Abstract
Purpose
Dorsal wrist ganglions are treated commonly with aspiration, or open or arthroscopic excision in operating room (OR) or procedure room (PR) settings. As it remains unclear which treatment strategy is most cost-effective in yielding cyst resolution, our purpose was to perform a formal cost-minimization analysis from the societal perspective in this context.
Methods
A microsimulation decision analytic model evaluating 5 treatment strategies for dorsal wrist ganglions was developed, ending in either resolution or a single failed open revision surgical excision. Strategies included immediate open excision in the OR, immediate open excision in the PR, immediate arthroscopic excision in the OR, or 1 or 2 aspirations before each of the surgical options. Recurrence and complications rates were pooled from the literature for each treatment type. One-way sensitivity and threshold analyses were performed.
Results
The most cost-minimal strategy was 2 aspiration attempts before open surgical excision in the PR setting ($1,603 ± 1,595 per resolved case), followed by 2 aspirations before open excision in the OR ($1,969 ± 2,165 per resolved case). Immediate arthroscopic excision was the costliest strategy ($6,539 ± 264 per resolved case). Single aspiration preoperatively was more cost-minimal than any form of immediate surgery ($2,918 ± 306 and $4,188 ± 306 per resolved case performed in the PR and OR, respectively).
Conclusions
From the societal perspective, performing 2 aspirations before surgical excision in the PR setting was the most cost-minimal treatment strategy, although in reference to surgeons who do not perform this procedure in the PR setting, open excision in the OR was nearly as cost-effective. As patient preferences may preclude routinely performing 2 aspirations, performing at least 1 aspiration before surgical excision improves the cost-effectiveness of dorsal wrist ganglions treatment.
Keywords: Cost-minimization analysis, dorsal wrist ganglion, ganglion cyst, ganglion cyst excision
Dorsal Wrist Ganglions (DWG) are common, and account for approximately 60%–80% of wrist ganglions.1–6 Though first line treatment generally is reassurance and observation, patients may elect for intervention. The most common treatments include aspiration, open excision, and arthroscopic excision. Despite the abundance of literature describing recurrence rates following these treatments, it is unclear which treatment strategy is most cost-effective.
Recently there has been an increased emphasis on healthcare cost, which is in part because of the policy changes that have included value-based payment models.7,8 In 2016, the United States spent nearly 18% of the gross domestic product on healthcare, with nearly double the mean spending per capita of the 10 other high-income countries studied.9 Physician practice variability in the setting of similar outcomes has been cited as a potential driver for increasing healthcare costs.10 When trying to determine the optimal treatment pathway for a given condition, it must be considered that different treatment options often are are associated with different index costs, complication and reoperation rates, duration of time off from work, and outcomes. A cost-minimization analysis framework is ideal for combining the effect of these parameters to determine the most cost-minimal treatment strategy. Cost-minimization analysis was chosen because health states or utilities, which are necessary for a cost-effectiveness analysis, are not reported commonly for DWG excision results in the literature. Cost-minimization analysis methods also are appropriate for DWG treatment, where the treatment goal is resolution, and the outcome is binary (resolution versus lack of resolution). Cost-minimization analysis methodology would apply to DWG treatment because, although arthroscopic DWG excision is considerably more expensive than open excision in the operating room (OR), this potentially could be offset by a lower recurrence rate.11 Similarly, aspiration is inexpensive relative to surgery and does not require time off work, but recurrence is more likely. Moreover, minor hand surgery procedures such as DWG excision, could be transitioned out of the operating room setting and into the procedure room (PR) setting using the wide-awake local anesthesia with no tourniquet technique.12–14 For a variety of small hand surgical procedures, use of the PR has been shown to reduce surgical costs through omission of an anesthesia team, by reducing facility charges, and through reduction in routine preoperative lab testing and facility costs.14,15 Though performing DWG treatments in the PR is likely to be more cost-effective than in the OR, we do not know how the cost-effectiveness would compare while factoring in 1 or 2 aspirations before surgical treatment.
Given the challenges and limitations in interpreting recurrence rates, complication rates, surgical or procedural costs, and time off work in isolation, the purpose of this study was to perform a cost-minimization analysis from a societal perspective that elucidates the least costly of 5 treatment strategies aimed at DWG resolution.
METHODS
Economic simulation
This cost-minimization analysis was performed in accord with the recommendations of the Second Panel on Cost-Effectiveness in Health and Medicine.16 A microsimulation decision analytic model comparing 5 different treatment strategies was developed, from the societal perspective, for patients undergoing treatment for DWGs. The effectiveness measure was resolution of the DWG. Model parameter inputs were obtained from the University of Utah Value-Driven Outcomes database, published literature, or from expert opinion (consensus of 4 fellowship-trained hand surgeons at our institution).7 The model was programmed in TreeAge Pro 2020 (TreeAge Software).
