Abstract
Previous work in our laboratory revealed that mice parenterally vaccinated with recombinantly attenuated staphylococcal enterotoxin (SE) or toxic shock syndrome toxin 1 develop protective antibodies against a lethal intraperitoneal (i.p.) toxin challenge. This study investigated the efficacy of nasal and oral immunizations with an SEB vaccine (SEBv) toward an i.p. or mucosal (via an aerosol) toxin challenge. Both vaccination routes, with the immunoadjuvant cholera toxin (CT), elicited comparable SEB-specific immunoglobulin A (IgA) and IgG levels in saliva. Nasal or oral inoculations also generated SEB-specific IgA, IgG, and IgM in the serum, but the nasal route yielded higher specific IgG titers. SEBv alone, when given nasally or orally, did not induce any detectable SEB-specific antibody. Mice vaccinated mucosally were protected against a 50% lethal dose of wild-type SEB given i.p. or mucosally, thus demonstrating that nasal or oral administration of this SEBv, with CT, elicits systemic and mucosal antibodies to SEB that protect against SEB-induced lethal shock.
Staphylococcal enterotoxins (SE), produced by the ubiquitous Staphylococcus aureus, are single-chain, 23- to 29-kDa proteins with potent immunomodulating properties (8, 27). These toxins (SEA to SEI) are commonly associated with a prevalent form of food poisoning and some cases of toxic shock. SE-induced shock is linked to greatly increased levels of proinflammatory cytokines following Vβ-specific stimulation of T lymphocytes. It is still unclear whether SE food poisoning is also associated with elevated cytokine levels, but Vβ8+ T cells found in the gut-associated lymphoid tissue of mice are activated by orally administered SEB (21). Overall, the SE are considered important virulence factors that enable S. aureus to survive and then flourish in various niches (11). Therefore, the SE represent an obvious vaccine target that may be useful for combating S. aureus infections (19), including those attributed to increasingly prevalent strains possessing vancomycin resistance (13).
Previous vaccine studies have shown that mice and nonhuman primates are effectively immunized against a lethal dose of SE (4, 15, 16, 26, 28–30). However, vomiting and/or diarrhea are still evident in orally, intratracheally, or intramuscularly vaccinated primates given an oral or aerosol toxin challenge (5, 15, 26). Repeated oral doses with a formaldehyde toxoid of SEB are not very efficacious against the enteric ill effects of orally given SEB (5). However, oral administration of an emetic or subemetic dose of wild-type SEB provides a temporary resistance that wanes over a week to a subsequent homologous toxin challenge (25). This transient protection is probably not mediated by antibodies, but clonal anergy of Vβ-specific lymphocytes likely plays a role (18). A method for generating potentially efficacious mucosal vaccines for SE involves carboxymethylation of histidines within SEA (22) and SEB (1), which effectively abrogates the enterotoxicity, but not mitogenicity, of these proteins when given orally to nonhuman primates.
This study explores the possibility of nasally and orally immunizing mice with a recombinantly attenuated SEB vaccine (SEBv) (28), with and without a potent mucosal adjuvant like cholera toxin (CT) (10). SEB-specific antibodies in the saliva and sera were detected by an enzyme-linked immunosorbent assay (ELISA), and the mice were finally challenged intraperitoneally (i.p.) or mucosally (via aerosol) with a lethal dose of wild-type SEB.
MATERIALS AND METHODS
Reagents.
Recombinantly attenuated SEBv was produced as described previously (28). The vaccine differed from wild-type toxin at residues 45 (leucine changed to arginine), 89 (tyrosine changed to alanine), and 94 (tyrosine changed to alanine), which prevents SEB binding to major histocompatibility complex II but maintains proper protein folding and antigenicity. CT and alum were purchased from List Biological Laboratories (Campbell, Calif.) and Pierce Chemical (Rockford, Ill.), respectively. Purified SEB was obtained from Toxin Technology (Sarasota, Fla.), and Escherichia coli O55:B5 lipopolysaccharide (LPS) was purchased from Difco Laboratories (Detroit, Mich.). All reagents were diluted in sterile, endotoxin-free phosphate-buffered saline, pH 7.4 (PBS).
