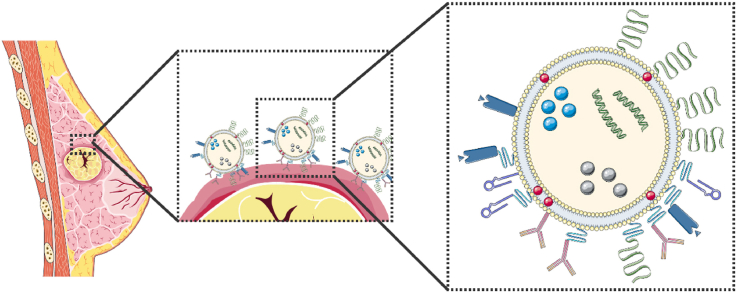
Abstract
Despite the exceptional progress in breast cancer pathogenesis, prognosis, diagnosis, and treatment strategies, it remains a prominent cause of female mortality worldwide. Additionally, although chemotherapies are effective, they are associated with critical limitations, most notably their lack of specificity resulting in systemic toxicity and the eventual development of multi-drug resistance (MDR) cancer cells. Liposomes have proven to be an invaluable drug delivery system but of the multitudes of liposomal systems developed every year only a few have been approved for clinical use, none of which employ active targeting. In this review, we summarize the most recent strategies in development for actively targeted liposomal drug delivery systems for surface, transmembrane and internal cell receptors, enzymes, direct cell targeting and dual-targeting of breast cancer and breast cancer-associated cells, e.g., cancer stem cells, cells associated with the tumor microenvironment, etc.
Keywords: Liposomes, Breast cancer, Anti-cancer agents, Targeted drug delivery, Surface functionalization, Receptor-targeted drug delivery
Graphical abstract
The latest strategies for the active targeting of breast cancer and breast cancer-associated cells using surface, transmembrane and internal cell receptors, enzymes, direct cell targeting and dual-targeting liposomal drug delivery nanocarriers are systematically summarized. This paper presents the non-targeted liposomes currently in clinical use for breast cancer treatment, as well as the targeted liposomes which have progressed to clinical trials. The major challenges in the development of actively targeted drug delivery systems for cancer is also highlighted.
Highlights
-
•
Breast cancer is the most diagnosed cancer for women worldwide.
-
•
Liposomes are immensely versatile and advantageous drug delivery tools.
-
•
Only four liposome-based treatments are in clinical use for breast cancer treatment and none make use of a targeting system.
-
•
Targeting liposome-based systems include surface, transmembrane and internal cell receptors, enzymes, and dual-targeting of breast cancer cells.
1. Introduction
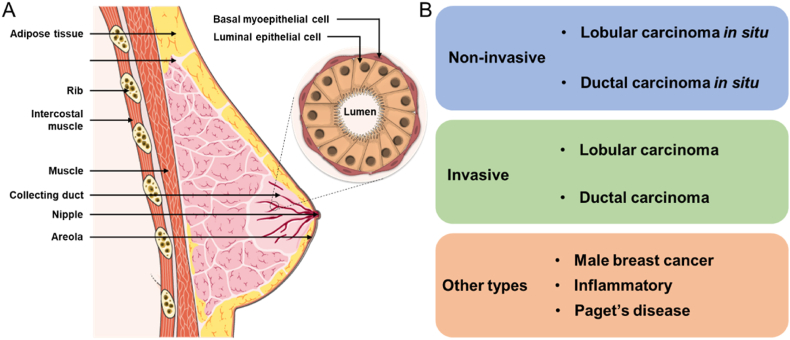
Cancer continues to be a complex, ubiquitous, and significant cause of mortality in humans. In 2021 the GLOBOCAN 2020 report, which serves to provide an analysis of the worldwide cancer burden, noted that the most diagnosed cancer in females shifted from lung to breast cancer with approximately 2.3 million cases and over 680,000 deaths recorded in 185 countries [1]. Although the incidence rate for breast cancer varies widely depending on race, ethnicity, socio-economic, location, and several other risk factors (such as reproductive, genetic, dietary, lifestyle-related, and environmental factors) [[2], [3], [4]], global trends over the last 25 years indicate a significant and continual increase in breast cancer incidence and mortality worldwide [5]. Breast cancer is caused by the malignant growth of cells in either the ductal or lobular epithelium of the breast. The classification of breast cancer, and its many subtypes and variants, is a multifaceted and contentious topic. However, a simplified categorization can be described as involving either lobular or ductal carcinomas which can present as either non-invasive or invasive (Fig. 1). In non-invasive breast cancer, wherein the affected cells remain bound within the duct or lobule they originated from (i.e., in situ), two forms are most commonly seen; ductal carcinoma in situ (DCIS) (90% of non-invasive cases) and lobular carcinoma in situ (LCIS) [6]. Notably, these in situ forms are relatively curable and stand in stark contrast with their invasive counterparts. Invasive breast cancer, wherein the affected cells break away from the ductal or lobular walls and access the fatty and connective tissue surrounding the breast, includes; ductal carcinoma (80% of invasive cases) and comprises medullary, mucinous, tubular, and papillary ductal carcinomas; lobular carcinoma; inflammatory breast cancer; and Paget's disease of the nipple and breast [7]. This last category also includes rare tumors such as the phyllodes tumor, which originates from the connective tissue of the breast, and breast carcinoma with neuroendocrine differentiation [8].
Fig. 1.
(A) Schematic representation of the ductal and lobular epithelium of the breast, and (B) a simplified classification of breast cancer types.
Great progress in etiology, clinical assessment, and molecular characterization has established several molecular subtypes of the above-mentioned ductal and lobular carcinomas [9]. The most well-known of which involves the presence (+) or absence (−) of three hormonal receptors, namely the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor-2 receptor (HER2), are used as markers of subtypes related to prognosis and are major determinants in treatment decision making [4,10]. The subtypes include Luminal A (ER+ and/or PR+, HER2−) which constitutes the majority of diagnosed breast cancer incidences, Luminal B (ER+ and/or PR+, HER2+), and basal-like (ER−, PR−, HER2−) [11]. Extensive gene expression profiling has identified Luminal A, Luminal B, HER2-enriched, claudin-low and basal-like, as the 5 most common intrinsic molecular subtypes of breast cancer [12,13]. This progress has also led to the discovery of the two most notable genes in breast cancer, BRCA1 and 2, the mutations of which are associated with a lifetime risk of 70 and 60%, respectively, of developing breast cancer [6,14]. Notably, the basal-like subtype (ER−, PR−, HER2−) is commonly referred to as triple-negative breast cancer (TNBC) and constitutes roughly 15–20% of diagnosed breast cancers and is distinguished by its invasiveness, poor differentiation, large tumor size and aggressive clinical progression [10,15,16].
Concurrently with our improved understanding of breast cancer pathogenesis, research regarding drug development, targeting, and delivery has advanced over the years. Treatment strategies for breast cancer are currently determined by; tumor size, proliferation, grade, molecular subtype, stage of progression, and lymph node involvement. For a detailed description of the diagnosis, treatment, and follow-up regimes for breast cancer the authors recommend Moo et al., 2018 [17]. In brief, treatment options include surgery, chemotherapy, endocrine therapy, radiation therapy, and immunotherapy, with recurrence rates highest amongst patients presenting basal-like and Luminal B subtypes than Luminal A [18]. Cytotoxic chemotherapy can be used as a neoadjuvant and/or adjuvant treatment with surgery with the most commonly used drugs being anthracyclines (e.g., doxorubicin (DOX) and epirubicin), taxanes (e.g. docetaxel and paclitaxel (PTX)), platinum-based compounds (e.g., cisplatin, carboplatin, and lobaplatin), gemcitabine and fluorouracil [19]. Due to the presence of hormone receptors, breast cancer can also be treated with targeted endocrine therapy drugs used in tandem with chemotherapy, e.g., tamoxifen, fulvestrant, letrozole [[20], [21], [22]]. Lastly, immunotherapy refers to the application of monoclonal antibodies (mAbs), adoptive cell transfer, cytokines, and vaccines in cancer treatment. In the case of breast cancer, this treatment is focused on mAbs and applies mainly to HER2+ breast cancers [23].
Chemotherapy is the treatment of choice for breast cancer but is associated with several limitations. Firstly, the most notable, is its lack of specificity resulting in systemic toxicity causing many well-documented short- and long-term side effects [24,25]. The overuse of chemotherapy, defined as the provision of neo- or adjuvant chemotherapies in situations where the specific treatment regime is not necessarily required or recommended, is also of growing concern with severe financial, physical, and psychological implications for patients [26,27]. Lastly, chemotherapy is associated with multidrug resistance (MDR) resulting in unresponsive, refractory, and recurrent cancers. MDR is linked to refractory or resistant cancers and involves the overexpression of specific ATP-binding cassette transporters which expel therapeutic agents before they can affect the cell. This is referred to as the tumor cells becoming resistant and involves not only the drug that is initially applied but also unrelated drugs with similar structures and mechanisms of action. This deficiency of an effective drug dose leads to the impairment of cell death mechanisms and the inhibition of certain apoptotic pathways, enhanced DNA repair, epigenetic alterations, deregulation of microRNAs, the progression of tumor microenvironment (TME) complexity, intratumoral heterogeneity, and cancer stem cell plasticity [[28], [29], [30]]. The most recognized MDR transporter is P-glycoprotein (P-gp) which resides in the plasma wall of tumoral cells and is overexpressed in 40–50% of breast cancer patients [31]. Other MDR proteins such as multidrug resistance-associated proteins 1 and 2, breast cancer resistance protein (BCRP), and certain cell signaling pathways have also been related to chemoresistance [7]. Notably, P-gp effluxes many chemotherapeutic drugs including PTX, docetaxel, vincristine, etoposide, and DOX. DOX is a non-selective anthracycline antineoplastic antibiotic used for early and advanced stage breast cancer. The cytotoxic mechanism of action for anthracyclines is due to two phenomena; firstly, the drug intercalates between the base pairs of DNA disrupting the function of the enzyme topoisomerase II inhibiting the formation of the DNA double helix thus halting replication and RNA transcription; and secondly, by triggering apoptosis due to the production of radicals and reactive oxygen species (ROS) able to damage the cell membrane, organelles and DNA [[32], [33], [34]]. Unfortunately, although anthracyclines are amongst the most clinically effective chemotherapeutic agents they are strongly correlated with MDR and cardiotoxicity [35,36]. Another hurdle in chemotherapeutic treatment is the metastatic progression of cancer cells to distant organs such as the lymph nodes, bones, lungs, liver, and brain. Metastatic breast cancer is a significant cause of concern as not only do 30–40% of patients develop metastatic tumors, but patients are also far more likely to succumb to the metastatic tumors than the primary tumor [37,38]. Thus, due to the currently incurable status of metastatic breast cancer with our current treatment strategies, it is considered a chronic disease [39].
To address these significant issues with chemotherapy, nano-based colloidal drug delivery systems such as lipid nanocapsules, dendrimers, micelles, and liposomes have become a research hotspot [40]. These delivery systems can be fine-tuned to have drugs dissolved, adsorbed, covalently bound, encapsulated, and embedded within the system, as well as incorporating functionalized peptides, antibodies, proteins, aptamers, ligands, and antigens that exploit the cell surface, intracellular and tumoral environment to target cancer. These advancements not only improve the pharmacokinetics of the drugs but also impede drug degradation, boost safety, provide sustained release, improve solubility, and reduce side effects and drug wastage [[41], [42], [43], [44], [45]]. The first nanomedicines approved by the FDA (US) and EMA (EU), polyethylene glycol (PEG) enrobed liposomal DOX (Doxil®□/Caelyx®□) and albumin-bound PTX (Abraxane®□), were not selective towards specific biological targets but rather exploited the enhanced permeability and retention (EPR) effect and are considered the first generation of nanomedicine [46,47]. Of particular interest are the liposomal drug delivery systems.
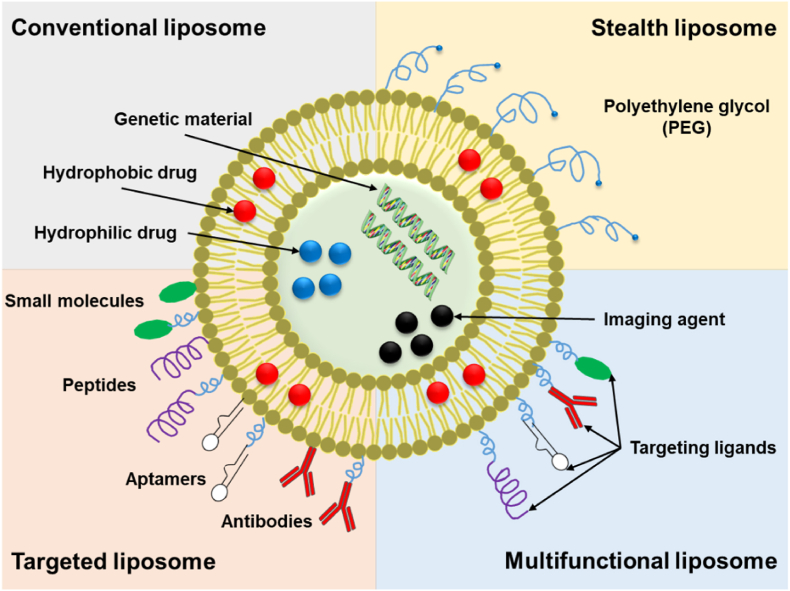
Liposomes, first introduced in 1965, are rounded vesicles composed of either single or multiple lipid bilayers with an aqueous center [[48], [49], [50]]. These vesicles can spontaneously form when amphiphilic lipids, such as phospholipids, are dispersed in water and closely resemble our cellular membranes [51,52]. This similarity serves as an immense advantage for drug delivery in terms of biocompatibility and biodegradation. Another advantage of liposomes is their ability to encapsulate hydrophilic, lipophilic, and amphiphilic compounds within their aqueous center and/or lipid bilayers (Fig. 2) [53,54].
Fig. 2.
Simplified representation of conventional, PEGylated, targeted, and multifunctional liposomes.
Notably, the specific lipid composition chosen to produce the liposomes can be easily modified and influences several factors including the method of preparation, bilayer fluidity, as well as surface charge and hydration [29,55]. The most commonly used lipids are phospholipids, which can be of natural or synthetic origin [56], wherein the addition of organic molecules to the phosphate head group can create a variety of phospholipid species such as phosphatidylethanolamine, phosphatidylserine, phosphatidylglycerol (PG), and phosphatidylcholine (PC) [57]. Liposomes consisting of only phospholipids, however, have a greatly reduced shelf-life and a limited ability to protect encapsulated drugs due to high permeability leading to drug leakage. To combat this, sterols are required to modulate membrane rigidity and stability [58,59]. Cholesterol is the most commonly used sterol and its insertion can result in major changes regarding liposome fluidity, penetrability, and stability [57,60,61]. Naturally, when encapsulating drugs, liposomes also serve as protective drug delivery systems which enhance the stability of the encapsulated compounds by protecting them from environmental, enzymatic, and chemical changes, and providing a shield against pH, temperature, and ion fluctuations [62]. Other components, such as vitamin E (or a derivative such as d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS)) and polymers (such as chitosan and PEG) can also be incorporated into liposomal membranes to improve stability, shelf-life, and biodistribution [63]. Taking all these properties into consideration, liposomes present several distinct advantages as a drug delivery system including; the ability to self-assemble; load hydrophilic, hydrophobic, and amphiphilic compounds; improve solubility; impart protection to the encapsulated drugs; provide biocompatibility and low toxicity at relative levels; to biodegrade, and to induce low immunogenicity [[64], [65], [66], [67], [68]]. Advancements in the engineering of liposomes, including the ability to respond to light, pH, temperature, redox, enzyme, ultrasound, and magnetic external stimuli, as well as their active site-specific functionalization (e.g., conjugation of mAbs to liposomes, referred to as immunoliposomes), have greatly increased the specificity and thus reduced the toxicity of the encapsulated compound by enabling controllable drug release and multi-drug encapsulation resulting in clinically favorable biodistribution profiles and reduced non-specific uptake [[69], [70], [71], [72], [73]].
Currently, only four liposome-based treatments are clinically proven for use as breast cancer therapies and have been approved for use: Doxil®/Caelyx®, Myocet liposomal (formerly Myocet®), Lipodox® and Lipusu® (Table 1).
Table 1.
Liposome-based therapies for breast cancer currently in clinical use.
| Product name | Active agent | Approval year | Indication | Description of liposome | Composition | References |
|---|---|---|---|---|---|---|
| Doxil® (US)/Caelyx ® (EU) | DOX HCl | 2003, USA; 2010, EU |
Metastatic breast cancer | PEGylated stealth liposomes,80–90 nm | HSPC, CHOL,DSPE-PEG (2000) | [[74], [75], [76]] |
| Myocet liposomal | DOX | 2000, EU; “Fast Track” status, USA |
HER2+ metastatic breast cancer | Non-PEGylated,150–250 nm | EPC, CHOL | [[77], [78], [79]] |
| Lipodox®a | DOX HCl | 2012, USAb | Breast cancer | PEGylated stealth liposomes, ∼100 nm |
DSPC, CHOL, DSPE-PEG (2000) | [80] |
| Lipusu® | PTX | 2003, China | HER2- metastatic breast cancer | Non-PEGylated,∼400 nm | Not available | [81,82] |
CHOL: cholesterol; DOX: doxorubicin; DSPE-PEG (2000): 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000]; EPC: egg yolk phosphatidylcoline; HCl: hydrochloride; HSPC: hydrogenated soybean phosphatidylcholine.
Not to be confused with Lipo-Dox. Lipodox® is manufactured by Sun Pharmaceuticals Industries Ltd. (India) and has been approved by the FDA as a generic equivalent of Doxil® since 2013. Lipo-Dox is manufactured by TTY Biopharm (Taiwan) [83].
Substitute during drug shortage [84].
Doxil®/Caelyx® (trade name depending on the country) is a PEGylated nanoliposomal drug delivery system that encapsulates DOX HCl for the primary treatment of AIDS-related Kaposi's sarcoma, multiple myeloma, treatment-resistant or refractory ovarian cancer, and metastatic breast cancer, and was the first chemotherapeutic nanosystem used clinically. The liposomal formulation, and its PEGylation, are considered revolutionary as they reduced the level of free DOX in the blood without limiting its anticancer effect, whilst simultaneously increasing the circulation time of the chemotherapeutic agent [85]. Myocet, on the other hand, is a non-PEGylated liposomal drug delivery system encapsulating DOX and has been used in the EU as a polytherapy treatment (in combination with cyclophosphamide) for metastatic breast cancer since 2000. In the US, “Fast Track” expedited status has been granted to Myocet as a starting treatment for HER2+ metastatic breast cancer [64]. Interestingly, this approval of Myocet was due to its ability to reduce drug-related cardiotoxicity rather than enhance antitumor efficacy [86]. Lipodox®, another PEGylated DOX HCl encapsulating liposomal formulation, was used as a substitute in 2012 during a critical shortage of Doxil® in the USA [87] and has since been considered a generic equivalent. Lastly is Lipusu®, a non-PEGylated liposomal system encapsulating PTX. PTX, a chemotherapeutic derived from the Pacific Yew tree, is often administered in the Kolliphor-EL solubilized form of Taxol® for the treatment of ovarian and breast cancers [88]. To combat Kolliphor-EL related toxicity, various nano-based drug carriers have been developed including polymeric micelles (Genexol®, Nanoxel®, and Paclical®), polymeric albumin-bound nanoparticles (Abraxane®), and liposomes (Lipusu®) [89]. Notably, although Lipusu® has been approved in China for HER2-metastatic breast cancer [81,82], its composition information is not publicly available. Also of note is DaunoXome®, a heat-activated liposome encapsulating daunorubicin [90,91], which has been investigated for the treatment of metastatic breast cancer but has not yet been clinically approved [92].
Notably, none of the liposomes mentioned in Table 1 make use of an active targeting system relying instead on their size for preferential accumulation in the interstitial spaces of tumors through passive accumulation or ‘passive targeting’ via the EPR effect [93,94]. The misnomer ‘passive targeting’ is used to describe the accumulation of particles, e.g., macromolecules, proteins, soluble particles, nanoparticles, etc., in tumoral interstitial spaces due to the hyperpermeable neo vasculature of these diseased tissues [95]. This permeable neo vasculature arises due to fast growing tumors exceeding the oxygen supply needed by the cells, causing tissue anoxia, and the consequent release of growth factors, e.g., vascular endothelial growth factor (VEGF), triggering rapid, dysregulated angiogenesis culminating in leaky neo vasculature and impaired lymphatic drainage. Consequently, particles <400 nm in size circulating in the blood tend to accumulate in these tumoral interstitial spaces and inflamed tissues. However, research has shown that the ‘passive targeting’ effect is largely absent in nascent tumors and non-vascularized diseased tissues and only evident in some solid tumors larger than 4.6 mm in diameter with tumor vessel pore size being highly dependent on tumor type and status [93,94].
Upon intravenous administration of non-PEGylated liposomal formulations, such as Myocet or Lipusu®, the nanocarrier travels through the vascular system of the body with eventual elimination and clearance by the renal and mononuclear phagocytic systems (MPS) (also referred to as the reticuloendothelial system (RES)) [96,97]. Liposomes and nanoparticles in general, approximately 8 nm in size, undergo minimal catabolism and are instead flushed through the kidneys and eliminated, whilst those larger than 8 nm are cleared by the MPS in a process referred to as opsonization [97]. Here, serum proteins (i.e., opsonins) accumulate on the surface of nanoparticle liposomes priming and targeting the nanocarriers for detection and phagocytosis [98]. This process can be negated by coating the nanocarrier in an inert polymer (e.g., PEG) leading to a ‘shielding’ effect on the nanoparticle's surface causing repulsive interactions between the particle and the blood components. This effect is referred to as ‘stealth’ [99,100]. Stealth hinders MPS clearance mechanisms resulting in improved vascular circulation time and pharmacokinetic properties of PEGylated delivery systems thus Doxil®/Caelyx® has an approximate ∼100-fold greater clearance half-life than free DOX [39,85,101].
2. Targeted nanoliposomes for breast cancer treatment
Actively-targeted liposomal drug delivery systems are a hugely promising concept, as it provides the advantage of specifically targeting cancer cells. This accurate targeting has many benefits, including; (i) selective cancer cell internalization and release of the therapeutic drug which results in less side effects in healthy tissues and mitigates the risk of MDR, (ii) the ability to across blood-brain barrier (BBB), and (iii) the ability to identify, image, and treat metastatic, relapsed and/or breast-cancer associated cells [102]. Both preclinical and clinical studies have demonstrated interest in using targeted nanomedicines as solid-tumor treatment.
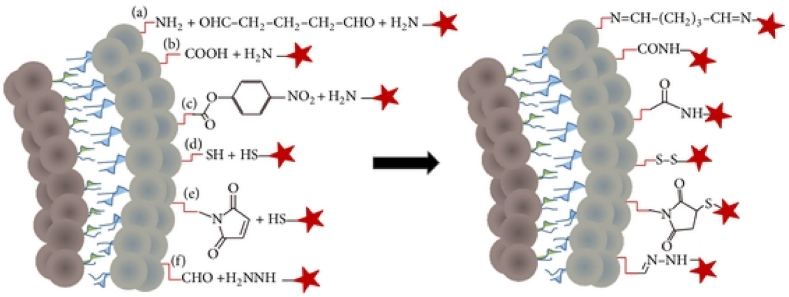
However, although the concept of developing targeted cancer therapy seems straightforward, in practice active targeting is exceedingly challenging. In addition to requiring the presence of viable targets, liposomes must be grafted with specific targeting moieties for optimum affinity without obscuring the needed stealth aspects. Commonly, the surface of liposomes is chemically modified with various reactive groups to functionalize it (i.e., covalently or non-covalently) with a large variety of targeting agents. Six main chemical functionalization strategies are generally used (Fig. 3), including, (a) imines-crosslinked using glutaraldehyde, (b and c) amide-crosslinked from primary amine and free or p-nitrophenylcarbonyl-activated carboxylic acid, respectively, (d) disulfide-crosslinked using thiol and pyridyldithiol groups, (e) thiol-maleimide click chemistry reactions, and (f) hydrazone-crosslinked from aldehyde and hydrazine groups [103,104].
Fig. 3.
Six main chemical strategies (a – f) for liposomal surface functionalization. Stars represent targeting ligands. Reprinted from Ref. [105].
Of these, the thiol-maleimide click chemistry reaction is one of the most popular methods with extensive literature available [106] showing the conjugation (with or without anchored PEG) between nanoparticles and antibodies, antibody fragments, peptides, aptamers, vitamins, etc. Alternative methods for liposomal surface functionalization includes adsorption or interpolation via electrostatic or hydrophobic interactions [105,107].
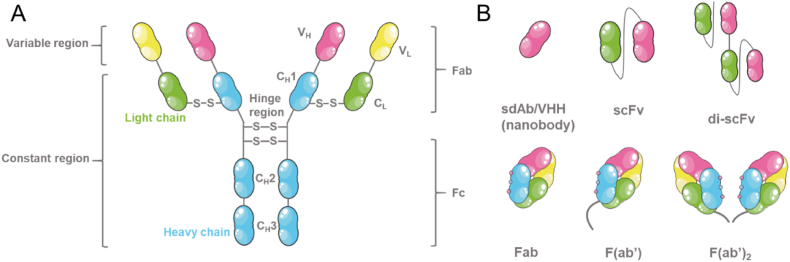
Targeting ligands, such as small molecules, mAbs, peptides, or aptamers, which can either directly bind to a target on or within the breast cancer or breast cancer-associated cell (e.g., a cell surface receptor or intracellular enzyme comparatively unique and abundant to the targeted cell) or be targeted to the nearby area of the tumor (e.g., acidic pH associated with the TME). Early drug-targeting studies focused on the use of whole mAbs, which are generally large, Y-shaped IgG antibodies consisting of two identical subunits of heavy and light protein chains joined by disulfide bonds. Although the whole mAbs possessed high affinity and specificity for their targets, they were also plagued with issues of poor permeability (due to their large size), immunogenicity, and high cost. Thus, it is now recognized that antibody fragments (e.g., fragment antigen-binding (Fab) units and single-chain variable fragments (scFv)) (Fig. 4) possess reduced immunogenicity and improved pharmacokinetic profiles [94,108]. Fab fragments consist of the variable and constant regions of the heavy and light protein chains which include the paratope region, i.e., the region that recognizes and binds to targets, but lacks the tail region of the antibody, i.e., the fragment crystallizable region (Fc region).
Fig. 4.
Schematic representations of (A) the general structure of an antibody and (B) some of the engineered antibody fragments currently in development.
Fab fragments can also be modified for easier immobilization with the addition of a thiol group and are then referred to as Fab’ fragments. Antibody Fv fragments, such as scFv fragments, are even smaller units as they consist of only the variable paratope region of the antibody. Another popular targeting ligand moieties are peptides due to their relatively simple and low cost preparation methods, and their powerful capacity to avoid non-specific binding, and opsonization [109,110]. It should be noted, however, that peptides are prone to proteolysis. Small molecules, such as sorafenib, have good permeability, and are easy and cheap to manufacture and synthesize but suffer from a lack of specificity. Lastly, aptamers are single-stranded DNA, RNA, or peptide sequences with incredible affinity and specificity towards targeted small molecules, proteins, viruses, or cells [[111], [112], [113]]. Compared to antibodies, aptamers are smaller, more stable, and are easier to manufacture and modify with markedly improved antigen recognition and specificity but are rapidly cleared and degraded [113].
In this manuscript, liposomal drug delivery developments employing the targeting of surface, transmembrane and internal cell receptors, enzymes, and dual-targeting of breast cancer and breast cancer-associated cells are presented in Table 2. Details regarding size, composition, etc. of each liposomal system discussed in this review is presented in Table 3. Moreover, the major hurdles regarding the targeted delivery approach to breast cancer are identified and future considerations are highlighted.
Table 2.
Targets and targeting moieties used in the design of targeted liposomal drug carriers for the treatment of breast cancer with associated in vitro and in vivo cell lines.
| Target type | Target | Targeting entity | Application | + Cell line used | - Cell line used | References |
|---|---|---|---|---|---|---|
| CELL SURFACE RECEPTORS | ||||||
| Chemokine Rc | CXCR4 | CXCL12/SDF-1 | MBC, TNBC | HCC1500, MDA-MB-175VII, MDA-MB-436, MDA-MB-231, 4T1 | MCF-10A, MCF-7 | [[114], [115], [116], [117]] |
| Cell surface nucleosomes | Antinuclear Abs | mAb 2C5 | Breast cancer, luminal, MDR, TNBC | BT-20, MCF-7, MDA-MB-231, SK-BR-3, 4T1 | [[118], [119], [120], [121]] | |
| Eph Rc | EphA2 | Anti-EphA2 scFv, YSA peptide |
TNBC, MBC | BT-549, MDA-MB-231, SUM-149PT, EMT-6 | MCF-7/S0.5 | [[122], [123], [124]] |
| Folate Rc | FRα | Folate, FA | MBC, MDR, TNBC | MDA-MB-231, MCF-7, MCF-7/ADR*, SK-BR-3, T-47D, 4T1, TUBO | MCF-10A, MCF-7, A549, JC, L929 | [[125], [126], [127], [128], [129], [130], [131], [132], [133]] |
| ICAM-1 | ICAM-1 | ICAM-1 Ab | TNBC | MDA-MB-231 | MCF-10A | [134] |
| LDL Rc-related protein | LRP1 | Angiopeptide LRP1 ligand | Breast cancer | MT-3c) | [135,136] | |
| Nucleolin | Nucleolin | F3 peptide, AS1411 aptamer | Breast cancer, TNBC, MDR | MDA-MB-231, Hs578T, MDA-MB-435Sb), MDA-MB-231-derived CSCs, MCF-7/ADRa) | T-47D, MCF-7, MCF-7-derived CSCs, MCF-10A, CHO | [[137], [138], [139], [140], [141], [142], [143]] |
| P-gp | P-gp, VM channels | TPGS | MDR | MDA-MB-435Sb), MCF-7, MCF-7/ADRa) | [[144], [145], [146]] | |
| Somatostatin receptor | Somatostatin receptor-2 | Octreotide, somatostatin analogs | Breast cancer, MBC, TNBC | MDA-MB-231, MDA-MB-436, MCF-7, MDA-MB-435Sb), MCF-12A | [[147], [148], [149]] | |
| Sigma Rc | Sigma-1 Rc, Sigma-2 Rc | Haloperidol, SV119 | Breast cancer | MCF-7 | HeLa, KB, HepG2, BEAS-2B, CHO | [150,151] |
| TfR | TfR1 | Transferrin | Breast cancer, luminal | MCF-7 | [152,153] | |
| uPA | uPAR | PAI-2 | TNBC, MBC | MDA-MB-231 | MCF-7 | [[154], [155], [156]] |
| TRANSMEMBRANE RECEPTORS | ||||||
| Biotin | SMVT | Biotin | Breast cancer, Luminal | MCF-7, 4T1 | B16, L929 | [[157], [158], [159]] |
| CD44 Rc | CD44 | HA | MBC, TNBC, Luminal | MDA-MB-231, MDA-MB-231-derived CSCs, MCF-7 CSCs, 4T1 | MCF-7, A549 | [[160], [161], [162], [163], [164], [165]] |
| HER Rc | HER1 | CET, anti-HER1 aptamers | TNBC | SK-BR-3, MDA-MB-468, MDA-MB-231, BT-20 | MCF-7, MDA-MB-453 | [[166], [167], [168]] |
| HER Rc | HER2 | Trastuzumab, HER2 Fab’ fragments, VHH, affibodies, tumor-targeting peptides | Breast cancer, luminal | SK-BR-3, HCC1954, BT‐474, MTSV1-7, MCF-7/Her18, TUBO | MDA-MB-231, MDA-MB-453, MDA-MB-468, MCF-10A, MCF-7, MCF-12A, Calu-6, A549, cE2 | [[169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183], [184], [185], [186], [187], [188], [189]] |
| Integrin Rc | αvβ3, αvβ1, αvβ1, αvβ5, VM channels | RGD, R8GD, cRGD fibronectin-mimetic peptide-amphiphile PR_b | Breast cancer, luminal A, CSCs, TNBC | MCF-7/ADRa), MDA-MB-435Sb), MDA-MB-468, HCC1806, MDA-MB-231, MDA-MB-231-derived CSCs, 4T1 | MCF-7, MCF-10A | [[190], [191], [192], [193], [194], [195], [196], [197]] |
| LHRH Rc | LHRH Rc | Gonadorelin | Breast cancer | MCF-7 | SK-OV-3 | [198,199] |
| MUC1 | Tumor-associated-MUC1 | hCTMO1, MUC1 antigenic peptide, MUC1 aptamer | Breast cancer, TNBC, Luminal | MDA-MB-435, MCF-7, 4T1 | MCF-10A, C33a, HepG2 | [[200], [201], [202], [203]] |
| NRP1 | NRP1 | A7RC peptide, PTD-3, TAT-PTD | Breast cancer, TNBC | MDA-MB-231, 4T1 | MCF-7, SUIT-2 | [204,205] |
| INTERNAL CELL RECEPTORS | ||||||
| Estrogen Rc | ERα | E1, E2, E3 | Luminal, ER+ | MCF-7, T-47D, ZR-75-1 | MDA-MB-231, HCC-1954 | [[206], [207], [208], [209], [210], [211]] |
| ENZYMES | ||||||
| MMP | MMP-2, MMP-9 | Chlorotoxin peptide, EGCG | MBC, breast cancer | MDA-MB-231, 4T1 | [212,213] | |
| Phospholipases | sPLA2 | sPLA2-triggered release | MBC, TNBC | MT-3c) | [214] | |
Cell line inclusion criteria: Breast cell lines included which expressed (i.e., +) the target and cell lines that did not express (i.e., -) the target according to the referenced literature. Cell lines that expressed the target but did not originate from breast tissue were not included. Negative controls not originating from mammary tissue are indicated in red, and rodent cell lines are underlined. It should be noted, that some articles indicated that the negative control cell lines used did express the targeted moiety but at ‘non-high expression’ levels.
Abs: antibodies; anti-EphA2 scFv: anti-erythropoietin-producing hepatocellular single-chain variable fragment; CD44: cluster of differentiation 44; CET: cetuximab; CSCs: cancer stem cells; CXCR4: C-X-C chemokine receptor type 4; CXCL12/SDF-1: CXCR4 receptor ligand; E1, E2, E3: estrone, estradiol, estriol; EGCG: epigallocatechin gallate; Eph: erythropoietin-producing hepatocellular receptor; EphA2: erythropoietin-producing hepatocellular receptor A2; ER+: expression of estrogen receptor; ERα: estrogen receptor α; FA: folic acid; Fab’: fragment antigen-binding with the addition of a thiol group; FRα: folate receptor α; HA: hyaluronic acid; hCTMO1: humanized anti-mucin 1 monoclonal antibodies; HER: human epidermal receptor; ICAM-1: intracellular adhesion molecule-1; LHRH: luteinizing hormone-releasing hormone; LRP1: low density lipoprotein receptor-related protein 1; MBC: metastatic breast cancer; MDR: multidrug resistant; MMP: matrix metalloproteinases; MUC1: mucin 1; NRP1: neuropilin 1; PAI-2: plasminogen activator inhibitor-2; P-gp: p-glycoprotein; Rc: receptor; RGD: arginylglycylaspartic acid, Arg-Gly-Asp; sPLA2: secretory phospholipase A2; SVMT: sodium-dependent multivitamin transporter; TfR: transferrin receptor; TNBC: triple negative breast cancer; TPGS: D-α-tocopheryl polyethylene glycol 1000 succinate; uPA: urokinase-type plasminogen activator; uPAR: urokinase-type plasminogen activator receptor; VHH: variable domain of the heavy chain antibody; VM: vascular mimicry.
Contaminated/misidentified cell line; renamed NCI/ADR-RES, possibly derived from OVCAR-8 [215].
Problematic cell line, derived from melanocytes [216].
Problematic cell line, contaminated with LS-174T cells.
Table 3.
