Abstract
For systemically delivered nanoparticles to reach target tissues, they must first circulate long enough to reach the target and extravasate there. A challenge is that the particles end up engaging with serum proteins and undergo immune cell recognition and premature clearance. The serum protein binding, also known as protein corona formation, is difficult to prevent, even with artificial protection via “stealth” coating. Protein corona may be problematic as it can interfere with the interaction of targeting ligands with tissue-specific receptors and abrogates the so-called active targeting process, hence, the efficiency of drug delivery. However, recent studies show that serum protein binding to circulating nanoparticles may be actively exploited to enhance their downstream delivery. This review summarizes known issues of protein corona and traditional strategies to control the corona, such as avoiding or overriding its formation, as well as emerging efforts to enhance drug delivery to target organs via nanoparticles. It concludes with a discussion of prevailing challenges in exploiting protein corona for nanoparticle development.
Keywords: Nanoparticles, protein corona, drug delivery, stealth, targeting
Graphical Abstract
1. Introduction
Nanoparticulate drug carriers are used to preserve drug stability in physiological conditions and improve drug delivery to target tissues. The premise of using nanoparticles (NPs) is that the nanosized particles have selective access to the tumor with leaky vasculature via the enhanced permeability and retention (EPR) effect [1]. Additionally, NPs may be coated with antibodies, peptides, and proteins that bind to the overexpressed receptors in target tissues, which further enhance target-specific retention and uptake. However, few nanoparticle formulations translated to clinical applications, with the majority still suffering from premature clearance by the mononuclear phagocyte system (MPS), resulting in suboptimal therapeutic benefits.
One of the critical challenges is that proteins in the physiological media bind to the NPs to form an assembly of various proteins on the surface, called protein corona (PC) [2]. The PC endows NPs with a new identity, distinct from the original physicochemical identity, driving the in-vivo fates of NPs in a manner that may not be consistent with the design [3–6]. Therefore, PC is considered a missing link between in vitro-in vivo correlation [7]. Understanding the nature of PC and its impact on the biological performance of NPs is critical to the successful development of NP products.
Since the initial recognition of PC and its impact on NP performance [8–12], numerous studies have investigated the relationship between PC profiles and NP attributes [9, 13–16]. The early focus has been to develop analytical methods, identify the components of PC, and understand NP properties that govern the PC profiles [4]. With increasing data linking the PC profiles and in vivo performance of NPs, recent efforts are made to exploit the PC, which may not be entirely avoided by existing approaches, by identifying serum proteins promoting target-specific interactions and co-opting those proteins by design [17]. Several preclinical studies support that PC can help deliver drugs via NPs to specific organs, including the brain, which remains a tempting target. Once considered a foe, the PC deserves new attention as a potential friend to help improve NP-based drug delivery.
With the new perspective on PC, we intend to compile recent literature to understand the current state of the art and explore new opportunities to take NP technology to the next level. We will first summarize known issues of PC formation and traditional approaches to control PC formation and discuss emerging strategies to exploit PC for NP delivery, focusing on articles published in the last five years. The review will conclude with a discussion of prevailing challenges in using PC for NP development.
2. Protein corona on nanoparticles
Once NPs enter the circulation, they interact with different components of biological fluid, especially serum proteins. The NP surface is first covered by proteins abundant in blood, forming a “soft corona,” which, over time, is replaced by high-affinity proteins to form a “hard corona” [18, 19]. A common in vitro method to profile PC on NPs is to incubate them with plasma or serum for a certain time, elute and digest the surface-bound proteins, and analyze them by gel electrophoresis and LS-MS/MS. Prior to the elution of PC, the NPs are typically separated from the excess solution by centrifugation and washing [20]; therefore, most in vitro results tend to focus on hard corona, which survives centrifugation and subsequent washing steps. Recognizing the significance of dynamic environment on PC formation, recent studies investigate the PC formed in vivo, where the NPs are injected intravenously and retrieved from blood [21]. Magnetic separation [22] or size exclusion chromatography [23] are employed to separate NPs from the blood for analysis. To investigate soft corona and temporal evolution of PC, in vitro methods are complemented by additional analytical tools, such as microscopy [24], spectroscopy [25] [26], zeta potential analysis [27, 28], or click chemistry that immobilizes soft corona in situ [29]. Techniques used to analyze PC have been thoroughly reviewed in recent literature [30].
The composition and quantity of proteins bound to NPs vary with their size and surface chemistry [3, 31]. A study with polystyrene NPs with differential sizes (50 and 100 nm) and surface chemistry (plain, carboxyl-modified, and amine-modified), incubated with human serum, detected > 80 plasma proteins in 88% of the data sets, commonly involving immunoglobulins, lipoproteins, and complement proteins [3]. Selected proteins may be clustered according to their preference of size and surface chemistry, where the identity, lipid affinity, and post-translational modification of each protein contribute to the preference [3]. PC also depends on plasma composition and concentration [32, 33]. In addition, the medical condition of a patient has a profound impact on PC profiles [34]. For example, PC-bound liposomes in pancreatic cancer patients are less negatively charged than those of other cancer patients or healthy individuals due to the enrichment of tumor antigen-specific autoantibodies [35]. These findings demonstrate the dependence of PC on biological factors and underscore the importance of simulating in vivo conditions (e.g., serum concentration, disease status, species) in PC analysis, which may be overlooked in conventional in vitro assays based on cell culture media with animal sera.
3. Effects of PC on NP performance
PC can alter the size and surface characters of NPs and confer different biological properties, causing unpredictable therapeutic outcomes. The following summarizes the influences of PC on NP functionality.
3.1. Colloid stability
There are mixed findings regarding the effects of PC on the colloidal stability of NPs. Some show that PC increases the risk of NP aggregation. Surfactant-stabilized gold nanorods (NR), precoated with PC by preincubation with equine serum, undergo differential degrees of aggregation depending on the stabilizing agents and the media in which they are tested [36]: With cationically stabilized gold NRs by cetyltrimethylammonium bromide (CTAB) or oligofectamine, the PC caused aggregation in deionized water but not in RPMI cell culture medium. On the other hand, the gold NRs stabilized with neutral surfactant Brij56 or anionic phosphatidylserine remained stable in both deionized water and cell culture medium [36]. Others report that the aggregation depends on the type and concentration of proteins: polystyrene NPs aggregated in medium containing cow serum, less so in cow serum depleted of immunoglobulin G (IgG), and did not aggregate in medium with fetal calf serum (very low on antibodies). Supplementing IgG or fibronectin to fetal serum caused the aggregation, indicating the two proteins to be responsible [37]. The diverse conclusions on the colloidal stability of PC-coated NPs may be attributable to the variability of test conditions: The interactions of PC-coated NPs vary with the pH and ionic strength of the medium, which influence the charges of NPs and proteins [38–42], types of ions [39, 43], and the protein to NP ratio (i.e., the protein concentration) [37, 44].
Proteins with relatively large molecular weights (> 40 kDa) and low isoelectric points (< pH 7.4), such as albumin, catalase, hemoglobin, glucose oxidase, and horseradish peroxidase, have shown to help disperse gold NPs, based on the steric hindrance and/or negative charges in physiological pH [45]. Therefore, serum albumin is actively exploited to improve the colloidal stability of NPs, such as gold NPs that can accommodate the protein via cysteine residues [45, 46]. The albumin-coated gold NPs maintained colloidal stability in hypertonic conditions, extreme pHs [45], high concentrations, and the lyophilization process [46]. Nevertheless, the protective effect of surface-bound albumin varies with the type of NPs and ions in the media [43] and may not be generalized.
3.2. Cellular interaction
PC affects the cellular uptake of NPs in various ways. In many cases, the presence of PC, such as serum albumin, on the NP surface lowers the surface energy and decreases the NP–cell membrane interaction, thereby reducing the NP uptake [47, 48]. The extent of NP uptake reduction varies with the NP size and the cell types. A study with gold NPs showed that the PC effect on particle uptake increased with the particle size, with 50 nm gold NPs being more affected than 5 nm and 20 nm gold NPs, and phagocytic cells were more affected than nonphagocytic cells [49]. In addition, the surface chemistry and softness of NPs influence the extent of the PC effect. In LNPs with two PEGylated acyl chains of different lengths (C18 vs. C14), PC decreased the HepG2 cell uptake of LNPs with the C18 acyl chain but increased the uptake of LNPs with the C14 chain [50]. Also, solid NPs showed a significant decrease in the uptake by human keratinocytes and endothelial cells upon PC binding, whereas soft nanogels made of dendritic polyglycerol were not affected by PC [51].
PC can also enhance the cellular uptake of NPs. PC can help negatively charged NPs to overcome the electrostatic repulsion between the cell membrane and NPs. For example, PC helps neutralize the surface charge of citrate-coated silver NPs and allows them to enter mouse embryonic fibroblasts, increasing cytotoxicity [52]. Fibrinogen, immunoglobulins, and complements bound to NPs respectively interact with integrin receptor, Fc receptor, and complement receptors of macrophages to facilitate phagocytosis of the NPs [53]. Apolipoproteins ApoE and ApoB-100 support the uptake of NPs by brain endothelial cells via the low-density lipoprotein receptor (LDLR) [54].
In addition, PC can change the uptake pathway of NPs. With gold NPs, PC interfered with classical caveolae- or scavenger receptor-mediated endocytosis or phagocytes but promoted clathrin-mediated endocytosis; in nonphagocytic hepatocellular carcinoma (HepG2) cells, clathrin-mediated endocytosis was favored in serum-containing medium, whereas caveolae-mediated endocytosis dominated in serum-free medium [49]. Our group has also observed a change in the endocytosis pathway from clathrin-mediated endocytosis to caveolae-mediated endocytosis due to the albumin binding to NPs via polydopamine surface (more in Section 6.1) [55].
The effect of PC on NP-cell interaction varies with the source of PC. For example, the adhesion of NPs to target cells was shown to be affected by PC differently according to the species from which the PC was obtained [56]. Here, organic and inorganic NPs (500 nm) were modified with sialyl Lewis A (sLeA)_to target selectin-expressing endothelial cells. When the NPs were added to selectin-expressing human umbilical vein endothelial cells (HUVEC) in laminar flow, their binding to the cells was significantly affected (almost eliminated) by porcine plasma but not by mouse plasma. The adhesion inhibition of PLGA particles was also observed in human plasma [57]. The differential effect of protein binding to HUVEC was attributed to the difference in PC composition: the sLeA-modified PLGA NPs exposed to porcine plasma showed the binding of 150 kDa protein (likely IgG), like in human plasma, as well as >250 kDa protein, but those exposed to mouse plasma did not. This study underscores that the effect of PC on cellular interactions and other behaviors of NPs discussed below should be interpreted in the context of species.
Given the species dependence, it is worthwhile to reconsider the reliability of animal models (typically mouse models) in predicting the clinical consequences of PC formation. Neutrophils are identified to be a major phagocytic cell population that contributes to NP clearance in blood [58, 59]. Neutrophils showed a differential NP uptake pattern according to the medium in which the NPs were incubated: polystyrene NPs in human plasma were taken up by human neutrophils far more than those in bovine serum [58]. PC profiling and incubation with selected proteins suggest that complement components in human plasma bind to the NPs and mediate their preferential uptake by neutrophils [58]. PEGylated polystyrene NPs were even more prone to neutrophil uptake compared to naked (carboxylated polystyrene) NPs. This difference is attributable, ironically, to the stealth function of PEG (Section 4): naked NPs were covered with benign PC, including albumin, whereas PEGylated NPs acquired less PC, leaving room for complement protein binding. This study suggests that the PC effect studied in one species may not be extrapolated to others. Species difference in the cell population (e.g., neutrophils representing 50–70% of circulating leukocytes in humans but 10–25% in mice [60]) brings an additional layer of complexity in interpreting the consequence of PC binding to NPs.
3.3. Cytotoxicity
NP introduced to the body continuously interact with various types of cells, including blood cells, endothelial cells, and cancerous cells. The carrier itself may have detrimental effects to the cell viability. PCs can affect the extent of cytotoxicity of the NPs either positively or negatively.
Many studies report that PC reduces the cellular toxicity of NPs [61–67]. The PC layer can block NPs from contacting and damaging cell membranes and reduce their cellular uptake. For instance, CTAB-coated gold NRs destabilize the cell membrane and cause necrosis; however, PC shielded the adverse effects of the cationic surfactant and reduced the toxicity on A549 cells [68]. Graphene oxide nanosheets induce reactive oxygen species (ROS) and directly damage the cell membrane via sharp edges [69, 70]; however, 10% FBS and bovine serum albumin PC reduced the damaging effect by making the edge of nanosheets dull and reducing their surface area available for lipid extraction [70]. Additionally, PC can scavenge ROS or reduce their production catalyzed by inorganic NPs. For example, titanium dioxide (TiO2) nanotubes induce phototoxicity upon UV irradiation by catalyzing ROS production. However, serum proteins bound to TiO2 nanotubes sequestered the photoinduced hydroxyl radicals and reduced phototoxicity [66]. Cadmium telluride quantum dots (CdTe QDs) impair mitochondrial activity by damaging the organelle membrane, resulting in ROS increase and ATP reduction. PC acquired in fetal bovine serum prevented the negative effects of QDs on mitochondria activities, alleviating the cytotoxicity to RAW 264.7 cells [71]. The PC-mediated reduction of ROS production and cytotoxicity has also been reported with ZnO NPs [72, 73] and Fe3O4 NPs [74]. Meanwhile, the protective effect of PC may be dependent on the protein identity. Lysozyme, immunoglobulin G, and bovine serum albumin are shown to reduce the cytotoxicity of the poly(acrylic acid)-block-polystyrene polymersomes on HeLa and HEK 293 cells [75]. Similarly, serum albumin corona attenuated the platelet aggregation caused by carboxylate-functionalized carbon nanotubes; however, IgG and histone H1 corona aggravated the platelet damage in distinct manners [76].
Conversely, PC can exert adverse effects on cell functions. For example, human serum albumin adsorbed on ultrasmall gold nanoparticles (AuNPs) underwent conformational change leading to increased α-helicity. The albumin with altered conformation damaged the cell membrane and increased cytotoxicity to BRL 3A rat liver cells and 293T embryonic kidney cells [77]. In another example, cadmium sulfide nanomaterials (CdS NMs) incubated with fetal bovine serum increased cytotoxicity to the cells while reducing macrophage uptake [78]. The apparently contradicting phenomenon was attributed to the PC-induced expression of FcγRIIB receptors [78], which activate protein kinase B/Caspase 3 pathway to induce apoptosis but also downregulate phagocytic activity of macrophages [79]. This study illustrates that cellular uptake is not always a prerequisite for increased cytotoxicity. PC formation can also lead to abnormal proliferation of the cells. PC formed on the nanoscale airborne particulate matter by serum incubation stimulated the generation of ROS, which activated α-smooth muscle actin expression, causing aberrant proliferation of human lung fibroblasts [80]. Similarly, SiO2 NPs recruited transforming growth factor β1 (TGF-β1) from the supernatant of lung tissue homogenate, enhancing the epithelialmesenchymal transition and the development of pulmonary fibrosis [81]. In blood, naked amorphous SiO2 NPs (70 nm) bound to coagulation factor XII, which activated the coagulation cascade and caused fatal toxicity in mice [82, 83].
3.4. Biodistribution
A prominent consequence of the altered NP-cell interaction due to PC is the loss of cell targeting function of the functional ligand. A seminal observation was made with Transferrin (Tf)-functionalized silica NPs, which lost the ability to specifically interact with cell Tf receptor (TfR) upon incubation in serum [84]. Similarly, polymeric NPs functionalized with HIV-1 trans-activating transcriptor (TAT) peptides (known to cross the blood-brain barrier, BBB) and antibodies to neural/glial antigen 2 (NG2) (known to target oligodendrocyte precursor cells, OPC) did not show enhanced brain delivery and OPC targeting, likely due to significant PC binding [85]. A study with sLeA -modified PLGA NPs suggests that IgA and IgM may be potential culprits [86]. As mentioned in Section 3.2, sLeA -modified PLGA NPs lose the ability to bind to E-selectin-expressing endothelial cells when incubated in human plasma [57]. The NPs incubated in Ig-depleted plasma maintained the binding ability, holding Igs accountable for the loss of ligand function [86]. Restoration of IgA and, to a lesser extent, IgM to the Ig-depleted plasma caused the inhibition of NP binding to the target cells, indicating that the two Igs are involved. Despite the abundance, IgG did not have a significant effect on NP-cell interaction [86].
On the other hand, PC with an affinity for specific cell receptors may provide an opportunity to increase NP biodistribution to specific organs. For example, lipid nanoparticles (LNPs) are envisioned to recruit ApoE in circulation, which binds to the LDLR of hepatocytes [87–89]. The ApoE-mediated liver tropism uniquely qualified LNPs for the delivery of nucleic acids addressing liver diseases, such as hereditary transthyretin amyloidosis [90, 91]. Serum proteins exploited for organ targeting are discussed in Section 6 with recent examples.
3.5. Immune responses
Some surface-bound proteins can activate immune systems, causing premature clearance of the NPs or promoting the release of proinflammatory cytokines [53, 92].
3.5.1. Complement activation
Several dextran-based magnetic resonance imaging agents (Sinerem, Combidex, Feridex) have been withdrawn from clinical use due to the complement-related side effects [93]. Dextran-coated ferrous NPs are known to activate the lectin complement pathway, starting with lectin binding, followed by the adsorption of mannose-binding lectin (MBL)-associated lectin serine protease 1 (MASP-1), and MASP-2, and complement protein C3, then converted to the activated C3b [93, 94]. A study with iron oxide NPs (IONPs) and liposomes describes that C3b binding involves immunoglobulins, which first bind to PC and are attacked by C3b. The initially bound C3b recruits convertases, which induce the cleavage of C3 near the surface, causing additional C3b binding [95]. The complement-bound NPs undergo increased binding/uptake by immune cells via corresponding receptors, such as complement receptor (CR)1/2 and CR3 [96].
Polymer coating of IONPs affects the complement protein deposition and corresponding production of proinflammatory cytokines [97]. Among IONPs with a bare surface, PEG, or PVP coating, incubated in human serum, IONP-PEG, but not the other two, showed increased C3a and C5a binding, indicating complement activation via the alternative pathway. IONP-PEG also augmented the IL-1β, TNF-α, and IL-6 levels and showed an increased presence in the spleen compared to the other IONPs. The differential effect of IONP-PEG was attributed to the pre-existing anti-PEG antibodies, which may have activated the immunoglobulin-mediated complement pathway [97]. Silica NPs show a similar dependence on polymer coating in complement activation and cellular uptake [98]. Poly(2-methyl-2-oxazoline) (PMOXA)-coated silica NPs showed more deposition of C3b and C3c α′ chain fragment 2 than the PEG-silica NPs or the uncoated NPs after human serum incubation. The C3 opsonized PMOXA-silica NPs promoted cellular uptake by human monocytes, polymorphonuclear granulocytes, and macrophages. However, the complement activation and macrophage uptake were not observed with mouse counterparts, indicating species dependence [98], consistent with the case shown in Section 3.3.