Vaccinations and toxin challenge.
BALB/c mice (18 to 22 g) were purchased from the National Cancer Institute (Frederick, Md.) and housed in a pathogen-free environment. Preimmune sera, collected from the tail vein, and saliva, collected in a caraway tube (Fisher Scientific, Pittsburgh, Pa.) following an i.p. injection (5 mg/kg of body weight) of pilocarpine (Sigma, St. Louis, Mo.), were obtained from each animal before vaccination. Mice were anesthetized with a ketamine (2.4 mg/kg)-acepromazine (0.024 mg/kg)-xylazine (0.27 mg/kg) mixture before nasal or oral inoculations (30 μl/dose) of SEBv with or without CT (5 μg nasally or 10 μg orally). Additional controls were given CT alone. Mice were also vaccinated with SEBv plus alum or alum alone (200 μl/i.p. dose). All groups received three vaccinations administered every 2 weeks. Sera and saliva were collected 1 week after the final immunization, and mice were then challenged 3 days later with a lethal mucosal (115 to 121 μg ∼7 to 8 50% lethal doses [LD50]) or i.p. (7.5 to 10 μg ∼25 to 30 LD50) dose of SEB and a potentiating amount of LPS (75 μg) administered i.p. (14, 23, 29, 30). SEB was administered mucosally via an aerosol generated by a Collison nebulizer (BGI Inc., Waltham, Mass.) in a temperature- and humidity-controlled, nose-only chamber (14). An independent-samples t test (SPSS/PC+; SPSS, Chicago, Ill.) was used to compare significant differences (P < 0.05) of survival between vaccinated groups and the appropriate adjuvant-only controls.
ELISA.
The serum or saliva samples from commonly vaccinated mice were pooled, and anti-SEB titers of each group were determined by an ELISA. Seroconversion of each animal was also tested by adsorbing 1 μg of SEB/ml of carbonate buffer (pH 9.6) onto Immulon II microtiter plates (Dynatech Laboratories, Chantilly, Va.). After overnight incubation at 4°C, plates were blocked for 1 h at 37°C with 3% skim milk in PBS. Serum or saliva samples were diluted in PBS containing 0.1% Tween 20 (PBST) plus 1% milk (PBSTM) and added to aspirated wells for 1 h at 37°C. Wells were again aspirated and then washed with PBST, and goat anti-mouse immunoglobulin A (IgA) or sheep anti-mouse IgG (Sigma) was diluted in PBSTM and added for 1 h at 37°C. Wells were finally washed with PBST and incubated with p-nitrophenyl phosphate substrate for 30 min (serum samples) or overnight (saliva samples) at room temperature. Data represent the mean absorbance (405 nm) of triplicate readings ± standard deviation.
SEB-specific antibodies were isotyped by an ELISA with a panel of rabbit anti-mouse immunoglobulin sera (Bio-Rad Laboratories, Hercules, Calif.) as described by the manufacturer. Results are presented as the mean reading of duplicate wells (± 10% deviation) minus the mean of negative control wells containing no sera but all other reagents.
RESULTS
SEBv inoculations and seroconversion.
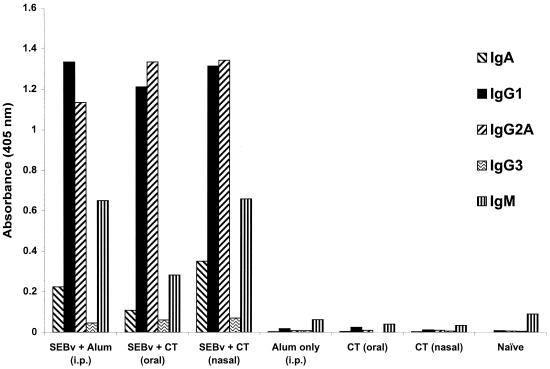
Earlier studies clearly demonstrate that antibodies elicited by parenterally administered vaccines for SEB, or premixing of SEB-specific antisera with toxin before injection into naive mice, protect animals against SEB-induced lethal shock (28–30). To extend these findings, we first determined if three nasal or oral doses of SEBv (20 or 50 μg each), without CT adjuvant, could effectively elicit SEB-specific antibodies. None of these animals (five per group) developed SEB-specific antibodies in their serum or saliva and were subsequently not protected against a lethal i.p. challenge of SEB. In contrast, there was SEB-specific IgA, IgG1, IgG2a, and IgM in sera after three inoculations (20 μg of SEBv each) given i.p. with alum, or nasally and orally with CT (Fig. 1). Control animals that received nasal or oral doses of CT alone, or alum i.p., did not develop SEB-specific antibodies.