Details of the cited targeted nanoliposomal systems for the treatment of breast cancer. Dynamic Light Scattering (DLS) results for size (nm) and charge/zeta potential (mV) at 25 °C and 7.4 pH were used as far as possible.
| Target type | Targeting entity | Reference | Composition and molar ratio | Size (nm) | Charge (mV) |
|---|---|---|---|---|---|
| CELL SURFACE RECEPTORS | |||||
| CXCR4 | CXCL12/SDF-1 | [114] | DOPC, DODAP, N-dod-PE 65:30:5 |
132 ± 4 | −5.4 ± 1.4 |
| CXCR4 | CXCL12/SDF-1 | [115] | DOPC, DSPE-PEG (2000)-DBCO 93:6 |
94.4 ± 0.6 to 100.1 ± 0.7 | −17.21 ± 1.26 to −6.03 ± 0.88 |
| CXCR4 | CXCL12/SDF-1 | [116] | DOPA, DOPC, CHOL 1:2:1 |
100.6 ± 6.8 | −10 ± 3.3 |
| CXCR4 | CXCL12/SDF-1 | [117] | DPPC, CHOL, DSPE-PEG 150:50:1 |
105 ± 0.6 | 17.8 |
| Cell surface nucleosome | mAb 2C5 | [119,120,120] | HSPC, CHOL, DSPE-mPEG (2000), DTPA-PE 3:2:0.3:0.3 |
90 to 120 | −25 to −23 |
| Cell surface nucleosome | mAb 2C5 | [121] | DOPE, DPPC, EPC, HSPC, DTPA-PE | 170 to 220 | −13 to −20 |
| Eph Rc | Anti-EphA2 scFv | [122] | CHOL, ESM, mPEG-DSG | 110 ± 10 | Negative |
| Eph Rc | YSA peptide | [124] | EPC, CHOL, NHS- DSPE-PEG (2000)-ligand 25:1.28:4.37:1.87 |
87.04 ± 0.80 | 1.73 ± 0.35 |
| Folate Rc | Folate | [132] | DSPC, CHOL, mPEG-DSPE 75.7:18.9:5.4 |
205 ± 2.2 | −13.6 ± 0.9 |
| Folate Rc | Folate | [133] | E80, CHOL, DSPE-PEG (1000), MAL-ligand-DSPE-PEG (2000) 40:25:3:2 |
138.5 ± 6.8 | −9.3 ± 0.8 |
| ICAM-1 | ICAM-1 Ab | [134] | DOPC, DODAP, DSPE-PEG-COOH 85:10:5 |
114 ± 51 | −14.8 ± 0.3 |
| LRP1 | Angiopeptide LRP1 ligand | [135] | PC, CHOL, DCP, OPP, DOPE 50:30:10:20:20 |
∼103 | |
| LRP1 | Angiopeptide LRP1 ligand | [136] | PC, CHOL, DCP, OPP, DOPE 50:30:10:20:20 |
173 ± 2 | |
| Nucleolin | F3 peptide | [137] | DOPE, CHEMS, HSPC, CHOL, DSPE-PEG, DSPE-PEG-MAL 4:2:2:2:0.18:0.12 |
170 ± 12 | |
| Nucleolin | F3 peptide | [138] | DOPE, CHEMS, DSPC, CHOL, DSPE-PEG (2000) 4:2:1:1:0.8:2 |
∼150 | Neutral |
| Nucleolin | AS1411 aptamer | [139] | HSPC, CHOL, DSPE-mPEG (2000) 2:1:0.16 |
210 ± 20 | −15 ± 5 |
| Nucleolin | AS1411 aptamer | [140] | DPPC, CHOL, DSPE-PEG (2000) 60:40:5 |
172.2 ± 43.9 | −7.8 ± 3.3 |
| Nucleolin | AS1411 aptamer | [141] | EPC, DPPC, CHOL 7:3:10 |
128.6 | −6.1 |
| P-gp, VM channels | TPGS | [144] | EPC, CHOL, TPGS 65:30:5 |
104.23 ± 3.32 | 0.24 ± 0.04 |
| P-gp, VM channels | TPGS | [145] | DSPC, DOPE, TPGS 13:3:5 |
∼230 | ∼17 |
| P-gp, VM channels | TPGS | [146] | CHOL, DSPC, DSPE-mPEG (2000), TPGS | 140.0 ± 6.0 | 0.196 ± 0.08 |
| Somatostatin receptor-2 | Somatostatin analogs | [147] | 129.0 ± 10.3 | −13.2 ± 2.1 | |
| Somatostatin receptor-2 | Octreotide | [148] | EPC, CHOL, DSPE-PEG (2000), DSPE-PEG (2000)-ligand, DHA 60:40:2:3:20 |
∼ 100 | 1.84 ± 0.54 |
| Somatostatin receptor-2 | Octreotide | [149] | DDAB, DSPE-PEG (2000)-ligand, DSPE-PEG (2000)-COOH, CHOL, TPGS | 95.3 to 256.6 | 7.2 to 11.3 |
| Sigma Rc | Haloperidol | [150] | DODEAC, CHOL, DSPE-PEG-MAL 1:1:0.05 |
||
| Sigma Rc | SV119 | [151] | SPC, CHOL, ligand-PEG-DOA 7:3:0.5:0.05 |
89.2 to 97.6 | −2.0 to −3.1 |
| TfR1 | Transferrin | [152] | SPC, CHOL, DSPG, DSPE-mPEG (2000) 60:30:8:2 |
133.2 ± 2.12 | −22.86 ± 1.6 |
| TfR1 | Transferrin | [153] | HSPC, CHOL, DSPG, DSPE-PEG (2000) 60:30:8:2 |
133.2 ± 2.12 | −22.86 ± 1.6 |
| uPAR | PAI-2 | [154] | SPC, DSPE-mPEG (2000), CHOL, N-alkylisatin | 141.1 ± 5.0 | −4.66 ± 0.52 |
| TRANSMEMBRANE RECEPTORS | |||||
| Biotin | Biotin | [157,159] | SPC, CHOL 62:33:6 |
∼110 | −2 to −3 |
| CD44 | HA | [160] | DPPC, 1-StePc, DSPE-PEG (2000) 86:10:4 |
90.30 ± 1.40 | −4.37 ± 1.72 |
| CD44 | HA | [161] | EPC, DOPE, CHOL 3:1:1 |
212 ± 15 | −19.0 ± 3.9 |
| CD44 | HA | [162] | EPC, CHOL 5:1 |
∼76 | ∼26 |
| CD44 | HA | [163] | LPC, CHOL, HPPH, drug, chitosan, oleic acid 20:2:2:6:3 |
128.7 ± 75.0 | 29.97 ± 3.5 |
| CD44 | HA | [164] | EDC, NHS, oleic acid 10:1:1 |
158.4 ± 3.3 | |
| CD44 | HA | [165] | DOPC, DOPE, CHOL 1:1:1 |
∼100 | |
| HER1 | CET | [166] | DSPE-mPEG-COOH, NHS, EDC 1:1:1 |
117.45 ± 3.52 | −18.21 ± 1.43 |
| HER1 | CET | [167] | DSPE-PEG (2000), CHOL 65:5 |
||
| HER1 | Anti-HER1 aptamer | [168] | DMKE, CHOL, DSPE-mPEG (2000) 46:46:4 |
165 | −2.7 |
| HER2 | HER2 Fab’ fragments | [174] | HSPC, CHOL, DSPE-mPEG (2000) 56.5:38.5:5 |
137.46 ± 1.35 | −13.2 ± 6.31 |
| HER2 | HER2 Fab’ fragments | [177] | DPPC, CHOL, DSPE-PEG, MAL-PEG-Glu2C18 5:5:0.03:0.03 |
154 ± 7.1 to 250 ± 8.3 | |
| HER2 | Trastuzumab | [178] | HSPC, DSPC, DSPE-PEG, CHOL 6.9:1.6:0.5:0.5 |
123.1 ± 3.1 | −11.1 ± 3.5 |
| HER2 | HER2 Fab’ fragments | [180] | DPPC, CHOL, DSPE-PEG, MAL-DSPE-PEG 1.36:1.36:0.28:0.1 |
120 ± 5 | −5 ± 0.04 |
| HER2 | HER2 Fab’ fragments | [181] | HSPC, CHOL, DSPE-PEG and MAL- DSPE-PEG 56.3:38.4:4.2:1. |
106.74 ± 6.37 | −7.3 ± 0.6 |
| HER2 | Trastuzumab | [183] | SPC, DSPC, CHOL, DSPE-PEG, DSPE-PEG-MAL, MAL-PEG-Glu2C18 4.37:1:0.3:0.3:0.05:0.63 |
119.2 ± 4.9 | −15.2 ± 1.1 |
| HER2 | Trastuzumab | [184] | PC, CHOL, PG, drug, MAL-PEG 50:19:15:1.7:1 |
140 | |
| HER2 | YCDGFYACY-MDV peptide | [186] | DSPC, CHOL, DSPE-mPEG (2000) | ∼80 | |
| HER2 | Anti-HER2 Ab | [188] | DSPC, CHOL, DSPE-PEG (2000)-MAL 70:25:5 |
48.79 ± 0.15 | −6.43 ± 0.45 |
| HER2 | TSA14 aptamer | [189] | HSPC, DSPE-mPEG (2000), MAL- DSPE-PEG (2000), CHOL, α-tocopherol 56.1:2.5:2:38.2:0.2 |
118 ± 2.2 | −20.2 ± 1.2 |
| Integrin Rc | cRGD | [195] | PC, DOTAP, CHOL, DSPE-PEG (2000) 58:7:30:5 |
112.2 ± 6.7 | 35.3 ± 3.1 |
| Integrin Rc | R8GD | [196] | EPC, CHOL, DSPE-PEG (2000), drug and DSPE-PEG (2000)-R8GD 100:25:8:6:40 |
103.33 ± 2.49 | 2.88 ± 0.47 |
| Integrin Rc | RGD | [197] | SPC, CHOL, ligand 62: 33: 3 |
121.9 ± 4.7 | −14.37 ± 4.85 |
| LHRH Rc | Gonadorelin | [198] | HSPC, CHOL, DSPE-mPEG (2000) 90:10:0.4 |
146.1 ± 0.94 | −14.4 ± 0.85 |
| LHRH Rc | Gonadorelin | [199] | HSPC, CHOL, DSPE-PEG (2000) 90:10:0.4 |
136.1 ± 0.94 | −14.4 ± 0.85 |
| Tumor-associated MUC1 | MUC1 aptamer | [200] | DPPC, HSPC, CHOL, DSPE-PEG (2000) 54:27:16:3 |
128.2 ± 1.6 | −28.0 ± 0.8 |
| Tumor-associated MUC1 | hCTMO1 | [201] | HSPC, CHOL, DSPE-PEG (2000) 56.3:38.2:5.5 |
131.3 ± 2.9 | −30.0 ± 1.5 |
| Tumor-associated MUC1 | hCTMO1 | [202] | PPC, CHOL, DSPE-PEG (2000), MAL- DSPE-PEG (2000) 60:40:2.5:2.5 |
215.2 ± 22.2 | −7.2 ± 0.5 |
| NRP1 | A7RC peptide | [204] | CHOL, EPC, DSPE-PEG (2000) 43:52:4.5 |
100 | −15 |
| INTERNAL CELL RECEPTORS | |||||
| Estrogen Rc | E1, E2, E3 | [206] | DOPE, HSPC, CHEMS, CHOL, ligand− DSPE-PEG | 151 ± 5.9 | −24 |
| Estrogen Rc | E1, E2, E3 | [210] | DPPC, CHOL, DSPE-PEG2000-NH2 65:30:5 |
97.1 ± 14.4 | |
| Estrogen Rc | E1, E2, E3 | [211] | SPC, CHOL, mPEG2000-DSPE, ligand- DSPE-PEG (2000) 9:6:0.75:0.075 |
137.93 ± 1.22 | −3.81 ± 0.31 |
| Estrogen Rc | E1, E2, E3 | [207] | PL90 G, PL90 H, CHOL 2.1:1 |
188.8 ± 2.2 | 47 |
| Estrogen Rc | Tamoxifen and QLPVM peptide | [208] | EPC, CHOL, DSPE-PEG (2000) 15.9:4.1:4.8 |
90.87 ± 2.26 | −12.1 ± 0.45 |
| Estrogen Rc | E1, E2, E3 | [209] | SPC, CHOL, SP-DSPE-PEG, ligand-DSPE-PEG 8:2:2:2 |
∼105 | −13.4 |
| ENZYMES | |||||
| MMP-2 | Chlorotoxin peptide | [212,380] | HSPC, CHOL, DSPE-PEG, DSPE-PEG-ligand 20:10:2:0.3 |
128.0 ± 0.99 | −1.76 ± 0.43 |
| MMP-2, MMP-9 | EGCG | [213] | CHOL, PC | 130.5 ± 3.2 | −36.77 |
| sPLA2 | sPLA2-triggered release | [214] | POPC, POPG, CHOL, DSPE-PEG (2000) | 129 ± 1 | −19 ± 1 |
| DUAL TARGETING | |||||
| P-gp and mitochondria | HA and TPGS | [358] | SPC, CHOL, TPGS 27:9:4 |
∼120 | −18 |
| Death receptors 4 and 5 | E-selectin and TRAIL | [359] | EPC, SM, CHOL | 120.3 ± 14 | |
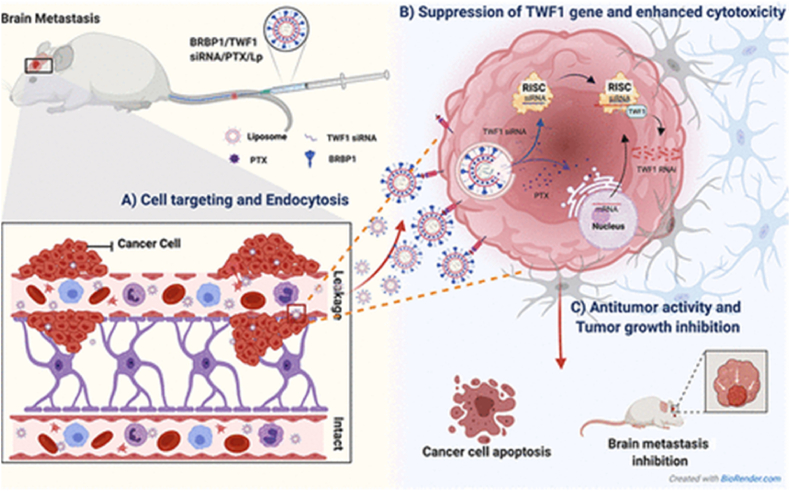
| MDA-MB-231BR cells and mitochondria | BRBP1 and KLA | [362] | DSPC, CHOL, DSPE-mPEG, ligand- DSPE-PEG, drug 20:10:1:1:2 |
123.9 ± 3.7 | −2.39 ± 0.28 |
| GLUT5and αvβ3integrin | RGD and fructose | [364] | CHOL, SPC, ligands 33:64:3 |
113.6 ± 2.1 | −4.20 ± 0.17 |
| Bone and hydroxyapatite | glutamic hexapeptide and FA | [365] | SPC, CHOL, ligands 62:33:3 |
114.2 ± 2.9 | −17.19 ± 2.59 |
| P-selectin and αvβ3integrin | c(RGDfC) and CDAEWVDVS | [366] | DPPC, CHOL, DSPE-PEG (2000) 55:40:5 |
104 ± 3.1 | 2 ± 0.17 |
| HER1 and αvβ3integrin | c(RGDfC) and CYHWYGYTPQNVI | [367] | DPPC, CHOL, DSPE-PEG (2000)-ligand 55:40:5 |
∼105 | ∼4 |
| ATB0,+ and LAT1 | glutamate, lysine, and tyrosine | [370] | DSPC, CHOL, DSPE-PEG (2000) | 110.9 ± 0.7 | −9.20 ± 0.52 |
| gC1qR and NRP1 | LinTT1 peptide | [375] | DPPC, CHOL, ganglioside, DSPE-mPEG (2000)-MAL 6:3:0.6:0.4 |
146 ± 4 | −32.6 ± 2.3 |
| ICAM-1 and HER1 | ICAM1- and EGFR-neutralizing Ab | [376] | DOPC, DSPE-PEG-COOH 95:5 |
130 ± 30 | Between −10 and −6 |
| MUC-1 and CD44 | MUC1- and CD44-aptamers | [377] | POPC, DSPE-PEG, DOPE, CHOL 2:0.1:0.03:1 |
157.8 | −19.57 |
| Extractable nuclear antigens and CPPs | TAT and mAb 2C5 | [378] | HSPC, DOPE, CHOL, PEG (2000)-Hz-PE, ligand-PEG (1000)-PE, ligand-PEG (3400)-PE | 80–100 | −41.00 ± 0.95 |
| Folate Rc and CPPs | FA and dNP2 | [379] | CHOL, SPC, DSPE-PEG (2000)-ligand | 104.1 ± 3.14 | −6.52 ± 1.34 |
Abbreviations: (pNP)2: bis(p-nitrophenyl carbonate); 1-StePc: 1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine; Ab: antibody; anti-EphA2 scFv: anti-erythropoietin-producing hepatocellular single-chain variable fragment; ATB0,+: amino acid transporter B0,+; CD44: cluster of differentiation 44; CET: cetuximab; CHEMS: cholesteryl hemisuccinate; CHOL: cholesterol; CPP: cell-penetrating peptide; cRGD: cyclo arginylglycylaspartic acid, Arg-Gly-Asp; CXCL12/SDF-1: CXCR4 receptor ligand; CXCR4: chemokine receptor type 4; DBCO: dibenzo-cyclooctyne; DCP: dicetylphosphate; DDAB: dimethyldioctadecylammonium bromide; DHA: dihydroartemisinin; DMKE: O,O′-dimyristyl-N-lysyl glutamate; dNP2: a cell-penetrating peptide; DOA: 3′,5′-dioleoyladenosine; DODAP: 1,2-dioleoyl-3-dimethylammonium-propane; DODEAC: N,N-di-n-tetradecyl-N,N-(2-hydroxyethyl)ammonium chloride; DOPA: dioleoyl phosphatidic acid; DOPC: 1,2-dioleoyl-sn-glycero-3-phosphocholine; DOPE: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP: dioleoyl-3-trimethylammonium propane; DPPC: dipalmitoylphosphatidylcholine; DSPC: distearylphosphatidylcholine; DSPE: 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine; DTPA: diethylenetriaminepentaacetic acide anhydride; E1, E2, E3: estrone, estradiol, estriol; E80: egg phosphatidylcholine; EDC: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydro-chloride; EGCG: epigallocatechin gallate; EMT: epithelial-to-mesenchymal; EPC: egg phosphatidylcholine; Eph: erythropoietin-producing hepatocellular receptor; ESM: sphingomyelin from egg; FA: folic acid; Fab’: fragment antigen-binding with the addition of a thiol group; HA: hyaluronic acid; hCTMO1: humanized anti-mucin 1 monoclonal antibodies; HER: human epidermal receptor; HPPH: 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a; HSPC: hydrogenated soy phosphatidylcholine; ICAM-1: intracellular adhesion molecule-1; KLA: acetyl-(KLAKLAK)2-NH2; LAT1: L-type amino acid transporter 1; LHRH: luteinizing hormone-releasing hormone; LPC: 1-palmitoyl-2-hydroxy sn-glycero-3-phosphocholine; LRP1: low density lipoprotein receptor-related protein 1; MAL: maleimide; MMP: matrix metalloproteinases; mPEG2000-DSPE: N-(carbonyl-methoxy-poly(ethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt; mPEG-DSG: 1,2-distearoyl-rac-glycero-3-methylpo-lyoxyethylene; MUC1: mucin 1; N-dod-PE: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-dodecanoyl; NHS: NHS ester; NRP1: neuropilin 1; OPP: octadecyl-1,1-dimethylpiperidin-1-ium-4-yl phosphate; PAI-2: plasminogen activator inhibitor-2; PC: phosphatidylcholine; PE: phosphatidylehtanolamine; PEG: polyethylene glycol; PG: phosphatidylglycerol; P-gp: p-glycoprotein; pNP: p-nitrophenylcarbonyl; POPC: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; POPG: 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) sodium salt; Rc: receptor; RGD: arginylglycylaspartic acid, Arg-Gly-Asp; SPC: soy phosphatidylcholine; sPLA2: secretory phospholipase A2; TAT: trans-activator of transcription cell-penetrating peptide; TfR1: transferrin receptor 1; TPGS: D-α-tocopheryl polyethylene glycol 1000 succinate; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; TUBO: cloned line established in vitro from a BALB-neuT mouse mammary carcinoma; uPAR: urokinase-type plasminogen activator receptor; VM: vascular mimicry.
2.1. Cell surface receptors
2.1.1. C-X-C chemokine receptor type 4
C-X-C chemokine receptor type 4 (CXCR4) is abundant in numerous tissues where they bind to G protein-coupled receptors to direct cell movement, traffic in developing embryos and specific adult tissues such as the extension of neurites and axons in neurons, and are involved in tumor metastasis and invasion [217]. CXCR4 is expressed on the plasma membrane of most cells, including hematopoietic and endothelial cells, neurons, stem cells, and cancer cells, and has been associated with hematological malignancies and poor prognosis in solid tumors such as breast cancer [218,219]. Recent advances have demonstrated the critical role that the CXCR4 receptor and its ligand CXCL12, or stromal cell-derived factor 1 (SDF-1) [220], play in breast cancer metastasis; as CXCL12 is a chemoattractant, when it's concentrated within a tissue it draws CXCR4+ tumor cells to the location thus establishing a secondary metastatic site. It is thus no surprise that breast cancer metastasis occurs in tissues with high levels of CXCL12, i.e., the lungs, bones, and lymph nodes [221].
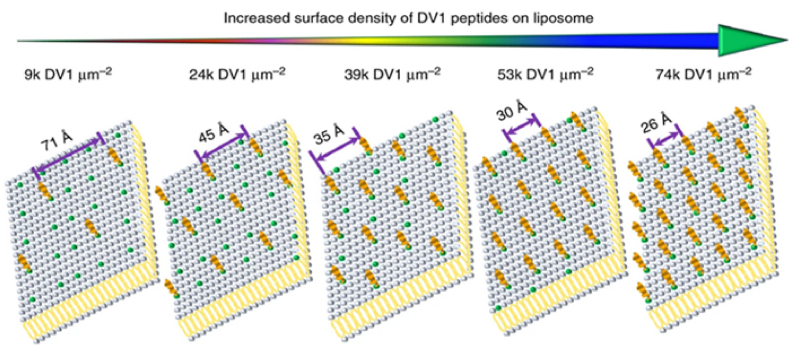
Using CXCR4-targeted, pH-responsive liposomes encapsulated with lipocalin 2 (Lcn2) small interfering RNA (siRNA), Guo et al. investigated the ability of the liposomal system to specifically inhibit cell migration of metastatic breast cancer [114]. In this approach, the team used receptor inhibition as well as the silencing of Lcn2, an upregulated protein in many human epithelial cancers associated with the epithelial-to-mesenchymal transition (EMT). The combination of CXCR4 coupling and Lcn2 silencing significantly reduced the migration of TNBC cells. In another example, Liu et al. demonstrated that different densities of a CXCR4 binding peptide (DV1) functionalized on liposomes, not only influenced the in vitro uptake of the nanocarrier but could also, via cell surface signaling, caused cell migration to cease in TNBC due to the down-regulation of cell-motility proteins [115]. The team demonstrated that the most favorable binding density of the DV1 peptide was 24k molecules μm−2 (Fig. 5) and, when mice were treated with these optimally targeted liposomes, five out of six mice demonstrated no metastases over 27 days.
Fig. 5.
Example of CXCR4 liposomal targeting of breast cancer. Illustration of a 2D CXCR4 binding peptide (DV1) array for 3D liposomal peptide density. Reprinted with permission from Ref. [114]. Copyright 2018 Springer Nature Limited.
As CXCR4 is associated with enhanced immunosuppression within the TME, Lu et al. [116] designed a CXCR4 targeting liposome to enhance the therapeutic efficacy of the CXCR4 antagonist, AMD3100. AMD3100 is currently the only CXCR4 antagonist and was approved by the FDA in 2008 for non-Hodgkin's lymphoma and multiple myeloma patients. In the team's unique design, AMD3100 was encapsulated in the liposome as well as coated onto the surface thus acting as a targeting moiety and treatment system by inhibiting CXCR4 activation both extracellularly (via the coating interaction) and intracellularly (via payload delivery). The AMD3100-functionalized and loaded system led to the reprogramming and remodeling of the immune and stromal TME. Zhang et al. developed a peptide-directed liposomal drug delivery system that combined both chemotherapy and photothermal therapy for the treatment of breast cancer [117]. The novel peptide, p12 (QGSRRRNTVDDWISRRRALC), was conjugated to PEGylated liposomes containing both DOX and indocyanine green (ICG), a commonly used photothermal sensitive molecule. The team demonstrated that the targeting p12 peptide helped route the liposomal system to preferentially accumulate in the tumor sites thus reducing DOX-associated side effects, i.e., cardiotoxicity and tumor metastasis. Moreover, the ICG molecule enabled the precise and controllable release of DOX upon activation at > 41 °C in the targeted zone.
Notably, although the CXCL12/CXCR4 biological axis is a promising pathway for cancer treatment, as demonstrated by the approval of AMD3100 for clinical use, the exact regulatory mechanisms of the axis and its antagonists are not fully understood. Furthermore, prolonged administration of CXCR4 antagonists has been associated with adverse cell mobilization effects (e.g., leukocytosis, thrombocytopenia, spleen enlargement, etc.) due to the ubiquitous presence of CXCR4 in the heart, spleen, liver, kidneys, etc. [222].
2.1.2. Cell surface nucleosomes
As cells undergo apoptosis or necrosis, the nuclear content of the cell is exposed to the extracellular milieu prompting the production of antinuclear antibodies which recognize these nuclear components and as such are considered biomarkers of systemic immune disorders. Several groups of antinuclear antibodies have been identified and associated with specific pathologies, for example, anti-double-stranded DNA antinuclear antibodies are considered a biomarker and a pre-clinical indicator of systemic lupus erythematosus [223]. Extractable nuclear antigens, a group of antinuclear antibodies named for their ability to be extracted from the cell nucleus with saline, recognize ribonucleoproteins and non-histone proteins (e.g., Smith (Sm), ribonucleoprotein, scleroderma 70 (Scl-70), etc.). These antigens have been used as biomarkers for cancer [224]; particularly for breast cancer where they are considered an aid in early diagnosis [225].
Over the course of several years, Torchilin and colleagues have developed an antinuclear antibody mAb specific for tumor-associated cell surface nucleosomes, referred to as 2C5 (mAb 2C5), which can recognize various types of tumors. The team developed mAb 2C5-targeted Doxil® liposomes to target and induce anticancer effects on several cell lines [118,119], and demonstrated a 3 to 8-fold increase in the binding and internalization, with significantly higher toxicity including those resistant to DOX. Following their in vitro success, the group studied mAb 2C5-targeted liposomes in vivo [120]. Using 111In-labeled liposomes and whole-body γ-scintigraphic imaging, the group showed the enhanced accumulation of mAb 2C5–targeted liposomes in tumors and significantly superior anticancer activity in the subcutaneous murine tumors of 4T1 nude mice models. In 2021, Narayanaswamy and Torchilin combined two chemotherapeutics, PTX and salinomycin, to simultaneously target and treat breast cancer cells and cancer stem cells (CSCs) to prevent cancer growth and metastases [121]. As of 2022, however, no clinical trials for the mAb 2C5 has been registered on clinical.trials.gov and the team have started exploring the use of a different drug carrier (micelles, dendrimers).
2.1.3. Erythropoietin-producing hepatocellular carcinoma (Eph) receptors
Membrane-bound erythropoietin-producing hepatocellular carcinoma (Eph) receptors are a large family of tyrosine kinase receptors, that play critical roles in cell-cell interactions, proliferation, differentiation, signaling, migration, and tissue morphogenesis, as well as in many pathological processes [226]. Of the 14 known Eph receptors, Eph class A2 (EphA2) receptors have the strongest links to cancer and have been detected in brain, bladder, breast, lung, skin, ovarian, and prostate cancers [227]. Specifically, EphA2 is involved in the proliferation, angiogenesis, drug resistance, progression, migration, and metastasis of breast cancer [228]. The development of EphA2 targeting systems is especially intriguing as highly aggressive breast cancer tumors with no ERα expression have shown consistently higher expression of EphA2 [228]. Thus, several research groups have developed EphA2-targeting agents.
One such group developed an EphA2-targeted nanoliposomal drug carrier loaded with docetaxel, named MM-310, for the treatment of assorted tumor types including TNBC [122], and have completed phase I clinical trials to determine the safety of the treatment strategy in humans (NCT03076372) [229]. As of 2022, no additional results from this trial has been published. The same team explored the combination of a chemo and immunotherapeutic strategy wherein the checkpoint inhibitors anti–programmed cell death receptor 1/programmed cell death ligand 1 and anti-T-lymphocyte-associated protein 4-antibodies, which are linked to tumor resistance and recurrence due to the low intratumoral presence of T cells, were combined with docetaxel which is known to increase the levels of T cells in TNBC [230]. In the TNBC tumor model, the combination of EphA2-functionalized docetaxel and anti-programmed cell death receptor 1-loaded liposomes showed a 60% response rate resistant to rechallenge and large immunomodulatory response. Stealth liposomes encapsulating DOX have also been conjugated with the homing peptide YSAYPDSVPMMSK and investigated both in vitro and in vivo [124]. Interestingly, the YSAYPDSVPMMSK-modified liposomes facilitated the efficacy of DOX by inducing cancer cell apoptosis, inhibiting tumor growth and CD31 expression, as well as diminishing the capacity of the tumoral cells to undergo angiogenesis and metastasis.
2.1.4. Folate receptor
Reduced folates are key components in the metabolism of amino acids and the synthesis of DNA/RNA, and are thus required for normal cell survival. Folate receptors are glycoprotein vitamin receptors with four known isoforms (α, β, γ, and δ) differentially expressed in several tissues [231]. Due to the high demand for folate in DNA repair during carcinogenesis, folate receptor-α is often overexpressed in tumors and has thus become a biomarker and therapeutic target for brain, lung, colorectal, ovarian, and breast cancers [232,233].
Folate-coated long circulating pH-sensitive liposomes have been thoroughly researched for the treatment of metastatic, MDR, and TNBC. An interesting example of folate receptor-targeted liposomes involves the work of Gazzano et al. who used DOX conjugated to NO-releasing groups to overcome P-gp drug efflux transporters in MDR breast cancer [132]. Folate was inserted onto the surface of the liposomes and upon uptake localized towards both the nucleus and the mitochondria where the DOX induced DNA damage, cell cycle arrest, and triggered mitochondria-dependent apoptosis. The team's liposomal system reduced the growth of P-gp and folate receptor-expressing breast cancer tumors in mice whereas DOX and Caelyx® failed. Most encouragingly, however, was that primary tumoral cells and cells derived from the exposed tumors remained responsive to the treatment over several treatment cycles. Another example is the work of Deng et al., who sought to exploit the use of matrix metalloproteinases (MMPs), specifically MMP-2, to cleave PEG chains over time from folate-functionalized and DOX-encapsulating liposomes to serve as a chemotherapy-induced ‘tumor vaccine’ [133]. Normally, this approach is challenging as chemotherapy induces immunogenic cell death, poor T cell activation and the general immunosuppressive environment of the TME, the team's approach was to target both 4T1 breast cancer cells and tumor promoting tumor-associated macrophages (M2-TAMs) via the folate receptor causing an enhanced immune response coupled with the elimination of M2-TAM whilst simultaneously using cytosine-phosphate-guanine therapy to improve T cell response. The team's combination therapy also considerably inhibited lung metastasis and the growth of metastasized nodes in the breast cancer models. Notably, because folates are so ubiquitous in cellular mechanisms, folate receptor targeting with a folate-functionalized nanocarrier can be impeded by circulating folates (i.e., due to the patient's diet) and, due to high expression of folate receptors in normal kidney tissues, persistant accumulation of nanocarriers is not uncommon.
2.1.5. Intercellular adhesion molecule-1
Intercellular adhesion molecule-1 (ICAM-1) is a cell surface receptor widely associated with cell adhesion and the recruitment of leukocytes to inflammation sites [234]. More recently, ICAM-1 has been linked to tumorigenesis via its promotion of tumor-immune cell adhesion and communication leading to more aggressive and invasive tumor phenotypes [235,236]. Although the specific mechanism of this communication and cell signaling pathways have not been fully defined, the interaction between ICAM-1 and mucin 1 (MUC1) causing the activation of the MAPK/ERK signaling cascade leading to migration in the surrounding tumoral cells has been implicated [[237], [238], [239]]. Thus, due to this interplay between the glycoprotein receptor and tumor metastasis and aggression, ICAM-1 has been investigated as a prognostic marker [235,240].
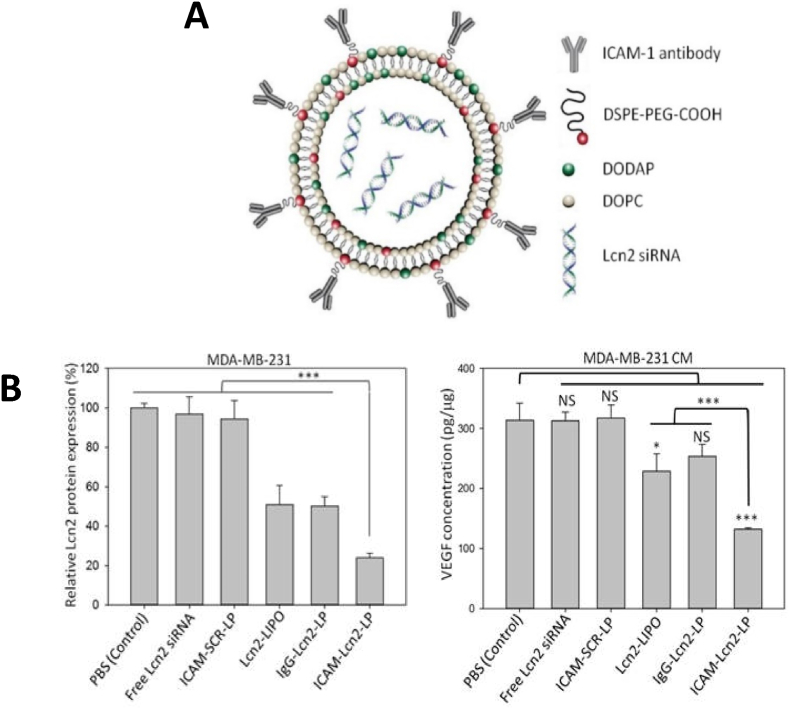
For example, Guo et al. developed a pH-sensitive liposomal drug delivery system to target and treat TNBC via anti-ICAM-1 Ab-functionalization and Lcn2 siRNA encapsulation [134] (Fig. 6). Lcn2 levels have been linked to breast cancer status and poor prognosis due to their affiliation with inducing EMT in breast cancer cells. In their study, ICAM-1-targeted liposomes bound significantly stronger to MDA-MB-231 cells as compared to the non-neoplastic MCF-10A cells, whilst efficient knockdown of Lnc2 by the siRNA encapsulation led to a reduction in VEGF production mitigating angiogenesis in both in vitro and in vivo TNBC models.
Fig. 6.
Example of ICAM-1 liposomal targeting for breast cancer. (A) Schematic of the Lnc2-encapsulating ICAM-1-functionalized liposomes (ICAM-1-Lnc2-LP). (B) Relative Lnc2 protein levels in MDA-MB-231 cells after Lnc2 gene knockdown by the ICAM-1-Lnc2-LPs, accompanied by VEGF concentration in the conditioned media (CM) collected from the knockdown MDA-MB-231 cells. Adapted from Ref. [134].
2.1.6. Lipoprotein receptor-related protein-1
The low-density receptor-related protein 1 (LRP1) (also referred to as apolipoprotein E receptor (APOER) or CD91) is a cell signaling receptor expressed on several cell types, including astrocytes and neurons, fibroblasts and smooth muscle cells, hepatocytes, macrophages, and carcinogenic cells. LRP1 is part of the LDL family of receptors which recognize a huge variety of ligands, including various proteases, matrix proteins, protease/inhibitor complexes, growth factors, and intracellular proteins [241], and as such is involved in several varied biological processes such as cell migration, the degradation of proteases, the activation of lysosomal enzymes, lipoprotein metabolism, the entry of viruses and bacterial toxins into cells, etc. Recently, LRP1 has also been reported to play key roles in tumorigenesis and tumor progression [242,243], with its discovery on the leading edge of breast cancer cells implying its involvement in cytoskeletal organization and cell-matrix interactions for protrusive structures used for cell migration [244].
To improve drug transport through the BBB, Orthman et al. prepared 19-mer angiopeptide (also referred to as angiopep-2 or ANG1005)-functionalized fluid or rigid membrane liposomes as a means to target LRP1 in both subcutaneously implanted breast cancer cells and intracerebrally implanted brain tumors in mice [135]. Fluid, ligand-bearing liposomes proved to have higher cellular uptake and were able to significantly reduce tumor volume, and brain metastasis, with a reduced drug toxicity effect. The group observed that ligand-modified liposomes had better endocytosis-induced cellular uptake but, more interestingly, that the presence of a receptor-ligand modification did not affect the intracellular transport (transcytosis) of the drugs; rather, the fluidity of the liposomes played a greater role in the dissemination of the drug throughout the cell.
In a continual effort to decrease side effects and boost the use of already established anticancer drugs, the same group used the drug oxaliplatin, a highly potent neurotoxic chemotherapeutic rarely used to treat breast cancer metastasis to the brain as it cannot cross the BBB, to treat primary breast cancer tumors and its brain metastasis [136]. LRP1-functionalized oxaliplatin-loaded liposomes were introduced in vitro MDCK cells and demonstrated a 12-fold higher uptake and 2.25-fold greater transcytosis than non-targeted liposomes. For in vivo experiments, both subcutaneous and intracerebral tumors were prepared with MT-3 cancer cells and treated with various iterations of oxaliplatin liposomes. Although the oxaliplatin-loaded liposomes with a fluid membrane caused significantly greater inhibition of both the subcutaneous and intracerebral MT-3 tumors than the free oxaliplatin, the LRP1-targeted liposome variations showed no superior effects in vivo. Furthermore, it should be noted that the MT-3 cell line is known to be contaminated with LS-174T colon adenocarcinoma cells (see Table 2).
