Abstract
Osteosarcoma and Ewing sarcoma (ES) are the most common pediatric bone cancers. Patients with metastatic disease at diagnosis have poorer outcomes compared with localized disease. Using the Surveillance, Epidemiology, and End Results registries, we identified children and adolescents diagnosed with osteosarcoma or ES between 2004 and 2015. We examined whether demographic and socioeconomic disparities were associated with a higher likelihood of metastatic disease at diagnosis and poor survival outcomes. In osteosarcoma, Hispanic patients and those living in areas of high language isolation were more likely to have metastatic disease at diagnosis. Regardless of metastatic status, osteosarcoma patients with public insurance had increased odds of death compared to those with private insurance. Living in counties with lower education levels increased odds of death for adolescents with metastatic disease. In ES, non-White adolescents had higher odds of death compared to white patients. Adolescents with metastatic ES living in higher poverty areas had increased odds of death compared to those living in less impoverished areas. Disparities in both diagnostic and survival outcomes based on race, ethnicity, and socioeconomic factors exist in pediatric bone cancers, potentially due to barriers to care and treatment inequities.
Keywords: Osteosarcoma, Ewing sarcoma, pediatric, disparities
Introduction
Osteosarcoma (OST) and Ewing sarcoma (ES) are the first and second most common malignancies of bone in children, respectively.1 They are most common in adolescents, an age at which issues with access to care and delays in seeking medical treatment are common.2 Both diseases have better outcomes when presenting with localized disease at diagnosis; prognosis is much worse when presenting with metastatic disease at diagnosis.3, 4
The existence of demographic and socioeconomic disparities in the diagnosis and outcome of cancer has been documented,5, 6 especially in adults.7-11 For ES, disparities have been documented in terms of access to care12 and worse outcomes13-15 for Hispanics and non-White patients. Socioeconomic factors and survival of ES have been examined, with mixed results as to presence of an association.16, 17 In OST, previous studies have documented a higher likelihood of metastatic disease18, 19 and poorer survival20 in individuals of lower socioeconomic status (SES). Most of these studies have examined mixed pediatric and adult populations. To our knowledge, only one study stratified survival outcomes of cancers based on patient age. ES adolescent and young adult (AYA) patients were more likely to present with metastasis and have poorer survival when compared to younger children.21, 22 These factors in the presentation and survival of childhood OST specifically have not been addressed in the medical literature. We aimed to further investigate metastasis and survival differences solely in pediatric patients based on demographic and socioeconomic characteristics.
In this population-based study, we used Surveillance, Epidemiology, and End Results (SEER) registries to determine the existence of disparities in the presentation and outcomes of pediatric ES and OST based on race, ethnicity, insurance status, and county-based socioeconomic indicators. Additionally, we investigated whether the presence of these factors varied based on metastatic disease and pediatric age of diagnosis. We hypothesized that demographic and socioeconomic risk factors are associated with both a greater likelihood of metastatic disease at diagnosis and poorer survival outcomes.
Materials and Methods
Study Population
This retrospective cohort study examined children (n=1,350) and adolescents (n=867) with ES (n=802) and OST (n=1,415) diagnosed from 2004 through 2015. Diagnosis was based on the International Classification of Childhood Cancer – 3rd edition (ICCC) (groups VIIIc and VIIIa, respectively), and characteristics were gathered using the SEER-18 dataset. SEER-18 registries represent approximately 28 percent of the United States population and are meant to accurately represent the geographic diversity of the country (https://seer.cancer.gov/registries/data.html). Pediatric patients with follow up information were included in this cohort, while death certificate/autopsy-only cases were excluded.
Individual-Level Variables of Interest
We obtained demographic patient data on age, sex, race, ethnicity, and payer status directly from the SEER-18 database. Individual demographic variables of interest were dichotomized for analysis due to lower sample size in some categories. For race, individuals classified as White were compared to those whose race was classified as non-White, while for ethnicity, the groups compared were Hispanic and non-Hispanic. Payer status was compared between private (insured or insured/no specifics) and non-private insurance (uninsured or any Medicaid), as many uninsured patients are enrolled in Medicaid when diagnosed with cancer.23 ES and OST diagnoses were considered non-metastatic when tumors were classified as localized or regional to the primary site of cancer based on SEER Historic Stage A. Metastatic was defined as tumors classified as having disease distant from the primary site. To explore additional differences due to age, adolescent (15-19 years old) were separated from childhood (0-14 years old) diagnoses.
County-Level Variables of Interest
To examine SES status, attributes of the patient’s county of residence at time of diagnosis were obtained from the SEER database. We chose three 2000 US Census variables, reflecting economic, educational, and primary language status within each county. For each SES characteristic, we divided our cohort into two groups of approximately equal sizes using the median value for that variable, allowing for comparison of outcomes between children and adolescents living in advantaged versus disadvantaged counties. We used the following cutoff values and definitions: percentage of people in the county with less than high school-level education (advantaged ≤18.3%, disadvantaged >18.3%), percentage of people below 150% of the poverty level (advantaged ≤12.1%, disadvantaged >12.1%), and percentage of households linguistically isolated, defined as having no proficient English-speaking adults (advantaged ≤5.3%, disadvantaged >5.3%).
Statistical Analysis
Socio-demographics were compared between ES and OST cohorts, as well as metastatic groups, using Pearson’s chi-squared test. Fisher’s exact test was used for any comparisons with frequencies less than five. Individual and county attributes of interest were then examined for exclusive and combined relationships with presentation of metastatic disease, relative survival, and hazard of death. When available, ES and OST outcomes were further stratified by metastatic status and age of diagnosis.
For adjusted analyses, Pearson’s R correlation was performed to remove any characteristics with strong correlations (R=+/−0.8) to prevent covariance in the final model. If demographic variables were missing, subjects were excluded from the stratum-specific analysis but not dropped from all analyses. However, for payer status, insurance information was only collected by SEER starting in 2007. Two models were examined in order to account for the large amount of missing payer information. The 2004-2015 cohort includes all patients, while the 2007-2015 model removes those diagnosed in 2004-2006 (n=549) but includes payer status. Diagnosis year was included to compare possible differences between these two cohorts.
For all statistical tests, results were considered statistically significant when the p-value was less than 0.05. Since this analysis was exploratory, we used this alpha level in order to investigate the direction of relationships despite multiple comparisons. All analyses were completed in SAS version 9.4 (SAS Institute Inc., Cary, NC).
Presentation of Metastasis at Diagnosis
Overall and age-stratified univariate and adjusted multivariable logistic regression models were used to explore the association of patient and county characteristics with metastasis at time of diagnosis. Odds ratios and confidence intervals were calculated in order to compare the odds of metastasis at diagnosis, given multiple risk factors.
Five Year Relative Survival
Five year relative survival rates (5yr RS) were determined using the actuarial life table with the Ederer II method, which is designed to be an accurate method of measuring survival of a cancer population compared to a matched cancer-free population.24 5yr RS between groups was determined using the actuarial life table method. RS rates were then calculated using the observed and expected survival rates. Expected survival rates were gathered from SEER-18 and age-matched to the respective population. RS risk factors were compared using z-score tests.
Hazard of Death
Univariate and multivariable Cox proportional hazards regressions were used to calculate hazard ratios and adjusted hazard ratios for the odds of death from all causes. The proportional hazards assumption was evaluated using scaled Schoenfeld residuals correlated with time. Metastatic disease and insurance status violated the proportional hazards assumption depending on the disease site and years studied. These violations were accounted for by including time-dependent interaction terms where appropriate in multivariable models.
Post Hoc Analysis
Primary tumor location was analyzed between racial and ethnic groups using Pearson’s Chi-squared analysis, since variation in tumor location could possibly confound results.15
Results
Cohort Characteristics
Demographic characteristics of the overall study cohort were similar between OST and ES groups (Table 1). The majority of each disease group presented with non-metastatic disease, although the proportion with metastatic disease was higher in ES (31.3%) than OST (22.4%). The majority of patients were non-Hispanic, White males between the ages of 10 and 19 years. There was a higher percentage of children between the ages of 0 and 9 years in ES (27.3% vs. 17.0%), while OST had higher proportion of non-White (25.0% vs. 11.4%) and Hispanic (30.7% vs. 25.2%) individuals.
Table 1.
Comparison of sociodemographic attributes among osteosarcoma and Ewing sarcoma diagnoses in pediatrics (0-19 years), SEER-18 registries 2004-2015
| Osteosarcoma (N=1415) | Ewing Sarcoma (N=802) | ||||
|---|---|---|---|---|---|
| n | % | n | % | p | |
| Diagnosis (year) | |||||
| 2004-2006 | 345 | 24.4 | 199 | 24.8 | 0.821 |
| 2007-2009 | 377 | 26.6 | 200 | 24.9 | |
| 2010-2012 | 340 | 24.0 | 193 | 24.1 | |
| 2013-2015 | 353 | 25.0 | 210 | 26.2 | |
| Age (years) | |||||
| 0-9 | 240 | 17.0 | 219 | 27.3 | <0.001 |
| 10-14 | 595 | 42.0 | 296 | 36.9 | |
| 15-19 | 580 | 41.0 | 287 | 35.8 | |
| Sex | |||||
| Male | 783 | 55.3 | 487 | 60.7 | 0.014 |
| Female | 632 | 44.7 | 315 | 39.3 | |
| Race | |||||
| White | 1051 | 75.0 | 707 | 88.6 | <0.001 |
| Non-White | 350 | 25.0 | 91 | 11.4 | |
| Ethnicity | |||||
| Non-Hispanic | 981 | 69.3 | 600 | 74.8 | 0.006 |
| Hispanic | 434 | 30.7 | 202 | 25.2 | |
| Insurance Status† | |||||
| Privately Insured | 647 | 62.2 | 390 | 65.8 | 0.151 |
| Non-Privately Insured | 393 | 37.8 | 203 | 34.2 | |
| Language Isolation | |||||
| Advantaged | 687 | 48.6 | 430 | 53.6 | 0.022 |
| Disadvantaged | 728 | 51.4 | 372 | 46.4 | |
| Education | |||||
| Advantaged | 689 | 48.7 | 423 | 52.7 | 0.067 |
| Disadvantaged | 726 | 51.3 | 379 | 47.3 | |
| Poverty | |||||
| Advantaged | 672 | 47.5 | 438 | 54.6 | 0.001 |
| Disadvantaged | 743 | 52.5 | 364 | 45.4 | |
| Metastatic Disease | |||||
| Non-Metastatic | 1098 | 77.6 | 551 | 68.7 | <0.001 |
| Metastatic | 317 | 22.4 | 251 | 31.3 | |
Individual-level attributes in standard typeface; county-level in italics. Bold indicates statistically significant difference in proportions.
Years 2007-2015 only.
Metastatic Disease at Diagnosis
Among children and adolescents with OST, there was a significantly higher percentage of metastatic disease in Hispanics compared to non-Hispanics (Table 2: 25.8% vs. 20.9%; p=0.041). In the 2004-2015 cohort that included all patients, children presenting with metastases were more likely to be Hispanic (Table 3: OR=1.4 [95% CI=1.0, 2.0]; p=0.042) and living in counties with high language isolation (1.4 [1.0, 1.9]; p=0.049). After adjusting for all risk factors of interest, these findings were no longer significant. In the 2007-2015 cohort that included payer status and when adjusting for risk factors of interest, only language isolation was a predictor of metastatic disease among children (Table 4: 1.6 [1.0, 2.4]; p=0.042).
