Abstract
Listeria monocytogenes is a gram-positive, facultative intracellular pathogen that can cause severe food-born infections in humans and animals. We have adapted signature-tagged transposon mutagenesis to L. monocytogenes to identify new genes involved in virulence in the murine model of infection. We used transposon Tn1545 carried on the integrative vector pAT113. Forty-eight tagged transposons were constructed and used to generate banks of L. monocytogenes mutants. Pools of 48 mutants were assembled, taking one mutant from each bank, injected into mice, and screened for those affected in their multiplication in the brains of infected animals. From 2,000 mutants tested, 18 were attenuated in vivo. The insertions harbored by these mutants led to the identification of 10 distinct loci, 7 of which corresponded to previously unknown genes. The properties of four loci involving putative cell wall components were further studied in vitro and in vivo. The data suggested that these components are involved in bacterial invasion and multiplication in the brain.
Listeria monocytogenes is a gram-positive bacterium that is widespread in nature and responsible for sporadic severe infections in humans and other animal species (reference 5 and references therein). This pathogen is a facultative intracellular microorganism capable of invading most host cells, including epithelial cells (20), hepatocytes (15, 22), fibroblasts (28), endothelial cells (16), and macrophages (31). Each step of the intracellular parasitism by L. monocytogenes is dependent upon the production of virulence factors (44). The major virulence genes (hly, plcA, plcB, mpl, actA, inlA, and inlB) are clustered into two distinct loci on the chromosome and are controlled by a single pleiotropic regulatory activator, PrfA, which is required for the virulence (10, 29, 41).
Transposon mutagenesis was the only successful strategy used so far to identify virulence genes in L. monocytogenes. In order to identify new genes involved in the infectious process, we have adapted signature-tagged mutagenesis (STM) to L. monocytogenes. The STM technique is a powerful method that allows a large number of mutants to be screened for attenuation. It was initially used to identify virulence genes in Salmonella enterica serovar typhimurium (23, 43) and more recently used with other bacterial species, including Staphylococcus aureus (13, 32), Vibrio cholerae (11), Streptococcus pneumoniae (35), Yersinia enterocolytica (14), Proteus mirabilis (52), Legionella pneumophila (17), and Brucella abortus (19), as well as in the fungus Aspergillus fumigatus (8).
We have adapted for the first time STM to L. monocytogenes. We focus here on the mutants affected in their multiplication in the brains of infected animals in order to identify genes possibly involved in passage across the blood-brain barrier. We have used transposon Tn1545, carried on the integrative vector pAT113 (47), to generate banks of L. monocytogenes mutants. Pools of mutants were assembled and screened in vivo in the mouse model of infection (Fig. 1). Because each transposon carried a unique sequence tag, the individual clones within each pool could be distinguished from one another by detection of these tags using hybridization. Two-thousand mutants of L. monocytogenes were screened for the loss of virulence. The transposon insertion sites were determined and compared with the genomic sequence of L. monocytogenes. This work was undertaken while the Listeria genome project was in progress. The sequence of the entire genome of EGD (1/2a serotype) is now completed, which allowed us to identify unambiguously all of the genes corresponding to the sequences that were determined.
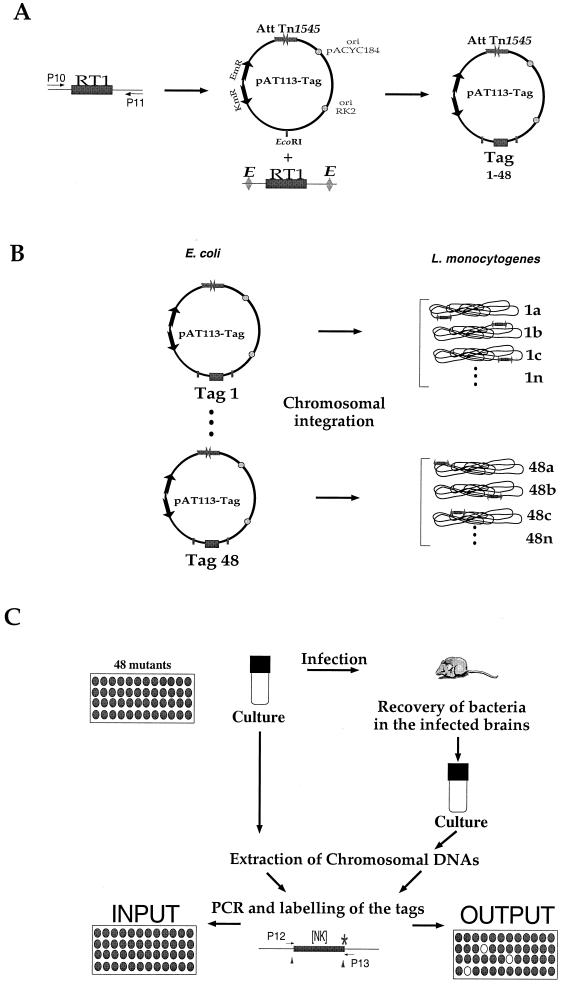
FIG. 1.
Plasmids, primers, and screening strategy. (A) Construction of the tagged transposons. Schematic map of RT1 (P10 and P11 are the primers used for amplification the random oligonucleotide signature tag) and of the integrative plasmid pAT113. After digestion with EcoRI, PCR-amplified RT1 was introduced into the EcoRI site of pAT113 (tags 1 to 48 symbolize the different tags cloned). The 48 tagged pAT113 plasmids were constructed in E. coli. Abbreviations: AttTn1545, attachment site of Tn1545; ori (RK2 and pACYC184), origin of replication; EmR, resistance to ERY encoded by the erm gene, KmR, KAN restance encoded by the aphA-3 gene. (B) Generation of the banks of L. monocytogenes mutants. The 48 tagged pAT113 plasmids were introduced individually into L. monocytogenes to generate 48 banks of mutants (numbered 1 to 48); a, b, c, and n represent the different clones within each bank (i.e., carrying the same tag). (C) Screening strategy. The basic STM schema was adapted. Pools of 48 L. monocytogenes mutants were screened in vivo. Four days after inoculation, the brains of infected mice were recovered and used to inoculate bacterial cultures. The extracted chromosomal DNAs were then used for PCR amplification of the tags. [NK], the random portion of the tag that was amplified using primers P12 and P13.
MATERIALS AND METHODS
Bacterial strains, phages, plasmids, media, and DNA techniques.
Escherichia coli recombinants were grown in Luria-Bertani (LB) medium, and L. monocytogenes was grown in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.) at 37°C. The wild-type virulent strain of L. monocytogenes EGD belongs to the serovar 1/2a (21). EGD was transformed with the different recombinant plasmids by electroporation as previously described (34). Antibiotics were used at the following concentrations: kanamycin (KAN), 50 μg ml−1, and erythromycin (ERY), 8 μg ml−1.
The phages used in this work have been described elsewhere (24, 51). The phages were propagated in strain EGD (serotype 1/2a). Infection assays and PFU determinations were performed as previously described (24).
Chromosomal DNA, plasmid isolation, restriction enzyme analyses, and PCR amplifications were performed according to standard protocols (2, 42). Oligonucleotides were synthesized by Genset (Paris, France). The AmpliTaq DNA polymerase of Thermus aquaticus from Finnzymes OY (Espoo, Finland) was used.
Production and cloning of the tags.
A pool of single-stranded 89-bp DNA molecules containing a central stretch of 40 random base pairs flanked by two invariant sequences was generated by oligonucleotide synthesis (5′-CTAGAAT TCTACAACCTCAAGCTT[NK]20AAGCTTGGTTAGAATGGAATTCATG- 3′). This oligonucleotide (RT1 in Fig. 1) is very similar to the RT1 designed by Hensel et al. (23) except that EcoRI sites were introduced at both extremities (the variable portion is flanked by HindIII restriction sites). Double-stranded DNA tags were generated by PCR amplification by using RT1 as a template and p10 (5′-CTAGAATTCTACAACCTCAAGCTT-3′) and p11 (5′-CATGAATTCCATTCTAACCAAGCTT-3′) as primers. The PCR was performed on a Perkin-Elmer DNA thermal cycler 2400 apparatus (Perkin-Elmer Corp., Norwalk, Conn.) as described previously (23) in a final volume of 100 μl containing 200 pg of RT1, 250 μM concentrations of each deoxynucleotide, 100 pmol of the primers and 2.5 U of DNA polymerase.