Model structure
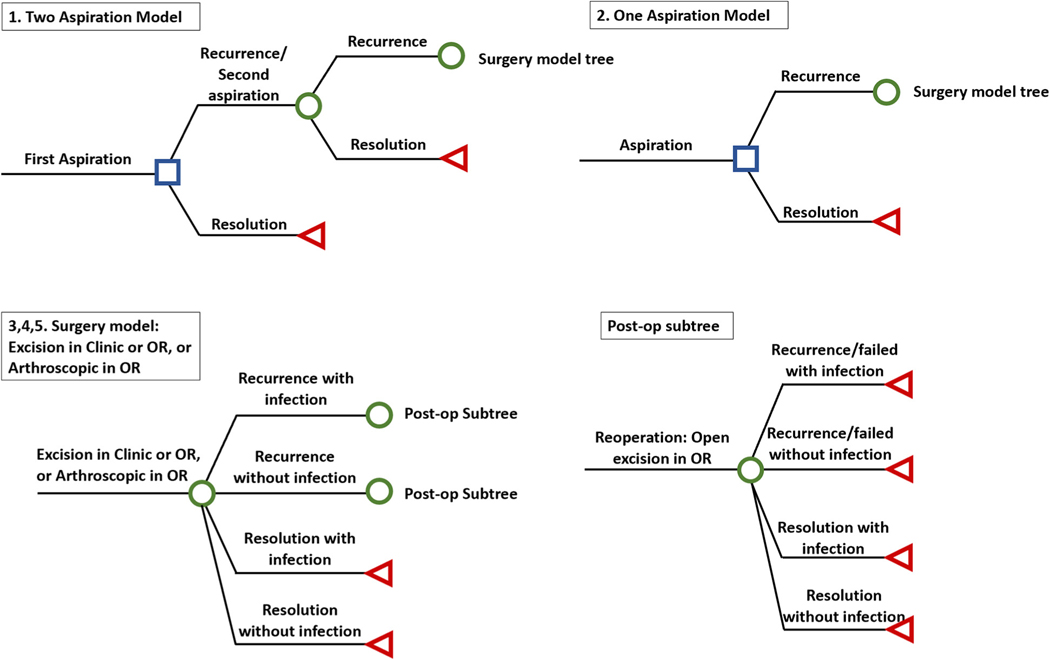
Figure 1 depicts the structure of the decision analytic model. Treatment strategies included open excision in the PR setting, open excision in the OR, arthroscopic excision in the OR, and each of these surgical options following either 1 or 2 attempts at aspiration. Patients enter the model having 1 of 5 treatments for DWGs. Each aspiration can succeed or fail to resolve the DWG, which leads the patient to have another aspiration, open excision in the OR/PR, or arthroscopic excision, depending on the treatment strategy. Each surgery also can succeed or fail to resolve the DWG, with or without infection. If there is failure after any surgical excision, revision reoperation is an open excision in the OR. Revision reoperation is offered only once and can succeed or fail to resolve the DWG, with or without infection. The model ends with resolution of the DWG, or after a single revision reoperation regardless of success or failure. We assumed that no patient experienced continued infection after irrigation and debridement. Further details of the model and its rationale are found in Appendix A (available online on the Journal’s website at www.jhandsurg.org).
FIGURE 1:
Decision analytic model of DWGs.
Input parameters: probabilities
The movement of hypothetical patients through the model was governed by probability input parameters. The model uses weighted averages based on the number of cohorts in each study for recurrence rate and complication rate data derived from available literature (Table 1).17–46 A full description of the literature inclusion criteria is found in Appendix B (available online on the Journal’s website at www.jhandsurg.org). Recurrence and infection rates of each procedure are summarized in Table 1. Weighted averages and standard deviations (SD) were calculated for the recurrence rates after procedure/surgery and complications resulting in revision surgery. Given a lack of available data, we assumed that the rate of recurrence after a revision procedure was the same as for the index excision procedure.
TABLE 1.
Recurrence and Complication Rates of Treatments
| Event | Value | SD | Source |
|---|---|---|---|
|
| |||
| Adverse Events Probabilities for recurrence | |||
| Aspiration | 53.9% | 2.1% | Literature17–19,28,29 |
| Open excision | 20.7% | 1.6% | Literature18,19,22,28,30–36 |
| Arthroscopic excision | 8.5% | 0.4% | Literature21,22,25–27,38—46 |
| Probabilities for infection/hematoma req. OR | |||
| Aspiration | None reported | - | Literature17–19,28,29 |
| Open excision (OR and PR) | 0.3% | - | Literature18,19,22,28,30–36 |
| Arthroscopic excision | 0.3% | - | Literature21,22,25–27,38–46 |
Input parameters: costs
Direct and indirect costs were included in the study to provide the cost-minimization result from a societal perspective. Direct costs related to ambulatory index surgical procedures and for surgical procedures related to complications were calculated as the sum of Medicare payments to the surgeon, anesthesia, and facility obtained through a query of respective current procedural terminology codes. Average 2016 Medicare standardized payments were used for surgeon costs, and ambulatory facility and anesthesia costs were obtained from the 2013 Florida State Ambulatory Surgery and Service Database as well as from the Value-Driven Outcomes tool at the University of Utah.47,48 Please see Appendix C (available online on the Journal’s website at www.jhandsurg.org) for further description of the Value-Driven Outcomes tool.7
Indirect costs were estimated using the lost income because of the time spent receiving medical care and the time out of work for recovery. We estimated a half day off work for aspiration and reaspiration, 7 days off of work for each excision, another half day off of work for an either preoperative or postoperative clinic visit, and 14 days off work for irrigation and debridement based on expert opinion of 4 fellowship trained hand surgeons at our institution. Days off work then were multiplied with average median earnings obtained from the Bureau of Labor Statistics of full-time wage and salary workers for age 35–44 years old.49 Total indirect costs for each treatment and infection were summarized in Table 2. All costs were adjusted to 2019 United States dollars using the personal consumption expenditures price index for healthcare services.50
TABLE 2.
