Introduction
Neuregulin-1 (NRG1) fusions are rare oncogenic driver alterations found in 0.2%-0.5% of solid tumors.1,2 In an analysis of approximately 22,000 solid tumors, 70% of tumor types harboring NRG1 fusions were adenocarcinomas.1 The frequency of NRG1 fusions identified in the pancreatic cancer subset was 0.5%1; however, other studies suggest enrichment (6%) in KRAS wild-type (wt) pancreatic ductal adenocarcinoma (PDAC). Most NRG1 fusion partners contain a transmembrane domain and sequester high levels of the NRG1 ligand with an intact epidermal growth factor receptor–like tyrosine kinase–binding domain near its primary receptor, the human epidermal growth factor receptor (HER)3/ERBB3.3,4 This results in overactivation of HER3 and downstream signaling pathways including the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) and mitogen-activated protein kinase (MAPK) pathways.2 In patient-derived xenograft (PDX) models harboring an NRG1 fusion, inhibition of ligand-dependent activation of HER3 by seribantumab resulted in blockade of the HER family and downstream signaling, producing tumor regression.5 Tumors harboring NRG1 fusions do not respond well to currently available therapies.1,2,6-10
Outcomes with PDAC are dismal with high mortality rates and a 5-year survival rate of < 10%.11-14 Chemotherapy with gemcitabine-based regimens or folinic acid, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) is recommended for patients with advanced PDAC.15-17 However, these regimens have shown limited efficacy18,19 and are often associated with toxicities that negatively affect patients' quality of life.11,19,20
The Cancer Molecular Screening and Therapeutics (MoST) program is an Australian precision oncology program using DNA- and RNA-based sequencing to identify targetable genomic alterations in treatment-refractory advanced cancers.21,22
Seribantumab is a fully human anti-HER3 IgG2 monoclonal antibody that blocks the ligand-dependent activation of HER3 and HER3-HER2 dimerization.5 This leads to reduced phosphorylation across the ERBB family and inhibition of the PI3K/AKT and MAPK downstream signaling pathways, resulting in tumor regression in preclinical models harboring an NRG1 fusion.5,23 Seribantumab dose-dependently inhibited phosphorylation of key signaling pathways in human pancreatic ductal epithelial cells expressing an ATP1B1-NRG1 fusion and inhibited tumor growth in a PDX model of PDAC harboring an APP-NRG1 fusion.24 Although preclinical data5,24 and case reports8,25-27 have previously described NRG1 fusions as a biomarker for other HER2-/HER3-directed therapies and there is active clinical investigation with seribantumab in this molecular subset,28 to our knowledge, this case report is the first characterization of seribantumab's clinical activity in an Australian patient with an ATP1B1-NRG1 fusion, treated through compassionate access.
Case Report
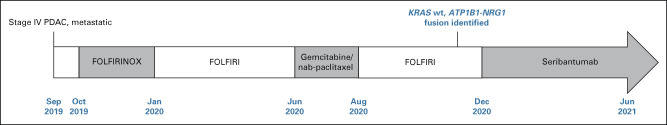
A 38-year-old woman was diagnosed with stage IV PDAC with metastases to the liver and peripancreatic lymph nodes in 2019. She presented with biliary obstruction, requiring biliary stenting. The patient received first-line treatment with FOLFIRINOX from October 2019 until January 2020 when she developed acute gangrenous cholecystitis, managed with cholecystectomy. Upon recommencement of the same regimen, she developed multiple allergic reactions to oxaliplatin requiring omission. The patient continued on folinic acid, fluorouracil, and irinotecan (FOLFIRI) with intermittent delays in treatment because of cumulative toxicities until June 2020. The patient then received gemcitabine/nab-paclitaxel as second-line treatment from June to August 2020, when she developed progressive disease in the liver. FOLFIRI was reinitiated with multiple dose interruptions because of myelotoxicity.
In November 2020, comprehensive genomic profiling through the MoST program (Fig 1) revealed an ATP1B1-NRG1 fusion in the RNA component joining ATP1B1 (exon 2) to NRG1 (exon 2). This fusion retains the immunoglobulin (Ig) domain of NRG1 and all downstream domains including the epidermal growth factor–like domain, as previously described.29
FIG 1.
