Abstract
Teichuronic acid (TUA), a component of the cell walls of the gram-positive organism Micrococcus luteus (formerly Micrococcus lysodeikticus), induced inflammatory cytokines in C3H/HeN mice but not in lipopolysaccharide (LPS)-resistant C3H/HeJ mice that have a defect in the Toll-like receptor 4 (TLR4) gene, both in vivo and in vitro, similarly to LPS (T. Monodane, Y. Kawabata, S. Yang, S. Hase, and H. Takada, J. Med. Microbiol. 50:4–12, 2001). In this study, we found that purified TUA (p-TUA) induced tumor necrosis factor alpha (TNF-α) in murine monocytic J774.1 cells but not in mutant LR-9 cells expressing membrane CD14 at a lower level than the parent J774.1 cells. The TNF-α-inducing activity of p-TUA in J774.1 cells was completely inhibited by anti-mouse CD14 monoclonal antibody (MAb). p-TUA also induced interleukin-8 (IL-8) in human monocytic THP-1 cells differentiated to macrophage-like cells expressing CD14. Anti-human CD14 MAb, anti-human TLR4 MAb, and synthetic lipid A precursor IVA, an LPS antagonist, almost completely inhibited the IL-8-inducing ability of p-TUA, as well as LPS, in the differentiated THP-1 cells. Reduced p-TUA did not exhibit any activities in J774.1 or THP-1 cells. These findings strongly suggested that M. luteus TUA activates murine and human monocytic cells in a CD14- and TLR4-dependent manner, similar to LPS.
The gram-positive organism Micrococcus luteus (formerly Micrococcus lysodeikticus) was initially isolated from the nasal secretion of a patient with acute coryza (6). Human skin is considered to be a primary habitat of the bacterium, and it has also been detected in the mucous membranes as well as in water and soil (18). Recently, this organism was recognized as an opportunistic pathogen and has been implicated in recurrent bacteremia (29, 44), septic shock (3), septic arthritis (45), endocarditis (5, 10, 35), meningitis (7), intracranial suppuration (36), and cavitating pneumonia in immunosuppressed patients (38).
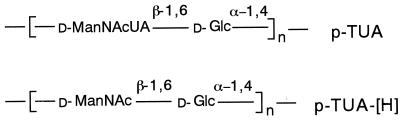
We found that M. luteus cells and cell walls induced serum cytokines in muramyldipeptide-primed mice (24). M. luteus cell walls consist of two polymers, i.e., peptidoglycans (9, 33) and teichuronic acids (TUA) (14, 26, 30), which are composed of N-acetyl-d-mannosaminuronic acid and d-glucose residues covalently bonded to each other (Fig. 1). Unlike the usual peptidoglycans from various gram-positive bacteria, those from M. luteus lacked immunomodulating activities such as immunoadjuvant activity (19), antitumor activity (11), mitogenic activity (12), and cytokine-inducing activity (21). We showed that the cytokine-inducing activity of M. luteus cell walls was attributable to the TUA portion and not to the peptidoglycan portion (25). Furthermore, the activity of TUA was detected in an in vivo experiment using C3H/HeN mice and in peritoneal macrophage cultures from C3H/HeN mice but not from C3H/HeJ mice (25).
FIG. 1.
Chemical structures of p-TUA and p-TUA-[H], based on previous reports (14, 26).
C3H/HeJ mice are genetically resistant to the immunobiological activities of lipopolysaccharides (LPS) (39). Recent studies indicated that a missense mutation in the third exon of the Toll-like receptor 4 (TLR4) gene in C3H/HeJ mice, which replaced proline with histidine at position 712 of TLR4, rendered the mice resistant to endotoxin (31, 32). Akira's group (16, 40, 41) then demonstrated that TLR2 was essential for the responses to peptidoglycans and lipopeptide, while TLR4 was essential for the responses to lipoteichoic acid as well as to LPS, in peritoneal macrophage cultures from TLR2 and TLR4 knockout mice.
In the present study, we first examined whether M. luteus TUA activated murine and human monocytic cells. We then examined the possible involvement of CD14 and TLR4 in the responses of the cells to TUA, which are similar to those to LPS.
MATERIALS AND METHODS
Reagents.
