Abstract
Aim
Approximately a third of patients with schizophrenia fail to adequately respond to antipsychotic medications, a condition known as treatment resistance (TR). We aimed to assess cognitive and cortical thickness deficits and their relationship to TR in schizophrenia.
Method
We recruited patients with schizophrenia (N=127), including patients at treatment initiation (N=45), treatment-responsive patients (N=40) and TR patients (N=42), and healthy controls (N=83). Clinical symptoms, neurocognitive function, and structural images were assessed. We performed group comparisons, and explored association of cortical thickness and cognition with TR.
Results
The TR patients showed significantly more severe clinical symptoms and cognitive impairment relative to the treatment-responsive group. Compared to healthy controls, 56 of 68 brain regions showed significantly reduced cortical thickness in patients with schizophrenia. Reductions in five regions were significantly associated with TR (reduction in TR relative to treatment-responsive patients), i.e., in the right caudal middle frontal gyrus, superior frontal cortex, fusiform gyrus, pars opercularis of the inferior frontal cortex, and supramarginal cortex. Cognition deficits were also significantly correlated with cortical thickness in these five regions in patients with schizophrenia. Cortical thickness of the right caudal middle frontal gyrus, superior frontal cortex and pars opercularis of the inferior frontal cortex also significantly mediated effects of cognitive deficits on TR.
Conclusion
Treatment resistance in schizophrenia was associated with reduced thickness in the right caudal middle frontal gyrus, superior frontal cortex, fusiform gyrus, pars opercularis of the inferior frontal cortex, and supramarginal cortex. Cortical abnormalities further mediate cognitive deficits known to be associated with TR.
Keywords: schizophrenia, treatment-resistant, cortical thickness, cognition, MRI
Introduction
Up to one third of patients with schizophrenia fail to adequately respond to antipsychotic medications, known as treatment resistance (TR) 1, showing lack of significant symptom improvement. In parallel, TR patients also show significantly higher neurocognitive deficits 2, 3. This clinical challenge is motivating development of more effective antipsychotics 4, but the attempts have been hindered by the lack of robust brain biomarkers for early identification of the risks for TR and insufficient understanding of the neurobiological mechanisms underlining it 5. Schizophrenia has been associated with significant cortical deficits including reduction in cortical gray matter thickness 6 and regional reduction in cortical thickness was linked to TR in prior studies 7–10.
Neuroimaging research has demonstrated that individuals with schizophrenia have robustly reduced global cortical thickness particularly in the prefrontal and temporal cortices 6. Several structural magnetic resonance imaging (MRI) studies have examined the relationship of cortical structures with TR. For example, significant and widespread cortical thickness reductions in 106 of 148 regions were found in TR groups compared with healthy controls 10. However, that study did not perform a comparison with treatment responsive patients, and this is an important consideration because patient with treatment-resistant auditory verbal hallucinations had signficantly lower medial and inferior frontal, insular and bilateral temporal gray matter volume than non-TR patients 11. Zugman et al. reported that TR patients (n=61) demonstrated significantly lower cortical thickness in the left dorsolateral prefrontal cortex (DLPFC) compared to non-TR patients (n=67) 7. Barry et al. found that TR patients (n=21) showed significant reductions in cortical volume and cortical thickness compared to non-TR patients (n=21) in the right frontal and precentral regions, right parietal and occipital cortex, left temporal cortex, and bilateral cingulate cortex 8. Another limitation is that TR patients are administered significantly higher antipsychotic doses than non-TR patients 7, 8 and therefore some of these findings may be attributable to differences in medication exposure. In this study, we evaluated whether identified cortical deficit findings in TR are also associated with schizophrenia with no to minimal antipsychotic drug administration early during the disease process, thus independent of chronic disease and treatment courses.
TR is linked to more severe neurocognitive deficits 2, 3, especially in the verbal learning and working memory domains 12–14. TR patients were reported to also have significantly poorer performance in attention 15, processing speed, verbal fluency, cognitive flexibility, and executive function 16. Methodological discrepancies existed in these studies, including low statistical power and variable cognitive task battery designs. A “gold standard” battery for cognitive assessment in schizophrenia, the MATRICS Consensus Cognitive Battery (MCCB) 17, 18, could provide comprehensive assessment of the cognitive domains associated with TR. Importantly, cortical thickness and cognitive deficits may be linked to treatment-resistant schizophrenia (TRS) as cortical structural measures have been closely related to cognitive dysfunctions in schizophrenia in general 19, 20.
In this study, our aim was to quantify cortical thickness deficits occurring in TRS by comparing TR patients with treatment-responsive patients, and then identify whether cortical deficit findings in TR are also associated with cognitive deficits in this group. Finally, we aimed to examine whether cortical thickness in specific regions and cognitive deficits, if found significantly related to TR, are impacting TR independently or through a mediation effect. We hypothesized that reduced cortical thickness in specific regions would be associated with cognitive deficits in TR.
Materials and Methods
Clinical characteristics
The study was performed with 210 participants, including 127 patients with a diagnosis of schizophrenia confirmed via clinical interview using the Diagnostic and Statistical Manual of Mental Disorders (73 M/54 F, average age=40.8±13.1 years) and 83 healthy controls (44 M/39 F, average age=38.6±12.2 years) (Table 1). The sample included patients with TRS (N=42, 29 M/13 F, average age=47.7±9.1 years). The primary comparison group comprised individuals with treatment-responsive schizophrenia (N=40, 26M/14F, average age=46.3±11.1 years). Additionally, we recruited a third group of patients with schizophrenia within the first 2 weeks of treatment initiation (N=45, 18M/27F, average age=29.6±10.0 years) to determine whether any structural deficit found to be related to TRS would also be associated with schizophrenia, independent of chronic disease and treatment courses. Healthy volunteers had no Axis-I psychiatric illness and no family history of psychotic illnesses according to the Family History Research Diagnostic Criteria. All participants had no current or past neurological conditions or substance (except nicotine) dependence. All participants signed institutional review board-approved consent forms according to a protocol approved by the Ethics Committee of the Beijing Huilongguan Hospital.
Table 1.