Clinical course of a patient with PDAC harboring an ATP1B1-NRG1 fusion. ATP1B1, ATPase Na+/K+ transporting subunit beta 1; FOLFIRI, fluorouracil, leucovorin, and irinotecan; FOLFIRINOX, folinic acid, fluorouracil, irinotecan, and oxaliplatin; KRAS, Kirsten rat sarcoma; NRG1, neuregulin-1; PDAC, pancreatic ductal adenocarcinoma; wt, wild-type.
A gain-of-function frameshift mutation in the oncogene JUN was the only other pathogenic genomic alteration identified. KRAS mutations were absent in this tumor (KRAS wt), consistent with mutual exclusivity of NRG1 fusions and other oncogenic drivers.30
On the basis of emerging preclinical data for seribantumab and clinical case reports of effective targeting of ATP1B1-NRG1 with anti–HER2-/HER3-directed therapies,27,31-33 access to seribantumab was pursued, requiring a collaborative effort with Elevation Oncology Inc to provide seribantumab compassionately through the Special Access Scheme (SAS) program. SAS allows patients to access treatments that are not included on the Australian Register of Therapeutic Goods34; with seribantumab only under clinical investigation in the United States at that time, and no appropriate clinical trials available in Australia. Approval to administer seribantumab required evidence from clinical case reports demonstrating effective targeting of NRG1 fusions with comparable agents in patients with pancreatic cancer (Table 1).25,32,33,35 In December 2020, the patient began seribantumab therapy.
TABLE 1.
Previous Studies/Case Reports Evaluating HER-Targeted Therapies in Patients With Pancreatic Cancer Harboring NRG1 Fusions
Clinical response was observed within the first week of treatment; carbohydrate antigen 19-9 (CA 19-9) decreased from 1,143 IU/mL to 818 IU/mL. Initial tumor assessment on seribantumab therapy revealed a 14% tumor reduction in target liver lesions (Fig 2). After 3 months of treatment, CA 19-9 levels reached a nadir of 60 IU/mL and, at 4 months, imaging demonstrated a partial response per Response Evaluation Criteria in Solid Tumors version 1.1, with a 39% reduction in target lesions (Fig 2). There was a gradual increase in CA 19-9 level to 85 IU/mL.
FIG 2.
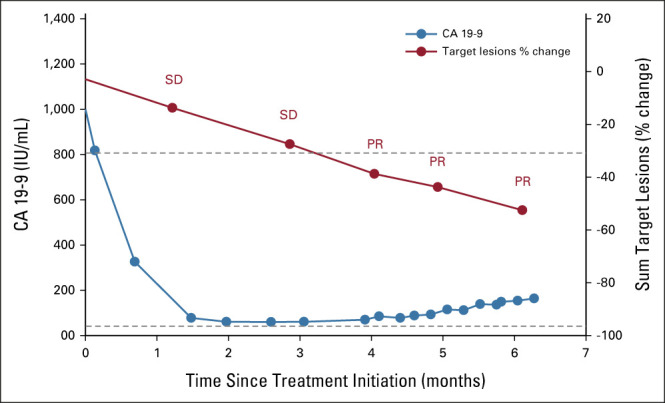
Seribantumab clinical activity in a patient with KRAS wt PDAC harboring an ATP1B1-NRG1 fusion. Plot of CA19-9 tumor marker (blue line) and sum of target lesions (red line). Dot represents time point of measurement. ATP1B1, ATPase Na+/K+ transporting subunit beta 1; CA 19-9, carbohydrate antigen 19-9; KRAS, Kirsten rat sarcoma; NRG1, neuregulin-1; PDAC, pancreatic ductal adenocarcinoma; PR, partial response; SD, stable disease; wt, wild-type.