FIG. 1.
Isotyping of SEB-specific antibodies in pooled sera after three vaccinations of SEBv given nasally, orally, or i.p. Mice were given 20 μg of SEBv and 5 or 10 μg of CT (nasal and oral routes, respectively) or alum (i.p.) per dose. Controls received adjuvant without SEBv by the same inoculation routes. Sera were pooled within each group (n = 10 mice) and diluted 1:100. Data represent the mean absorbance of duplicate wells ± 10%.
Additional studies determined the anti-SEB serum titers (IgG) of vaccine groups administered two and three mucosal doses consisting of 2.5 or 20 μg of SEBv plus CT (Fig. 2). SEB-specific IgG levels noticeably increased in each group following three (Fig. 2B), versus two (Fig. 2A), vaccinations. Either dose of SEBv plus CT given nasally, after two or three inoculations, elicited higher serum IgG titers than doses given by the oral or i.p. route. There was no evidence of SEB-specific IgG after two oral doses of 2.5 μg of SEBv plus CT, and neither route elicited detectable levels of SEB-specific IgA, IgG, or IgM in sera or saliva after a single vaccination. Mice (eight per group) vaccinated nasally or orally with 0.625 μg of SEBv plus CT did not develop SEB-specific antibodies in serum or saliva after three inoculations (data not shown), which established that the minimal dose of mucosally administered SEBv necessary for generating toxin-specific antibodies was between 2.5 and 0.625 μg.
FIG. 2.
Comparison of serum anti-SEB IgG titers among nasally and orally vaccinated mice after two (A) and three (B) inoculations with 2.5 or 20 μg of SEBv plus CT. Pooled sera from commonly immunized mice (n = 10 per group) and control animals (CT oral shown, although CT nasal and alum i.p.-only controls also resulted in similar data) were diluted serially (1:50 to 1:156,250). Data represent the mean absorbance of triplicate wells ± standard deviation.
In addition to the pooled sera data (Fig. 2), serum from each mouse was also diluted 1:100 and tested for anti-SEB IgG after two and three vaccinations (Table 1). There were a higher percentage of seropositive mice following two or three nasal, versus oral, inoculations at either SEBv dose. Particularly striking, after two doses of SEBv (2.5 μg) plus CT, was the different seroconversion rates among nasal (77%) and oral (0%) vaccinees.
TABLE 1.
Seroconversion (anti-SEB IgG) following two and three nasal or oral vaccinations with SEBv + CT
| Vaccination | % Seroconversiona
|
|
|---|---|---|
| 2 vaccinations | 3 vaccinations | |
| Oral | ||
| 2.5 μg of SEBv + CT (n = 10) | 0 | 30 |
| 20 μg of SEBv + CT (n = 10) | 20 | 80 |
| Nasal | ||
| 2.5 μg of SEBv + CT (n = 9) | 77 | 100 |
| 20 μg of SEBv + CT (n = 10) | 60 | 100 |
The serum from each mouse was collected 7 days after the second and third vaccinations, diluted 1:100, and tested by an ELISA for SEB-specific IgG. Sera were considered positive if the mean A405 of triplicate wells was at least 10-fold greater than the mean A405 for preimmune sera from the same animal.
