Abstract
Background and study aims Concomitant hiatal hernia (HH) repair with transoral incisionless fundoplication (TIF) is a therapeutic option for patients with HH > 2 cm and gastroesophageal reflux disease (GERD). Data comparing this approach with laparoscopic Nissen fundoplication (LNF) are lacking. We performed an exploratory analysis to compare these two approaches' adverse events (AEs) and clinical outcomes.
Patients and methods This was a multicenter retrospective cohort study of HH repair followed by LNF versus HH repair followed by TIF in patients with GERD and moderate HH (2–5 cm). AEs were assessed using the Clavien-Dindo classification. Symptoms (heartburn/regurgitation, bloating, and dysphagia) were compared at 6 and 12 months.
Results A total of 125 patients with HH repair with TIF and 70 with HH repair with LNF were compared. There was no difference in rates of discontinuing or decreasing proton pump inhibitor use, dysphagia, esophagitis, disrupted wrap, and HH recurrence between the two groups ( P > 0.05). The length of hospital stay (1 day vs. 2 days), 30-day readmission rate (0 vs. 4.3 %), early AE rate (0 vs. 18.6 %), and early serious AE rate (0 vs. 4.3 %) favored TIF (all P < 0.05). The rate of new or worse than baseline bloating was lower in the TIF group at 6 months (13.8 % vs. 30.0 %, P = 0.009).
Conclusions Concomitant HH repair with TIF is feasible and associated with lower early and serious AEs compared to LNF. Further comparative efficacy studies are warranted.
Introduction
Gastroesophageal reflux disease (GERD) is a common chronic disease, affecting approximately 8.8 % to 27.8 % of the Western population 1 . Although this condition is non-malignant, untreated GERD can significantly impair quality of life (QoL) and potentially lead to Barrett’s esophagus (BE) and esophageal adenocarcinoma 2 3 . Proton pump inhibitors (PPIs) are the mainstay of treatment; however, medical treatment frequently requires a long-term commitment and is associated with a substantial cost burden 4 . Several associated risks have been linked to chronic PPI use 5 6 . Despite taking standard-dose PPIs, up to 45 % continued to experience persistent reflux symptoms 7 , and one-third continued to have abnormal acid exposure 8 . In those who achieved an initial response, up to 35 % later experienced a relapse requiring a higher PPI dose or ultimately required anti-reflux surgery 9 .
Anti-reflux surgery has proven effective in managing GERD with a randomized trial indicating over 85 % of patients experiencing “good” satisfaction without further need of medical therapy at 5 years 10 . Long-term studies have also demonstrated the superior efficacy of anti-reflux surgery to medical therapy in patient satisfaction, clinical outcomes, and healthcare costs 11 12 13 . However, this is to be weighed against the side effects, mainly gas-bloat syndrome and dysphagia reportedly occurring in 31.2 % and 12.6 % of patients, respectively, from a meta-analysis of randomized controlled trials (RCTs) 14 . Transoral incisionless fundoplication (TIF) is an endoscopic intervention to restore the position of the distal esophagus to a subdiaphragmatic intragastric position providing a high-pressure zone functionally and anatomically similar to the surgical fundoplication, but with minimal anatomical alterations of the gastroesophageal junction, the fundus, and diaphragmatic hiatus 15 . This procedure provided a safe and sustained relief of reflux symptoms with no serious adverse outcomes based on a 5-year study 16 . A meta-analysis of three RCTs of 233 refractory GERD patients also demonstrated a significant reduction in PPI use, improvement in esophagitis, esophageal pH, and QoL at 3 years after TIF compared to sham or PPI therapy 17 .
Hiatal hernia (HH) frequently coexists with GERD and further facilitates GERD development by compromising the anti-reflux barrier 18 . The relationships between HH severity and reflux symptoms, esophagitis, and BE are well-established 19 20 21 . GERD patients with HH larger than 2 cm traditionally undergo laparoscopic HH repair, followed by anti-reflux surgery if clinically warranted. Similarly, while TIF alone is adequate for HH < 2 cm, in cases with larger HH, TIF can be combined with cruroplasty. This combined approach of HH and TIF has demonstrated safety and efficacy in patients with moderate HH 3 cm or larger 22 . Another retrospective study of 46 patients who underwent concomitant HH repair and TIF found significant GERD-HRQL score improvement at a mean follow-up of 14.5 months, with only minor complications observed 23 . Another study of 99 GERD patients with HH 2 to 5 cm undergoing this combined approach showed a considerable improvement in reflux symptoms with no long-term bloating or dysphagia, which are well-known side effects after LNF at 12 months 24 . A recent retrospective study of 33 patients also demonstrated that over 80 % of patients were off PPI, and over 90 % of patients achieved greater than 75 % satisfaction after this combined approach at a median follow-up of 9 months 25 . The U.S. Food and Drug Administration expanded the indication for TIF to use in patients with HH larger than 2 cm in whom laparoscopic HH repair is performed. However, data comparing this approach with conventional LNF are lacking. Therefore, we performed an exploratory analysis to compare clinical outcomes and adverse events (AEs) between HH repair with TIF and HH repair with LNF.
Patients and methods
This was a US multicenter comparative study of HH repair with TIF versus HH repair with LNF. The TIF cohort was derived from one published retrospective study of one center (San Angelo Medical Center, Texas) and one previously published prospective study of two centers (Affinity Health Systems and Methodist Hospitals, Indiana Wisconsin) 24 26 . The LNF cohort was from the Mayo Clinic (Rochester, Minnesota).
