Abstract
The objective of the present study was to enhance the transdermal permeation of cannabinoids: cannabidiol (CBD), cannabigerol (CBG) and tetrahydrocannabivarin (THCV) using chemical permeation enhancer approach and evaluate them for their anti-inflammatory effect in vivo in a paw edema model in rats. Cannabinoids gel formulations were developed using FDA approved inactive ingredients: lactic acid (LA), polyethylene glycol-400 (PEG-400), N-methyl-2 pyrrolidone (NMP), dimethyl sulfoxide (DMSO). In vitro skin permeation testing (IVPT) showed flux of ~ 13.25 μg/cm2/h for CBD, ~9.38 μg/cm2/h for CBG and ~ 51.74 μg/cm2/h for THCV. Additionally, IVPT study showed cumulative drug permeation of 610.96 ± 88.92 μg/cm2, 432.09 ± 35.59 μg/cm2 and 2384.44 ± 42.22 μg/cm2 from CBD, CBG and THCV gel formulations respectively. Further, effect of excipients on cannabinoid permeation showed that, formulation containing lactic acid, NMP and DMSO showed significantly (p < 0.0001) enhanced flux of cannabinoids as compared to formulation without LA, NMP and DMSO. In vivo studies showed that paw edema was significantly (p < 0.0001) reduced in the groups containing CBD, CBG, THCV as compared to control and placebo formulation. In conclusion, flux of CBD, CBG and THCV was significantly enhanced using chemical permeation enhancers approach which helped in reducing rat paw edema.
Keywords: Cannabidiol, Cannabigerol, Tetrahydrocannabivarin, Permeation enhancer, Transdermal
1. Introduction
Transdermal drug delivery (TDD) is poised with significant advantages over other routes of administration; notably the ability to bypass first pass effect which can prematurely metabolize drugs, especially in oral delivery. TDD is a non-invasive drug delivery system unlikely to hypodermic injections (Karande et al., 2005, Carvalho et al., 2018). There are three main generations of TDD systems; the first generation TDD systems include drugs that have low molecular weights, are lipophilic and are efficient at low doses. Generally, the transdermal delivery profile of these drugs is more attractive than the countering oral profiles due to low bioavailability, and other factors. In the second generation TDD systems, enhancement of skin permeability is necessary for expansion of transdermal drugs, therefore disruption of the stratum corneum, provision of driving force into the skin, and avoidance of injury to deeper living tissues are all essential considerations for this generation of TDD systems. A major mechanism utilized in this generation of TDD is chemical enhancers. In employing this strategy, the highly ordered bilayer structures of the intracellular lipids which are seen in the stratum corneum are disrupted as when amphiphilic molecules are introduced into these highly structured bilayers, which results in disorganization of the molecular packing or alternatively extraction of lipids. (Mathur et al., 2014, Mali, 2015). Finally, there is the third generation TDD systems which focuses on the disruption and bypass of the SC barrier, leading to a more effective system. Examples in this class include novel chemical enhancers, cavitational ultrasound, electroporation, and most recently thermal ablation, microdermabrasion, and microneedles (Carvalho et al., 2018; Somagoni et al., 2014; Marepally et al., 2014; Desai et al., 2013; Boakye et al., 2015).
Overcoming the barrier functions using chemical permeation enhancer approach has been widely used by numerous researchers. These enhancers can reversibly alter the stratum corneum layer and enhance the transport of low permeable APIs across the skin layers into the systemic circulation. Numerous permeation enhancers have been reported to improve the TDD of APIs including alcohols, poly-alcohols, pyrrolidones, amines, amides, fatty acids, alkanes, sulphoxides, esters, phospholipids, surfactants and terpenes and much more (Casiraghi et al., 2020).
Due to the presence of natural moisturizing factor (NMF), the skin is capable of maintaining a constant level of hydration. The principal humectant in NMF, pyrrolidone carboxylic acid, demonstrates abilities to increase the water-binding capacity of the stratum corneum (SC) (Babu and Chen 2015). N-Methyl-2-Pyrrolidone has been utilized in numerous studies to enhance the penetration of various compounds. In a study by Babu et al, NMP was used at 5% concentration in a gel and solution for the permeation enhancement of bupranolol across rat skin resulted in 1.5 and 2.4 times increase in the drug permeation respectively (Babu and Pandit 2005). In another study evaluating the effect of DMSO on in-vitro skin permeation of acyclovir (ACV) in a transdermal microemulsion formulation, DMSO and ethanol enhanced the skin flux of ACV up to 107 times that of its aqueous solution (Kumar et al., 2011). Lactic acid which belongs to a class of α-hydroxy acids has been widely used in cosmetic products as a moisturizer, emollient and exfoliant. Because of its exfoliant property, it increases the flexibility in the stratum corneum by absorbing into the polar sites of keratin molecules which results in weakening the interaction between the keratin molecules (Mizukoshi 2020). Chessa et al has reported the use of PEG400 as a permeation enhancer for increasing the percutaneous absorption of quercetin (Chessa et al., 2011).
Recently, cannabinoids have raised significant attention from the scientific community due to claims and findings of its therapeutic beneficial potentials. Studies have shown anti-inflammatory, anti-necrotic, and anti-oxidative effects which indicate significant therapeutic utilization for patients with multiple sclerosis, Alzheimer’s disease, epilepsy, and Parkinson’s disease (Crippa, Guimarães et al., 2018; Millar et al., 2018; Millar et al., 2020). Currently there are four United States Food and Drug Administration (U.S. FDA) approved cannabinoid products on the market; two of these are Phyto cannabinoids (CBD based) including Epididiolex® (an oral solution of pure CBD) and Sativex® (a (1:1) CBD and Δ−9 THC oral mucosal spray) (Romero-Sandoval et al., 2015) and the other two, synthetic cannabinoids: Dronabinol “Marinol/Syndros” (synthetic Δ9 −THC) and Nabilone “Cesamet” (a Δ9 −THC analogue) (Prausnitz and Langer 2008). However, due to low aqueous solubility and first pass metabolism, low bioavailability (13–19%) has been reported with CBD oral products (Millar et al., 2018). TDD system can be an ideal route for APIs with low bioavailability as it bypasses the first pass metabolism unlike the oral route (Casiraghi et al., 2020). However, Due to the high lipophilicity, cannabinoids tend to retain more in the lipophilic epidermal region (stratum corneum) compared to hydrophilic dermal region which results in limited or no transdermal permeation. So far, various reports are published on the skin permeation of cannabinoids including Lodzki et al who developed a reservoir system for transdermal delivery of CBD using ethosomal carriers (Lodzki et al., 2003), Stinchcomb et al. who reported quantification of in-vitro human skin transdermal flux of Δ8-tetrahydrocannabinol (Δ8-THC), CBD and cannabinol (CBN) (Stinchcomb, Valiveti et al. 2004), and Casiraghi et al. who studied influence of vehicle-related aspects on skin permeation of CBD (Valiveti et al., 2004, Casiraghi et al., 2020). The objective of the present study is to enhance the permeation of cannabinoids by using combinational permeation enhancer approach. In this study we have systematically explored the permeation of three cannabinoids, CBG, CBD and THCV using various formulation strategies to enhance their delivery across the skin. Further their pharmacological effect in vivo was also investigated in a rat paw edema model.
2. Materials and methods
2.1. Materials
CBD and CBG were obtained from Purisys (GA, USA). THCV was obtained from Open books (NC, USA). N-Methyl-2-pyrrolidone (NMP), Dimethyl sulfoxide (DMSO), Polyethylene glycol (PEG) 400, λ-Carrageenan (plant-mucopolysaccharide) and Oleic acid were purchased from Sigma-Aldrich (USA). Lactic Acid was purchased from Merck (Kenilworth, NJ). Hydroxyethyl cellulose (H.E.C.) was purchased from The Dow Chemical Company (Midland, MI). Isopropyl myristate (IPM), Ethyl Oleate and Polyoxyethylene Oleyl Ether were gifts from Croda (Edison, NJ). Transcutol P, Labrasol and Labrafil were gifts from Gattefossé (France).
