Abstract
An analog of 1α,25-dihydroxyvitamin D3, 22-oxyacalcitriol (OCT), differentiated human monocytic THP-1 and U937 cells to express membrane CD14 and rendered the cells responsive to bacterial cell surface components. Both THP-1 and U937 cells expressed Toll-like receptor 4 (TLR4) on the cell surface and TLR4 mRNA in the cells, irrespective of OCT treatment. In contrast, OCT-treated U937 cells scarcely expressed TLR2 mRNA, while OCT-treated THP-1 cells expressed this transcript. Muramyldipeptide (MDP) by itself exhibited only a weak ability to induce secretion of inflammatory cytokines such as interleukin-8 (IL-8) in the OCT-differentiated THP-1 cells but showed marked synergistic effects with Salmonella lipopolysaccharide (LPS) or lipoteichoic acid (LTA) from Staphylococcus aureus, both of which exhibited strong activities. Combinatory stimulation with LPS plus LTA did not show a synergistic effect on OCT-differentiated THP-1 cells. Similar results were observed in OCT-differentiated U937 cells, although combination experiments were carried out only with MDP plus LPS. Anti-CD14 monoclonal antibody (MAb) MY4, anti-TLR4 MAb HTA125, and the synthetic lipid A precursor LA-14-PP almost completely inhibited the IL-8-inducing activities of LTA as well as LPS on OCT-treated THP-1 cells, but these treatments increased MDP activity. OCT-treated THP-1 cells primed with MDP exhibited enhanced production of IL-8 upon stimulation with LPS, while the cells primed with LPS showed no change in production upon stimulation with MDP. MDP up-regulated mRNA expression of an adapter molecule to TLRs, MyD88, to an extent similar to that for LPS in OCT-treated THP-1 cells. These findings suggested that LTA as well as LPS activated human monocytic cells in a CD14- and TLR4-dependent manner, whereas MDP exhibited activity in a CD14-, TLR4-, and probably TLR2-independent manner and exhibited synergistic and priming effects on the cells for cytokine production in response to various bacterial components.
In bacterial infectious diseases, host cells should first recognize and respond to bacterial cell surface components via the innate immune system and develop inflammatory and immunological reactions. Macrophages may play key roles in antibacterial host defense by producing various inflammatory cytokines in response to bacterial components. Lipopolysaccharide (LPS), an amphiphilic material in the outer membrane of gram-negative bacteria, is a well-known potent activator of macrophages (30). Lipoteichoic acid (LTA), another amphiphilic material distributed in the cell surface of various gram-positive bacteria, has been suggested to be a counterpart of LPS in these bacteria (8). Peptidoglycans are essential constituents of all bacterial cell walls and are therefore distributed ubiquitously in both gram-positive and gram-negative bacteria except mycoplasma, although their contents are high and low in gram-positive and gram-negative bacteria, respectively. The minimal essential structure of peptidoglycan for bioactivity was chemically synthesized and designated as muramyldipeptide (MDP; N-acetylmuramyl-l-alanyl-d-isoglutamine) (reviewed in references 42 and 43).
A number of studies suggested that all of these bacterial components are recognized by membrane CD14 (mCD14) on macrophages and other cells. mCD14 is a glycosylphosphatidylinositol-anchored protein and thus lacks the intracellular domain for signaling (reviewed in reference 49). Recently, the Toll-like receptor (TLR) family was shown to play a key role in signaling of host cells in response to bacterial cell surface components (reviewed in references 2, 16, 21, and 52). Takeuchi et al. (44) demonstrated that TLR2 and TLR4 are essential for the responses to peptidoglycan and LTA as well as LPS, respectively, in peritoneal macrophage cultures from TLR2 and TLR4 knockout mice. However, the usage of TLRs for individual bacterial components is still controversial. Early studies using human TLR-transfected cells suggested that human TLR2, but not TLR4, is involved in the response of cells to LPS (14, 53), probably because of the lack of information concerning MD-2, another essential molecule conferring LPS responsiveness on TLR4 in human and murine cells (3, 33), and of possible contamination of LPS specimens with LPS-associated material (11) such as lipopeptide, the activity of which is dependent on TLR2 (45). In fact, TLR4 mutations are associated with endotoxin hyporesponsiveness in humans (4). Schwandner et al. (31) suggested the involvement of TLR2, but not TLR4, in signaling of the LTA response in human cells. Recently, Takeuchi et al. (46) demonstrated that MyD88, an adapter molecule for the TLR/interleukin-1 (IL-1) receptor family, is essential for the cellular responses to bacterial cell surface components including various LPS specimens, gram-positive bacterial cell walls, and peptidoglycans. The receptor and signaling systems for MDP are not yet clear, although the competition of MDP with soluble peptidoglycan for CD14 was reported (51).
Intravenous injection of a mixture of LPS and MDP resulted in marked lethality in guinea pigs and mice (7, 29). To sensitize mice with MDP for lethal toxicity of LPS, pretreatment of mice around 4 h prior to LPS administration is more effective than simultanous administration of MDP and LPS, where MDP alone is not lethal in mice (38). In MDP-primed mice, high levels of serum cytokines were induced upon stimulation with LPS (26). MDP priming is also effective for inducing inflammatory cytokines in mice in response to various bacterial components, including LTA (22, 39, 41). Kengatharan et al. (13) reported enhancement of nitric oxide formation by MDP in the murine macrophage cell line J774.2 in response to Staphylococcus aureus LTA, although MDP alone is not effective in this respect. In human monocyte cultures, MDP is a powerful cytokine inducer. Therefore, synergistic effects on induction of cytokines were observed only at relatively low concentration of MDP (48).
In this study, we found that MDP exhibited only weak ability to induce cytokines in human monocytic cell lines THP-1 and U937 in culture even after differentiation by treatment with an active form of vitamin D3. Therefore, we examined the synergistic effects of MDP with LPS or LTA on human monocytic cells in relation to modulation of the CD14/TLR system by MDP in the cells.
MATERIALS AND METHODS
Cell culture.
The human monocytic leukemia cell line THP-1 and human monocytic U937 cells prepared from diffuse histiocytic lymphoma, both of which were supplied by the Health Science Research Resources Bank (Tokyo, Japan), were cultured in RPMI 1640 medium with 10% fetal calf serum (Flow Laboratories, Inc., McLean Va.) in 100-mm-diameter tissue culture dishes (Falcon; Becton Dickinson Labware, Lincoln Park, N.J.) at 37°C in a 5% CO2 atmosphere. The cells were maintained in logarithmic growth (2 × 105 to 1 × 106/ml) by passage every 3 to 4 days. Cells (2 × 105/ml) were treated with 0.1 μM 22-oxyacalcitriol (OCT), an analogue of 1α,25-dihydroxyvitamin D3 (Chugai Pharmaceutical Co., Tokyo, Japan) (17), for 3 days to induce differentiation to macrophage-like cells (Fig. 1).