SEB-specific antibodies were also detected within the pooled saliva from nasal and oral vaccinees after three (Fig. 3), but not two (data not shown), inoculations. Only the nasally and orally vaccinated mice developed SEB-specific IgA and IgG in their saliva, unlike animals injected i.p. with 20 μg of SEBv plus alum. However, the anti-SEB titers found in the saliva of nasally and orally vaccinated mice were low and undetectable beyond a 1:10 dilution.
FIG. 3.
Comparison of anti-SEB-specific IgA and IgG in salivary secretions among nasally and orally vaccinated mice given three inoculations with 2.5 or 20 μg of SEBv plus CT. Pooled saliva from each vaccine group (n = 10 mice) and controls given CT was diluted 1:5. Data represent the mean absorbance ± standard deviation of triplicate wells.
Toxin challenge studies.
After determining that mice given SEBv plus CT nasally or orally developed systemic and mucosal antibodies toward SEB, animals vaccinated three times were then challenged with a lethal i.p. or mucosal (aerosolized) dose of toxin (Table 2). The percent survival among i.p. or mucosally challenged mice was comparable for groups previously vaccinated i.p. (SEBv plus alum) or nasally (SEBv plus CT), yet these results were significantly different from those for the alum or CT (nasal) controls. In contrast, mice inoculated orally (20 or 2.5 μg of SEBv plus CT) were not protected against an i.p. toxin challenge, as evidenced by statistical comparisons with the 10 μg of CT (oral) control group. However, oral vaccinations were statistically effective toward a mucosal SEB challenge. Overall, these results demonstrate that this SEBv is efficacious when given mucosally and that the nasal vaccination route provided better seroconversion rates and protection against a lethal toxin challenge than did the oral route.
TABLE 2.
Protection among vaccinated mice against an i.p. or mucosal SEB challenge
| Vaccination dose (route) | No. survived/no. challengeda (% survival)
|
|
|---|---|---|
| i.p. challenge | Mucosal challenge | |
| 20 μg of SEBv + alum (i.p.) | 10/10 (100)b | 8/8 (100)b |
| 10 μg of SEBv + alum (i.p.) | ND | 5/8 (63)b |
| 5 μg of SEBv + alum (i.p.) | 10/10 (100)b | ND |
| Alum only (i.p.) | 0/10 (0) | 0/12 (0) |
| 20 μg of SEBv + 10 μg of CT (oral) | 3/8 (38)c | 6/8 (75)d |
| 10 μg of SEBv + 10 μg of CT (oral) | ND | 5/6 (83)d |
| 2.5 μg of SEBv + 10 μg of CT (oral) | 1/8 (13)c | ND |
| 10 μg of CT (oral) | 0/7 (0) | 0/12 (0) |
| 20 μg of SEBv + 5 μg of CT (nasal) | 8/8 (100)e | 8/8 (100)e |
| 10 μg of SEBv + 5 μg of CT (nasal) | ND | 7/7 (100)e |
| 2.5 μg of SEBv + 5 μg of CT (nasal) | 6/7 (86)e | ND |
| 5 μg of CT (nasal) | 0/6 (0) | 1/10 (10) |
| None | ND | 0/11 (0) |
The i.p. challenge consisted of 25 to 30 LD50 of SEB; the mucosal challenge consisted of 7 to 8 LD50 of aerosolized SEB. ND, not determined.
Significantly different (P < 0.05) from alum-only control.
Not significantly different (P > 0.05) from 10 μg of CT (oral) control.
Significantly different (P < 0.05) from 10 μg of CT (oral) control.
Significantly different (P < 0.05) from 5 μg of CT (nasal) control.
DISCUSSION
Recombinant SEA (4), SEB (29, 30), or toxic shock syndrome toxin 1 (24) vaccines, when given parenterally to mice using the model described in this study (23), have proven quite efficacious against an i.p. challenge with homologous toxin. Intramuscular vaccinations of nonhuman primates with SEBv plus alum confirm our previous murine findings that vaccinated animals develop SEB-specific antibody and are protected against SEB-induced lethal shock (28). However, all previous nonhuman primate studies suggest that a better vaccine, and/or delivery system, is necessary to prevent the enteric ill effects of mucosally administered SEB (5, 15, 26). Although mice lack an emetic reflex, these animals afford a reasonable model to (i) investigate whether a target antigen given mucosally elicits an antibody response and (ii) determine if there is protection against the lethal effects of wild-type toxin given mucosally (via aerosol) or parenterally (i.p.). At this time, our study is the first to use a recombinantly attenuated SE as a mucosal vaccine in any animal model.
