Abstract
Withaferin A (WA), which is a small molecule derived from a medicinal plant (Withania somnifera), inhibits growth of human breast cancer xenografts and mammary tumor development in rodent models without any toxicity. However, the mechanism underlying inhibition of mammary cancer development by WA administration is not fully understood. Herein, we demonstrate that the fatty acid synthesis pathway is a novel target of WA in mammary tumors. Treatment of MCF-7 and MDA-MB-231 cells with WA resulted in suppression of fatty acid metabolizing enzymes, including ATP-citrate lyase (ACLY), acetyl-CoA carboxylase 1 (ACC1), fatty acid synthase (FASN), and carnitine palmitoyltransferase 1A (CPT1A). Expression of FASN and CPT1A was significantly higher in N-methyl-N-nitrosourea-induced mammary tumors in rats when compared to normal mammary tissues. WA-mediated inhibition of mammary tumor development in rats was associated with a statistically significant decrease in expression of ACC1 and FASN and suppression of plasma and/or mammary tumor levels of total free fatty acids and phospholipids. WA administration also resulted in a significant increase in percentage of natural killer cells in the spleen. The protein level of sterol regulatory element binding protein 1 (SREBP1) was decreased in MDA-MB-231 cells after WA treatment. Overexpression of SREBP1 in MDA-MB-231 cells conferred partial but significant protection against WA-mediated downregulation of ACLY and ACC1. In conclusion, circulating and/or mammary tumor levels of fatty acid synthesis enzymes and total free fatty acids may serve as biomarkers of WA efficacy in future clinical trials.
Keywords: breast cancer, withaferin A, fatty acid synthesis
Introduction
Withaferin A (WA) is a small molecule derived from the root of a medicinal plant (Withania somnifera) that is still used in Ayurvedic medicine formulations in India and surrounding countries (1). Clinical trials have investigated the effects of Withania somnifera on various conditions, including maximum oxygen consumption, neurodegenerative diseases, anxiety, cognitive dysfunction etc. (2–5). Preclinical studies have determined the effects of Withania somnifera on different solid tumors including breast cancer (6,7). The root extract of Withania somnifera contains multiple compounds, but WA is the predominant anticancer phytochemical in this plant (8,9). For example, while WA treatment significantly inhibited proliferation of MCF-7 and MDA-MB-231 human breast cancer cells, such an effect was not observed with withanone or withanolide A that are also present in Withania somnifera (9). Mechanistic studies have revealed G2/M phase and mitotic arrest and induction of apoptosis as well as autophagy in breast cancer cells after WA treatment (9–13). At the molecular level, the mitotic arrest by WA treatment in breast cancer cells was accompanied by downregulation as well as covalent binding at cysteine 303 of β-tubulin (9). The apoptosis induction in breast cancer cells following WA treatment was triggered by reactive oxygen species-mediated activation of Bax and Bak (12). The SV40 immortalized embryonic fibroblasts derived from Bax and Bak double knockout mice were more resistant to WA-induced apoptosis compared with fibroblasts derived from wild-type mice (12). On the other hand, inhibition of autophagy did not have a meaningful impact on growth inhibition by WA treatment at least in human breast cancer cells (13). WA is known to inhibit other oncogenic transcription factors including estrogen receptor-α, signal transducer and activator of transcription-3 (STAT3), forkhead box (Fox) Q1, and FoxO3a (14–17). A much higher concentration of WA is required to induce apoptosis in the normal human mammary epithelial cell line MCF-10A when compared to breast cancer cells (14).
Our laboratory was the first to investigate in vivo effects of WA treatment using rodent models of breast cancer (14,18,19). Intraperitoneal administration of 4 mg WA/kg body weight 5 times/week reduced the growth of MDA-MB-231 xenografts by about 45% (14). Administration of 100 μg WA/mouse (three times/week) for 28 weeks significantly decreased macroscopic mammary tumor size, microscopic mammary tumor area, and the incidence of pulmonary metastasis in MMTV-neu transgenic mice (18). The area of invasive cancer was lower by about 95% in the WA-treated MMTV-neu mice when compared with the solvent-treated control mice (18). In a rat model of luminal-type breast cancer induced by a single injection of N-methyl-N-nitrosourea (MNU), WA administration (4 mg/kg or 8 mg/kg, five times/week) decreased the incidence, multiplicity, and tumor burden (19). As an example, the wet tumor weight in the 8 mg/kg group was decreased by about 68% compared with control rats (19). In each of the rodent study, WA treatment did not cause weight loss or any other toxicity (14,18,19). WA-mediated inhibition of breast cancer growth in mice and rats was associated with a significant decrease in proliferation marker like proliferating cell nuclear antigen as well as apoptosis induction (14,18,19). Inhibition of breast cancer development in MMTV-neu mice and rats was accompanied by accumulation of mitotic cells (19).
Recently, we performed RNA-seq analysis to identify novel mechanistic targets of WA (20). The RNA-seq data revealed downregulation of fatty acid synthesis genes. The present study was undertaken to determine the effect of WA treatment on fatty acid synthesis using human breast cancer cell lines (MCF-7 and MDA-MB-231) and plasma and mammary tumor tissues of control and WA-treated rats.
Materials and Methods
Ethics statement
The use of rats was approved by the University of Pittsburgh Institutional Animal Care and Use Committee (protocol numbers 21100115, 20128342, and 20128342).
Reagents
WA (purity 95.8%) was purchased from ChromaDex (Los Angeles, CA), dissolved in dimethyl sulfoxide (DMSO study), and stored at −80°C. Cell culture media were from MediaTech (Manassas, VA). Fetal bovine serum was obtained from Atlanta Biologicals (Norcross, GA). Antibiotic mixture and other cell culture reagents were purchased from Life Technologies-Thermo Fisher Scientific (Waltham, MA). The antibodies were purchased from the following vendors: anti-acetyl-CoA carboxylase 1 (ACC1) antibody was from Proteintech Group (Rosemont, IL); antibody against fatty acid synthase (FASN) was from Cell Signaling Technology (Beverly, MA); anti-ATP citrate lyase (ACLY), anti-carnitine palmitoyltransferase 1A (CPT1A), and anti-sterol regulatory element binding protein 1 (SREBP1) antibodies were from Abcam (Cambridge, MA); anti-ACC1 antibody for immunohistochemistry was from Sigma-Aldrich (St. Louis, MO); Alexa fluor 488-conjugated goat anti-rabbit antibody was from Life Technologies; fluorescein isothiocyanate-conjugated anti-CD3 antibody and allophycocyanin-conjugated anti-CD161 antibody were from BioLegend (San Diego, CA); BV711-conjugated anti-CD4 antibody was from BD Biosciences (San Jose, CA); and anti-granzyme B antibody and anti-perforin 1 antibody for immunohistochemistry were from Lifespan Biosciences (Seattle, WA). Kits for determination of total free fatty acids and total phospholipids, and anti-ACC1 antibody for immunohistochemistry were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture
The MCF-7 and MDA-MB-231 cell lines were procured from the American Type Culture Collection (Manassas, VA) and each cell line was maintained as suggested by the supplier. These cell lines were last authenticated by us in March of 2017.