Eligible subjects were adults aged 18 to 75 years with chronic GERD (at least 6 months) and HH between 2 and 5 cm in length. The TIF cohort was accrued from January 2014 to December 2017. The LNF cohort was accrued from January 2001 to December 2018. Exclusion criteria included patients with severe esophagitis with Los Angeles classification grade C or D, BE, prior esophageal surgery, major esophageal motility disorders or gastroparesis. Fundoplication was performed according to the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) guidelines 27 in patients who have failed medical management due to inadequate symptom control, developing GERD-related complications including BE, developing drug-related side effects, or patients who opt for surgery despite successful medical management due to QoL considerations, lifelong need for medication, or costs. GERD was diagnosed based on symptoms and endoscopy with an adjunct of an esophageal pH study if clinically indicated. HH size was measured on upper endoscopy and/or esophagram. Subjects who had an open surgical approach, prior anti-reflux surgery, a follow-up of fewer than 6 months, or insufficient medical records were excluded. Patient baseline characteristics, symptoms, and endoscopic findings were collected from electronic medical record systems.
This study was approved by the Mayo Clinic Institutional Review Board (IRB 19-008262) and was conducted in accordance with the Declaration of Helsinki.
Hiatal hernia repair and TIF
All patients underwent the procedure under general anesthesia. In the TIF cohort, first, hernia repair was performed laparoscopically via four ports with an optional fifth port to facilitate retraction and exposure. A hiatal dissection was performed until 2 to 3 cm of the intraabdominal tension-free esophagus was visualized. Both vagus nerves were identified and preserved throughout the procedure. The hiatal defect was then repaired using interrupted posterior sutures to reapproximate the crura of the esophagus with a bougie ranging from 48F to 54F based on the patient size, mostly using 50F bougie or endoscope in place to prevent esophageal constriction. The abdomen was then closed. Patients were then placed in the partial left lateral decubitus position by tilting the operating table for the TIF procedure. The TIF procedure was performed using the TIF 2.0 iteration with the EsophyX device (EndoGastric Solutions, Redmond, Washington, United States) described by Bell and Cadière 29 and first published by Jobe, et al 30 . The valve was created approximately 270 degrees around the esophagus. All TIF procedures were performed by experienced endoscopists. Fig. 1 shows the images of preoperative and postoperative endoscopy and esophagram.
Fig. 1 a.
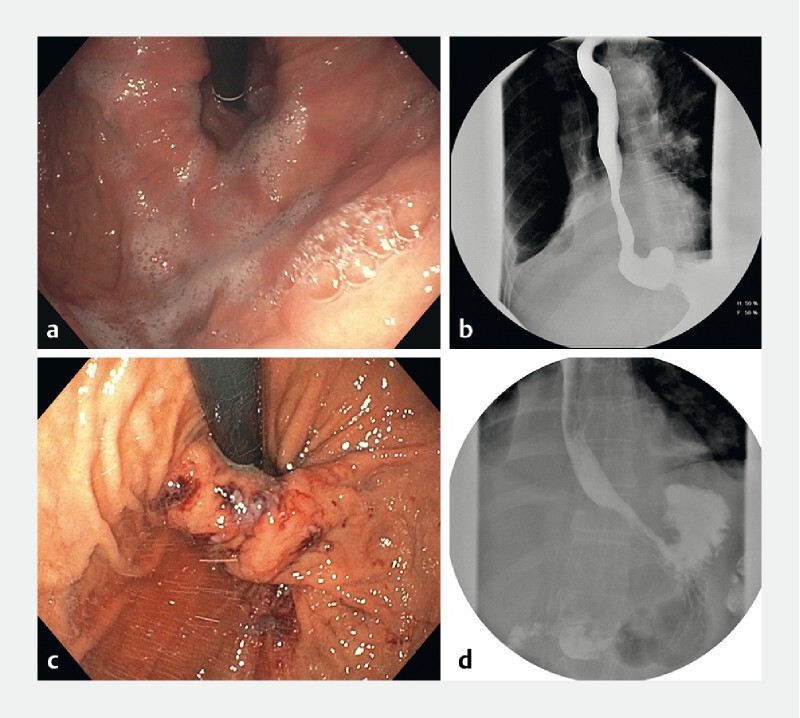
Preoperative endoscopy demonstrating Hill grade IV. b Preoperative esophagram demonstrating hiatal hernia. c Postoperative endoscopy demonstrating a successful creation of the gastroesophageal flap valve. d Postoperative esophagram showing 3-cm high-pressure zone with an absence of hiatal hernia.
Hiatal hernia repair and Nissen fundoplication
In the LNF cohort, patients first underwent laparoscopic HH repair with techniques similar to those used in the TIF cohort. Following this, Nissen fundoplication was done by creating 360-degree wrapping of the fundus around the distal, subdiaphragmatic, and intraabdominal esophagus. All patients were monitored in the hospital overnight after the procedure and discharged if the pain was adequately controlled with no signs of infection, with adequate oral intake.