PCR products were purified on agarose gels, digested with EcoRI, and ligated into the EcoRI site of pAT113. The ligation mixture was introduced in E. coli by electroporation, and transformants were selected on LB medium containing KAN. Clones carrying tagged pAT113 plasmids were screened by colony PCR by using primers APH-CDS and RK2-F. A series of eight randomly chosen tagged plasmids was rapidly checked by sequencing by using the primers APH-CDS (5′-CTGCGTCCGGTCGATCAGGGA-3′) and RK2-F (5′-ACACCCGCTCGCGGGTGGGCC-3′) flanking the EcoRI insertion site. Sequence comparison of the eight tags confirmed the hypervariability of the 40-bp central portion (data not shown).
Integration into the chromosome of L. monocytogenes.
A total of 2 μg of Qiagen purified recombinant plasmid was used per transformation. The recipient strain was EGD carrying pAT145. Transformants were selected on BHI-ERY solid medium at 37°C. Although the transposition event of Tn1545 is expected to occur at a high frequency (10−1 to 10−2), the yield of transformants obtained was lower than that obtained with plasmid DNA able to replicate in L. monocytogenes. In these conditions, generally 50 transformants were obtained per transformation. In order to check that pAT113 had no hot spot of insertion in the chromosome of L. monocytogenes, 12 randomly chosen mutants from the same bank (i.e., carrying the same tag) were tested by Southern blot. The assay showed that there was only one Tn1545 insertion in each of the mutants tested and that the insertions were apparently randomly distributed in the chromosome of L. monocytogenes (not shown).
Probe labeling and purification.
Double-stranded DNA probes were radioactively labeled by incorporation of 32P-labeled dCTP. A total of 50 pmol of primers P12 (5′-ATTCTACAACCTCAAGC-3′) and P13 (5′-ATTCCATTCTAACCAAGC-3′) and 50 μCi of radiolabeled dCTP were included in each reaction, using the Amersham Megaprime kit. Probes were then purified by passage through Sephadex Nick Columns (Amersham Pharmacia Biotech) and restricted with HindIII in order to eliminate the invariant arms (see Fig. 1).
Dot blot hybridization.
A series of membranes containing the 48 different tags was produced by amplifying the different plasmid-borne tags with primers APH-CDS and RK2-F (producing an 800-bp product). The amplified fragments were first denatured for 10 min at 100°C and then neutralized before transfer onto Hybond N+ membranes (Amersham) using a BioDot Apparatus (Bio-Rad). The membranes were finally fixed by incubation for 2 h at 80°C before hybridization.
The specificity of the hybridization signal was checked by spotting onto each input membrane plasmid DNA from five additional tags that had not been used for the generation of the mutant bank, as well as DNA from pAT113 (plasmid without tag). In all the assays, no nonspecific cross-hybridization was observed with these spots. However, since we did not systematically check each probe with the 47 other tags, we cannot exclude that, for some of them, a certain level of cross-hybridization existed which might have hindered the identification of attenuated mutants.
Identification of attenuated mutants. (i) PCR amplification.
Chromosomal DNA from each culture generated after in vivo passage were prepared, and the tags were amplified by PCR with primers P12 and P13. PCR products were purified on 2% agarose gels and radioactively labeled as described above.
(ii) Dot blot analysis.
Membranes coated with the 48 different tags were hybridized with the labeled probes amplified from each of the output pools. Only the mutants that gave a negative signal were further tested (the labeled probe amplified from the input pool served as a positive control). Two different labeled probes were prepared for several output pools. In each case, the two probes gave similar hybridization signals, thus confirming the reproducibility of the assay.
Southern blot analysis.
L. monocytogenes chromosomal DNAs were prepared as previously described (37). Briefly, chromosomal DNAs were digested with different enzymes (i.e., BglII, PstI, and SacI) and transferred onto nylon Hybond N+ membranes (Amersham) under denaturing conditions. Hybridizations were realized under high-stringency conditions (prehybridization at 65°C for 3 h in 6× SSC [1× SSC is NaCl at 0.15 M plus sodium citrate at 15 mM]–0.5% sodium dodecyl sulfate [SDS]–0.05% Régilait buffer); hybridization was carried out overnight at 65°C in 6× SSC–0.1% SDS–0.05% Régilait buffer, with two 30-min washes at room temperature in 2× SSC–0.1% SDS, followed by two 30-min washes at 65°C in 0.2× SSC–0.1% SDS. Hybridization was detected on Hyperfilm-MP films (Amersham).
The probe used corresponds to a 0.7-kb internal portion of the plasmid pAT113 located between the ori RK2 and the multiple cloning site (MCS) (Fig. 1). The fragment was amplified by PCR using primer RK2-F and the forward primer of mp18-pUC18 (sequencing primer 1211; NEN/Biolabs), digested with HindIII (downstream of the MCS) to remove the region of the polylinker, and 32P labeled using the Megaprime Kit (Amersham).
RT-PCR.
Total RNA was extracted from L. monocytogenes cultures grown overnight in BHI broth at 37°C. The primers used to amplify the different mRNA from EGD by reverse transcription-PCR (RT-PCR) are listed below. The procedure used was that described in the kit SuperScript One-Step RT-PCR System (Life Technologies, Paisley, Scotland). Prior to RT-PCR, total RNA samples were incubated for 1 h at 37°C with DNase I-RNase-free (Boehringer, Mannheim, Germany) to eliminate any DNA contamination. The following primers were used: Pbpx (5′-ATGAATCGACGCGAAAGACG-3′ and 5′-TTCCGAATGAAGCAATGTAG-3′), ORF626 (5′-GGAGTTTCCCATGGTGATATTCCAATTACC-3′ and 5′-CTACATTACCAGTTAAGTTATCGGAAG-3′), Ptb (5-GCAGTTGCCGGCGCAGATGA-3′ and 5′-CTTACAGTTAGTATGAATGAAGC-3′), YtgP (5′-CGGAATGCAAGAGGTTAGTGGTGGGAC-3′ and 5′-GTAGTAACATCGAACCATCTGTATTATAAC-3′), ORF483 (5-GGATTATCACTAATCGAATCGTCCGC-3′ and 5′-GCTGTTGATTTTCTCAGCTCTTGCATATAC-3′), CelR (5-GGCGAGATGAAAGTATCACTTAAAAAC-3′ and 5′-CATGCAACTTCTCCTCACGCTGATTAC-3′), RecQ (5′-GGTAGGTTCATTCAAGAAGCCAAATTT-3′ and 5′-GTTTTGCGTTGTTCAGTCGTCAGATTC-3′), and GtcA (5′-CGGAGAGAAAGAAGACATAGT-3′ and 5′-TTATTTTTCCACTTTGAAAATGATCC-3′).
Sequence analysis and identification of the transposon insertion site in the Listeria genome. (i) Inverse PCR.
Chromosomal DNA from Listeria cells was digested with Sau3A. The digestion products were ligated, generating circular molecules containing either the right end or the left end of Tn1545. DNA was amplified by PCR using the primers SeqR (5′-CGTGAAGTATCTTCCTACAGT-3′) and SeqR2 (5′-CTACTACTAAGCAACAAGACGC-3′).
(ii) PCR sequencing.
The PCR products were sequenced with the automated ABI Prism 310 sequencer (Perkin-Elmer/Applied Biosystems) using the BigDye Terminator Cycle Sequencing Ready Reaction kit. Sequences were analyzed with the Sequence Navigator software program (Perkin-Elmer). Similarity searches were then performed via the Internet with BLAST software (1) from the National Center for Biotechnology Information (www.ncbi.nlm.gov./BLAST/) by launching the sequences in the complete 2,900,000-bp Listeria genome database (BLASTn search). The identified open reading frames (ORFs) were then launched in the general databases (nonredundant BLASTp search).
Infection of mice. (i) In vivo screening.
In order to limit a possible counterselection of some mutants in a pool upon simultaneous growth, the pools were prepared in two steps: first, each mutant was inoculated individually in 200 μl of BHI-ERY and grown overnight at 37°C without agitation in microtitration plates. Then, a 100-μl fraction from each culture was collected and mixed, and the pool was used to inoculate an 8-h culture in BHI-ERY at 37°C with agitation. Each pool was injected into two mice at a dose of 106 bacteria per mouse. The brains of the infected animals were recovered 4 days later and pooled. Half of the brain homogenate was then used to inoculate an overnight culture in BHI-ERY medium at 37°C with agitation for chromosomal DNA preparation (see above).
(ii) Attenuated mutants.
Bacteria were grown in BHI medium overnight at 37°C with agitation. Attenuation was evaluated by injecting individually each candidate clone at the lethal dose of 106 bacteria per mouse (four mice per mutant). Only 18 of the 60 candidates tested appeared to be attenuated, i.e., the animals showed 75 to 100% survival.
The 50% lethal dose (LD50) was determined by the probit method by inoculating serial tenfold dilutions of each mutant.