Analysis of RNA-seq data for expression of ACLY, ACC1, FASN, and CTP1A1
The RNA-seq data presented in this study have been submitted to the Gene Expression Omnibus of NCBI (GSE158085).
Analysis of breast cancer The Cancer Genome Atlas (TCGA) dataset
The expression of ACLY, ACC1 (also known as ACACA), FASN or CPT1A in normal breast as well as in different subtypes of breast cancer was determined from the TCGA dataset using the following link from the University of California Santa Cruz (https://tcga.xenahubs.net).
Microscopy
The MCF-7 and MDA-MB-231 cells were plated in triplicate on coverslips in 24-well plates. After overnight incubation, the cells were treated with DMSO (control) or WA for 24 hours. The cells were then fixed and permeabilized with 2% paraformaldehyde and 0.5% Triton X-100, respectively. After blocking with phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin and 0.15% glycine, the cells were treated overnight at 4°C with the anti-ACLY, anti-ACC1 or anti-FASN antibody followed by incubation with Alexa fluor 488-conjugated goat anti-rabbit antibody for 1 hour. Corrected total cell fluorescence (CTCF) was determined using ImageJ software. For microscopic analysis of CPT1A, the cells were treated with DMSO or WA in triplicate on coverslips in 24-well plates. The cells were then stained with 100 nmol/L MitoTracker Red in complete medium at 37°C for 1 hour followed by washing and fixation of the cells with 4% formaldehyde in complete medium at 37°C for 15 minutes. The cells were permeabilized with 0.5% Triton X-100 for 5 minutes and incubated for 1 hour with blocking solution consisting of PBS, 0.5% bovine serum albumin, and 0.15% glycine, and then stained with anti-CPT1A antibody at 4°C overnight followed by Alexa fluor 488-conjugated goat anti-mouse secondary antibody for 1 hour at room temperature.
Comparison of normal mammary glands and mammary tumors for expression of fatty acid synthesis enzymes
Thirty female Sprague Dawley rats (20 days old) were purchased from Charles River Laboratories (Wilmington, MA). On 21 days of age, the rats were randomly divided into two groups. The MNU group of rats (n = 15) received single intraperitoneal injection of 50 mg/kg of MNU in 0.9% sodium chloride solution, while control group of rats (n = 15) received single injection of 0.9% sodium chloride solution (IACUC protocol number: 21100115). All rats were fed with regular 5P76 -Prolab® Isopro® RMH 3000 diet and sacrificed after 8 weeks post-injection. Thirteen rats from the MNU group developed mammary tumors at the end of study. No tumors were found in the control rats. After sacrifice, blood was collected from all rats to prepare plasma. Mammary tumors from MNU group of rats and normal mammary glands from the control rats were collected and processed for the determination of fatty acid levels and western blot analysis. In a separate study (IACUC protocol number: 20128342), a total of twenty MNU-treated rats were divided into two groups and the rats of the control group were treated intraperitoneally with vehicle, consisting of 10% dimethyl sulfoxide (DMSO), 40% Cremophor EL/ethanol (3:1), and 50% PBS, five times/week for 10 weeks. The rats in the WA treatment groups were treated with vehicle containing 4 WA/kg body weight five times/week for 10 weeks. This study was used to determine the in vivo effect of WA administration on plasma levels granzyme B and perforin 1 and tumor expression of Granzyme B as well as immunophenotyping of tumor and spleen tissues. Effect of WA administration on expression of fatty acid metabolizing proteins (ACLY, ACC1, FASN, and CPT1A) as well as levels of total free fatty acids and total phospholipids were determined using the fresh frozen plasma and tumor tissues as well as paraffin embedded tumor tissue from our previously published study (19) (IACUC protocol number: 14064037).
Immunoblotting
The lysates from cultured cells and tissues were prepared as described by us previously (18,19,21). Western blotting was per4formed as described by us previously (18,21). Western blots were stripped and re-probed with anti-β-Actin antibody to correct for protein loading. Immunoreactive bands were visualized by enhanced chemiluminescence method and quantified by densitometric scanning using UN-SCAN-IT gel analysis software (Version 7.1, Silk Scientific Corporation, Orem, UT).
Determination of total free fatty acids and phospholipids
Levels of total free fatty acids (C8 and longer) and total phospholipids in the plasma or tissue supernatants of normal mammary glands and mammary tumors were determined using commercially available kits as described by us previously (22).
Immunohistochemistry
Mammary tumor sections from our previous study (19) were de-paraffinized, hydrated, and immersed in boiling citrate retrieval buffer solution (pH 6.0) for 20–30 minutes followed by treatment with 0.3% hydrogen peroxide in 100% methanol for 20 minutes at room temperature. Sections were exposed to blocking buffer (PBS consisting of 5% normal goat serum) for 1 hour at room temperature followed by overnight incubation with anti-ACLY (1:200 dilution), anti-ACC1 (1:150 dilution), anti-FASN (1:250 dilution) or anti-CPT1A (1:50 dilution) antibody but 24 hour incubation with anti-granzyme B (1:200 dilution) and anti-perforin (1:44 dilution) antibody in humidified chambers at 4°C. Sections were washed with PBS, incubated with horseradish peroxidase-conjugated secondary antibody (1:150~400) for 2 hours at room temperature. Color was developed by incubation with 3,3’-diaminobenzidine tetrahydrochloride. Sections were counterstained with hematoxylin and examined under Leica microscope equipped with DFC 450C digital camera. The H-score was determined as described previously by us previously (18).
Determination of plasma granzyme B and perforin 1 level
Plasma level of granzyme B and perforin 1 was measured using ELISA kits commercially available from LifeSpan BioSciences and by following supplier’s instructions.
Preparation of single cell suspension from rat mammary tumors
Tumor tissues from rats were cut into small pieces, minced with a scalpel, and incubated in digesting medium containing collagenase/hyaluronidase/DNase at 37°C for 30 minutes with gentle stirring. Tissue homogenates were sieved through a 70-μm cell strainer to obtain single cell suspension. Red blood cells lysis buffer (BD Biosciences) was added to remove these cells from single cell suspensions. The cells were counted and incubated with desired fluorochrome-conjugated antibodies and then processed for multicolor flowcytometry using Cytek® Aurora flow cytometer (Cytek Biosciences, Fremont, CA).
Isolation of splenocytes from rat spleen
Harvested spleen tissues from rats were placed in 70-μm cell strainer, minced using 1 mL syringe, and then flushed with cold PBS. Red blood cells were removed. The splenocytes were counted and stained with desired fluorochrome-conjugated antibody and then processed for flowcytometry using Cytek® Aurora flow cytometer.
Transient overexpression of SREBP1
The MDA-MB-231 cell line was transiently transfected with empty vector (pcDNA3) (hereafter abbreviated as EV) or pcDNA3.1-2xFLAG-SREBP1C plasmid (hereafter abbreviated as SREBP1) using FuGENE HD transfection reagent (Promega). The pcDNA3.1–2xFLAG-SREBP-1c was a gift from Timothy Osborne (Addgene plasmid # 26802) (23). The cells were treated for 24 hours with either DMSO or 2 μmol/L of WA.