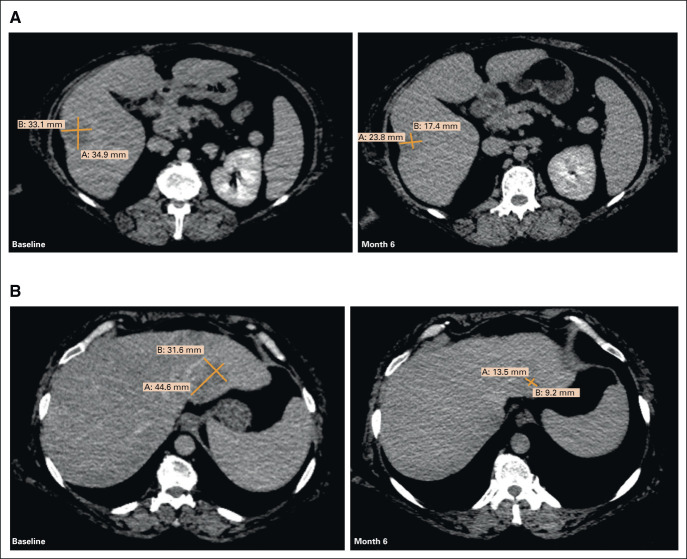
The patient initiated treatment with seribantumab following the dosing schema explored in the ongoing CRESTONE study consisting of a loading dose of seribantumab 3,000 mg administered as a single 1-hour intravenous (IV) infusion once on week 1, followed by 2,000 mg, 1-hour IV infusion once weekly (week 2-4), and then maintenance dosing consisting of a fixed dose of 3,000 mg IV once every 2 weeks. Given the favorable safety and tolerability profile of seribantumab and to align with the updated dosing regimen of the CRESTONE study, in April 2021, the patient was transitioned to seribantumab 3,000 mg IV once weekly. At the June 11, 2021, data cutoff, imaging showed further tumor shrinkage with a 53% reduction in the target lesions within the liver (Figs 2 and 3), reflecting disease control of 6 months and a duration of response of 2 months, which is ongoing.
FIG 3.
Confirmed partial response in a patient with KRAS wt PDAC harboring an ATP1B1-NRG1 fusion: (A) target lesion 1 and (B) target lesion 2, at baseline and after 6 months on treatment with seribantumab. ATP1B1, ATPase Na+/K+ transporting subunit beta 1; KRAS, Kirsten rat sarcoma; NRG1, neuregulin-1; PDAC, pancreatic ductal adenocarcinoma; wt, wild-type.
Seribantumab was generally well tolerated. She reported improved energy levels, which allowed resumption of social activities and hobbies. During treatment with seribantumab, the patient reported intermittent grade 1/2 diarrhea, managed with loperamide. Diarrhea may have been confounded by a new diagnosis of type II diabetes requiring initiation of metformin before commencing seribantumab treatment and metformin uptitration during seribantumab therapy. The patient experienced a flare of her pre-existing seasonal fungal rash over the axilla, breast, and umbilicus, which resolved with topical antifungal treatment. This was the presumed cause for the reactive axillary lymph node enlargement seen on imaging. Six months after starting seribantumab, the patient experienced a grade 2 rise in ALT and grade 1 rise in AST. This was in the context of two courses of oral antibiotics for paronychia of the big toe. As a precautionary measure, the seribantumab dose was reduced by 25% (2,250 mg for one dose for 1 week) followed by a 1-week treatment delay. ALT and AST levels returned to baseline, and the patient resumed seribantumab treatment at full dose once weekly. There were no other adverse events.
The authors obtained informed consent to publish the report and clinical images from the patient.
Patient Consent
The authors obtained informed consent to publish the report and clinical images from the patient. This case study treatment falls under research exemption policies and thus, no ethics committee/institutional review board approval was required.
Discussion
This case report demonstrates proof of clinical activity of seribantumab in a patient with a treatment-refractory, metastatic KRAS wt PDAC harboring an ATP1B1-NRG1 fusion.1,33,36 The patient was treated with seribantumab under the SAS program following the dosing schema used in the CRESTONE study. When the study was amended to evaluate a weekly dosing regimen, the patient transitioned to the new dosing frequency, which may have contributed to continued clinical benefit. After 6 months of treatment, the patient's tumor continues to show radiologic response and good tolerability with only grade 1/2 adverse events, even on a weekly dosing regimen. This was consistent with the previously established safety profile of seribantumab monotherapy and combination therapy.37 The patient also reported improved energy levels and had resumed some of her normal activities, suggesting improvements in quality of life, which is remarkable for a patient with treatment-refractory advanced cancer.38,39
In this case study, standard-of-care therapies had been exhausted before the patient's tumor was sequenced through the MoST program. The patient benefited from biomarker-based therapy with seribantumab on the basis of the NRG1 fusion, highlighting the importance of comprehensive genomic profiling at diagnosis, including an RNA component that is particularly crucial for detecting gene fusions.40-42 Earlier testing provides an opportunity to ensure appropriate targeted treatments are initiated without delay.42-44
Although only 7%-10% of pancreatic cancers are KRAS wt, this is more common in patients younger than 50 years.36 This case study provides rationale for further evaluation of HER3-directed therapies such as seribantumab in these patient subsets, with the added advantage of less toxicities compared with standard therapies.2,11,19,20
Several studies are underway to assess the efficacy of HER3-targeting therapies in tumors harboring NRG1 fusions.2,28,45,46 The phase II CRESTONE (ClinicalTrials.gov identifier: NCT04383210) study of seribantumab is enrolling patients with solid tumors harboring an NRG1 fusion. The confirmed overall response rate (ORR) across tumor types was 33% including two complete responses and a disease control rate of 92%.47 Similarly, zenocutuzumab, a HER2/HER3 bispecific antibody, demonstrated an ORR of 34% across tumor types and 42% in PDACs, with no complete responses and 76% of patients maintaining an objective response at 6 months.35 The CRESTONE study is enrolling patients in Australia in collaboration with the University of Sydney and Omico through MoST CRESTONE.
