Abstract
The long-term clinical efficacy of SARS-CoV-2 vaccines according to antibody response in immunosuppressed patients such as hematological patients has been little explored. A prospective multicenter registry-based cohort study conducted from December 2020 to July 2022 by the Spanish Transplant and Cell Therapy group, was used to analyze the relationship of antibody response over time after full vaccination (at 3–6 weeks, 3, 6 and 12 months) (2 doses) and of booster doses with breakthrough SARS-CoV-2 infection in 1551 patients with hematological disorders. At a median follow-up of 388 days after complete immunization, 266 out of 1551 (17%) developed breakthrough SARS-CoV-2 infection at median of 86 days (range 7–391) after full vaccination. The cumulative incidence was 18% [95% confidence interval (C.I.), 16–20%]. Multivariate analysis identified higher incidence in chronic lymphocytic leukemia patients (29%) and with the use of corticosteroids (24.5%), whereas female sex (15.5%) and more than 1 year after last therapy (14%) were associated with a lower incidence (p < 0.05 for all comparisons). Median antibody titers at different time points were significantly lower in breakthrough cases than in non-cases. A serological titer cut-off of 250 BAU/mL was predictive of breakthrough infection and its severity. SARS-CoV-2 infection-related mortality was encouragingly low (1.9%) in our series. Our study describes the incidence of and risk factors for COVID-19 breakthrough infections during the initial vaccination and booster doses in the 2021 to mid-2022 period. The level of antibody titers at any time after 2-dose vaccination is strongly linked with protection against both breakthrough infection and severe disease, even with the Omicron SARS-CoV-2 variant.
Subject terms: Medical research, Haematological diseases
Introduction
Patients with hematological disorders (HD) are at high risk of severe coronavirus infectious disease 2019 (COVID-19) caused by the new coronavirus (SARS-CoV-2) [1]. The dramatic impact of COVID-19 on hematological patients was observed during the early phase of the pandemic, with mortality rates then exceeding 25% [2–7]. However, encouraging preliminary data suggest that SARS-CoV-2 vaccination has played a major role in reducing the severity of breakthrough COVID-19 in these immunocompromised patients, with mortality dropping to less than 10% during 2022 [8]. A recent retrospective case series supports the reduced mortality (12.4%) of breakthrough COVID-19 (mainly with the alfa variants of concern (VOC) after vaccination in hematological patients, but with high burden of hospitalization (66.4%) and intensive care unit (ICU) admission (21.3%) irrespective of the vaccine doses administered or serological response [9].
The first generation of SARS-CoV-2 vaccines failed to prevent community transmission of the virus, in particular the most recent Omicron VOC, but protection still remained high against severe COVID-19 [10]. In fact, it is expected that most HD patients worldwide will be infected several times with different SARS-CoV-2 VOC despite vaccination and boosters, and a significant number of them could still experience a severe course. The poor humoral response and impaired cellular immunity in HD patients contribute to a higher incidence of post-vaccination SARS-CoV-2 infection and its severity [11, 12]. To date, there is a lack of studies evaluating the kinetics of breakthrough SARS-CoV-2 infection in a prospective fashion as well as its severity in vaccinated HD patients. Thus, we still do not know to what extent these patients will develop breakthrough SARS-COV-2 infection after vaccination, their potential severity and the risk factors for these breakthrough infections.
In view of these gaps, the current study analyzes the incidence, characteristics, severity and clinical and immune factors associated with breakthrough SARS-CoV-2 infections through a prospective observational registry conducted by the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH-TC) aimed at monitoring the humoral response to SARS-CoV-2 vaccines through 1 year after complete vaccination in a large cohort of 1551 HD patients.
Patients and methods
Study population
In December 2020 the Infectious Complications Subcommittee (GRUCINI) of the GETH-TC in collaboration with the Spanish Society of Hematology and Hemotherapy (SEHH) launched a national prospective multicenter registry aimed at evaluating the humoral response for SARS-CoV-2 vaccination and its duration through one year after full vaccination (defined as two vaccine doses for RNA vaccines or one dose for adenoviral vector-based vaccines). Characteristics of this registry have been described in detail elsewhere [13, 14]. The registry included consecutive HD patients who were vaccinated against SARS-CoV-2 between December 30, 2020 to June 30, 2021 in 21 participating Spanish centers. In June 2021 a specific form for booster doses and breakthrough SARS-CoV-2 infection was implemented in the registry. Patients were followed as initially planned and monitored for the development of SARS-CoV-2 reactive IgG antibodies (SCoV2-R-A) and breakthrough SARS-CoV-2 infection at different time points (3–6 weeks, 3, 6, and 12 months) after the complete vaccination schedule. The status of all included patients was updated on July 28, 2022. All patients included in this registry gave their signed informed consent in accordance with the declaration of Helsinki. The local Research Ethical Committee of the Hospital Clínico Universitario of Valencia approved the registry and study protocol (reference code 35.21).
Inclusion criteria, cohort selection and data check
As of July 28, 2022, the GETH-TC registry recruited 1783 patients with different hematological disorders who were fully vaccinated against SARS-CoV-2. With the aim of assessing (1) the incidence and severity of breakthrough SARS-CoV-2 infection at one year after full vaccination (2) the effect of serological response at different time points and (3) the effect of booster doses, the current study focused on patients with complete demographic and survival data. We excluded 232 patients from 3 centers, initially included with limited data (only affiliation data), that did not obtain the institutional approval for serological testing leaving 1551 hematological patients for final study analysis. At the time of this analysis none of the patients had received pre-exposure prophylaxis with monoclonal antibodies.
Out of 1551 HD patients, 1398 (90.1%), 1174 (76%), 1023 (66%) and 849 (55%) had available serological assessment at 3–6 weeks, 3 months, 6 months, and 12 months after full vaccination, respectively. Of these 1250 (89%), 1070 (91%), 900 (88%) and 820 (97%) had quantitative assessment in BAU/mL available, respectively, reflecting a high rate of missing serology data over time. To analyze whether there were relevant unknown biases which led to not having serological testing, we compared the clinical characteristics of patients and the survival of those with and without complete serological data at different time points and we did not find any significant differences (See supplementary Table S1), suggesting random reasons for missing serological data. Accordingly, we used a listwise deletion analysis to evaluate the effect of antibody titers on the risk of breakthrough infection at each pre-specified time point. A major reason for having missing serologies, from our personal experience and through our interactions with all participating physicians/centers, the most common reasons involved the patients’ and investigators’ exhaustion and/or the difficulties in scheduling serological tests outside the routine schedule for outpatient visits in long-term survivors, as well as the institutional refusal for serological monitoring through academic non-funded studies while the patient’s death before the scheduled serological test was a logical reason for missing serological follow-up.
Technical considerations and Definitions
We assessed humoral response at the specified time points using serological ELISA or chemiluminescence immunoassay according to their availability at the microbiology services of each participating center. Antibody detection or seropositivity was defined as detectable SARS-CoV-2-reactive IgG antibodies (SCoV2-R-A) at any level above the lower limit of detection level for each test used. As recommended by the SEHH in vaccinated individuals, serological testing included the detection of IgG against both the nucleocapsid (N) and surface (S) proteins (anti-N and anti-S IgG, respectively) [15]. Antibody levels in patients with quantitative assessment were normalized according to the WHO standards and results were reported as SCoV2-R-A binding antibody units per milliliter (BAU/mL). Supplementary Table S2 summarizes the technical characteristics of the serological tests used and the normalization of antibody titers to BAU/mL according to WHO standards.
Complete vaccination (full primary immunization) schedules were defined as per marketing authorization at the time of study conduct and included two doses for full primary immunization (except COVID-19 Vaccine Janssen®). An additional dose after completion of full primary immunization was considered as a booster dose.
Pre-vaccination SARS-CoV-2 infection was defined as patients with prior history of PCR-proven COVID-19 and/or positive SARS-CoV-2 serostatus (IgG and/or IgM) before the first vaccine dose.
Breakthrough SARS-CoV-2 infection was defined as molecular (PCR test), antigenic or serological (IgG anti-N seroconversion between two consecutive serological tests) evidence of SARS-CoV-2 infection 7 days after full vaccination and until the last follow-up. Commercial PCR test used for diagnosis was provided in Supplementary Table S3. Corticosteroids use was defined as ≥15 mg per day of prednisone or equivalent doses of methylprednisolone, dexamethasone or hydrocortisone started at least 2 weeks before vaccination.
Although we did not sequence SARS-CoV-2 strains in any case, the inference of VOC on each episode was based on the likelihood of having a specific VOC according to the Spanish sequencing epidemiological data (see Supplementary Fig. S1) [16]. We inferred a specific VOC when the infection was diagnosed during the predominance (>50% of all sequenced VOC) of a specific VOC in our country. For example, Alfa-Beta VOC was considered in SARS-CoV-2 infections diagnosed from April 2021 to June 20 2021. Delta was considered from June 21 to December 26, 2021 and Omicron from December 27, 2021 to the end of the study in July 2022.