2.2. Pre-formulation studies: Solubility of CBD, THCV and CBG
The solubility of APIs including CBD, CBG and THCV was determined in various solvents including dimethyl sulfoxide (DMSO), N-Methyl-2-pyrrolidone (NMP), and PEG 400. Briefly, 100 mg of each API was dissolved in 0.5 mL of DMSO, NMP, PEG400 and vortexed until it dissolved completely. All the solutions were then visually observed for any undissolved particles. Further, all the solubility samples were centrifuged, and the supernatant were collected. Samples were then analyzed using HPLC for the amount of drug solubilized in each solvent.
2.3. Cannabinoid gel formulation
CBD, THCV and CBG gels (10% w/w) were prepared. Briefly, APIs were weighed and dissolved in dimethyl sulfoxide (DMSO) or N-Methyl-2-pyrrolidone (NMP). PEG400, lactic acid, cosolvents (DMSO or NMP) and penetration enhancers were pipetted appropriately as required. Hydroxy-ethyl cellulose was weighed separately and dissolved in the PEG400/lactic-acid/cosolvent/penetration-enhancer mixture. Drug solutions were transferred into the gel mixture and vortexed for 5 min or until completely homogenous (Table 1).
Table 1.
CBD, CBG and THCV gel formulation with the compositions.
| Composition (%w/w) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Components | A | B | C | D | E | F | G | H | I | J | K |
| CBD | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |||
| CBG | 10 | ||||||||||
| THCV | 10 | ||||||||||
| DMSO | 43 | 43 | 43 | 43 | – | 43 | 41.5 | 39 | 43 | 43 | 45 |
| NMP | 25 | 34 | 41 | – | 25 | 25 | 25 | 25 | 25 | 25 | 27 |
| PEG400 | 12 | 12 | – | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 17 |
| Lactic Acid | 9 | – | 5 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 10 |
| H.E.C. | 1 | 1 | 1 | 1 | 1 | 1 | 2.5 | 5 | 1 | 1 | 1 |
| Water | – | – | – | 25 | 25 | – | – | – | – | – | |
2.4. Drug content assay using HPLC
All the formulations were analyzed for the drug content using HPLC. HPLC analysis was conducted with a Waters 1525 Binary Pump and a Waters 717plus Autosampler system (Waters Technology Corporation, USA). Methanol/water (85:15 v/v) was used as the mobile phase at a flow rate of 1 mL min−1 with injection volume of 20uL. Data was only collected from 220 nm channel. A reverse phase C18 column (Symmetry®, 5 μm, 4.6 × 250 mm; Waters Technology Corporation, USA) with a guard column (Symmetry®, reversed phase, C18) was used for the elution of samples. The retention times were 9.226 mins, 13.13 mins, and 9.713 mins for CBD, THCV and CBG respectively. The calibration curve (peak area vs concentration) was generated over the range of 2–100 μg/ml and was found to be linear with a correlation coefficient of 0.9998.
2.5. Rheology of Topical/Transdermal hydrogels
Viscosity of the gel formulation was measured using AR 1500 Rheometer (New Castle, DE). Approximately 0.4 mL of gel formulation was loaded onto the lower plate of the rheometer and flow sweep measurements with 0.5 mm gap, 0.1 S−1 to 100 S−1 shear rate and parallel plate geometry (20 mm diameter) were employed in obtaining the viscosity of each formulation. Rheological study was conducted at standard room temperature (25 °C). Results for viscosity were expressed in (Pa•s).
2.6. Differential scanning calorimetry (DSC)
APIs including CBD, THCV and CBG and their gel formulations were characterized by DSC, (DSCQ100, TA Instruments, New Castle, DE). Data analysis was performed using a thermal analysis software (Universal Analysis 2000, TA Instruments). In general, heating rates of 10 °C/min were employed over a temperature range of 10–100 °C for CBD/CBG, and 10–400 °C for THCV. DSC analysis was performed using hermetically sealed pan configuration. CBD, THCV, and CBG or their gel formulations were pre-weighed in an aluminum sample pan for DSC and each measurement was performed in triplicate on different samples (Lodzki et al., 2003).
2.7. In-Vitro skin permeation study
The in-vitro permeation and deposition studies were performed under occlusive conditions using jacketed Franz diffusion cells with a 0.636 cm2 permeation area and 5 mL receptor chamber volume. Human skin used was obtained from The New York Fire Fighter’s skin bank and originated from the right and left posterior leg regions. Dermatomed, cryopressed split thickness skin with epidermis and dermis were delivered in plastic bags and stored at −80 °C until further use.
Skin was thawed in water for 45 min and was cut into small pieces and mounted on Franz cells. Receptor medium containing PBS with 25% (v/v) ethanol and 5% (v/v) Tween 80 was used for the IVPT study. 0.01% (w/v) sodium azide was added in the receiving media and sonicated for 20 mins to degasify it. To ensure the absence of bubbles in the receptor chamber, each cell was turned upside down to allow release of air though the sampling port. The cells were then left to equilibrate for about 10 mins before application of hydrogel samples. To begin the permeation experiment, 0.2 mL (contains 20 mg of an API) samples of each formulation was pipetted onto the donor compartment. The donor compartment of each cell was sealed with parafilm and perforated with a needle and the fastened to the receiving compartment using a clamp. Following the completion of the experimental setup, the Franz diffusion cells were blanketed to prevent photo-degradation of samples and the system kept at 32.3 °C with the use of its circulating water bath to maintain the skin surface temperature of 32 °C throughout the duration of the experiment. A 72 h experimentation duration was employed with sampling times set at 24 h increments. 1 mL samples were withdrawn from the receptor chamber via the sampling port and analyzed by HPLC, and the entire remaining receiving media in the receptor chamber was discarded and replaced with a fresh batch. The cumulative amount of drug permeated through the skin per unit area (Qp) was calculated from drug concentration in the receptor fluid and plotted as a function of time. The steady-state flux (J) was determined as the slope of the linear portion of the plot.
2.8. In-Vitro skin deposition study
Upon completion of the in-vitro permeation experiment, the residue hydrogel samples on each skin were carefully wiped off with ethanol/water (50:50) solution and the skin treated further to remove the unabsorbed drugs. Subsequently, the skin samples were removed from the Franz diffusion cells and the area exposed to the donor compartment and receiving compartment of the cells were excised, thinly sliced into fine sections, and placed into a 1 mL solution of methanol/RM (50:50). Each 1 mL suspension was vortexed for 1–2 mins, sonicated for 1 h and centrifuged at 14000 rpm at 24 °C for 15mins. After centrifugation, the 0.2 mL of the supernatant was collected and placed in vials for HPLC analysis. The deposited drug amounts (Qd,72) were expressed as micrograms per unit area of the excised skin with average results expressed from experiments conducted with at least three sample groups.
2.9. In vivo anti-inflammatory study in SD rats
The anti-inflammatory effect of the gel formulations was assessed using carrageenan-induced paw edema model (Whiteley and Dalrymple 1998, Lee and Crosby 1999, Sammons et al., 2000, Cong et al., 2015) using Sprague Dawley (SD) rats. This model was deduced suitable for the evaluation of anti-inflammatory and anti-hyperalgesic drugs (Whiteley and Dalrymple 1998, Lee and Crosby 1999, Sammons et al., 2000, Cong et al., 2015). Edema was induced in male Sprague Dawley (SD) rats by sub-plantar injection of 50uL of 1% λ-carrageenan (22049 SIGMA λ-Carrageenan plant-mucopolysaccharide, Sigma-Aldrich) gel in nuclease free water into the left hind paw of the animals. All the animals were treated with 0.2 mL (contains 20 mg of API) of gel formulation. We evaluated the intensity, development, and duration of the induced inflammation by measurements of rat paw volume using a digital plethysmometer (LE 7500, Panlab, Harvard Apparatus). Measurements were made prior to edema induction (base-line volume) and at 1, 2, 4, 6, 8, 12, and 24 h after sub-plantar carrageenan injection. The intensity of inflammation was assessed by calculating the percentage of increase in paw volume against the base-line measurements. Animals were divided into 5 groups (4 rats each), 1: control (no treatment); group 2: placebo gel- (Gel K) (without CBD/CBG/THCV), group 3: CBD gel (Gel A); group 4: CBG gel (Gel I) and group 5: THCV gel (Gel J).
