Abstract
Background
Delirium is an acute neuropsychiatric condition associated with unfavourable outcomes, frequent in older hospitalized people. In the context of the SARS-CoV-2 pandemic, few studies have specifically focused on the inflammatory status of older, frail patients with hyperactive delirium (HD) hospitalized for COVID-19.
Aim
To identify biological correlates of HD at hospital admission and to assess the independent effect of delirium and physical frailty on in-hospital mortality.
Methods
Data were retrospectively extracted by the multicenter registry GeroCovid Observational Study. Individuals aged ≥ 60 years were included if the information on the presence of HD, frailty based on the modified Fried criteria and inflammatory status had been collected. The risk of mortality was evaluated using a Kaplan–Meier estimator, according to frailty and delirium. Logistic and restricted cubic-spline regressions were employed to assess the relationship between inflammatory markers and HD.
Results
Three-hundred-thirty-seven older adults were included in the analysis [mean age (SD) 77.1 (9.5) years, 50.1% females], and 11.5% presented with HD. A significant association of both PaO2/FiO2 ratio (p = 0.015) and serum lactate dehydrogenase (p = 0.04) with delirium was observed. By Cox multivariable regression, frail and non-frail patients with HD had a 4.42 and 2.85 higher mortality risk compared with non-frail, non-delirious patients.
Conclusions
Hyperactive delirium at hospital admission is related with markers of lung failure among older adults, especially when physical frailty coexists. Delirium is associated with increased in-hospital mortality risk, which is doubled by the coexistence of physical frailty.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40520-022-02328-0.
Keywords: COVID-19, Delirium, Inflammation, Frailty, Aging
Introduction
After the identification, in China at the end of 2019, of the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) as the aetiologic agent of a new type of viral pneumonia, the World Health Organization (WHO) declared the unprecedented COronaVIrus Disease 2019 (COVID-19) a pandemic on March 11, 2020 [1]. The disease suddenly and unpredictably evolved, causing a catastrophe in terms of mortality and morbidity, especially among older subjects, with direct and indirect effects worldwide, difficult to estimate [2].
Delirium is a neuropsychiatric syndrome characterized by a disorder of attention and other cognitive functions, with acute onset and fluctuating course, which is usually triggered by acute illness, surgery, drugs and environmental factors [3]. Despite being often underestimated, especially in its hypoactive form, overall prevalence in hospital setting exceeds 20% of older subjects, and is even higher in intensive care units (ICU), proportional to the severity of illness [4, 5]. Previous studies reported a prevalence of delirium between 20 and 30% among patients hospitalized for COVID-19, but these results may underestimate the real data due to the challenges of performing an adequate assessment of this condition at admission [6]. Systemic inflammation and oxidative stress, together with the hypoxemia caused by the “COVID-19 cytokine-storm” [7], could represent triggers for delirium onset, particularly in frail patients. Most of the studies which have evaluated the presence of delirium in COVID-19 patients did not report a distinction between the different phenotypes of this multifaceted condition. Particularly, to the best of our knowledge, there is no evidence assessing hyperactive delirium, probably because it is more susceptible to sedative treatments and the acute phase of the disease. Furthermore, there is a lack of evidence focusing on the evaluation of both outcomes and clinical or biochemical correlates in patients affected by hyperactive delirium according to physical frailty status.
Following these premises, the main hypothesis underlying the present research is that SARS-CoV-2 patients developing delirium suffer from more severe disease, increased inflammatory status, and greater comorbidities burden. Accordingly, the primary aim of the study is to describe the clinical features of hospitalized COVID-19 older patients experiencing hyperactive delirium at disease onset; furthermore, we will focus on both clinical and biochemical profile and outcomes of these patients according to their physical frailty status.
Methods
Study population
The multicenter registry GeroCovid Observational Study (ClinicalTrials.gov registration: NCT04379440) endorsed by the Italian Society of Gerontology and Geriatrics (SIGG) and the Norwegian Geriatrics Society was intended to investigate the SARS-CoV-2 infection in older patients [8]. Patients aged ≥ 60 years from a different setting of care were retrospectively and prospectively included since March 1st, 2020. Enrollment ended on December 31st, 2020. Local Ethical Committees approved the participation of 66 investigational sites in the Registry. For the present study, we considered the patients enrolled during the first wave of the COVID-19 pandemic within the 19 centers participating in the “GeroCovid acute wards” cohort of the registry, comprising first-time hospitalized older adults with positive diagnostic tests for SARS-CoV-2 infection (nasopharyngeal swab and/or serological tests). As delirium at admission and inflammatory markers were not available for all subjects included in the protocol, we selected a sub-sample including all the GeroCovid Observational Centers that reported a common minimum data set of variables such as: (a) main comorbidities; (b) physical frailty and delirium assessment at admission, c) inflammatory markers (hs-CRP, LDH and PaO2/FiO2 ratio) (see Supplemental Fig. S1). Participants’ clinical status at hospitalization and discharge were divided into five-clinical categories ordinal scale referred to hospitalized patients according to the classification recommended by the WHO R&D Blueprint expert group [9], which has already been used in several studies in COVID-19 patients [10–12]: (1) patients not requiring oxygen therapy; (2) patients requiring oxygen by mask or nasal prongs; (3) with high-flow oxygen or non-invasive ventilation (HF/NIV); (4) needing intubation and mechanical ventilation; (5) death. The severity of respiratory failure was assessed by calculating the PaO2/FIO2 ratio [13] (i.e., partial pressure arterial oxygen/fraction of inspired oxygen ratio) of the first arterial blood gas analysis performed at ward admission (in the case of using Venturi mask, the FIO2 indicated in the swivel connector was utilized). All patients (or their legal caregivers) gave their informed consent to participate in the study.