Purified TUA (p-TUA) were prepared from M. luteus NCTC 2665 as described previously (14). Briefly, the cell walls from stationary-phase M. luteus cells were digested with egg white lysozyme and L11 enzyme and then fractionated through an ECTEOLA-cellulose column. To obtain TUA with aminohexose residues (p-TUA-[H]) instead of the original aminohexuronic acid residues, p-TUA was acetylated with acetic anhydride, reduced with diborane, and treated with mild alkali to remove O-acetyl groups. The structures of p-TUA and p-TUA-[H] are shown in Fig. 1. The possible contaminating LPS contents in p-TUA and p-TUA-[H] were calculated as 0.091 and 0.070 ng/mg, respectively, based on the results of the colorimetric Limulus test using Escherichia coli O111:B4 LPS as a control (25). Ultrapurified LPS prepared from Salmonella choleraesuis subsp. choleraesuis serovar Abortus-equi (Novo-Pyrexal) (8), a gift from C. Galanos (Max Planck Institut für Immunbiologie, Freiburg, Germany), was used as reference LPS. A synthetic lipid A precursor IVA (LA-14-PP or compound 406) was obtained from Daiichi Chemical Co. (Tokyo, Japan), and an anti-mouse CD14 monoclonal antibody (MAb) (4C1; rat immunoglobulin G2b [IgG2b]) was prepared as described previously (1). The isotype-matched rat IgG2b, anti-human CD14 MAb MY4, and isotype-matched mouse IgG2b were obtained from Coulter Co., (Miami, Fla). Anti-human TLR4 MAb HTA125 was prepared as described previously (37). Unless otherwise indicated, other reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Cell culture.
The murine macrophage-like cell line J774.1 and the LPS-resistant mutant LR-9 (27) were maintained in Ham's F12 medium (Gibco BRL, Life Technologies, Grand Island, N.Y.) supplemented with 10% fetal calf serum (FCS) in tissue culture dishes at 37°C in a humidified 5% CO2 atmosphere. Cells of the human monocytic leukemia cell line THP-1 were cultured in RPMI 1640 medium with 10% FCS (Flow Laboratories, Inc., McLean, Va.) in tissue culture dishes (Falcon; Becton Dickinson Labware, Lincoln Park, N.J.) at 37°C in a humidified 5% CO2 atmosphere. The THP-1 cells were maintained in logarithmic growth phase (2 × 105 to 1 × 106/ml) by passage every 3 to 4 days. Cells (2 × 105/ml) were then treated with 0.1 μM 22-oxyacalcitriol (OCT), an analogue of 1α, 25-dihydroxy-vitamin D3 (Chugai Pharmaceutical Co., Tokyo, Japan) (20), for 3 days. OCT treatment induced differentiation of THP-1 cells to macrophage-like cells strongly expressing membrane CD14 (mCD14) (45a).
Flow cytometry.
The confluent J774.1 and LPS-resistant mutant LR-9 cells were analyzed for expression of mCD14 by flow cytometry. The cells were collected and washed once with phosphate-buffered saline. The cells were then stained with fluorescein isothiocyanate-conjugated anti-mouse CD14 MAb (rmC5-3) (PharMingen, San Diego, Calif.) at 4°C for 30 min. Flow cytometric analyses were performed with a fluorescence-activated cell sorter (FACScan; Becton Dickinson).
Cytokine assay.
The confluent J774.1, LR-9, and OCT-treated THP-1 cells were collected and washed twice with phosphate-buffered saline. The J774.1 and LR-9 cells (105/200 μl per well) were incubated in Ham's F12 medium with 1% FCS, and the THP-1 cells (105/200 μl per well) were incubated in RPMI 1640 medium with 1% FCS, with or without stimulants for 24 h. In antibody blocking and antagonist-inhibitory experiments, cells were preincubated with MAbs or inhibitor (LA-14-PP) for 30 min and then incubated with stimulants. After incubation, the cytokine levels in the culture supernatants were determined using enzyme-linked immunosorbent assay (ELISA) kits (Pharmingen). Tumor necrosis factor alpha (TNF-α) production by J774.1 and LR-9 cells and interleukin-8 (IL-8) production by OCT-treated THP-1 cells were measured. The concentrations of cytokines in the supernatants were determined using the Softmax data analysis program (Molecular Devices Corp., Menlo Park, Calif.). Each assay was carried out in triplicate.
Statistical analysis.
All experiments were performed at least three times. The data shown are representative results and are means ± standard deviations. The statistical significance of differences between each test group and its respective control was examined by Student's t test.
RESULTS
Expression of mCD14 by J774.1 and LR-9 cells in culture.