2.10. In vivo skin irritation study
Sprague–Dawley rats weighing about 250 g were used for the skin irritation study. The animals were acclimatized to laboratory conditions for 7 days before the experiment. To prevent fur from interfering with dermal contact of the gel formulation, animals were anesthetized using 2–4% isoflurane in oxygen, 24 h before experimentation, and the hair was removed with an animal hair clipper. Briefly, animals were divided into 4 groups: Animals were divided into 4 groups (3 rats each), group 1: placebo gel- (Gel K) (without CBD/CBG/THCV), group 2: CBD gel (Gel A); group 3: CBG gel (Gel I) and group 4: THCV gel (Gel J). 0.25 mL of gel formulation was applied on the back of the SD rats. Further, the treated area was covered with the help of 3 M tegaderm adhesive patch to prevent rub-off and oral ingestion of the formulations. Gel was applied once a day for 3 days. Rats were inspected for dermal reactions including erythema, edema and papules (Castro et al., 2011, Patel et al., 2013, Ramezanli and Michniak-Kohn 2018). Further score was given depending on the skin appearance as shown in the Table 2.
Table 2.
Score for in vivo skin irritation study.
| Skin appearance | Score |
|---|---|
| No evidence of irritation | 0 |
| Minimal erythema that is barely perceptible | 1 |
| Definite erythema that is readily visible and minimal edema or minimal papular response | 2 |
| Erythema and papules | 3 |
| Definite edema | 4 |
| Erythema, edema, and papules | 5 |
| Vesicular eruption | 6 |
| Strong reaction spreading beyond the application site | 7 |
2.11. Stability study
All cannabinoid gel formulations including CBD gel (Gel A), CBG gel (Gel I) and THCV gel (Gel J) were stored in the fridge at 2–4 °C for a month in a transparent glass vial. At the end of a month, all gel formulations were analyzed for drug content assay, particle sedimentation and phase separation.
2.12. Statistical analysis
Tests for significant differences among means were performed using the one-way and two-way analysis of variance (ANOVA) accompanied by Tukey and Dunnett post-analysis tests. Differences between groups were considered significant at the p < 0.05 level. Statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA).
3. Results
3.1. Pre-formulation and formulation
All the cannabinoids including CBD, THCV and CBG showed high solubility in DMSO, NMP, PEG 400 and lactic acid. Further, CBD, THCV and CBG gels were formulated using HEC as a gelling agent. Resultant formulations were transparent in appearance with no precipitation of drug. Drug content assay of CBD, THCV and CBG gels showed 98.23 ± 1.34 %, 98.89 ± 0.78 % and 97.45 ± 1.76 % of drug in all the formulations with no significant drug loss in formulation process.
3.2. Rheological studies
The rheological properties of the gels were assessed. The viscoelasticity data of all sample gels showed non-Newtonian fluid characteristics at room temperature (25 °C). 1% gel formulations of CBD, THCV and CBG showed decrease in viscosity on increase in shear rate suggesting the shear thinning property of the gel.
3.3. Differential scanning calorimetry (DSC)
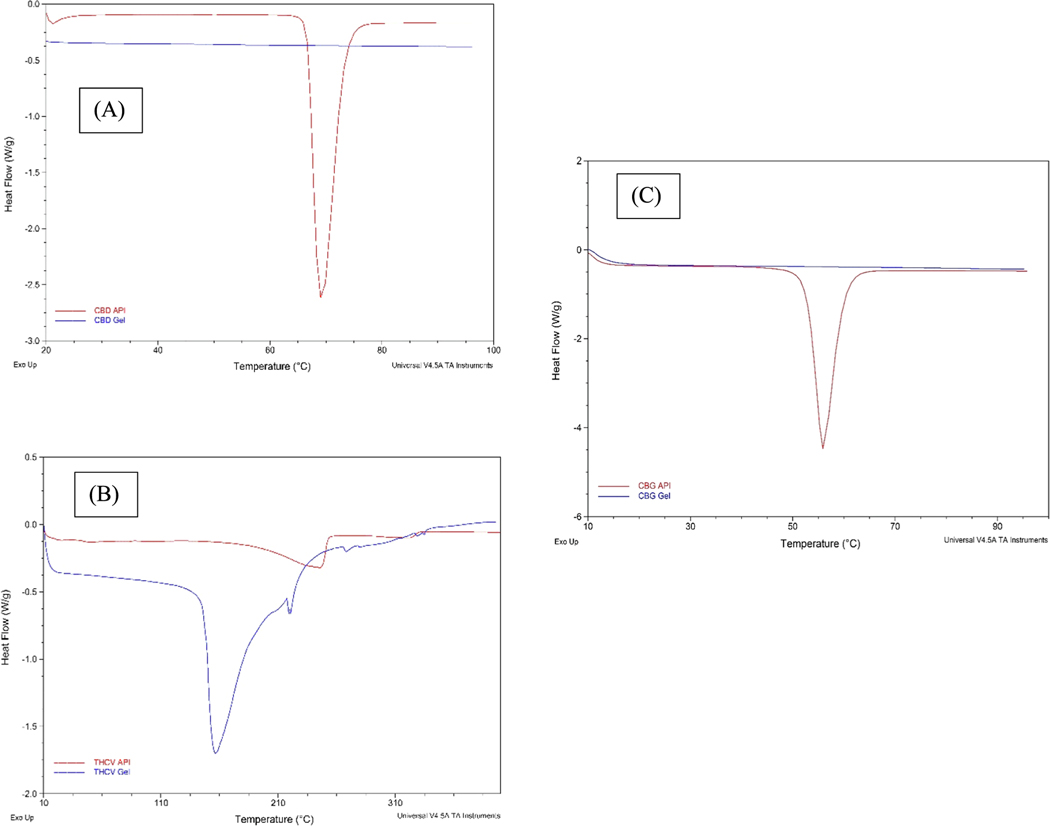
Fig. 1 shows DSC thermograms of CBD, THCV and CBG API as compared to drug mixtures in the gel formulation with DMSO, NMP, PEG400, lactic acid and H.E.C. DSC thermogram of CBD API showed endothermic peak at its melting point 67.5 0C, whereas CBD gel formulation thermogram didn’t show any peak at the melting point temperature of API. DSC thermogram of CBG API also showed endothermic peak at its melting point 56.5 °C, whereas CBG gel formulation thermogram didn’t show any peak at the melting point temperature of API. DSC thermogram of THCV API showed endothermic peak at its melting point 247.5 °C, however THCV gel formulation thermogram showed a shifted endothermic peak approximately 100 °C less than the melting point (Fig. 1).
Fig. 1.
DSC thermograms of: (A) CBD API and CBD gel, (B) THCV API and THCV gel and (C) CBG API and CBG gel.
3.4. In-Vitro permeation testing (IVPT) of CBD gel formulation
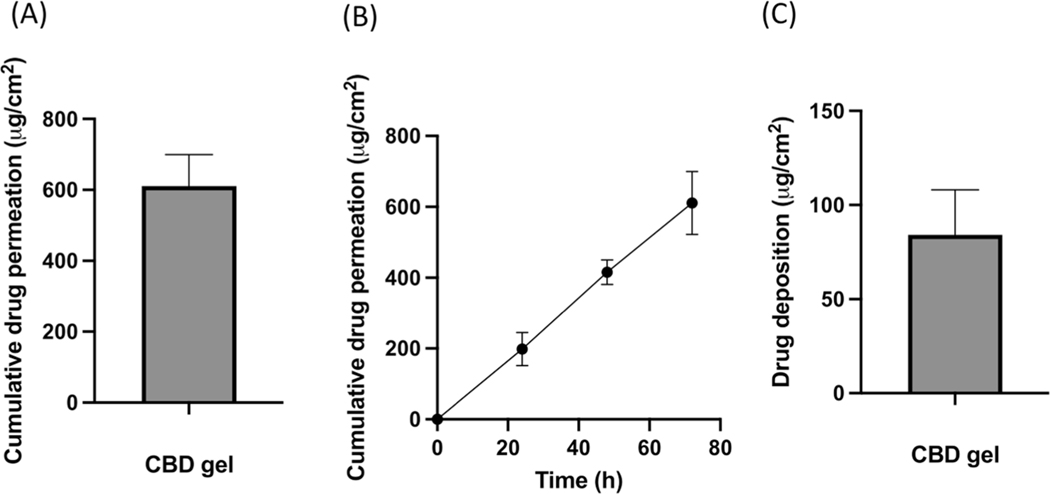
Data showed that 198.40 ± 46.91 μg/cm2, 415.42 ± 34.90 μg/cm2 and 610.96 ± 88.92 μg/cm2 of cumulative CBD was permeated in the receiving media from the gel formulation at 24, 48 and 72 h respectively. The average flux of CBD was found to be 13.26 μg/cm2/h at the end of 72 h. Skin deposition study showed that 84.24 ± 23.77 μg/cm2 of CBD was deposited from CBD formulation at the end of 72 h study (Fig. 2).