Follow-up and outcome assessment
After surgery, a multidisciplinary team routinely follows patients, including the thoracic surgeon and gastroenterologist, at progressive intervals. Postsurgical outcomes, including the length of hospital stay, 30-day readmission, 1-year mortality, and AEs, were reviewed. The primary endpoint was the incidence of early (< 30 days) and late (30 days to 12 months) AEs, as assessed using the Clavien-Dindo classification 31 , in which severe AEs were defined as grade III-V. AEs were defined as any untoward medical occurrence that presented after treatment. Secondary endpoints included postoperative symptoms, including reflux symptoms (heartburn/ acid regurgitation), bloating, and dysphagia assessed using the GERD Health-Related Quality of Life questionnaire (GERD-HRQL) in the TIF cohort involving 11 heartburn/regurgitation questions, two dysphagia questions, and one bloating question with a score of 0 to 5 in each item at 6 and 12 months after surgery and by a chart review in the LNF cohort. Improvement in reflux symptoms was defined as at least 1-point decrease in the GERD-HRQL questionnaire of combined reflux and heartburn questions in the TIF cohort. New or worse than baseline of bloating and dysphagia were defined as at least 1-point increase in the GERD-HRQL questionnaire. In the LNF group, symptoms at 6 and 12 months were assessed by reviewing the medical records to determine any new or worsening symptoms of acid regurgitation, heartburn, dysphagia, and bloating. The rates of discontinued and decreased use of PPI were also assessed.
PPIs were routinely continued for 2 to 4 weeks postoperatively and then titrated off based on patient symptoms. Patients routinely underwent an upper endoscopy before the procedure to assess the grading of HH and esophagitis. Routine upper endoscopy was performed in the TIF cohort at San Angelo Medical Center at 6 months and was performed if clinically indicated in the rest of the cohort. Patients who had a paired pre-and post-surgery endoscopy (within 6 to 12 months after surgery) were assessed for improvement in esophagitis reported as the Los Angeles (LA) classification, the appearance of the wrap, and recurrence of HH ( Fig. 2a ). The hiatal repair was considered intact if the hiatal opening was less than 3 cm of the greatest transverse diameter. The fundoplication was considered intact if the wrap was at least 200 degrees in rotation and at least 2 cm in length ( Fig. 2b ).
Fig. 2 a.
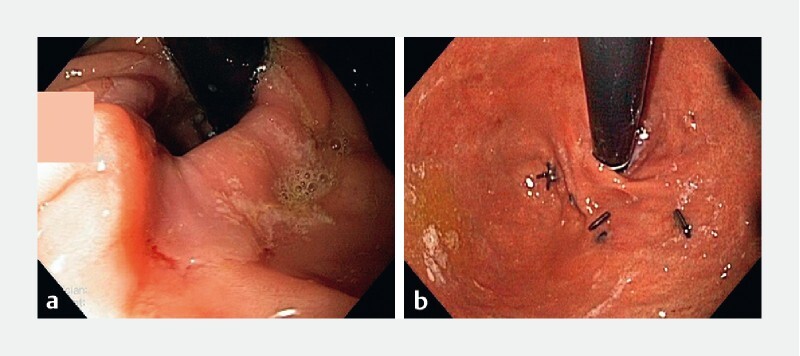
Image showing intact wrap with recurrent hiatal hernia. b Image showing disrupted wrap with no hiatal hernia.
Statistical analysis
Data were expressed as mean and standard deviation (SD) for continuous variables with normal distribution or median and interquartile range (IQR) for skewed data and proportions for categorical variables. Continuous data were compared using an unpaired Student t -test and nonparametric Mann-Whitney U test when appropriate. Categorical data were analyzed using a Chi-square test or Fisher’s exact test when cells had expected counts of less than 5. The multivariable logistic regression analysis was performed to find the association between TIF and the occurrence of AE after adjusting for potential confounders including age, sex, and body mass index (BMI). P < 0.05 was considered significant. The analysis was performed using JMP Pro 14.1. (SAS Institute, Cary, North Carolina, United States).
Results
Patients
A total of 125 patients with HH repair and TIF and 70 with HH repair and LNF were included. The mean age was 57.2 ± 14.3 years, 58.5 % were women, and the mean BMI was 29.2 ± 4.7 kg/m 2 . The baseline characteristics, including BMI and sex, were similar between the two groups, except that patients in the TIF cohort were younger than the LNF cohort (55.1 ± 14.5 years vs. 60.9 ± 13.4 years, P = 0.005) ( Table 1 ).
Table 1. Baseline characteristics and clinical outcomes.
|
TIF group
(N = 125) |
LNF group
(N = 70) |
P value | |
| Baseline/procedure | |||
| Age (years, mean ± SD) | 55.1 ± 14.5 | 60.9 ± 13.4 | 0.005 |
| Female [n (%)] | 71 (56.8) | 43 (61.4) | 0.53 |
| BMI (kg/m 2 , mean ± SD) | 29.1 ± 5.0 | 29.2 ± 4.2 | 0.97 |
| PPI use [n (%)] | 119 (95.2) | 66 (94.3) | 0.78 |
| Length of hospital stay (days, median [IQR]) | 1 (1–1) | 2 (1–2) | < 0.001 |
| Readmission in 30 days [n (%)] | 0 | 3 (4.3) | 0.013 |
| Adverse event [n (%)] | |||
|
0 | 13 (18.6) | < 0.001 |
|
0 | 3 (4.3) | < 0.001 |
|
0 | 0 | – |
|
0 | 0 | – |
|
0 | 0 | – |
| At 6 months [n (%)] | |||
|
76 (73.8) | 40 (60.6) | 0.07 |
|
88 (85.4) | 55 (83.3) | 0.71 |
|
0 | 0 | – |
|
15 (13.8) | 21 (30.0) | 0.009 |
|
9 (8.3) | 10 (14.3) | 0.21 |
|
65 (60.8) | 44 (62.9) | 0.78 |
|
14 (13.1) | 3 (4.3) | 0.04 |
| At 12 months [n (%)] | |||
|
50 (73.5) | 35 (58.3) | 0.07 |
|
57 (83.8) | 47 (78.3) | 0.43 |
|
0 | 0 | – |
|
10 (14.9) | 15 (24.2) | 0.18 |
|
7 (10.1) | 8 (12.9) | 0.62 |
|
32 (52.5) | 36 (58.1) | 0.53 |
|
8 (13.1) | 3 (4.8) | 0.10 |
BMI, body mass index; IQR, interquartile range; LNF, laparoscopic Nissen fundoplication; PPI, proton pump inhibitor; SD, standard deviation; TIF, transoral incisionless fundoplication.
