Abstract
Background
Plain language summaries (PLSs) are intended for a non-expert audience in order to make health research accessible and understandable to the public. This is important because most research is written with jargon and at a high reading level. However, there is a high degree of variability in the instructions for writing PLSs, which may impede their usefulness as a tool for communicating health research to the public.
Objective
The aim of this scoping review was to conduct a detailed analysis of the author instructions for PLSs provided by leading biomedical and health journals.
Method
We screened 534 health journals covering 11 categories selected from the InCites Journal Citation Reports linked to the top 10 non-communicable diseases. We included journals published in English that recommended the inclusion of a PLS (as defined by the National Institute for Health Research) and provided authors with text-based instructions on how it should be written. Two independent reviewers extracted data pertaining to common elements identified in author instructions, such as word count/PLS length, content, structure, purpose, wording to support plain language, and the use of jargon, acronyms and abbreviations. Other aspects of PLSs were recorded, such as the label used (e.g., plain language summary, lay summary, and patient summary), journal publisher, consumer involvement and whether the PLS is optional or mandatory. We recorded the frequency of each element and qualitative details of specific instructions. A consumer representative provided ongoing and iterative feedback on the methods, results, and reporting of this study
Results
Despite reviewing 534 journals across 10 non-communicable disease areas and 11 journal categories, we found only 27 (5.1%) contained text-based instructions for PLS. Of the 27 journals included in this review, most (70%) did not require a PLS. Approximately 70% of journals with PLS instructions included advice about the use of jargon, abbreviations, and acronyms. Only one journal recommended the use of a readability tool, however five noted that the reading level of the audience or readability of the PLS should be considered. Author instructions were highly heterogeneous between journals. There was inconsistency regarding the word count/PLS length (e.g., between 100 and 850 words), structure (e.g., paragraphs or bullet points), and varying levels of detail for other elements in the instructions. Although only one journal recommended consumer involvement in the development of PLSs, many recommended authors consult those who are not an expert in their field to review their summary prior to submission.
Conclusion
The development of consistent author instructions could enhance the effectiveness and use of PLSs. Such instructions should be developed with consumers to ensure they met the needs of a lay non-expert audience.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40271-022-00606-7.
Plain Language Summary
Plain language summaries (PLSs) are short summaries of research articles written in clear, easy-to-understand language. This makes them a useful way of getting health research to a non-expert reader. Many journals suggest authors write a PLS with their article, but the instructions for them vary from journal to journal. The aim of this study was to review the author instructions for writing PLSs from health journals. We looked at 534 journals and only found 27 (5.1%) had a PLS aimed at a lay reader. We looked at the author instructions from these 27 journals and noted common details of a PLS. For example, we checked word count/PLS length, content, structure, and wording. We also recorded the label used, publisher and whether the PLS was required or not. We found most (70%) journals did not require a PLS. The instructions were different from journal to journal. For example, word count/PLS length ranged from 100 to 850 words and the suggested structure was a mix of paragraph format and bullet points. About 70% of journals gave advice about the use of jargon, abbreviations and acronyms. Only one journal suggested the use of a readability tool, but five thought the reading level of the reader or readability of the PLS was important. Only one journal suggested consumers be involved in writing a PLS, however many suggested the PLSs be checked by someone who is not an expert in the field. PLSs could be improved with help from consumers to make instructions that are more standard.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40271-022-00606-7.
Key Points for Decision Makers
| In this review, we searched 534 biomedical and health journals and conducted a detailed analysis of their author instructions for writing plain language summaries (PLSs). |
| Only 27 journals (5.1%) met our inclusion criteria, in that they had author instructions for writing PLSs aimed at a lay non-expert audience. |
| We found variation between journals in the content and detail of instructions provided, for example the word count/PLS length, content, structure and recommendations regarding the use of jargon in the author instructions for PLS. |
| PLS could be improved with consistent instructions developed with the assistance of consumers. |
Introduction
Most health research is not written with the public in mind as it contains jargon and acronyms and is usually written at a high reading level [1]. Plain language summaries (PLSs) are condensed summaries of research articles written in plain, easy-to-understand language aimed at a non-scientific audience [1]. Ideally, PLSs contain no jargon or technical language [2], which makes them an ideal tool for disseminating reliable health information in a way that a lay audience can understand [3]. PLSs are of particular importance to people with chronic health conditions as this audience consider journals a valuable source of information about their health condition, using information from journal articles to inform health decisions [4].