3.5.2. Effects on immune cells
Several studies report that PC can increase NP interaction with immune cells to increase proinflammatory cytokine production. For example, PC on black phosphorus nanosheets (BPNSs) and black phosphorus quantum dots (BPQDs), mostly (70–76%) consisting of immune-relevant proteins, facilitated macrophage uptake of these NPs and the production of pro-inflammatory cytokines from the NP-treated macrophages [99]. Likewise, PC formed on PEGylated carbon nanotubes (CNT) induced the production of ROS and pro-inflammatory cytokines in macrophages and activated the immune cells in the spleen [100]. Such immunostimulatory effects depended on the conformation of the bound proteins. IgG and α1 acid-glycoprotein underwent unfolding on the surface CNT, causing the production of inflammatory mediators, whereas fibrinogen and vitronectin - with no structural changes - had negligible effects on ROS production [100].
The effects on immune cells also depend on the surface chemistry of NPs. Gold NRs were modified with cetyltrimethyl ammonium bromide (CTAB), poly(diallyldimethyl ammonium chloride) (PDDAC), polyethyleneimine (PEI), polystyrene sulfonate (PSS), and PEG. Of these, PEI- or PDDAC-coated gold NRs increased the IL-1β production in macrophages upon PC binding, enriched with acute-phase proteins and complements [101].
PC may also reduce the proinflammatory effects. For example, PC-covered nanosilica (SiO2) showed lower cytotoxicity and reduced production of IL-8 in human epithelial cells and TNFα in macrophages, compared to bare SiO2 particles, despite the increase of cellular uptake [102]. Another study reports the dependence on the environment in which PC was formed [103]. Poly(methacrylic acid) (PMA) hollow capsules (CAPs) and core-shell particles (CSPs) were incubated with a cell-conditioned medium or medium supplemented with specific proteins (FBS, human serum, or human plasma) to form PC. Upon incubation with the PC-NP combinations, the production of pro-inflammatory cytokines in macrophages or monocytes changed in either direction compared to PC-free NPs, depending on the source of PC, i.e., the type of proteins bound to the NPs [103].
4. Strategies to avoid protein corona formation
Given the aberrant effects of PC on nanoparticle performance, drug delivery scientists have made diverse efforts to manage protein corona formation. One of the approaches is to prevent the formation of PC on NPs. A common method is to modify the NP surface by grafting hydrophilic polymers [104].
Polyethylene glycol (PEG) is most widely used for modifying the NP surface due to its hydrophilicity and low toxicity [105]. PEG chains grafted on the NP surface provide a hydrated layer to prevent protein adsorption [106], a phenomenon so-called the “stealth” effect. The surface density, molecular weight, and chain architecture of the polymer affect the PEG conformation on the NP surface, hence the effectiveness of the stealth effect [107]. It was shown that PEG density determines the extent of PC formation on gold NPs and the composition of PC [108]. With the fixed PEG grafting density, relatively small NPs form greater PC formation due to the high curvature that leaves more room for each PEG to spread out and lowers the thermodynamic barrier to protein adsorption [108]. The examples and the effects of PEGylated NPs have been thoroughly reviewed in recent literature [109].
Poly(2-oxazoline)s (POx) is a potential alternative to PEG. POx is synthesized by cationic ring-opening polymerization of 2-oxazolines, amenable to variations in architecture and hydrophilic-lipophilic balance [110]. Due to the versatile synthesis, POx has been applied to various formulations, including drug-polymer conjugates, polymeric micelles, complexes, and hydrogels [111–113]. In a head-to-head comparison with PEG (2000 Da), poly(2-ethyl-2-oxazoline) was comparable to PEG in reducing protein adsorption to poly(organosiloxane) NPs in serum and non-specific uptake of the NPs by macrophage-like cells and endothelial cells [114].
Another alternative stealth polymer is zwitterionic polymers, represented by polybetaines such as poly(sulfobetaine) or poly(carboxybetaine) [115]. As zwitterionic molecules, the polymers bind to water molecules via electrostatic interactions, more strongly than other hydrophilic polymers relying on hydrogen bonding [115]. When grafted on hydrophobic substrates, zwitterionic polymers form a well-hydrated layer, which resists protein adsorption [116].
Polyglycerols are nonionic aliphatic polyether polyols with high protein resistance and have been used as stealth coating [117, 118]. In a recent study, polyglycerol was compared with PEG as stealth coating to prevent serum protein binding to supraparamagnetic IONPs [119]. At the same weight content, polyglycerol reduced protein adsorption to the NPs in serum and NP uptake by macrophages more efficiently than PEG [119].
Polymers of natural origin, such as polysaccharides, have also been explored as potential stealth polymers [120]. For example, hyaluronic acid, a glycosaminoglycan consisting of (1➔4)-β-D-glucuronic acid and (1➔3)-β-N-acetyl-D-glucosamine, was used for coating chitosan NPs to reduce PC formation in comparison with alginic acid [121]. Hyaluronic acid coating reduced the extent of protein adsorption to chitosan NPs upon incubation in serum solution. Despite the overall low protein binding, the hyaluronic acid coating allowed the binding of anti-inflammatory proteins, suggesting the potential to reduce the immunogenicity of NPs [121]. Similarly, polyacrylamide-grafted guar gum (PAm-g-GG) was used to mitigate PC formation on ZnO NPs [122]. The PAm-g-GG-coated ZnO NPs reduced the binding of complement protein C3 and IgG compared to bare ZnO NPs. Consequently, PAm-g-GG-coated ZnO NPs experienced lower immune cell recognition and caused less liver inflammation than bare ZnO NPs, resulting in an extended circulation half-life and systemic exposure [122]. Chitosan, especially low molecular weight chitosan or water-soluble derivatives, is another natural polymer used for stealth function [120]. Coated on PLGA NPs, low molecular weight chitosan reduced protein binding to the NPs and macrophage uptake [123]. In a recent study, chitosan coating, together with albumin, prevented a negative effect of PC on the interaction of sLeA-modified PLGA NPs with the target, selectin-expressing endothelium [124]. Chitosan or albumin alone was not as effective as their combination (or not effective at all) in counteracting the PC effect. It is postulated that the chitosan coating may have enhanced the binding of albumin, which helps interact with endothelium via albumin-specific receptors, such as glycoprotein 60 (gp60). On the other hand, the analysis of PCs on chitosan/albumin-dual coated NPs showed the enrichment of histidine-rich glycoprotein, which has a dysopsonin function. This study suggests that the stealth function of chitosan coating may be ascribed to promoting the albumin binding followed by dysopsonin binding, which is in line with Section 6 – the piggyback strategy.
Another approach that has gained significant interest in the last decade is to coat NPs with cell membranes (“cloaking”) [125]. The rationale of the cloaking approach is that the cell membrane-coated NPs may simulate the cell’s interactions with the physiological environment, including blood proteins, other cells, and tissues. Therefore, this method was mainly adopted to promote the delivery of NPs to target tissues [126–129] but has also proven to be effective in reducing PC formation [130]. A study with inorganic NPs for cancer imaging demonstrated that coating with red blood cell membrane protected the NPs from protein adsorption in 100% human plasma, showing no change in size or surface property, unlike the uncoated counterpart [130].
The examples shown here are only a limited subset of diverse approaches employed to prevent PC formation. Interested readers are referred to more comprehensive reviews dedicated to stealth modification of NP surfaces [131–133].
5. Pre-functionalization: Strategies to override protein corona
While the stealth polymers help reduce protein adsorption to the NP surface, they do not completely prevent it. Major efforts have been made to modify the NP surface with antibodies, ligands, and other functional proteins that may override protein corona and facilitate NP distribution to specific organs or tissues.
5.1. Functionalization with antibodies or ligands
Recent efforts for functionalizing NP surfaces with cell-interactive ligands have been thoroughly reviewed elsewhere [134]. These approaches help retain the NPs in target tissues and have achieved a varying degree of success in improving target-specific drug delivery with preclinical models. However, PC may shield the ligands and interfere with the intended cell-ligand interactions, as mentioned earlier. For example, silica NPs modified with a model ligand bicyclononyne, which undergoes click reaction with an azide counterpart, did not react with the corresponding substrate after incubation with serum solution [135]. Similarly, Tf-functionalized silica NPs, covered with PC, lost the specificity for TfR [84]. The protein binding was reduced by PEG linkers between the NP surface and Tf but not completely prevented [84]. The extent of ligand-receptor binding interference varies with the type of ligands and the origin of serum [136, 137]. Nevertheless, due to the prevalence, PC is often considered one of the reasons for the lack of clinical success in the so-called “active targeting.”
Accordingly, efforts have been made to overcome the interference of PC with the ligand/antibody recognition of the target receptors. One approach is to “backfill” the NP surface unoccupied by targeting ligands with extra PEG. For example, gold NPs were modified with Herceptin, an ErbB2 receptor-targeting antibody, via a 5 kDa PEG linker, and the remaining surface was saturated with methoxy-PEG (mPEG) [138]. mPEG shorter than 5 kDa (1 or 2 kDa), but not 5 kDa or 10 kDa mPEGs, allowed the antibody to retain the receptor binding ability by preventing the formation of PC by reducing the nonspecific protein adsorption and PC formation [138]. Another study showed the reduction of PC formation by blocking residual maleimide groups on NP surface with 2-mercaptoethanol [139]. The authors argue that the small molecular weight and neutral charge of 2-mercaptoethanol were important because other thiolated compounds with charges or larger molecular weights were less efficient in reducing PC formation. Here, the charged groups would still interact with serum proteins via electrostatic interactions, and the steric hindrance of PEG limit the number of maleimides that can be blocked [139].
As envisioned from these examples, the likelihood of PC formation depends largely on the NP surface underlying the functional layer. Unlike polystyrene NPs, hydroxyethyl starch NPs were already resistant to protein binding [140]. When the hydroxyethyl starch NPs were modified with mannose via a PEG linker, they withstood the preincubation with human plasma and retained the ability to interact with the target C-type lectin and lectin-expressing immature dendritic cells [140].
These studies illustrate that backfilling the surface with a short stealth layer or using inherently nonfouling NPs as a platform may reduce the effect of PC on the ligand/antibody-mediated recognition of target cells. Nevertheless, the effectiveness of these approaches needs to be rigorously measured since the common practice of isolating PC-bound NPs may underestimate the function of loosely bound proteins. With a similar rationale, cysteine was used as a zwitterionic blocking agent [141]. Here, silica NPs were functionalized with cysteine in tandem with a model ligand biotin. The presence of zwitterionic amino acids reduced protein adsorption to NPs, allowing the NPs preincubated in serum to retain the ability to interact with the corresponding substrate (streptavidin-modified surface) or biotin receptor-positive cells. However, the cysteine modification was not enough to overcome the effect of the soft corona, which bound to the NPs relatively weakly (and hence were removed during NP washing). Accordingly, the cysteine/biotin-modified NPs, upon simultaneous incubation with serum, did not bind to biotin receptor-positive cells as effectively as those preincubated in serum and washed [141].
5.2. Pre-coating with functional proteins
5.2.1. Albumin
Albumin is the most abundant plasma protein with a molecular weight of 66.5 kDa, responsible for the transport of nutrients and hydrophobic drugs in circulation [142]. Albumin has attracted significant interest as a carrier or a component of an NP carrier in recent years [143–145]. In NP formulation, albumin is employed to improve colloidal stability and drug loading, reduce nonspecific cellular uptake, and prolong the circulation half-life of the NPs [146–151]. In addition, albumin may promote NP delivery to target tissues via proteins naturally interacting with albumin, such as glycoproteins (gp60) or secreted protein acidic and rich in cysteine (SPARC) [145, 152, 153], and/or by taking advantage of pathological conditions that increase albumin consumption [154]. As such, various NPs have been pre-coated with albumin [155], most commonly by incubating the NPs with albumin to let it physically adsorb to the NP surface (physisorption) [151, 156].
Our group has reported that pre-coating paclitaxel nanocrystals with albumin increases the colloidal stability of the nanocrystals and their cellular uptake by SPARC+ melanoma cells compared to a non-albumin coated counterpart [157]. The albumin-coated nanocrystals took advantage of NP configuration that allowed for the EPR effect, showing greater bioavailability, tumor distribution, and antitumor efficacy than Abraxane at the same dose of paclitaxel in a murine model of melanoma [157, 158]. Similarly, albumin has been used to coat silver NPs for anticancer applications [159]. The albumin-coated silver NPs showed more selective toxicity to cancer cells and generated greater ROS than bare silver NPs. While not explicitly explained, the difference between bare and albumin-coated silver NPs may be attributable to the changes in NP interaction with the cell membrane due to the albumin coating.
The pre-coated albumin layer is not static and may attract or be replaced by other serum proteins. In a serum-free medium, the pre-coated albumin reduced cellular uptake of gelatin-oleic NPs irrespective of cell types, suggesting the interference of albumin with cell-NP interaction [160]. However, in a serum-supplemented medium, the albumin-coated gelatin-oleic NPs showed a consistent reduction in cellular uptake by A549 cells compared to bare gelatin-oleic NPs, whereas the opposite was observed in HEK293 cells. This result suggests that the pre-coated albumin attracted or was replaced by other serum proteins, which may exert differential effects depending on the cell types [160].
A caveat of pre-coating albumin on NPs is the potential to perturb the protein structure [155]. We have modified the surface of poly(lactic-co-glycolic acid) (PLGA) nanoparticles with albumin by two methods (interfacial embedding and polydopamine-mediated physisorption) and compared albumin functionality (Fig. 1a) [161]. The interfacial embedding method caused denaturation of surface-bound albumin, offering no particular benefit to the interaction with cancer cells but rather promoting macrophage uptake via interactions with scavenger receptor A. In contrast, albumin adsorbed to the polymerized dopamine, serving as an adhesive layer on PLGA NPs, retained the native structure, evident from the resistance to thermolysin digestion and retention of the esterase-like activity (Fig. 1b). With the native conformation, the albumin pre-coating helped PLGA NPs avoid macrophage uptake, travel across the endothelial layer, and interact with SPARC-expressing tumors (Fig. 1c) to deliver more drugs to tumors than the NPs with denatured albumin (Fig. 1d) [161]. The differential protein conformation, although often overlooked, may explain some of the conflicting reports on the effects of surface-bound albumin on NP performance (e.g., enhancement of particle uptake by phagocytes [59] vs. prevention of liver accumulation [162]).
Figure 1.
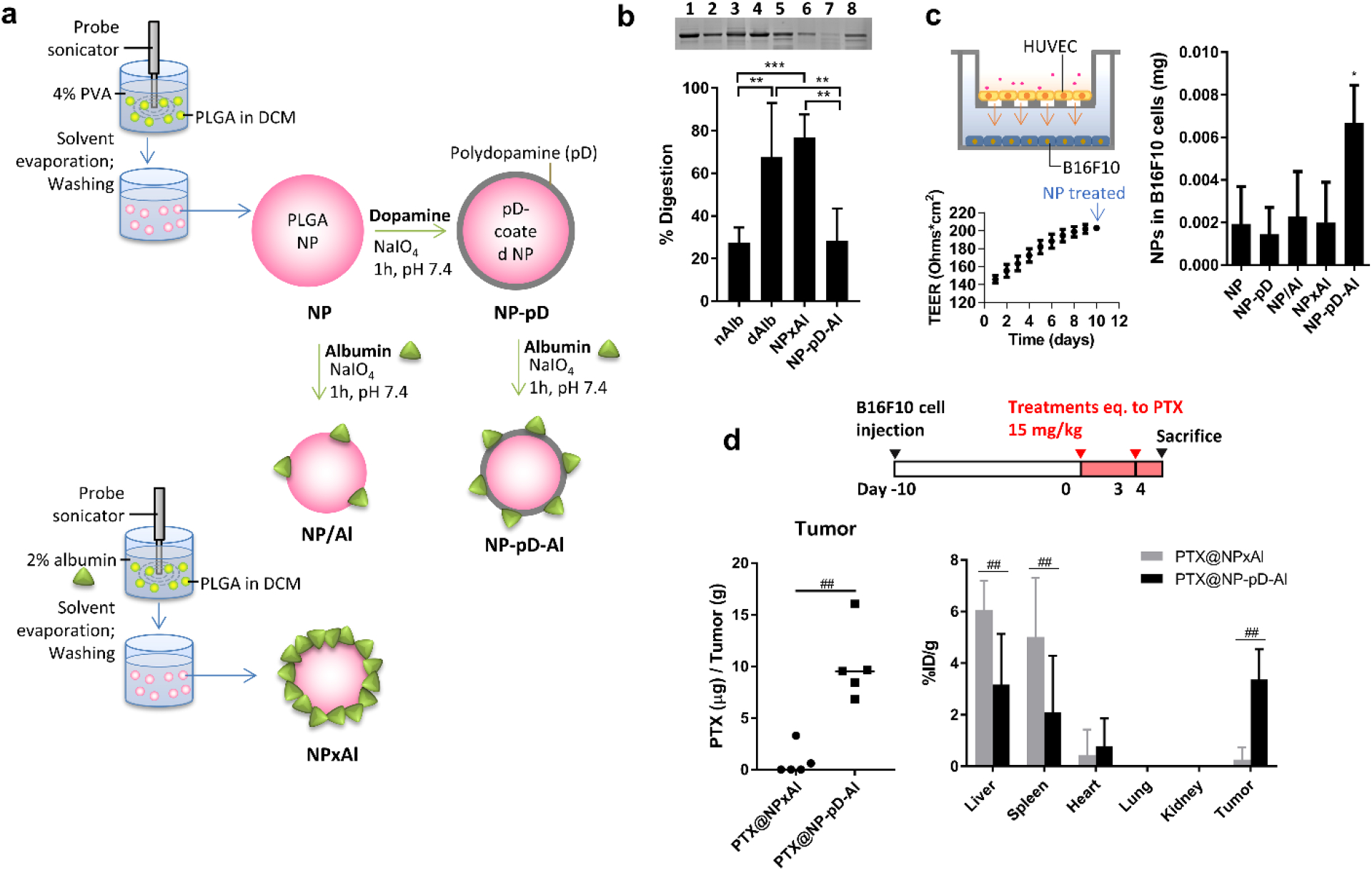
(a) Different methods to coat PLGA NPs with albumin. NP×Al: interfacial embedding; NP-pD-Al: polydopamine-mediated physisorption. (b) Top: Representative SDS-PAGE gel image of albumin after pulse proteolysis. Native albumin (nAlb), denatured albumin (dAlb), NP×Al, and NP-pD-Al were treated with thermolysin for 3 min. Lane 1: nAlb; Lane 2: dAlb; Lane 3: NP×Al; Lane 4: NP-pD-Al; Lane 5: nAlb + thermolysin; Lane 6: dAlb + thermolysin; Lane 7: NP×Al + thermolysin; and Lane 8: NP-pD-Al + thermolysin. Bottom: % digestion albumin was defined as (1-albumin band intensity after proteolysis/albumin band intensity prior to proteolysis) × 100. (c) Schematic of a Transwell co-culture system with HUVEC in the insert and B16F10 cells in the bottom of the basolateral side (left top); Transendothelial electrical resistance (TEER) indicating the confluence of HUVEC layer at the time of NP application (left bottom); NP associated with B16F10 cells, measured at 24 h after 6 h incubation with a Transwell containing NPs and the confluent HUVEC layer. (d) Dosing schedule of PTX-loaded NPs (top); PTX content in B16F10 tumors treated with PTX-loaded NP×Al (PTX@NP×Al) or PTX-loaded NP-pD-Al (PTX@NP-pD-Al) (bottom left); % injected PTX dose per gram of each tissues (%ID/g) of PTX@NP×Al or PTX@NP-pD-Al in B16F10 tumor bearing mice 24 h after i.v. injection. %ID/g is defined as percentage of injected dose per gram of tissue weight. Reprinted from [161] with permission.