Delirium and physical frailty assessment
Demographics, comorbidity, clinical and laboratory data were collected for all patients, including a comprehensive geriatric assessment (CGA). Given the complexity of assessing physical performance tests (lack of dynamometers and enough space/time to assess walking speed) in the acute COVID-19 wards during the first and second pandemic waves, physical frailty status was reported according to the modified Fried Criteria [14]. More in depth, we assessed the criteria that do not require performance measures (weight loss, exhaustion, and low activity). Unintentional weight loss was defined as a reduction in weight more than 4.5 kg in the past 12 months. Exhaustion was defined as a feeling of needing the effort to do everything and was considered present if the participant reported it for more than 3 or 4 days in the last week. Reduced physical activity was defined as having performed less than 2–4 h of light exercise per week. Physical frailty was defined by the presence of ≥ 2 criteria of an anamnestic frailty phenotype (AFP). The validity and reliability of AFP were already confirmed in the previous paper [14]. The presence of hyperactive delirium at admission was clinically diagnosed and recorded as a categorical variable (yes/no). In particular, patients were considered delirious if they showed increased motor activity, restlessness, agitation, aggression, wandering, hyper alertness, hallucinations and delusions, and inappropriate behaviour [15].
Inflammatory status
Inflammatory markers including White Blood Cell (WBC) count, high-sensitivity C-reactive protein (hs-CRP) levels, serum D-dimer and LDH at hospital admission were extracted from the E-Registry. Neutrophils/Lymphocyte ratio (NLR) at admission was used as a marker of systemic inflammation, as previously reported in COVID-19 [16]. Gas exchange impairment was evaluated using arterial partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2) at admission [17].
Statistical analysis
Continuous variables were expressed as mean and standard deviation or, for non-normally skewed distributions, as median and interquartile (IQR); categorical data were expressed as absolute observation and percentage. The normality of distribution was assessed for each variable using Shapiro’s test. Comparisons were performed with Student T-test or Kruskal–Wallis test for continuous variables, as appropriate, and with χ2 test for categorical variables. A graphical approach was used to fit a non-linear curve smoothing across five quintiles (knots 0.2, 0.4, 0.6, 0.8) of the continuous variables for NLR, LDH, P/F ratio and Hs-CRP, applying the restricted cubic-spline logistic regression in the case of non-linear associations and logistic regression for the linear association. Multiple adjustments were performed for both the linear and non-linear regression accounting for age, sex, history of heart failure, diabetes type 1 or type 2, Chronic Obstructive Pulmonary Disease (COPD), Chronic Renal Failure, history of stroke, obesity and WHO severity status. (R-packages: “splines”, “ggplot2”, “survival” and “mgcv). The in-hospital mortality risk was evaluated using a Kaplan–Meier estimator. After checking the Cox proportional hazards assumption using Schöenfeld residuals, the hazard ratio (HR) and 95% confidence interval (95% CI) of mortality was calculated for four categories, according to the presence of AFP and delirium or not (FD: frail with delirium; FND: Frail not-delirious, NFD: Not frail with delirium, NFND: Not frail not-delirious) using the NFND category as a reference. Hence, we evaluated the univariable analysis between frailty/delirium categories and mortality. The first model was then adjusted for demographic factors (age, sex). Model 2 was additionally adjusted for comorbidities, including a history of heart failure, diabetes type 1 or type 2, Chronic Obstructive Pulmonary Disease (COPD), Chronic Renal Failure, history of stroke, obesity. All analyses were performed using RStudio software, Version 4.1.0 (RStudio, Inc., Boston, MA, USA) and statistical significance was considered for p < 0.05, with False Discovery Rate (FDR) adjusted p-values also reported.
Results
Baseline clinical characteristics of the study population
Table 1 lists the characteristics of 337 patients included in the study. The mean age (SD) was 77.1 (9.5) years; 169 (50.1%) were females. More than two-third (68.3%) of the study population had hypertension, 36.4% had a history of cardiomyopathy, 25.3% were affected by diabetes. At hospital admission, fever, dyspnea and cough resulted the three most common symptoms (68.7%, 57.4% and 49.0%, respectively). Compared to the 471 patients excluded due to missing data, enrolled participants were younger [77.1 (9.5) vs. 78.5 (9.1), p = 0.03], with higher prevalence of fever, dyspnea and in-hospital mortality (Supplemental Table S1).
Table 1.