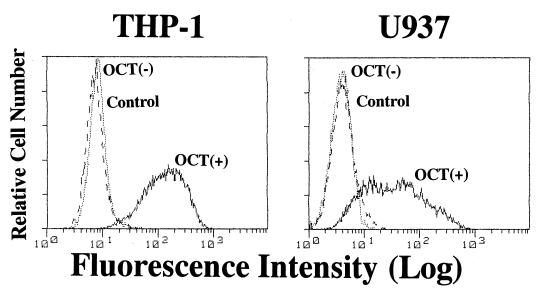
FIG. 1.
Up-regulation of mCD14 expression by OCT in THP-1 and U937 cells in culture. THP-1 and U937 cells were cultured in the presence or absence of OCT (0.1 μM/ml) for 3 days. Cells were then collected and stained with the second antibody (control) or anti-CD14 MAb (MY4) and analyzed by FACS. The results are representative of three different experiments.
Reagents and cDNA.
Ultrapurified LPS prepared from Salmonella abortus-equi (Novo-Pyrexal) (10) was a gift from C. Galanos (Max Planck Institut für Immunbiologie, Freiburg, Germany). A synthetic MDP was supplied by Daiichi Pharmaceutical Co. (Tokyo, Japan), and a synthetic lipid A precursor IVA, LA-14-PP (compound 406), was obtained from Daiichi Chemical Co. (Tokyo, Japan). Anti-CD14 monoclonal antibody (MAb) MY4 (mouse immunoglobulin G2b [IgG2b]) and isotype-matched mouse IgG2b were obtained from Coulter Co. (Miami, Fla). Anti-TLR4 MAb HTA125 was prepared as described previously (33). Other reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.). A human MyD88 cDNA clone was prepared as described previously (1). A human glyceraldehyde-3-phosphate dehydrogenase (GADPH) cDNA clone (47) was provided by I. Sakiyama (Chiba Cancer Center Research Institute and Hospital, Chiba, Japan).
Cytokine assay.
OCT-treated THP-1 cells were collected and washed twice in phosphate-buffered saline (PBS). The cells (105/200 μl per well) were incubated with or without stimulant in RPMI 1640 medium with 1% fetal calf serum for 24 h in 96-well round-bottomed plates (Falcon). In some experiments, cells were preincubated with inhibitor (LA-14-PP) or MAbs for 30 min and then incubated with stimulant. In most combination assay experiments, two kinds of bacterial components were added to the cultures simultaneously. After cultivation, the culture supernatants were collected and the levels of cytokines were determined by enzyme-linked immunosorbent assay (ELISA) kits (Pharmingen, San Diego, Calif.). The concentrations of cytokines in the supernatants were determined using the Softmax data analysis program (Molecular Devices Corp., Menlo Park, Calif.).
Flow cytometry.
THP-1 cells pretreated with OCT and then treated with MDP were analyzed for the expression of mCD14 and TLR4 by flow cytometry. To analyze the expression of mCD14, the cells were stained with fluorescein isothiocyanate-labeled anti-CD14 MAb MY4 at 4°C for 30 min. To analyze the expression of TLR4, the cells were stained with anti-TLR4 MAb HTA125 or isotype-matched mouse IgG1 at 4°C for 30 min, followed by fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Biosource International Inc., Camarillo, Calif.) at 4°C for a further 30 min. Fluorescence-activated cell sorting (FACS) was performed with a FACScan (Becton Dickinson, Mountain View, Calif.).
RT-PCR assay.
As described above, THP-1 and U937 cells were precultured in 0.1 μM OCT or medium alone for 3 days; then total RNA was extracted from the cells by Isogen (Nippon Gene, Tokyo, Japan) according to the manufacturer's instructions. Reverse transcription of the RNA samples to cDNA was performed with avian myeloblastosis virus reverse transcriptase (RT) XL (Life Sciences, St. Petersburg, Fla.) and Random primer (nonadeoxyribonucleotide mixture; Takara, Tokyo, Japan). For cDNA preparation, 1.0 μg of RNA, 50 pmol of random primer, 5 mM MgCl2, 2 μl of 10× RNA PCR buffer (Takara), 1.0 mM each deoxynucleoside triphosphate (Takara), 5 U of avian myeloblastosis virus RT XL, and 20 U of RNase inhibitor (Takara) were added to a total volume of 20 μl. The reaction mixture was incubated (at 30°C for 10 min, at 42°C for 30 min, and then at 99°C for 5 min to inactive the RT), cooled at 5°C for 5 min, and stored at -20°C. The primers used for PCR had the following sequences: TLR2, 5′- TCACCTACATTAGCAACAG-3′ (forward) and 5′-GATCTGAAGCATCAATCTC-3′ (reverse); TLR4, 5′-TGGATACGTTTCCTTATAAG-3′ (forward) and 5′- GAAATGGAGGCACCCCTT C-3′ (reverse); and human GAPDH, 5′- TGAAGGTCGGAGTCAACGGATTTGGT-3′ (forward) and 5′-CATGTGGGCCATGAGGTCCACCAC-3′ (reverse). The primers for TLR2, TLR4, and GAPDH were constructed to generate fragments of 368, 506, and 983 bp, respectively. The PCR mixture contained 4 μl of the cDNA mixture, 1.2 μl of 10× Ex Taq buffer, 2.5 mM MgCl2, and 0.1 μl of Takara Ex Taq in a total of volume of 20 μl. Amplification was performed in a model MP TP3000 PCR thermal cycler (Takara) as follows: with TLR2, 33 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 90 s; with TLR4, 28 cycles at 95°C for 40 s, 54°C for 40 s, and 72°C for 1 min; and with GAPDH, 25 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min and then a final extension at 72°C for 3 min. Amplified products were visualized on 2.0% agarose gels stained with ethidium bromide and photographed under UV light.
Northern blotting analysis.