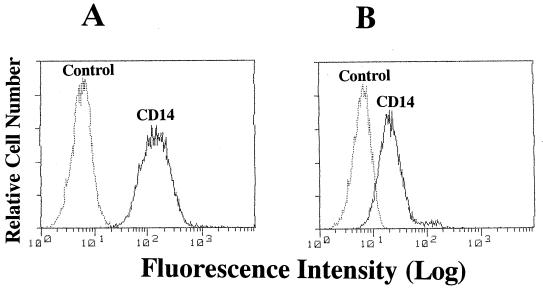
LR-9 cells were originally isolated from J774.1 cells as an LPS-resistant mutant with a defect in LPS binding (13) and later were reported to lack mCD14 (27). Therefore, we first compared mCD14 expression by J774.1 cells with that by LR-9 cells by flow cytometry. The J774.1 cells were clearly stained with anti-mouse CD14 MAb, whereas LR-9 cells were only weakly stained (Fig. 2). These results indicated that the parent J774.1 and the mutant LR-9 cells express mCD14 at high and low levels, respectively.
FIG. 2.
mCD14 expression in J774.1 and LR-9 cells in culture. J774.1 (A) and LR-9 (B) cells were stained with fluorescein isothiocyanate-labeled anti-mouse CD14 MAb (rmC5-3) and analyzed by fluorescence-activated cell sorter analysis. The results shown are representative of those from three different experiments.
Induction of TNF-α production by p-TUA in J774.1 and LR-9 cells in culture.
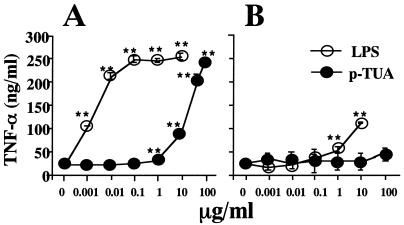
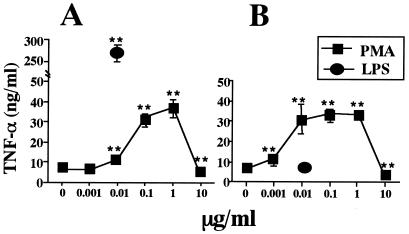
First, we examined the potency of p-TUA to induce TNF-α production in J774.1 and LR-9 cells in culture. In J774.1 cells, p-TUA exhibited a definite activity to induce TNF-α release in a concentration-dependent manner from 1 to 100 μg/ml (Fig. 3A). In the same experiment, the reference LPS induced TNF-α production in a concentration-dependent manner from 1 to 100 ng/ml and exhibited a plateau response at 100 ng/ml to 10 μg/ml. In accordance with a previous report by Nishijima et al. (27), LR-9 cells did not respond to the lower concentrations of LPS (1 to 100 ng/ml) and showed only a slight response to the higher concentrations (1 and 10 μg/ml) (Fig. 3B). In LR-9 cell cultures, p-TUA showed almost no ability to induce TNF-α; no activity was seen 1 ng/ml to 50 μg/ml, and slight but negligible activity was observed at 100 μg/ml. In contrast, both J774.1 and LR-9 cells responded to a CD14-independent stimulant, phorbol myristate acetate, to similar extents (Fig. 4). These findings strongly suggested that p-TUA activates J774.1 cells preferentially in an mCD14-dependent manner.
FIG. 3.
Induction of TNF-α by p-TUA in J774.1 and LR-9 cells. J774.1 (A) and LR-9 (B) cells were stimulated with p-TUA and reference Salmonella serovar Abortus-equi LPS at the indicated concentrations for 24 h in triplicate. The TNF-α levels in the culture supernatants were determinated by ELISA, and are expressed as means ± standard deviations. ∗, P < 0.05; ∗∗, P < 0.01 (versus medium alone). The results are representative of those from three different experiments.
FIG. 4.
Induction of TNF-α by PMA in J774.1 and LR-9 cells. J774.1 (A) and LR-9 (B) cells were stimulated with phorbol myristate acetate (PMA) and reference Salmonella serovar Abortus-equi LPS at the indicated concentrations for 24 h in triplicate. The TNF-α levels in the culture supernatants were determinated by ELISA and are expressed as means ± standard deviations. ∗∗, P < 0.01 versus medium alone. The results are representative of those from two different experiments.
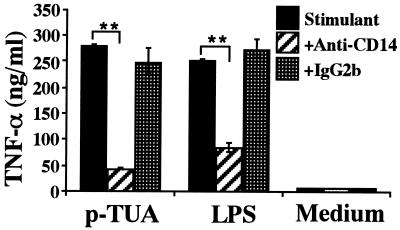
Blocking effect of anti-mouse CD14 MAb on TNF-α secretion by J774.1 cells in response to p-TUA.
