Abstract
Background:
The efficacy and safety of direct oral anticoagulants (DOACs) for patients with thrombotic antiphospholipid syndrome (APS) remain controversial.
Objectives:
We performed a systematic review and meta-analysis of randomized controlled trials (RCTs) that compared DOACs with vitamin-K antagonists (VKAs).
Methods:
We searched PubMed, EMBASE, and Cochrane Central Register of Controlled Trials through April 9, 2022. The two main efficacy outcomes were a composite of arterial thrombotic events, and venous thromboembolic events (VTE). The main safety outcome was major bleeding. Random effects models with inverse variance were used.
Results:
Our search retrieved 253 studies. Four open-label RCTs involving 472 patients were included (mean control-arm time-in-therapeutic-range: 60%). All had proper random sequence generation and adequate allocation concealment. Overall, use of DOACs compared with VKAs was associated with increased odds of subsequent arterial thrombotic events (OR 5.43, 95% confidence interval [CI] 1.87–15.75, p < 0.001, I2 = 0%), especially stroke, and composite of arterial thrombotic events or VTE (OR 4.46, 95% CI 1.12–17.84, p = 0.03, I2 = 0%). The odds of subsequent VTE (OR 1.20, 95% CI 0.31–4.55, p = 0.79, I2 = 0%), or major bleeding (OR 1.02, 95% CI 0.42–2.47, p = 0.97, I2 = 0%) were not significantly different between the two groups. Most findings were consistent within subgroups.
Conclusions:
Patients with thrombotic APS randomized to DOACs compared to VKAs appear to have increased risk for arterial thrombosis. No significant differences were observed between patients randomized to DOACs vs VKAs in the risk of subsequent VTE or major bleeding.
Keywords: Arterial thrombosis, bleeding, mortality, stroke, venous thromboembolism
CONDENSED ABSTRACT:
While vitamin-K antagonists (VKAs) are effective for the management of thrombotic antiphospholipid syndrome, the role of direct oral anticoagulants (DOACs) remains uncertain. We conducted a systematic review and meta-analysis of randomized controlled trials that compared DOACs and VKAs in patients with thrombotic antiphospholipid syndrome. DOACs were associated with a higher odds of arterial thrombotic events compared to VKAs (OR: 5.43), while there was no significant change in the odds of subsequent venous thromboembolism or major bleeding. These findings support the preferential use of VKAs in patients with thrombotic antiphospholipid syndrome.
INTRODUCTION
Thrombotic antiphospholipid syndrome (APS) is a systemic autoimmune disorder characterized by recurrent arterial and/or venous thrombotic events, with heterogeneous laboratory and clinical manifestations. The pathogenesis of thrombotic APS involves a complex interplay between inflammatory and coagulation pathways, with activation of vascular and immune cells, inhibition or down-regulation of antithrombotic factors such as protein C and plasminogen, and upregulation of procoagulant molecules such as tissue factor, and factors V and VIII(1,2).
Direct oral anticoagulants (DOACs) are now the standard treatment for many patients with venous thromboembolism (VTE)(3–11) or for those who require prevention of stroke and systemic embolism, particularly in the setting of non-valvular atrial fibrillation(3,12–15). Such decisions are based on several randomized clinical trials (RCTs) showing at least non-inferior efficacy for DOACs and superior safety for bleeding events, especially intracranial bleeding, compared with vitamin-K antagonists (VKAs). Although VKAs are effective for patients with thrombotic APS, the use of DOACs as potential alternatives remains controversial. Recently, a few RCTs were reported and compared DOACs versus VKAs for the management of patients with thrombotic APS(16–19).
However, most of these RCTs were relatively small and although some of these studies raised concern for excess thrombotic events with DOACs compared with VKAs, particularly arterial thrombosis, they were not sufficiently powered to assess individual thrombotic outcomes or to analyze key subgroups. Therefore, the treatment tradeoffs between DOACs and VKAs in patients with thrombotic APS remain uncertain. For this reason, we conducted a systematic review and meta-analysis of RCTs to compare the efficacy and safety of DOACs with VKAs for the prevention of subsequent venous and/or arterial thrombosis in patients with thrombotic APS.
METHODS
Search strategy and data extraction
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (Table 1 in the Supplement)(20). The study protocol was registered on the International Prospective Register of Systematic Reviews, PROSPERO (Registration No. CRD42022268035). We conducted a search in PubMed, EMBASE, and the Cochrane Central Register of Clinical Trials (CENTRAL) for RCTs that compared DOACs to VKAs in patients with thrombotic APS through April 9, 2022. We also searched ClinicalTrials.gov to identify any ongoing RCTs. No language restriction was imposed. The search was complemented by manual search of the reference list of relevant articles and published guideline statements by professional societies. We included RCTs that studied patients older than 18 years with thrombotic APS and reported cardiovascular outcomes in patients receiving DOACs versus VKAs. Thrombotic APS was defined in the individual trials according to standard criteria as reported history of arterial or venous thrombosis with documented positivity of at least one antiphospholipid antibody (lupus anticoagulant, IgG and/or IgM anticardiolipin antibodies, anti-beta-2 glycoprotein 1 antibodies) and verified at least 12 weeks apart. We excluded clinical trials that were not randomized, used a crossover design, focused on APS without thrombosis, and clinical trials of pediatric populations.
Central Illustration. Use of DOACs versus VKAs in thrombotic APS.