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Total RNA from EV and SREBP1 overexpressing cells was isolated using RNeasy kit and cDNA was synthesized using Superscript Reverse Transcriptase (Invitrogen-Life Technologies) with oligo dT20 primer. The RT-PCR was performed using 2x SYBR green qPCR Kit (Thermo Fisher Scientific). The primers used in this study are listed in Supplementary Table S1. The PCR conditions were as follows: 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds, 60°C for 1 minute, and 72°C for 30 seconds. Relative gene expression was calculated using the method of Livak and Schmittgen (24).
Statistical analysis
GraphPad Prism (version 8.0.0) was used to perform statistical analyses. Statistical significance of difference for two sample comparison was determined by unpaired Student’s t-test. One-way analysis of variance (ANOVA) followed by Dunnett’s test or Bonferroni’s correction were used for multiple group comparisons. A P value of < 0.05 was considered statistically significant.
Data availability
Data would be made available upon written request to the corresponding author.
Results
WA treatment downregulated expression of fatty acid synthesis proteins
As summarized in Supplementary Figure S1A, citrate is the precursor of fatty acid synthesis. Citrate is converted to acetyl-CoA through catalytic mediation of ACLY. Acetyl-CoA is further converted to malonyl-CoA by a reaction catalyzed by ACC1. One molecule of acetyl-CoA and seven molecules of malonyl-CoA are then utilized to synthesize saturated fatty acids (palmitic acid, myristic acid, and stearic acid) by the FASN complex. The CPT1A is a mitochondrial protein that is responsible for formation of acyl carnitines. This reaction is critical for mitochondrial uptake of fatty acids for β-oxidation. The RNA-seq data from our previously published study (20) revealed downregulation of ACLY, ACC1, and/or FASN in MCF-7 and/or MDA-MB-231 cells after WA treatment (Supplementary Figure S1B). The RNA-seq data was validated by qRT-PCR for the mRNA expression of ACLY, ACC1, FASN and CPT1A in MCF-7 and MDA-MB-231 cells after WA treatment (Supplementary Figure S2).
In this study, we used WA concentrations of 1 or 2 μmol/L based on pharmacokinetic considerations. The maximal plasma concentration of WA in rats was about 4.9 μmol/L after intravenous administration of 5 mg WA/kg body weight (25). The plasma half-life in rats after intravenous treatment with 5 mg WA/kg body weight was about 4.5 hours (25). A lower steady state concentration of WA was observed in our chemoprevention study in rats because the blood samples were collected only 1 hour after last injection (19). Figure 1A depicts confocal microscopic images of ACLY and ACC1 proteins in control and WA-treated MCF-7 and MDA-MB-231 cells. The expression of ACLY protein was decreased by 59.2% and 39.6% in MCF-7 and MDA-MB-231 cells, respectively upon treatment with 2 μmol/L WA (Figure 1B). A respective decrease of 54.4 and 56% in protein level of ACC1 was observed in MCF-7 and MDA-MB-231 cells following treatment with 2 μmol/L WA (Figure 1B). The effects of WA treatment on protein expression of FASN and CPT1A are shown in Figure 2A. Expression of both FASN and CPT1A proteins were also decreased significantly upon WA treatment in MCF-7 and MDA-MB-231 cells (Figure 2B). These results indicated inhibitory effect of WA treatment on protein levels of fatty acid synthesis enzyme proteins.
Figure 1.
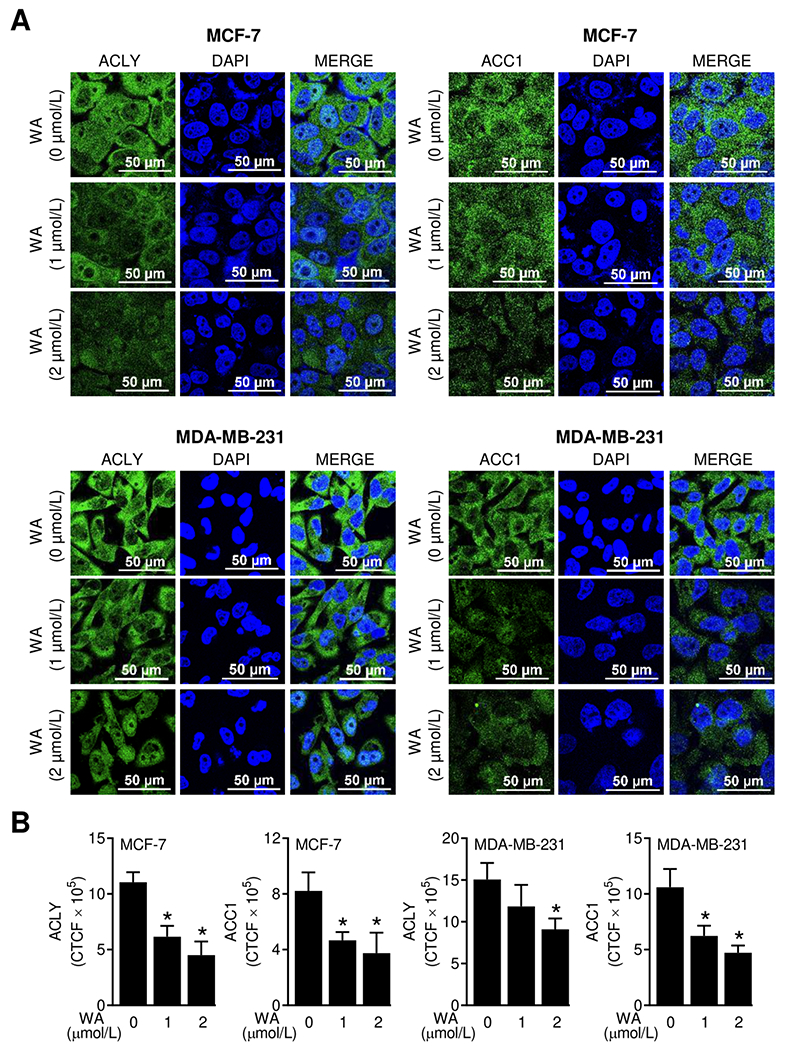
WA treatment decreased protein levels of ACLY and ACC1 in human breast cancer cells. (A) Representative confocal images (60× oil objective magnification, scale bar = 50 μm) for ACLY and ACC1 proteins (green fluorescence) in MCF-7 or MDA-MB-231 cells following 24-hour treatment with DMSO (control) or WA. Nucleus was stained with DAPI (blue fluorescence). (B) Quantitation of corrected total cell fluorescence (CTCF) using ImageJ software. The results shown are mean ± SD (n = 3). Statistically significant (*P < 0.05) compared with the DMSO-treated control by one-way ANOVA followed by Dunnett’s test. The experiment was repeated two times and the results were consistent.
Figure 2.
