strategies such as pooled procurement, negotiation at the national level, group contracting, stringent methods to identify quality generics and biosimilars, strengthening the regulatory processes to ensure maintenance of drug quality and supply beyond the marketing approval, and fair pricing will cumulatively serve to mitigate the problems identified in our study.32 Research efforts will identify new cancer therapeutics to improve the outcome of future patients. However, we must devote an equal amount of energy and attention to ensuring that all current patients have access to the treatments that we already know work. Ensuring access and affordability of cancer care in India and other LMICs should be one of the top priorities for governments and policymakers.
TABLE 5.
Recommendations to Improve Access and Affordability to High-Priority Cancer Medicines in India

APPENDIX
TABLE A1.
List of Drugs
TABLE A2.
Access to the 20 Most Frequently Selected High-Priority Medicines Identified by 67 Oncologists From LMICs Other Than India
C.S. Pramesh
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: Aurobindo
Dorothy Lombe
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Bishal Gyawali
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Vivio Health
Felipe Roitberg
Honoraria: Boehringer Ingelheim, AstraZeneca, Oncologia Brasil
Consulting or Advisory Role: MSD Oncology
Research Funding: Roche (Inst), Boehringer Ingelheim (Inst), MSD (Inst), Bayer (Inst), AstraZeneca (Inst), Takeda (Inst)
Elisabeth G.E. De Vries
Consulting or Advisory Role: Daiichi Sankyo (Inst), NSABP Foundation (Inst), Crescendo Biologics (Inst)
Research Funding: Amgen (Inst), Synthon (Inst), CytomX Therapeutics (Inst), Regeneron (Inst), G1 Therapeutics (Inst), Bayer (Inst), Roche (Inst), Genentech (Inst), Servier (Inst), Crescendo Biologics (Inst)
Uncompensated Relationships: ESMO (Inst), World Health Organization (Inst), RECIST (Inst)
Richard Sullivan
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Pfizer
Consulting or Advisory Role: Pfizer (Inst)
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Manju Sengar, Adam Fundytus, Dorothy Lombe, Matthew Jalink, Bishal Gyawali, Felipe Roitberg, Lorenzo Moja, Richard Sullivan, Christopher M. Booth
Administrative support: Christopher M. Booth
Provision of study materials or patients: Christopher M. Booth
Collection and assembly of data: Manju Sengar, Adam Fundytus, Wilma Hopman, C.S. Pramesh, Venkatraman Radhakrishnan, Matthew Jalink, Dario Trapani, Christopher M. Booth
Data analysis and interpretation: Manju Sengar, Adam Fundytus, Wilma Hopman, C.S. Pramesh, Venkatraman Radhakrishnan, Prasanth Ganesan, Aju Mathew, Matthew Jalink, Bishal Gyawali, Dario Trapani, Felipe Roitberg, Elisabeth G.E. De Vries, Lorenzo Moja, André Ilbawi, Richard Sullivan, Christopher M. Booth
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
C.S. Pramesh
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: Aurobindo
Dorothy Lombe
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Bishal Gyawali
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Vivio Health
Felipe Roitberg
Honoraria: Boehringer Ingelheim, AstraZeneca, Oncologia Brasil
Consulting or Advisory Role: MSD Oncology
Research Funding: Roche (Inst), Boehringer Ingelheim (Inst), MSD (Inst), Bayer (Inst), AstraZeneca (Inst), Takeda (Inst)
Elisabeth G.E. De Vries
Consulting or Advisory Role: Daiichi Sankyo (Inst), NSABP Foundation (Inst), Crescendo Biologics (Inst)
Research Funding: Amgen (Inst), Synthon (Inst), CytomX Therapeutics (Inst), Regeneron (Inst), G1 Therapeutics (Inst), Bayer (Inst), Roche (Inst), Genentech (Inst), Servier (Inst), Crescendo Biologics (Inst)
Uncompensated Relationships: ESMO (Inst), World Health Organization (Inst), RECIST (Inst)
Richard Sullivan
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Pfizer
Consulting or Advisory Role: Pfizer (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Palumbo MO, Kavan P, Miller WH Jr, et al. : Systemic cancer therapy: Achievements and challenges that lie ahead. Front Pharmacol 4:57, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grössmann N, Wolf S, Rothschedl E, et al. : Twelve years of European cancer drug approval—A systematic investigation of the “magnitude of clinical benefit”. ESMO Open 6:100166, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saluja R, Arciero VS, Cheng S, et al. : Examining trends in cost and clinical benefit of novel anticancer drugs over ime. J Oncol Pract 14:e280-e294, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Del Paggio JC, Berry JS, Hopman WM, et al. : Evolution of the randomized clinical trial in the era of precision oncology. JAMA Oncol 7:728-734, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Paggio JC, Sullivan R, Schrag D, et al. : Delivery of meaningful cancer care: A retrospective cohort study assessing cost and benefit with the ASCO and ESMO frameworks. Lancet Oncol 18:887-894, 2017 [DOI] [PubMed] [Google Scholar]
