SUMMARY
Innovative non-pharmacological lifestyle strategies to treat non-alcoholic fatty liver disease (NAFLD) are critically needed. This study compared the effects of alternate day fasting (ADF) combined with exercise, to fasting alone, or exercise alone, on intrahepatic triglyceride (IHTG) content. Adults with obesity and NAFLD (n = 80, 81% female, age 23-65 y) were randomized to 1 of 4 groups for 3 months: combination of ADF (600 kcal/2500 kJ “fast day” alternated with an ad libitum intake “feast day”) and moderate-intensity aerobic exercise (5 session per week, 60 min/session); ADF alone; exercise alone; or a no-intervention control group. By month 3, IHTG content was significantly reduced in the combination group (−5.48%; 95% CI, −7.77 to −3.18), compared to the exercise group (−1.30%; 95% CI, −3.80 to 1.20; P = 0.02) and the control group (−0.17%; 95% CI, −2.17 to 1.83; P < 0.01), but was not significantly different versus the ADF group (−2.25%; 95% CI, −4.46 to −0.04; P = 0.05). Body weight, fat mass, waist circumference, and ALT levels significantly decreased, while insulin sensitivity significantly increased, in the combination group compared to the control group. Lean mass, AST, HbA1c, blood pressure, plasma lipids, liver fibrosis score, and hepatokines (fetuin-A, FGF-21, selenoprotein P) did not differ between groups. Combining intermittent fasting with exercise is effective for reducing hepatic steatosis in patients with NAFLD but may offer no additional benefit versus fasting alone.
Trial registration:
Graphical Abstract
IN BRIEF
Ezpeleta et al. examined the effect of alternate day fasting combined with aerobic exercise on hepatic steatosis in adults with non-alcoholic fatty liver disease (NAFLD). After 3 months, the combination therapy produced significant decreases in hepatic steatosis, body weight, fat mass, waist circumference, and ALT levels, and increases in insulin sensitivity.
INTRODUCTION
The prevalence of non-alcoholic fatty liver disease (NAFLD) has reached epidemic proportions worldwide. 1 NAFLD is characterized by an accumulation of fat in the liver not resulting from excessive alcohol consumption. Approximately 65% of adults with obesity have NAFLD, 2 and this condition is strongly related to the development of insulin resistance and type 2 diabetes. 3,4 While certain pharmacological agents have been shown to reduce hepatic steatosis, i.e. thiazolidinediones, there is mounting concern regarding the safety and weight-gaining effects of these compounds. 5,6 In light of this, recent research has focused on non-pharmacological interventions to reduce hepatic steatosis.
Physical activity is a powerful lifestyle therapy that can improve several parameters of NAFLD. 7 Data from a recent systematic review of 24 exercise-only trials in patients with NAFLD, show that structured exercise (moderate to vigorous intensity, 3-5 days per week) produces a 20-30% relative reduction in hepatic steatosis. 8 Physical activity also decreases pro-inflammatory and oxidative stress markers, which in turn, improves liver enzyme profile. 9 In addition, exercise enhances hepatic and peripheral insulin sensitivity, 10 which can slow NAFLD progression and reduce cardiovascular disease risk. Physical activity can also improve quality of life, mood, and mental health in individuals with obesity and NAFLD. 11 Although exercise alone has several benefits, current NAFLD treatment guidelines 12,13 suggest that physical activity combined with a weight reducing diet is the most effective non-pharmacological therapy to treat this condition.
Recently, it has been shown that alternate day fasting (ADF) may be an effective dietary weight loss therapy for patients with NAFLD. 14,15 ADF has greatly increased in popularity over the past decade and is currently one of the most researched diets on the internet.16 This diet involves a “fast day” where individuals consume approximately 600 kcal (2500 kJ), alternated with a “feast day” where individuals are permitted to consume food ad libitum. 17 Evidence from two randomized controlled trials show that ADF is effective for reducing liver steatosis score, circulating levels of alanine transaminase (ALT), and body weight in patients with NAFLD.14,15 While these studies show promise for the use of ADF in treating this condition, they are limited in that changes in intrahepatic triglyceride (IHTG) content were not quantified. Moreover, these studies did not include an exercise intervention in their design. Thus, they were not able to assess whether exercise combined with ADF, would yield superior improvements in NAFLD activity markers, versus diet or exercise alone.
Accordingly, the current study aimed to compare the effects of ADF combined with aerobic exercise, to ADF alone, or exercise alone, on IHTG content and metabolic risk factors in patients with NAFLD. We hypothesized that the combination intervention would produce superior reductions in IHTG content when compared to each intervention alone and compared to control.
RESULTS
We conducted a 3-month randomized parallel-arm trial to compare the effects of ADF combined with aerobic exercise, to ADF alone, exercise alone, and a control group, on IHTG content in adults with obesity and NAFLD. Participants were randomized by a stratified random sample (based on age, sex, BMI, and IHTG content) into 1 of 4 groups: combination, ADF, exercise, or a no-intervention control group. Participants randomized to the combination group and ADF group were instructed to consume 600 kcal (2500 kJ) as a dinner (between 5:00 pm and 8:00 pm) on fast days and eat food as desired on alternating feast days. Subjects randomized to the combination group and exercise group participated in a moderate-intensity aerobic exercise program five times per week for 3 months. Control participants were instructed to maintain their body weight throughout the trial, and not to change their eating or physical activity habits. Controls did not receive any dietary advice, but they visited the research center at the same frequency as the intervention groups for clinical measurements. The primary outcome measure was change in IHTG content from baseline to month 3 (measured by MRI-PDFF). Secondary outcome measures were body weight, body composition, liver enzymes, glucoregulatory factors, blood pressure, plasma lipids, hepatokines, adherence, dietary intake, and habitual activity.
Participants
As shown in Figure 1, 132 individuals expressed interest in the study. Of these participants, 52 were excluded as they did not meet one or more inclusion criteria. Inclusion and exclusion criteria are listed in the STAR Methods section. A total of 80 participants were randomized to the combination group (n = 20), ADF group (n = 20), exercise group (n = 20), or the control group (n = 20). At the conclusion of the 3-month trial, there were 20 completers in the combination group, 19 completers in the ADF group, 15 completers in the exercise group, and 20 completers in the control group. On average, 93% of participants randomized to the interventions completed the trial. Participants withdrew from the study due to schedule conflicts, inability to contact, personal reasons, or a car accident. Notably, no one dropped out of the study due to dislike of the ADF diet or the exercise intervention. Table 1 displays the baseline characteristics of the completers, dropouts, and a pooled analysis of all participants. At baseline, there were no significant differences between groups for the primary outcome measure (IHTG content) or any secondary outcome measure. Participants were primarily middle-aged, Hispanic and Black females with obesity and NAFLD.
Figure 1. Subject flow chart.
Table 1.
Baseline characteristics
| Combination | Alternate day fasting | Exercise | Control | All participants | Completers | Dropouts | |
|---|---|---|---|---|---|---|---|
| n | 20 | 20 | 20 | 20 | 80 | 74 | 6 |
| Age (y) | 44 ± 13 | 44 ± 16 | 44 ± 13 | 44 ± 12 | 44 ± 13 | 44 ± 13 | 39 ± 15 |
| Sex | |||||||
| Female | 17 (85%) | 16 (80%) | 16 (80%) | 16 (80%) | 65 (81%) | 61 (82%) | 4 (67%) |
| Male | 3 (15%) | 4 (20%) | 4 (20%) | 4 (20%) | 15 (19%) | 13 (18%) | 2 (33%) |
| Race or ethnic group | |||||||
| Black | 7 (35%) | 7 (35%) | 6 (30%) | 4 (20%) | 24 (30%) | 23 (31%) | 1 (17%) |
| Hispanic | 10 (50%) | 8 (40%) | 10 (50%) | 12 (60%) | 40 (50%) | 35 (47%) | 5 (83%) |
| White | 2 (10%) | 2 (10%) | 4 (20%) | 1 (5%) | 9 (11%) | 9 (12%) | 0 (0%) |
| Asian | 1 (5%) | 3 (15%) | 0 (0%) | 3 (15%) | 7 (9%) | 7 (9%) | 0 (0%) |
| Liver parameters | |||||||
| IHTG (%) | 18 ± 8 | 16 ± 6 | 17 ± 6 | 17 ± 9 | 17 ± 8 | 17 ± 8 | 15 ± 6 |
| ALT (U/L) | 28 ± 15 | 31 ± 33 | 24 ± 18 | 22 ± 8 | 26 ± 20 | 26 ± 20 | 32 ± 32 |
| AST (U/L) | 23 ± 8 | 23 ± 15 | 21 ± 12 | 18 ± 5 | 21 ± 11 | 21 ± 10 | 25 ± 21 |
| Fibrosis (FIB-4) | 0.91 ± 0.36 | 0.93 ± 0.57 | 0.86 ± 0.35 | 0.76 ± 0.22 | 0.87 ± 0.39 | 0.87 ± 0.40 | 0.78 ± 0.32 |
| Body composition | |||||||
| Body weight (kg) | 101 ± 20 | 96 ± 21 | 100 ± 21 | 100 ± 17 | 99 ± 19 | 98 ± 19 | 111 ± 26 |
| Fat mass (kg) | 46 ± 12 | 40 ± 8 | 45 ± 13 | 45 ± 11 | 44 ± 11 | 44 ± 11 | 49 ± 13 |
| Lean mass (kg) | 51 ± 12 | 51 ± 9 | 52 ± 9 | 51 ± 10 | 51 ± 10 | 51 ± 10 | 57 ± 13 |
| Visceral fat mass (kg) | 1.6 ± 0.8 | 1.6 ± 0.8 | 1.7 ± 0.8 | 1.7 ± 0.8 | 1.6 ± 0.8 | 1.6 ± 0.8 | 1.7 ± 1.2 |
| Waist circumference (cm) | 111 ± 13 | 107 ± 17 | 111 ± 14 | 109 ± 12 | 109 ± 14 | 109 ± 14 | 114 ± 18 |
| Height (cm) | 166 ± 9 | 164 ± 9 | 165 ± 8 | 165 ± 8 | 165 ± 8 | 165 ± 8 | 165 ± 10 |
| BMI (kg/m2) | 37 ± 5 | 36 ± 8 | 37 ± 6 | 37 ± 5 | 36 ± 6 | 36 ± 6 | 41 ± 7 |
| Glucoregulatory factors | |||||||
| Fasting insulin (μIU/mL) | 31 ± 35 | 17 ± 10 | 26 ± 25 | 19 ± 15 | 23 ± 24 | 23 ± 25 | 21 ± 11 |
| Insulin resistance (HOMA-IR) | 7.1 ± 7.3 | 3.8 ± 2.3 | 5.8 ± 5.3 | 4.5 ± 3.8 | 5.3 ± 5.1 | 5.3 ± 5.2 | 5.0 ± 3.3 |
| Insulin sensitivity (QUICKI) | 0.29 ± 0.03 | 0.31 ± 0.03 | 0.30 ± 0.04 | 0.31 ± 0.03 | 0.30 ± 0.03 | 0.30 ± 0.03 | 0.31 ± 0.03 |
| Fasting glucose (mg/dl) | 94 ± 12 | 90 ± 15 | 91 ± 14 | 98 ± 38 | 93 ± 22 | 93 ± 23 | 97 ± 14 |
| HbA1c (%) | 5.7 ± 0.5 | 5.8 ± 0.5 | 5.6 ± 1.0 | 5.9 ± 0.4 | 5.8 ± 0.9 | 5.7 ± 0.8 | 6.1 ± 1.7 |
| Blood pressure, heart rate | |||||||
| Systolic BP (mm Hg) | 127 ± 18 | 126 ± 16 | 130 ± 17 | 126 ± 22 | 127 ± 18 | 127 ± 18 | 131 ± 20 |
| Diastolic BP (mm Hg) | 87 ± 9 | 87 ± 10 | 85 ± 10 | 84 ± 9 | 86 ± 9 | 86 ± 9 | 86 ± 9 |
| Heart rate (bpm) | 75 ± 13 | 78 ± 18 | 74 ± 14 | 75 ± 8 | 76 ± 13 | 76 ± 13 | 74 ± 18 |
| Plasma lipids | |||||||
| Total cholesterol (mg/dl) | 192 ± 29 | 175 ± 34 | 168 ± 47 | 186 ± 37 | 180 ± 38 | 182 ± 37 | 159 ± 45 |
| LDL cholesterol (mg/dl) | 116 ± 24 | 97 ± 29 | 87 ± 40 | 108 ± 35 | 102 ± 34 | 103 ± 33 | 90 ± 43 |
| HDL cholesterol (mg/dl) | 53 ± 13 | 50 ± 13 | 55 ± 18 | 51 ± 13 | 52 ± 14 | 53 ± 15 | 45 ± 7 |
| Triglycerides (mg/dl) | 116 ± 37 | 141 ± 72 | 129 ± 63 | 109 ± 40 | 124 ± 55 | 124 ± 56 | 117 ± 41 |
| Hepatokines | |||||||
| Fetuin-A (ng/ml) | 285 ± 602 | 310 ± 323 | 279 ± 362 | 370 ± 624 | 309 ± 480 | 316 ± 499 | 233 ± 93 |
| FGF-21 (ng/ml) | 0.55 ± 0.74 | 0.82 ± 1.23 | 1.36 ± 1.59 | 0.58 ± 1.04 | 0.83 ± 1.21 | 0.83 ± 1.17 | 0.88 ± 1.79 |
| Selenoprotein P (ng/ml) | 1175 ± 129 | 1168 ± 124 | 1203 ± 123 | 1191 ± 384 | 1184 ± 211 | 1184 ± 219 | 1177 ± 44 |
Data are expressed as mean (SD) unless otherwise indicated. Abbreviations: ALT: alanine transaminase; AST: aspartate transaminase; BP: Blood pressure; FGF-21: Fibroblast growth factor-21, FIB-4: Fibrosis-4, IHTG: Intrahepatic triglyceride content; HbA1c: Glycated hemoglobin; HDL, high-density lipoprotein; HOMA, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; QUICKI: Quantitative insulin sensitivity check index.
