Abstract
AIM
To examine the effect of pioglitazone on epicardial (EAT) and paracardial (PAT) adipose tissue and measures of diastolic function and insulin sensitivity in T2DM patients.
METHODS
12 T2DM without clinically manifest cardiovascular disease and 12 NGT subjects underwent cardiac magnetic resonance imaging (MRI) to quantitate EAT and PAT and diastolic function before and after pioglitazone treatment for 24 weeks. Whole body insulin sensitivity was measured with euglycemic insulin clamp and Matsuda Index (OGTT).
RESULTS
Pioglitazone reduced HbA1c by 0.9% (p < 0.05), increased HDL cholesterol by 7% (p < 0.05), reduced triacylglycerol by 42% (p<0.01) and increased whole-body insulin-stimulated glucose uptake by 71% (p < 0.01) and Matsuda Index by 100% (p<0.01). In T2DM, EAT (p<0.01) and PAT (p<0.01) areas were increased versus NGT and decreased by 9% (p=0.03) and 9% (p=0.09), respectively, following pioglitazone. Transmitral E/A flow rate and peak left ventricular flow rate (PLVFR) were reduced in T2DM versus NGT (p<0.01) and increased following pioglitazone (p<0.01–0.05). At baseline normalized PLVFR inversely correlated with EAT (r=−0.45, p=0.03) but not PAT (r=−0.29, p=0.16). E/A was significantly and inversely correlated with EAT (r=−0.55, p=0.006) and PAT (r=−0.40, p=0.05). EAT and PAT were inversely correlated with whole-body insulin-stimulated glucose uptake (r=−0.68, p<0.001) and with Matsuda Index (r=0.99, p<0.002).
CONCLUSION/INTERPRETATION
Pioglitazone reduced EAT and PAT areas and improved LV diastolic function in T2DM. EAT is (PAT less strongly) inversely correlated with LV diastolic functionand both EAT and PAT are inversely correlated with measures of insulin sensitivity.
Keywords: Type 2 diabetes, pioglitazone, epicardial and paracardial fat, insulin sensitivity, diastolic function
INTRODUCTION
Pericardial adipose tissue represents ectopic fat in the mediastinum and is associated with metabolic syndrome, severity of coronary artery disease, and cardiovascular events (1–5). Pericardial adipose tissue is comprised of two compartments: (i) epicardial adipose tissue (EAT), defined as the visceral fat deposited between the epicardium and pericardium and (ii) paracardial adipose tissue (PAT), which represents the fat deposited on the external surface of the pericardium within the mediastinum (6). Excess adipose tissue surrounding the heart has been associated with insulin resistance and impaired myocardial function in T2DM and nondiabetic individuals (1,2,7–9). Increased release of inflammatory adipokines and accelerated lipolysis with release of fatty acids by EAT has been linked to myocardial lipotoxicity, leading to pathological cardiac remodeling and LV diastolic dysfunction (10–15).
Pioglitazone is a potent insulin sensitizer in skeletal and cardiac muscle, liver, and adipose tissue (16–22) and exerts its beneficial effects via the peroxisome proliferator-activated receptor gamma (PPARγ) pathway (23). In adipocytes pioglitazone inhibits lipolysis, leading to a marked reduction in plasma free fatty acid (FFA) concentration (24), and redistributes fat from muscle, liver, and visceral fat depots to subcutaneous fat depots (18,25–29). In the heart we have shown that pioglitazone improves LV ejection fraction and multiple measures of diastolic function in T2DM patients (13,15) and attenuates pathological remodeling (17). In a previous study, we demonstrated that pioglitazone treatment for 6 months improved LV diastolic dysfunction in T2DM patients in close association with enhanced insulin-stimulated myocardial glucose uptake (13,15). Early-to-late transmitral flow ratio (E/A), deceleration time, peak LV flow rate (PLVFR), ejection fraction (EF), and myocardial blood flow all increased and were correlated with improved myocardial insulin sensitivity (13,15). Additionally, pioglitazone reduces recurrent CV events in diabetic and nondiabetic individuals with a prior CV event (30–33). Surprisingly, only one previous study (34) has examined the effect of pioglitazone on the adipose tissue surrounding the heart. In the present study we have examined the effect of pioglitazone on EAT and PAT in T2DM subjects and their relationship to changes in LV diastolic function.
MATERIALS AND METHODS
Subjects.
The participants and methods used in this study were described previously (15). Briefly, 12 T2DM patients without clinically manifest CVD (age = 51 ± 9 years, 2 female/10 male, BMI = 30.8 ± 4.3 kg/m2, HbA1c = 6.8 ± 1.6%, diabetes duration = 4.0 ± 3.1 years, 7 Mexican-American/5 Caucasian) were studied (Table 1). Other than diabetes, subjects were in good health as established by medical history, physical exam, routine blood chemistries, hematology, urinalysis, and electrocardiogram. All subjects were normally active, and none participated in exercise programs involving excessive exertion. Body weight was stable (±3 lbs.) for at least 3 months prior to study. T2DM patients either were drug naïve (n = 4) or treated with metformin/sulfonylurea (n = 1) or metformin alone (n = 7). Other than these antidiabetic agents, no subject was taking any medication known to alter glucose metabolism. The effect of pioglitazone on diastolic function and whole-body insulin sensitivity were similar in subjects treated with metformin and those who were drug naïve. Ten patients were taking an antihypertensive medication (ACE inhibitor in 7, ARB in 3, calcium channel blocker in 1), and ten were taking a statin.
Table 1.