5.2.2. Apolipoproteins
Apolipoproteins are responsible for regulating plasma lipid levels [163]. As part of lipoproteins, apolipoproteins mediate the intracellular transport of the lipoproteins via LDLR or heparan sulfate proteoglycans [163]. ApoE is often identified to be one of the main components of PC that forms on NPs in human serum and affects their cellular uptake [164, 165].
Apolipoproteins have been pre-coated on NPs to enhance their transport across the brain endothelium. For example, ApoE [166] or ApoA-I [167] were covalently attached to albumin NPs, increasing endocytosis and transcytosis of the NPs by brain endothelial cells. Based on early observations of preferential ApoE binding to surfactant-stabilized NPs [168] and their brain tropism [169], ApoE4 was also pre-adsorbed on polysorbate 80-stabilized lipid NPs by simple mixing before the administration [170]. The ApoE4-bound NPs crossed the blood-brain barrier via an LDLR and reached the brain parenchyma. This approach is amenable to extemporaneous preparation; however, excess ApoE4 rather interfered with brain delivery due to the competition for the receptor [170]. Thus, a tight titration of ApoE4 would be critical to the successful delivery to the brain. An ApoE-coating on gold NPs has also been used for carrying hydrophobic sensitizer chlorin e6 (Ce6), achieving greater loading efficiency than albumin-coated gold NPs [171] and intracellular delivery of Ce6 [172].
On the other hand, ApoJ, also known as clusterin, is shown to be a PC component with a stealth effect [173, 174]. Polystyrene NPs were modified with PEG or poly(ethyl ethylene phosphate) (PEEP) to reduce protein adsorption [173]. The NPs showed a difference in macrophage uptake only in the presence of serum protein, indicating that the surface-bound protein was necessary for the stealth effect. A proteomic analysis found that the PC, albeit reduced, was enriched with clusterin and it was responsible for reducing nonspecific cellular uptake of the NPs [173]. Clusterin corona is similarly credited with silver- and silica NPs [174]. Like the previous study, the silver and silica NPs showed reduced monocyte uptake after incubation with human serum or plasma. Clusterin was identified as a protein accountable for the stealth effect [174]. These results prompted the consideration of clusterin for the stealth coating of NPs [173]. However, clusterin is also associated with microglia activation leading to inflammation and neurotoxicity [175]; therefore, its utility as a stealth coating remains to be determined.
5.2.3. Cluster of Differentiation 47 (CD47)
CD47 is a highly glycosylated ~50 kDa plasma membrane protein, consisting of a single IgV-like domain at the N-terminus, a hydrophobic membrane-spanning domain, and a cytoplasmic C-terminus, broadly expressed in all tissues [176]. As a marker of “self,” CD47 interacts with signal-regulatory protein alpha (SIRPα, also known as CD172a) expressed at high densities on macrophages to prevent their recognition and engulfment of host cells [177–179]. The deficiency of CD47 in red blood cells leads to lethal hemolytic anemia in nonobese diabetic mice [180]; conversely, CD47 is exploited by most human tumor cells to evade macrophage surveillance [181].
Due to its role in preventing phagocytosis, CD47 or its derivatives have been explored as a stealth (self) ligand [182, 183]. For example, polystyrene NPs were surface-modified with the extracellular domain of CD47 or a small peptide (“self-peptide”) with a sequence most responsible for the interaction with SIRPα [183]. The CD47 or self-peptide-coated NPs persisted in circulation and accumulated in the tumor better than the control peptide-coated NPs [183].
6. Join them if you can’t beat them: Strategies to piggyback on PC in situ
As the tenacity of PC formation is increasingly recognized, recent efforts have focused on designing NPs such that they attract PC specifically recognized by the target cells or tissues during circulation.
6.1. Attracting albumin
Recently, our group has reported soft polydopamine nanocapsules called Nanosac for siRNA delivery [55]. Nanosac is produced by sequential attachment of siRNA and polydopamine on sacrificial mesoporous silica NPs (MSN), followed by the removal of the MSN core (Fig. 2a). Unlike the bare MSN core, Nanosac enters cells via caveolae-mediated endocytosis, bypassing lysosomal sequestration (Fig. 2b), which occurs with traditional NPs and is detrimental to siRNA stability. Gel electrophoresis showed that the polydopamine surface reduced protein adsorption. Proteomics analysis identified albumin to be the dominant protein of the few proteins binding to the Nanosac (Fig. 2c), suggesting selective recruitment of albumin by polydopamine surface. Systemically administered, Nanosac delivered siRNA targeting PD-L1 to CT26 tumor, resulting in significant suppression of CT26 tumor growth compared to the monoclonal PDL1 antibody-treated group (Fig. 2d) [55]. However, it remains to be determined whether the albumin co-option occurred in vivo and contributed to Nanosac distribution in tumors.
Figure 2.
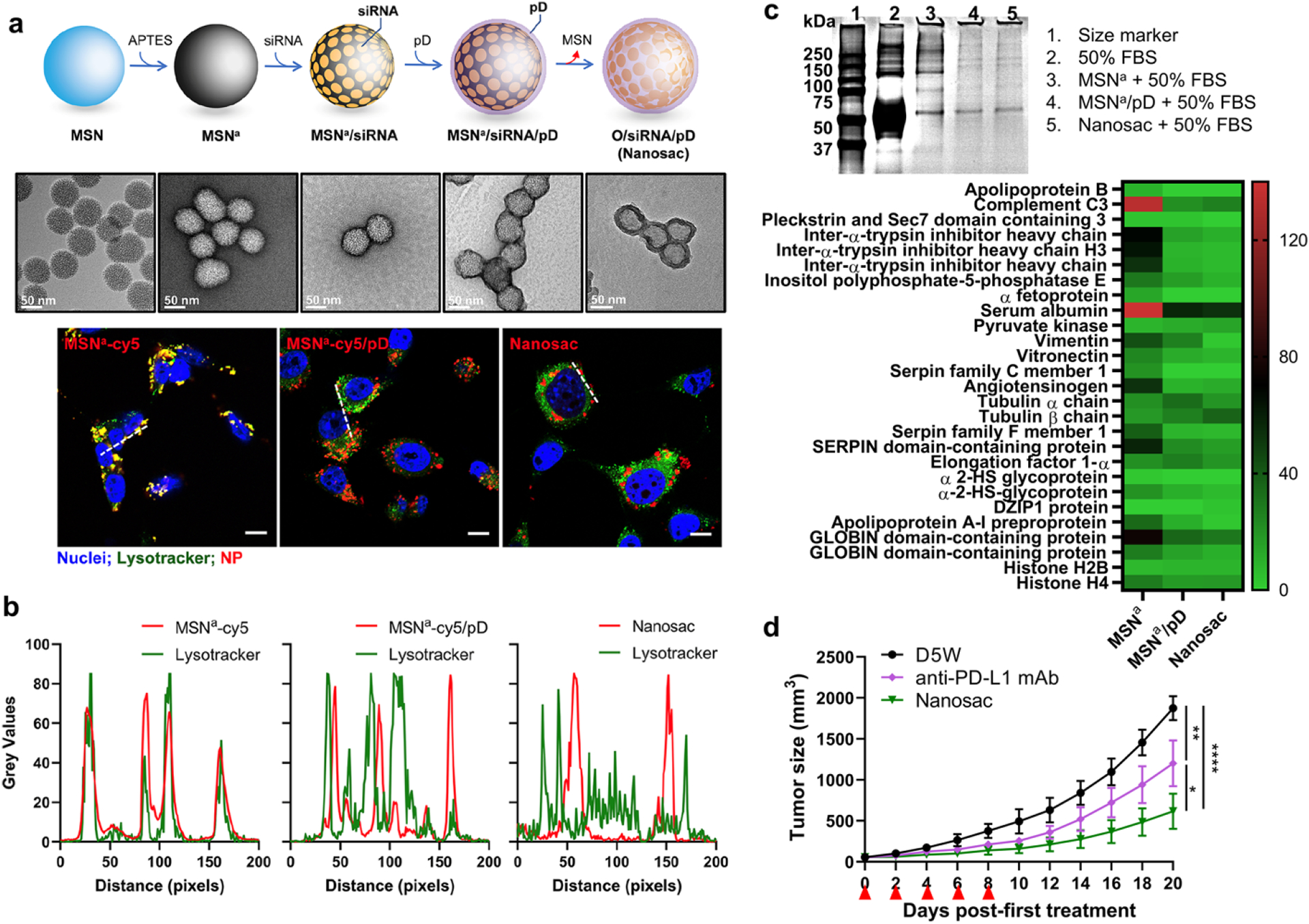
(a) Schematic illustration of Nanosac (MSNa/siRNA/pD) preparation and transmission electron microscopy images of Nanosac and Nanosac precursors. Nanosac is produced by sequential coating of mesoporous silica nanoparticles (MSNs) with siRNA and polydopamine, followed by removal of the sacrificial MSN core. (b) Confocal microscope images of cy5-labeled MSNa, MSNa/pD, and Nanosac relative to lysosomes in CT26 cells and fluorescence intensity profiles along the white lines in confocal images. Green: Lysotracker (lysosome); red: cy5- labeled NPs; and blue: Hoechst 33342 (nuclei). Scale bars: 10 μm. (c) SDS-PAGE of protein corona composition formed on MSNa, MSNa/pD, and Nanosac. The protein corona bound on each NPs were further analyzed by LC-MS/MS for most abundant proteins. 4 mg/mL of each NPs were incubated in 50% FBS for 2 h and rinsed with PBS twice. (d) Average tumor size after treatment of anti-PD-L1 antibody and siPD-L1-Nanosac to Balb/c mice bearing CT26 tumors. (anti-PD-L1 antibody: 200 μg/mouse/time, intraperitoneal injection; siPD-L1:1.5 mg/kg/time, IV injection; q2d × 5). Reprinted from [55] with permission.
In another example, PLGA NPs were deliberately decorated with maleimide for albumin binding in circulation [184]. The distance between NP surface and maleimide was varied by the length of the PEG linker (none, 500 Da, 2000 Da) to control the avidity of albumin binding. PEG 2000 Da was needed to avoid excessive protein binding, and the maleimide terminus helped attract albumin conjugating with Cys-34 residue. The albumin binding, maleimide-PEGylated PLGA NPs underwent reduced accelerated blood clearance phenomenon upon the second administration and improved tumor accumulation via interactions with SPARC and CD31 as compared to PEGylated PLGA NPs (with no maleimide) [184].
For the same purpose, NPs were prepared by molecular imprinting using albumin as a template [162]. NPs were produced by polymerizing albumin binding monomer (pyrrolidyl acrylate) and acrylamide monomers in the presence of albumin, followed by the removal of albumin by size-exclusion chromatography. The molecularly imprinted NPs captured circulating albumin in situ, as demonstrated by in vivo fluorescence resonance energy transfer microscopy, and accumulated in tumors taking advantage of the dysopsonin function of albumin [162].
6.2. Attracting apolipoproteins
As mentioned earlier, apolipoproteins bind to polysorbate-stabilized NPs and promote their delivery to the brain via the LDLR [168]. Accordingly, subsequent studies employed polysorbate 80 as a surface modifier of an NP carrier to improve drug delivery to the brain [185]. The amount of apolipoproteins binding to NPs depends on the hydrophilic-lipophilic balance of polysorbate and the corresponding NP surface: more lipophilic polysorbate attracted more ApoE to the lipid NP surface. However, this trend may not be generalized to other NPs [168].
Alternatively, there has been an attempt to control the apolipoprotein binding to LNPs by the length and amount of the surface-exposed PEG [186]. LNPs were made with PEGylated acyl chains in two different lengths (C18 vs. C14) in two amounts (6 mol% vs. 3 mol%). Upon incubation in mouse serum, LNPs with 3% PEG acquired more ApoA4 than those with 6% PEG. LNPs with C18 PEG showed greater accumulation in LDLR-expressing HepG2 than those with C14 PEG. However, there appears to be no explicit relationship between PC and biodistribution [186].
Another example shows biodegradable nonionic poly(phosphoester) (PPE)-surfactants adsorbed on polystyrene NPs helping attract apolipoproteins [187]. The PPE-surfactants consist of hydrophilic and hydrophobic blocks, where the lengths and side chains are varied to control protein adsorption and aggregation. The PPE-surfactant-coated polystyrene NPs were enriched with clusterin and ApoA-I upon incubation with human plasma. The NPs coated with the PPE surfactant and plasma showed reduced macrophage uptake, supporting the potential of the polymer surfactant to recruit “stealth” apolipoprotein in circulation [187].
A recent study uses dihydroartemisinin (DHA) to decorate PLGA NPs and attract ApoE to the NP surface [188]. DHA is a metabolite of artemisinin, an antimalarial drug known for its affinity for albumin and transferrin. When conjugated to PLGA NPs, DHA rather recruited ApoE (than albumin or transferrin) in serum, which helped the NP uptake by LDLR+ 4T1 cells, and extended the NP circulation half-life and delivery to 4T1 tumors compared to plain PLGA NPs [188].
6.3. Attracting vitronectin
Vitronectin is a glycoprotein with a molecular weight of 75 kDa, abundant in blood and extracellular matrix [189]. The Arg-Gly-Asp (RGD) sequence of vitronectin binds to cell surface integrins such as ανβ3, ανβ5, ανβ1, αIIββ3, ανβ6, and ανβ8, facilitating cellular adhesion, spreading, and migration [189]. Of these, ανβ3 has gained particular interest in cancer therapy due to its implication in angiogenesis and metastasis [190] and has been targeted by various RGD-based peptides [191–195].
Liposome/DNA complexes (lipoplexes) comprising 1,2-dioleoyl-3- trimethylammonium propane (DOTAP) were shown to acquire a PC enriched with vitronectin and albumin upon preincubation with human plasma [196]. Similarly, LNPs containing 3β-[N-(N’,N’-dimethylaminoethane)-carbamoyl]cholesterol (DC-cholesterol) showed vitronectin enrichment in PC upon incubation with mouse serum [186]. In both cases, the binding of vitronectin to the lipid particles, which is attributed to charge interaction (negatively charged vitronectin vs. cationic lipids) [197], increased the particle uptake by ανβ3-positive cells (but not by the ανβ3-negative cells) [196]. Accordingly, cationic lipids have been incorporated in LNPs to facilitate their delivery to the αvβ3-expressing tumor via vitronectin corona [198]. Nevertheless, the presence of serum was detrimental to the cellular uptake of LNPs irrespective of DOTAP inclusion [198]. In vivo, DOTAP-containing LNPs showed higher accumulation in the αvβ3-expressing A375.S2 tumor than in the ανβ3-negative HepG2 tumor. However, DOTAP LNPs did not deliver the RNA payload to the tumor cells, suggesting that the accumulation might have occurred in the αvβ3-expressing endothelial cells rather than in the tumor cells [198].
6.4. Attracting other proteins
Transferrin (Tf) is a 79 kDa glycoprotein supplying irons to cells. Due to the high demand for irons, TfR is overexpressed in malignant cells, making an important target for cancer therapy [199, 200]. To enable in-situ binding of Tf to NPs, a Tf-binding peptide was designed by iterative multiscale-modeling coupled with quantitative structure-activity relationship analysis and evolutionary algorithms [201]. Gold NPs conjugated with the Tf-binding peptide attracted serum Tf, which in turn increased the NP uptake by TfR+ Mia PaCa-2 cells in a Tf-specific manner [201] (Fig. 3).
Figure 3.