The combination intervention produces superior reductions in intrahepatic triglyceride (IHTG) content versus exercise alone, but not fasting alone
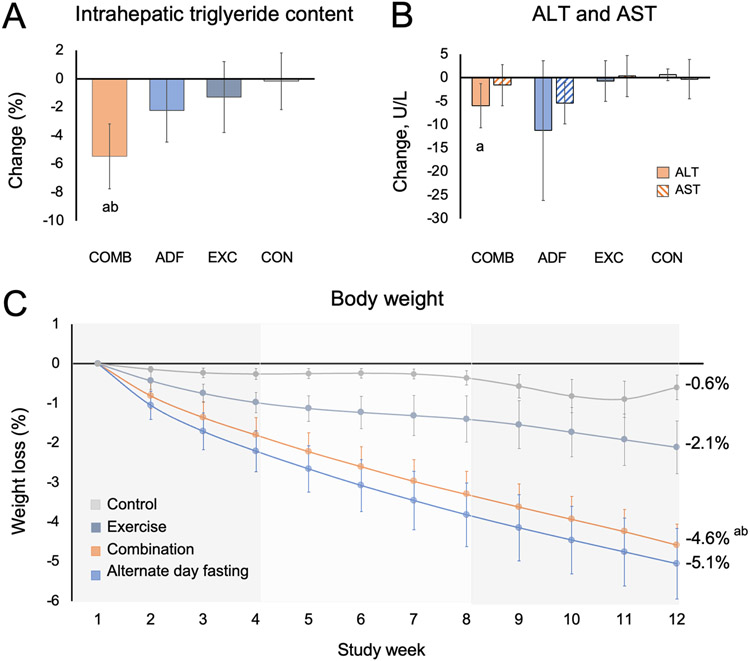
By month 3, IHTG content (expressed as absolute percent change) was significantly reduced in the combination group (−5.48%; 95% CI, −7.77 to −3.18%), compared to the exercise group (−1.30%; 95% CI, −3.80 to 1.20%; P = 0.02), and the control group (−0.17%; 95% CI, −2.17 to 1.83%; P < 0.01) (Figure 2A, Table 2). However, the reduction in IHTG content in the combination group was not significantly different compared to the ADF group (−2.25%; 95% CI, −4.46 to −0.04%; P = 0.05). Change in serum ALT by month 3 in the combination group (−5.97 U/L; 95% CI, −10.66 to −1.28 U/L) was significantly different compared to the control group (0.65 U/L; 95% CI, −1.90 to 3.20 U/L; P = 0.01), but not significantly different compared to the ADF group (−11.24 U/L; 95% CI, −26.12 to 3.64 U/L; P = 0.48) or exercise group (−0.70 U/L; 95% CI, −5.03 to 3.64 U/L; P = 0.09) (Figure 2B, Table 2). Change in serum AST by month 3 did not significantly differ among the four groups (Figure 2B, Table 2). Changes in hepatic fibrosis score (estimated using the FIB-4 index) did not significantly differ between groups by month 3 (Table 2).
Figure 2. Intrahepatic triglyceride content, liver enzymes and body weight.
a P < 0.01 vs Control group; b P < 0.05 vs Exercise group. Data were included for 80 participants; means were estimated using an intention-to-treat analysis using a linear mixed model.
A. Error bars indicate 95% confidence intervals for intrahepatic triglyceride content from baseline by diet group.
B. Error bars indicate 95% confidence intervals for alanine transaminase (ALT) and aspartate transaminase (AST) concentrations from baseline by diet group.
C. Error bars indicate 95% confidence intervals for weight loss from baseline by diet group.
Table 2.
Liver parameters, body composition, and metabolic risk factors
| Change by month 3 (95% CI) | P-values | ||||||
|---|---|---|---|---|---|---|---|
| Outcomes | Combination | Alternate day fasting | Exercise | Control | Combination vs ADF |
Combination vs Exercise |
Combination vs Control |
| Liver parameters | |||||||
| IHTG (%) | −5.48 (−7.77, −3.18) | −2.25 (−4.46, −0.04) | −1.30 (−3.80, 1.20) | −0.17 (−2.17, 1.83) | 0.05 | 0.02 | <0.01 |
| ALT (U/L) | −5.97 (−10.66, −1.28) | −11.24 (−26.12, 3.64) | −0.70 (−5.03, 3.64) | 0.65 (−1.90, 3.20) | 0.48 | 0.09 | 0.01 |
| AST (U/L) | −1.59 (−5.98, 2.79) | −5.39 (−9.8, −0.98) | 0.34 (−4.71, 5.39) | −0.33 (−4.51, 3.85) | 0.23 | 0.56 | 0.68 |
| Fibrosis (FIB-4) | 0.16 (−0.22, 0.55) | −0.08 (−0.31, 0.15) | 0.06 (−0.02, 0.13) | −0.01 (−0.06, 0.05) | 0.26 | 0.58 | 0.36 |
| Body weight, composition | |||||||
| Body weight (%) | −4.58 (−5.62, −3.55) | −5.06 (−6.81, −3.32) | −2.11 (−3.41, −0.80) | −0.60 (−2.04, 0.83) | 0.62 | < 0.01 | < 0.01 |
| Body weight (kg) | −4.18 (−4.65, −3.71) | −4.45 (−4.93, −3.97) | −1.79 (−2.33, −1.26) | −0.52 (−1.00, −0.05) | 0.43 | < 0.01 | < 0.01 |
| Fat mass (kg) | −3.24 (−4.31, −2.16) | −3.32 (−4.39, −2.24) | −1.34 (−2.52, −0.16) | −0.62 (−1.69, 0.46) | 0.92 | 0.02 | < 0.01 |
| Lean mass (kg) | −0.83 (−1.57, −0.09) | −1.20 (−1.95, −0.46) | −0.90 (−1.71, −0.08) | 0.05 (−0.69, 0.79) | 0.48 | 0.91 | 0.10 |
| Visceral fat mass (kg) | −0.20 (−0.33, −0.07) | −0.21 (−0.34, −0.09) | −0.08 (−0.23, 0.06) | −0.02 (−0.15, 0.10) | 0.86 | 0.25 | 0.06 |
| Waist circumference (cm) | −5.02 (−6.85, −3.19) | −4.59 (−6.37, −2.80) | −3.24 (−5.25, −1.24) | −0.52 (−2.30, 1.26) | 0.73 | 0.20 | < 0.01 |
| BMI (kg/m2) | −1.63 (−2.35, −0.92) | −1.88 (−2.62, −1.15) | −0.86 (−1.69, −0.04) | −0.67 (−1.39, 0.04) | 0.63 | 0.16 | 0.06 |
| Glucoregulatory factors | |||||||
| Fasting insulin (μIU/mL) | −9.59 (−15.16, −4.02) | −7.41 (−13.58, −1.24) | −3.93 (−8.87, 1.01) | 1.22 (−2.48, 4.92) | 0.58 | 0.11 | < 0.01 |
| Insulin resistance (HOMA-IR) | −2.55 (−4.03, −1.08) | −1.80 (−3.07, −0.54) | −1.25 (−2.76, 0.27) | 0.49 (−0.83, 1.80) | 0.41 | 0.19 | < 0.01 |
| Insulin sensitivity (QUICKI) | 0.04 (0.02, 0.05) | 0.02 (0.00, 0.03) | 0.02 (0.00, 0.03) | 0.00 (−0.02, 0.01) | 0.05 | 0.07 | < 0.01 |
| Fasting glucose (mg/dl) | −5.28 (−11.76, 1.21) | −5.14 (−11.64, 1.35) | −2.12 (−9.75, 5.50) | 0.62 (−5.67, 6.90) | 0.98 | 0.53 | 0.20 |
| HbA1c (%) | −0.08 (−0.20, 0.05) | −0.12 (−0.24, 0.01) | 0.02 (−0.13, 0.16) | 0.04 (−0.08, 0.16) | 0.66 | 0.33 | 0.17 |
| Blood pressure, heart rate | |||||||
| Systolic BP (mm Hg) | −3.78 (−8.67, 1.12) | −3.85 (−8.86, 1.17) | −3.19 (−8.77, 2.39) | 1.95 (−2.95, 6.85) | 0.98 | 0.88 | 0.10 |
| Diastolic BP (mm Hg) | −4.25 (−7.48, −1.02) | −1.82 (−5.13, 1.49) | −1.03 (−4.70, 2.63) | 0.10 (−3.13, 3.33) | 0.30 | 0.19 | 0.06 |
| Heart rate (bpm) | −3.78 (−8.2, 0.65) | −0.87 (−5.38, 3.65) | 1.16 (−3.84, 6.17) | 0.25 (−4.17, 4.67) | 0.36 | 0.14 | 0.20 |
| Plasma lipids | |||||||
| Total cholesterol (mg/dl) | −6.32 (−15.72, 3.07) | −2.24 (−11.65, 7.17) | 0.01 (−11.61, 11.64) | −7.81 (−16.63, 1.00) | 0.54 | 0.40 | 0.82 |
| LDL cholesterol (mg/dl) | −3.88 (−12.33, 4.57) | 2.54 (−5.92, 11.00) | 8.89 (−1.57, 19.35) | −2.52 (−10.45, 5.41) | 0.29 | 0.06 | 0.82 |
| HDL cholesterol (mg/dl) | 0.24 (−3.62, 4.10) | 3.74 (−0.12, 7.61) | −4.64 (−9.41, 0.13) | −1.54 (−5.16, 2.09) | 0.20 | 0.12 | 0.50 |
| Triglycerides (mg/dl) | −13.28 (−36.53, 9.96) | −41.29 (−63.75, −18.83) | −18.75 (−46.92, 9.43) | 3.07 (−10.37, 16.51) | 0.07 | 0.74 | 0.20 |
Data were included for 80 participants; means were estimated using an intention-to-treat analysis using a linear mixed model. Error bars indicate 95% confidence intervals for each parameter from baseline by diet group. Abbreviations: ALT: Alanine transaminase; AST: aspartate transaminase; BP: Blood pressure; FIB-4: Fibrosis-4, HDL, high-density lipoprotein; HOMA, homeostasis model assessment of insulin resistance; IHTG: Intrahepatic triglyceride content; LDL, low-density lipoprotein; QUICKI: Quantitative insulin sensitivity check index.