Cost Input Data for the Markov Model
| Direct Cost | Days Off Work | Total Cost (2019 dollars) | |
|---|---|---|---|
|
|
|
|
|
| Normalized Mean | Mean | Mean | |
|
| |||
| Clinic visit/aspiration | 0.00 | 0.5 | $444 |
| Open excision: OR | 0.49 | 7.0 | $4,140 |
| Open excision: PR | 0.22 | 7.0 | $2,870 |
| Arthroscopic excision: OR | 1.00 | 7.0 | $6,513 |
| Postoperative | |||
| Infection | 0.25 | 14.0 | $4,585 |
| Recurrence/reoperation | 0.73 | 7.5 | $5,344 |
| Recurrence (after second surgery) | 0.09 | 0.5 | $847 |
| Median usual weekly earnings of full-time wage and salary workers: 35—44 years old (in 2019 dollars) | $1,066 | ||
Sensitivity analysis
Point estimates for each input parameter value were used in the base case analysis. To determine model robustness, we performed 1-way sensitivity analyses to measure and evaluate the uncertainly among key input parameters and those obtained via expert opinion. The results from 1-way sensitivity analyses assessed the impact of the change in a certain parameter on the results of the analysis. Key input parameters analyzed were aspiration failure rate, open excision recurrence rate, days out of work after procedures, total arthroscopic resection cost, total aspiration cost, and total costs for open excision in the OR and PR. We also performed 1-way sensitivity analyses on direct cost and indirect cost inputs separately and in a probability sensitivity analysis.
RESULTS
Literature reported recurrence rates
From the literature, the weighted average and standard deviation for recurrence is 53.9% ± 2.1% after aspiration, 20.7% ± 1.6% after open resection, and 9.5% ± 0.4% following arthroscopic resection (Table 1).17–46 The mean follow-up periods for the included studies were 32.7 months for arthroscopy, 37.1 months for open resection, and 19.6 months for aspiration.
Microsimulation results
Based on our microsimulation, we found that performing 2 attempts at aspiration before undergoing any given surgical intervention was the most cost-minimal strategy with surgical excision in the PR being the dominant strategy (Table 3). The combined direct and indirect costs from this strategy were $1,603 ± 1,595 per resolved case. The most expensive strategy was immediate arthroscopic excision in the OR, with an average projected cost of $6,539 ± 264 (Table 3). Notably, although performing 2 aspirations before surgical excision is less costly than 1 aspiration before surgical excision, performing 1 aspiration before considering surgery is less costly than performing surgery as the initial treatment.
TABLE 3.
Total Costs of Each Treatment Strategy
| Mean Cost (2019 dollars) | SD | |
|---|---|---|
|
| ||
| 1) Two aspirations followed by (when needed) | ||
| Open excision in PR | $1,603 | $1,595 |
| Open excision in OR | $1,969 | $2,165 |
| Arthroscopic excision in OR | $2,648 | $3,226 |
| 2) Single aspiration followed by (when needed) | ||
| Open excision in PR | $2,159 | $1,614 |
| Open excision in OR | $2,841 | $2,242 |
| Arthroscopic excision in OR | $4,102 | $3,409 |
| 3) Open excision in PR | $2,918 | $306 |
| 4) Open excision in OR | $4,188 | $306 |
| 5) Arthroscopic excision in OR | $6,539 | $264 |
One-way, threshold analyses, and probability sensitivity analysis
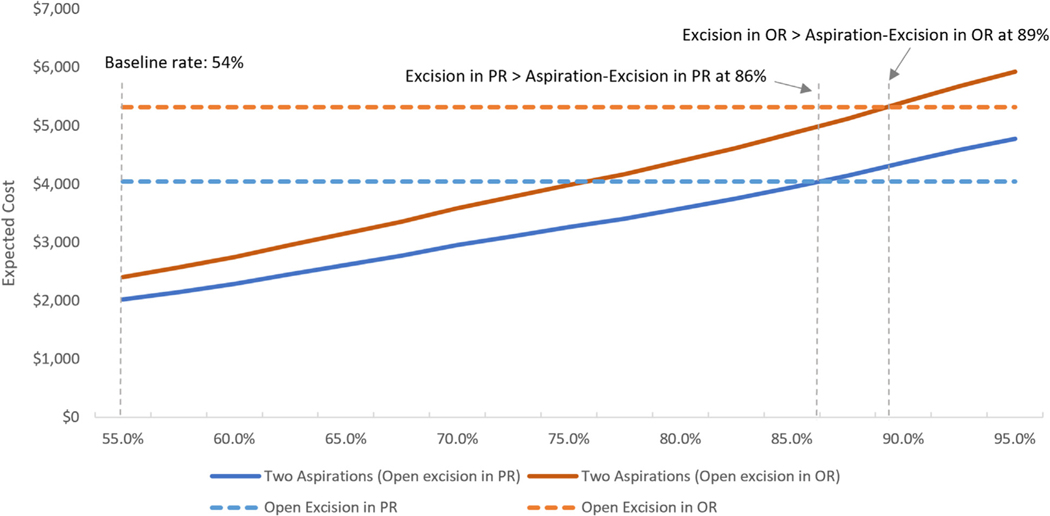
Our findings were robust in 1-way and threshold analyses. In the PR and OR, the failure rate of aspiration would need to increase from 54% to 86% or 89%, respectively, for it to be less expensive to proceed to surgical excision as the index procedure than 2 aspirations before a surgical excision (Fig. 2). Similarly, even in the setting of the open excision recurrence rate approaching 0% instead of 20.7%, 2 aspirations before surgical excision would still be the dominant strategy.