Endpoints and statistical analysis
The primary objective of the study was to assess the incidence, severity and risk factors of breakthrough SARS-CoV-2 infection in vaccinated HD patients. Secondary endpoints include the effect of SCoV2-R-A titers on the development and severity of breakthrough SARS-CoV-2 infection at each pre-specified serological time points. We also evaluated the clinical benefit of booster vaccine doses as compared to those without boosters in terms of breakthrough infection incidence. Finally, we evaluated the effect of breakthrough SARS-CoV-2 infection in all-cause mortality.
The main patient characteristics were reported by descriptive statistics on the total available information: medians and ranges were used for continuous variables, while absolute and percentage frequencies were used for categorical variables. For comparisons, Fisher exact test or Mann–Whitney’s U test were used when appropriate.
Breakthrough SARS-CoV-2 infection after vaccination was estimated by the cumulative incidence method considering death without SARS-CoV-2 infection as the competing event [17, 18]. Univariate and multivariate analyses of risk factors for breakthrough infection were calculated using the Fine and Gray test [19]. For multivariate analysis, only variables with parameter estimates showing a p ≤ 0.10 in the univariate analysis were finally included. We did not include in the multivariate analysis time-dependent covariates (serological assessments and/or booster doses) since the circulation burden of SARS-CoV-2 in the community was not constant over time [20] which may lead to a misleading assumption (i.e. the booster vaccine doses were given after the second dose and just before the increase of community SARS-CoV-2 circulation and its inclusion as a time-depend covariate could lead to a misleading significant increase in the risk of breakthrough infection). To address the clinical benefit of booster doses as compared to those who only received 2 doses in terms of breakthrough infection incidence we performed two landmark analyses, the first one selecting a control group of patients (only 2 doses) alive without breakthrough infection and whose observation period started at the median time of booster administration in the study group (median time from second to third vaccine dose). The second landmark analysis started the observation period in December 27, 2021 in both groups (booster and non-booster) including patients alive and COVID-free at the beginning of the observation period to assure that booster and non-booster patients did not differ in the rate at which they came in contact with SARS-CoV-2 [21]. A median test analysis to check the protective effect of the amount of SCoV2-R-A was carried out in patients with available quantitative SCoV2-R-A titers normalized to BAU/mL, whether they developed or not breakthrough infections. A p-value <0.05 was considered statistically significant. All p-values are two-sided. Analyses were performed using the statistical software SPSS v. 25(IBM SPSS Statistics, Armonk, New York, USA) and R software, version 4.0.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. Most patients (n = 1496, 96%) received complete vaccination with mRNA vaccines, and their median age was 63 years (range 16–97). Overall, the most common hematological diseases were B-cell non-Hodgkin’s lymphoma (B-cell NHL) (n = 260, 16.8%), plasma cell disorders (PCD) (n = 164, 10.6%), chronic lymphocytic leukemia (CLL) (n = 155, 10%) and chronic myeloproliferative neoplasms (cMPN) (n = 127, 8.2%). Among the cell therapy procedures, the most common was allo-HSCT (n = 429, 27.7%) followed by autologous stem cell transplantation (n = 121, 7.8%) and chimeric antigen receptor of T cell (CAR-T) therapy (n = 22, 1.4%). Pre-vaccine SARS-CoV-2 serologies were available in 514 patients (33%) and 29 of them (6%) were positive. Note that this series included 129 patients (8.3%) with prior evidence of SARS-CoV-2 infection before being vaccinated. Median follow-up after the second vaccine dose was 388 days (range 47–561), and for the 83% who received the 1st booster dose it was 189 days (range 0–302).
Table 1.
Patient characteristics.
| Characteristics | (n = 1551) |
|---|---|
| Prior COVID-19, n (%) | 129 (8.3) |
| • Median time from COVID-19 to vaccination, days (range) | 203 (11–422) |
| Serological status prior to vaccination, n (%) | |
| • Positive | 64 (4) |
| • Negative | 485 (31) |
| • Not tested | 1002 (65) |
| Type of 1st and/or 2nd vaccine dose, n (%) | |
| • Moderna mRNA-1273 | 1086 (70) |
| • Pfizer-BioNTech BNT162b2 | 410 (26) |
| • Adenoviral vector-based | 55 (4) |
| Type of 3rd vaccine, n (%) | 1275 (82%) |
| • Moderna mRNA-1273 | 910 (71.3) |
| • Pfizer-BioNTech BNT162b2 | 361 (28) |
| • Adenoviral vector-based | 4 (0.7) |
| Age (years), median (range) | 63 (16–97) |
| • 18–40 years, n (%) | 167 (11) |
| • 41–60 years, n (%) | 551 (35.5) |
| • 61–70 years, n (%) | 407 (26) |
| • >71 years, n (%) | 426 (27.5) |
| Male, n (%) | 871 (56.2) |
| Baseline disease, n (%) | |
| • AML | 50 (3.2) |
| • ALL | 5 (0.3) |
| • MDS | 117 (7.5) |
| • B cell NHL | 260 (16.8) |
| • T cell NHL | 15 (1) |
| • Plasma cell disorders | 164 (10.6) |
| • CLL | 155 (10) |
| • HD | 65 (4.2) |
| • cMPN | 127 (8.2) |
| • Aplastic anemia | 4 (0.3) |
| • Non-malignant disorders | 17 (1) |
| • Allo-HSCT | 429 (27.7) |
| • ASCT | 121 (7.8) |
| • CAR-T | 22 (1.4) |
| Status disease at vaccination, n (%) | |
| • Complete remission | 823 (59.3) |
| • Partial remission | 179 (11.5) |
| • Active disease | 453 (29.2) |
| Time last treatment to COVID-19 vaccine, months (range) | |
| • Untreated | 252 (16.2) |
| • Active treatment | 441 (28.4) |
| • ≥6 months to 1 year | 148 (9.5) |
| • ≥1 year | 710 (45.8) |
| Immunosuppressant drugs at vaccination, n (%) | 322 (20.8) |
| Corticosteroids at vaccination, n (%) | 278 (17.9) |
| Daratumumab, n (%) | 52 (3.4) |
| Venetoclax, n (%) | 16 (1) |
| Anti-CD-20 moAb, n (%) | 270 (17.4) |
| • <6 months before 1st vaccine dose | 97 (6.3) |
| • 6 to 1 year before 1st vaccine dose | 25 (1.6) |
| • >1 year before 1st vaccine dose | 148 (9.5) |
| BTK inhibitor therapy, n (%) | 67 (4.3) |
| TKI therapy, n (%) | 49 (3.2) |
| Lenalidomide maintenance, n (%) | 129 (8.3) |
| Ruxolitinib therapy, n (%) | 15 (1) |
| Blood count before vaccination (×109/mL) | |
| • Absolute neutrophile counts, median (range) | 3.1 (0–46.7) |
| • Absolute lymphocyte counts, median (range) | 1.73 (0.14–262.1) |
| • Absolute lymphocyte counts <1 × 109/L | 288 (18.6) |
| Median time between 1st and 2nd vaccine doses, median days (range) | 28 (17–115) |
| Time from 2nd dose to first serologies, median days (range) | 21 (12–62) |
| Serological response at 3 weeks after 2 doses, n (%) | |
| • Positive | 1092 (70.4) |
| • negative | 306 (19.7) |
| • Not available | 153 (10) |
| Third vaccine dose given, n (%) | 1284 (82.8) |
| Time from 2nd dose to 3rd dose, days (range) | 168 (31–538) |
| Median follow-up after 3 doses, days (range) | 189 (0–302) |
| Serological response after 3 doses, n (%) | |
| • Positive | 251 (19) |
| • negative | 72 (5.6) |
| • Not available | 961 (75) |
| Forth vaccine dose, n (%) | 430 (27.7) |
| Time from 3rd dose to 4th dose, days (range) | 159 (31–266) |
| Median follow-up after 4 doses, days (range) | 12 (0–95) |
| SCoV2-R-A detection at 3–6 weeks after 2 vaccine doses, n/evaluable (%) | 1090/1398 (78) |
| • Antibody titers in BAU/mL, n (%) | 1250 (89) |
| SCoV2-R-A detection at 3 months after 2 vaccine doses, n (%) | 922/1174 (78) |
| • Antibody titers in BAU/mL, n (%) | 1070 (91) |
| SCoV2-R-A detection at 6 months after full vaccination, n (%) | 842/1023 (82) |
| • Antibody titers in BAU/mL, n (%) | 900 (88) |
| SCoV2-R-A detection at 1 year after full vaccination, n (%) | 740/849 (87) |
| • Antibody titers in BAU/mL, n (%) | 820 (97) |
| Breakthrough COVID-19, n (%) | 266 (17) |
| Median time from 2 doses of vaccine to SARS-CoV-2 infection, days (range) | 86 (7–391) |
| Median follow-up whole cohort, days (range) | 388 (47–561) |
| All-cause mortality at median follow-up, n (%) | 89 (5.7) |
PCR polimerase chain reaction, AML acute myeloid leukemia, ALL acute lymphoblastic leukemia, MDS myelodysplastic syndrome, B-cell NHL B-cell non-Hodgkin lymphoma, T cell NHL T cell non-hodgkin lymphoma, CLL chronic lymphocytic leukemia, HD Hodgkin disease, MPN chronic myeloproliferative neoplasm, Allo-HSCT allogeneic stem cell transplantation, ASCT autologous stem cell transplantation, CAR-T T-cell chimeric antigen receptor, moAb monoclonal antibody, BTK inhibitor Bruton’s tyrosine kinase inhibitor, TKIs tyrosine kinase inhibitors, SCoV2-R-A SARS-CoV-2-reactive IgG antibodies.