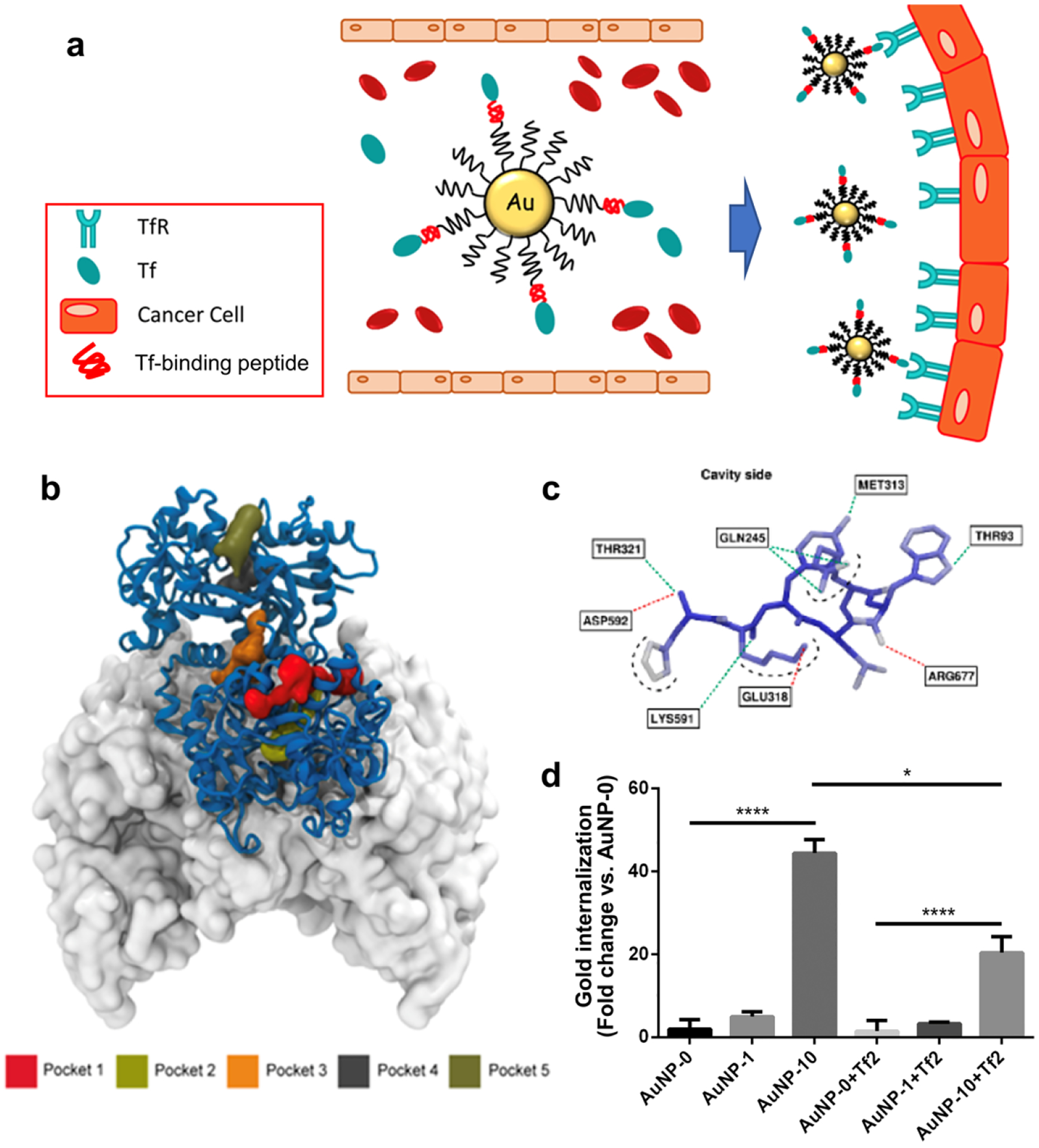
(a) Schematics Illustration of gold NPs targeting TfR overexpressed on cancer cells. (b) Five potential binding sites, Pocket 1 through 5, identified on the human Tf predicted by Fpocket. Tf is represented with the blue-ribbon diagram, and part of the ectodomain of the transferrin receptor dimer23 is rendered by its accessible surface. Due to the adequate volume for peptide and the distance from transferrin or iron binding site, Pocket 3 (orange) is chosen to dock the peptide. (c) 3D docked pose of the synthesized Tf-binding peptide (Tf2) created by coarse-grained molecular dynamics simulation. Atoms are colored according to their root mean squared displacement. Blue: rigid regions; red: flexible regions; green dashed lines: hydrogen bonds; red dashed lines: salt bridges; black dashed lines: solvent exposed atoms. (d) Cell uptake of gold NPs conjugated with Tf2. Various gold NPs were prepared using different percentage of PepN-Tf2 (0, 1, and 10% w/w). NPs were incubated in human plasma for 1 h at 37 °C and then with Mia PaCa-2 cells for 1 h at 37 °C in DMEM with (right, suffix +Tf2) or without (left) a Tf2. The results were normalized to the amount of internalized gold in AuNP-0. Reprinted from [201] with permission.
Another example of PC-binding NPs is a retinol-conjugated polyethylenimine (RcP) NP, developed for the delivery of antisense oligonucleotide (ASO) [202]. The RcP NP recruited retinol-binding protein 4 (RBP) as well as albumin, where the protein binding prevented lethal aggregation of the NPs and led to the cellular uptake profile skewed to hepatic stellate cells (HSC). Controlled in vitro studies identified that the two proteins had distinct functions: albumin preventing macrophage uptake and RBP promoting HSC uptake of RcP NPs. Consistently, in a mouse model of liver fibrosis, the RcP NPs entered HSC in the liver, unlike free ASO that was stuck in endothelial cells and macrophages, and delivered ASO downregulating collagen I to HSC to reduce hepatic fibrosis. Of note, the retinol ligand of RcP played a significant role in controlling the functions of two proteins: as a hydrophobic compound, it helped keep the conformation of albumin, unlike cationic PEI; retinol was also critical to maintaining the functionality of RBP (uptake by HSC) [202].
7. Application of protein corona to organ/disease-specific nanoparticle delivery
7.1. Brain
Based on the early observation of brain tropism of apolipoprotein-coated NPs, the NP surface was deliberately engineered to co-opt apolipoproteins to enhance NP delivery to the brain [203]. Here, liposomes were decorated with a short peptide (Aβ25–35 with amide form in the C-terminal, SP) derived from β-amyloid peptides, which captured plasma ApoA1, ApoE, and ApoJ. On the surface of liposomes (SP-sLip), SP interacted with the lipid-binding domain of the apolipoproteins, exposing their receptor-binding domains to respective receptors (SR-B1, LRP1, and LRP2) and inducing brain transport of liposomes via LRP1/LRP2/SR-B1 mediated transcytosis. As a carrier of doxorubicin (DOX), SP-sLip showed significant improvement in drug delivery to the brain and extended the survival of mice bearing intracranial tumors compared to plain liposomes [203]. A similar approach was applied to polymeric micelles, with the amide-terminated Aβ25–35 as a ligand to bind to the lipid-binding domain of ApoE [204]. The ApoE binding increased the uptake of micelles by C6 glioma cells and human umbilical endothelial cells. The Aβ25–35-modified micelles increased paclitaxel (PTX) delivery to the orthotopic glioma in the mouse brain, extending the survival of glioma-bearing mice, compared to the micelles without the peptide [204].
TfR is another receptor highly expressed in glioma cells and may be targeted by Tf. Since Tf is abundant in blood, NPs were designed to bind Tf in circulation for the improvement of brain delivery (Fig. 4a) [205]. Covalent organic framework (COF) NPs were decorated with T10 peptide, which interacts with Tf without affecting its binding to TfR. The T10 ligand of COF helped recruit Tf-rich PC, facilitating the NP uptake by TfR+ U87 cells (Fig. 4b). The T10-coated COF NPs delivered more DOX to the orthotopic glioma (Fig. 4c) and extended the survival of mice than unmodified COF NPs [205].
Figure 4.
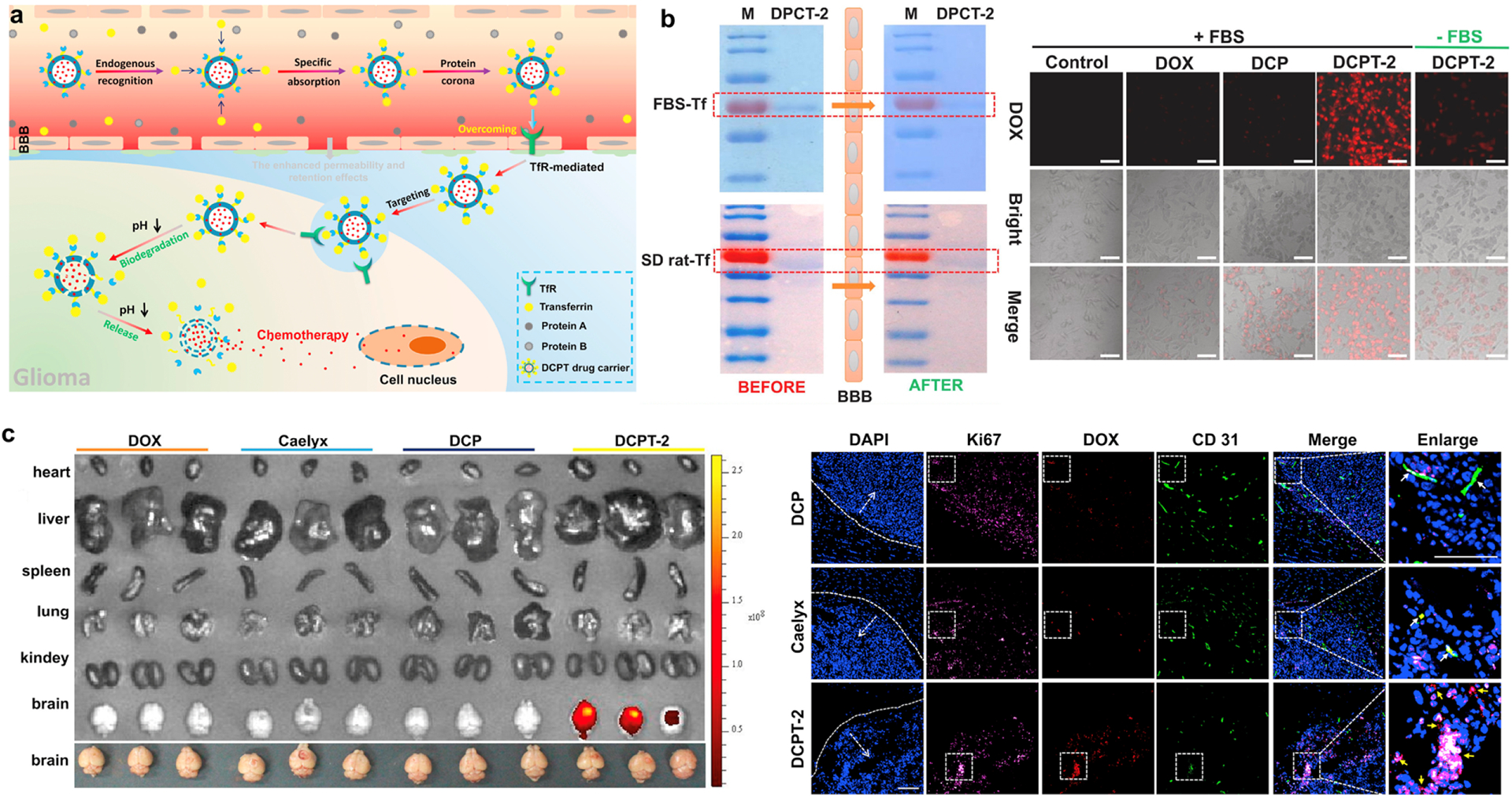
Tf targeting in glioma by DOX-loaded, T10-coated COF NPs (DCPT) NPs. (a) Schematic illustration of endogenous Tf corona-mediated DCPT delivery across the BBB. (b) SDS-PAGE analysis of PC on DCPT-2 before and after passage through the in vitro BBB model (left). FBS-Tf: Formation of Tf corona on the surface of DCPT-2 mediated by Tf from the FBS. SD rat-Tf: Formation of Tf corona on the surface of DCPT-2 mediated by Tf from the SD rat serum. Cellular uptake of DOX and COF formulations incubated with U87 cells under different conditions (right). (c) Ex vivo imaging of DOX in main organs of glioma-bearing mice after intravenous injection of DOX, Caelyx, DCP (DOX-loaded COF, no T10) and DCPT-2 at 12 h (left). Immunofluorescence images of brain sections from orthotopic glioma mice after 12 h post-injection of DCP, Caelyx and DCPT-2, respectively. Blue: nuclei; purple: U87 cells; red: DOX; green: anti-CD31 labeled blood vessels. White arrows: co-localization of DOX and blood vessels; Yellow arrows: co-localization of DOX and glioma cells. Bar: 200 μm. Reprinted from [205] with permission.
A recent study reported that exosome mimetics (EMs) decorated with angiopep-2 (Ang) peptide, a ligand specifically interacting with lipoprotein receptor-related protein 1 (LRP1) to facilitate BBB transport, can retain the ligand function despite PC and deliver docetaxel (DTX) to the glioblastoma (GBM) in mice [206]. Here, Ang peptide was integrated into EM as DSPE-PEG-Ang. The Ang-decorated EM (Ang-EM) showed relatively low protein binding compared to Ang-liposomes and enhanced DTX delivery to orthotopic GBM compared to Ang-liposomes. However, due to the lack of comparison with the unmodified EM, it is unclear whether the improved brain delivery is due to the ligand effect of Ang or other attributes of EMs, such as CD47 [206].
7.2. Tumor
In controlling PC for enhancing NP delivery to tumors, the focus has been to increase the circulation time for the EPR-based tumor accumulation and/or to improve the interaction with tumor cells. The former may be achieved by decreasing opsonin binding or promoting the binding of dysopsonin, whereas the latter depends on the binding to specific proteins interactive with tumor-specific receptors.
Surface components are the primary variable to control the PC profile. For example, DNA coating on cationic liposome/DNA complexes (lipoplexes) formed opsonin-deficient PC in low concentration (5%) plasma, which helped the lipoplexes to avoid immune cell uptake [207]. The DNA-coated lipoplexes, then coated with plasma proteins before injection, outperformed traditional PEGylated lipoplexes in avoiding RES uptake in mice [207]. When PEG is used as a surface stabilizer, its molecular weight plays a key role [208]. For example, 2.4 nm gold particles were modified with 350, 550, and 1000 Da PEG, forming 100–200 nm NP assemblies. The PC profile varied with the PEG size, thereby hydrophilicity of the surface. The NPs with 550 Da PEG had more Tf in PC than those with 350 or 1000 Da PEG. Conjugated with DOX, the 550 Da PEG-bound gold NPs delivered more drugs to TfR+ HepG2 tumors than the other two NPs [208]. Albumin was another protein enriched on the 500 Da PEG-covered surface; though the increased albumin binding did not explicitly increase the circulation, it may have contributed to tumor accumulation via interactions with albumin-binding proteins (Section 5.2.1). A cabazitaxel nanocrystal study also shows that a surface stabilizer alters the pharmacokinetics/biodistribution profiles and antitumor effect of the drug via PC [209]. Here, the nanocrystals were stabilized with D-α-Tocopherol polyethylene glycol 1000 succinate (TPGS), which formed a PC enriched with albumin, ApoA-IV, ApoE, and Tf upon incubation with mouse plasma. The TPGS-stabilized nanocrystals showed a longer circulation half-life and greater tumor accumulation in 4T1 orthotopic tumor-bearing mice than those covered with an additional layer of lipid. The prolonged circulation was attributed to the reduced binding to IgM, and the enhanced tumor distribution to Tf, ApoA-IV, and ApoE in the PC [209].
NP components have also been varied to control the PC. With carbonate apatite NPs, a pH-sensitive drug carrier consisting of Ca2+, PO43−, and CO32−, it was found that partial replacement of Ca2+ with Mg2+ and Fe3+ changes the PC formation in both quantity and components [210]. The PC change resulted in reduced NP aggregation in serum-containing medium, increased cancer cell uptake, and changes in biodistribution profile [210]. Alternatively, the variation of NP components may help recruit specific proteins that interact with cancer cells via overexpressed receptors. For example, NPs were produced with pheophytin carbon dots (PCD), a lipoid compound derived from natural chlorophyll, to impart the affinity for apolipoproteins [211]. Coated with an optimal amount of DSPE-mPEG, the PCD NPs attracted major apolipoproteins (ApoA-I, ApoC-III, and ApoE) better than those without a lipoid component, showed enhanced uptake by LDLR+ cancer cells, and distributed more in the lungs with metastatic LDLR+ MDA-MB-231 tumors than in normal lungs [211].
7.3. MPS organs
The FDA-approved LNP products deliver siRNA mainly to the liver due to the binding of ApoE, which interacts with LDLR of hepatocytes [88, 89]. A similar principle was applied to DNA tetrahedrons, which, upon conjugation with trivalent cholesterol, bound to various apolipoproteins and showed liver-selective delivery, achieving comparable activity to a trivalent N-acetylgalactosamine-conjugated system in a liver fibrosis model [212]. Meanwhile, a series of recent studies inform that the structure of a lipid or a lipidoid (lipid-like) component of NPs resulted in the variation of the PC profile and altered the organ tropism: LNPs containing imidazole-based lipidoids target the spleen [213], LNPs containing lipidoids with an ester bond in the tail (O-series LNPs) tend to go to the liver [214], whereas those with an amide bond in the tail (N-series LNPs) showed predominant deposition in the lung [215]. The analysis of PC on O-series and N-series LNPs revealed a difference that may be responsible for the differential distribution (Fig. 5): following albumin (the most abundant protein for both LNPs), ApoE and complement 1 constituted dominant PC components in O-series LNPs, whereas N-series LNPs were enriched with fibrinogen beta and gamma chains, which enhance endothelial cell adhesion [215].
Figure 5.