The combination intervention produces superior reductions in body weight and fat mass versus exercise alone, but not fasting alone
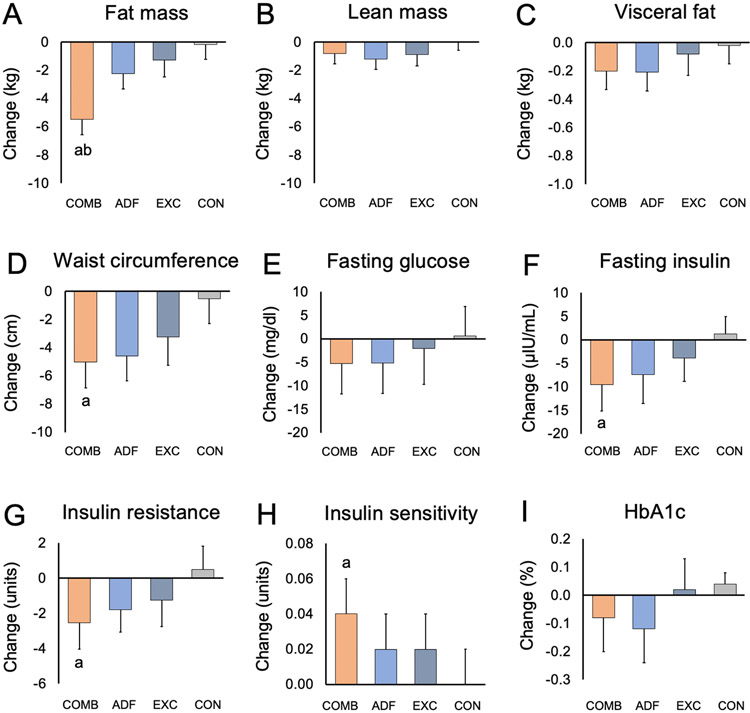
During the 3-month intervention, weight loss was significantly greater in the combination group (−4.58%; 95% CI, −5.62 to −3.55%), compared to the exercise group (−2.11%; 95% CI, −3.41 to −0.80%; P < 0.01) and the control group (−0.60%; 95% CI, −2.04 to 0.83%; P < 0.01) (Figure 2C, Table 2). However, weight loss did not significantly differ in the combination group compared to the ADF group (−5.06%; 95% CI, −6.81 to −3.32%; P = 0.62). Likewise, fat mass loss was significantly greater in the combination group (−3.24 kg; 95% CI, −4.31 to −2.16 kg), compared to the exercise group (−1.34 kg; 95% CI, −2.52 to −0.16 kg; P = 0.02) and the control group (−0.62 kg; 95% CI, −1.69 to 0.46 kg; P < 0.01) but not the ADF group (−3.32 kg; 95% CI, −4.39 to −2.24 kg; P = 0.92) (Figure 3A, Table 2). Change in waist circumference by month 3 in the combination group (−5.02 cm; 95% CI, −6.85 to −3.19 cm) was significantly different compared to the control group (−0.52 cm; 95% CI, −2.30 to −1.26 cm; P < 0.01), but not significantly different compared to the ADF group (−4.59 cm; 95% CI, −6.37 to −2.80 cm; P = 0.73) or exercise group (−3.24 cm; 95% CI, −5.25 to −1.24 cm; P = 0.20) (Figure 3D, Table 2). Change in lean mass, visceral fat mass, and body mass index (BMI) did not significantly differ among the four groups (Figure 3, Table 2).
Figure 3. Body composition and glucoregulatory factors.
a P < 0.01 vs Control group; b P < 0.05 vs Exercise group. Data were included for 80 participants; means were estimated using an intention-to-treat analysis using a linear mixed model. Error bars indicate 95% confidence intervals for each parameter from baseline by diet group. Insulin resistance measured by HOMA-IR (Homeostasis model assessment of insulin resistance). Insulin sensitivity measured by QUICKI (Quantitative insulin sensitivity check index).
The combination intervention improved insulin resistance and insulin sensitivity versus controls, but not versus exercise alone or fasting alone
By month 3, fasting insulin was significantly reduced in the combination group (−9.59 μIU/mL; 95% CI, −15.16 to −4.02 μIU/mL) compared to the control group (1.22 μIU/mL; 95% CI, −2.48 to 4.92 μIU/mL; P < 0.01), but was not significantly different compared to the ADF group (−7.41 μIU/mL; 95% CI, −13.58 to −1.24 μIU/mL; P = 0.58) or the exercise group (−3.93 μIU/mL; 95% CI, −8.87 to 1.01 μIU/mL; P = 0.11) (Figure 3F, Table 2). Likewise, insulin resistance (absolute change in HOMA-IR) was significantly reduced in the combination group (−2.55; 95% CI, −4.03 to −1.08) compared to the control group (0.49; 95% CI, −0.83 to 1.80; P < 0.01), but was not significantly different compared to the ADF group (−1.80; 95% CI, −3.07 to −0.54; P = 0.41) or the exercise group (−1.25; 95% CI, −2.76 to 0.27; P = 0.19) (Figure 3G, Table 2). Insulin sensitivity (absolute change in QUICKI) was significantly increased in the combination group (0.04; 95% CI, 0.02 to 0.05) compared to the control group (0.00; 95% CI, −0.02 to 0.01; P < 0.01), but was not significantly different compared to the ADF group (0.02; 95% CI, 0.00 to 0.03; P = 0.05) or the exercise group (0.02; 95% CI, 0.00 to 0.03; P = 0.07) (Figure 3H, Table 2). Change in fasting glucose and HbA1c did not significantly differ among the four groups by the end of the trial (Figure 3, Table 2).
The combination intervention does not affect blood pressure, heart rate, LDL cholesterol, HDL cholesterol, or triglycerides
As shown in Table 2, changes in blood pressure and heart rate did not significantly differ when the combination group was compared to the ADF group, exercise group, or control group. Likewise, change in total cholesterol, LDL cholesterol, HDL cholesterol and triglyceride concentrations did not significantly differ among the four groups by the end of the study.
Adherence to the interventions was high
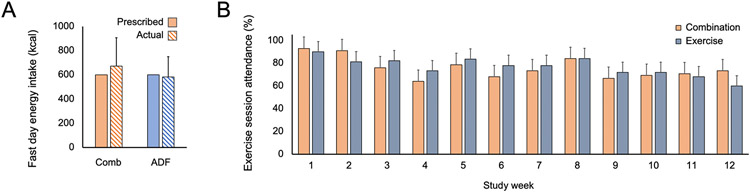
Actual energy intake on the fast day in the combination group (673 ± 234 kcal; 2815 ± 979 kJ) and ADF group (581 ± 168 kcal; 2430 ± 702 kJ) was not significantly different from that of the prescribed fast day energy goal (600 kcal; 2500 kJ) (Figure 4A). Thus, participants were adherent with the intermittent fasting protocol during the 3-month trial. Adherence to the exercise program, defined as participating in 80% or more of the supervised exercise sessions, was very good. Participants in the combination group attended 80 ± 18% of the exercise sessions (Figure 4B). Likewise, participants in the exercise group attended 81 ± 18% of the exercise sessions. Willingness to continue with the diet intervention was assessed via survey on the last day of the trial. Results reveal that 95% of combination subjects (19/20) and 85% of ADF subjects (17/20) were interested in continuing the ADF diet on their own at home. Unfortunately, we did not collect data on whether the exercise group was willing to continue with their intervention after the study was over.
Figure 4. Adherence to the alternate fasting diet and exercise program.
Data are expressed as mean (SD); only observed values included.
A. Actual energy intake on the fast day in the combination (Comb) and alternate day fasting (ADF) groups was not significantly different from that of the prescribed fast day energy goal (600 kcal).
B. Adherence to the exercise program was defined as participating in 80% or more the supervised exercise sessions (a mean of 4 of 5 each week). Participants in the combination group attended 80 ± 18% of the exercise sessions. Participants in the exercise group attended 81 ± 18% of the exercise sessions.
The combination intervention produces similar reductions in energy intake compared to fasting alone
As shown in Table 3, energy, fiber, cholesterol, and macronutrient intake at baseline was similar when the combination group was compared to the ADF group, exercise group, and control group. Energy and fiber intake significantly decreased (P < 0.05) in the combination group and ADF group by month 3, on the fast day and feast day, versus baseline. In addition, cholesterol intake significantly decreased (P < 0.05) in the combination and ADF groups at month 3, on the fast day, versus baseline. In contrast, energy, fiber, and cholesterol intake did not change from baseline to month 3 in the exercise group or control group. Percent intake of macronutrients was not significantly different by month 3 in any of the groups, versus baseline. At baseline, regular physical activity measured as steps/d was not significantly different between the four groups. By month 3, regular physical activity (excluding the exercise intervention program) did not change in any of the groups, when compared to baseline.
Table 3.