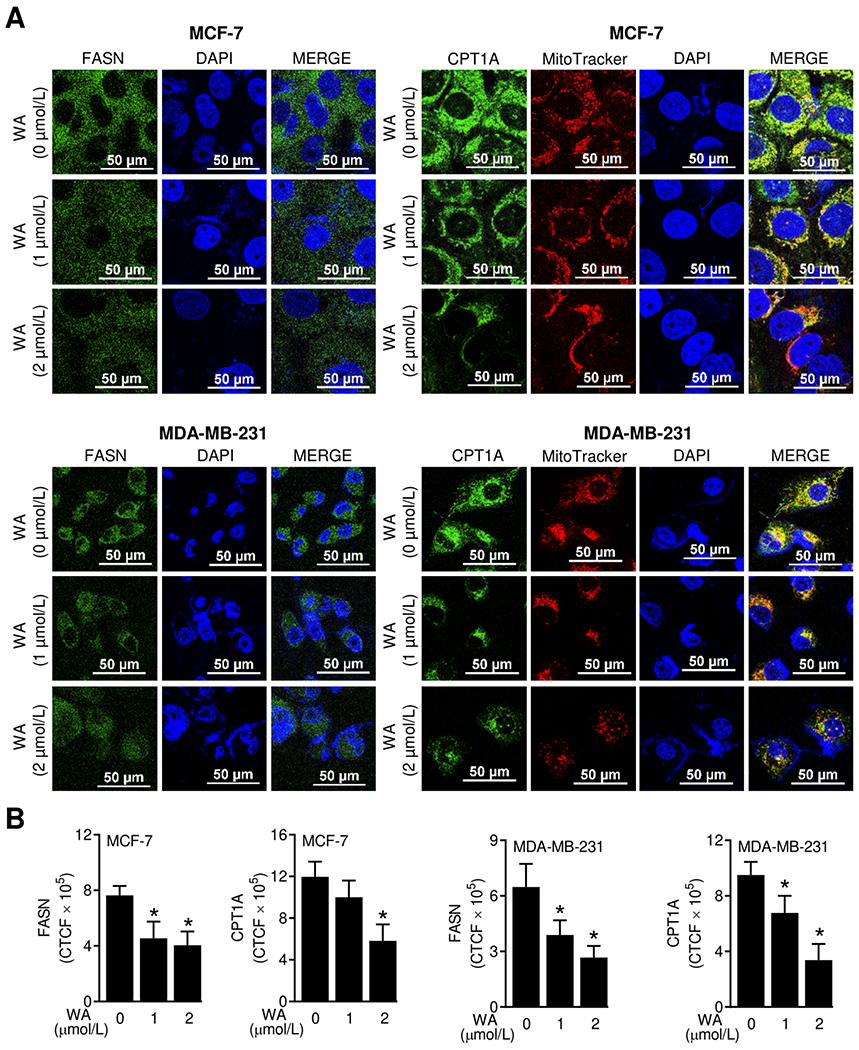
WA treatment decreased protein levels of FASN and CPT1A in human breast cancer cells. (A) Representative confocal images (60× oil objective magnification, scale bar = 50 μm) for FASN and CPT1A proteins (green fluorescence) in MCF-7 or MDA-MB-231 cells following 24-hour treatment with DMSO (control) or WA. Nuclei and mitochondria were visualized by staining with DAPI (blue fluorescence) and MitoTracker Red (red fluorescence), respectively. (B) Quantitation of corrected total cell fluorescence (CTCF) using ImageJ software. The results shown are mean ± SD (n = 3). Statistically significant (*P < 0.05) compared with the DMSO-treated control by one-way ANOVA followed by Dunnett’s test. The experiment was repeated two times and the results were consistent.
Analysis of the breast cancer TCGA data
The expression of ACLY was significantly higher in luminal-type, Her2+, and basal-like subtypes of breast cancer when compared to normal mammary gland (Supplementary Figure S3). The expression of ACC1 was significantly lower in basal-like breast cancer in comparison with normal mammary gland (Supplementary Figure S3). Only Her2+ mammary tumors exhibited upregulation of FASN when compared to normal mammary gland. Finally, the expression of CPT1A was significantly higher in Luminal B type breast cancer (Supplementary Figure S3).
Fatty acid synthesis was increased in MNU-induced mammary tumors than in normal mammary gland
Initially, we conducted a study to determine expression of fatty acid synthesis pathway proteins in normal mammary glands and MNU-induced breast tumors of rats. The expression of ACLY, FASN, and CPT1A protein was very low or undetectable in normal mammary glands (Figure 3A). On the other hand, expression of FASN and CPT1A proteins was higher by about 4.98 and 7.8-fold, respectively, in mammary tumors when compared to normal mammary glands (Figure 3B). Total free fatty acid levels in mammary tumors and plasma were significantly higher compared to those of normal mammary glands (Figure 3C). Similarly, the plasma and mammary tumors from MNU-treated rats had significantly elevated levels of total phospholipids in comparison with normal mammary tissues (Figure 3D). These results indicated elevation of fatty acid synthesis in MNU-induced mammary tumors in rats when compared to normal mammary tissues.
Figure 3.