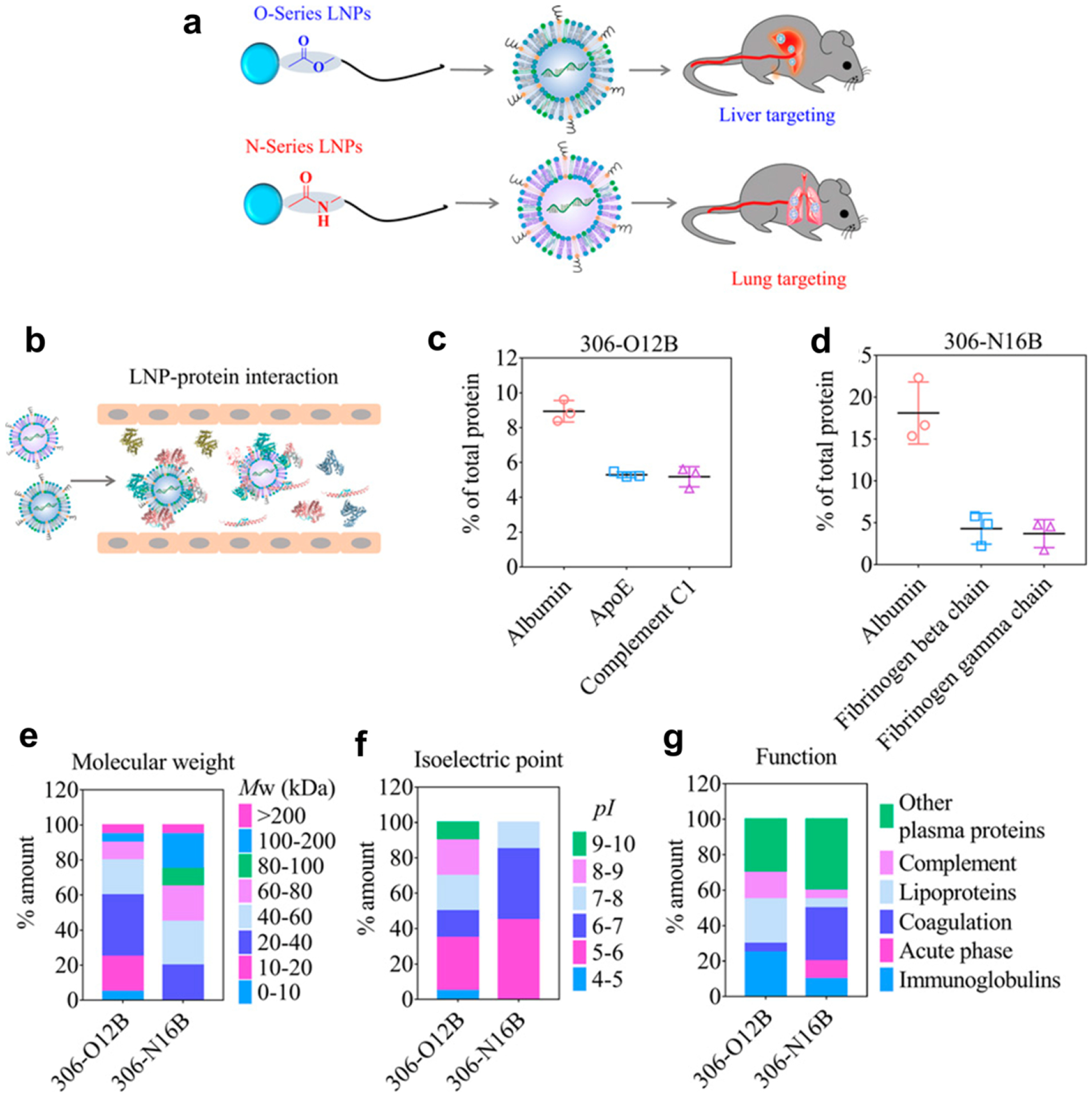
Schematic illustrations of (a) differential organ distribution of O- and N-series LNPs and (b) interaction of LNPs with proteins in the blood vessel. Quantification of the percentage of total proteins of the top three protein components in the protein corona of (c) the O-series LNPs (306-O12B) and (d) the N-series LNPs (306-N16B). Top 20 most abundant corona proteins based on (e) their calculated molecular weight, (f) isoelectric point, and (g) biological function. Reprinted from [215] with permission.
7.4. Inflammation
While opsonin binding followed by immune cell engagement is traditionally considered an undesirable event, it may be exploited to deliver drugs to active immune cells and control inflammation. For example, dextran-coated ferrous NPs (DEX-NPs), which activate the lectin complement pathway, resulting in the binding of C3b and its cleavage products, can target B cells in the spleen via the interaction of C3b with CR1/2 [94]. As such, DEX-NPs delivered a model antigen ovalbumin (OVA) and immunostimulant CpG to splenic B cells by systemic administration, eliciting OVA-specific IgG2a production better than soluble OVA/CpG mixture and preventing anaphylactic shock and asthma in mice [94]. Similarly, liposomes made of 2-((2,3-bis(oleoyloxy)propyl)dimethylammonio)ethyl hydrogen phosphate (DOCP), an anionic lipid, were shown to target active neutrophils by binding to C3 fragment iC3b, which interacts with CR3 of the cells. The neutrophils took up DOCP liposomes (but not the control DOPC liposomes), extravasated in the inflamed lungs, and delivered drugs intracellularly or to neutrophil extracellular traps [216].
Other anionic liposomes comprising 1,2-distearoyl-sn-glycero-3-phosphoglycerol (DSPG) are shown to recruit C1q, which facilitates their uptake by BMDCs via the scavenger receptor, leading to the induction of immunosuppressive regulatory T cells (Tregs) [217]. The DSPG liposomes were superior to another anionic liposomes based on phosphatidylserine or cationic liposomes in inducing antigen-specific Tregs. As a carrier of atherosclerosis-related antigen (p3500), DSPG liposomes suppressed plaque formation in a mouse model compared to PBS or free antigen, indicating the potential utility in Treg-mediated treatment of atherosclerosis [217].
PC has also been used to guide IONPs, a magnetic resonance imaging agent, to plaques in the inflamed arteries for the diagnosis of atherosclerosis [218]. Here, iron oxide magnetites were clustered in a phosphatidylcholine coat, forming 75 nm NP. The phosphatidylcholine coat binds to ApoB-100, which takes the NPs to the plaque, where the polar head of the coat is cleaved by phosphatidylcholine-specific phospholipase C upregulated in the plaque endothelium. The cleavage induces the aggregation of NPs, increasing T2 contrast in the plaque sites [218].
7.5. Eyes
PC can affect the activity of NPs in the ocular application. Gold NPs have been explored as an antiangiogenic agent in treating ocular neovascularization based on the affinity for vascular endothelial growth factor (VEGF). Bare NPs nonspecifically bind to proteins in the vitreous, losing the ability to bind to VEGF. Pre-functionalizing the gold NPs with the top five vitreous PC proteins helped gold NPs to maintain the VEGF-binding properties in the vitreous compared to bare gold NPs [219]. In a recent application of PC to ocular delivery, cationic lipoplexes were prefunctionalized with artificial PCs, such as fibronectin or Val-Gly-Asp (VGA) tripeptide, to improve the uptake by corneal epithelial cells [220]. Bare lipoplexes bound to mucin, losing the ability to enter corneal epithelial cells, whereas fibronectin or VGA peptide-coated lipoplexes were taken up by the cells via epithelial receptors. However, it remains to be seen whether the artificial PCs retain such ligand functions despite mucin [220].
8. Future perspectives
There are numerous cases where uncontrolled PC interferes with the intended function of NPs, leading to MPS accumulation, immunological responses, and loss of target-specific interactions. Meanwhile, emerging evidence indicates that specific proteins in the blood, such as albumin, transferrin, and apolipoproteins, can enhance NP delivery to particular organs, as shown with ApoE-bound LNPs trafficking to hepatocytes [87–91]. Therefore, it is reasonable to envision pre-functionalizing NPs with desirable serum proteins or engineering the surface to co-opt those proteins preferentially in situ. Preclinical studies reviewed in this article demonstrate the feasibility of this approach in different disease models. At the same time, one must be aware of the remaining challenges that may complicate the maneuver of PC formation.
First, there is a considerable variation in specific protein content according to the medical conditions and diseases [221]. The interpatient variability generates an opportunity to develop a personalized diagnostic tool based on the protein fingerprint [222–224]. On the other hand, the variability may undermine the utility of PC identified by population-based studies. A potential alternative is to couple with a computer-assisted prediction of PC-NP interaction [225] and tailor the NP design to each patient’s condition.
Second, there is limited understanding of the roles non-protein biomolecules play in NP performance [92]. For example, lipid corona can be highly relevant when the NPs are introduced via the lungs, where pulmonary surfactants form the first layer that inhaled NPs encounter [226]. Therefore, future studies are needed to understand the functions of non-protein corona and the interplay of corona components.
Third, while PC profiling has become a common practice in the characterization of NPs, most studies rely on in vitro incubation of NPs in sera of animal origin. The current method is limited in many ways: (i) blood protein composition varies with the species; thus, the information obtained with animal serum may not be extrapolated to humans; (ii) the in vitro studies are performed in static conditions and do not involve shear stress in blood flow that may affect the NP-PC interactions [227]. (iii) Moreover, the typical protocol of PC analysis [20] focuses on hard corona and does not reflect the contribution of soft corona that is constantly remodeled in circulation [228]. A recent review elaborates on additional limitations of the current methodology [229]. Robust in vitro methodologies reflecting dynamic in vivo conditions are critical to accurate prediction of NP-PC interactions and corresponding design of NPs.
Highlights.
Protein corona interferes with the intended function of nanoparticles, such as target-specific interactions.
Previous efforts have focused on avoiding protein corona formation or overriding the effect of the protein corona.
Specific proteins in the blood, such as albumin or apolipoproteins, can enhance nanoparticle delivery to particular organs.
To exploit the functions of specific serum proteins, nanoparticles are prefunctionalized with the proteins or designed to attract them in situ.
The remaining challenges include interpatient variability in serum protein contents, poor understanding of non-protein corona, and limited research methodologies.
Acknowledgments
This work was supported by NIH R01 CA232419 and NIH R01 CA258737. The authors also acknowledge the support from the Purdue Center for Cancer Research, NIH grant P30 CA023168.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Matsumura Y, Maeda H, A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs, Cancer Res, 46 (1986) 6387–6392. [PubMed] [Google Scholar]
- [2].Singh AK, Chapter 6 - Nanoparticle Pharmacokinetics and Toxicokinetics, in: Singh AK (Ed.) Engineered Nanoparticles, Academic Press, Boston, 2016, pp. 229–293. [Google Scholar]
- [3].Zhang H, Burnum KE, Luna ML, Petritis BO, Kim J-S, Qian W-J, Moore RJ, Heredia-Langner A, Webb-Robertson B-JM, Thrall BD, Camp DG, Smith RD, Pounds JG, Liu T, Quantitative proteomics analysis of adsorbed plasma proteins classifies nanoparticles with different surface properties and size, Proteomics, 11 (2011) 4569–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Monopoli MP, Aberg C, Salvati A, Dawson KA, Biomolecular coronas provide the biological identity of nanosized materials, Nat Nanotechnol, 7 (2012) 779–786. [DOI] [PubMed] [Google Scholar]
- [5].Sepand MR, Ghavami M, Zanganeh S, Stacks S, Ghasemi F, Montazeri H, Corbo C, Derakhshankhah H, Ostad SN, Ghahremani MH, Mahmoudi M, Impact of plasma concentration of transferrin on targeting capacity of nanoparticles, Nanoscale, 12 (2020) 4935–4944. [DOI] [PubMed] [Google Scholar]
- [6].Xiao W, Wang Y, Zhang H, Liu Y, Xie R, He X, Zhou Y, Liang L, Gao H, The protein corona hampers the transcytosis of transferrin-modified nanoparticles through blood-brain barrier and attenuates their targeting ability to brain tumor, Biomaterials, 274 (2021) 120888. [DOI] [PubMed] [Google Scholar]
- [7].Jain P, Pawar RS, Pandey RS, Madan J, Pawar S, Lakshmi PK, Sudheesh MS, In-vitro in-vivo correlation (IVIVC) in nanomedicine: Is protein corona the missing link?, Biotechnol Adv, 35 (2017) 889–904. [DOI] [PubMed] [Google Scholar]
- [8].Corbo C, Molinaro R, Parodi A, Toledano Furman NE, Salvatore F, Tasciotti E, The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. [DOI] [PMC free article] [PubMed]
- [9].Lynch I, Cedervall T, Lundqvist M, Cabaleiro-Lago C, Linse S, Dawson K.A.J.A.i.c., i. science, The nanoparticle–protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century, 134 (2007) 167–174. [DOI] [PubMed] [Google Scholar]
- [10].Ahsan SM, Rao CM, Ahmad MJC, M.T.o. Nanoparticles, Nanoparticle-protein interaction: the significance and role of protein corona, (2018) 175–198. [DOI] [PubMed] [Google Scholar]
- [11].Capjak I, Goreta SŠ, Jurašin DD, Vrček I.V.J.A.o.I.H., Toxicology, How protein coronas determine the fate of engineered nanoparticles in biological environment, 68 (2017) 245–253. [DOI] [PubMed] [Google Scholar]
- [12].Chen D, Ganesh S, Wang W, Amiji MJN, Plasma protein adsorption and biological identity of systemically administered nanoparticles, 12 (2017) 2113–2135. [DOI] [PubMed] [Google Scholar]
- [13].Lundqvist M, Stigler J, Cedervall T, Berggard T, Flanagan MB, Lynch I, Elia G, Dawson K.J.A.n., The evolution of the protein corona around nanoparticles: a test study, 5 (2011) 7503–7509. [DOI] [PubMed] [Google Scholar]
- [14].Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA, Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts, Proc Natl Acad Sci U S A, 105 (2008) 14265–14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lynch I, Dawson KA, Linse S.J.S.s.S., Detecting cryptic epitopes created by nanoparticles, 2006 (2006) pe14–pe14. [DOI] [PubMed] [Google Scholar]
- [16].Lynch I, Dawson K.A.J.N.t., Protein-nanoparticle interactions, 3 (2008) 40–47. [Google Scholar]
- [17].Li H, Wang Y, Tang Q, Yin D, Tang C, He E, Zou L, Peng Q, The protein corona and its effects on nanoparticle-based drug delivery systems, Acta Biomater, 129 (2021) 57–72. [DOI] [PubMed] [Google Scholar]
- [18].Ke PC, Lin S, Parak WJ, Davis TP, Caruso F.J.A.n., A decade of the protein corona, 11 (2017) 11773–11776. [DOI] [PubMed] [Google Scholar]
- [19].Casals E, Pfaller T, Duschl A, Oostingh GJ, Puntes V, Time Evolution of the Nanoparticle Protein Corona, ACS Nano, 4 (2010) 3623–3632. [DOI] [PubMed] [Google Scholar]
- [20].Monopoli MP, Pitek AS, Lynch I, Dawson KA, Formation and characterization of the nanoparticle-protein corona, Methods Mol Biol, 1025 (2013) 137–155. [DOI] [PubMed] [Google Scholar]
- [21].Bai X, Wang J, Mu Q, Su G, In vivo Protein Corona Formation: Characterizations, Effects on Engineered Nanoparticles’ Biobehaviors, and Applications, Front Bioeng Biotechnol, 9 (2021) 646708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sakulkhu U, Maurizi L, Mahmoudi M, Motazacker M, Vries M, Gramoun A, Ollivier Beuzelin MG, Vallee JP, Rezaee F, Hofmann H, Ex situ evaluation of the composition of protein corona of intravenously injected superparamagnetic nanoparticles in rats, Nanoscale, 6 (2014) 11439–11450. [DOI] [PubMed] [Google Scholar]
- [23].Garcia-Alvarez R, Hadjidemetriou M, Sanchez-Iglesias A, Liz-Marzan LM, Kostarelos K, In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape, Nanoscale, 10 (2018) 1256–1264. [DOI] [PubMed] [Google Scholar]
- [24].Sanchez-Guzman D, Giraudon-Colas G, Marichal L, Boulard Y, Wien F, Degrouard J, Baeza-Squiban A, Pin S, Renault JP, Devineau S, In Situ Analysis of Weakly Bound Proteins Reveals Molecular Basis of Soft Corona Formation, ACS Nano, 14 (2020) 9073–9088. [DOI] [PubMed] [Google Scholar]
- [25].Shang L, Nienhaus GU, In Situ Characterization of Protein Adsorption onto Nanoparticles by Fluorescence Correlation Spectroscopy, Acc Chem Res, 50 (2017) 387–395. [DOI] [PubMed] [Google Scholar]
- [26].Velasco-Rodriguez B, Soltero-Martinez JF, Rosales-Rivera LC, Macias-Balleza ER, Landazuri G, Larios-Duran ER, Adsorption and Interaction of Bovine Serum Albumin and Pluronic P103 Triblock Copolymer on a Gold Electrode: Double-Layer Capacitance Measurements, ACS Omega, 5 (2020) 17347–17355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kopac T, Bozgeyik K, Equilibrium, Kinetics, and Thermodynamics of Bovine Serum Albumin Adsorption on Single-Walled Carbon Nanotubes, Chemical Engineering Communications, 203 (2016) 1198–1206. [Google Scholar]
- [28].Bozgeyik K, Kopac T, Adsorption Properties of Arc Produced Multi Walled Carbon Nanotubes for Bovine Serum Albumin, International Journal of Chemical Reactor Engineering, 14 (2016) 549–558. [Google Scholar]
- [29].Mohammad-Beigi H, Hayashi Y, Zeuthen CM, Eskandari H, Scavenius C, Juul-Madsen K, Vorup-Jensen T, Enghild JJ, Sutherland DS, Mapping and identification of soft corona proteins at nanoparticles and their impact on cellular association, Nat Commun, 11 (2020) 4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kopac T, Protein corona, understanding the nanoparticle-protein interactions and future perspectives: A critical review, Int J Biol Macromol, 169 (2021) 290–301. [DOI] [PubMed] [Google Scholar]
- [31].Tenzer S, Docter D, Rosfa S, Wlodarski A, Kuharev J.r., Rekik A, Knauer SK, Bantz C, Nawroth T, Bier C.J.A.n., Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: a comprehensive quantitative proteomic analysis, 5 (2011) 7155–7167. [DOI] [PubMed] [Google Scholar]
- [32].Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Baldelli Bombelli F, Dawson K.A.J.J.o.t.A.C.S., Physical− chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles, 133 (2011) 2525–2534. [DOI] [PubMed] [Google Scholar]
- [33].Ghavami M, Saffar S, Abd Emamy B, Peirovi A, Shokrgozar MA, Serpooshan V, Mahmoudi MJRA, Plasma concentration gradient influences the protein corona decoration on nanoparticles, RSC Advances, 3 (2013) 1119–1126. [Google Scholar]
- [34].Corbo C, Molinaro R, Tabatabaei M, Farokhzad OC, Mahmoudi M.J.B.s., Personalized protein corona on nanoparticles and its clinical implications, Biomaterials Science, 5 (2017) 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Colapicchioni V, Tilio M, Digiacomo L, Gambini V, Palchetti S, Marchini C, Pozzi D, Occhipinti S, Amici A, Caracciolo G, Personalized liposome–protein corona in the blood of breast, gastric and pancreatic cancer patients, International Journal of Biochemistry & Cell Biology, 75 (2016) 180–187. [DOI] [PubMed] [Google Scholar]
- [36].Kah JCY, Grabinski C, Untener E, Garrett C, Chen J, Zhu D, Hussain SM, Hamad-Schifferli KJAN, Protein coronas on gold nanorods passivated with amphiphilic ligands affect cytotoxicity and cellular response to penicillin/streptomycin, 8 (2014) 4608–4620. [DOI] [PubMed] [Google Scholar]
- [37].Cukalevski R, Ferreira SA, Dunning CJ, Berggård T, Cedervall TJNR, IgG and fibrinogen driven nanoparticle aggregation, 8 (2015) 2733–2743. [Google Scholar]
- [38].Dewald I, Isakin O, Schubert J, Kraus T, Chanana M.J.T.J.o.P.C.C., Protein identity and environmental parameters determine the final physicochemical properties of protein-coated metal nanoparticles, 119 (2015) 25482–25492. [Google Scholar]