Dietary intake and physical activity
| Variable | Combination | Alternate day fasting | Exercise | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Feast day | Fast day | Feast day | Fast day | |||||||
| Baseline | Month 3 | Month 3 | Baseline | Month 3 | Month 3 | Baseline | Month 3 | Baseline | Month 3 | |
| Nutrient intake | ||||||||||
| Energy (kcal) | 2062 ± 1111 | 1356 ± 628 * | 673 ± 234 * | 1940 ± 478 | 1285 ± 469 * | 581 ± 168 * | 1808 ± 637 | 1779 ± 656 | 1810 ± 438 | 1833 ± 497 |
| Protein (% kcal) | 18 ± 5 | 18 ± 6 | 21 ± 6 | 17 ± 4 | 20 ± 3 | 21 ± 7 | 19 ± 4 | 18 ± 4 | 18 ± 5 | 18 ± 5 |
| Carbohydrates (% kcal) | 44 ± 8 | 43 ± 12 | 44 ± 12 | 44 ± 9 | 38 ± 11 | 46 ± 8 | 39 ± 9 | 43 ± 13 | 43 ± 9 | 41 ± 9 |
| Fat (% kcal) | 38 ± 7 | 39 ± 10 | 35 ± 10 | 39 ± 8 | 42 ± 7 | 33 ± 11 | 42 ± 7 | 39 ± 11 | 39 ± 6 | 41 ± 9 |
| Cholesterol (mg) | 324 ± 178 | 246 ± 182 | 174 ± 111 * | 336 ± 132 | 323 ± 160 | 112 ± 97 * | 294 ± 156 | 308 ± 97 | 280 ± 131 | 255 ± 121 |
| Fiber (g) | 19 ± 12 | 15 ± 10 * | 10 ± 5 * | 18 ± 7 | 11 ± 8 * | 9 ± 3 * | 15 ± 8 | 17 ± 12 | 14 ± 4 | 12 ± 4 |
| Physical activity | ||||||||||
| Steps/d | 7434 ± 3889 | 7455 ± 3847 | 7528 ± 4340 | 7041 ± 3740 | 6754 ± 2446 | 6936 ± 2476 | 6497 ± 2688 | 6004 ± 2377 | ||
Data are expressed as mean (SD); only observed values included. At baseline, there were no differences in nutrient intake or steps/d between the combination group, alternate day fasting group, exercise group, or control group. Significant changes (P < 0.05) from baseline to month 3 indicated by (*).
The combination intervention does not affect hepatokine concentrations
Changes in hepatokines from baseline to month 3 are reported in Figure S2. Change in fetuin-A, FGF-21, and selenoprotein P did not significantly differ among the four groups by the end of the study. Correlations between hepatokines and metabolic disease risk factors are displayed in Table S1. A positive correlation was observed between change in Fetuin-A and fasting glucose concentrations (r = 0.465, P = 0.006). Similarly, a positive correlation was noted between change in Fetuin-A and HbA1c (r = 0.432, P = 0.012). No correlations were observed for FGF-21 or selenoprotein P and any metabolic outcome.
DISCUSSION
The results of this randomized clinical trial demonstrate that 3-months of ADF combined with aerobic exercise is an effective lifestyle therapy to reduce IHTG content versus exercise alone or a no-intervention control group. However, further research will be required to elucidate whether the combination intervention produces greater reductions in IHTG content versus fasting alone. Our findings also indicate that the combination intervention was effective for reducing body weight, fat mass, waist circumference, ALT, fasting insulin, insulin resistance, and increasing insulin sensitivity, among patients with obesity and NAFLD, versus controls.
This is the first study to examine the effect of intermittent fasting combined with exercise on NAFLD outcomes. After 3 months, significant reductions in hepatic steatosis (5.5%) were observed in the combination group. These beneficial changes in IHTG content were significantly different compared to the exercise group (1.3%, P = 0.02), but not compared to the ADF group (2.3%, P = 0.05). Since the latter comparison was marginally significant (P = 0.05), more research will be needed to inform whether the combination intervention produces greater reductions in IHTG content versus fasting alone. The changes noted for IHTG content are on par with what has been observed in previous lifestyle intervention trials. 18-20 For instance, Cheng and colleagues 18 observed a 5.5% absolute reduction from baseline in IHTG content after 8 months of calorie restriction combined with aerobic exercise (150 min per week) in adults with NAFLD and prediabetes. Likewise, Kantartzis et al 20 demonstrated a 4.6% absolute decrease from baseline in IHTG content when patients with NAFLD followed a low calorie diet combined with exercise (180 min per week) for 9 months. Improvements in liver fat content and fibrosis scores have also been observed with intermittent fasting alone. 14,15,21 Moreover, Holmer et al 21 showed that fasting two days per week produced comparable reductions from baseline in absolute IHTG content (6.1%) when compared to daily calorie restriction (7.2%) after 3 months. In view of these findings, it is possible that intermittent fasting may be a suitable alternative to daily energy restriction for patients with NAFLD who struggle with calorie counting. More studies that directly compare the effects of intermittent fasting versus daily calorie restriction on NAFLD outcomes are well warranted.
We also examined the effect of these interventions on hepatic fibrosis scores and liver enzymes. Degree of liver fibrosis was estimated by the fibrosis-4 (FIB-4) index. A FIB-4 score below 1.30 is considered as low risk for advanced fibrosis, while a score over 2.67 is considered as high risk for advanced fibrosis. At baseline, the mean FIB-4 score for all participants was 0.87, indicated low risk for advanced fibrosis. By the end of the study, changes in liver fibrosis score did not differ significantly between the groups. As for liver enzymes, ALT decreased by 6 U/L in the combination group versus controls. However, AST was not significantly different between groups by the end of the trial. Our findings for liver enzymes are consistent with other fasting studies conducted in patients with NAFLD. Holmer et al 21 observed decreases in ALT (−17 U/L) but no change in AST, despite a 6.1% decrease in absolute IHTG content, after 12 weeks of fasting on two days per week. Similarly, Johari et al 15 reported reductions in ALT (−25 U/L) without any change in AST, after 8 weeks of ADF, even though liver fat and fibrosis scores were improved (measured by ultrasound). While our findings are in line with previous reports, the general relevance of liver enzymes as diagnostic tests for NAFLD has been debated. 22 In particular, ALT has been highly scrutinized since there is no clear link between ALT levels and degree of hepatic steatosis. For instance, in large cohort studies conducted in the US and Italy, 55-79% of adults with fatty liver had normal ALT levels. 23,24 Moreover, another study in patients with NAFLD showed no relationship between ALT concentrations and changes in steatosis, inflammation, hepatocyte ballooning or degree of fibrosis over time. 25 Contrary to these findings, others have shown that elevated ALT concentrations are significantly correlated with incidence of steatohepatitis and fibrosis in patients, with obesity, NAFLD, and type 2 diabetes. 26-28 In view of these equivocal findings, it remains unknown if ALT has enough sensitivity and specificity to reliably diagnose fatty liver disease.
Body weight decreased in the combination group (−4.6%) versus the exercise group (−2.1%) and controls (−0.6%) but was not significantly different compared to the ADF group (−5.1%). The degree of weight loss noted with ADF (~5%) is on par with what has been reported in other short-term trials conducted in people with obesity 17,29 and those with NAFLD. 14,15 Interestingly, the combination group did not lose more weight than the ADF group. This is somewhat surprising since these subjects were expending more energy than the ADF group by exercising 5 times per week but eating approximately the same number of calories. Thus, it would be expected that the net energy deficit by the combination group would be greater than that of the ADF group, which would in turn, produce greater weight loss. It is unclear why this did not occur. However, it is possible that our participants were underreporting energy intake and that our food record data is inaccurate. For instance, the average daily caloric deficit reported by the combination and ADF groups was approximately 50%, based on food record data. If the study participants were truly restricting energy intake by 50% per day, their weight loss would have been much greater (10-15% from baseline). Since weight loss was closer to 5% by month 3, it is quite likely that our subjects were underreporting food intake, which is common in diet intervention trials. 30,31 It is also interesting that our participants did not compensate for the lack of food on the fast day, by eating more on the feast day. This phenomenon has been reported in several other ADF trials. 32-35 More specifically, it has been shown that participants eat either marginally less on the feast day, 35 or just slightly more, 17 relative to baseline intake. Future trials in this area should use gold-standard methods, such as the doubly-labeled water technique, 36 to assess changes in energy expenditure. This will help to elucidate how these interventions truly impact energy balance and weight regulation.
Whether these beneficial changes in hepatic steatosis are mediated by fasting or merely just weight loss, remains unclear. Body weight reductions of 5-10% have been shown to reduce, and even resolve, fatty liver disease. 37,38 Since the combination group achieved ~5% weight loss after 3 months, it can be speculated that these changes in weight played a role in the benefits observed. However, these subjects were also fasting for approximately 17-22 hours on the fast day (i.e., midnight to 5:00-8:00 pm). Fasting diets induce the metabolic switch from glucose to fatty acid-derived ketone bodies for energy. 39 Ketones regulate the expression of many proteins that optimize physiological function, thereby slowing aging and disease processes. 39 However, in NAFLD, the role of ketones is less clear. It has been hypothesized that the combination of elevated liver fat and insulin resistance may predispose patients with NAFLD to increased ketogenesis by providing more substrate for ketone body production. But increased levels of ketones may pose risk, as noted in a recent cohort study,40 which shows that augmented ketones were independently associated with higher all-cause mortality in NAFLD. While these findings are concerning, it is unknown if ketones are the culprit in this association, or merely a reflection of an underlying mechanism. 40 Moreover, how higher levels of ketones impact degree of hepatic steatosis is still unclear, with some studies showing increases in liver fat, 41,42 decreases in liver fat, 43,44 or no effect. 45 Unfortunately, we did not measure circulating ketone bodies in the present trial, so we were not able to further explore these relationships.
Changes in body composition were also assessed. By the end of the trial, the combination group lost significantly more fat mass than the exercise group or control group. However, no change in lean mass was observed in the combination group relative to the other groups. Our findings are complementary to previous intermittent fasting studies. When fasting is applied alone, lean mass typically decreases by 10-30% from baseline. 17,29 However, when fasting is combined with endurance or resistance exercise, lean mass is maintained 46,47 or increased. 48,49 The maintenance or accretion of lean mass, or more specifically, skeletal muscle mass, has important implications for NAFLD. In a recent cohort study of approximately 13,000 subjects, skeletal muscle mass was negatively associated with NAFLD incidence and positively associated with NAFLD resolution. 50 Skeletal muscle is also independently associated with the severity of hepatic steatosis and fibrosis in a dose-dependent manner in patients with NAFLD. 51-53 Taken together, the lack of significant lean mass loss by the combination group likely played a role in the improvements noted in IHTG content. However, our study is limited in that it measured lean mass by DXA, instead of skeletal muscle mass by MRI or computed tomography. 54
The type, intensity, and duration of exercise necessary to elicit beneficials changes in NAFLD parameters is still uncertain. Both endurance and resistance exercise reduce hepatic steatosis by 20-40% from baseline, independent of weight loss. 55,56 At present, no definitive exercise guidelines have been established for NAFLD. However, general recommendations include a structured exercise program, involving >150 min/week of moderate-intensity aerobic exercise, or >75 min/week of high-intensity exercise training for at least 3 months. 57 Higher exercise intensity may be more effective for reducing liver fat, 58-60 but findings are not consistent. 61-63 Moreover, it is currently unknown if adding resistance training to aerobic exercise would be more effective for improving NAFLD parameters, versus aerobic exercise alone. 55-57 In the absence of clear guidelines, clinicians should emphasize the importance of exercise, but leave type and intensity of the training regimen to the patient. Allowing patients with NAFLD to individualize their exercise programs may help increase long-term adherence to these lifestyle therapies.