Morphometric, metabolic, and cardiac parameters by group and treatment.
| Metric | NGT Control | T2D Baseline | T2D After Pioglitazone |
|---|---|---|---|
|
| |||
| Sex | 8M/4F | 10M/2F | 10M/2F |
| Age (years) | 47.7 ± 10.5 | 50.7 ± 9.1 | 51.3 ± 9.1 |
| Body surface area (m2) | 1.87 ± 0.24 | 2.06 ± 0.18 | 2.07 ± 0.18 |
| BMI (kg/m2) | 28.4 ± 4.4 | 30.8 ± 4.3 | 31.3 ± 4.2 |
| Body Fat (%) | 29.3 ± 8.6% | 31.9 ± 5.7 % | 33.4 ± 6.1% |
| Metabolic Parameters | |||
| HbA1c (%) | 5.5 ± 0.4% | 6.7 ± 1.3% | 5.6 ± 0.8%* |
| Fasting plasma glucose (mg/dL) | 93 ± 6 | 149 ± 48 | 112 ± 23* |
| Fasting FFAs (mol/L) | 0.32 ± 0.10 | 0.52 ± 0.17 | 0.30 ± 0.14** |
| HDL cholesterol (mg/dL) | 55.7 ± 9.8 | 38.8 ± 11.9 | 41.5 ± 9.7* |
| Triacylglycerol (mg/dL) | 128 ± 94 | 265 ± 155 | 153 ± 74** |
| Matsuda index | 8.7 ± 4.8 | 2.8 ± 1.9 | 5.6 ± 3.3** |
| Glucose infusion rate (mg/kg/min) | 7.5 ± 2.8 | 3.4 ± 1.3 | 5.8 ± 2.1** |
| Cardiac Parameters | |||
| Epicardial adipose tissue (cm2) | 8.5 ± 2.8 | 15.3 ± 3.9 | 14.0 ± 3.9* |
| Paracardial adipose tissue (cm2) | 17.9 ± 8.9 | 33.5 ± 13.2 | 30.4 ± 14.1* |
| Normalized EAT (cm2/m2) | 4.5 ± 1.3 | 7.4 ± 1.7 | 6.8 ± 1.7* |
| Normalized PAT (cm2/m2) | 9.4 ± 4.3 | 16.2 ± 6.1 | 14.6 ± 6.5 |
| Transmitral E/A flow ratio | 1.46 ± 0.36 | 0.96 ± 0.27 | 1.39 ± 0.49** |
| PLVFR/BSA (mL/s ÷ m2) | 212 ± 35 | 164 ± 46 | 188 ± 64** |
Values are means ± 1 standard deviation.
Indicates p<0.05
p<0.01 after pioglitazone.
Twelve healthy normal glucose tolerant (NGT) subjects (age = 48 ±10 years, 4 female/8 male, BMI = 28.4 ± 0.4 kg/m2, HbA1c = 5.5 ± 0.4%, 9 Mexican American/3 Caucasian) served as the control group (Table 1). Data for control subjects were collected at a single timepoint, during the baseline assessment. The protocol was approved by the Institutional Review Board of the University of Texas Health, San Antonio, TX, and written informed consent was obtained from all subjects prior to participation.
Study Design
Prior to treatment with pioglitazone HbA1c, fasting plasma glucose (FPG), and plasma lipid concentrations were measured, and a 2-hour oral glucose tolerance test (OGTT) (75g) was performed in the morning following 10-hour overnight fast. During OGTT, plasma glucose, insulin, and C-peptide concentrations were measured every 15 minutes. Total body fat and lean mass were measured with DEXA (Hologic, Waltham, MA). Subjects returned within 3–7 days for euglycemic insulin clamp study (35) to measure whole-body (primarily reflects muscle) insulin sensitivity and for a cardiac MRI study to provide quantitative measures of cardiac function. T2DM patients then were treated with pioglitazone for 24 weeks, and all baseline tests were repeated.
Cardiac MRI Studies
Cardiac MRI was performed on 3.0 T system (TIM Trio; Siemens Healthcare, Malvern, PA) with 6-channel anterior phased-array torso coil combined with the spine coil. Axial, sagittal and 4-chamber localizer views were obtained using gradient echo sequence (2.2 × 1.3 mm2 pixel area). Images of mediastinal fat were acquired at end-diastole using a fat-saturated 2D inversion recovery (IR) sequence (TR/TE/TI = 721/1.3/370 ms) with a 1.5 ×1.5 mm2 or smaller pixel area. IR images were acquired using a 300 ms delay from triggering to acquire image data during diastole with 7 mm slice thickness. Cine imaging was performed with a balanced steady-state free precession sequence (TR/TE = 2.44/1.22 ms) with retrospective gating, acquiring 25–30 phases of one complete heart cycle (matrix= 224 × 288, FOV = 336 × 430 mm2, 1.5 ×1.5 mm2 pixel area), which varied slightly by subject body size and heart rate. Each phase of the cine acquisitions consisted of short-axis slices obtained during repetitive breath-holds at end expiration. A phase-contrast gradient-echo sequence with through-plane velocity encoding (Venc = 100 cm/s) was used to capture mitral inflow at the valve plane (flip angle 10°, TR/TE = 5.8/3.6 ms) with 8 mm slice thickness and typical FOV = 228 × 430 mm2, matrix = 192 × 102, producing 2.89 × 2.89 × 8.0 mm3 voxels.
Euglycemic Insulin Clamp
Euglycemic insulin clamp (35) was performed at Texas Diabetes Institute CRC at 7:00 AM following 10-hour overnight fast. Prior to the procedure, a catheter (for blood withdrawal) was placed in a vein on dorsum of the hand, which rested in a box heated to 60°C. A second catheter was inserted into an antecubital vein for infusion of test substances. Three baseline blood samples were drawn at 15-minute intervals beginning 30 minutes prior to start of the study. At time t = 0, a primed-continuous insulin (40 mU/m2/min1) infusion was initiated for 150 minutes. Blood samples were taken at 5-minute intervals for measurement of plasma glucose concentration which was allowed to decline to 100 mg/dL, at which time a variable infusion of 20% glucose was started to maintain plasma glucose concentration constant. The glucose infusion rate (GIR) was stable for the last 30 minutes of all studies and averaged to obtain a measure of whole-body insulin-mediated glucose disposal.
Pioglitazone Treatment
Prior to initiation of pioglitazone, patients received dietary counseling and were advised to consume a standard weight-maintaining American Diabetes Association diet throughout the treatment period. For the first two weeks, patients received pioglitazone 15 mg/day, which was increased to 30 mg/day for two weeks, and then to 45 mg/day until end of study at week 24. Subjects returned for follow-up visit every 2 to 4 weeks.