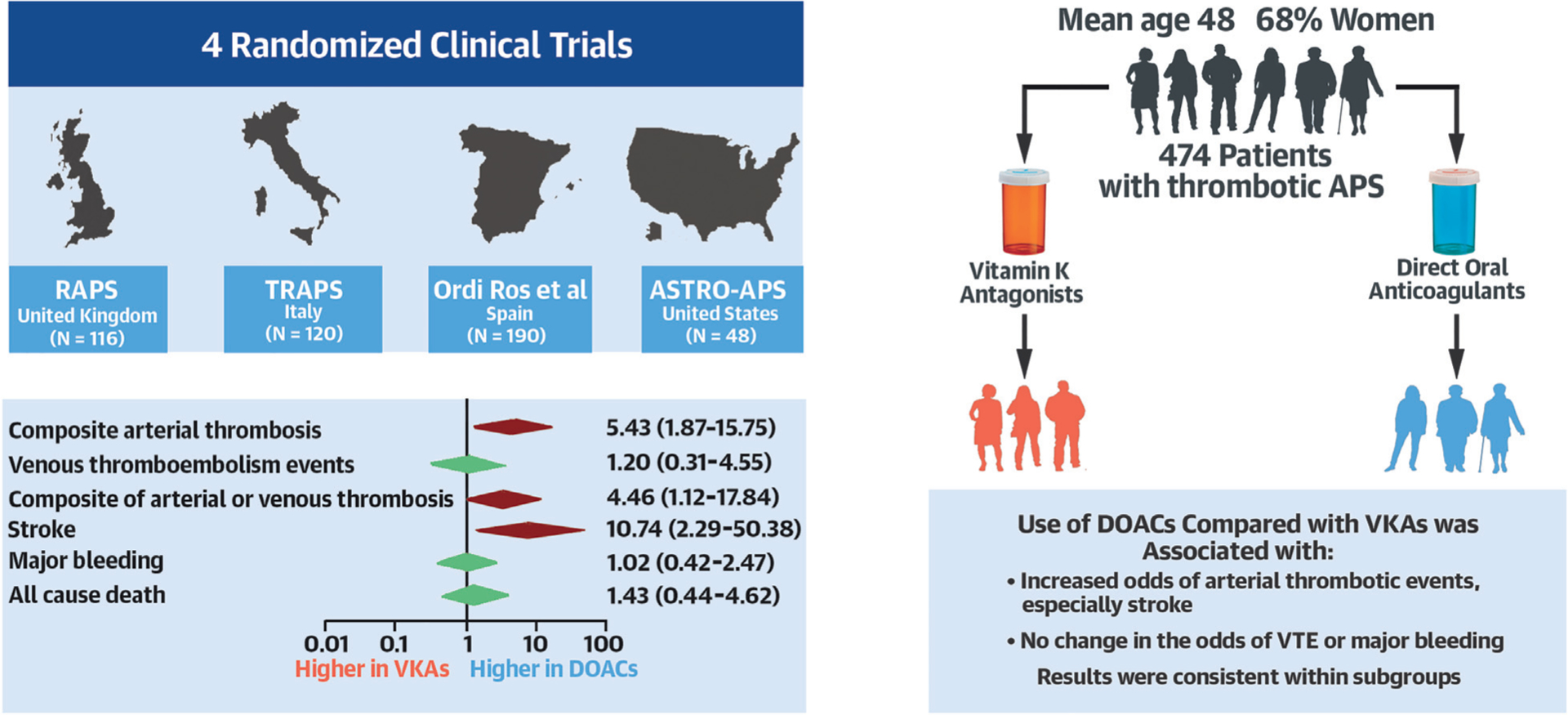
In this systematic review and meta-analysis of RCTs comparing DOACs to VKAs in patients with thrombotic APS, the results were limited by the small number of patients (472 among 4 studies) and the open-label status of the trials. Overall results indicated that in patients with thrombotic APS, DOACs were associated with significantly increased odds of subsequent arterial thrombotic events, an effect that appeared mostly driven by the increased rate of stroke, compared with VKAs. No significant differences were observed with regards to the odds of VTE or major bleeding. There was no major modification of the effect across subgroups. These results would favor the use of VKAs in patients with thrombotic APS. APS = antiphospholipid syndrome; DOACs = direct oral anticoagulants; RCTs = randomized controlled trials; VKAs = vitamin-K antagonists, VTE = venous thromboembolism.
The protocol was drafted by three authors (C.D.K, A.B, and B.B) and reviewed by all co-authors. All records identified through database and hand-searching were imported into Covidence (www.covidence.org), a software platform to facilitate a collaborative screening process and maintenance of systematic reviews. Two authors (C.D.K and A.B) independently screened the studies and extracted the data. Potential discrepancies were discussed with the senior author (B.B). If the pre-specified data elements were not found during the review of published trial results, we contacted the trialists of these publications to obtain additional study-level summary information. Investigators from three trials (V.P., S.C.W, and J.C.H) provided additional data upon request.
Outcomes
The two main efficacy outcomes were: 1. composite of arterial thrombotic events, and 2. venous thromboembolic events. Other efficacy outcomes included acute myocardial infarction, ischemic stroke or transient ischemic attack, acute major limb events, pulmonary embolism (PE), deep vein thrombosis (DVT), all-cause death, and a composite of any arterial or venous thromboembolic events. The main safety outcome was major bleeding, as defined by International Society on Thrombosis and Haemostasis (ISTH)(21). Clinically relevant non-major bleeding (CRNMB), according to the ISTH definition, was also assessed(22). Definitions in individual trials were reviewed, and a harmonizing definition was used across the trials to the extent possible (Supplemental Table 2).
We used the Cochrane Collaboration criteria to determine the risk of bias for each included study(23). We then used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) profiler tool to assess the reporting quality of major study outcomes(24).
Statistical analysis
For the primary analysis, random-effects models with inverse variance weights were used to calculate pooled odds ratio (OR) estimates with the related 95% confidence intervals (CIs). The proportion of variability due to heterogeneity between studies was assessed using visual inspection of forest plots and calculation of the Higgin’s index (I2). An I2 value of 75–100% was interpreted as high heterogeneity(25). Statistical analyses were performed using Stata version 17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC). Figures were prepared using Stata and GraphPad Prism version 9.4.0 for Windows (GraphPad Software, San Diego, California, USA, www.graphpad.com). Since no prospective patient enrollment was planned, an a priori power calculation was not performed. The choice of the main outcomes and other outcomes were determined according to consensus in the authors’ group prior to conducting the analyses. No adjustment for multiplicity of comparisons was planned, and the study results, albeit pre-specified, were not definitive.
Subgroup and Sensitivity Analyses
The treatment effect of DOACs versus VKAs was explored in patients with triple-positive APS versus those with any other combination of APS. The results were descriptively reported separately for dual positive and single positive APS. Additional subgroup analyses compared the efficacy and safety results in women versus men, and in patients with a history of arterial thrombotic events versus those without. To assess the robustness of the findings, inverse variance fixed-effects models with Peto odds ratio (OR) and Mantel-Haenszel fixed effects models with relative risk (RR) were used for sensitivity analysis.
RESULTS
Our search in PubMed, Cochrane Central Register for Controlled Trials, and EMBASE identified 253 studies (Supplemental Figure 1) including five full-text articles reporting 4 RCTs, which were assessed for eligibility. These RCTs include RAPS (rivaroxaban in antiphospholipid syndrome), TRAPS (trial on rivaroxaban in antiphospholipid syndrome), rivaroxaban versus VKA in APS: a randomized non-inferiority trial, and ASTRO-APS (apixaban for the secondary prevention of thrombosis among patients with antiphospholipid syndrome) (16–19). The fifth article, which addressed the two-year outcomes of the TRAPS trial, was excluded from the current meta-analysis since randomization was broken for the two-year analysis, and the only intervention was warfarin(26). The original report from TRAPS was included in the analysis. Search of ClinicalTrial.gov identified one ongoing RCT (the RISAPS trial [Rivaroxaban for Stroke Patients with AntiPhospholipid Syndrome]; ClinicalTrial.gov identifier: NCT03684564).
In the end, 4 RCTs involving 474 patients, comprising 234 patients assigned to DOACs and 240 patients assigned to VKAs, were included in the analysis (Figure 1). The RCTs were conducted in the United Kingdom(16), Italy(17), Spain(18), and the United States(19). All RCTs were industry-funded; two of them were investigator-initiated. The average age of participants across the trials was 48.0 years, and the mean BMI was 28.3 kg/m2. Women accounted for 68% of the total study participants, and 56.5% of the study participants had triple positive APS. Across the four included RCTs, the mean percent time in the therapeutic range for patients in the VKAs arm was 60%, and the mean follow-up time was 19 months.
Figure 1. Graphical summary of trial characteristics.