Fig. 2.
(A) IVPT study showing cumulative drug permeation from gel formulation at the end of 72 h., (B) IVPT study showing cumulative drug permeation from gel formulation at 24,48 and 72 h., (C) In vitro skin deposition study showing CBD deposition from gel formulation at the end of 72 h.,
3.5. Effect of gelling concentration on CBD permeation
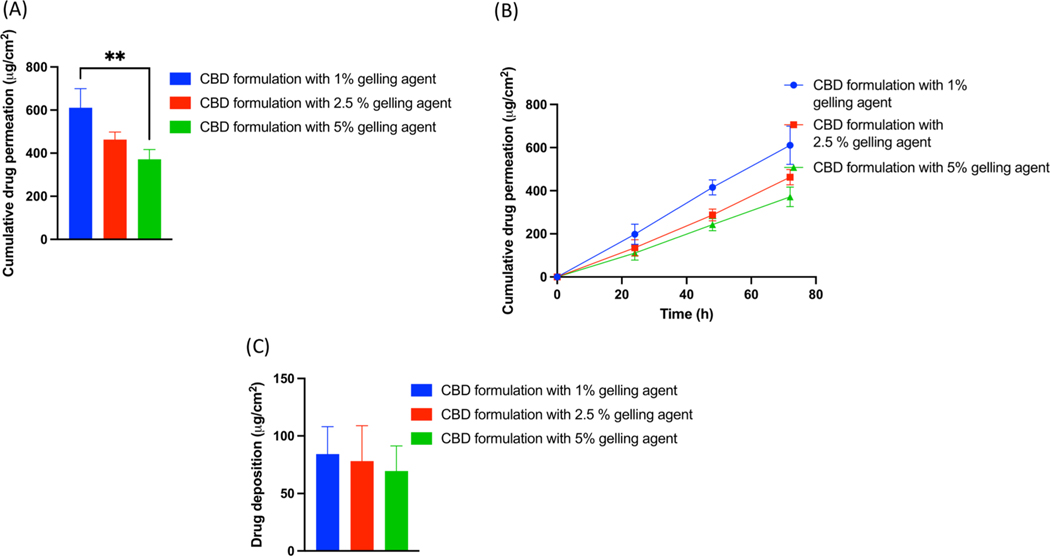
Results revealed that formulation with gelling agent concentration of 1, 2.5 and 5% showed 610.96.
± 88.92 μg/cm2, 462.94 ± 35.65 μg/cm2 and 371.61 ± 45.11 μg/cm2 of cumulative drug permeation respectively. Gel formulation with 1% gelling agent concentration resulted in increased cumulative drug permeation by 1.32 folds as compared to 2.5 and 1.64-fold as compared to 5% gelling agent concentration. However, there was no significant difference in drug permeation between the groups with 2.5% and 5% gelling agent. Results from skin deposition study showed that 84.24 ± 23.77 μg/cm2, 78.06 ± 30.94 μg/cm2 and 69.37 ± 21.82 μg/cm2 of CBD was deposited from CBD formulation containing 1, 2.5 and 5% gelling agent (Fig. 3).
Fig. 3.
(A) IVPT study showing cumulative drug permeation from gel formulation with gelling agent concentration of 1, 2.5 and 5% at the end of 72 h., (B) IVPT study showing cumulative drug permeation from gel formulation with gelling agent concentration of 1, 2.5 and 5% at 24,48 and 72 h., (C) In vitro skin deposition study showing CBD deposition from gel formulation gelling agent concentration of 1, 2.5 and 5% at the end of 72 h.
3.6. Effect of lactic acid and PEG400 on CBD permeation
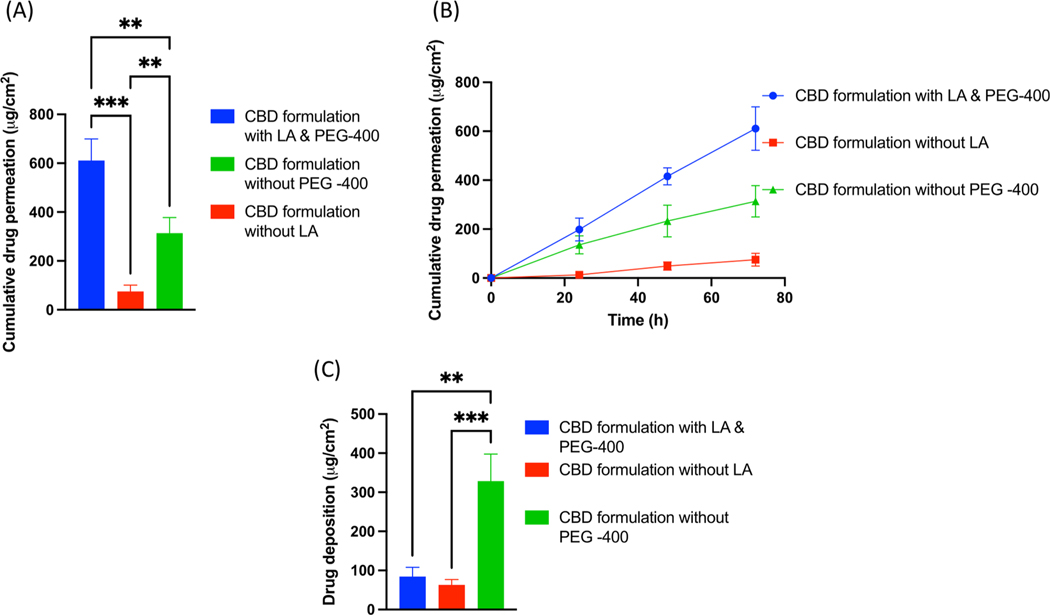
Data showed that CBD formulation with and without lactic acid showed 610.96 ± 88.92 μg/cm2 and 74.80 ± 26.36 μg/cm2 of cumulative drug permeation respectively at the end of 72 h study. It was observed that, cumulative drug permeation from a formulation without lactic acid was significantly (p < 0.0001) decreased by 8.24-fold as compared to gel formulation with lactic acid at the end of 72 h IVPT study. Similarly, formulation with lactic acid showed average flux of 13.26 ± 1.92 μg/cm2/h as compared to a formulation without lactic acid which showed average flux of 1.62 ± 0.57 μg/cm2/h. Formulation with lactic acid showed 8.17-fold increase in average flux as compared to without lactic acid. Skin deposition study showed that 84.24 ± 23.77 μg/cm2 of CBD was deposited from CBD formulation with lactic acid as compared to formulation without lactic acid which showed 63.08 ± 13.88 μg/cm2 of CBD deposition at the end of 72 h study. On the other hand, formulations with PEG400 or without PEG 400 showed 610.96 ± 88.92 μg/cm2 and 313.41 ± 64.53 μg/cm2 of cumulative drug permeation respectively with significant difference at the end of 72 h IVPT study. Formulation without PEG-400 showed 1.94-fold decrease in permeation at the end of the study. Moreover, formulation without PEG 400 demonstrated decreased average flux of 6.80 ± 1.40 μg/cm2/h as compared to formulation with PEG 400 (13.26 ± 1.92 μg/cm2/h). Skin deposition study showed that 84.24 ± 23.77 μg/cm2 μg/cm2 of CBD was deposited from CBD formulation with PEG 400 as compared to formulation without PEG 400 which showed 328.51 ± 69.31 μg/cm2 of CBD deposition at the end of 72 h study (Fig. 4).
Fig. 4.