Scientists are accustomed to describing their work using jargon and technical concepts and may find it difficult to describe their research in a way that is accessible to a non-expert lay audience [2]. To guide authors, many journals and organisations provide instructions for writing PLSs. However, the level of guidance they provide varies, and the advice is not always clear and/or thorough [5]. Despite the availability of instructions for writing lay summaries, research suggests that most PLSs are difficult for consumers to understand [6, 7]. As such, they may misinterpret the messages they contain [1]. Journals should provide clear instructions to guide the author to write the PLS with the intended audience in mind. Such instructions could include the avoidance of jargon and the use of accessible language [1]. Another strategy is recommending the PLS is reviewed by a member of the lay public. This helps build capacity and confidence in the lay public through active participation in the research process [8].
Previous reviews of PLSs and the author instructions for writing them have been conducted, however they report limited findings and often in minimal detail [9–12]. One review focused primarily on PLS author instructions from consumer advocacy groups, including only one list of instructions from a scientific paper and one published by a university [11]. Another review of PLS author instructions included over 50 data sources, such as journals and scientific organisations [12]; however, the only element of PLS author instructions noted was word count/PLS length [12].
This review aims to provide a better understanding of the PLS instructions currently available to authors. We see it as an important step in the development of evidence-based, consistent, and uniform instructions for writing PLSs. Although further research is needed to determine what those instructions should include, a thorough understanding of the situation and potential challenges to address is vital.
Methods
Definition of Terms
We will use the acronym PLS to refer to the singular form and PLSs to refer to the plural form of the term ‘plain language summary’. For this review, we define a consumer as a member of the lay public not possessing any expert or technical health expertise. We will use the terms consumer, patient, and public synonymously.
Protocol and Registration
This review was conducted according to the Joanna Briggs Institute Reviewers’ Manual for scoping reviews [13]. The Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR) [14] was used as a guide. The protocol for this review was completed prior to data analysis and is registered on Open Science Framework [15].
Information Sources and Search
In our pilot, our information sources included journals, global health organisations (WHO, etc.), professional associations and multidisciplinary organisation, consumer advocacy groups, and funding bodies. Of those that published PLS and author instructions for writing PLSs, 79% were journals. Although organisations such as those included in our pilot are stakeholders in the area of PLS, they are not high producers of PLSs and PLS instructions. Therefore, we made the decision to limit our information sources to journals for this review.
As previous reviews that investigated PLS author instructions were limited in scope and number of journals searched, we designed this study to be a comprehensive analysis of a large number of high impact health journals. We developed our search strategy to ensure we analysed a large number of journals from a wide range of health areas. We determined that the top 10 non-communicable diseases responsible for the greatest burden of disease [16] was an appropriate framework to apply. Diseases from these categories tended to be chronic and we know from previous research that people with chronic diseases or illnesses are high users of health information, listing journals in the top three preferred sources of health information [4].
We searched biomedical and health journals from 11 journal categories selected from the InCites Journal Citation Reports. Journals from the category of medicine (general and internal) were added to increase the scope of our search. Using data from the 2020 Incites Journal Citation Reports, we compiled a list of 50 journals for each journal category, selecting those with the highest impact factor. The category of Rheumatology only had 34 journals that met our inclusion criteria, giving us a total of 534 journals searched. We compiled our list of journals from each of the 11 categories on 18 August 2021. Non-communicable diseases and journal categories, with the number of each journal per category, are outlined in Table 1.