2.1.7. Nucleolin
Nucleolin is a major protein component of the nucleolus and is involved in a cell's regulation of transcription, proliferation, and growth [245]. Whilst approximately 90% of nucleolin is found in the nucleolus, the protein is detectable on the cell surface and in the cytoplasm of various cancer cells [246]. Once at the cell surface, the protein is involved in shuttling molecules between the nucleus, cytosol, and the cell surface, and has been implicated in mechanisms involving leukocyte trafficking and inflammation, cell adhesion and differentiation, as well as angiogenesis and tumor development [[247], [248], [249]]. Nucleolin overexpression on the cell membrane is linked to tumor progression, metastasis, and drug resistance of various cancers [246,250,251], and has been found to interact with tumor-promoting proteins and receptors such as VEGF and HER2 [251,252].
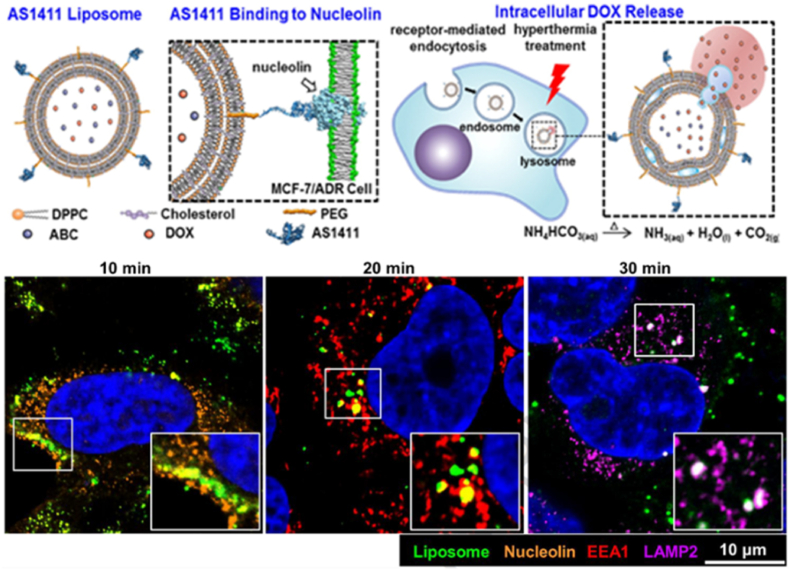
Moura et al. assessed the ability of a nucleolin tumor-homing F3 peptide (KDEPQRRSARLSAKPAPPKPEPKPKKAPAKK) to target two different cell populations, namely endothelial and cancer cells from angiogenic blood vessels, functionalized on a PEG stabilized pH-sensitive liposome containing DOX [137]. The team identified the nucleolin receptor in the neovascularization of 30 tumor samples of patients diagnosed with invasive breast cancer but not in mature blood vessels or the mammary ducts. Cells with positive staining for the nucleolin receptor were harvested and, after incubation with both F3-functionalized and non-targeted liposomes, were able to bind and internalize the functionalized nanocarrier. F3-targeted liposomes also demonstrated a 9.7 to 17-fold greater accumulation in nucleolin receptor overexpressing breast cancer cell lines (MDA-MB-231 and Hs578T) and a 10.4-fold increased accumulation in an angiogenic blood vessel cell line (HMEC-1) overexpressing the nucleolin receptor as compared with low-level expression (T47D and MCF-7) breast cancer cell lines. It should be noted, however, that although the group designated their use of the MDA-MB-435S as a breast cancer cell line, the origin of these cells has been disputed and related to melanoma instead [216]. The F3-targeted liposomes also improved the cytotoxicity of DOX by 177- and 162-fold towards breast cancer and endothelial cells, respectively, relative to generic non-targeted pH-sensitive liposomes. The same team explored the use of the F3 peptide-targeted liposomes to identify a common receptor for both CSCs and certain non-stem cancer cell lines (from which CSCs are thought to originate via EMT) [138]. Their results demonstrated a clear link between nucleolin expression and the stem cell-like phenotype often seen in TNBC. Furthermore, a synergistic relationship between the dual-loaded drugs, DOX and C6-ceramide, was shown to increase cellular toxicity against CSCs, non-stem cancer cells, and tumoral angiogenic blood vessels. Subsequently, the team has further developed and applied the F3-liposome targeted system to target various other cancers, the tumor microenvironment, and other CSCs [[253], [254], [255], [256]]. AS1411 is a nucleolin-targeting, anti-proliferative DNA aptamer developed in the late 1990s [257] which has undergone phase I and II clinical trials (NCT01034410, NCT00881244, NCT00740441, NCT00512083) for the treatment of advanced solid tumors, acute myeloid leukemia, and renal cell carcinoma. Naturally, the AS1411 aptamer has also been employed as a nuclear-targeted liposomal drug delivery system enabling the intranuclear release of anticancer drugs for various cancers, including breast cancer. In 2013, Xing et al. showed that increased cellular internalization and cytotoxicity of MCF-7 tumors in athymic nude mice could be achieved with AS1411-functionalized liposomes [139]. To address the need for treatment and targeting strategies for MDR, Liao et al. prepared AS1411-functionalized liposomes loaded with DOX and the bubble-generating agent ammonium bicarbonate to target DOX-resistant breast cancer cells (MCF-7/ADR) (Fig. 7) [140]. Molecular dynamic simulation studies indicated that the contact between the non-targeted liposomes and the nucleolin receptors was not favorable, whilst the G-quadruplex structure of the AS1411–nucleolin complex was spontaneous and exceptionally stable as demonstrated by the large negative binding energy. In vivo studies in MCF-7/ADR tumor-bearing, nude mice demonstrated AS1411-functionalized liposomes had greater cellular internalization concentrations of DOX in tumor tissue than free DOX or PEGylated liposomes and were able to greatly decrease tumor growth and reduce systemic effects such as cardiotoxicity. Similarly, Li et al. designed DOX-loaded liposomes which circumvented the MDR P-gp action by utilizing AS1411 targeting to directly deliver DOX into the cell nuclei thus having AS1411 serve as an intracellular targeting system [141]. Treatment systems with and without combination chemotherapeutics have also been developed using AS1411 aptamer-functionalized liposomes for breast cancer treatment [142,143].
Fig. 7.
Example of nucleolin liposomal targeting in breast cancer. Schematic showing the structure of an AS1411-functionalized liposome and its binding to nucleolin on the cell surface, followed by receptor-mediated endocytosis, and intracellular DOX accumulation. DOX release is activated by the formation of CO2 bubbles via the reduction of encapsulated ammonium bicarbonate by heat. Adapted and reprinted with permission from Ref. [140]. Copyright 2015 Elsevier.
2.1.8. P-glycoprotein
P-gp is a cell surface ATP-dependent efflux pump responsible for the elimination of various substances in the cell. In normal tissue, P-gp is expressed on the apical membranes of hepatocytes, enterocytes, and brain endothelial cells where they recognize a variety of substrates and serve to limit the absorption of harmful substances, including toxins and drugs, into the liver, kidneys, and intestine as well as limiting penetration across the BBB of orally administered drugs [258]. As such, P-gp overexpression is infamously known to lower the intracellular concentrations of several anticancer agents to sub-therapeutic levels and has been described as one of the main mechanisms of MDR [259,260].
Many P-gp modulators and inhibitors, e.g., TPGS, have been developed for use alongside nanocarriers. TPGS is formed by an esterification reaction between vitamin E and PEG and, due specifically to the vitamin E component, possesses enhanced cellular internalization abilities via membrane receptors [261,262]. TPGS has also been shown to cause the extravasation of electrons from the mitochondrial respiratory complex II resulting in the generation of ROS [261]. Not surprisingly, TPGS has been used in the development of targeted liposomes in combination with both conventional chemotherapeutics and other compounds for breast cancer treatment. For example, Han et al. loaded PTX, a known substrate of P-gp, into TPGS-functionalized liposomes to assess whether the nanocarrier could produce a controlled, long-term release of PTX [145]. In MCF-7/ADR cells, the uptake of TPGS-functionalized PTX-loaded liposomes was increased 3.56-fold at 2 h and 5.75-fold at 4 h as compared to non-targeted PTX-loaded liposomes at similar times. Further analysis also confirmed an inhibitory effect on the P-gp pumps and an accompanying cytotoxic effect on the cells. Li et al. attempted to reverse MDR by developing a synergistic TPGS-functionalized docetaxel-loaded liposomal system for breast cancer [146]. The TPGS-functionalized liposomes demonstrated enhanced intracellular accumulation and cytotoxicity in wild-type MCF-7 and drug-resistant MCF-7 breast cancer cells whilst simultaneously inhibiting the function of the P-gp pump. Another interesting application of TPGS-functionalized liposomes relates to the phenomenon of vascular mimicry (VM) channels. For instance, Shi et al. encapsulated sunitinib (a targeted chemotherapeutic for tyrosine kinase receptors) and vinorelbine within TPGS-coated liposomes to treat invasive breast cancer and its associated VM channels [144]. The team demonstrated in both in vitro MCF-7 and in vivo MDA-MB-435S models that TPGS-functionalized liposomes had enhanced internalization and accumulation within the mitochondria of VM neovascular cells. Once there, the dual-loaded chemotherapeutics induced cell death via the upregulation of caspase 9 and caspase 3 and downregulated several proteins associated with VM channel formation, e.g., EphA2, MMP-9, vascular endothelial (VE)-cadherin, and hypoxia-inducible factor 1-alpha (HIF-1α). It should be noted, however, that whilst the team did observe increased cellular uptake with the TPGS-functionalized liposomes in vivo, they partially credited this phenomenon to the liposomes being of favorable size for long-term circulation. Furthermore, there is some debate regarding the tissue origin of the MDA-MB-435S cell line.
2.1.9. Somatostatin receptor
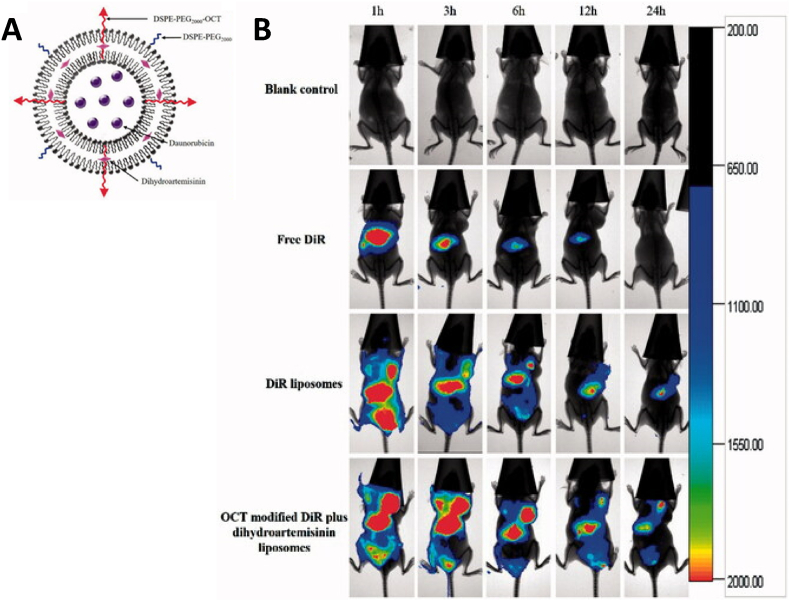
Somatostatin receptors are G protein-coupled receptors most commonly expressed by the pancreas, cerebrum, kidneys, jejunum, colon, and liver. Five receptor subtypes, termed somatostatin receptors 1–5, along with their ligands, somatostatin, somatostatin analogs, and octreotide, are currently known [263]. All five receptors are to some extent involved in the regulation of cell division, secretion, proliferation, and apoptosis. Somatostatin receptor-2 is found almost exclusively at the plasma membrane of central and myenteric neurons, neuroendocrine cells of the gastric antrum, anterior pituitary pancreatic islets, and tumors, such as breast cancer [264,265]. Bharti et al. used the synthetic somatostatin analog 2 targeting agent diacerein, a drug used to treat swelling and pain in the joints, loaded in liposome nanocarriers for the treatment of TNBC [147]. The targeted system showed improved circulation times as compared to non-targeted controls and was able to suppress the IL-6/IL-6R/STAT3/MAPK/Akt signaling pathways involved in cancer development and growth, as well as suppressing angiogenesis and cancer cell invasion. Similarly, octreotide-modified liposomes loaded with both daunorubicin and dihydroartemisinin, a poorly water-soluble drug used to treat malaria, were evaluated for their ability to treat breast cancer [148]. In vitro results showed good cellular uptake of the octreotide-functionalized liposomes with the combinatorial treatment enhancing the cytotoxicity, and blocking tumor cell wound healing and migration. In vivo experiments performed on MDA-MB-435S xenograft mice showed prolonged circulation times leading to enhanced accumulation of the targeted liposomes at the tumor sites, and thus an excellent overall antitumor efficacy and no obvious side effects (Fig. 8).
Fig. 8.
Example of somatostatin receptor liposomal targeting in breast cancer. (A) Schematic representation of octreotide (OCT)-modified liposomes dual-loaded with daunorubicin and dihydroartemisinin. (B) Increased accumulation of the OCT-modified liposomes loaded with a dye (DiR) and dihydroartemisinin in MDA-MB-435S xenograft tumors in mice after intravenous administration. Adapted and reprinted with permission from Ref. [148]. Copyright 2018 Elsevier.
Gote & Pal also engineered an octreotide-functionalized PEGylated liposomal system but encapsulated Lcn2 siRNAs for the selective targeting and treatment of metastatic and TNBC [149]. In vitro uptake and intracellular studies indicated a higher uptake at 6 h in both MCF-7 and MDA-MB-231 cells accompanied by the silencing of the Lnc2 mRNA (55–60%).
2.1.10. Sigma receptor
Although discovered over four decades ago, sigma receptors remain largely obscure and poorly understood. Sigma receptors are membrane-bound proteins, originally thought to be related to the opioid receptor family but are now recognized as distinct proteins found in the plasma, endoplasmic reticulum, and mitochondrial membranes of the brain, kidneys, and liver [266,267]. Two receptor subtypes, sigma-1 and sigma-2 receptors have been discovered and linked to critical roles in the nervous system with steroid hormones (especially progesterone), sphingolipid-derived amines, and haloperidol (an antipsychotic medication) identified as ligands [268].
Both sigma-1 and sigma-2 receptors are overexpressed in cancer cells, including breast cancer, and have been implicated as possible drug delivery targets [269,270], however few of these strategies have involved liposomes. Mukherjee et al. modified haloperidol-linked lipids with cationic lipid-DNA complexes to specifically target the sigma-1 receptors of breast cancer cells [150]. Haloperidol was conjugated to the distal end of PEG and incorporated into cationic liposomes known for their ability to deliver genes intracellularly. The resulting haloperidol-functionalized lipoplexes produced a 10-fold greater reporter gene expression in MCF-7 cells than in control lipoplexes. The Mukherjee et al. study demonstrated for the first time that haloperidol-functionalized delivery systems could be used to deliver genes in breast cancer cells via overexpressed sigma receptors thus introducing a new class of therapeutics for cancer treatment [271]. More recent work regarding sigma receptor targeting was the development of SV119; a synthetically engineered small molecule able to bind exclusively to sigma-2 receptors with high affinity and specificity. Zhang et al. explored the potential of using SV119 conjugated to liposomes as a targeting ligand and apoptosis-inducing peptide for several cancer cell lines, including prostate, lung, and breast cancers [151]. Their results demonstrated that the incorporation of the peptide on the PEGylated liposome significantly increased cellular uptake in all the cancer cell lines but not normal cells. As expected, the SV119-functionalized DOX-encapsulating liposomes demonstrated increased cytotoxicity as compared to non-targeted and unloaded liposomes.
2.1.11. Transferrin receptor
Iron is an essential component of cancer cell proliferation making its receptor, the transferrin receptor (TfR), an attractive avenue for targeted drug delivery. TfR is a membrane glycoprotein with two isoforms, TfR1 and TfR2. TfR1, also known as CD71, cell surface receptor expressed at low levels in normal tissues while TfR2 is largely restricted to hepatocytes [272]. TfR1 has been correlated with cancer proliferation, migration, invasion, apoptosis, and metastasis [273] and is abundantly expressed in liver, breast, lung, and colon cancer cells [274].
Gandhi et al. formulated transferrin-functionalized epirubicin-HCl liposomes able to target breast cancer cells with improved in vitro uptake and excellent safety and distribution profiles capable of minimizing the cardiotoxicity normally associated with the chemotherapeutic [152]. Similarly, Fu et al. developed a transferrin-functionalized co-loaded liposomal delivery system to deliver both sorafenib and DOX for an enhanced antitumor effect [153]. Sorafenib, a hydrophobic drug loaded in the phospholipid bilayer of the liposome carrier, inhibited tumor cell proliferation and blocked angiogenesis. In vitro experiments verified that transferrin-functionalized liposomes demonstrated the highest uptake and that the combination of the two drugs inhibited tumor growth more effectively than the monotherapy controls.
2.1.12. Urokinase plasminogen activator receptor
The plasminogen activator system is an extracellular enzymatic cascade system consisting of the urokinase-type plasminogen activator (uPA), its receptor (uPAR), and plasminogen activator inhibitors-1 and -2 (PAI-1 and PAI-2, also referred to as serpinE1 or B2). Once the ligand uPA binds to its receptor uPAR, it activates the conversion of plasminogen to plasmin which is an essential component of the proteolytic cascade needed for normal tissue reorganization such as the remodeling of the extracellular matrix (ECM) and cell surface, wound healing, and mammary gland involution [275]. Unfortunately, this same system is usurped by tumor cells for migration to secondary locations. Although several protease systems have been implicated in this process, the uPA system has been identified as a central player implicated in tumor progression, metastasis, angiogenesis, cancer cell adhesion, migration, and EMT [276]. As such, the system's components are considered a diagnostic biomarker for several malignancies and cancers, including TNBC where its elevation is correlated to poor clinical outcomes, more aggressive primary tumors, metastasis, and recurrence [277].
An excellent example of uPAR used as an active targeting agent includes the work of Belfiore et al. who encapsulated N-alkylisatin, a potent microtubule destabilizing agent capable of evading P-gp drug efflux in cancer cell lines, in PAI-2-functionalized liposomes. Previous work by the group confirmed that uPA could be efficiently and specifically inhibited by PAI-2 as well as rapidly internalized [155,156]. In their recent work, the authors showed that the in vitro uptake of PAI-2-functionalized liposomes was greater in the uPA/uPAR overexpressing MDA-MB-231 than in the relatively lower expressing MCF-7 cells [154]. Further in vivo testing determined that a maximum of 0.5% of the initial injected dose of PAI-2-functionalized N-alkylisatin-loaded liposomes was detectable within primary tumors compared to the 0.02% for non-targeted N-alkylisatin-loaded liposomes (Fig. 9). It is interesting to note, however, that Doxil® (a non-targeted liposome) typically has a 1% or less accumulation rate in primary tumors.
Fig. 9.
Example of uPAR liposomal targeting in breast cancer. Biodistribution and pharmacokinetics of the radiolabeled N-alkylisatin-loaded liposomes (N-AI) and plasminogen activator inhibitor-2 (PAI-2)-functionalized N-alkylisatin-loaded liposomes (N-AI PAI-2) over time. Measurements were taken in the plasma, primary tumor, kidneys, liver, spleen, and lungs of mice after one intravenous bolus injection. Note that 0.5% of the dose of PAI-2-functionalized liposomes was observed in the primary tumor soon after injection. Adapted from Ref. [154].
2.2. Transmembrane receptors
2.2.1. Biotin receptor
Biotin is an essential vitamin required for carbohydrate, amino acid, and lipid metabolism. At extracellular concentrations exceeding 25 μM, biotin passively diffuses across cell membranes but carrier-mediated uptake via biotin receptors and sodium-dependent multivitamin transporters (SMVTs) predominates below 5 μM [278]. SMVTs are found in the absorptive tissues of biotin, for example, the intestinal mucosa, kidneys, liver, brain, lung, heart, skeletal muscles, and placenta, and serves to translocate vitamins and other essential cofactors [279]. Some evidence suggests that biotin receptors and SMVTs are correlated with cancer, including breast cancer [280], and can be used to target cancer for diagnostic and therapeutic use [281].
To investigate if ligand orientation and density affect the uptake of targeted liposomal systems into breast cancer cells, Lu et al. strayed from the more tradition single branched ligand design of targeting systems and instead developed liposomes with double-branched biotin with varying densities [157]. Here, the team showed in both their in vitro uptake and in vivo biodistribution experiments, that double-branched biotin modified liposomes exhibited a significantly improved ability to target SMVT-expressing breast cancer. Additionally, the similarly targeted PTX-loaded liposomes exhibited better therapeutic effects, indicating that increasing the density of the targeting ligand on the surface of liposomes could significantly improve targeting ability. The group expanded upon their work by investigating the effects of increasing the number of branched ligands on the liposomes, e.g., tri- and tetra-branched biotin [158] (Fig. 10).
Fig. 10.
Example of biotin receptor liposomal targeting using tri-branched biotin-functionalized PTX-loaded liposomes to show that ligand density and spatial orientation affects the uptake of liposomal systems into breast cancer cells. Reprinted with permission from Ref. [158]. Copyright 2020 Elsevier.
Here cellular uptake and cytotoxicity assays indicated that the PTX-loaded tri-branched liposomes possessed the strongest internalization and anti-proliferative activity. Moreover, imaging of 4T1 tumor-bearing mice demonstrated the same results in vivo, indicating that the density and spatial distance of biotin residues influence the affinity between the targeted liposomes and the SMVT receptors. Biotin has also been used for the dual targeting of breast cancer by Huang et al. [159] who designed a liposomal system to simultaneously target both biotin and glucose on a double-branched surface functionalized attachment and showed the improved uptake of the drug carrier both in vitro and in vivo when compared to mono-targeting ligand-modified liposomes.
2.2.2. Cluster of differentiation 44
The cluster of differentiation 44 (CD44) is a ubiquitously expressed transmembrane glycoprotein found throughout the human body and is a multifunctional receptor with diverse roles in cell proliferation, aggregation, migration, hematopoiesis, hyaluronic metabolism, and lymphocyte response. This diversity in function has been linked to the many distinct CD44 variants found in specific cells including endothelial and epithelial cells, fibroblasts, keratinocytes, and leucocytes [282]. Moreover, CD44 has been linked to tumor growth, migration, invasion, angiogenesis, and bone metastasis and is overexpressed in various cancer types including TNBC [283,284]. The ECM plays an essential role in tissue growth, function, and disease, and thus its major component hyaluronic acid (HA) plays a similarly important role in cellular interactions and physiological functions [285]. As HA is the principal ligand of CD44, unsurprisingly, the CD44-HA signaling pathway has been linked to tumorigenesis, antiangiogenic and anti-inflammatory responses [286,287]. In addition to HA, CD44 also interacts with several other ECM proteins, including MMPs, fibronectin, collagens, osteopontin, as well as several growth factors, cytokines, and chemokines.
Over the years, many HA-conjugated liposomes for targeted breast cancer therapy have been designed. An interesting example includes the work of Lv et al. who loaded lysolipid-containing thermosensitive liposomes, which are stable at 37 °C but deteriorate when exposed to mild hyperthermia (42 °C), with marimastat, a synthetic inhibitor of collagenases, gelatinases, and MMPs [160]. The use of hyperthermia is a well-established method to improve the accumulation of targeted entities in tumors and has been shown to improve blood perfusion and increase the pore size between endothelial cells of tumor microvessels thus enhancing the extravasation of the nanoparticles into the interstitial spaces of the tumoral tissue [288]. The marimastat-loaded thermosensitive liposomes were combined with an HA-PTX prodrug to form hybrid nanoparticles for the targeting and treatment of the TME and breast cancer cells. Once the hybrid nanoparticles were injected intravenously in 4T1 tumor-bearing mouse models, HA-PTX and marimastat release were triggered by the application of hyperthermia at the tumor site, whereafter the marimastat interacted with the TME and the released HA-PTX entered the cells via CD44-HA coupling. The team reported that the hybrid nanoparticles promoted deep tumor penetration and accumulation and significantly inhibited tumor growth and angiogenesis by 10-fold with complete metastatic suppression. Another example by Han et al. used HA to dual target breast cancer cells and CSCs with gemcitabine-loaded liposomes [161]. For many years the prevailing theory of cancer initiation and progression has been that cancer arises from genetic mutations that build up over several divisions in normal somatic cells. This theory is now considered overly simplistic as the concept of CSCs as key contributors to therapy failure has gained considerable recognition [289]. The team's liposomes targeted breast cancer cells and CSCs, reduced the systemic toxicity of the loaded chemotherapeutic, and improved the cellular uptake of gemcitabine into CSCs. Jiang et al. [162] showcased the unique versatility of liposomes by engineering a “nanodepot” with a liposomal core surrounded by a crosslinked gelatinous shell for the delivery of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and DOX (Fig. 11).
Fig. 11.
Illustration of the TRAIL/DOX-Gelipo design, showing the HA crosslinked outer shell encapsulating DOX and TRAIL, and the proposed multistage delivery of TRAIL to the cell surface and DOX to the nucleus as an example of CD44 liposomal targeting of breast cancer. Reprinted with permission from Ref. [162]. Copyright 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
In this strategy, DOX was loaded into the aqueous core of the liposomes whilst TRAIL, which acts on cell death receptors, was confined to the outer shell of the crosslinked HA shell. The targeting and subsequent anticancer effect of the DOX-liposomes depended on the degradation of the HA shell by hyaluronidases, concentrated within the TME, causing the release of TRAIL and thus liposome internalization. In an excellent study, Yang et al. demonstrated the biological and molecular basis for the ability of chitosan to target CD44 and showed that the receptor is overexpressed in breast CSC, 3D mammospheres, and patient TNBC tissue samples [164]. The team synthesized chitosan and 89Zr-labeled gambogic acid-loaded liposomes able to bind to the active site of CD44 on TNBC CSCs, accumulate in tumors of xenograft-bearing mice with good radiochemical stability, and show excellent antitumor efficacy in vivo. In a similar multifunctional approach, Ding et al. conjugated chitosan oligosaccharide liposomes containing the photosensitizer HPPH and the hypoxia-activated prodrug TH302 to enable simultaneous CD44 targeting, imaging, and photodynamic and hypoxia-activated therapy of TNBC [163]. Photodynamic therapy is based on the use of photosensitizers triggered by specific light wavelengths to produce ROS which induces cytotoxicity. The effectiveness of chemotherapy and photodynamic therapy is often limited due to the prevalence of hypoxia in solid tumors, however, in this study the team used the hypoxic environment created by the photodynamic therapy to activate the hypoxia-activated prodrug TH302 to produce a synergistic anticancer effect in vitro and in vivo. Specifically, the group demonstrated that the combination of using HPPH- and TH302-loaded liposomes functionalized with the CD44 targeting chitosan oligosaccharides had a significantly enhanced effect on reducing tumor growth as compared to monotherapies or non-targeted liposomes. The group also showed that the chitosan oligosaccharides-functionalized dual-loaded liposomes demonstrated more necrosis and severe morphology changes in the tumors. Lastly, Guo et al. developed a liposomal drug delivery system loaded with anti-interleukin 6 receptor (IL6R) antibodies to target the TME of CD44+ breast cancer cells in TNBC and luminal breast cancer mouse models [165]. The binding of IL6 to its receptor (IL6R) regulates the expression of VEGF and MMPs, the activation of which promotes the metastatic ability of tumoral cells. The team's liposomal system blocked IL6R signaling in cancer cells and macrophages, thus suppressing the stemness and angiogenesis of 4T1 tumor-bearing mouse models. Even more interestingly, the treatment had an inhibitory effect on the lung metastatic potential of the breast cancer stem cells.
2.2.3. Human epidermal growth factor receptors 1 and 2
The transmembrane epidermal growth factor receptor family are tyrosine kinase receptors encompassing the human epidermal growth factor receptors 1 to 4 (HER1 - 4, also known as ErbB1 - 4). As the name suggests, most of the epidermal growth factor receptors are linked to the epidermal growth factor family of extracellular proteins and are involved in cell growth, differentiation, organ development, and the repair of healthy cells and tissues [290]. The exception is HER2 for, although it plays an integral role in several signaling pathways, its endogenous ligand is currently unknown. Both HER1 and HER2 are frequently overexpressed in cancer cells and induce erroneous development, unrestricted proliferation, decreased apoptosis, drug resistance, and increased metastasis and angiogenesis in several cancers including breast cancer [291,292].
In mammary tissue, HER1 is essential for ductal morphogenesis but its overexpression has been correlated to several breast cancer subtypes, including TNBC, and its presence is correlated to worse clinical outcomes [291]. HER1 therapy (or anti-epidermal growth factor receptor therapy) includes the use of mAbs which bind to the extracellular domain of HER1 to inhibit ligand binding and block downstream receptor signaling. These mAbs include; panitumumab originally developed to treat metastatic colorectal cancer but has recently been studied as a combination neoadjuvant therapy for TNBC; zalutumumab for the treatment of squamous cell carcinoma of the head and neck; and cetuximab (CET) [293].
CET is a chimeric (mouse/human) mAb able to target HER1 and produce anticancer effects by impeding the binding of epidermal growth factors thus causing an interruption to cell proliferation, angiogenesis, and metastasis [294]. Not surprisingly, targeted drug delivery systems using CET have been thoroughly pursued by HER1-expressing breast cancer cells. For example, Dorjsuren et al. developed CET-functionalized thermo-sensitive liposomes, encapsulating both DOX and citric acid-covered iron oxide magnetic nanoparticles for pH-sensitive chemotherapy release and near-infrared (NIR)-triggered photothermal therapy [166]. The team observed that after 2 h the uptake of CET-coated liposomes was considerably higher compared to uncoated liposomes in both the HER1+ SKBR-3 breast cancer cell line and the low HER1 expressing MCF-7 cell line. This uptake was correlated to reduced breast cancer cell viability in vitro and increased tumor temperatures in vivo sufficient for a therapeutic effect whilst remaining biocompatible and safe. Although multiple whole mAb CET-coated nanoparticle drug delivery systems have been investigated their use poses several disadvantages as previously mentioned. To overcome these drawbacks antibody fragments, such as Fab’ and scFv, have been developed. Su et al. investigated an intriguing use for such fragments by developing a pre-targeting system that prepared cells for the conditional uptake of nanocarriers specifically functionalized with PEG [167]. In their study, the team combined an anti-PEG Fab’ fragment with an anti-HER1 Fv fragment and named it a PEG-engager. This PEG-engager is designed to bind to the HER1 on TNBC cells but remains inert on the receptor until it interacts with a specific PEGylated liposomal DOX nanocarrier after which it quickly triggers internalization (Fig. 12A). The PEG-engager pre-targeting system significantly increased the internalization, retention, and antitumor activity of the PEGylated liposomal DOX in human TNBC xenografts in mice whilst reducing off-target system effects.
Fig. 12.
Example of HER1 and HER2 liposomal targeting in breast cancer. (A) Schematic of the PEG engager approach which relies on (1) PEG engagers (anti-PEG Fab’ fragment with an anti-HER1 Fv fragment) binding to target receptors, (2) PEG engagers remaining inert on the cell surface until contact with (3) PEGylated liposomal DOX nanocarriers which triggers internalization. Reprinted with permission from Ref. [167]. Copyright 2017 Springer Nature Limited. (B) Illustration showing how HER2 receptors would be conferred onto the surface of TNBC cells via the fusion of HER2+ extracellular vesicles. This is followed by the treatment of the cells with anti-HER2 Ab-conjugated liposomes. Reprinted from Ref. [188].
Aptamers are another excellent targeting system for anti-HER1 therapy. Kim et al. developed theranostic liposomes, containing CdSe/ZnS quantum dots for tumor bio-imaging and siRNA molecules for TNBC treatment, coupled with a commercially available anti-HER1 aptamer [168]. The aptamer-targeted liposomes delivered the siRNA directly into the cytoplasm of the target cells, while the untargeted siRNA were found at the marginal edge of the outer leaflet of the plasma membrane, demonstrating the efficiency of the targeting approach. Tumor-bearing mice treated with the HER1-targeted nanocarrier demonstrated a greater accumulation in the tumors than in mice treated with the untargeted nanocarrier.
In normal tissue HER2 aids in breast cell growth, division, and repair. In the 20% of breast cancer tumors which overexpress the receptor, it is a major driver of uncontrolled cell growth and is associated with metastasis and a poor prognosis [295,296]. The first recombinant humanized mAb, trastuzumab (tradename Herceptin®), was approved in 1998 for HER2+ targeted breast cancer therapy and was followed by pertuzumab (tradenames Omnitarg and Perjeta®) which was approved in 2012 for first-line and neoadjuvant treatment of metastatic HER2+ breast cancer [297,298]. Both of these mAbs act by binding to the extracellular region of the HER2 receptor to block downstream signaling which induces the internalization of the antibody-receptor complex and causes cell growth and proliferation inhibition. Unfortunately, both de novo and acquired resistance to this treatment occurs in ∼70% of HER2+ breast cancers with tumors restarting their growth within 1 year thus emphasizing the need for new HER2+ treatments [299].
One method to combat trastuzumab resistance has been to encapsulate docetaxel in TPGS liposomes conjugated with trastuzumab to enable a more direct, sustained, and targeted chemotherapy [170]. The efficacy of trastuzumab-functionalized docetaxel encapsulating liposomes was compared to the antibody-drug conjugate trastuzumab-emtansine (T-DM1, Kadcyla™) in 3D spheroids and xenografted mice [184]. T-DM1 is composed of trastuzumab and the anti-microtubule chemotherapeutic agent emtansine (also known as DM1) and, although originally designed for the treatment of drug-resistant HER2+ breast cancer, was approved in 2013 as a HER2-targeted treatment for metastatic breast cancer. Interestingly, the trastuzumab and TPGS-conjugated liposomes resulted in better treatment efficacy as compared with all other treatments, both in vitro and in vivo, but did not demonstrate enhanced tumor biodistribution. This discrepancy was hypothesized to be due to the trastuzumab and TPGS-conjugated liposomes improving cellular internalization rather than improving tumoral targeting. Another approach to overcome trastuzumab resistance has been to combine the targeting mAb with novel cancer treatment strategies. For example, simvastatin, a drug used to decrease the risk of heart problems by lowering elevated lipids levels, was encapsulated within trastuzumab-functionalized liposomes for targeting HER2+ breast cancer cells [178]. Here, Matusewicz et al. demonstrated that simvastatin disorganized the lipid-rich membrane rafts that HER2 is attached to thus disturbing HER2-dependent signaling pathways. In 2019, the group further tested their system with trastuzumab-functionalized simvastatin-loaded stealth liposomes to determine whether the system could effectively treat TNBC [183]. In vitro experiments conducted with MDA-MB-231 cells demonstrated an increase in membrane disorganization and an inhibition of the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathway involved in the cell cycle, and in vivo experiments indicated effective antitumor results.
In regards to clinical development another liposomal system that made use of trastuzumab, named MM-302, was of great clinical interest. MM-302 is a trastuzumab-conjugated PEGylated liposomal DOX developed to treat HER2+ metastatic breast cancer (NCT01304797, NCT02213744, NCT02735798) by Merrimack Pharmaceuticals, and was designed to deliver DOX to cells whilst sparing healthy cardiomyocytes [300]. Unfortunately, although phase I studies [[301], [302], [303]] indicated that MM-302 had an acceptable safety profile and was well tolerated, the randomized phase II/III trial (referred to as HERMIONE) in HER2+ metastatic breast cancer patients previously treated with pertuzumab, trastuzumab, and T-DM1 failed to show satisfactory clinical results [304].
HER2 Fab’ fragments have also been conjugated to pH-sensitive PEGylated liposomes [177]. In this study, the HER2 Fab’ fragment targeting on the liposomal carrier enhanced its efficiency to HCC1954 cells 10-fold as compared to non-targeted liposomes. As previously noted, engineered antibody fragments (Fab’, scFv, and Fv) have demonstrated improved penetration of tumors, as compared to the much larger mAbs. One such fragment, referred to as the nanobody, comprises the variable domain of the heavy chain antibodies (VHH) and has become an intriguing area of interest as they are highly stable with sub-nanomolar target affinity. Nikkhoi et al. demonstrated the use of VHH-PEG-liposomes as targeting moieties specific to several extracellular regions on the HER2 receptors of breast cancer [180,181]. The group developed and compared four non-overlapping monoclonal VHHs for their ability to target HER2 in vitro as compared to VHH-decorated nanocarriers coated at a similar density. The tetra-specific VHHs showed improved targeting, binding affinity, and intracellular drug release. Similarly, Alavizadeh et al. investigated whether small proteins that mimic mAbs, referred to as affibodies, with anti-HER2 targeting conjugated to cisplatin PEGylated liposomes could efficiently enhance the therapeutic effectiveness of the targeted liposome [174]. The team showed an increased intracellular accumulation of cisplatin which caused cell death at much lower concentrations using their targeted affibody-liposome system (referred to as affisomes). Equally promising, the affisome-treated HER2+ TUBO tumor mouse models showed improved therapeutic efficiency and an extended survival time.