Table 2.
Comparison of sociodemographic attributes of metastatic disease at diagnosis among osteosarcoma and Ewing sarcoma diagnoses in pediatrics (0-19 years), SEER-18 registries 2004-2015
| Osteosarcoma | Ewing Sarcoma | ||||||
|---|---|---|---|---|---|---|---|
| Metastasis at diagnosis | p | Metastasis at diagnosis | p | ||||
| No | Yes | No | Yes | ||||
| n (%) | n (%) | n (%) | n (%) | ||||
| Diagnosis (years) | 2004-2006 | 270 (78.3) | 75 (21.7) | 0.909 | 143 (71.9) | 56 (28.1) | 0.214 |
| 2007-2009 | 296 (78.5) | 81 (21.5) | 145 (72.5) | 55 (27.5) | |||
| 2010-2012 | 261 (76.8) | 79 (23.2) | 126 (65.3) | 67 (34.7) | |||
| 2013-2015 | 271 (76.8) | 82 (23.2) | 137 (65.2) | 73 (34.8) | |||
| Age (years) | 0-9 | 194 (80.8) | 46 (19.2) | 0.164 | 164 (74.9) | 55 (25.1) | 0.006 |
| 10-14 | 448 (75.3) | 147 (24.7) | 209 (70.6) | 87 (29.4) | |||
| 15-19 | 456 (78.6) | 124 (21.4) | 178 (62.0) | 109 (38.0) | |||
| Sex | Male | 604 (77.1) | 179 (22.9) | 0.646 | 338 (69.4) | 149 (30.6) | 0.594 |
| Female | 494 (78.2) | 138 (21.8) | 213 (67.6) | 102 (32.4) | |||
| Race | White | 815 (77.5) | 236 (22.5) | 0.876 | 485 (68.6) | 222 (31.4) | 0.737 |
| Non-White | 270 (77.1) | 80 (22.9) | 64 (70.3) | 27 (29.7) | |||
| Ethnicity | Non-Hispanic | 776 (79.1) | 205 (20.9) | 0.041 | 422 (70.3) | 178 (29.7) | 0.086 |
| Hispanic | 322 (74.2) | 112 (25.8) | 129 (63.9) | 73 (36.1) | |||
| Insurance Status† | Privately Insured | 510 (78.8) | 137 (21.2) | 0.111 | 273 (70.0) | 117 (30.0) | 0.051 |
| Non-Privately Insured | 293 (74.5) | 100 (25.5) | 126 (62.1) | 77 (37.9) | |||
| Language Isolation | Advantaged | 542 (78.9) | 145 (21.1) | 0.256 | 305 (70.9) | 125 (29.1) | 0.144 |
| Disadvantaged | 556 (76.4) | 172 (23.6) | 246 (66.1) | 126 (35.9) | |||
| Education | Advantaged | 536 (77.8) | 153 (22.2) | 0.863 | 295 (69.7) | 128 (30.3) | 0.504 |
| Disadvantaged | 562 (77.4) | 164 (22.6) | 256 (67.5) | 123 (32.5) | |||
| Poverty | Advantaged | 525 (78.1) | 147 (21.9) | 0.651 | 309 (70.5) | 129 (29.5) | 0.216 |
| Disadvantaged | 573 (77.1) | 170 (22.9) | 242 (66.5) | 122 (33.5) | |||
Individual-level attributes in standard typeface; county-level in italics. Bold indicates statistically significant findings.
Years 2007-2015 only.
Table 3.
Odds ratios of metastatic disease presentation by sociodemographic attributes among osteosarcoma and Ewing sarcoma diagnoses in children (0-14 years) and adolescents (15-19 years), SEER-18 registries 2004-2015
| Overall | Children | Adolescents | Overall | Children | Adolescents | ||
|---|---|---|---|---|---|---|---|
| Predictor | OR (95% CI) | OR (95% CI) | OR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Osteosarcoma | (n=1415) | (n=835) | (n=580) | (n=1415) | (n=835) | (n=580) | |
| Diagnosis Year | 2004-2006 | 0.92 (0.64, 1.31) | 0.84 (0.52, 1.36) | 1.02 (0.60, 1.72) | 0.94 (0.66, 1.35) | 0.90 (0.55, 1.47) | 0.97 (0.57, 1.65) |
| 2007-2009 | 0.90 (0.64, 1.28) | 1.12 (0.71, 1.77) | 0.66 (0.38, 1.15) | 0.91 (0.64, 1.29) | 1.17 (0.74, 1.85) | 0.64 (0.37, 1.12) | |
| 2010-2012 | 1.00 (0.70, 1.42) | 1.32 (0.85, 2.07) | 0.60 (0.33, 1.09) | 1.01 (0.71, 1.44) | 1.42 (0.90, 2.23) | 0.55 (0.30, 1.00) | |
| Age | 10-14 years | 1.38 (0.95, 2.01) | - | - | 1.35 (0.93, 1.97) | - | - |
| 15-19 years | 1.15 (0.79, 1.67) | - | - | 1.13 (0.77, 1.66) | - | - | |
| Sex | Female | 0.94 (0.73, 1.21) | 1.01 (0.73, 1.39) | 0.80 (0.52, 1.23) | 0.92 (0.72, 1.20) | 1.01 (0.72, 1.39) | 0.78 (0.51, 1.21) |
| Race | Non-White | 1.02 (0.77, 1.37) | 1.10 (0.76, 1.59) | 0.93 (0.59, 1.47) | 1.15 (0.84, 1.56) | 1.27 (0.84, 1.90) | 0.93 (0.57, 1.52) |
| Ethnicity | Hispanic | 1.32 (1.01, 1.72) * | 1.42 (1.01, 1.98) * | 1.16 (0.75, 1.79) | 1.30 (0.96, 1.78) | 1.44 (0.96, 2.14) | 1.19 (0.72, 1.96) |
| Language Isolation | Disadvantaged | 1.16 (0.90, 1.49) | 1.39 (1.00, 1.92) * | 0.88 (0.59, 1.30) | 1.11 (0.83, 1.47) | 1.42 (0.98, 2.06) | 0.73 (0.46, 1.16) |
| Education | Disadvantaged | 1.02 (0.80, 1.31) | 0.93 (0.67, 1.28) | 1.20 (0.80, 1.79) | 0.92 (0.68, 1.25) | 0.83 (0.56, 1.22) | 1.03 (0.62, 1.72) |
| Poverty | Disadvantaged | 1.06 (0.82, 1.36) | 0.91 (0.66, 1.26) | 1.35 (0.90, 2.03) | 1.05 (0.78, 1.42) | 0.85 (0.58, 1.25) | 1.50 (0.90, 2.51) |
| Ewing Sarcoma | (n=802) | (n=515) | (n=287) | (n=802) | (n=515) | (n=287) | |
| Diagnosis Year | 2004-2006 | 0.73 (0.48, 1.12) | 0.68 (0.39, 1.18) | 0.66 (0.34, 1.29) | 0.69 (0.45, 1.06) | 0.66 (0.37, 1.17) | 0.64 (0.33, 1.27) |
| 2007-2009 | 0.71 (0.47, 1.08) | 0.80 (0.47, 1.36) | 0.52 (0.26, 1.06) | 0.68 (0.44, 1.05) | 0.78 (0.46, 1.35) | 0.48 (0.23, 1.00) | |
| 2010-2012 | 1.00 (0.66, 1.50) | 0.99 (0.59, 1.66) | 0.93 (0.46, 1.86) | 1.00 (0.66, 1.52) | 1.03 (0.61, 1.74) | 0.89 (0.44, 1.80) | |
| Age | 10-14 years | 1.24 (0.84, 1.84) | - | - | 1.24 (0.83, 1.86) | - | - |
| 15-19 years | 1.83 (1.24, 2.69) ** | - | - | 1.94 (1.31, 2.88) ** | - | - | |
| Sex | Female | 1.09 (0.80, 1.47) | 1.29 (0.88, 1.91) | 0.94 (0.56, 1.57) | 1.13 (0.82, 1.54) | 1.30 (0.87, 1.94) | 0.89 (0.52, 1.50) |
| Race | Non-White | 0.92 (0.57, 1.49) | 0.82 (0.40, 1.66) | 0.88 (0.46, 1.72) | 0.84 (0.51, 1.39) | 0.77 (0.37, 1.62) | 0.90 (0.45, 1.80) |
| Ethnicity | Hispanic | 1.34 (0.96, 1.88) | 1.55 (1.01, 2.37) * | 1.09 (0.63, 1.88) | 1.21 (0.83, 1.76) | 1.34 (0.82, 2.19) | 0.99 (0.54, 1.80) |
| Language Isolation | Disadvantaged | 1.25 (0.93, 1.69) | 1.42 (0.96, 2.09) | 0.98 (0.61, 1.58) | 1.19 (0.84, 1.68) | 1.40 (0.88, 2.20) | 0.93 (0.53, 1.63) |
| Education | Disadvantaged | 1.11 (0.82, 1.49) | 1.07 (0.72, 1.57) | 1.13 (0.70, 1.82) | 0.92 (0.62, 1.35) | 0.84 (0.51, 1.39) | 0.99 (0.53, 1.86) |
| Poverty | Disadvantaged | 1.21 (0.90, 1.63) | 1.19 (0.81, 1.75) | 1.19 (0.74, 1.92) | 1.16 (0.79, 1.69) | 1.10 (0.67, 1.79) | 1.32 (0.72, 2.44) |
Includes all diagnoses from 2004-2015 and does not include insurance status as a predictor
Individual attributes in standard typeface and references are 2013-2015, 0-9 years, males, white, and non-Hispanic; county attributes in italics and reference is advantaged
OR=Odds Ratio, aOR=Adjusted Odds Ratio, CI=95% Confidence Intervals
Bold indicates statistically significant findings
indicates a p-value <0.05, and
indicates a p-value <0.01
Table 4.