- [39].Chanana M, Correa‐Duarte MA, Liz‐Marzán LMJS, Insulin‐Coated Gold Nanoparticles: A Plasmonic Device for Studying Metal–Protein Interactions, 7 (2011) 2650–2660. [DOI] [PubMed] [Google Scholar]
- [40].Chanana M, Rivera_Gil P, Correa‐Duarte MA, Liz‐Marzán LM, Parak WJJACIE, Physicochemical properties of protein‐coated gold nanoparticles in biological fluids and cells before and after proteolytic digestion, 52 (2013) 4179–4183. [DOI] [PubMed] [Google Scholar]
- [41].Strozyk MS, Chanana M, Pastoriza‐Santos I, Pérez‐Juste J, Liz‐Marzán LMJAFM, Protein/polymer‐based dual‐responsive gold nanoparticles with pH‐dependent thermal sensitivity, 22 (2012) 1436–1444. [Google Scholar]
- [42].Ashraf S, Abbasi AZ, Pfeiffer C, Hussain SZ, Khalid ZM, Gil PR, Parak WJ, Hussain IJC, Biointerfaces SB, Protein-mediated synthesis, pH-induced reversible agglomeration, toxicity and cellular interaction of silver nanoparticles, 102 (2013) 511–518. [DOI] [PubMed] [Google Scholar]
- [43].Li X, He E, Jiang K, Peijnenburg WJ, Qiu HJWR, The crucial role of a protein corona in determining the aggregation kinetics and colloidal stability of polystyrene nanoplastics, 190 (2021) 116742. [DOI] [PubMed] [Google Scholar]
- [44].Moerz ST, Kraegeloh A, Chanana M, Kraus T.J.A.n., Formation mechanism for stable hybrid clusters of proteins and nanoparticles, 9 (2015) 6696–6705. [DOI] [PubMed] [Google Scholar]
- [45].Wu R, Peng H, Zhu J-J, Jiang L-P, Liu J.J.F.i.c., Attaching DNA to gold nanoparticles with a protein corona, 8 (2020) 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tebbe M, Kuttner C, Männel M, Fery A, Chanana M.J.A.a.m., interfaces, Colloidally stable and surfactant-free protein-coated gold nanorods in biological media, 7 (2015) 5984–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yan Y, Gause K, Kamphuis M, Ang CJAN, O, Brien-Simpson NM; Lenzo JC; Reynolds EC; Nice EC; Caruso F Differential roles of the protein corona in the cellular uptake of nanoporous polymer particles by monocyte and macrophage cell lines, 7 (2013) 10960–10970. [DOI] [PubMed] [Google Scholar]
- [48].Lesniak A, Fenaroli F, Monopoli MP, Åberg C, Dawson KA, Salvati A.J.A.n., Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells, 6 (2012) 5845–5857. [DOI] [PubMed] [Google Scholar]
- [49].Cheng X, Tian X, Wu A, Li J, Tian J, Chong Y, Chai Z, Zhao Y, Chen C, Ge C.J.A.a.m., interfaces, Protein corona influences cellular uptake of gold nanoparticles by phagocytic and nonphagocytic cells in a size-dependent manner, 7 (2015) 20568–20575. [DOI] [PubMed] [Google Scholar]
- [50].Chen D, Ganesh S, Wang W, Amiji MJN, The role of surface chemistry in serum protein corona-mediated cellular delivery and gene silencing with lipid nanoparticles, 11 (2019) 8760–8775. [DOI] [PubMed] [Google Scholar]
- [51].Obst K, Yealland G, Balzus B, Miceli E, Dimde M, Weise C, Eravci M, Bodmeier R, Haag R, Calderón MJB, Protein corona formation on colloidal polymeric nanoparticles and polymeric nanogels: impact on cellular uptake, toxicity, immunogenicity, and drug release properties, 18 (2017) 1762–1771. [DOI] [PubMed] [Google Scholar]
- [52].Barbalinardo M, Caicci F, Cavallini M, Gentili DJS, Protein corona mediated uptake and cytotoxicity of silver nanoparticles in mouse embryonic fibroblast, 14 (2018) 1801219. [DOI] [PubMed] [Google Scholar]
- [53].Cai R, Chen C, The Crown and the Scepter: Roles of the Protein Corona in Nanomedicine, Adv Mater, 31 (2019) e1805740. [DOI] [PubMed] [Google Scholar]
- [54].Kreuter J.J.A.d.d.r., Nanoparticulate systems for brain delivery of drugs, 47 (2001) 65–81. [DOI] [PubMed] [Google Scholar]
- [55].Kim H, Yuk SA, Dieterly AM, Kwon S, Park J, Meng F, Gadalla HH, Cadena MJ, Lyle LT, Yeo Y, Nanosac, a Noncationic and Soft Polyphenol Nanocapsule, Enables Systemic Delivery of siRNA to Solid Tumors, ACS Nano, 15 (2021) 4576–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Namdee K, Sobczynski DJ, Onyskiw PJ, Eniola-Adefeso O, Differential Impact of Plasma Proteins on the Adhesion Efficiency of Vascular-Targeted Carriers (VTCs) in Blood of Common Laboratory Animals, Bioconjug Chem, 26 (2015) 2419–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sobczynski DJ, Charoenphol P, Heslinga MJ, Onyskiw PJ, Namdee K, Thompson AJ, Eniola-Adefeso O, Plasma protein corona modulates the vascular wall interaction of drug carriers in a material and donor specific manner, PLoS One, 9 (2014) e107408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kelley WJ, Fromen CA, Lopez-Cazares G, Eniola-Adefeso O, PEGylation of model drug carriers enhances phagocytosis by primary human neutrophils, Acta Biomater, 79 (2018) 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bisso PW, Gaglione S, Guimaraes PPG, Mitchell MJ, Langer R, Nanomaterial Interactions with Human Neutrophils, ACS Biomater Sci Eng, 4 (2018) 4255–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mestas J, Hughes CC, Of mice and not men: differences between mouse and human immunology, J Immunol, 172 (2004) 2731–2738. [DOI] [PubMed] [Google Scholar]
- [61].Docter D, Bantz C, Westmeier D, Galla HJ, Wang Q, Kirkpatrick JC, Nielsen P, Maskos M, Stauber R.H.J.B.j.o.n., The protein corona protects against size-and dose-dependent toxicity of amorphous silica nanoparticles, 5 (2014) 1380–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ge C, Du J, Zhao L, Wang L, Liu Y, Li D, Yang Y, Zhou R, Zhao Y, Chai Z.J.P.o.t.N.A.o.S., Binding of blood proteins to carbon nanotubes reduces cytotoxicity, 108 (2011) 16968–16973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlenk F, Fischer D, Kiouptsi K, Reinhardt C.J.N.n., Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology, 8 (2013) 772–781. [DOI] [PubMed] [Google Scholar]
- [64].Ortega M, Riviere J, Choi K, Monteiro-Riviere NJTIV, Biocorona formation on gold nanoparticles modulates human proximal tubule kidney cell uptake, cytotoxicity and gene expression, 42 (2017) 150–160. [DOI] [PubMed] [Google Scholar]
- [65].Lu N, Sui Y, Ding Y, Tian R, Li L, Liu F.J.C.-b.i., Adsorption of human serum albumin on functionalized single-walled carbon nanotubes reduced cytotoxicity, 295 (2018) 64–72. [DOI] [PubMed] [Google Scholar]
- [66].Garvas M, Testen A, Umek P, Gloter A, Koklic T, Strancar JJPO, Protein corona prevents TiO2 phototoxicity, 10 (2015) e0129577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chandran P, Riviere JE, Monteiro-Riviere NAJN, Surface chemistry of gold nanoparticles determines the biocorona composition impacting cellular uptake, toxicity and gene expression profiles in human endothelial cells, 11 (2017) 507–519. [DOI] [PubMed] [Google Scholar]
- [68].Wang L, Li J, Pan J, Jiang X, Ji Y, Li Y, Qu Y, Zhao Y, Wu X, Chen C.J.J.o.t.A.C.S., Revealing the binding structure of the protein corona on gold nanorods using synchrotron radiation-based techniques: understanding the reduced damage in cell membranes, 135 (2013) 17359–17368. [DOI] [PubMed] [Google Scholar]
- [69].Mbeh DA, Akhavan O, Javanbakht T, Mahmoudi MJASS, Cytotoxicity of protein corona-graphene oxide nanoribbons on human epithelial cells, 320 (2014) 596–601. [Google Scholar]
- [70].Duan G, Kang S.-g., Tian X, Garate JA, Zhao L, Ge C, Zhou RJN, Protein corona mitigates the cytotoxicity of graphene oxide by reducing its physical interaction with cell membrane, 7 (2015) 15214–15224. [DOI] [PubMed] [Google Scholar]
- [71].Liu N, Liang Y, Wei T, Zou L, Bai C, Huang X, Wu T, Xue Y, Tang M, Zhang T, Protein corona mitigated the cytotoxicity of CdTe QDs to macrophages by targeting mitochondria, NanoImpact, 25 (2022) 100367. [DOI] [PubMed] [Google Scholar]
- [72].Žūkienė R, Snitka VJC, S.B. Biointerfaces, Zinc oxide nanoparticle and bovine serum albumin interaction and nanoparticles influence on cytotoxicity in vitro, 135 (2015) 316–323. [DOI] [PubMed] [Google Scholar]
- [73].Yin H, Chen R, Casey PS, Ke PC, Davis TP, Chen C, Reducing the cytotoxicity of ZnO nanoparticles by a pre-formed protein corona in a supplemented cell culture medium, RSC Advances, 5 (2015) 73963–73973. [Google Scholar]
- [74].Escamilla-Rivera V, Uribe-Ramirez M, Gonzalez-Pozos S, Lozano O, Lucas S, De Vizcaya-Ruiz A.J.T.l., Protein corona acts as a protective shield against Fe3O4-PEG inflammation and ROS-induced toxicity in human macrophages, 240 (2016) 172–184. [DOI] [PubMed] [Google Scholar]
- [75].de Oliveira FA, Albuquerque LJC, Castro CE, Riske KA, Bellettini IC, Giacomelli FC, Reduced cytotoxicity of nanomaterials driven by nano-bio interactions: Case study of single protein coronas enveloping polymersomes, Colloids Surf B Biointerfaces, 213 (2022) 112387. [DOI] [PubMed] [Google Scholar]
- [76].De Paoli SH, Diduch LL, Tegegn TZ, Orecna M, Strader MB, Karnaukhova E, Bonevich JE, Holada K, Simak J, The effect of protein corona composition on the interaction of carbon nanotubes with human blood platelets, Biomaterials, 35 (2014) 6182–6194. [DOI] [PubMed] [Google Scholar]
- [77].Yang H, Wang M, Zhang Y, Li F, Yu S, Zhu L, Guo Y, Yang L, Yang S, Conformational-transited protein corona regulated cell-membrane penetration and induced cytotoxicity of ultrasmall Au nanoparticles, RSC Adv, 9 (2019) 4435–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wu G, Jiang C, Zhang T, FcgammaRIIB receptor-mediated apoptosis in macrophages through interplay of cadmium sulfide nanomaterials and protein corona, Ecotoxicol Environ Saf, 164 (2018) 140–148. [DOI] [PubMed] [Google Scholar]
- [79].Tridandapani S, Siefker K, Teillaud JL, Carter JE, Wewers MD, Anderson CL, Regulated expression and inhibitory function of Fcgamma RIIb in human monocytic cells, J Biol Chem, 277 (2002) 5082–5089. [DOI] [PubMed] [Google Scholar]
- [80].Li Y, Wang P, Hu C, Wang K, Chang Q, Liu L, Han Z, Shao Y, Zhai Y, Zuo ZJSR, Protein corona of airborne nanoscale PM2. 5 induces aberrant proliferation of human lung fibroblasts based on a 3D organotypic culture, 8 (2018) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang Z, Wang C, Liu S, He W, Wang L, Gan J, Huang Z, Wang Z, Wei H, Zhang J, Dong L, Specifically Formed Corona on Silica Nanoparticles Enhances Transforming Growth Factor β1 Activity in Triggering Lung Fibrosis, ACS Nano, 11 (2017) 1659–1672. [DOI] [PubMed] [Google Scholar]
- [82].Nabeshi H, Yoshikawa T, Matsuyama K, Nakazato Y, Arimori A, Isobe M, Tochigi S, Kondoh S, Hirai T, Akase T, Yamashita T, Yamashita K, Yoshida T, Nagano K, Abe Y, Yoshioka Y, Kamada H, Imazawa T, Itoh N, Kondoh M, Yagi K, Mayumi T, Tsunoda S.-i., Tsutsumi Y, Amorphous nanosilicas induce consumptive coagulopathy after systemic exposure, Nanotechnology, 23 (2012) 045101. [DOI] [PubMed] [Google Scholar]
- [83].Yoshida T, Yoshioka Y, Morishita Y, Aoyama M, & Tochigi S Hira i T, Tanaka K, Nagano K, Kamada H, Tsunoda S, Nabeshi H, Yoshikawa T, Higashisaka K, Tsutsumi Y, Protein corona change s mediated by surface modification of amorphous silica nanoparticle s suppress acute toxicity and activation of intrinsic coagulation cascade in mice, Nanotechnology, (2015) 245101. [DOI] [PubMed] [Google Scholar]
- [84].Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, Dawson KA, Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface, Nat Nanotechnol, 8 (2013) 137–143. [DOI] [PubMed] [Google Scholar]
- [85].Naidu PSR, Gavriel N, Gray CGG, Bartlett CA, Toomey LM, Kretzmann JA, Patalwala D, McGonigle T, Denham E, Hee C, Ho D, Taylor NL, Norret M, Smith NM, Dunlop SA, Iyer KS, Fitzgerald M, Elucidating the Inability of Functionalized Nanoparticles to Cross the Blood-Brain Barrier and Target Specific Cells in Vivo, ACS Appl Mater Interfaces, 11 (2019) 22085–22095. [DOI] [PubMed] [Google Scholar]
- [86].Sobczynski DJ, Eniola-Adefeso O, IgA and IgM protein primarily drive plasma corona-induced adhesion reduction of PLGA nanoparticles in human blood flow, Bioeng Transl Med, 2 (2017) 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhang X, Goel V, Robbie GJ, Pharmacokinetics of Patisiran, the First Approved RNA Interference Therapy in Patients With Hereditary Transthyretin-Mediated Amyloidosis, J Clin Pharmacol, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Du X, Hope MJ, Madden TD, Mui BL, Semple SC, Tam YK, Ciufolini M, Witzigmann D, Kulkarni JA, van der Meel R, Cullis PR, The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs, Nat Nanotechnol, 14 (2019) 1084–1087. [DOI] [PubMed] [Google Scholar]
- [89].Witzigmann D, Kulkarni JA, Leung J, Chen S, Cullis PR, van der Meel R, Lipid nanoparticle technology for therapeutic gene regulation in the liver, Adv Drug Deliv Rev, 159 (2020) 344–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, Lin KP, Vita G, Attarian S, Plante-Bordeneuve V, Mezei MM, Campistol JM, Buades J, Brannagan TH 3rd, Kim BJ, Oh J, Parman Y, Sekijima Y, Hawkins PN, Solomon SD, Polydefkis M, Dyck PJ, Gandhi PJ, Goyal S, Chen J, Strahs AL, Nochur SV, Sweetser MT, Garg PP, Vaishnaw AK, Gollob JA, Suhr OB, Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis, N Engl J Med, 379 (2018) 11–21. [DOI] [PubMed] [Google Scholar]
- [91].Gillmore JD, Gane E, Taubel J, Kao J, Fontana M, Maitland ML, Seitzer J, O’Connell D, Walsh KR, Wood K, Phillips J, Xu Y, Amaral A, Boyd AP, Cehelsky JE, McKee MD, Schiermeier A, Harari O, Murphy A, Kyratsous CA, Zambrowicz B, Soltys R, Gutstein DE, Leonard J, Sepp-Lorenzino L, Lebwohl D, CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis, New England Journal of Medicine, (2021). [DOI] [PubMed] [Google Scholar]
- [92].Digiacomo L, Pozzi D, Palchetti S, Zingoni A, Caracciolo G, Impact of the protein corona on nanomaterial immune response and targeting ability, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 12 (2020) e1615. [DOI] [PubMed] [Google Scholar]
- [93].Banda NK, Mehta G, Chao Y, Wang G, Inturi S, Fossati-Jimack L, Botto M, Wu L, Moghimi SM, Simberg D, Mechanisms of complement activation by dextran-coated superparamagnetic iron oxide (SPIO) nanoworms in mouse versus human serum, Particle and Fibre Toxicology, 11 (2014) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shen L, Tenzer S, Storck W, Hobernik D, Raker VK, Fischer K, Decker S, Dzionek A, Krauthauser S, Diken M, Nikolaev A, Maxeiner J, Schuster P, Kappel C, Verschoor A, Schild H, Grabbe S, Bros M, Protein corona-mediated targeting of nanocarriers to B cells allows redirection of allergic immune responses, J Allergy Clin Immunol, 142 (2018) 1558–1570. [DOI] [PubMed] [Google Scholar]
- [95].Vu VP, Gifford GB, Chen F, Benasutti H, Wang G, Groman EV, Scheinman R, Saba L, Moghimi SM, Simberg D, Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles, Nat Nanotechnol, 14 (2019) 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bednarczyk M, Medina-Montano C, Fittler FJ, Stege H, Roskamp M, Kuske M, Langer C, Vahldieck M, Montermann E, Tubbe I, Rohrig N, Dzionek A, Grabbe S, Bros M, Complement-Opsonized Nano-Carriers Are Bound by Dendritic Cells (DC) via Complement Receptor (CR)3, and by B Cell Subpopulations via CR-1/2, and Affect the Activation of DC and B-1 Cells, Int J Mol Sci, 22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Escamilla-Rivera V, Solorio-Rodriguez A, Uribe-Ramirez M, Lozano O, Lucas S, Chagolla-Lopez A, Winkler R, De Vizcaya-Ruiz A, Plasma protein adsorption on Fe3O4-PEG nanoparticles activates the complement system and induces an inflammatory response, Int J Nanomedicine, 14 (2019) 2055–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Tavano R, Gabrielli L, Lubian E, Fedeli C, Visentin S, Polverino De Laureto P, Arrigoni G, Geffner-Smith A, Chen F, Simberg D, Morgese G, Benetti EM, Wu L, Moghimi SM, Mancin F, Papini E, C1q-Mediated Complement Activation and C3 Opsonization Trigger Recognition of Stealth Poly(2-methyl-2-oxazoline)-Coated Silica Nanoparticles by Human Phagocytes, ACS Nano, 12 (2018) 5834–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mo J, Xie Q, Wei W, Zhao J, Revealing the immune perturbation of black phosphorus nanomaterials to macrophages by understanding the protein corona, Nat Commun, 9 (2018) 2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Park JY, Park SJ, Park JY, Kim SH, Kwon S, Jung Y, Khang D, Unfolded Protein Corona Surrounding Nanotubes Influence the Innate and Adaptive Immune System, Adv Sci (Weinh), 8 (2021) 2004979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Cai R, Ren J, Ji Y, Wang Y, Liu Y, Chen Z, Farhadi Sabet Z, Wu X, Lynch I, Chen C, Corona of Thorns: The Surface Chemistry-Mediated Protein Corona Perturbs the Recognition and Immune Response of Macrophages, ACS Appl Mater Interfaces, 12 (2020) 1997–2008. [DOI] [PubMed] [Google Scholar]