Sample features according to the presence of hyperactive delirium at admission
| All patients | Hyperactive delirium | Controls | p-value | |
|---|---|---|---|---|
| N = 337 | N = 39 | N = 298 | ||
| Gender (F) | 169 (50.1) | 21 (53.8) | 148 (49.6) | 0.628 |
| Age (years) | 77.1 (9.5) | 84.2 (7.8) | 76.2 (9.3) | < 0.001 |
| Fever | 231 (68.7) | 30 (76.6) | 201 (67.6) | 0.362 |
| Cough | 162 (49) | 20 (52.6) | 142 (48.6) | 0.643 |
| Dyspnea | 193 (57.4) | 30 (76.9) | 163 (54.8) | 0.008 |
| Physical frailty | 129 (38.3) | 19 (48.7) | 110 (36.9) | 0.15 |
| Cognitive impairment | 62 (18.5) | 28 (73.6) | 34 (11.4) | < 0.001 |
| Diuresis contraction | 13 (3.9) | 4 (11.1) | 9 (3) | 0.019 |
| Urine/fecal incontinence | 22 (6.5) | 12 (32.4) | 10 (3.3) | < 0.001 |
| Cardiomyopathy | 120 (36.4) | 17 (43.5) | 103 (35.5) | 0.338 |
| Atrial fibrillation | 75 (22.5) | 14 (36.8) | 61 (20.7) | 0.026 |
| Peripheral arterial disease | 46 (13.9) | 7 (18.4) | 39 (13.3) | 0.381 |
| Hypertension | 228 (68.4) | 29 (74.3) | 199 (67.6) | 0.599 |
| Heart failure | 39 (11.8) | 8 (21.6) | 31 (10.5) | 0.091 |
| Depression | 28 (8.4) | 4 (10.8) | 24 (8.1) | 0.306 |
| Diabetes | 85 (25.3) | 14 (35.8) | 71 (23.9) | 0.573 |
| Stroke | 29 (8.7) | 9 (24.3) | 20 (6.8) | < 0.001 |
| COPD | 42 (12.6) | 4 (10.5) | 38 (12.9) | 0.789 |
| Chronic renal failure | 48 (14.4) | 13 (34.2) | 35 (11.8) | 0.002 |
| Chronic liver disease | 7 (2.1) | 3 (7.8) | 4 (1.3) | 0.017 |
| Obesity | 48 (14.4) | 8 (21) | 40 (13.6) | 0.384 |
| Poor nutritional status | 41 (12.4) | 10 (26.3) | 31 (10.6) | 0.017 |
| PaO2 at admission | 73.8 (25.4) | 66 (19.7) | 74.7 (25.8) | 0.239 |
| Mean P/F at admission | 284 (106.6) | 236.4 (102.9) | 290.4 (105.3) | 0.057 |
| WBC/mcL at admission | 7127 (4159) | 9088 (4773) | 7006 (4054) | 0.018 |
| Lymphocytes count (%) | 16.3 (10) | 10.7 (5.6) | 16.9 (10.5) | 0.033 |
| Neutrophils count (%) | 73 (13.6) | 80.8 (9.2) | 73 (13.1) | 0.027 |
| NLR at admission | 8.3 (10) | 10.1 (6.1) | 8.1 (10.4) | 0.514 |
| LDH (U/L) at admission | 415 (246) | 518.2 (280.5) | 404.5 (240.7) | 0.175 |
| Hs-CRP (mg/L) at admission | 72 (75) | 92.9 (85.4) | 70.2 (74.8) | 0.486 |
| Serum creatinine (mg/dL) at admission | 1.1 (0.7) | 1.26 (0.6) | 1.11 (0.7) | 0.493 |
| D-dimer at admission | 2384 (6531) | 3733 (8468) | 2243 (6325) | 0.682 |
| Death (%) | 65 (19.2) | 19 (48.7) | 46 (15.4) | < 0.001 |
Data are expressed as mean (standard deviation) and number (%) as appropriate. Significant false detection rate (FDR) adjusted p values are marked in bold
COPD, Chronic Obstructive Pulmonary Disease; PaO2, arterial oxygen partial; WBC, White blood count; P/F, ratio of arterial oxygen partial pression to fractional inspired oxygen; NLR, Neutrophil/Lymphocytes Ratio; Hs-CRP, High-sensitivity C-reactive protein
Thirty-nine (11.5%) patients presented with hyperactive delirium at admission. Patients with delirium were significantly older [mean age 84.2 (7.8) vs. 76.2 (9.3) than non-delirious ones, p < 0.001], with a higher prevalence of cognitive impairment (73.6% vs. 11.4% respectively, p < 0.001), atrial fibrillation (36.8% vs. 20.7%, respectively, p = 0.026), chronic renal failure (34.2% vs. 11.8%, respectively, p = 0.002) and chronic liver disease (7.8% vs. 1.3%, respectively, p = 0.017). No significant association was observed between hyperactive delirium and AFP.
Biological correlates of delirium
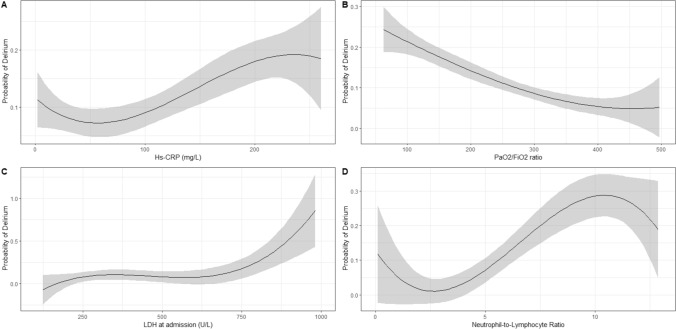
Mean PaO2/FiO2 at baseline was lower in the delirium group compared to controls [236.4 (102) vs. 290.4 (105.3), p = 0.057]. Regarding white cell blood count, patients with delirium showed a lower proportion of lymphocytes [10.7 (5.6) vs. 16.9 (10.5), p = 0.033] and an increased proportion of neutrophils at admission [80.8 (9.2) vs. 73 (13.1), respectively, p = 0.027], with no significant difference in NLR, hs-CPR and LDH (Table 1). As shown in Fig. 1, we found an inverse linear association between P/F ratio and delirium (Beta Coefficient ± Standard Error = − 0.0004 ± 0.0002, p = 0.015, see Supplemental Table S2), whereas by cubic spline regression, only LDH showed a significant non-linear association with delirium at admission, with a quasi-exponential increase for LDH values higher than 500 U/L (p = 0.004) (Fig. 1).