FIGURE 2:
Results from 1-way sensitivity analysis on the recurrence rate of aspiration.
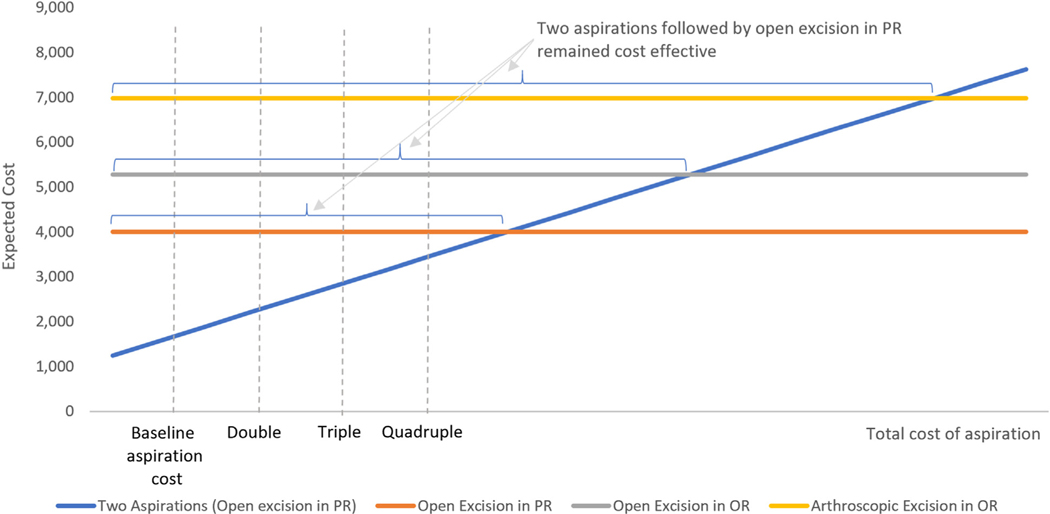
One-way sensitivity analysis shows 2 aspirations followed by open excision in the PR remained cost-effective unless the total cost of aspiration becomes twice, triple, or more than quadruple, compared with open excision in PR, open excision in OR, or arthroscopic excision in OR, respectively (Fig. 3). The results on the direct costs remained consistent.
FIGURE 3:
Results from 1-way sensitivity analysis on the total cost of aspiration.
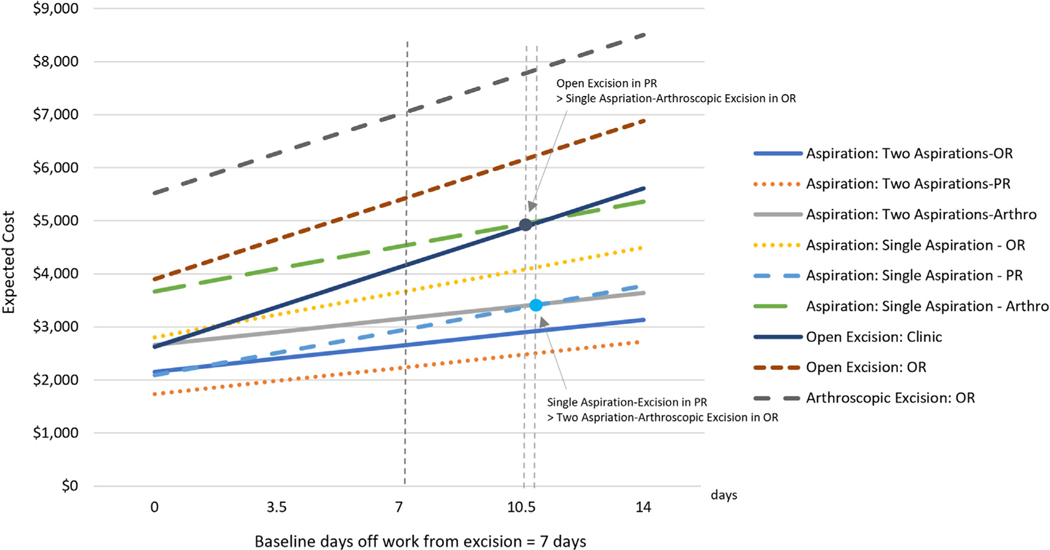
The cost of arthroscopic excision would have to be lowered by 61% and 34% to become equally cost-effective as primary open excision in the PR and OR, respectively. Results from 1-way sensitivity analyses on direct cost of arthroscopic resection were consistent with the results for total cost. One-way sensitivity analysis also showed that in the hypothetical scenario with 0 days off work following arthroscopic resection, it still would be the most expensive strategy ($5,021 ± 1,955). One-way sensitivity analysis for days out of work showed that the results are robust to this parameter. Specifically, the treatment strategy involving 2 aspirations before excision in the PR remained the most cost-effective, and the strategy involving initial treatment with arthroscopic excision remained the least cost-effective, regardless of days out of work (Fig. 4).