Demographic and clinical characteristics of patients with schizophrenia and healthy controls
| Schizophrenia patients |
Comparision |
|||||||
|---|---|---|---|---|---|---|---|---|
| Healthy Controls (n=83) | Treatment Initiation (n=45) | Treatment Responsive (n=40) | Treatment Resistant (n=42) | Four groups | Treatment Resistant Versus Treatment Responsive | |||
|
| ||||||||
| mean ± s.d. | mean ± s.d. | mean ± s.d. | mean ± s.d. | F/χ2 | p | T/χ2 | p | |
| Demographic information | ||||||||
| Sex(M/F) | 44/39 | 18/27 | 26/14 | 29/13 | 5.8 | 0.12 | 0.15 | 0.82 |
| Age (years) | 38.6 ± 12.2 | 29.6 ± 10.0 | 46.4 ± 11.1 | 47.7 ± 9.1 | 25.1 | 7.3×10−14 | −0.6 | 0.55 |
| Age range | 19~59 | 15~54 | 21~60 | 25~62 | ||||
| Education (years) | 13.1 ± 2.6 | 12.4 ± 3.6 | 12.1 ± 3.1 | 12.1 ± 2.9 | 1.8 | 0.16 | −0.1 | 0.91 |
| Illness duration (years) | 1.1±1.2 | 22.2±12.9 | 23.6±10.2 | −0.5 | 0.59 | |||
| Onset age | 28.2±9.8 | 24.0±5.8 | 22.6±5.9 | 1.1 | 0.27 | |||
| Symptoms | ||||||||
| PANSS positive | 21.9 ± 5.5 | 10.9± 4.0 | 23.4 ± 4.4 | −13.5 | 1.6×10−22 | |||
| PANSS negative | 14.8 ± 5.5 | 14.5 ± 5.8 | 24. 0 ± 7.3 | −6.5 | 2.3×10−10 | |||
| PANSS total | 74.1 ± 13.6 | 49.4 ± 10.7 | 86.2 ± 12.6 | −14.2 | 8.5×10−26 | |||
| Antipsychotic dosage (chlorpromazine equivalents) | 306.6 ± 183.2 | 447.8 ± 220.1 | 733.8 ± 231.1 | −5.7 | 1.69×10−7 | |||
Note: PANSS=Positive and Negative Syndrome Scale
The TR and treatment-responsive groups were defined based on consensus guidelines 1. Patients with TRS met the criteria of 1) little response to treatment with at least two different antipsychotic medications with a dosage of chlorpromazine equivalent (CPZ) ≥600 mg/day for at least 6 weeks, 2) a Brief Psychiatric Rating Scale (BPRS) score ≥45, and 3) a Clinical Global Impressions Severity Scale (CGI-S) score ≥4 during the current assessment. The treatment-responsive group was defined by periods of good clinical response to antipsychotic medications as indicated by a CGI-S score <3 over at least 12 weeks. The TR and treatment-responsive groups were frequency matched on age, sex, years of education, and duration of illness. The treatment-initiation group was included to identify whether TR biomarkers, if found, would be present at the onset of the disorder with minimal antipsychotics exposure. Imaging data were collected within 2 weeks of the initiation of the antipsychotic medication treatment. Details of demographic information are presented in Table 1.
Five patients were medication-free (three in the treatment-initiation group, two in the treatment-responsive group, and zero in the TRS group). Seven (4, 3, 0) patients were respectively using first generation antipsychotic medications. The remaining patients were respectively using the following second-generation antipsychotics: risperidone (21, 11, 10); clozapine (0, 9, 23); olanzapine (11, 14, 13); aripiprazole (8, 7, 4); paliperidone (2, 1, 5); and amisulpride, iloperisone, lurazidone, or quetiapine (1, 3, 5); among these patients, 45 (4, 7, 34) were respectively using more than one antipsychotic medication. The dosage of antipsychotic medication (CPZ) was calculated for each patient 21, 22. Of the 210 participants, 200 were also included in a previous diffusion tensor imaging study that also reported the cognitive data 5; the cortical thickness data are newly reported here.
Clinical symptom evaluations
Patients were evaluated using the Positive and Negative Syndrome Scale (PANSS), BPRS, Auditory Hallucination Rating Scale and CGI-S by one of three attending psychiatrists who maintained inter-rater reliability (intraclass correlation coefficient) above 0.80. The BPRS and CGI-S were used for group-definitions only, and the PANSS was used for symptom assessment.
Neurocognitive assessment
The neurocognitive assessments were performed using the MCCB validated Chinese versions 23–25, covering seven domains including speed of processing, attention and vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition. All raw scores were transformed into a T score with a mean of 50 and SD of 10 according to Chinese norms 25; higher T-values represent better cognitive performance. The composite MCCB score was used as the assessment measure for cognitive function.
Imaging and data preprocessing
Imaging data were collected on a Siemens Prisma 3 T MRI scanner with a 64-channel head coil. Head motions were minimized by foam pads. Parameters for structural MRI were acquired by covering the whole brain with a sagittal 3D-magnetization prepared rapid acquisition gradient echo sequence: echo time =2.98 ms, inversion time =1100 ms, repetition time =2530 ms, flip angle =7°, field of view =256×224 mm2, matrix size = 256×224, thickness/gap = 1/0 mm.
For each participant, structural T1 image preprocessing was carried out using Freesurfer (version 6.0) 26. Following the ENIGMA protocol (http://enigma.ini.usc.edu/), cortical thickness was extracted for 70 Desikan-Killiany atlas regions 6, 27 (35 regions per hemisphere and the total left and right hemisphere mean thicknesses were measured) (Table S1). The intracranial volume was obtained. For quality control, we followed the ENIGMA pipeline: visual checking of the cortical segmentations and region-by-region removal of values for segmentations found to be incorrect (http://enigma.usc.edu/protocols/imaging-protocols); no data were excluded.
Statistical analysis
All group comparisons of cortical thickness were performed using a general linear model controlling for age, sex (model A) or age, sex, and global mean cortical thickness (model B). Statistical analysis was conducted in the following four steps: 1. We compared all patients to all healthy controls to identify cortical thickness in brain regions showing significant differences. 2. From these significant brain regions, we compared patients at the first 2 weeks of treatment initiation with controls to identify those cortical regions already significantly associated with schizophrenia but with minimal impact from chronic antipsychotic medication treatment. 3. Separately, we compared the TR and treatment-responsive groups to identify those cortical regions significantly associated with TR by comparing with age, sex, and illness duration matched treatment-responsive patients. From this step we obtained results representing regional abnormalities associated with TRS but potentially confounded by large differences in antipsychotic medication use between these groups. 4. The last step involved conducting a conjunction analysis of steps 2 and 3 to identify cortical regions associated with schizophrenia at treatment initiation with minimal antipsychotic medication exposure and TRS. In steps 1, 2, and 3, we used a nominally significant statistical threshold at uncorrected p<0.05 for screening purposes. In step 4, we used false discovery rate correction for multiple comparisons at q<0.05. Associations of clinical measures, cognition and cortical thickness in brain regions showing significant differences between the TR and treatment-responsive groups were examined with partial correlation analyses controlling for age and sex. Finally, a mediation analysis was performed using PROCESS (Version 2.16.3) for SPSS to test whether cognition may contribute to TRS through the mediating effect of cortical thickness thinning, with a 5000 bias-corrected bootstrap sample for significance testing. All statistical tests were two-tailed.