Fig. 1.
Non-linear relationship between Hs-CRP (a), P/F ratio (b), LDH (c), NLR (d) and delirium at admission in the smooth curve fitting after adjustment for age, sex and WHO severity status
Features of the study population after stratification for physical frailty
Among the 129 patients with physical frailty (AFP+), 19 (14.7%) experienced hyperactive delirium, while 20 (9.6%) of 208 participants without AFP (AFP−) had hyperactive delirium. Features of subjects with and without delirium within AFP subgroups are reported in Table 2. In both subgroups patients with delirium were significantly older and more often had a history of cognitive impairment and stroke than those without delirium. Moreover, in AFP+ subgroup, patients with delirium had lower PaO2/FiO2 and higher LDH and more frequently displayed fever and dyspnea at admission than those without delirium. Conversely, in AFP− subgroup, subjects with delirium showed significantly higher levels of NLR and were more often affected by chronic renal failure.
Table 2.
Sample features according to the presence of hyperactive delirium at admission, after stratification for physical frailty
| Frail patients | Controls | Delirium | p-value | Non-frail patients | Controls | Delirium | p-value | |
|---|---|---|---|---|---|---|---|---|
| N = 129 | N = 110 | N = 19 | N = 208 | N = 188 | N = 20 | |||
| Gender F/M | 51 (39.5)/78 (60.5) | 43 (39.1)/67 (60.9) | 8 (42.1)/11 (57.9) | 0.80 | 117 (56.2)/91 (43.7) | 107 (56.9)/10 (50) | 10 (50)/10 (50) | 0.55 |
| Age (years) | 81.4 (8.1) | 80.8 (8.4) | 85 (5.2) | 0.039 | 74.5 (9.3) | 73.5 (8.8) | 83.5 (9.7) | < 0.001 |
| Hospital stay (days) | 23.5 (20.5) | 25.7 (21.2) | 11 (10.1) | 0.006 | 17.6 (17.1) | 17.7 (17.8) | 17.2 (9.0) | 0.89 |
| PaO2/FiO2 | 316 (117) | 331 (111) | 220 (116) | 0.041 | 271 (98) | 273 (98) | 246 (97) | 0.59 |
| NLR | 7.9 (7.0) | 7.8 (7.2) | 8.5 (5.4) | 0.80 | 8.5 (11.3) | 8.3 (11.7) | 11.0 (6.5) | 0.18 |
| Hs-CRP (mg/L) | 63.3 (82.8) | 61.9 (83.1) | 74.5 (84.5) | 0.80 | 77 (72) | 74 (70) | 105 (87) | 0.59 |
| D-dimer | 4766 (11,016) | 4128 (10,471) | 10,725 (16,769) | 0.41 | 1614 (3975) | 1636 (4134) | 1402 (1964) | 0.89 |
| LDH (U/L) | 368 (181) | 346 (163) | 538 (236) | 0.041 | 432 (264) | 425 (260) | 508 (309) | 0.59 |
| Serum creatinine | 1.22 (0.94) | 1.21 (0.95) | 1.32 (0.85) | 0.78 | 1.08 (0.58) | 1.06 (0.59) | 1.23 (0.48) | 0.56 |
| Cognitive impairment | 40 (31.0) | 24 (21.8) | 16 (84.2) | < 0.001 | 22 (16.7) | 10 (5.3) | 12 (63.1) | < 0.001 |
| Arterial hypertension | 97 (76.4) | 81 (75) | 16 (84.2) | 0.56 | 131 (63.5) | 118 (63.4) | 13 (65) | 0.89 |
| Atrial fibrillation | 40 (31.5) | 31 (28.4) | 9 (50.0) | 0.22 | 35 (17.0) | 30 (16.2) | 5 (25) | 0.48 |
| Peripheral arterial disease | 26 (20.6) | 23 (21.3) | 6 (16.7) | 0.69 | 20 (9.8) | 16 (8.7) | 4 (20) | 0.12 |
| Obesity | 23 (17.9) | 21 (19.1) | 2 (11.1) | 0.57 | 25 (12.2) | 19 (10.3) | 6 (30) | 0.017 |
| Stroke | 17 (13.4) | 12 (10.9) | 5 (29.4) | 0.14 | 12 (5.8) | 8 (4.3) | 4 (20) | 0.050 |
| Diabetes | 29 (22.5) | 22 (20) | 7 (36.8) | 0.29 | 56 (27.1) | 49 (26.3) | 7 (35) | 0.53 |
| COPD | 23 (18.1) | 20 (18.3) | 3 (16.7) | 0.86 | 19 (9.3) | 18 (9.7) | 1 (5.3) | 0.489 |
| Chronic renal failure | 25 (19.5) | 20 (18.1) | 5 (27.8) | 0.57 | 23 (11.2) | 15 (8.1) | 8 (40) | < 0.001 |
| Fever at admission | 67 (52.3) | 53 (48.6) | 14 (73.7) | 0.12 | 164 (78.8) | 148 (78.7) | 16 (80) | 0.894 |
| Cough at admission | 52 (41.6) | 43 (40.6) | 9 (47.4) | 0.69 | 110 (53.6) | 99 (53.2) | 11 (57.9) | 0.893 |