Clinical outcomes
Rates of discontinuation of PPIs (73.8 % vs. 60.6 % at 6 months and 73.5 % vs. 58.3 % at 12 months) and decrease in PPI use (85.4 % vs. 83.3 % at 6 months and 83.8 % vs. 78.3 % at 12 months) were not significantly different between the two groups at 6 and 12 months. In both groups, PPI non-users at baseline did not need to start using PPIs. The rate of reflux symptom-free patients with no PPI use was similar between the two groups at both time points (60.8 % vs. 62.9 %, P = 0.78 at 6 months and 52.5 % vs. 58.1 %, P = 0.53). The rate of patients with continued reflux symptoms with PPI use was significantly higher in the TIF cohort at 6 months (13.1 % vs. 4.3 %, P = 0.04) and numerically higher at 12 months (13.1 % vs. 4.8 %, P = 0.10).
The rate of new or worse than baseline bloating was lower in the TIF cohort at 6 months (13.8 % vs. 30.0 %, P = 0.009) and numerically lower at 12 months (14.9 % vs. 24.2 %, P = 0.18). In the 109 TIF patients with preoperative and 6-month postoperative scores using the GERD-HRQL questionnaire, the overall mean score changed from 2.8 ± 1.7 to 1.5 ± 1.7, reflecting an overall decrease in bloating. However, 15 of 109 patients reported an increase in bloating score of at least 1 point. Five patients had a preoperative bloating score of zero that increased at least 1 point at 6 months, placing the rate of de novo bloating at 4.6 % (5 of 109).
The rate of new or worse than baseline dysphagia was not significantly different between the two groups at either time point (8.3 % vs. 14.3 %, P = 0.10 at 6 months and 10.1 % vs. 12.9%, P = 0.62 at 12 months). The length of hospital stay was shorter in the TIF group (1 [IQR 1–2] days vs. 2 [IQR 1–2] days, P < 0.001). The 30-day readmission rate was lower in the TIF group (0 % vs. 4.3 %, P = 0.013). One-year mortality was not observed in both groups. Table 1 and Fig. 3 summarize clinical outcomes.
Fig. 3 a.
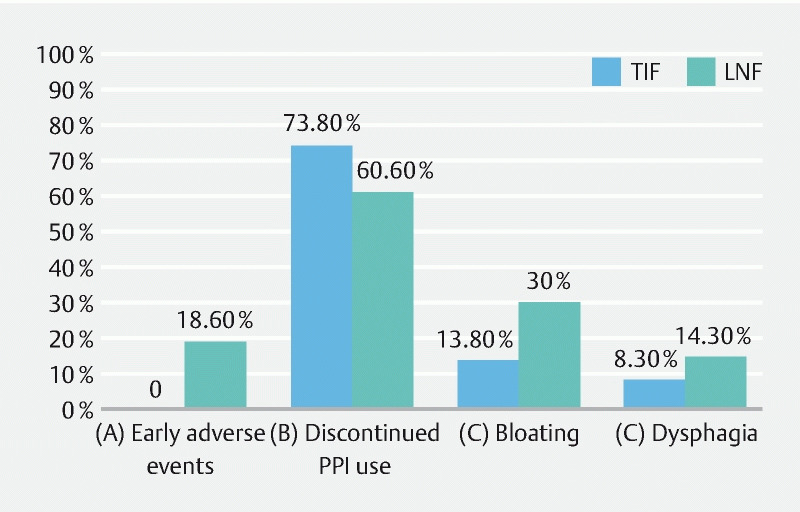
Percentage of patients with early AEs. b Percentage of patients who discontinued PPI use at 6 months. c Percentage of patients with new or worse than baseline bloating at 6 months. d Percentage of patients with new or worse than baseline dysphagia at 6 months. TIF, transoral incisionless fundoplication; LNF, laparoscopic Nissen fundoplication.
Endoscopic assessment
Of the total, 29 TIF patients and 19 LNF patients had presurgical and postsurgical upper endoscopy. Patients in the TIF group had a higher grade of esophagitis at baseline than the LNF group (any grade of esophagitis: 79.3 % vs. 42.1 %, P = 0.008). At follow-up, the rate of improvement of esophagitis (lower LA grade of esophagitis) was equally observed in both groups (95.7 % vs. 87.5 %, P = 0.45), and the rate of resolution of esophagitis was also similar between the two groups (82.6 % vs. 87.5%, P = 0.45). The rates of HH recurrence (17.2 % vs. 10.5 %, P = 0.51) and disrupted wrap (3.5 % vs. 6.3 %, P = 0.33) were comparable between the two groups. Table 2 outlines endoscopy findings.