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of the participants are shown in Table 1 and Figure 1. Illness duration, age of illness onset, age, sex, and years of education were not significantly different between the TR and treatment-responsive groups. TR patients had nearly twice the CPZ of treatment-responsive patients (p<0.001). The TR group showed significantly more severe PANSS total, positive, and negative symptoms than the treatment-responsive group. Negative symptoms, but not positive symptoms, were significantly more severe in the TR group than in both the treatment-initiation and treatment responsive groups (Figure 1).
Figure 1.
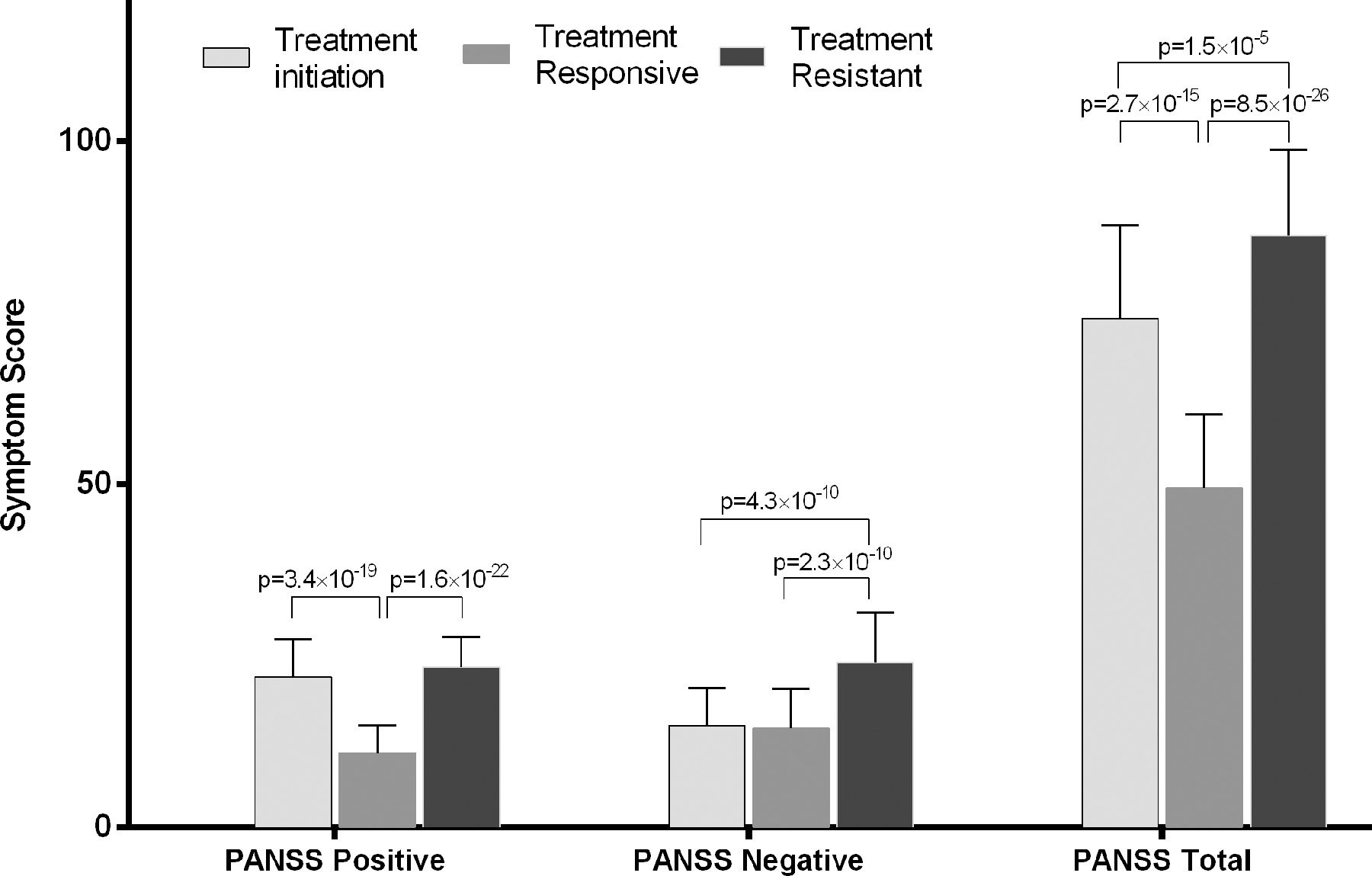
Clinical symptoms severity assessed by the Positive and Negative Syndrome Scale (PANSS) in schizophrenia patient groups.
Cortical thickness: all patients vs. controls
In comparison with healthy controls, patients with schizophrenia (treatment-initiation, treatment-responsive, and TR groups combined) had a nominally significantly thinner cortex in 56 of 68 examined regions (Figure 2A, a list of the 56 regions is included in Supplemental Table S1). Only one region–the right pericalcarine cortex–was thicker in patients than in controls.
Figure 2.