Table 1.
Journal categories and corresponding top 10 non-communicable disease categories
| Non-communicable disease categorya | Journal category | Journals |
|---|---|---|
| Not applicable | Medicine, general and internal | 50 |
| Cardiovascular disease | Cardiac and cardiovascular systems | 50 |
| Neoplasms | Oncology | 50 |
| Musculoskeletal | Rheumatology | 34 |
| Mental disorders | Psychology | 50 |
| Diabetes and chronic kidney disease | Urology and nephrology | 50 |
| Chronic respiratory | Respiratory system | 50 |
| Neurological disorders | Clinical neurology | 50 |
| Digestive diseases | Gastroenterology and hepatology | 50 |
| Sense organ diseases | Ophthalmology | 50 |
| Skin diseases | Dermatology | 50 |
| Total | 534 | |
aBased on category listings from the Incites Journal Citation Reports as at 18 August 2021 [17]
Information Selection: Inclusion Criteria
We included journals published in English that recommended the inclusion of a PLS and provide authors with instructions on how it should be written. We identified a PLS as being a summary of a research article that is not the abstract and is in alignment with the National Institute for Health and Care Research (NIHR) definition of a PLS, which states “A plain English summary is a clear, brief summary of the research that has been written for members of the public, rather than researchers or professionals. It should be written clearly and simply, without jargon and with an explanation of any technical terms” [2]. We excluded the following. (1) Journals that recommend the use of a PLS but did not include any information on how the PLS should be written in their author instructions. These were excluded because our review was focused on an analysis of the author instructions for PLSs, and the information contained within them comprised the data for our study. (2) Journals that recommended a PLS but only provided a link to a third-party service that provides authors with a PLS for a fee. (3) Other formats for PLSs, such as graphical and video, as these formats are a reasonably new option for authors and are offered in addition to text-based PLSs.
Information Selection: Screening
To determine the intended audience for the PLSs, one reviewer (KG) checked the author instructions of each journal. A journal was included if the author instructions described the intended audience of a PLS with labels such as consumer, public, patient, lay, people living with ‘X’ (e.g., people living with diabetes), and similar.
One reviewer (KG) searched the author instructions of all 534 journals in October 2021. If the journal recommended a PLS, the intended audience was recorded. To ensure reliability, a second reviewer (JS) checked a random sample of 10% of journals from each journal category. This sample was selected using a random number generator on 20 October 2021. Any conflicts were resolved through discussion.
Data Charting and Collation
We conducted a pilot study to revise our screening and search strategy. We inspected the author instructions for writing PLSs and noted components or ‘elements’ and features or ‘characteristics’ that were common across many journals. We defined ‘elements’ as aspects in the author instructions that guide authors how to write the PLS and how it should appear. We identified elements such as word count/PLS length, structure, purpose, content, wording to support plain language, guidance on the use of jargon, acronyms and abbreviations, and resources for writing a PLS. We defined ‘characteristics’ of a PLS as those items in the author instructions that related to a PLS but were not elements. These were the label used for the PLS (e.g., ‘patient summary’, ‘lay summary’), whether the PLS was mandatory or optional for each journal, and any recommendations for consumer involvement in the development of the PLS. We also recorded the journal publisher to note any patterns in journals published by the same group and the term used by the journal to designate their author instructions/submission guidelines. We refer to these throughout as ‘author instructions’. Aspects of PLSs not covered by other categories were grouped together under the heading of ‘miscellaneous’. These included the option to use graphical PLS/abstracts, images, whether the PLS is freely available to readers (i.e., no paywall), whether the journal translated PLSs into languages other than English, and information about the empirical foundation on which PLS author instructions were developed. Using an iterative process, reviewers also extracted any other information about PLSs they discovered and determined to be relevant.