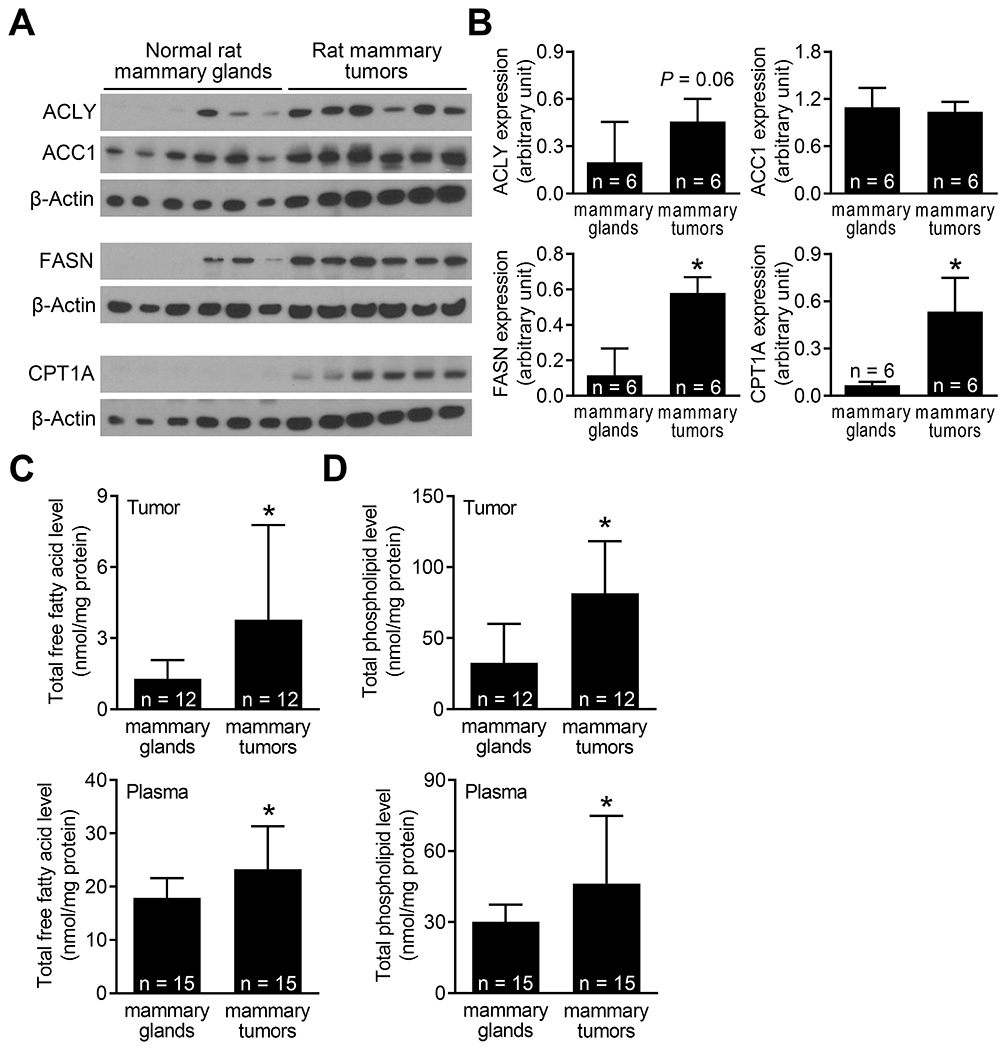
Expression of fatty acid metabolism proteins and total free fatty acids and total phospholipids in mammary tumors and normal mammary glands of rats. (A) Western blotting for ACLY, ACC1, FASN, CPT1A, and β-Actin proteins using supernatants from mammary glands (n = 6) and mammary tumors (n = 6). (B) Densitometric quantitation of the ACLY, ACC1, FASN, and CPT1A protein expression. The results shown are mean ± SD (n = 6). Statistical significance (*P < 0.05) was determined by Student’s t-test. Levels of (C) total free fatty acids and (D) total phospholipids in the plasma and mammary tissues from control rats and MNU-treated rats. The results shown are mean ± SD (n = 12 for mammary tissues; n = 15 for plasma). Statistical significance (*P < 0.05) was determined by Student’s t-test.
Administration of WA inhibited fatty acid synthesis in mammary tumors of rats
Next, we determined the expression of fatty acid metabolism proteins using mammary tumors from control and WA-treated rats (Figure 4A). A decrease in expression of each protein was observed in the WA treatment group compared to control group but the difference was statistically significant only for ACC1 and FASN (Figure 4B). The WA treatment resulted in a significant decrease in circulating levels of total and free fatty acids and total phospholipids (Figure 4C). These results indicated in vivo inhibition of fatty acid synthesis by WA treatment.
Figure 4.
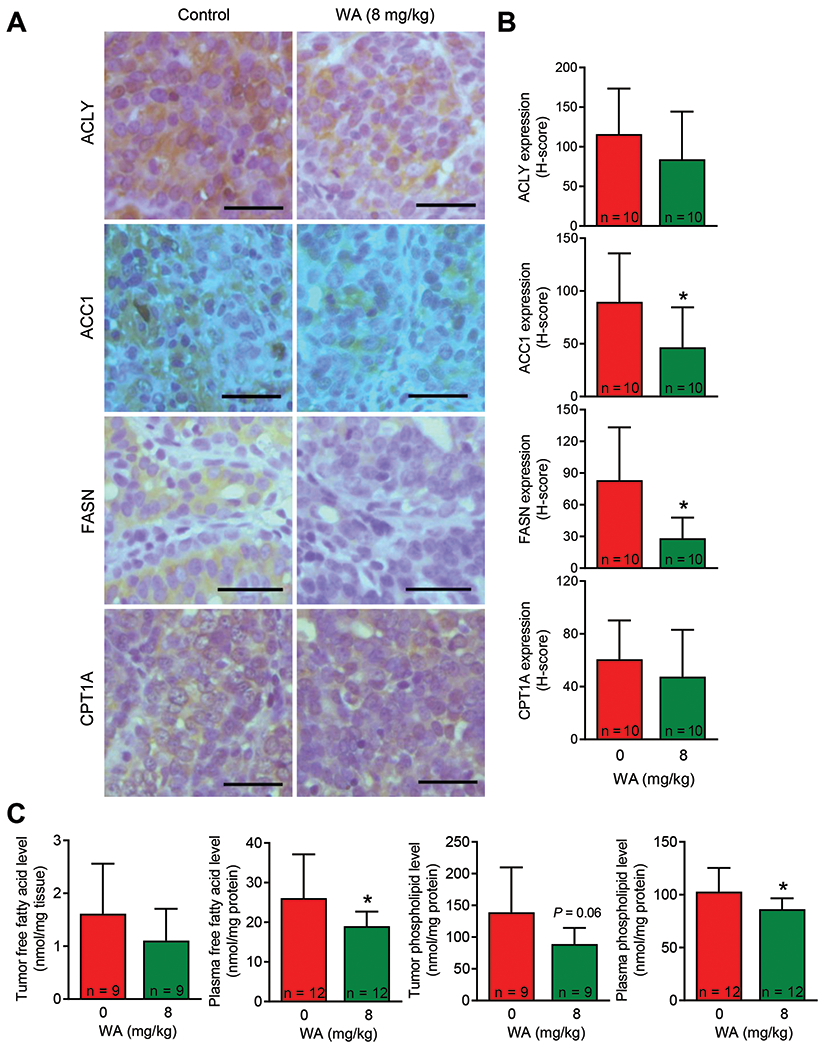
Effect of WA treatment on expression of fatty acid synthesis proteins and total free fatty acid and total phospholipid levels in the plasma and mammary tumors of rats. (A) Representative immunohistochemical images for ACLY, ACC1, FASN, and CPT1A protein expression in mammary tumors from control and WA-treated rats (40× objective magnification, scale bar = 50 μm). (B) H-score for ACLY, ACC1, FASN, and CPT1A protein expression. The results shown are mean ± SD (n = 10). Statistical analysis (*P < 0.05) was performed by Student’s t-test. (C) Levels of total free fatty acids and total phospholipids in the mammary tumors or plasma of control and WA-treated rats. The results shown are mean ± SD (n = 9 for mammary tumors or n = 12 for plasma). Statistical significance (*P < 0.05) was determined by Student’s t-test.
WA treatment increased fraction of CD161+ natural killer (NK) cells in the spleen
Because lipids are known to influence tumor microenvironment and immune cells (26), we determined the effect of WA treatment on markers of T cells (Granzyme B and perforin 1) and fraction of CD3+CD4+ T cells and CD161+ NK cells. The level of Granzyme B was higher in both plasma and the mammary tumors of WA-treated rats in comparison with vehicle-treated control rats, but the difference did not reach significance due large data scatter (Figure 5A,B). Therefore, the CD8+ T cells were not quantitated. The fraction of CD3+CD4+ T cells did not differ between control and the WA treatments groups (Figure 5C). However, the fraction of CD161+ NK cells was significantly higher in the spleen of WA-treated rats when compared to controls (Figure 5C). These results indicated that inhibition of mammary tumor development by WA treatment was accompanied by an increase in fraction of CD161+ NK cells at least in the spleen. Mammary tumor infiltration of CD161+ NK cells was also modestly higher in the WA treatment group when compared to the control group but the difference was not significant
Figure 5.