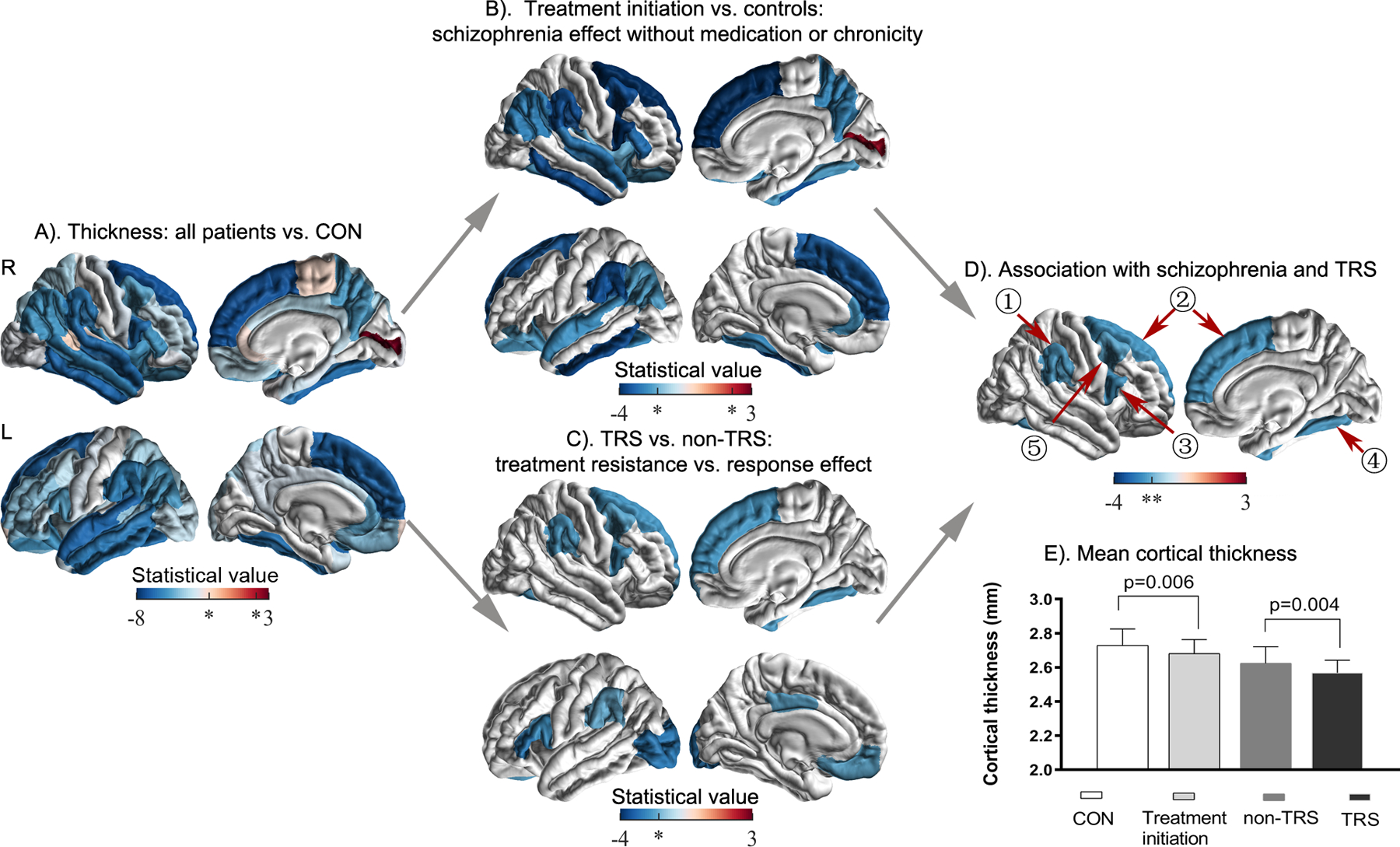
Cortical thickness in treatment resistance schizophrenia. A). Comparison between all the patients and healthy controls (CON); B). Comparison between patients at treatment initiation and healthy controls; C). Comparison between treatment resistant patients (TRS) and treatment responsive patients (non-TRS); D). Conjunction of B and C to identify cortical thinning that is associated with both schizophrenia at treatment initiation and schizophrenia treatment resistance in four regions including ①right supramarginal gyrus, ②right superior frontal cortex, ③pars opercularis, ④fusiform gyrus, and ⑤caudal middle frontal gyrus. E). Mean cortical thickness values of the five regions in D) for each subgroup. * p <0.05, uncorrected; ** q <0.05, FDR corrected.
In the context of a widespread thinner cortex in schizophrenia, we assessed the regional specificity of these cortical thickness differences. When controlling for individual differences in global mean cortical thickness, 11 of the 68 regions showed a significantly thinner cortex (e.g., fusiform, parahippocampal, frontal gyrus, and temporal gyri), and another12 regions showed a significantly thicker cortex (e.g., cuneus, paracentral lobule, and lingual gyrus) in patients with schizophrenia than in healthy controls (model B) (Figure S2A, Table S2 in Supplemental Material).
Cortical thickness: treatment-initiation patient group vs. controls
The treatment-initiation group had nominally significant cortical thinning in 21 of the 68 regions compared to healthy controls (Figure 2B, a list of the 21 regions is included in Supplemental Table S1).
When controlling for individual differences in global mean cortical thickness, seven regions showed a significantly thinner cortex (e.g., inferior temporal gyrus, and frontal gyrus), and another three regions showed a significantly thicker cortex (e.g., paracentral lobule and temporal gyrus) in patients with treatment-initiation schizophrenia than in healthy controls (model B) (Figure S2B, Table S2 in Supplemental Material).
Cortical thickness: treatment-resistant group vs. treatment-responsive group
The TR group had nominally significant cortical thinning in 9 of the 68 regions compared to the treatment-responsive group (Figure 2C, a list of the nine regions is included in Supplemental Table S1).
When controlling for individual differences in global mean cortical thickness, four regions showed a significantly thicker cortex (e.g., entorhinal cortex and transverse temporal gyrus) in patients with TRS than in those who were treatment responsive (model B) (Figure S2C, Table S2 in Supplemental Material).
Conjunction analysis of the group results described above showed significantly reduced thickness in five cortical areas, i.e., the right caudal middle frontal gyrus, superior frontal cortex, fusiform gyrus, pars opercularis, and supramarginal cortex, after correction for multiple comparisons (Figure 2D).
Associations of cortical thickness with cognition
Compared with healthy controls, patients at treatment initiation had a significantly reduced total MCCB score (p=2.63×10−9) (Figure 3A, Figure S1). Compared with patients in the non-TRS group, TRS patients showed significant impairment on the MCCB total score (p=0.01). Therefore, a reduced MCCB score was simultaneously associated with schizophrenia with minimal antipsychotic medication confounders and with TRS. The cortical thickness of the right caudal middle frontal gyrus, superior frontal cortex, fusiform gyrus, pars opercularis of the inferior frontal cortex, and supramarginal cortex was also significantly correlated with the MCCB score in the combined patient sample (Figure 3B–F).
Figure 3.