ACKNOWLEDGMENT
The authors would like to thank the patient, her family, and all investigators and staff involved in this study. Medical writing support was provided by Shavonn Harper, PhD, and editorial support was provided by Michelle Seddon, DipPsych, all of Paragon Knutsford, UK, supported by Elevation Oncology, Inc., according to Good Publication Practice guidelines (Link). The patient was treated through the Australian SAS program; seribantumab was provided by Elevation Oncology, Inc.
Subotheni Thavaneswaran
Research Funding: Roche/Genentech (Inst), AstraZeneca (Inst), Elevation Oncology (Inst)
Maegan Deegan
Employment: Elevation Oncology, Biohaven Pharmaceuticals
Stock and Other Ownership Interests: Biohaven Pharmaceuticals
Travel, Accommodations, Expenses: Elevation Oncology
Valerie M. Jansen
Employment: Mersana, Elevation Oncology
Stock and Other Ownership Interests: Elevation Oncology
David M. Thomas
Employment: Australian Unity
Honoraria: Roche
Research Funding: Pfizer, Amgen, AstraZeneca, Elevation Oncology, Roche, Bayer, Microba (Inst), Seattle Genetics (Inst), Sun Pharma (Inst), Lilly (Inst), George Clinical (Inst), InterVenn Biosciences
Travel, Accommodations, Expenses: Amgen
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Data from this case study patient were presented at the AGITG 2021 virtual meeting.
SUPPORT
Supported by Elevation Oncology, Inc.
AUTHOR CONTRIBUTIONS
Conception and design: Subotheni Thavaneswaran, Ray Asghari, Valerie M. Jansen, David M. Thomas
Financial support: David M. Thomas
Provision of study materials or patients: Subotheni Thavaneswaran, Ray Asghari
Collection and assembly of data: Subotheni Thavaneswaran, Wei Yen Chan, John P Grady, David M. Thomas
Data analysis and interpretation: Subotheni Thavaneswaran, Wei Yen Chan, John P Grady, Maegan Deegan, Valerie M. Jansen, David M. Thomas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Subotheni Thavaneswaran
Research Funding: Roche/Genentech (Inst), AstraZeneca (Inst), Elevation Oncology (Inst)
Maegan Deegan
Employment: Elevation Oncology, Biohaven Pharmaceuticals
Stock and Other Ownership Interests: Biohaven Pharmaceuticals
Travel, Accommodations, Expenses: Elevation Oncology
Valerie M. Jansen
Employment: Mersana, Elevation Oncology
Stock and Other Ownership Interests: Elevation Oncology
David M. Thomas
Employment: Australian Unity
Honoraria: Roche
Research Funding: Pfizer, Amgen, AstraZeneca, Elevation Oncology, Roche, Bayer, Microba (Inst), Seattle Genetics (Inst), Sun Pharma (Inst), Lilly (Inst), George Clinical (Inst), InterVenn Biosciences
Travel, Accommodations, Expenses: Amgen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Jonna S, Feldman RA, Swensen J, et al. : Detection of NRG1 gene fusions in solid tumors. Clin Cancer Res 25:4966-4972, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laskin J, Liu SV, Tolba K, et al. : NRG1 fusion-driven tumors: Biology, detection, and the therapeutic role of afatinib and other ErbB-targeting agents. Ann Oncol 31:1693-1703, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Cuesta L, Thomas RK: Molecular pathways: Targeting NRG1 fusions in lung cancer. Clin Cancer Res 21:1989-1994, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Dimou A, Camidge DR: Detection of NRG1 fusions in solid tumors: Rare gold? Clin Cancer Res 25:4865-4867, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Odintsov I, Lui AJW, Sisso WJ, et al. : The anti-HER3 mAb seribantumab effectively inhibits growth of patient-derived and isogenic cell line and xenograft models with oncogenic NRG1 fusions. Clin Cancer Res 27:3154-3166, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang JC, Offin M, Falcon CJ, et al. : Comprehensive molecular and clinicopathologic analysis of 200 pulmonary invasive mucinous adenocarcinomas identifies distinct characteristics of molecular subtypes. Clin Cancer Res 27:4066-4076, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drilon A, Duruisseaux M, Han J-Y, et al. : Clinicopathologic