Abstract
Early life development and its divergence is influenced by multiple genetic, neurological, and environmental factors. Atypical neurodevelopment, such as that observed in autism spectrum disorder, likely begins in early gestation during a period of entwined growth between the brain and epithelial barriers of the skin, gastrointestinal tract, and airway. This review coalesces epidemiological and neuroinflammatory evidence linking cutaneous atopic disease with both reduced skin barrier integrity and determinants of neurodivergence. We consider the shared developmental origin of epidermal and neural tissue with related genetic and environmental risk factors to evaluate potential pre- and postnatal modifiers of the skin-brain connection. Initial postnatal skin barrier integrity may provide a useful marker for both cortical integrity and meaningful subgroups of children showing early neurodevelopmental delays. It may also modify known risk factors to neurodevelopment, such as pathogen caused immune system activation. These novel insights of a skin-brain-neurodevelopment connection may advance detection and intervention opportunities.
Subject terms: Psychology, Predictive markers
Introduction
Early neurodevelopmental delays are underpinned by a complex interplay between heterogeneous genetic, neurological, and environmental factors. Individuals with Autism Spectrum Disorder (ASD), for instance, show marked social delay, characterised by a continuum of difficulties during social interactions, communication, and the presence of restrictive and repetitive behaviours [1]. This neurodevelopmental divergence occurs amongst a backdrop of other physical and medical comorbidities [2, 3]. Neurocutaneous syndromes, such as tuberous sclerosis complex (TSC) and neurofibromatosis type 1 (NF1), and atopic syndromes, including atopic dermatitis, allergic rhinitis, and asthma, are all frequently reported [2, 3]. Interestingly, the global prevalence of these ‘atopic diseases’ has risen in parallel to childhood-onset ASD, recently reported to affect one in 54 children in the United States [4, 5]. This temporal synchroneity in diagnostic rates of atopic diseases and ASD has prompted investigations into the association between the two.
In this paper, we propose a novel hypothesis, that there is an antenatal link between skin and neurodevelopment, partially underpinned by the tissue’s shared ectodermal origin with common molecular factors. Additionally, we evaluate postnatal mediators of this skin-brain co-vulnerability, considering the role of epidermal keratinocytes and the cutaneous microbiota in cortical development. Accordingly, we hypothesise that skin barrier integrity may represent an accessible and novel biomarker to aid in the early detection of neurodevelopmental divergence. We further propose that skin barrier integrity may play a crucial role in mediating the relationship between environmental triggers of infection, immune processes, and neurodevelopment, with potential to reduce the impact of such environmental triggers by improving skin barrier integrity.
Atopic comorbidities in ASD
Accumulating evidence supports an association between atopic dermatitis (AD) and neurodevelopmental divergence. This chronic, inflammatory disease is the most common paediatric skin condition in developed countries, characterised by dry and scaly cutaneous lesions, itch, pain and oozing, that manifest during the first year of life in 60% of those affected [6–8]. Interestingly, AD’s perceived link with psychopathology has been long appreciated, referred to as “neuro-dermatitis” in the early 1900s and a “psycho-somatic” disorder in the 1950s [9]. As anticipated, the observed association between AD and neurodevelopmental divergence has been extensively investigated, yet scant attention has been directed towards understanding its underlying mechanistic basis.
Population-based epidemiological studies consistently support a link between atopic eczema and ASD. Using the 2007 U.S. National Survey of Children’s Health, for instance, Yaghmai et al. [10] reported eczematic children to be 2.73 times more likely to meet the criteria for ASD, relative to their non-atopic peers (Table 1). Data from Taiwan’s National Health Insurance Database similarly indicated that atopic diseases likely precede and predict ASD onset, with 118 atopic children diagnosed with ASD at follow-up, compared to only 12 non-atopic participants [3]. Notably, ASD risk appeared to further increase alongside the number and severity of reported atopic comorbidities [3, 7].
Table 1.
Summary of study characteristics.
| Study ID, ref. | Study design | Sample size | Age (years) | Main findings | Limitations |
|---|---|---|---|---|---|
| Bakkaloglu et al. [73] | Case–control study | N = 71 | 2–4 | 7/30 autistic children and 7/39 age and sex-matched controls reported allergic symptoms (p = ns). | A small sample size of 32 children with autism and 39 controls. |
| Chen et al. [3] | Longitudinal follow-up study | N = 21 756 | 0–3 |
Early childhood atopic disease increased the risk of ASD (HR 3.40, 95% CI: 1.95–5.93). HR of ASD increased with the number of atopic comorbidities. 1: HR 2.14 (95% CI: 0.90–5.11) 2: HR: 2.70 (95% CI: 1.44–5.05) 3: HR: 4.08 (95% CI: 2.24–7.43) 4: HR: 4.29 (95% CI: 2.25–8.19) |
ASD incidence was likely underestimated as the sample only included those who sought medical attention. |
| Gurney et al. [12] | Population-based cross-sectional study | N = 85 272 | 3–17 |
Atopic prevalence in ASD cohort vs. control Hay fever: 27% vs. 16.2% OR: 1.6 (95% CI: 1.1–2.4) Food allergy: 14.1% vs. 3.2% OR: 4.5 (95% CI: 3.0–7.0) Eczema or skin allergy: 14.9% vs. 9.2% OR: 1.4 (95% CI 1.0–2.1) |
Unvalidated parental report of atopic manifestations and no subtype analysis of ASD. |
| Liao et al. [7] | Population-based case–control study | N = 32 956 | 0.08–3 |
HR of ASD increased with the number of eczema-related doctor visits. 1: aHR 1.15 (95% CI 1.06–1.24) 2–3: aHR 1.18 (95% CI 1.07–1.29) 4+: aHR 1.40 (95% CI 1.25–1.56) |
Data may be confounded by patterns of help-seeking behaviour. |
| Magalhães et al. [74] | Case–control study | N = 45 | 7–18 | 86.6% of Asperger patients were atopic (allergic symptoms, elevated serum IgE, high eosinophil count or positive skin prick test), compared to <7% of controls | A small sample size of 15 Asperger patients, 15 atopic neurotypicals and 15 non-atopic neurotypicals |
| Miyazaki et al. [11] | Systematic review and meta-analysis | N = 10 380 | 2.5–18 | Prevalence of asthma and allergic rhinitis were significantly increased in the ASD cohort (OR 1.69; 95% CI: 1.11–2.59 and OR 1.66; 95% CI: 1.49–1.85 respectively) | ASD diagnostic criteria varied across studies (e.g. DSM-V vs. ADOS vs. ICD-10) |
| Tsai et al. [75] | Systematic review and meta-analysis | N = 1 055 837 | 0–18 |
Prevalence of AD was significantly higher in individuals with ASD than the control group OR: 1.485 (95% CI: 1.20–1.83) |
Heterogeneity in diagnostic criteria, age, race and disease severity across studies. |
| Xu et al. [5] | Population-based, cross-sectional study | N = 199 520 | 3–17 | Children with ASD were more likely to report food allergy (11.25% vs. 4.25%), respiratory allergy (18.73% vs. 12.15%) & skin allergy (16.81% vs. 9.84%) than a non-autistic comparison group. | Retrospectively collected self-reported data may have enabled misreporting due to recall bias |
| Yaghmai et al. [10] | Cross-sectional study | N = 92 642 | 0–17 |
Prevalence of ASD was significantly increased in children with eczema (2.19%) compared to non-autistic controls (0.89%). OR ASD: 2.73 (95% CI 1.94–3.84) |
Parental reporting subject to misclassification bias |
| Zerbo et al. [76] | Case–control study | N = 33 390 | 3–26 |
Allergies were more frequently diagnosed in ASD cohort (20.6%) vs. controls (17.7%) OR: 1.22 (95% CI 1.13–1.31) |
Did not account for heterogeneous ASD subtypes. |
Given that AD often marks the onset of the “atopic march”, facilitating subsequent allergic sensitisation, various atopic manifestations should co-occur in neurodiverse populations. Accordingly, a meta-analysis including 10 380 children [11] found increased rates of asthma and allergic rhinitis in participants with ASD relative to non-autistic populations. Similarly, using the 2004 U.S. National Survey of Children’s Health, Gurney et al. [12] observed eczema, hay fever and food allergy to be significantly more prevalent amongst 483 children with autism compared to their neurotypical peers. Additionally, analysing data from the U.S. National Health Interview Scheme, Xu et al. [5] reported across a large and multi-racial sample that autistic children were more likely to experience food allergy (11.25% vs. 4.25%), respiratory allergy (18.73% vs. 12.15%) and skin allergy (16.81% vs. 9.84%) relative to matched controls. More recently, one recent study in children with ASD suggested that the presence of comorbid atopic conditions was associated with more severe ASD symptoms, with the prensence of atopic diseases more than doubling the likelihood of severe ASD symptoms and severe social impairment scores [13].