All patients were fully vaccinated, whereas 1284 (82.8%) received a first booster dose at a median of 168 days after complete vaccination schedule, and 430 (27.7%) received a second booster dose at a median of 159 days after the first booster.
Incidence, severity, and risk factors of breakthrough SARS-CoV-2 infection
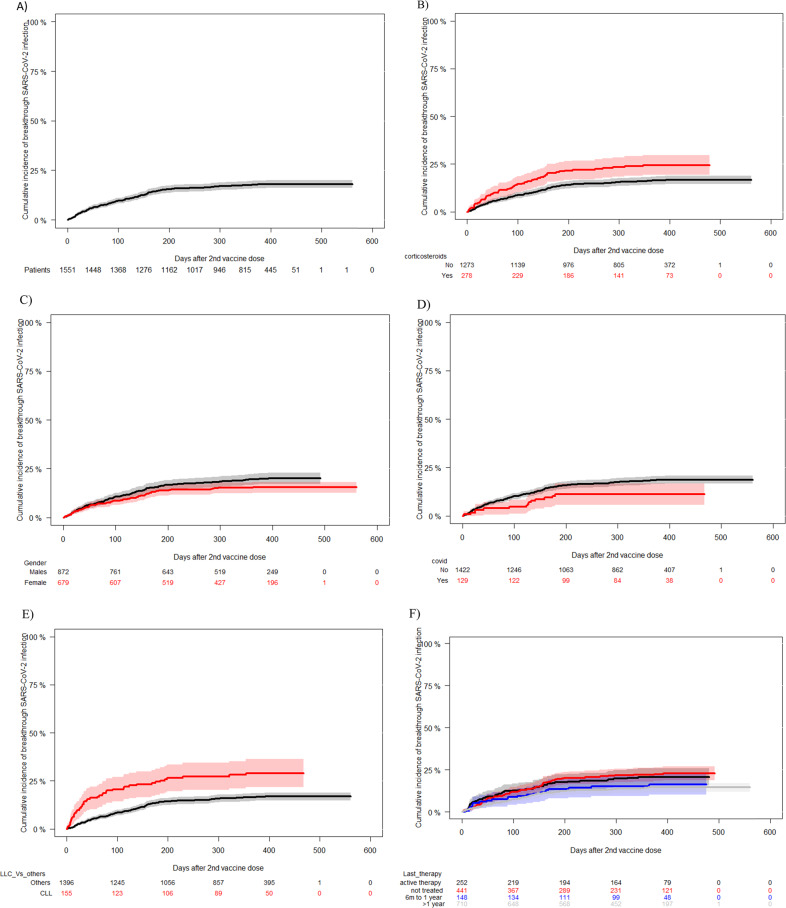
We identified 266 patients (17%) with breakthrough SARS-CoV-2 infection at a median of 158 days (range 7–391) after complete vaccination. Fourteen out of 266 (5%) episodes occurred in patients with pre-vaccination COVID-19 being detected by PCR (14/129, 11%). Characteristics of patients and SARS-CoV-2 breakthrough infection are summarized in Table 2. The distribution of breakthrough cases during the study period and type of probable VOC are provided in Fig. 1. Spanish epidemiological curve of SARS-CoV-2 infection during the study period was provided in Supplementary Fig. S2. The one-year cumulative incidence of breakthrough SARS-CoV-2 infection in the whole cohort from the start of vaccination was 18% [95% confidence interval (C.I.), 16–20] (Fig. 2A). Seventy-six (28.5%) developed SARS-CoV-2 infection after full primary vaccination and before the booster dose, whereas 165 (62% of breakthrough infections and 12.8% of those who received the 1st booster) and 25 (9.4% of breakthrough infections and 5.8% of those who received the 2nd booster) were infected after the 1st booster (before 2nd booster) and after the 2nd booster, respectively.
Table 2.
Characteristics of patients with breakthrough SARS-CoV-2 infection.
| Characteristics | SARS-CoV-2 infection (n = 266) |
|---|---|
| Prior COVID-19, n/n evaluable (%) | 14/129 (10.8) |
| Type of 1st and 2nd vaccines, n/n evaluable (%) | |
| • Moderna mRNA-1273 | 189/1086 (17.4) |
| • Pfizer-BioNTech BNT162b2 | 68/410 (16.6) |
| • Adenoviral vector-based | 9/55 (16.4) |
| Type of 3rd vaccines before SARS-CoV-2 breakthrough, n evaluable (%) | 185 (69) |
| • Moderna mRNA-1273 | 133 (71.8) |
| • Pfizer-BioNTech BNT162b2 | 50 (27) |
| • Adenoviral vector-based | 2 (1) |
| Likely SARS-CoV-2 variantsa, n (%) | |
| • Alfa or Beta | 18 (6.7) |
| • Delta | 45 (17) |
| • Omicron | 203 (76.3) |
| Age (years), median (range) | 62 (19–97) |
| • 16–40 years, n/n evaluable (%) | 32/167 (19.1) |
| • 41–60 years, n/n evaluable (%) | 97/551 (17.6) |
| • 61–70 years, n/n evaluable (%) | 60/407 (14.7) |
| • >71 years, n/n evaluable (%) | 77/426 (18) |
| Male, n (%)/n evaluable (%) | 165/871 (18.9) |
| Baseline disease, n/n evaluable (%) | |
| • AML | 9/50 (18) |
| • ALL | 0/5 (0) |
| • MDS | 22/117 (18.8) |
| • B cell NHL | 49/260 (18.8) |
| • T cell NHL | 5/15 (33.3) |
| • Plasma cell disorders | 32/164 (19.5) |
| • CLL | 44/155 (26.8) |
| • HD | 9/65 (13.8) |
| • cMPN | 12/127 (9.4) |
| • Aplastic anemia | 0/4 (0) |
| • Non-malignant disorders | 5/17 (29) |
| • Allo-HSCT | 53/429 (12.3) |
| • ASCT | 23/121 (19) |
| • CAR-T | 3/22 (13.6) |
| Immunosuppressant drugs at vaccination, n /n evaluable (%) | 64/322 (19.8) |
| Corticosteroids at vaccination, n /n evaluable (%) | 65/278 (23.3) |
| Daratumumab, n /n evaluable (%) | 11/52 (21.1) |
| Venetoclax, n /n evaluable (%) | 6/16 (37.5) |
| Anti-CD-20 moAb, n /n evaluable (%) | 50/270 (18.5) |
| • <6 months before 1st vaccine dose | 22/97 (22.6) |
| • 6 to 1 year before 1st vaccine dose | 4/25 (16) |
| • >1 year before 1st vaccine dose | 24/148 (16.2) |
| BTK inhibitor therapy, n /n evaluable (%) | 17/67 (25.3) |
| TKI therapy, n /n evaluable (%) | 12/49 (24.5) |
| Lenalidomide, n /n evaluable (%) | 31/129 (24) |
| Ruxolitinib therapy, n /n evaluable (%) | 1/15 (6) |
| Timing and characteristics of breakthrough SARS-CoV-2 infection | |
| SARS-CoV-2 infection after 2 vaccine doses and before booster/s, n (%) | 76 (30) |
| • Median time, days (range) | 158 (7–391) |
| SARS-CoV-2 infection after 3 vaccine doses and before the 4th dose, n (%) | 165 (62) |
| • Median time, days (range) | 121 (1–280) |
| SARS-CoV-2 infection after 4 vaccine doses, n (%) | 25 (8) |
| • Median time, days (range) | 24 (1–115) |
| Number of evaluable SARS-CoV-2 infections after each serological time point, n (%) | |
| • After 3–6 weeks from 2 doses | 240 (90) |
| • After 3 months from 2 doses | 207 (77.8) |
| • After 6 months from 2 doses | 166 (62) |
| • After 1 year from 2 doses | 37 (14) |
| SCoV2-R-A detection at 3–6 weeks, n /n evaluable (%) | 170/240 (70.8) |
| Median SCoV2-R-A titer at 3–6 weeks, BAU/mL (range) | 202 (0–10400) |
| SCoV2-R-A detection at 3 months, n /n evaluable (%) | 137/200 (68.5) |
| Median SCoV2-R-A titer at 3 months, BAU/mL (range) | 99 (0–15846) |
| SCoV2-R-A detection at 6 months, n /n evaluable (%) | 120/161 (74) |
| Median SCoV2-R-A titer at 6 months, BAU/mL (range) | 302 (0–48856) |
| SCoV2-R-A detection at 1 year, n /n evaluable (%) | 27/37 (73) |
| Median SCoV2-R-A titer at 1 year, BAU/mL (range) | 1017 (0–6423) |
| Symptomatic SARS-CoV-2 infection, n /n evaluable (%) | 145/266 (54.5) |
| Pneumonia, n /n evaluable (%) | 49/266 (18.4) |
| Hospital admission, n /n evaluable (%) | 48/266 (18) |
| Oxygen requirement, n /n evaluable (%) | 36/266 (13.5) |
| ICU admission, n /n evaluable (%) | 5/266 (1.9) |
| COVID-related Death, n /n evaluable (%) | 5/266 (1.9) |
| All-cause mortality at median follow-up, n /n evaluable (%) | 12/266 (4.5) |
AML acute myeloid leukemia, ALL acute lymphoblastic leukemia, MDS myelodysplastic syndrome, B-cell NHL B-cell non-Hodgkin lymphoma, T cell NHL T cell non-Hodgkin lymphoma, CLL chronic lymphocytic leukemia, HD Hodgkin disease, MPN chronic myeloproliferative neoplasm, Allo-HSCT allogeneic stem cell transplantation, ASCT autologous stem cell transplantation, CAR-T T-cell chimeric antigen receptor, moAb monoclonal antibody, BTK inhibitor Bruton’s tyrosine kinase inhibitor, TKIs tyrosine kinase inhibitors, SCoV2-R-A SARS-CoV-2-reactive IgG antibodies, Anti-N SARS-CoV-2 nucleocapsid antibodies, ICU intensive care unit.