(iii) Kinetics studies.
Twenty-five mice were inoculated intravenously (i.v.) in the lateral tail vein per mutant. At days 1, 2, 3, 4, and 6, groups of five mice were sacrificed, and the organs (spleen, liver, and brain) were aseptically removed and separately homogenized in 0.15 M NaCl. Bacterial numbers in organs homogenates were determined at various intervals on BHI plates containing ERY. Five-hundred microliters of bacterial suspension containing 106 bacteria was injected per mouse.
In all the assays, bacteria were stored in 2-ml fractions at −80°C after growth, and the number of bacterial per milliliter in each defrosted culture was determined before inoculation.
Hemolysis.
The clones from each bank in L. monocytogenes were screened for hemolytic phenotype onto horse blood agar plates (BioMerieux). Only the hemolysin-positive clones were further used for in vivo studies.
Adhesion and invasion assays. (i) Culture of cell lines.
The human colon carcinoma cell line Caco-2 (ATCC HTB37), the human hepatocellular carcinoma cell line HepG-2 (ATCC HB 8065; obtained from S. Dramsi and P. Cossart, Institut Pasteur, Paris, France), the human cervical epithelial cell line HeLa, and the African green monkey kidney cell line Vero were propagated as previously described (33) in Dulbecco modified Eagle medium (DMEM; Gibco) plus 25 mM glucose. The mouse macrophage cell line J774 was grown as described elsewhere (45). All incubations were carried out in a 10% CO2 atmosphere at 37°C. Cells were seeded at 8 × 104 cells cm−2 in 24-well tissue culture plates (Falcon). Monolayers were used 24 h after seeding.
(ii) Invasion assays.
The invasion assays were carried out essentially as described earlier (33). Briefly, cells were inoculated with bacteria at a multiplicity of infection of approximately 200 bacteria per cell. Cells were incubated for 1 h to allow the adherent bacteria to enter and were then washed twice with phosphate-buffered saline (PBS) and overlaid with fresh DMEM containing gentamicin (10 mg liter−1) to kill the extracellular bacteria. At intervals, cells were washed twice and processed to count the infecting bacteria. For that, cells were lysed by adding cold distilled water. The titer of viable bacteria released from the cells was determined by spreading them onto BHI-ERY plates. Each experiment was carried out in triplicate and repeated two to three times.
RESULTS
We have used pAT113, a system that takes advantage of the transposition properties of Tn1545 carried on a plasmid containing the origin of replication of pACYC184 (enabling it to replicate in E. coli), the attachment site of Tn1545 and ERY and KAN resistance-encoding genes (47). Chromosomal integration of the tagged transposons was achieved by the presence, in the recipient L. monocytogenes strain, of the transposon-encoded integrase provided in trans by plasmid pAT145.
Construction of tagged L. monocytogenes mutant libraries and screening of the attenuated mutants. (i) Construction of the libraries.
Double-stranded DNA tags (Fig. 1) were cloned into the EcoRI restriction site of plasmid pAT113. The E. coli recombinant clones carrying tagged pAT113 plasmids were screened by colony PCR by using a pair of primers flanking the EcoRI insertion site (see Materials and Methods for details). As previously reported (11, 23), we found that the different tags do not amplify evenly. Therefore, in order to select 48 tags that amplified and labeled efficiently, we first constructed 96 transposon-tagged recombinant plasmids and tested them by dot blot hybridization (not shown). The 48 tagged plasmids selected, each carrying a different tag, were introduced individually by electroporation into EGD carrying pAT145, thus generating 48 banks of L. monocytogenes mutants.
(ii) In vivo screening.
We chose here to eliminate from the banks the nonhemolytic clones, which are likely to correspond to the disruption of the hly gene encoding listeriolysin O (21). Therefore, the mutant banks of L. monocytogenes were first screened on horse blood agar, and only the clones showing a normal hemolytic pattern were further used in the in vivo screening.
One important limitation for applying STM to in vivo screening is that the inoculated (or input) pool must have a dose such that each mutant is well represented and is able to establish an infection. Likewise, the recovered (or output) pool requires an adequate amount of each insertion mutant to be recovered and be represented in order to avoid a false-negative result (3, 11). We used an infecting dose of 106 bacteria per mouse to ensure a starting inoculum of approximately 2 × 104 bacteria per mutant in each pool. Forty-one pools of 48 mutants of L. monocytogenes were generated, and each pool was injected into two mice at a dose of 106 bacteria per mouse (see Materials and Methods). At this dose, the mice infected with the virulent strain (EGD) die 4 to 5 days after infection (4), and at day 4 the average number of bacteria reaches up to 105 per brain. Infected brains were collected, and overnight cultures in BHI-ERY inoculated with brain homogenates were prepared. Chromosomal DNA from the resulting bacterial cultures (from the output pool) were extracted (Fig. 1). In parallel, chromosomal DNA from the input pool was also prepared. The chromosomally inserted tags were amplified by PCR with primers P12 and P13 (see above and Fig. 1) from chromosomal DNAs of the input and output pools. The two types of probes were then radiolabeled and used for hybridization on nylon membranes coated with the 48 tags.
Sixty putative candidates that showed no or only a weak reaction with the probe from the output pool (ca. 3% of the clones tested) were identified by this procedure and further tested individually for attenuation in vivo. Attenuation was evaluated by injecting each mutant at the lethal dose of 106 bacteria per mouse (four mice per mutant). Of the 60 candidates tested, 18 led to 75 to 100% of survival and were considered to be attenuated. The 18 clones are listed in Table 1.
TABLE 1.
Analysis of attenuated strains of L. monocytogenes identified by STM
| Mutanta | Disrupted or adjacent ORFb | Homologc | Putative functiond | Expression (RT)e | Phage sensitivityf | LD50 (log mean CFU)g |
|---|---|---|---|---|---|---|
| H41 | 1669.1 | GtcA | Cell wall decoration | + | R | 6.4 |
| S22 | 1669.1 | GtcA | Cell wall decoration | |||
| K′28 | 1669.1 | GtcA | Cell wall decoration | |||
| Y6 | 1669.1 | GtcA | Cell wall decoration | |||
| Q11 | 1669.1 | GtcA | Cell wall decoration | |||
| H′49 | 2808.1 | YtgP | Integral membrane protein | + | S | 6.4 |
| D3 | 2808.1 | YtgP | Integral membrane protein | |||
| V4 | 2808.1 | YtgP | Integral membrane protein | |||
| E26 | 2808.1 | YtgP | Integral membrane protein | |||
| A23 | 1012.1 | PbpX | Membrane-anchored protein | + | S | 6.6 |
| I′2 | 2504.1 | ORF626 | Membrane-anchored protein | + | S | 6.1 |
| D22 | 4183.1 | Dfp | Adhesin | NT | S | 5.3 |
| A′24 | 1518.1 | CelR | Transcription regulation | + | S | 6 |
| O′15 | 1518.1 | CelR | Transcription regulation | |||
| C43 | 3360.1 | RecQ | Recombination | + | S | 6 |
| D38 | 835.1 | AttM | Antigenic peptide | NT | S | 5.7 |
| D′4 | 835.1 | AttM | Antigenic peptide | |||
| F′25 | 2271.1 | Ptb | Metabolism | + | S | 5.8 |
| D7 | 156.1 | ORF483 | Unknown | + | S | 5.6 |
Names of the L. monocytogenes Tn1545 insertion mutants.
Number attributed to the ORF in the Listeria genome web site.
Name of the ORF sharing the highest similarity with the identified ORF.
Proposed function for the ORF on the basis of sequence similarity.
+, Detection of a band by RT-PCR (Fig. 5); NT, not tested.
Phage sensitivity toward LMUP121 assayed by the spot test procedure. R, resistant; S, sensitive.
LD50 (in log mean CFU) assays performed on female Swiss mice.
Sequence analyses.
The mutation leading to the attenuated phenotype was mapped precisely using amplification of the region adjacent to the Tn1545 insertion by inverse PCR, sequencing, and comparison with the L. monocytogenes genome sequence. It has been shown that integration of Tn1545 occurs via reciprocal site-specific recombination between nonhomologous DNA sequences that are designated overlap sequences (48). Although Tn1545 exhibits no apparent preference for a particular target sequence, it shows a strong preference for insertion in AT-rich regions and integrates into attachment sites bearing short segments of homology with the end of the element (36, 48). Our sequence analyses fully supported this notion, revealing in each case a transposon insertion at a TAAAA site. Unexpectedly, several independently isolated insertions were in the same genes (Table 1). This might reflect the existence of hot spots of insertion, but it could also be due to a bias inherent to the STM. Indeed, this observation has been repeatedly reported with STM, like for examples with Mycobacterium tuberculosis (9) and Y. enterocolitica (14).