Historically, an attempt to abrogate the enteric ill effects of SEB in nonhuman primates was first reported by Bergdoll (5). However, repeated oral doses of formaldehyde-inactivated SEB toxoid provide limited protection against SEB-induced emesis. Evidently, there was no attempt to determine the presence of SEB-specific antibodies in serum or at various mucosal sites. These disappointing findings may be linked to inefficient processing and/or presentation of formaldehyde-treated proteins by antigen-presenting cells (9). Furthermore, inactivation of pertussis toxin by formaldehyde significantly diminishes mucosal immunogenicity versus a recombinantly attenuated version of this molecule (7). It is possible that the SE, like pertussis toxin, do not represent optimal mucosal immunogens after formaldehyde treatment.
In slight contrast to the results for oral dosing, subcutaneous vaccinations of nonhuman primates with the same SEB toxoid adsorbed onto alum are more effective at preventing emesis, but neither vaccination route unequivocally prevents SEB-induced emesis (5). Additionally, early SE vaccine studies must be carefully interpreted, as “homogeneous” toxin preparations were relatively impure and likely contaminated with other enterotoxins and/or biologically active proteins. More recent vaccine studies in nonhuman primates have used a highly purified SEB toxoid (formaldehyde inactivated) incorporated into meningococcal proteosomes with and without alum (15) or microspheres (26). SEB toxoid with proteosomes (no alum) or microspheres, when given orally or intratracheally to nonhuman primates, effectively elicits systemic and mucosal antibodies toward SEB that prevent lethal shock following an aerosolized toxin challenge. Intramuscular injections of a SEB toxoid-proteosome-alum mixture generates systemic, but not mucosal, antibodies toward SEB that also protect against SEB-induced lethal shock (15). However, with any of these SEB toxoid preparations given via different routes, enteric effects like emesis and/or diarrhea are still evident in vaccinated nonhuman primates after an aerosol SEB challenge.
In addition to their nonhuman primate study (15), Lowell et al. (16) nasally vaccinated mice using SEB toxoid with and without meningococcal proteosomes. Although their results reveal very low levels of SEB-specific antibody in the sera, lungs, and intestines of mice vaccinated twice with toxoid alone (50- or 100-μg dose), we did not detect any SEB-specific antibody among animals nasally vaccinated three times with 20 or 50 μg of SEBv alone. A possible explanation may be antigen aggregation after chemical (formaldehyde) inactivation of SEB, which may fortuitously generate a more immunogenic target for the mucosal immune system. We did test a heat-generated aggregate of SEBv as an intranasal antigen (50 μg/dose), but there were no detectable SEB-specific antibodies, with or without CT adjuvant, after three inoculations (B. G. Stiles, unpublished data). Potential epitopes on the SEBv molecule were likely destroyed during the heating process. Additionally, the results from Lowell et al. (16) reveal only 40 and 53% survival among mice immunized nasally with proteosomes containing SEB toxoid (50 to 100 μg) and respectively challenged mucosally and intramuscularly with homologous toxin. In contrast, our studies showed that nasally administered SEBv (20 μg) plus CT (5 μg) was 100% protective against an i.p. or mucosal toxin challenge. However, results from this study or that of Lowell et al. (16) clearly show no protective efficacy toward SEB among mice mucosally vaccinated with SEBv or SEB toxoid without CT or proteosomes, respectively. These data strongly suggest the importance of discovering a new adjuvant, or antigen display vehicle, to effectively stimulate mucosal immunity toward SEB and other SE.