Data Analysis
MRI cine and flow data were analyzed using a commercial cardiac postprocessing package (cvi42; Circle Cardiovascular Imaging, Calgary, AB, Canada). Functional analyses were performed in cvi42 function module on short-axis cine slices of the left ventricle. The cvi42 flow module computed velocity and flow of blood across the mitral valve. Measurements of flow rate in early diastole were divided by flow rates during the atrial wave to obtain E/A measurement. PLVFR was assessed based on the maximal upslope of volume time curves obtained from the volumetric model produced in the summation of short-axis slices.
Analyses of pericardial fat were performed using MATLAB (Mathworks, Natick, MA). Manual ROI contours were placed on water suppressed, 4-chamber long-axis views of the heart and surrounding adipose tissue within the thorax. The EAT was defined by the region included inside contours of the epicardium and pericardium (Figure 1). PAT was defined by the ROI bordered by the outside of the pericardium and the margins of surrounding adipose tissue (Supplemental Figure 1). Summation of the pixels included in the contours was recorded and multiplied by the per-pixel dimensions to obtain an area measurement. Dimensional parameters were normalized to body surface area (BSA) using the Mosteller equation (36).
Figure 1.
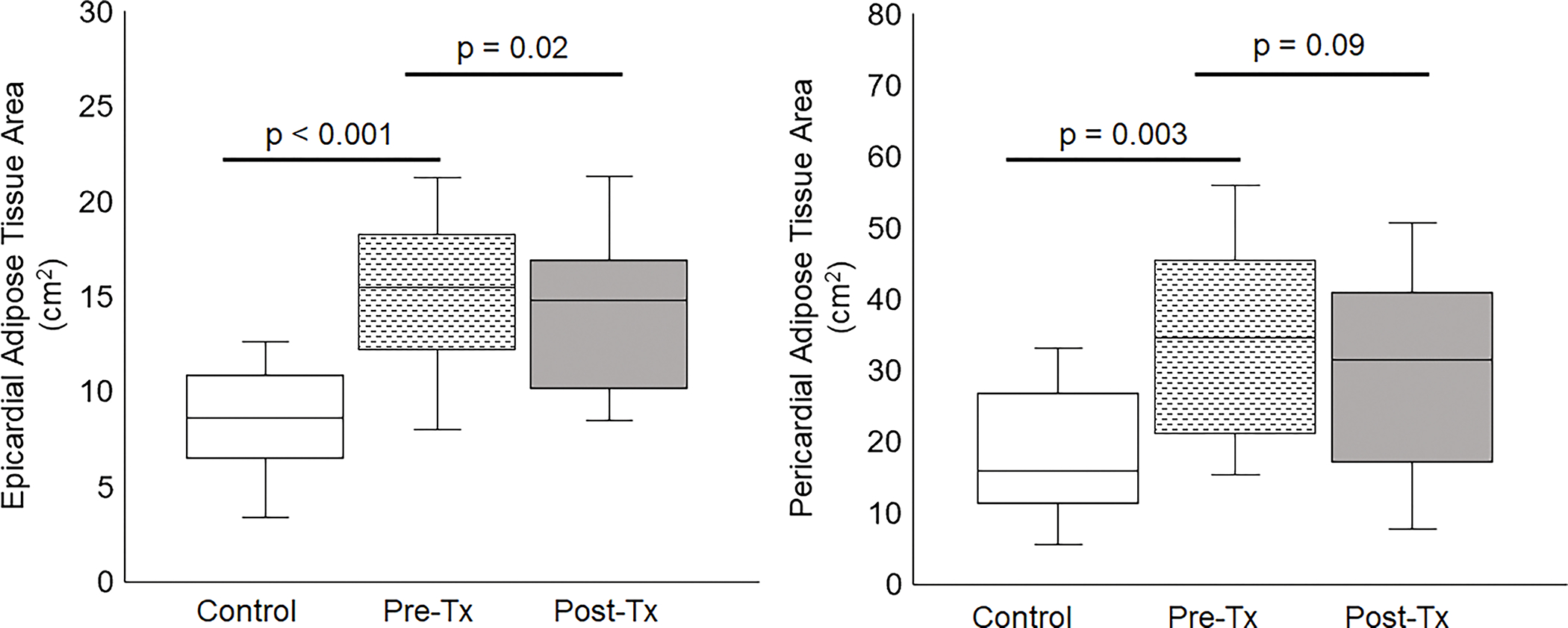
Epicardial and paracardial adipose tissue area in NGT controls and T2DM patients before (Pre-Pio) and after (Post-Pio) pioglitazone treatment. A significant (p = 0.03) decrease in EAT area was noted Post-Pio.
Statistical Analysis
Data are expressed as mean ± SD or as percentages unless otherwise stated. Analyses were performed using R version 4.0.2 statistical software in the integrated development environment RStudio version 1.2.5042. Paired two-sided Student’s t-test was used to evaluate changes between baseline and post-treatment against the null hypothesis of no difference. Unpaired two-sided Student’s t-tests were used to evaluate differences between NGT and T2DM groups. Correlations of fat quantification against other heart and metabolic parameters were computed using linear regression analysis. A value of p<0.05 was deemed significant.
RESULTS
Control and T2DM groups were similar in age, BMI, body fat and sex (Table 1). HbA1c and plasma FPG, FFA, and triglyceride concentrations were lower in the control versus T2DM group at baseline (p < 0.05) (Table 1). Plasma HDL, Matsuda index, and GIR during insulin clamp were higher in the control versus T2DM group at baseline (p < 0.05) (Table 1).
After pioglitazone treatment, EAT area decreased by 1.3 cm2 (15.3 ± 3.9 to 14.0 ± 3.9 cm2, p = 0.03), and PAT area decreased by 3.2 cm2 (33.5 ± 13.2 to 30.4 ± 14.1 cm2, p = 0.04) (Table 1). BSA-normalized EAT area decreased by 0.64 cm2/m2 (7.4 ± 1.7 to 6.8 ± 1.7 cm2/m2, p = 0.03), while the BSA-normalized PAT area decreased by 1.6 cm2/m2 (16.2±6.1 to 14.6±6.5, P=0.09) (Table 1). When corrected for age and sex, the significance of the fat area measurement differences did not change. EAT and PAT areas remained significantly higher than those of the control group before and after treatment (p < 0.005) (Figure 1).