| Dyspnoea at admission | 63 (48.8) | 48 (43.6) | 15 (78.9) | 0.004 | 130 (62.8) | 115 (61.5) | 15 (75) | 0.597 |
| Death (%) | 38 (29.5) | 28 (25.4) | 10 (52.6) | 0.012 | 27 (19.9) | 18 (9.5) | 20 (45) | < 0.001 |
Data are expressed as mean (standard deviation) and number (%) as appropriate. Significant FDR-adjusted p values are marked in bold
COPD, Chronic Obstructive Pulmonary Disease; PaO2, arterial oxygen partial pression; WBC, White blood count. P/F, ratio of arterial oxygen partial pression to fractional inspired oxygen; NLR, Neutrophil/Lymphocytes Ratio; Hs-CRP, High-sensitivity C-reactive protein
Mortality according to delirium at admission and physical frailty
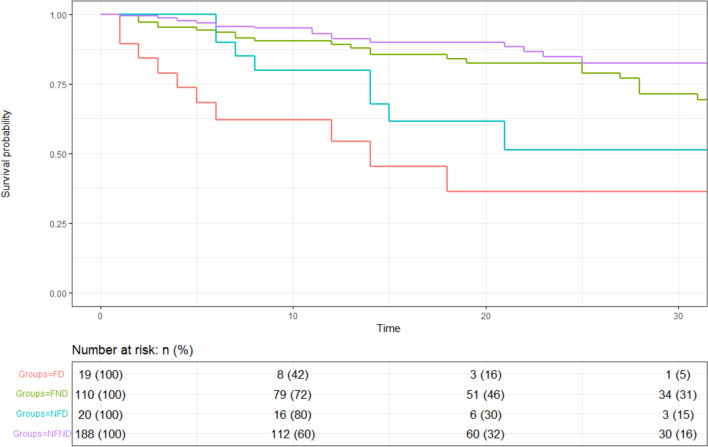
Patients with hyperactive delirium had a threefold risk of in-hospital mortality, compared with patients without delirium (48.7% vs. 15.4%, respectively, p < 0.001). Accordingly, as shown in Fig. 2 at Kaplan–Meier analysis, both in AFP+ and in AFP− subgroup patients experiencing delirium at admission showed a significantly lower mean survival time (± standard error) compared to their counterparts [18.8 ± 3.9 and 24.1 ± 3.1 vs. 59.8 ± 4.7 and 80.1 ± 4.1, respectively; p-value for log-rank test < 0.001)]. The bivariate and multivariate associations between frailty status, delirium and mortality are shown in Table 3. In the fully adjusted model, frail patients with hyperactive delirium had a 4.42 higher mortality risk and non-frail patients with delirium had a 2.85 higher mortality risk as compared with non-frail, non-delirious patients. Mortality of frail non-delirious patients did not differ from non-frail non-delirious controls in adjusted models.
Fig. 2.
Relationship between delirium at admission and mortality among hospitalized older patients with COVID-19 according to frailty status—FD, frail patients with Delirium; FND, frail patients non-delirious; NFD, non-frail patients with delirium; NFND, non-frail patients non-delirious
Table 3.
Physical frailty, hyperactive delirium and their combinations as independent mortality predictors by univariate and multivariate cox regression analysis
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
|---|---|---|---|---|---|---|
| Model 1 | 5.52 | 2.40–12.66*** | 1.54 | 0.82–2.87 | 3.11 | 1.33–7.28*** |
| Model 2 | 4.79 | 1.93–11.95*** | 1.38 | 0.71–2.66 | 2.68 | 1.07–6.72** |
| Model 3 | 4.42 | 1.75–11.13*** | 1.66 | 0.85–3.25 | 2.85 | 1.18–6.87** |
FD, frail with delirium; FND, frail non delirious, NFD, not-frail with delirium
NFND as reference
Model 1: Age + sex adjusted
Model 2: Model 1 plus history of heart failure, diabetes type 1 or type 2, Chronic Obstructive Pulmonary Disease, Chronic Renal Failure, Stroke and Obesity
Model 2 + WHO status
*p-value < 0.05; **p-value < 0.01; ***p-value < 0.001
Discussion
Among older people hospitalized for COVID-19, the analysis of the GeroCovid Registry detected an 11.5% prevalence of patients suffering from hyperactive delirium at admission. Delirium was associated with lower PaO2/FiO2 and with higher LDH levels in the case of frail patient; in constrast, a significant non-linear relationship with higher levels of NLR was demonstrated among non-frail subjects with COVID-19. Hyperactive delirium was identified as a predictor of mortality, independent of demographics and comorbidity, and the mortality risk was almost doubled if delirium was superimposed on physical frailty.