Research into HER2-targeting aptamers and peptides has also emerged. Moosavian et al. developed an RNA aptamer (TSA14) able to bind to external HER2-associated proteins specifically on the TUBO cell line [189]. In vitro experiments with the TSA14-functionalized PEGylated DOX-loaded liposomes indicated that the targeting system improved cellular uptake leading to improved cytotoxicity of the TUBO cell line. Similarly, DOX accumulated within the TUBO breast tumors in mice at a significantly higher rate when introduced via targeted liposomes than in non-targeted. Kim et al. engineered a YCDGFYACY-MDV peptide-targeted DOX-encapsulating liposomal system highly specific to HER2+ breast tumor cells [186]. To increase HER2+ tumor cell selectivity the group optimized peptide density and linker length with methodical in vitro uptake and in vivo tumor accumulation studies. Their research demonstrated that ∼1% peptide density was optimal to enhance the in vitro HER2+ cellular uptake whilst only a 0.5% peptide density was sufficient in vivo. Another interesting strategy for receptor targeting was the approach of Quinn et al., who purposefully conferred the HER2 receptor onto the surface of TNBC MDA-MB-231 cells via the fusion of HER2+ extracellular vesicles extracted from HER2 overexpressing BT-474 cells to convert TNBC cells to an anti-HER2 treatable phenotype [188] (Fig. 12B). The team demonstrated that MDA-MB-231 cells normally harbored approximately 4 × 104 HER2 receptors but that an additional 5.9 × 104 HER2 receptors could be conferred, and maintained for 24 h, after 1 × 108 BT-474 extracellular vesicle incubation for 12 h. The team acknowledged that there is as-of-yet unknown equilibrium between the ability of the MDA-MB-231 cells to take up foreign and release of self-derived extracellular vesicles which could pose as a limiting factor. Subsequently, anti-HER2 antibody-conjugated PTX-loaded liposomes were used for HER2-targeted drug delivery both in vitro and in vivo, and showed significantly enhanced cytotoxicity and reduced tumoral volume in mice. Of note, the ability to insert antigens onto cell surfaces in this manner is limited to our knowledge harvestable extracellular vesicles, and that the intravenous administration of such therapies are not enitrely practical.
2.2.4. Integrin receptors
Integrin receptors are comprised of α- and β-subunits from which an array of unique isoforms can be built, e.g., the integrin alpha V and beta 3 (αvβ3) receptor. Roughly 24 distinct isoforms of the transmembrane receptor are responsible for the signal transduction of several pathways related to cell attachment, survival, differentiation, intracellular organization, and motility. Due to this abundance of isoforms and universality of function the dysregulation of several integrin receptors, e.g., αvβ3, αvβ1 and αvβ6, have been connected to the initiation and progression of cancer, including stages of vasculature invasion, promotion of circulating tumor cells, metastatic niche preparation and colonization, and the development of drug resistance [305,306]. Thus for decades, liposomes have been functionalized to target various cancer-related integrin receptors using arginyl glycyl aspartic acid (also referred to as Arg-Gly-Asp or RGD) [307]. This targeting strategy has been applied in multiple ways to various cancer types and has most recently been applied to targeting breast CSCs. Vakhshiteh et al. conjugated cyclic RGD (cRGD) to PEGylated liposomes encapsulating miR-34a, a dysregulated microRNA associated with tumor suppression found in several cancers, to target TNBC and CSCs [195]. The team found that the cRGD-functionalized miR-34a-encapsulating liposomes internalized into breast cancer cells at a 1.8-fold higher rate than untargeted liposomes, inhibited cell growth and migration, and caused a significant decrease in the CD44+/CD24−/low cancer stem cell-like population thus impeding the invasion of the cells. Other RGD targeting systems have also been developed to address the issue of breast cancer metastasis to the bone. Zhao et al. synthesized bone-targeting glutamic oligopeptide-RGD peptides for the delivery of PTX to bone metastases [197]. The glutamic and RGD-conjugated liposomes showed superior targeting towards MDA-MB-231 cells both in vitro and in vivo, and the glutamic-RGD peptides acted as a vector to enhance the drug delivery to bone metastasis.
Another interesting use of integrin targeting has been for VM channels wherein CSCs transdifferentiate into endothelial-like cells and induce neovascularization in highly aggressive tumors without the presence of endothelial cells [308]. VM channels mediate the early growth and metastasis of cancer cells and are thus associated with invasive and drug-resistant breast cancers. Unfortunately, VM channels are common in breast cancer and their presence is associated with a poor prognosis [309]. To promote the destruction of these channels and thus curb metastasis, Fu et al. developed two RGD-functionalized liposome types to encapsulate either daunorubicin or emodin (an active component of several plants used in traditional Chinese medicine) [196]. Both of the RGD-targeted liposome types accumulated in sufficient amounts at the tumor site to produce a cytotoxic effect on the cancer cells as well as inhibit and destroy the formation of VM channels. The team also demonstrated that the specific combination of the two RGD-modified liposomes, i.e., the use of both the daunorubicin and the emodin liposomes, caused the downregulation of MMP-2, VE-cadherin, transforming growth factor beta 1 (TGF-β1) and HIF-1α proteins associated with metastasis.
2.2.5. Luteinizing hormone-releasing hormone receptor
The luteinizing hormone-releasing hormone (LHRH) receptor (sometimes referred to as the gonadotropin-releasing hormone receptor, GnRHR) is a major regulator in reproduction. As the name suggested, the LHRH receptor responds to the binding of the gonadotropin-releasing hormone to stimulate the release of luteinizing hormone (LH), gonadotropins, and follicle-stimulating hormone (FSH), which brings about gametogenesis, germ cell proliferation and the production of sex steroids [310]. LHRH receptor has been demonstrated to be largely absent in healthy visceral organs but highly expressed in the cell membranes of several reproductive solid tumors, including breast, endometrial, ovarian, and prostate cancers [311]. Analogs of LHRH, such as gonadorelin, have also been used as targeting ligands for drug carriers to facilitate internalization.
Mitoxantrone is a chemotherapeutic agent used to treat advanced-stage prostate cancer associated with severe side effects including myelosuppression and cardiotoxicity. In an attempt to improve the drug's efficacy, diversify its uses, and prevent these adverse effects, He et al. developed gonadorelin-functionalized liposomes by co-incubating the micelles of DSPE-PEG-peptide with pre-formed liposomes [198]. Cellular uptake studies indicated that the gonadorelin-functionalized liposomes had better uptake into targeted cells than non-targeted liposomes thus the group expanded upon their work by co-loading the gonadorelin-functionalized liposomes with both magnetic iron oxide nanoparticles (for T2-weighted MRI imaging) and mitoxantrone for cancer therapy [199]. The uptake of the gonadorelin-functionalized liposomes was higher in LHRH receptor overexpressing cells (MCF-7) than in cells with negligible expression of the receptor (SK-OV-3 cells). Correspondingly, mitoxantrone accumulation increased within the MCF-7 cells demonstrating the ability of the liposomes to deliver their chemotherapeutic cargo within only the targeted cells. When the gonadorelin-functionalized mitoxantrone-loaded liposomes were applied to MCF-7 tumor-bearing mice, tumor reduction only became evident after two weeks and the group attributed this to the passive accumulation of the system within the tissues via the EPR effect with the liposomes serving as a depot for sustained release of mitoxantrone over time. Whilst the biodistribution studies indicated a reduced toxicity as compared to free mitoxantrone, MRI imaging at 2 h post-injection showed a decrease of T2 signal in the tumors exposed to either targeted or untargeted liposomes indicating a washout of the signal at this time.
2.2.6. Mucin 1
In healthy tissue, the transmembrane glycoprotein MUC1 is expressed in the luminal epithelial cells of the esophagus, duodenum, stomach, pancreas, lungs, prostate, mammary glands, and uterus where it provides protection and anti-adhesion properties to underlying epithelia by creating a physical barrier of negatively charged sugar branches preventing bacterial colonization [312]. Tumor-associated-MUC1 arises when MUC1 becomes aberrantly glycosylated and cells undergo a loss of polarity, causing the redistribution of the glycoprotein on the cell surface and cytoplasm which contributes to cancer metabolism, proliferation, angiogenesis, invasion, chemoresistance and metastasis [313].
Al-Ahmady et al. developed a MUC1 targeted delivery vector by conjugating humanized anti-MUC1 mAbs (hCTMO1) to temperature-sensitive liposomes to augment the accumulation of the targeted liposomes and to trigger the release of the encapsulated DOX using mild hyperthermia [200]. Although a two-fold increase of the targeted thermosensitive liposome was observed in the tumoral cells, only a moderate improvement in tumor growth inhibition and animal survival was noted and was attributed to insufficient bioavailable drug concentrations of a single injection. MUC1-functionalized PEGylated liposomes, dual-loaded with ICG and DOX, have also been studied as a targeted theranostic system using multispectral optoacoustic tomography in fast-growing (4T1) and slow-growing (HT-29, human colorectal adenocarcinoma) MUC1 positive cell line tumor models [201]. After an intravenous injection both the targeted and untargeted liposomes accumulated in the tumor tissue but, interestingly, the targeted liposomes were observed to accumulate more rapidly along the periphery of tumors whilst the untargeted liposomes accumulated at the center of the tumors at later times (Fig. 13A).
Fig. 13.
Examples of MUC1 liposomal targeting of breast cancer. (A) In vivo multispectral optoacoustic tomography imaging of 4T1 murine breast tumor models after intravenous injections of either the non-targeted or targeted liposomes. Although both non-targeted and targeted liposomes accumulated in the tumors over time, the targeted liposomes accumulated more quickly and along the periphery of the tumors (rather than penetrating at the center). Adapted and reprinted with permission from Ref. [201]. Copyright 2015 Elsevier. (B) In vitro cellular uptake of h-MUC1 gold nanocage liposomes (h-MCU1 AuNG-Lip) in MCF-7 (MUC1 positive cells) over time. In vivo time-dependent fluorescence revealing the tumor accumulation of the h-MUC1-functionalized liposomes over time. Adapted and reprinted with permission from Ref. [202]. Copyright 2016 Elsevier.
The use of fluorescent-modified MUC1 aptamers as multifunctional targeting and imaging sensors has recently been explored. In an excellent example, Chuang et al. modified MUC1 aptamers with two FRET fluorophores (FITC–MUC1 aptamer and Cy3–DNA) to develop a multifunctional liposomal system [202]. The targeted liposomal system showed enhanced internalization into the MCF-7 cells where the FRET fluorophores acted as molecular beacons to signal the optimal time for photothermal application. Gold nanocages encapsulated within the liposomes converted the NIR into localized heat triggering the ammonium bicarbonate to form CO2 bubbles, causing the carriers to release DOX. Using both in vitro and in vivo models, the group demonstrated that whilst the FRET imaging technique did allow for real-time dynamic in vitro monitoring of the liposomal system as it accumulated in the endosomes/lysosomes near the nucleus, due to the rapid attenuation of light the FRET signal was weak in deep tissues thus the approach could only be used for superficial tumors (Fig. 13B).
Notably, a therapeutic peptide vaccine, ONT-10, combining the MUC1 antigen (M40Tn6) and a novel synthetic toll-like receptor 4 agonists (PET Lipid A) with a liposomal carrier is under development for the generation of a targeted response to several solid tumors. Phase I clinical studies (NCT01556789, NCT02270372) demonstrated the vaccine was well-tolerated with a promising immune response [314,315] and was followed up by a phase Ib study (NCT01978964) in combination with varlilumab (an anti-CD27 agonistic antibody) in advanced ovarian and breast cancers, and a phase III study (NCT00409188) on non-small cell lung cancers where no significant difference in survival was found [271].
2.2.7. Neuropilin 1
Neuropilin 1 (NRP1) is a transmembrane glycoprotein co-receptor for certain isoforms of semaphorins, VEGF, and TGF-β [316]. As its wide array of ligands suggests, NRP1 is involved in angiogenesis, cardiovascular development, cell migration, neuronal guidance, immunity, EMT, and chemoresistance [317]. As such, the expression of NRP1 has been extensively studied in various cancers, including breast cancer, and has been identified as a possible drug delivery target.
Cao et al. functionalized a modified version of the A7R peptide (ATWLPPR, herein known as the cysteine modified version A7RC), a known ligand of NRP1, to PTX-loaded liposomes to attempt the targeted inhibition of tumor growth and angiogenesis in vivo [204]. The team showed that A7RC-functionalized liposomes had good in vitro and in vivo uptake in NRP + cells (MDA-MB-231) accompanied by a strong cytotoxic effect.
Cell-penetrating peptides (CPPs) are small, positively charged peptides consisting of 5–30 amino acids characterized by their ability to penetrate cell membranes and translocate into cells. Since the discovery of the first cell-penetrating proteins, e.g., trans-activator of transcription (TAT) and penetratin, numerous CPPs have been developed and used as a means to improve drug efficacy [318]. CPPs cannot specifically target cells or receptors, relying instead on entering cells either by direct penetration, endocytosis-mediated entry, or through transitory inverted micelles [318]. Kadonosono et al. examined the ability of two CPPs, PTD-3 and TAT-PTD, to bind to NRP1 receptors and accumulate in tumors [205]. To prove the specificity of the targeting system, the group pretreated cells with NRP1 neutralizing antibodies or iRGD, a tumor-homing peptide with an affinity for NRP1, to demonstrate the reduction in uptake is related to the ability of the CPPs to bind to NRP1.
2.3. Internal cell receptors
2.3.1. Estrogen receptors
A large proportion of breast cancers overexpress ERs and are dependent on intact estrogen signaling. ERs are part of the nuclear hormone receptor superfamily, consisting of G protein-coupled ERs, extra-nuclear ERs, and nuclear ERs, with three known endogenous ligands; estrone (E1), estradiol (E2) and estriol (E3). Nuclear ERs mediate the internalization and multidirectional effects of the estrogen analogs into the cell nucleus whilst extra-nuclear ERs are situated in the plasma membrane. G protein-coupled ERs can be found in both the plasma membrane and cytoplasm and are strongly associated with drug resistance, metastasis, and extracellular remodeling in cancer [[319], [320], [321]]. Two ER subtypes have been identified, ERα and Erβ, with ERα found mainly on the mammary glands and uterus and associated with the proliferation of ER + breast cancer, whilst Erβ is principally found in the prostate [322,323]. Consequently, only ERα is used as a target in breast cancer. Due to endocrine therapy, ER + breast cancer generally has a good prognosis but is also partially responsible for the occurrence of patient relapse [324].
Needless to say, both E1 and E2-targeted liposomes have been extensively researched and developed. Cationic liposomes incorporating E2 in their structure have been used to protect and deliver antisense oligonucleotides against mRNA encoding ERα and β for chemosensitization of cells for enhanced treatment effectivity [325]. E1-targeted liposomes for the intracellular delivery of therapeutic agents have also been developed using stimuli-responsive liposomes, including pH-triggered liposomes [206] and ultrasound-triggered liposomes [210]. pH-triggered DOX liposomes were able to translocate the chemotherapeutic agent to the cell nuclei and were more cytotoxic to ER + breast cancer. E1-fragments conjugated to liposomes as targeting moieties were utilized by the group of Han et al. who developed an ER-targeting, PEGylated, and PTX-loaded liposome drug delivery system [211]. The E1-targeted long-acting PTX liposomes demonstrated an inhibitory effect in vitro on ER + MCF-7 cells which was 12-times lower than the IC50 value of PTX-loaded PEGylated liposomes with no targeting system. In vivo studies indicated that the accumulation of the E1-targeted long-acting liposomes in some of the more common areas of nanoparticle accumulation, such as the kidneys, spleen, liver, and the tumor site within 1 h of administration and peaking at 12 h (Fig. 14A). The E1-targeted liposomal system also showed an excellent ability to slow tumor growth in vivo as compared to PTX-loaded PEGylated liposomes with no targeting.
Fig. 14.
Examples of estrogen receptor liposomal targeting for breast cancer. (A) Imaging of MCF-7 tumor-bearing mice and organs after a tail vein injection of either non-targeted or E1-functionalized DiR-encapsulated long-circulating liposomes (ES-SSL-DiR). Accumulation of the ES-SSL-DiR liposomes could be seen in the liver, spleen, kidneys and the tumor site within 1 h of administration peaking at 12 h. Adapted and reprinted with permission from Ref. [211]. Copyright 2019 Dovepress. (B) Schematic of the synthesis and internalization process of the DOX-encapsulating synergistic targeted liposome carrier system (SELS DOX). Reprinted with permission from Ref. [209]. Copyright 2019 Royal Society of Chemistry.
As previously mentioned, tamoxifen is an ER antagonist and is used as both a treatment and a chemo-preventative for ER+ and metastatic breast cancer [324]. Tamoxifen itself has a weak affinity for ERs and acts as a prodrug converted to functional metabolites which outcompete and displace estrogen and E2 to strongly binding to ERs causing the inhibition of tumor cell growth [326]. Thus, to determine if a breast cancer patient is suitable for treatment with tamoxifen, the patient must be both ER+ and have ERα present on the tumoral cells. ER-breast cancer cells still have ER expression but are classified as negative due to the relatively low expression of ERα as compared to other ER + types but up to 10% of ER-classified breast cancers have some sensitivity for tamoxifen treatment [327]. Although tamoxifen is widely used in breast cancer treatment, 20–30% of tumors are resistant [328] with long-term use strongly associated with endometrial and liver cancers. Side effects with tamoxifen are dose and concentration-dependent thus low dosing is the preferred treatment regime. This was demonstrated by Jain et al. who synthesized liposomes with tamoxifen loaded in the outer lipid bilayer with DOX entrapped in the aqueous compartment to target ER + cells [207]. Internalization studies confirmed that the DOX from the non-targeted liposomal formulations accumulated in the cytosol and was not taken up as significantly in the cell's nucleus as compared to the targeted formulations. The team of Wang et al. (2016) sought to further improve the targeting and uptake ability of tamoxifen-targeted liposomes by combining the ER antagonist with the pentapeptide QLPVM onto DOX-loaded stealth liposomes for luminal A breast cancer therapy [208]. The team demonstrated that the QLPVM peptide, a relatively weak CPP consisting of no positively charged side chain amino acids, enhanced the uptake of the liposomal system in MCF-7 cells whilst not damaging the cellular membranes. QLPVM also increased the cytotoxicity of tamoxifen and DOX by translocating the drugs quickly through the cell membrane and to the nucleus. The targeted liposome exhibited excellent antitumor activity with improved accumulation and cellular internalization in vivo.
Wang et al. (2019) used the high surface area-to-volume ratio of nanoparticles to develop a synergistic targeted liposome carrier system (referred to as SELS) functionalized with both anti-ER antibodies (to target ER + breast cancer cells) and an immune targeting ‘self-peptide’ recognized by macrophages to reduce the phagocytic clearance of the nanocarrier [209]. This self-peptide moiety is related to the transmembrane glycoprotein CD47; an important tumor biomarker that acts as a shield for cancer cells against the MPS. Its action is linked to the binding of signal-regulatory protein alpha (SIRPa) to the critical segment of CD47, acting as ‘don't eat me’ signals to the MPS thus avoiding elimination. Interestingly, cellular uptake studies in MCF-7 demonstrated a 2.4 times greater fluorescence intensity of DOX with the self-peptide and ER targeting than ER-only targeting even though the two liposome formulations both targeted ER. This anomaly was attributed to density differences in the targeting ligands (Fig. 14B).
2.3.2. Progesterone receptors
PRs are expressed in approximately 75% of ER + breast cancers. However as up to 30% of advanced staged tumors lose their PR expression [329], the vast majority of targeted treatment has been developed for ERs [330]. PRs are located on the surface and in the nuclear membrane of cells, much like ERs, and their activity is triggered by the hormone progesterone. Two nuclear receptor isoforms, PR-A and PR-B, have been identified and tumors expressing either respond to endocrine treatment [331,332]. Unlike the nuclear PRs, which mediate their effects via genomic mechanisms, membrane PRs (mPRs) mediate physiological functions in the immune system, neuroendocrine tissues, liver, and reproductive systems, as well as in breast and ovarian cancer. Subtypes include mPRα, mPRβ, mPRγ, mPRδ, and mPRε but only mPRα and mPRδ are found in breast tissue [333]. Notably, PR and mPRα expression are not synonymous, for example, TNBC is associated with low PR but high mPRα expression levels [333]. It has also been demonstrated that progesterone can impede TNBC cell growth and brain metastasis via the progesterone/mPRα pathway suggesting that progesterone could be a viable treatment strategy. Whilst a few compounds have been developed to specifically target PRs for breast cancer treatment, e.g., mifepristone, telapristone, and onapristone, no new agents have been reported since 1990 [334]. The required PR expression levels needed for this type of targeting are unknown, however, tumors with low ER + cells (1–10%) do not respond to endocrine therapies, and those with PR + cells >10% may benefit from progestin (synthetic progesterone) therapy [335]. Whether or not PRs and ER + tumors could be co-targeted remains unknown and evidence has linked PRs to endocrine-resistant tumors [336].
2.4. Enzymes
2.4.1. Matrix metalloproteinases
MMPs are a family of enzymes whose catalytic activity relies on zinc to function. MMPs facilitate angiogenesis and wound healing via their ability to break down and remodel almost every component of the ECM in normal tissues, and their dysregulation has been implicated in cardiovascular diseases, inflammatory disorders, and cancer [337]. Each type of MMP is distinct and can recognize and cleave specific amino acid sequences, which is used to classify the MMPs depending on the ECM component it degrades, e.g., the collagenases (MMP-1, MMP-8, MMP-13), the membrane-types (MMP-14, MMP-15, MMP-16, MMP-17, MMP-24 MMP-25), the gelatinases (MMP-2, MMP-9), etc. [338,339]. The gelatinases have been proven to possess tumorigenic activity due to their profound participation in tumor growth, migration, invasion, and metastasis [340]. Although elevated levels of MMP in primary and metastatic tumors have been associated with increased differentiation, recurrence, invasion, lymph node, and brain metastases leading to worse prognosis and patient outcomes, these enzymes have also been recognized as potent biomarkers and targets for controlled release drug delivery systems [341,342].
MMPs have been used as both tumoral and tumor microenvironment targets and are often utilized to trigger the release of drugs from specifically formulated liposomes. Qin et al. functionalized the surface of liposomes with chlorotoxin (a peptide derived from scorpion venom and a known ligand for MMP-2 previously used for targeting brain tumors) to target MMP-2s expressed in metastatic breast cancer [212]. The chlorotoxin-functionalized liposomes had increased cellular uptake in the murine metastatic 4T1 cell line as compared to non-targeted liposomes, and demonstrated enhanced cytotoxicity, antitumor, and antimetastatic effects in tumor-bearing mice with low systemic toxicity. In an example of using high concentrations of MMPs as an internal targeting system, Ramadass et al. designed a novel liposomal co-delivery system by co-loading epigallocatechin gallate (EGCG) (an MMP inhibitor) with the chemotherapeutic agent PTX [213]. Their combinatorial system outperformed the individual drug-loaded liposomes in all measured parameters, including invasion assays, MMP-2 and -9 inhibition, cytotoxicity, and caspase-3 activity thus demonstrating the system's ability to promote apoptosis and inhibit cell invasion.
2.4.2. Secretory phospholipase A2
Phospholipases are a diverse group of enzymes with over 30 isoforms all involved in the cleaving of phospholipids [343] and are divided into several classes depending on their catalytic mechanism, evolutionary relationships, structure, and localization (whether extracellular or intracellular). Of these isoforms, secretory phospholipase A2 (sPLA2) has been associated with several inflammatory pathologies and atherosclerosis, as well as promoting the tumor proliferation of prostate, pancreatic, and breast cancers [[344], [345], [346]]. sPLA2 is also known to have a strong preference for cleaving negatively charged phospholipid head groups such as phosphatidylserine [347] and lipid bilayers over free lipids making it an excellent candidate as a target for liposomal drug delivery. Consequently, sPLA2-responsive liposomes in which sPLA2 activity provides a controlled and localized drug release have been developed.
Oxaliplatin is a potent platinum-based inhibitor of DNA synthesis and is currently used as an anticancer treatment of colorectal cancer but has shown some activity in metastatic and TNBC patients with prior exposure to anthracyclines and/or taxanes in phase II clinical trials [[348], [349], [350], [351], [352]]. In an attempt to reduce the myelotoxicity, peripheral neuropathy, and gastrointestinal toxicities commonly associated with this chemotherapeutic, PEGylated oxaliplatin-loaded liposomes have been extensively studied. A phase I clinical trial for the first cisplatin-encapsulating liposomal formulation with a sPLA2-trigger, LiPlaCis®, was performed in advanced and/or refractory solid tumors including prostate, skin, and metastatic breast cancer (NCT01861496). In 2009 trials were halted due to safety issues requiring reformulation [353], after which the efficacy, dosage, and tolerability of LiPlaCis® continued to be assessed pre-clinically and in phase I/II clinical trials in advanced solid tumors such as head and neck, skin, colorectal, gastric, and breast cancers [214,[354], [355], [356]]. Østrem et al. developed a liposomal delivery system optimized for sPLA2-triggered drug release by adjusting the fluidity and cholesterol level in the liposomes (Fig. 15A) [214]. Unfortunately, although the in vitro studies showed enzyme-specific release of the encapsulated oxaliplatin, when the formulation was injected intravenously into sPLA2-releasing MT-3 tumor-bearing mice the experiment had to be prematurely halted due to severe systemic toxicity (Fig. 15B). The team cautioned that several outbred mouse stocks are known to have active sPLA2 genes causing abnormally high serum sPLA2 levels and similar findings have been noted for cancer patients, thus causing the premature activation of the liposomes in circulation. More recently, an mRNA-based LiPlaCis® drug response predictor for heavily pretreated-platin metastatic breast cancer is scheduled to undergo randomized phase II trials after the phase I clinical trial [357] showed promising efficacy in metastatic breast cancer patients (NCT01861496).
Fig. 15.
Examples of sPLA2 liposomal targeting in breast cancer. (A) Schematic of the proposed mechanism of action of sPLA2; oxaliplatin-encapsulating liposomes extravasates from the fenestrated capillaries of tumors where elevated levels of sPLA2 cause the release of the drug in a localized area. (B) Although the in vivo treatment of sPLA2-sensitive oxaliplatin-encapsulating liposomes showed an improvement in the survival of nude mice bearing MT-3 xenograft tumors, the skin of the mice exhibited subcutaneous bleeding, and liver sections showed multiple areas of necrosis in hepatocytes, vacuolar degeneration and an acute inflammatory reaction with an accumulation of granulocytes (black arrows). Reprinted with permission from Ref. [214]. Copyright 2017 Elsevier.
2.5. Dual targeting
Tumoral tissue is exceptionally heterogeneous and dynamic and is known to undergo both micro and macro spatio-temporal changes. An example of this phenomenon is the ever-changing presence or absence of targetable cell-surface biomarkers. As drug delivery platforms have progressed towards systems able to improve the effectiveness and reduce the side effects of chemotherapy, so too has the understanding that our mono-targeted nanocarriers are simply not sufficient. Thus, it has become apparent that the development of multi-functional and multi-targeted drug delivery systems must be designed to target several receptors and ligands on breast cancer and its associated cells, as well as the TME.
Cationic liposomes that are dual-functionalized with TPGS and HA for the delivery of the chemosensitizing agent lonidamine and PTX were developed by Assanhou et al. to treat MDR breast and lung cancers [358]. The developed HA-functionalized cationic liposomes incorporated synthetic positively charged 1,5-dioctadecyl-N-histidyl-l-glutamate (HG2C18) lipids which facilitated the endo-lysosomal escape of liposomes after internalization whilst the TPGS aided in mitochondrial delivery. The team demonstrated that once internalized, the liposomes disrupted mitochondrial function and ATP production and resensitized the xenograft MCF-7/MDR tumors to PTX. Although surgical excision of primary tumors is a common treatment strategy for several cancers, postsurgical metastasis is a known risk. To help combat this, Jyotsana et al. developed dual E-selectin and TRAIL-functionalized liposomes to inhibit metastasis in mouse models similar to clinical representations of TNBC [359]. The group demonstrated that minimally administrated co-functionalized E-selectin and TRAIL liposomes could target and inhibit metastasis in TNBC mouse models after tumor resection.
A major factor in chemotherapeutic resistance are EMT-associated genes, such as the cytoskeleton gene twinfilin 1 (TWF1) gene which has been reported to be involved in cell migration, cancer progression, and drug sensitivity [360]. Du et al. developed a novel brain-seeking breast carcinoma cell peptide (BRBP1) able to target the TWF1-overexpressing triple-negative MDA-MB-231BR (or 231-BR) cell line. The peptide, BRBP1-TAT-KLA, is composed of i) the MDA-MB-231BR-targeting peptide BRBP1, ii) the pro-apoptotic KLA (acetyl-(KLAKLAK)2-NH2) peptide to target mitochondria and disrupt their membranes [361], and iii) the CPP TAT for enhanced cell penetration [362]. As MDA-MB-231BR cells are resistant to PTX, the team used the BRBP1-TAT-KLA-functionalized liposomes, dual-loaded with PTX and an siRNA to intefere with TWF1 gene expression, to resensitize the cells to PTX (Fig. 16). In vitro results indicated that the targeted liposomes could penetrate through a BBB-transwell model resulting in efficient cellular uptake in the MDA-MB-231BR cells. Subsequent siRNA delivery caused TWF1 gene suppression and enhanced PTX cytotoxicity in the PTX-resistant cells. In vivo results showed the inhibition of tumor growth in subcutaneous MDA-MB-231BR-xenograft tumors after intravenous injections of the liposomes, and excellent reduction and inhibition of MDA-MB-231BR-brain metastatic micro- and macro-lesions over three weeks. Currently, however, the exact target and mechanism of the BRBP1-peptide is unknown.
Fig. 16.
Example of a dual-ligand liposomal system targeting both MDA-MB-231BR cells and their mitochondria via BRBP1-TAT-KLA-functionalized liposomes. The illustration demonstrates how the targeted liposomes are expected to bypass through the blood-brain barrier (BBB) to accumulate in brain metastatic breast cancer tumors to delivery EMT-associated TWF1 gene therapy. Reprinted with permission from Ref. [362]. Copyright 2021 American Chemical Society.
A feature of tumor metabolism distinguishing it from normal cells is the increased uptake and use of glucose for energy production in aerobic conditions, known as the Warburg effect [363], accompanied by increased proliferation and rapid depletion of glucose in the microenvironment. To circumvent this depletion several cancer types, including breast cancer cells, have been demonstrated to mitigate this reduced availability by using the highly abundant monosaccharide d-fructose. Thus, not surprisingly, a hexose transport (GLUT) known to be able to transport fructose, GLUT5, is overexpressed in breast cancer cells. Pu et al. identified GLUT5 as a potential biomarker for breast cancer and developed a dual-targeted GLUT5 and integrin αvβ3-functionalized liposome for targeting TNBC [364]. For the targeting ligand, the team used an innovative Y-shaped structure wherein RGD and fructose were attached to the branches, and cholesterol was used on the distal end as the anchor into the lipid material. The system proved to be highly efficient for internalization, accumulation in tumor sites, and cytotoxicity likely due to the dual recognition by GLUT5 and αvβ3 causing rapid endocytosis in MDA-MB-231 and 4T1 cells. Similarly, Yang et al. designed a dual-targeted PTX-loaded liposomal system using glutamic hexapeptide and folic acid (Glu6-FA)-coated liposomes for targeting breast cancer metastasis in bone [365]. This strategy performed well both in vitro and in vivo as it enhanced PTX cytotoxicity by enabling high binding affinity and efficiency towards both the tumoral cells using FA and hydroxyapatite via Glu6. The team of Doolittle et al. investigated the use of a dual ligand system to target both P-selectin (CDAEWVDVS peptide) and αvβ3 integrin (cRGD) with peptide-functionalized liposomes to target the development of metastasis in aggressive tumors [366]. As a case study, the team used two TNBC mouse models with either MDA-MB-231 or 4T1 cells and demonstrated that their dual ligand system outperformed single-ligand systems by identifying and targeting several different metastatic sites in the same animal with 22% of the total injected volume accumulating in the metastatic sites 2 h postinjection (Fig. 17).
Fig. 17.
Example of a dual-ligand liposomal system targeting both P-selectin via the CDAEWVDVS peptide and the αvβ3 integrin receptor via the cRGD peptide in metastatic breast cancer. Bioluminescence imaging (BLI) showed mice before and after resection of the primary tumor and in vivo fluorescence molecular tomography imaging showed the spread and location of the metastatic cells visualized using the dual-targeted liposomes. Adapted and reprinted with permission from Ref. [366]. Copyright 2015 American Chemical Society.
In a similar trend to Doolittle et al., the team of Covarraubias et al. used a two-ligand system to target HER1 and αvβ3 integrin to address the issue of variable micrometastatic regions in tumors [367]. The team showed that the targeting of both HER1 and αvβ3 integrin was needed to reliably target breast cancer metastasis in mouse models and, using advanced imaging techniques, could visualize the evolution and growth of the metastasis as it spread. Doubtless due to this targeting excellency, DOX treatment enabled complete inhibition of primary and metastatic site growth resulting in high survival rates of the mouse models.
Some lesser known receptors have also been used for the dual targeting of liposomes in breast cancer. For example, amino acid transporter B0,+ (ATB0,+) and L-type amino acid transporter 1 (LAT1) are both, as their names suggest, plasma membrane transporters responsible for providing cells with essential and cationic amino acids. ATB0,+ is a broad-spectrum amino acid transporter able to pump against a high concentration of all the essential amino acids, except glutamate and aspartate, whilst LAT1 transports only cationic amino acids [368]. Notably, these transporters are overexpressed in glioma, pancreatic, prostate, and breast cancers [369]. This overexpression is not universal in all breast cancers types; for example, ATB0,+ is expressed more in ER + breast cancers such as MCF-7 (LAT1+) and T-47D (LAT1-) than in ER-breast cancers such as MDA-MB-231 (LAT1+). Thus, dual targeting of both amino acid transporters has been explored as promising targets. Wang et al. designed glutamate, lysine, and tyrosine-functionalized liposomes to target LAT1 and ATB0,+ in breast cancer cells both in vitro and in vivo [370]. The enhanced targeting showed superior cellular uptake, tumor site accumulation, and cytotoxicity compared to Onivyde®, an irinotecan-loaded liposomal medication used to treat non-small lung cancer and metastatic pancreatic lung cancer, or single-targeted liposomes.
Another lesser known receptor for breast cancer targeting is the globular head of the complement component receptor (gC1qR). The gC1qR is a multifunctional and multicompartmental protein and, due to its simultaneous discovery by three different laboratories, is known also known as hyaluronan-binding protein 1 (HABP1), p33, and p32 [371,372]. Notably, the gC1qR is not only a cell surface receptor but is also an intracellular and secreted protein, and is able to bind a large variety of plasma and cellular molecules [373]. The gC1qR is normally expressed in all tissues, from the heart to the thalamus, and is involved in the function of the mitochondria, the regulation of dendritic cells and the cell cycle, as well as immunological functions such as bacterial and parasitic interactions, inflammation, etc. When overexpressed, the gC1qR has been associated with adenocarcinomas including thyroid, colon, pancreatic, gastric, esophageal, lung, and breast adenocarcinomas [374], as well as cancer-associated fibroblasts and TAMs. Thus, gC1qR has been identified as a possible tumor biomarker due to its involvement in cancer progression and metastasis [371]. D'Avanzo et al. made use of the LinTT1 (AKRGARSTA) peptide, functionalized to DOX and sorafenib-loaded liposomes, to target TNBC cells [375]. The LinTT1 peptide is able to bind to both the gC1qR and to NRP1, via the cleavage of the peptide by uPa exposing the C-end moiety (AKRGAR), leading to improved penetration of the LinTT1-functionalized liposomes. In the team's study, the LinTT1-functionalized DOX/sorafenib-loaded liposomes showed enhanced therapeutic activity in 2D and 3D cell models of TNBC (MDA-MB-231). Interestingly, the LinTT1-functionalized liposomes were not wholly taken into the M2 macrophages but were partly internalized and partly associated to the cell surface of the macrophages.
Regarding the ideal selection of complementary targeting for drug delivery, Guo et al. sought to identify and select the optimal target combinations for TNBC and designed a targeted DOX-loaded liposomal system [376]. The team used an exhaustive methodology to identify ICAM-1 and HER1 as the most suitable candidates for TNBC targeting amongst a selection of 68 cancer targets in three human TNBC cell lines. Dual targeting liposomes were engineered with the precise ratio and organization of ICAM-1 and HER1-specific ligands to the cell surface of TNBC. In vitro studies demonstrated that the group's targeting system had far superior binding, internalization, and antitumor efficacy and activity in breast cancer and lung metastatic in vivo models.
Whilst the targeting of breast cancer cells, primary tumors, and metastatic sites are worthwhile avenues to pursue, in recent years the targeting of cancer-associated cells, CSCs, and the TME have also been explored. For example, Kim et al. designed DOX-loaded liposomes dual-functionalized with MUC-1 and CD44 aptamers to target both breast cancer cells and CSCs [377]. The targeted design was effective against in vitro 3D-cultured cells and athymic nude mice with metastatic breast cancer tumors showing enhanced cytotoxicity and inhibitory activity. In another example, Koren et al. developed a multistage targeting system whereby the pH-triggered PEGylated liposomes shielded the TAT and mAb 2C5 targeting until an acidic environment, i.e., the tumor site, was encountered and enabled mAb 2C5 to target and TAT to encourage penetration into MCF-7 and 4T1 cells to deliver the DOX cargo [378]. To simultaneously target cancer-associated fibroblasts and overcome the BBB, both great challenges in metastatic cancer treatment, Li et al. designed PTX-loaded liposomes with pH-sensitive FA and dNP2, a CPP able to penetrate through the BBB, for the targeting and treatment of primary breast cancer tumors, its associated TME, and brain metastasis [379]. The liposomal system was able to infiltrate the BBB and, due to the acid-cleavable FA and the subsequent release of PTX, was able to target the folate receptors on cancer-associated fibroblasts leading to decreased tumor activity.