The sequence of the 18 attenuated clones led to the identification of 10 distinct loci (Table 1). Five loci correspond to putative cell wall components, and five involve proteins participating in a variety of cellular processes, such as recombination, transcriptional regulation, or metabolism. These two sets of genes will be described separately below. Only three of these loci correspond to genes of L. monocytogenes previously sequenced (AttM, Dfp, and GtcA). The mutants were designated by the name of the ORF product with the highest similarity in the databases. When no homologous protein was found, it was named ORFn, where n is the number of predicted residues.
Insertions into genes encoding putative cell wall components. (i) Locus gtcA.
We found five independent Tn1545 insertions in gtcA (Fig. 2): three mutants (H41, S22, and K′28) were identical, one mutant (Q11) corresponded to a Tn1545 insertion at the same site but in the opposite orientation, and the fifth mutant (Y6) had an insertion 4 bp downstream from the site of H41 and S22. The five mutants showed an attenuated phenotype in vivo. Strikingly, Kathariou and coworkers (39) recently identified the gtcA gene of L. monocytogenes serotype 4b by screening a bank of mutants (generated by Tn916 mutagenesis) for loss of serotype-specific monoclonal antibody recognition.
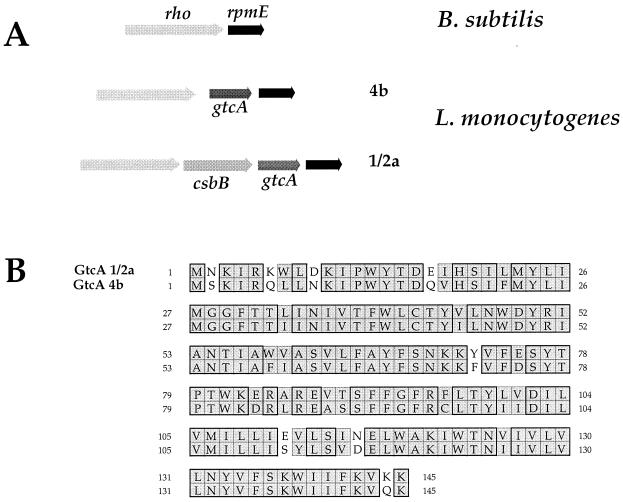
FIG. 2.
Locus gtcA. (A) Schematic organization of the gtcA region. (Upper part) rpmE-rho region of B. subtilis. The rpmE is shaded in dark gray; the rho is shaded in light gray. (Middle part) rpmE-gtcA-rho region of L. monocytogenes serotype 4b. (Lower part) rpmE-csbB-gtcA-rho region of L. monocytogenes serotype 1/2a. (B) Sequence alignment of the GtcA from serotype 4b and 1/2a strains. Sequence alignment was performed using the CLUSTALw algorithm (www.infobiogen.fr/services/analyseq/cgi-bin/clustalw_in.pl). Similar residues are shaded, and identical residues are boxed (thick lines). The numbers refer to amino acid length.
Sequence comparison between the GtcA proteins of serotypes 4b and 1/2a revealed a high degree of homology. Both proteins, which are composed of 145 residues, share 82% identity (Fig. 2). Of particular interest, the gtcA gene is preceded by an ORF (csbB) only in the 1/2a serotype, encoding a putative dolichol phosphate mannose synthase. This ORF shares 43% identity with CsbB of B. subtilis, a 329-residue protein possibly involved in peptidoglycan biosynthesis (25). In the 4b serotype, the G+C content of gtcA was found to be noticeably lower (ca. 30%) than the average G+C content of L. monocytogenes and in particular lower than that of the two flanking genes rho and rpmE (ca. 40%) (39). In serotype 1/2a, both csbB and gtcA have lower G+C contents (33 and 31%, respectively) than the flanking genes (39% for the two upstream ORFs and 40% for the downstream one). In B subtilis, rho is immediately followed by rpmE (Fig. 2). It thus appears that some Listeria strains have acquired (by an as-yet-unknown mechanism) gtcA alone and that others have acquired both csbB and gtcA. The role of this additional gene remains to be elucidated.
(ii) Locus ytgP.
We found four identical independent Tn1545 insertions in an ORF which determines a putative 537-residue protein. This protein shares 42% identity over its entire sequence with YtgP, a putative transmembrane protein of B. subtilis, which is possibly involved in polysaccharide biosynthesis. Of interest, immediately downstream of the inactivated gene (200 bp), a second ORF encodes another YtgP-like protein of identical size, sharing 40% identity with YtgP. The two ORFs are also highly similar with one another (57% identity). The Toppred2 algorithm (http://www.sbc.su.se/∼erikw/toppred2/toppredServer.cgi) predicts with certainty 14 transmembrane segments for the two ORFs, suggesting that they are integral membrane proteins.
(iii) Locus pbpX.
Mutant A23 corresponds to an insertion immediately upstream of a gene encoding a putative penicillin-binding protein of 397 residues sharing 47% identity with PbpX of B. subtilis. This ORF shared also 31% identity with FmtA of S. aureus, which is identical in size. This non-penicillin-binding protein was shown to be located in the membrane and responsible for alterations in methicillin resistance. Moreover, an FmtA mutation affected the cell wall structure (27). Hence, PbpX of L. monocytogenes protein might be involved in cell wall synthesis.
(iv) Locus orf626.
Mutant I′2 corresponds to an insertion immediately upstream of an ORF encoding a 626-residue protein of unknown function. The putative ORF that did not show any significant similarity with other proteins in the databases contains five leucine-rich repeats and a membrane-anchor LPxTG motif (residues 589 to 593) in its C-terminal part, preceding a predicted hydrophobic membrane-spanning region (residues 599 to 619). Such motifs are generally found in membrane-anchored adhesins of gram-positive bacteria. In particular, in Listeria spp., they are found in proteins of the internalin family (12). As in the membrane-anchored protein, the SignalP program (http://www.cbs.dtu.dk/services/SignalP/) predicts a putative signal sequence in the 27 N-proximal residues of this ORF. ORF626 might thus be a new type of cell-wall-anchored protein involved in the binding and entry of L. monocytogenes.
(v) Locus dfp.
The Tn1545 had inserted 29 bp upstream of an ORF similar to the dfp gene from B. subtilis (60% identity at the DNA level). Strikingly, Milohanic et al. (33) have very recently identified an adhesion-defective mutant of L. monocytogenes (G48) generated by Tn1545 mutagenesis of a inlAB deletion mutant and isolated after in vitro screening that was also located immediately upstream from the dfp gene (only 10 bp downstream from the dfp gene located in mutant D22). In E. coli, the dfp genes encodes a 45-kDa flavoprotein involved in DNA synthesis.
Other insertion sites. (i) Locus celR.
In mutants A′24 and O′15 the Tn1545 was located in the proximal part of an ORF encoding a 641-amino-acid putative transcriptional regulator which shared moderate similarity (23% identity over the entire sequence) with CelR, the putative cel operon regulator of B. subtilis. In L. monocytogenes, this ORF is followed by three consecutive ORFs encoding putative IIA, IIC, and IIB components of a fructose-like transporter (Fig. 3). In B. subtilis, the phosphoenolpyruvate-sugar phosphotransferase system (PTS)-specific fructose transporter includes three permeases each containing the three domains IIA, IIB, and IIC that reside on a single polypeptide chain: MtlA, ManP, and FruA (40). Strikingly, in L. monocytogenes, the three proteins encoded by the genes located immediately downstream of celR are closely related to FruA of B. subtilis, each of them corresponding to a single domain of the permease (with 34, 44, and 52% identities between the IIA, IIC, and IIB domains, respectively).
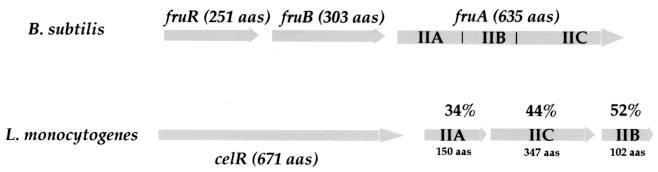
FIG. 3.
Locus celR. (Upper part) Schematic organization of the celR locus in B. subtilis. The three domains comprised within the FruA polypeptide are indicated (IIA, IIB, and IIC). (Lower part) Schematic organization of the celR locus in L. monocytogenes. The three genes downstream of celR determine three ORFs that share 34, 44, and 52% identities, respectively, with the corresponding domains of the FruA protein of B. subtilis. The arrows indicate the approximate size and orientation of the different genes.