Our results suggest a pivotal role exerted by lung failure as a predictor of delirium. It is well known that conditions like acute respiratory distress syndrome (ARDS) or prolonged ‘iatrogenic hypoxaemia’ in Intensive Unite Care (ICU) constitute triggers for delirium [18, 19]. COVID-19 has been related to capillary flow disturbance, concurring to altered systemic oxygen uptake. It is worth mentioning that this mechanism may worsen the pre-existing age-related asymptomatic microvascular dysfunction typical of late life, thus worsening brain perfusion and facilitating the development of acute conditions as delirium, especially on an altered substrate such as the presence of cognitive decline [20]. Moreover, many patients in the acute ward during COVID-19 infection undergo severe hypoxemia without showing dyspnea and/or impaired ventilator mechanics. This so-called ‘silent hypoxemia’ has been described as a paradox of this syndrome, and may be explained by a central nervous system viral invasion [21], which in turn may provide neuronal injury, thus allowing us to speculate on the subsequent development of delirium [22, 23].
Among studied laboratory biomarkers, LDH levels at admission showed the strongest association with delirium. LDH is an intracellular enzyme almost ubiquitarian in all organ systems which catalyses the interconversion of pyruvate and lactate in hypoxic conditions. High levels of serum LDH have been previously associated with an increased risk of severe disease development and mortality in COVID-19 patients [24, 25]. Indeed, LDH increases as a result of extensive tissue damage due to inflammation, and for this reason it has been listed among inflammatory biomarkers of COVID-19 [26], and more specifically it may represent a marker of lung damage. For these reasons, the correlation of LDH with delirium could be explained both by the severity of lung damage and as a result of a cytokine dysregulation leading to neuroinflammation.
The role played by inflammation and the widespread concept of ‘citokine storm’ in the onset of delirium in COVID-19 is still difficult to estimate, and the findings of the present study failed to demonstrate the relationship between hs-CPR and delirium at admission. Indeed, levels of hs-CPR, NLR and D-dimer of delirious and non-delirious patients did not differ significantly in the whole sample. Yet, the difference was more evident in the subgroup without physical frailty, and it reached statistical significance for NLR. This result might partly be explained by the selection of delirium cases at hospital admission. In fact, patients at admission might have been in a variable stage of the disease course, with only a limited number of cases of severe systemic inflammation. This might be particularly true for frail subjects, that may show a blunted inflammatory response to the infection. This concept is well-characterized in the cubic-spline regression in which a progressive increase of delirium probability was observed moving from the second hs-CRP quintile to the fourth (equals from 75 to 200 mg/L) but was not confirmed for the last quintile (hs-CRP higher than 20 mg/L), presumably due to the small number of subjects with high values. Conversely, in non-frail subgroup, delirium was significantly associated with a higher NLR and, at a trend level, with higher hs-CRP.
The results regarding the relationship between physical frailty and delirium are partly unexpected. In the whole population, only a non-significant trend in terms of higher prevalence of this complex geriatric syndrome emerged in the delirium group. Conversely, Zazzara and colleagues demonstrated a significant association between frailty and delirium as the presenting symptom of COVID-19, in both hospital and community settings [27]. Yet, in the cited study frailty in the hospital setting was defined according as a score 5+ at the Clinical Frailty Scale, which is largely a measure of disability [28], thus being obviously associated with cognitive impairment and delirium risk. Moreover, the vast majority of studies showing the prognostic role of frailty in delirium adopted the Clinical Frailty Scale [29]. The only one, as far as we know, that tested a measure of physical frailty phenotype included a 10-year old measure, with a limited prognostic ability [30]. This suggests that different measures of frailty capture different constructs, with physical frailty being relatively separate from delirium. As expected, irrespective of frailty phenotype, delirium was related to chronological age, history of cognitive impairment and other chronic conditions typical of unsuccessful aging, such as previous stroke [31], cardiac diseases (atrial fibrillation and heart failure) and chronic conditions typical of late life, such as kidney and liver failure.
Regarding prognosis, the multivariate model confirms the association between delirium and mortality in COVID-19 hospitalized patients, irrespectively of demographics and comorbidity, which is consistent with previous evidence [32, 33]. Physical frailty, which does not appear to be associated with in-hospital mortality among patients without delirium, seems to act as a risk multiplier when associated with hyperactive delirium, almost doubling in-hospital mortality risk in comparison with non-frail subjects with delirium.
Limitations
The main limitation of the present study is represented by the lack of a validated delirium assessment instrument. Moreover, the need for unprecedented precautions such as patient isolation and distance from families, with the impossibility of direct contact with family caregivers, may have led to the under-detection of delirium [34, 35]. The prevalence of delirium reported in other pieces of literature exploring the specific clinical setting of elderly hospitalized for COVID-19 widely varies according to the setting of care and the state of the disease (between 28% in the emergency department and 78% in intensive care units) [36–39]. The lower prevalence of delirium reported is likely due to the exclusion of the hypoactive forms, which have been reported to be more common than hyperactive ones, especially in patients burdened with greater severity of disease [5]. A second limitation is represented by the limited sample size, which reduces the statistical power of the analysis. Moreover, we could not consider the different viral variants' role in the study outcomes since only a few centers systematically detected SARS-CoV-2 variants at the time of study enrollment. Finally, a single determination of the inflammatory status and of delirium, as required by the study protocol, constitute further criticalities that do not allow an estimate of the effect of inflammation on delirium over time.