3. Challenges and future considerations in targeted nanoliposomal drug delivery
As previously noted, only four liposome-based treatments have been approved for clinical use in breast cancer and none make use of a targeting system. Instead, the drug delivery systems rely on the increased protection, stability, and circulation time provided by liposomes and their PEGylation for accumulation of the therapeutic in the tumor tissue, i.e., the EPR effect. Of all the targeted-liposomal drug delivery systems discussed in this review, only five (MM-310, MM-302, ONT-10, AS1411 and LiPlaCis®) have progressed to the clinical phase and have registered clinicaltrial.gov identifiers (Table 4). Notably, although ONT-10 is a liposomal system which makes use of targeting system it is a cancer vaccine and not a direct treatment.
Table 4.
The targeted liposomal systems for breast cancer which have progressed to clinical trials.
| Name | Identifier | Target | Composition | Size (nm) | Last updated | Clinical phase | Status | Reference |
|---|---|---|---|---|---|---|---|---|
| MM-310 | NCT03076372 | EphA2, TNBC | ESM, CHOL, DSPE-mPEG (2000) | ∼100 | 2018 | I | Unknown* | [122] |
| MM-302 | NCT01304797, NCT02213744, NCT02735798 | HER2 | PC, CHOL, PEG, DSPE-PEG | 75–110 | 2017 | I, II, III | Unknown*, Terminated, Withdrawn |
[302,[381], [382], [383], [384]] |
| ONT-10 |
NCT01556789, NCT02270372, NCT01978964 |
MUC1, PET Lipid A | CHOL, DMPG, DPPC | 2018 | I, Ib, III | Completed, Completed, Completed |
[314,385,386] | |
| AS1411 | NCT01034410, NCT00881244, NCT00740441,NCT00512083 | Nucleolin | HSPC, CHOL, DSPE-mPEG (2000) | ∼200 | 2009, 2011 | I, II Not BC specifically but solid tumors |
Completed, Unknown*, Completed, Terminated |
[139] |
| LiPlaCis® | NCT01861496 | sPLA2-trigger | POPC, POPG, CHOL, DSPE-PEG (2000) | ∼130 | 2021 | I, II Issues with formulation |
Completed | [214,356,387,388] |
Eph: erythropoietin-producing hepatocellular receptor A2; CHOL: cholesterol; DMPG: dimyristoyl phosphatidylglycerol; DPPC: dipalmitoylphosphatidylcholine; DSPE: 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine; ESM: sphingomyelin from egg; HER2: human epidermal receptor 2; HSPC: hydrogenated soybean phosphatidylcholine; MUC1: mucin 1; PC: phosphatidylcholine; PEG; polyethylene glycol; POPC: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; POPG: 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) sodium salt; sPLA2: secretory phospholipase A2; TNBC: triple negative breast cancer. * Study has passed its completion date and status has not been updated in over two years.
Key features in this bench-to-bedside failure of nanoformulations involve; issues with laboratory-scale batch-to-batch variation, complex large-scale manufacturing processes resulting in limitations in terms of fabrication costs and throughput speeds, intellectual property disputes, lack of clear and consistent governmental guidelines, and the cost-effectiveness of these formulations when compared to current therapies [94,[389], [390], [391], [392], [393], [394]]. Although there are several methods for synthesizing liposomes at the laboratory-scale (e.g., lipid film hydration, solvent dispersion, reverse phase evaporation, etc.), only one – ethanol injection followed by extrusion – is the preferred method for large-scale commercial production as it provides the necessary reproducibility and quality. Large-scale manufacturing is a complex and laborious process with various in-operation controls and associated tests (e.g., buffer preparation, phospholipid solution preparation, filtration, dilution, freeze drying, etc.) which take time and increase costs. New large-scale liposome production methods, e.g., microfluidics and self-assembling technologies, are able to cut-down on costs, energy and time by avoiding certain steps in the manufacturing process all together (e.g., extrusion) and not using organic solvents thus eliminating the need to remove them and the associated quality controls to ensure it [395]. Although there are well-established good manufacturing processes with safety and pharmaceutical criteria (e.g., ingredient purity, stability, administration route, etc.) for the development and production of drugs, nanoformulations used for drug delivery must undergo an additional litany of highly detailed trials. For example; the key components of the nanoformulation must be identified and each related to the performance of the nanomedicine; furthermore, the immune response, endotoxicity, biocompatibility, and genotoxicity, etc. of the nanoparticle and its components must be stipulated; as well as the ability to replicate and sterilize the nanoformulation; and, lastly, a thorough understanding of the in vivo biodistribution, metabolism, absorption, and excretion of every component of the nanoformulation is required [396,397]. To expedite nanomedicines through the clinical pipeline, a shift towards “safety-by-design”, wherein the risks to human health and the environment are foreseen and reduced during the early stages of product development, have become the norm. Projects such as the EU's NANoREG and GoNanoBioMat are aimed towards installing standardized approaches to nanomedicine design and includes several aspects of the development of nanomedicines (e.g., environmental impact, safe transport, specific methods of production, etc.) [398].
The issue with nanomedicine and drug targeting, however, is not just a problem of pharmaceutical manufacturing or governmental red tape but also a lack of the right knowledge and tools. Currently, we have an imperfect understanding of tumor biology and drug delivery design which causes a domino effect of ineffectual nanoformulations and physiologically and clinically irrelevant animal models leading to failures in clinical trials [[399], [400], [401]]. A perfect example of this is the major gaps in our understanding of passive targeting and the EPR effect. The extent and heterogeneity of the EPR effect in solid and metastatic tumors are highly debated and are hypothesized as a reason for the inconsistent response of passive and targeted therapeutic nanomedicines in clinical studies [402,403]. The EPR effect is influenced by several factors, including; the presence and extent of the vascular and lymphatic networks in tumoral tissue and their associated interstitial pressure; the patient's age and gender, tumor type, size, and location; whether the tumor is a primary or metastatic lesion; and the degree of immune involvement regarding the MPS, macrophages, etc. [404,405]. In the coming years, our understanding and use of the EPR effect in cancer nanoliposomal drug delivery will require an overhaul, including the use of strategies to; standardize and analyze the EPR effect in solid tumors; identify, image and track tumor vascularization; and reduce the clearance of therapeutic nanomedicines by the liver, kidneys, lungs, and spleen thus allowing enhanced accumulation in the tumor [406].
Although there are many pre-clinical reports of enhanced cellular internalization and tumor accumulation – some of which are discussed in this review – the clinical boon of nanomedicines has, so far, been limited. Reasons for this discrepancy could be due to formulation stability or the accessibility and availability of targets (e.g., the distinction between ‘low’, ‘normal’, and ‘overexpression’ of surface receptors and proteins). Inter-and intra-heterogeneity of tumors and the presence of metastasis and metastatic supporting cells (CSCs, fibroblasts, TAMs, etc.) drive the complexity of cancer and confound our ability to target it. The majority of breast cancer-specific liposomal formulations are mono-targeted towards a single receptor on the surface of tumor cells which may result in a positive response initially but could promote clone selection leading to MDR and resistant tumor relapse [407]. Other considerations include optimal ligand density on targeted liposomes which will invariably be affected by the target's expression, accessibility, and internalization ability; as well as tumor location, stage, location, immunogenicity, etc. [[408], [409], [410]]. Indeed, it is also important to consider that there is a misunderstanding regarding the potential usefulness of active targeting in drug delivery [411]. Although active targeting can improve the poor tissue and tumor penetration of nanomedicines, it has become abundantly clear that simply modifying liposomes to target receptors, enzymes, macromolecules, or the TME is not enough to produce enhanced drug delivery and efficient cancer treatment in the clinic. Several of the studies mentioned herein highlight a common problem in active targeting, in that targeted drug nanocarriers do not seem to display enhanced ‘tumor homing’ abilities or superior pharmacodynamics resulting in increased anticancer efficacy, but rather 1) increased accumulation in the required zone, i.e., tumoral area, before drug release, and/or 2) improved cellular internalization once the nanocarrier has reached the tumor cells via the EPR effect [200,412,413]. Several reasons for this phenomenon have been proposed, including our definition of the ‘overexpression’ of cell receptors being a major oversimplification, the administration of nanomedicines causing opsonization and obstruction of targeted liposomes thus creating mistargeted or altered pharmacokinetics, and the choice of targets, e.g., HER-2, whilst being clinically relevant for targeting might not be suitable for efficient internalization and either allow for escape from the intracellular endosome or bypass the destructive endocytic pathway altogether [407].
To combat this oversimplification of targeting, researchers are using bio-inspired designs with multimodal targeting and stimuli-responsive drug release. For example, the work of Ma et al. involves the use of a biomimetic membrane via the merging of the erythrocyte membrane with pH-responsive HER2-functionalized liposomes for breast cancer treatment [414]. Other innovative strategies to target and physically localize treatments into breast cancer cells include the use of biomaterials such as framework nucleic-acid-based nanostructures and nanorobots for targeted HER2 breast cancer treatment [415,416]. Liposomes are continually innovated for use in tissue engineering as liposome-scaffold composite systems [417]; programmable, multi-staged or stimuli-triggered multi-drug eluting systems [418]; and in cancer immunotherapy to deliver targeted immune modulators within tumors and the TME, as well as improving CAR-T treatments and enhancing vaccine efficiency in both primary and metastatic tumors [[419], [420], [421], [422]]. Targeting strategies are also being improved by the use of dual-targeted combination therapies which treat several aspects of the tumoral cells (e.g., targeting the mitochondria and MDR mechanisms) [423]. Furthermore, the medical model of personalized medicine which matches patients to the most appropriate treatment via screening for genes, proteins, receptors, vascularization, etc. is also an interesting avenue for targeted liposomal cancer drug delivery.
4. Summary
In summary, this review provides a comprehensive description of the current advancements in liposomal drug delivery which actively targets surface, transmembrane, internal cell receptors, and enzymes, of breast cancer and breast cancer-associated cells. These liposomal targets are involved in; cell movement, proliferation, aggregation, protection, attachment, cell-cell interactions and signaling; as well as vitamin and macromolecule metabolism; and hormonal regulation of breast cancer cells. For several decades liposomes have been continually developed and improved to overcome their issues with reproducibility, scale-ability, production cost, solubility, and stability (i.e., sedimentation, sensitivity to oxidation, leakage, etc.), sterilization, and short circulation time. Although the appeal of liposomes in cancer drug delivery was initially due to their biocompatability and reduced drug clearance, their ability to lower systemic with stealth modifications and active targeting has gained immense interest. Several liposomal formulations have recently been approved for clinical use, e.g., Onpattro®, Vyxeos®, Onivyde®, COVID-19 vaccines, etc., and although none are targeted, they highlight the clinical relevance of liposomes in drug delivery. Furthermore, as liposomes can be easily functionalized with several state-of-the-art targeting entities, e.g., peptides, aptamers, various Ab fragments, etc., they are promising nanocarriers for targeted drug delivery in breast cancer.
Ethics approval and consent to participate
Not applicable.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to acknowledge the Lorraine Université d’Excellence (LUE) and the Mirabelle + funding organizations. Graphical abstract image was been made with Servier Medical Art (smart.servier.com).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Stéphanie Grandemange, Email: stephanie.grandemange@univ-lorraine.fr.
Elmira Arab-Tehrany, Email: elmira.arab-tehrany@univ-lorraine.fr.
Abbreviations
- αvβ3
integrin alpha V and beta 3
- (pNP)2
bis(p-nitrophenyl carbonate)
- 1-StePc
1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine
- Ab
antibody
- anti-EphA2 scFv
anti-erythropoietin-producing hepatocellular single-chain variable fragment
- ATB0,+
amino acid transporter B0+
- BCRP
breast cancer resistance protein
- BBB
blood-brain barrier
- CD44
cluster of differentiation 44
- CDK
cyclin-dependent kinase
- CET
cetuximab
- CHEMS
cholesteryl hemisuccinate
- CHOL
cholesterol
- CPP
cell-penetrating peptide
- cRGD
cyclic arginyl glycyl aspartic acid, Arg-Gly-Asp
- CSCs
cancer stem cells
- CXCR4
C-X-C chemokine receptor type 4
- DBCO
dibenzo-cyclooctyne
- DCIS
ductal carcinoma in situ
- DCP
dicetylphosphate
- DDAB
dimethyldioctadecylammonium bromide
- DHA
dihydroartemisinin
- DLS
dynamic light scattering
- DMKE
O,O′-dimyristyl-N-lysyl glutamate
- DMPG
dimyristoyl phosphatidylglycerol
- dNP2
a type of cell-penetrating peptide
- DOA
3′,5′-dioleoyladenosine
- DODAP
1,2-dioleoyl-3-dimethylammonium-propane
- DODEAC
N,N-di-n-tetradecyl-N,N-(2-hydroxyethyl)ammonium chloride
- DOPA
dioleoyl phosphatidic acid
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DOTAP
dioleoyl-3-trimethylammonium propane
- DOX
doxorubicin
- DPPC
dipalmitoylphosphatidylcholine
- DSPC
distearylphosphatidylcholine
- DSPE
1,2-distearoyl-sn-glycero-3-phosphorylethanolamine
- DTPA
diethylenetriaminepentaacetic acide anhydride
- E1, E2, E3
estrone, estradiol, estriol
- E80
egg phosphatidylcholine
- EDC
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydro-chloride
- EGCG
epigallocatechin gallate
- EMT
epithelial to mesenchymal transition
- EPC
egg yolk phosphatidylcholine
- Eph
erythropoietin-producing hepatocellular carcinoma receptor
- EPR
enhanced permeability and retention effect
- ER
estrogen receptor
- ESM
sphingomyelin from egg
- FA
folic acid
- Fab
fragment antigen-binding unit
- Fc region
fragment crystallizable region
- GLUTs
hexose transporters
- HA
hyaluronic acid
- HABP1
hyaluronan-binding protein 1
- hCTMO1
humanized anti-MUC1 mAbs
- HER
human epidermal receptor
- HG2C18
1,5-dioctadecyl-N-histidyl-l-glutamate
- HIF-1α
hypoxia-inducible factor 1-alpha
- HPPH
2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a
- HSPC
hydrogenated soybean phosphatidylcholine
- ICAM-1
intracellular adhesion molecule-1
- ICG
indocyanine green
- IL6R
interleukin 6 receptor
- KLA
acetyl-(KLAKLAK)2-NH2 peptide
- LAT1
L-type amino acid transporter 1
- LCIS
lobular carcinoma in situ
- Lcn2
lipocalin 2
- LDL
low-density lipoprotein
- LHRH
luteinizing hormone-releasing hormone
- LPC
1-palmitoyl-2-hydroxy sn-glycero-3-phosphocholine
- LRP1
lipoprotein receptor-related protein 1
- mAb
monoclonal antibody
- mAb 2C5
tumor-associated cell surface nucleosome antibody
- MAL
maleimide
- MAPK/ERK
mitogen-activated protein kinases/extracellular signal-regulated kinases
- MDR
multidrug resistance
- mGPER30
membrane-bound G-protein-coupled estrogen receptor
- MMPs
matrix metalloproteinases
- mPEG-DSG
1,2-distearoyl-rac-glycero-3-methylpo-lyoxyethylene
- mPRs
membrane progesterone receptors
- MPS
mononuclear phagocytic system
- MUC1
mucin 1
- N-dod-PE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-dodecanoyl
- NHS
NHS ester
- NIR
near-infrared
- NRP1
neuropilin 1
- OPP
octadecyl-1,1-dimethylpiperidin-1-ium-4-yl phosphate
- PAI
plasminogen activator inhibitor
- PARP
poly ADP-ribose polymerase
- PC
phosphatidylcholine
- PE
phosphatidylehtanolamine
- PEG
polyethylene glycol
- PG
phosphatidylglycerol
- P-gp
p-glycoprotein
- PI3K/Akt
phosphoinositide 3-kinase/protein kinase B
- pNP
p-nitrophenylcarbonyl
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- POPG
1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) sodium salt
- PR
progesterone receptor
- PTX
paclitaxel
- Rc
receptor
- RES
reticuloendothelial system
- RGD
arginylglycylaspartic acid, Arg-Gly-Asp
- ROS
reactive oxygen species
- scFv
single-chain variable fragments
- SDF-1
stromal cell-derived factor 1
- siRNA
small interfering RNA
- SIRPa
signal-regulatory protein alpha
- SMVT
sodium-dependent multivitamin transporter
- SPC
soy phosphatidylcholine
- sPLA2
secretory phospholipase A2
- TAM
tumor-associated macrophage
- TAT
trans-activator of transcription
- T-DM1
antibody-drug conjugate trastuzumab-emtansine
- TfR
transferrin receptor
- TGF-β1
transforming growth factor beta 1
- TME
tumor microenvironment
- TNBC
triple-negative breast cancer
- TNF
tumor necrosis factor
- TPGS
d-α-tocopheryl polyethylene glycol 1000 succinate
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- uPA
urokinase-type plasminogen activator
- uPAR
urokinase-type plasminogen activator receptor
- VEGF
vascular endothelial growth factor
- VHH
variable domain of the heavy chain antibody
- VM
vasculogenic mimicry
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/CAAC.21660. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N., Altekruse S.F., Li C.I., Chen V.W., Clarke C.A., Ries L.A.G., Cronin K.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. JNCI J. Natl. Cancer Inst. 2014;106 doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akinyemiju T.F., Pisu M., Waterbor J.W., Altekruse S.F. Socioeconomic status and incidence of breast cancer by hormone receptor subtype. SpringerPlus. 2015;4:1–8. doi: 10.1186/s40064-015-1282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Momenimovahed Z., Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer. 2019;11:151–164. doi: 10.2147/BCTT.S176070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azamjah N., Soltan-Zadeh Y., Zayeri F. Global trend of breast cancer mortality rate: a 25-year study. Asian Pac. J. Cancer Prev. APJCP. 2019;20:2015–2020. doi: 10.31557/APJCP.2019.20.7.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akram M., Iqbal M., Daniyal M., Khan A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017;50:33. doi: 10.1186/s40659-017-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Núñez C., Capelo J.L., Igrejas G., Alfonso A., Botana L.M., Lodeiro C. An overview of the effective combination therapies for the treatment of breast cancer. Biomaterials. 2016;97:34–50. doi: 10.1016/j.biomaterials.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Trevisi E., La Salvia A., Daniele L., Brizzi M.P., De Rosa G., Scagliotti G.V., Di Maio M. Neuroendocrine breast carcinoma: a rare but challenging entity. Med. Oncol. Northwood Lond. Engl. 2020;37:70. doi: 10.1007/S12032-020-01396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis C., Shah S.P., Chin S.F., Turashvili G., Rueda O.M., Dunning M.J., Speed D., Lynch A.G., Samarajiwa S., Yuan Y., Gräf S., Ha G., Haffari G., Bashashati A., Russell R., McKinney S., Aparicio S., Brenton J.D., Ellis I., Huntsman D., Pinder S., Murphy L., Bardwell H., Ding Z., Jones L., Liu B., Papatheodorou I., Sammut S.J., Wishart G., Chia S., Gelmon K., Speers C., Watson P., Blamey R., Green A., MacMillan D., Rakha E., Gillett C., Grigoriadis A., De Rinaldis E., Tutt A., Parisien M., Troup S., Chan D., Fielding C., Maia A.T., McGuire S., Osborne M., Sayalero S.M., Spiteri I., Hadfield J., Bell L., Chow K., Gale N., Kovalik M., Ng Y., Prentice L., Tavaré S., Markowetz F., Langerød A., Provenzano E., Purushotham A., Børresen-Dale A.L., Caldas C. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leong A.S.-Y., Zhuang Z. The changing role of pathology in breast cancer diagnosis and treatment. Pathobiology. 2011;78:99–114. doi: 10.1159/000292644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldhirsch A., Wood W.C., Coates A.S., Gelber R.D., Thürlimann B., Senn H.-J. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the st gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann. Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prat A., Parker J.S., Karginova O., Fan C., Livasy C., Herschkowitz J.I., He X., Perou C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:1–18. doi: 10.1186/BCR2635/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prat A., Pineda E., Adamo B., Galván P., Fernández A., Gaba L., Díez M., Viladot M., Arance A., Muñoz M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24:S26–S35. doi: 10.1016/J.BREAST.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Narod S.A. BRCA mutations in the management of breast cancer: the state of the art. Nat. Rev. Clin. Oncol. 2010;7:702–707. doi: 10.1038/nrclinonc.2010.166. [DOI] [PubMed] [Google Scholar]
- 15.Onitilo A.A., Engel J.M., Greenlee R.T., Mukesh B.N. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin. Med. Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malorni L., Shetty P.B., De Angelis C., Hilsenbeck S., Rimawi M.F., Elledge R., Osborne C.K., De Placido S., Arpino G. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res. Treat. 2012;136:795–804. doi: 10.1007/s10549-012-2315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moo T.A., Sanford R., Dang C., Morrow M. Overview of breast cancer therapy. Pet. Clin. 2018;13:339–354. doi: 10.1016/j.cpet.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowery A.J., Kell M.R., Glynn R.W., Kerin M.J., Sweeney K.J. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res. Treat. 2012;133:831–841. doi: 10.1007/s10549-011-1891-6. [DOI] [PubMed] [Google Scholar]
- 19.Denduluri N., Chavez-MacGregor M., Telli M.L., Eisen A., Graff S.L., Hassett M.J., Holloway J.N., Hurria A., King T.A., Lyman G.H., Partridge A.H., Somerfield M.R., Trudeau M.E., Wolff A.C., Giordano S.H. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO clinical practice guideline focused update. J. Clin. Oncol. 2018;36:2433–2443. doi: 10.1200/JCO.2018.78.8604. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein S.R., Siddhanti S., Ciaccia A.V., Plouffe L. A pharmacological review of selective oestrogen receptor modulators. Hum. Reprod. Update. 2000;6:212–224. doi: 10.1093/humupd/6.3.212. [DOI] [PubMed] [Google Scholar]
- 21.Smith I.E., Dowsett M. Aromatase inhibitors in breast cancer. N. Engl. J. Med. 2003;348:2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 22.Ali S., Mondal N., Choudhry H., Rasool M., Pushparaj P.N., Khan M.A., Mahfooz M., Sami G.A., Jarullah J., Ali A., Jamal M.S. Current management strategies in breast cancer by targeting key altered molecular players. Front. Oncol. 2016;6 doi: 10.3389/fonc.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alavi M., Hamidi M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019;34 doi: 10.1515/dmpt-2018-0032. [DOI] [PubMed] [Google Scholar]
- 24.Mokhatri-Hesari P., Montazeri A. Health-related quality of life in breast cancer patients: review of reviews from 2008 to 2018. Health Qual. Life Outcome. 2020;18:1–25. doi: 10.1186/S12955-020-01591-X/TABLES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teixeira S., Carvalho M.A., Castanheira E.M.S. Functionalized liposome and albumin-based systems as carriers for poorly water-soluble anticancer drugs: an updated review. Biomedicines. 2022;10:486. doi: 10.3390/biomedicines10020486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schleicher S.M., Bach P.B., Matsoukas K., Korenstein D. Medication overuse in oncology: current trends and future implications for patients and society. Lancet Oncol. 2018;19:e200–e208. doi: 10.1016/S1470-2045(18)30099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wadhwani N., Jatoi I. Overuse of neo-adjuvant chemotherapy for primary breast cancer. Indian J. Surg. Oncol. 2020;11:12–14. doi: 10.1007/S13193-019-01002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y.J., Lei Y.H., Yao N., Wang C.R., Hu N., Ye W.C., Zhang D.M., Chen Z.S. Autophagy and multidrug resistance in cancer. Chin. J. Cancer. 2017;36:52. doi: 10.1186/s40880-017-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh S.K., Singh S., Wlillard J., Singh R. Drug delivery approaches for breast cancer. Int. J. Nanomed. 2017;12:6205–6218. doi: 10.2147/IJN.S140325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assaraf Y.G., Brozovic A., Gonçalves A.C., Jurkovicova D., Linē A., Machuqueiro M., Saponara S., Sarmento-Ribeiro A.B., Xavier C.P.R., Vasconcelos M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updates. 2019;46 doi: 10.1016/j.drup.2019.100645. [DOI] [PubMed] [Google Scholar]
- 31.Giacomini K.M., Huang S.M., Tweedie D.J., Benet L.Z., Brouwer K.L.R., Chu X., Dahlin A., Evers R., Fischer V., Hillgren K.M., Hoffmaster K.A., Ishikawa T., Keppler D., Kim R.B., Lee C.A., Niemi M., Polli J.W., Sugiyama Y., Swaan P.W., Ware J.A., Wright S.H., Wah Yee S., Zamek-Gliszczynski M.J., Zhang L. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tacar O., Sriamornsak P., Dass C.R. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013;65:157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 33.He H., Liu C., Wu Y., Zhang X., Fan J., Cao Y. A multiscale physiologically-based pharmacokinetic model for doxorubicin to explore its mechanisms of cytotoxicity and cardiotoxicity in human physiological contexts. Pharm. Res. (N. Y.) 2018;35:1–10. doi: 10.1007/s11095-018-2456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang N., Shu G., Qiao E., Xu X., Shen L., Lu C., Chen W., Fang S., Yang Y., Song J., Zhao Z., Tu J., Xu M., Chen M., Du Y., Ji J. DNA-functionalized liposomes in vivo fusion for NIR-II/MRI guided pretargeted ferroptosis therapy of metastatic breast cancer. ACS Appl. Mater. Interfaces. 2022;14:20603–20615. doi: 10.1021/acsami.2c01105. [DOI] [PubMed] [Google Scholar]
- 35.Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 36.Mao Z., Shen K., Zhu L., Xu M., Yu F., Xue D., Li H., Xue C. Comparisons of cardiotoxicity and efficacy of anthracycline-based therapies in breast cancer: a network meta-analysis of randomized clinical trials. Oncol. Res. Treat. 2019;42:405–413. doi: 10.1159/000500204. [DOI] [PubMed] [Google Scholar]
- 37.Caswell-Jin J.L., Plevritis S.K., Tian L., Cadham C.J., Xu C., Stout N.K., Sledge G.W., Mandelblatt J.S., Kurian A.W. vol. 2. JNCI Cancer Spectr; 2018. (Change in Survival in Metastatic Breast Cancer with Treatment Advances: Meta-Analysis and Systematic Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardoso F., Spence D., Mertz S., Corneliussen-James D., Sabelko K., Gralow J., Cardoso M.J., Peccatori F., Paonessa D., Benares A., Sakurai N., Beishon M., Barker S.J., Mayer M. Global analysis of advanced/metastatic breast cancer: decade report (2005–2015) Breast. 2018;39:131–138. doi: 10.1016/J.BREAST.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Li A., Schleicher S.M., Andre F., Mitri Z.I. Genomic alteration in metastatic breast cancer and its treatment. Am. Soc. Clin. Oncol. Educ. Book. 2020:1–14. doi: 10.1200/edbk_280463. [DOI] [PubMed] [Google Scholar]
- 40.Liu R., Zhou J., Yang S., Zhang Z. Efficacy and safety of pegylated liposomal doxorubicin-based chemotherapy of AIDS-related Kaposiʼs sarcoma. Am. J. Ther. 2018;25:e719–e721. doi: 10.1097/MJT.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 41.Di Paolo A. Liposomal anticancer therapy: pharmacokinetic and clinical aspects. J. Chemother. 2004:90–93. doi: 10.1179/joc.2004.16.supplement-1.90. [DOI] [PubMed] [Google Scholar]
- 42.Miele E., Spinelli G.P., Miele E., Tomao F., Tomao S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009;4:99–105. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eloy J.O., Claro de Souza M., Petrilli R., Barcellos J.P.A., Lee R.J., Marchetti J.M. Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery. Colloids Surf. B Biointerfaces. 2014;123:345–363. doi: 10.1016/j.colsurfb.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 44.Ansari L., Shiehzadeh F., Taherzadeh Z., Nikoofal-Sahlabadi S., Momtazi-Borojeni A.A., Sahebkar A., Eslami S. The most prevalent side effects of pegylated liposomal doxorubicin monotherapy in women with metastatic breast cancer: a systematic review of clinical trials. Cancer Gene Ther. 2017;24:189–193. doi: 10.1038/cgt.2017.9. [DOI] [PubMed] [Google Scholar]
- 45.Fang X., Cao J., Shen A. Advances in anti-breast cancer drugs and the application of nano-drug delivery systems in breast cancer therapy. J. Drug Deliv. Sci. Technol. 2020;57 doi: 10.1016/j.jddst.2020.101662. [DOI] [Google Scholar]
- 46.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hare J.I., Lammers T., Ashford M.B., Puri S., Storm G., Barry S.T. Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv. Drug Deliv. Rev. 2017;108:25–38. doi: 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 48.Bangham A.D. A correlation between surface charge and coagulant action of phospholipids. Nature. 1961;192:1197–1198. doi: 10.1038/1921197a0. [DOI] [PubMed] [Google Scholar]
- 49.Bangham A.D. Liposomes: the Babraham connection. Chem. Phys. Lipids. 1993;64:275–285. doi: 10.1016/0009-3084(93)90071-A. [DOI] [PubMed] [Google Scholar]
- 50.Elkhoury K., Koçak P., Kang A., Arab-Tehrany E., Ellis Ward J., Shin S.R. Engineering smart targeting nanovesicles and their combination with hydrogels for controlled drug delivery. Pharmaceutics. 2020;12:849. doi: 10.3390/pharmaceutics12090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velot É., Elkhoury K., Kahn C., Kempf H., Linder M., Arab-Tehrany E., Bianchi A. Efficient TGF-β1 delivery to articular chondrocytes in vitro using agro-based liposomes. Int. J. Mol. Sci. 2022;23:2864. doi: 10.3390/ijms23052864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Passeri E., Bun P., Elkhoury K., Linder M., Malaplate C., Yen F.T., Arab-Tehrany E. Transfer phenomena of nanoliposomes by live imaging of primary cultures of cortical neurons. Pharmaceutics. 2022;14:2172. doi: 10.3390/pharmaceutics14102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elkhoury K., Sanchez-Gonzalez L., Lavrador P., Almeida R., Gaspar V., Kahn C., Cleymand F., Arab-Tehrany E., Mano J.F. Gelatin methacryloyl (GelMA) nanocomposite hydrogels embedding bioactive Naringin liposomes. Polymers. 2020;12:2944. doi: 10.3390/polym12122944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arab-Tehrany E., Elkhoury K., Francius G., Jierry L., Mano J.F., Kahn C., Linder M. Curcumin loaded nanoliposomes localization by nanoscale characterization. Int. J. Mol. Sci. 2020;21:7276. doi: 10.3390/ijms21197276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Webster D.M., Sundaram P., Byrne M.E. Injectable nanomaterials for drug delivery: carriers, targeting moieties, and therapeutics. Eur. J. Pharm. Biopharm. 2013;84:1–20. doi: 10.1016/j.ejpb.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 56.Shende P., Ture N., Gaud R.S., Trotta F. Lipid- and polymer-based plexes as therapeutic carriers for bioactive molecules. Int. J. Pharm. 2019;558:250–260. doi: 10.1016/j.ijpharm.2018.12.085. [DOI] [PubMed] [Google Scholar]
- 57.Vemuri S., Rhodes C.T. Preparation and characterization of liposomes as therapeutic delivery systems: a review. Pharm. Acta Helv. 1995;70:95–111. doi: 10.1016/0031-6865(95)00010-7. [DOI] [PubMed] [Google Scholar]
- 58.Elizondo E., Moreno E., Cabrera I., Córdoba A., Sala S., Veciana J., Ventosa N. Prog. Mol. Biol. Transl. Sci. Elsevier B.V.; 2011. Liposomes and other vesicular systems: structural characteristics, methods of preparation, and use in nanomedicine; pp. 1–52. [DOI] [PubMed] [Google Scholar]
- 59.Gregoriadis G., Davis C. Stability of liposomes invivo and invitro is promoted by their cholesterol content and the presence of blood cells. Biochem. Biophys. Res. Commun. 1979;89:1287–1293. doi: 10.1016/0006-291X(79)92148-X. [DOI] [PubMed] [Google Scholar]
- 60.Drummond D.C., Noble C.O., Hayes M.E., Park J.W., Kirpotin D.B. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J. Pharm. Sci. 2008;97:4696–4740. doi: 10.1002/jps.21358. [DOI] [PubMed] [Google Scholar]
- 61.Laouini A., Jaafar-Maalej C., Limayem-Blouza I., Sfar S., Charcosset C., Fessi H. Preparation, characterization and applications of liposomes: state of the art. J. Colloid Sci. Biotechnol. 2012;1:147–168. doi: 10.1166/jcsb.2012.1020. [DOI] [Google Scholar]
- 62.Chaudhry Q., Watkins R., Castle L. RSC Nanosci. Nanotechnol. Royal Society of Chemistry; 2017. Nanotechnologies in food: what, Why and how? pp. 1–19. [DOI] [Google Scholar]
- 63.Khorasani S., Danaei M., Mozafari M.R. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018;79:106–115. doi: 10.1016/j.tifs.2018.07.009. [DOI] [Google Scholar]
- 64.Bulbake U., Doppalapudi S., Kommineni N., Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9:12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saraf S., Jain A., Tiwari A., Verma A., Panda P.K., Jain S.K. Advances in liposomal drug delivery to cancer: an overview. J. Drug Deliv. Sci. Technol. 2020;56 doi: 10.1016/J.JDDST.2020.101549. [DOI] [Google Scholar]
- 66.Large D.E., Abdelmessih R.G., Fink E.A., Auguste D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/J.ADDR.2021.113851. [DOI] [PubMed] [Google Scholar]