(ii) Locus recQ. Mutant C43 corresponds to an insertion into the distal portion of an ORF encoding a putative protein of 467 amino acids sharing 43% identity with RecQ (or RecS) of B. subtilis, an ATP-dependent DNA helicase involved in DNA repair and intramolecular recombination (P50729) (18). This ORF also shares 39% with RecQ, the DNA helicase of E. coli which was shown to participate in the RecF recombination pathway (49).
(iii) Locus attM. Two independent insertions were found to lie upstream of the lapA gene (138 and 228 bp upstream of the first predicted ATG start codon, respectively). Of particular interest, Princiotta et al., (38) recently identified a 23-amino-acid antigenic polypeptide of L. monocytogenes (named AttM) that is presented by the nonpolymorphic murine major histocompatibility complex class Ib molecule H2-M3 (38). The AttM polypeptide is encoded by an ORF located immediately upstream of lapA and is preceded by an RNA polymerase binding site consensus sequence. Analysis of the mRNA secondary structure of this region suggested that this polypeptide could be a leader peptide encoded by a transcriptional attenuator. The AttM polypeptide did not appear to be either a membrane protein or specifically targeted for secretion by L. monocytogenes. However, the fact that antigenic activity could be recovered from the culture medium indicated that AttM was released by the bacteria. The two Tn1545 insertion mutants identified fell into the promoter region of attM. The LapA protein did not show any striking similarities with other proteins in the databases, except for a modest similarity (23% identity over the entire sequence) with Fnr, a transcription regulator of Bacillus licheniformis.
(iv) Locus ptb.
Tn1545 had inserted into the predicted Shine-Dalgarno sequence (AGGAGT) an ORF encoding a putative protein sharing 44% identity over its entire sequence with Ptb, a putative phosphotransacylase of B. subtilis, and 42% identity with the Ptb protein of Enterococcus faecalis. Of interest, analysis of the genes adjacent to ptb suggests that, as in E. faecalis, the ptb gene of L. monocytogenes could be the first gene of a cluster of six genes encoding the enzymes that constitute a catabolic pathway for the branched-chain α-keto acid (50). In that study Ward et al. demonstrated that this cluster formed an operon, and they identified its transcriptional start site upstream of the ptb gene. A putative promoter sequence was predicted upstream of ptb in L. monocytogenes (with a score of 1/1) with the Promoter Prediction by Neural Network program (http://www.dot.imgen.bcm.tmc.edu:9331/seq-search/gene-search).
(v) Locus orf483.
Tn1545 had inserted immediately upstream of an ORF encoding a putative protein of 483 residues of unknown function that did not show any homology with other proteins in the databases.
Characterization of the attenuated mutants.
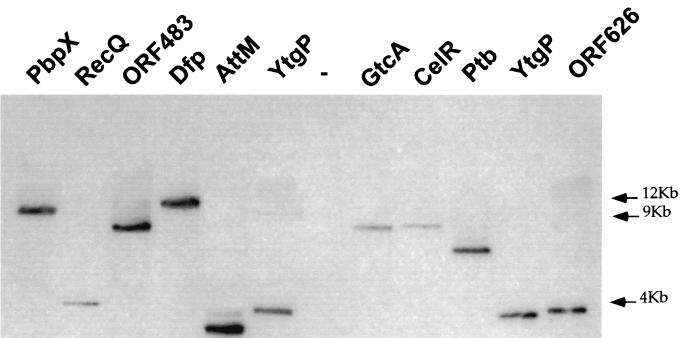
One mutant per locus was chosen for further analyses. As shown in Fig. 4, Southern blot hybridization showed that each of these 10 mutants corresponded to a single transposon insertion.
FIG. 4.
Southern blot of PstI-digested genomic DNA from the different mutant strains. A total of 5 μg of digested DNA was applied to 0.8% agarose gel, electrophoresed, blotted on nylon filter, and subjected to Southern hybridization. The 32P-labeled probe used was obtained after PCR amplification of a 0.7-kb fragment of pAT113. In the case of mutant YtgP, two identical clones were tested (E26 and H′49, in the 6th and last lanes, respectively). As a negative control (−), we used chromosomal DNA from EGD (wild type). The positions of the molecular weight markers are indicated in kilobases preceded by arrows on the right of the figure.
(i) Growth in liquid culture.
Growth in BHI medium of each of the 10 mutants was compared with that of EGD. None of them showed any growth defect since growth rates were identical in exponential phase and since all of the cultures reached the same optical density at 600 nm in the stationary phase, thus indicating that the transposon insertions had no major deleterious effect on bacterial multiplication in vitro (data not shown). This result is consistent with the fact that none of the mutated genes identified exhibited similarity to essential housekeeping genes from other bacteria.
(ii) Transcriptional analysis.
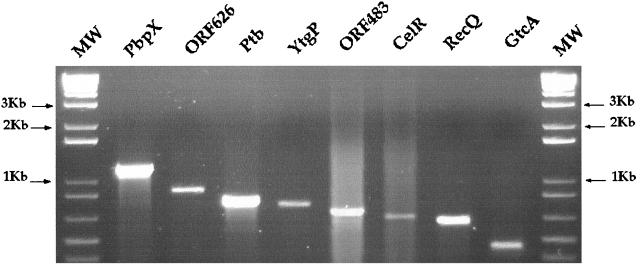
We then tested whether each of the genes identified was transcribed in the wild-type strain (EGD) grown in laboratory conditions (see Materials and Methods for details). As shown in Fig. 5, RT-PCR analysis demonstrated that all of the genes tested (i.e., all of the genes except attM and dfp, previously identified) were transcribed in vitro, strongly suggesting that these genes encode functional proteins.
FIG. 5.
RT-PCR transcriptional analysis. Expression of the 10 loci identified was examined in EGD grown in BHI medium. Appropriate pairs of primers internal to each gene were used for RT-PCR. Amplified DNA fragments were detected after electrophoresis in 1% agarose gels by ethidium bromide staining. One microgram of molecular weight DNA ladder (MW; Gibco-BRL) was loaded at both sides of the gel. The fragments were arbitrarily loaded from the largest to the smallest (left to right).
(iii) Phage sensitivity.
The cell wall of L. monocytogenes contains large amounts of the anionic polymer teichoic acid (TA) covalently linked to peptidoglycan. The TA of L. monocytogenes is a polyribitol phosphate polymer carrying glycosidic substitutions of the ribitol phosphate unit. TA-associated glycosidic substituents have been shown to serve as receptor for phages in L. monocytogenes serotype 1/2a (51). In particular, bacterial resistance to the serotype 1/2-specific phage A118 was shown to be associated with a lack of N-acetylglucosamine (GlcNAc) (46), strongly suggesting that this sugar serves as a receptor for phage A118.
Sensitivity to four L. monocytogenes-specific phages (A118, A502, LMUP35, and LMUP121 [24, 30, 51]) was also evaluated with of all the mutants. Nine of the mutants showed a wild-type phage sensitivity pattern toward the four phages tested (Table 1). We found that Tn1545 inactivation of the gtcA gene of EGD (of serotype 1/2a) did not affect the adsorption of phages A118 and A502 but conferred tight resistance to LMUP121, a phage that forms clear plaques on all serotype 1/2 and 4b strains tested (24). The adsorption of phage LMUP35 was also strongly affected in this mutant; its efficiency of plating was of 10−4.
LD50.
The contribution to virulence of the 10 mutants was studied by determining the LD50 by i.v. inoculation of Swiss mice (Table 1). Four mutants had an LD50 that was greater than 106 bacteria (ranging between 106.1 and 106.6, i.e., 1.5 to 2 logs higher than that of the parental strain [104.6]). Five mutants had an LD50 of between 105.6 and 106. One mutant (Dfp) was only mildly attenuated, with an LD50 of 105.3.
In vivo and in vitro properties of mutants in GtcA, YtgP, PbpX, and ORF626.
The four mutants that showed the highest LD50 values (>106; Table 1) were analyzed in greater detail. Strikingly, they all corresponded to genes involved in cell wall components (that is, GtcA, YtgP, PbpX, and ORF626). In vivo, the kinetics of infection in the organs of infected mice was monitored over a 10-day period and, in vitro, intracellular multiplication was monitored in different cell lines.
(i) In vivo properties.