- [102].Leibe R, Hsiao IL, Fritsch-Decker S, Kielmeier U, Wagbo AM, Voss B, Schmidt A, Hessman SD, Duschl A, Oostingh GJ, Diabaté S, Weiss C, The protein corona suppresses the cytotoxic and pro-inflammatory response in lung epithelial cells and macrophages upon exposure to nanosilica, Archives of toxicology, 93 (2019) 871–885. [DOI] [PubMed] [Google Scholar]
- [103].Dai Q, Guo J, Yan Y, Ang CS, Bertleff-Zieschang N, Caruso F, Cell-Conditioned Protein Coronas on Engineered Particles Influence Immune Responses, Biomacromolecules, 18 (2017) 431–439. [DOI] [PubMed] [Google Scholar]
- [104].Amoozgar Z, Yeo Y, Recent advances in stealth coating of nanoparticle drug delivery systems, Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 4 (2012) 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Mozar FS, Chowdhury EH, Impact of PEGylated Nanoparticles on Tumor Targeted Drug Delivery, Curr Pharm Des, 24 (2018) 3283–3296. [DOI] [PubMed] [Google Scholar]
- [106].Jeon SI, Lee JH, Andrade JD, De Gennes PG, Protein—surface interactions in the presence of polyethylene oxide, Journal of Colloid and Interface Science, 142 (1991) 149–158. [Google Scholar]
- [107].Perry JL, Reuter KG, Kai MP, Herlihy KP, Jones SW, Luft JC, Napier M, Bear JE, DeSimone JM, PEGylated PRINT Nanoparticles: The Impact of PEG Density on Protein Binding, Macrophage Association, Biodistribution, and Pharmacokinetics, Nano Letters, 12 (2012) 5304–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Walkey CD, Olsen JB, Guo H, Emili A, Chan WC, Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake, J Am Chem Soc, 134 (2012) 2139–2147. [DOI] [PubMed] [Google Scholar]
- [109].Hussain Z, Khan S, Imran M, Sohail M, Shah SWA, de Matas M, PEGylation: a promising strategy to overcome challenges to cancer-targeted nanomedicines: a review of challenges to clinical transition and promising resolution, Drug Deliv Transl Res, 9 (2019) 721–734. [DOI] [PubMed] [Google Scholar]
- [110].de la Rosa VR, Poly(2-oxazoline)s as materials for biomedical applications, J Mater Sci Mater Med, 25 (2014) 1211–1225. [DOI] [PubMed] [Google Scholar]
- [111].Luxenhofer R, Schulz A, Roques C, Li S, Bronich TK, Batrakova EV, Jordan R, Kabanov AV, Doubly amphiphilic poly(2-oxazoline)s as high-capacity delivery systems for hydrophobic drugs, Biomaterials, 31 (2010) 4972–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lorson T, Lubtow MM, Wegener E, Haider MS, Borova S, Nahm D, Jordan R, Sokolski-Papkov M, Kabanov AV, Luxenhofer R, Poly(2-oxazoline)s based biomaterials: A comprehensive and critical update, Biomaterials, 178 (2018) 204–280. [DOI] [PubMed] [Google Scholar]
- [113].Simon L, Marcotte N, Devoisselle JM, Begu S, Lapinte V, Recent advances and prospects in nano drug delivery systems using lipopolyoxazolines, Int J Pharm, 585 (2020) 119536. [DOI] [PubMed] [Google Scholar]
- [114].Koshkina O, Westmeier D, Lang T, Bantz C, Hahlbrock A, Wurth C, Resch-Genger U, Braun U, Thiermann R, Weise C, Eravci M, Mohr B, Schlaad H, Stauber RH, Docter D, Bertin A, Maskos M, Tuning the Surface of Nanoparticles: Impact of Poly(2-ethyl-2-oxazoline) on Protein Adsorption in Serum and Cellular Uptake, Macromol Biosci, 16 (2016) 1287–1300. [DOI] [PubMed] [Google Scholar]
- [115].Jiang S, Cao Z, Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications, Adv Mater, 22 (2010) 920–932. [DOI] [PubMed] [Google Scholar]
- [116].Schlenoff JB, Zwitteration: coating surfaces with zwitterionic functionality to reduce nonspecific adsorption, Langmuir, 30 (2014) 9625–9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Pranantyo D, Xu LQ, Neoh KG, Kang E-T, Teo SL-M, Antifouling Coatings via Tethering of Hyperbranched Polyglycerols on Biomimetic Anchors, Industrial & Engineering Chemistry Research, 55 (2016) 1890–1901. [Google Scholar]
- [118].Zhao L, Xu YH, Akasaka T, Abe S, Komatsu N, Watari F, Chen X, Polyglycerol-coated nanodiamond as a macrophage-evading platform for selective drug delivery in cancer cells, Biomaterials, 35 (2014) 5393–5406. [DOI] [PubMed] [Google Scholar]
- [119].Zou Y, Ito S, Yoshino F, Suzuki Y, Zhao L, Komatsu N, Polyglycerol Grafting Shields Nanoparticles from Protein Corona Formation to Avoid Macrophage Uptake, ACS Nano, 14 (2020) 7216–7226. [DOI] [PubMed] [Google Scholar]
- [120].Doh K-O, Yeo Y, Application of polysaccharides for surface modification of nanomedicines, Therapeutic Delivery, 3 (2012) 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Almalik A, Benabdelkamel H, Masood A, Alanazi IO, Alradwan I, Majrashi MA, Alfadda AA, Alghamdi WM, Alrabiah H, Tirelli N, Alhasan AH, Hyaluronic Acid Coated Chitosan Nanoparticles Reduced the Immunogenicity of the Formed Protein Corona, Sci Rep, 7 (2017) 10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Srivastav AK, Dhiman N, Khan H, Srivastav AK, Yadav SK, Prakash J, Arjaria N, Singh D, Yadav S, Patnaik S, Kumar M, Impact of Surface-Engineered ZnO Nanoparticles on Protein Corona Configuration and Their Interactions With Biological System, J Pharm Sci, 108 (2019) 1872–1889. [DOI] [PubMed] [Google Scholar]
- [123].Amoozgar Z, Park J, Lin Q, Yeo Y, Low Molecular-Weight Chitosan as a pH-Sensitive Stealth Coating for Tumor-Specific Drug Delivery, Molecular Pharmaceutics, 9 (2012) 1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lopez-Cazares G, Eniola-Adefeso O, Dual Coating of Chitosan and Albumin Negates the Protein Corona-Induced Reduced Vascular Adhesion of Targeted PLGA Microparticles in Human Blood, Pharmaceutics, 14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Dash P, Piras AM, Dash M, Cell membrane coated nanocarriers - an efficient biomimetic platform for targeted therapy, J Control Release, 327 (2020) 546–570. [DOI] [PubMed] [Google Scholar]
- [126].Zhuang J, Gong H, Zhou J, Zhang Q, Gao W, Fang RH, Zhang L, Targeted gene silencing in vivo by platelet membrane–coated metal-organic framework nanoparticles, Science Advances, 6 (2020) eaaz6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Rao L, Bu LL, Meng QF, Cai B, Deng WW, Li A, Li KY, Guo SS, Zhang WF, Liu W, Sun ZJ, Zhao XZ, Antitumor Platelet-Mimicking Magnetic Nanoparticles, Adv Funct Mater, 27 (2017). [Google Scholar]
- [128].Wang D, Dong H, Li M, Cao Y, Yang F, Zhang K, Dai W, Wang C, Zhang X, Erythrocyte-Cancer Hybrid Membrane Camouflaged Hollow Copper Sulfide Nanoparticles for Prolonged Circulation Life and Homotypic-Targeting Photothermal/Chemotherapy of Melanoma, ACS Nano, 12 (2018) 5241–5252. [DOI] [PubMed] [Google Scholar]
- [129].Kang T, Zhu QQ, Wei D, Feng JX, Yao JH, Jiang TZ, Song QX, Wei XB, Chen HZ, Gao XL, Chen J, Nanoparticles Coated with Neutrophil Membranes Can Effectively Treat Cancer Metastasis, Acs Nano, 11 (2017) 1397–1411. [DOI] [PubMed] [Google Scholar]
- [130].Rao L, Meng QF, Bu LL, Cai B, Huang Q, Sun ZJ, Zhang WF, Li A, Guo SS, Liu W, Wang TH, Zhao XZ, Erythrocyte Membrane-Coated Upconversion Nanoparticles with Minimal Protein Adsorption for Enhanced Tumor Imaging, ACS Appl Mater Interfaces, 9 (2017) 2159–2168. [DOI] [PubMed] [Google Scholar]
- [131].Rampado R, Crotti S, Caliceti P, Pucciarelli S, Agostini M, Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials, Front Bioeng Biotechnol, 8 (2020) 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Fam SY, Chee CF, Yong CY, Ho KL, Mariatulqabtiah AR, Tan WS, Stealth Coating of Nanoparticles in Drug-Delivery Systems, Nanomaterials (Basel), 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sanchez-Cano C, Carril M, Recent Developments in the Design of Non-Biofouling Coatings for Nanoparticles and Surfaces, Int J Mol Sci, 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Manzari MT, Shamay Y, Kiguchi H, Rosen N, Scaltriti M, Heller DA, Targeted drug delivery strategies for precision medicines, Nature Reviews Materials, 6 (2021) 351–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Mirshafiee V, Mahmoudi M, Lou K, Cheng J, Kraft ML, Protein corona significantly reduces active targeting yield, Chemical Communications, 49 (2013) 2557–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zarschler K, Prapainop K, Mahon E, Rocks L, Bramini M, Kelly PM, Stephan H, Dawson KA, Diagnostic nanoparticle targeting of the EGF-receptor in complex biological conditions using single-domain antibodies, Nanoscale, 6 (2014) 6046–6056. [DOI] [PubMed] [Google Scholar]
- [137].Dai Q, Yan Y, Ang CS, Kempe K, Kamphuis MM, Dodds SJ, Caruso F, Monoclonal antibody-functionalized multilayered particles: targeting cancer cells in the presence of protein coronas, ACS Nano, 9 (2015) 2876–2885. [DOI] [PubMed] [Google Scholar]
- [138].Dai Q, Walkey C, Chan WC, Polyethylene glycol backfilling mitigates the negative impact of the protein corona on nanoparticle cell targeting, Angew Chem Int Ed Engl, 53 (2014) 5093–5096. [DOI] [PubMed] [Google Scholar]
- [139].D’Hollander A, Jans H, Velde GV, Verstraete C, Massa S, Devoogdt N, Stakenborg T, Muyldermans S, Lagae L, Himmelreich U, Limiting the protein corona: A successful strategy for in vivo active targeting of anti-HER2 nanobody-functionalized nanostars, Biomaterials, 123 (2017) 15–23. [DOI] [PubMed] [Google Scholar]
- [140].Kang B, Okwieka P, Schottler S, Winzen S, Langhanki J, Mohr K, Opatz T, Mailander V, Landfester K, Wurm FR, Carbohydrate-Based Nanocarriers Exhibiting Specific Cell Targeting with Minimum Influence from the Protein Corona, Angew Chem Int Ed Engl, 54 (2015) 7436–7440. [DOI] [PubMed] [Google Scholar]
- [141].Safavi-Sohi R, Maghari S, Raoufi M, Jalali SA, Hajipour MJ, Ghassempour A, Mahmoudi M, Bypassing Protein Corona Issue on Active Targeting: Zwitterionic Coatings Dictate Specific Interactions of Targeting Moieties and Cell Receptors, ACS Appl Mater Interfaces, 8 (2016) 22808–22818. [DOI] [PubMed] [Google Scholar]
- [142].Kratz F, Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles, Journal of Controlled Release, 132 (2008) 171–183. [DOI] [PubMed] [Google Scholar]
- [143].Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ, Structure-based programming of lymph-node targeting in molecular vaccines, Nature, 507 (2014) 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Hawkins MJ, Soon-Shiong P, Desai N, Protein nanoparticles as drug carriers in clinical medicine, Advanced Drug Delivery Reviews, 60 (2008) 876–885. [DOI] [PubMed] [Google Scholar]
- [145].Elsadek B, Kratz F, Impact of albumin on drug delivery — New applications on the horizon, Journal of Controlled Release, 157 (2012) 4–28. [DOI] [PubMed] [Google Scholar]
- [146].Quan Q, Xie J, Gao H, Yang M, Zhang F, Liu G, Lin X, Wang A, Eden HS, Lee S, Zhang G, Chen X, HSA coated iron oxide nanoparticles as drug delivery vehicles for cancer therapy, Mol Pharm, 8 (2011) 1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Xia B, Zhang W, Shi J, Xiao SJ, Engineered stealth porous silicon nanoparticles via surface encapsulation of bovine serum albumin for prolonging blood circulation in vivo, ACS Appl Mater Interfaces, 5 (2013) 11718–11724. [DOI] [PubMed] [Google Scholar]
- [148].Ogawara K.-i., Furumoto K, Nagayama S, Minato K, Higaki K, Kai T, Kimura T, Pre-coating with serum albumin reduces receptor-mediated hepatic disposition of polystyrene nanosphere: implications for rational design of nanoparticles, Journal of Controlled Release, 100 (2004) 451–455. [DOI] [PubMed] [Google Scholar]
- [149].Furumoto K, Yokoe J-I, Ogawara K.-i., Amano S, Takaguchi M, Higaki K, Kai T, Kimura T, Effect of coupling of albumin onto surface of PEG liposome on its in vivo disposition, International Journal of Pharmaceutics, 329 (2007) 110–116. [DOI] [PubMed] [Google Scholar]
- [150].Beukers H, Deierkauf FA, Blom CP, Deierkauf M, Riemersma JC, Effects of albumin on the phagocytosis of polystyrene spherules by rabbit polymorphonuclear leucocytes, J Cell Physiol, 97 (1978) 29–36. [DOI] [PubMed] [Google Scholar]
- [151].Peng Q, Zhang S, Yang Q, Zhang T, Wei XQ, Jiang L, Zhang CL, Chen QM, Zhang ZR, Lin YF, Preformed albumin corona, a protective coating for nanoparticles based drug delivery system, Biomaterials, 34 (2013) 8521–8530. [DOI] [PubMed] [Google Scholar]
- [152].Trieu V, Damascelli B, Soon-Shiong P, Desai N, SPARC expression in head and neck cancer correlates with tumor response to nanoparticle albumin-bound paclitaxel (nab-paclitaxel, ABI-007, Abraxane), Cancer Research, 66 (2006) 1050. [Google Scholar]
- [153].Iglesias J, nab-Paclitaxel (Abraxane(®)): an albumin-bound cytotoxic exploiting natural delivery mechanisms into tumors, Breast Cancer Research : BCR, 11 (2009) S21–S21.20030873 [Google Scholar]
- [154].Stehle G, Sinn H, Wunder A, Schrenk HH, Stewart JC, Hartung G, Maier-Borst W, Heene DL, Plasma protein (albumin) catabolism by the tumor itself--implications for tumor metabolism and the genesis of cachexia, Critical reviews in oncology/hematology, 26 (1997) 77–100. [DOI] [PubMed] [Google Scholar]
- [155].Mariam J, Sivakami S, Dongre PM, Albumin corona on nanoparticles - a strategic approach in drug delivery, Drug Deliv, 23 (2016) 2668–2676. [DOI] [PubMed] [Google Scholar]
- [156].Peng Q, Wei XQ, Yang Q, Zhang S, Zhang T, Shao XR, Cai XX, Zhang ZR, Lin YF, Enhanced biostability of nanoparticle-based drug delivery systems by albumin corona, Nanomedicine (Lond), 10 (2015) 205–214. [DOI] [PubMed] [Google Scholar]
- [157].Park J, Sun B, Yeo Y, Albumin-coated nanocrystals for carrier-free delivery of paclitaxel, J Control Release, 263 (2017) 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Park J, Park JE, Hedrick VE, Wood KV, Bonham C, Lee W, Yeo Y, A Comparative In Vivo Study of Albumin‐Coated Paclitaxel Nanocrystals and Abraxane, Small, 14 (2018) 1703670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Azizi M, Ghourchian H, Yazdian F, Bagherifam S, Bekhradnia S, Nyström B, Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line, Sci Rep, 7 (2017) 5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Nguyen VH, Meghani NM, Amin HH, Tran TTD, Tran PHL, Park C, Lee BJ, Modulation of serum albumin protein corona for exploring cellular behaviors of fattigation-platform nanoparticles, Colloids Surf B Biointerfaces, 170 (2018) 179–186. [DOI] [PubMed] [Google Scholar]
- [161].Hyun H, Park J, Willis K, Park JE, Lyle LT, Lee W, Yeo Y, Surface modification of polymer nanoparticles with native albumin for enhancing drug delivery to solid tumors, Biomaterials, 180 (2018) 206–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Takeuchi T, Kitayama Y, Sasao R, Yamada T, Toh K, Matsumoto Y, Kataoka K, Molecularly Imprinted Nanogels Acquire Stealth In Situ by Cloaking Themselves with Native Dysopsonic Proteins, Angew Chem Int Ed Engl, 56 (2017) 7088–7092. [DOI] [PubMed] [Google Scholar]
- [163].Phillips MC, Apolipoprotein E isoforms and lipoprotein metabolism, IUBMB Life, 66 (2014) 616–623. [DOI] [PubMed] [Google Scholar]
- [164].Yallapu MM, Chauhan N, Othman SF, Khalilzad-Sharghi V, Ebeling MC, Khan S, Jaggi M, Chauhan SC, Implications of protein corona on physico-chemical and biological properties of magnetic nanoparticles, Biomaterials, 46 (2015) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Kim HR, Andrieux K, Gil S, Taverna M, Chacun H, Desmaële D, Taran F, Georgin D, Couvreur P, Translocation of poly(ethylene glycol-co-hexadecyl)cyanoacrylate nanoparticles into rat brain endothelial cells: role of apolipoproteins in receptor-mediated endocytosis, Biomacromolecules, 8 (2007) 793–799. [DOI] [PubMed] [Google Scholar]
- [166].Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Wagner S, Buchel C, von Briesen H, Kreuter J, Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones, J Control Release, 137 (2009) 78–86. [DOI] [PubMed] [Google Scholar]