- 67.Elkhoury K., Chen M., Koçak P., Enciso-Martínez E., Bassous N.J., Lee M.C., Byambaa B., Rezaei Z., Li Y., Ubina López M.E., Gurian M., Sobahi N., Hussain M.A., Sanchez-Gonzalez L., Leijten J., Hassan S., Arab-Tehrany E., Ward J.E., Shin S.R. Hybrid extracellular vesicles-liposome incorporated advanced bioink to deliver microRNA. Biofabrication. 2022;14 doi: 10.1088/1758-5090/ac8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elkhoury K., Russell C.S., Sanchez‐Gonzalez L., Mostafavi A., Williams T.J., Kahn C., Peppas N.A., Arab‐Tehrany E., Tamayol A. Soft‐nanoparticle functionalization of natural hydrogels for tissue engineering applications. Adv. Healthc. Mater. 2019;8 doi: 10.1002/adhm.201900506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antoniou A.I., Giofrè S., Seneci P., Passarella D., Pellegrino S. Stimulus-responsive liposomes for biomedical applications, Drug Discov. Today Off. 2021;26:1794–1824. doi: 10.1016/J.DRUDIS.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 70.Bilal M., Qindeel M., Raza A., Mehmood S., Rahdar A. Stimuli-responsive nanoliposomes as prospective nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021;66 doi: 10.1016/J.JDDST.2021.102916. [DOI] [Google Scholar]
- 71.Belfiore L., Saunders D.N., Ranson M., Thurecht K.J., Storm G., Vine K.L. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: challenges and opportunities. J. Contr. Release. 2018;277:1–13. doi: 10.1016/j.jconrel.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 72.Sonju J.J., Dahal A., Singh S.S., Jois S.D. Peptide-functionalized liposomes as therapeutic and diagnostic tools for cancer treatment. J. Contr. Release. 2021;329:624–644. doi: 10.1016/J.JCONREL.2020.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alsawaftah N., Pitt W.G., Husseini G.A. Dual-targeting and stimuli-triggered liposomal drug delivery in cancer treatment. ACS Pharmacol. Transl. Sci. 2021;4:1028–1049. doi: 10.1021/acsptsci.1c00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gabizon A., Peretz T., Sulkes A., Amselem S., Ben-Yosef R., Ben-Baruch N., Catane R., Biran S., Barenholz Y. Systemic administration of doxorubicin-containing liposomes in cancer patients: a phase I study. Eur. J. Cancer Clin. Oncol. 1989;25:1795–1803. doi: 10.1016/0277-5379(89)90350-7. [DOI] [PubMed] [Google Scholar]
- 75.Gabizon A., Catane R., Uziely B., Kaufman B., Safra T., Cohen R., Martin F., Huang A., Barenholz Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987–992. [PubMed] [Google Scholar]
- 76.Keller A.M., Mennel R.G., Georgoulias V.A., Nabholtz J.-M., Erazo A., Lluch A., Vogel C.L., Kaufmann M., von Minckwitz G., Henderson C., Mellars L., Alland L., Tendler C. Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J. Clin. Oncol. 2004;22:3893–3901. doi: 10.1200/JCO.2004.08.157. [DOI] [PubMed] [Google Scholar]
- 77.Batist G., Ramakrishnan G., Rao C.S., Chandrasekharan A., Gutheil J., Guthrie T., Shah P., Khojasteh A., Nair M.K., Hoelzer K., Tkaczuk K., Park Youn Choi, Lee L.W. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J. Clin. Oncol. 2001;19:1444–1454. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 78.Batist G., Barton J., Chaikin P., Swenson C., Welles L. Myocet (liposome-encapsulated doxorubicin citrate): a new approach in breast cancer therapy. Expet Opin. Pharmacother. 2002;3:1739–1751. doi: 10.1517/14656566.3.12.1739. [DOI] [PubMed] [Google Scholar]
- 79.Chan S., Davidson N., Juozaityte E., Erdkamp F., Pluzanska A., Azarnia N., Lee L.W. Phase III trial of liposomal doxorubicin and cyclophosphamide compared with epirubicin and cyclophosphamide as first-line therapy for metastatic breast cancer. Ann. Oncol. 2004;15:1527–1532. doi: 10.1093/annonc/mdh393. [DOI] [PubMed] [Google Scholar]
- 80.Burade V., Bhowmick S., Maiti K., Zalawadia R., Ruan H., Thennati R. Lipodox® (generic doxorubicin hydrochloride liposome injection): in vivo efficacy and bioequivalence versus Caelyx® (doxorubicin hydrochloride liposome injection) in human mammary carcinoma (MX-1) xenograft and syngeneic fibrosarcoma (WEHI 164) mouse models. BMC Cancer. 2017;17:405. doi: 10.1186/s12885-017-3377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu X., Wang L., Xu H., Huang X., Qian Y., Xiang J. Clinical comparison between paclitaxel liposome (Lipusu®) and paclitaxel for treatment of patients with metastatic gastric cancer. Asian Pac. J. Cancer Prev. APJCP. 2013;14:2591–2594. doi: 10.7314/APJCP.2013.14.4.2591. [DOI] [PubMed] [Google Scholar]
- 82.Wang H., Cheng G., Du Y., Ye L., Chen W., Zhang L., Wang T., Tian J., Fu F. Hypersensitivity reaction studies of a polyethoxylated castor oil-free, liposome-based alternative paclitaxel formulation. Mol. Med. Rep. 2013;7:947–952. doi: 10.3892/MMR.2013.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chou H., Lin H., Liu J.M. A tale of the two PEGylated liposomal doxorubicins. OncoTargets Ther. 2015;8:1719–1720. doi: 10.2147/OTT.S79089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.FDA approves generic version of Doxil; expected to help resolve shortage. Oncol. Times. 2013;35:25. doi: 10.1097/01.cot.0000428636.40337.70. [DOI] [Google Scholar]
- 85.Barenholz Y. Doxil® - the first FDA-approved nano-drug: lessons learned. J. Contr. Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 86.Eloy J.O., Petrilli R., Trevizan L.N.F., Chorilli M. Immunoliposomes: a review on functionalization strategies and targets for drug delivery. Colloids Surf. B Biointerfaces. 2017;159:454–467. doi: 10.1016/j.colsurfb.2017.07.085. [DOI] [PubMed] [Google Scholar]
- 87.Gaspar R.S., Florindo H.F., Silva L.C., Videira M.A., Corvo M.L., Martins B.F., Silva-Lima B. Springer; Cham: 2014. Regulatory Aspects of Oncologicals: Nanosystems Main Challenges; pp. 425–452. [DOI] [Google Scholar]
- 88.Zhu L., Chen L. Progress in research on paclitaxel and tumor immunotherapy, Cell. Mol. Biol. Lett. 2019;24:1–11. doi: 10.1186/S11658-019-0164-Y. 2019 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bernabeu E., Cagel M., Lagomarsino E., Moretton M., Chiappetta D.A. Paclitaxel: what has been done and the challenges remain ahead. Int. J. Pharm. 2017;526:474–495. doi: 10.1016/J.IJPHARM.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 90.Gill P.S., Wernz J., Scadden D.T., Cohen P., Mukwaya G.M., von Roenn J.H., Jacobs M., Kempin S., Silverberg I., Gonzales G., Rarick M.U., Myers A.M., Shepherd F., Sawka C., Pike M.C., Ross M.E. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi's sarcoma. J. Clin. Oncol. 1996;14:2353–2364. doi: 10.1200/JCO.1996.14.8.2353. [DOI] [PubMed] [Google Scholar]
- 91.Bellott R., Auvrignon A., Leblanc T., Pérel Y., Gandemer V., Bertrand Y., Méchinaud F., Bellenger P., Vernois J., Leverger G., Baruchel A., Robert J. Pharmacokinetics of liposomal daunorubicin (DaunoXome) during a phase I-II study in children with relapsed acute lymphoblastic leukaemia. Cancer Chemother. Pharmacol. 2000;47:15–21. doi: 10.1007/S002800000206. 2000 471. [DOI] [PubMed] [Google Scholar]
- 92.O'Byrne K.J., Thomas A.L., Sharma R.A., DeCatris M., Shields F., Beare S., Steward W.P. A phase I dose-escalating study of DaunoXome, liposomal daunorubicin, in metastatic breast cancer. Br. J. Cancer. 2002;87:15–20. doi: 10.1038/sj.bjc.6600344. 2002 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Danhier F., Feron O., Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Contr. Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 94.Sawant R.R., Torchilin V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012;14:303–315. doi: 10.1208/s12248-012-9330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reineke J. Terminology matters: there is no targeting, but retention. J. Contr. Release. 2018;273:180–183. doi: 10.1016/j.jconrel.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 96.Scherphof G.L., Dijkstra J., Spanjer H.H., Derksen J.T., Roerdink F.H. Uptake and intracellular processing of targeted and nontargeted liposomes by rat kupffer cells in vivo and in vitro. Ann. N. Y. Acad. Sci. 1985;446:368–384. doi: 10.1111/j.1749-6632.1985.tb18414.x. [DOI] [PubMed] [Google Scholar]
- 97.Longmire M., Choyke P.L., Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomed. 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moghimi S.M., Farhangrazi Z.S. Nanomedicine and the complement paradigm. Nanomed. Nanotechnol. Biol. Med. 2013;9:458–460. doi: 10.1016/j.nano.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 99.Torchilin V.P. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007;9:e128–e147. doi: 10.1208/aapsj0902015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Charrois G.J.R., Allen T.M. Multiple injections of pegylated liposomal doxorubicin: pharmacokinetics and therapeutic activity. J. Pharmacol. Exp. Therapeut. 2003;306:1058–1067. doi: 10.1124/jpet.103.053413. [DOI] [PubMed] [Google Scholar]
- 102.Gu W., Meng F., Haag R., Zhong Z. Actively targeted nanomedicines for precision cancer therapy: concept, construction, challenges and clinical translation. J. Contr. Release. 2021;329:676–695. doi: 10.1016/j.jconrel.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 103.Almeida B., Nag O.K., Rogers K.E., Delehanty J.B. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Mol. Basel Switz. 2020;25:5672. doi: 10.3390/molecules25235672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Lima P.H.C., Butera A.P., Cabeça L.F., Ribeiro-Viana R.M. Liposome surface modification by phospholipid chemical reactions. Chem. Phys. Lipids. 2021;237 doi: 10.1016/j.chemphyslip.2021.105084. [DOI] [PubMed] [Google Scholar]
- 105.Marqués-Gallego P., de Kroon A.I.P.M. Ligation strategies for targeting liposomal nanocarriers. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/129458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taiariol L., Chaix C., Farre C., Moreau E. Click and bioorthogonal chemistry: the future of active targeting of nanoparticles for nanomedicines? Chem. Rev. 2022;122:340–384. doi: 10.1021/acs.chemrev.1c00484. [DOI] [PubMed] [Google Scholar]
- 107.Riaz M., Riaz M., Zhang X., Lin C., Wong K., Chen X., Zhang G., Lu A., Yang Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: a review. Int. J. Mol. Sci. 2018;19:195. doi: 10.3390/ijms19010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crivianu-Gaita V., Thompson M. Aptamers, antibody scFv, and antibody Fab’ fragments: an overview and comparison of three of the most versatile biosensor biorecognition elements. Biosens. Bioelectron. 2016;85:32–45. doi: 10.1016/J.BIOS.2016.04.091. [DOI] [PubMed] [Google Scholar]
- 109.Forssen E., Willis M. Ligand-targeted liposomes. Adv. Drug Deliv. Rev. 1998;29:249–271. doi: 10.1016/S0169-409X(97)00083-5. [DOI] [PubMed] [Google Scholar]
- 110.Ruoslahti E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv. Mater. 2012;24:3747–3756. doi: 10.1002/adma.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu Y., Phillips J.A., Yan J., Li Q., Fan Z.H., Tan W. Aptamer-based microfluidic device for enrichment, sorting, and detection of multiple cancer cells. Anal. Chem. 2009;81:7436–7442. doi: 10.1021/ac9012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dickey D.D., Giangrande P.H. Oligonucleotide aptamers: a next-generation technology for the capture and detection of circulating tumor cells. Methods. 2016;97:94–103. doi: 10.1016/j.ymeth.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou Z., Liu M., Jiang J. The potential of aptamers for cancer research. Anal. Biochem. 2018;549:91–95. doi: 10.1016/j.ab.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 114.Guo P., You J.-O., Yang J., Jia D., Moses M.A., Auguste D.T. Inhibiting metastatic breast cancer cell migration via the synergy of targeted, pH-triggered siRNA delivery and chemokine Axis blockade. Mol. Pharm. 2014;11:755–765. doi: 10.1021/MP4004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu D., Guo P., McCarthy C., Wang B., Tao Y., Auguste D. Peptide density targets and impedes triple negative breast cancer metastasis. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-05035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu G., Qiu Y., Su X. Targeting CXCL12-CXCR4 signaling enhances immune checkpoint blockade therapy against triple negative breast cancer. Eur. J. Pharmaceut. Sci. 2021;157 doi: 10.1016/J.EJPS.2020.105606. [DOI] [PubMed] [Google Scholar]
- 117.Zhang K., Fang X., You Q., Lin Y., Ma L., Xu S., Ge Y., Xu H., Yang Y., Wang C. Novel peptide-directed liposomes for targeted combination therapy of breast tumors, Mater. Adv. 2020;1:3483–3495. doi: 10.1039/D0MA00536C. [DOI] [Google Scholar]
- 118.Lukyanov A.N., Elbayoumi T.A., Chakilam A.R., Torchilin V.P. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J. Contr. Release. 2004;100:135–144. doi: 10.1016/J.JCONREL.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 119.Elbayoumi T., Torchilin V. Enhanced cytotoxicity of monoclonal anticancer antibody 2C5-modified doxorubicin-loaded PEGylated liposomes against various tumor cell lines. Eur. J. Pharmaceut. Sci. 2007;32:159–168. doi: 10.1016/J.EJPS.2007.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Elbayoumi T., Torchilin V.P. Tumor-targeted nanomedicines: enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clin. Cancer Res. 2009;15:1973–1980. doi: 10.1158/1078-0432.CCR-08-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Narayanaswamy R., Torchilin V.P. Targeted delivery of combination therapeutics using monoclonal antibody 2C5-modified immunoliposomes for cancer therapy. Pharm. Res. (N. Y.) 2021;38:429–450. doi: 10.1007/S11095-021-02986-1. [DOI] [PubMed] [Google Scholar]
- 122.Kamoun W.S., Kirpotin D.B., Huang Z.R., Tipparaju S.K., Noble C.O., Hayes M.E., Luus L., Koshkaryev A., Kim J., Olivier K., Kornaga T., Oyama S., Askoxylakis V., Pien C., Kuesters G., Dumont N., Lugovskoy A.A., Schihl S.A., Wilton J.H., Geddie M.L., Suchy J., Grabow S., Kohli N., Reynolds C.P., Blaydes R., Zhou Y., Sawyer A.J., Marks J.D., Drummond D.C. Antitumour activity and tolerability of an EphA2-targeted nanotherapeutic in multiple mouse models. Nat. Biomed. Eng. 2019;3:264–280. doi: 10.1038/s41551-019-0385-4. [DOI] [PubMed] [Google Scholar]
- 123.Kamoun W.S., Dugast A.-S., Suchy J.J., Grabow S., Fulton R.B., Sampson J.F., Luus L., Santiago M., Koshkaryev A., Sun G., Askoxylakis V., Tam E., Huang Z.R., Drummond D.C., Sawyer A.J. Synergy between EphA2-ILs-DTXp, a novel EphA2-targeted nanoliposomal taxane, and PD-1 inhibitors in preclinical tumor models. Mol. Cancer Therapeut. 2020;19:270–281. doi: 10.1158/1535-7163.MCT-19-0414. [DOI] [PubMed] [Google Scholar]
- 124.Guo Z., He B., Yuan L., Dai W., Zhang H., Wang X., Wang J., Zhang X., Zhang Q. Dual targeting for metastatic breast cancer and tumor neovasculature by EphA2-mediated nanocarriers. Int. J. Pharm. 2015;493:380–389. doi: 10.1016/J.IJPHARM.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 125.Barbosa M.V., Monteiro L.O.F., Carneiro G., Malagutti A.R., Vilela J.M.C., Andrade M.S., Oliveira M.C., Carvalho-Junior A.D., Leite E.A. Experimental design of a liposomal lipid system: a potential strategy for paclitaxel-based breast cancer treatment. Colloids Surf. B Biointerfaces. 2015;136:553–561. doi: 10.1016/J.COLSURFB.2015.09.055. [DOI] [PubMed] [Google Scholar]
- 126.Monteiro L.O.F., Fernandes R.S., Oda C.M.R., Lopes S.C., Townsend D.M., Cardoso V.N., Oliveira M.C., Leite E.A., Rubello D., de Barros A.L.B. Paclitaxel-loaded folate-coated long circulating and pH-sensitive liposomes as a potential drug delivery system: a biodistribution study, Biomed. Pharma. 2018;97:489–495. doi: 10.1016/J.BIOPHA.2017.10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Oliveira Silva J., Fernandes R.S., Ramos Oda C.M., Ferreira T.H., Machado Botelho A.F., Martins Melo M., de Miranda M.C., Assis Gomes D., Dantas Cassali G., Townsend D.M., Rubello D., Oliveira M.C., de Barros A.L.B. Folate-coated, long-circulating and pH-sensitive liposomes enhance doxorubicin antitumor effect in a breast cancer animal model. Biomed. Pharmacother. 2019;118 doi: 10.1016/J.BIOPHA.2019.109323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen Y., Cheng Y., Zhao P., Zhang S., Li M., He C., Zhang X., Yang T., Yan R., Ye P., Ma X., Xiang G. Co-delivery of doxorubicin and imatinib by pH sensitive cleavable PEGylated nanoliposomes with folate-mediated targeting to overcome multidrug resistance. Int. J. Pharm. 2018;542:266–279. doi: 10.1016/J.IJPHARM.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 129.Soe Z.C., Thapa R.K., Ou W., Gautam M., Nguyen H.T., Jin S.G., Ku S.K., Oh K.T., Choi H.G., Yong C.S., Kim J.O. Folate receptor-mediated celastrol and irinotecan combination delivery using liposomes for effective chemotherapy. Colloids Surf. B Biointerfaces. 2018;170:718–728. doi: 10.1016/J.COLSURFB.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 130.Du Nguyen V., Min H.K., Kim C.S., Han J., Park J.O., Choi E. Folate receptor-targeted liposomal nanocomplex for effective synergistic photothermal-chemotherapy of breast cancer in vivo. Colloids Surf. B Biointerfaces. 2019;173:539–548. doi: 10.1016/j.colsurfb.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 131.Sneider A., Jadia R., Piel B., Van Dyke D., Tsiros C., Rai P. Engineering remotely triggered liposomes to target triple negative breast cancer. Oncomedicine. 2017;2:1–13. doi: 10.7150/ONCM.17406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gazzano E., Rolando B., Chegaev K., Salaroglio C., Kopecka J., Pedrini I., Saponara S., Sorge M., Buondonno I., Stella B., Marengo A., Valoti M., Brancaccio M., Fruttero R., Gasco A., Arpicco S., Riganti C. Folate-targeted liposomal nitrooxy-doxorubicin: an effective tool against P-glycoprotein-positive and folate receptor-positive tumors. J. Contr. Release. 2018;270:37–52. doi: 10.1016/J.JCONREL.2017.11.042. [DOI] [PubMed] [Google Scholar]
- 133.Deng C., Zhang Q., Jia M., Zhao J., Sun X., Gong T., Zhang Z. Tumors and their microenvironment dual-targeting chemotherapy with local immune adjuvant therapy for effective antitumor immunity against breast cancer. Adv. Sci. 2019;6 doi: 10.1002/ADVS.201801868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guo P., Yang J., Jia D., Moses M.A., Auguste D.T. ICAM-1-Targeted, Lcn2 siRNA-encapsulating liposomes are potent anti-angiogenic agents for triple negative breast cancer. Theranostics. 2016;6:1. doi: 10.7150/THNO.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Orthmann A., Zeisig R., Süss R., Lorenz D., Lemm M., Fichtner I. Treatment of experimental brain metastasis with MTO-liposomes: impact of fluidity and LRP-targeting on the therapeutic result. Pharm. Res. (N. Y.) 2012;29:1949–1959. doi: 10.1007/S11095-012-0723-7. [DOI] [PubMed] [Google Scholar]
- 136.Orthmann A., Peiker L., Fichtner I., Hoffmann A., Hilger R., Zeisig R. Improved treatment of MT-3 breast cancer and brain metastases in a mouse xenograft by LRP-targeted oxaliplatin liposomes. J. Biomed. Nanotechnol. 2016;12:56–68. doi: 10.1166/JBN.2016.2143. [DOI] [PubMed] [Google Scholar]
- 137.Moura V., Lacerda M., Figueiredo P., Corvo M., Cruz M., Soares R., de Lima M., Simões S., Moreira J. Targeted and intracellular triggered delivery of therapeutics to cancer cells and the tumor microenvironment: impact on the treatment of breast cancer. Breast Cancer Res. Treat. 2012;133:61–73. doi: 10.1007/S10549-011-1688-7. [DOI] [PubMed] [Google Scholar]
- 138.Fonseca N.A., Rodrigues A.S., Rodrigues-Santos P., Alves V., Gregório A.C., Valério-Fernandes Â., Gomes-da-Silva L.C., Rosa M.S., Moura V., Ramalho-Santos J., Simões S., Moreira J.N. Nucleolin overexpression in breast cancer cell sub-populations with different stem-like phenotype enables targeted intracellular delivery of synergistic drug combination. Biomaterials. 2015;69:76–88. doi: 10.1016/J.BIOMATERIALS.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 139.Xing H., Tang L., Yang X., Hwang K., Wang W., Yin Q., Wong N., Dobrucki L., Yasui N., Katzenellenbogen J., Helferich W., Cheng J., Lu Y. Selective delivery of an anticancer drug with aptamer-functionalized liposomes to breast cancer cells in vitro and in vivo. J. Mater. Chem. B. 2013;1:5288–5297. doi: 10.1039/C3TB20412J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liao Z.X., Chuang E.Y., Lin C.C., Ho Y.C., Lin K.J., Cheng P.Y., Chen K.J., Wei H.J., Sung H.W. An AS1411 aptamer-conjugated liposomal system containing a bubble-generating agent for tumor-specific chemotherapy that overcomes multidrug resistance. J. Contr. Release. 2015;208:42–51. doi: 10.1016/J.JCONREL.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 141.Li X., Wu X., Yang H., Li L., Ye Z., Rao Y. A nuclear targeted Dox-aptamer loaded liposome delivery platform for the circumvention of drug resistance in breast cancer, Biomed. Pharma. 2019;117 doi: 10.1016/J.BIOPHA.2019.109072. [DOI] [PubMed] [Google Scholar]
- 142.Yu S., Bi X., Yang L., Wu S., Yu Y., Jiang B., Zhang A., Lan K., Duan S. Co-delivery of paclitaxel and PLK1-targeted siRNA using aptamer-functionalized cationic liposome for synergistic anti-breast cancer effects in vivo. J. Biomed. Nanotechnol. 2019;15:1135–1148. doi: 10.1166/JBN.2019.2751. [DOI] [PubMed] [Google Scholar]
- 143.Abnous K., Danesh N.M., Ramezani M., Alibolandi M., Bahreyni A., Lavaee P., Moosavian S.A., Taghdisi S.M. A smart ATP-responsive chemotherapy drug-free delivery system using a DNA nanostructure for synergistic treatment of breast cancer in vitro and in vivo. J. Drug Target. 2020;28:852–859. doi: 10.1080/1061186X.2020.1712407. [DOI] [PubMed] [Google Scholar]
- 144.Shi J., Sun M., Li X., Zhao Y., Ju R., Mu L., Yan Y., Li X., Zeng F., Lu W. A combination of targeted sunitinib liposomes and targeted vinorelbine liposomes for treating invasive breast cancer. J. Biomed. Nanotechnol. 2015;11:1568–1582. doi: 10.1166/JBN.2015.2075. [DOI] [PubMed] [Google Scholar]
- 145.Han S., Baek J., Kim M., Hwang S., Cho C. Surface modification of paclitaxel-loaded liposomes using d-α-tocopheryl polyethylene glycol 1000 succinate: enhanced cellular uptake and cytotoxicity in multidrug resistant breast cancer cells. Chem. Phys. Lipids. 2018;213:39–47. doi: 10.1016/J.CHEMPHYSLIP.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 146.Li N., Fu T., Fei W., Han T., Gu X., Hou Y., Liu Y., Yang J. Vitamin E D-alpha-tocopheryl polyethylene glycol 1000 succinate-conjugated liposomal docetaxel reverses multidrug resistance in breast cancer cells. J. Pharm. Pharmacol. 2019;71:1243–1254. doi: 10.1111/JPHP.13126. [DOI] [PubMed] [Google Scholar]
- 147.Bharti R., Dey G., Banerjee I., Dey K.K., Parida S., Kumar B.N.P., Das C.K., Pal I., Mukherjee M., Misra M., Pradhan A.K., Emdad L., Das S.K., Fisher P.B., Mandal M. Somatostatin receptor targeted liposomes with Diacerein inhibit IL-6 for breast cancer therapy. Cancer Lett. 2017;388:292–302. doi: 10.1016/J.CANLET.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 148.Ju R.-J., Cheng L., Peng X.-M., Wang T., Li C.-Q., Song X.-L., Liu S., Chao J.-P., Li X.-T. Octreotide-modified liposomes containing daunorubicin and dihydroartemisinin for treatment of invasive breast cancer. Artif. Cell Nanomed. Biotechnol. 2018;46:616–628. doi: 10.1080/21691401.2018.1433187. [DOI] [PubMed] [Google Scholar]
- 149.Gote V., Pal D. Octreotide-targeted Lcn2 siRNA PEGylated liposomes as a treatment for metastatic breast cancer. Bioengineering. 2021;8:44. doi: 10.3390/BIOENGINEERING8040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mukherjee A., Prasad T.K., Rao N.M., Banerjee R. Haloperidol-associated stealth liposomes: a potent carrier for delivering genes to human breast cancer cells. J. Biol. Chem. 2005;280:15619–15627. doi: 10.1074/jbc.M409723200. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Y., Huang Y., Zhang P., Gao X., Gibbs R.B., Li S. Incorporation of a selective sigma-2 receptor ligand enhances uptake of liposomes by multiple cancer cells. Int. J. Nanomed. 2012;7:4473. doi: 10.2147/IJN.S31981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gandhi R., Khatri N., Baradia D., Vhora I., Misra A. Surface-modified Epirubicin-HCl liposomes and its in vitro assessment in breast cancer cell-line: MCF-7. Drug Deliv. 2015;23:1152–1162. doi: 10.3109/10717544.2014.999960. [DOI] [PubMed] [Google Scholar]
- 153.Fu J., Li W., Xin X., Chen D., Hu H. Transferrin-modified nanoliposome codelivery strategies for enhancing the cancer therapy. J. Pharm. Sci. 2020;109:2426–2436. doi: 10.1016/J.XPHS.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 154.Belfiore L., Saunders D., Ranson M., Vine K. N-Alkylisatin-Loaded liposomes target the urokinase plasminogen activator system in breast cancer. Pharmaceutics. 2020;12:1–16. doi: 10.3390/PHARMACEUTICS12070641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cochran B.J., Croucher D.R., Lobov S., Saunders D.N., Ranson M. Dependence on endocytic receptor binding via a minimal binding motif underlies the differential prognostic profiles of SerpinE1 and SerpinB2 in cancer. J. Biol. Chem. 2011;286:24467–24475. doi: 10.1074/JBC.M111.225706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stutchbury T.K., Al-ejeh F., Stillfried G.E., Croucher D.R., Andrews J., Irving D., Links M., Ranson M. Preclinical evaluation of 213Bi-labeled plasminogen activator inhibitor type 2 in an orthotopic murine xenogenic model of human breast carcinoma. Mol. Cancer Therapeut. 2007;6:203–212. doi: 10.1158/1535-7163.MCT-06-0264. [DOI] [PubMed] [Google Scholar]
- 157.Lu R., Zhou L., Yue Q., Liu Q., Cai X., Xiao W., Hai L., Guo L., Wu Y. Liposomes modified with double-branched biotin: a novel and effective way to promote breast cancer targeting. Bioorg. Med. Chem. 2019;27:3115–3127. doi: 10.1016/j.bmc.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 158.Tang B., Peng Y., Yue Q., Pu Y., Li R., Zhao Y., Hai L., Guo L., Wu Y. Design, preparation and evaluation of different branched biotin modified liposomes for targeting breast cancer. Eur. J. Med. Chem. 2020;193 doi: 10.1016/J.EJMECH.2020.112204. [DOI] [PubMed] [Google Scholar]
- 159.Huang M., Pu Y., Peng Y., Fu Q., Guo L., Wu Y., Zheng Y. Biotin and glucose dual-targeting, ligand-modified liposomes promote breast tumor-specific drug delivery. Bioorg. Med. Chem. Lett. 2020;30 doi: 10.1016/J.BMCL.2020.127151. [DOI] [PubMed] [Google Scholar]
- 160.Lv Y., Xu C., Zhao X., Lin C., Yang X., Xin X., Zhang L., Qin C., Han X., Yang L., He W., Yin L. Nanoplatform assembled from a CD44-targeted prodrug and smart liposomes for dual targeting of tumor microenvironment and cancer cells. ACS Nano. 2018;12:1519–1536. doi: 10.1021/ACSNANO.7B08051. [DOI] [PubMed] [Google Scholar]
- 161.Han N.-K., Shin D.H., Kim J.S., Weon K.Y., Jang C.-Y., Kim J.-S. Hyaluronan-conjugated liposomes encapsulating gemcitabine for breast cancer stem cells. Int. J. Nanomed. 2016;11:1413. doi: 10.2147/IJN.S95850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jiang T., Mo R., Bellotti A., Zhou J., Gu Z. Gel–liposome-mediated Co-delivery of anticancer membrane-associated proteins and small-molecule drugs for enhanced therapeutic efficacy. Adv. Funct. Mater. 2014;24:2295–2304. doi: 10.1002/ADFM.201303222. [DOI] [Google Scholar]
- 163.Ding Y., Yang R., Yu W., Hu C., Zhang Z., Liu D., An Y., Wang X., He C., Liu P., Tang Q., Chen D. Chitosan oligosaccharide decorated liposomes combined with TH302 for photodynamic therapy in triple negative breast cancer. J. Nanobiotechnol. 2021;19:1–17. doi: 10.1186/S12951-021-00891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang R., Lu M., Ming L., Chen Y., Cheng K., Zhou J., Jiang S., Lin Z., Chen D. 89Zr-Labeled multifunctional liposomes conjugate chitosan for PET-trackable triple-negative breast cancer stem cell targeted therapy. Int. J. Nanomed. 2020;15:9061. doi: 10.2147/IJN.S262786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guo C., Chen Y., Gao W., Chang A., Ye Y., Shen W., Luo Y., Yang S., Sun P., Xiang R., Li N. Liposomal nanoparticles carrying anti-IL6R antibody to the tumour microenvironment inhibit metastasis in two molecular subtypes of breast cancer mouse models. Theranostics. 2017;7:775. doi: 10.7150/THNO.17237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dorjsuren B., Chaurasiya B., Ye Z., Liu Y., Li W., Wang C., Shi D., Evans C.E., Webster T.J., Shen Y. Cetuximab-coated thermo-sensitive liposomes loaded with magnetic nanoparticles and doxorubicin for targeted EGFR-expressing breast cancer combined therapy. Int. J. Nanomed. 2020;15:8201. doi: 10.2147/IJN.S261671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Su Y.-C., Burnouf P.-A., Chuang K.-H., Chen B.-M., Cheng T.-L., Roffler S.R. Conditional internalization of PEGylated nanomedicines by PEG engagers for triple negative breast cancer therapy. Nat. Commun. 2017;8:1–12. doi: 10.1038/ncomms15507. 2017 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim M.W., Jeong H.Y., Kang S.J., Choi M.J., You Y.M., Im C.S., Lee T.S., Song I.H., Lee C.G., Rhee K.-J., Lee Y.K., Park Y.S. Cancer-targeted nucleic acid delivery and quantum dot imaging using EGF receptor aptamer-conjugated lipid nanoparticles. Sci. Rep. 2017;7 doi: 10.1038/S41598-017-09555-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kullberg M., Mann K., Owens J.L. A two-component drug delivery system using Her-2-targeting thermosensitive liposomes. J. Drug Target. 2009;17:98–107. doi: 10.1080/10611860802471562. [DOI] [PubMed] [Google Scholar]
- 170.Raju A., Muthu M.S., Feng S.-S. Trastuzumab-conjugated vitamin E TPGS liposomes for sustained and targeted delivery of docetaxel. Expet Opin. Drug Deliv. 2013;10:747–760. doi: 10.1517/17425247.2013.777425. [DOI] [PubMed] [Google Scholar]
- 171.Kullberg M., McCarthy R., Anchordoquy T.J. Gene delivery to Her-2 + breast cancer cells using a two-component delivery system to achieve specificity. Nanomed. Nanotechnol. Biol. Med. 2014;10:1253–1262. doi: 10.1016/J.NANO.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Khosroshahi M.E., Hassannejad Z., Firouzi M., Arshi A.R. Nanoshell-mediated targeted photothermal therapy of HER2 human breast cancer cells using pulsed and continuous wave lasers: an in vitro study. Laser Med. Sci. 2015;30:1913–1922. doi: 10.1007/s10103-015-1782-x. [DOI] [PubMed] [Google Scholar]
- 173.Li Q., Tang Q., Zhang P., Wang Z., Zhao T., Zhou J., Li H., Ding Q., Li W., Hu F., Du Y., Yuan H., Chen S., Gao J., Zhan J., You J. Human epidermal growth factor receptor-2 antibodies enhance the specificity and anticancer activity of light-sensitive doxorubicin-labeled liposomes. Biomaterials. 2015;57:1–11. doi: 10.1016/J.BIOMATERIALS.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 174.Alavizadeh S., Akhtari J., Badiee A., Golmohammadzadeh S., Jaafari M. Improved therapeutic activity of HER2 Affibody-targeted cisplatin liposomes in HER2-expressing breast tumor models. Expet Opin. Drug Deliv. 2016;13:325–336. doi: 10.1517/17425247.2016.1121987. [DOI] [PubMed] [Google Scholar]
- 175.Zahmatkeshan M., Gheybi F., Rezayat S., Jaafari M. Improved drug delivery and therapeutic efficacy of PEgylated liposomal doxorubicin by targeting anti-HER2 peptide in murine breast tumor model. Eur. J. Pharmaceut. Sci. 2016;86:125–135. doi: 10.1016/J.EJPS.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 176.Nguyen H., Tran T., Thapa R., Phung C., Shin B., Jeong J., Choi H., Yong C., Kim J. Targeted co-delivery of polypyrrole and rapamycin by trastuzumab-conjugated liposomes for combined chemo-photothermal therapy. Int. J. Pharm. 2017;527:61–71. doi: 10.1016/J.IJPHARM.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 177.Li T., Amari T., Semba K., Yamamoto T., Takeoka S. Construction and evaluation of pH-sensitive immunoliposomes for enhanced delivery of anticancer drug to ErbB2 over-expressing breast cancer cells. Nanomed. Nanotechnol. Biol. Med. 2017;13:1219–1227. doi: 10.1016/J.NANO.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 178.Matusewicz L., Podkalicka J., Sikorski A.F. Immunoliposomes with simvastatin as a potential therapeutic in treatment of breast cancer cells overexpressing HER2—an in vitro study. Cancers. 2018;10:418. doi: 10.3390/CANCERS10110418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang L., Wang Q., Gao M., Fu L., Li Y., Quan H., Lou L. STAT3 activation confers trastuzumab-emtansine (T-DM1) resistance in HER2-positive breast cancer. Cancer Sci. 2018;109:3305–3315. doi: 10.1111/CAS.13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Khoshtinat Nikkhoi S., Rahbarizadeh F., Ahmadvand D., Moghimi S.M. Multivalent targeting and killing of HER2 overexpressing breast carcinoma cells with methotrexate-encapsulated tetra-specific non-overlapping variable domain heavy chain anti-HER2 antibody-PEG-liposomes: in vitro proof-of-concept. Eur. J. Pharmaceut. Sci. 2018;122:42–50. doi: 10.1016/j.ejps.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 181.Farasat A., Rahbarizadeh F., Ahmadvand D., Ranjbar S., Khoshtinat Nikkhoi S. Effective suppression of tumour cells by oligoclonal HER2-targeted delivery of liposomal doxorubicin. J. Liposome Res. 2019;29:53–65. doi: 10.1080/08982104.2018.1430829. [DOI] [PubMed] [Google Scholar]
- 182.Singh M.K., Pindiprolu S.K.S.S., Sanapalli B.K.R., Yele V., Ganesh G.N.K. Tumor homing peptide modified liposomes of capecitabine for improved apoptotic activity and HER2 targeted therapy in breast cancer: in vitro studies. RSC Adv. 2019;9:24987–24994. doi: 10.1039/C9RA04814F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Matusewicz L., Filip-Psurska B., Psurski M., Tabaczar S., Podkalicka J., Wietrzyk J., Ziółkowski P., Czogalla A., Sikorski A.F. EGFR-targeted immunoliposomes as a selective delivery system of simvastatin, with potential use in treatment of triple-negative breast cancers. Int. J. Pharm. 2019;569 doi: 10.1016/J.IJPHARM.2019.118605. [DOI] [PubMed] [Google Scholar]
- 184.Rodallec A., Sicard G., Giacometti S., Carre M., Maia T., Valette M., Bouquet F., Savina A., Lacarelle B., Ciccolini J., Fanciullino R. Tumor uptake and associated greater efficacy of anti-Her2 immunoliposome does not rely on Her2 expression status: study of a docetaxel-trastuzumab immunoliposome on Her2+ breast cancer model (SKBR3) Anti Cancer Drugs. 2020;31:463–472. doi: 10.1097/CAD.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 185.Chowdhury N., Chaudhry S., Hall N., Olverson G., Zhang Q.-J., Mandal T., Dash S., Kundu A. Targeted delivery of doxorubicin liposomes for Her-2+ breast cancer treatment. AAPS PharmSciTech. 2020;21:202. doi: 10.1208/S12249-020-01743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim B., Shin J., Wu J., Omstead D.T., Kiziltepe T., Littlepage L.E., Bilgicer B. Engineering peptide-targeted liposomal nanoparticles optimized for improved selectivity for HER2-positive breast cancer cells to achieve enhanced in vivo efficacy. J. Contr. Release. 2020;322:530–541. doi: 10.1016/J.JCONREL.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Elamir A., Ajith S., Al Sawaftah N., Abuwatfa W., Mukhopadhyay D., Paul V., Al-Sayah M.H., Awad N., Husseini G.A. Ultrasound-triggered herceptin liposomes for breast cancer therapy. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-021-86860-5. 2021 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Quinn Z., Mao W., Xia Y., John R., Wan Y. Conferring receptors on recipient cells with extracellular vesicles for targeted drug delivery. Bioact. Mater. 2021;6:749–756. doi: 10.1016/J.BIOACTMAT.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Moosavian S.A., Abnous K., Badiee A., Jaafari M.R. Improvement in the drug delivery and anti-tumor efficacy of PEGylated liposomal doxorubicin by targeting RNA aptamers in mice bearing breast tumor model. Colloids Surf. B Biointerfaces. 2016;139:228–236. doi: 10.1016/J.COLSURFB.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 190.Kangarlou S., Ramezanpour S., Balalaie S., Mohammadi S.R., Haririan I. Curcumin-loaded nanoliposomes linked to homing peptides for integrin targeting and neuropilin-1-mediated internalization. 2016. Https://Doi.Org/10.1080/13880209.2016.1261301 55. 277–285. [DOI] [PMC free article] [PubMed]