Odds ratios of metastatic disease presentation by sociodemographic characteristics of osteosarcoma and Ewing sarcoma diagnoses in children (0-14 years) and adolescents (15-19 years), SEER-18 registries 2007-2015
| Overall | Children | Adolescents | Overall | Children | Adolescents | ||
|---|---|---|---|---|---|---|---|
| Attribute | Predictor | OR (95% CI) | OR (95% CI) | OR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) |
| Osteosarcoma | (n=1070) | (n=636) | (n=434) | (n=1070) | (n=636) | (n=434) | |
| Diagnosis Year | 2007-2009 | 0.90 (0.64, 1.28) | 1.12 (0.71, 1.77) | 0.66 (0.38, 1.15) | 0.94 (0.66, 1.35) | 1.25 (0.78, 2.00) | 0.63 (0.35, 1.10) |
| 2010-2012 | 1.00 (0.70, 1.42) | 1.32 (0.85, 2.07) | 0.60 (0.33, 1.09) | 1.02 (0.71, 1.46) | 1.45 (0.91, 2.31) | 0.54 (0.29, 1.01) | |
| Age | 10-14 years | 1.21 (0.81, 1.81) | - | - | 1.20 (0.80, 1.82) | - | - |
| 15-19 years | 0.89 (0.59, 1.35) | - | - | 0.94 (0.61, 1.44) | - | - | |
| Sex | Female | 0.96 (0.72, 1.29) | 0.99 (0.69, 1.42) | 0.82 (0.49, 1.36) | 0.92 (0.68, 1.24) | 0.95 (0.65, 1.38) | 0.86 (0.51, 1.44) |
| Race | Non-White | 1.11 (0.80, 1.54) | 1.23 (0.81, 1.85) | 0.96 (0.56, 1.64) | 1.17 (0.81, 1.69) | 1.27 (0.79, 2.03) | 1.00 (0.54, 1.83) |
| Ethnicity | Hispanic | 1.21 (0.89, 1.64) | 1.18 (0.80, 1.73) | 1.23 (0.74, 2.05) | 1.13 (0.77, 1.66) | 1.09 (0.67, 1.76) | 1.29 (0.69, 2.43) |
| Insurance Status | Non-Private | 1.27 (0.95, 1.71) | 1.22 (0.84, 1.78) | 1.34 (0.83, 2.17) | 1.24 (0.90, 1.71) | 1.32 (0.87, 1.99) | 1.13 (0.66, 1.93) |
| Language Isolation | Disadvantaged | 1.08 (0.81, 1.44) | 1.31 (0.91, 1.88) | 0.78 (0.49, 1.25) | 1.12 (0.80, 1.55) | 1.56 (1.02, 2.38) * | 0.62 (0.36, 1.09) |
| Education | Disadvantaged | 0.90 (0.67, 1.20) | 0.81 (0.57, 1.17) | 1.07 (0.67, 1.70) | 0.80 (0.56, 1.15) | 0.72 (0.46, 1.12) | 0.84 (0.45, 1.57) |
| Poverty | Disadvantaged | 0.98 (0.74, 1.31) | 0.82 (0.57, 1.18) | 1.35 (0.84, 2.16) | 1.05 (0.74, 1.50) | 0.83 (0.54, 1.28) | 1.79 (0.94, 3.40) |
| Ewing Sarcoma | (n=603) | (n=401) | (n=202) | (n=603) | (n=401) | (n=202) | |
| Diagnosis Year | 2007-2009 | 0.71 (0.47, 1.08) | 0.80 (0.47, 1.36) | 0.52 (0.26, 1.06) | 0.69 (0.45, 1.07) | 0.78 (0.45, 1.34) | 0.56 (0.26, 1.19) |
| 2010-2012 | 1.00 (0.66, 1.50) | 0.99 (0.59, 1.66) | 0.93 (0.46, 1.86) | 1.02 (0.66, 1.55) | 1.01 (0.60, 1.71) | 1.02 (0.49, 2.11) | |
| Age | 10-14 years | 1.22 (0.78, 1.89) | - | - | 1.19 (0.75, 1.87) | - | - |
| 15-19 years | 1.77 (1.14, 2.76) * | - | - | 1.86 (1.17, 2.94) ** | - | - | |
| Sex | Female | 1.17 (0.83, 1.66) | 1.18 (0.77, 1.83) | 1.33 (0.73, 2.42) | 1.16 (0.81, 1.67) | 1.14 (0.73, 1.78) | 1.20 (0.63, 2.26) |
| Race | Non-White | 0.84 (0.49, 1.46) | 0.88 (0.41, 1.87) | 0.70 (0.31, 1.59) | 0.71 (0.39, 1.27) | 0.80 (0.36, 1.78) | 0.59 (0.25, 1.41) |
| Ethnicity | Hispanic | 1.34 (0.91, 1.95) | 1.52 (0.95, 2.46) | 1.05 (0.56, 1.98) | 1.02 (0.65, 1.60) | 1.20 (0.68, 2.12) | 0.77 (0.37, 1.63) |
| Insurance Status | Non-Private | 1.43 (1.00, 2.04) | 1.38 (0.89, 2.16) | 1.65 (0.89, 3.03) | 1.39 (0.93, 2.08) | 1.25 (0.76, 2.05) | 1.64 (0.81, 3.32) |
| Language Isolation | Disadvantaged | 1.32 (0.94, 1.86) | 1.37 (0.89, 2.11) | 1.21 (0.68, 2.12) | 1.30 (0.87, 1.94) | 1.30 (0.78, 2.18) | 1.22 (0.62, 2.38) |
| Education | Disadvantaged | 1.21 (0.86, 1.70) | 1.14 (0.74, 1.76) | 1.27 (0.72, 2.24) | 0.88 (0.56, 1.38) | 0.83 (0.48, 1.46) | 0.95 (0.43, 2.09) |
| Poverty | Disadvantaged | 1.36 (0.96, 1.91) | 1.39 (0.90, 2.14) | 1.25 (0.71, 2.20) | 1.23 (0.78, 1.92) | 1.26 (0.73, 2.19) | 1.19 (0.54, 2.64) |
Includes all diagnoses from 2007-2015 and insurance status as a predictor
Individual attributes in standard typeface and references are 2013-2015, 0-9 years, males, white, and non-Hispanic; county attributes in italics and reference is advantaged
OR=Odds Ratio, aOR=Adjusted Odds Ratio, CI=95% Confidence Intervals
Bold indicates statistically significant findings
indicates a p-value <0.05, and
indicates a p-value<0.01
In the ES group, age had a significant association with metastatic disease at diagnosis. Adolescents had a higher percentage than children (Table 2: 0-9 years, 25.1%; 10-14 years, 29.4%, 15-19 years, 38.0%; p=0.006), and adolescence remained a significant predictor in adjusted models with (Table 3: p=0.001) and without insurance status (Table 4: p=0.008). Similar to OST, Hispanic children diagnosed between 2004 and 2015 had higher odds of metastatic disease at diagnosis (Table 3: 1.6 [1.0, 2.4]; p=0.045), but ethnicity was no longer a predictor of metastatic disease when early diagnosis years were excluded (Table 4).
Five Year Relative Survival
For OST, stratifying by metastatic status found age of diagnosis to be associated with survival. 5yr RS was higher for patients with metastatic disease age 10 to 14 years old when compared to children between 0 and 9 years (Table 5: 44.4% vs. 22.3%; p=0.001).
Table 5.
Five-year relative survival rates by sociodemographic attributes of metastatic disease at diagnosis among osteosarcoma and Ewing sarcoma diagnoses in pediatrics (0-19 years), SEER-18 registries 2004-2015
| Attribute | Category | Osteosarcoma | Ewing Sarcoma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Metastatic | Metastatic | Total | Non-Metastatic | Metastatic | Total | ||||||||
| 5y RS | p | 5y RS | p | 5y RS | p | 5y RS | p | 5y RS | p | 5y RS | p | ||
| Age (years)* | 0-9 | 78.9 | 0.861 0.203 |
22.3 |
0.001 0.292 |
68.9 | 0.746 0.348 |
96.0 |
0.001
<0.001 |
54.8 | 0.170 0.028 |
85.2 |
0.010
<0.001 |
| 10-14 | 79.9 | 44.4 | 71.0 | 80.0 | 45.1 | 70.1 | |||||||
| 15-19 | 71.7 | 28.8 | 62.6 | 72.3 | 39.3 | 60.5 | |||||||
| Sex | Male | 74.5 | 0.494 | 35.1 | 0.836 | 65.3 | 0.516 | 79.6 | 0.289 | 45.7 | 0.722 | 69.1 | 0.586 |
| Female | 78.6 | 36.5 | 69.6 | 85.3 | 43.2 | 72.6 | |||||||
| Race | White | 76.5 | 0.934 | 34.8 | 0.587 | 67.0 | 0.904 | 82.1 | 0.705 | 46.6 | 0.012 | 71.1 | 0.395 |
| Non-White | 76.0 | 38.5 | 67.8 | 80.0 | 29.4 | 65.5 | |||||||
| Ethnicity | Non-Hispanic | 77.3 | 0.576 | 32.0 | 0.147 | 68.0 | 0.708 | 83.7 | 0.150 | 42.5 | 0.275 | 71.8 | 0.409 |
| Hispanic | 73.9 | 41.9 | 65.5 | 75.5 | 50.2 | 66.4 | |||||||
| Insurance Status† | Privately Insured | 77.3 | 0.973 | 31.4 | 0.123 | 67.7 | 0.952 | 82.3 | 0.366 | 49.5 | 0.756 | 72.4 | 0.937 |
| Non-Privately Insured | 77.1 | 41.9 | 68.1 | 86.9 | 47.3 | 72.9 | |||||||
| Language Isolation | Advantaged | 74.1 | 0.454 | 36.6 | 0.768 | 66.4 | 0.810 | 84.0 | 0.363 | 46.0 | 0.744 | 73.6 | 0.307 |
| Disadvantaged | 78.6 | 34.6 | 68.0 | 79.0 | 43.7 | 67.0 | |||||||
| Foreign Born | Advantaged | 74.7 | 0.595 | 34.1 | 0.700 | 66.0 | 0.729 | 83.9 | 0.382 | 48.5 | 0.306 | 74.2 | 0.211 |
| Disadvantaged | 77.9 | 36.7 | 68.3 | 79.1 | 41.3 | 66.1 | |||||||
| Education | Advantaged | 77.2 | 0.777 | 39.0 | 0.330 | 68.8 | 0.640 | 81.4 | 0.296 | 45.0 | 0.943 | 70.8 | 0.926 |
| Disadvantaged | 75.5 | 32.4 | 65.7 | 82.4 | 44.5 | 70.2 | |||||||
| Poverty | Advantaged | 75.1 | 0.702 | 37.0 | 0.701 | 66.9 | 0.940 | 83.1 | 0.584 | 45.3 | 0.865 | 72.3 | 0.536 |
| Disadvantaged | 77.4 | 34.4 | 67.4 | 80.1 | 44.1 | 68.3 | |||||||
P-values reflect comparisons of the age group below to the reference population, 0-9 year-olds. 5y RS = five-year relative survival; Individual-level attributes in standard typeface; county level in italics. Bold indicates statistically significant findings.
Years 2007-2015 only.
In ES, age was associated with survival in both non-metastatic and metastatic groups. Children between the ages of 0 and 9 had higher 5yr RS when compared to older ages in the metastatic (54.8% vs. 15-19 years: 39.3%, p=0.028) and non-metastatic (96.0% vs. 10-14 years: 80.0%, p=0.001; 15-19 years: 72.3%, p=<0.001) disease groups. Race was associated with survival in metastatic groups. Non-White individuals with metastases at diagnosis had significantly lower 5yr RS when compared to White subjects (29.4% vs. 46.6%; p=0.012), but this difference did not hold true for children and adolescents with non-metastatic disease (80.0% vs. 82.1%; p=0.705).