Conclusive remarks
In the present sample of older patients hospitalized with COVID-19, greater severity of lung parenchymal disease, as measured by lower PaO2/FiO2 and higher LDH levels, seems to be the main clinical correlate of hyperactive delirium, especially in subjects with physical frailty. The presence of hyperactive delirium carries an increased mortality risk, which is doubled by the coexistence of physical frailty. Further studies are needed to better explain the pathways shared between inflammation markers and delirium onset.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The Authors wish to thank Dr. Susanna Del Signore and Dr. Gianluca Zia (Bluecompanion Ltd, London, UK) for their helpful advice on the technical issues during the data collection.
YES Group:
Ilaria Parrotta, Leonardo Bencivenga, Chukwuma Okoye, Alberto Zucchelli, Francesco Tonarelli, Antonio D’Errico, Riccardo Calvani, Benedetta Govoni, Lorenzo Maestri, Liliana Mazza, Valeria Prestipino Giaritta, Bruno Michael Zanforlini, Chiara Ceolin, Federica Bellone, Giulia Perfetti, Antonella Giordano, Daniela Florio, Laura Petraglia, Sara Rogani, Nicola Acampora, Alice Margherita Ornago, Marco Salvi, Mariya Muzyka, Edoardo Saporiti, Irene Zucchini, Giuseppe Armentaro, Beatrice Zazzara, Andrea Volpini, Vanessa Nunziata, Maria Cristina Ferrara, Lavinia Patetta, Serena Iuorio, Giordana Gava, Elena Pinardi, Keti Barbara, Alessia Maria Calabrese, Giuseppe Dario Testa, Riccardo Franchi, Luca Tagliafico, Federico Bellelli Luca Soraci, Stefano Cacciatore, Enrico Brunetti, Davide Montini, Caterina Trevisan, Panaiotis Finamore, Matteo Candeloro, Diana Lelli.
Author contributions
Conceptualization, IP, LB, CO; Data curation: Formal analysis, CO, EM; Methodology: RAI, GB, EM; Supervision, RAI, GB, EM, SF; Writing—original draft, IP, LB, CO; Writing—review and editing, RAI, GB, EM. All authors have read and agreed to the published version of the manuscript.
Funding
Dr. Leonardo Bencivenga has been supported by the research grant provided by the Cardiopath Ph.D. program, the research grant provided by the FDIME and the STAR PLUS Research Grant provided by the University of Naples Federico II and Compagnia San Paolo.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no conflict of interest to declare.
Consent to participate
Informed consent was obtained from all individuals included in this study.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. The study was approved by the BIO-CAMPUS Ethic Committee, University of Rome—Prot. Number: 22.5(20).20 OSS ComEt-UCBM. Each center, moreover, had the approval of its own Ethic Committee.
Informed consent
All patients (or their legal caregivers) provided informed consent to participation before entering the study.
Footnotes
Dr. Ilaria Parrotta, Dr. Leonardo Bencivenga and Dr. Chukwuma Okoye belong to the Young Epidemiologists of the Italian Society of Gerontology and Geriatrics (SIGG) (YES) working group (all members are listed in the acknowledgements).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ilaria Parrotta and Leonardo Bencivenga have equally contributed to this article.
References
- 1.WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020 [Internet]. [Cited 2021 Dec 28]. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 2.Mallah SI, Ghorab OK, Al-Salmi S, et al. COVID-19: breaking down a global health crisis. Ann Clin Microbiol Antimicrob. 2021;20:1. doi: 10.1186/s12941-021-00438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hshieh TT, Inouye SK, Oh ES. Delirium in the Elderly. Psychiatr Clin North Am. 2018;41:1–17. doi: 10.1016/j.psc.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Gibb K, Seeley A, Quinn T, et al. The consistent burden in published estimates of delirium occurrence in medical inpatients over four decades: a systematic review and meta-analysis study. Age Ageing. 2020;49:352. doi: 10.1093/ageing/afaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krewulak KD, Stelfox HT, Leigh JP, et al. Incidence and prevalence of delirium subtypes in an adult ICU: a systematic review and meta-analysis. Crit Care Med. 2018;46:2029–2035. doi: 10.1097/CCM.0000000000003402. [DOI] [PubMed] [Google Scholar]
- 6.O’Hanlon S, Inouye SK. Delirium: a missing piece in the COVID-19 pandemic puzzle. Age Ageing. 2020;49:497–498. doi: 10.1093/ageing/afaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machado-Curbelo C, González-Quevedo A. Hypoxemia and cytokine storm in COVID-19: clinical implications. MEDICC Rev. 2021;23:54–59. doi: 10.37757/MR2021.V23.N3.10. [DOI] [PubMed] [Google Scholar]
- 8.Trevisan C, Del Signore S, Fumagalli S et al (2021) Assessing the impact of COVID-19 on the health of geriatric patients: The European GeroCovid Observational Study. Eur J Intern Med [Internet]. [cited 2021 Apr 4]; Available from: https://pubmed.ncbi.nlm.nih.gov/33573885/ [DOI] [PMC free article] [PubMed]
- 9.R&D Blueprint and COVID-19 [Internet]. [cited 2021 Apr 9]. Available from: https://www.who.int/teams/blueprint/covid-19
- 10.Beigel JH, Tomashek KM, Dodd LE et al (2020) Remdesivir for the treatment of Covid-19—Final Report. N Engl J Med [Internet]. [cited 2021 Apr 4]; 383(19):1813–1826. Available from: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed]
- 11.Goldman JD, Lye DCB, Hui DS et al (2020) Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med [Internet]. [cited 2021 Apr 4]; 383(19):1827–1837. Available from: 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed]