- 191.Veneti E., Tu R.S., Auguste D.T. RGD-targeted liposome binding and uptake on breast cancer cells is dependent on elastin linker secondary structure. Bioconjugate Chem. 2016;27:1813–1821. doi: 10.1021/ACS.BIOCONJCHEM.6B00205. [DOI] [PubMed] [Google Scholar]
- 192.Wen X., Li J., Cai D., Yue L., Wang Q., Zhou L., Fan L., Sun J., Wu Y. Anticancer efficacy of targeted shikonin liposomes modified with RGD in breast cancer cells. Molecules. 2018;23:268. doi: 10.3390/MOLECULES23020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mahmoudi R., Ashraf Mirahmadi-Babaheidri S., Delaviz H., Fouani M., Alipour M., Jafari Barmak M., Christiansen G., Bardania H. RGD peptide-mediated liposomal curcumin targeted delivery to breast cancer cells. J. Biomater. Appl. 2021;35:743–753. doi: 10.1177/0885328220949367. [DOI] [PubMed] [Google Scholar]
- 194.Zhou B., Li M., Xu X., Yang L., Ye M., Chen Y., Peng J., Xiao L., Wang L., Huang S., Zhang L., Lin Q., Zhang Z. Integrin α2β1Targeting DGEA-modified liposomal doxorubicin enhances antitumor efficacy against breast cancer. Mol. Pharm. 2021;18:2634–2646. doi: 10.1021/acs.molpharmaceut.1c00132. [DOI] [PubMed] [Google Scholar]
- 195.Vakhshiteh F., Khabazian E., Atyabi F., Ostad S.N., Madjd Z., Dinarvand R. Peptide-conjugated liposomes for targeted miR-34a delivery to suppress breast cancer and cancer stem-like population. J. Drug Deliv. Sci. Technol. 2020;57 doi: 10.1016/J.JDDST.2020.101687. [DOI] [Google Scholar]
- 196.Fu M., Tang W., Liu J.-J., Gong X.-Q., Kong L., Yao X.-M., Jing M., Cai F.-Y., Li X.-T., Ju R.-J. Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer. J. Drug Target. 2019;28:245–258. doi: 10.1080/1061186X.2019.1656725. [DOI] [PubMed] [Google Scholar]
- 197.Zhao Z., Zhao Y., Xie C., Chen C., Lin D., Wang S., Cui X., Guo Z., Zhou J. Dual-active targeting liposomes drug delivery system for bone metastatic breast cancer: synthesis and biological evaluation. Chem. Phys. Lipids. 2019;223 doi: 10.1016/J.CHEMPHYSLIP.2019.104785. [DOI] [PubMed] [Google Scholar]
- 198.He Y., Zhang L., Song C. Luteinizing hormone-releasing hormone receptor-mediated delivery of mitoxantrone using LHRH analogs modified with PEGylated liposomes. Int. J. Nanomed. 2010;5:697–705. doi: 10.2147/IJN.S12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.He Y., Zhang L., Zhu D., Song C. Design of multifunctional magnetic iron oxide nanoparticles/mitoxantrone-loaded liposomes for both magnetic resonance imaging and targeted cancer therapy. Int. J. Nanomed. 2014;9:4055–4066. doi: 10.2147/IJN.S61880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Al-Ahmady Z.S., Chaloin O., Kostarelos K. Monoclonal antibody-targeted, temperature-sensitive liposomes: in vivo tumor chemotherapeutics in combination with mild hyperthermia. J. Contr. Release. 2014;196:332–343. doi: 10.1016/J.JCONREL.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 201.Lozano N., Al-Ahmady Z.S., Beziere N.S., Ntziachristos V., Kostarelos K. Monoclonal antibody-targeted PEGylated liposome-ICG encapsulating doxorubicin as a potential theranostic agent. Int. J. Pharm. 2015;482:2–10. doi: 10.1016/J.IJPHARM.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 202.Chuang E.Y., Lin C.C., Chen K.J., Wan D.H., Lin K.J., Ho Y.C., Lin P.Y., Sung H.W. A FRET-guided, NIR-responsive bubble-generating liposomal system for in vivo targeted therapy with spatially and temporally precise controlled release. Biomaterials. 2016;93:48–59. doi: 10.1016/J.BIOMATERIALS.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 203.Wang D.E., Gao X., You S., Chen M., Ren L., Sun W., Yang H., Xu H. Aptamer-functionalized polydiacetylene liposomes act as a fluorescent sensor for sensitive detection of MUC1 and targeted imaging of cancer cells. Sens. Actuators B Chem. 2020;309 doi: 10.1016/J.SNB.2020.127778. [DOI] [Google Scholar]
- 204.Cao J., Wang R., Gao N., Li M., Tian X., Yang W., Ruan Y., Zhou C., Wang G., Liu X., Tang S., Yu Y., Liu Y., Sun G., Peng H., Wang Q. A7RC peptide modified paclitaxel liposomes dually target breast cancer. Biomater. Sci. 2015;3:1545–1554. doi: 10.1039/C5BM00161G. [DOI] [PubMed] [Google Scholar]
- 205.Kadonosono T., Yamano A., Goto T., Tsubaki T., Niibori M., Kuchimaru T., Kizaka-Kondoh S. Cell penetrating peptides improve tumor delivery of cargos through neuropilin-1-dependent extravasation. J. Contr. Release. 2015;201:14–21. doi: 10.1016/J.JCONREL.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 206.Paliwal S., Paliwal R., Pal H., Saxena A., Sharma P., Gupta P., Agrawal G., Vyas S. Estrogen-anchored pH-sensitive liposomes as nanomodule designed for site-specific delivery of doxorubicin in breast cancer therapy. Mol. Pharm. 2012;9:176–186. doi: 10.1021/MP200439Z. [DOI] [PubMed] [Google Scholar]
- 207.Jain A.S., Goel P.N., Shah S.M., Dhawan V.V., Nikam Y., Gude R.P., Nagarsenker M.S. Tamoxifen guided liposomes for targeting encapsulated anticancer agent to estrogen receptor positive breast cancer cells: in vitro and in vivo evaluation. Biomed. Pharmacother. 2014;68:429–438. doi: 10.1016/j.biopha.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 208.Wang X., Chen X., Yang X., Gao W., He B., Dai W., Zhang H., Wang X., Wang J., Zhang X., Dai Z., Zhang Q. A nanomedicine based combination therapy based on QLPVM peptide functionalized liposomal tamoxifen and doxorubicin against Luminal A breast cancer. Nanomed. Nanotechnol. Biol. Med. 2016;12:387–397. doi: 10.1016/J.NANO.2015.12.360. [DOI] [PubMed] [Google Scholar]
- 209.Wang Y., Wang Z., Qian Y., Fan L., Yue C., Jia F., Sun J., Hu Z., Wang W. Synergetic estrogen receptor-targeting liposome nanocarriers with anti-phagocytic properties for enhanced tumor theranostics. J. Mater. Chem. B. 2019;7:1056–1063. doi: 10.1039/c8tb03351j. [DOI] [PubMed] [Google Scholar]
- 210.Salkho N.M., Paul V., Kawak P., Vitor R.F., Martins A.M., Al Sayah M., Husseini G.A. Ultrasonically controlled estrone-modified liposomes for estrogen-positive breast cancer therapy. Artif. Cell Nanomed. Biotechnol. 2018;46:462–472. doi: 10.1080/21691401.2018.1459634. [DOI] [PubMed] [Google Scholar]
- 211.Han B., Yang Y., Chen J., Tang H., Sun Y., Zhang Z., Wang Z., Li Y., Li Y., Luan X., Li Q., Ren Z., Zhou X., Cong D., Liu Z., Meng Q., Sun F., Pei J. Preparation, characterization, and pharmacokinetic study of a novel long-acting targeted paclitaxel liposome with antitumor activity. Int. J. Nanomed. 2020;15:553–571. doi: 10.2147/IJN.S228715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Qin C., He B., Dai W., Zhang H., Wang X., Wang J., Zhang X., Wang G., Yin L., Zhang Q. Inhibition of metastatic tumor growth and metastasis via targeting metastatic breast cancer by chlorotoxin-modified liposomes. Mol. Pharm. 2014;11:3233–3241. doi: 10.1021/MP400691Z. [DOI] [PubMed] [Google Scholar]
- 213.Ramadass S., Anantharaman N., Subramanian S., Sivasubramanian S., Madhan B. Paclitaxel/epigallocatechin gallate coloaded liposome: a synergistic delivery to control the invasiveness of MDA-MB-231 breast cancer cells. Colloids Surf. B Biointerfaces. 2015;125:65–72. doi: 10.1016/J.COLSURFB.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 214.Østrem R.G., Parhamifar L., Pourhassan H., Clergeaud G., Nielsen O.L., Kjær A., Hansen A.E., Andresen T.L. Secretory phospholipase A2 responsive liposomes exhibit a potent anti-neoplastic effect in vitro, but induce unforeseen severe toxicity in vivo. J. Contr. Release. 2017;262:212–221. doi: 10.1016/J.JCONREL.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 215.Liscovitch M., Ravid D. A case study in misidentification of cancer cell lines: MCF-7/AdrR cells (re-designated NCI/ADR-RES) are derived from OVCAR-8 human ovarian carcinoma cells. Cancer Lett. 2007;245:350–352. doi: 10.1016/J.CANLET.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 216.Prasad V.V.T.S., Gopalan R.O.G. Continued use of MDA-MB-435, a melanoma cell line, as a model for human breast cancer, even in year. NPJ Breast Cancer. 2015;1 doi: 10.1038/NPJBCANCER.2015.2. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Oshiro-Júnior J., Rodero C., Hanck-Silva G., Sato M., Alves R., Eloy J., Chorilli M. Stimuli-responsive drug delivery nanocarriers in the treatment of breast cancer. Curr. Med. Chem. 2020;27:2494–2513. doi: 10.2174/0929867325666181009120610. [DOI] [PubMed] [Google Scholar]
- 218.Müller A., Homey B., Soto H., Ge N., Catron D., Buchanan M.E., McClanahan T., Murphy E., Yuan W., Wagner S.N., Barrera J.L., Mohar A., Verástegui E., Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 219.Zlotnik A. Chemokines and cancer. Int. J. Cancer. 2006;119:2026–2029. doi: 10.1002/IJC.22024. [DOI] [PubMed] [Google Scholar]
- 220.Bleul C.C., Fuhlbrigge R.C., Casasnovas J.M., Aiuti A., Springer T.A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J. Exp. Med. 1996;184:1101–1109. doi: 10.1084/JEM.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mukherjee D., Zhao J. The role of chemokine receptor CXCR4 in breast cancer metastasis. Am. J. Cancer Res. 2013;3:46–57. [PMC free article] [PubMed] [Google Scholar]
- 222.Wang Y., Xie Y., Oupický D. Potential of CXCR4/CXCL12 chemokine Axis in cancer drug delivery. Curr. Pharmacol. Rep. 2016;2:1–10. doi: 10.1007/s40495-015-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Robertson J.M., James J.A. Preclinical systemic lupus erythematosus. Rheum. Dis. Clin. 2014;40:621–635. doi: 10.1016/J.RDC.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vlagea A., Falagan S., Gutiérrez-Gutiérrez G., Moreno-Rubio J., Merino M., Zambrana F., Casado E., Sereno M. Antinuclear antibodies and cancer: a literature review. Crit. Rev. Oncol. Hematol. 2018;127:42–49. doi: 10.1016/J.CRITREVONC.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 225.Nisihara R., Machoski M., Neppel A., Maestri C., Messias-Reason I., Skare T. Anti-nuclear antibodies in patients with breast cancer. Clin. Exp. Immunol. 2018;193:178–182. doi: 10.1111/CEI.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wilson K., Shiuan E., Brantley-Sieders D.M. Oncogenic functions and therapeutic targeting of EphA2 in cancer. Oncogene. 2021;40:2483. doi: 10.1038/S41388-021-01714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Xiao T., Xiao Y., Wang W., Tang Y.Y., Xiao Z., Su M. Targeting EphA2 in cancer. J. Hematol. Oncol.J Hematol Oncol. 2020;13:1–17. doi: 10.1186/S13045-020-00944-9/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhao P., Jiang D., Huang Y., Chen C. EphA2: a promising therapeutic target in breast cancer. J. Genet. Genomics. 2021;48:261–267. doi: 10.1016/J.JGG.2021.02.011. [DOI] [PubMed] [Google Scholar]
- 229.Ernstoff M.S., Ma W.W., Tsai F.Y.-C., Munster P.N., Zhang T., Kamoun W., Pipas J.M., Chen S., Santillana S., Askoxylakis V. A phase 1 study evaluating the safety, pharmacology and preliminary activity of MM-310 in patients with solid tumors. J. Clin. Oncol. 2018;36 doi: 10.1200/JCO.2018.36.15_SUPPL.TPS2604. TPS2604–TPS2604. [DOI] [Google Scholar]
- 230.Kamoun W.S., Dugast A.-S., Suchy J.J., Grabow S., Fulton R.B., Sampson J.F., Luus L., Santiago M., Koshkaryev A., Sun G., Askoxylakis V., Tam E., Huang Z.R., Drummond D.C., Sawyer A.J. Synergy between EphA2-ILs-DTXp, a novel EphA2-targeted nanoliposomal taxane, and PD-1 inhibitors in preclinical tumor models. Mol. Cancer Therapeut. 2020;19:270–281. doi: 10.1158/1535-7163.MCT-19-0414. [DOI] [PubMed] [Google Scholar]
- 231.Kumar P., Huo P., Liu B. Formulation strategies for folate-targeted liposomes and their biomedical applications. Pharmaceutics. 2019;11 doi: 10.3390/PHARMACEUTICS11080381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Xu L., Bai Q., Zhang X., Yang H. Folate-mediated chemotherapy and diagnostics: an updated review and outlook. J. Contr. Release. 2017;252:73–82. doi: 10.1016/J.JCONREL.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tagde P., Kulkarni G.T., Mishra D.K., Kesharwani P. Recent advances in folic acid engineered nanocarriers for treatment of breast cancer. J. Drug Deliv. Sci. Technol. 2020;56 doi: 10.1016/J.JDDST.2020.101613. [DOI] [Google Scholar]
- 234.Hubbard A., Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic. Biol. Med. 2000;28:1379–1386. doi: 10.1016/S0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 235.Bui T.M., Wiesolek H.L., Sumagin R. ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020;108:787. doi: 10.1002/JLB.2MR0220-549R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kong D., Kim Y., Kim M., Jang J., Lee S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int. J. Mol. Sci. 2018;19 doi: 10.3390/IJMS19041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hattrup C., Gendler S. MUC1 alters oncogenic events and transcription in human breast cancer cells. Breast Cancer Res. 2006;8 doi: 10.1186/BCR1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ihermann-Hella A., Lume M., Miinalainen I., Pirttiniemi A., Gui Y., Peränen J., Charron J., Saarma M., Costantini F., Kuure S. Mitogen-activated protein kinase (MAPK) pathway regulates branching by remodeling epithelial cell adhesion. PLoS Genet. 2014;10 doi: 10.1371/JOURNAL.PGEN.1004193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hayashi T., Takahashi T., Motoya S., Ishida T., Itoh F., Adachi M., Hinoda Y., Imai K. MUC1 mucin core protein binds to the domain 1 of ICAM-1. Digestion. 2001;63(Suppl 1):87–92. doi: 10.1159/000051917. [DOI] [PubMed] [Google Scholar]
- 240.Rosette C., Roth R.B., Oeth P., Braun A., Kammerer S., Ekblom J., Denissenko M.F. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis. 2005;26:943–950. doi: 10.1093/carcin/bgi070. [DOI] [PubMed] [Google Scholar]
- 241.Lillis A.P., Van Duyn L.B., Murphy-Ullrich J.E., Strickland D.K. The low density lipoprotein receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 2008;88:887. doi: 10.1152/PHYSREV.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Song H., Li Y., Lee J., Schwartz A., Bu G. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res. 2009;69:879–886. doi: 10.1158/0008-5472.CAN-08-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bian W., Tang M., Jiang H., Xu W., Hao W., Sui Y., Hou Y., Nie L., Zhang H., Wang C., Li N., Wang J., Qin J., Wu L., Ma X., Chen J., Wang W., Li X. Low-density-lipoprotein-receptor-related protein 1 mediates Notch pathway activation. Dev. Cell. 2021;56:2902–2919. doi: 10.1016/J.DEVCEL.2021.09.015. e8. [DOI] [PubMed] [Google Scholar]
- 244.Xing P., Liao Z., Ren Z., Zhao J., Song F., Wang G., Chen K., Yang J. Roles of low-density lipoprotein receptor-related protein 1 in tumors. Chin. J. Cancer. 2016;35:1–8. doi: 10.1186/S40880-015-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Losfeld M.E., El Khoury D., Mariot P., Carpentier M., Krust B., Briand J.P., Mazurier J., Hovanessian A.G., Legrand D. The cell surface expressed nucleolin is a glycoprotein that triggers calcium entry into mammalian cells. Exp. Cell Res. 2009;315:357–369. doi: 10.1016/J.YEXCR.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 246.Chen Z., Xu X. Roles of nucleolin: focus on cancer and anti-cancer therapy. Saudi Med. J. 2016;37:1312. doi: 10.15537/SMJ.2016.12.15972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Koutsioumpa M., Papadimitriou E. Cell surface nucleolin as a target for anti-cancer therapies. Recent Pat. Anti-Cancer Drug Discov. 2014;9:137–152. doi: 10.2174/1574892808666131119095953. [DOI] [PubMed] [Google Scholar]
- 248.Jia W., Yao Z., Zhao J., Guan Q., Gao L. New perspectives of physiological and pathological functions of nucleolin (NCL) Life Sci. 2017;186:1–10. doi: 10.1016/J.LFS.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 249.Carvalho L.S., Gonçalves N., Fonseca N.A., Moreira J.N. Cancer stem cells and nucleolin as drivers of carcinogenesis. Pharmaceuticals. 2021;14:60. doi: 10.3390/PH14010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hu J., Lin M., Liu T., Li J., Chen B., Chen Y. DIGE-based proteomic analysis identifies nucleophosmin/B23 and nucleolin C23 as over-expressed proteins in relapsed/refractory acute leukemia. Leuk. Res. 2011;35:1087–1092. doi: 10.1016/J.LEUKRES.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 251.Wolfson E., Goldenberg M., Solomon S., Frishberg A., Pinkas-Kramarski R. Nucleolin-binding by ErbB2 enhances tumorigenicity of ErbB2-positive breast cancer. Oncotarget. 2016;7 doi: 10.18632/ONCOTARGET.11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Berger C.M., Gaume X., Bouvet P. The roles of nucleolin subcellular localization in cancer. Biochimie. 2015;113:78–85. doi: 10.1016/J.BIOCHI.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 253.Fonseca N.A., Moura V., Colelli F., Pesce D., Cardile F., Pisano C., Simões S., Moreira J.N. Abstract 5155: targeting nucleolin with doxorubicin-containing nanoparticle induces a significant tumor growth inhibition in an orthotopic animal model of standard of care-resistant mesothelioma. Cancer Res. 2017;77 doi: 10.1158/1538-7445.AM2017-5155. 5155–5155. [DOI] [Google Scholar]
- 254.Balça-Silva J., do Carmo A., Tão H., Rebelo O., Barbosa M., Moura-Neto V., Sarmento-Ribeiro A.B., Lopes M.C., Moreira J.N. Nucleolin is expressed in patient-derived samples and glioblastoma cells, enabling improved intracellular drug delivery and cytotoxicity. Exp. Cell Res. 2018;370:68–77. doi: 10.1016/J.YEXCR.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 255.Lopes R., Shi K., Fonseca N.A., Gama A., Ramalho J.S., Almeida L., Moura V., Simões S., Tidor B., Moreira J.N. Modelling the impact of nucleolin expression level on the activity of F3 peptide-targeted pH-sensitive pegylated liposomes containing doxorubicin. Drug Deliv. Transl. Res. 2021;2021:1–18. doi: 10.1007/S13346-021-00972-Z. [DOI] [PubMed] [Google Scholar]
- 256.Cruz A.F., Caleiras M.B., Fonseca N.A., Gonçalves N., Mendes V.M., Sampaio S.F., Moura V., Melo J.B., Almeida R.D., Manadas B., Simões S., Moreira J.N. The enhanced efficacy of intracellular delivery of doxorubicin/C6-ceramide combination mediated by the F3 peptide/nucleolin system is supported by the downregulation of the PI3K/Akt pathway. Cancers. 2021;13:3052. doi: 10.3390/CANCERS13123052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bates P.J., Laber D.A., Miller D.M., Thomas S.D., Trent J.O. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009;86:151–164. doi: 10.1016/J.YEXMP.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Alfarouk K.O., Stock C.-M., Taylor S., Walsh M., Muddathir A.K., Verduzco D., Bashir A.H.H., Mohammed O.Y., Elhassan G.O., Harguindey S., Reshkin S.J., Ibrahim M.E., Rauch C. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:1–13. doi: 10.1186/S12935-015-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhang H., Xu H., Ashby C.R., Assaraf Y.G., Chen Z.S., Liu H.M. Chemical molecular-based approach to overcome multidrug resistance in cancer by targeting P-glycoprotein (P-gp) Med. Res. Rev. 2021;41:525–555. doi: 10.1002/MED.21739. [DOI] [PubMed] [Google Scholar]
- 260.Lund M., Petersen T.S., Dalhoff K.P. Clinical implications of P-glycoprotein modulation in drug–drug interactions. Drugs. 2017;77:859–883. doi: 10.1007/S40265-017-0729-X. [DOI] [PubMed] [Google Scholar]
- 261.Tan S., Zou C., Zhang W., Yin M., Gao X., Tang Q. Recent developments in d-α-tocopheryl polyethylene glycol-succinate-based nanomedicine for cancer therapy. Drug Deliv. 2017;24:1831–1842. doi: 10.1080/10717544.2017.1406561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Constantinou C., Charalambous C., Kanakis D. Vitamin E and cancer: an update on the emerging role of γ and δ tocotrienols. Eur. J. Nutr. 2020;59:845–857. doi: 10.1007/S00394-019-01962-1. [DOI] [PubMed] [Google Scholar]
- 263.Kumar U., Grant M. Somatostatin and somatostatin receptors. Results Probl. Cell Differ. 2009;50:97–120. doi: 10.1007/400_2009_29. [DOI] [PubMed] [Google Scholar]
- 264.Kumar U., Grigorakis S.I., Watt H.L., Sasi R., Snell L., Watson P., Chaudhari S. Somatostatin receptors in primary human breast cancer: quantitative analysis of mRNA for subtypes 1-5 and correlation with receptor protein expression and tumor pathology. Breast Cancer Res. Treat. 2005;92:175–186. doi: 10.1007/S10549-005-2414-0. [DOI] [PubMed] [Google Scholar]
- 265.Günther T., Tulipano G., Dournaud P., Bousquet C., Csaba Z., Kreienkamp H.-J., Lupp A., Korbonits M., Castaño J.P., Wester H.-J., Culler M., Melmed S., Schulz S. International union of basic and clinical pharmacology. CV. Somatostatin receptors: structure, function, ligands, and new nomenclature. Pharmacol. Rev. Pharmacol Rev. 2018;70:763–835. doi: 10.1124/pr.117.015388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.van Waarde A., Rybczynska A.A., Ramakrishnan N.K., Ishiwata K., Elsinga P.H., Dierckx R.A.J.O. Potential applications for sigma receptor ligands in cancer diagnosis and therapy. Biochim. Biophys. Acta - Biomembr. 2015;1848:2703–2714. doi: 10.1016/j.bbamem.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 267.Zeng C., Mach R.H. The evolution of the sigma-2 (σ2) receptor from obscure binding site to bona fide therapeutic target. Adv. Exp. Med. Biol. 2017;964:49–61. doi: 10.1007/978-3-319-50174-1_5. [DOI] [PubMed] [Google Scholar]
- 268.van Waarde A., Rybczynska A.A., Ramakrishnan N.K., Ishiwata K., Elsinga P.H., Dierckx R.A.J.O. Potential applications for sigma receptor ligands in cancer diagnosis and therapy. Biochim. Biophys. Acta - Biomembr. 2015;1848:2703–2714. doi: 10.1016/j.bbamem.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 269.Georgiadis M.-O., Karoutzou O., Foscolos A.-S., Papanastasiou I. Sigma receptor (σR) ligands with antiproliferative and anticancer activity. Molecules. 2017;22:1408. doi: 10.3390/MOLECULES22091408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Borde P. Royal College of Surgeons in Ireland; 2022. The Sigma-1 Receptor – an Investigation of its Potential as a Prognostic Marker and Cancer Selective Target in Breast Cancer, Thesis. [DOI] [Google Scholar]
- 271.Butts C., Socinski M.A., Mitchell P.L., Thatcher N., Havel L., Krzakowski M., Nawrocki S., Ciuleanu T.-E., Bosquée L., Trigo J.M., Spira A., Tremblay L., Nyman J., Ramlau R., Wickart-Johansson G., Ellis P., Gladkov O., Pereira J.R., Eberhardt W.E.E., Helwig C., Schröder A., Shepherd F.A. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68. doi: 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 272.Dufès C., Robaian M.A., Somani S. Transferrin and the transferrin receptor for the targeted delivery of therapeutic agents to the brain and cancer cells. Ther. Deliv. 2013;4:629–640. doi: 10.4155/tde.13.21. [DOI] [PubMed] [Google Scholar]
- 273.Singh M., Mugler K., Hailoo D., Burke S., Nemesure B., Torkko K., Shroyer K. Differential expression of transferrin receptor (TfR) in a spectrum of normal to malignant breast tissues: implications for in situ and invasive carcinoma. Appl. Immunohistochem. Mol. Morphol. AIMM. 2011;19:417–423. doi: 10.1097/PAI.0B013E318209716E. [DOI] [PubMed] [Google Scholar]
- 274.Shen Y., Li X., Dong D., Zhang B., Xue Y., Shang P. Transferrin receptor 1 in cancer: a new sight for cancer therapy. Am. J. Cancer Res. 2018;8:916. [PMC free article] [PubMed] [Google Scholar]
- 275.Mahmood N., Mihalcioiu C., Rabbani S.A. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): diagnostic, prognostic, and therapeutic applications. Front. Oncol. 2018;8:24. doi: 10.3389/FONC.2018.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Duffy M. The urokinase plasminogen activator system: role in malignancy. Curr. Pharmaceut. Des. 2005;10:39–49. doi: 10.2174/1381612043453559. [DOI] [PubMed] [Google Scholar]
- 277.Smaradhania N., Rahman S., Ardi Syamsu S., Prihantono P. Urokinase type plasminogen activator receptor (uPAR) and human epidermal growth factor receptor 2 (HER2) expression in metastasis of breast cancer. Breast Dis. 2021;40:S1–S7. doi: 10.3233/BD-219001. [DOI] [PubMed] [Google Scholar]
- 278.Zempleni J., Wijeratne S., Hassan Y. Biotin, BioFactors Oxf. Engl. 2009;35:36–46. doi: 10.1002/BIOF.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Vadlapudi A., Vadlapatla R., Mitra A. Sodium dependent multivitamin transporter (SMVT): a potential target for drug delivery. Curr. Drug Targets. 2012;13:994–1003. doi: 10.2174/138945012800675650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Vadlapudi A.D., Vadlapatla R.K., Pal D., Mitra A.K. Biotin uptake by T47D breast cancer cells: functional and molecular evidence of sodium-dependent multivitamin transporter (SMVT) Int. J. Pharm. 2013;441:535–543. doi: 10.1016/J.IJPHARM.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 281.Ren W.X., Han J., Uhm S., Jang Y.J., Kang C., Kim J.-H., Kim J.S. Recent development of biotin conjugation in biological imaging, sensing, and target delivery. Chem. Commun. 2015;51:10403–10418. doi: 10.1039/C5CC03075G. [DOI] [PubMed] [Google Scholar]
- 282.Chen C., Zhao S., Karnad A., Freeman J.W. The biology and role of CD44 in cancer progression: therapeutic implications. J. Hematol. Oncol. 2018;11:1–23. doi: 10.1186/S13045-018-0605-5. 2018 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zheng Z., Shao N., Weng H., Li W., Zhang J., Zhang L., Yang L., Ye S. Correlation between epidermal growth factor receptor and tumor stem cell markers CD44/CD24 and their relationship with prognosis in breast invasive ductal carcinoma. Med. Oncol. Northwood Lond. Engl. 2015;32:1–11. doi: 10.1007/S12032-014-0275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Jin J., Krishnamachary B., Mironchik Y., Kobayashi H., Bhujwalla Z.M. Phototheranostics of CD44-positive cell populations in triple negative breast cancer. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep27871. 2016 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Jiang D., Liang J., Noble P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011;91:221–264. doi: 10.1152/PHYSREV.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yang C., He Y., Zhang H., Liu Y., Wang W., Du Y., Gao F. Selective killing of breast cancer cells expressing activated CD44 using CD44 ligand-coated nanoparticles in vitro and in vivo. Oncotarget. 2015;6 doi: 10.18632/ONCOTARGET.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Gupta R., Lall R., Srivastava A., Sinha A. Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019;6 doi: 10.3389/FVETS.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Seynhaeve A.L.B., Amin M., Haemmerich D., van Rhoon G.C., ten Hagen T.L.M. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020;163–164:125–144. doi: 10.1016/J.ADDR.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 289.Crabtree J.S., Miele L. Breast cancer stem cells. Biomedicines. 2018;6:77. doi: 10.3390/biomedicines6030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wu J.M., Flynn J.F., Wong C. Anti-EGFR therapy: mechanism and advances in clinical efficacy in breast cancer. JAMA Oncol. 2009 doi: 10.1155/2009/526963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lee H.J., Seo A.N., Kim E.J., Jang M.H., Kim Y.J., Kim J.H., Kim S.W., Ryu H.S., Park I.A., Im S.A., Gong G., Jung K.H., Kim H.J., Park S.Y. Prognostic and predictive values of EGFR overexpression and EGFR copy number alteration in HER2-positive breast cancer. Br. J. Cancer. 2015;112:103–111. doi: 10.1038/bjc.2014.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Luiz M.T., Dutra J.A.P., Tofani L.B., de Araújo J.T.C., Di Filippo L.D., Marchetti J.M., Chorilli M. Targeted liposomes: a nonviral gene delivery system for cancer therapy. Pharmaceutics. 2022;14:821. doi: 10.3390/pharmaceutics14040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bou-Assaly W., Mukherji S. Cetuximab (erbitux) Am. J. Neuroradiol. 2010;31:626–627. doi: 10.3174/AJNR.A2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Brockhoff G., Heckel B., Schmidt-Bruecken E., Plander M., Hofstaedter F., Vollmann A., Diermeier S. Differential impact of Cetuximab, Pertuzumab and Trastuzumab on BT474 and SK-BR-3 breast cancer cell proliferation. Cell Prolif. 2007;40:488–507. doi: 10.1111/j.1365-2184.2007.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Carlsson J., Nordgren H., Sjöström J., Wester K., Villman K., Bengtsson N.O., Ostenstad B., Lundqvist H., Blomqvist C. HER2 expression in breast cancer primary tumours and corresponding metastases. Br. J. Cancer. 2004;90:2344–2348. doi: 10.1038/sj.bjc.6601881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Iqbal B., Buch A. Hormone receptor (ER, PR, HER2/neu) status and proliferation index marker (Ki-67) in breast cancers: their onco-pathological correlation, shortcomings and future trends. Med. J. Dr Patil Univ. 2016;9:674. doi: 10.4103/0975-2870.194180. [DOI] [Google Scholar]
- 297.Tiwari S., Mishra P., Raska P., Calhoun B., Abraham J., Moore H., Budd G., Fanning A., Valente S., Stewart R., Grobmyer S., Montero A. Retrospective study of the efficacy and safety of neoadjuvant docetaxel, carboplatin, trastuzumab/pertuzumab (TCH-P) in nonmetastatic HER2-positive breast cancer. Breast Cancer Res. Treat. 2016;158:189–193. doi: 10.1007/S10549-016-3866-0. [DOI] [PubMed] [Google Scholar]
- 298.Nahta R., Hung M., Esteva F. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.CAN-03-3856. [DOI] [PubMed] [Google Scholar]
- 299.Wang C., Wang L., Yu X., Zhang Y., Meng Y., Wang H., Yang Y., Gao J., Wei H., Zhao J., Lu C., Chen H., Sun Y., Li B. Combating acquired resistance to trastuzumab by an anti-ErbB2 fully human antibody. Oncotarget. 2017;8:42742–42751. doi: 10.18632/oncotarget.17451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Espelin C., Leonard S., Geretti E., Wickham T., Hendriks B. Dual HER2 targeting with trastuzumab and liposomal-encapsulated doxorubicin (MM-302) demonstrates synergistic antitumor activity in breast and gastric cancer. Cancer Res. 2016;76:1517–1527. doi: 10.1158/0008-5472.CAN-15-1518. [DOI] [PubMed] [Google Scholar]
- 301.LoRusso P., Krop I., Miller K., Ma C., Siegel B.A., Shields A.F., Molnar I., Wickham T., Reynolds J., Campbell K., Hendriks B., McClure T., Moyo V., Munster P. Abstract CT234: A phase I study of MM-302, a HER2-targeted PEGylated liposomal doxorubicin, in patients with HER2+ metastatic breast cancer. Cancer Res. 2015;75 doi: 10.1158/1538-7445.AM2015-CT234. CT234–CT234. [DOI] [Google Scholar]
- 302.Munster P., Krop I.E., LoRusso P., Ma C., Siegel B.A., Shields A.F., Molnár I., Wickham T.J., Reynolds J., Campbell K., Hendriks B.S., Adiwijaya B.S., Geretti E., Moyo V., Miller K.D. Safety and pharmacokinetics of MM-302, a HER2-targeted antibody–liposomal doxorubicin conjugate, in patients with advanced HER2-positive breast cancer: a phase 1 dose-escalation study. Br. J. Cancer. 2018;119:1086–1093. doi: 10.1038/s41416-018-0235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kirpotin D.B., Drummond D.C., Shao Y., Shalaby M.R., Hong K., Nielsen U.B., Marks J.D., Benz C.C., Park J.W. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 304.Miller K., Cortes J., Hurvitz S., Krop I., Tripathy D., Verma S., Riahi K., Reynolds J., Wickham T., Molnar I., Yardley D. HERMIONE: a randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician's choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer. 2016;16 doi: 10.1186/S12885-016-2385-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hamidi H., Pietilä M., Ivaska J. The complexity of integrins in cancer and new scopes for therapeutic targeting. Br. J. Cancer. 2016;115:1017–1023. doi: 10.1038/bjc.2016.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hanker A., Estrada M., Bianchini G., Moore P., Zhao J., Cheng F., Koch J., Gianni L., Tyson D., Sánchez V., Rexer B., Sanders M., Zhao Z., Stricker T., Arteaga C. Extracellular matrix/integrin signaling promotes resistance to combined inhibition of HER2 and PI3K in HER2 + breast cancer. Cancer Res. 2017;77:3280–3292. doi: 10.1158/0008-5472.CAN-16-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Weis S.M., Cheresh D.A. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 308.Andonegui-Elguera M.A., Alfaro-Mora Y., Cáceres-Gutiérrez R., Caro-Sánchez C.H.S., Herrera L.A., Díaz-Chávez J. An overview of vasculogenic mimicry in breast cancer. Front. Oncol. 2020:220. doi: 10.3389/FONC.2020.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Shirakawa K., Kobayashi H., Sobajima J., Hashimoto D., Shimizu A., Wakasugi H. Inflammatory breast cancer: vasculogenic mimicry and its hemodynamics of an inflammatory breast cancer xenograft model. Breast Cancer Res. 2003;5:1–4. doi: 10.1186/BCR585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Flanagan C.A., Manilall A. Gonadotropin-releasing hormone (GnRH) receptor structure and GnRH binding. Front. Endocrinol. 2017;8:274. doi: 10.3389/FENDO.2017.00274/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Schally A.V., Szepeshazi K., Nagy A., Comaru-Schally A.M., Halmos G. New approaches to therapy of cancers of the stomach, colon and pancreas based on peptide analogs, Cell. Mol. Life Sci. 2004;61:1042–1068. doi: 10.1007/s00018-004-3434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Kufe D. Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer. 2009;9:874–885. doi: 10.1038/NRC2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Jing X., Liang H., Hao C., Yang X., Cui X. Overexpression of MUC1 predicts poor prognosis in patients with breast cancer. Oncol. Rep. 2019;41:801–810. doi: 10.3892/OR.2018.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Nemunaitis J., Bedell C., Klucher K., Vo A., Whiting S. Phase 1 dose escalation of ONT-10, a therapeutic MUC1 vaccine, in patients with advanced cancer. J. Immunother. Cancer. 2013;1 doi: 10.1186/2051-1426-1-S1-P240. 1–1. [DOI] [Google Scholar]