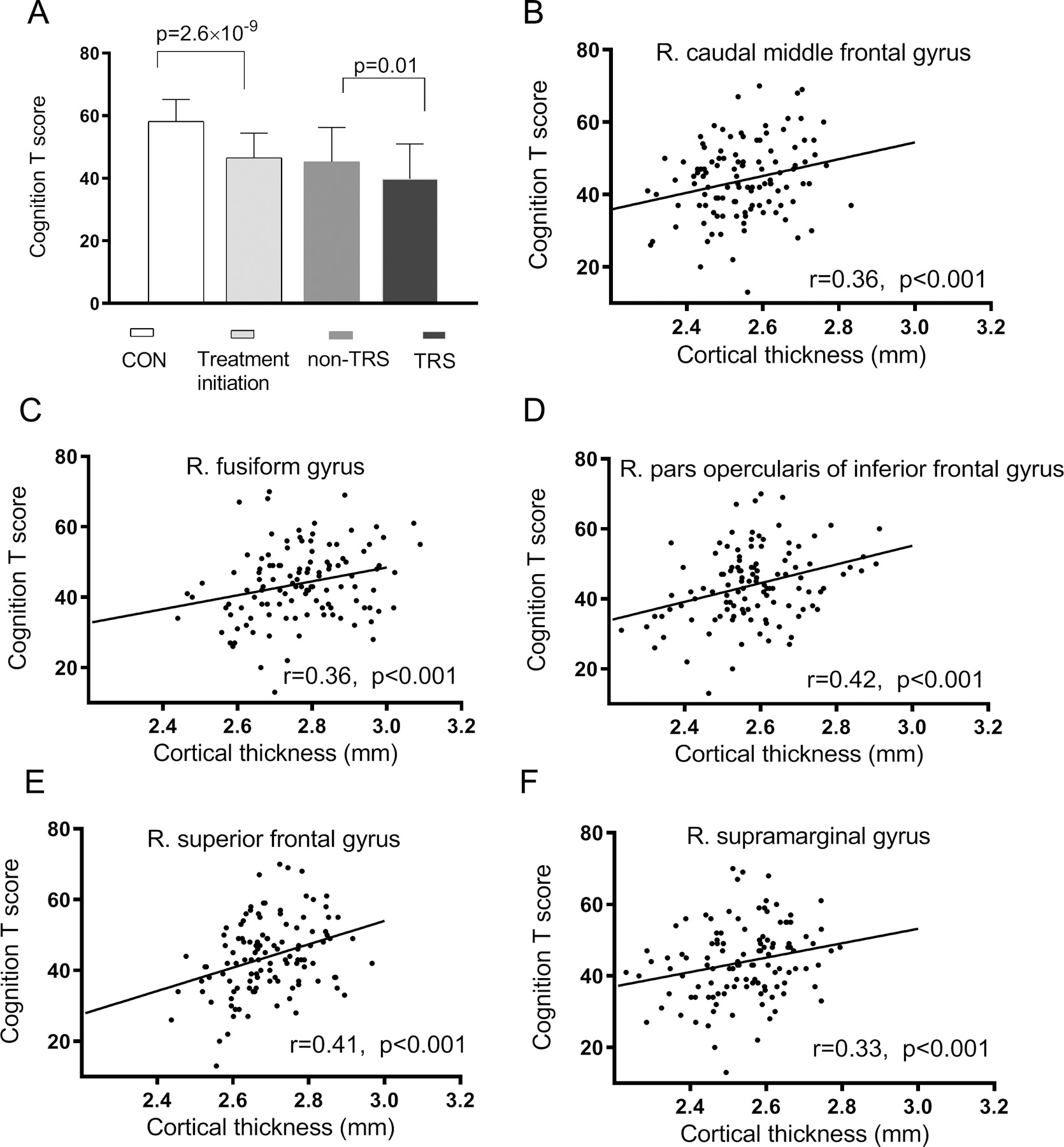
Cognition in schizophrenia treatment initiation and treatment resistance (A), and the relationship between cognition and mean cortical thickness in regions (significant regions in Figure 2D) showing significant associations with both treatment initiation and treatment resistance in patients (B-F).
Cortical thickness and cognition mediation analyses
Furthermore, we assessed whether cognition may contribute to TRS through the mediating effect of cortical thinning of the five significant regions. The indirect effect of cognition (MCCB total score) on the diagnosis of TRS (Path AB) was significant in all three models (Figure 4A–C), and the direct effect of cognition on the diagnosis of TRS (Path C) was not significant. The results showed that the cognitive effect on the diagnosis of TRS was almost entirely mediated by the cortical thickness of the right caudal middle frontal gyrus (β=−0.01, 95% Cl [−0.04 to −0.0009]) (Figure 4A), pars opercularis of the inferior frontal cortex (β=−0.01, 95% Cl [−0.04 to −0.0005]) (Figure 4B), and superior frontal cortex (β=−0.02, 95% Cl [−0.05 to −0.004]) (Figure 4C). The effects of age and sex in the above mediation analyses were not significant (all p>0.05).
Figure 4.
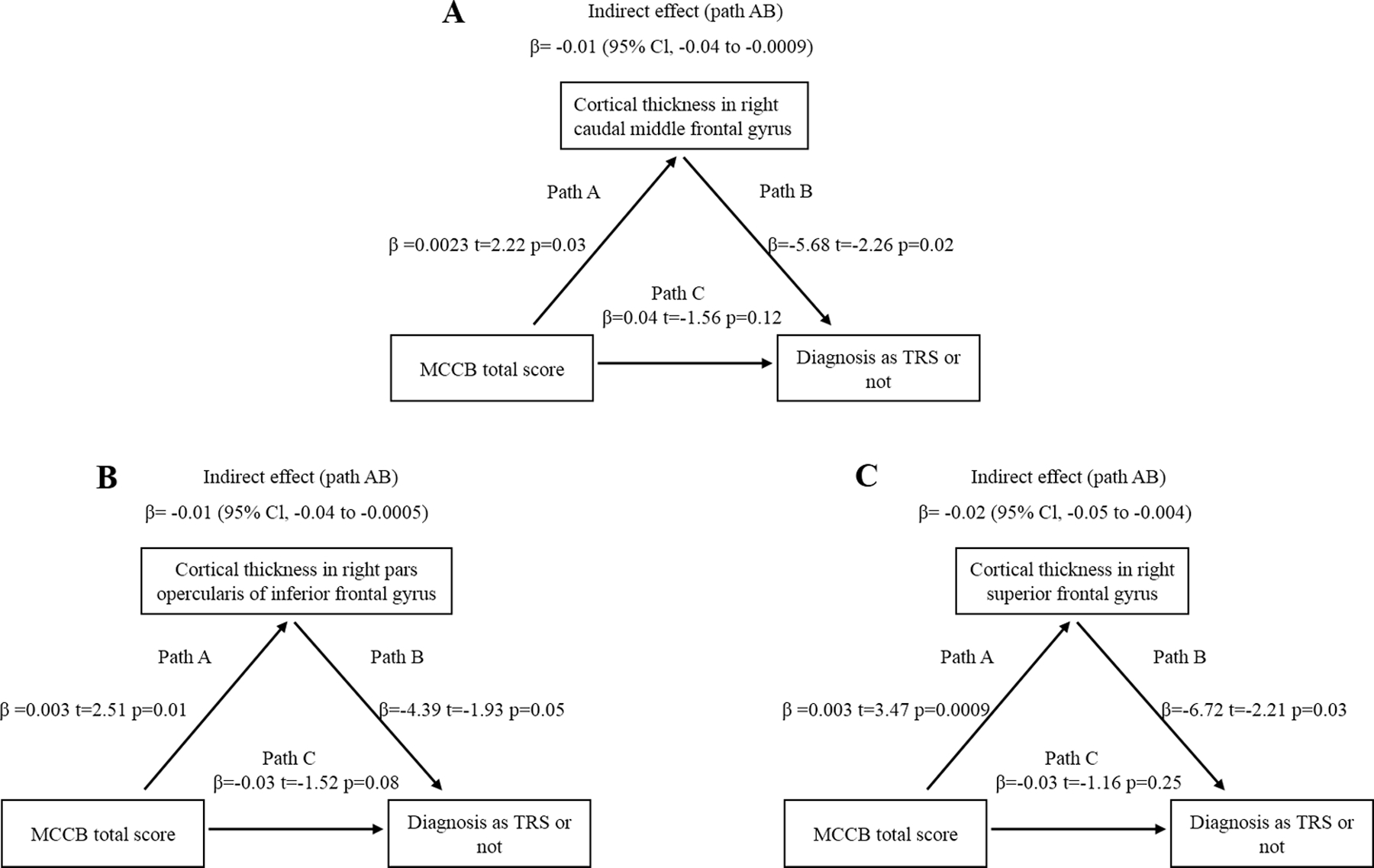
Cortical thickness mediated the cognition effect on treatment resistance, in the right caudal middle frontal gyrus (A), pars opercularis of inferior frontal gyrus (B) and superior frontal gyrus (C). Path AB is the mediation effect and is significant based on confidence interval.