Hazard of Death
For OST, sex was correlated with odds of death in both disease stage cohorts. Among adolescents with metastatic disease at diagnosis, females had lower hazard ratios of death (Table 6: 0.5 [0.3, 0.8]; p=0.011) compared to males. In the 2007-2015 cohort, children with non-private insurance had higher odds of death compared to adolescents (Table 7: 2.4 [1.2, 4.7]; p=0.030). Among adolescents with metastatic disease, there was a two-fold increase in odds of death for those living in educationally disadvantaged compared to advantaged counties (Table 7: 2.3 [1.1, 4.7]; p=0.034). For non-metastatic patients, those living in disadvantaged counties by language isolation had a lower HR of death (0.6 [0.4, 0.9]; p=0.016). Once stratified by age, this correlation was only maintained for adolescents (0.49 [0.3, 0.9]; p=0.013).
Table 6.
Hazard ratios of death by sociodemographic characteristics of osteosarcoma diagnoses in children (0-14 years) and adolescents (15-19 years), SEER-18 registries 2004-2015
| Overall | Non-Metastatic | Metastatic | Overall | Non-Metastatic | Metastatic | ||
|---|---|---|---|---|---|---|---|
| Attribute | Predictor | HR (95% CI) | HR (95% CI) | HR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) |
| Pediatric Combined | (n=1415) | (n=1098) | (n=317) | (n=1415) | (n=1098) | (n=317) | |
| Diagnosis Year | 2004-2006 | 0.80 (0.56, 1.14) | 0.82 (0.49, 1.37) | 0.86 (0.52, 1.42) | 0.84 (0.59, 1.21) | 0.83 (0.49, 1.39) | 0.84 (0.50, 1.39) |
| 2007-2009 | 0.79 (0.55, 1.12) | 0.83 (0.50, 1.38) | 0.81 (0.49, 1.34) | 0.85 (0.59, 1.21) | 0.83 (0.49, 1.38) | 0.82 (0.49, 1.37) | |
| 2010-2012 | 0.80 (0.56, 1.15) | 0.72 (0.42, 1.23) | 1.00 (0.61, 1.63) | 0.87 (0.60, 1.25) | 0.71 (0.42, 1.22) | 1.03 (0.62, 1.70) | |
| Age | 10-14 years | 0.97 (0.72, 1.30) | 0.96 (0.64, 1.42) | 0.73 (0.47, 1.14) | 0.84 (0.62, 1.14) | 0.91 (0.61, 1.35) | 0.76 (0.48, 1.19) |
| 15-19 years | 1.25 (0.94, 1.67) | 1.39 (0.95, 2.03) | 0.97 (0.62, 1.51) | 1.18 (0.88, 1.58) | 1.33 (0.91, 1.95) | 1.01 (0.63, 1.62) | |
| Sex | Female | 0.88 (0.72, 1.07) | 0.88 (0.68, 1.15) | 0.91 (0.68, 1.23) | 0.92 (0.75, 1.12) | 0.93 (0.71, 1.21) | 0.95 (0.70, 1.29) |
| Race | Non-White | 1.00 (0.80, 1.26) | 1.05 (0.78, 1.42) | 0.93 (0.66, 1.33) | 1.00 (0.78, 1.26) | 1.06 (0.77, 1.46) | 0.87 (0.60, 1.26) |
| Ethnicity | Hispanic | 1.13 (0.92, 1.39) | 1.14 (0.86, 1.51) | 0.91 (0.66, 1.25) | 1.07 (0.84, 1.35) | 1.29 (0.92, 1.79) | 0.87 (0.62, 1.24) |
| Disease | Metastatic | 3.94 (3.23, 4.80) ** | - | - | 6.27 (4.38, 8.96) ** | - | - |
| Language Isolation | Disadvantaged | 0.98 (0.81, 1.20) | 0.85 (0.66, 1.11) | 1.10 (0.81, 1.48) | 0.93 (0.75, 1.16) | 0.76 (0.56, 1.03) | 1.14 (0.82, 1.58) |
| Education | Disadvantaged | 1.10 (0.91, 1.34) | 1.04 (0.81, 1.35) | 1.20 (0.89, 1.62) | 1.17 (0.92, 1.48) | 1.14 (0.83, 1.58) | 1.19 (0.83, 1.69) |
| Poverty | Disadvantaged | 1.03 (0.84, 1.25) | 0.96 (0.74, 1.24) | 1.08 (0.80, 1.45) | 0.93 (0.73, 1.17) | 0.93 (0.68, 1.28) | 0.96 (0.68, 1.36) |
| Children | (n=835) | (n=642) | (n=193) | (n=835) | (n=642) | (n=193) | |
| Diagnosis Year | 2004-2006 | 0.77 (0.46, 1.26) | 0.65 (0.33, 1.29) | 1.09 (0.52, 2.28) | 0.84 (0.51, 1.39) | 0.68 (0.34, 1.35) | 1.04 (0.49, 2.21) |
| 2007-2009 | 0.87 (0.53, 1.41) | 0.73 (0.37, 1.43) | 1.01 (0.50, 2.06) | 0.90 (0.55, 1.48) | 0.75 (0.38, 1.48) | 0.99 (0.48, 2.05) | |
| 2010-2012 | 0.83 (0.51, 1.37) | 0.54 (0.27, 1.11) | 1.16 (0.58, 2.33) | 0.82 (0.49, 1.34) | 0.56 (0.27, 1.16) | 1.18 (0.59, 2.39) | |
| Sex | Female | 1.02 (0.78, 1.33) | 0.86 (0.59, 1.23) | 1.36 (0.92, 2.02) | 1.06 (0.81, 1.39) | 0.86 (0.59, 1.24) | 1.42 (0.95, 2.12) |
| Race | Non-White | 1.09 (0.80, 1.48) | 1.24 (0.82, 1.89) | 0.85 (0.54, 1.36) | 1.06 (0.76, 1.47) | 1.36 (0.87, 2.12) | 0.76 (0.46, 1.24) |
| Ethnicity | Hispanic | 1.15 (0.87, 1.52)) | 1.19 (0.80, 1.75) | 0.80 (0.53, 1.21) | 1.01 (0.73, 1.40) | 1.32 (0.82, 2.15) | 0.76 (0.49, 1.18) |
| Disease | Metastatic | 4.21 (3.22, 5.52** | - | - | 5.69 (3.58, 9.07)** | - | - |
| Language Isolation | Disadvantaged | 1.03 (0.79, 1.35) | 0.95 (0.66, 1.37) | 0.85 (0.57, 1.26) | 0.88 (0.65, 1.19) | 0.86 (0.56, 1.33) | 0.86 (0.57, 1.32) |
| Education | Disadvantaged | 1.07 (0.82, 1.40) | 1.14 (0.79, 1.65) | 1.09 (0.74, 1.60) | 1.29 (0.94, 1.78) | 1.32 (0.84, 2.08) | 1.22 (0.76, 1.95) |
| Poverty | Disadvantaged | 0.90 (0.69, 1.17) | 0.86 (0.60, 1.24) | 0.96 (0.65, 1.43) | 0.83 (0.61, 1.12) | 0.74 (0.48, 1.15) | 0.97 (0.62, 1.50) |
| Adolescents | (n=580) | (n=456) | (n=124) | (n=580) | (n=456) | (n=124) | |
| Diagnosis Year | 2004-2006 | 0.85 (0.51, 1.41) | 1.11 (0.50, 2.46) | 0.67 (0.34, 1.33) | 0.83 (0.50, 1.38) | 1.06 (0.48, 2.36) | 0.65 (0.33, 1.30) |
| 2007-2009 | 0.70 (0.42, 1.17) | 0.97 (0.44, 2.15) | 0.66 (0.32, 1.38) | 0.78 (0.46, 1.31) | 0.96 (0.43, 2.12) | 0.73 (0.34, 1.56) | |
| 2010-2012 | 0.80 (0.47, 1.36) | 1.02 (0.45, 2.31) | 1.09 (0.52, 2.25) | 0.91 (0.53, 1.57) | 0.96 (0.42, 2.18) | 1.12 (0.52, 2.39) | |
| Sex | Female | 0.78 (0.58, 1.07) | 1.02 (0.70, 1.49) | 0.49 (0.28, 0.84) * | 0.78 (0.57, 1.06) | 1.03 (0.70, 1.51) | 0.49 (0.28, 0.85) * |
| Race | Non-White | 0.89 (0.64, 1.25) | 0.85 (0.55, 1.31) | 1.08 (0.64, 1.84) | 0.95 (0.67, 1.35) | 0.85 (0.54, 1.34) | 0.95 (0.53, 1.67) |
| Ethnicity | Hispanic | 1.14 (0.83, 1.55) | 1.10 (0.73, 1.65) | 1.16 (0.71, 1.90) | 1.11 (0.78, 1.58) | 1.20 (0.76, 1.89) | 0.96 (0.54, 1.73) |
| Disease | Metastatic | 3.78 (2.81, 5.08)** | - | - | 7.13 (4.06, 12.52) ** | - | - |
| Language Isolation | Disadvantaged | 0.94 (0.70, 1.25) | 0.75 (0.52, 1.08) | 1.66 (1.05, 2.62) * | 0.96 (0.69, 1.33) | 0.68 (0.44, 1.04) | 1.51 (0.90, 2.54) |
| Education | Disadvantaged | 1.13 (0.85, 1.51) | 0.95 (0.66, 1.37) | 1.36 (0.85, 2.18) | 1.07 (0.75, 1.53) | 0.97 (0.62, 1.54) | 1.50 (0.83, 2.71) |
| Poverty | Disadvantaged | 1.19 (0.89, 1.59) | 1.05 (0.73, 1.53) | 1.21 (0.75, 1.95) | 1.09 (0.76, 1.56) | 1.17 (0.75, 1.84) | 0.82 (0.46, 1.48) |
Includes all diagnoses from 2004-2015 and does not include insurance status as a predictor
Individual attributes in standard typeface and references are 2013-2015, 0-9 years, males, white, and non-Hispanic; county attributes in italics and reference is advantaged
HR=Hazards Ratio, aHR=Adjusted Hazards Ratio, CI=95% Confidence Intervals
Bold indicates statistically significant findings
indicates a p-value <0.05, and
indicates a p-value <0.01
Table 7.