aAccording to the Spanish epidemiological data regarding the predominance of each SARS-CoV-2 variant during the time of the study period, we considered Alfa or Beta VOC the episodes of SARS-CoV-2 infection diagnosed between April 1, 2021 and July 26, 2021, Delta VOC between July 27, 2021 and Omicron between December 27, 2021 to July 31, 2022.
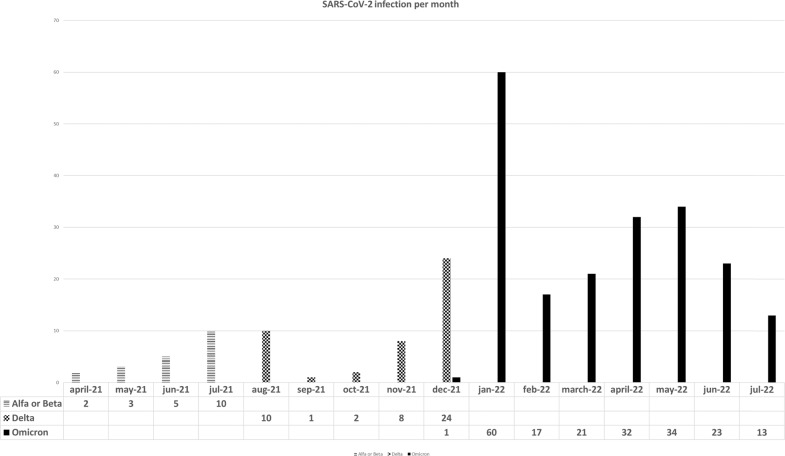
Fig. 1.
Epidemiological distribution of reported SARS-CoV-2 breakthrough infections in the whole cohort according to the likelihood of SARS-CoV-2 variants.
Fig. 2. 1-year Incidence of SARS-CoV-2 breakthrough infection.
A In the whole cohort [cumulative incidence of 18% (95% confidence interval 95%, 16–20%)]. B According to the use of corticosteroids at the time of first vaccine dose. [Under corticosteroids cumulative incidence of 24.5% (95% confidence interval, 19–30%) vs without 16.7%, 95% C.I. 14–18 (p < 0.001)]. C According to the patient’s gender. [Male 19.6% (95% confidence interval, 17–22.4%) vs. female 15.5%, 95% C.I. 13–18 (p = 0.04)]. D According to development of COVID-19 before the first vaccine dose. [Prior COVID-19 11% (95% confidence interval, 6–17%) vs without 18.4%, 95% C.I. 16–20.5, (p = 0.04)]. E According to chronic lymphocytic leukemia vs others. [CLL 29% (95% confidence interval, 22–36%) vs. others16.8%, 95% C.I. 15–18.9 (p < 0.001)]. F According to timing of last therapy. [No treated 21% (C.I. 95%, 16–26%) vs. active therapy 20.5%, 95% C.I. 18–24 vs 6 months to 1 year 16% (C.I. 95%, 10–22) vs. >1 year 14% (95% C.I., 12–17) (p = 0.04)].
Out of 266 cases, 145 (54.5%) had COVID-19 whereas 121 (45.5%) were asymptomatic infections. Pneumonia was documented in 49 cases (18.4%) whereas the SARS-CoV-2 infection-related hospital admission rate was 18% (n = 48). There were 5 COVID-19-related deaths (1.9%) at a median of 28 days (range 7–50) after SARS-CoV-2 detection.
Univariate and multivariate analyses of risk factors associated with the incidence of breakthrough SARS-CoV-2 infection are shown in Table 3. CLL patients (29%) and use of corticosteroids (24.5%) at the time of vaccination were associated with a higher cumulative incidence of breakthrough infection whereas female gender (15.5%), and vaccination at least 1 year after the end of HD therapy (14%) were protective in the multivariate analysis. As shown in Table 3, diagnosis of a cMPN and having had COVID-19 before vaccination showed a trend for a protective effect. The incidences of SARS-CoV-2 infection according to the aforementioned risk factors are shown in Fig. 2B–E. To analyze whether similar risk factors existed for breakthrough infection by the omicron variants, we performed a separate multivariate analysis in those infected by these VOC and found the same risk factors as in the entire cohort. We also performed a multivariate analysis of risk factor for symptomatic breakthrough SARS-CoV-2 infection and risk factors remain unchanged except for gender which was no longer significant (details of both analyses in the footnote to Table 3).
Table 3.
Logistic regression univariate and multivariate analyses of factors predicting SARS-CoV-2 breakthrough infection after full vaccination.
| SARS-CoV-2 infection | P-Value | SARS-CoV-2 infection | P-value | |
|---|---|---|---|---|
| Characteristics | Univariate Fine and Gray (95% C.I.) | Regression Fine and Graya/b HR (95% C.I.) | ||
| Prior to vaccination COVID-19 | 0.04 | |||
| • Yes | 11 (6–17) | 0.6 (0.35–1.02) | 0.06 | |
| • No | 18.4 (16–2) | |||
| Type of vaccine | 0.5 | |||
| • Moderna mRNA-1273 | 17.9 (16–20) | |||
| • Pfizer-BionTech BNT162b2 | 17.6 (14–21) | |||
| • Adenoviral vector-based | 18 (7–30) | |||
| Age (years) | 0.5 | |||
| • 18–40 years | 20.7 (14–27) | |||
| • 41–60 years | 18 (15–22) | |||
| • 61–70 years | 15 (12–19) | |||
| • >71 years | 18.4 (15–22) | |||
| Sex | 0.067 | |||
| • Male | 19.7 (15–20) | |||
| • Female | 15.5 (13–18) | 0.75 (0.58–0.96) | 0.025 | |
| Baseline disease | 0.005 | |||
| • ALL | NT | |||
| • AML | 20 (8–32) | |||
| • MDS | 19.4 (12–27) | |||
| • B cell NHL | 20.2 (15–25) | |||
| • T cell NHL | 33 (9–57) | |||
| • Plasma cell disorders | 20 (14–27) | |||
| • CLL | 29 (20–34) | 1.96 (1.37–2.79) | 0.0002 | |
| • HD | 14 (6–23) | |||
| • cMPN | 9 (3.9–14) | 0.56 (0.31–1) | 0.054 | |
| • Aplastic anemia or non-malignant disorders | 28 (7–49) | |||
| • Allo-HSCT | 13 (10–16) | |||
| • ASCT | 21 (13–28) | |||
| • CAR-T | 14 (0–28) | |||
| Disease status at vaccination | 0.2 | |||
| • Complete remission | 17.2 (15–19.7) | |||
| • Partial remission | 22.5 (16–29) | |||
| • Active disease | 17 (14–21) | |||
| Time from last treatment to COVID-19 vaccine | 0.004 | |||
| • Untreated | 21 (16–26) | |||
| • Under treatment | 22 (18–26) | |||
| • >6 months to 1 year | 16 (10–22) | |||
| • ≥1 year | 14.5 (12–1) | 0.92 (0.85–0.99) | 0.04 | |
| SCoV2-R-A at 3–6 weeks | 0.002 | NT | ||
| • Positive | 16 (14–18) | |||
| • Negative | 25 (20–30) | |||
| SCoV2-R-A in BAU/mL at 3–6 weeks | <0.0001 | NT | ||
| • >250 | 15 (12–18) | |||
| • <250 | 25 (21–28) | |||
| Corticosteroids at vaccination | 0.001 | |||
| • Yes | 24.5 (19–30) | 1.41 (1.05–1.93) | 0.02 | |
| • No | 16.7 (15–19) | |||
| Daratumumab | 0.4 | |||
| • Yes | 21 (10–33) | |||
| • No | 18 (16–20) | |||
| Venetoclax | 0.01 | ns | ||
| • Yes | 38 (14–61) | |||
| • No | 17.9 (16–19.6) | |||
| Anti-CD-20 moAb | 0.5 | |||
| • Yes | 19.5 (15–24) | |||
| • No | 17.5 (14–17.7) | |||
| Bruton’s TKI therapy | 0.07 | ns | ||
| • Yes | 26 (15–37) | |||
| • No | 17.4 (16–19.7) | |||
| TKI therapy | 0.23 | |||
| • Yes | 25 (13–37) | |||
| • No | 18 (16–19.8) | |||
| Lenalidomide | 0.02 | ns | ||
| • Yes | 25 (17–33) | |||
| • No | 17.4 (15–19) | |||
| Ruxolitinib therapy | 0.3 | |||
| • Yes | 7 (0–19) | |||
| • No | 18.2 (16–20) | |||
| Lymphocyte count < 0.5 × 109/L | 0.5 | |||
| • Yes | 16 (6–26) | |||
| • No | 18 (16–20) | |||
| Lymphocyte count < 1.0 × 109/L | 0.3 | |||
| • Yes | 20.6 (16–25) | |||
| • No | 17.4 (15–19.5) |
AL acute leukemia, MDS myelodysplastic syndrome, B-cell NHL B-cell non-Hodgkin lymphoma, MM multiple myeloma, CLL chronic lymphocytic leukemia, HD Hodgkin disease, MPN chronic myeloproliferative neoplasm, Allo-HSCT allogeneic stem cell transplantation, ASCT autologous stem cell transplantation, moAb monoclonal antibody, TKIs tyrosine kinase inhibitors, SCoV2-R-A SARS-CoV-2-reactive IgG antibodies.