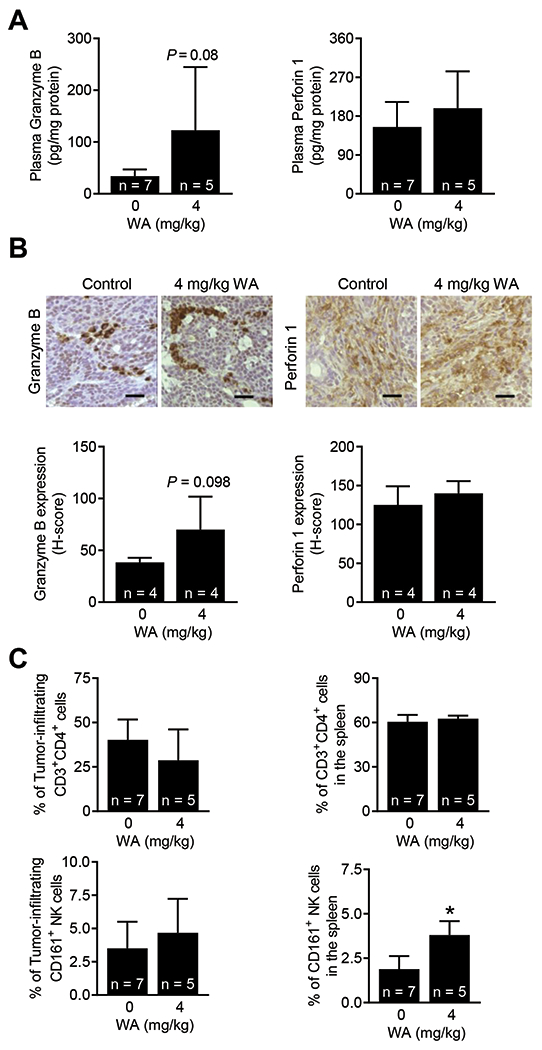
Effect of WA administration on immune cells/markers. (A) Quantification of Granzyme B and perforin 1 in the plasma of control and WA-treated rats. The results shown are mean ± SD (n = 5-7). Statistical significance (*P < 0.05) was determined by Student’s t-test. (B) Representative immunohistochemical images (200× magnification; scale bar = 50 μm) showing the expression of granzyme B and perforin 1 proteins in mammary tumors from a rat administered vehicle or WA. Quantification of tumor-infiltrating Granzyme B and perforin 1 are shown in lower panels. The results shown are mean ± SD (n = 4). Statistical significance (*P < 0.05) by Student’s t-test. (C) Quantification of immune cells in mammary tumors (left) and spleen (right). The results shown are mean ± SD (n = 5-7). Statistical analysis was done by Student’s t-test (*P < 0.05).
Overexpression of SREBP1 conferred partial protection against WA-mediated downregulation of ACLY and ACC1
The SREBP1 is known to play an important role in regulation of fatty acid synthesis genes (27). The expression of precursor and mature SREBP1 was decreased upon treatment of MDA-MB-231 cells with WA (Figure 6A). The level of SREBP1 protein was also lower in the mammary tumors of WA-treated rats than that of control rats but the difference was not significant (Figure 6B). Overexpression of SREBP1 (Figure 6C) conferred partial protection against WA-mediated downregulation of ACLY and ACC1 (Figure 6D). On the other hand, SREBP1 overexpression has no meaningful impact on FASN expression.
Figure 6.
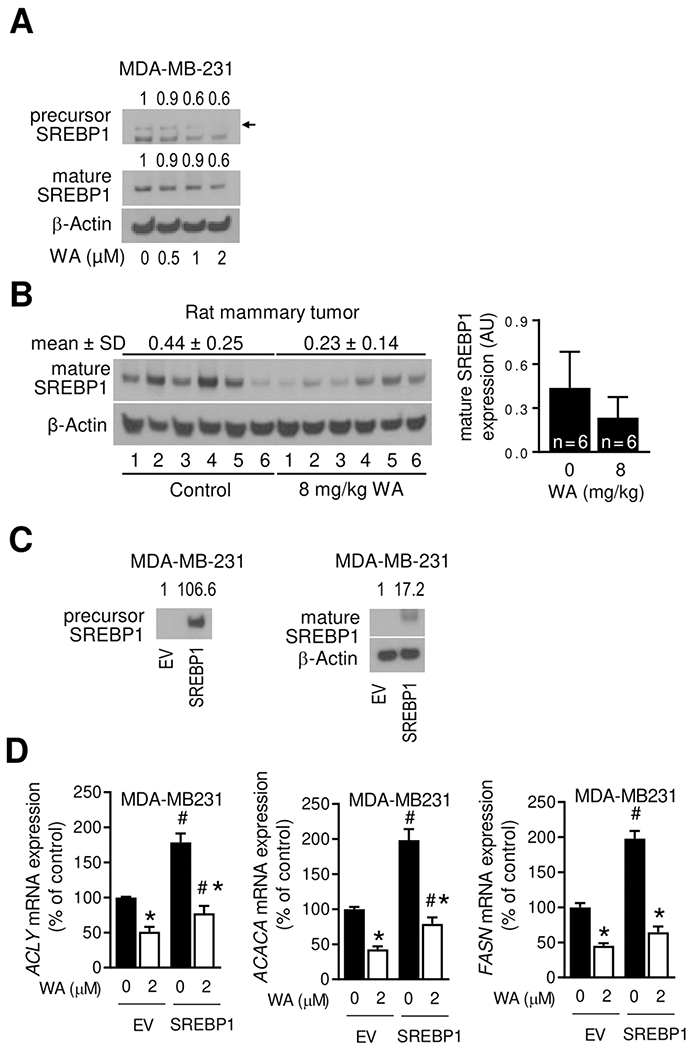
WA administration inhibited SREBP1 expression. (A) Immunoblotting for precursor and mature forms of SREBP1 using lysates from MDA-MB-231 cells treated for 24 hours with DMSO (control) or the indicated doses of WA. The numbers above bands represent fold change of each protein relative to DMSO-treated control. (B) Immunoblotting for mature form of SREBP1 protein using mammary tumor lysates from control and WA-treated rats. The results shown are mean ± SD (n = 6). Statistical significance was determined by Student’s t-test. AU, arbitrary unit. (C) Immunoblotting for precursor and mature forms of SREBP1 using lysates from empty vector (EV) transfected or SREBP1 overexpressing MDA-MB-231 cells. (D) Real-time RT-PCR for ACLY, ACACA and FASN mRNA expression in EV and SREBP1 transfected MDA-MB-231 cells after 24 hours treatment with DMSO or 2 μmol/L WA. Results shown are mean ± SD (n = 3). Significantly different (P < 0.05) compared with DMSO-treated control (*) or between EV and SREBP1 cells at the same dose of WA (#) by one-way ANOVA followed by Bonferroni’s multiple comparisons test. The results were reproducible from replicate experiments.