Associations of cortical thickness with clinical symptoms
There were no significant associations between the cortical thickness of the five regions and the PANSS total, positive, and negative symptom scores in the combined sample of patients with schizophrenia (all p values > 0.05). Illness duration was significantly correlated with cortical thickness of the right superior frontal cortex, fusiform gyrus, pars opercularis of the inferior frontal cortex, and supramarginal cortex in the combined sample of patients (Figure S3), controlling for age, suggesting continuous thinning as a function of illness duration in these regions. Medication dose was significantly correlated with cortical thickness of the five regions (Figure S4). Finally, age of onset was positively correlated with the cortical thickness of the right superior frontal cortex, fusiform gyrus and supramarginal cortex (Figure S5) in the combined sample of patients, controlling for age.
Discussion
In this study we explored specific regional reductions in cortical thickness as a biomarker for TRS. We found that 84% of the 68 explored brain regions showed significant reduction in cortical thickness in patients with schizophrenia versus controls, thus replicating previous findings. Reductions in only five cortical regions were shown to be significantly associated with TR (reduction in TRS patients relative to treatment-responsive patients), i.e., the right caudal middle frontal gyrus, superior frontal cortex, fusiform gyrus, pars opercularis of the inferior frontal cortex, and supramarginal cortex. These regions showed significantly lower thickness at treatment initiation suggesting the prodromal nature of TR. We used the MCCB to examine the implicated cognitive domains and replicate previous findings of higher cognitive deficits in TR patients. The effects of cognitive deficits on the TR status were largely mediated by cortical thickness in these regions. Furthermore, cortical thickness in these regions was significantly associated with more severe hallucination symptoms.
TR patients showed lower cortical thickness in the right caudal middle frontal gyrus, superior frontal cortex and pars opercularis of the inferior frontal cortex, consistent with previous reports of reduced gray matter volume 11, 28 and functional frontal dysfunction in TR 29, 30. A thinner cortex in the right pars opercularis in patients with schizophrenia than in healthy controls was reported by the ENIGMA study on schizophrenia 6. Moreover, reduced gray matter volume in the right superior frontal cortex was found in TR patients compared to patients responsive to atypical antipsychotics 28. Besides the above described structural abnormalities, TR patients with hallucinations presented with hypermetabolism in the right superior frontal gyrus 29 and lower word generation related activation in the bilateral inferior frontal gyrus than healthy controls 30. Our findings are consistent with those of Zugman et al. 7 where cortical thickness reduction in the DLPFC was associated with TR compared to a non-TR group, a finding that was partly overlapping with the DLPFC region in the TR vs. non-TR comparison in the current study (Figure 2C). However, when considering medication exposure, we observed no differences in the left DLPFC between treatment-initiation patients and healthy controls, suggesting that alterations in the left DLPFC are not significantly related to schizophrenia pathology at least during the first episode period and might be related to medication or illness duration. Barry et al. further showed significant reductions in cortical volume and cortical thickness in TR compared to non-TR (n=21 in each group) patients in the right frontal and precentral regions, right parietal and occipital cortex, left temporal cortex and bilateral cingulate cortex 8. None of these regions overlapped with the regions found to be implicated in the current study. However, TR patients were only treated with first-generation antipsychotics in that study 8. Differences in medication type, dose, illness duration and sample size between these studies may have contributed to the inconsistent findings 7, 8. Nevertheless, these studies together suggested that cortical structural deficits might underlie TRS.
The finding related to the right supramarginal gyrus was consistent with previous findings of reduced gray matter volume in the right supramarginal gyrus in TR patients compared to those responsive to atypical antipsychotics 28. Previous studies have shown that the supramarginal cortex plays an important role in directional tuning, proprioception, and emotion regulation 31–33. This region has also been repeatedly found to be abnormal in structural imaging studies in schizophrenia 34, but not significant in direct comparisons of TR and non-TR patients 8. However, this region has been associated with treatment-resistant auditory verbal hallucinations (AVH), for example, increased regional cerebral blood flow in the right supramarginal gyrus was found in treatment resistant AVH compared to those patients without AVH 35.
The reduced cortical thickness of the right fusiform gyrus in TR patients was a novel finding. However, previous studies have shown progressive reduction of the fusiform volume 36–38 and fusiform dysfunction in schizophrenia 38–40. Alterations in the fusiform gyrus were related to deficits in the visual processing of faces 37, 41 and facial emotion processing 42, which may be linked to more severe negative symptoms 43 in schizophrenia, and more severe negative symptoms have been associated with TRS in the literature 5 and in the current study (Figure 1). Cognitive deficits have also been linked to TR 2, 3, especially verbal learning and working memory deficits 2, 12. Consistent with our previous findings 12, TR patients showed significant impairment on the MCCB score compared to non-TR patients. The novelty of this study lies in the addition of the treatment initiation group comparisons, which allowed studying cognitive deficits in patients with a minimal antipsychotic medication confound. The mediation analyses demonstrated that cognition contributed to TR through the mediating effect of cortical thickness thinning of these regions (Figure 4). Therefore, this study further supported that cortical thickness is associated with TR and provided new evidence that cortical thinning mediates cognitive deficit effects on treatment resistance.