To further determine whether p-TUA activated J774.1 cells in an mCD14-dependent manner, J774.1 cells were preincubated with anti-mouse CD14 MAb 4C1 (50 μg/ml) for 30 min and then stimulated with p-TUA (50 μg/ml) or reference LPS (10 ng/ml) for 24 h. Pretreatment of J774.1 cells with the anti-mouse CD14 MAb almost completely inhibited the p-TUA-induced TNF-α release (Fig. 5). In accordance with the results shown in Fig. 3, the blocking effect of the MAb on TNF-α release induced by p-TUA was observed more clearly than that induced by the reference LPS. These findings shown in Fig. 3 to 5 clearly indicate that p-TUA activated J774.1 cells preferentially in an mCD14-dependent manner.
FIG. 5.
Blocking effect of anti-mouse CD14 MAb on TNF-α secretion by J774.1 cells in response to p-TUA. J774.1 cells were preincubated with anti-mouse CD14 MAb 4C1 or isotype-matched control antibody (50 μg/ml) for 30 min and then stimulated with p- TUA (50 μg/ml) and reference LPS (10 ng/ml) for 24 h. The levels of TNF-α in the culture supernatants were determined by ELISA and are expressed as means ± standard deviations for triplicate cultures. ∗∗, P < 0.01 versus the respective control. The results are representative of those from four different experiments.
Induction of IL-8 secretion by p-TUA in THP-1 cells in culture.
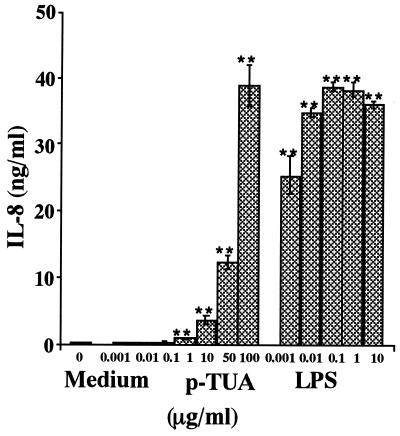
We further examined the cytokine-inducing activity of p-TUA in OCT-differentiated human monocytic THP-1 cells. p-TUA induced IL-8 in a concentration-dependent manner from 1 to 100 μg/ml in the THP-1 cells. The maximum IL-8 level induced by p-TUA (100 μg/ml) was comparable to that induced by reference LPS (0.01 to 10 μg/ml), although considerably higher concentrations of p-TUA were required to exert sufficient activity compared with LPS (Fig. 6).
FIG. 6.
Induction of IL-8 release by p-TUA in THP-1 cells in culture. The OCT-differentiated THP-1 cells were stimulated by p-TUA and reference Salmonella serovar Abortus-equi LPS at the indicated concentrations for 24 h in triplicate. The IL-8 levels in THP-1 cell culture supernatants were determined by ELISA and are expressed as means ± standard deviations. ∗∗, P < 0.01 versus medium alone. The results are representative of those from three different experiments.
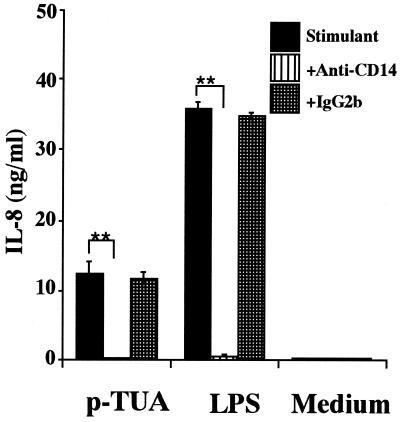
Blocking effect of anti-human CD14 MAb on IL-8 secretion by THP-1 cells in response to p-TUA.
To determine whether p-TUA also activated THP-1 cells in a CD14-dependent manner, OCT-differentiated THP-1 cells were preincubated with anti-human CD14 MAb MY4 (10 μg/ml) or isotype-matched antibody (IgG2b) for 30 min and then stimulated with p-TUA (50 μg/ml) or reference LPS (10 ng/ml). Pretreatment of the THP-1 cells with anti-human CD14 MAb inhibited IL-8 production induced by p-TUA to almost the same level as observed with medium alone (Fig. 7). This result suggested that p-TUA activated OCT-differentiated THP-1 cells in a CD14 (probably mCD14)-dependent manner similarly to LPS.
FIG. 7.
Blocking effect of anti-human CD14 MAb on IL-8 secretion by THP-1 cells in response to p-TUA. The OCT-differentiated THP-1 cells were preincubated with anti-human CD14 MAb MY4 or isotype-matched control antibody (10 μg/ml) for 30 min and then stimulated with p-TUA (50 μg/ml) or reference LPS (10 ng/ml) for 24 h in triplicate. The IL-8 levels in the culture supernatants were determined by ELISA and are expressed as means ± standard deviations. ∗∗, P < 0.01 versus the respective control. The results are representative of those from three different experiments.