- [167].Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Buchel C, Kreuter J, Human serum albumin nanoparticles modified with apolipoprotein A-I cross the blood-brain barrier and enter the rodent brain, J Drug Target, 18 (2010) 842–848. [DOI] [PubMed] [Google Scholar]
- [168].Göppert TM, Müller RH, Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: Comparison of plasma protein adsorption patterns, Journal of Drug Targeting, 13 (2005) 179–187. [DOI] [PubMed] [Google Scholar]
- [169].Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA, Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles), Brain Research, 674 (1995) 171–174. [DOI] [PubMed] [Google Scholar]
- [170].Dal Magro R, Albertini B, Beretta S, Rigolio R, Donzelli E, Chiorazzi A, Ricci M, Blasi P, Sancini G, Artificial apolipoprotein corona enables nanoparticle brain targeting, Nanomedicine, 14 (2018) 429–438. [DOI] [PubMed] [Google Scholar]
- [171].Yeo ELL, Thong PSP, Soo KC, Kah JCY, Protein corona in drug delivery for multimodal cancer therapy in vivo, Nanoscale, 10 (2018) 2461–2472. [DOI] [PubMed] [Google Scholar]
- [172].Yeo ELL, Cheah JUJ, Thong PSP, Soo KC, Kah JCY, Gold Nanorods Coated with Apolipoprotein E Protein Corona for Drug Delivery, ACS Applied Nano Materials, 2 (2019) 6220–6229. [Google Scholar]
- [173].Schöttler S, Becker G, Winzen S, Steinbach T, Mohr K, Landfester K, Mailänder V, Wurm FR, Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers, Nat Nanotechnol, 11 (2016) 372–377. [DOI] [PubMed] [Google Scholar]
- [174].Aoyama M, Hata K, Higashisaka K, Nagano K, Yoshioka Y, Tsutsumi Y, Clusterin in the protein corona plays a key role in the stealth effect of nanoparticles against phagocytes, Biochem Biophys Res Commun, 480 (2016) 690–695. [DOI] [PubMed] [Google Scholar]
- [175].Xie Z, Harris-White ME, Wals PA, Frautschy SA, Finch CE, Morgan TE, Apolipoprotein J (clusterin) activates rodent microglia in vivo and in vitro, J Neurochem, 93 (2005) 1038–1046. [DOI] [PubMed] [Google Scholar]
- [176].Brown EJ, Frazier WA, Integrin-associated protein (CD47) and its ligands, Trends in Cell Biology, 11 (2001) 130–135. [DOI] [PubMed] [Google Scholar]
- [177].Oldenborg P-A, Zheleznyak A, Fang Y-F, Lagenaur CF, Gresham HD, Lindberg FP, Role of CD47 as a Marker of Self on Red Blood Cells, Science, 288 (2000) 2051–2054. [DOI] [PubMed] [Google Scholar]
- [178].Tsai RK, Rodriguez PL, Discher DE, Self inhibition of phagocytosis: The affinity of [`]marker of self’ CD47 for SIRP[alpha] dictates potency of inhibition but only at low expression levels, Blood Cells, Molecules, and Diseases, 45 (2010) 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Sosale NG, Spinler KR, Alvey C, Discher DE, Macrophage engulfment of a cell or nanoparticle is regulated by unavoidable opsonization, a species-specific ‘Marker of Self’ CD47, and target physical properties, Curr Opin Immunol, 35 (2015) 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Oldenborg P-A, Gresham HD, Chen Y, Izui S, Lindberg FP, Lethal autoimmune hemolytic anemia in CD47-deficient nonobese diabetic (NOD) mice, Blood, 99 (2002) 3500–3504. [DOI] [PubMed] [Google Scholar]
- [181].Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua MS, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PO, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NN, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL, The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors, Proc Natl Acad Sci U S A, 109 (2012) 6662–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Qie Y, Yuan H, von Roemeling CA, Chen Y, Liu X, Shih KD, Knight JA, Tun HW, Wharen RE, Jiang W, Kim BY, Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes, Sci Rep, 6 (2016) 26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Rodriguez T Fau - Harada Pl, Harada DA Fau - Christian T, Christian Da Fau - Pantano DA, Pantano Da Fau - Tsai RK, Tsai Rk Fau - Discher DE, Discher DE, Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles, (2013) 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Li Z, Li D, Li Q, Luo C, Li J, Kou L, Zhang D, Zhang H, Zhao S, Kan Q, Liu J, Zhang P, Liu X, Sun Y, Wang Y, He Z, Sun J, In situ low-immunogenic albumin-conjugating-corona guiding nanoparticles for tumor-targeting chemotherapy, Biomater Sci, 6 (2018) 2681–2693. [DOI] [PubMed] [Google Scholar]
- [185].Prabhakar K, Afzal SM, Surender G, Kishan V, Tween 80 containing lipid nanoemulsions for delivery of indinavir to brain, Acta Pharm Sin B, 3 (2013) 345–353. [Google Scholar]
- [186].Chen D, Parayath N, Ganesh S, Wang W, Amiji M, The role of apolipoprotein- and vitronectin-enriched protein corona on lipid nanoparticles for in vivo targeted delivery and transfection of oligonucleotides in murine tumor models, Nanoscale, 11 (2019) 18806–18824. [DOI] [PubMed] [Google Scholar]
- [187].Muller J, Bauer KN, Prozeller D, Simon J, Mailander V, Wurm FR, Winzen S, Landfester K, Coating nanoparticles with tunable surfactants facilitates control over the protein corona, Biomaterials, 115 (2017) 1–8. [DOI] [PubMed] [Google Scholar]
- [188].Li Z, Zhu J, Wang Y, Zhou M, Li D, Zheng S, Yin L, Luo C, Zhang H, Zhong L, Li W, Wang J, Gui S, Cai B, Wang Y, Sun J, In situ apolipoprotein E-enriched corona guides dihydroartemisinin-decorating nanoparticles towards LDLr-mediated tumor-homing chemotherapy, Asian J Pharm Sci, 15 (2020) 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Schvartz I, Seger D, Shaltiel S, Vitronectin, The International Journal of Biochemistry & Cell Biology, 31 (1999) 539–544. [DOI] [PubMed] [Google Scholar]
- [190].Foubert P, Varner JA, Integrins in tumor angiogenesis and lymphangiogenesis, Methods Mol Biol, 757 (2012) 471–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Sebak AA, Gomaa IEO, ElMeshad AN, Farag MH, Breitinger U, Breitinger HG, AbdelKader MH, Distinct Proteins in Protein Corona of Nanoparticles Represent a Promising Venue for Endogenous Targeting - Part I: In vitro Release and Intracellular Uptake Perspective, Int J Nanomedicine, 15 (2020) 8845–8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Sebak AA, Gomaa IEO, ElMeshad AN, Farag MH, Breitinger U, Breitinger HG, AbdelKader MH, Distinct Proteins in Protein Corona of Nanoparticles Represent a Promising Venue for Endogenous Targeting - Part II: In vitro and in vivo Kinetics Study, Int J Nanomedicine, 15 (2020) 9539–9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Jiang X, Sha X, Xin H, Xu X, Gu J, Xia W, Chen S, Xie Y, Chen L, Chen Y, Fang X, Integrin-facilitated transcytosis for enhanced penetration of advanced gliomas by poly(trimethylene carbonate)-based nanoparticles encapsulating paclitaxel, Biomaterials, 34 (2013) 2969–2979. [DOI] [PubMed] [Google Scholar]
- [194].Jiang X, Xin H, Ren Q, Gu J, Zhu L, Du F, Feng C, Xie Y, Sha X, Fang X, Nanoparticles of 2-deoxy-D-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment, Biomaterials, 35 (2014) 518–529. [DOI] [PubMed] [Google Scholar]
- [195].Choi J, Rustique E, Henry M, Guidetti M, Josserand V, Sancey L, Boutet J, Coll JL, Targeting tumors with cyclic RGD-conjugated lipid nanoparticles loaded with an IR780 NIR dye: In vitro and in vivo evaluation, Int J Pharm, 532 (2017) 677–685. [DOI] [PubMed] [Google Scholar]
- [196].Caracciolo G, Cardarelli F, Pozzi D, Salomone F, Maccari G, Bardi G, Capriotti AL, Cavaliere C, Papi M, Laganà A, Selective targeting capability acquired with a protein corona adsorbed on the surface of 1,2-dioleoyl-3-trimethylammonium propane/DNA nanoparticles, ACS Appl Mater Interfaces, 5 (2013) 13171–13179. [DOI] [PubMed] [Google Scholar]
- [197].Caracciolo G, Pozzi D, Capriotti AL, Cavaliere C, Piovesana S, Amenitsch H, Laganà A, Lipid composition: a “key factor” for the rational manipulation of the liposome–protein corona by liposome design, RSC Advances, 5 (2015) 5967–5975. [Google Scholar]
- [198].Chen D, Ganesh S, Wang W, Lupieri A, Amiji M, Role of vitronectin-rich protein corona on tumor-specific siRNA delivery and transfection with lipid nanoparticles, Nanomedicine (London, England), 16 (2021) 535–551. [DOI] [PubMed] [Google Scholar]
- [199].Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML, The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer, Clin Immunol, 121 (2006) 144–158. [DOI] [PubMed] [Google Scholar]
- [200].Daniels TR, Delgado T, Helguera G, Penichet ML, The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells, Clin Immunol, 121 (2006) 159–176. [DOI] [PubMed] [Google Scholar]
- [201].Santi M, Maccari G, Mereghetti P, Voliani V, Rocchiccioli S, Ucciferri N, Luin S, Signore G, Rational Design of a Transferrin-Binding Peptide Sequence Tailored to Targeted Nanoparticle Internalization, Bioconjug Chem, 28 (2017) 471–480. [DOI] [PubMed] [Google Scholar]
- [202].Zhang Z, Wang C, Zha Y, Hu W, Gao Z, Zang Y, Chen J, Zhang J, Dong L, Corona-directed nucleic acid delivery into hepatic stellate cells for liver fibrosis therapy, ACS Nano, 9 (2015) 2405–2419. [DOI] [PubMed] [Google Scholar]
- [203].Zhang Z, Guan J, Jiang Z, Yang Y, Liu J, Hua W, Mao Y, Li C, Lu W, Qian J, Zhan C, Brain-targeted drug delivery by manipulating protein corona functions, Nat Commun, 10 (2019) 3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Zhang ZA, Xin X, Liu C, Liu YH, Duan HX, Qi LL, Zhang YY, Zhao HM, Chen LQ, Jin MJ, Gao ZG, Huang W, Novel brain-targeted nanomicelles for anti-glioma therapy mediated by the ApoE-enriched protein corona in vivo, J Nanobiotechnology, 19 (2021) 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Huo T, Yang Y, Qian M, Jiang H, Du Y, Zhang X, Xie Y, Huang R, Versatile hollow COF nanospheres via manipulating transferrin corona for precise glioma-targeted drug delivery, Biomaterials, 260 (2020) 120305. [DOI] [PubMed] [Google Scholar]
- [206].Wu JY, Li YJ, Wang J, Hu XB, Huang S, Luo S, Xiang DX, Multifunctional exosome-mimetics for targeted anti-glioblastoma therapy by manipulating protein corona, J Nanobiotechnology, 19 (2021) 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Giulimondi F, Vulpis E, Digiacomo L, Giuli MV, Mancusi A, Capriotti AL, Laganà A, Cerrato A, Zenezini Chiozzi R, Nicoletti C, Amenitsch H, Cardarelli F, Masuelli L, Bei R, Screpanti I, Pozzi D, Zingoni A, Checquolo S, Caracciolo G, Opsonin-Deficient Nucleoproteic Corona Endows UnPEGylated Liposomes with Stealth Properties In Vivo, ACS Nano, 16 (2022) 2088–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Cui T, Ma Y, Yang JY, Liu S, Wang Z, Zhang F, Wang J, Cai T, Dong L, Hong J, Qian H, Zhang C, Ding Y, Protein corona-guided tumor targeting therapy via the surface modulation of low molecular weight PEG, Nanoscale, 13 (2021) 5883–5891. [DOI] [PubMed] [Google Scholar]
- [209].Luo Z, Lu L, Xu W, Meng N, Wu S, Zhou J, Xu Q, Xie C, Liu Y, Lu W, In vivo self-assembled drug nanocrystals for metastatic breast cancer all-stage targeted therapy, J Control Release, 346 (2022) 32–42. [DOI] [PubMed] [Google Scholar]
- [210].Haque ST, Karim M, Abidin SAZ, Othman I, Holl MMB, Chowdhury EH, Fe/Mg-Modified Carbonate Apatite with Uniform Particle Size and Unique Transport Protein-Related Protein Corona Efficiently Delivers Doxorubicin into Breast Cancer Cells, Nanomaterials, 10 (2020) 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Peng Y, Cong Y, Lei Y, Sun F, Xu M, Zhang J, Fang L, Hong H, Cai T, Transforming Passive into Active: Multimodal Pheophytin-Based Carbon Dots Customize Protein Corona to Target Metastatic Breast Cancer, Adv Healthc Mater, 11 (2022) e2102270. [DOI] [PubMed] [Google Scholar]
- [212].Kim KR, Kim J, Back JH, Lee JE, Ahn DR, Cholesterol-Mediated Seeding of Protein Corona on DNA Nanostructures for Targeted Delivery of Oligonucleotide Therapeutics to Treat Liver Fibrosis, ACS Nano, (2022). [DOI] [PubMed] [Google Scholar]
- [213].Zhao X, Chen J, Qiu M, Li Y, Glass Z, Xu Q, Imidazole-Based Synthetic Lipidoids for In Vivo mRNA Delivery into Primary T Lymphocytes, Angew Chem Int Ed Engl, 59 (2020) 20083–20089. [DOI] [PubMed] [Google Scholar]
- [214].Qiu M, Glass Z, Chen J, Haas M, Jin X, Zhao X, Rui X, Ye Z, Li Y, Zhang F, Xu Q, Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3, Proc Natl Acad Sci U S A, 118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Qiu M, Tang Y, Chen J, Muriph R, Ye Z, Huang C, Evans J, Henske EP, Xu Q, Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis, Proc Natl Acad Sci U S A, 119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Li S, Li M, Huo S, Wang Q, Chen J, Ding S, Zeng Z, Zhou W, Wang Y, Wang J, Voluntary‐Opsonization‐Enabled Precision Nanomedicines for Inflammation Treatment, Advanced Materials, 33 (2021) 2006160. [DOI] [PubMed] [Google Scholar]
- [217].Benne N, van Duijn J, Lozano Vigario F, Leboux RJT, van Veelen P, Kuiper J, Jiskoot W, Slutter B, Anionic 1,2-distearoyl-sn-glycero-3-phosphoglycerol (DSPG) liposomes induce antigen-specific regulatory T cells and prevent atherosclerosis in mice, J Control Release, 291 (2018) 135–146. [DOI] [PubMed] [Google Scholar]
- [218].Lechuga-Vieco AV, Groult H, Pellico J, Mateo J, Enríquez JA, Ruiz-Cabello J, Herranz F, Protein corona and phospholipase activity drive selective accumulation of nanomicelles in atherosclerotic plaques, Nanomedicine, 14 (2018) 643–650. [DOI] [PubMed] [Google Scholar]
- [219].Jo DH, Kim JH, Son JG, Dan KS, Song SH, Lee TG, Kim JH, Nanoparticle-protein complexes mimicking corona formation in ocular environment, Biomaterials, 109 (2016) 23–31. [DOI] [PubMed] [Google Scholar]
- [220].Astarita C, Palchetti S, Massaro-Giordano M, Di Domenico M, Petrillo F, Boffo S, Caracciolo G, Giordano A, Artificial Protein Coronas Enable Controlled Interaction with Corneal Epithelial Cells: New Opportunities for Ocular Drug Delivery, Pharmaceutics, 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].García Vence M, Chantada-Vázquez MDP, Vázquez-Estévez S, Manuel Cameselle-Teijeiro J, Bravo SB, Núñez C, Potential clinical applications of the personalized, disease-specific protein corona on nanoparticles, Clin Chim Acta, 501 (2020) 102–111. [DOI] [PubMed] [Google Scholar]
- [222].Caputo D, Papi M, Coppola R, Palchetti S, Digiacomo L, Caracciolo G, Pozzi D, A protein corona-enabled blood test for early cancer detection, Nanoscale, 9 (2017) 349–354. [DOI] [PubMed] [Google Scholar]
- [223].Ren J, Cai R, Wang J, Daniyal M, Baimanov D, Liu Y, Yin D, Liu Y, Miao Q, Zhao Y, Chen C, Precision Nanomedicine Development Based on Specific Opsonization of Human Cancer Patient-Personalized Protein Coronas, Nano Lett, 19 (2019) 4692–4701. [DOI] [PubMed] [Google Scholar]
- [224].Corbo C, Li AA, Poustchi H, Lee GY, Stacks S, Molinaro R, Ma P, Platt T, Behzadi S, Langer R, Farias V, Farokhzad OC, Analysis of the Human Plasma Proteome Using Multi-Nanoparticle Protein Corona for Detection of Alzheimer’s Disease, Adv Healthc Mater, 10 (2021) e2000948. [DOI] [PubMed] [Google Scholar]
- [225].Lu X, Xu P, Ding HM, Yu YS, Huo D, Ma YQ, Tailoring the component of protein corona via simple chemistry, Nat Commun, 10 (2019) 4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Raesch SS, Tenzer S, Storck W, Rurainski A, Selzer D, Ruge CA, Perez-Gil J, Schaefer UF, Lehr C-M, Proteomic and Lipidomic Analysis of Nanoparticle Corona upon Contact with Lung Surfactant Reveals Differences in Protein, but Not Lipid Composition, ACS Nano, 9 (2015) 11872–11885. [DOI] [PubMed] [Google Scholar]
- [227].Caracciolo G, Farokhzad OC, Mahmoudi M, Biological Identity of Nanoparticles In Vivo: Clinical Implications of the Protein Corona, Trends Biotechnol, 35 (2017) 257–264. [DOI] [PubMed] [Google Scholar]
- [228].Abbina S, Takeuchi LE, Anilkumar P, Yu K, Rogalski JC, Shenoi RA, Constantinescu I, Kizhakkedathu JN, Blood circulation of soft nanomaterials is governed by dynamic remodeling of protein opsonins at nano-biointerface, Nat Commun, 11 (2020) 3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Mahmoudi M, The need for improved methodology in protein corona analysis, Nat Commun, 13 (2022) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]