A previous study examined different structural alterations in subcortical brain regions between TRS and responders to first-line antipsychotics (FL-Resp), and found that the FL-Resp group showed larger striatal and globus pallidus volumes than TR patients who were non-responders to both first-line antipsychotics and clozapine 44. In the current study, we explored specific regional reductions in cortical thickness as a biomarker for TRS. Moreover, we compared cortical thickness between TRS patients using first-line antipsychotics (i.e., one or more atypical or typical non-clozapine antipsychotics) and TRS patients using clozapine. We found that the TRS patients using clozapine had a thinner super frontal cortex (Table S4 in Supplemental Material). Taken together with the previous subcortical study 44, these findings suggest both brain cortical and subcortical abnormalities to elucidate the pathophysiological mechanism of TRS.
TR more frequently occurs in male patients who on average have poorer premorbid functioning and an earlier age of onset 45. The significant correlation between younger age of onset and thinner cortical thickness in regions associated with treatment resistance identified here thus provides an anatomic interpretation of the association between earlier age of onset and treatment resistance. Longer illness duration and drug dose were also negatively correlated with the cortical thickness of these regions (e.g., the five implicated regions) in patients with schizophrenia (Figure S2, S3). As the deficits were already apparent at treatment initiation in the current study, the above correlation further suggests that these regions might continue to show abnormal thinning as the disease progresses.
Several issues need to be further considered. First, this was a cross-sectional study, limiting our ability to examine whether cortical thickness can predict TR. Longitudinal studies are needed to achieve accurate TRS prediction. Second, in the current study, we did not record the treatment-resistant illness duration, which limited exploring the differences between early- and late-onset TRS 1. Third, we found regional structural abnormalities in TRS, however, the structural or functional connections between these brain regions remain unclear. Network based analysis is needed to interpret the interaction among these structures. Fourth, notably, for the comparisons of cortical thickness, we used a nominally significant statistical threshold at uncorrected p<0.05 for screening purposes, similar to our previous study 5. This would increase the probability of false positives, especially in a relatively small sample. We need to repeat the investigation with a larger sample and stricter multiple comparison correction. Finally, TR patients were exposed to higher antipsychotic medication doses than non-TR patients. Although we considered the medication dose as a covariate in the statistical analysis, and our design accounted for this limitation by restricting the findings in TR vs. non-TR patients to be contingent on significant findings also in patients with minimal antipsychotic medication exposure. However, a residual effect of medication may still exist, and this issue requires the development of advanced strategies for medication correction or a more appropriate design.
Conclusion
Treatment resistance in schizophrenia was found to be associated with reduced thickness in five frontal and temporal/parietal cortical areas, and the cognitive impairment effects on treatment resistance was largely mediated by cortical thickness of these areas. The effort for developing more effective therapeutics for treatment resistant schizophrenia should consider cortical thinning in these areas as an important biomarker and target.
Supplementary Material
Acknowledgments
Support was received from the National Natural Science Foundation of China (No. 81761128021, 81401115, 31671145), National Institute of Health grant (R01MH112180), Beijing Hospitals Authority Youth Programme (QML20202001), the Beijing Municipal Natural Science Foundation (7182074), Capital’s Funds for Health Improvement and Research (No.2018-4-2133), Cultivation Program of Beijing Municipal Hospital Administration (PX2018069).
Footnotes
Data Access and Responsibility: Fengmei Fan has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicting interests: LEH has received or plans to receive research funding or consulting fees on research projects from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho, Heptares, Pfizer, Luye Pharma, Sound Pharma, Takeda, and Regeneron. Other authors have declared that no conflicting interests.
References
- 1.Howes OD, McCutcheon R, Agid O et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 2017; 174: 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bartolomeis A, Balletta R, Giordano S, Buonaguro EF, Latte G, Iasevoli F. Differential cognitive performances between schizophrenic responders and non-responders to antipsychotics: correlation with course of the illness, psychopathology, attitude to the treatment and antipsychotics doses. Psychiatry Res 2013; 210: 387–95. [DOI] [PubMed] [Google Scholar]
- 3.Frydecka D, Beszlej JA, Goscimski P, Kiejna A, Misiak B. Profiling cognitive impairment in treatment-resistant schizophrenia patients. Psychiatry Res 2016; 235: 133–8. [DOI] [PubMed] [Google Scholar]
- 4.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry 1988; 45: 789–96. [DOI] [PubMed] [Google Scholar]
- 5.Kochunov P, Huang J, Chen S et al. White Matter in Schizophrenia Treatment Resistance. Am. J. Psychiatry 2019: appiajp201918101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Erp TGM, Walton E, Hibar DP et al. Cortical Brain Abnormalities in 4474 Individuals With Schizophrenia and 5098 Control Subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol. Psychiatry 2018; 84: 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zugman A, Gadelha A, Assuncao I et al. Reduced dorso-lateral prefrontal cortex in treatment resistant schizophrenia. Schizophr. Res 2013; 148: 81–6. [DOI] [PubMed] [Google Scholar]
- 8.Barry EF, Vanes LD, Andrews DS et al. Mapping cortical surface features in treatment resistant schizophrenia with in vivo structural MRI. Psychiatry Res 2019; 274: 335–344. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima S, Takeuchi H, Plitman E et al. Neuroimaging findings in treatment-resistant schizophrenia: A systematic review: Lack of neuroimaging correlates of treatment-resistant schizophrenia. Schizophr. Res 2015; 164: 164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wannan CMJ, Cropley VL, Chakravarty MM et al. Evidence for Network-Based Cortical Thickness Reductions in Schizophrenia. Am. J. Psychiatry 2019; 176: 552–563. [DOI] [PubMed] [Google Scholar]