(A) IVPT study showing cumulative drug permeation from gel formulation with and without PEG-400 and Lactic acid at the end of 72 h., (B) IVPT study showing cumulative drug permeation from gel formulation with and without PEG-400 and Lactic acid at 24,48 and 72 h., (C) In vitro skin deposition study showing CBD deposition from gel formulation with and without PEG-400 and Lactic acid at the end of 72 h.
3.7. Effect of NMP and DMSO with water on CBD permeation
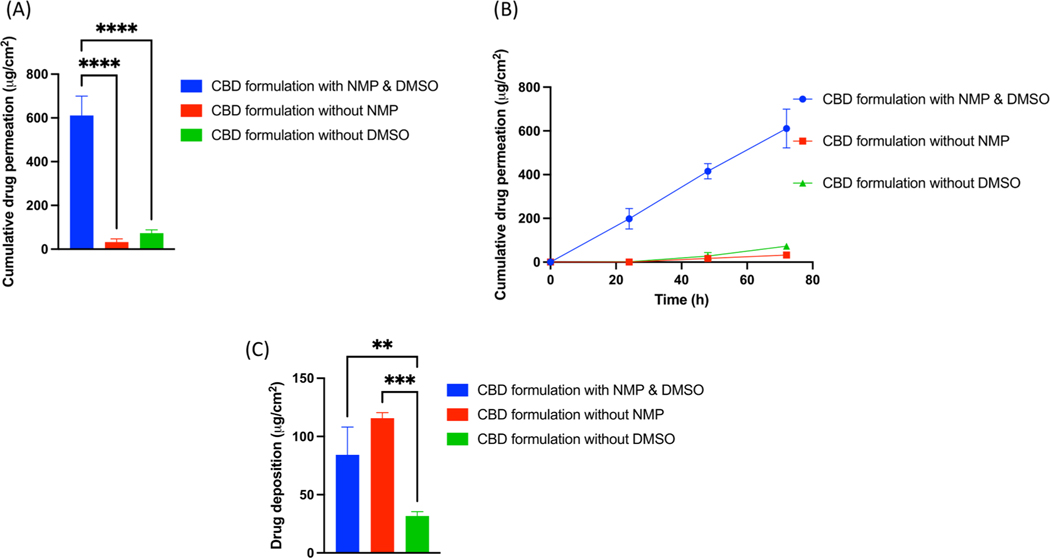
Our results revealed that gels with and without NMP showed 610.96 ± 88.92 μg/cm2 and 32.61 ± 14.67 μg/cm2 of cumulative drug permeation respectively at the end of 72 h study. It was observed that, cumulative drug permeation from a formulation without NMP was significantly (p < 0.0001) decreased by 19.06-fold as compared to gel formulation with NMP at the end of 72 h IVPT study. Similarly, formulation with NMP showed average flux of 13.26 ± 1.93 μg/cm2/h as compared to a formulation without NMP which showed average flux of 0.71 ± 0.32 μg/cm2/h. Skin deposition study showed that 84.24 ± 23.77 μg/cm2 of CBD was deposited from CBD formulation with NMP as compared to formulation without NMP which showed 115.63 ± 4.78 μg/cm2 of CBD deposition at the end of 72 h study. Moreover, formulations with or without DMSO showed 610.96 ± 88.92 μg/cm2 and 72.76 ± 15.58 μg/cm2 of cumulative drug permeation respectively with (p < 0.0001) significant difference at the end of 72 h IVPT study. Formulation without DMSO demonstrated average flux of 1.57 ± 0.34 μg/cm2/h at the end of 72 h study. Skin deposition study showed that 84.24 ± 23.77 μg/cm2 of CBD was deposited from CBD formulation with DMSO as compared to formulation without DMSO which showed 31.63 ± 3.82 μg/cm2 of CBD deposition at the end of 72 h study. In combination with water, formulations without NMP, failed to permeate into the receptor media within the first 24 h. (Fig. 5).
Fig. 5.
(A) IVPT study showing cumulative drug permeation from gel formulation with and without NMP and DMSO at the end of 72 h., (B) IVPT study showing cumulative drug permeation from gel formulation with and without NMP and DMSO at 24,48 and 72 h., (C) In vitro skin deposition study showing CBD deposition from gel formulation with and without NMP and DMSO at the end of 72 h.
3.8. IVPT study of CBG and THCV gel formulation
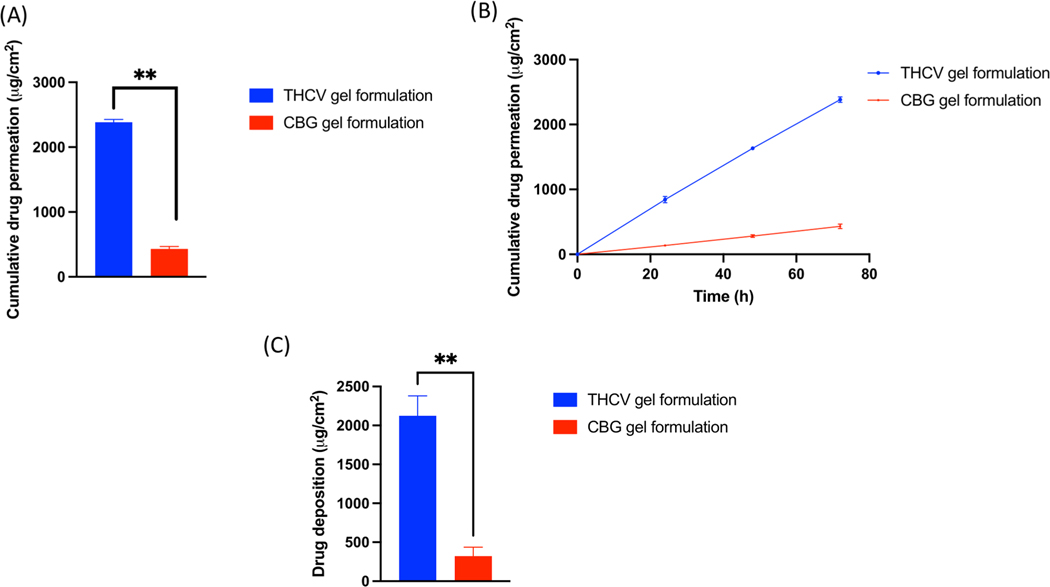
Our skin permeation of CBG results showed that 136.06 ± 0.77 μg/cm2, 281.30 ± 20.61 μg/cm2 and 432.09 ± 35.59 μg/cm2 of CBG was permeated in the receiving media from the gel formulation at 24, 48 and 72 h respectively. The average flux of CBG was found to be 9.38 ± 1.40 μg/cm2/h. Skin deposition study showed that 321.91 ± 113.86 μg/cm2 of CBG was deposited from CBG formulation at the end of 72 h study. Similarly, 843.19 ± 48.98 μg/cm2, 1613.19 ± 27.09 μg/cm2 and 2384.44 ± 42.22 μg/cm2 of cumulative THCV was permeated in the receiving media from the gel formulation at 24, 48 and 72 h respectively. The average flux of THCV was found to be 51.74 ± 3.42 μg/cm2/h. Skin deposition study showed that 2124.40 ± 256.35 μg/cm2 of THCV was deposited from initial gel A formulation at the end of 72 h study (Fig. 6).
Fig. 6.
(A) IVPT study showing cumulative drug permeation from CBG and THCV gel formulation at the end of 72 h., (B) IVPT study showing cumulative drug permeation from CBG and THCV gel formulation at 24,48 and 72 h., (C) In vitro skin deposition study showing CBG and THCV deposition from gel formulation at the end of 72 h.