The efficacy of various recombinant SE vaccines administered parenterally, and the protective effects of toxin-specific antibodies in serum, have been clearly established using an i.p. challenge model in mice (4, 29, 30). For the present study, vaccinated mice were also challenged with mucosally administered SEB (14). We found that mice injected i.p. with 20 μg of SEBv plus alum, which had no detectable SEB-specific IgA or IgG in their saliva, were protected against a lethal mucosal challenge with SEB. In contrast, there was SEB-specific IgA and IgG in the saliva of nasally or orally vaccinated mice as well as SEB-specific IgA, IgG1, IgG2a, and IgM in the sera. Like the results from previous SE vaccine studies in mice with parenteral inoculations and a subsequent i.p. toxin challenge (4, 29, 30), systemic antibodies alone afforded enough protection against a lethal mucosal dose of SEB in this mouse model.
In addition to the demonstrated efficacy of SE vaccines toward a lethal toxin challenge, subcutaneous injections of mice with recombinantly attenuated SEA also afford protection from S. aureus infections (19). The SE represent an ideal vaccine target, as they are important virulence factors that down regulate the immune system via anergy of specific T-cell populations (11). These results are also very timely, as there are increasing concerns regarding the emergence of vancomycin-resistant strains of S. aureus (13) and exacerbation of influenza symptoms by the SE (17), which further imperil the elderly and immunocompromised.
Mucosal vaccines for the SE are also logical, as S. aureus actively colonizes various mucosal sites, including the gastrointestinal tract. A recent clinical report by Gravet et al. (12) shows that a significant number of patients with antibiotic-associated diarrhea suffer from SEA-producing strains of S. aureus that colonize the gut mucosa. Mucosal vaccination with a recombinantly attenuated SEA molecule may afford enteric relief, especially among patients with recurring diarrheic episodes. Recent data from our laboratory reveal that a recombinant SEA vaccine, when given nasally or orally with CT, elicits systemic antibodies to SEA and protection against an i.p. toxin challenge (Stiles, unpublished data).
Finally, the use of CT as a mucosal adjuvant for humans has not been approved by the Food and Drug Administration. However, CT represents the “gold standard” for mucosal immunoadjuvants and enables experimental testing of the immunogenic and protective potential of any mucosally administered antigen (10). Various laboratories are attempting to recombinantly diminish the enterotoxic effects of CT (31), or the closely related E. coli heat-labile enterotoxin (6), and still retain immunostimulatory properties. For our study, we simply sought to determine if mucosally administered SEBv was sufficiently potent to elicit protective antibodies, with or without CT as an adjuvant. Another adjuvant like CT, with similar immunopotentiating properties but fewer side effects, or perhaps chimeric virus-like particles that are naturally enterotropic (2, 3, 20) and fused to a recombinant SE vaccine, may provide better protection against the systemic and enteric ill effects of these toxins. This laboratory is currently exploring various methods to more effectively present the SEBv, and other SE vaccines, to the mucosal immune system.
ACKNOWLEDGMENTS
The technical expertise of Yvette Campbell and Christina Gargan was much appreciated with the animal studies and ELISAs. Afroz Sultana and Beverly Dyas were invaluable for providing a continual supply of purified SEBv for these, and many other, studies. Kathy Kenyon's insightful editorial comments were instrumental during the final stages of the manuscript. Many thanks are again extended to Sara A. Grove of Shippensburg University (Center for Applied Research and Policy Analysis) for timely statistical analysis.