Reviewers noted links to two kinds of resources for writing PLSs: one written by the journal itself and one from a third-party source. For example, some Elsevier journals linked to one of their own resources titled “In a nutshell: how to write a lay summary” [20] and journals from multiple publishers provided a link to the plain English summaries resource published by the NIHR [2]. We only collated data from resources provided by the journal itself, not third parties. This is because it is reasonable to expect an author will consult all instructions for writing a PLS provided directly by the journal itself, whereas third-party resources may only be consulted if the author wants extra guidance.
Author instructions varied and not all journals provided information on each element, therefore we recorded the frequency of each item and qualitative details of specific instructions. During November and December 2021, two reviewers (KG and JS) independently extracted data, each reviewing 50% of the author instructions of the included journals. Reviewers checked each other’s data and resolved conflicts through discussion. Both the coding framework and data charts were developed over time throughout the data collection and analysis process. Changes were made to ensure both were capturing all qualitative and quantitative data relevant to the study aims. During piloting, we tested our data charts and developed them using an iterative process until finalised.
Consumer Involvement
We consulted a consumer representative (SC), who was engaged as part of the research team, to provide input on the study. SC provided ongoing feedback on the study methods and results, offering insight from the perspective of an end-user of PLSs. Specifically, SC helped with iterative development of the data charts and the ‘4.2’ section of the discussion, suggesting changes to improve clarity. SC also reviewed the full manuscript and co-wrote the PLS for the review
Results
Selection of Sources of Evidence
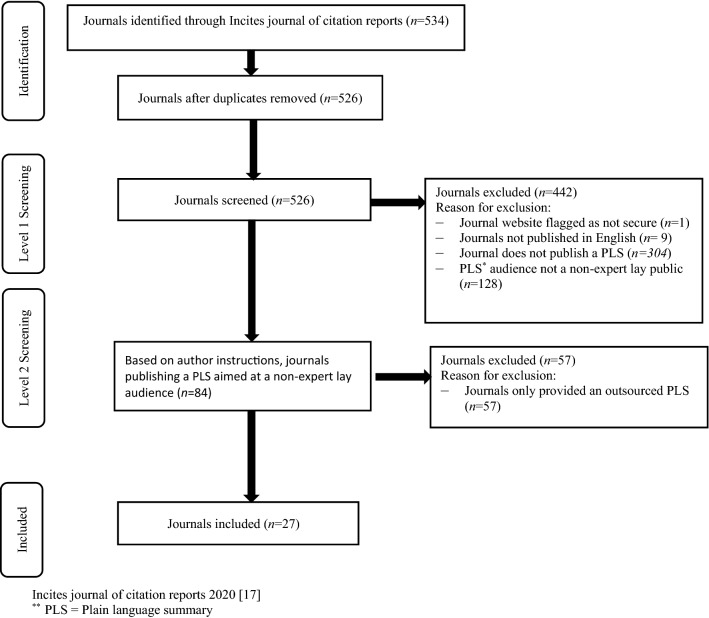
We identified 534 journals through the 2020 Incites Journal Citation Reports. After screening, 27 (5.1%) journals met our inclusion criteria in that they contained author instructions for writing PLS, published by six different publishing groups (see Table 2 for a breakdown of the key characteristics, and Table 3 for a summary of the elements of instructions for PLSs). A PLS was mandatory in 8 (30%) journals and optional in 19 (70%) journals. We found three labels used to designate a PLS; plain language summary, lay summary, patient summary. Henceforth, these will all be referred to as plain language summaries (PLSs). About half (51.9%) of journals used the terms guidelines or guide when referring to their author instructions, while the second most common term used was ‘instructions’ (33.3%). One journal recommended the involvement of consumers in the development of PLSs. However, several journals recommended PLSs be reviewed prior to submission, for example by someone without knowledge in the subject area. Only one journal (Cochrane Database of Systematic Reviews) provided information on the development of their instructions for writing PLSs (Fig. 1).
Table 2.