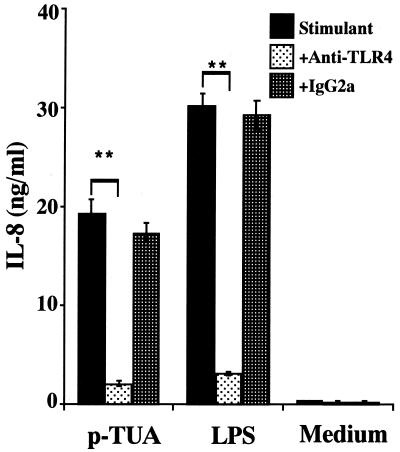
Blocking effect of anti-TLR4 MAb on IL-8 secretion by THP-1 cells in response to p-TUA.
Next, we examined whether TLR4 is involved in IL-8 production by THP-1 cells in response to p-TUA similarly to LPS. OCT-treated THP-1 cells were preincubated with anti-human TLR4 MAb HTA125 (5 μg/ml) or isotype-matched antibody IgG2a for 30 min and then stimulated with p-TUA (50 μg/ml) or reference LPS (10 ng/ml). The IL-8 release induced by p-TUA was markedly inhibited by HTA125 to an extent similar to that for LPS (Fig. 8). This result suggested that p-TUA activated THP-1 cells in the same (i.e., TLR4-dependent) manner as LPS.
FIG. 8.
Blocking effect of anti-human TLR4 MAb on IL-8 secretion by THP-1 cells in response to p-TUA. The OCT-differentiated THP-1 cells were preincubated with anti-human TLR4 MAb HTA125 or isotype-matched control antibody (5 μg/ml) for 30 min and then stimulated with p-TUA (50 μg/ml) or reference LPS (10 ng/ml) for 24 h in triplicate. The IL-8 levels in the culture supernatants were determined by ELISA and are expressed as means ± standard deviations. ∗∗, P < 0.01 versus the respective control. The results are representative of those from three different experiments.
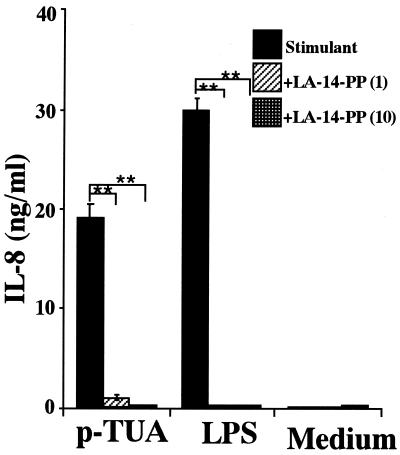
Inhibitory effect of LA-14-PP on IL-8 secretion by THP-1 cells in response to p-TUA.
LA-14-PP is an endotoxin antagonist in human cells and has been suggested to antagonize LPS at multiple sites in the LPS recognition pathway (17, 22). Therefore, we examined the possible antagonistic effects of LA-14-PP against p-TUA in OCT-differentiated THP-1 cells. The IL-8 release induced by p-TUA was markedly inhibited by LA-14-PP in a concentration-dependent manner (Fig. 9). Complete inhibition was observed at 10 μg of LA-14-PP per ml versus 50 μg of p-TUA per ml and at 1 μg LA-14-PP versus 10 ng of LPS per ml.
FIG. 9.
Inhibitory effect of LA-14-PP on IL-8 secretion by THP-1 cells in response to p-TUA. The OCT-differentiated THP-1 cells were preincubated with 1 or 10 μg of LA-14-PP per ml for 30 min and then stimulated with p-TUA (50 μg/ml) or reference LPS (10 ng/ml) for 24 h in triplicate. The IL-8 levels in the culture supernatants were determined by ELISA and are expressed as means ± standard deviations. ∗∗, P < 0.01 versus the respective control. The results are representative of those from three different experiments.
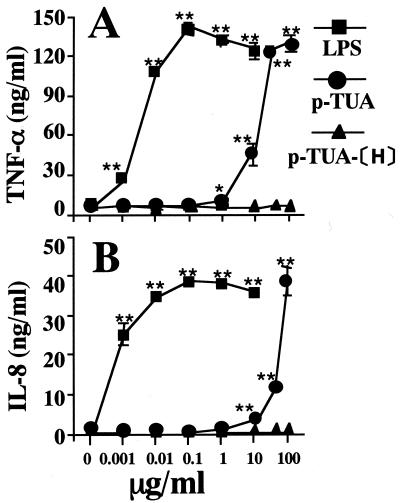
Inactivity of p-TUA-[H] in both J774.1 and THP-1 cells.