Table 2. Endoscopy findings.
|
TIF group
(N = 29) |
LNF group (N = 19) | P value | |
| Baseline EGD [n (%)] | 0.008 | ||
|
6 (20.7) | 11 (57.9) | |
|
10 (34.5) | 2 (10.5) | |
|
9 (31.0) | 1 (5.3) | |
|
3 (10.3) | 2 (10.5) | |
|
1 (3.5) | 3 (15.8) | |
| Improvement of esophagitis [n (%)] | 22 (95.7) | 7 (87.5) | 0.45 |
| Resolution of esophagitis [n (%)] | 19 (82.6) | 7 (87.5) | 0.74 |
| Disrupted wrap | 1 (3.5) | 2 (6.3) | 0.33 |
| Recurrence of hiatal hernia [n (%)] | 5 (17.2) | 2 (10.5) | 0.51 |
EGD, esophagogastroduodenoscopy; LA class, Los Angeles Classification; LNF, laparoscopic Nissen fundoplication; TIF, transoral incisionless fundoplication.
Adverse events
Rates of early AEs (0 % vs. 18.6 %, P < 0.001) and serious early AEs (0 % vs. 4.3 %, P < 0.001) were lower in the TIF group than in the LNF group. Three serious early AEs in the LNF group were dysphagia requiring endoscopic dilation (n = 1) and food impaction requiring endoscopic disimpaction, followed by dilation (n = 2). Ten early non-serious AEs were abdominal distension/ileus (n = 4), urinary retention (n = 3), nausea/vomiting (n = 2), and wound infection (n = 1). There were no late AEs observed in either group at 12 months. The multivariate logistic regression analysis could not be performed given that no AE occurred in the TIF group.
Discussion
Despite the increasing incidence of GERD, there is an established trend toward decreased utilization of surgical therapies, while the use of medical therapies that only ameliorate symptoms has been increasing. This could partly be due to patient preference to avoid surgery or fear of increased bloating and dysphagia post-surgery. Given the shortcomings of medical therapy as a predominant management option for GERD, an alternative anatomic correction is appealing. Combining TIF with HH repair could be offered to patients with GERD and moderate HH. This study provided comparative evidence of TIF versus LNF in patients with a moderate HH with a predominant focus on AE profiles in the short term.
Our study demonstrates that HH repair with TIF resulted in a comparable decreased or discontinued rate of PPI use and a comparable rate of symptom-free patients off PPI to HH repair with LNF up to 12 months. Objectively, the healing rates of erosive esophagitis were similar between the two groups. We have previously reported our experience with a separate cohort of 18 patients undergoing concomitant HH repair and TIF and demonstrated the feasibility and symptomatic improvement at 6 months 22 . Another unpublished trial of 27 PPI-refractory patients demonstrated that HH repair with TIF offered an 85 % PPI discontinuation rate with high patient satisfaction at up to 11 months 31 . In studies of TIF with no concomitant HH repair, comparative evidence overall is lacking. Only one nonrandomized prospective trial was available comparing TIF with LNF in 10 patients each 32 . This study showed a higher prevalence of reflux esophagitis and more acid exposure in the TIF cohort than LNF at 3 months. The follow-up interval was relatively short. No grading of esophagitis and the change from their baseline were reported. A recent network meta-analysis comparing TIF versus LNF favored LNF in terms of improvements in reflux physiologic parameters and durability 33 . However, in addition to the indirect comparison nature of this meta-analysis, there were several clinical and methodological issues affecting its validity, and it did not investigate the effects of concomitant TIF and HH repair 34 .
Our TIF cohort experienced no AEs up to 12 months after surgery. Three serious AEs were observed in the LNF cohort. The two previous studies of HH repair with TIF 2.0 also demonstrated no serious AEs 22 31 . It should be noted that experienced endoscopists performed all TIFs in our study. Based on our review of all available RCTs of almost 200 patients total for TIF with no HH repair, only one AE was reported, which was pneumoperitoneum that was successfully managed with needle decompression 16 35 36 37 . The rest of the AEs were self-limiting and short-lived, including nausea, bloating, and dysphagia. The continuous evolution of the device could further improve procedural consistency and reproducibility with less operator dependence, e. g., the EsophyX-Z device was introduced in 2016 and was used in this study 15 .
TIF potentially offers a lower rate of post-fundoplication syndrome than LNF, including bloating and dysphagia. In studies with no concomitant HH repair, this finding has also been observed across previous RCTs 16 36 37 38 and in long-term studies of over 5 years 16 35 36 37 . Our study demonstrated significantly less bloating up to 12 months after surgery with a comparable rate of dysphagia in the TIF group versus the LNF group. Of note, unlike LNF, dysphagia after TIF appeared to be milder and did not require additional interventions. This could be due to creating a partial rather than a circumferential 360-degree wrap. A previous meta-analysis comparing LNF with laparoscopic Toupet fundoplication (270-degree wrap) supported this plausible explanation, demonstrating that LNF resulted in 1.6-times and 2.8-times higher prevalences of bloating and dysphagia, respectively, as compared to Toupet 41 . This may have been attributable to more procedural standardization of TIF. It should be noted that in our institution, LNF historically has been the most commonly performed type of surgical fundoplication. Our study compared TIF with LNF but not the Toupet fundoplication, which is also a partial 270-degree wrap that could be more comparable with the TIF.