Antenatal parallels between cortical and epidermal development
Overall, whilst the positive association between atopic disorders and ASD is well-established, the biological basis of this association has not been elucidated. Here, we consider the potential prenatal genesis of this link, reviewing the shared in utero development of skin and neural tissue alongside common genetic susceptibility variants.
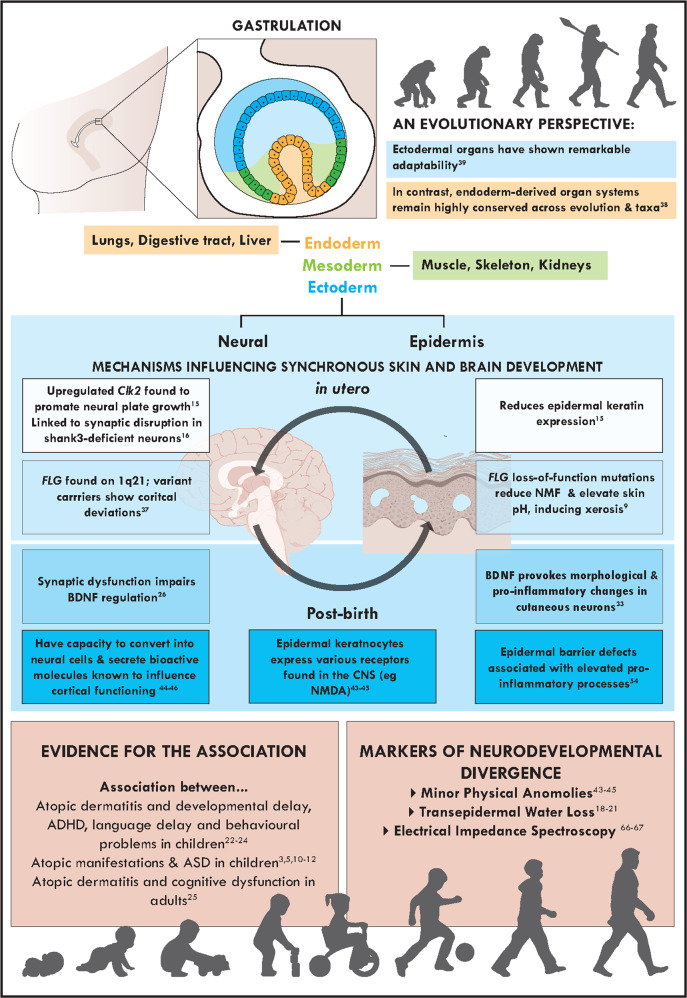
Gastrulation is a fundamental process in early embryonic development, beginning at three post-conception weeks (PCW) to induce the formation of three distinct cell layers: the mesoderm, endoderm, and ectoderm [14]. Over the following 5 weeks, these primary germ layers undergo organogenesis, differentiating into the body’s organs [14]. Importantly, the brain, skin and skin appendages develop in synchronisation, all originating from the embryonic ectoderm [15]. Accordingly, from 3 to 4 PCW, the ventral ectoderm differentiates into a monolayer epidermis whilst the dorsal ectoderm becomes the neuroectoderm, from which early derivatives of the brain develop [14]. It is therefore plausible that early deviations to neural development may influence patterns of epidermal differentiation.
Preliminary evidence supporting this hypothesis can be derived from a study [16] investigating Xenopus laevis embryonic development. The researchers demonstrated that upregulated Cdc2-like kinase 2 (Clk2), found in neural tissue to augment signalling pathways regulating organogenesis, promotes neural plate growth at the expense of epidermal development. Their findings revealed that Clk2 overexpression both induced the neural gene markers sip1 and sox3 whilst reducing epidermal keratin expression compared to controls. This is striking in the context of neurodevelopmental disorders, such as ASD, as elevated Clk2 has been linked to synaptic disruption in Shank3-deficient neurons in mouse models of ASD [17]. Further, the Clk2-induced neural plate expansion observed during Xenopus embryogenesis holds similarities with the prenatal cortical overgrowth often observed in autistic children [18]. The transferability of these findings is also strengthened by Clk2 being the most abundantly expressed Clk family member in the mammalian brain [16]. Previous literature, therefore, indicates that abnormal neural development can alter epidermal differentiation in utero.
Further supporting a fundamental link between skin and neurodevelopment are studies investigating ectoderm-derived minor physical anomalies (MPAs) in neurodivergent populations. MPAs are subtle phenotypic deviations to the craniofacial or limb regions, observed to occur significantly more frequently in those with neurodevelopmental delay (up to 60%), such as that seen in fetal alcohol syndrome, attention-deficit/hyperactivity disorder (ADHD) and ASD [19–21]. Consequently, prior literature has posited MPAs to represent surface biomarkers that may reflect atypical neurodevelopment arising during the first trimester of pregnancy [19, 20]. In support of this hypothesis, Miles et al. [22] reported that MPAs strongly correlate with structural brain anomalies, with 19 adults showing MPAs being twice as likely (29%) to record abnormal MRIs relative to 51 adults without morphological deviations (14%). Their findings were later replicated in children, where inter-orbital MPAs were observed to positively correlate with bilateral amygdalae volume in 36 children with ASD [19]. Overall, evidence correlating ectodermal-derived MPAs with neurodevelopmental divergence further supports a link between cortical and epidermal development.