At baseline, E/A and normalized PLVFR were significantly lower in T2DM versus controls (p < 0.005 and p = 0.008, respectively) (Table 1). Pioglitazone increased both E/A (0.96 ± 0.27 to 1.39 ± 0.49, p = 0.01) and BSA-normalized PLVFR (164 ± 46 to 188 ± 64 mL/s*m2, p = 0.05) in T2DM subjects (Table 1). After pioglitazone treatment, E/A and PLVFR were not significantly different from values in NGT subjects (p > 0.05) (Table 1).
In T2DM subjects, pioglitazone reduced HbA1c (6.7 ± 1.3% to 5.6 ± 0.8%, p = 0.02), FPG (149 ± 48 to 112 ± 23 mg/dL, p = 0.04), fasting FFA (0.52 ± 0.17 to 0.30 ± 0.14 mg/dL, p < 0.005), and triglycerides (265 ± 155 to 153 ± 74 mg/dL, p = 0.005) (Table 1) and increased the Matsuda Index of insulin sensitivity (2.8 ± 1.9 to 5.6 ± 3.3, p < 0.005) and insulin-stimulated GIR (3.4 ± 1.3 to 5.8 ± 2.1 mg/kg/min, p = 0.007).
Following 24 weeks of pioglitazone treatment, HbA1c, plasma FFA and triglyceride concentrations, Matsuda Index, and insulin-stimulated GIR in T2DM patients were not significantly different from the NGT control group (p > 0.05 for all). HDL remained significantly lower (p = 0.001) and FPG significantly higher in T2DM versus control subjects (p = 0.02).
EAT and PAT were negatively correlated with Matsuda index (r = −0.68, p < 0.001 and r = −0.59, p = 0.002, respectively) (Figure 2) and GIR (r = −0.69, p < 0.001 and r = −0.68, p < 0.001, respectively) (Figure 3). Both EAT and PAT were positively correlated with BMI (r = 0.66, p < 0.001 and r = 0.69, p < 0.001, respectively), while only PAT was significantly correlated with percent body fat (r = 0.40, p = 0.049). The correlation between EAT and percent body fat was did not reach statistical significance (r = 0.33, p = 0.11).
Figure 2.
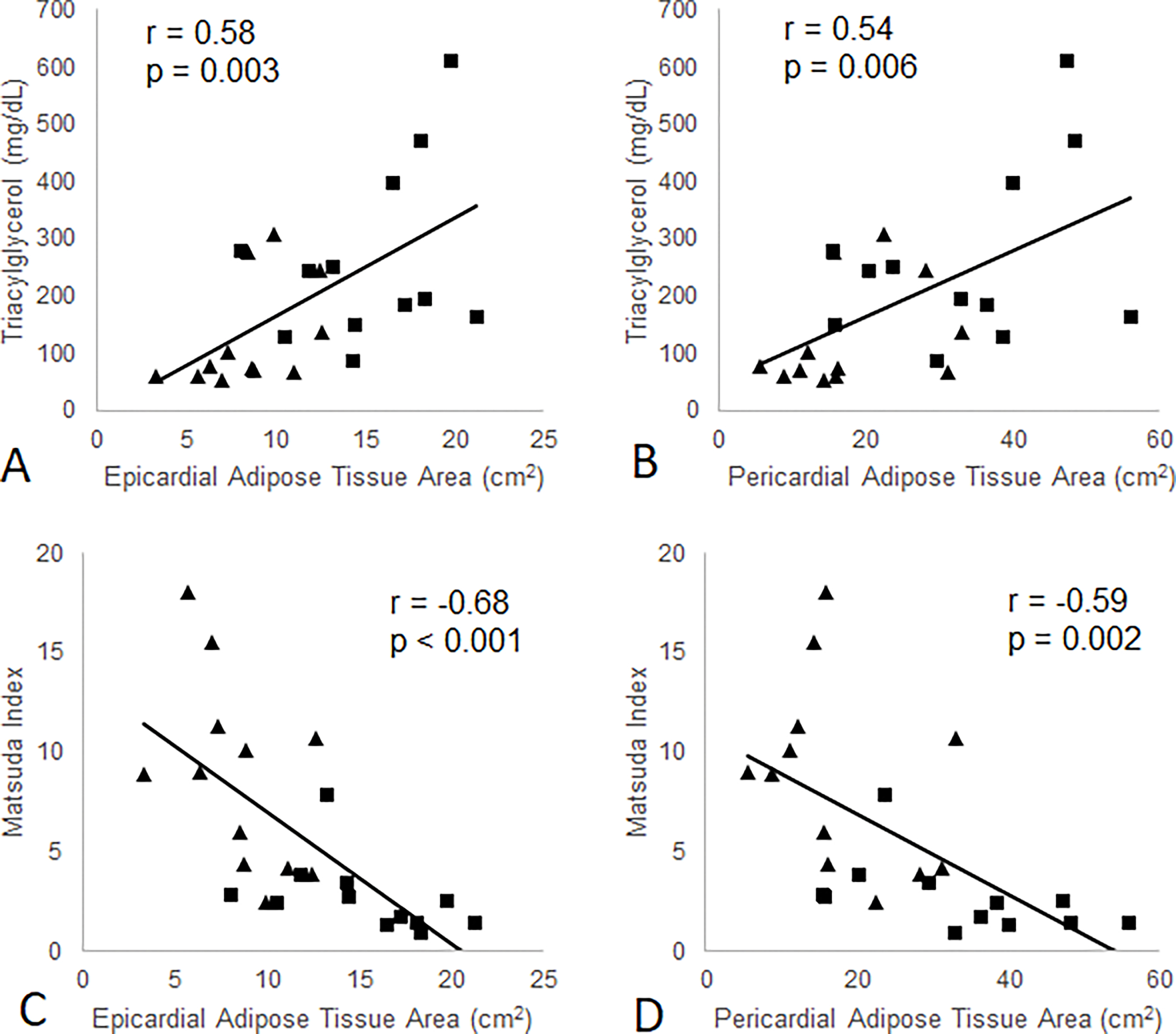
Association between EAT and PAT areas and Matsuda Index of insulin sensitivity and plasma triglyceride concentrations in NGT control (diamonds) and T2DM patients (squares) at baseline.