FIGURE 4:
Results from 1-way sensitivity analysis on the days off work from excision.
Finally, 2 aspirations followed by open excision in the PR remains cost-effective as compared with open excision in the OR regardless of the direct cost of open excision or unless the total cost drops to less than one-fifth (approximately $800) of our estimate. Two aspirations followed by open excision in the PR also remains cost-effective as compared with arthroscopic excision in the OR regardless of the direct cost of arthroscopic excision unless the total arthroscopic cost drops to less than one-fourth (approximately $1,500) of our estimate.
The results from the probability sensitivity analysis are consistent with our baseline microsimulation results.
DISCUSSION
The principle finding of this cost-minimization analysis is that, from a societal perspective, the least expensive strategy for DWG resolution is to attempt 2 aspirations followed by open excision of the DWG in the PR. Moreover, 2 attempts at aspiration outperformed a single aspiration attempt as well as immediate surgical excision in each respective surgical technique (open excision in the PR, open excision in the OR, and arthroscopic excision in the OR; Table 3). It is important to emphasize that the total cost from each strategy is not just the cost of the single procedure, but in fact, a combination of direct and indirect costs of each treatment strategy. These values represent the cost of the treatment strategy including possible revision surgeries for recurrence, irrigation and debridement for infections, lost wages related to recovery, and the expected preoperative and postoperative clinic visits along the treatment course. The most expensive strategy according to our microsimulation was immediate arthroscopic excision despite its lower recurrence rate (9.5% ± 0.4% arthroscopically, versus 20.7% ± 1.6% for open excision and 53.9% ± 2.1% for aspiration).
As the healthcare market has evolved to increase its focus on medical spending and payments, treatment strategies have evolved as well. With the popularization of wide-awake local anesthesia with no tourniquet surgery and similar techniques, an increasing number of hand and wrist soft tissue procedures are now being performed in a PR without formal aid from an anesthesiologist13,15,51,52 To this effect, cost analyses recently have been used to evaluate treatment strategies for pathologies, such as wrist arthritis, carpal tunnel syndrome, and trigger finger.11,13,14,53–57 Interestingly, the concept of 2 attempts at a nonoperative treatment strategy (corti-costeroid injection) preoperatively also was seen to be the dominant strategy in a recent trigger finger management cost-effective analysis.57
Arthroscopy was the most expensive surgical strategy even in isolated scenarios of no time off work or decreased cost by 34%. For immediate open surgical excision in the PR to be less expensive than 2 attempts at aspiration before open excision, the failure rate after ganglion aspiration would need to be greater than 86%, which is 1.6 times its currently accepted rate of 53.9 ± 2.1%. Similarly, if open excision is planned for the OR, the aspiration recurrence rate would need to be >89% (or 1.65 times the currently accepted rate) for immediate surgery to be less expensive. Rephrased, if the success rate after aspiration is >11%, 2 aspiration attempts before surgical intervention is the dominant strategy. The sensitivity analysis also shows that even in the setting of the open excision recurrence rate being 5% instead of 20.7%, 2 aspirations before surgical excision still would be the dominant strategy.