Moreover, if an ectodermal link exists between skin and neurodevelopment, epidermal abnormalities should co-occur with various neurodevelopmental conditions. Using data from the Danish National Patient Registry, Vittrup et al. [23] reported that eczema was significantly associated with ADHD in 157 113 children (Table 2). This coincides with data from the U.S. National Health Interview Survey spanning 2008–2018, observing in 109 483 children that AD was associated with caregiver-reported developmental delay and ADHD [24]. These findings further align with an earlier cross-sectional study, which found that 36% of 639 children showing language delay and problems in internalisation and externalisation symptoms presented with comorbid AD [25]. Interestingly, the association between neurodevelopmental delay and altered skin development appears to persist beyond paediatric populations. In an adult prospective study, for instance, 66.8% of 366 AD patients self-reported at least one symptom of cognitive dysfunction, with 10.6% showing moderate to severe cognitive impairments, determined by their PROMIS Cognitive Function scores [26].
Table 2.
Summary of study characteristics.
| Study ID, ref. | Study design | Sample size | Age (years) | Main findings | Limitations |
|---|---|---|---|---|---|
| Jackson-Cowan et al. [24] | Population-based cross-sectional study | N = 109 483 | 2–17 | Childhood AD was significantly associated with developmental delay (aOR: 1.54; 95% CI: 1.40–1.40) (p < 0.001) and ADD/ADHD (aOR: 1.31; 95% CI: 1.20–1.42) (p < 0.001) | Did not control for sedating medications that may have influenced cognitive function (e.g. antihistamines) |
| Kandelaki et al. [25] | Cross-sectional study | N = 639 | 5–6 | 36% of children with language delays and problems in internalisation and externalisation reported comorbid AD | Cross-sectional design prevented the inclusion of follow-up data |
| Silverberg et al. [26] | Prospective study |
Baseline: N = 366 Follow-up: N = 245 |
18–88 |
Baseline: 66.8% self-reported ≥1 symptom of cognitive dysfunction (e.g. slowed thinking, difficulty concentrating) Follow-up: 5.4% showed moderate and 5.2% showed severe PROMIS cognitive function T-scores |
The baseline cognitive function questionnaire was self-administered and may be subject to recall bias |
| Vittrup et al. [23] | Population-based longitudinal study | 157 113 | 0–18 |
Childhood AD was significantly associated with ADHD HR: 1.89 (95% CI: 1.56–2.29) |
AD severity was classified based on the proxy of medication use, allowing for misclassification bias |
Molecular factors common to brain and skin development
The association between skin and neural development is further strengthened by common molecular factors, including brain-derived neurotrophic factor (BDNF) and filaggrin, which appear abnormally expressed in both ASD and AD.
Brain-derived neurotrophic factor
BDNF is an activity-dependent neurotrophin regulating synaptic plasticity and the development of cortical connections [27]. BDNF acts via the pan-neurotrophin receptor (p75NTR), to which all mature neurotrophins bind, or the tropomyosin receptor kinase B (TrkB), to which it shows high affinity and specificity [28]. Numerous studies have detected elevated serum and plasma concentrations of BDNF in children with autism, compared to non-autistic controls [27, 29, 30]. BDNF has therefore been proposed to contribute to altered neurodevelopment in ASD pathology. In neurotypical individuals, for instance, BDNF increases pre-synaptic glutamate release and post-synaptically augments NMDA receptors, inducing excitatory activity that is dampened by neighbouring receptors [29]. In those with ASD, however, this negative regulation appears to be impaired by synaptic dysfunction, provoking cytotoxicity associated with neurodevelopmental divergence [27].
Interestingly, BDNF may also mediate the interaction between immune cells and neurons in chronic atopic conditions [28]. For instance, elevated BDNF has been observed in bronchoalveolar lavage samples of 8 male asthmatics [31] and the nasal mucosa of 9 allergic rhinitis patients following allergen exposure [32]. Numerous studies have also identified significantly increased serum BDNF concentration in eczematic children relative to non-atopic participants [28, 33]. Notably, in those with AD, BDNF appears to be expressed by cutaneous eosinophils, which promote axonal outgrowth and neurite branching in dorsal root ganglion neurons when stimulated by a plate-activating factor [34]. Further, BDNF has been observed to elicit chemotaxis and halt eosinophil apoptosis exclusively in AD patients [33]. Taken together, these findings support BDNF’s role in provoking morphological and pro-inflammatory changes in the cutaneous neurons of those with AD [34]. BDNF’s neurotrophic and immunomodulatory activities may therefore increase susceptibility to both atypical neurodevelopment and AD-associated cutaneous inflammation.
Filaggrin
Additionally, the FLG gene has garnered attention, with common loss-of-function mutations determined as susceptibility variants for both ASD and AD [35–37]. FLG is part of the epidermal differentiation gene complex encoding the precursor for filaggrin [6]. Mature filaggrin units themselves are fundamental to the stratum corneum, aggregating keratin intermediary filaments and regulating terminal differentiation via disulphide bond formation [37]. When proteolysed, filaggrin also assists in epidermal water retention by contributing to the stratum corneum’s Natural Moisturising Factors (NMF) [37]. Interestingly, FLG is located on chromosome 1q21, a region of the genome where deletion and duplication carriers have been observed to be at a higher risk for ASD [38]. For example, a large-scale neuroimaging study of over 37 000 participants found that brain structural abnormalities detected in 1q21.1 variant carriers overlapped with cortical deviations often observed in those with ASD [38]. Recently, FLG itself was also identified as a possible genetic risk factor for ASD [35, 36]. For instance, Shi et al.’s [36] genome-wide association study found seven candidate genes shared by two siblings with autism, one of which was FLG with two deleterious mutations. Similarly, Chang et al. [35] later identified FLG as one of seven pathogenic variants found in four probands, observing a p.E2322X mutations in the FLG2 gene.
Unsurprisingly, FLG loss-of-function variants have been long-identified as major risk factors for AD in children [35–37]. Flohr et al. [6] for instance, observed in 88 Caucasian infants that those carrying a common FLG mutation were four times more likely to experience eczema by 3 months of age than those without the variant. This association between FLG mutations and xerosis appears to be underpinned by a reduction in NMF and elevations in skin pH that provoke downstream effects, including abnormal lipid transport to the stratum corneum [9].