Figure 3.
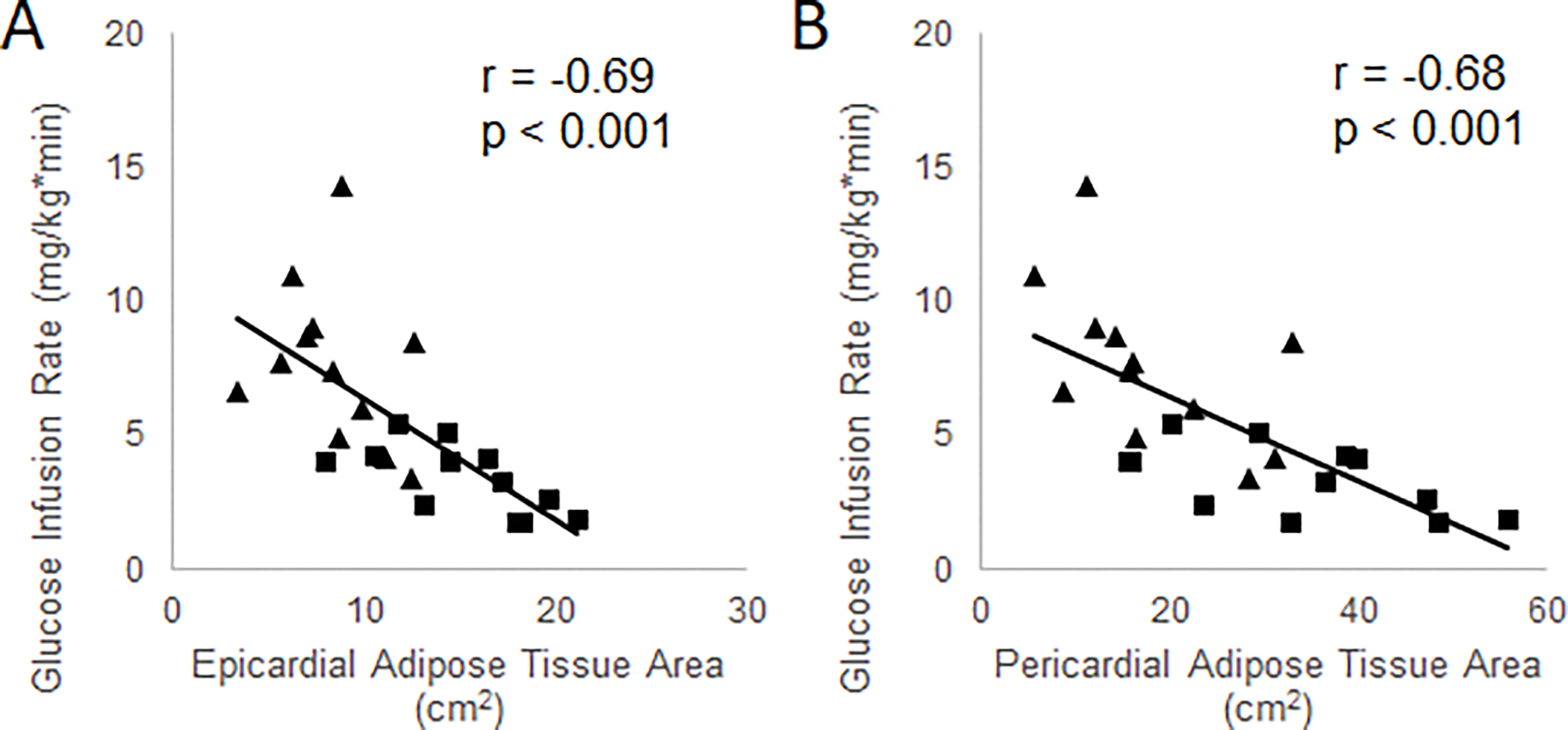
Associations between EAT and PAT areas and insulin-stimulated whole body glucose disposal (glucose infusion rate) in NGT controls (diamonds) and T2DM patients (squares) at baseline.
EAT area was negatively correlated with E/A (r = −0.55, p = 0.006) and normalized PLVFR (r = −0.45, p = 0.03) (Figure 4). PAT area was significantly correlated with E/A but not with PLVFR (Figure 4). Both EAT and PAT were positively correlated with the plasma triglyceride concentration (r = 0.58, p = 0.003 and r = 0.54, p = 0.006, respectively) (Figure 2).
Figure 4.