- 12.Cao B, Wang Y, Wen D et al (2020) A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med [Internet]. [cited 2021 Apr 9]; 382(19):1787–1799. Available from: 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed]
- 13.Boatright JJM. Therapeutic gases: management and administration. In: Hess DMIN, editor. Respiratory care: principles and practice. 4. Burlington: Jones and Bartlett Learning; 2020. p. 298. [Google Scholar]
- 14.Pedone C, Costanzo L, Cesari M et al (2016) Are performance measures necessary to predict loss of independence in elderly people? Journals Gerontol Ser A Biol Sci Med Sci [Internet]. [cited 2021 Apr 26];71(1):84–9. Available from: 10.1093/gerona/glv096 [DOI] [PMC free article] [PubMed]
- 15.First MB. DSM-5® Handbook of Differential Diagnosis. DSM-5® Handb Differ Diagnosis. 2013 Nov
- 16.Li X, Liu C, Mao Z, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Crit Care. 2020;24:1–10. doi: 10.1186/s13054-020-03374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA J Am Med Assoc. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 18.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:1–3. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bein T, Grasso S, Moerer O, et al. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med. 2016;42:699–711. doi: 10.1007/s00134-016-4325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol Rep. 2021;9:1. doi: 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akoumianaki E, Vaporidi K, Bolaki M, et al. Happy or silent hypoxia in COVID-19—a misnomer born in the pandemic era. Front Physiol. 2021;12:1783. doi: 10.3389/fphys.2021.745634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nouri-Vaskeh M, Sharifi A, Khalili N, et al. Dyspneic and non-dyspneic (silent) hypoxemia in COVID-19: possible neurological mechanism. Clin Neurol Neurosurg. 2020;198:1. doi: 10.1016/j.clineuro.2020.106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202:356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han Y, Zhang H, Mu S, et al. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: a retrospective and observational study. Aging (Albany, NY) 2020;12:11245–11258. doi: 10.18632/aging.103372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38:1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sen A, Nigam A, Vachher M. Role of polypeptide inflammatory biomarkers in the diagnosis and monitoring of COVID-19. Int J Pept Res Ther. 2022;28:1. doi: 10.1007/s10989-022-10366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zazzara MB, Penfold RS, Roberts AL, et al. Probable delirium is a presenting symptom of COVID-19 in frail, older adults: a cohort study of 322 hospitalised and 535 community-based older adults. Age Ageing. 2021;50:40–48. doi: 10.1093/ageing/afaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockwood K, Song X, MacKnight C et al (2005) A global clinical measure of fitness and frailty in elderly people. C Can Med Assoc J = J l’Association medicale Can 173(5):489–495 [DOI] [PMC free article] [PubMed]
- 29.Zou Y, Han M, Wang J, et al. Predictive value of frailty in the mortality of hospitalized patients with COVID-19: a systematic review and meta-analysis. Ann Transl Med. 2022;10:166–166. doi: 10.21037/atm-22-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mak JKL, Kuja-Halkola R, Wang Y et al (2021) Frailty and comorbidity in predicting community COVID-19 mortality in the UK Biobank: the effect of sampling. J Am Geriatr Soc 69(5):1128–1139 [DOI] [PMC free article] [PubMed]
- 31.She Q, Chen B, Liu W, et al. Frailty pathogenesis, assessment, and management in older adults with COVID-19. Front Med. 2021;8:1048. doi: 10.3389/fmed.2021.694367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rebora P, Rozzini R, Bianchetti A, et al. Delirium in patients with SARS-CoV-2 infection: a multicenter study. J Am Geriatr Soc. 2021;69:293–299. doi: 10.1111/jgs.16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marengoni A, Zucchelli A, Grande G, et al. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age Ageing. 2020;49:923–926. doi: 10.1093/ageing/afaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellelli G, Nobili A, Annoni G, et al. Under-detection of delirium and impact of neurocognitive deficits on in-hospital mortality among acute geriatric and medical wards. Eur J Intern Med. 2015;26:696–704. doi: 10.1016/j.ejim.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Baker HA, Safavynia SA, Evered LA. The “third wave”: impending cognitive and functional decline in COVID-19 survivors. Br J Anaesth. 2021;126:44–47. doi: 10.1016/j.bja.2020.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pun BT, Badenes R, Heras La Calle G et al (2021) Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med 9(3):239–250 [DOI] [PMC free article] [PubMed]
- 37.Martinotti G, Bonanni L, Barlati S, et al. Delirium in COVID-19 patients: a multicentric observational study in Italy. Neurol Sci. 2021;42:3981–3988. doi: 10.1007/s10072-021-05461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennedy M, Helfand BKI, Gou RY, et al. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Netw Open. 2020;3:e2029540–e2029540. doi: 10.1001/jamanetworkopen.2020.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ragheb J, McKinney A, Zierau M, et al. Delirium and neuropsychological outcomes in critically Ill patients with COVID-19: a cohort study. BMJ Open. 2021;11:e050045. doi: 10.1136/bmjopen-2021-050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.