Hazard ratios of death by sociodemographic characteristics of osteosarcoma diagnoses in children (0-14 years) and adolescents (15-19 years), SEER-18 registries 2007-2015
| Overall | Non-Metastatic | Metastatic | Overall | Non-Metastatic | Metastatic | ||
|---|---|---|---|---|---|---|---|
| Attribute | Predictor | HR (95% CI) | HR (95% CI) | HR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) |
| Pediatric Combined | (n=1070) | (n=828) | (n=242) | (n=1070) | (n=828) | (n=242) | |
| Diagnosis Year | 2007-2009 | 0.80 (0.56, 1.15) | 0.82 (0.48, 1.38) | 0.85 (0.51, 1.41) | 0.94 (0.64, 1.36) | 0.89 (0.52, 1.55) | 0.93 (0.55, 1.58) |
| 2010-2012 | 0.81 (0.56, 1.17) | 0.71 (0.41, 1.21) | 1.02 (0.62, 1.68) | 0.89 (0.61, 1.31) | 0.72 (0.41, 1.27) | 1.09 (0.64, 1.83) | |
| Age | 10-14 years | 1.01 (0.71, 1.43) | 1.08 (0.65, 1.77) | 0.83 (0.51, 1.35) | 0.93 (0.64, 1.33) | 1.07 (0.64, 1.78) | 0.76 (0.45, 1.28) |
| 15-19 years | 1.20 (0.86, 1.69) | 1.44 (0.89, 2.31) | 1.16 (0.70, 1.91) | 1.29 (0.90, 1.85) | 1.47 (0.90, 2.40) | 1.19 (0.70, 2.02) | |
| Sex | Female | 0.85 (0.67, 1.08) | 0.85 (0.61, 1.18) | 0.87 (0.61, 1.25) | 0.89 (0.69, 1.14) | 0.92 (0.65, 1.29) | 0.91 (0.63, 1.34) |
| Race | Non-White | 1.05 (0.79, 1.38) | 1.11 (0.77, 1.61) | 0.91 (0.60, 1.37) | 0.96 (0.70, 1.31) | 1.04 (0.69, 1.58) | 0.82 (0.51, 1.31) |
| Ethnicity | Hispanic | 1.04 (0.80, 1.35) | 1.07 (0.75, 1.52) | 0.88 (0.60, 1.29) | 1.08 (0.79, 1.48) | 1.39 (0.89, 2.15) | 0.85 (0.53, 1.34) |
| Insurance Status | Non-Private | 1.08 (0.84, 1.38) | 1.08 (0.76, 1.53) | 0.92 (0.64, 1.33) | 1.62 (0.99, 2.63) | 1.72 (0.84, 3.51) | 1.75 (0.88, 3.47) |
| Disease | Metastatic | 4.14 (3.26, 5.27) ** | - | - | 7.31 (4.53, 11.82) ** | - | - |
| Language Isolation | Disadvantaged | 0.91 (0.71, 1.15) | 0.77 (0.55, 1.06) | 1.01 (0.71, 1.43) | 0.81 (0.61, 1.06) | 0.61 (0.41, 0.91) * | 1.04 (0.70, 1.55) |
| Education | Disadvantaged | 1.14 (0.90, 1.44) | 1.14 (0.83, 1.58) | 1.29 (0.91, 1.84) | 1.38 (1.03, 1.85) * | 1.29 (0.85, 1.94) | 1.46 (0.96, 2.21) |
| Poverty | Disadvantaged | 0.96 (0.76, 1.22) | 0.94 (0.68, 1.29) | 0.95 (0.67, 1.35) | 0.83 (0.62, 1.11) | 0.90 (0.60, 1.36) | 0.82 (0.53, 1.25) |
| Children | (n=636) | (n=481) | (n=155) | (n=636) | (n=481) | (n=155) | |
| Diagnosis Year | 2007-2009 | 0.87 (0.53, 1.43) | 0.70 (0.35, 1.40) | 1.03 (0.50, 2.11) | 0.90 (0.54, 1.51) | 0.66 (0.33, 1.34) | 1.10 (0.51, 2.35) |
| 2010-2012 | 0.80 (0.48, 1.33) | 0.49 (0.24, 1.02) | 1.15 (0.57, 2.33) | 0.73 (0.44, 1.24) | 0.44 (0.21, 0.93) * | 1.17 (0.56, 2.46) | |
| Sex | Female | 0.89 (0.65, 1.23) | 0.73 (0.46, 1.16) | 1.19 (0.76, 1.87) | 0.94 (0.67, 1.30) | 0.75 (0.47, 1.20) | 1.24 (0.77, 2.01) |
| Race | Non-White | 1.13 (0.78, 1.64) | 1.24 (0.74, 2.09) | 0.89 (0.53, 1.50) | 0.96 (0.63, 1.46) | 1.20 (0.67, 2.17) | 0.73 (0.39, 1.33) |
| Ethnicity | Hispanic | 1.02 (0.72, 1.44) | 1.14 (0.70, 1.85) | 0.78 (0.48, 1.28) | 0.95 (0.62, 1.47) | 1.30 (0.69, 2.48) | 0.73 (0.41, 1.32) |
| Insurance Status | Non-Private | 1.19 (0.85, 1.66) | 1.34 (0.83, 2.15) | 1.01 (0.62, 1.63) | 2.36 (1.18, 4.71) * | 2.90 (0.96, 8.72) | 2.25 (0.89, 5.70) |
| Disease | Metastatic | 4.22 (3.06, 5.83) ** | - | - | 6.19 (3.24, 11.84) ** | - | - |
| Language Isolation | Disadvantaged | 1.00 (0.72, 1.37) | 0.88 (0.56, 1.39) | 0.86 (0.55, 1.36) | 0.84 (0.57, 1.22) | 0.76 (0.43, 1.34) | 0.89 (0.53, 1.48) |
| Education | Disadvantaged | 1.09 (0.79, 1.50) | 1.33 (0.84, 2.11) | 1.11 (0.71, 1.75) | 1.34 (0.91, 1.98) | 1.32 (0.75, 2.31) | 1.35 (0.77, 2.34) |
| Poverty | Disadvantaged | 0.85 (0.62, 1.17) | 0.87 (0.55, 1.38) | 0.88 (0.56, 1.39) | 0.78 (0.54, 1.13) | 0.73 (0.43, 1.26) | 0.86 (0.51, 1.43) |
| Adolescents | (n=434) | (n=347) | (n=87) | (n=434) | (n=347) | (n=87) | |
| Diagnosis Year | 2007-2009 | 0.72 (0.43, 1.23) | 0.99 (0.44, 2.23) | 0.74 (0.35, 1.57) | 0.99 (0.56, 1.73) | 1.27 (0.51, 3.17) | 0.91 (0.42, 1.99) |
| 2010-2012 | 0.86 (0.50, 1.47) | 1.09 (0.48, 2.49) | 1.15 (0.55, 2.39) | 1.18 (0.66, 2.09) | 1.29 (0.51, 3.28) | 1.44 (0.66, 3.18) | |
| Sex | Female | 0.83 (0.57, 1.21) | 1.09 (0.68, 1.74) | 0.50 (0.25, 0.99) * | 0.85 (0.57, 1.26) | 1.20 (0.73, 1.96) | 0.48 (0.24, 0.99) * |
| Race | Non-White | 0.92 (0.61, 1.39) | 0.95 (0.57, 1.60) | 0.98 (0.50, 1.93) | 1.03 (0.65, 1.65) | 0.92 (0.51, 1.69) | 1.00 (0.46, 2.18) |
| Ethnicity | Hispanic | 1.09 (0.74, 1.62) | 1.03 (0.62, 1.73) | 1.07 (0.58, 1.98) | 1.28 (0.79, 2.07) | 1.44 (0.77, 2.68) | 0.86 (0.37, 2.00) |
| Insurance Status | Non-Private | 0.95 (0.65, 1.40) | 0.86 (0.51, 1.45) | 0.76 (0.42, 1.36) | 1.02 (0.50, 2.06) | 1.09 (0.40, 2.96) | 1.28 (0.42, 3.89) |
| Disease | Metastatic | 4.40 (3.04, 6.35) ** | - | - | 9.06 (4.31, 19.05) ** | - | - |
| Language Isolation | Disadvantaged | 0.80 (0.56, 1.15) | 0.65 (0.41, 1.04) | 1.37 (0.78, 2.42) | 0.74 (0.48, 1.13) | 0.49 (0.28, 0.86) * | 1.31 (0.65, 2.65) |
| Education | Disadvantaged | 1.20 (0.84, 1.71) | 0.99 (0.63, 1.56) | 1.59 (0.89, 2.85) | 1.39 (0.87, 2.23) | 1.06 (0.57, 1.99) | 2.26 (1.08, 4.73) * |
| Poverty | Disadvantaged | 1.12 (0.78, 1.60) | 1.02 (0.64, 1.60) | 0.98 (0.54, 1.75) | 0.93 (0.56, 1.53) | 1.26 (0.66, 2.38) | 0.54 (0.24, 1.21) |
Includes all diagnoses from 2007 -2015 and insurance status as a predictor
Individual attributes in standard typeface and references are 2013-2015, 0-9 years, males, white, non-Hispanic, and private; county attributes in italics and reference is advantaged
HR= Hazards Ratio, a HR=Adjusted Hazards Ratio, CI=95% Confidence Intervals
Bold indicates statistically significant findings
indicates a p-value <0.05, and
indicates a p-value <0.01
For ES, non-White adolescents had higher odds of death than their White counterparts (Table 8: 2.0 [1.2, 3.3]; p=0.005), and this disadvantage worsened for those with metastatic disease at diagnosis (2.8 [1.4, 5.8]; p=0.004). Hispanic children without metastasis had increased odds of death compared to non-Hispanics (2.1 [1.1, 3.8]; p=0.049). These findings were also observed from 2007 to 2015 (Table 9). Adolescents diagnosed in 2004 through 2006 had a two-fold increase (Table 8: 2.6 [1.0, 6.8]; p=0.047) in odds of death when compared to those diagnosed between 2007 and 2015.
Table 8.