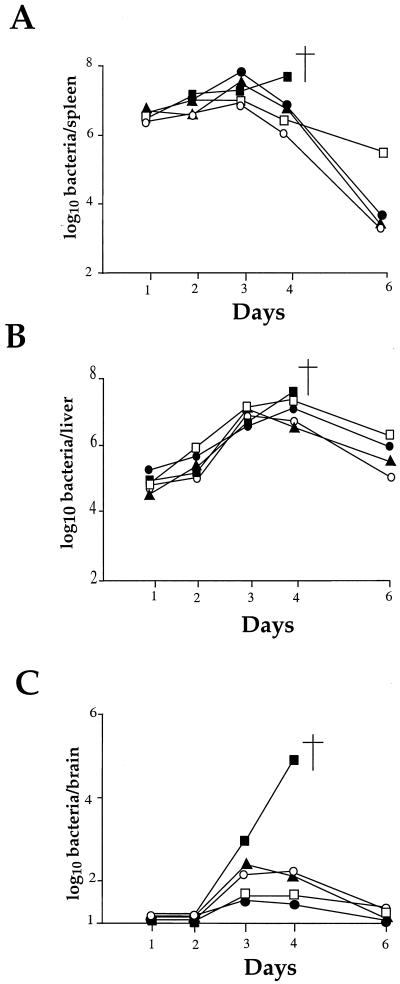
As shown in Fig. 6A and B, the kinetics of infection in the spleen and the liver for the four mutants tested were similar to that of the wild-type strain until day 3. However, in the spleens the bacterial counts fell at day 4 in the four mutants, whereas with the wild-type strain multiplication continued (ultimately leading to death). At day 6, bacteria were rapidly eliminated for three mutants. For the fourth mutant (ORF626), the counts recorded at day 6 remained significantly higher (2 logs). In the livers, the bacterial counts fell significantly for the four mutants only at day 6, and the bacterial counts remained higher (ca. 2 logs) than in the spleens in the four mutants. All of the mice fully recovered from the infection. These data indicate that attenuation of the four mutants did not result in a defect in the early stage of multiplication in the spleen or in the liver.
FIG. 6.
In vivo survival of the attenuated strains. The kinetics of bacterial growth was monitored in the organs of mice infected with the different Tn1545 insertion mutants and compared with EGD as a positive control. Mice were inoculated with 106 bacteria (indicated by an arrow to the left of the ordinate). Bacterial survival was monitored in the spleen (A), liver (B), and brain (C) over a 6-day period. Symbols: ■, EGD; □, ORF626; ●, PbpX; ▴, GtcA; ○, YtgP. The point of death is indicated by a cross symbol.
In no case was invasion of the brain completely abolished (Fig. 6C). However, in the four mutants, a significant reduction of bacterial counts was observed in the brain, with a peak at day 3, followed by complete elimination by day 6. The multiplication of mutants PbpX and ORF626 appeared to be slightly more affected than that of mutants GtcA and YtgP.
(ii) In vitro properties.
The ability of the mutants to penetrate into and to replicate within cells was studied in different types of mammalian cell lines, i.e., epithelial cells (Caco-2, Vero, and HeLa) and hepatocytes (HepG-2), previously used as model systems to study infection by L. monocytogenes (6, 20, 26).
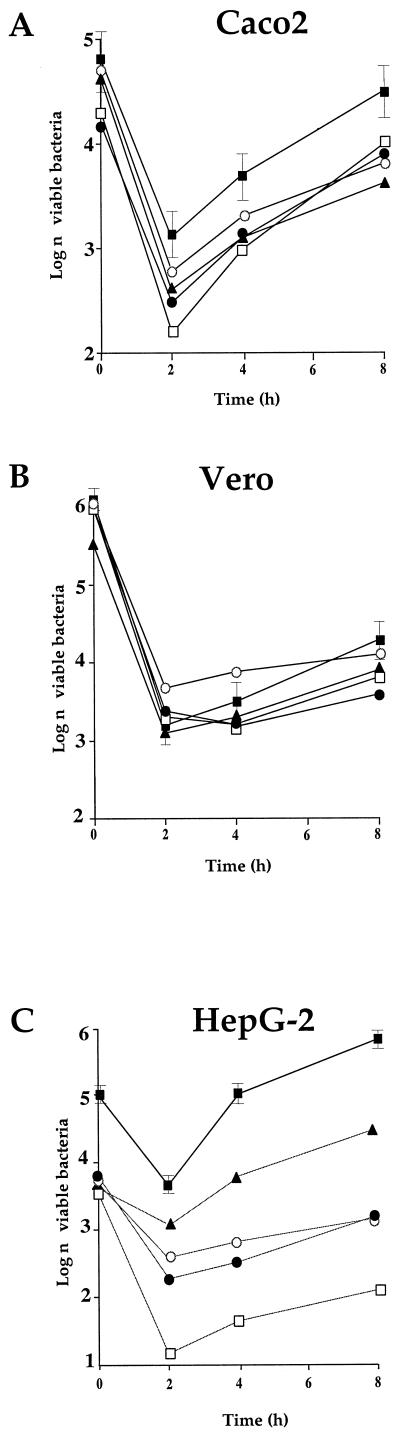
In Caco-2 cells (Fig. 7A), a 1-log difference was recorded after 2 h between mutant ORF626 and wild-type EGD, and half-log difference was recorded for GtcA and PbpX. After 8 h, intracellular multiplication of the GtcA mutant was the most impaired (almost 1 log lower than for EGD). The growth of the three other mutants was quite similar. The intracellular multiplication of the mutants in Vero cells was only slightly altered (Fig. 7B). We also tested the four mutants in HeLa cells. The intracellular growth of the four mutants appeared to be essentially indistinguishable from that of EGD (not shown). In contrast, in HepG-2 cells (Fig. 7C) growth defects were recorded in all of the mutants. Intracellular multiplication of mutant ORF626 was particularly affected: after 2 h a 2.5-log difference was recorded between the mutant and EGD, and this difference increased to ca. 3.8 log after 4 and 8 h. The intracellular multiplication of mutants YtgP and PbpX was slightly less affected but was significantly lower than that of EGD (ca. 1 log after 2 h and 2.5 logs after 8 h). Mutant GtcA was more moderately affected than the three other mutants (1.3 logs lower than EGD after 8 h).
FIG. 7.
Invasiveness of the attenuated strains. Invasiveness was evaluated in three different types of cell lines (A, Caco2 cells; B, Vero cells; and C, HepG-2 cells). Cell monolayers were incubated for 1 h at 37°C with approximately 200 bacteria per cell. After being washed, the cells were reincubated for 8 h in fresh culture medium containing gentamicin (10 mg liter−1). At intervals, the cells were washed again and lysed, and viable bacteria were counted on BHI-ERY plates. Datum points and error bars (indicated only for the wild-type strain for clarity) represent the mean and standard deviation of the number of bacteria per well. Three different assays were performed. Symbols: ■, EGD; □, ORF626; ●, PbpX; ▴, GtcA; ○, YtgP.
Finally, we tested the intracellular multiplication of the four mutants in the macrophage cell line J774 (45). Growth of the four mutants was essentially indistinguishable from that of the wild-type strain (not shown).
DISCUSSION
We have used transposon Tn1545 carried on the integrative vector pAT113 to generate banks of L. monocytogenes mutants. Approximately 2,000 mutants were screened for those affected in their multiplication in the brains of infected animals. We chose the brain as a target organ because it could lead to the identification of not only mutants with impaired growth in the spleen and liver and so subsequently unable to infect the brain but also mutants specifically altered in their capacity to cross the blood-brain barrier and to replicate in the brain (i.e., mutants with normal growth capacity in the spleen and liver). At present, the molecular mechanisms of passage across the blood-brain barrier by L. monocytogenes and the kinetics of central nervous system infection remain largely unknown. It is likely that a number of as-yet-undiscovered L. monocytogenes gene products may participate to this complex process.
Of the 2,000 mutants tested, 18 candidates showed decreased virulence in the mouse model. The insertions harbored by the identified mutants corresponded to 10 distinct loci. At four loci, several mutants were found (see Table 1). Strikingly, half of the mutated loci appeared to involve putative cell wall components.
Cell wall components (GtcA, YtgP, PbpX, ORF626, and Dfp).
Different types of cell wall components were identified, including (i) an integral membrane protein (YtgP), (ii) membrane-anchored proteins (PbpX and ORF626), and (iii) a protein involved in the glycosylation of TA (GtcA).