OCT-pretreated THP-1 cells were treated with stimulants for various intervals, and then total cellular RNA was isolated from 50 × 105 cells every 4 h using Isogen. For each sample, 20 μg of RNA was fractionated on 1.2% agarose gels containing 2 M formaldehyde and transferred onto nylon membranes (Zeta-Probe; Bio-Rad Laboratories, Richmond, Calif.). The membranes were prehybridized overnight at 42°C in 50% formamide-based hybridization buffer. Hybridization was performed at 42°C for 16 h in hybridization buffer with 32P-labeled cDNA probes (106 cpm/ml). Probes were labeled by using random primers with Klenow fragment (Roche Molecular Biochemicals, Mannheim, Germany) and [α-P32] dCTP (NEN, Wilmington, Del.). The membranes were exposed to imaging plates at room temperature overnight. cDNA specific for GAPDH was used as a control for quantification. Radioactivity was determined by autoradiography on the imaging plate and analyzed with a bioimage analyzer (BAS 1500 Mac; Fuji Photo Film Co., Tokyo, Japan).
Statistical analysis.
All experiments were performed at least twice. The data shown are representative results as the means ± standard deviation of triplicate cultures. The statistical significance of the differences between each test and its control was examined by a one-way analysis of variance (ANOVA), using the Bonferroni or Dunn method. In combinatory stimulation experiments, ANOVA on the interaction between bacterial components was carried out to examine the synergistic effects of the agents.
RESULTS
Influence of OCT treatment on the CD14/TLR system in monocytic cells.
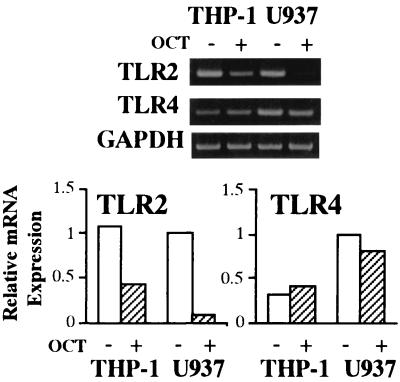
The OCT-treated THP-1 and U937 cells strongly expressed mCD14, whereas the nontreated cells showed little expression of mCD14 on flow cytometry (Fig. 1). Absence of TLR2 protein expression in the differentiated U937 cells was reported, although the cells expressed TLR2 mRNA (6). We found that TLR2 mRNA expression by U937 cells was down-regulated by OCT treatment, while the treatment did not change TLR4 mRNA expression by U937 cells or TLR2 and TLR4 mRNA expression by THP-1 cells (Fig. 2).
FIG. 2.
Influence of OCT treatment on TLR2 and TLR4 mRNA expression in THP-1 and U937 cells. THP-1 and U937 cells were cultured in the presence or absence of OCT (0.1 μM/ml) for 3 days. Total RNA was extracted from the cells and analyzed for TLR2, TLR4, and GAPDH by RT-PCR (top). Densities of the bands of TLR2 and TLR4 mRNA shown at the top were analyzed with NIH Image and expressed as mRNA expression relative to that of GADPH mRNA as an internal standard. The results are representative of three different experiments.
Induction of IL-8 secretion by bacterial cell surface components in monocytic cell cultures.
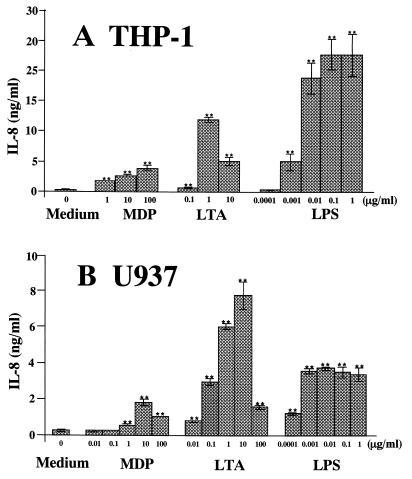
We examined the ability of MDP, S. aureus LTA, and S. abortus-equi LPS to induce IL-8 in OCT-treated THP-1 (Fig. 3A) and U937 (Fig. 3B) cell cultures. LPS induced IL-8 production in a dose-dependent manner from 1 to 100 ng/ml in THP-1 cells (Fig. 3A) and 0.1 to 1 ng/ml in U937 cells (Fig. 3B) and then reached a plateau. LTA exhibited bell-shaped dose-response curves where peak responses were observed at 1 μg/ml in both cells. MDP also exhibited definite induction of IL-8 production in a dose-dependent manner from 1 to 100 μg/ml in THP-1 cells (Fig. 3A) and with a bell-shaped dose-response curve where the peak response was observed at 10 μg/ml in U937 cells (Fig. 3B), although the levels of IL-8 secretion were considerably lower than those induced by LPS and LTA.
FIG. 3.
Induction of IL-8 secretion by bacterial cell surface components in human monocytic cell cultures. OCT-differentiated THP-1 and U937 cells were stimulated with MDP, S. aureus LTA, and S. abortus-equi LPS at the indicated concentrations for 24 h in triplicate. IL-8 levels in the culture supernatants were determined by ELISA and expressed as means ± standard deviation. ∗∗, P < 0.01 versus medium alone. The results are representative of three different experiments.
Synergistic effect of MDP with LPS or LTA on induction of cytokine secretion in THP-1 cell cultures.
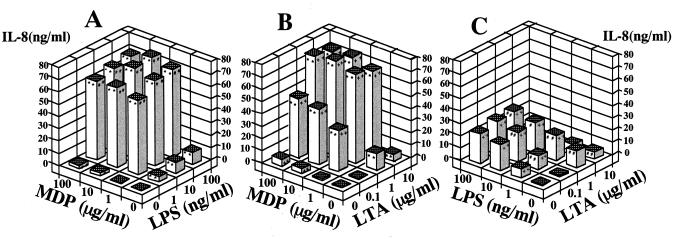
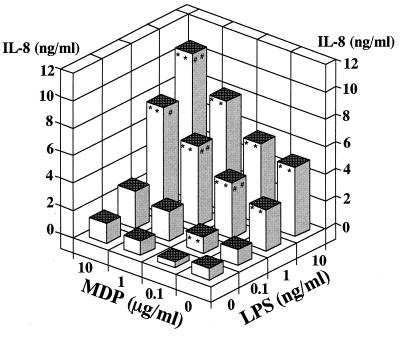
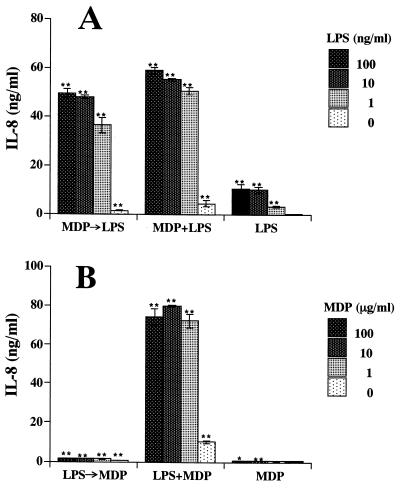
We examined the combinatory effects of MDP, LPS, and LTA in THP-1 cells in culture. A combination of MDP and LPS induced marked IL-8 secretion (Fig. 4A). The data indicated a clear synergistic effect of MDP and LPS based on statistical analysis. In this experiment, IL-6 and IL-1β levels in the culture supernatants were also measured, and similar synergistic effects were noted (data not shown). With a combination of MDP and LTA, a synergistic effect on IL-8 secretion was also observed in THP-1 cell cultures (Fig. 4B). In contrast, no synergistic effect was observed in cultures treated with a combination of LPS and LTA (Fig. 4C). Furthermore, LTA at the excessive concentration (10 μg/ml) abolished the activity of LPS, and IL-8 secretion in cells treated with combination of 10 μg of LTA per ml with various concentrations of LPS was equivalent to that induced by 10 μg of LTA per ml alone.