Discussion
Metabolic reprogramming, including increased glycolysis and fatty acid synthesis is a hallmark of cancer (28). While increased glycolysis provides energy, the fatty acids are utilized for β-oxidation in the mitochondria to not only produce energy but also in cell signaling (e.g., palmitylation and myristylation of oncogenic proteins) or incorporation into complex lipids for membrane integrity (29,30). Studies have implicated fatty acid synthesis enzyme proteins in breast cancer progression (31–35). The expression of ACLY protein was higher in breast tumors in comparison with adjacent normal mammary tissue (31). Knockdown of ACLY protein in MCF-7 cells decreased cell viability due to apoptosis induction (31). The enzymatic activity of ACLY was higher by about 150-fold in breast cancer compared to normal mammary tissue (32). Another study showed physical interaction of ACLY with post-translationally cleaved low molecular weight cyclin E thereby promoting breast cancer growth (33). Both ACC1 and FASN proteins were expressed at higher level in breast carcinoma in situ than in histologically normal mammary tissue (34). The BRCA1 physically interacts with phosphorylated ACC1 to control lipid metabolism in breast cancer cells (35). Even though studies overwhelmingly support an oncogenic role of ACLY, ACC1, and FASN, their inhibitors are not available for cancer therapy (36). Prior efforts in clinical development of FASN inhibitors have been hampered due to a variety of reasons, including stability (Cerulenin), poor bioavailability (Orlistat), and side effects (appetite suppression and weight loss through direct activation of CPT1A) (36). We have shown previously that inhibition of breast cancer development in MMTV-neu mice is accompanied by suppression of glycolysis (18). The present study is the first to demonstrate inhibition of fatty acid synthesis by WA treatment in vivo in a rodent model of breast cancer. Therefore, plasma and tumor levels of total free fatty acids or immunohistochemical analyses of ACLY, ACC1, and/or FASN protein expression may be integrated as biomarkers of WA efficacy in future clinical trials. In this context, a placebo-controlled (n=13) clinical study with daily oral administration of one 400 mg Withania somnifera capsule (three times/day) for 1 month in patients with schizophrenia (n=12) showed a significant decrease (P < 0.05) in circulating levels of triglycerides (37). Because triglycerides are a by-product of fatty acid synthesis, we are quite optimistic for suppression of circulating and possibly tumor levels of total free fatty acids by WA administration in breast cancer patients.
Progression of cancer, including mammary carcinoma, is regulated by cells of innate immunity (e.g., NK cells) and adaptive immunity (CD4+ and CD8+ T cells) in the tumor microenvironment (38). Tumor-infiltrating lymphocytes like CD8+ cytotoxic T cells serve as prognostic indicators for response to chemotherapy and for survival (38). On the other hand, Treg and myeloid derived suppressor cells cause immune evasion (39,40). The level of myeloid derived suppressor cells in the blood correlates with tumor burden, stage and with poor prognosis in multiple cancer types (39). A meta-analysis showed a negative effect of FoxP3+ Treg on overall survival (41). Previous studies have shown immunomodulatory effects of Withania somnifera as well as WA. The WA treatment was shown to inhibit mitogen induced T and B cell proliferation in vitro in association with upregulation of T cell markers CD25, CD69, CD71 and CD54 and B cell activation markers CD80, CD86 and MHC-II (42). Function of myeloid derived suppressor cells was inhibited by WA (43). Dietary administration of Withania somnifera increased NK cell function in a laying hen model of spontaneous ovarian cancer (44). Our results also showed an increase in CD161+ NK cells in the spleen by WA administration to tumor bearing rats. The mammary tumor level of CD161+ NK cells was higher in the WA treatment group, but the difference was insignificant.
We found that overexpression of SREBP1 only partially attenuates WA-mediated downregulation of fatty acid synthesis genes. It is possible that additional mechanisms are involved in WA-mediated suppression of fatty acid genes. For example, we have shown previously that c-Myc is another regulator of fatty acid synthesis at least in prostate cancer (22). The c-Myc was recruited at the promoter of ACLY, ACC1, and FASN (22). It is possible that WA treatment suppresses c-Myc to inhibit fatty acid synthesis in breast cancer. The STAT3 was also shown to regulate fatty acid metabolism in breast cancer (45). However, further work is necessary to determine the role of c-Myc or STAT3 in WA-mediated downregulation of fatty acid synthesis enzyme proteins.
The key conclusions from the present study include: (a) fatty acid synthesis and expression of key enzymes of this pathway are elevated in mammary tumors resulting from MNU injection and this model may be useful for future screening of fatty acid synthesis inhibitors; (b) WA treatment inhibits fatty acid synthesis in vivo by downregulating expression of fatty acid synthesis proteins; and (c) inhibition of breast cancer development by WA administration in the MNU-rat model is accompanied by a statistically significant increase in CD161+ NK cells in the spleen.
Supplementary Material
Prevention relevance.
The present study shows that breast cancer prevention by WA in rats is associated with suppression of fatty acid synthesis.
Funding
This study was supported in part by the National Cancer Institute grants R01 CA219180 (S.V. Singh) and CA142604 (S.V. Singh). This study used the UPMC Hillman Cancer Center Core Facilities, including the Animal Facility and Tissue and Research Pathology Facility. The funders had no role in the design of the study, data collection, analysis or interpretation of the data, manuscript preparation or decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: The authors declare no potential conflict of interest.