Earlier studies (4) have shown that, upon i.v. inoculation of 106 wild-type EGD, bacterial growth in the spleen and liver reach a peak at day 3 (with up to 108 bacteria per organ). In contrast, bacteria are not detected in the brain before day 2 of infection. The fact that the increase of bacterial counts in the brain (from day 2 until death) is associated with prolonged bacteremia strongly suggested that the invasion of the brain was a consequence of a hematogenous dissemination of L. monocytogenes. For the four mutants tested here, the kinetics of infection in the spleen was not different from that of the wild-type strain until day 3, but from day 4 the bacterial counts started to diminish significantly. The kinetics of infection of the liver (Fig. 6B) also showed a continuous increase of bacterial counts until days 3 to 4 (reaching <107 bacteria per liver) and then started to diminish (reaching ca. 105 at day 6). Thus, attenuation of these mutants is unlikely to be due to a defect in multiplication in the spleen and liver. Invasion of the brain was in no case completely abolished. However, in the four mutants, a significant reduction in the bacterial counts was observed, and bacteria were completely cleared from the brains by day 6. In vitro assays showed that the four mutants did not present major invasion defects in most of the model systems tested, except in the hepatocytes (the HepG-2 cell line), where intracellular multiplication was altered to various extents. In particular, the transposon insertion in orf626 caused a strong inhibition of intracellular multiplication (>3 logs lower than that of EGD). Remarkably, ORF626 contains an LPxTG sequence preceding a hydrophobic region, which is typical of sortase-dependent surface anchored adhesins (12). The participation of this protein in the entry and multiplication of L. monocytogenes will be addressed experimentally in a future study. The fact that the defect observed in vitro was not correlated with a marked defect of growth in the liver indicates that intrahepatic bacterial multiplication depends on additional parameters other than those encountered in cultured hepatocytes.
Since the four mutations correspond to genes encoding putative cell wall components, it is tempting to speculate that the significant reduction in bacterial counts in the brain could be due to a defect in the efficiency of crossing the blood-brain barrier. The mutations might, for example, alter (directly or indirectly) the proper interaction between the free bacteria released in the bloodstream from day 3 (4) and the endothelial cells at the blood-brain barrier. Direct invasion of the vascular endothelium by bacteria may not be the only means of cellular infection and L. monocytogenes-infected cells (e.g., macrophages) might also constitute potential listerial carriers for crossing the blood-brain barrier. Experimental data had already demonstrated dissemination of intracellular L. monocytogenes (16), indicating that phagocyte-facilitated invasion has a role in central nervous system infection. However, we found no difference in vitro in the intracellular multiplication of the four mutants in mouse macrophages, thus favoring the idea of a direct interaction. These hypotheses will have to be addressed experimentally.
One of the insertions (GtcA) lies in a gene likely to be involved in TA decoration. In serogroup 1/2, the TA consists of polyribitol phosphate with GlcNAc and rhamnose. In contrast, in serogroup 4 GlcNAc is incorporated in the TA chains, and serotype 4b bears both galactose and glucose substituents on the GlcNAc of TA (reference 39 and references therein). It was recently shown that, in the 4b serotype of L. monocytogenes, gtcA was essential for the presence of normal amounts of both galactose and glucose (39), strongly suggesting that it was specifically involved in the glycosylation of TA domains. The phage sensitivity pattern of our GtcA mutant (see Results) suggests that, in serotype 1/2a, gtcA is involved in the glycosylation of TA with rhamnose and that this sugar substituent is the primary receptor for phage LMUP121.
Our in vivo data indicate that the integrity of this major cell wall component is required for bacterial virulence. TAs might directly influence the capacity of the bacteria to invade and multiply in the brain. However, attenuation might also be due to an indirect effect on other components such as, for example, cell-wall-associated proteins involved in virulence.
Other functions (RecQ, AttM, CelR, ORF483, and Ptb).
The second set of genes identified in this study encode putative proteins involved in different cellular processes. In most cases, their effect on virulence is likely to be indirect. For example, CelR is a putative repressor of PTS genes. Several studies have shown that in L. monocytogenes the expression of certain genes belonging to the PTS regulates the expression of the PrfA-dependent virulence genes (reference 7 and references therein). Thus, CelR might also influence the expression of genes required for virulence by as-yet-unknown mechanisms. RecQ is involved in DNA repair and intramolecular recombination. Its expression might modulate the expression of other genes required for bacterial virulence. Sequence analysis suggests that Ptb could be one of the enzymes of a catabolic pathway for branched-chain α-keto acids. The role of AttM and ORF483 in virulence remains to be elucidated.
Our results demonstrate that STM successfully identifies previously unknown genes of L. monocytogenes that participate in bacterial virulence. None of the selected mutants were impaired for growth in standard liquid medium. Thus, the mutated loci seem to be important only in specific environments, such as those encountered in vivo. Several explanations can account for the fact that previously identified virulence genes were not identified: (i) our screening of the bank for hemolytic clones on blood agar eliminated mutants in either the prfA or the hly gene; (ii) our screen cannot have been exhaustive (2,000 mutants have been tested); and (iii) the Tn1545 insertions appeared not to be totally randomly distributed in the Listeria chromosome, implying that some genes might never be mutated. Attenuated mutants occurred at a frequency close to the values previously reported by STM with other bacterial pathogens (ca. 1%). At this stage we cannot exclude that some of the transposon insertions may have been accompanied by independent spontaneous mutations or may have had polar effects on genes located downstream from the interrupted genes. The construction of nonpolar null mutations and complementation experiments will have to be conducted to establish the direct link between gene inactivation and the attenuated phenotype. Adaptation of the STM method to L. monocytogenes opens the way to more extensive studies to target additional genes involved in the infectious process.
ACKNOWLEDGMENTS
We are grateful to Martin Loessner and David Hodgson for providing us with phages A118, 1502, LMUP35, and LMUP121. We thank the European Listeria Genome Consortium for access to the Listeria genome sequence. We also thank Vladimir Pelicic for helpful technical advice and Fred Heffron for critical reading of the manuscript.
This work was supported by CNRS, INSERM, Université Paris V, Université Paris VII, and the EEC (BMH-4 CT 960659). Nicolas Autret received a fellowship from the Ministère de l'Education Nationale de la Recherche et de la Technologie.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1990. [Google Scholar]
- 3.Badger J L, Wass C A, Weissman S J, Kim K S. Application of signature-tagged mutagenesis for identification of Escherichia coli K1 genes that contribute to invasion of human brain microvascular endothelial cells. Infect Immun. 2000;68:5056–5061. doi: 10.1128/iai.68.9.5056-5061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berche P. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb Pathog. 1995;18:323–336. doi: 10.1006/mpat.1995.0029. [DOI] [PubMed] [Google Scholar]
- 5.Berche P, Gaillard J L, Sansonetti P J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987;138:2266–2271. [PubMed] [Google Scholar]
- 6.Braun L, Dramsi S, Dehoux P, Bierne H, Lindahl G, Cossart P. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol Microbiol. 1997;25:285–294. doi: 10.1046/j.1365-2958.1997.4621825.x. [DOI] [PubMed] [Google Scholar]
- 7.Brehm K, Ripio M T, Kreft J, Vazquez-Boland J A. The bvr locus of Listeria monocytogenes mediates virulence gene repression by beta-glucosides. J Bacteriol. 1999;181:5024–5032. doi: 10.1128/jb.181.16.5024-5032.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown J S, Aufauvre-Brown A, Brown J, Jennings J M, Arst H, Jr, Holden D W. Signature-tagged and directed mutagenesis identify PABA synthetase as essential for Aspergillus fumigatus pathogenicity. Mol Microbiol. 2000;36:1371–1380. doi: 10.1046/j.1365-2958.2000.01953.x. [DOI] [PubMed] [Google Scholar]
- 9.Camacho L R, Ensergueix D, Perez E, Gicquel B, Guilhot C. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol. 1999;34:257–267. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty T, Leimeister-Wachter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang S L, Mekalanos J J. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol Microbiol. 1998;27:797–805. doi: 10.1046/j.1365-2958.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- 12.Cossart P, Jonquieres R. Sortase, a universal target for therapeutic agents against gram-positive bacteria? Proc Natl Acad Sci USA. 2000;97:5013–5015. doi: 10.1073/pnas.97.10.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulter S N, Schwan W R, Ng E Y, Langhorne M H, Ritchie H D, Westbrock-Wadman S, Hufnagle W O, Folger K R, Bayer A S, Stover C K. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol Microbiol. 1998;30:393–404. doi: 10.1046/j.1365-2958.1998.01075.x. [DOI] [PubMed] [Google Scholar]