The rates of recurrence seen in this study are similar to that reported in the 2015 systematic review and meta-analysis, with 6% recurrence for arthroscopic excision, 21% for open excision, and 59% for aspiration.2 The cost-based study by Pang et al11 expressed a similar result, concluding that arthroscopic excision was significantly more expensive than open excision in the OR at a cost per payer of $3,668 compared with $1,821. Though also a cost-minimization analysis, its focus was primarily on the direct cost of the 2 procedures under the supposition that there was nonsuperiority of arthroscopic resection over open resection in terms of recurrence rates.53–57
There are several limitations to this study. The recurrence rates are based on weighted averages from the literature, which does not necessarily account for certain variables; therefore we are unable to factor in patient activity levels, handedness, or variations in surgical skill. Moreover, these studies are subject to potential publication bias. Although there is uncertainty, the robustness of our 1-way sensitivity analysis supports our results even in extreme cases. As open excision is the most common procedure, the research impetus is placed upon evaluating alternatives to open excision, potentially favoring aspiration or arthroscopic techniques. All variations of aspiration attempts were grouped together because there have not been randomized controlled trials demonstrating superiority of aspiration plus injection or with ultrasound over aspiration alone.17–21,23,24 The retrospective payment data used for our calculations were collected from a single institution, which may potentially affect the generalizability of the cost data. As arthroscopic ganglion excision is not performed commonly at our institution, diagnostic wrist arthroscopy with or without synovial biopsy (current procedural terminology 29840) was used. This was similar to the method used by Pang et al11 in evaluating the payer cost of arthroscopic excision. However, our 1-way sensitivity analysis showed that our findings were robust to direct costs of surgeries and aspiration. For irrigation and debridement following ganglion excision, wrist arthrotomy current procedural terminology was used. As a result, there may be more variability in the cost data acquired from the associated codes, and this variability is likely an underestimation of these costs, although this is a rare complication following DWG excision. Some patients may attend physical or occupational therapy after DWG surgery or wrist irrigation and debridement, and associated costs were not included in the model. Despite recommendations for cost-minimization analysis studies to be performed from a societal perspective, it is possible that other patient-related factors were not included in our calculations,16 and costs from the perspective of the patient could be interpreted differently. Lastly, our study results do not factor in patient preferences e these results may help guide shared decision-making between the surgeon and patient that reviews chances of DWG resolution, costs, time off work, and convalescence.
Despite its limitations, this study has several key strengths. Owing to the inclusion of 3 different surgical options and effectively 9 different treatment strategies (Fig. 1), these results can be applied to any surgeon based on their preferences or facilities’ capabilities in regard to arthroscopic and PR cases. Moreover, because of the relatively simple treatment techniques advocated in this study (e.g., aspiration, open excision) there is less of a barrier for surgeons to change their current treatment strategy based on the results of the study. Lastly, the sensitivity analysis highlights the robustness of our results between different treatment strategies.
In conclusion, performing 2 aspirations before surgical excision in the PR setting was the most cost-minimal treatment strategy in our study for DWG treatment. In reference to surgeons who do not use the PR setting, open excision in the OR was nearly as cost-effective. Realizing that patient preferences may preclude routinely performing 2 aspirations, we advocate for performing at least 1 aspiration before surgical excision because this will improve the cost-effectiveness of DWG treatment.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR002538 (formerly 5UL1TR00106705, 8UL1TR000105 and UL1RR025764).
Footnotes
No benefits in any form have been received or will be received related directly or indirectly to the subject of this article.
Type of study/level of evidence Economic decision analysis II.
REFERENCES
- 1.Mathoulin C, Gras M. Arthroscopic management of dorsal and volar wrist ganglion. Hand Clin. 2017;33(4):769–777. [DOI] [PubMed] [Google Scholar]
- 2.Head L, Gencarelli JR, Allen M, Boyd KU. Wrist ganglion treatment: systematic review and meta-analysis. J Hand Surg Am. 2015;40(3): 546–553 e8. [DOI] [PubMed] [Google Scholar]
- 3.Dermon A, Kapetanakis S, Fiska A, Alpantaki K, Kazakos K. Ganglionectomy without repairing the bursal defect: long-term results in a series of 124 wrist ganglia. Clin Orthop Surg. 2011;3(2):152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young L, Bartell T, Logan SE. Ganglions of the hand and wrist. Southern Med J. 1988;81(6):751–760. [DOI] [PubMed] [Google Scholar]