aWe carry out the multivariate analysis for Omicron VOC and found that risk factors remain unchanged:
Female gender HR 0.71 (C.I. 95%, 0.53–0.95), p-value 0.024.
LLC Vs others HR 2.01 (C.I. 95%, 1.33–3.04), p-value 0.0009.
Last therapy >1 year HR 0.88 (C.I. 95%, 0.77–1.00), p-value 0.051.
Corticosteroid use HR 1.681 (C.I. 95%, 1.16–2.41), p-value 0.005.
bMultivariate analysis for symptomatic breakthrough SARS-CoV-2 infection:
LLC Vs others HR 2.5 (C.I. 95%, 1.59–3.94), p-value 0.0007.
Last therapy >1 year HR 0.82 (C.I. 95%, 0.71–0.96), p-value 0.018.
Corticosteroid use HR 1.83 (C.I. 95%, 1.2 2.8), p-value 0.005.
Antibody level titers and severity of breakthrough SARS-CoV-2 infection
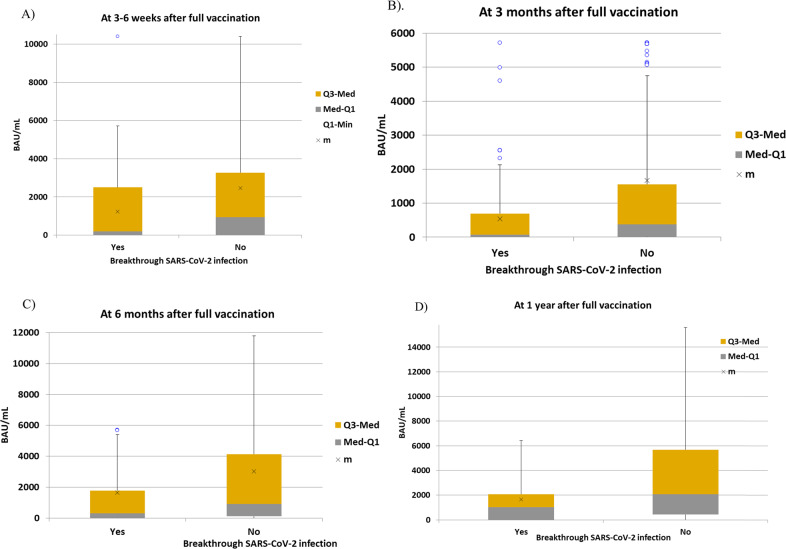
For this purpose, we excluded patients with breakthrough SARS-CoV-2 infection before each serological testing point. Median antibody titers according to the later development of breakthrough SARS-CoV-2 infection at different time points are shown in Table 4. As expected, the magnitude of SCoV2-R-A responses was significantly lower in patients with breakthrough SARS-CoV-2 infection as compared to those without at each of the serological points analyzed (Fig. 3A–D). Results were similar when analyzing cases attributed to the Omicron VOC (data not shown). Note that median antibody titers increased over time in both groups, reflecting the effect of booster doses, especially after 6 months from full vaccination. A serological cut-off value of 250 BAU/mL was able to discriminate the risk of being infected and the disease severity (Table 5).
Table 4.
Median test analyses of the SARS-CoV-2-R-A titers in BAU/mL at different serological time point assessments according to occurrence of breakthrough SARS-CoV-2 infectiona.
| Variables | SARS-CoV-2 infection | No infection | P-value |
|---|---|---|---|
| SARS-CoV-2-R-A at 3–6 weeks (n = 1250) | 229 | 1021 | |
| • Median Ab titers in BAU/mL (range) | 202 (0–10400) | 937 (0–56800) | <0.001 |
| SARS-CoV-2-R-A at 3 months (n = 910) | 136 | 774 | |
| • Median Ab titers in BAU/mL (range) | 64.9 (0–5714) | 380 (0–30460) | <0.001 |
| SARS-CoV-2-R-A at 6 months (n = 841) | 144 | 697 | |
| • Median Ab titers in BAU/mL (range) | 302 (0–48842) | 897.35 (0–95334) | <0.001 |
| SARS-CoV-2-R-A at 12 months (n = 674) | 36 | 638 | |
| • Median Ab titers in BAU/mL (range) | 1017 (0–6423) | 2080 (0–87200) | 0.003 |
SARS-CoV-2-R-A SARS-CoV-2 reactive antibodies, Ab antibodies.
aResults of median test remain unchanged when we only include evaluable cases for Omicron VOC.
Fig. 3. Median anti-SARS-CoV-2 IgG reactive antibodies titers measured in binding antibody units/mL (BAU/mL) at 3–6 weeks, 3 months, 6 months and 1 year after the 2nd dose according to the development of breakthrough SARS-CoV-2 infection.
A Patients with breakthrough SARS-CoV-2 infection had a median of 202.57 BAU/mL (range 0–5714) vs those without SARS-CoV-2 infection median 937 BAU/mL (range 0–10400) (p < 0.001). B Q3-med means median interquartil 75%; Med-Q1, median interquartil 25%; m, mean. C Patients with breakthrough SARS-CoV-2 infection had a median of 70.14 BAU/mL (range 0–2126) vs those without SARS-CoV-2 infection median 379 BAU/mL (range 0–4746) (p < 0.001). D Patients with breakthrough SARS-CoV-2 infection had a median of 302 BAU/mL (range 0–5408) vs those with SARS-CoV-2 infection median 907 BAU/mL (range 0–11800) (p < 0.001). E Patients with breakthrough SARS-CoV-2 infection had a median of 1017 BAU/mL (range 0–6423) vs those without SARS-CoV-2 infection median 2080 (range 0–15600) (p < 0.001).
Table 5.