The above findings suggested that the receptor system is shared by p-TUA and LPS, i.e., is mCD14 and TLR4 dependent. As mentioned in Materials and Methods, the Limulus test suggested that the p-TUA preparations might have been slightly contaminated with LPS (0.091 ng/mg). To exclude the possibility that the activities of p-TUA were due to contaminating LPS in the preparation, the activity of the reduced p-TUA (p-TUA-[H]) compared with that of the parent p-TUA was examined in J774.1 and OCT-treated THP-1 cells in culture, because the activity of p-TUA-[H] might be similarly affected by contamination with LPS (0.070 ng/mg). In contrast to p-TUA, p-TUA-[H] was completely devoid of cytokine-inducing activity in J774.1 (Fig. 10A) and THP-1 (Fig. 10B) cells in culture. These results support the conclusion that the activities of p-TUA were inherent and not attributable to contaminating LPS in the p-TUA preparations.
FIG. 10.
Inability of p-TUA-[H] to induce cytokines in J774.1 and THP-1 cells in culture. J774.1 (A) and the OCT-differentiated THP-1 (B) cells were stimulated with p-TUA, p-TUA-[H], and reference LPS at the indicated concentrations for 24 h in triplicate. The TNF-α and IL-8 levels in the culture supernatants of J774.1 and THP-1 cells, respectively, were determinated by ELISA and are expressed as means ± standard deviations. ∗∗, P < 0.01; ∗, P < 0.05 (versus medium alone). The results are representative of those from three different experiments.
DISCUSSION
In this study, we demonstrated that the cell wall TUA from the gram-positive organism M. luteus activated human monocytic THP-1 cells in a CD14- and TLR4-dependent manner and that the TUA also activated murine monocytic J774.1 cells in a CD14-dependent manner. CD14 is known to be a pattern recognition molecule and to recognize common structures on various bacterial cell surfaces (43). The TUA is a special structure of the M. luteus cell wall, and therefore this finding is clear evidence that this special structure is also recognized by CD14. TLR4 is involved mainly in recognition of gram-negative bacteria, probably through recognition of the LPS portion, while TLR2 is involved mainly in recognition of gram-positive bacteria, probably through recognition of the peptidoglycan portion (2, 23, 42). However, this is not necessarily the case, because (i) peptidoglycans, which are recognized by TLR2 (34, 40, 46), are distributed ubiquitously in both gram-positive and gram-negative bacteria, although their contents in the cell wall are high and low, respectively; (ii) another ubiquitous component, lipoprotein (or lipopeptide), which frequently coexists with LPS as endotoxin protein, is recognized by TLR2 (15, 41); and (iii) lipoteichoic acid, which is widely distributed in gram-positive bacteria, is recognized by TLR4 (40), although the converse result was also reported (34). This is the first report of a TLR4-recognized chemically defined cell surface component prepared from gram-positive bacteria. Furthermore, immunobiological activities of this bacterium are attributable mainly to TUA (25), because peptidoglycans of this bacterium lacked bioactivities, as described above. Thus, it is possible that whole cells of this gram-positive bacterium might be recognized by TLR4, not by TLR2, unlike the case for most gram-positive bacteria. Further experiments to investigate this possibility using various micrococcal cells and cell walls are in progress.
The possible unique recognition system for M. luteus might be of some advantage for the bacterium to colonize tissues by escaping from the innate immune system of the host. Hosts carrying TLR4 mutations (4) might be sensitive to infection by M. luteus as well as gram-negative bacteria. Furthermore, hosts in a state of endotoxin tolerance, where function of TLR4 is down-regulated (28), probably to avoid harmful effects of endotoxic activity, would be easily infected with M. luteus.
ACKNOWLEDGMENTS
We thank D. Mrozek (Medical English Service, Kyoto, Japan) for reviewing the paper.