Hazard ratios of death by sociodemographic characteristics of Ewing sarcoma diagnoses in children (0-14 years) and adolescents (15-19 years), SEER-18 registries 2004-2015
| Overall | Non-Metastatic | Metastatic | Overall | Non-Metastatic | Metastatic | ||
|---|---|---|---|---|---|---|---|
| Attribute | Predictor | HR (95% CI) | HR (95% CI) | HR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) |
| Pediatric Combined | (n=802) | (n=551) | (n=251) | (n=802) | (n=551) | (n=251) | |
| Diagnosis Year | 2004-2006 | 1.47 (0.85, 2.52) | 2.01 (0.70, 5.79) | 1.73 (0.91, 3.28) | 1.81 (1.05, 3.12) * | 1.91 (0.66, 5.55) | 1.92 (1.00, 3.67) |
| 2007-2009 | 0.96 (0.55, 1.69) | 1.33 (0.45, 3.93) | 0.99 (0.50, 1.95) | 1.13 (0.64, 1.98) | 1.16 (0.39, 3.43) | 1.05 (0.53, 2.09) | |
| 2010-2013 | 1.20 (0.69, 2.09) | 1.67 (0.56, 4.95) | 1.01 (0.52, 1.96) | 1.22 (0.70, 2.13) | 1.60 (0.54, 4.76) | 1.07 (0.55, 2.08) | |
| Age | 10-14 years | 2.27 (1.47, 3.53) ** | 4.32 (1.93, 9.70) ** | 1.48 (0.87, 2.54) | 2.20 (1.42, 3.42) ** | 4.29 (1.91, 9.67) ** | 1.46 (0.84, 2.54) |
| 15-19 years | 3.30 (2.16, 5.03) ** | 6.84 (3.10, 15.08) ** | 1.63 (0.98, 2.71) | 2.63 (1.71, 4.04) ** | 6.45 (2.92, 14.28) ** | 1.45 (0.86, 2.46) | |
| Sex | Female | 0.85 (0.64, 1.13) | 0.63 (0.41, 0.98) * | 1.10 (0.76, 1.59) | 0.93 (0.69, 1.23) | 0.68 (0.43, 1.06) | 1.10 (0.74, 1.61) |
| Race | Non-White | 1.47 (1.00, 2.17) * | 1.41 (0.80, 2.49) | 2.11 (1.24, 3.58) ** | 1.78 (1.19, 2.67) ** | 1.33 (0.72, 2.42) | 2.16 (1.24, 3.74) ** |
| Ethnicity | Hispanic | 1.32 (0.98, 1.78) | 1.55 (1.00, 2.41) | 0.96 (0.64, 1.44) | 1.26 (0.89, 1.80) | 1.74 (1.06, 2.84) * | 0.95 (0.57, 1.57) |
| Disease | Metastatic | 3.97 (3.02, 5.22) ** | - | - | 6.40 (3.89, 10.52) ** | - | - |
| Language Isolation | Disadvantaged | 1.26 (0.96, 1.65) | 1.33 (0.89, 1.98) | 0.98 (0.68, 1.40) | 0.94 (0.69, 1.27) | 1.02 (0.63, 1.66) | 0.87 (0.57, 1.32) |
| Education | Disadvantaged | 1.15 (0.88, 1.50) | 1.08 (0.72, 1.62) | 1.17 (0.81, 1.68) | 1.01 (0.72, 1.41) | 0.94 (0.55, 1.61) | 1.19 (0.76, 1.87) |
| Poverty | Disadvantaged | 1.29 (0.98, 1.69) | 1.24 (0.83, 1.86) | 1.24 (0.86, 1.78) | 1.20 (0.86, 1.67) | 1.16 (0.70, 1.92) | 1.33 (0.86, 2.05) |
| Children | (n=515) | (n=373) | (n=142) | (n=515) | (n=373) | (n=142) | |
| Diagnosis Year | 2004-2006 | 1.04 (0.52, 2.07) | 1.86 (0.41, 8.50) | 1.22 (0.55, 2.72) | 1.32 (0.66, 2.66) | 1.83 (0.40, 8.37) | 1.39 (0.62, 3.15) |
| 2007-2009 | 0.60 (0.29, 1.23) | 1.09 (0.23, 5.19) | 0.49 (0.21, 1.17) | 0.59 (0.28, 1.22) | 0.97 (0.20, 4.62) | 0.48 (0.20, 1.17) | |
| 2010-2013 | 0.89 (0.44, 1.79) | 1.77 (0.38, 8.18) | 0.66 (0.29, 1.49) | 0.84 (0.41, 1.69) | 1.93 (0.42, 8.97) | 0.58 (0.25, 1.36) | |
| Sex | Female | 1.06 (0.72, 1.56) | 0.79 (0.43, 1.47) | 1.15 (0.69, 1.91) | 0.89 (0.60, 1.33) | 0.81 (0.44, 1.51) | 0.96 (0.57, 1.61) |
| Race | Non-White | 0.97 (0.47, 1.99) | 0.83 (0.26, 2.68) | 1.31 (0.53, 3.29) | 1.28 (0.61, 2.69) | 0.90 (0.27, 3.01) | 1.74 (0.66, 4.55) |
| Ethnicity | Hispanic | 1.62 (1.08, 2.43) * | 2.05 (1.11, 3.78) * | 1.04 (0.60, 1.79) | 1.45 (0.90, 2.35) | 2.03 (1.00, 4.13) * | 1.15 (0.59, 2.25) |
| Disease | Metastatic | 5.11 (3.45, 7.57) ** | - | - | 9.91 (4.65, 21.12) ** | - | - |
| Language Isolation | Disadvantaged | 1.38 (0.94, 2.03) | 1.60 (0.88, 2.89) | 0.82 (0.50, 1.37) | 0.94 (0.61, 1.46) | 1.30 (0.65, 2.63) | 0.74 (0.41, 1.33) |
| Education | Disadvantaged | 1.24 (0.84, 1.82) | 1.26 (0.70, 2.28) | 1.18 (0.71, 1.97) | 1.20 (0.75, 1.91) | 1.05 (0.47, 2.32) | 1.40 (0.75, 2.61) |
| Poverty | Disadvantaged | 1.17 (0.80, 1.73) | 1.21 (0.66, 2.20) | 1.04 (0.63, 1.74) | 0.97 (0.61, 1.53) | 0.88 (0.40, 1.92) | 1.09 (0.60, 1.99) |
| Adolescents | (n=287) | (n=178) | (n=109) | (n=287) | (n=178) | (n=109) | |
| Diagnosis Year | 2004-2006 | 2.14 (0.83, 5.50) | 1.69 (0.38, 7.42) | 3.25 (0.95, 11.06) | 2.62 (1.01, 6.78) * | 1.80 (0.40, 8.06) | 3.32 (0.96, 11.51) |
| 2007-2009 | 1.74 (0.67, 4.56) | 1.31 (0.29, 5.91) | 2.93 (0.84, 10.25) | 2.11 (0.80, 5.56) | 1.11 (0.24, 5.12) | 2.91 (0.83, 10.24) | |
| 2010-2013 | 1.82 (0.69, 4.81) | 1.41 (0.30, 6.64) | 2.12 (0.61, 7.39) | 1.85 (0.70, 4.91) | 1.23 (0.26, 5.89) | 2.32 (0.65, 8.23) | |
| Sex | Female | 0.78 (0.51, 1.19) | 0.57 (0.30, 1.08) | 1.21 (0.68, 2.15) | 0.83 (0.54, 1.28) | 0.52 (0.27, 1.01) | 1.02 (0.54, 1.93) |
| Race | Non-White | 1.58 (0.99, 2.53) | 1.39 (0.71, 2.70) | 2.85 (1.46, 5.57) ** | 2.02 (1.23, 3.32) ** | 1.53 (0.74, 3.16) | 2.84 (1.39, 5.79) ** |
| Ethnicity | Hispanic | 1.10 (0.70, 1.73) | 1.25 (0.65, 2.39) | 0.91 (0.49, 1.69) | 1.17 (0.69, 1.96) | 1.54 (0.75, 3.16) | 0.83 (0.38, 1.83) |
| Disease | Metastatic | 2.75 (1.87, 4.04) ** | - | - | 4.65 (2.38, 9.09) ** | - | - |
| Language Isolation | Disadvantaged | 1.11 (0.76, 1.62) | 1.02 (0.59, 1.76) | 1.24 (0.73, 2.09) | 0.99 (0.64, 1.52) | 0.87 (0.45, 1.67) | 1.11 (0.59, 2.09) |
| Education | Disadvantaged | 1.04 (0.71, 1.52) | 0.93 (0.53, 1.61) | 1.12 (0.66, 1.90) | 0.82 (0.50, 1.34) | 0.69 (0.33, 1.43) | 1.08 (0.55, 2.12) |
| Poverty | Disadvantaged | 1.37 (0.93, 2.00) | 1.22 (0.70, 2.11) | 1.48 (0.87, 2.51) | 1.50 (0.93, 2.42) | 1.54 (0.76, 3.12) | 1.60 (0.84, 3.03) |
Includes all diagnoses from 2004-2015 and does not include insurance status as a predictor
Individual attributes in standard typeface and references are 2013-2015, 0-9 years, males, white, and non-Hispanic; county attributes in italics and reference is advantaged
HR= Hazards Ratio, aHR=Adjusted Hazards Ratio, CI=95% Confidence Intervals
Bold indicates statistically significant findings
indicates a p-value <0.05, and
indicates a p-value <0.01
Table 9.
Hazard ratios of death by sociodemographic characteristics of Ewing sarcoma diagnoses in children (0-14 years) and adolescents (15-19 years), SEER-18 registries 2007-2015
| Overall | Non-Metastatic | Metastatic | Overall | Non-Metastatic | Metastatic | ||
|---|---|---|---|---|---|---|---|
| Attribute | Predictor | HR (95% CI) | HR (95% CI) | HR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) |
| Pediatric Combined | (n=603) | (n=408) | (n=195) | (n=603) | (n=408) | (n=195) | |
| Diagnosis Year | 2007-2009 | 0.91 (0.51, 1.62) | 1.38 (0.46, 4.18) | 0.90 (0.45, 1.80) | 1.09 (0.60, 1.99) | 1.10 (0.36, 3.37) | 1.13 (0.54, 2.34) |
| 2010-2012 | 1.11 (0.63, 1.96) | 1.68 (0.56, 5.05) | 0.91 (0.47, 1.79) | 1.17 (0.65, 2.10) | 1.58 (0.53, 4.77) | 1.07 (0.53, 2.17) | |
| Age | 10-14 years | 1.89 (1.11, 3.23) * | 4.07 (1.40, 11.86) * | 1.29 (0.68, 2.43) | 1.87 (1.09, 3.20) * | 3.79 (1.30, 11.09) * | 1.40 (0.72, 2.70) |
| 15-19 years | 2.90 (1.73, 4.85) ** | 6.80 (2.38, 19.43) ** | 1.53 (0.83, 2.82) | 2.47 (1.46, 4.17) ** | 5.94 (2.06, 17.12) ** | 1.64 (0.88, 3.08) | |
| Sex | Female | 0.95 (0.66, 1.37) | 0.75 (0.41, 1.34) | 1.12 (0.71, 1.78) | 1.04 (0.72, 1.51) | 0.83 (0.46, 1.53) | 1.18 (0.73, 1.92) |
| Race | Non-White | 1.21 (0.72, 2.04) | 0.96 (0.41, 2.25) | 2.09 (1.07, 4.07) * | 1.51 (0.86, 2.65) | 0.88 (0.35, 2.18) | 2.05 (0.99, 4.24) |
| Ethnicity | Hispanic | 1.14 (0.77, 1.67) | 1.44 (0.80, 2.59) | 0.78 (0.46, 1.30) | 1.12 (0.69, 1.82) | 1.44 (0.75, 2.75) | 0.89 (0.44, 1.82) |
| Insurance Status | Non-Private | 0.97 (0.67, 1.42) | 0.71 (0.37, 1.35) | 1.03 (0.64, 1.66) | 0.85 (0.55, 1.32) | 0.61 (0.30, 1.23) | 1.08 (0.60, 1.96) |
| Disease | Metastatic | 4.03 (2.82, 5.75) ** | - | - | 3.93 (2.71, 5.69) ** | - | - |
| Language Isolation | Disadvantaged | 1.10 (0.78, 1.56) | 1.07 (0.61, 1.85) | 0.85 (0.54, 1.34) | 0.82 (0.55, 1.22) | 0.99 (0.51, 1.92) | 0.80 (0.47, 1.36) |
| Education | Disadvantaged | 1.08 (0.76, 1.53) | 1.10 (0.64, 1.90) | 0.89 (0.57, 1.40) | 0.80 (0.52, 1.24) | 0.74 (0.34, 1.63) | 0.80 (0.46, 1.40) |
| Poverty | Disadvantaged | 1.41 (1.00, 2.00) | 1.50 (0.87, 2.59) | 1.12 (0.71, 1.77) | 1.46 (0.95, 2.25) | 2.01 (0.96, 4.19) | 1.23 (0.72, 2.11) |
| Children | (n=401) | (n=285) | (n=116) | (n=401) | (n=285) | (n=116) | |
| Diagnosis Year | 2007-2009 | 0.56 (0.26, 1.19) | 1.07 (0.22, 5.30) | 0.48 (0.20, 1.17) | 0.54 (0.25, 1.17) | 0.87 (0.18, 4.35) | 0.50 (0.20, 1.26) |
| 2010-2012 | 0.83 (0.41, 1.70) | 1.73 (0.36, 8.19) | 0.61 (0.27, 1.42) | 0.80 (0.39, 1.65) | 1.95 (0.41, 9.31) | 0.62 (0.26, 1.48) | |
| Sex | Female | 1.10 (0.68, 1.79) | 1.11 (0.51, 2.46) | 1.04 (0.56, 1.94) | 0.98 (0.60, 1.62) | 1.22 (0.54, 2.76) | 0.98 (0.51, 1.89) |
| Race | Non-White | 0.65 (0.24, 1.80) | N/A | 1.28 (0.45, 3.59) | 0.78 (0.27, 2.22) | N/A | 1.29 (0.43, 3.82) |
| Ethnicity | Hispanic | 1.45 (0.87, 2.42) | 2.19 (0.98, 4.88) | 0.82 (0.42, 1.61) | 1.48 (0.77, 2.85) | 2.73 (1.04, 7.15) * | 0.96 (0.38, 2.44) |
| Insurance Status | Non-Private | 0.88 (0.52, 1.47) | 0.51 (0.19, 1.36) | 0.94 (0.50, 1.76) | 0.63 (0.35, 1.14) | 0.32 (0.11, 0.93) * | 0.92 (0.42, 2.04) |
| Disease | Metastatic | 5.15 (3.13, 8.49) ** | - | - | 5.00 (2.99, 8.35) ** | - | - |
| Language Isolation | Disadvantaged | 1.20 (0.74, 1.94) | 1.36 (0.62, 2.98) | 0.75 (0.40, 1.38) | 0.86 (0.49, 1.52) | 1.26 (0.47, 3.35) | 0.79 (0.38, 1.66) |