- 5.Angelides AC, Wallace PF. The dorsal ganglion of the wrist: its pathogenesis, gross and microscopic anatomy, and surgical treatment. J Hand Surg Am. 1976;1(3):228–235. [DOI] [PubMed] [Google Scholar]
- 6.Rocchi L, Canal A, Fanfani F, Catalano F. Articular ganglia of the volar aspect of the wrist: arthroscopic resection compared with open excision. A prospective randomised study. Scand J Plast Reconstr Surg Hand Surg. 2008;42(5):253–259. [DOI] [PubMed] [Google Scholar]
- 7.Lee VS, Kawamoto K, Hess R, et al. Implementation of a valuedriven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316(10):1061–1072. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto K, Martin CJ, Williams K, et al. Value driven outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inform Assoc. 2015;22(1):223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10): 1024–1039. [DOI] [PubMed] [Google Scholar]
- 10.Fisher ES, Bynum JP, Skinner JS. Slowing the growth of health care costs–lessons from regional variation. N Engl J Med. 2009;360(9): 849–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang EQ, Zhang S, Harris AHS, Kamal RN. Cost minimization analysis of ganglion cyst excision. J Hand Surg Am. 2017;42(9):750 e1–750 e4. [DOI] [PubMed] [Google Scholar]
- 12.Van Demark RE Jr, Smith VJS, Fiegen A. Lean and green hand surgery. J Hand Surg Am. 2018;43(2):179–181. [DOI] [PubMed] [Google Scholar]
- 13.Alter TH, Warrender WJ, Liss FE, Ilyas AM. A cost analysis of carpal tunnel release surgery performed wide awake versus under sedation. Plast Reconstr Surg. 2018;142(6):1532–1538. [DOI] [PubMed] [Google Scholar]
- 14.Kazmers NH, Presson AP, Xu Y, Howenstein A, Tyser AR. Cost implications of varying the surgical technique, surgical setting, and anesthesia type for carpal tunnel release surgery. J Hand Surg Am. 2018;43(11):971–977 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davison PG, Cobb T, Lalonde DH. The patient’s perspective on carpal tunnel surgery related to the type of anesthesia: a prospective cohort study. Hand (N Y). 2013;8(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 17.Hatchell A, Meathrel K, Farrokhyar F, Hynes N. A prospective randomized controlled trial of aspiration and fibrin sealant use versus aspiration alone in the treatment of dorsal wrist ganglia. Plast Surg (Oakv). 2019;27(1):22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan PS, Hayat H. Surgical excision versus aspiration combined with intralesional triamcinolone acetonide injection plus wrist immobilization therapy in the treatment of dorsal wrist ganglion; a randomized controlled trial. J Hand Microsurg. 2011;3(2): 55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Limpaphayom N, Wilairatana V. Randomized controlled trial between surgery and aspiration combined with methylprednisolone acetate injection plus wrist immobilization in the treatment of dorsal carpal ganglion. J Med Assoc Thai. 2004;87(12):1513–1517. [PubMed] [Google Scholar]
- 20.Nasab SAM, Mashhadizadeh E, Sarrafan N. Comparative study between three methods of aspiration alone, aspiration plus steroid injection and aspiration plus ethanol injection for treatment of dorsal wrist ganglions. Pak J Med Sci. 2012;28(3):404–407. [Google Scholar]
- 21.Nishikawa S, Toh S, Miura H, Arai K, Irie T. Arthroscopic diagnosis and treatment of dorsal wrist ganglion. J Hand Surg Br. 2001;26(6): 547–549. [DOI] [PubMed] [Google Scholar]
- 22.Kang L, Akelman E, Weiss AP. Arthroscopic versus open dorsal ganglion excision: a prospective, randomized comparison of rates of recurrence and of residual pain. J Hand Surg Am. 2008;33(4): 471–475. [DOI] [PubMed] [Google Scholar]
- 23.Rathod CM, Nemade AS, Badole CM. Treatment of dorsal wrist ganglia by transfixation technique. Niger J Clin Pract. 2011;14(4): 445–448. [DOI] [PubMed] [Google Scholar]
- 24.Kurkis G, Anastasio A, DeVos M, Gottschalk MB. Ultrasound-guided aspiration does not reduce the recurrence rate of ganglion cysts of the wrist. J Wrist Surg. 2019;8(2):100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aslani H, Najafi A, Zaaferani Z. Prospective outcomes of arthroscopic treatment of dorsal wrist ganglia. Orthopedics. 2012;35(3): e365–e370. [DOI] [PubMed] [Google Scholar]
- 26.Gallego S, Mathoulin C. Arthroscopic resection of dorsal wrist ganglia: 114 cases with minimum follow-up of 2 years. Arthroscopy. 2010;26(12). 1675–82. [DOI] [PubMed] [Google Scholar]
- 27.Rocchi L, et al. Results and complications in dorsal and volar wrist Ganglia arthroscopic resection. Hand Surg. 2006;11(1–2): 21–26. [DOI] [PubMed] [Google Scholar]
- 28.Dias JJ, Dhukaram V, Kumar P. The natural history of untreated dorsal wrist ganglia and patient reported outcome 6 years after intervention. J Hand Surg Eur. 2007;32(5):502–508. [DOI] [PubMed] [Google Scholar]
- 29.Zeidenberg J, et al. Ultrasound-guided aspiration of wrist ganglions: a follow-up survey of patient satisfaction and outcomes. Acta Radiol. 2016;57(4). 481–6. [DOI] [PubMed] [Google Scholar]
- 30.Craik JD, Walsh SP. Patient outcomes following wrist ganglion excision surgery. Hand Surg Eur Vol. 2012;37(7). 673–7. [DOI] [PubMed] [Google Scholar]