This work was supported in part by Grants-in-Aid for Scientific Research (no. 10470378, 11671796, and 12470380) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Adachi Y, Satokawa C, Saeki M, Ohno N, Tamura H, Tanaka S, Yadomae T. Inhibition by a CD14 monoclonal antibody of lipopolysaccharide binding to murine macrophages. J Endotoxin Res. 1999;5:139–146. [Google Scholar]
- 2.Aderem A, Ulevitch R J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 3.Albertson D, Natsios G A, Gleckman R. Septic shock with Micrococcus luteus. Arch Intern Med. 1978;138:487–488. [PubMed] [Google Scholar]
- 4.Arbour N C, Lorenz E, Schutte B C, Zabner J, Kline J N, Jones M, Frees K, Watt J L, Schwartz D A. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 5.Dürst U N, Bruder E, Egloff L, Wust J, Schneider J, Hirzel H O. Micrococcus luteus: Ein seltener Erreger einer Klappenprothesenendokarditis. Z Kardiol. 1991;80:294–298. [PubMed] [Google Scholar]
- 6.Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc B. 1922;93:306–317. [Google Scholar]
- 7.Fosse T, Peloux Y, Granthil C, Toga B, Bertrando J, Sethian M. Meningitis due to Micrococcus luteus. Infection. 1985;13:280–281. doi: 10.1007/BF01645439. [DOI] [PubMed] [Google Scholar]
- 8.Galanos C, Lüderitz O, Westphal O. Preparation and properties of a standardized lipopolysaccharide from Salmonella abortus equi (Novo-Pyrexal) Zentbl Bakteriol Hyg Abt 1. 1979;243:226–244. [PubMed] [Google Scholar]
- 9.Ghuysen J-M, Bricas E, Lache M, Leyh-Bouille M. Structure of the cell walls of Micrococcus lysodeikticus. III. Isolation of a new peptide dimer, Nα-[l-alanyl-γ-(α-d-glutamyl-glycine)]-l-lysyl-d-alanyl-Nα-[l-alanyl-(α-d-glutamyl-glycine)]-l-lysyl-d-alanine. Biochemistry. 1968;7:1450–1460. doi: 10.1021/bi00844a030. [DOI] [PubMed] [Google Scholar]
- 10.Glupezynski Y, Lagast H, Van der Auwera P, Thys J P, Crokaert F, Yourassowsky E, Meunier-Carpentier F, Klastersky J, Kanins J P, Serruys-Schoutens E, Legrand J C. Clinical evaluation of teicoplanin for therapy of severe infections caused by gram-positive bacteria. Antimicrob Agents Chemother. 1986;29:52–57. doi: 10.1128/aac.29.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goguel A-F, Lespinats G, Nauciel C. Peptidoglycans extracted from gram-positive bacteria: expression of antitumor activity according to peptide structure and route of injection. JNCI. 1982;68:657–663. [PubMed] [Google Scholar]
- 12.Guenounou M, Goguel A-F, Nauciel C. Study of adjuvant and mitogenic activities of bacterial peptidoglycans with different structures. Ann Immunol (Paris) 1982;133:3–13. [PubMed] [Google Scholar]
- 13.Hara-Kuge S, Amano F, Nishijima M, Akamatsu Y. Isolation of a lipopolysaccharide (LPS)-resistant mutant, with defective LPS binding, of cultured macrophage-like cells. J Biol Chem. 1990;265:6606–6610. [PubMed] [Google Scholar]
- 14.Hase S, Matsushima Y. Structural studies on a glucose-containing polysaccharide obtained from cell walls of Micrococcus lysodeikticus. III. Determination of the structure. J Biochem (Tokyo) 1972;72:1117–1128. doi: 10.1093/oxfordjournals.jbchem.a129999. [DOI] [PubMed] [Google Scholar]
- 15.Hirschfeld M, Ma Y, Weis J H, Vogel S N, Weis J J. Repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 17.Kitchens R L, Munford R S. Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J Biol Chem. 1995;270:9904–9910. doi: 10.1074/jbc.270.17.9904. [DOI] [PubMed] [Google Scholar]
- 18.Kocur M, Kloos W E, Schleifer K-H. The genus Micrococcus. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. II. New York, N.Y: Springer Verlag; 1992. pp. 1300–1311. [Google Scholar]
- 19.Kotani S, Narita T, Stewart-Tull D E S, Shimono T, Watanabe Y, Kato K, Iwata S. Immunoadjuvant activities of cell walls and their water-soluble fractions prepared from various gram-positive bacteria. Biken J. 1975;18:77–92. [PubMed] [Google Scholar]
- 20.Kubodera N, Sato K, Nishii Y. Characteristics of 22-oxacalcitriol (OCT) and 2β-(3-hydroxypropoxy)-calcitriol (ED-71) In: Feldman D, Glorieux F H, Pike J W, editors. Vitamin D. San Diego, Calif: Academic Press; 1997. pp. 1071–1086. [Google Scholar]
- 21.Lawrence C, Nauciel C. Production of interleukin-12 by murine macrophages in response to bacterial peptidoglycan. Infect Immun. 1998;66:4947–4949. doi: 10.1080/00222936700770321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynn W A, Golenbock D T. Lipopolysaccharide antagonists. Immunol Today. 1992;13:271–276. doi: 10.1016/0167-5699(92)90009-V. [DOI] [PubMed] [Google Scholar]