- 6.WHO Expert Committee on the Selection of Essential Drugs & World Health Organization : The Selection of Essential Drugs: Report of a WHO Expert Committee [Meeting Held in Geneva from 17 to 21 October 1977]. World Health Organization, 1977. https://apps.who.int/iris/handle/10665/41272 [Google Scholar]
- 7.World Health Organization : Selection of Essential Medicines at Country Level: Using the WHO Model List of Essential Medicines to Update a National Essential Medicines List. 2019. https://apps.who.int/iris/bitstream/handle/10665/330668/9789241210300-eng.pdf?ua=1 [Google Scholar]
- 8.World Health Organization : The Selection and Use of Essential Medicines: Report of the WHO Expert Committee on Selection and Use of Essential Medicines. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 [Google Scholar]
- 9.World Health Assembly, 70 : Cancer Prevention and Control in the Context of an Integrated Approach. World Health Organization, 2017. https://apps.who.int/iris/handle/10665/275676 [Google Scholar]
- 10.Mathur P, Sathishkumar K, Chaturvedi M, et al. : Cancer statistics, 2020: Report from National Cancer Registry Programme, India. JCO Glob Oncol 6:1063-1075, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallath MK, Taylor DG, Badwe RA, et al. : The growing burden of cancer in India: Epidemiology and social context. Lancet Oncol 15:e205-e212, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Report of the core-committee for revision of National List of Essential Medicines: https://main.mohfw.gov.in/sites/default/files/Recommendations.pdf
- 13.Drug Price control order: https://pharmaceuticals.gov.in/act
- 14.Fundytus A, Sengar M, Lombe D, et al. : Access to cancer medicines deemed essential by oncologists in 82 countries: An international, cross-sectional survey. Lancet Oncol 22:1367-1377, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajpal S, Kumar A, Joe W: Economic burden of cancer in India: Evidence from cross-sectional nationally representative household survey, 2014. PLoS One 13:e0193320, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qualtrics online survey software: https://www.qualtrics.com/au/core-xm/survey-software/
- 17.Cancer Care Ontario: Drugs. 2021. https://www.cancercareontario.ca/en/drugformulary/drugs [Google Scholar]
- 18.Cherny N, Sullivan R, Torode J, et al. : ESMO European Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in Europe. Ann Oncol 27:1423-1443, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Ekman B: Catastrophic health payments and health insurance: Some counterintuitive evidence from one low-income country. Health Policy 83:304-313, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hsu J, Flores G, Evans D, et al. : Measuring financial protection against catastrophic health expenditures: Methodological challenges for global monitoring. Int J Equity Health 17:69, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fundytus A, Sullivan R, Vanderpuye V, et al. : Delivery of global cancer care: An International Study of Medical Oncology Workload. J Glob Oncol 4:1-11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Grid: https://tmc.gov.in/ncg/
- 23.The World Bank : World Bank Country and Lending Group. 2021. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [Google Scholar]
- 24.Cheung WY, Kornelsen EA, Mittmann N, et al. : The economic impact of the transition from branded to generic oncology drugs. Curr Oncol 26:89-93, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Health Benefit Package 2.0: https://pmjay.gov.in/sites/default/files/2020-10/HBP-2-0-User-Guidelines-vFinal.pdf
- 26.Caduff C, Booth CM, Pramesh CS, et al. : India's new health scheme: What does it mean for cancer care? Lancet Oncol 20:757-758, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Dehury RK, Samal J, Coutinho S, et al. : How does the largely unregulated private health sector impact the Indian mass? J Health Manag 21:383-393, 2019 [Google Scholar]
- 28.Boby JM, Rajappa S, Mathew A: Financial toxicity in cancer care in India: A systematic review. Lancet Oncol 22:e541-e549, 2021 [DOI] [PubMed] [Google Scholar]
- 29.Ramalingam SS, Vansteenkiste J, Planchard D, et al. : Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382:41-50, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Goodall S, Liew D: Health technology assessment challenges in oncology: 20 years of value in health. Value Health 22:593-600, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Pramesh CS, Badwe RA, Borthakur BB, et al. : Cancer burden and health systems in India 3: Delivery of affordable and equitable cancer care in India. Lancet Oncol 15:e223-e233, 2014 [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization : Pricing of Cancer Medicines and Its Impacts 2018, https://apps.who.int/iris/bitstream/handle/10665/277190/9789241515115-eng.pdf?sequence=1&isAllowed=y [Google Scholar]