REFERENCES
- 1.Alber G, Hammer D K, Fleischer B. Relationship between enterotoxic- and T lymphocyte-stimulating activity of staphylococcal enterotoxin B. J Immunol. 1990;144:4501–4506. [PubMed] [Google Scholar]
- 2.Ball J M, Graham D Y, Opekun A R, Gilger M A, Guerrero R A, Estes M K. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology. 1999;117:40–48. doi: 10.1016/s0016-5085(99)70548-2. [DOI] [PubMed] [Google Scholar]
- 3.Ball J M, Hardy M E, Atmar R L, Conner M E, Estes M K. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J Virol. 1998;72:1345–1353. doi: 10.1128/jvi.72.2.1345-1353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bavari S, Dyas B, Ulrich R G. Superantigen vaccines: a comparative study of genetically attenuated receptor-binding mutants of staphylococcal enterotoxin A. J Infect Dis. 1996;174:338–345. doi: 10.1093/infdis/174.2.338. [DOI] [PubMed] [Google Scholar]
- 5.Bergdoll M S. Immunization of rhesus monkeys with enterotoxoid B. J Infect Dis. 1966;116:191–196. doi: 10.1093/infdis/116.2.191. [DOI] [PubMed] [Google Scholar]
- 6.Cheng E, Cardenas-Freytag L, Clements J D. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT) Vaccine. 2000;18:38–49. doi: 10.1016/s0264-410x(99)00168-1. [DOI] [PubMed] [Google Scholar]
- 7.Cropley I, Douce G, Roberts M, Chatfield S, Pizza M, Marsili I, Rappuoli R, Dougan G. Mucosal and systemic immunogenicity of a recombinant, non-ADP-ribosylating pertussis toxin: effects of formaldehyde treatment. Vaccine. 1995;13:1643–1648. doi: 10.1016/0264-410x(95)00134-m. [DOI] [PubMed] [Google Scholar]
- 8.Dinges M M, Orwin P M, Schlievert P M. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Tommaso A, De Magistris M T, Bugnoli M, Marsili I, Rappuoli R, Abrignani S. Formaldehyde treatment of proteins can constrain presentation to T cells by limiting antigen processing. Infect Immun. 1994;62:1830–1834. doi: 10.1128/iai.62.5.1830-1834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elson C. Cholera toxin as a mucosal adjuvant. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. San Diego, Calif: Academic Press; 1996. pp. 59–72. [Google Scholar]
- 11.Fleischer B. Superantigens produced by infectious pathogens: molecular mechanism of action and biological significance. Int J Clin Lab Res. 1994;24:193–197. doi: 10.1007/BF02592461. [DOI] [PubMed] [Google Scholar]
- 12.Gravet A, Rondeau M, Harf-Monteil C, Grunenberger F, Monteil H, Scheftel J-M, Prevost G. Predominant Staphylococcus aureus isolated from antibiotic-associated diarrhea is clinically relevant and produces enterotoxin A and the bicomponent toxin LukE-LukD. J Clin Microbiol. 1999;37:4012–4019. doi: 10.1128/jcm.37.12.4012-4019.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiramatsu K. Reduced susceptibility of Staphylococcus aureus to vancomycin-Japan, 1996. Morbid Mortal Weekly Rep. 1997;46:624–626. [PubMed] [Google Scholar]
- 14.LeClaire R D, Hunt R E, Bavari S, Estep J E, Nelson G O, Wilhelmsen C L. Potentiation of inhaled staphylococcal enterotoxin B-induced toxicity by lipopolysaccharide in mice. Toxicol Pathol. 1996;24:619–626. doi: 10.1177/019262339602400513. [DOI] [PubMed] [Google Scholar]
- 15.Lowell G H, Colleton C, Frost D, Kaminiski R W, Hughes M, Hatch J, Hooper C, Estep J, Pitt L, Topper M, Hunt R E, Baker W, Baze W B. Immunogenicity and efficacy against lethal aerosol staphylococcal enterotoxin B challenge in monkeys by intramuscular and respiratory delivery of proteosome-toxoid vaccines. Infect Immun. 1996;64:4686–4693. doi: 10.1128/iai.64.11.4686-4693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowell G H, Kaminski R W, Grate S, Hunt R E, Charney C, Zimmer S, Colleton C. Intranasal and intramuscular proteosome-staphylococcal enterotoxin B (SEB) toxoid vaccines: immunogenicity and efficacy against lethal SEB intoxication in mice. Infect Immun. 1996;64:1706–1713. doi: 10.1128/iai.64.5.1706-1713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald K L, Osterholm M T, Hedberg C W, Schrock C G, Peterson G F, Jentzen J M, Leonard S A, Schlievert P M. Toxic shock syndrome. A newly recognized complication of influenza and influenza-like illness. JAMA. 1987;257:1053–1058. doi: 10.1001/jama.257.8.1053. [DOI] [PubMed] [Google Scholar]