From an evolutionary perspective, the variability profiles of genes underlying ectodermal and endodermal organ development are strikingly distinct. Whilst the genetic mechanisms regulating endoderm-derived organ development have shown a high degree of evolutionary conservation across various vertebrate classes [39], gene families coordinating ectodermal development appear to be strongly driven by adaptive evolution [40]. The human brain, for example, has evolved extensively throughout history, responding to changes in our environment and climate, as evidenced by a rapid expansion of higher-order functional networks [40, 41]. Notably, the skin has shown analogous evolutionary potential, with the loss of body hair provoking the evolution of genes regulating keratinisation and epidermal differentiation to enhance cutaneous barrier function [42]. To date, the complete repeats of FLG continue to show high nucleotide diversity and copy number variation across primates [43]. Interestingly, this similarity in neural and epidermal evolvability may arise from the decentralised neurological system of primitive multicellular animals, including Cnidarians, possibly marking the origins of the surface ectoderm’s link with brain development [44]. Therefore, whilst endoderm-derived organ systems remain highly conserved across evolution and taxa, the genetic basis of ectodermal development has shown remarkable adaptability, which may partially underly the basal link between skin aberrations and neurodevelopmental divergence.
Postnatal modifiers of the skin-brain connection
Epidermal keratinocytes
The principal link between epidermal and neurodevelopment has further been observed to persist postnatally, with the notion of a skin-brain or brain-skin axis growing in popularity. Epidermal keratinocytes appear to be central to this association, with these ‘information and sensory processing cells’ expressing numerous receptors found within the central nervous system (CNS) [44, 45]. NMDA receptors, for instance, are expressed in both epidermal keratinocytes, influencing epidermal proliferation and barrier maintenance, and the hippocampus, where their dysfunction has been associated with ASD-related pathology in mice [45, 46]. Keratinocytes also appear to release oxytocin, a fundamental hormone in behaviour and social bonding, in the presence of an ATP analogue [47]. This is significant in the context of ASD, where oxytocin may improve social cognition and development [48]. Notably, epidermal cells also seem to have the capacity to convert into neural cells, with Tenorio-Mina et al. [49] transdifferentiating human keratinocytes into neural progenitors in vitro that produce neuronal proteins when transplanted into a developing rat brain. Taken together, prior research suggests that epidermal keratinocytes may house sensory systems and secrete bioactive molecules able to postnatally influence neural functioning.
HPA-axis mediated brain-skin crosstalk
Persistent neuro-epidermal communication further appears to be coordinated by the sympathetic nervous system (SNS), whose sensory neuron cell bodies occupy dorsal root ganglia innervating both the skin and CNS and the hypothalamic-pituitary-adrenal (HPA)-axis [18, 50]. Interestingly, mammalian epidermal keratinocytes express all HPA-axis components, which function to regulate cutaneous anti-microbial defence [18, 51]. Consequently, environmental factors disrupting axis activity, including stress, have been associated with epidermal abnormalities, inducing the activation of cutaneous mast cells and corticotrophin-releasing hormone, stimulating cytokine secretion [50]. The neural modulation of epidermal functioning is additionally supported by Hunter et al. [52] observations of slowed cutaneous wound healing during periods of heightened stress, perhaps attributable to the SNS release of noradrenaline and adrenaline associated with inhibited fibroblast growth and proliferation [53]. In summary, previous literature supports a skin-brain connection modified by the HPA-axis postnatally, pointing to the epidermal barrier as possibly representing a “diagnostic window into the brain” from which simple biomarkers of neurodevelopment could be identified [50].
Childhood inflammation
The cutaneous microbiota and postnatal skin inflammation may represent additional early life modifiers of neurodevelopment. After birth, infant skin is rapidly colonised by diverse microbiota, prompting epidermal maturation to protect against the transcutaneous invasion of external irritants [54]. In instances of skin barrier deficiency, the colonising microbiome may provoke cutaneous infection, transforming the largest organ of the body into a potential source of systemic inflammation [55]. This phenomenon has been observed in an N-WASPCK14-Cre KO mouse model, where induced epidermal barrier defects were associated with increased mast cell and eosinophil influx to the dermis as well as a significantly increased serum concentration of pro-inflammatory cytokines, including interleukin (IL)-1α, IL-6 and IL-17 [55]. Interestingly, elevated IL-6 and IL-17A have similarly been detected in the plasma and serum of children with ASD, with prior research indicating that these mediators may traverse the BBB and provoke neuroinflammation in circuitry regulating developmental outcomes [56]. Upregulated inflammatory processes provoked by the cutaneous microbiota’s colonisation of a damaged epidermal barrier may therefore act as a postnatal modifier of neurodevelopment.
Thermoregulation
To conclude our review of the postnatal skin-brain connection, we turn our attention towards the skin’s thermoregulatory function. Following the loss of fur, hominin’s capacity for thermoregulation advanced, shifting from thermal panting to high-density eccrine sweat glands [42]. In the same period, our cognitive capacity increased emphatically, spurring interest as to whether thermoregulation may influence neural development. Preliminary evidence for this hypothesis stems from recent zebrafish models, where the ambient water temperature was observed to drive persistent changes in exploratory behaviour [57]. These findings indicate that thermoregulatory mechanisms may drive intra-individual variability in vertebrate kinematics, likely by influencing neural circuits that regulate locomotor control [57]. In humans, anecdotal evidence supporting a link between thermoregulation and neurodevelopment can be derived from parental reports of improved social interaction and language ability during febrile states in autistic children [58]. Additionally, daily skin-to-skin care in preterm infants, which provides thermoregulatory stimulation, has been observed to improve children’s cognitive development and executive functioning at 10 years of age, relative to no-contact infants [59]. In summary, prior literature indicates that thermoregulation may modify neural development, providing further evidence for a fundamental skin-brain connection persisting postnatally.
Plausible skin barrier integrity markers for neurodevelopmental divergence
Transepidermal water loss
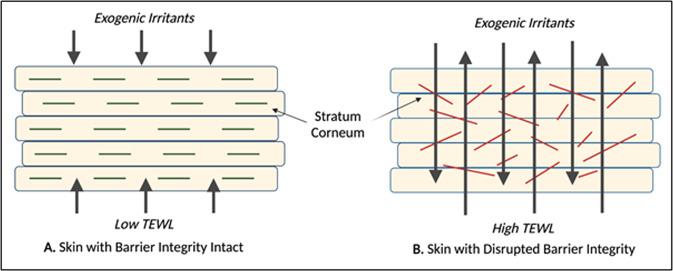
Thus far, this paper has reviewed accumulating evidence supporting a principal link between cortical and epidermal development, underpinned by their common in utero origin and genetic susceptibility variants, that endures postnatally (see Fig. 1). Consequently, we propose that skin barrier integrity might represent an early indicator for neurodevelopmental divergence. The most widely used tool to measure epidermal barrier function in vivo is transepidermal water loss (TEWL), a non-invasive technique that calculates water’s flux density as it diffuses from the dermis and epidermis, through the stratum corneum, to the skin’s surface (Fig. 2) [60]. An elevated TEWL indicates a disrupted epidermal barrier, increasing the ease with which microbes and immunogens can penetrate the skin [60]. Given the proposed skin-brain co-vulnerability and frequent reports of cutaneous disorders in neurodiverse populations, we consider whether TEWL may serve as a novel biomarker for detecting early neurodevelopmental delay [61].