Our findings also show the combination intervention improved glycemic control by lowering fasting insulin, insulin resistance, and increasing insulin sensitivity. The insulin-sensitizing effects of intermittent fasting have been well documented. For instance, Gabel and colleagues 64 showed that fasting insulin and insulin resistance decreased by 52% and 53% from baseline, respectively, after 6 months of ADF in adults with insulin resistance and obesity. Likewise, Paravesh et al 65 reported 22% reductions in insulin resistance after 2 months of ADF in people with metabolic syndrome, relative to baseline. Aerobic exercise also has profound impacts on glycemic control. In a meta-analysis of 54 studies, aerobic exercise was shown to significantly reduce insulin resistance (measured by HOMA-IR) by 0.33 units in adults with obesity. 66 Exercise also helps improve insulin sensitivity in patients with NAFLD. 67-69 HbA1c, on the other hand, was not different between groups in the present study. These findings are somewhat surprising considering the improvements in insulin sensitivity. However, it is likely that our intervention period was too short to see significant modifications, as HbA1c generally takes 3 months to change. 70
Blood pressure was not significantly altered by the interventions in this 3-month trial. These findings are unexpected as blood pressure generally improves by both intermittent fasting 17 and aerobic exercise. 71 However, it is possible that the degree of weight loss experienced by the combination group (4.6%), ADF group (5.1%), and exercise group (2.1%), was not sufficient to observe a significant change in blood pressure. Body weight reductions of 7-10% from baseline are usually necessary to see significant improvements in blood pressure with diet and exercise interventions. 72,73
Plasma lipids were also not significantly different between groups by the end of the study. Intermittent fasting generally has little effect on in LDL cholesterol, HDL cholesterol, or triglyceride concentrations in individuals with obesity 17 or those with NAFLD, 14,15 so our findings are in line with previous reports. On the other hand, aerobic exercise (>120 min/week) generally increases HDL cholesterol concentrations in individuals with obesity 74,75 and NAFLD, 76,77 so our findings are not in accordance with those studies. The lack of improvement in HDL cholesterol may be partly explained by baseline lipid levels. Improvements in HDL cholesterol with exercise are more likely to occur when HDL cholesterol is low (<36 mg/dl) at baseline. 78 None of the groups had low HDL cholesterol at the onset of treatment (range 50-55 mg/dl), which may explain why no change in HDL cholesterol was observed.
We also examined the underlying role of hepatokines in mediating the effects observed. Hepatokines are proteins secreted by the liver that are known to directly affect glucose and insulin metabolism. 79 In individuals with NAFLD, hepatokine secretion is altered, which adversely affects glucoregulatory systems and exacerbates hepatic steatosis. 79 Fetuin-A, fibroblast growth factor-21 (FGF-21), and selenoprotein P have recently emerged as hepatokines of interest due to their effects on insulin sensitivity. Fetuin-A is a glycoprotein secreted by the liver that is positively correlated with hepatic steatosis, NAFLD severity, and insulin resistance. 80 This hepatokine disrupts tyrosine kinase receptor activity leading to impaired insulin signaling. 80 FGF-21, on the other hand, has been shown to improve insulin sensitivity and glycemic control due to augmented basal glucose disposal and increased Akt phosphorylation (a key step in hepatic insulin and FGF-21 signaling). 81 Considering these effects, FGF-21 analogs have recently emerged as potential therapeutic agents to treat hyperglycemia. 82 Lastly, Selenoprotein P is a hepatokine that disrupts glucose sensing in the beta-cell of the pancreas, inhibiting the secretion of insulin. In animal studies, inhibiting selenoprotein P expression was shown to improve insulin sensitivity and augment glucose tolerance. 83,84
In the present study, hepatokines did not change in any group after 3 months of intervention. These findings are somewhat surprising as the combination group experienced favorable reductions in hepatic steatosis, insulin resistance, and body weight. Nonetheless, it is possible that the degree of weight loss and liver fat reduction was not great enough to see changes in these hepatokines. Indeed, recent evidence suggests that a minimum of 7-10% weight loss may be required. 85,86 However, a positive correlation was observed between fetuin-A and HbA1c, and fetuin-A and fasting glucose levels. These results are in accordance with previous reports. For instance, in a sample of 3790 men, fetuin A was higher (309 ng/ml) in men with HbA1c greater than 7%, compared with those who had an HbA1c lower than 6.5% (290 ng/ml). 87 Results from other trials, demonstrate that fetuin A is positively correlated with fasting blood glucose in adults with new onset type 2 diabetes, 88 and those with NAFLD. 89 Results from our study and others, further highlight the relationship between increased fetuin A levels and the risk for developing type 2 diabetes and NAFLD. Future well powered RCTs that examine how lifestyle interventions impact hepatokines, are warranted.
Adherence to the ADF diet and exercise interventions was very good. Fast day energy intake in the combination group was 673 kcal/d (2815 kJ), on average, which was 73 kcal (305 kJ) more than that prescribed. Likewise, fast day energy intake in the ADF group was 581 kcal/d (2430 kJ/d), on average, which was 19 kcal (79 kJ) less than that prescribed. Our findings are complementary to what has been reported previously for short-term adherence to ADF. 17,29,32 In addition, a high percent of subjects in the combination group (95%) and ADF group (85%) said that they would like to continue with the fasting protocol after the study was over. This bodes well for the longer-term feasibility of ADF in patients with NAFLD, but a >6-month RCT would be needed to confirm these assumptions. As for exercise adherence, attendance at the supervised sessions was consistently high, with participants attending 4 out of 5 sessions on average, over 3 months. Taken together, these findings suggest that patients with NAFLD may not find it difficult to follow a lifestyle intervention that combines ADF with exercise for short durations (up to 3 months). However, previous trials have shown that adherence to both intermittent fasting 32,90 and aerobic exercise 91 wanes after 6 months. Thus, it will be important to examine if the high level of adherence can be sustained for longer periods of time in this population group.
Dropout rates in the combination group (0%) and ADF group (5%) were minimal, but the dropout rate in the exercise group was moderately high (25%). This dropout rate may have induced selection bias. 92 Upon closer examination of the exercise dropouts, these individuals tended to be heavier (BMI 41 kg/m2) than the rest of the sample (BMI 36 kg/m2). This excess body weight may have made it more difficult for these participants to perform the exercise, which could have contributed to them dropping out. It is also possible that some participants in the exercise group may have wanted to participate in the intermittent fasting intervention. After finding out that they were not randomized to the fasting protocol, they may have lost motivation to participate in the study, which may have led them to drop out. However, since no formal exit survey was performed in our trial, the precise reason for the greater dropouts in the exercise group remains unknown. This highlights the need for future studies to perform a comprehensive exit interview in dropout subjects to see what led them to terminate their participation.
In conclusion, the results of this randomized controlled trial demonstrate that ADF combined with aerobic exercise is an effective lifestyle therapy to reduce IHTG content versus exercise alone and controls. However, more research will be required to inform whether the combination intervention produces greater reductions in IHTG content versus fasting alone. The combination therapy also produced significant decreases in body weight, fat mass, waist circumference, ALT levels, fasting insulin, insulin resistance, and significant increases in insulin sensitivity, versus controls. Compliance to the ADF and exercise protocols was shown to be very good and 93% of subjects randomized completed the full protocol. While these preliminary findings offer promise for the use of ADF combined with aerobic exercise to improve NAFLD outcomes, future trials will be needed to examine whether these benefits can be sustained over longer durations of time.
Limitations
Our study has several limitations. First, the combination intervention produced beneficial changes in key NAFLD parameters, but it did not achieve a reduction in IHTG content and ALT levels into the normal range. Second, the intervention period was short (3 months). The long-term effects of intermittent fasting alone or combined with exercise on NAFLD outcomes warrants investigation. Third, we did not follow up with the participants to see if they were still following the interventions after the study was over. Fourth, the generalizability of our findings is questionable. Based on baseline IHTG content (range: 16-18%, where 5-33% is considered mild steatosis 93,94), and liver fibrosis score, it would appear as though our sample was at an early stage of NAFLD development. Thus, it is unclear if our findings can be extrapolated to patients with a more severe form of the disease. Our participants were also highly adherent to both the diet and exercise interventions. This suggests that our subjects were perhaps a highly motivated subgroup of patients with NAFLD. Whether or not individuals with a more advanced stage of the disease would be just as compliant with this intensive lifestyle therapy, remains unknown. Fifth, baseline values for certain secondary outcomes were numerically different between groups. More specifically, energy intake, ALT and insulin resistance were all higher numerically (but not statistically) at the beginning of the study in the combination group compared to controls. This could have increased the likelihood for observing larger mean absolute differences in the combination group. Thus, these findings should be interpreted with caution, as regression to the mean could be a possible cause of this observed change. 95
Lastly, since our study began in January 2020, it was impacted by the COVID pandemic. It is likely that the regular activity level of our participants decreased since they had to stay at home for several months. Based on the pedometer data, there were no significant changes in steps per day in any group from the beginning to the end of the trial. However, our study is limited in that the pedometer was only worn at baseline and month 3, so we failed to capture weekly changes in activity levels. In addition, our supervised exercise program changed considerably because of the pandemic. At the beginning of the study (early 2020), the exercise sessions were conducted at the research center and supervised by the study coordinator. However, when the pandemic began, we had to shift all the exercise sessions to home-based programs where subjects used their own exercise equipment or watched online exercise videos. Even though the sessions were still supervised via video conference, using different modalities of exercise may have introduced some variability into the intervention.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Krista Varady (varady@uic.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All other data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials, see Data S1.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human subjects
Participants were recruited from the Hepatology Clinic at the University of Illinois Chicago Medical Center. Subjects (mean age 44 ± 13 y; 81% female, 19% male) were enrolled in the study between January 2020 and March 2022. Participants were recruited in four nonoverlapping rounds (approximately 20 subjects per round). Individuals between the ages of 18 and 65 years with obesity (body mass index (BMI) between 30 and 60 kg/m2) were screened via a questionnaire and ALT blood test. Women who had an ALT greater than 17 U/L 96 and men who had an ALT greater than 25 U/L 96 were invited to have their IHTG content quantified by magnetic resonance imaging proton density fat fraction (MRI-PDFF). 97 Those who had ultra-sonography or biopsy diagnosed NAFLD were also invited to confirm their diagnosis by MRI-PDFF. Participants were included in the study if the MRI-PDFF exam revealed that IHTG content was at least 5% of liver weight. 12 Participants were excluded if they had a history of acute or chronic viral hepatitis, drug-induced liver diseases, or autoimmune hepatitis. Women who consumed more than a mean of 70 g of ethanol (5 alcoholic drinks per week) and men who consumed more than 140 g of ethanol (10 drinks per week) in the past 6 months were also excluded. In addition, patients were excluded if they had a history of diabetes, cardiovascular disease, or chronic kidney disease. Patients who were weight unstable, i.e., more than 4 kg weight loss or gain in the past 3 months, or who had a medical condition that would preclude them from participating in an exercise program, were also excluded. The protocol was approved by the Office for the Protection of Research Subjects at the University of Illinois at Chicago, and informed consent was obtained from all participants.
Experimental design
The study was a 3-month randomized, controlled, parallel-arm trial designed to compare the effects of ADF combined with aerobic exercise, to each intervention alone, on IHTG content and metabolic disease risk factors in patients with NAFLD. Participants were randomized in a 1:1:1:1 ratio to one of four intervention groups: ADF combined with exercise, ADF alone, exercise alone, or no-intervention control group (Figure S1 in the Supplementary Appendix).