SARS-CoV-2 infection severity according to anti-SARS-CoV-2 IgG reactive antibody cut-off before the occurrence of SARS-CoV-2 infection in evaluable patients.
| Variable | <250 BAU/mL At 3–6 weeks (n = 504) | ≥250 BAU/mL At 3–6 weeks (n = 746) | P value | <250 BAU/mL At 3 months (n = 430) | ≥250 BAU/mL At 3 months (n = 480) | P value | <250 BAU/mL At 6 months (n = 300) | ≥250 BAU/mL At 6 months (n = 541) | P value |
|---|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 infection | 119 (23.6%) | 110 (14.7) | <0.001 | 86 (20%) | 50 (10%) | <0.001 | 67 (22%) | 77 (14%) | 0.004 |
| Symptomatic SARS-CoV-2 | 77/119 (65%) | 45/110 (41%) | <0.001 | 56/86 (65%) | 18/50 (36%) | 0.001 | 43/67 (64%) | 28/77 (36%) | 0.001 |
| Pneumonia | 38/119 (32%) | 7/110 (6%) | <0.001 | 21/86 (24%) | 1/50 (2%) | <0.001 | 13/67 (19%) | 3/77 (3.8%) | 0.004 |
| Hospital admission | 38/119 (32%) | 6/110 (5%) | <0.001 | 22/86 (25.5%) | 0 | <0.001 | 16/67 (23.8%) | 3/77 (3.8%) | <0.001 |
| Oxygen requirement | 31/119 (33%) | 3/110 (2.7%) | <0.001 | 19/86 (22%) | 0 | <0.001 | 13/67 (19%) | 1/77 (1.2%) | <0.001 |
| ICU admission | 4/119 (3.8%) | 1/110 (0.9%) | 0.3 | 1/86 (1.2%) | 0 | 0.9 | 0 | 1/77 (1.2%) | 0.9 |
| Death | 5/119 (4.2%) | 0 | 0.06 | 2/86 (2.4%) | 0 | 0.5 | 3/67 (4.5%) | 0 | 0.09 |
The median SCoV2-R-A levels at 3–6 weeks after full vaccination were markedly higher in asymptomatic breakthrough SARS-CoV-2 infection (n = 107) than in those with COVID-19 (n = 122) [870.67 BAU/mL (range 0–10400) vs 0 BAU/mL (range 0–5714.93), p < 0.001]. In addition, the median SCoV2-R-A levels were lower in those requiring hospital admission vs those who did not [0 BAU/mL (range 0–5456.88) vs 467.89 BAU/mL (range 0–10400), respectively, p < 0.001] and in those with pneumonia vs those without [0 BAU/mL (range 0–5456) vs 458 BAU/mL (range 0–10400), p < 0.001]. These findings were also true when we compared median SCoV2-R-A levels at 3 and 6 months after complete vaccination (data not shown).
Infection severity, number of vaccine doses, and type of SARS-CoV-2 VOC
We analyzed potential differences in severity whether the patient had the breakthrough infection after the second, third, or fourth dose of vaccine (Table 6) and we did not find relevant differences, except for a trend to a lower rate of ICU admission after the third dose. In contrast, differences were more obvious according to the type of VOC (alfa-beta vs delta vs omicron) which was inferred from our National epidemiological sequencing data. Omicron causes milder cases of COVID-19 requiring less ICU admission than Alfa-Beta or Delta (p < 0.01 for both comparisons). There was a trend towards a lower pneumonia rate with Delta and Omicron. However, death occurred with Delta and Omicron but with very few cases (3/45 and 2/203, respectively).
Table 6.
Severity of SARS-CoV-2 infection according to the likelihood of SARS-CoV-2 variant and the timing of the infection with regards to the vaccine dose.
| Variable | After 2 doses (n = 81) | After 3 doses (n = 160) | After 4 doses (n = 25) | P value | Alfa or Beta (n = 18) | Delta (n = 45) | Omicron (n = 203) | P value |
|---|---|---|---|---|---|---|---|---|
| Symptomatic SARS-CoV-2 | 40 (49.5%) | 90 (56%) | 15 (60%) | 0.5 | 8 (44%) | 15 (33%) | 122 (60%) | 0.003 |
| Pneumonia | 19 (23%) | 27 (17%) | 3 (12%) | 0.3 | 7 (38%) | 8 (17.7%) | 34 (16.7%) | 0.06 |
| Hospital admission | 18 (22%) | 28 (18%) | 2 (8%) | 0.26 | 6 (33%) | 7 (15%) | 35 (17%) | 0.2 |
| Oxygen requirement | 11 (13%) | 23 (14%) | 2 (8%) | 0.6 | 4 (22%) | 5 (11%) | 27 (13%) | 0.48 |
| ICU admission | 4 (5%) | 1 (0.6%) | 0 | 0.051 | 2 (11%) | 2 (4%) | 1 (0.5%) | 0.002 |
| Death | 2 (2.4%) | 3 (1.8%) | 0 | 0.7 | 0 | 3 (6.6%) | 2 (1.9%) | 0.03 |
Booster doses and breakthrough SARS-CoV-2 infection
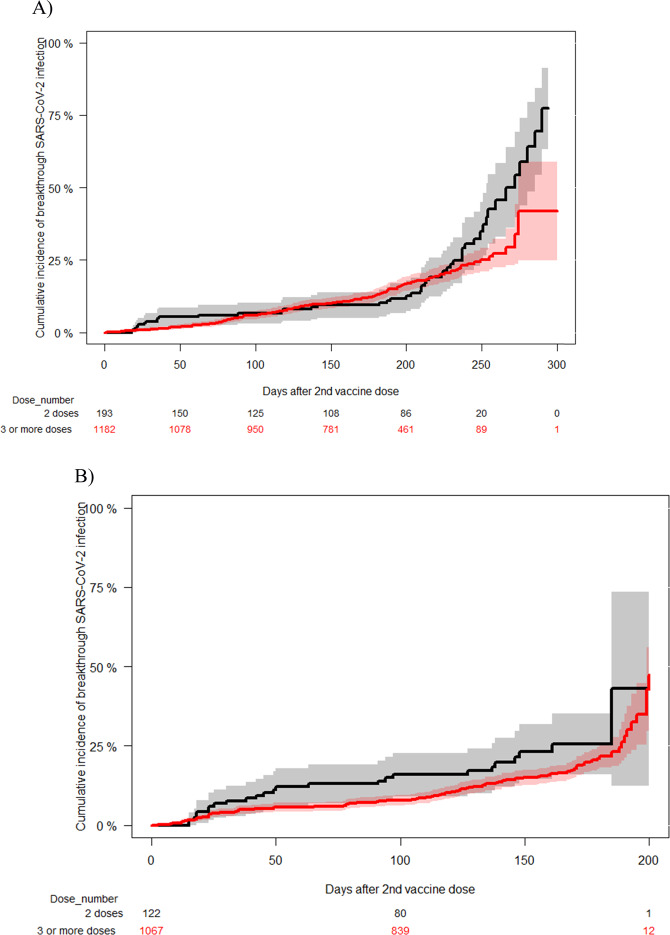
In order to evaluate the potential clinical benefit of receiving a booster dose, we performed a landmark analysis comparing the incidence of breakthrough SARS-CoV-2 infection in patients with or without a booster dose. The inclusion criterion in the booster group was no history of SARS-CoV-2 infection before the 1st booster dose (n = 1182), which was day 0 for the landmark analysis. The non-booster cohort included patients who were alive and COVID-19-free at the median time interval (plus 3 extra weeks) after the 2nd dose and the 1st booster dose in the booster cohort. There were no significant differences among groups regarding baseline diagnosis, corticosteroid use, time from last treatment to first COVID-19 vaccine or gender (data not shown). The cumulative incidence of breakthrough SARS-CoV-2 infection was similar among groups (booster group 18%, 95% C.I., 16–21 vs. non-booster group 18%, 95% C.I. 12–25, p = 0.1) (Fig. 4A). However, in this landmark analysis we observed that there was a progressive separation of both curves at the end of the observation period which coincided with a remarkable increase of SARS-CoV-2 circulation in the community (From December 26, 2021, see Figs. 1 and S2). To figure out if there was a significant difference among groups in the incidence of SARS-CoV-2 infection at the time of increased prevalence in the community we performed an additional landmark analysis where the observation period started on December 26, 2021 for both groups. For this analysis we included patients alive and without prior history of SARS-CoV-2 infection before that date and with at least 3 weeks of follow-up (n = 1067 in the booster group and n = 122 in the non-booster group). We observed a marginal benefit in the booster group (16%, 95% C.I., 13–18%) as compared to the non-booster group (23%, 95% C.I., 14–32) (p = 0.06) (Fig. 4B).
Fig. 4. Landmark analyses of cumulative incidence of SARS-CoV-2 breakthrough infection according to the vaccine dose status.
A Estimated from the time of third vaccine dose for booster cases and from median time between the 2nd and 3rd vaccine dose for the control group. [3rd dose 18% (95% confidence interval 16–21%) vs 2 doses 18%, 95% C.I. 12–25 (p = 0.1)]. B Estimated from December 26, 2021 in patients alive and without SARS-CoV-2 infection before the 3rd vaccine dose or before this date for the control group. [3rd dose 16% (95% confidence interval, 13–18%) vs 2 doses 23%, 95% C.I. 14–32 (p = 0.06)].
Cause of death and all-cause mortality
Eighty-nine patients died (5.7%) in the whole cohort at a median of 233 days (range 48–442) after the first vaccine dose. Causes of death included progression or relapse of the underlying disease (n = 43, 48%), infections (n = 18, 20%), transplant-related complications (n = 9, 11%) and others (n = 19, 21%). Among those who developed breakthrough SARS-CoV-2 infection there were 12 deaths (4.5%) as compared to 77 (6%) deaths in those who did not (p = 0.35). Five patients (3 CLL and 2 NHL) died as a direct consequence of SARS-CoV-2 infection (1.9%). Three of them died after a third vaccine dose whereas 2 patients died after 2 doses.