- 11.Kubera KM, Sambataro F, Vasic N et al. Source-based morphometry of gray matter volume in patients with schizophrenia who have persistent auditory verbal hallucinations. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014; 50: 102–9. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Zhu Y, Fan F et al. Hippocampus and cognitive domain deficits in treatment-resistant schizophrenia: A comparison with matched treatment-responsive patients and healthy controls. Psychiatry Res Neuroimaging 2020; 297: 111043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joober R, Rouleau G, Lal S et al. Neuropsychological impairments in neuroleptic-responder vs. -nonresponder schizophrenic patients and healthy volunteers. Schizophrenia research 2002; 53: 229–38. [DOI] [PubMed] [Google Scholar]
- 14.de Bartolomeis A, Balletta R, Giordano S, Buonaguro E, Latte G, Iasevoli F. Differential cognitive performances between schizophrenic responders and non-responders to antipsychotics: correlation with course of the illness, psychopathology, attitude to the treatment and antipsychotics doses. Psychiatry Res 2013; 210: 387–95. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez P, Ojeda N, Elizagarate E et al. [Attention deficits and response to drug therapy in patients with treatment-resistant schizophrenia: results through confirmatory factor analysis]. Revista de psiquiatria y salud mental 2010; 3: 40–9. [DOI] [PubMed] [Google Scholar]
- 16.Frydecka D, Beszlej J, Goscimski P, Kiejna A, Misiak B. Profiling cognitive impairment in treatment-resistant schizophrenia patients. Psychiatry Res 2016; 235: 133–8. [DOI] [PubMed] [Google Scholar]
- 17.Keefe R, Davis V, Harvey P et al. Placebo Response and Practice Effects in Schizophrenia Cognition Trials. JAMA Psychiatry 2017; 74: 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smelror RE, Jorgensen KN, Lonning V et al. Healthy Adolescent Performance With Standardized Scoring Tables for the MATRICS Consensus Cognitive Battery: A Multisite Study. Schizophr. Bull 2019; 45: 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eack SM, Hogarty GE, Cho RY et al. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch. Gen. Psychiatry 2010; 67: 674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penades R, Franck N, Gonzalez-Vallespi L, Dekerle M. Neuroimaging Studies of Cognitive Function in Schizophrenia. Adv. Exp. Med. Biol 2019; 1118: 117–134. [DOI] [PubMed] [Google Scholar]
- 21.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry 2010; 67: 255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am. J. Psychiatry 2010; 167: 686–93. [DOI] [PubMed] [Google Scholar]
- 23.Kern RS, Nuechterlein KH, Green MF et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am. J. Psychiatry 2008; 165: 214–20. [DOI] [PubMed] [Google Scholar]
- 24.Nuechterlein KH, Green MF, Kern RS et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry 2008; 165: 203–13. [DOI] [PubMed] [Google Scholar]
- 25.Zou YZ, Cui JF, Wang J et al. Clinical reliability and validity of the version of Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery. Chin J Psychiatry 2009; 42: 29–33. [Google Scholar]
- 26.Fischl B FreeSurfer. Neuroimage 2012; 62: 774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desikan RS, Segonne F, Fischl B et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–80. [DOI] [PubMed] [Google Scholar]
- 28.Anderson VM, Goldstein ME, Kydd RR, Russell BR. Extensive gray matter volume reduction in treatment-resistant schizophrenia. Int. J. Neuropsychopharmacol 2015; 18: pyv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klirova M, Horacek J, Novak T et al. Individualized rTMS neuronavigated according to regional brain metabolism ((18)FGD PET) has better treatment effects on auditory hallucinations than standard positioning of rTMS: a double-blind, sham-controlled study. Eur. Arch. Psychiatry Clin. Neurosci 2013; 263: 475–84. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald PB, Sritharan A, Benitez J et al. A preliminary fMRI study of the effects on cortical activation of the treatment of refractory auditory hallucinations with rTMS. Psychiatry Res 2007; 155: 83–8. [DOI] [PubMed] [Google Scholar]
- 31.Barbaro MF, Kramer DR, Nune G et al. Directional tuning during reach planning in the supramarginal gyrus using local field potentials. J. Clin. Neurosci 2019; 64: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDowell T, Holmes NP, Sunderland A, Schurmann M. TMS over the supramarginal gyrus delays selection of appropriate grasp orientation during reaching and grasping tools for use. Cortex 2018; 103: 117–129. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Shabat E, Matyas TA, Pell GS, Brodtmann A, Carey LM. The Right Supramarginal Gyrus Is Important for Proprioception in Healthy and Stroke-Affected Participants: A Functional MRI Study. Front. Neurol 2015; 6: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong L, Herold CJ, Cheung EFC, Chan RCK, Schroder J. Neurological Soft Signs and Brain Network Abnormalities in Schizophrenia. Schizophr. Bull 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf ND, Gron G, Sambataro F et al. Magnetic resonance perfusion imaging of auditory verbal hallucinations in patients with schizophrenia. Schizophr. Res 2012; 134: 285–7. [DOI] [PubMed] [Google Scholar]
- 36.Lee CU, Shenton ME, Salisbury DF et al. Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch. Gen. Psychiatry 2002; 59: 775–81. [DOI] [PubMed] [Google Scholar]
- 37.Onitsuka T, Shenton ME, Kasai K et al. Fusiform gyrus volume reduction and facial recognition in chronic schizophrenia. Arch. Gen. Psychiatry 2003; 60: 349–55. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi T, Zhou SY, Nakamura K et al. A follow-up MRI study of the fusiform gyrus and middle and inferior temporal gyri in schizophrenia spectrum. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011; 35: 1957–64. [DOI] [PubMed] [Google Scholar]
- 39.Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biol. Psychiatry 2003; 53: 1099–112. [DOI] [PubMed] [Google Scholar]
- 40.Maher S, Ekstrom T, Ongur D et al. Functional disconnection between the visual cortex and right fusiform face area in schizophrenia. Schizophr. Res 2019; 209: 72–79. [DOI] [PubMed] [Google Scholar]
- 41.Onitsuka T, Nestor PG, Gurrera RJ et al. Association between reduced extraversion and right posterior fusiform gyrus gray matter reduction in chronic schizophrenia. Am. J. Psychiatry 2005; 162: 599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim DW, Kim HS, Lee SH, Im CH. Positive and negative symptom scores are correlated with activation in different brain regions during facial emotion perception in schizophrenia patients: a voxel-based sLORETA source activity study. Schizophr. Res 2013; 151: 165–74. [DOI] [PubMed] [Google Scholar]
- 43.Demjaha A, Weinstein S, Stahl D et al. Formal thought disorder in people at ultra-high risk of psychosis. BJPsych Open 2017; 3: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Plitman E, Iwata Y et al. Neuroanatomical profiles of treatment-resistance in patients with schizophrenia spectrum disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020; 99: 109839. [DOI] [PubMed] [Google Scholar]
- 45.Meltzer HY. Treatment-resistant schizophrenia--the role of clozapine. Curr. Med. Res. Opin 1997; 14: 1–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