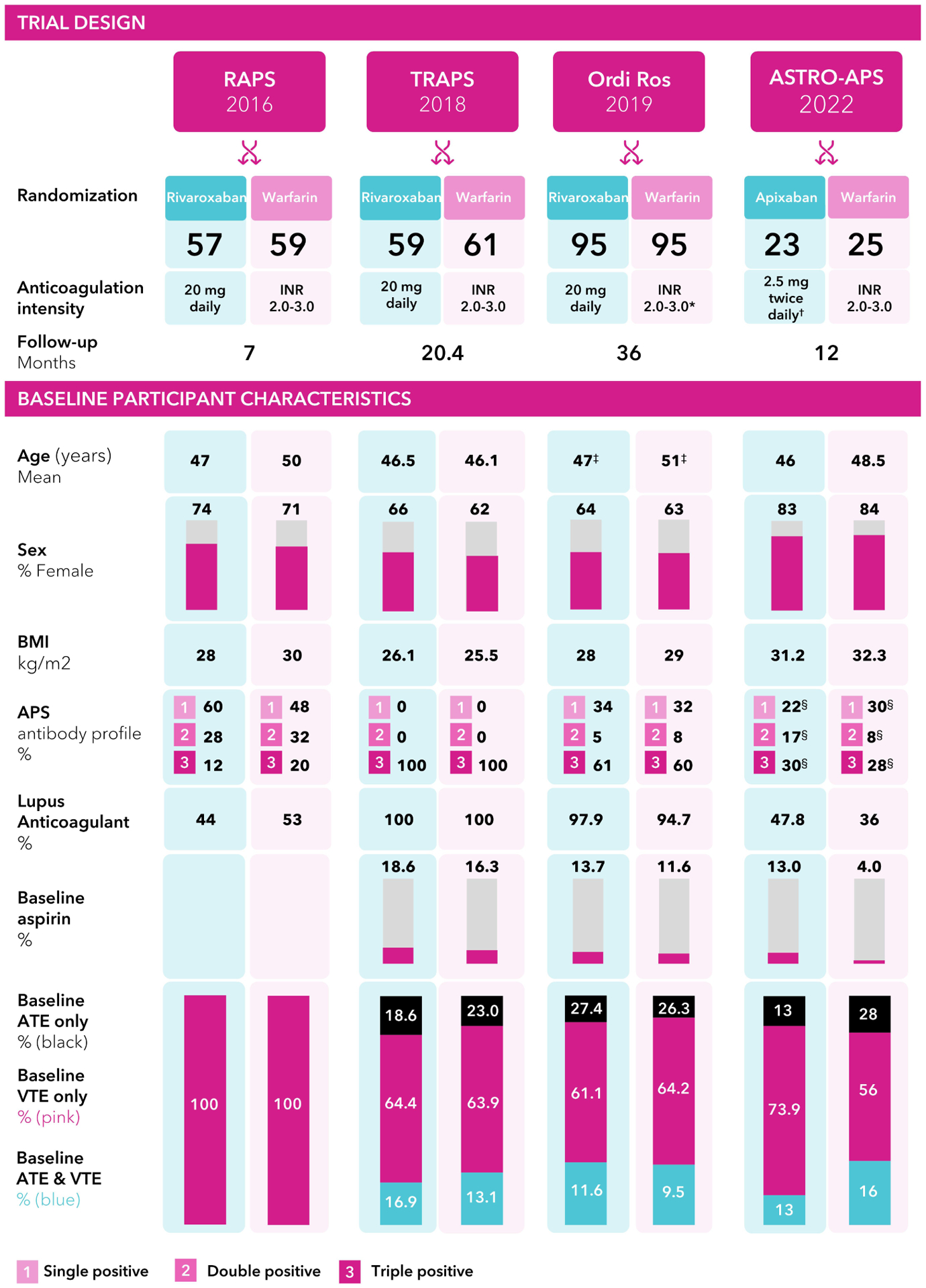
*Patients with a history of recurrent thrombosis were assigned to an INR of 3.1 to 4 in the VKA arm. ✝In ASTRO-APS, after 25 patients were randomized, all patients in the apixaban arm had their dose increased to 5 mg twice daily. ‡Ordi-Ros et al. used median and IQR for age. §In the ASTRO-APS trial, 31% had historical APS in the apixaban group, and 34% had historical APS in the VKA group. APS = antiphospholipid syndrome; ATE = arterial thrombotic events; BMI = body mass index; INR = international normalized ratio; IQR = interquartile range; VKA = vitamin-K antagonist; VTE = venous thromboembolism.
Clinical outcomes
Overall, the use of DOACs compared with VKAs was associated with increased odds of the composite of arterial thrombotic events (10.3% vs. 1.3%, OR 5.43, 95% CI 1.87–15.75, p < 0.001, I2 = 0%) (Figure 2A). Among the arterial thrombotic events, the odds of subsequent stroke were significantly higher in patients assigned to DOACs, compared with VKAs (8.6% vs 0%, OR 10.74, 95% CI 2.29–50.38, p < 0.001, I2 = 0%) (Figure 2B). There was no significant difference between DOACs and VKAs in the odds of myocardial infarction (1.3% vs 0%, OR 2.15, 95% CI 0.35–13.11, p = 0.41, I2 = 0%) (Figure 2C) or in the odds of major acute limb events (0.4% vs 1.3%, OR 0.58, 95% CI 0.12–2.92, p = 0.51, I2 = 0%) (Supplemental figure 2).
Figure 2. Composite of arterial thrombotic events (A), MI (B), and stroke (C).
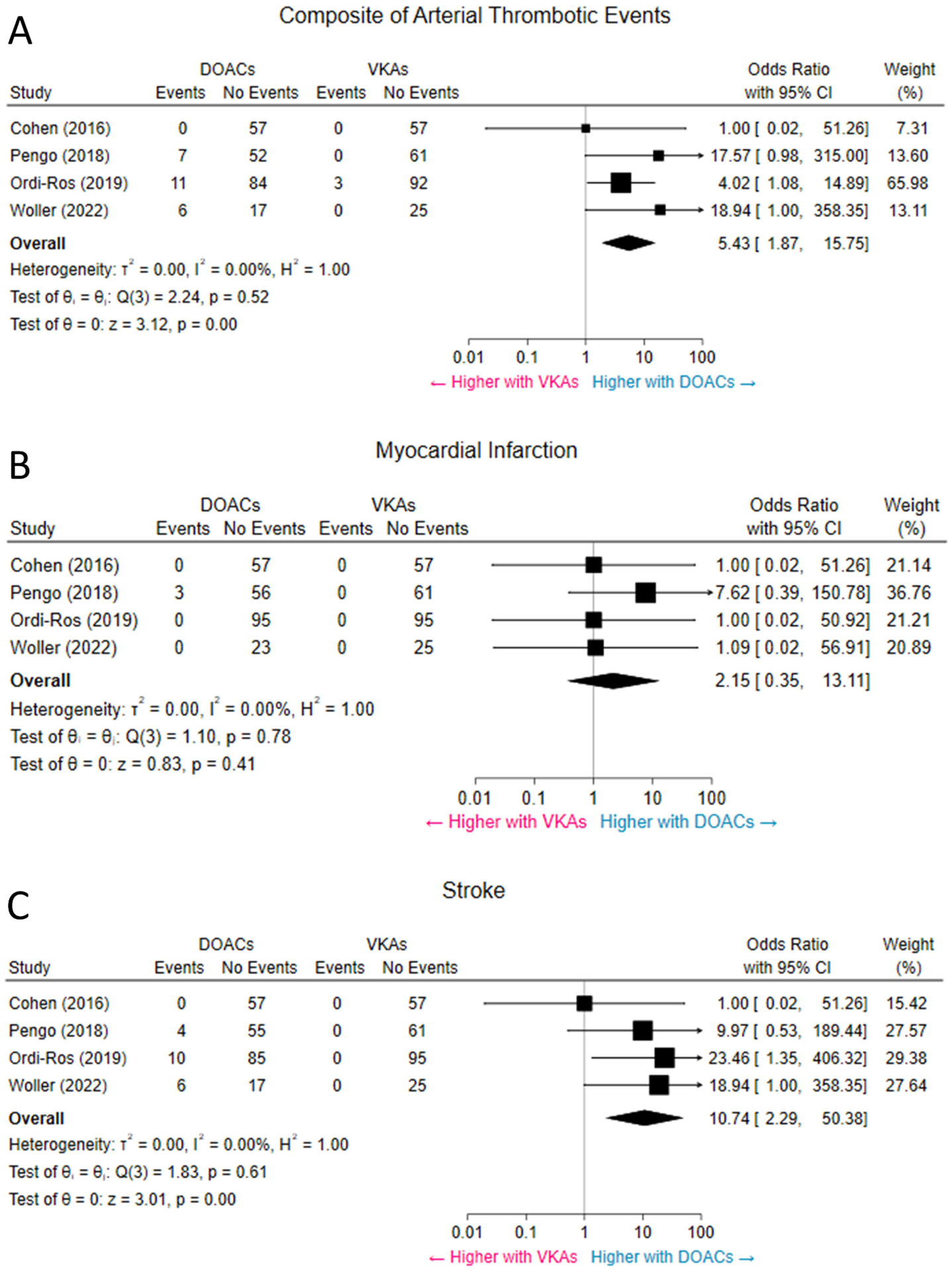
Pooled results revealed higher odds of arterial thrombotic events in patients assigned to DOACs. The rate of stroke was higher with DOACs, while the rate of MI was not significantly different between the two groups. CI = confidence interval; DOACs = direct oral anticoagulants; MI = myocardial infarction; VKAs = vitamin-K antagonists.