3.9. In vivo anti-inflammatory study in SD rats
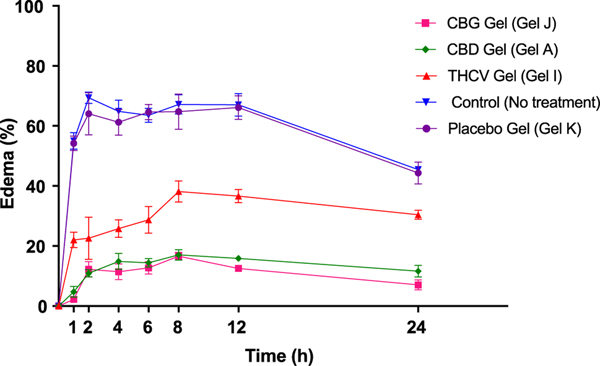
Administration of carrageenan in rat’s paw by sub-planter injection resulted in a time-dependent gradual augmentation of paw volume with maximal values observed between 2 and 12 h post injection. Edema rates for control (no treatment) group, placebo gel (Gel K), CBD gel (Gel A), CBG gel (Gel I) and THCV gel (Gel J) were 55.02 ± 2.78 %, 54.23 ± 2.40 %, 4.74 ± 1.80 %, 2.17 ± 0.82 % and 22.04 ± 2.53 % respectively in the first hour of inflammation induction. Our results showed that edema rate was reduced by ~ 3.92-fold, ~6.46-fold and ~ 1.5-fold as compared to no treatment group at the end of 24 h after application of CBD, CBG and THCV gel formulation respectively. Placebo gel didn’t reduce edema at all time points. It was also observed that all cannabinoid formulations considerably (p < 0.001) reduced paw edema rate at all time points post carrageenan injection as compared to control (no treatment and placebo group). CBD and CBG gel applied groups showed higher anti-inflammatory activity compared to THCV gel (Fig. 7).
Fig. 7.
Inflammatory rate of untreated and treated groups after induction of edema using sub-planter injection of carrageenan showing significant reduction in edema after application of CBD, CBG and THCV gel formulations.
3.10. In vivo skin irritation study
All groups including placebo gel (Gel K), CBD gel (Gel A), CBG gel (Gel I) and THCV gel (Gel J) didn’t show any dermal reaction post application of the gel formulation at any time points. No irritation, edema or papules was observed after applying the gel formulation in all the four animal groups at 24, 48 and 72 h. Therefore, score “0′′ was given at 24, 48 and 72 h to all the animal groups including placebo gel (Gel K), CBD gel (Gel A), CBG gel (Gel I) and THCV gel (Gel J).
3.11. Stability study
Drug content assay showed 98.76 ± 1.32 % of CBD, 98.18 ± 0.65 % THCV and 97.22 ± 0.85 % in the gel formulations with no significant drug degradation at 2–4 °C for a month. Additionally, no sedimentation or phase separation was observed in the gel formulation.
4. Discussion
Over the years, clinical and preclinical studies have demonstrated the therapeutic significance of cannabinoids for diverse indications including schizophrenia, chronic pain, chemotherapeutic induced neuropathic pain, sickle cell disease and Huntington’s disease (Bruni et al., 2018; Crippa et al., 2018; Tijani et al., 2021). For transdermal drug delivery, ideally API should have low molecular weight (<1000 Da), affinity towards lipophilic and hydrophilic phases, short half-life and low melting point. CBD, CBG and THCV have molecular weight < 400 Da, melting point of 66–67 °C, log P values of > 5 which makes them highly lipophilic (Tijani et al., 2021). However, they are insoluble in water (2–10 μg/ml in water) (Koch et al., 2020). Because of high lipophilicity, they tend to retain more in the lipophilic epidermal region (stratum corneum) compared to hydrophilic dermal region which prevents their systemic absorption (Tijani et al., 2021). Few researchers have worked on improving the systemic absorption of cannabinoids including Jiri et al who patented different CBD formulations including aerosol, emulgel and ointment and evaluated them on human subjects for the treatment of inflammatory diseases (Skalicky et al., 2012). Jackson et al also patented CBD formulation with no IVPT results (Jackson and Hyatt 2013). Lowe et al formulated and patented transdermal CBD formulation by solubilizing it in volatile oils (eucalyptus oil and emu oil) with no in vitro or in vivo data (Tijani et al., 2021). Similarly, employment of oils: sunflower, mineral and argan for improving the transdermal delivery of CBD has been reported in the literature (Tijani et al., 2021). However, all these studies lack characterization of formulation including flux determination which is important to know the drug permeation profile across the skin after application of the formulation. Lodzki et al developed a reservoir system for transdermal delivery of CBD using ethosomal carriers, in which they achieved ~ 110.07 μg /cm2 CBD permeation in abdominal skin, ~11.54 μg (μg/g muscle) in abdominal muscle and ~ 0.67 μg /ml concentration in the systemic circulation in their in vivo study in mice (Lodzki et al., 2003). However, API may leak out from the reservoir patch as reported for fentanyl patch and may affect the systemic absorption of API (Pastore et al., 2015). Stinchcomb et al. who developed the cannabinoids solutions in propylene glycol, water and ethanol combination showed flux of Δ8-tetrahydrocannabinol (Δ8-THC), CBD and cannabinol (CBN) in 48 h IVPT study. Their IVPT results showed maximum flux of ~ 1.47 μg/cm2/h CBD permeation and cumulative amount of ~ 78.61 μg CBD permeation at the end of 48 h (Stinchcomb, Valiveti et al. 2004). Witowski et al patented transdermal encapsulated cannabinoid formulation (patent no. LU101384) in which they achieved flux of 2.7 μg/cm2/h for CBD formulation in 72 h in vivo study (Witowski and Salm 2020). Paudel et al developed CBD and THC gel formulation using propylene glycol and transcutol P as permeation enhancers where they showed enhanced bioavailability in Transcutol containing gel formulation compared to a formulation without Transcutol in PK study on on hairless guinea pigs (Paudel et al., 2010). Hannon et al demonstrated that cannabidolic acid (CBDA) and tetrahydrocannabinolic acid (THCA) were absorbed better in systemic circulation compared to CBD and THC after transdermal application of low-THC cannabis extract on heathy dogs (Hannon et al., 2020). Sharkawy et al also developed topical pickering emulsion of CBD to study the effect of degree of deacetylation of chitosan on the stability of the emulsion along with other parameters (Sharkawy et al., 2022). Vanti el developed 1% CBD microemulgel containing transcutol, IPM and solutol HS 15 which showed about 3 μg/cm2 of CBD permeation in 24 h IVPT studies (Vanti et al., 2021). Junaid et al investigated the effect of CBD concentration, essential oils and chemical enhancers, on the flux of CBD in which they found significant difference in CBD permeation in the receiver compartment with formulations containing 1 and 5 % CBD with no significant difference between 5 and 10% CBD in IVPT study. Their results showed 242.41 ± 12.17 μg/cm2 CBD permeation in 24 h IVPT study using human skin (Junaid et al., 2022). Hammell et al formulated CBD gel with ethanol (72.5 % w/w) and isopropyl myristate and evaluated anti-inflammatory effect of CBD gel in an arthritis rat model. In this study, they mainly investigated the in vivo effect of CBD gel on arthritis induced rats (Hammell et al., 2016). In the present study, using combinational permeation enhancers approach, we could significantly increase the flux of ~ 13.25 μg/cm2/h for CBD, ~9.38 μg/cm2/h for CBG and ~ 51.74 μg/cm2/h for THCV with formulations containing combination of NMP, DMSO, lactic acid and PEG 400 as compared to formulations without combination of these enhancers. To the best of our knowledge, ours is the first study to show significantly high transdermal permeation of cannabinoids from a gel formulation using combinational approach of permeation enhancers with significant decrease in inflammation in rat paw in carrageenan induced rat paw edema study (as compared to no treatment group).