features and response to therapy of NRG1 fusion–driven lung cancers: The eNRGy1 global multicenter registry. J Clin Oncol 39:2791-2802, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drilon A, Somwar R, Mangatt BP, et al. : Response to ERBB3-directed targeted therapy in NRG1-rearranged cancers. Cancer Discov 8:686-695, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin DH, Lee D, Wan Hong D, et al. : Oncogenic function and clinical implications of SLC3A2-NRG1 fusion in invasive mucinous adenocarcinoma of the lung. Oncotarget 7:69450-69465, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stalbovskaya V, Wasserman E, Fryzek J, et al. : NRG1 fusion-driven cancers: A systematic literature review and meta-analysis. J Clin Oncol 38, 2020. (suppl 15; abstr e15605) [Google Scholar]
- 11.Mashayekhi V, Mocellin O, Fens MH, et al. : Targeting of promising transmembrane proteins for diagnosis and treatment of pancreatic ductal adenocarcinoma. Theranostics 11:9022-9037, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2021. CA Cancer J Clin 71:7-33, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Orth M, Metzger P, Gerum S, et al. : Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat Oncol 14:141, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh RR, O'Reilly EM: New treatment strategies for metastatic pancreatic ductal adenocarcinoma. Drugs 80:647-669, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohal DPS, Kennedy EB, Khorana A, et al. : Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol 36:2545-2556, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NCCN : NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®)—Pancreatic Adenocarcinoma, 2020. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf [Google Scholar]
- 18.Tiwari A, Kumar L: Pancreatic ductal adenocarcinoma: Role of chemotherapy & future perspectives. Indian J Med Res 148:254-257, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conroy T, Desseigne F, Ychou M, et al. : FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817-1825, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Von Hoff DD, Ervin T, Arena FP, et al. : Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691-1703, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thavaneswaran S, Sebastian L, Ballinger M, et al. : Cancer Molecular Screening and Therapeutics (MoST): A framework for multiple, parallel signal-seeking studies of targeted therapies for rare and neglected cancers. Med J Aust 209:354-355, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Chan WY, Thavaneswaran S, Lin F, et al. : Comprehensive Genomic Profiling Reveals Novel Opportunities for Treatment-Refractory Gastrointestinal Cancers. Presented at the AGITG, Virtual, 2021
- 23.Schoeberl B, Faber AC, Li D, et al. : An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res 70:2485-2494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odintsov I, Kurth RI, Lui AJW, et al. : Preclinical activity of seribantumab in gastrointestinal cancers with NRG1 fusions. Presented at the AACR Annual Meeting 2021, Virtual, April 10-15, 2021, 2021 (abstr 935)
- 25.Gan HK, Millward M, Jalving M, et al. : A phase I, first-in-human study of GSK2849330, an anti-HER3 monoclonal antibody, in HER3-expressing solid tumors. Oncologist 26:e1844-e1853, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schram A, O'Reilly E, O'Kane G, et al. : Efficacy and safety of zenocutuzumab in advanced pancreatic cancer and other solid tumors harboring NRG1 fusions. J Clin Oncol 39, 2021. (suppl 15; abstr 3003) [Google Scholar]
- 27.Schram AM, Odintsov I, Espinosa-Cotton M, et al. : Zenocutuzumab, a HER2 x HER3 bispecific antibody, is effective therapy for tumors driven by NRG1 gene rearrangements. Cancer Discov 12:1233-1247, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov : Study of Seribantumab in Adult Patients with NRG1 Gene Fusion Positive Advanced Solid Tumors [NCT04383210], 2020. https://clinicaltrials.gov/ct2/show/NCT04383210?term=seribantumab&draw=2&rank=9 [Google Scholar]