- 14.Darwin A J, Miller V L. Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol Microbiol. 1999;32:51–62. doi: 10.1046/j.1365-2958.1999.01324.x. [DOI] [PubMed] [Google Scholar]
- 15.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes into hepatocytes requires expression of InIB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 16.Drevets D A, Sawyer R T, Potter T A, Campbell P A. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelstein P H, Edelstein M A, Higa F, Falkow S. Discovery of virulence genes of Legionella pneumophila by using signature-tagged mutagenesis in a guinea pig pneumonia model. Proc Natl Acad Sci USA. 1999;96:8190–8195. doi: 10.1073/pnas.96.14.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez S, Sorokin A, Alonso J C. Genetic recombination in Bacillus subtilis 168: effects of recU and recS mutations on DNA repair and homologous recombination. J Bacteriol. 1998;180:3405–3409. doi: 10.1128/jb.180.13.3405-3409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foulongne V, Bourg G, Cazevieille C, Michaux-Charachon S, O'Callaghan D. Identification of Brucella suis genes affecting intracellular survival in an in vitro human macrophage infection model by signature-tagged transposon mutagenesis. Infect Immun. 2000;68:1297–1303. doi: 10.1128/iai.68.3.1297-1303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaillard J L, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaillard J L, Berche P, Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986;52:50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaillard J L, Jaubert F, Berche P. The inlAB locus mediates the entry of Listeria monocytogenes into hepatocytes in vivo. J Exp Med. 1996;183:359–369. doi: 10.1084/jem.183.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hensel M, Shea J E, Gleeson C, Jones M D, Dalton E, Holden D W. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 24.Hodgson D A. Generalized transduction of serotype 1/2 and serotype 4b strains of Listeria monocytogenes. Mol Microbiol. 2000;35:312–323. doi: 10.1046/j.1365-2958.2000.01643.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang X, Helmann J D. Identification of target promoters for the Bacillus subtilis sigma X factor using a consensus-directed search. J Mol Biol. 1998;279:165–173. doi: 10.1006/jmbi.1998.1765. [DOI] [PubMed] [Google Scholar]
- 26.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial invasion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 27.Komatsuzawa H, Ohta K, Labischinski H, Sugai M, Suginaka H. Characterization of fmtA, a gene that modulates the expression of methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:2121–2125. doi: 10.1128/aac.43.9.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhn M, Goebel W. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989;57:55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leimeister-Wachter M, Haffner C, Domann E, Goebel W, Chakraborty T. Identification of a gene that positively regulates expression of listeriolysin, the major virulence factor of Listeria monocytogenes. Proc Natl Acad Sci USA. 1990;87:8336–8340. doi: 10.1073/pnas.87.21.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loessner M J, Inman R B, Lauer P, Calendar R. Complete nucleotide sequence, molecular analysis and genome structure of bacteriophage A118 of Listeria monocytogenes: implications for phage evolution. Mol Microbiol. 2000;35:324–340. doi: 10.1046/j.1365-2958.2000.01720.x. [DOI] [PubMed] [Google Scholar]
- 31.Mackaness G B. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 32.Mei J M, Nourbakhsh F, Ford C W, Holden D W. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol Microbiol. 1997;26:399–407. doi: 10.1046/j.1365-2958.1997.5911966.x. [DOI] [PubMed] [Google Scholar]
- 33.Milohanic E, Pron B, Berche P, Gaillard J L The European Listeria Genome Consortium. Identification of new loci involved in adhesion of Listeria monocytogenes to eukaryotic cells. Microbiology. 2000;146:731–739. doi: 10.1099/00221287-146-3-731. [DOI] [PubMed] [Google Scholar]
- 34.Park S F, Stewart G S. High efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene. 1990;94:129–132. doi: 10.1016/0378-1119(90)90479-b. [DOI] [PubMed] [Google Scholar]
- 35.Polissi A, Pontiggia A, Feger G, Altieri M, Mottl H, Ferrari L, Simon D. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun. 1998;66:5620–5629. doi: 10.1128/iai.66.12.5620-5629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poyart-Salmeron C, Trieu-Cuot P, Carlier C, Courvalin P. The integration-excision system of the conjugative transposon Tn1545 is structurally and functionally related to those of lambdoid phages. Mol Microbiol. 1990;4:1513–1521. doi: 10.1111/j.1365-2958.1990.tb02062.x. [DOI] [PubMed] [Google Scholar]
- 37.Poyart-Salmeron C, Trieu-Cuot P, Carlier C, MacGowan A, McLauchlin J, Courvalin P. Genetic basis of tetracycline resistance in clinical isolates of Listeria monocytogenes. Antimicrob Agents Chemother. 1992;36:463–466. doi: 10.1128/aac.36.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Princiotta M F, Lenz L L, Bevan M J, Staerz U D. H2–M3 restricted presentation of a Listeria-derived leader peptide. J Exp Med. 1998;187:1711–1719. doi: 10.1084/jem.187.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Promadej N, Fiedler F, Cossart P, Dramsi S, Kathariou S. Cell wall teichoic acid glycosylation in Listeria monocytogenes serotype 4b requires gtcA, a novel, serogroup-specific gene. J Bacteriol. 1999;181:418–425. doi: 10.1128/jb.181.2.418-425.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reizer J, Bachem S, Reizer A, Arnaud M, Saier M H, Jr, Stulke J. Novel phosphotransferase system genes revealed by genome analysis—the complete complement of PTS proteins encoded within the genome of Bacillus subtilis. Microbiology. 1999;145:3419–3429. doi: 10.1099/00221287-145-12-3419. [DOI] [PubMed] [Google Scholar]
- 41.Renzoni A, Cossart P, Dramsi S. PrfA, the transcriptional activator of virulence genes, is upregulated during interaction of Listeria monocytogenes with mammalian cells and in eukaryotic cell extracts. Mol Microbiol. 1999;34:552–561. doi: 10.1046/j.1365-2958.1999.01621.x. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Expression of cloned genes in Escherichia coli. In: Nolan C, editor. Molecular cloning: a laboratory manual. 3. Cold Spring Harbor Laboratory Press. N.Y: Cold Spring Harbour; 1989. pp. 17.37–17.41. [Google Scholar]
- 43.Shea J E, Hensel M, Gleeson C, Holden D W. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheehan B, Kocks C, Dramsi S, Gouin E, Klarsfeld A D, Mengaud J, Cossart P. Molecular and genetic determinants of the Listeria monocytogenes infectious process. Curr Top Microbiol Immunol. 1994;192:187–216. doi: 10.1007/978-3-642-78624-2_9. [DOI] [PubMed] [Google Scholar]
- 45.Tilney L G, Portnoy D A. Actin filaments and the growth movement and spread of the intracellular bacterial parasite Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran H L, Fiedler F, Hodgson D A, Kathariou S. Transposon-induced mutations in two loci of Listeria monocytogenes serotype 1/2a result in phage resistance and lack of N-acetylglucosamine in the teichoic acid of the cell wall. Appl Environ Microbiol. 1999;65:4793–4798. doi: 10.1128/aem.65.11.4793-4798.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trieu-Cuot P, Carlier C, Poyart-Salmeron C, Courvalin P. An integrative vector exploiting the transposition properties of Tn1545 for insertional mutagenesis and cloning of genes from gram-positive bacteria. Gene. 1991;106:21–27. doi: 10.1016/0378-1119(91)90561-o. [DOI] [PubMed] [Google Scholar]
- 48.Trieu-Cuot P, Poyart-Salmeron C, Carlier C, Courvalin P. Sequence requirements for target activity in site-specific recombination mediated by the Int protein of transposon Tn1545. Mol Microbiol. 1993;8:179–185. doi: 10.1111/j.1365-2958.1993.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 49.Umezu K, Nakayama H. RecQ DNA helicase of Escherichia coli. Characterization of the helix-unwinding activity with emphasis on the effect of single-stranded DNA-binding protein. J Mol Biol. 1993;230:1145–1150. doi: 10.1006/jmbi.1993.1231. [DOI] [PubMed] [Google Scholar]
- 50.Ward D E, van Der Weijden C C, van Der Merwe M J, Westerhoff H V, Claiborne A, Snoep J L. Branched-chain alpha-keto acid catabolism via the gene products of the bkd operon in Enterococcus faecalis: a new, secreted metabolite serving as a temporary redox sink. J Bacteriol. 2000;182:3239–3246. doi: 10.1128/jb.182.11.3239-3246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wendlinger G, Loessner M J, Scherer S. Bacteriophage receptors on Listeria monocytogenes cells are the N-acetylglucosamine and rhamnose substituents of teichoic acids or the peptidoglycan itself. Microbiology. 1996;142:985–992. doi: 10.1099/00221287-142-4-985. [DOI] [PubMed] [Google Scholar]
- 52.Zhao H, Li X, Johnson D E, Mobley H L. Identification of protease and rpoN-associated genes of uropathogenic Proteus mirabilis by negative selection in a mouse model of ascending urinary tract infection. Microbiology. 1999;145:185–195. doi: 10.1099/13500872-145-1-185. [DOI] [PubMed] [Google Scholar]