ADF protocol
Participants assigned to the combination group and ADF group were instructed to consume 600 calories as a dinner (between 5:00 pm and 8:00 pm) on fast days and eat food as desired on alternating feast days. The feast and fast days began at midnight each day. As such, on fast days, subjects fasted for approximately 17-20 hours (from midnight to 5:00 pm or 8:00 pm). During the fasting period on fast days, participants were encouraged to drink plenty of water and were permitted to consume calorie free drinks such as black coffee, tea, and diet soda in moderation. These participants were provided with fast day meals during the first month of the trial and received dietary counseling thereafter. The provided fast day meals were in accordance with the American Diabetes Association guidelines 98 for macronutrient intake, with 30%, 55%, and 15% of energy as fat, carbohydrate and protein, respectively. The exercise group and control group participants were asked to maintain their regular eating habits and were not provided with any food or dietary counseling.
Exercise protocol
Participants assigned to the combination group and exercise group participated in a moderate-intensity aerobic exercise program five times per week for 3 months. All exercise sessions were supervised by study staff. Exercise was performed using treadmills, stationary bikes, or elliptical machines at the research center. The maximum predicted heart rate was calculated as 210/min (220/min for men) minus the participant’s age. 99 Heart rates were monitored by an activity monitor worn on the wrist. Training intensity gradually increased over the first four weeks of the study from 65 to 80% of their maximum predicted heart rate (equivalent to 8.0 to 10.0 metabolic equivalents). The participants were instructed to exercise at this intensity for 60 minutes per session. During the peak of the COVID-19 pandemic, participants transitioned to an at-home exercise program using their own treadmills, stationary bikes, elliptical machines, or by watching online aerobic exercise videos. These at-home sessions were supervised by study staff via video conference. Participants in the ADF and control groups did not participate in the exercise intervention and were instructed to not change their physical activity habits.
Control group protocol
Control participants were instructed to maintain their body weight throughout the trial, and not to change their eating or physical activity habits. Controls received no food or dietary counseling but visited the research center at the same frequency as the intervention participants to provide outcome measurements. Controls who completed the 3-month trial received free weight loss counseling at the end of the study.
METHOD DETAILS
Intrahepatic triglyceride (IHTG) content by MRI-PDFF
The primary outcome of the study was absolute percent change in IHTG content from baseline to month 3. The IHTG content was measured using MRI-PDFF. 97 MRI scans were performed at the Center for Magnetic Resonance Research at the University of Illinois Medical Center. MRI-PDFF was utilized with a 3.0-Tesla MRI scanner (SIEMENS) for the baseline and follow-up liver fat estimations. Fat-water separation images of the liver were acquired using a T1volumetric interpolated breath-hold examination (VIBE) Dixon sequence with the following parameter settings: TE1 2.5ms; TE2 3.7ms; repetition time 5.47ms; 5° flip angle; ±504.0kHz per pixel receiver bandwidth; and a slice thickness of 3.0 mm. The fat content was calculated in an irregular-shaped ROI covering the entire liver in 21 consecutive slices (max-area centered) of each participant placed by a trained radiologist manually. MRI-PDFF maps for all segments were generated by placing circular ROIs with diameter of 20mm centrally in each of the eight liver segments. The average fat content values were calculated for the entire liver. The technician performing the MRI-PDFF measurements was blinded to participant group assignment.
Body weight and body composition
Body weight measurements were taken with subjects wearing light clothing and without shoes using a digital scale at home each week. Body weight was also measured at the research center at baseline and month 3. Height was assessed using a wall-mounted stadiometer at baseline. BMI will be assessed as kg/m2. Dual energy X-ray absorptiometry (DXA) was performed on all subjects at baseline and month 3 to assess fat mass, fat free mass, and visceral fat mass (iDXA, GE Inc).
Metabolic disease risk factors:
Twelve-hour fasting blood samples were collected at baseline and month 3. Blood was centrifuged for 15 min at 520 x g and 4°C to separate plasma from red cells and was stored at −80°C. Plasma metabolic disease risk factors were assessed at baseline and month 3. Plasma total cholesterol, LDL cholesterol, HDL cholesterol, triglyceride, fasting glucose, fasting insulin, HbA1c, ALT, and AST concentrations were measured by a commercial lab (Medstar, IL). Insulin resistance (IR) was calculated by the HOMA (Homeostasis Model Assessment) method: [HOMA-IR = Fasting insulin (μlU/ml) × Fasting glucose (mg/dL) / 405].100 Insulin sensitivity was measured by the Quantitative insulin sensitivity check index (QUICKI) and calculated as: 1 / [log [Insulin (μlU/ml)] + log [Glucose (mg/dl)].101 Blood pressure and heart rate was assessed after a 10-minute rest at baseline and month 3 using a blood pressure cuff. Degree of liver fibrosis was estimated using the Fibrosis-4 (FIB-4) index: Age (years) × AST (IU/L)/(√ALT (IU/L) × Platelet count (109/L)).102
Hepatokines
Plasma levels of fetuin-A, FGF-21, and selenoprotein P were measured by ELISA (Invitrogen, Frederick, MD; RayBio Tech, Norcross, GA, and MyBioSource Inc., San Diego, CA) on a Bio Rad Microplate reader (Bio-Rad Laboratories; Hercules, CA) in duplicate.
Adherence to the intervention and dietary intake
Adherence with the diet intervention and dietary intake was assessed at baseline and month 3 using the NIH web-based system, Automated Self-administered 24-hour Dietary Assessment Tool (ASA24).103 Participants following the ADF diet were considered “adherent” when actual energy intake on the fast day, determined via food records, was not significantly different from the prescribed energy goal for that day (600 kcal; 2500 kJ). Participants following the exercise program were considered adherent if they attended 80% or more of the supervised exercise sessions (a mean of 4 of 5 per week). All participants were required to wear a pedometer (Fitbit Alta) for 7 days to record their regular physical activity (excluding the exercise intervention program) at baseline and at month 3. On the last of the study, the combination and ADF group were asked if they would be willing to continue with the ADF diet after the study was over. The percent of subjects willing to continue the fasting intervention was calculated based on these survey findings.
QUANTIFICATION AND STATISTICAL ANALYSIS
Power and sample size
For the sample size calculation, we estimated that IHTG content would be reduced by 5.0% in the combination group, 2.5% in the ADF group, 2.5% in the exercise group, and 0% in the control group, by month 3. These estimations were derived from a pilot trial conducted by our lab. We calculated that 17 participants per group would provide 90% power to detect a significant difference in IHTG content between the combination group and the three other groups using one-way ANOVA with α = 0.05. We anticipated a dropout rate of 15%. Thus, we aimed to recruit 80 participants (20 per group) assuming that 68 participants (17 per group) would complete the trial.
Randomization
Participants were randomized in a 1:1:1:1 ratio to one of four intervention groups: ADF combined with exercise, ADF alone, exercise alone, or no-intervention control group (Figure S1 in the Supplementary Appendix). Randomization was performed by a stratified random sampling procedure by sex, age (18-42 y/ 43-65 y), BMI (30.0-45.0 kg/m2/45.1-60.0 kg/m2), and IHTG content (5.0-17.5%/ 17.6-30.0%). Due to the nature of the interventions, the study could not be blinded. However, study staff involved in outcome ascertainment were blinded as to the subjects’ group assignments.
Statistical analyses
Data are shown as mean (95% CI) unless otherwise noted. A two-tailed P value of less than 0.05 was considered statistically significant. We conducted an intention-to-treat analysis, which included data from all 80 participants who underwent randomization. Results are reported by intention-to-treat analysis unless indicated otherwise.
A linear mixed model was used to assess time, group, and time*group effects for each outcome. Linear mixed models for longitudinal data analysis account for missing outcome data using maximum likelihood principles. Thus, these models provide unbiased estimates of time and treatment effects under a missing at random assumption. Group is included in the model to account for baseline differences in the outcome variable between treatment groups. The inclusion of time in the model allows for changes in the outcome over time that are unrelated to the intervention (e.g., regression to the mean, Hawthorne effect). In models for body weight, which was measured at 12 time points, time was modeled with cubic splines. This strategy allowed for estimation of time and group effects (and their interaction) without imposing a linear time trend. The decision to model time with splines was based on a priori rationale for possibly non-linear intervention effects over time (e.g., weight plateau effect, declining compliance) rather than empirical evidence of non-linearity. The interaction terms provide estimates of the 12-week intervention effects, here defined as the effect of assignment to the ADF, exercise, or control compared to the combination group. Estimated changes from baseline and P values for intervention effects based on the linear mixed models are shown in Table 2.
For each outcome variable, linear modelling assumptions were assessed with residual diagnostics. To account for the potential for non-uniform variances (heteroskedasticity) between treatment groups due to random chance, CIs and P values (i.e., body weight percent, ALT, and HOMA-IR) were calculated using robust variance estimators (sandwich estimators) as needed. 104-106 The analyses were performed using R software (version 4.3.1).
Supplementary Material
Unprocessed data underlying the display items in the manuscript, related to Figures 2, 3 and 4
aa
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical commercial assays | ||
| Fetuin A Human ELISA Kit | Thermofisher-Invitrogen | EHAHSG |
| FGF-21 Human ELISA Kit | Ray Biotech | ELH-FGF21-1 |
| Selenoprotein P Human ELISA Kit | MyBioSource | MBS760712 |
| Software and algorithms | ||
| R software | R Foundation for Statistical Computing | https://www.r-project.org |
HIGHLIGHTS.
Adults with NAFLD followed an intermittent fasting plus exercise protocol for 3 months
Hepatic steatosis was significantly reduced by 5.5% versus controls
Body weight, fat mass, and waist circumference also decreased versus controls
ALT was reduced, while insulin sensitivity increased, versus controls, by month 3
ACKNOWLEDGMENTS
The authors would like to thank the study participants for their time and effort in participating in the trial. This study was supported by R01DK119783 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sources of funding:
National Institutes of Health, NIDDK (R01DK119783).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ADDITIONAL RESOURCES
Prior to enrolling participants, the trial was preregistered on clinicaltrials.gov (NCT04004403).
INCLUSION AND DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
DECLARATION OF INTERESTS
K.A.V. received author fees from Hachette Book Group for the book The Every Other Day Diet. The other authors declare no competing interests.