A question remains about whether this concomitant procedure is cost-effective. Although not explicitly addressed, our preliminary data showed a significantly shorter length of hospital stay and lower 30-day readmission rate in the TIF group than in the LNF group. Even though conclusive evidence is not available at this time, these positive findings are encouraging. A formal cost-effectiveness study should be pursued focusing on the work productivity related to reflux disease, procedure-related AEs, and reintervention for failure.
There are limited data regarding the long-term durability of TIF. A few studies with follow-up of 5 years or longer demonstrated the sustained benefits of TIF for symptomatic control, improvement in QoL, and PPI discontinuation in those without HH repair 16 41 42 43 . In our study, rates of recurrent HH and disrupted wrap were comparable between the two groups, although there was a numerically higher rate of recurrent HH in the TIF group. Further studies are needed to determine how to select ideal candidates for this concomitant approach. GERD is a chronic disease, and a subset of patients may require a redo fundoplication. Redo fundoplication after LNF could result in higher morbidity than primary fundoplication 42 . In contrast, previous case series showed that redo fundoplication after TIF is feasible without a significant increase in surgical morbidity 43 44 45 .
Our study has several limitations, first and foremost being a nonparallel retrospective study design with an indirect comparison of clinical outcomes derived from medical records and registries recruiting patients at different time intervals. In particular, the way we measure and define a HH has changed significantly from HH length/axial displacement to its transverse diameter. Assessment of outcomes was also performed using different methods in these two cohorts. However, defining clinical changes by the GERD-HRQL score in the TIF group would detect a more subtle change in symptoms than changes in symptoms being reported in the patient medical records in the LNF group and the clinical symptoms were still in favor of TIF. Second, the follow-up duration of 12 months also limits our findings. Long-term data are needed. Third, there were only 70 patients in the LNF group because we included only patients with HH of measuring 2 to 5 cm. Fourth, as with other retrospective studies, there were uncontrollable confounders, including follow-up intervals and data collection by different investigators that could give rise to study bias. Fifth, because only a small subset of patients underwent esophagogastroduodenoscopy after the procedure, the status of the anti-reflux wrap, HH, and erosive esophagitis is unknown in the patients who did not undergo EGD. Sixth, PPI use served as a surrogate marker of symptom improvement in our study but an esophageal pH study would be more objective and cannot be provided. Finally, the sample size is limited for objective comparisons to provide any conclusive evidence. However, it should be noted that our study provides the only direct evidence of concomitant HH repair with TIF versus traditional LNF, which is needed to guide future prospective studies and inform clinical practice.
Conclusions
In this retrospective exploratory cohort study, we demonstrate that a concomitant HH repair with TIF is feasible and safe in a select cohort of patients with moderate HH. A RCT is warranted to validate our findings.
Acknowledgement
This study was sponsored in part by a research grant from Endogastric Solutions.
Footnotes
Competing interests Dr. Abu Dayyeh is a consultant for Metamodix, BFKW, DyaMx, Boston Scientific, USGI medical, Hemostasis, and Endo-TAGSS. He has received research support from Apollo Endosurgery, USGI, Spatz Medical, Boston Scientific, GI Dynamics, Cairn Diagnostics, Aspire Bariatrics, and Medtronic. He has served as a speaker for Johnson & Johnson, EndoGastric Solutions, and Olympus. Dr. Janu is a consultant for EndoGastric Solutions, Ethicon/J&J, and Olympus. Dr. Murray is a consultant for Boston Scientific Corp, Pentax America. He has received research support from Pentax America. He has served as a speaker for AbbVie. He is an advisory board member for Colubris Rx. He has received royalties from UpToDate. Dr. Chang is a consultant for and has received educational research grants from EndoGastric Solutions, is a consultant for and has received educational grants from Cook and Olympus. Dr. Canto has received research support from EndoGastric Solutions and Pentax Medical Corporation and has received royalties from UpToDate.