- 29.Jonna S, Feldman RA, Ou S-HI, et al. : Characterization of NRG1 gene fusion events in solid tumors. J Clin Oncol 38, 2020. (suppl 15; abstr 3113) [Google Scholar]
- 30.Nakaoku T, Tsuta K, Ichikawa H, et al. : Druggable oncogene fusions in invasive mucinous lung adenocarcinoma. Clin Cancer Res 20:3087-3093, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones MR, Lim H, Shen Y, et al. : Successful targeting of the NRG1 pathway indicates novel treatment strategy for metastatic cancer. Ann Oncol 28:3092-3097, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Heining C, Horak P, Uhrig S, et al. : NRG1 fusions in KRAS wild-type pancreatic cancer. Cancer Discov 8:1087-1095, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Jones MR, Williamson LM, Topham JT, et al. : NRG1 gene fusions are recurrent, clinically actionable gene rearrangements in KRAS wild-type pancreatic ductal adenocarcinoma. Clin Cancer Res 25:4674-4681, 2019 [DOI] [PubMed] [Google Scholar]
- 34.Australian Government/Department of Health/Therapeutic Goods Administration: Special Access Scheme. 2021. https://www.tga.gov.au/form/special-access-scheme [Google Scholar]
- 35.Schram AM, Goto K, Kim DW, et al. : Efficacy and Safety of Zenocutuzumab, a HER2 X HER3 Bispecific Antibody, across Advanced NRG1 Fusion (NRG1+) Cancers. Presented at the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2022
- 36.Lee MS, Pant S: Personalizing medicine with germline and somatic sequencing in advanced pancreatic cancer: Current treatments and novel opportunities. Am Soc Clin Oncol Ed Book 41:1-13, 2021 [DOI] [PubMed] [Google Scholar]
- 37.Denlinger CS, Keedy VL, Moyo V, et al. : Phase 1 dose escalation study of seribantumab (MM-121), an anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Invest New Drugs 39:1604-1612, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hubner RA, Cubillo A, Blanc J-F, et al. : Quality of life in metastatic pancreatic cancer patients receiving liposomal irinotecan plus 5-fluorouracil and leucovorin. Eur J Cancer 106:24-33, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Marschner N, Zacharias S, Lordick F, et al. : Association of disease progression with health-related quality of life among adults with breast, lung, pancreatic, and colorectal cancer. JAMA Netw Open 3:e200643, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heyer EE, Blackburn J: Sequencing strategies for fusion gene detection. Bioessays 42:e2000016, 2020 [DOI] [PubMed] [Google Scholar]
- 41.Solomon JP, Linkov I, Rosado A, et al. : NTRK fusion detection across multiple assays and 33,997 cases: Diagnostic implications and pitfalls. Mod Pathol 33:38-46, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colomer R, Mondejar R, Romero-Laorden N, et al. : When should we order a next generation sequencing test in a patient with cancer? EClinicalMedicine 25:100487, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garinet S, Laurent-Puig P, Blons H, et al. : Current and future molecular testing in NSCLC, what can we expect from new sequencing technologies? J Clin Med 7:144, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krzyszczyk P, Acevedo A, Davidoff EJ, et al. : The growing role of precision and personalized medicine for cancer treatment. Technology 06:79-100, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ClinicalTrials.gov : HMBD-001 in Advanced HER3 Positive Solid Tumours [NCT05057013], 2021. https://clinicaltrials.gov/ct2/show/NCT05057013?cond=NRG1+fusion&draw=2&rank=8 [Google Scholar]
- 46.ClinicalTrials.gov : A study of zenocutuzumab (MCLA-128) in patients with solid tumors harboring an NRG1 fusion [NCT02912949]. 2016. https://clinicaltrials.gov/ct2/show/NCT02912949?cond=NRG1+fusion&draw=2&rank=1
- 47.Carrizosa DR, Burkard ME, Elamin YY, et al. : CRESTONE: Inital efficacy and safety of seribantumab in solid tumors harboring NRG1 fusions. Presented at the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2022