Fig. 1. The ectoderm and neurodevelopmental divergence.
This figure describes the evolutionary and developmental skin-brain connection, common molecular factors that underpin this relationship in utero and post-birth, the existing evidence for associations between atopic disease and neurodevelopmental delay, and potential early life markers that could identify neurodevelopmental divergence.
Fig. 2.
A schematic diagram showing how the integrity of the skin barrier may influence allergen penetration and TEWL measurements in (A) Healthy skin and (B) Disturbed skin.
Epidermal barrier function in atopic dermatitis has been extensively investigated. For instance, Seidenari and Giusti [62] observed elevated TEWL at eczematous skin of the forehead, cheek, volar and dorsal forearm, and abdomen of 100 children with AD compared to 21 healthy participants (Table 3). Their findings were later replicated across a spectrum of measurement sites, with significant increases in TEWL also detected at lesions of the postauricle, thigh, and popliteal fossa of 25 AD patients [63]. Further, this association remains perceptible in infant cohorts [64], with elevated TEWL at the non-lesional upper arm being significantly associated with an eczema diagnosis at follow-up. Increased TEWL, therefore, appears to precede and predict AD onset, and is indicative of widespread epidermal barrier dysfunction, with Montero-Vilchez et al. [65] measuring heightened TEWL at the non-lesional skin of eczema patients relative to non-atopic controls.
Table 3.
Summary of study characteristics.
| Study ID, ref. | Study design | Sample size | Age (years) | Main findings | Limitations |
|---|---|---|---|---|---|
| Berents et al. [77] | Population-based cohort study | N = 116 | 1.2–13.4 |
TEWL > 9.33 g/m2h at visit 1 was significantly associated with a diagnosis of AD at visit 2 (p = 0.03) AD OR: 3.32 (95% CI: 1.15–9.60) |
TEWL was measured by multiple clinicians at two medical services across a range of humidity |
| Kim et al. [63] | Case–control study | N = 50 | 12–39 | The mean TEWL value measured across AD participants was significantly greater than that of age-matched healthy controls at all evaluation sites (postauricle, forearm, abdomen, thigh, and popliteal fossa) | TEWL was measured using an open-chamber device (vulnerable to ambient airflow) |
| Montero-Vilchez et al. [65] | Cross-sectional study | N = 130 | X̅ = 32.05 | Mean TEWL was elevated at the uninvolved skin of AD patients (13.15 g/m2h) compared to healthy controls (11.60 g/m2h) | Cross-sectional design prevented participant follow-up |
| Seidenari and Guisti [62] | Cross-sectional study | N = 121 | 3–12 | TEWL was increased at the eczematous lesions of children with AD compared to control subjects (p < 0.05) | TEWL was measured using an open-chamber device (vulnerable to ambient airflow) |
| Shin et al. [66] | Randomised controlled trial |
N = 20 first-generation N = 100 pups |
Neo-natal |
Cutaneous scaling was observed in VPA mice. TEWL levels measured 4 days post-partum were elevated in VPA mice compared to controls |
The unique physiology of the human brain limits the transferability of mouse model findings |
| Shin et al. [66] | Randomly selected cohort study | N = 25 | X̅ = 19 | Abnormalities in skin barrier permeability and skin hydration were observed in the ASD cohort, yet these did not reach statistical significance (p = 0.2) |
A small sample size (n = 25) Differences in TEWL may be more difficult to discern in adolescent populations (influenced by age-associated changes in the stratum corneum) |
Investigations into skin barrier integrity in neurodivergent children, however, are scarce. In animals, Shin et al. [66] conducted a randomised controlled trial to evaluate epidermal integrity in a valproic acid (VPA)-induced mouse model of autism. As anticipated, the VPA-exposed neo-natal mice displayed both neural and cutaneous structural abnormalities, displaying vacuolisation exclusively in the brain and outer nucleated layer of the epidermis. Interestingly, the VPA mice additionally showed significantly elevated TEWL measurements, taken 4 days post-partum, relative to the controls. Whilst this rodent model offers preliminary insight into the role of cutaneous permeability in ASD, the unique physiology of the human brain limits the transferability of their findings. Shin et al. [67] also investigated skin barrier function in a cohort of 25 adolescents with ASD. In line with their mouse model findings, TEWL appeared likely elevated in individuals with ASD, however, differences did not reach statistical significance in this small sample size, with adolescent-associated corneocyte flattening and heightened sebum secretion also possibly reducing the ability to discern differences between groups [60].
Electrical impedance spectroscopy
Electrical impedance spectroscopy (EIS) has recently emerged as a promising alternative measurement of skin barrier function. EIS is a non-invasive indicator of epidermal integrity, rapidly measuring the skin’s resistance to the flow of alternating imperceptible currents [67, 68]. Considering the likely differences in cell size and orientation between typical and abnormal skin, EIS has been suggested as a useful proxy for disease status [67, 68]. Currently, EIS has been observed to successfully differentiate between healthy, lesional and non-lesional skin in adults with AD, with these readings inversely correlating to TEWL [68]. To date, no study has measured EIS in neurodevelopmentally diverse populations. Preliminary evidence from eczematic patients, however, has shown EIS to negatively correlate with serum proteins associated with the inflammatory response, indicating that upregulated inflammatory processes, as observed in ASD, may be associated with weaker barrier function [69]. Of particular interest, EIS measured at the non-lesional skin of adults with AD was found to correlate with the number of tandem repeats in the FLG gene, an established susceptibility variant for both ASD and skin abnormalities [38, 68]. EIS may therefore represent an alternate clinical measure of skin barrier integrity that may function as a surrogate for neurodevelopmental divergence.
Tactile sensitivity
Sensory processing atypicality is increasingly recognised as an important diagnostic and common feature of ASD [69], which may include both hyper- and hypo-sensitivity to touch [70]. The current biological mechanisms proposed to underpin this touch-sensitivity skin-brain connection is largely independent to what has been discussed in this review [70–72]. While it is beyond the scope of this review to speculate about any commonalities, future research is required to understand common biological mechanisms that could contribute to both the development of skin barrier integrity and adaptive tactile sensory processing.
Conclusion
In summary, this paper reviewed epidemiological and clinical evidence supporting a fundamental link between skin and neurodevelopment, antenatally mediated by a shared developmental origin and genetic susceptibility variants. Moreover, we consider how the skin, being the body’s largest organ, is a fundamental barrier to diseases, viruses, and infections, and may therefore have a neuroprotective effect by reducing systemic inflammation associated with atypical neurodevelopment. Subsequent to evaluating postnatal modifiers of this skin-brain co-vulnerability, we propose that skin barrier integrity might represent an early indicator of atypical neurodevelopment. Our approach addresses the need for easily accessible clinical tools that support the detection of neural diversity and offers plausible biological and environmental contributors to early life development.