Subsequent VTE events were infrequent and occurred in 4 patients receiving DOACs (1 case of PE and 3 cases of DVT) and 3 patients receiving VKAs (3 cases of DVT). The odds of VTE risk were not significantly different between patients assigned to DOACs versus VKAs (1.7% vs. 1.3%, OR 1.20, 95% CI 0.31–4.55, p = 0.79, I2 = 0%) (Figure 3A). Similarly, the odds of PE (0.4% vs 0%, OR 1.49, 95% CI 0.23–9.53, p = 0.68, I2 = 0%) and DVT (1.3% vs 1.3%, OR 1.03, 95% CI 0.23–4.57, p = 0.97, I2 = 0%) were not significantly different between patients assigned to DOACs versus VKAs (Figures 3B and 3C).
Figure 3. VTE (A), DVT (B), and PE (C).
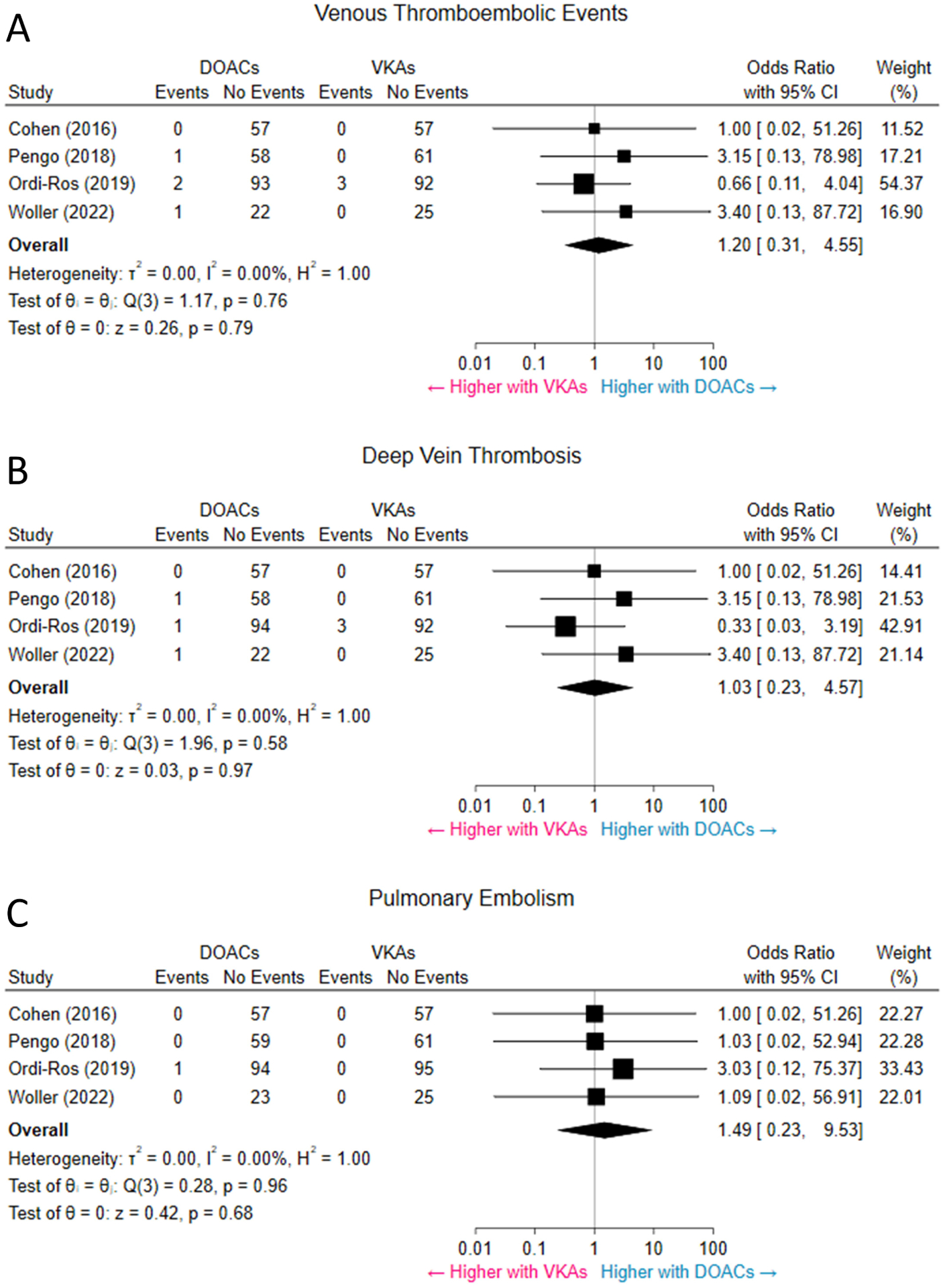
Pooled results showed no significant difference in the rate of VTE between DOACs and VKAs. The rates of DVT and PE were not significantly different among the two groups, either. CI = confidence interval; DOACs = direct oral anticoagulants; DVT = deep vein thrombosis; PE = pulmonary embolism; VKAs = vitamin-K antagonists; VTE = venous thromboembolism.
Twenty cases of major bleeding were reported across the 4 studies, equally split among the 2 therapeutic groups. Overall, the odds of major bleeding were not significantly different between patients randomized to DOACs versus VKAs (4.3% vs. 4.2%, OR 1.02, 95% CI 0.42–2.47, p = 0.97, I2 = 0%) (Figure 4A). The odds of CRNMB were not significantly different between patients on DOACs versus VKAs (6.0% vs 2.9%, OR 1.90, 95% CI: 0.78–4.66, p = 0.16, I2 = 0%, respectively) (Figure 4B).
Figure 4. Major bleeding and clinically relevant non-major bleeding.