- 18.Migata K, Ochi A. Induction of clonal anergy by oral administration of staphylococcal enterotoxin B. Eur J Immunol. 1994;24:2081–2086. doi: 10.1002/eji.1830240922. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson I-M, Verdrengh M, Ulrich R G, Bavari S, Tarkowski A. Protection against Staphylococcus aureus sepsis by vaccination with recombinant staphylococcal enterotoxin A devoid of superantigenicity. J Infect Dis. 1999;180:1370–1373. doi: 10.1086/315023. [DOI] [PubMed] [Google Scholar]
- 20.O'Neal C M, Crawford S E, Estes M K, Conner M E. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707–8717. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiekermann G M, Nagler-Anderson C. Oral administration of the bacterial superantigen staphylococcal enterotoxin B induces activation and cytokine production by T cells in murine gut-associated lymphoid tissue. J Immunol. 1998;161:5825–5831. [PubMed] [Google Scholar]
- 22.Stelma G N, Bergdoll M S. Inactivation of staphylococcal enterotoxin A by chemical modification. Biochem Biophys Res Commun. 1982;105:121–126. doi: 10.1016/s0006-291x(82)80019-3. [DOI] [PubMed] [Google Scholar]
- 23.Stiles B G, Bavari S, Krakauer T, Ulrich R G. Toxicity of staphylococcal enterotoxin potentiated by lipopolysaccharide: major histocompatibility complex class II molecule dependency and cytokine release. Infect Immun. 1993;61:5333–5338. doi: 10.1128/iai.61.12.5333-5338.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stiles B G, Krakauer T, Bonventre P F. Biological activity of toxic shock syndrome toxin 1 and a site-directed mutant, H135A, in a lipopolysaccharide-potentiated mouse lethality model. Infect Immun. 1995;63:1229–1234. doi: 10.1128/iai.63.4.1229-1234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiyama H, Bergdoll M S, Dack G M. Early development of a temporary resistance to the emetic action of staphylococcal enterotoxin. J Infect Dis. 1962;111:233–238. doi: 10.1093/infdis/111.3.233. [DOI] [PubMed] [Google Scholar]
- 26.Tseng J, Komisar J L, Trout R N, Hunt R E, Chen J Y-J, Johnson A J, Pitt L, Ruble D L. Humoral immunity to aerosolized staphylococcal enterotoxin B (SEB), a superantigen, in monkeys vaccinated with SEB toxoid-containing microspheres. Infect Immun. 1995;63:2880–2885. doi: 10.1128/iai.63.8.2880-2885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulrich R G. Evolving superantigens of Staphylococcus aureus. FEMS Immunol Med Microbiol. 2000;27:1–7. doi: 10.1111/j.1574-695X.2000.tb01404.x. [DOI] [PubMed] [Google Scholar]
- 28.Ulrich R G, Olson M A, Bavari S. Development of engineered vaccines effective against structurally related bacterial superantigens. Vaccine. 1998;16:1857–1864. doi: 10.1016/s0264-410x(98)00176-5. [DOI] [PubMed] [Google Scholar]
- 29.Woody M A, Krakauer T, Stiles B G. Staphylococcal enterotoxin B mutants (N23K and F44S): biological effects and vaccine potential in a mouse model. Vaccine. 1997;15:133–139. doi: 10.1016/s0264-410x(96)00166-1. [DOI] [PubMed] [Google Scholar]
- 30.Woody M A, Krakauer T, Ulrich R G, Stiles B G. Differential immune responses to staphylococcal enterotoxin B mutations in a hydrophobic loop dominating the interface with major histocompatibility complex class II receptors. J Infect Dis. 1998;177:1013–1022. doi: 10.1086/515250. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto S, Takeda Y, Yamamoto M, Kurazono H, Imaoka K, Yamamoto M, Fujihashi K, Noda M, Kiyono H, McGhee J R. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J Exp Med. 1997;185:1203–1210. doi: 10.1084/jem.185.7.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]