REFERENCES
- 1.Mitra S, De A, and Chowdhury A (2020). Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol 5, 16. 10.21037/tgh.2019.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godoy-Matos AF, Silva Junior WS, and Valerio CM (2020). NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol Metab Syndr 12, 60. 10.1186/s13098-020-00570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Targher G, Corey KE, Byrne CD, and Roden M (2021). The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol 18, 599–612. 10.1038/s41575-021-00448-y. [DOI] [PubMed] [Google Scholar]
- 4.Zhang CH, Zhou BG, Sheng JQ, Chen Y, Cao YQ, and Chen C (2020). Molecular mechanisms of hepatic insulin resistance in non-alcoholic fatty liver disease and potential treatment strategies. Pharmacol Res 159, 104984. 10.1016/j.phrs.2020.104984. [DOI] [PubMed] [Google Scholar]
- 5.Lipscombe LL, Gomes T, Levesque LE, Hux JE, Juurlink DN, and Alter DA (2007). Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA 298, 2634–2643. 10.1001/jama.298.22.2634. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca V (2003). Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med 115 Suppl 8A, 42S–48S. 10.1016/j.amjmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Cigrovski Berkovic M, Bilic-Curcic I, Mrzljak A, and Cigrovski V (2021). NAFLD and Physical Exercise: Ready, Steady, Go! Front Nutr 8, 734859. 10.3389/fnut.2021.734859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, Takano Y, Ueno T, Koga H, George J, et al. (2017). Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J Hepatol 66, 142–152. 10.1016/j.jhep.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Farzanegi P, Dana A, Ebrahimpoor Z, Asadi M, and Azarbayjani MA (2019). Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur J Sport Sci 19, 994–1003. 10.1080/17461391.2019.1571114. [DOI] [PubMed] [Google Scholar]
- 10.Sargeant JA, Gray LJ, Bodicoat DH, Willis SA, Stensel DJ, Nimmo MA, Aithal GP, and King JA (2018). The effect of exercise training on intrahepatic triglyceride and hepatic insulin sensitivity: a systematic review and meta-analysis. Obes Rev 19, 1446–1459. 10.1111/obr.12719. [DOI] [PubMed] [Google Scholar]
- 11.Carraca EV, Encantado J, Battista F, Beaulieu K, Blundell JE, Busetto L, van Baak M, Dicker D, Ermolao A, Farpour-Lambert N, et al. (2021). Effect of exercise training on psychological outcomes in adults with overweight or obesity: A systematic review and meta-analysis. Obes Rev 22 Suppl 4, e13261. 10.1111/obr.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ando Y, and Jou JH (2021). Nonalcoholic Fatty Liver Disease and Recent Guideline Updates. Clin Liver Dis (Hoboken) 17, 23–28. 10.1002/cld.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, and Sanyal AJ (2018). The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357. 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 14.Cai H, Qin YL, Shi ZY, Chen JH, Zeng MJ, Zhou W, Chen RQ, and Chen ZY (2019). Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol 19, 219. 10.1186/s12876-019-1132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johari MI, Yusoff K, Haron J, Nadarajan C, Ibrahim KN, Wong MS, Hafidz MIA, Chua BE, Hamid N, Arifin WN, et al. (2019). A Randomised Controlled Trial on the Effectiveness and Adherence of Modified Alternate-day Calorie Restriction in Improving Activity of Non-Alcoholic Fatty Liver Disease. Sci Rep 9, 11232. 10.1038/s41598-019-47763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GoogleTrends (2022). Google top trending diets of 2022. https://trends.google.com/trends/explore?q=intermittent%20fasting&geo=US. [Google Scholar]
- 17.Varady KA, Cienfuegos S, Ezpeleta M, and Gabel K (2022). Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Rev Endocrinol. 10.1038/s41574-022-00638-x. [DOI] [PubMed] [Google Scholar]
- 18.Cheng S, Ge J, Zhao C, Le S, Yang Y, Ke D, Wu N, Tan X, Zhang X, Du X, et al. (2017). Effect of aerobic exercise and diet on liver fat in pre-diabetic patients with non-alcoholic-fatty-liver-disease: A randomized controlled trial. Sci Rep 7, 15952. 10.1038/s41598-017-16159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez T, Vinuela M, Vidal C, and Barrera F (2022). Lifestyle changes in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. PLoS One 17, e0263931. 10.1371/journal.pone.0263931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantartzis K, Thamer C, Peter A, Machann J, Schick F, Schraml C, Konigsrainer A, Konigsrainer I, Krober S, Niess A, et al. (2009). High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut 58, 1281–1288. 10.1136/gut.2008.151977. [DOI] [PubMed] [Google Scholar]
- 21.Holmer M, Lindqvist C, Petersson S, Moshtaghi-Svensson J, Tillander V, Brismar TB, Hagstrom H, and Stal P (2021). Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet - a randomised controlled trial. JHEP Rep 3, 100256. 10.1016/j.jhepr.2021.100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadizadeh F, Faghihimani E, and Adibi P (2017). Nonalcoholic fatty liver disease: Diagnostic biomarkers. World J Gastrointest Pathophysiol 8, 11–26. 10.4291/wjgp.v8.i2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Browning JD (2006). Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology 44, 466–471. 10.1002/hep.21248. [DOI] [PubMed] [Google Scholar]
- 24.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, and Bellentani S (2005). Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 42, 44–52. 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 25.Charatcharoenwitthaya P, Lindor KD, and Angulo P (2012). The spontaneous course of liver enzymes and its correlation in nonalcoholic fatty liver disease. Dig Dis Sci 57, 1925–1931. 10.1007/s10620-012-2098-3. [DOI] [PubMed] [Google Scholar]
- 26.Prashanth M, Ganesh HK, Vima MV, John M, Bandgar T, Joshi SR, Shah SR, Rathi PM, Joshi AS, Thakkar H, et al. (2009). Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India 57, 205–210. [PubMed] [Google Scholar]
- 27.Gholam PM, Flancbaum L, Machan JT, Charney DA, and Kotler DP (2007). Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol 102, 399–408. 10.1111/j.1572-0241.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 28.Kashyap SR, Diab DL, Baker AR, Yerian L, Bajaj H, Gray-McGuire C, Schauer PR, Gupta M, Feldstein AE, Hazen SL, and Stein CM (2009). Triglyceride levels and not adipokine concentrations are closely related to severity of nonalcoholic fatty liver disease in an obesity surgery cohort. Obesity (Silver Spring) 17, 1696–1701. 10.1038/oby.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson RE, and Sears DD (2017). Metabolic Effects of Intermittent Fasting. Annu Rev Nutr 37, 371–393. 10.1146/annurev-nutr-071816-064634. [DOI] [PubMed] [Google Scholar]
- 30.Kretsch MJ, Fong AK, and Green MW (1999). Behavioral and body size correlates of energy intake underreporting by obese and normal-weight women. J Am Diet Assoc 99, 300–306; quiz 307-308. 10.1016/S0002-8223(99)00078-4. [DOI] [PubMed] [Google Scholar]
- 31.Goris AH, Westerterp-Plantenga MS, and Westerterp KR (2000). Undereating and underrecording of habitual food intake in obese men: selective underreporting of fat intake. Am J Clin Nutr 71, 130–134. 10.1093/ajcn/71.1.130. [DOI] [PubMed] [Google Scholar]
- 32.Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J, et al. (2017). Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern Med 177, 930–938. 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, Hoddy KK, and Calvo Y (2013). Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J 12, 146. 10.1186/1475-2891-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klempel MC, Bhutani S, Fitzgibbon M, Freels S, and Varady KA (2010). Dietary and physical activity adaptations to alternate day modified fasting: implications for optimal weight loss. Nutr J 9, 35. 10.1186/1475-2891-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harvey J, Howell A, Morris J, and Harvie M (2018). Intermittent energy restriction for weight loss: Spontaneous reduction of energy intake on unrestricted days. Food Sci Nutr 6, 674–680. 10.1002/fsn3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westerterp KR (2017). Doubly labelled water assessment of energy expenditure: principle, practice, and promise. Eur J Appl Physiol 117, 1277–1285. 10.1007/s00421-017-3641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, Chim AM, Lai JW, Li LS, Sea MM, et al. (2013). Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 59, 536–542. 10.1016/j.jhep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, and Romero-Gomez M (2015). Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 149, 367–378 e365; quiz e314-365. 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 39.de Cabo R, and Mattson MP (2019). Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med 381, 2541–2551. 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 40.Post A, Garcia E, van den Berg EH, Flores-Guerrero JL, Gruppen EG, Groothof D, Westenbrink BD, Connelly MA, Bakker SJL, and Dullaart RPF (2021). Nonalcoholic fatty liver disease, circulating ketone bodies and all-cause mortality in a general population-based cohort. Eur J Clin Invest 51, e13627. 10.1111/eci.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, and Rizzetto M (2005). Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia 48, 634–642. 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 42.Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, and Crabb DW (2003). Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 37, 544–550. 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 43.Mey JT, Erickson ML, Axelrod CL, King WT, Flask CA, McCullough AJ, and Kirwan JP (2020). beta-Hydroxybutyrate is reduced in humans with obesity-related NAFLD and displays a dose-dependent effect on skeletal muscle mitochondrial respiration in vitro. Am J Physiol Endocrinol Metab 319, E187–E195. 10.1152/ajpendo.00058.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fletcher JA, Deja S, Satapati S, Fu X, Burgess SC, and Browning JD (2019). Impaired ketogenesis and increased acetyl-CoA oxidation promote hyperglycemia in human fatty liver. JCI Insight 5. 10.1172/jci.insight.127737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotronen A, Seppala-Lindroos A, Vehkavaara S, Bergholm R, Frayn KN, Fielding BA, and Yki-Jarvinen H (2009). Liver fat and lipid oxidation in humans. Liver Int 29, 1439–1446. 10.1111/j.1478-3231.2009.02076.x. [DOI] [PubMed] [Google Scholar]
- 46.Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, and Varady KA (2013). Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity (Silver Spring) 21, 1370–1379. 10.1002/oby.20353. [DOI] [PubMed] [Google Scholar]
- 47.Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, and Paoli A (2016). Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med 14, 290. 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tinsley GM, Moore ML, Graybeal AJ, Paoli A, Kim Y, Gonzales JU, Harry JR, VanDusseldorp TA, Kennedy DN, and Cruz MR (2019). Time-restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr 110, 628–640. 10.1093/ajcn/nqz126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moro T, Tinsley G, Pacelli FQ, Marcolin G, Bianco A, and Paoli A (2021). Twelve Months of Time-restricted Eating and Resistance Training Improves Inflammatory Markers and Cardiometabolic Risk Factors. Med Sci Sports Exerc 53, 2577–2585. 10.1249/MSS.0000000000002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim G, Lee SE, Lee YB, Jun JE, Ahn J, Bae JC, Jin SM, Hur KY, Jee JH, Lee MK, and Kim JH (2018). Relationship Between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7-Year Longitudinal Study. Hepatology 68, 1755–1768. 10.1002/hep.30049. [DOI] [PubMed] [Google Scholar]
- 51.Chung GE, Park HE, Kim MJ, Kwak MS, Yang JI, Chung SJ, Yim JY, and Yoon JW (2021). The Association between Low Muscle Mass and Hepatic Steatosis in Asymptomatic Population in Korea. Life (Basel) 11. 10.3390/life11080848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo W, Zhao X, Miao M, Liang X, Li X, Qin P, Lu J, Zhu W, Wu J, Zhu C, et al. (2022). Association Between Skeletal Muscle Mass and Severity of Steatosis and Fibrosis in Non-alcoholic Fatty Liver Disease. Front Nutr 9, 883015. 10.3389/fnut.2022.883015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kouvari M, Polyzos SA, Chrysohoou C, Skoumas J, Pitsavos CS, Panagiotakos DB, and Mantzoros CS (2022). Skeletal muscle mass and abdominal obesity are independent predictors of hepatic steatosis and interact to predict ten-year cardiovascular disease incidence: Data from the ATTICA cohort study. Clin Nutr 41, 1281–1289. 10.1016/j.clnu.2022.03.022. [DOI] [PubMed] [Google Scholar]