References
- 1.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev 2000;5:334–46. [PubMed] [Google Scholar]
- 2.Pérez-Gómez J, Villafaina S, Adsuar JC, Merellano-Navarro E, Collado-Mateo D. Effects of Ashwagandha (Withania somnifera) on VO2max: A systematic review and meta-analysis. Nutrients 2020;12:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuboyama T, Tohda C, Komatsu K. Effects of Ashwagandha (roots of Withania somnifera) on neurodegenerative diseases. Biol Pharm Bull 2014;37:892–7. [DOI] [PubMed] [Google Scholar]
- 4.Pratte MA, Nanavati KB, Young V, Morley CP. An alternative treatment for anxiety: a systematic review of human trial results reported for the Ayurvedic herb ashwagandha (Withania somnifera). J Altern Complement Med. 2014;20:901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng QX, Loke W, Foo NX, Tan WJ, Chan HW, Lim DY, et al. A systematic review of the clinical use of Withania somnifera (Ashwagandha) to ameliorate cognitive dysfunction. Phytother Res 2020;34:583–90. [DOI] [PubMed] [Google Scholar]
- 6.Dutta R, Khalil R, Green R, Mohapatra SS, Mohapatra S. Withania Somnifera (Ashwagandha) and withaferin A: potential in integrative oncology. Int J Mol Sci 2019;20:5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palliyaguru DL, Singh SV, Kensler TW. Withania somnifera: from prevention to treatment of cancer. Mol Nutr Food Res 2016;60:1342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahm ER, Kim SH, Singh KB, Singh K, Singh SV. A comprehensive review and perspective on anticancer mechanisms of withaferin A in breast cancer. Cancer Prev Res (Phila) 2020;13:721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antony ML, Lee J, Hahm ER, Kim SH, Marcus AI, Kumari V, et al. Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of β-tubulin. J Biol Chem 2014;289:1852–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahm ER, Lee J, Singh SV. Role of mitogen-activated protein kinases and Mcl-1 in apoptosis induction by withaferin A in human breast cancer cells. Mol Carcinog 2014;53:907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahm ER, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is associated with suppression of inhibitor of apoptosis family protein expression. Cancer Lett. 2013;334:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One 2011;6:e23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahm ER, Singh SV. Autophagy fails to alter withaferin A-mediated lethality in human breast cancer cells. Curr Cancer Drug Targets 2013;13:640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res 2008;68:7661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Hahm ER, Singh SV. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis 2010;31:1991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahm ER, Lee J, Huang Y, Singh SV. Withaferin a suppresses estrogen receptor-α expression in human breast cancer cells. Mol Carcinog 2011;50:614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SH, Singh KB, Hahm ER, Singh SV. The role of forkhead box Q1 transcription factor in anticancer effects of withaferin A in breast cancer. Cancer Prev Res (Phila) 2021;14:421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahm ER, Lee J, Kim SH, Sehrawat A, Arlotti JA, Shiva SS, et al. Metabolic alterations in mammary cancer prevention by withaferin A in a clinically relevant mouse model. J Natl Cancer Inst 2013;105:1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samanta SK, Sehrawat A, Kim SH, Hahm ER, Shuai Y, Roy R, et al. Disease subtype-independent biomarkers of breast cancer chemoprevention by the ayurvedic medicine phytochemical withaferin A. J Natl Cancer Inst 2016;109:djw293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahm ER, Kim SH, Singh KB, Singh SV. RNA-seq reveals novel cancer-selective and disease subtype-independent mechanistic targets of withaferin A in human breast cancer cells. Mol Carcinog 2021;60:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis 2003;24:891–7. [DOI] [PubMed] [Google Scholar]
- 22.Singh KB, Hahm ER, Kim SH, Wendell SG, Singh SV. A novel metabolic function of Myc in regulation of fatty acid synthesis in prostate cancer. Oncogene 2021;40:592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toth JI, Datta S, Athanikar JN, Freedman LP, Osborne TF. Selective coactivator interactions in gene activation by SREBP-1a and −1c. Mol Cell Biol 2004;24:8288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 25.Dai T, Jiang W, Guo Z, Wang Z, Huang M, Zhong G, et al. Studies on oral bioavailability and first-pass metabolism of withaferin A in rats using LC-MS/MS and Q-TRAP. Biomed Chromatogr. 2019;33:e4573. [DOI] [PubMed] [Google Scholar]
- 26.Corn KC, Windham MA, Rafat M. Lipids in the tumor microenvironment: from cancer progression to treatment. Prog Lipid Res 2020;80:101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horton JD, Goldstein JL, Brown MS. SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb Symp Quant Biol 2002;67:491–8. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 29.Harrelson JP, Lee MW. Expanding the view of breast cancer metabolism: promising molecular targets and therapeutic opportunities. Pharmacol Ther 2016;167:60–73. [DOI] [PubMed] [Google Scholar]
- 30.Kinlaw WB, Baures PW, Lupien LE, Davis WL, Kuemmerle NB. Fatty acids and breast cancer: make them on site or have them delivered. J Cell Physiol 2016;231:2128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, Yin L, Wei J, Yang Z, Jiang G. ATP citrate lyase is increased in human breast cancer, depletion of which promotes apoptosis. Tumour Biol 2017;39:1010428317698338. [DOI] [PubMed] [Google Scholar]
- 32.Szutowicz A, Kwiatkowski J, Angielski S. Lipogenetic and glycolytic enzyme activities in carcinoma and nonmalignant diseases of the human breast. Br J Cancer 1979;39:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucenay KS, Doostan I, Karakas C, Bui T, Ding Z, Mills GB, Hunt KK, et al. Cyclin E associates with the lipogenic enzyme ATP-citrate lyase to enable malignant growth of breast cancer cells. Cancer Res 2016;76:2406–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res 1997;3:2115–20. [PubMed] [Google Scholar]
- 35.Moreau K, Dizin E, Ray H, Luquain C, Lefai E, Foufelle F, et al. BRCA1 affects lipid synthesis through its interaction with acetyl-CoA carboxylase. Biol Chem 2006;281:3172–81. [DOI] [PubMed] [Google Scholar]
- 36.Angeles TS, Hudkins RL. Recent advances in targeting the fatty acid biosynthetic pathway using fatty acid synthase inhibitors. Expert Opin Drug Discov 2016;11:1187–99. [DOI] [PubMed] [Google Scholar]
- 37.Agnihotri AP, Sontakke SD, Thawani VR, Saoji A, Goswami VS. Effects of Withania somnifera in patients of schizophrenia: a randomized, double blind, placebo controlled pilot trial study. Indian J Pharmacol 2013;45:417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goff SL, Danforth DN. The role of immune cells in breast tissue and immunotherapy for the treatment of breast cancer. Clin Breast Cancer 2021;21:e63–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Cicco P, Ercolano G, Ianaro A. The new era of cancer immunotherapy: targeting myeloid-derived suppressor cells to overcome immune evasion. Front Immunol. 2020;11:1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe MA, Oda JM, Amarante MK, Cesar Voltarelli J. Regulatory T cells and breast cancer: implications for immunopathogenesis. Cancer Metastasis Rev 2010;29:569–79. [DOI] [PubMed] [Google Scholar]
- 41.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep 2015;5:15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gambhir L, Checker R, Sharma D, Thoh M, Patil A, Degani M, et al. Thiol dependent NF-κB suppression and inhibition of T-cell mediated adaptive immune responses by a naturally occurring steroidal lactone withaferin A. Toxicol Appl Pharmacol 2015;289:297–312. [DOI] [PubMed] [Google Scholar]
- 43.Sinha P, Ostrand-Rosenberg S. Myeloid-derived suppressor cell function is reduced by Withaferin A, a potent and abundant component of Withania somnifera root extract. Cancer Immunol Immunother 2013;62:1663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barua A, Bradaric MJ, Bitterman P, Abramowicz JS, Sharma S, Basu S, et al. Dietary supplementation of Ashwagandha (Withania somnifera, Dunal) enhances NK cell function in ovarian tumors in the laying hen model of spontaneous ovarian cancer. Am J Reprod Immunol 2013;70:538–50. [DOI] [PubMed] [Google Scholar]
- 45.Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, et al. JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab 2018;27:136–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data would be made available upon written request to the corresponding author.