Our pre-formulation studies showed that all 3 cannabinoids: CBD, CBG and THCV are readily soluble in all the excipients: NMP, DMSO, PEG 400 and lactic acid used in the formulation. All these excipients are FDA approved inactive ingredients. Additionally, acceptable concentrations levels (As per the FDA inactive ingredient database) were used for the formulation development. All the liquid excipients are completely miscible in each other. DSC data demonstrated that API endothermic peak disappeared in gel formulation for CBD and CBG. However, THCV endothermic peak was shifted in case of the gel formulation. This indicates that cannabinoids might have entrapped in the gel formulation and converted from crystalline to amorphous form in the formulation process. Elsenosy et al also showed the absence of endothermic peak of duloxetine HCl in cubosome gel formulation DSC thermogram suggesting the API is encapsulated in the cubosomes (Elsenosy et al., 2020). Cannabinoid gel formulation with NMP, DMSO, lactic acid and PEG 400 showed significantly high average flux of 13.25 g/cm2/h for CBD, 9.38 g/cm2/h for CBG and 51.74 g/cm2/h for THCV for 72 h. Numerous studies including enhancing the flux of lidocaine free base, lidocaine HCl, prilocaine HCl using NMP as a permeation enhancer by Lee et al (Lee et al., 2005), transdermal delivery of highly ionized fat insoluble drug using NMP as a permeability enhancer by ALZA corporation (US Patent No. 4,645,502) have been reported in the literature (Gale and Enscore 1987). Similarly, use of DMSO as an effective permeation enhancer has been reported in multiple studies including Marren et al who demonstrated enhancement of topical NSAID delivery using DMSO (Marren 2011) and Lamprecht et al who studied the enhancement of transdermal delivery of estradiol using DMSO as a permeation enhancer (Otterbach and Lamprecht 2021). Lactic acid and PEG 400 have also been studied for their permeability enhancement effect for the topical and transdermal drug delivery (Sebastiani et al., 2005; Chessa et al., 2011). Effect of gelling agent concentration results revealed that cumulative drug permeation and flux decreased significantly (p < 0.0001) when the gelling agent concentration increased from 1 to 2.5 and 5% suggesting that viscosity of a formulation has a critical role to play in skin permeation of API. Siemiradzka et al studied the influence of gelling agent concentration in a semisolid formulation on the permeation of peptide-corticotropin in which they observed decrease in permeation in higher gelling agent concentration in the formulation (Siemiradzka et al., 2020).
Additionally, effect of excipients on skin permeation of cannabinoids permeation across the skin has also been explored in the present study. Our results showed that presence of lactic acid in the formulation helped in enhancing the flux significantly (p < 0.0001). It is reported that lactic acid which belongs to a class of α hydroxy acid may decrease the cohesion between corneocytes in the epidermal layer of the skin by interfering with ionic bonding which may lead to enhancement of API permeation across the skin (Sebastiani et al., 2005). Results also showed that cannabinoid formulations with NMP, DMSO, lactic acid with or without PEG400 showed significant effect on cumulative drug permeation at the end of 72 h of IVPT study. This could be due to the presence of NMP, DMSO and lactic acid in the formulation which helped in enhancing the skin permeation. Additionally, substituting water for either NMP or DMSO in the formulation affected the percutaneous absorption and flux of cannabinoids. DMSO acts by penetrating between the polar head groups of phospholipid molecules without interaction, penetrating the lipid/water interphase of the lipid bilayer resulting in loss of interaction between the lipid head groups, and accumulating in the head group region resulting in an increase in the area per head group, weakening the lateral forces between ceramide molecules and increasing the fluidity of the lipids in the SC (Marren 2011). Results also showed that formulation with DMSO and water resulted in higher drug retention and significantly lower drug diffusion than the formulation with NMP and water. This is likely due to the mimicking factor of NMP to the NMF, pyrrolidone carboxylic acid (Babu and Chen 2015) and indicates that the addition of NMP as a cosolvent was essential to the effectiveness of our formulations.. Further, THCV formulation showed significantly (p < 0.0001) high flux compared to CBD with same combinations of permeation enhancers. In addition to this, CBG formulation also showed flux of ~ 9.38 μg/cm2/h with the combinational approach of permeation enhancers. This suggests that permeation enhancers could successfully overcome the barrier function of the skin by disrupting the stratum corneum layer of the skin. Our in vivo anti-inflammatory study of cannabinoids formulations on SD rats showed significant (p < 0.0001) decrease in the paw edema inflammation in the group containing CBG followed by CBD and THCV at all time points as compared to no treatment group and placebo formulation. Lodzki et al studied the anti-inflammatory effect of CBD transdermal reservoir patch on mice in which they showed significant decrease in edema induced paw thickness after the pretreatment of CBD patch which was applied 19 h before the study (Lodzki et al., 2003). However, in our study, CBD gel formulation was applied right after the edema induction in paw. This indicates that our locally applied gel formulation reduced edema significantly faster than the transdermal reservoir patch studied by Lodzki et al. This could be due to the combination of permeation enhancers used in the formulation to enhance the systemic absorption of CBD. Additionally, in vivo skin irritation study showed no adverse reaction on animal’s skin. Overall, transdermal permeation of cannabinoids CBD, CBG and THCV was significantly enhanced using combinational permeation enhancer approach as shown in our IVPT results with significant reduction in edema when applied on carrageenan induced inflamed rat paw. Although our work focused on three cannabinoids: CBD, THCV and CBG, our formulation approach can be applied to any other minor cannabinoids and result in significant increase in flux.
5. Conclusion
Transdermal gel formulations of cannabinoids: CBD, CBG and THCV were formulated using combination of permeation enhancers and solvents. Our IVPT results showed significantly high permeation of these cannabinoids with controlled release for 72 h. Results also showed that NMP DMSO and lactic acid played a critical role in overcoming the stratum corneum barrier of skin which eventually resulted in enhancing the flux. In vivo studies showed that our formulation approach led to an enhanced the permeation in inflamed rat paw and alleviated the inflammation significantly as compared to no treatment group and placebo formulation.
Acknowledgements
Authors are thankful to the Consortium for Medical Marijuana Clinical Outcomes Research, Grant/Award number: SUB00002097, National Institute on Minority Health and Health Disparities of National Institutes of Health, Grant/Award Number: U54 MD007582 and NSFCREST Center for Complex Materials Design for Multidimensional Additive Processing (CoManD), Grant/Award Number:1735968 for providing the funding for this research work.
Footnotes
CRediT authorship contribution statement
Oluwaseyi Salau: Investigation, Writing – original draft, Methodology, Visualization, Software. Arvind Bagde: Conceptualization, Methodology, Writing – original draft, Investigation, Software, Visualization. Anil Kalvala: Investigation. Mandip Singh: Supervision, Resources, Writing – review & editing, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper: [Mandip Singh reports financial support was provided by Consortium for Medical Marijuana Clinical Outcomes Research,National Institute on Minority Health and Health Disparities of National Institutes of Health.].