- 23.Means T K, Golenbock D T, Fenton M J. The biology of Toll-like receptors. Cytokine Growth Factor Rev. 2000;11:219–232. doi: 10.1016/s1359-6101(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 24.Monodane T, Kawabata Y, Takada H. Micrococcus luteus cells and cell walls induce anaphylactoid reactions accompanied by early death and serum cytokines in mice primed with muramyl dipeptide. FEMS Immunol Med Microbiol. 1997;17:49–55. doi: 10.1111/j.1574-695X.1997.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 25.Monodane T, Kawabata Y, Yang S, Hase S, Takada H. Induction of tumour necrosis factor-α and interleukin-6 in mice in vivo and in murine peritoneal macrophages and human whole blood cells in vitro by Micrococcus luteus teichuronic acids. J Med Microbiol. 2001;50:4–12. doi: 10.1099/0022-1317-50-1-4. [DOI] [PubMed] [Google Scholar]
- 26.Nasir-ud-Din R W, Jeanloz The chemical structure of a fragment of Micrococcus lysodeikticus cell-wall. Carbohydr Res. 1976;47:245–260. doi: 10.1016/s0008-6215(00)84190-7. [DOI] [PubMed] [Google Scholar]
- 27.Nishijima M, Hara-Kuge S, Takasuka N, Akagawa K, Setouchi M, Matsuura K, Yamamoto S, Akamatsu Y. Identification of a biochemical lesion, and characteristic response to lipopolysaccharide (LPS) of a cultured macrophage-like cell mutant with defective LPS-binding. J Biochem (Tokyo) 1994;116:1082–1087. doi: 10.1093/oxfordjournals.jbchem.a124631. [DOI] [PubMed] [Google Scholar]
- 28.Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J Immunol. 2000;164:3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 29.Peces R, Gago E, Tejada F, Laures A S, Alvarez-Grande J. Relapsing bacteremia due to Micrococcus luteus in a haemodialysis patient with a Perm-Cath catheter. Nephrol Dial Transplant. 1997;12:2428–2429. doi: 10.1093/ndt/12.11.2428. [DOI] [PubMed] [Google Scholar]
- 30.Perkins H R. A polymer containing glucose and aminohexuronic acid isolated from the cell walls of Micrococcus lysodeikticus. Biochem J. 1963;86:475–483. doi: 10.1042/bj0860475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poltorak A, He X, Smirnova I, Liu M-Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi S T, Larivière L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schleifer K H, Kandler O. Micrococcus lysodeikticus: a new type of cross-linkage of murein. Biochem Biophys Res Commun. 1967;28:965–972. doi: 10.1016/0006-291x(67)90074-5. [DOI] [PubMed] [Google Scholar]
- 34.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 35.Seifert H, Kaltheuner M, Perdreau-Remington F. Micrococcus luteus endocarditis: case report and review of the literature. Int J Med Microbiol. 1995;282:431–435. doi: 10.1016/s0934-8840(11)80715-2. [DOI] [PubMed] [Google Scholar]
- 36.Selladurai B M, Sivakumaran S, Aiyar S, Mohamad A R. Intracranial suppuration caused by Micrococcus luteus. Br J Neurosurg. 1993;7:205–208. doi: 10.3109/02688699309103481. [DOI] [PubMed] [Google Scholar]
- 37.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souhami L, Feld R, Tuffnell P G, Feller T. Micrococcus luteus pneumonia: a case report and review of the literature. Med Pediatr Oncol. 1979;7:309–314. doi: 10.1002/mpo.2950070404. [DOI] [PubMed] [Google Scholar]
- 39.Sultzer B M. Genetic control of leucocyte responses to endotoxin. Nature. 1968;219:1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 41.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Mühlradt P F, Akira S. Preferentially the S-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 42.Ulevitch R J. Toll gates for pathogen selection. Nature. 1999;401:755–756. doi: 10.1038/44490. [DOI] [PubMed] [Google Scholar]
- 43.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 44.Von Eiff C, Kuhn N, Herrmann M, Weber S, Peters G. Micrococcus luteus as a cause of recurrent bacteremia. Pediatr Infect Dis J. 1996;15:711–713. doi: 10.1097/00006454-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Wharton M, Rice J R, McCallum R, Gallis H A. Septic arthritis due to Micrococcus luteus. J Rheumatol. 1986;13:659–660. [PubMed] [Google Scholar]
- 45a.Yang S, Tamai R, Akashi S, Takeuchi O, Akira S, Sugawara S, Takada H. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect Immun. 2001;69:2045–2053. doi: 10.1128/IAI.69.4.2045-2053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshimura A, Lien E, Ingalls R R, Tuomanen E, Dziarski R, Golenbock D. Recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]