Author contributions
Author CJ, RN, AG were involved in the conception and design of the work; author CJ conducted the review of the literature and initial draft of the paper, authors KB, NS, RN, AG provided substantive edits and revisions to the drafted work and all authors approved the submitted version. This work was partially completed for fulfilment of an honours research thesis to CJ, under the supervision of KB, AG, and RN.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Competing interests
Author AG and NS declare fee for service support of pharmaceutical clinical trials for autism spectrum disorder in their clinic services (including a current and past trial with Axial Therapeutics of a gut-based therapy). These authors receive no personal financial gain from this contracted work. Authors CJ, RN, and KB report no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: R. Nanan, A. J. Guastella.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Publishing; 2013.
- 2.Accordino RE, Lucarelli J, Yan AC. Cutaneous disease in autism spectrum disorder: a review. Pediatr Dermatol. 2015;32:455–60. doi: 10.1111/pde.12582. [DOI] [PubMed] [Google Scholar]
- 3.Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, et al. Is atopy in early childhood a risk factor for ADHD and ASD? a longitudinal study. J Psychosom Res. 2014;77:316–21. doi: 10.1016/j.jpsychores.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Maenner MJ, Shaw KA, Baio J, EdS, Washington A, Patrick M, et al. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill Summ. 2020;69:1–12. doi: 10.15585/mmwr.ss6904a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu G, Snetselaar LG, Jing J, Liu B, Strathearn L, Bao W. Association of food allergy and other allergic conditions with autism spectrum disorder in children. JAMA Netw Open. 2018;1:e180279. doi: 10.1001/jamanetworkopen.2018.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flohr C, England K, Radulovic S, McLean WH, Campbel LE, Barker J, et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br J Dermatol. 2010;163:1333–6. doi: 10.1111/j.1365-2133.2010.10068.x. [DOI] [PubMed] [Google Scholar]
- 7.Liao TC, Lien YT, Wang S, Huang SL, Chen CY. Comorbidity of atopic disorders with autism spectrum disorder and attention deficit/hyperactivity disorder. J Pediatr. 2016;171:248–55. doi: 10.1016/j.jpeds.2015.12.063. [DOI] [PubMed] [Google Scholar]
- 8.Silverberg JI. Selected comorbidities of atopic dermatitis: Atopy, neuropsychiatric, and musculoskeletal disorders. Clin Dermatol. 2017;35:360–6. doi: 10.1016/j.clindermatol.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson EL. Comorbidity in atopic dermatitis. Curr Dermatol Rep. 2012;1:29–38. doi: 10.1007/s13671-011-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428–33. doi: 10.1016/j.jaci.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazaki C, Koyama M, Ota E, Swa T, Amiya RM, Mlunde LB, et al. Allergies in children with autism spectrum disorder: a systematic review and meta-analysis. Rev J Autism Dev Disord. 2015;2:374–401. [Google Scholar]
- 12.Gurney JG, McPheeters ML, Davis MM. Parental report of health conditions and health care use among children with and without autism: National Survey of Children’s Health. Arch Pediatr Adolesc Med. 2006;160:825–30. doi: 10.1001/archpedi.160.8.825. [DOI] [PubMed] [Google Scholar]
- 13.Jameson C, Boulton KA, Silove N, Guastella AJ. Eczema and related atopic diseases are associated with increased symptom severity in children with autism spectrum disorder. Transl Psychiatry. 2022;12:415. doi: 10.1038/s41398-022-02185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donovan MF, Cascella M. Embryology, weeks 6-8. Treasure Island (FL): StatPearls; 2021. [PubMed]
- 15.Rewane A, Munakomi S. Embryology, central nervous system, malformations. Treasure Island (FL): StatPearls; 2021. [PubMed]
- 16.Virgirinia RP, Jahan N, Okada M, Takebayashi-Suzuki K, Yoshida H, Nakamura M, et al. Cdc2-like kinase 2 (Clk2) promotes early neural development in Xenopus embryos. Dev Growth Differ. 2019;61:365–77. doi: 10.1111/dgd.12619. [DOI] [PubMed] [Google Scholar]
- 17.Bidinosti M, Botta P, Kruttner S, Proenca CC, Stoehr N, Bernhard M, et al. CLK2 inhibition ameliorates autistic features associated with SHANK3 deficiency. Science. 2016;351:1199–203. doi: 10.1126/science.aad5487. [DOI] [PubMed] [Google Scholar]
- 18.Donovan AP, Basson MA. The neuroanatomy of autism—a developmental perspective. J Anat. 2017;230:4–15. doi: 10.1111/joa.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung C, McAlonan GM, Fung YY, Fung G, Yu KK, Tai KS, et al. MRI study of minor physical anomaly in childhood autism implicates aberrant neurodevelopment in infancy. PLoS ONE. 2011;6:e20246. doi: 10.1371/journal.pone.0020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manouilenko I, Eriksson JM, Humble MB, Bejerot S. Minor physical anomalies in adults with autism spectrum disorder and healthy controls. Autism Res Treat. 2014;2014:743482. doi: 10.1155/2014/743482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sreeraj VS, Puzhakkal JC, Holla B, Nadella RK, Sheth S, Balachander S, et al. Cross-diagnostic evaluation of minor physical anomalies in psychiatric disorders. J Psychiatr Res. 2021;142:54–62. doi: 10.1016/j.jpsychires.2021.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Miles JH, Hillman RE. Value of a clinical morphology examination in autism. Am J Med Genet. 2000;91:245–53. [PubMed] [Google Scholar]
- 23.Vittrup I, Andersen YMF, Droitcourt C, Skov L, Egeberg A, Fenton MC, et al. Association between hospital-diagnosed atopic dermatitis and psychiatric disorders and medication use in childhood. Br J Dermatol. 2021;185:91–100. doi: 10.1111/bjd.19817. [DOI] [PubMed] [Google Scholar]
- 24.Jackson-Cowan L, Cole EF, Silverberg JI, Lawley LP. Childhood atopic dermatitis is associated with cognitive dysfunction: A National Health Interview Survey study from 2008 to 2018. Ann Allergy Asthma Immunol. 2021;126:661–5. doi: 10.1016/j.anai.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Kandelaki E, Kavlashvili N, Kherkheulidze M, Chkhaidze I. Prevalence of atopic dermatitis symptoms in children with developmental and behavioral problems. Georgian Med N. 2015;243:29–33. [PubMed] [Google Scholar]