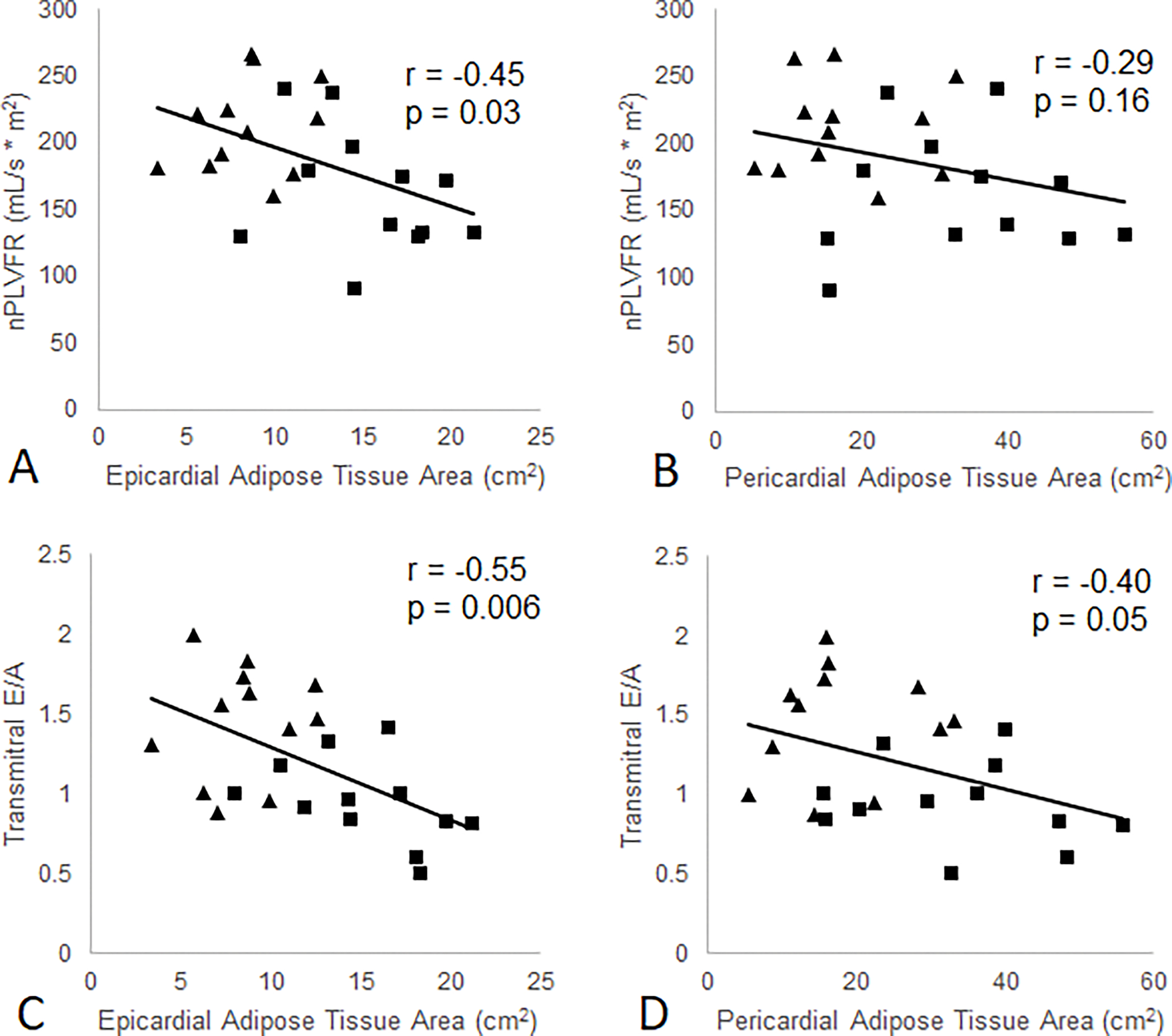
Association between EAT and PAT areas and measures of diastolic function in NGT control (diamonds) and T2DM patients (squares) at baseline.
DISCUSSION
The current study provides three important novel findings: (i) this is the first study to simultaneously quantitate EAT and insulin sensitivity in the same subject and demonstrate a correlation between the two; (ii) the results demonstrate that pioglitazone reduces EAT and that the decrease in EAT is correlated with the improvement in insulin sensitivity by the gold standard euglycemic insulin clamp; (iii) the pioglitazone-mediated improvements in LV diastolic function (E/A and PLVFR) are closely related to reduced EAT after pioglitazone treatment in T2DM patients without clinical cardiovascular disease. Our results also demonstrated that PAT was inversely associated with measures of insulin sensitivity (Matsuda and GIR) and less strongly than EAT with LV diastolic function. Based on these results we speculate that proximity of epicardial adipose tissue to the heart muscle, with secretion of adipocytokines and lipotoxic molecules by EAT, exerts a negative effect on myocardial function and myocardial insulin sensitivity.
Consistent with our findings, a number of studies have suggested that excess fat surrounding the heart exerts a deleterious effect on myocardial function (37–41). Of note, there is a close physiological relationship between EAT and the heart muscle since both tissues are fed by the coronary arteries and EAT serves as a readily available supply of triglycerides and FFA for the myocardium (37,40). Consistent with this, the present results document that increased EAT is associated with elevated levels of plasma triglycerides. Our results of show that PAT was more closely associated with percent body fat, which bolsters assertions in the literature that EAT is anatomically and physiologically less comparable to other visceral fat stores than PAT (37, 40).
In contrast to the present study, an earlier study suggested that PAT area correlated better with cardiometabolic risk factors than EAT (42); however, insulin sensitivity was not measured in this study. More recently EAT, but not PAT, was found to be increased in T2DM versus control subjects and was associated with echocardiographic measures of impaired diastolic function (43). In a recent study (41), higher EAT thickness was found to be associated with lower peak oxygen uptake during exercise and lower E/A ratio at rest. However, all parameters of diastolic function were within the normal range during a maximal cardiopulmonary test. This same group found that elevated EAT was associated with the composite of incident cardiovascular disease (CVD) and mortality in T2D patients after adjustment for CVD risk factors (44). These results are consistent with those of the present study in which we demonstrated that diastolic function was closely linked to EAT but less so to PAT. Excessive EAT volume also has been associated with increased coronary plaque, quantitated by computed tomography calcium scores (45). Using magnetic resonance spectroscopy (MRS) and MRI to measure myocardial extracellular volume, Ng et al. showed that insulin resistance and EAT volume index were independently associated with high intramyocardial lipid levels and the development of diffuse LV fibrosis (39). Taken together with the current study, EAT emerges as an important component of myocardial insulin resistance.