- 31.Faithfull DK, Seeto BG. The simple wrist ganglion–more than a minor surgical procedure? Hand Surg. 2000;5(2). 139–43. [DOI] [PubMed] [Google Scholar]
- 32.Finsen V, Haberg O, Borchgrevink GE. Surgery for wrist Ganglia: one-hundred and twenty-two patients reviewed 8 years after operation. Orthop Rev (Pavia). 2014;6(1):5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gundes H, et al. Prognosis of wrist ganglion operations. Acta Orthop Belg. 2000;66(4). 363–7. [PubMed] [Google Scholar]
- 34.Kulinski S, et al. Dorsal and volar wrist ganglions: The results of surgical treatment. Adv Clin Exp Med. 2019;28(1):95–102. [DOI] [PubMed] [Google Scholar]
- 35.Lidder S, Ranawat V, Ahrens P. Surgical excision of wrist ganglia; literature review and nine-year retrospective study of recurrence and patient satisfaction. Orthop Rev (Pavia). 2009;1(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jagers Op Akkerhuis M, Van Der Heijden M, Brink PR. Hyaluronidase versus surgical excision of ganglia: a prospective, randomized clinical trial. J Hand Surg Br. 2002;27(3). 256–8. [DOI] [PubMed] [Google Scholar]
- 37.Edwards SG, Johansen JA. Prospective outcomes and associations of wrist ganglion cysts resected arthroscopically. J Hand Surg Am. 2009;34(3):395–400. [DOI] [PubMed] [Google Scholar]
- 38.Fernandes CH, et al. Arthroscopic Resection of Dorsal Wrist Ganglion: Results and Rate of Recurrence Over a Minimum Follow-up of 4 Years. Hand (N Y). 2019;14(2):236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho PC, et al. Current treatment of ganglion of the wrist. Hand Surg. 2001;6(1):49–58. [DOI] [PubMed] [Google Scholar]
- 40.Kim JP, et al. Arthroscopic excision of dorsal wrist ganglion: factors related to recurrence and postoperative residual pain. Arthroscopy. 2013;29(6). 1019–24. [DOI] [PubMed] [Google Scholar]
- 41.Luchetti R, et al. Arthroscopic resection of dorsal wrist ganglia and treatment of recurrences. J Hand Surg Br. 2000;25(1):38–40. [DOI] [PubMed] [Google Scholar]
- 42.Mathoulin C, Hoyos A, Pelaez J. Arthroscopic resection of wrist ganglia. Hand Surg. 2004;9(2). 159–64. [DOI] [PubMed] [Google Scholar]
- 43.Osterman AL, Raphael J. Arthroscopic resection of dorsal ganglion of the wrist. Hand Clin. 1995;11(1):7–12. [PubMed] [Google Scholar]
- 44.Rizzo M, et al. Arthroscopic resection in the management of dorsal wrist ganglions: results with a minimum 2-year follow-up period. J Hand Surg Am. 2004;29(1):59–62. [DOI] [PubMed] [Google Scholar]
- 45.Shih JT, et al. Dorsal ganglion of the wrist: results of treatment by arthroscopic resection. Hand Surg. 2002;7(1):1–5. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto M, et al. Sonography-guided arthroscopic excision is more effective for treating volar wrist ganglion than dorsal wrist ganglion. Acta Orthop Belg. 2018;84(1):78–83. [PubMed] [Google Scholar]
- 47.Services. CfMM. Medicare provider utilization and payment data: physician and other supplier. Accessed May, 2020. Available from: https://www.cms.gov/research-statistics-data-and-systems/statisticstrends-and-reports/medicare-provider-charge-data/physician-and-othersupplier.html
- 48.(HCUP) HCaUP. HCUP State Ambulatory Surgery and Services Databases (SASD). Rockville: Agency for Healthcare Research and Quality. Accessed May, 2020. Available from: www.hcup-us.ahrq.gov/sasdoverview.jsp
- 49.Statistics BoL. Occupational outlook handbook - “Usual weekly earnings of wage and salary workers: first quarter 2020. ”. Accessed May 19, 2020. Available from: https://www.bls.gov/news.release/pdf/wkyeng.pdf
- 50.Analysis USBoE. Table 2.3.4. Price indexes for personal consumption expenditures by major type of product: health care. Accessed May 29, 2020. Available from: https://apps.bea.gov/iTable/iTable.cfm?reqid=19&step=2#reqid=19&step=2&isuri=1&1921=survey
- 51.Lalonde D, Eaton C, Amadio P, Jupiter J. Wide-awake hand and wrist surgery: a new horizon in outpatient surgery. Instr Course Lect. 2015;64:249–259. [PubMed] [Google Scholar]
- 52.Lalonde D, Martin A. Tumescent local anesthesia for hand surgery: improved results, cost effectiveness, and wide-awake patient satisfaction. Arch Plast Surg. 2014;41(4):312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kazmers NH, Stephens AR, Presson AP, Xu Y, Feller RJ, Tyser AR. Comparison of direct surgical costs for proximal row carpectomy and four-corner arthrodesis. J Wrist Surg. 2019;8(1):66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luther GA, Murthy P, Blazar PE. Cost of immediate surgery versus non-operative treatment for trigger finger in diabetic patients. J Hand Surg Am. 2016;41(11):1056–1063. [DOI] [PubMed] [Google Scholar]
- 55.Yoo M, Nelson RE, Illing DA, Martin BI, Tyser AR, Kazmers NH. Cost-effectiveness analysis comparing proximal row carpectomy and four-corner arthrodesis. JB JS Open Access. 2020;5(2):e0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maliha SG, Cohen O, Jacoby A, Sharma S. A cost and efficiency analysis of the WALANT technique for the management of trigger finger in a procedure room of a major city hospital. Plast Reconstr Surg Glob Open. 2019;7(11):e2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerrigan CL, Stanwix MG. Using evidence to minimize the cost of trigger finger care. J Hand Surg Am. 2009;34(6):997–1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.