FIG. 4.
Synergistic effect of MDP with LPS or LTA on induction of IL-8 secretion in THP-1 cell cultures. OCT-differentiated THP-1 cells were stimulated with MDP plus S. abortus-equi LPS (A), MDP plus S. aureus LTA (B), and LPS plus LTA (C) at the indicated concentrations in triplicate. IL-8 levels in the culture supernatants were determined by ELISA, and the mean values are shown. ∗∗, ##, and ++, P < 0.01 versus control; ∗, #, and +, P < 0.05 versus control. Significant (P < 0.01) synergistic effects were detected in panel A (P < 0.01) and B (P < 0.01), but not in panel C, by ANOVA including an interaction term. The results are representative of two different experiments.
Synergistic effect of MDP and LPS on IL-8 secretion in U937 cell cultures.
The combinatory effect of MDP and LPS was also examined in OCT-treated U937 cells, another human monocytic cell line that probably lacks TLR2 on the cell surface (6). Results similar to those for THP-1 cells were observed in U937 cell cultures (Fig. 5), although the response of U937 cells to the combination of stimuli was weaker than that of THP-1 cells.
FIG. 5.
Synergistic effect of MDP with LPS on induction of IL-8 secretion in U937 cell cultures. OCT-differentiated U937 cells were stimulated with MDP plus S. abortus-equi LPS at the indicated concentrations in triplicate. The IL-8 levels in the culture supernatants were determined by ELISA, and the mean values are shown. ∗∗ and ##, P < 0.01 versus control; #, P < 0.05 versus control. A significant (P < 0.01) synergistic effect of MDP and LPS on IL-8 production was detected by ANOVA including an interaction term. The results are representative of two different experiments.
Effects of anti-CD14 MAb on IL-8 secretion by THP-1 cells in response to bacterial cell surface components.
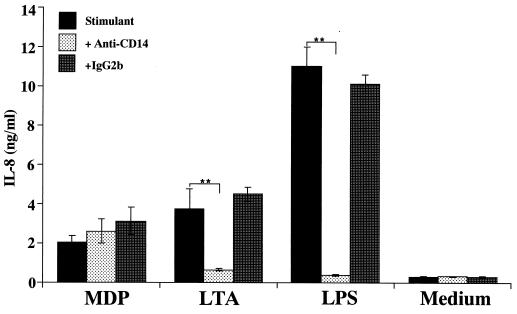
To determine whether the test stimulant activated THP-1 cells in an mCD14-dependent manner, OCT-treated THP-1 cells were preincubated with anti-CD14 MAb MY4 (10 μg/ml) or isotype-matched control antibody (IgG2b) for 30 min and then stimulated with MDP (10 μg/ml), LTA (1 μg/ml), or LPS (10 ng/ml) for 24 h. Pretreatment of THP-1 cells with the anti-CD14 MAb inhibited LPS- and LTA-induced IL-8 release to the control (medium alone) and to almost control levels, respectively (Fig. 6). In contrast, the MDP-inducing IL-8 secretion was not reduced at all. These findings suggested that MDP activates THP-1 cells in a CD14-independent manner, whereas LTA as well as LPS activate the cells in a CD14-dependent manner.
FIG. 6.
Effects of anti-CD14 MAb on IL-8 secretion by THP-1 cells in response to bacterial cell surface components. OCT-differentiated THP-1 cells were preincubated with anti-CD14 MAb MY4 (10 μg/ml) or isotype-matched control antibody for 30 min and then stimulated with MDP (10 μg/ml), S. aureus LTA (1 μg/ml), or S. abortus-equi LPS (10 ng/ml) for 24 h in triplicate. IL-8 levels in the culture supernatants were determined by ELISA and expressed as means ± standard deviation. ∗∗, P < 0.01 versus control. The results are representative of two different experiments.
Influence of LA-14-PP on IL-8 secretion by THP-1 cells in response to MDP, LTA, and LPS.
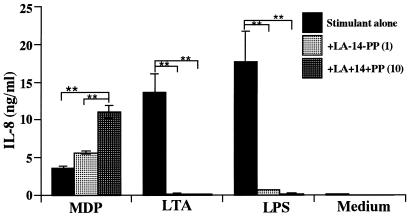
LA-14-PP is an endotoxin antagonist in human cells (reviewed in reference 20). Therefore, we examined the possible inhibitory effect of LA-14-PP on IL-8 secretion by THP-1 cells in response to MDP, LTA, and LPS. IL-8 secretion induced by LTA (1 μg/ml) and LPS (10 ng/ml) was markedly inhibited in the presence of LA-14-PP in a concentration-dependent manner (Fig. 7). LA-14-PP at 10 μg/ml completely inhibited IL-8 secretion to the control (medium alone) level. In contrast, IL-8 secretion by the THP-1 cells upon stimulation with MDP (10 μg/ml) was not inhibited by LA-14-PP. Conversely, LA-14-PP significantly increased IL-8 secretion induced by MDP in a concentration-dependent manner (1.5-fold at 1 μg and 3-fold at 10 μg respectively, of LA-14-PP, per ml), although LA-14-PP by itself did not induce IL-8 production in THP-1 cell cultures.
FIG. 7.