- 54.Faron A, Sprinkart AM, Kuetting DLR, Feisst A, Isaak A, Endler C, Chang J, Nowak S, Block W, Thomas D, et al. (2020). Body composition analysis using CT and MRI: intra-individual intermodal comparison of muscle mass and myosteatosis. Sci Rep 10, 11765. 10.1038/s41598-020-68797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brouwers B, Hesselink MK, Schrauwen P, and Schrauwen-Hinderling VB (2016). Effects of exercise training on intrahepatic lipid content in humans. Diabetologia 59, 2068–2079. 10.1007/s00125-016-4037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romero-Gomez M, Zelber-Sagi S, and Trenell M (2017). Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 67, 829–846. 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 57.Thyfault JP, and Rector RS (2020). Exercise Combats Hepatic Steatosis: Potential Mechanisms and Clinical Implications. Diabetes 69, 517–524. 10.2337/dbi18-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsunoda K, Kai Y, Kitano N, Uchida K, Kuchiki T, and Nagamatsu T (2016). Impact of physical activity on nonalcoholic steatohepatitis in people with nonalcoholic simple fatty liver: A prospective cohort study. Prev Med 88, 237–240. 10.1016/j.ypmed.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 59.Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB, and Group NCR (2011). Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol 106, 460–468; quiz 469. 10.1038/ajg.2010.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamasaki H (2019). Perspectives on Interval Exercise Interventions for Non-Alcoholic Fatty Liver Disease. Medicines (Basel) 6. 10.3390/medicines6030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keating SE, Hackett DA, Parker HM, O'Connor HT, Gerofi JA, Sainsbury A, Baker MK, Chuter VH, Caterson ID, George J, and Johnson NA (2015). Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol 63, 174–182. 10.1016/j.jhep.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 62.Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, Chen Z, Han HW, Chen S, Sun Q, et al. (2016). Effects of Moderate and Vigorous Exercise on Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. JAMA Intern Med 176, 1074–1082. 10.1001/jamainternmed.2016.3202. [DOI] [PubMed] [Google Scholar]
- 63.Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Elnegamy TE, Soliman GS, and Ibrahim AA (2020). Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: A comparative randomized controlled trial. Medicine (Baltimore) 99, e19471. 10.1097/MD.0000000000019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gabel K, Kroeger CM, Trepanowski JF, Hoddy KK, Cienfuegos S, Kalam F, and Varady KA (2019). Differential Effects of Alternate-Day Fasting Versus Daily Calorie Restriction on Insulin Resistance. Obesity (Silver Spring) 27, 1443–1450. 10.1002/oby.22564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parvaresh A, Razavi R, Abbasi B, Yaghoobloo K, Hassanzadeh A, Mohammadifard N, Safavi SM, Hadi A, and Clark CCT (2019). Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: A randomized clinical trial. Complement Ther Med 47, 102187. 10.1016/j.ctim.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 66.Battista F, Ermolao A, van Baak MA, Beaulieu K, Blundell JE, Busetto L, Carraca EV, Encantado J, Dicker D, Farpour-Lambert N, et al. (2021). Effect of exercise on cardiometabolic health of adults with overweight or obesity: Focus on blood pressure, insulin resistance, and intrahepatic fat-A systematic review and meta-analysis. Obes Rev 22 Suppl 4, e13269. 10.1111/obr.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, Day CP, and Trenell MI (2011). Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 60, 1278–1283. 10.1136/gut.2011.242073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, and Arslanian S (2012). Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes 61, 2787–2795. 10.2337/db12-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, Jones H, Pugh CJ, Richardson P, Kemp GJ, Barrett M, Jackson NC, Thomas EL, et al. (2016). Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 130, 93–104. 10.1042/CS20150447. [DOI] [PubMed] [Google Scholar]
- 70.Eyth E, Naik R (2022). NIH StatPearls. Hemoglobin A1C. https://www.ncbi.nlm.nih.gov/books/NBK549816/. [PubMed] [Google Scholar]
- 71.Whelton SP, Chin A, Xin X, and He J (2002). Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 136, 493–503. 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 72.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L, and Look ARG (2011). Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 34, 1481–1486. 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williamson DA, Bray GA, and Ryan DH (2015). Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring) 23, 2319–2320. 10.1002/oby.21358. [DOI] [PubMed] [Google Scholar]
- 74.Palazon-Bru A, Hernandez-Lozano D, and Gil-Guillen VF (2021). Which Physical Exercise Interventions Increase HDL-Cholesterol Levels? A Systematic Review of Meta-analyses of Randomized Controlled Trials. Sports Med 51, 243–253. 10.1007/s40279-020-01364-y. [DOI] [PubMed] [Google Scholar]
- 75.Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K, et al. (2007). Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med 167, 999–1008. 10.1001/archinte.167.10.999. [DOI] [PubMed] [Google Scholar]
- 76.Ghamarchehreh ME, Shamsoddini A, and Alavian SM (2019). Investigating the impact of eight weeks of aerobic and resistance training on blood lipid profile in elderly with non-alcoholic fatty liver disease: a randomized clinical trial. Gastroenterol Hepatol Bed Bench 12, 190–196. [PMC free article] [PubMed] [Google Scholar]
- 77.Whyte MB, Shojaee-Moradie F, Sharaf SE, Cuthbertson DJ, Kemp GJ, Barrett M, Jackson NC, Herring RA, Wright J, Thomas EL, et al. (2020). HDL-apoA-I kinetics in response to 16 wk of exercise training in men with nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab 318, E839–E847. 10.1152/ajpendo.00019.2020. [DOI] [PubMed] [Google Scholar]
- 78.Thompson PD, and Rader DJ (2001). Does exercise increase HDL cholesterol in those who need it the most? Arterioscler Thromb Vasc Biol 21, 1097–1098. 10.1161/hq0701.092147. [DOI] [PubMed] [Google Scholar]
- 79.Watt MJ, Miotto PM, De Nardo W, and Montgomery MK (2019). The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr Rev 40, 1367–1393. 10.1210/er.2019-00034. [DOI] [PubMed] [Google Scholar]
- 80.Trepanowski JF, Mey J, and Varady KA (2015). Fetuin-A: a novel link between obesity and related complications. Int J Obes (Lond) 39, 734–741. 10.1038/ijo.2014.203. [DOI] [PubMed] [Google Scholar]
- 81.Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, and Wasserman DH (2009). Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 150, 4084–4093. 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rader DJ, Maratos-Flier E, Nguyen A, Hom D, Ferriere M, Li Y, Kompa J, Martic M, Hinder M, Basson CT, et al. (2022). LLF580, an FGF21 Analog, Reduces Triglycerides and Hepatic Fat in Obese Adults With Modest Hypertriglyceridemia. J Clin Endocrinol Metab 107, e57–e70. 10.1210/clinem/dgab624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mita Y, Nakayama K, Inari S, Nishito Y, Yoshioka Y, Sakai N, Sotani K, Nagamura T, Kuzuhara Y, Inagaki K, et al. (2017). Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models. Nat Commun 8, 1658. 10.1038/s41467-017-01863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, Ishikura K, Ando H, Takeshita Y, Ota T, et al. (2010). A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab 12, 483–495. 10.1016/j.cmet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 85.Brix JM, Stingl H, Hollerl F, Schernthaner GH, Kopp HP, and Schernthaner G (2010). Elevated Fetuin-A concentrations in morbid obesity decrease after dramatic weight loss. J Clin Endocrinol Metab 95, 4877–4881. 10.1210/jc.2010-0148. [DOI] [PubMed] [Google Scholar]
- 86.Lim J, Park HS, Lee SK, Jang YJ, Lee YJ, and Heo Y (2016). Longitudinal Changes in Serum Levels of Angiopoietin-Like Protein 6 and Selenoprotein P After Gastric Bypass Surgery. Obes Surg 26, 825–832. 10.1007/s11695-015-1808-2. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y, Xu M, Xu Y, Li M, Wang T, Chen Y, and Bi Y (2012). Positive correlation between chronic hyperglycemia and serum fetuin-A levels in middle-aged and elderly Chinese. J Diabetes 4, 351–358. 10.1111/1753-0407.12000. [DOI] [PubMed] [Google Scholar]
- 88.Yin L, Cai WJ, Chang XY, Li J, Su XH, Zhu LY, Wang XL, and Sun K (2014). Association between fetuin-A levels with insulin resistance and carotid intima-media thickness in patients with new-onset type 2 diabetes mellitus. Biomed Rep 2, 839–842. 10.3892/br.2014.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ou HY, Yang YC, Wu HT, Wu JS, Lu FH, and Chang CJ (2012). Increased fetuin-A concentrations in impaired glucose tolerance with or without nonalcoholic fatty liver disease, but not impaired fasting glucose. J Clin Endocrinol Metab 97, 4717–4723. 10.1210/jc.2012-2414. [DOI] [PubMed] [Google Scholar]
- 90.Carter S, Clifton PM, and Keogh JB (2018). Effect of Intermittent Compared With Continuous Energy Restricted Diet on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Noninferiority Trial. JAMA Netw Open 1, e180756. 10.1001/jamanetworkopen.2018.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Linke SE, Gallo LC, and Norman GJ (2011). Attrition and adherence rates of sustained vs. intermittent exercise interventions. Ann Behav Med 42, 197–209. 10.1007/s12160-011-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hernan MA, Hernandez-Diaz S, and Robins JM (2004). A structural approach to selection bias. Epidemiology 15, 615–625. 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 93.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. (2005). Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321. 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 94.Nassir F, Rector RS, Hammoud GM, and Ibdah JA (2015). Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol Hepatol (N Y) 11, 167–175. [PMC free article] [PubMed] [Google Scholar]
- 95.Barnett AG, van der Pols JC, and Dobson AJ (2005). Regression to the mean: what it is and how to deal with it. Int J Epidemiol 34, 215–220. 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- 96.Miyake T, Kumagi T, Hirooka M, Koizumi M, Furukawa S, Ueda T, Tokumoto Y, Ikeda Y, Abe M, Kitai K, et al. (2012). Metabolic markers and ALT cutoff level for diagnosing nonalcoholic fatty liver disease: a community-based cross-sectional study. J Gastroenterol 47, 696–703. 10.1007/s00535-012-0534-y. [DOI] [PubMed] [Google Scholar]
- 97.Gu J, Liu S, Du S, Zhang Q, Xiao J, Dong Q, and Xin Y (2019). Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol 29, 3564–3573. 10.1007/s00330-019-06072-4. [DOI] [PubMed] [Google Scholar]
- 98.Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KHK, MacLeod J, Mitri J, Pereira RF, Rawlings K, Robinson S, et al. (2019). Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 42, 731–754. 10.2337/dci19-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hill J, and Timmis A (2002). Exercise tolerance testing. BMJ 324, 1084–1087. 10.1136/bmj.324.7345.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, and Turner RC (1985). Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 101.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, and Quon MJ (2000). Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85, 2402–2410. 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 102.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, and Pol S (2007). FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 46, 32–36. 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 103.Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, Thompson FE, Bingley C, Willis G, Islam NG, Baranowski T, McNutt S, and Potischman N (2012). The Automated Self-Administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet 112, 1134–1137. 10.1016/j.jand.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mansournia MA, Nazemipour M, Naimi AI, Collins GS, and Campbell MJ (2021). Reflection on modern methods: demystifying robust standard errors for epidemiologists. Int J Epidemiol 50, 346–351. 10.1093/ije/dyaa260. [DOI] [PubMed] [Google Scholar]
- 105.Huber P (1967). Under nonstandard conditions. In Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. [Google Scholar]
- 106.White H (1980). A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica: journal of the Econometric Society, 817–838. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Unprocessed data underlying the display items in the manuscript, related to Figures 2, 3 and 4
aa
Data Availability Statement
All other data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials, see Data S1.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.