- 315.Nemunaitis J.J., Adams N., Bedell C., Klucher K., Taylor J., Whiting S.H. Tolerability, humoral immune response, and disease control in phase 1 patients receiving ONT-10, a MUC1 liposomal vaccine. J. Clin. Oncol. 2014;32 doi: 10.1200/jco.2014.32.15_suppl.3091. 3091–3091. [DOI] [Google Scholar]
- 316.Chaudhary B., Khaled Y.S., Ammori B.J., Elkord E. Neuropilin 1: function and therapeutic potential in cancer. Cancer Immunol. Immunother. 2013;63:81–99. doi: 10.1007/S00262-013-1500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Abdullah A., Akhand S.S., Paez J.S.P., Brown W., Pan L., Libring S., Badamy M., Dykuizen E., Solorio L., Andy Tao W., Wendt M.K. Epigenetic targeting of neuropilin-1 prevents bypass signaling in drug-resistant breast cancer. Oncogene. 2020;40:322–333. doi: 10.1038/s41388-020-01530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Derakhshankhah H., Jafari S. Cell penetrating peptides: a concise review with emphasis on biomedical applications. Biomed. Pharmacother. 2018;108:1090–1096. doi: 10.1016/J.BIOPHA.2018.09.097. [DOI] [PubMed] [Google Scholar]
- 319.Wu Y., Zhang Z., Cenciarini M.E., Proietti C.J., Amasino M., Hong T., Yang M., Liao Y., Chiang H.C., Kaklamani V.G., Jeselsohn R., Vadlamudi R.K., Huang T.H.M., Li R., De Angelis C., Fu X., Elizalde P.V., Schiff R., Brown M., Xu K. Tamoxifen resistance in breast cancer is regulated by the EZH2–ERa–GREB1 transcriptional axis. Cancer Res. 2018;78:671–684. doi: 10.1158/0008-5472.CAN-17-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Jallow F., O'Leary K.A., Rugowski D.E., Guerrero J.F., Ponik S.M., Schuler L.A. Dynamic interactions between the extracellular matrix and estrogen activity in progression of ER+ breast cancer. Oncogene. 2019;38:6913–6925. doi: 10.1038/s41388-019-0941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Liu Y., Ma H., Yao J. ERα, a key target for cancer therapy: a review. OncoTargets Ther. 2020;13:2183–2191. doi: 10.2147/OTT.S236532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Omoto Y., Iwase H. Clinical significance of estrogen receptor β in breast and prostate cancer from biological aspects. Cancer Sci. 2015;106:337–343. doi: 10.1111/CAS.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Arnal J.F., Lenfant F., Metivier R., Flouriot G., Henrion D., Adlanmerini M., Fontaine C., Gourdy P., Chambon P., Katzenellenbogen B., Katzenellenbogen J. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol. Rev. 2017;97:1045–1087. doi: 10.1152/physrev.00024.2016. [DOI] [PubMed] [Google Scholar]
- 324.Abe O., Abe R., Enomoto K., Kikuchi K., Koyama H., Masuda H., Nomura Y., Sakai K., Sugimachi K., Tominaga T., Uchino J., Yoshida M., Haybittle J.L., Davies C., Harvey V.J., Holdaway T.M., Kay R.G., Mason B.H., Forbes J.F., Wilcken N., Gnant M., Jakesz R., Ploner M., Yosef H.M.A., Focan C., Lobelle J.P., Peek U., Oates G.D., Powell J., Durand M., Mauriac L., Di Leo A., Dolci S., Piccart M.J., Masood M.B., Parker D., Price J.J., Hupperets P.S.G.J., Jackson S., Ragaz J., Berry D., Broadwater G., Cirrincione C., Muss H., Norton L., Weiss R.B., Abu-Zahra H.T., Portnoj S.M., Baum M., Cuzick J., Houghton J., Riley D., Gordon N.H., Davis H.L., Beatrice A., Mihura J., Naja A., Lehingue Y., Romestaing P., Dubois J.B., Delozier T., Mace-Lesec’h J., Rambert P., Andrysek O., Barkmanova J., Owen J.R., Meier P., Howell A., Ribeiro G.C., Swindell R., Alison R., Boreham J., Clarke M., Collins R., Darby S., Davies C., Elphinstone P., Evans V., Godwin J., Gray R., Harwood C., Hicks C., James S., MacKinnon E., McGale P., McHugh T., Mead G., Peto R., Wang Y., Albano J., De Oliveira C.F., Gervásio H., Gordilho J., Johansen H., Mouridsen H.T., Gelman R.S., Harris J.R., Henderson I.C., Shapiro C.L., Andersen K.W., Axelsson C.K., Blichert-Toft M., Møller S., Mouridsen H.T., Overgaard J., Overgaard M., Rose C., Carstensen B., Palshof T., Trampisch H.J., Dalesio O., De Vries E.G.E., Rodenhuis S., Van Tinteren H., Comis R.L., Davidson N.E., Gray R., Robert N., Sledge G., Tormey D.C., Wood W., Cameron D., Chetty U., Forrest P., Jack W., Rossbach J., Klijn J.G.M., Treurniet-Donker A.D., Van Putten W.L.J., Costa A., Veronesi U., Bartelink H., Duchateau L., Legrand C., Sylvester R., Van Der Hage J.A., Van De Velde C.J.H., Cunningham M.P., Catalano R., Creech R.H., Bonneterre J., Fargeot P., Fumoleau P., Kerbrat P., Namer M., Jonat W., Kaufmann M., Schumacher M., Von Minckwitz G., Bastert G., Rauschecker H., Sauer R., Sauerbrei W., Schauer A., Schumacher M., De Schryver A., Vakaet L., Belfiglio M., Nicolucci A., Pellegrini F., Sacco M., Valentini M., McArdle C.S., Smith D.C., Galligioni E., Boccardo F., Rubagotti A., Dent D.M., Gudgeon C.A., Hacking A., Erazo A., Medina J.Y., Izuo M., Morishita Y., Takei H., Fentiman I.S., Hayward J.L., Rubens R.D., Skilton D., Graeff H., Jänicke F., Meisner C., Scheurlen H., Kaufmann M., Von Fournier D., Dafni U., Fountzilas G., Klefstrom P., Blomqvist C., Saarto T., Margreiter R., Asselain B., Salmon R.J., Vilcoq J.R., Arriagada R., Hill C., Laplanche A., Lê M.G., Spielmann M., Bruzzi P., Montanaro E., Rosso R., Sertoli M.R., Venturini M., Amadori D., Benraadt J., Kooi M., Van De Velde A.O., Van Dongen J.A., Vermorken J.B., Castiglione M., Cavalli F., Coates A., Collins J., Forbes J., Gelber R.D., Goldhirsch A., Lindtner J., Price K.N., Rudenstam C.M., Senn H.J., Bliss J.M., Chilvers C.E.D., Coombes R.C., Hall E., Marty M., Borovik R., Brufman G., Hayat H., Robinson E., Wigler N., Bonadonna G., Camerini T., De Palo G., Del Vecchio M., Formelli F., Valagussa P., Martoni A., Pannuti F., Cocconi G., Colozza A., Camisa R., Aogi K., Takashima S., Abe O., Ikeda T., Inokuchi K., Kikuchi K., Sawa K., Sonoo H., Korzeniowski S., Skolyszewski J., Ogawa M., Yamashita J., Bonte J., Christiaens R., Paridaens R., Van Den Boegart W., Martin P., Romain S., Hakes T., Hudis C.A., Norton L., Wittes R., Giokas G., Kondylis D., Lissaios B., De La Huerta R., Sainz M.G., Altemus R., Cowan K., Danforth D., Lichter A., Lippman M., O’Shaughnessy J., Pierce L.J., Steinberg S., Venzon D., Zujewski J.A., Paradiso A., De Lena M., Schittulli F., Myles J.D., Pater J.L., Pritchard K.I., Nomura Y., Anderson S., Bass G., Brown A., Bryant J., Costantino J., Dignam J., Fisher B., Redmond C., Wieand S., Wolmark N., Baum M., Jackson I.M., Palmer M.K., Ingle J.N., Suman V.J., Bengtsson N.O., Jonsson H., Larsson L.G., Lythgoe J.P., Swindell R., Kissin M., Erikstein B., Hannisdal E., Jacobsen A.B., Varhaug J.E., Erikstein B., Gundersen S., Hauer-Jensen M., Høst H., Jacobsen A.B., Nissen-Meyer R., Blamey R.W., Mitchell A.K., Morgan D.A.L., Robertson J.F.R., Di Palma M., Mathé G., Misset J.L., Clark R.M., Levine M., Morimoto K., Sawa K., Takatsuka Y., Crossley E., Harris A., Talbot D., Taylor M., Cocconi G., Di Blasio B., Ivanov V., Semiglazov V., Brockschmidt J., Cooper M.R., Ueo H., Falkson C.I., A’Hern R., Ashley S., Powles T.J., Smith I.E., Yarnold J.R., Gazet J.C., Corcoran N., Deshpande N., Di Martino L., Douglas P., Hacking A., Høst H., Lindtner A., Notter G., Bryant A.J.S., Ewing G.H., Firth L.A., Krushen-Kosloski J.L., Nissen-Meyer R., Foster L., George W.D., Stewart H.J., Stroner P., Malmström P., Möller T.R., Rydén S., Tengrup I., Tennvall-Nittby L., Carstenssen J., Dufmats M., Hatschek T., Nordenskjöld B., Söderberg M., Carpenter J.T., Albain K., Crowley J., Green S., Martino S., Osborne C.K., Ravdin P.M., Glas U., Johansson U., Rutqvist L.E., Singnomklao T., Wallgren A., Castiglione M., Goldhirsch A., Maibach R., Senn H.J., Thürlimann B., Brenner H., Hercbergs A., Yoshimoto M., DeBoer G., Paterson A.H.G., Pritchard K.I., Meakin J.W., Panzarella T., Pritchard K.I., Shan Y., Shao Y.F., Wang X., Zhao D.B., Boreham J., Chen Z.M., Pan H.C., Peto R., Bahi J., Reid M., Spittle M., Deutsch G.P., Senanayake F., Kwong D.L.W., Bianco A.R., Carlomagno C., De Laurentiis M., De Placido S., Buzdar A.U., Smith T., Bergh J., Holmberg L., Liljegren G., Nilsson J., Seifert M., Sevelda P., Zielinsky C.C., Buchanan R.B., Cross M., Royle G.T., Dunn J.A., Hills R.K., Lee M., Morrison J.M., Spooner D., Litton A., Chlebowski R.T., Caffier H. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 325.Heger Z., Gumulec J., Cernei N., Tmejova K., Kopel P., Balvan J., Masarik M., Zitka O., Beklova M., Adam V., Kizek R. 17β-estradiol-containing liposomes as a novel delivery system for the antisense therapy of ER-positive breast cancer: an in vitro study on the MCF-7 cell line. Oncol. Rep. 2015;33:921–929. doi: 10.3892/OR.2014.3627. [DOI] [PubMed] [Google Scholar]
- 326.Higgins M.J., Stearns V. CYP2D6 polymorphisms and tamoxifen metabolism: clinical relevance. Curr. Oncol. Rep. 2010;12:7–15. doi: 10.1007/s11912-009-0076-5. [DOI] [PubMed] [Google Scholar]
- 327.Manna S., Holz M.K. Tamoxifen action in ER-negative breast cancer. Signal Transduct. Insights. 2016;5:1–7. doi: 10.4137/sti.s29901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Droog M., Beelen K., Linn S., Zwart W. Tamoxifen resistance: from bench to bedside. Eur. J. Pharmacol. 2013;717:47–57. doi: 10.1016/j.ejphar.2012.11.071. [DOI] [PubMed] [Google Scholar]
- 329.Tomlinson I.P., Strickland J.E., Lee A.S., Bromley L., Evans M.F., Morton J., McGee J.O. Loss of heterozygosity on chromosome 11 q in breast cancer. J. Clin. Pathol. 1995;48:424–428. doi: 10.1136/JCP.48.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Horwitz K.B., Sartorius C.A. 90 years of progesterone: progesterone and progesterone receptors in breast cancer: past, present, future. J. Mol. Endocrinol. 2020;65:T49–T63. doi: 10.1530/JME-20-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Giulianelli S., Molinolo A., Lanari C. Targeting progesterone receptors in breast cancer. Vitam. Horm. 2013;93:161–184. doi: 10.1016/B978-0-12-416673-8.00009-5. [DOI] [PubMed] [Google Scholar]
- 332.Pandit A., Khare L., Devarajan P.V., Jain R., Dandekar P. Breast cancer receptors and targeting strategies. AAPS Adv. Pharm. Sci. Ser. 2019;39:79–108. doi: 10.1007/978-3-030-29168-6_3. [DOI] [Google Scholar]
- 333.Lv Y., Ma X., Du Y., Feng J. Understanding patterns of brain metastasis in triple-negative breast cancer and exploring potential therapeutic targets. OncoTargets Ther. 2021;14:589. doi: 10.2147/OTT.S293685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Chabbert-Buffet N., Kolanska K., Daraï E., Bouchard P. Selective progesterone receptor modulators: current applications and perspectives. Climacteric. 2018;21:375–379. doi: 10.1080/13697137.2017.1386650. [DOI] [PubMed] [Google Scholar]
- 335.Yi M., Huo L., Koenig K.B., Mittendorf E.A., Meric-Bernstam F., Kuerer H.M., Bedrosian I., Buzdar A.U., Symmans W.F., Crow J.R., Bender M., Shah R.R., Hortobagyi G.N., Hunt K.K. Which threshold for ER positivity? a retrospective study based on 9639 patients. Ann. Oncol. 2014;25:1004–1011. doi: 10.1093/ANNONC/MDU053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Knutson T.P., Lange C.A. Tracking progesterone receptor-mediated actions in breast cancer. Pharmacol. Ther. 2014;142:114–125. doi: 10.1016/J.PHARMTHERA.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Yan C., Boyd D. Regulation of matrix metalloproteinase gene expression. J. Cell. Physiol. 2007;211:19–26. doi: 10.1002/JCP.20948. [DOI] [PubMed] [Google Scholar]
- 338.Cathcart J., Pulkoski-Gross A., Cao J. Targeting matrix metalloproteinases in cancer: bringing new life to old ideas. Genes Dis. 2015;2:26–34. doi: 10.1016/J.GENDIS.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Alaseem A., Alhazzani K., Dondapati P., Alobid S., Bishayee A., Rathinavelu A. Matrix Metalloproteinases: a challenging paradigm of cancer management. Semin. Cancer Biol. 2019;56:100–115. doi: 10.1016/J.SEMCANCER.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 340.Tauro M., Lynch C.C. Cutting to the chase: how matrix metalloproteinase-2 activity controls breast-cancer-to-bone metastasis. Cancers. 2018;10:185. doi: 10.3390/CANCERS10060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Medina O.P., Haikola M., Tahtinen M., Simpura I., Kaukinen S., Valtanen H., Zhu Y., Kuosmanen S., Cao W., Reunanen J., Nurminen T., Saris P.E.J., Smith-Jones P., Bradbury M., Larson S., Kairemo K. Liposomal tumor targeting in drug delivery utilizing MMP-2- and MMP-9-binding ligands. J. Drug Deliv. 2011;2011:1–9. doi: 10.1155/2011/160515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Isaacson K.J., Jensen M.M., Subrahmanyam N.B., Ghandehari H. Matrix-metalloproteinases as targets for controlled delivery in cancer: an analysis of upregulation and expression. J. Contr. Release. 2017;259:62. doi: 10.1016/J.JCONREL.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Quach N.D., Arnold R.D., Cummings B.S. Secretory phospholipase A2 enzymes as pharmacological targets for treatment of disease. Biochem. Pharmacol. 2014;90:338. doi: 10.1016/J.BCP.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yamashita S., Yamashita J., Ogawa M. Overexpression of group II phospholipase A2 in human breast cancer tissues is closely associated with their malignant potency. Br. J. Cancer. 1994;69:1166–1170. doi: 10.1038/BJC.1994.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Dennis E., Cao J., Hsu Y., Magrioti V., Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011;111:6130–6185. doi: 10.1021/CR200085W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Jespersen S.S., Stovgaard E.S., Nielsen D., Christensen T.D., Buhl A.S.K., Christensen I.J., Balslev E. Expression of secretory phospholipase A2 group IIa in breast cancer and correlation to prognosis in a cohort of advanced breast cancer patients. Appl. Immunohistochem. Mol. Morphol. 2021;29:E5–E9. doi: 10.1097/PAI.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 347.Singer A.G., Ghomashchi F., Le Calvez C., Bollinger J., Bezzine S., Rouault M., Sadilek M., Nguyen E., Lazdunski M., Lambeau G., Gelb M.H. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 2002;277:48535–48549. doi: 10.1074/JBC.M205855200. [DOI] [PubMed] [Google Scholar]
- 348.André T., Boni C., Mounedji-Boudiaf L., Navarro M., Tabernero J., Hickish T., Topham C., Zaninelli M., Clingan P., Bridgewater J., Tabah-Fisch I., de Gramont A. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2009;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 349.Zhang J., Wang L., Wang Z., Hu X., Wang B., Cao J., Lv F., Zhen C., Zhang S., Shao Z. A phase II trial of biweekly vinorelbine and oxaliplatin in second- or third-line metastatic triple-negative breast cancer. Cancer Biol. Ther. 2015;16:225–232. doi: 10.4161/15384047.2014.986973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Garufi C., Nisticò C., Brienza S., Vaccaro A., D'Ottavio A., Zappalà A.R., Aschelter A.M., Terzoli E. Single-agent oxaliplatin in pretreated advanced breast cancer patients: a phase II study. Ann. Oncol. 2001;12:179–182. doi: 10.1023/A:1008386419047. [DOI] [PubMed] [Google Scholar]
- 351.Fei F., Chen C., Xue J., Di G.H., Lu J.S., Liu G.Y., Shao Z.M., Wu J. Efficacy and safety of docetaxel combined with oxaliplatin as a neoadjuvant chemotherapy regimen for Chinese triple-negative local advanced breast cancer patients : a prospective, open, and unicentric phase II clinical trial. Am. J. Clin. Oncol. Cancer Clin. Trials. 2013;36:545–551. doi: 10.1097/COC.0B013E31825D5317. [DOI] [PubMed] [Google Scholar]
- 352.Zelek L., Cottu P., Tubiana-Hulin M., Vannetzel J.-M., Chollet Ph, Misset J.-L., Chouaki N., Marty M., Gamelin E., Culine S., Dieras V., Mackenzie S., Spielmann M. Phase II study of oxaliplatin and fluorouracil in taxane- and anthracycline-pretreated breast cancer patients. J. Clin. Oncol. 2002;20:2551–2558. doi: 10.1200/JCO.2002.06.164. [DOI] [PubMed] [Google Scholar]
- 353.De Jonge M.J.A., Slingerland M., Loos W.J., Wiemer E.A.C., Burger H., Mathijssen R.H.J., Kroep J.R., Den Hollander M.A.G., Van Der Biessen D., Lam M.H., Verweij J., Gelderblom H. Early cessation of the clinical development of LiPlaCis, a liposomal cisplatin formulation. Eur. J. Cancer. 2010;46:3016–3021. doi: 10.1016/J.EJCA.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 354.Pourhassan H., Clergeaud G., Hansen A., Østrem R., Fliedner F., Melander F., Nielsen O., O'Sullivan C., Kjær A., Andresen T. Revisiting the use of sPLA 2-sensitive liposomes in cancer therapy. J. Contr. Release. 2017;261:163–173. doi: 10.1016/J.JCONREL.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 355.Lassen U., Mau-Sørensen M., Buhl U.H., Madsen M.W., Balslev E., Pluim D., Schellens J.H.M., Knudsen S., Jensen P.B. Abstract CT154: phase I dose-escalating PoC study to evaluate the safety and tolerability of LiPlaCis (liposomal cisplatin formulation) in patients with advanced or refractory tumors. Cancer Res. 2016;76 doi: 10.1158/1538-7445.AM2016-CT154. CT154–CT154. [DOI] [Google Scholar]
- 356.Lassen U.N., Knudsen S., Hertel P.B., Kumler I., Nielsen D., Ejlertsen B., Soerensen M.M., Brunner N., Buhl U.H., Madsen M.W., Buhl I.K., Hansen A., Jensen T., Balslev E., Askaa J., Vestlev P.M., Laenkholm A.-V., Jensen P.B. J. Clin. Oncol. American Society of Clinical Oncology; 2014. Use of microRNA to identify stage IV breast cancer patients to be targeted with phospholipase A2 disrupted cisplatin carrying liposomes: an ongoing phase I trial. TPS1139–TPS1139. [DOI] [Google Scholar]
- 357.Jakobsen E.H., Nielsen D., Danoe H., Linnet S., Hansen J., Lassen U.N., Balslev E., Glavicic V., Bogovic J., Knudsen S., Ejlertsen B., Knoop A.S., Buhl U.H., Madsen M.W., Buhl I.K., Hansen A., Jensen T., Rasmussen A., Jensen P.B., Langkjer S.T. Breast Cancer - Metastatic. American Society of Clinical Oncology; 2018. Liposomal cisplatin response prediction in heavily pretreated breast cancer patients: a multigene biomarker in a prospective phase 2 study. e13077–e13077. [DOI] [Google Scholar]
- 358.Assanhou A.G., Li W., Zhang L., Xue L., Kong L., Sun H., Mo R., Zhang C. Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials. 2015;73:284–295. doi: 10.1016/J.BIOMATERIALS.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 359.Jyotsana N., Zhang Z., Himmel L.E., Yu F., King M.R. Minimal dosing of leukocyte targeting TRAIL decreases triple-negative breast cancer metastasis following tumor resection. Sci. Adv. 2019;5:eaaw4197. doi: 10.1126/SCIADV.AAW4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Bockhorn J., Dalton R., Nwachukwu C., Huang S., Prat A., Yee K., Chang Y.-F., Huo D., Wen Y., Swanson K.E., Qiu T., Lu J., Young Park S., Eileen Dolan M., Perou C.M., Olopade O.I., Clarke M.F., Greene G.L., Liu H. MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nat. Commun. 2013;4:1393. doi: 10.1038/ncomms2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Chen T., Chen H., Jiang Y., Yan Q., Zheng S., Wu M. Co-delivery of 5-fluorouracil and paclitaxel in mitochondria-targeted KLA-modified liposomes to improve triple-negative breast cancer treatment. Pharmaceuticals. 2022;15:881. doi: 10.3390/ph15070881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Du J., Shao Y., Hu Y., Chen Y., Cang J., Chen X., Pei W., Miao F., Shen Y., Muddassir Mohd, Zhang Y., Zhang J., Teng G. Multifunctional liposomes enable active targeting and twinfilin 1 silencing to reverse paclitaxel resistance in brain metastatic breast cancer. ACS Appl. Mater. Interfaces. 2021;13:23396–23409. doi: 10.1021/acsami.1c02822. [DOI] [PubMed] [Google Scholar]
- 363.Pathania D., Millard M., Neamati N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv. Drug Deliv. Rev. 2009;61:1250–1275. doi: 10.1016/j.addr.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 364.Pu Y., Zhang H., Peng Y., Fu Q., Yue Q., Zhao Y., Guo L., Wu Y. Dual-targeting liposomes with active recognition of GLUT5 and αvβ3 for triple-negative breast cancer. Eur. J. Med. Chem. 2019;183 doi: 10.1016/J.EJMECH.2019.111720. [DOI] [PubMed] [Google Scholar]
- 365.Yang Y., Zhao Z., Xie C., Zhao Y. Dual-targeting liposome modified by glutamic hexapeptide and folic acid for bone metastatic breast cancer. Chem. Phys. Lipids. 2020;228 doi: 10.1016/J.CHEMPHYSLIP.2020.104882. [DOI] [PubMed] [Google Scholar]
- 366.Doolittle E., Peiris P., Doron G., Goldberg A., Tucci S., Rao S., Shah S., Sylvestre M., Govender P., Turan O., Lee Z., Schiemann W., Karathanasis E. Spatiotemporal targeting of a dual-ligand nanoparticle to cancer metastasis. ACS Nano. 2015;9:8012–8021. doi: 10.1021/ACSNANO.5B01552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Covarrubias G., He F., Raghunathan S., Turan O., Peiris P.M., Schiemann W.P., Karathanasis E. Effective treatment of cancer metastasis using a dual-ligand nanoparticle. PLoS One. 2019;14 doi: 10.1371/JOURNAL.PONE.0220474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Müller A., Chiotellis A., Keller C., Ametamey S.M., Schibli R., Mu L., Krämer S.D. Imaging tumour ATB0,+ transport activity by PET with the cationic amino acid O-2((2-[18F]fluoroethyl)methyl-amino)ethyltyrosine. Mol. Imag. Biol. 2013;16:412–420. doi: 10.1007/S11307-013-0711-2. [DOI] [PubMed] [Google Scholar]
- 369.Yan R., Zhao X., Lei J., Zhou Q. Structure of the human LAT1–4F2hc heteromeric amino acid transporter complex. Nature. 2019;568:127–130. doi: 10.1038/s41586-019-1011-z. [DOI] [PubMed] [Google Scholar]
- 370.Wang Z., Chi D., Wu X., Wang Y., Lin X., Xu Z., Liu H., Sun J., He Z., Wang Y. Tyrosine modified irinotecan-loaded liposomes capable of simultaneously targeting LAT1 and ATB 0,+ for efficient tumor therapy. J. Contr. Release. 2019;316:22–33. doi: 10.1016/j.jconrel.2019.10.037. [DOI] [PubMed] [Google Scholar]
- 371.Saha P., Datta K. Multi-functional, multicompartmental hyaluronan-binding protein 1 (HABP1/p32/gC1qR): implication in cancer progression and metastasis. Oncotarget. 2018;9 doi: 10.18632/ONCOTARGET.24082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Chen Y.-B., Jiang C.-T., Zhang G.-Q., Wang J.-S., Pang D. Increased expression of hyaluronic acid binding protein 1 is correlated with poor prognosis in patients with breast cancer. J. Surg. Oncol. 2009;100:382–386. doi: 10.1002/JSO.21329. [DOI] [PubMed] [Google Scholar]
- 373.Ghebrehiwet B., Geisbrecht B.V., Xu X., Savitt A.G., Peerschke E.I.B. The C1q Receptors: focus on gC1qR/p33 (C1qBP, p32, HABP-1)1, Semin. Immunol. 2019;45 doi: 10.1016/j.smim.2019.101338. [DOI] [PubMed] [Google Scholar]
- 374.Rubinstein D.B., Stortchevoi A., Boosalis M., Ashfaq R., Ghebrehiwet B., Peerschke E.I.B., Calvo F., Guillaume T. Receptor for the globular heads of C1q (gC1q-R, p33, hyaluronan-binding protein) is preferentially expressed by adenocarcinoma cells. Int. J. Cancer. 2004;110:741–750. doi: 10.1002/ijc.20105. [DOI] [PubMed] [Google Scholar]
- 375.d'Avanzo N., Torrieri G., Figueiredo P., Celia C., Paolino D., Correia A., Moslova K., Teesalu T., Fresta M., Santos H.A. LinTT1 peptide-functionalized liposomes for targeted breast cancer therapy. Int. J. Pharm. 2021;597 doi: 10.1016/J.IJPHARM.2021.120346. 120346. [DOI] [PubMed] [Google Scholar]
- 376.Guo P., Yang J., Liu D., Huang L., Fell G., Huang J., Moses M.A., Auguste D.T. Dual complementary liposomes inhibit triple-negative breast tumor progression and metastasis. Sci. Adv. 2019;5:1–14. doi: 10.1126/sciadv.aav5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kim D.-M., Kim M., Park H.-B., Kim K.-S., Kim D.-E. Anti-MUC1/CD44 dual-aptamer-conjugated liposomes for cotargeting breast cancer cells and cancer stem cells. ACS Appl. Bio Mater. 2019;2:4622–4633. doi: 10.1021/ACSABM.9B00705. [DOI] [PubMed] [Google Scholar]
- 378.Koren E., Apte A., Jani A., Torchilin V.P. Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity. J. Contr. Release. 2012;160:264–273. doi: 10.1016/J.JCONREL.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Li M., Shi K., Tang X., Wei J., Cun X., Long Y., Zhang Z., He Q. Synergistic tumor microenvironment targeting and blood–brain barrier penetration via a pH-responsive dual-ligand strategy for enhanced breast cancer and brain metastasis therapy. Nanomed. Nanotechnol. Biol. Med. 2018;14:1833–1843. doi: 10.1016/J.NANO.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 380.Xiang Y., Liang L., Wang X., Wang J., Zhang X., Zhang Q. Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicine-loaded liposomes. J. Contr. Release. 2011;152:402–410. doi: 10.1016/j.jconrel.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 381.Espelin C., Leonard S., Geretti E., Wickham T., Hendriks B. Dual HER2 targeting with trastuzumab and liposomal-encapsulated doxorubicin (MM-302) demonstrates synergistic antitumor activity in breast and gastric cancer. Cancer Res. 2016;76:1517–1527. doi: 10.1158/0008-5472.CAN-15-1518. [DOI] [PubMed] [Google Scholar]
- 382.LoRusso P., Krop I., Miller K., Ma C., Siegel B.A., Shields A.F., Molnar I., Wickham T., Reynolds J., Campbell K., Hendriks B., McClure T., Moyo V., Munster P. Abstract CT234: A phase I study of MM-302, a HER2-targeted PEGylated liposomal doxorubicin, in patients with HER2+ metastatic breast cancer. Cancer Res. 2015;75 doi: 10.1158/1538-7445.AM2015-CT234. CT234–CT234. [DOI] [Google Scholar]
- 383.Kirpotin D.B., Drummond D.C., Shao Y., Shalaby M.R., Hong K., Nielsen U.B., Marks J.D., Benz C.C., Park J.W. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 384.Miller K., Cortes J., Hurvitz S., Krop I., Tripathy D., Verma S., Riahi K., Reynolds J., Wickham T., Molnar I., Yardley D. HERMIONE: a randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician's choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer. 2016;16 doi: 10.1186/S12885-016-2385-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.J.J. Nemunaitis, N. Adams, C. Bedell, K. Klucher, J. Taylor, S.H. Whiting, Tolerability, humoral immune response, and disease control in phase 1 patients receiving ONT-10, a MUC1 liposomal vaccine., Https://Doi.Org/10.1200/Jco.2014.32.15_suppl.3091. 32 (2014) 3091–3091. 10.1200/JCO.2014.32.15_SUPPL.3091. [DOI]
- 386.Butts C., Socinski M.A., Mitchell P.L., Thatcher N., Havel L., Krzakowski M., Nawrocki S., Ciuleanu T.-E., Bosquée L., Trigo J.M., Spira A., Tremblay L., Nyman J., Ramlau R., Wickart-Johansson G., Ellis P., Gladkov O., Pereira J.R., Eberhardt W.E.E., Helwig C., Schröder A., Shepherd F.A. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68. doi: 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 387.De Jonge M.J.A., Slingerland M., Loos W.J., Wiemer E.A.C., Burger H., Mathijssen R.H.J., Kroep J.R., Den Hollander M.A.G., Van Der Biessen D., Lam M.H., Verweij J., Gelderblom H. Early cessation of the clinical development of LiPlaCis, a liposomal cisplatin formulation. Eur. J. Cancer. 2010;46:3016–3021. doi: 10.1016/J.EJCA.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 388.Lassen U., Mau-Sørensen M., Buhl U.H., Madsen M.W., Balslev E., Pluim D., Schellens J.H.M., Knudsen S., Jensen P.B. Abstract CT154: phase I dose-escalating PoC study to evaluate the safety and tolerability of LiPlaCis (liposomal cisplatin formulation) in patients with advanced or refractory tumors. Cancer Res. 2016;76 doi: 10.1158/1538-7445.AM2016-CT154. CT154–CT154. [DOI] [Google Scholar]
- 389.Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14:282–295. doi: 10.1208/S12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Allen T.M., Cullis P.R. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 391.Narang A.S., Chang R.K., Hussain M.A. Pharmaceutical development and regulatory considerations for nanoparticles and nanoparticulate drug delivery systems. J. Pharm. Sci. 2013;102:3867–3882. doi: 10.1002/JPS.23691. [DOI] [PubMed] [Google Scholar]
- 392.Zhang L., Gu F.X., Chan J.M., Wang A.Z., Langer R.S., Farokhzad O.C. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 2008;83:761–769. doi: 10.1038/SJ.CLPT.6100400. [DOI] [PubMed] [Google Scholar]
- 393.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/J.ADDR.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 394.Barz M., Luxenhofer R., Schillmeier M. Quo vadis nanomedicine? Nanomed. 2015;10:3089–3091. doi: 10.2217/nnm.15.156. [DOI] [PubMed] [Google Scholar]
- 395.Shah S., Dhawan V., Holm R., Nagarsenker M.S., Perrie Y. Liposomes: advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020;154–155:102–122. doi: 10.1016/j.addr.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 396.Dobrovolskaia M.A., McNeil S.E. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J. Contr. Release. 2013;172:456–466. doi: 10.1016/J.JCONREL.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Tinkle S., Mcneil S.E., Mühlebach S., Bawa R., Borchard G., Barenholz Y.C., Tamarkin L., Desai N. Nanomedicines: addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014;1313:35–56. doi: 10.1111/NYAS.12403. [DOI] [PubMed] [Google Scholar]
- 398.Schmutz M., Borges O., Jesus S., Borchard G., Perale G., Zinn M., Sips Ä.A.J.A.M., Soeteman-Hernandez L.G., Wick P., Som C. A methodological safe-by-design approach for the development of nanomedicines. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00258. https://www.frontiersin.org/articles/10.3389/fbioe.2020.00258 accessed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Salvioni L., Rizzuto M.A., Bertolini J.A., Pandolfi L., Colombo M., Prosperi D. Thirty years of cancer nanomedicine: success, frustration, and hope. Cancers. 2019;11 doi: 10.3390/CANCERS11121855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Sun D., Zhou S., Gao W. What went wrong with anticancer nanomedicine design and how to make it right. ACS Nano. 2020;14:12281–12290. doi: 10.1021/acsnano.9b09713. [DOI] [PubMed] [Google Scholar]
- 401.Hafeez M.N., Celia C., Petrikaite V. Challenges towards targeted drug delivery in cancer nanomedicines. Processes. 2021;9 doi: 10.3390/PR9091527. [DOI] [Google Scholar]
- 402.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015;91:3–6. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 403.Lee H., Shields A.F., Siegel B.A., Miller K.D., Krop I., Ma C.X., LoRusso P., Munster P.N., Campbell K., Gaddy D.F., Leonard S.C., Geretti E., Blocker S.B., Kirpotin D.B., Moyo V., Wickham T.J., Hendriks B.S. 64 Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. 2017;23:4190–4202. doi: 10.1158/1078-0432.CCR-16-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015;91:3–6. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 405.Islam R., Maeda H., Fang J. Factors affecting the dynamics and heterogeneity of the EPR effect: pathophysiological and pathoanatomic features, drug formulations and physicochemical factors. Expet Opin. Drug Deliv. 2021;19:199–212. doi: 10.1080/17425247.2021.1874916. [DOI] [PubMed] [Google Scholar]
- 406.Fang J. EPR effect-based tumor targeted nanomedicine: a promising approach for controlling cancer. J. Personalized Med. 2022;12:95. doi: 10.3390/jpm12010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Rosenblum D., Joshi N., Tao W., Karp J.M., Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Puri A., Loomis K., Smith B., Lee J.H., Yavlovich A., Heldman E., Blumenthal R. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit. Rev. Ther. Drug Carrier Syst. 2009;26:523–580. doi: 10.1615/CRITREVTHERDRUGCARRIERSYST.V26.I6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Hua S., Wu S.Y. The use of lipid-based nanocarriers for targeted pain therapies. Front. Pharmacol. 2013;21:143. doi: 10.3389/fphar.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Kraft J.C., Freeling J.P., Wang Z., Ho R.J.Y. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014;103:29–52. doi: 10.1002/JPS.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Lammers T., Kiessling F., Hennink W.E., Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J. Contr. Release. 2012;161:175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 412.Kirpotin D., Drummond D., Shao Y., Shalaby M., Hong K., Nielsen U., Marks J., Benz C., Park J. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 413.Mamot C., Drummond D.C., Noble C.O., Kallab V., Guo Z., Hong K., Kirpotin D.B., Park J.W. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65:11631–11638. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 414.Ma W., Yang Y., Zhu J., Jia W., Zhang T., Liu Z., Chen X., Lin Y. Biomimetic nanoerythrosome-coated aptamer–DNA tetrahedron/maytansine conjugates: pH-responsive and targeted cytotoxicity for HER2-positive breast cancer. Adv. Mater. 2022;34 doi: 10.1002/adma.202109609. [DOI] [PubMed] [Google Scholar]
- 415.Zhang T., Tian T., Lin Y. Functionalizing framework nucleic-acid-based nanostructures for biomedical application. Adv. Mater. 2022;34 doi: 10.1002/adma.202107820. [DOI] [PubMed] [Google Scholar]
- 416.Ma W., Zhan Y., Zhang Y., Shao X., Xie X., Mao C., Cui W., Li Q., Shi J., Li J., Fan C., Lin Y. An intelligent DNA nanorobot with in vitro enhanced protein lysosomal degradation of HER2. Nano Lett. 2019;19:4505–4517. doi: 10.1021/acs.nanolett.9b01320. [DOI] [PubMed] [Google Scholar]
- 417.Cheng R., Liu L., Xiang Y., Lu Y., Deng L., Zhang H., Santos H.A., Cui W. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials. 2020;232 doi: 10.1016/J.BIOMATERIALS.2019.119706. 119706. [DOI] [PubMed] [Google Scholar]
- 418.Ding Y., Li W., Zhang F., Liu Z., Zanjanizadeh Ezazi N., Liu D., Santos H.A. Electrospun fibrous architectures for drug delivery, tissue engineering and cancer therapy. Adv. Funct. Mater. 2019;29 doi: 10.1002/ADFM.201802852. [DOI] [Google Scholar]
- 419.Gao A., li Hu X., Saeed M., fan Chen B., ping Li Y., jun Yu H. Overview of recent advances in liposomal nanoparticle-based cancer immunotherapy. Acta Pharmacol. Sin. 2019;40:1129–1137. doi: 10.1038/s41401-019-0281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Najibi A.J., Mooney D.J. Cell and tissue engineering in lymph nodes for cancer immunotherapy. Adv. Drug Deliv. Rev. 2020;161–162:42–62. doi: 10.1016/J.ADDR.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Irvine D.J., Dane E.L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020;20:321–334. doi: 10.1038/s41577-019-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Chen M., Miao Y., Qian K., Zhou X., Guo L., Qiu Y., Wang R., Gan Y., Zhang X. Detachable liposomes combined immunochemotherapy for enhanced triple-negative breast cancer treatment through reprogramming of tumor-associated macrophages. Nano Lett. 2021;21:6031–6041. doi: 10.1021/acs.nanolett.1c01210. [DOI] [PubMed] [Google Scholar]
- 423.Yang M., Li J., Gu P., Fan X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact. Mater. 2021;6:1973–1987. doi: 10.1016/j.bioactmat.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]