| Education | Disadvantaged | 1.22 (0.75, 1.98) | 1.42 (0.65, 3.10) | 0.94 (0.51, 1.74) | 1.13 (0.63, 2.01) | 1.18 (0.40, 3.44) | 1.04 (0.50, 2.14) |
| Poverty | Disadvantaged | 1.21 (0.75, 1.97) | 1.20 (0.54, 2.64) | 0.95 (0.52, 1.76) | 1.02 (0.59, 1.78) | 1.08 (0.38, 3.08) | 1.03 (0.52, 2.02) |
| Adolescents | (n=202) | (n=123) | (n=79) | (n=202) | (n=123) | (n=79) | |
| Diagnosis Year | 2007-2009 | 1.67 (0.63, 4.45) | 1.49 (0.32, 6.88) | 2.53 (0.71, 9.04) | 2.43 (0.81, 7.33) | 0.88 (0.18, 4.37) | 4.55 (0.93, 22.19) |
| 2010-2012 | 1.70 (0.64, 4.54) | 1.47 (0.31, 7.01) | 1.85 (0.52, 6.54) | 2.04 (0.69, 6.09) | 1.20 (0.25, 5.86) | 3.31 (0.70, 15.71) | |
| Sex | Female | 0.97 (0.55, 1.70) | 0.56 (0.21, 1.49) | 1.40 (0.69, 2.84) | 0.94 (0.52, 1.69) | 0.43 (0.15, 1.20) | 1.19 (0.50, 2.80) |
| Race | Non-White | 1.48 (0.79, 2.78) | 1.29 (0.52, 3.19) | 4.14 (1.64, 10.47) * | 1.90 (0.93, 3.90) | 1.00 (0.36, 2.79) | 4.00 (1.36, 11.80) * |
| Ethnicity | Hispanic | 0.85 (0.47, 1.55) | 0.88 (0.35, 2.17) | 0.74 (0.34, 1.65) | 0.90 (0.43, 1.89) | 0.77 (0.29, 2.08) | 0.93 (0.25, 3.41) |
| Insurance Status | Non-Private | 1.33 (0.76, 2.31) | 1.18 (0.50, 2.81) | 1.36 (0.66, 2.83) | 1.17 (0.59, 2.31) | 1.07 (0.41, 2.82) | 1.34 (0.46, 3.87) |
| Disease | Metastatic | 2.87 (1.72, 4.79) ** | - | - | 3.30 (1.90, 5.75) ** | - | - |
| Language Isolation | Disadvantaged | 1.02 (0.62, 1.69) | 0.84 (0.38, 1.83) | 1.05 (0.54, 2.06) | 0.89 (0.49, 1.60) | 0.94 (0.38, 2.31) | 0.98 (0.43, 2.25) |
| Education | Disadvantaged | 0.92 (0.55, 1.52) | 0.86 (0.40, 1.86) | 0.81 (0.41, 1.59) | 0.58 (0.30, 1.11) | 0.28 (0.09, 0.87) * | 0.71 (0.26, 1.93) |
| Poverty | Disadvantaged | 1.56 (0.93, 2.59) | 1.67 (0.78, 3.61) | 1.37 (0.69, 2.72) | 1.89 (0.96, 3.75) | 4.46 (1.47, 13.50) ** | 1.16 (0.45, 3.03) |
Includes all diagnoses from 2007-2015 and insurance status as a predictor
Individual attributes in standard typeface and references are 2013-2015, 0-9 years, males, white, non-Hispanic, and private; county attributes in italics and reference is advantaged
HR=Hazards Ratio, aHR=Adjusted Hazards Ratio, CI=95% Confidence Intervals
Bold indicates statistically significant findings
indicates a p-value <0.05, and
indicates a p-value <0.01
Similar to OST, the inclusion of insurance status and removal of 2004-2006 cases modified the importance of county level attributes. For adolescents with non-metastatic disease, living in disadvantaged counties by poverty level was associated with 4.5-fold (Table 9 [1.5, 13.5]; p=0.008) increased odds of death, but living in disadvantaged counties educationally was protective (OR 0.3 [0.1, 0.9]; p=0.035) when compared to adolescents in advantaged counties for these measures. In children with non-metastatic disease, non-private insurance was protective (0.3 [0.1, 0.9]; p=0.04) compared to private insurance.
Post Hoc Analysis
To examine the possibility that differences in tumor location based on race and ethnicity could account for some disparities seen, we analyzed tumor location differences based on these factors. There was no significant variability in primary tumor location for either OST or ES based on race or ethnicity (Supplemental Table 1).
Discussion
In the present study, we document the impact of demographic and socioeconomic factors on stage at presentation and survival outcomes in pediatric OST and ES. In OST, there was an association between Hispanic ethnicity and metastatic disease at presentation. Living in counties with language isolation was also found to be a predictor of metastatic disease at diagnosis. Regardless of metastatic status, children with OST with public insurance had increased odds of death compared to those with private insurance. Living in disadvantaged education counties increased odds of death for adolescents with metastatic disease. In ES, non-White adolescents had higher odds of death compared to white patients. Adolescents with metastatic ES living in counties with higher poverty levels showed an increase in odds of death compared to those living in counties with less poverty.
Tumor biology irrespective of race and ethnicity can certainly affect disease aggressiveness and outcomes. However, it is unlikely that differences in tumor biology based on race and ethnicity are responsible for the outcomes disparities documented here, although we are not able to fully exclude this possibility. Previous studies that showed a different age distribution of ES patients by ethnicity12, 17 may give some credence to a biological explanation, as this could mean that biological drivers impacting metastasis or survival of ES vary by ethnicity; however, we found no significant difference in age distribution of our ES study population based on ethnicity (chi2 p=0.666, Supplemental Table 2). The lack of variability in primary location for either tumor based on race and ethnicity also argues against a biological etiology for the observed disparities (Supplemental Table 1), as do the continued influence of some social disadvantages on multivariable analysis. Instead, the presence of disparities both in presentation as well as in survival of these tumors suggests that both access to care and quality of care issues may be playing separate roles in creating poorer outcomes for disadvantaged groups. The social disparities we studied may cause a higher likelihood of metastatic disease at diagnosis through impaired access to care, which in turn leads to delayed diagnosis. These disadvantages could also independently result in poorer quality treatment after diagnosis, leading to lower survival rates.
A previous study of time to diagnosis in ES patients showed no impact of a longer time to diagnosis on the presence of metastases at diagnosis, or on survival.25 This study’s findings correspond with our data showing a lack of significant differences in metastatic rate at diagnosis for ES. The prior study’s findings also support the conclusion that the survival disparities we observed in ES are due more to disparities in quality of care after diagnosis than access to care prior.
We believe it is possible for barriers to care to increase the likelihood of metastatic disease at diagnosis, however, and that this is the most likely explanation for our findings in OST. Our census-based findings, especially the comparisons based on Hispanic ethnicity and language-isolated populations, suggest that decreased English proficiency may be a barrier to diagnosis leading to poorer outcomes. This may be further supported by the difference noted for children in language-isolated populations that was not seen in adolescents. Adolescents are more likely to have developed English proficiency in school adequate to communicate in a medical setting, even if their parents have not. They are also more likely to be personally involved in their medical care compared to children, who still need their parents to communicate and advocate for them, potentially causing delays in diagnosis if their parents are not proficient in English.
When longer time to diagnosis (generally defined as duration from first symptoms to definitive diagnosis and used interchangeably with the term “delayed diagnosis”) has been previously studied in osteosarcoma, it has been found to have no impact on outcome,26 or even to be associated with better outcomes.27 We would assert that the explanation for these findings may lie in the distinction between time to diagnosis and delayed diagnosis.
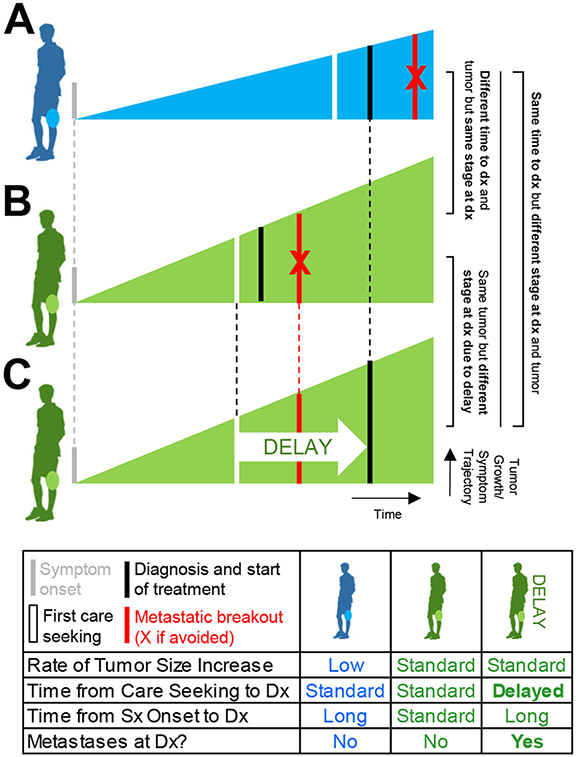
As an illustrative example, take two patients who both develop OST (Figure 1). If one patient’s tumor grows faster (potentially due to more aggressive biology, a possibility previously raised by distinct lab findings between patients with different stage tumors at diagnosis27), that patient is likely to have a faster progression of symptoms and present to a diagnosing provider sooner than the patient whose symptoms are not as severe (Figure 1B vs. 1A). If they are able to access care smoothly, however, both patients are likely to present with early stage disease, even though their time to diagnosis from symptom onset is different. If that same patient with a faster-growing tumor has difficulty accessing care, however, and the patient with a slower-growing tumor does not, their time to diagnosis may be equal, but the first patient may have greater extent of disease at diagnosis because of his delay in diagnosis (i.e. longer time from care-seeking to definitive diagnosis) not experienced by the second patient (Figure 1C vs. 1A). In this example, a study looking only at time to diagnosis would conclude (misleadingly) that time does not influence extent of disease at diagnosis. We believe delayed diagnosis, using this definition, has been less studied than time to diagnosis, likely because it requires more detailed history that is best ascertained directly from patients.