As reported previously by our group and others, pioglitazone is a potent insulin sensitizer in skeletal muscle, liver, and adipocytes (16–24) and improves myocardial insulin resistance and function in T2DM (13,15). Pioglitazone is a PPAR-γ agonist that acts as an adipogenic agent (24). PPAR-γ is highly expressed in adipose tissue and its expression is reduced in insulin resistant conditions (46). An ex vivo analysis of EAT from T2DM patients found an increase in the expression of proinflammatory genes (IL-1β, IL-1Rα, and IL-10) in EAT compared to subcutaneous adipose tissue (14). The increased expression of EAT proinflammatory genes was markedly reduced by pioglitazone. In T2DM and other insulin resistant conditions we have shown that there is a coordinated reduction of genes involved in oxidative metabolism and decreased expression of PGC-1 (47), which is the master regulator of mitochondrial biogenesis. Further, in T2DM patients pioglitazone treatment markedly increased the expression of PGC-1 and genes involved in adiponectin signaling, mitochondrial function and fat oxidation (48). Taken collectively, these results suggest that the beneficial effect of pioglitazone on myocardial function and metabolism, as well as protection against CV events (30–33), may, in part, be mediated by its effect to reduce EAT mass and proinflammatory gene expression. To the best of our knowledge only one previous study (34) has examined the effect of pioglitazone on cardiac adipose tissue following pioglitazone treatment in T2DM and found a modest increase in pericardial fat. Of note, this study did not utilize the water-suppression MRI technique employed in the present study and total pericardial fat was measured without distinguishing EAT from PAT. Also, subjects were titrated to a lower pioglitazone dose (30 mg) than in the current study. However, consistent with our results the same group (49) found a significant improvement in left ventricular diastolic function with pioglitazone treatment.
The present study has several limitations. First, T2DM patients who were enrolled did not have clinically manifest heart disease. Whether similar results would be obtained in subjects with confirmed cardiac dysfunction warrants further investigation. Second, the number of subjects recruited was relatively small which likely explains why a number of parameters (i.e. decreases in PAT) and correlations trended toward, but did not reach statistical significance. Third, area measurements of cardiac adipose tissue only were obtained in one long axis view. While studies have shown that volumetric measures of cardiac adiposity correlate well with area measurements, there is some loss of accuracy when only one image plane is analyzed (50). While cine acquisitions can be used to assess tissue volumes, the pericardium is not as easily distinguishable in images without water suppression. In similar studies which either did not utilize water-suppression or separate the two adipose tissue compartments, results were largely inconclusive (34, 42). Finally, we did not measure inflammatory markers which could have provided a potential mechanism to explain the negative correlation between EAT and diastolic dysfunction.
CONCLUSION
In summary, compared to NGT control subjects both EAT and PAT are increased in T2DM without clinically manifest CV disease and correlate positively with measures of diastolic dysfunction (E/A ratio and peak LV flow rate) and whole-body insulin resistance measured with the insulin clamp technique and Matsuda Index. Following 24 weeks of pioglitazone treatment both EAT and PAT decreased in association with improved measures of LV diastolic function.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01DK24093. Pioglitazone tablets were provided by Takeda Pharmaceuticals North America.
Footnotes
Declaration of Interests
RAD is supported by grants from Boehringer Ingelheim, Astra Zeneca, Merck; is a member of the Advisory Board of Boehringer Ingelheim, Astra Zeneca, Novo Nordisk, Intarcia; is a member of the Astra Zeneca Speakers Bureau. EC is a member of the Advisory Board for Bayer Pharmaceuticals. No other authors have a conflict of interest to declare.
Guarantor. RAD is the guarantor of the data and takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. Dr. Ralph DeFronzo is the senior author and Drs. Geoffrey Clarke and Muhammad Abdul-Ghani had direct access to and verified all of the data.
Data Available.
The data from the current study are available on reasonable request from RAD.
References
- 1.Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab 2003; 88:5163–5168. [DOI] [PubMed] [Google Scholar]
- 2.Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab 2005; 90:6300–6302. [DOI] [PubMed] [Google Scholar]
- 3.Gorter PM, de Vos AM, van der Graaf Y, et al. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol. 2008;102:380–385. [DOI] [PubMed] [Google Scholar]
- 4.Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta-analysis. Metab Syndr Relat Disord 2014; 12:31–42. [DOI] [PubMed] [Google Scholar]
- 6.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. [DOI] [PubMed] [Google Scholar]
- 7.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 2008; 117:605–613. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Gona P, Hoffmann U, et al. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation 2009; 119:1586–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J 2008; 30:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 2007; 115:3213–3223. [DOI] [PubMed] [Google Scholar]
- 11.Kankaanpää M, Lehto HR, Pärkkä JP, et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab 2006; 91:4689–4695. [DOI] [PubMed] [Google Scholar]
- 12.Sharma AM, Chetty VT. Obesity, hypertension and insulin resistance. Acta Diabetol 2005; 42(S1):s3–s8. [DOI] [PubMed] [Google Scholar]
- 13.Clarke GD, Molina-Wilkins M, Solis-Herrera C, et al. Impaired left ventricular diastolic function in T2DM patients is closely related to glycemic control. Endocrinol Diabetes Metab 2018; 1:e00014. doi: 10.1002/edm2.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks HS, Fain JN, Cheema P, Bahouth SW, Garrett E, Wolf RY, Wolford D, Samaha J. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: changes associated with pioglitazone. Diabetes Care 2011;34:730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke GD, Solis-Herrera C, Molina-Wilkins M, et al. Pioglitazone improves left ventricular diastolic function in subjects with diabetes. Diabetes Care 2017; 40:1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Inzucchi S, Abdul-Ghani M, Nissen SE. Pioglitazone: The forgotten, cost-effective cardioprotective drug for type 2 diabetes. Diab Vasc Dis Res. 2019; 16:133–143. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj M, Baig R, Suraamornkul S, Hardies LJ, Coletta DK, Cline GW, Monroy A, Koul S, Sriwijitkamol A, Musi N, Shulman GI, DeFronzo RA. Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2010;95:1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazaki Y, Mahankali A, Matsuda M, Glass L, Mahankali S, Ferrannini E, Cusi K, Mandarino LJ, DeFronzo RA. Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care 2001;24:710–719 [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki Y, DeFronzo RA. Rosiglitazone and pioglitazone similarly improve insulin sensitivity and secretion, glucose tolerance and adipocytokines in type 2 diabetic patients. Diabetes Obes Metab 2008;10:1204–1211 [DOI] [PubMed] [Google Scholar]