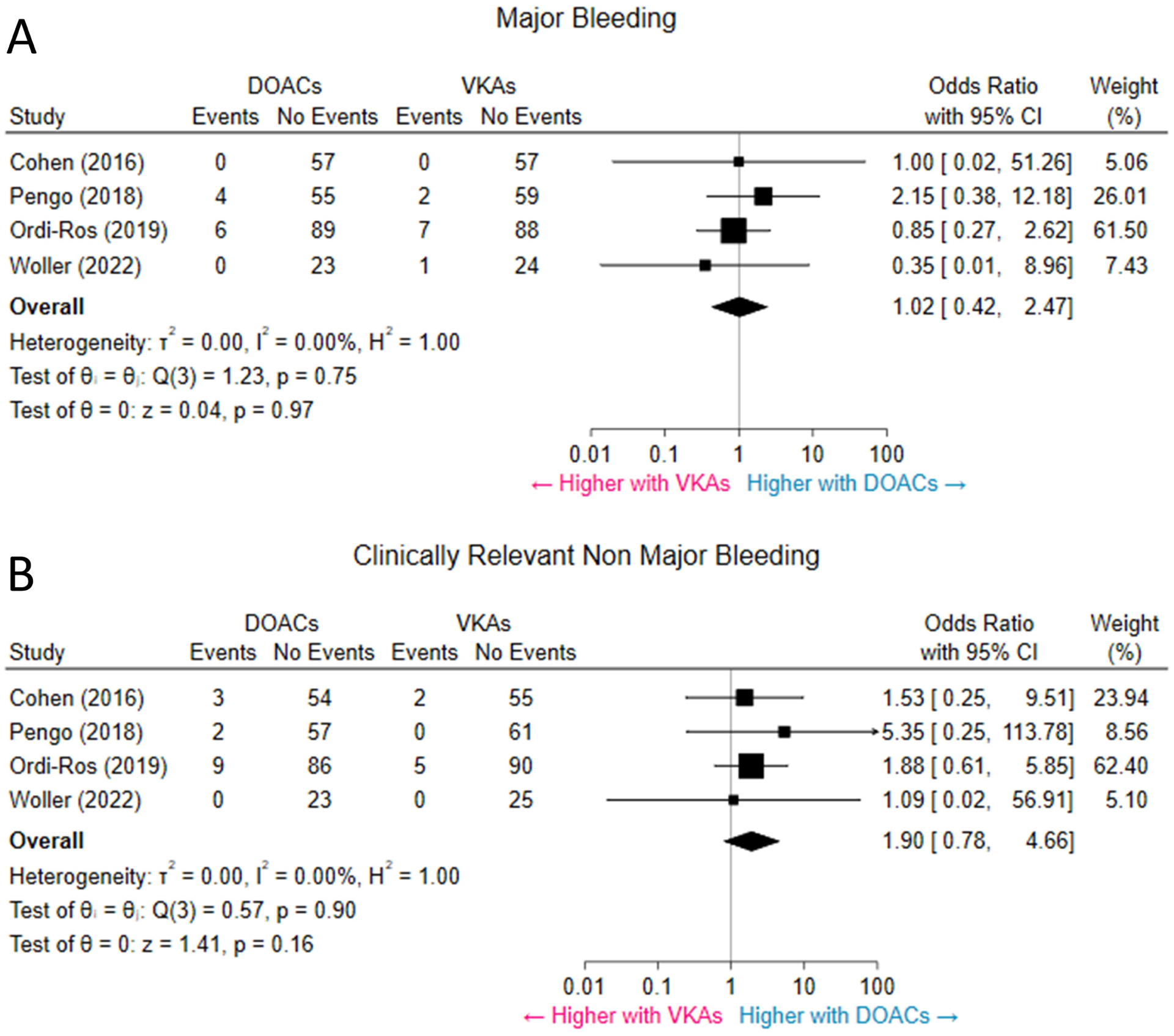
Pooled results suggest no significant difference in the rates of major bleeding (A) and CRNMB (B) between patients receiving DOACs and patients receiving VKAs. CI = confidence interval; CRNMB = clinically relevant non-major bleeding; DOACs = direct oral anticoagulants; VKAs = vitamin-K antagonists. Abbreviations as in other Figures.
DOACs were associated with an increased odds of composite of arterial thrombotic events or VTE compared to VKAs (11.5% vs 2.5%, OR 4.46, 95% CI 1.12–17.84, p = 0.03, I2 = 0%) (Supplemental figure 3). All-cause mortality was not significantly different between patients randomized to DOACs versus VKAs (2.6% vs 1.7%, OR 1.43, 95% CI 0.44–4.62, p = 0.55, I2 = 0%) (Supplemental figure 4).
Subgroup analysis
We conducted a pre-specified subgroup analysis of patients with different types of thrombotic APS: 249 had triple-positive APS (124 were assigned to DOACs and 125 to VKAs) and 93 had any other combination of APS (46 were assigned to DOACs and 47 to VKAs). Those receiving DOACs, compared with VKAs, had increased odds of a composite of arterial thrombotic events, with no change in the odds of subsequent VTE or major bleeding whether they had triple APS or any other combination of APS. Test for subgroup differences indicated that there was no statistically significant subgroup effect (p = 0.80 for arterial thrombotic events, p = 0.85 for VTE, p = 0.45 for major bleeding). These results indicate that the type of APS, as included in the trials, did not modify the effect of DOACs in comparison to VKAs on the odds of these outcomes (Figure 5).
Figure 5. Pre-specified subgroup analysis.
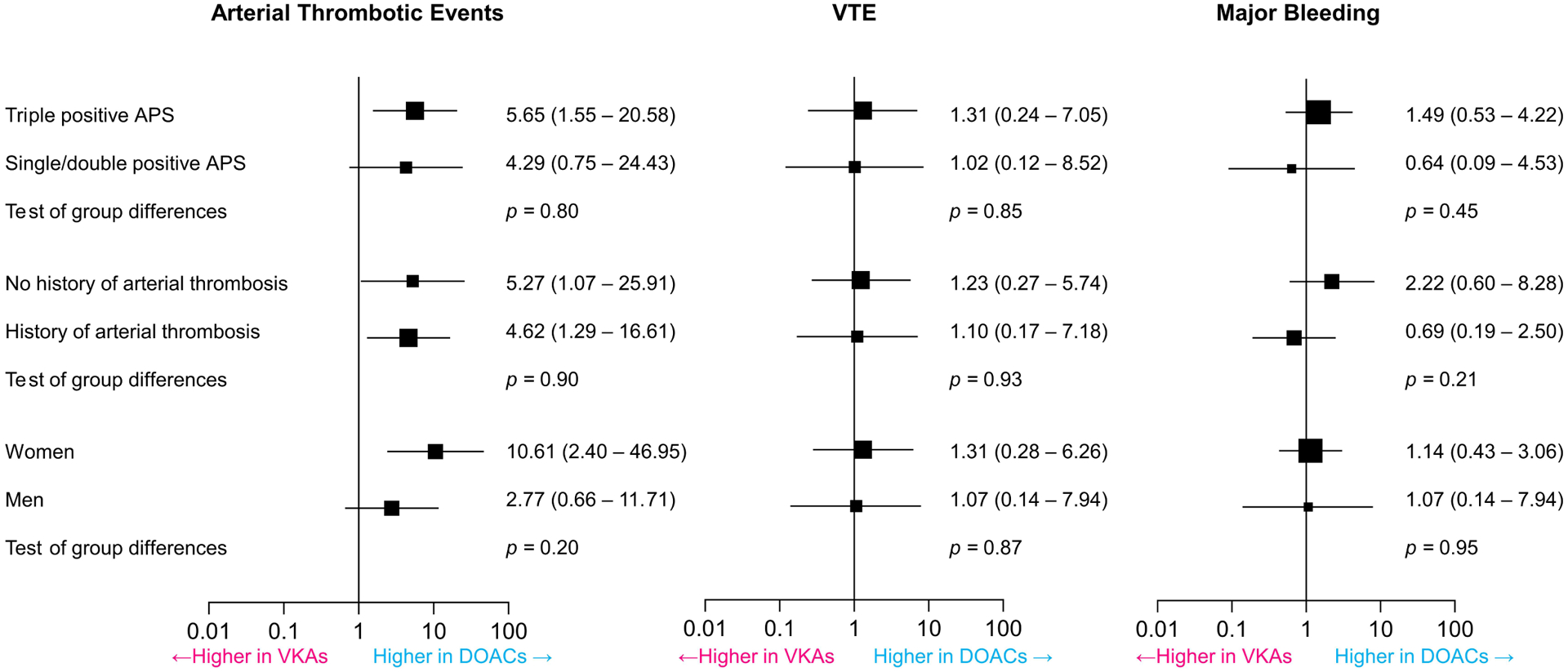
Pooled results indicate that patients assigned to DOACs compared with patients assigned to VKAs had higher odds of developing arterial thrombotic events without clear effect modification based on the type of APS (triple-positive vs any other combination), sex, or history of arterial thrombosis (vs no prior arterial thrombosis). The rates of VTE and major bleeding were not significantly different between the two treatment arms, regardless of the subgroup category.