Discussion
The current study provides a real picture of SARS-CoV-2 breakthrough infections in patients with HD one year after the implementation of mass vaccination and boosters. The first observation was that the epidemiology of breakthrough SARS-CoV-2 infection in HD patients mirrored that of the community. Secondly, we identified conditions associated with higher incidence of breakthrough infection, such as CLL and corticosteroid use at the time of vaccination, whereas female gender and vaccination more than one year after the last treatment were associated with a reduced incidence. Quantitative humoral responses correlated with both the risk of developing breakthrough SARS-CoV-2 infection and its severity, irrespective of when it was evaluated. Unexpectedly, we observed an encouraging low mortality rate (1.9%) of breakthrough SARS-COV-2 infection in HD patients. In addition, breakthrough SARS-CoV-2 infections did not increase all-cause mortality in this large series of HD patients suggesting a favorable evolution of the pandemic in terms of virulence.
The prospective design of our registry along with the large sample size, the longitudinal serological monitoring and the long follow-up enable us to provide an accurate estimate of several critical issues, such as real-life epidemiology of SARS-CoV-2 infection in vaccinated HD patients and cumulative incidence estimates. In addition, our design limited the likely risk of bias of reporting only severe cases, which is characteristic of retrospective registry studies, providing a precise picture of clinical characteristics and severity of SARS-CoV-2 infection in this particular scenario.
Despite intensive transmission prevention measures counseling our patients and caregivers, we still observed that SARS-CoV-2 infection in hematological patients mirrored national epidemiological data [2, 20], since the three-peak prevalence of breakthrough SARS-CoV-2 infection observed in our series (July-August 2021, January 2022 and April-May 2022, see Fig. 1) fully coincided with our national epidemiological data (see Fig. 1 and Supplementary Fig. S2). Preventive transmission measures (hand washing, social distancing, wearing mask, etc) are still highly recommended for patients but are clearly far from being satisfactory. It looks suitable that such measures would be applied not only to immunosuppressed patients and their caregivers but also to those who have a close contact with these patients (friends, other relatives, health staff, etc). We already learned that the more thorough preventive transmission measures are, the lesser the incidence of respiratory virus infections in immunocompromised patients [22].
We report an overall cumulative incidence of breakthrough infection of 18% at one year after the start of mass vaccination. Although there is no available data in other scenarios for comparing cumulative incidence estimates, it is likely that HD patients have an increased breakthrough infection incidence as was the case in patients with solid tumors [23]. In fact, there is an evident increased risk of significant breakthrough infection immediately after vaccination in HD patients as compared to the general population [11], however, the incidence is not necessarily the same for all HD. Our findings suggest that CLL disease and receiving corticosteroid therapy at the time of vaccination experienced a significantly higher infection incidence. Several prior studies revealed that among HD patients, CLL patients are characterized by a severe cellular immune dysfunction which translates into a low humoral response rate [24, 25] and a severe course of the disease [26, 27], although a significant reduction in mortality has been observed in the Omicron era [28]. On the other hand, corticosteroid use has been associated with a lower probability of mounting an adequate humoral response [29]. Both conditions may facilitate the virus entry and in particular its spread. Although the use of anti-CD20 monoclonal antibodies has been consistently associated with a lower amount of SARS-CoV-2-R-A after vaccination [29–32], being treated with anti-CD20 monoclonal antibodies was not associated with an increased incidence of breakthrough infection in our study. It is likely that the number of events and in particular of patients who received anti-CD20 monoclonal antibodies within 6 months before starting vaccination (n = 97, 36%) was too low to observe significant differences. The male gender has been already associated with a more severe course of the disease [33]. In accordance, our multivariate analysis revealed that males were prone to higher incidence of breakthrough infection as compared to females. Although we do not have a likely explanation for this difference, we can speculate that hormonal differences and/or differences in social behaviors between males and females may explain such differences. Finally, patients free of treatment for at least one year before vaccination experienced a lower incidence, most likely because the probabilities of having a serological response after vaccination are consistently higher [29]. In light of these findings, it seems reasonable to maximize humoral vaccine responses according to the disease status and treatment as recently suggested by the SEHH [15] and that low-evidence-based pre-exposure prophylaxis recommendations in vaccinated patients with long-lasting monoclonal antibodies should be restricted to patients harboring these conditions instead of its unselective use. Of note, most of emergent Omicron BA. 4 and BA. 5 subvariants showed a loss of neutralization activity to almost all monoclonal antibodies but bebtelovimab [34].
Our prior preliminary analysis with only 37 cases of breakthrough infection, showed a higher rate of breakthrough infection and higher disease severity in patients without SARS-CoV-2-RA at 3–6 weeks of full vaccination [8]. With a significantly larger number of cases, we hereby confirm this observation and extended this finding beyond 3–6 weeks after full vaccination to later times points from vaccination and irrespective of the number of booster doses given. In this sense, higher levels of both binding and neutralizing anti-SARS-CoV-2 antibodies may protect against COVID-19 and are correlated with each other [35, 36]. Data in the general population also support this statement [37, 38]. Thus, the quantitative assessment of anti-SARS-CoV-2 antibodies at any time after vaccination could be justified for a benefit/risk calculation before additional vaccine doses or anti-SARS-CoV-2 monoclonal antibodies are given in these immunosuppressed population.
The severity of SARS-CoV-2 infection in patients with HD appeared to decrease over time most likely due to the implementation of mass vaccination programs. Initial reports, including ours, described high rates of pneumonia (>70%), hospital admission (>50%), ICU requirement (>20%) and overall mortalities exceeding 25% in unvaccinated HD patients [2–7]. A recent study focusing on breakthrough infections in vaccinated HD patients found a reduced severity but still a significant burden of hospitalizations (66%), ICU admissions (21.3%) and SARS-CoV-2-related mortality (12.4%) [9]. In contrast to this retrospective study, our data indicate that the severity is lower than previously reported in terms of hospitalizations (18%), ICU admissions (1.9%) and especially, mortality (1.9%) which was encouragingly low. These observations could be justified by several factors; first, the prospective design of the current study led to the capture of both symptomatic and asymptomatic infections, reducing the bias of registry-based retrospective studies. Second, the vast majority of cases (76%) were attributed to the Omicron VOC, which has a lower intrinsic virulence [28, 39]. Third, efforts in maintaining a high titers of anti-SARS-CoV-2 antibody titers in this vulnerable population may have paid off, as higher levels of antibody titers were consistently associated with lower/less disease severity. In fact, the cut-off of 250 BAU/mL was able to predict the risk of breakthrough infection as well as the disease severity.
Finally, we did not observe any significant differences according to the number of booster doses administered, which may merely indicate that more important than the number of booster doses is the amount of anti-SARS-CoV-2 antibody titers. In fact, no patient with SCoV2-R-A > 250 BAU/mL died from SARS-CoV-2 in our cohort irrespective of the serological time points assessed and the number of booster doses. The monitoring period during which our study was conducted (from early 2021 to end July 2022) spanned between the fifth, sixth and seventh COVID-19 waves in Spain, which were sequentially caused by Alfa-Beta, Delta and Omicron VOC according to our national epidemiological sequencing data. Current data show that infections by the Omicron VOC are more frequently symptomatic but less severe in term of ICU support compared to Alfa-Beta or Delta VOC. Our findings are in line with prior studies in the general population [39, 40].
The limitations of this study comprise the use of different serological tests, absence of neutralizing antibody testing, the absence of cellular immune response analyses and the lack of molecular data regarding the SARS-CoV-2 variants in patients with breakthrough infections. In addition, although a significant drop out at the 12 months after full vaccination period was observed and could be regarded as a limitation, all patients alive at the given follow-up time points were clinically assessed, and there were thus no patients who were alive and lost to follow-up during the study period. In this sense, the random appearance of serological missing data along with a still large number of evaluable cases and consistent results at each time point assessment may support a limited potential bias. The use of anti-N IgG seroconversion in defining breakthrough SARS-CoV-2 infection could be regarded as an additional limitation given the possibility of passive immunization transfer in patients receiving blood products support from seropositive donors. However, only 7.2% of patients with anti-N IgG seroconversion were on transfusion and/or immunoglobulin support at the time of vaccination.
Conclusion
CLL patients and those under corticosteroids showed a higher incidence of breakthrough SARS-CoV-2 infection one year after the start of mass vaccination. The amount of antibody titers appears useful in identifying HD patients at higher risk of breakthrough SARS-CoV-2 infection by any VOC and its severity, and titers of SCoV2-R-A above 250 BAU/mL appear to be a good cut-off value. Last but not least, we report a reassuring reduction of SARS-CoV-2 mortality in this vulnerable population.