Influence of LA-14-PP on IL-8 secretion by THP-1 cells in response to MDP, LTA, and LPS. OCT-differentiated THP-1 cells were stimulated with MDP (10 μg/ml), S. aureus LTA (1 μg/ml), or S. abortus-equi LPS (10 ng/ml) in the presence or absence of LA-14-PP (1 or 10 μg/ml) for 24 h in triplicate. IL-8 levels in the culture supernatants were determined by ELISA and expressed as means ± standard deviation. ∗∗, P < 0.01 versus control. The results are representative of two different experiments.
Effects of anti-TLR4 MAb on IL-8 secretion by THP-1 cells in response to MDP, LTA, and LPS.
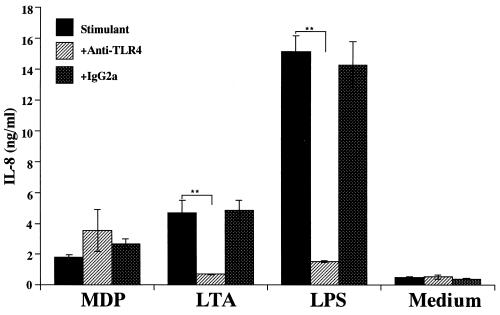
Both TLR4 and CD14 have been suggested to be involved in the antagonistic effect of LA-14-PP on LPS activity (15). Therefore, we examined the possible involvement of TLR4 in IL-8 secretion by THP-1 cells in response to LTA as well as LPS. OCT-treated THP-1 cells were preincubated with anti-TLR4 MAb HTA125 (5 μg/ml) or isotype-matched control antibody IgG2a for 30 min and then stimulated with MDP (10 μg/ml), LTA (1 μg/ml), or LPS (10 ng/ml). The IL-8 release induced by LTA as well as that induced by LPS was almost completely blocked by MAb HTA125 (Fig. 8). However, MDP-induced IL-8 release was not suppressed but rather enhanced by the MAb. These results suggested that MDP activates THP-1 cells in a TLR4- and CD14-independent manner, through a pathway different from those of LPS and LTA.
FIG. 8.
Effects of anti-TLR4 MAb on IL-8 secretion by THP-1 cells in response to bacterial cell surface components. OCT-differentiated THP-1 cells were preincubated with anti-TLR4 MAb HTA125 (5 μg/ml) or isotype-matched antibody for 30 min and then stimulated with MDP (10 μg/ml), S. aureus LTA (1 μg/ml), or S. abortus-equi LPS (10 ng/ml) for 24 h in triplicate. IL-8 levels in the culture supernatants were determined by ELISA and expressed as means ± standard deviation. ∗∗, P < 0.01 versus control. The results are representative of two different experiments.
Priming effect of MDP on IL-8 secretion by THP-1 cells on stimulation with LPS.
To examine the mechanism of the synergistic effect of MDP and LPS, we examined whether MDP exerts a priming effect on THP-1 cells. OCT-treated THP-1 cells were preincubated with MDP (10 μg/ml) for 4 h and then washed in PBS three times. The cells were further stimulated with serial concentrations of LPS for 24 h. Pretreatment of the cells with MDP markedly augmented LPS-induced IL-8 secretion, although the IL-8 levels were lower than those induced by LPS and MDP simultaneously (Fig. 9A). In contrast, pretreatment of the cells with LPS only slightly augmented MDP-induced IL-8 secretion (Fig. 9B). The results suggest that MDP primed THP-1 cells to respond to LPS at a higher sensitivity.
FIG. 9.
Priming effect of MDP or LPS on IL-8 secretion by OCT-treated THP-1 cells upon stimulation with LPS or MDP, respectively. OCT-differentiated THP-1 cells were preincubated with MDP (10 μg/ml) (A) or S. abortus-equi LPS (10 ng/ml) (B) for 4 h, washed three times, and then stimulated with S. abortus-equi LPS (A) or MDP (B), respectively, at the indicated concentrations for 24 h. As reference experiments, OCT-differentiated THP-1 cells without pretreatment were stimulated with MDP (10 μg/ml) plus LPS, LPS alone (A), or MDP alone (B) at the indicated concentrations for 24 h. IL-8 levels in the culture supernatants were determined by ELISA and expressed as the mean ± standard deviation of triplicate assays. ∗∗, P < 0.01 versus control, LPS or medium alone (A) and MDP or medium alone (B). The results are representative of two different experiments.
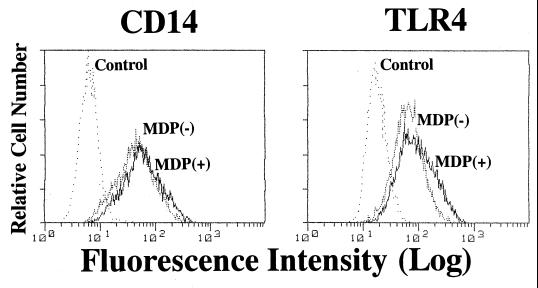
Expression of mCD14 and TLR4 in THP-1 cells treated with MDP.
To elucidate the mechanism of the synergistic and priming effects of MDP for stimulation with LPS or LTA on THP-1 cells, we first examined the effect of MDP treatment of THP-1 cells on the expression of mCD14 and TLR4 by the cells. OCT-differentiated THP-1 cells were treated with MDP (10 μg/ml) for 4 to 24 h. Thereafter, the cells were collected and stained with anti-CD14 MAb or anti-TLR4 MAb, and then CD14 and TLR4 expression was analyzed by flow cytometry. MDP treatment scarcely increased mCD14; only a slight increase in mCD14 expression was noted 12 h after treatment (Fig. 10). MDP treatment exerted only a slight effect on TLR4 expression at 24 h (Fig. 10). These results suggested that the degree of up-regulation noted could not account for the degree of synergistic action.
FIG. 10.
Expression of mCD14 and TLR4 in THP-1 cells treated with MDP. OCT-differentiated THP-1 cells were incubated with MDP (10 μg/ml) for 4 to 24 h. The cells were collected and stained with anti-CD14 MAb MY4 and anti-TLR4 MAb HTA125, and then mCD14 and TLR4 expression was analyzed by FACS. Representative results of mCD14 expression at 12 h and TLR4 expression at 24 h are shown. The results are representative of two different experiments.
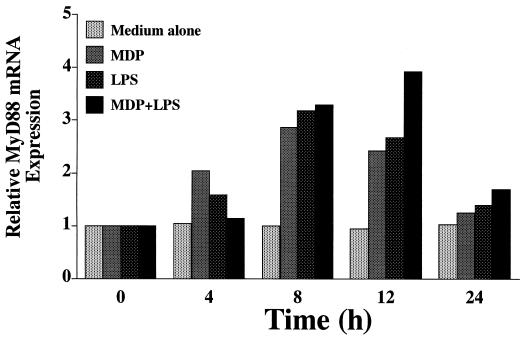
MyD88 mRNA expression induced by MDP and LPS in THP-1 cells.