Another subgroup analysis was performed in patients with a history of prior arterial thrombosis versus those with no history of arterial thrombosis at the time of enrollment. The odds of a composite of arterial thrombotic events in the DOACs arms compared to the VKAs arms were increased with no significant change in the odds of subsequent VTE or major bleeding. Test for subgroup differences indicated that there was no statistically significant subgroup effect (p = 0.90 for arterial thrombotic events, p = 0.93 for VTE, p = 0.21 for major bleeding). These data suggest that a history of arterial thrombosis did not modify the effect of DOACs in comparison to VKAs on the odds of these outcomes (Figure 5).
A third pre-specified subgroup analysis was according to sex: 120 men, of whom 58 were assigned to DOACs and 62 to VKAs, and 238 women, of whom 119 were assigned to each treatment arm were included. Both men and women receiving DOACs compared with VKAs had increased odds of developing a composite of arterial thrombotic events, with no significant change in the odds of VTE or major bleeding. Test for subgroup differences did not show statistically significant subgroup effects (p = 0.20 for arterial thrombotic events, p = 0.87 for VTE, p = 0.95 for major bleeding) (Figure 5).
Sensitivity analysis
Sensitivity analysis using inverse variance fixed-effects model and Peto OR yielded similar results; there were significant increased odds in the pooled effects of arterial thrombotic events in patients receiving DOACs compared to patients receiving VKAs (Peto OR 5.51, 95% CI 2.52–12.07, p < 0.001, I2 = 0%), but no significant difference in the odds of subsequent VTE (Peto OR 1.36, 95% CI 0.31–6.05, p = 0.69, I2 = 7.52%), or major bleeding (Peto OR 1.02, 95% CI 0.41–2.50, p = 0.97, I2 = 0%) (Supplemental figure 5). There was also no significant difference in other efficacy and safety outcomes. Sensitivity analysis using Mantel-Haenszel fixed effects models with RR as the effect measure yielded similar results (Supplemental figure 6).
Risk of bias and quality assessment of outcomes
All RCTs were open-label. However, their outcomes were adjudicated by expert committees blinded to treatment allocation. All studies had proper random sequence generation and adequate allocation concealment (Table 1).
Table 1.
Risk of bias table for the included trials.
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| RAPS 2016 | + | + | − | + | − | − | − |
| TRAPS 2018 | + | + | − | + | − | − | − |
| Ordi-Ros 2019 | + | + | − | + | − | − | − |
| ASTRO-APS 2022 | + | + | − | + | − | − | − |
Note: +, present; −, absent; green, low risk of bias; red, high risk of bias.
Our confidence in the main outcomes assessment, using the GRADE criteria,(27) was variable (Table 2). For the composite of arterial thrombotic events, the observed effect was large (OR >5). Thus, despite the wide CIs, the quality of evidence was graded as high according to the GRADE criteria. With regards to VTE and major bleeding, the CIs included no clear benefit or harm with the use of DOACs along with a low number of events; this led us to downgrade for imprecision, and the overall quality of evidence was moderate. A funnel plot was not generated to assess for publication bias since fewer than ten studies were included(28).
Table 2.
GRADE Assessment
| Certainty assessment | No of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other consideration | DOACs | VKAs | Relative (95% CI) | Absolute (95% CI) | ||
| Composite of arterial thrombotic events | ||||||||||||
| 4 | Randomized trials | Not serious | Not serious | Not serious | Not serious | Very strong association | 24/234 (10.3%) |
3/238 (1.3%) |
OR 5.43 (1.87 to 15.75) |
5 more per 100 (from 1 more to 15 more) |
⨁⨁⨁⨁ High |
CRITICAL |
| Venous thromboembolic events | ||||||||||||
| 4 | Randomized trials | Not serious | Not serious | Not serious | Seriousa | None | 4/234 (1.7%) |
3/238 (1.3%) |
OR 1.20 (0.31 to 4.55) |
0 fewer per 100 (from 1 fewer to 4 more) |
⨁⨁⨁◯ Moderate |
IMPORTANT |
| Major bleeding | ||||||||||||
| 4 | Randomized trials | Not serious | Not serious | Not serious | Seriousa | None | 10/234 (4.3%) |
10/238 (4.2%) |
OR 1.02 (0.42 to 2.47) |
0 fewer per 100 (from 2 fewer to 6 more) |
⨁⨁⨁◯ Moderate |
IMPORTANT |
Factors contributing to the certainty of evidence include the risk of bias, inconsistency, indirectness, imprecision, publication bias, and the strength of association. CI = confidence interval; DOACs = direct oral anticoagulants; GRADE = Grading of Recommendations, Assessment, Development and Evaluation; OR = odds ratio; VKAs = vitamin-K antagonists.
Explanations
Low event rate and wide CI with no clear harm or benefit with the use of direct oral anticoagulants.
DISCUSSION
This systematic review of the efficacy and safety of DOACs compared with VKAs in patients with thrombotic APS retrieved 4 existing RCTs with a total of 474 patients. We found that among patients with thrombotic APS, treatment with DOACs compared with VKAs is associated with an increase in the odds of arterial thrombotic events, while the odds of VTE and major bleeding are not significantly different between the 2 groups (Central Illustration). Our subgroups results suggest an increased odds of subsequent arterial thrombotic events, especially stroke, in patients taking DOACs compared to VKAs, regardless of the type of thrombotic APS (triple positive vs others), sex, and history of prior arterial thrombosis (vs VTE).