Supplementary information
Acknowledgements
REDCap is developed and supported by the Vanderbilt Institute for Clinical and Translational Research. We thank the Spanish Society of Hematology (SEHH) for its support of the study diffusion. We also offer our sincere thanks to the invaluable microbiology services for their commitment to SARS-CoV-2-reactive IgG antibody monitoring in these highly immunosuppressed patients from all participating centers, in particular to Santiago Muñoz Criado from Microbiological Division of Hospital Clínico Universitario of Salamanca and to Tamar Talaván from microbiological Division of Hospital Infanta Leonor of Madrid. Special thanks to all hematology units from participating centers for their commitment to the current study. Finally, we also want to thank patients, nurses and study coordinators for their foremost contributions to this study.
Author contributions
Authors responsible for the conception and the design of the study: JLP, RM, DN, and AC. Authors who performed the data analysis and generated the tables and figures: JLP and RM Authors responsible for patient recruitment: JLP, LL-C, MC, LV, GM-M, LV, GS-L, VC-G, AS-S, BG, AF, IR-G, MTO, IE, JL, JÁH-R, SM-C, IA, EF, IG-C, CGS, ARP, MS-L, AC, DC. Authors responsible for writing the manuscript: JLP and RM. were responsible for writing and supervising the writing of the manuscript. All co-authors were responsible for reviewing the analysis interpretation, suggesting modifications to the text, critically reviewing the manuscript, and final approval of the manuscript.
Data availability
Data available upon formal request by email to the Spanish hematopoietic transplant and cell therapy group (GETH-TC).
Competing interests
The authors declare no competing interests.
Ethics approval
The local research ethics committee of the Hospital Clínico Universitario of Valencia approved the registry and study protocol (reference code 35.21).
Patient consent
All patients included in this registry gave their sign informed consent in accordance with the declaration of Helsinki.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-022-00778-3.
References
- 1.Langerbeins P, Hallek M. COVID-19 in patients with hematologic malignancy. Blood. 2022;140:236–52. doi: 10.1182/blood.2021012251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piñana JL, Martino R, García-García I, Parody R, Morales MD, Benzo G, et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21.. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Suárez J, de la Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V, et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. 2020;13:133. doi: 10.1186/s13045-020-00970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagano L, Salmanton-García J, Marchesi F, Busca A, Corradini P, Hoenigl M, et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA) J Hematol Oncol. 2021;14:168. doi: 10.1186/s13045-021-01177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;S2352-3026:30429–4. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ljungman P, de la Camara R, Mikulska M, Tridello G, Aguado B, Zahrani MA, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;2:1–10. doi: 10.1038/s41375-021-01302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribera JM, Morgades M, Coll R, Barba P, López-Lorenzo JL, Montesinos P, et al. Frequency, Clinical Characteristics and Outcome of Adults With Acute Lymphoblastic Leukemia and COVID 19 Infection in the First vs. Second Pandemic Wave in Spain. Clin Lymphoma Myeloma Leuk. 2021;21:e801–e809. doi: 10.1016/j.clml.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piñana JL, López-Corral L, Martino R, Vazquez L, Pérez A, Martin-Martin G, et al. SARS-CoV-2 vaccine response and rate of breakthrough infection in patients with hematological disorders. J Hematol Oncol. 2022;15:54. doi: 10.1186/s13045-022-01275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagano L, Salmanton-García J, Marchesi F, López-García A, Lamure S, Itri F, et al. COVID-19 in vaccinated adult patients with hematological malignancies: preliminary results from EPICOVIDEHA. Blood. 2022;139:1588–92. doi: 10.1182/blood.2021014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ssentongo P, Ssentongo AE, Voleti N, Groff D, Sun A, Ba DM, et al. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2022;22:439. doi: 10.1186/s12879-022-07418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mittelman M, Magen O, Barda N, Dagan N, Oster HS, Leader A, et al. Effectiveness of the BNT162b2mRNA COVID-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood. 2022;139:1439–51. doi: 10.1182/blood.2021013768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La J, Wu JT, Branch-Elliman W, Huhmann L, Han SS, Brophy MT, et al. Increased COVID-19 breakthrough infection risk in patients with plasma cell disorders. Blood. 2022:blood.2022016317. 10.1182/blood.2022016317. [DOI] [PMC free article] [PubMed]
- 13.Piñana JL, López-Corral L, Martino R, Montoro J, Vazquez L, Pérez A, et al. SARS-CoV-2-reactive antibody detection after SARS-CoV-2 vaccination in hematopoietic stem cell transplant recipients: prospective survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. Am J Hematol. 2022;97:30–42. doi: 10.1002/ajh.26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piñana JL, Garcia-Sanz R, Martino R, Garcia-Roa M, Martin-Martin GA, Risco-Gálvez I, et al. Booster effect after SARS-CoV-2 vaccination in immunocompromised hematology patients with prior COVID-19. Blood Adv. 2022;6:848–53. doi: 10.1182/bloodadvances.2021006326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piñana JL, Vázquez L, Martino R, de la Cámara R, Sureda A, Rodríguez-Veiga R, et al. Spanish Society of Hematology and Hemotherapy expert consensus opinion for SARS-CoV-2 vaccination in onco-hematological patients. Leuk Lymphoma. 2021. 10.1080/10428194.2021.1992619. [DOI] [PMC free article] [PubMed]
- 16.https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Actualizacion_variantes_20220523.pdf
- 17.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 2012;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 19.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. doi: 10.1214/aos/1176350951. [DOI] [Google Scholar]
- 20.https://cnecovid.isciii.es/covid19/#evoluci%C3%B3n-pandemia.
- 21.World Health Organization. Evaluation of influenza vaccine effectiveness: a guide to the design and interpretation of observational studies. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO.ISBN 978-92-4-151212-1 © World Health Organization 2017.
- 22.De la Puerta R, Montoro J, Aznar C, Lorenzo I, González-Barberá EM, Balaguer-Roselló A, et al. Common seasonal respiratory virus infections in allogeneic stem cell transplant recipients during the SARS-COV-2 pandemic. Bone Marrow Transpl. 2021;56:2212–20. doi: 10.1038/s41409-021-01319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Q, Bates B, Shao YR, Hsu FC, Liu F, Madhira V, et al; Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-world evidence from the National COVID Cohort Collaborative. J Clin Oncol. 2022:JCO2102419. 10.1200/JCO.21.02419. [DOI] [PMC free article] [PubMed]
- 24.Ehmsen S, Asmussen A, Jeppesen SS, Nilsson AC, Østerlev S, Vestergaard H, et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021;39:1034–6. doi: 10.1016/j.ccell.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–73. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatzikonstantinou T, Kapetanakis A, Scarfò L, Karakatsoulis G, Allsup D, Cabrero AA, et al. COVID-19 severity and mortality in patients with CLL: an update of the international ERIC and Campus CLL study. Leukemia. 2021;35:3444–54. doi: 10.1038/s41375-021-01450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–92. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niemann CU, da Cunha-Bang C, Helleberg M, Ostrowski SR, Brieghel C. Patients with CLL have a lower risk of death from COVID-19 in the Omicron era. Blood. 2022;140:445–50. doi: 10.1182/blood.2022016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piñana JL, Rodríguez-Belenguer P, Caballero D, Martino R, Lopez-Corral L, Terol MJ, et al. Applicability of probabilistic graphical models for early detection of SARS-CoV-2 reactive antibodies after SARS-CoV-2 vaccination in hematological patients. Ann Hematol. 2022. 10.1007/s00277-022-04906-8. [DOI] [PubMed]
- 30.Perry C, Luttwak E, Balaban R, Shefer G, Morales MM, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5:3053–61. doi: 10.1182/bloodadvances.2021005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shree T, Shankar V, Lohmeyer JJK, Czerwinski DK, Schroers-Martin JG, Rodriguez GM, et al. CD20-targeted therapy ablates de novo antibody response to vaccination but spares pre-established immunity. Blood Cancer Discov. 2022. 10.1158/2643-3230.BCD-21-0222. [DOI] [PMC free article] [PubMed]
- 32.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39:1031–3. doi: 10.1016/j.ccell.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen NT, Chinn J, De Ferrante M, Kirby KA, Hohmann SF, Amin A. Male gender is a predictor of higher mortality in hospitalized adults with COVID-19. PLoS ONE. 2021;16:e0254066. doi: 10.1371/journal.pone.0254066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jian F, Yu Y, Song W, Yisimayi A, Yu L, Gao Y, et al. Further humoral immunity evasion of emerging SARS-CoV-2 BA.4 and BA.5 subvariants. Lancet Infect Dis. 2022. 10.1016/S1473-3099(22)00642-9. [DOI] [PMC free article] [PubMed]
- 35.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial. Lancet. 2020;396:467–78. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, et al. Efcacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–62. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–40. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing: update on hospitalisation and vaccine effectiveness for omicron VOC-21NOV-01 (B.1.1.529). https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1044481/Technical-Briefing-31-Dec-2021-Omicron_severity_update.pdf
- 40.Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022. 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon formal request by email to the Spanish hematopoietic transplant and cell therapy group (GETH-TC).