MyD88 is an adapter molecule essential for signaling of bacterial cell surface components via the TLR family (46). We next examined whether the stimulation of monocytic cells by cell surface components up-regulates MyD88 mRNA expression in the cells. OCT-differentiated THP-1 cells were treated with MDP (10 μg/ml), S. abortus-equi LPS (10 ng/ml), and MDP plus LPS for 4 to 24 h, and then Northern blot analysis was carried out to detect MyD88 mRNA expression. As shown in Fig. 11, MDP and LPS definitely enhanced MyD88 mRNA expression in a time-dependent manner where peak expression was noted at 8 h. The effect of MDP was comparable to that of LPS in this respect. We observed no synergistic up-regulation of MyD88 mRNA in cells stimulated with MDP plus LPS. S. aureus LTA (10 μg/ml) also up-regulated MyD88 mRNA expression in OCT-treated THP-1 cells, although the activity was less than that of either MDP or LPS (data not shown).
FIG. 11.
MDP and LPS up-regulated MyD88 mRNA expression in THP-1 cells. OCT-differentiated THP-1 cells were treated with MDP (10 μg/ml), S. abortus-equi LPS (10 ng/ml), and MDP plus LPS for 2 to 24 h. Total cellular RNA (20 μg/lane) extracted from the cells was electrophoresed, transferred to a nylon membrane, and then analyzed by Northern blotting using a 32P-labeled cDNA probe for MyD88. Relative induction of MyD88 mRNA expression were determined with a BAS 1500 Mac image analyzer (Fuji Photo Film) after normalization using GAPDH. The results are representative of two different experiments.
DISCUSSION
As described above, the usage of TLRs by individual bacterial components is still controversial. TLR4 has been clearly demonstrated to be essential for LPS signaling in murine cells, because cells from C3H/HeJ and C57BL/10ScCr mice that carry a point mutation and null deletion in the TLR4 gene, respectively (27, 28, 50), and TLR4 gene knockout mice show no or a reduced response to LPS (12). In contrast, some early studies suggested that TLR2 is mainly associated with cellular responses to LPS in human cells (14, 53). Furthermore, TLR4-deficient mice still responded to some LPS preparations (12, 44). Recently, Hirschfeld et al. (11) demonstrated that repurification of LPS to remove contaminating endotoxin protein eliminated signaling through TLR2 in both human and murine cells. In this study, we also demonstrated that LPS activated human monocytic cells specifically via TLR4, because anti-TLR4 MAb almost completely blocked the activity of ultrapurified LPS from S. abortus-equi (Fig. 8).
Takeuchi et al. (44) demonstrated the essential role of TLR4 in LTA signaling using TLR4 gene knockout mice and purified LTA from S. aureus. In contrast, Schwandner et al. (31) reported that TLR2 was a signal transducer for LTA as well as LPS in a gene-transfected human cell system, although in their report the bacterial source of LTA was not specified. The bioactive structure of LTA has not yet been determined (35, 39), and the bioactivities of LTA differ between bacterial species (36, 37). Regarding the S. aureus LTA fraction (Sigma), Kusunoki et al. (18) separated purified LTA and non-LTA fractions and showed the inactivity of the former and the activity of the latter in human astrocytoma U373 cells in culture. In contrast, Kengatharan et al. (13) reported the reverse results: the LTA fraction was active, but the non-LTA fraction was inactive in murine macrophage J774.2 cells in culture. In this study, the net LTA fraction of S. aureus activated human monocytic cells specifically via TLR4, because anti-TLR4 MAb completely blocked the activity of the LTA fraction (Fig. 8). LA-14-PP, which exerted antagonistic effects in association with TLR4 as well as mCD14 (15), completely blocked the activities of LTA as well as LPS (Fig. 7). Anti-CD14 MAb also completely blocked activities of LTA as well as LPS (Fig. 6). These finiding suggested that LTA as well as LPS activated human monocytic cells in a CD14- and TLR4-dependent manner.
The involvement of TLR2 in peptidoglycan signaling has been reported (31, 44, 54). MDP is the minimal structure essential for bioactivities of peptidoglycan (reviewed in references 42 and 43). However, MDP signaling in relation to CD14 and TLRs has not been elucidated. In this study, the activity of MDP on human monocytic cells was not inhibited by anti-CD14 MAb, anti-TLR4 MAb, or LA-14-PP (Fig. 6 to 8). Furthermore, MDP also activated OCT-differentiated U937 cells (Fig. 3), which were probably devoid of TLR2 on their cell surface. These findings suggested that MDP activated human monocytic cells in a CD14-, TLR4-, and TLR2-independent manner. Moreover, we found unique modification of MDP activity by the anti-CD14 MAb, anti-TLR4 MAb, and LA-14-PP: these three possible inhibitors, especially LA-14-PP, increased MDP activity. In some experiments, MDP exerted powerful activities different from those of peptidoglycan (25, 40). The interaction of MDP with peripheral serotonin receptors including those on macrophages has been suggested by some investigators (32, 34). Further studies are required to elucidate the recognition and signaling mechanism of MDP by host cells.
Synergistic effects of MDP with endotoxin or priming effects of MDP to enhance the activities of endotoxin have been studied mainly in murine in vivo systems, and MDP alone was inactive in the experiments (5, 26, 38). Nagao et al. (23, 24) reported species dependency of in vitro macrophage activation by peptidoglycans and MDP; guinea pig and rat cells were responsive, but murine cells were not. In human peripheral blood mononuclear cell cultures, MDP is a powerful cytokine inducer comparable to LPS. Therefore, synergistic effects were observed only at relatively low concentrations of MDP (48). Recently, Flak et al. (9) reported synergistic effects of endotoxin and a peptidoglycan fragment from Bordetella pertussis, N-acetylglucosaminyl-1,6-anhydro-N-acetylmuramyl-l-alanyl-γ-d-glutamyl-meso-diaminopimelyl-d-alanine, on hamster trachea epithelial cells, induction of IL-1α, and NO production. The fragment alone had some effect on the cells, but MDP was completely inactive (19). Therefore, this study is the first report of the synergistic and priming effects of MDP for LPS activity in vitro. We also found clear synergistic effects of MDP with a LTA specimen in this study. Kengatharan et al. (13) reported that MDP and S. aureus LTA exerted synergistic effects inducing NO formation in the murine macrophage cell line J774.2, although the effect of MDP was considerably weaker than that of the whole peptidoglycan from S. aureus and N-acetylglucosamine-β-[1-4]-N-acetylmuramyl-l-alanyl-d-isoglutamine. As mentioned above, in the murine in vivo system the priming effect of MDP augments the activities of not only endotoxin but also of other bacterial components including LTA (39).