References
- 1.El-Serag H B, Sweet S, Winchester C C et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ronkainen J, Aro P, Storskrubb T et al. Gastro-oesophageal reflux symptoms and health-related quality of life in the adult general population--the Kalixanda study. Aliment Pharmacol Ther. 2006;23:1725–1733. doi: 10.1111/j.1365-2036.2006.02952.x. [DOI] [PubMed] [Google Scholar]
- 3.Sontag S J, Sonnenberg A, Schnell T G et al. The long-term natural history of gastroesophageal reflux disease. J Clin Gastroenterol. 2006;40:398–404. doi: 10.1097/00004836-200605000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bruley des Varannes S, Lofman H G, Karlsson M et al. Cost and burden of gastroesophageal reflux disease among patients with persistent symptoms despite proton pump inhibitor therapy: an observational study in France. BMC Gastroenterol. 2013;13:39. doi: 10.1186/1471-230X-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nehra A K, Alexander J A, Loftus C G et al. Proton pump inhibitors: review of emerging concerns. Mayo Clinic proceedings. 2018;93:240–246. doi: 10.1016/j.mayocp.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Scholten T. Long-term management of gastroesophageal reflux disease with pantoprazole. Ther Clin Risk Manag. 2007;3:231–243. doi: 10.2147/tcrm.2007.3.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther. 2010;32:720–737. doi: 10.1111/j.1365-2036.2010.04406.x. [DOI] [PubMed] [Google Scholar]
- 8.Grigolon A, Cantu P, Savojardo D et al. Esophageal acid exposure on proton pump inhibitors in unselected asymptomatic gastroesophageal reflux disease patients. J Clin Gastroenterol. 2008;42:969–973. doi: 10.1097/MCG.0b013e31814b8fc2. [DOI] [PubMed] [Google Scholar]
- 9.Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut. 2009;58:295–309. doi: 10.1136/gut.2007.145581. [DOI] [PubMed] [Google Scholar]
- 10.Nijjar R S, Watson D I, Jamieson G G et al. Five-year follow-up of a multicenter, double-blind randomized clinical trial of laparoscopic Nissen vs anterior 90 degrees partial fundoplication. Arch Surg. 2010;145:552–557. doi: 10.1001/archsurg.2010.81. [DOI] [PubMed] [Google Scholar]
- 11.Cookson R, Flood C, Koo B et al. Short-term cost effectiveness and long-term cost analysis comparing laparoscopic Nissen fundoplication with proton-pump inhibitor maintenance for gastro-oesophageal reflux disease. Br J Surgery. 2005;92:700–706. doi: 10.1002/bjs.4933. [DOI] [PubMed] [Google Scholar]
- 12.Lundell L, Miettinen P, Myrvold H E et al. Seven-year follow-up of a randomized clinical trial comparing proton-pump inhibition with surgical therapy for reflux oesophagitis. Br J Surgery. 2007;94:198–203. doi: 10.1002/bjs.5492. [DOI] [PubMed] [Google Scholar]
- 13.Mahon D, Rhodes M, Decadt B et al. Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton-pump inhibitors for treatment of chronic gastro-oesophageal reflux. Br J Surgery. 2005;92:695–699. doi: 10.1002/bjs.4934. [DOI] [PubMed] [Google Scholar]
- 14.Tian Z C, Wang B, Shan C X et al. A meta-analysis of randomized controlled trials to compare long-term outcomes of Nissen and Toupet fundoplication for gastroesophageal reflux disease. PLoS One. 2015;10:e0127627. doi: 10.1371/journal.pone.0127627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bazerbachi F, Krishnan K, Abu Dayyeh B K. Endoscopic GERD therapy: a primer for the transoral incisionless fundoplication procedure. Gastrointest Endosc. 2019;90:370–383. doi: 10.1016/j.gie.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 16.Trad K S, Barnes W E, Prevou E R et al. The TEMPO Trial at 5 Years: transoral fundoplication (tif 2.0) is safe, durable, and cost-effective. Surg Innov. 2018;25:149–157. doi: 10.1177/1553350618755214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerson L, Stouch B, Lobontiu A. Transoral incisionless fundoplication (TIF 2.0): a meta-analysis of three randomized, controlled clinical trials. Chirurgia (Bucur) 2018;113:173–184. doi: 10.21614/chirurgia.113.2.173. [DOI] [PubMed] [Google Scholar]
- 18.Pandolfino J E. Hiatal hernia and the treatment of acid-related disorders. Gastroenterol Hepatol (NY) 2007;3:92–94. [PMC free article] [PubMed] [Google Scholar]
- 19.Avidan B, Sonnenberg A, Schnell T G et al. Hiatal hernia size, Barrett's length, and severity of acid reflux are all risk factors for esophageal adenocarcinoma. Am J Gastroenterol. 2002;97:1930–1936. doi: 10.1111/j.1572-0241.2002.05902.x. [DOI] [PubMed] [Google Scholar]
- 20.Cameron A J. Barrett's esophagus: prevalence and size of hiatal hernia. Am J Gastroenterol. 1999;94:2054–2059. doi: 10.1111/j.1572-0241.1999.01277.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones M P, Sloan S S, Rabine J C et al. Hiatal hernia size is the dominant determinant of esophagitis presence and severity in gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:1711–1717. doi: 10.1111/j.1572-0241.2001.03926.x. [DOI] [PubMed] [Google Scholar]
- 22.Ihde G M, Besancon K, Deljkich E.Short-term safety and symptomatic outcomes of transoral incisionless fundoplication with or without hiatal hernia repair in patients with chronic gastroesophageal reflux disease Am J Surgery 2011202740–746.; discussion 746–747 [DOI] [PubMed] [Google Scholar]
- 23.Chang C G, Thackeray L. Laparoscopic hiatal hernia repair in 221 patients: outcomes and experience. JSLS. 2016;20:e2015.00104. doi: 10.4293/JSLS.2015.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janu P, Shughoury A B, Venkat K et al. Laparoscopic hiatal hernia repair followed by transoral incisionless fundoplication with EsophyX device (HH + TIF): efficacy and safety in two community hospitals. Surg Innov. 2019;26:675–686. doi: 10.1177/1553350619869449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gisi C, Wang K, Khan F et al. Efficacy and patient satisfaction of single-session transoral incisionless fundoplication and laparoscopic hernia repair. Surg Endosc. 2020;35:921–927. doi: 10.1007/s00464-020-07796-x. [DOI] [PubMed] [Google Scholar]