Figure 1:
Comparison of the consequences of differing time to diagnosis versus delayed diagnosis in two patients with osteosarcoma: (A) a patient with slow-growing disease; (B) a patient with faster-growing disease; and (C) the same patient as in (B) but with a delay in diagnosis from first care seeking. Dx = diagnosis; Sx = symptoms.
There may be particular challenges for ES patients in accessing quality care compared to OST patients. ES has a wider variety of phenotypes than OST, given that it commonly presents in soft tissue or in the axial skeleton,28 whereas the great majority of osteosarcoma occurs in the appendicular skeleton, especially in children;29 which our dataset also reflects (Supplemental Table 1). This can create more challenging surgical cases in ES that may depend on higher quality orthopedic care, or, if inoperable, on high quality radiation oncology care, which could create disparities in outcomes. This same variety of presenting locations also may cause more subtle symptoms, including visceral versus parietal pain, potentially increasing the likelihood of delayed presentation and/or diagnosis.
The impact of insurance status on survival differed between OST and ES. In OST, pediatric patients with public insurance had greater odds of death versus those with private insurance. This could be due to an increase in options for care with private insurance, allowing more freedom to find expert providers and a care team that aligns well with the child. However, in ES, non-private insurance was found to be protective in children with non-metastatic disease. At a non-metastatic stage of disease in ES, differences in providers may not have as great of an impact. Further study should be conducted to investigate the role that insurance plays in the outcomes of these pediatric populations.
Our population-based study has both strengths and weaknesses. The findings of significant disparities based on census-derived, county-level data give validation to the patient-specific ethnic disparities. While the association between area-based and individual analyses is well-studied and fairly strong,30 findings can often differ,31 and thus having positive findings from both analyses lends validity to our conclusions. Although the use of SEER data gives our study population validation as being representative of the U.S. as a whole, we acknowledge that the use of population-based data limits our ability to posit explanations for our findings. SEER does not include such information as immigration status or distance to referral center, which could help clarify our findings. There may also be differences among subpopulations of Latinos that we are unable to find with this database. In addition, the size of the dataset, especially in terms of patients with metastatic disease, patients with available insurance information, and 5yr RS analysis, limits our statistical power to show some potential disparities.
Future work should focus on primary data collection, in order to expand on these findings and better determine reasons for the disparities found. This would allow patient-specific questions about time to presentation, barriers to care (including language proficiency) leading to delayed diagnosis, and potential biological differences in tumors to be answered. With better explanations, interventions to address true socioeconomic disparities for particular groups, such as increasing access to care and timely subspecialty referral to experienced centers, could be undertaken.
Supplementary Material
Supplemental Table 1: Location of tumors by race and ethnicity
Supplemental Table 2: Ages of study subjects with ES by ethnicity
Support:
This work was supported in part by the Population Health Shared Resource of the Colorado cancer center support grant P30CA046934.
Footnotes
Conflict of Interest Statement
The authors declare that they have no conflict of interest with this work.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA: a cancer journal for clinicians. Jan-Feb 2000;50(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Ford CA, Millstein SG, Halpern-Felsher BL, Irwin CE Jr. Influence of physician confidentiality assurances on adolescents' willingness to disclose information and seek future health care. A randomized controlled trial. Jama. Sep 24 1997;278(12):1029–34. [PubMed] [Google Scholar]
- 3.Terrier P, Llombart-Bosch A, Contesso G. Small round blue cell tumors in bone: prognostic factors correlated to Ewing's sarcoma and neuroectodermal tumors. Seminars in diagnostic pathology. Aug 1996;13(3):250–7. [PubMed] [Google Scholar]
- 4.Ferguson WS, Goorin AM. Current treatment of osteosarcoma. Cancer investigation. 2001;19(3):292–315. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia S Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer. Jun 2011;56(6):994–1002. doi: 10.1002/pbc.23078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Truong B, Green AL, Friedrich P, Ribeiro KB, Rodriguez-Galindo C. Ethnic, Racial, and Socioeconomic Disparities in Retinoblastoma. JAMA pediatrics. Oct 5 2015:1–9. doi: 10.1001/jamapediatrics.2015.2360 [DOI] [PubMed] [Google Scholar]
- 7.Tannenbaum SL, Koru-Sengul T, Zhao W, Miao F, Byrne MM. Survival disparities in non-small cell lung cancer by race, ethnicity, and socioeconomic status. Cancer journal. Jul-Aug 2014;20(4):237–45. doi: 10.1097/PPO.0000000000000058 [DOI] [PubMed] [Google Scholar]
- 8.Bristow RE, Powell MA, Al-Hammadi N, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. Journal of the National Cancer Institute. Jun 5 2013;105(11):823–32. doi: 10.1093/jnci/djt065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. Aug 1 2007;110(3):660–9. doi: 10.1002/cncr.22826 [DOI] [PubMed] [Google Scholar]
- 10.Du XL, Liu CC. Racial/Ethnic disparities in socioeconomic status, diagnosis, treatment and survival among medicare-insured men and women with head and neck cancer. Journal of health care for the poor and underserved. Aug 2010;21(3):913–30. doi: 10.1353/hpu.0.0331 [DOI] [PubMed] [Google Scholar]
- 11.Yu XQ. Socioeconomic disparities in breast cancer survival: relation to stage at diagnosis, treatment and race. BMC cancer. 2009;9:364. doi: 10.1186/1471-2407-9-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharib J, Horvai A, Gray Hazard FK, et al. Comparison of Latino and non-Latino patients with Ewing sarcoma. Pediatric blood & cancer. Feb 2014;61(2):233–7. doi: 10.1002/pbc.24745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koohbanani B, Han G, Reed D, et al. Ethnicity and age disparities in Ewing sarcoma outcome. Fetal and pediatric pathology. Jul 2013;32(4):246–52. doi: 10.3109/15513815.2012.721480 [DOI] [PubMed] [Google Scholar]
- 14.Karski EE, McIlvaine E, Segal MR, et al. Identification of Discrete Prognostic Groups in Ewing Sarcoma. Pediatric blood & cancer. Jan 2016;63(1):47–53. doi: 10.1002/pbc.25709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG. Ethnic and racial differences in patients with Ewing sarcoma. Cancer. Feb 15 2010;116(4):983–8. doi: 10.1002/cncr.24865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung MR. Optimization of predictors of Ewing sarcoma cause-specific survival: a population study. Asian Pacific journal of cancer prevention : APJCP. 2014;15(10):4143–5. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Hoang BH, Ziogas A, Zell JA. Analysis of prognostic factors in Ewing sarcoma using a population-based cancer registry. Cancer. Apr 15 2010;116(8):1964–73. doi: 10.1002/cncr.24937 [DOI] [PubMed] [Google Scholar]
- 18.Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer epidemiology. Aug 2015;39(4):593–9. doi: 10.1016/j.canep.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 19.Miller BJ, Cram P, Lynch CF, Buckwalter JA. Risk factors for metastatic disease at presentation with osteosarcoma: an analysis of the SEER database. The Journal of bone and joint surgery American volume. Jul 3 2013;95(13):e89. doi: 10.2106/JBJS.L.01189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan SS, Healey JH. Making a case for the socioeconomic determinacy of survival in osteosarcoma. Clinical orthopaedics and related research. Mar 2013;471(3):784–91. doi: 10.1007/s11999-012-2575-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen I, Pasalic D, Fischer-Valuck B, et al. Disparity in Outcomes for Adolescent and Young Adult Patients Diagnosed With Pediatric Solid Tumors Across 4 Decades. Am J Clin Oncol. May 2018;41(5):471–475. doi: 10.1097/coc.0000000000000304 [DOI] [PubMed] [Google Scholar]
- 22.Shi J, Yang J, Ma X, Wang X. Risk factors for metastasis and poor prognosis of Ewing sarcoma: a population based study. J Orthop Surg Res. Mar 4 2020;15(1):88. doi: 10.1186/s13018-020-01607-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawes AJ, Louie R, Nguyen DK, et al. The impact of continuous Medicaid enrollment on diagnosis, treatment, and survival in six surgical cancers. Health Serv Res. Dec 2014;49(6):1787–811. doi: 10.1111/1475-6773.12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. Sep 1961;6:101–21. [PubMed] [Google Scholar]
- 25.Brasme JF, Chalumeau M, Oberlin O, Valteau-Couanet D, Gaspar N. Time to diagnosis of Ewing tumors in children and adolescents is not associated with metastasis or survival: a prospective multicenter study of 436 patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Jun 20 2014;32(18):1935–40. doi: 10.1200/JCO.2013.53.8058 [DOI] [PubMed] [Google Scholar]
- 26.Brasme JF, Morfouace M, Grill J, et al. Delays in diagnosis of paediatric cancers: a systematic review and comparison with expert testimony in lawsuits. The Lancet Oncology. Oct 2012;13(10):e445–59. doi: 10.1016/S1470-2045(12)70361-3 [DOI] [PubMed] [Google Scholar]
- 27.Bacci G, Ferrari S, Longhi A, et al. High-grade osteosarcoma of the extremity: differences between localized and metastatic tumors at presentation. Journal of pediatric hematology/oncology. Jan 2002;24(1):27–30. [DOI] [PubMed] [Google Scholar]
- 28.Applebaum MA, Worch J, Matthay KK, et al. Clinical features and outcomes in patients with extraskeletal Ewing sarcoma. Cancer. Jul 1 2011;117(13):3027–32. doi: 10.1002/cncr.25840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. Dec 1 1995;76(11):2253–9. [DOI] [PubMed] [Google Scholar]
- 30.Diez-Roux AV, Kiefe CI, Jacobs DR Jr, et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Annals of epidemiology. Aug 2001;11(6):395–405. [DOI] [PubMed] [Google Scholar]
- 31.Marra CA, Lynd LD, Harvard SS, Grubisic M. Agreement between aggregate and individual-level measures of income and education: a comparison across three patient groups. BMC Health Serv Res. Mar 31 2011;11:69. doi: 10.1186/1472-6963-11-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Location of tumors by race and ethnicity
Supplemental Table 2: Ages of study subjects with ES by ethnicity