In this study, we observed a priming effect of MDP on IL-8 secretion by THP-1 cells upon stimulation with LPS (Fig. 9A). This model in human monocytic cells might be useful to elucidate the activity of MDP, especially the priming effect of MDP. The marked up-regulation of MyD88 by MDP (Fig. 11) might be partially responsible for the synergistic or priming effects of MDP with LPS or LTA. Other factors, however, might be also involved in the priming effect of MDP, because LPS also up-regulated MyD88 mRNA in the cells (Fig. 11) but did not exhibit priming effects for the following MDP stimulation (Fig. 9B). Anyway, our findings suggested possible synergistic effects of natural peptidoglycan fragments, which are ubiquitous in bacterial cell walls, with both gram-positive and gram-negative bacterial cell surface components, i.e., LPS and LTA. If this is the case, bacterial cell surface components in various combinations might exert more powerful activities on host cells than the expected levels based on the activities of individual components. In fact, bacterial cell envelopes sometimes show activities stronger than those of any individual components.
ACKNOWLEDGMENTS
We thank K. Miyake (Saga Medical School, Saga, Japan) for generously supplying anti-TLR4 MAb HTA125. We also thank D. Mrozek (Medical English Service, Kyoto, Japan) for reviewing the paper.
This work was supported in part by Grants-in-Aid for Scientific Research (no. 10470378 and 12470380) from the Ministry of Education, Culture, Sports, Science, and Culture of Japan.
REFERENCES
- 1.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 2.Aderem A, Ulevitch R J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 3.Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cell surface expression and lipopolysaccharide signaling via the Toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 4.Arbour N C, Lorenz E, Schutte B C, Zabner J, Kline J N, Jones M, Frees K, Watt J L, Schwartz D A. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 5.Bloksma N, Hofhuis F M A, Willers J M N. Endotoxin-induced antitumor activity in the mouse is highly potentiated by muramyl dipeptide. Cancer Lett. 1984;23:159–165. doi: 10.1016/0304-3835(84)90149-6. [DOI] [PubMed] [Google Scholar]
- 6.Cario E, Rosenberg I M, Brandwein S L, Beck P L, Reinecker H-C, Podolsky D K. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 7.Chedid L A, Parant M A, Audibert F M, Riveau G J, Parant F J, Lederer E, Choay J P, Lefrancier P L. Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect Immun. 1982;35:417–424. doi: 10.1128/iai.35.2.417-424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer W. Bacterial phosphoglycolipids and lipoteichoic acids. Handb Lipid Res. 1990;6:123–234. [Google Scholar]
- 9.Flak T A, Heiss L A, Engle J T, Goldman W E. Synergistic epithelial responses to endotoxin and a naturally occurring muramyl peptide. Infect Immun. 2000;68:1235–1242. doi: 10.1128/iai.68.3.1235-1242.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galanos C, Lüderitz O, Westphal O. Preparation and properties of a standardized lipopolysaccharide from Salmonella abortus equi (Novo-Pyrexal) Zentbl Bakteriol Hyg I Abt Orig Reihe A. 1979;243:226–244. [PubMed] [Google Scholar]
- 11.Hirschfeld M, Ma Y, Weis J H, Vogel S N, Weis J J. Repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 13.Kengatharan K M, De Kimpe S, Robson C, Foster S J, Thiemermann C. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J Exp Med. 1998;188:305–315. doi: 10.1084/jem.188.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirschning C J, Wesche H, Ayres T M, Rothe M. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitchens R L, Munford R S. Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J Biol Chem. 1995;270:9904–9910. doi: 10.1074/jbc.270.17.9904. [DOI] [PubMed] [Google Scholar]
- 16.Kopp E B, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 17.Kubodera N, Sato K, Nishii Y. Characteristics of 22-oxacalcitriol (OCT) and 2β-(3-hydroxypropoxy)-calcitriol (ED-71) In: Feldman D, Glorieux F H, Pike J W, editors. Vitamin D. San Diego, Calif: Academic Press; 1997. pp. 1071–1086. [Google Scholar]
- 18.Kusunoki T, Hailman E, Juan T S-C, Lichenstein H S, Wright S D. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J Exp Med. 1995;182:1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luker K E, Collier J L, Kolodziej E W, Marshall G R, Goldman W E. Bordetella pertussis tracheal cytotoxin and other muramyl peptides: distinct structure-activity relationships for respiratory epithelial cytopathology. Proc Natl Acad Sci USA. 1993;90:2365–2369. doi: 10.1073/pnas.90.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynn W A, Golenbock D T. Lipopolysaccharide antagonists. Immunol Today. 1992;13:271–276. doi: 10.1016/0167-5699(92)90009-V. [DOI] [PubMed] [Google Scholar]
- 21.Means T K, Golenbock D T, Fenton M J. The biology of Toll-like receptors. Cytokine Growth Factor Rev. 2000;11:219–232. doi: 10.1016/s1359-6101(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 22.Monodane T, Kawabata Y, Takada H. Micrococcus luteus cells and cell walls induce anaphylactoid reactions accompanied by early death and serum cytokines in mice primed with muramyl dipeptide. FEMS Immunol Med Microbiol. 1997;17:49–55. doi: 10.1111/j.1574-695X.1997.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 23.Nagao S, Akagawa K S, Okada F, Harada Y, Yagawa K, Kato K, Tanigawa Y. Species dependency of in vitro macrophage activation by bacterial peptidoglycans. Microbiol Immunol. 1992;36:1155–1171. doi: 10.1111/j.1348-0421.1992.tb02119.x. [DOI] [PubMed] [Google Scholar]
- 24.Nagao S, Akagawa K S, Yamada K, Yagawa K, Tokunaga T, Kotani S. Lack of response of murine peritoneal macrophages to in vitro activation by muramyl dipeptide (MDP). I. Macrophage activation by MDP is species dependent. Microbiol Immunol. 1990;34:323–335. doi: 10.1111/j.1348-0421.1990.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 25.Nagao S, Takada H, Yagawa K, Kutsukake H, Shiba T, Kusumoto S, Kawata S, Hasegawa A, Kiso M, Azuma I, Tanaka A, Kotani S. Structural requirements of muramylpeptides for induction of necrosis at sites primed with Mycobacterium tuberculosis in guinea pigs. Infect Immun. 1987;55:1279–1288. doi: 10.1128/iai.55.5.1279-1288.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parant M, Parant F, Vinit M-A, Jupin C, Noso Y, Chedid L. Priming effect of muramyl peptides for induction by lipopolysaccharide of tumor necrosis factor production in mice. J Leukoc Biol. 1990;47:164–169. doi: 10.1002/jlb.47.2.164. [DOI] [PubMed] [Google Scholar]