These findings provide pooled estimates from high-quality studies to guide clinical decision-making. In patients with thrombotic APS, some current guidelines recommend against the use of DOACs in those with either triple-positive APS or a history of arterial thrombosis (1,29–33). Our results extend the existing knowledge on this topic by providing pooled estimates from four RCTs that increased statistical power and by providing subgroup-specific analyses. None of the tests for subgroup differences was statistically significant to indicate effect modification in such a way that reassures about the safety of DOACs for patients with thrombotic APS. The difference in point estimates for arterial thrombotic events among women versus men could suggest an effect modification, such that even higher odds of thrombotic events are observed for women with APS who receive DOACs compared to men (OR: 10.6 vs 2.8). Nonetheless, patients of both sexes manifested increased odds of arterial thrombotic events on DOACs compared to VKAs.
There are other recently published studies that examined the effect of various anticoagulant regimens in patients with thrombotic APS. Two RCTs compared conventional warfarin therapy to higher intensity warfarin (INR 3–4) in patients with thrombotic APS but failed to show improved outcomes with a higher INR target(34,35). The details about the potential explanations for failure of high-intensity INR in preventing recurrent thrombotic arterial or venous events are complex and beyond the scope of the current study. Briefly, lack of benefit from high-intensity warfarin therapy may have been, at least in part, related to suboptimal treatment adherence in the active intervention arms in those trials, or difficulty with INR monitoring in the setting of APS. The rate of a composite of arterial thrombotic events or VTE in our meta-analysis was 2.5% in the warfarin arms over a mean duration of follow up of 1.6 years. The event rates in the INR 2–3 groups were slightly higher in the trials by Crowther et al.(34) and Finazzi et al.(35), compared to randomized trials included in our study. However, factors that could explain these findings include longer duration of follow-up in both trials compared to our study and also a smaller sample size in these 2 trials, that may have brought imprecision. Visual estimates from the Kaplan-Meier curves of thrombotic events in the study by Crowther et al.(34), suggest that the control arm event rate was close to 1.7% (1/58), which is within range with findings in our study. Previous prospective cohort studies showed rates of VTE and arterial thrombotic events directionally similar with the pooled rates in our meta-analysis. In a prospective cohort study by Malec et al.(36), reviewing the provided Kaplan-Meier curves, there were no arterial thrombotic events or VTE in the DOACs (82 patients) and VKAs arms (94 patients) over follow-up duration of 1.6 years. However, over a median duration of follow-up of 4.2 years, the rate of recurrent thrombosis (arterial or venous) was 3.54 per 100 patient-years for patients on DOACs and 2.65 per 100 patient-years for patients on VKAs. In a single-arm cohort study of rivaroxaban(37), over a median follow-up of 1.7 years, the rates of VTE and arterial thrombotic events were both 2.4% (2/82). In the trial by Crowther et al.(34), the rates of VTE and arterial thrombotic events in the INR 2–3 group were both approximately 1.7% (1/58) during a follow-up interval similar to average follow-up in the current meta-analysis. In the WAPS trial(35), there was no VTE and the rate of arterial thrombotic events was 5.5% (3/55) in the INR 2–3 group. After TRAPS was prematurely discontinued because of a high incidence of stroke in the rivaroxaban arm, 109 patients were given warfarin while 6 patients remained on DOACs. In a subsequent analysis of TRAPS participants, the rate of recurrent thrombosis was significantly higher among those who continued receiving DOACs upon trial discontinuation compared to those receiving warfarin 2 years after study closure(26). It is also interesting to note that patients with a history of venous thrombosis only and assigned to DOACs developed subsequent arterial thrombotic events. These findings go against the dogma that patients with thrombotic APS and VTE develop recurrent VTE, while patients with thrombotic APS and prior arterial thrombosis develop recurrent arterial thrombotic events.
The reason why DOACs are less effective than VKAs in prevention of arterial thrombosis, particularly stroke, in patients with thrombotic APS remains elusive. DOACs are targeted drugs. In the RAPS trial, patients in the rivaroxaban arm had higher endogenous thrombin generation compared to those receiving VKAs, which inhibit multiple sites in the coagulation cascade(16). In the context of APS, it is possible that VKAs prove to be more effective because of suppressing multiple pathways leading into thrombosis, compared with DOACs. Whether inhibition of only one coagulation factor could explain these treatment failures remains to better assessed. Another potential reason could be related to the shorter half-life of DOACs compared to VKAs and short periods of non-adherence may significantly increase the risk of thrombotic events. However, in the ASTRO-APS trial, patients’ reported adherence to apixaban was 97.3%(19). The ongoing RISAPS trial (NCT03684564) is investigating the efficacy and safety of high-intensity rivaroxaban (15 mg twice daily) compared to high-intensity warfarin (INR 3–4) for 24 months in stroke patients with thrombotic APS. The trial’s primary outcome, however, will involve using a surrogate marker of ischemic damage by evaluating the change in brain white matter hyperintensity.
Study limitations
This study has several limitations. First, only 4 relevant RCTs were included with a pooled sample size of 472 patients. The limited number of participants and events observed in the different RCTs resulted in wide CIs. However, this constitutes the current pool of evidence on this subject, and the certainty of evidence was high with regards to the odds of arterial thrombotic events, according to the GRADE criteria. Second, subgroup analysis must be interpreted with caution because they are not based on randomized comparisons, but rather they are observational in nature and are also subject to type II error(38,39). However, the completed analyses did not identify a major departure from the pooled results and showed relatively similar results, supporting the robustness of the findings. Third, we did not compare rivaroxaban and apixaban to each other. This question could have been potentially addressed using a network meta-analysis to explore whether the excess in arterial thrombotic events is a class effect, or specific to an individual DOAC agent. However, a network meta-analysis would not be clinically informative in this case since only 1 prematurely terminated and relatively small study used apixaban. Nevertheless, the direction of results was similar in trials of rivaroxaban and the trial using apixaban, hinting that the excess in arterial thrombotic events in patients with thrombotic APS receiving DOACs compared with VKAs is likely a class effect. Lastly, a positive lupus anticoagulant antibody is known to be the most strongly associated with thrombosis and many participants in these trials had positive lupus anticoagulants(40). Therefore, it is still possible that in patients with other forms of low-titer single positive APS, treatment with DOACs would be reasonable. However, in ASTRO-APS, less than half of participants had a positive lupus anticoagulant result, including only 3 out of the 6 who developed arterial thrombosis. Therefore, until further studies are available and prove the safety of DOACs, caution should be exercised for treating thrombotic APS with DOACs.
CONCLUSIONS
This systematic review and meta-analysis of four RCTs that compared the safety and efficacy of DOACs versus VKAs in patients with thrombotic APS indicated an increased odd of arterial thrombotic events, specifically stroke, and no significant change in the odds of VTE or major bleeding in patients receiving DOACs compared to those receiving VKAs. Subgroup analysis according to sex, type of APS (triple versus any other combination), and history of arterial thrombosis (versus no history of arterial thrombosis) did not show evidence of effect modification. Collectively, the findings of this study do not support the routine use of existing DOAC regimens in patients with thrombotic APS. Further RCTs will be required to elucidate whether higher doses of DOACs can offer convenience to patients and clinicians, while ensuring efficacy and safety.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in patient care and procedural skills:
In patients with thrombotic APS, DOACs are associated with a higher rate of arterial thrombotic events, without a significant change in the odds of subsequent VTE or major bleeding compared to VKAs.
Translational outlook:
Additional research is needed to explore the potential efficacy and safety of higher doses of DOACs and their use across various subgroups in patients with thrombotic APS.
Acknowledgments:
The schematic diagram of included randomized clinical trials in the Central Illustration was created using BioRender.com.