- 26.Silverberg JI, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, et al. Association of atopic dermatitis severity with cognitive function in adults. J Am Acad Dermatol. 2020;83:1349–59. doi: 10.1016/j.jaad.2020.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Robinson-Agramonte MLA, Michalski B, Fernandez LG, Vidal-Martinez B, Cuesta HV, Rizo CM, et al. Effect of non-invasive brain stimulation on behavior and serum brain-derived neurotrophic factor and insulin-like growth factor-1 levels in autistic patients. Drug Dev Res. 2021;82:716–23. doi: 10.1002/ddr.21808. [DOI] [PubMed] [Google Scholar]
- 28.Folster-Holst R, Papakonstantinou E, Rudrich U, Buchner M, Pite H, Gehring M, et al. Childhood atopic dermatitis-Brain-derived neurotrophic factor correlates with serum eosinophil cationic protein and disease severity. Allergy. 2016;71:1062–5. doi: 10.1111/all.12916. [DOI] [PubMed] [Google Scholar]
- 29.Saghazadeh A, Rezaei N. Brain-derived neurotrophic factor levels in autism: a systematic review and meta-analysis. J Autism Dev Disord. 2017;47:1018–29. doi: 10.1007/s10803-016-3024-x. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Z, Zhang L, Zhu T, Huang J, Qu Y, Mu D. Peripheral brain-derived neurotrophic factor in autism spectrum disorder: a systematic review and meta-analysis. Sci Rep. 2016;6:31241. doi: 10.1038/srep31241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virchow JC, Julius P, Lommatzsch M, Luttmann W, Renz H, Braun A. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am J Respir Crit Care Med. 1998;158:2002–5. doi: 10.1164/ajrccm.158.6.9803023. [DOI] [PubMed] [Google Scholar]
- 32.Raap U, Fokkens W, Bruder M, Hoogsteden H, Kapp A, Braunstahl GJ. Modulation of neurotrophin and neurotrophin receptor expression in nasal mucosa after nasal allergen provocation in allergic rhinitis. Allergy. 2008;63:468–75. doi: 10.1111/j.1398-9995.2008.01626.x. [DOI] [PubMed] [Google Scholar]
- 33.Raap U, Goltz C, Deneka N, Bruder M, Renz H, Kapp A, et al. Brain-derived neurotrophic factor is increased in atopic dermatitis and modulates eosinophil functions compared with that seen in nonatopic subjects. J Allergy Clin Immunol. 2005;115:1268–75. doi: 10.1016/j.jaci.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Guseva D, Rudrich U, Kotnik N, Gehring M, Patsinakidis N, Agelopoulos K, et al. Neuronal branching of sensory neurons is associated with BDNF-positive eosinophils in atopic dermatitis. Clin Exp Allergy. 2020;50:577–84. doi: 10.1111/cea.13560. [DOI] [PubMed] [Google Scholar]
- 35.Chang YS, Lin CY, Huang HY, Chang JG, Kuo HT. Chromosomal microarray and whole-exome sequence analysis in Taiwanese patients with autism spectrum disorder. Mol Genet Genom Med. 2019;7:e996. doi: 10.1002/mgg3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi L, Zhang X, Golhar R, Otieno FG, He M, Hou C, et al. Whole-genome sequencing in an autism multiplex family. Mol Autism. 2013;4:8. doi: 10.1186/2040-2392-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Z, Hansmann B, Meyer-Hoffert U, Glaser R, Schroder JM. Molecular identification and expression analysis of filaggrin-2, a member of the S100 fused-type protein family. PLoS ONE. 2009;4:e5227. doi: 10.1371/journal.pone.0005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonderby IE, van der Meer D, Moreau C, Kaufmann T, Walters GB, Ellegaard M, et al. 1q21.1 distal copy number variants are associated with cerebral and cognitive alterations in humans. Transl Psychiatry. 2021;11:182. doi: 10.1038/s41398-021-01213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwek J, De Iongh R, Nicholas K, Familari M. Molecular insights into evolution of the vertebrate gut: focus on stomach and parietal cells in the marsupial, macropus eugenii. J Exp Zool B Mol Dev Evol. 2009;312:613–24. doi: 10.1002/jez.b.21227. [DOI] [PubMed] [Google Scholar]
- 40.Ma Y, Cao C, Zhao M, Liu X, Cheng F, Wang J. Evolutionary changes in pathways and networks of genes expressed in the brains of humans and macaques. J Mol Neurosci. 2021;71:1825–37. doi: 10.1007/s12031-021-01874-y. [DOI] [PubMed] [Google Scholar]
- 41.Wei Y, de Lange SC, Scholtens LH, Watanabe K, Ardesch DJ, Jansen PR, et al. Genetic mapping and evolutionary analysis of human-expanded cognitive networks. Nat Commun. 2019;10:4839. doi: 10.1038/s41467-019-12764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jablonski NG, Chaplin G. The colours of humanity: the evolution of pigmentation in the human lineage. Philos Trans R Soc. 2017;372:1–8. doi: 10.1098/rstb.2016.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero V, Hosomichi K, Nakaoka H, Shibata H, Inoue I. Structure and evolution of the filaggrin gene repeated region in primates. BMC Evol Biol. 2017;17:10. doi: 10.1186/s12862-016-0851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denda M, Nakanishi S. Do epidermal keratinocytes have sensory and information processing systems? Exp Dermatol. 2021;0:1–16. doi: 10.1111/exd.14494. [DOI] [PubMed] [Google Scholar]
- 45.Denda M, Nakatani M, Ikeyama K, Tsutsumi M, Denda S. Epidermal keratinocytes as the forefront of the sensory system. Exp Dermatol. 2006;16:157–61. doi: 10.1111/j.1600-0625.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- 46.Lee E, Lee S, Shin J, Choi W, Chung C, Lee S, et al. Excitatory synapses and gap junctions cooperate to improve Pv neuronal burst firing and cortical social cognition in Shank2-mutant mice. Nat Commun. 2021;12:1–20. doi: 10.1038/s41467-021-25356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denda M, Takei K, Denda S. How does epidermal pathology interact with mental state? Med Hypotheses. 2013;80:194–6. doi: 10.1016/j.mehy.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 48.Mota-Rojass D, Titto CG, Oriheula A, Martinez-Burnes J, Gomez-Prado J, Torres-Bernal F, et al. Physiological and behavioural mechanisms of thermoregulation in mammals. Animals. 2021;11:1–27. doi: 10.3390/ani11061733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tenorio-Mina A, Cortes D, Esquivel-Estudillo J, Lopez-Orenelas A, Cabrera-Wrooman A, Lara-Rodarte R et al. Human keratinocytes adopt neuronal fates after in utero transplantation in developing rat brain. Cell Transplant. 2019;30:1–13. [DOI] [PMC free article] [PubMed]
- 50.Mueller SM, Hogg S, Mueller JM, McKie S, Itin P, Reinhardt J, et al. Functional magnetic resonance imaging in dermatology: The skin, the brain and the invisible. Exp Dermatol. 2017;26:845–53. doi: 10.1111/exd.13305. [DOI] [PubMed] [Google Scholar]