- 20.Gastaldelli A, Miyazaki Y, Mahankali A, Berria R, Pettiti M, Buzzigoli E, Ferrannini E, DeFronzo RA. The effect of pioglitazone on the liver: role of adiponectin. Diabetes Care 2006;29:2275–2281 [DOI] [PubMed] [Google Scholar]
- 21.Gastaldelli A, Casolaro A, Ciociaro D, Frascerra S, Nannipieri M, Buzzigoli E, Ferrannini E. Decreased whole body lipolysis as a mechanism of the lipid-lowering effect of pioglitazone in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2009;297:E225–230. [DOI] [PubMed] [Google Scholar]
- 22.DeFronzo RA, Banerji M, Clement SC, Kitabchi AE, Ratner RE, Musi N. Pioglitazone for Diabetes Prevention in Impaired Glucose Tolerance. N Engl J Med 2011; 364: 1104–1115. [DOI] [PubMed] [Google Scholar]
- 23.Thiazolidinediones Yki-Järvinen H. N Engl J Med 2004; 351:1106–1118. [DOI] [PubMed] [Google Scholar]
- 24.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab 2004;89:463–478. [DOI] [PubMed] [Google Scholar]
- 25.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010;53:1270–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2002;87:2784–2791. [DOI] [PubMed] [Google Scholar]
- 27.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297–2307. [DOI] [PubMed] [Google Scholar]
- 28.Bajaj M, Suraamornkul S, Hardies LJ, Glass L, Musi N, DeFronzo RA. Effects of peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ agonists on glucose and lipid metabolism in patients with type 2 diabetes mellitus. Diabetologia 2007; 50:1723–1731. [DOI] [PubMed] [Google Scholar]
- 29.Nesti L, Tricò D, Mengozzi A, Natali A. Rethinking pioglitazone as a cardioprotective agent: a new perspective on an overlooked drug. Cardiovasc Diabetol 2021; 20:1–7. doi: 10.1186/s12933-021-01294-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. [DOI] [PubMed] [Google Scholar]
- 31.Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. New Engl Med 2016; 374:1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Jong M, Vander Worp HB, Vander Graaf Y, et al. Pioglitazone and the secondary prevention of cardiovascular disease. A meta-analysis of randomized-controlled trials. Cardiovasc Diabetol 2017; 16:134. 10.1186/s12933-017-0617-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strongman H, Christopher S, Majak M, Williams R, Bahmanyar S, Linder M, Heintjes EM, Bennett D, Korhonen P, Hoti F. Pioglitazone and cause-specific risk of mortality in patients with type 2 diabetes: extended analysis from a European multidatabase cohort study. BMJ Open Diabetes Res Care 2018;6:e000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonker JT, Lamb HJ, van der Meer RW, et al. Pioglitazone compared with metformin increases pericardial fat volume in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2010; 95:456–460 [DOI] [PubMed] [Google Scholar]
- 35.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237: E214–223 [DOI] [PubMed] [Google Scholar]
- 36.Mosteller RD. Simplified calculation of body-surface area. Engl J Med 1987; 317: 1098. [DOI] [PubMed] [Google Scholar]
- 37.Berg G, Miksztowicz V, Morales C, Barchuk M. Epicardial adipose tissue in cardiovascular disease. Chapter 9 in: Trostchansky A and Rubbo H, eds. Bioactive Lipids in Health and Disease 2019; 1127:131–143. [DOI] [PubMed] [Google Scholar]
- 38.Guglielmi V, Sbraccia P. Epicardial adipose tissue: at the heart of the obesity complications. Acta Diabetol. 2017; 54:805–812. [DOI] [PubMed] [Google Scholar]
- 39.Ng ACT, Strudwick M, van der Geest RJ, et al. Impact of epicardial adipose tissue, left ventricular myocardial fat content, and interstitial fibrosis on myocardial contractile function. Circ Cardiovasc Imag 2018; 11:e007372. [DOI] [PubMed] [Google Scholar]
- 40.Gastaldelli A, Morales MA, Marraccini P, Sicari R. The role of cardiac fat in insulin resistance: Curr Opin Clin Nutr Metab Care 2012; 15:523–528. [DOI] [PubMed] [Google Scholar]
- 41.Nesti L, Pugliese NR, Chiriacò M, Trico D, Baldi S, Natali A. Epicardial adipose tissue thickness is associated with reduced peak oxygen consumption and systolic reserve in patients with type 2 diabetes and normal heart function. Diabetes Obes Metab 2022. doi.org/ 10.1111/dom.14861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sicari R, Sironi AM, Petz R, et al. Pericardial rather than epicardial fat is a cardiometabolic risk marker: an MRI vs echo study. J Am Soc Echocardiogr 2011; 24:1156–1162. [DOI] [PubMed] [Google Scholar]
- 43.Christensen RH, Hansen CS, von Scholten BJ, et al. Epicardial and pericardial adipose tissues are associated with reduced diastolic and systolic function in type 2 diabetes. Diabetes Obes Metab 2019; 21: 2006–2011. [DOI] [PubMed] [Google Scholar]
- 44.Christensen RH, Scholten BJ Von, Hansen CS, et al. Epicardial adipose tissue predicts incident cardiovascular disease and mortality in patients with type 2 diabetes. Cardiovasc Diabetol 2019; 18: 114. 10.1186/s12933-019-0917-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakanishi R, Rajani R, Cheng VY, Gransar H, Nakazato R, Shmilovich H, Otaki Y, Hayes SW, Thomson LE, Friedman JD, Slomka PJ. Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: a serial study using non-contrast cardiac CT. Atherosclerosis 2011; 218:363–368. [DOI] [PubMed] [Google Scholar]
- 46.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 2013; 19:557–566. 10.1038/nm.3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 2003;100:8466–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coletta DK, Sriwijitkamol A, Wajcberg E, Tantiwong P, Li M, Prentki M, Madiraju M, Jenkinson CP, Cersosimo E, Musi N, Defronzo RA. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia 2009;52:723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Meer RW, Rijzewijk LJ, de Jong HWAM, et al. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation 2009; 119:2069–2077. [DOI] [PubMed] [Google Scholar]
- 50.Sicari R, Sironi AM, Petz R, et al. Pericardial Rather Than Epicardial Fat is a Cardiometabolic Risk Marker: An MRI vs Echo Study. Journal of the American Society of Echocardiography. 2011;24(10):1156–1162. doi: 10.1016/j.echo.2011.06.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from the current study are available on reasonable request from RAD.