References
- Babu RJ, Chen L, 2015. Pyrrolidones as penetration enhancers. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement, Springer: 291–299. [Google Scholar]
- Babu RJ, Pandit JK, 2005. Effect of penetration enhancers on the transdermal delivery of bupranolol through rat skin. Drug Delivery 12 (3), 165–169. [DOI] [PubMed] [Google Scholar]
- Boakye CH, et al. , 2015. Doxorubicin liposomes as an investigative model to study the skin permeation of nanocarriers. Int. J. Pharm. 489 (1–2), 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni N, et al. , 2018. Cannabinoid delivery systems for pain and inflammation treatment. Molecules 23 (10), 2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho ALM, Silva JA, Lira AAM, Almeida EDP, Nunes R.d.S., Sarmento VHV, Veras LMC, de Almeida Leite JR, Leal LB, de Santana DP, 2018. Third-generation transdermal delivery systems containing zidovudine: effect of the combination of different chemical enhancers and a microemulsion system. AAPS PharmSciTech 19 (7), 3219–3227. [DOI] [PubMed] [Google Scholar]
- Casiraghi A, et al. , 2020. Topical administration of cannabidiol: influence of vehicle-related aspects on skin permeation process. Pharmaceuticals 13 (11), 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro GA, Oliveira CA, Mahecha GAB, Ferreira LAM, 2011. Comedolytic effect and reduced skin irritation of a new formulation of all-trans retinoic acid-loaded solid lipid nanoparticles for topical treatment of acne. Arch. Dermatol. Res. 303 (7), 513–520. [DOI] [PubMed] [Google Scholar]
- Chessa M, Caddeo C, Valenti D, Manconi M, Sinico C, Fadda AM, 2011. Effect of penetration enhancer containing vesicles on the percutaneous delivery of quercetin through new born pig skin. Pharmaceutics 3 (3), 497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong H, et al. , 2015. Rat paw oedema modeling and NSAIDs: Timing of effects. Int. J. Risk Saf. Med. 27 (s1), S76–S77. [DOI] [PubMed] [Google Scholar]
- Crippa JA, et al. , 2018. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front. Immunol. 9, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai PR, Shah PP, Hayden P, Singh M, 2013. Investigation of follicular and non-follicular pathways for polyarginine and oleic acid-modified nanoparticles. Pharm. Res. 30 (4), 1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenosy FM, et al. , 2020. Brain targeting of duloxetine HCL via intranasal delivery of loaded cubosomal gel: In vitro characterization, ex vivo permeation, and in vivo biodistribution studies. Int. J. Nanomed. 15, 9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale RM, Enscore DJ, 1987. Transdermal delivery of highly ionized fat insoluble drugs. Google Patents. [Google Scholar]
- Hammell DC, Zhang LP, Ma F, Abshire SM, McIlwrath SL, Stinchcomb AL, Westlund KN, 2016. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur. J. Pain 20 (6), 936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon MB, Deabold KA, Talsma BN, Lyubimov A, Iqbal A, Zakharov A, Gamble LJ, Wakshlag JJ, 2020. Serum cannabidiol, tetrahydrocannabinol (THC), and their native acid derivatives after transdermal application of a low-THC Cannabis sativa extract in beagles. J. Vet. Pharmacol. Ther. 43 (5), 508–511. [DOI] [PubMed] [Google Scholar]
- Jackson DK, Hyatt K, 2013. Silicone and Hylauronic Acid (HLA) Delivery Systems for Products by Sustainable Processes for Medical Uses Including Wound Management, Google Patents. [Google Scholar]
- Junaid MSA, et al. , 2022. In vitro percutaneous absorption studies of cannabidiol using human skin: exploring the effect of drug concentration, chemical enhancers, and essential oils. Int. J. Pharm. 616, 121540. [DOI] [PubMed] [Google Scholar]
- Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S, 2005. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc. Natl. Acad. Sci. 102 (13), 4688–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch N, et al. , 2020. Cannabidiol aqueous solubility enhancement: Comparison of three amorphous formulations strategies using different type of polymers. Int. J. Pharm. 589, 119812. [DOI] [PubMed] [Google Scholar]
- Kumar B, Jain SK, Prajapati SK, 2011. Effect of penetration enhancer DMSO on in-vitro skin permeation of acyclovir transdermal microemulsion formulation. Int. J. Drug Delivery 3 (1), 83–94. [Google Scholar]
- Lee IO, Crosby G, 1999. Halothane effect on formalin-induced paw edema and flinching in rat. J. Korean Med. Sci. 14 (1), 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PJ, Langer R, Shastri VP, 2005. Role of n-methyl pyrrolidone in the enhancement of aqueous phase transdermal transport. J. Pharm. Sci. 94 (4), 912–917. [DOI] [PubMed] [Google Scholar]
- Lodzki M, Godin B, Rakou L, Mechoulam R, Gallily R, Touitou E, 2003. Cannabidiol—transdermal delivery and anti-inflammatory effect in a murine model. J. Control. Release 93 (3), 377–387. [DOI] [PubMed] [Google Scholar]
- Mali AD, 2015. An updated review on transdermal drug delivery systems. Int. J. Adv. Sci. Res. 1 (6), 244. 10.7439/ijasr.v1i6.2243. [DOI] [Google Scholar]
- Marepally S, Boakye CHA., Patel AR, Godugu C, Doddapaneni R, Desai PR, Singh M, 2014. Topical administration of dual siRNAs using fusogenic lipid nanoparticles for treating psoriatic-like plaques. Nanomedicine 9 (14), 2157–2174. [DOI] [PubMed] [Google Scholar]
- Marren K, 2011. Dimethyl sulfoxide: an effective penetration enhancer for topical administration of NSAIDs. Phys. Sportsmed. 39 (3), 75–82. [DOI] [PubMed] [Google Scholar]
- Mathur V, et al. , 2014. Physical and chemical penetration enhancers in transdermal drug delivery system. Asian J. Pharm. (AJP): Free full text articles Asian J. Pharm. 4 (3). [Google Scholar]
- Millar SA, et al. , 2020. Towards better delivery of cannabidiol (CBD). Pharmaceuticals 13 (9), 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SA, et al. , 2018. A systematic review on the pharmacokinetics of cannabidiol in humans. Front. Pharmacol. 9, 1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukoshi K, 2020. Effects of lactic acid on the flexibility of the stratum corneum. Skin Research and Technology 26 (4), 599–607. [DOI] [PubMed] [Google Scholar]
- Otterbach A, Lamprecht A, 2021. Enhanced skin permeation of estradiol by dimethyl sulfoxide containing transdermal patches. Pharmaceutics 13 (3), 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore MN, Kalia YN, Horstmann M, Roberts MS, 2015. Transdermal patches: history, development and pharmacology. Br. J. Pharmacol. 172 (9), 2179–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HK, et al. , 2013. Topical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo: ex vivo permeation and skin irritation studies. Colloids Surf., B: Biointerfaces 102, 86–94. [DOI] [PubMed] [Google Scholar]
- Paudel KS, Hammell DC, Agu RU, Valiveti S, Stinchcomb AL, 2010. Cannabidiol bioavailability after nasal and transdermal application: effect of permeation enhancers. Drug Dev. Ind. Pharm. 36 (9), 1088–1097. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR, Langer R, 2008. Transdermal drug delivery. Nat. Biotechnol. 26 (11), 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezanli T, Michniak-Kohn BB, 2018. Development and characterization of a topical gel formulation of adapalene-tyrospheres and assessment of its clinical efficacy. Mol. Pharm. 15 (9), 3813–3822. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval EA, Asbill S, Paige CA, Byrd-Glover K, 2015. Peripherally restricted cannabinoids for the treatment of pain. Pharmacotherapy: J. Hum. Pharmacol. Drug Therapy 35 (10), 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons MJ, et al. , 2000. Carrageenan-induced thermal hyperalgesia in the mouse: role of nerve growth factor and the mitogen-activated protein kinase pathway. Brain Res. 876 (1–2), 48–54. [DOI] [PubMed] [Google Scholar]
- Sebastiani P, et al. , 2005. Effect of lactic acid and iontophoresis on drug permeation across rabbit ear skin. Int. J. Pharm. 292 (1–2), 119–126. [DOI] [PubMed] [Google Scholar]
- Sharkawy A, et al. , 2022. Pickering emulsions stabilized with chitosan/gum Arabic particles: effect of chitosan degree of deacetylation on the physicochemical properties and cannabidiol (CBD) topical delivery. J. Mol. Liq. 355, 118993. [Google Scholar]
- Siemiradzka W, et al. , 2020. Influence of concentration on release and permeation process of model peptide substance-corticotropin-from semisolid formulations. Molecules 25 (12), 2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalicky J, et al. , 2012. A composition for the treatment of inflammatory diseases comprising boswellic acids and cannabidiol. European Patent Application (2444081A1). [Google Scholar]
- Somagoni J, et al. , 2014. Nanomiemgel-a novel drug delivery system for topical application-in vitro and in vivo evaluation. PLoS ONE 9 (12), e115952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb AL, Valiveti S, Hammell DC, Ramsey DR, 2004. Human skin permeation of Δ8-tetrahydrocannabinol, cannabidiol and cannabinol. J. Pharm. Pharmacol. 56 (3), 291–297. [DOI] [PubMed] [Google Scholar]
- Tijani AO, et al. , 2021. Delivering therapeutic cannabinoids via skin: Current state and future perspectives. J. Control. Release 334, 427–451. [DOI] [PubMed] [Google Scholar]
- Valiveti S, Hammell DC, Earles DC, Stinchcomb AL, 2004. Transdermal delivery of the synthetic cannabinoid WIN 55,212–2: in vitro/in vivo correlation. Pharm. Res. 21 (7), 1137–1145. [DOI] [PubMed] [Google Scholar]
- Vanti G, et al. , 2021. Development and optimisation of biopharmaceutical properties of a new microemulgel of cannabidiol for locally-acting dermatological delivery. Int. J. Pharm. 607, 121036. [DOI] [PubMed] [Google Scholar]
- Whiteley PE, Dalrymple SA, 1998. Models of inflammation: Carrageenan air pouch in the rat. Curr. Protocols Pharmacol. 5 (6), 5.6. 1–5.6. 6. [Google Scholar]
- Witowski CG, Salm JL, 2020. Encapsulated cannabinoid formulations for transdermal delivery, Google Patents. [Google Scholar]