- 51.Slominski A, Wortsman J, Tuckey R, Paus R. Differential expression of HPA axis homolog in skin. Mol Cell Endocrinol. 2007;265-266:143–9. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunter HJ, Momen SE, Kleyn CE. The impact of psychosocial stress on healthy skin. Clin Exp Dermatol. 2015;40:540–6. doi: 10.1111/ced.12582. [DOI] [PubMed] [Google Scholar]
- 53.Maarouf M, Maarouf CL, Yosipovitch G, Shi VY. The impact of stress on epidermal barrier function: an evidence-based review. Br J Dermatol. 2019;181:1129–37. doi: 10.1111/bjd.17605. [DOI] [PubMed] [Google Scholar]
- 54.Onaolap O, Onaolap AY, Olowe AO. The neurobehavioral implications of the brain and microbiota interaction. Front Biosci. 2020;1:363–97. doi: 10.2741/4810. [DOI] [PubMed] [Google Scholar]
- 55.Kalailingam P, Tan H, Jain N, Sng M, Chan J, Tan N, et al. Conditional knock out of N-WASP in keratinocytes causes skin barrier defects and atopic dermatitis-like inflammation. Sci Rep. 2017;7:1–15. doi: 10.1038/s41598-017-07125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacome M, Chacon L, Cuesta H, Rizo C, Santiesteban M, Hernandez L, et al. Peripheral inflammatory markers contributing to comorbidities in autism. Behav Sci. 2016;6:1–14. doi: 10.3390/bs6040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Goc G, Lafaye J, Karpenko S, Bormuth V, Candelier R, Debrgeas G. Thermal modulation of Zebrafish exploratory statistics reveals constraints on individual behavioural variability. BMC Biol. 2021;19:1–17. doi: 10.1186/s12915-021-01126-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curran L, Newschaffer C, Lee L, Crawford S, Johnston M, Zimmerman A. Behaviours associated with fever in children with autism spectrum disorders. Paediatrics. 2007;120:e1386–92. doi: 10.1542/peds.2007-0360. [DOI] [PubMed] [Google Scholar]
- 59.Feldman R, Rosenthal Z, Eidelman A. Maternal-preterm skin-to-skin contact enhances child physiological organisation and cognitive control across first 10 years of life. Biol Psychiatry. 2014;75:56–64. doi: 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Alexander H, Brown S, Danby S, Flohr C. Research techniques made simple: transepidermal water loss measurement as a research tool. J Investig Dermatol. 2018;138:2295–300. doi: 10.1016/j.jid.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Jansen van Rensburg S, Franken A, Du Plessis JL. Measurement of transepidermal water loss, stratum corneum hydration and skin surface pH in occupational settings: a review. Ski Res Technol. 2019;25:595–605. doi: 10.1111/srt.12711. [DOI] [PubMed] [Google Scholar]
- 62.Seidenari S, Giusti G. Objective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm Venereol. 1995;75:429–33. doi: 10.2340/0001555575429433. [DOI] [PubMed] [Google Scholar]
- 63.Kim DW, Park JY, Na GY, Lee SJ, Lee WJ. Correlation of clinical features and skin barrier function in adolescent and adult patients with atopic dermatitis. Int J Dermatol. 2006;45:698–701. doi: 10.1111/j.1365-4632.2005.02644.x. [DOI] [PubMed] [Google Scholar]
- 64.Berents TL, Lodrup Carlsen KC, Mowinckel P, Skjerven HO, Rolfsjord LB, Bradley M, et al. Transepidermal water loss in infancy associated with atopic eczema at 2 years of age: a population-based cohort study. Br J Dermatol. 2017;177:e35–7. doi: 10.1111/bjd.15157. [DOI] [PubMed] [Google Scholar]
- 65.Montero-Vilchez T, Segura-Fernández-Nogueras MV, Pérez-Rodríguez I, Soler-Gongora M, Martinez-Lopez A, Fernández-González A, et al. Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J Clin Med. 2021;10:1–12. doi: 10.3390/jcm10020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin K, Crumrine D, Kim S, Lee Y, Kim B, Abuabara K, et al. Phenotypic overlap between atopic dermatitis and autism’. BMC Neuroscience. 2021;22:1–14. doi: 10.1186/s12868-021-00645-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitamura Y, Ogulur I, Pat Y, Rinaldi A, Ardicli O, Cevhertas L, et al. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermatitis. 2021;85:615–626. doi: 10.1111/cod.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rinaldi AO, Korsfeldt A, Ward S, Burla D, Dreher A, Gautschi M, et al. Electrical impedance spectroscopy for the characterization of skin barrier in atopic dermatitis. Allergy. 2021;76:3066–79. doi: 10.1111/all.14842. [DOI] [PubMed] [Google Scholar]
- 69.Tomchek SD, Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am J Occup Ther. 2007;61:190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- 70.Schaffler MD, Middleton LJ, Abdus-Saboor I. Mechanisms of tactile sensory phenotypes in autism: current understanding and future directions for research. Cur Psych Rep. 2019;21:134. doi: 10.1007/s11920-019-1122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orefice LL, Mosko JR, Morency DT, Wells MF, Tasnim A, Mozeika SM, et al. Targeting peripheral somatosensory neurons to improve tactile-related phenotypes in ASD models. Cell. 2019;178:867–86. [DOI] [PMC free article] [PubMed]
- 72.Abraira Victoria E, Ginty David D. The sensory neurons of touch. Neuron. 2013;79:618–39. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bakkaloglu B, Anlar B, Anlar F, Öktem F, Pehlivantürk B, Ünal F, et al. Atopic features in early childhood autism. Eur J Paediatr Neurol. 2008;12:476–479. doi: 10.1016/j.ejpn.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Magalhães E, Pinto-Mariz F, Bastos-Pinto S, Pontes A, Prado E, deAzevedo L. Immune allergic response in Asperger syndrome’. J Neuroimmunol. 2009;216:108–112. doi: 10.1016/j.jneuroim.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 75.Tsai T, Chao Y, Hsieh C, Huang Y. Association Between Atopic Dermatitis and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Acta Derm Venereol. 2020;100:1–2. doi: 10.2340/00015555-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zerbo O, Leong A, Barcellos L, Bernal P, Fireman B, Croen L. Immune Mediated Conditions in Autism Spectrum Disorders. Brain Behav Immun. 2015;46:232–236. doi: 10.1016/j.bbi.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berents T, Carlsen K, Mowinckel P, Skjerven H, Rolfsjord L, Bradley M, et al. Transepidermal water loss in infancy associated with atopic eczema at 2 years of age: a population-based cohort study. Br J Dermatol. 2016;177:35–37. doi: 10.1111/bjd.15157. [DOI] [PubMed] [Google Scholar]