Funding:
No specific funding was sought for this investigation. Dr. Bikdeli is supported by the Scott Schoen and Nancy Adams IGNITE Award from the Mary Horrigan Connors Center for Women’s Health and Gender Biology at Brigham and Women’s Hospital and a Career Development Award from the American Heart Association and VIVA Physicians (#938814).
Disclosures:
Dr. Piazza has received research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen and Boston Scientific Corporation, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific Corporation, Janssen, Namsa, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen. Dr. Jiménez has received research support from Daiichi-Sankyo and Sanofi, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Daiichi-Sankyo, Leo Pharma, Rovi and Sanofi. Dr. Monreal has received research support from Sanofi, Leo and Rovi, and consulting fees from Sanofi. Dr. Pengo received lecture fees from Werfen Group (Milan, Italy). Dr Cortes-Hernandez received institutional research funding from Bayer Hispania to conduct the APS RCT. Dr. Connors reports research funding to her institution from CSL Behring; consulting fees from Abbott; honoraria for lectures from Bristol Myers Squibb, Roche, and Sanofi; and participated in the advisory board of Abbott, Alnylam, Anthos, Bristol Myers Squibb, Sanofi, and Takeda, outside the submitted work. Dr. Kanthi is an inventor on a pending patent application by the University of Michigan on the use of biogases in vascular disease (US202201670756A1) and is supported by the NHLBI Intramural Research Program and the Lasker Foundation. Dr. Krumholz reports receiving (within the last 3 years) expenses and/or personal fees from UnitedHealth, Element Science, Aetna, Reality Labs, Tesseract/4Catalyst, F-Prime, the Siegfried and Jensen Law Firm, the Arnold and Porter Law Firm, and the Martin/Baughman Law Firm; being a co-founder of Refactor Health, an enterprise health care artificial intelligence-augmented data management company, and HugoHealth, a personal health information platform; and is associated with contracts through Yale University from Johnson & Johnson. Dr. Middeldorp reports grants and personal fees from Daiichy Sankyo, grants and personal fees from Bayer, grants and personal fees from Pfizer, grants and personal fees from Boehringer-Ingelheim, personal fees from Portola/Alexion, personal fees from Abbvie, personal fees from BMS Pfizer, personal fees from Norgine, personal fees from Viatris, all paid to her institution. Dr. Falanga has received honorarium/consulting fees from Stago, Sanofi, Daiichi-Sankyo, Leo Pharma, Pfizer. Dr. Goldhaber has received research support from Bayer, BMS, Boston Scientific BTG EKOS, Janssen, NHLBI, Pfizer, and consulting fees from Agile, Bayer, and Pfizer. Dr. Bikdeli reports that he is a consulting expert, on behalf of the plaintiff, for litigation related to two specific brand models of IVC filters. All other authors report no potential conflicts of interest to disclose.
ABBREVIATIONS
- APS
antiphospholipid syndrome
- CRNMB
clinically relevant non-major bleeding
- DOACs
direct oral anticoagulants
- DVT
deep vein thrombosis
- OR
odds ratio
- PE
pulmonary embolism
- RCT
randomized controlled trial
- VKAs
vitamin-K antagonists
- VTE
venous thromboembolism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sayar Z, Moll R, Isenberg D, Cohen H. Thrombotic antiphospholipid syndrome: A practical guide to diagnosis and management. Thromb Res 2021;198:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia D, Erkan D. Diagnosis and Management of the Antiphospholipid Syndrome. N Engl J Med 2018;378:2010–2021. [DOI] [PubMed] [Google Scholar]
- 3.Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 4.Agnelli G, Buller HR, Cohen A et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708. [DOI] [PubMed] [Google Scholar]
- 5.Investigators E, Bauersachs R, Berkowitz SD et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510. [DOI] [PubMed] [Google Scholar]
- 6.Investigators E-P, Buller HR, Prins MH et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97. [DOI] [PubMed] [Google Scholar]
- 7.Hokusai VTEI, Buller HR, Decousus H et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15. [DOI] [PubMed] [Google Scholar]
- 8.Schulman S, Kearon C, Kakkar AK et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18. [DOI] [PubMed] [Google Scholar]
- 9.Schulman S, Kearon C, Kakkar AK et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342–52. [DOI] [PubMed] [Google Scholar]
- 10.Schulman S, Kakkar AK, Goldhaber SZ et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764–72. [DOI] [PubMed] [Google Scholar]
- 11.Weitz JI, Lensing AWA, Prins MH et al. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. N Engl J Med 2017;376:1211–1222. [DOI] [PubMed] [Google Scholar]
- 12.Granger CB, Alexander JH, McMurray JJ et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 13.Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 14.Giugliano RP, Ruff CT, Braunwald E et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 15.Eikelboom JW, Connolly SJ, Bosch J et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 16.Cohen H, Hunt BJ, Efthymiou M et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol 2016;3:e426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pengo V, Denas G, Zoppellaro G et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018;132:1365–1371. [DOI] [PubMed] [Google Scholar]
- 18.Ordi-Ros J, Saez-Comet L, Perez-Conesa M et al. Rivaroxaban Versus Vitamin K Antagonist in Antiphospholipid Syndrome: A Randomized Noninferiority Trial. Ann Intern Med 2019;171:685–694. [DOI] [PubMed] [Google Scholar]
- 19.Woller SC, Stevens SM, Kaplan D et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv 2022;6:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- 22.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of A. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:2119–26. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gotzsche PC et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt G, Oxman AD, Akl EA et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pengo V, Hoxha A, Andreoli L et al. Trial of Rivaroxaban in AntiPhospholipid Syndrome (TRAPS): Two-year outcomes after the study closure. J Thromb Haemost 2021;19:531–535. [DOI] [PubMed] [Google Scholar]
- 27.Balshem H, Helfand M, Schunemann HJ et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- 28.Sterne JA, Sutton AJ, Ioannidis JP et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 29.Cohen H, Cuadrado MJ, Erkan D et al. 16th International Congress on Antiphospholipid Antibodies Task Force Report on Antiphospholipid Syndrome Treatment Trends. Lupus 2020;29:1571–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tektonidou MG, Andreoli L, Limper M et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis 2019;78:1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuily S, Cohen H, Isenberg D et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: Guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2020;18:2126–2137. [DOI] [PubMed] [Google Scholar]
- 32.Stevens SM, Woller SC, Baumann Kreuziger L et al. Executive Summary: Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest 2021;160:2247–2259. [DOI] [PubMed] [Google Scholar]
- 33.Fazili M, Stevens SM, Woller SC. Direct oral anticoagulants in antiphospholipid syndrome with venous thromboembolism: Impact of the European Medicines Agency guidance. Res Pract Thromb Haemost 2020;4:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowther MA, Ginsberg JS, Julian J et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003;349:1133–8. [DOI] [PubMed] [Google Scholar]
- 35.Finazzi G, Marchioli R, Brancaccio V et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost 2005;3:848–53. [DOI] [PubMed] [Google Scholar]
- 36.Malec K, Broniatowska E, Undas A. Direct oral anticoagulants in patients with antiphospholipid syndrome: a cohort study. Lupus 2020;29:37–44. [DOI] [PubMed] [Google Scholar]
- 37.Legault K, Blostein M, Carrier M et al. A single-arm feasibility cohort study of rivaroxaban in antiphospholipid syndrome. Pilot Feasibility Stud 2020;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). 2022. [Google Scholar]
- 39.Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci 2013;14:134–43. [DOI] [PubMed] [Google Scholar]
- 40.Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood 2003;101:1827–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