- 27.Poltorak A, He X, Smirnova I, Liu M-Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 28.Qureshi S T, Larivière L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribi E E, Cantrell J L, Von Eschen K B, Schwartzman S M. Enhancement of endotoxic shock by N-acetylmuramyl-l-alanyl-(l-seryl)-d-isoglutamine (muramyl dipeptide) Cancer Res. 1979;39:4756–4759. [PubMed] [Google Scholar]
- 30.Rietschel E T, Brade H. Bacterial endotoxins. Sci Am. 1992;267:54–61. doi: 10.1038/scientificamerican0892-54. [DOI] [PubMed] [Google Scholar]
- 31.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 32.Ševčík J, Mašek K. The interaction of immunomodulator muramyl dipeptide with peripheral 5-HT receptors: overview of the current state. Int J Immunopharmacol. 1999;21:227–232. doi: 10.1016/s0192-0561(98)00079-4. [DOI] [PubMed] [Google Scholar]
- 33.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverman D H S, Wu H, Karnovsky M L. Muramyl peptides and serotonin intereract at specific binding sites on macrophages and enhance superoxide release. Biochem Biophys Res Commun. 1985;131:1160–1167. doi: 10.1016/0006-291x(85)90212-8. [DOI] [PubMed] [Google Scholar]
- 35.Suda Y, Tochio H, Kawano K, Takada H, Yoshida T, Kotani S, Kusumoto S. Cytokine-inducing glycolipids in the lipoteichoic acid fraction from Enterococcus hirae ATCC 9790. FEMS Immunol Med Microbiol. 1995;12:97–112. doi: 10.1111/j.1574-695X.1995.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 36.Sugawara S, Arakaki R, Rikiishi H, Takada H. Lipoteichoic acids as an antagonist and an agonist of lipopolysaccharide on human gingival fibroblasts and monocytes in a CD14-dependent manner. Infect Immun. 1999;67:1623–1632. doi: 10.1128/iai.67.4.1623-1632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugiyama A, Arakaki R, Ohnishi T, Arakaki N, Daikuhara Y, Takada H. Lipoteichoic acid and interleukin 1 stimulate synergistically production of hepatocyte growth factor (scatter factor) in human gingival fibroblasts in culture. Infect Immun. 1996;64:1426–1431. doi: 10.1128/iai.64.4.1426-1431.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takada H, Galanos C. Enhancement of endotoxin lethality and generation of anaphylactoid reactions by lipopolysaccharides in muramyl-dipeptide-treated mice. Infect Immun. 1987;55:409–413. doi: 10.1128/iai.55.2.409-413.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takada H, Kawabata Y, Arakaki R, Kusumoto S, Fukase K, Suda Y, Yoshimura T, Kokeguchi S, Kato K, Komuro T, Tanaka N, Saito M, Yoshida T, Sato M, Kotani S. Molecular and structural requirements of lipoteichoic acid from Enterococcus hirae ATCC 9790 for cytokine-inducing, antitumor, and antigenic activties. Infect Immun. 1995;63:57–65. doi: 10.1128/iai.63.1.57-65.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takada H, Kawabata Y, Kawata S, Kusumoto S. Structural characteristics of peptidoglycan fragments required to prime mice for induction of anaphylactoid reactions by lipopolysaccharides. Infect Immun. 1996;64:657–659. doi: 10.1128/iai.64.2.657-659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takada H, Kawabata Y, Tamura M, Matsushita K, Igarashi H, Ohkuni H, Todome Y, Uchiyama T, Kotani S. Cytokine induction by extracellular products of oral viridans group streptococci. Infect Immun. 1993;61:5252–5260. doi: 10.1128/iai.61.12.5252-5260.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takada H, Kotani S. Immunopharmacological activities of synthetic muramyl-peptides. In: Stewart-Tull D E S, Davies M, editors. Immunology of the bacterial cell envelope. Chichester, United Kingdom: John Wiley & Sons; 1985. pp. 119–152. [Google Scholar]
- 43.Takada H, Kotani S. Muramyl dipeptide and derivatives. In: Stewart-Tull D E S, editor. The theory and practical application of adjuvants. Chichester, United Kingdom: John Wiley & Sons; 1995. pp. 171–202. [Google Scholar]
- 44.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:431–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Mühlradt P F, Akira S. Preferentially the S-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi O, Takeda K, Hoshino K, Adachi O, Ogawa T, Akira S. Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int Immunol. 2000;12:113–117. doi: 10.1093/intimm/12.1.113. [DOI] [PubMed] [Google Scholar]
- 47.Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, Sakiyama S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987;47:5616–5619. [PubMed] [Google Scholar]
- 48.Tsuchida K, Takemoto Y, Yamagami S, Edney H, Niwa M, Tsuchiya M, Kishimoto T, Shaldon S. Detection of peptidoglycan and endotoxin in dialysate, using silkworm larvae plasma and limulus amebocyte lysate methods. Nephron. 1997;75:438–443. doi: 10.1159/000189582. [DOI] [PubMed] [Google Scholar]
- 49.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 50.Vogel S N, Johnson D, Perera P-Y, Medvedev A, Larivière L, Qureshi S T, Malo D. Functional characterization of the effect of the C3H/HeJ defect in mice that lack an LPSn gene: in vivo evidence for a dominant negative mutation. J Immunol. 1999;162:5666–5670. [PubMed] [Google Scholar]
- 51.Weidemann B, Schletter J, Dziarski R, Kusumoto S, Stelter F, Rietschel E T, Flad H-D, Ulmer A J. Specific binding of soluble peptidoglycan and muramyldipeptide to CD14 on human monocytes. Infect Immun. 1997;65:858–864. doi: 10.1128/iai.65.3.858-864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright S D. Toll, a new piece in the puzzle of innate immunity. J Exp Med. 1999;189:605–609. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang R-B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura A, Lien E, Ingalls R R, Tuomanen E, Dziarski R, Golenbock D. Recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]