- 26.Ihde G M, 2nd, Pena C, Scitern C et al. pH scores in hiatal repair with transoral incisionless fundoplication. JSLS. 2019;23:e2018.00087. doi: 10.4293/JSLS.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefanidis D, Hope W W, Kohn G P et al. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24:2647–2669. doi: 10.1007/s00464-010-1267-8. [DOI] [PubMed] [Google Scholar]
- 28.Bell R C, Cadiere G B. Transoral rotational esophagogastric fundoplication: technical, anatomical, and safety considerations. Surg Endosc. 2011;25:2387–2399. doi: 10.1007/s00464-010-1528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jobe B A, O'Rourke R W, McMahon B P et al. Transoral endoscopic fundoplication in the treatment of gastroesophageal reflux disease: the anatomic and physiologic basis for reconstruction of the esophagogastric junction using a novel device. Ann Surg. 2008;248:69–76. doi: 10.1097/SLA.0b013e31817c9630. [DOI] [PubMed] [Google Scholar]
- 30.Dindo D, Demartines N, Clavien P A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang K, Khan F, Hou L A et al. Efficacy of combined transoral incisionless fundoplication and laparaoscopic hiatal hernia repair. Gastrointest Endosc. 2018;87:AB254–AB255. [Google Scholar]
- 32.Frazzoni M, Conigliaro R, Manta R et al. Reflux parameters as modified by EsophyX or laparoscopic fundoplication in refractory GERD. Aliment Pharmacol Ther. 2011;34:67–75. doi: 10.1111/j.1365-2036.2011.04677.x. [DOI] [PubMed] [Google Scholar]
- 33.Richter J E, Kumar A, Lipka S et al. Efficacy of laparoscopic nissen fundoplication vs transoral incisionless fundoplication or proton pump inhibitors in patients with gastroesophageal reflux disease: a systematic review and network meta-analysis. Gastroenterology. 2018;154:1298–1308. doi: 10.1053/j.gastro.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Abu Dayyeh B, Murad M H, Bazerbachi F et al. Efficacy of laparoscopic Nissen fundoplication vs transoral incisionless fundoplication or proton pump inhibitors in patients with gastroesophageal reflux disease: misleading ranking probabilities in network meta-analysis. Gastroenterology. 2018;155:935–936. doi: 10.1053/j.gastro.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 35.Hakansson B, Montgomery M, Cadière G B et al. Randomised clinical trial: transoral incisionless fundoplication vs. sham intervention to control chronic GERD. Aliment Pharmacol Ther. 2015;42:1261–1270. doi: 10.1111/apt.13427. [DOI] [PubMed] [Google Scholar]
- 36.Hunter J G, Kahrilas P J, Bell R C et al. Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a randomized controlled trial. Gastroenterology. 2015;148:324–333. doi: 10.1053/j.gastro.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Witteman B P, Conchillo J M, Rinsma N F et al. Randomized controlled trial of transoral incisionless fundoplication vs. proton pump inhibitors for treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2015;110:531–542. doi: 10.1038/ajg.2015.28. [DOI] [PubMed] [Google Scholar]
- 38.Chimukangara M, Jalilvand A D, Melvin W S et al. Long-term reported outcomes of transoral incisionless fundoplication: an 8-year cohort study. Surg Endosc. 2019;33:1304–1309. doi: 10.1007/s00464-018-6403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefanidis G, Viazis N, Kotsikoros N et al. Long-term benefit of transoral incisionless fundoplication using the esophyx device for the management of gastroesophageal reflux disease responsive to medical therapy. Dis Esophagus. 2017;30:1–8. doi: 10.1111/dote.12525. [DOI] [PubMed] [Google Scholar]
- 40.Testoni P A, Testoni S, Mazzoleni G et al. Long-term efficacy of transoral incisionless fundoplication with Esophyx (Tif 2.0) and factors affecting outcomes in GERD patients followed for up to 6 years: a prospective single-center study. Surg Endosc. 2015;29:2770–2780. doi: 10.1007/s00464-014-4008-6. [DOI] [PubMed] [Google Scholar]
- 41.Du X, Hu Z, Yan C et al. A meta-analysis of long follow-up outcomes of laparoscopic Nissen (total) versus Toupet (270 degrees ) fundoplication for gastro-esophageal reflux disease based on randomized controlled trials in adults. BMC Gastroenterol. 2016;16:88. doi: 10.1186/s12876-016-0502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furnee E J, Draaisma W A, Broeders I A et al. Surgical reintervention after failed antireflux surgery: a systematic review of the literature. J Gastrointest Surg. 2009;13:1539–1549. doi: 10.1007/s11605-009-0873-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashfaq A, Rhee H K, Harold K L. Revision of failed transoral incisionless fundoplication by subsequent laparoscopic Nissen fundoplication. World J Gastroenterol. 2014;20:17115–17119. doi: 10.3748/wjg.v20.i45.17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bell R C, Kurian A A, Freeman K D. Laparoscopic anti-reflux revision surgery after transoral incisionless fundoplication is safe and effective. Surg Endosc. 2015;29:1746–1752. doi: 10.1007/s00464-014-3897-8. [DOI] [PubMed] [Google Scholar]
- 45.Perry K A, Linn J G, Eakin J L et al. Transoral incisionless fundoplication does not significantly increase morbidity of subsequent laparoscopic Nissen fundoplication. J Laparoendosc Adv Surg Tech A. 2013;23:456–458. doi: 10.1089/lap.2012.0525. [DOI] [PubMed] [Google Scholar]