Characteristics of plain language summaries from author instructions
| Characteristic of plain language summaries | Frequency (%) [n = 27] | |
|---|---|---|
| Publisher | Elsevier | 9 (33.3) |
| Springer Nature | 7 (25.9) | |
| Taylor and Francis | 4 (14.8) | |
| Wiley | 3 (11.1) | |
| SAGE | 2 (7.4) | |
| Dove Medical Press | 2 (7.4) | |
| Term for author instructions | Submission guidelines/guide for authors | 14 (51.9) |
| Instructions for authors/author instructions | 9 (33.3) | |
| Author information | 3 (11.1) | |
| Handbook | 1 (3.7) | |
| Inclusion | Optional | 19 (70.3) |
| Mandatory | 8 (29.6) | |
| Label | Plain language summary | 18 (66.7) |
| Lay summary | 5 (18.5) | |
| Patient summary | 4 (14.8) | |
| Recommended consumer involvement | Role in co-writing the PLS | 1 (3.7) |
| Role in reviewing the PLS | 11 (40.7) | |
| Miscellaneous | Graphical PLS/abstracts | 11 (40.7) |
| Freely available to readers | 5 (18.5) | |
| Inclusion of images recommended | 2 (7.4) | |
| Translated into a language other than English | 2 (7.4) | |
| Information on the development of PLS author instructions | 1 (3.7) | |
PLS plain language summary
Table 3.
Elements of plain language summaries from author instructions
| Element | Description | Frequency (%) | Example(s) |
|---|---|---|---|
| Audience | How the audience is described | 27 (100) | “Non-medical audience”a |
| “Non-specialists in the field, including members of the public and non-academics”b | |||
| Word count/length | The recommended or maximum word count or length | 27 (100) | “Two or three short sentences”c |
| “No more than 250 words”d | |||
| Structure | How the summary should be structured | 26 (96.3) | “3 brief bullet points”e |
|
“Formatted as a single paragraph”f “Should be short, clear sentences broken up into relevant sections”f | |||
| Content | The information that should be included in the summary | 27 (100) |
“Provide an accurate representation of the article”g “The summary should be based on the abstract of the paper”g |
|
“Descriptions of the paper that are easily understandable”h “True reflection of the research presented”h “Both merits and limitations should be discussed”h “Define the who, what, why, when, where and how of the research. Provide answers to the following questions: Why was this study done? What did the researchers do? What did the researchers find? What do the findings mean?”h “Ensure that your conclusion/take home message is clear”h | |||
| Purpose | Reason(s) for writing the summary and what it should convey to the reader | 24 (88.9) |
“Make the research findings presented in the article accessible to those outside the scientific community” i “Help you reach the people who may directly benefit from your research. These are the people who are affected by your discoveries—whose lives have the potential to improve because of your analyses and conclusions”i “A lay summary can be a valuable tool to tell the story of your research. And stories are what we all connect to most. In a lay summary, your research team is the hero, and your passion is the answer to the question of why”i “They make it quick and easy for people outside the research community to understand why your work matters”i |
|
“Plain language summaries (PLSs) communicate the significance of scientific research evidence to a broad audience, including patients and professionals in nearby disciplines, in jargon-free and clear language. As an author, expanding the reach of your work by engaging with a wider audience can help you: Enable the reader to capture the content quickly and bookmark the paper for in-depth reading. Crucially, PLS improve public engagement with science and medical research. By helping the public to understand biomedical research, researchers can contribute to raising awareness of its value and attracting further public support, engagement, and involvement. Attract more readers, increasing access to the article and its associated metrics Connect with patients, caregivers, policy makers, and other decision makers Connect with non-specialist healthcare professionals from other fields Improve access to scientific data in a format that is easy to understand Translate complex science into practical knowledge and initiatives Expand your professional network and enhance your reputation Crucially, PLSs improve public engagement with science and medical research. By helping the public to understand biomedical research, researchers can contribute to raising awareness of its value and attracting further public support, engagement, and involvement” j | |||
| Wording | Specific recommendations regarding wording | 25 (92.6) |
“Should be written in an easy-to-understand manner, using language that is accessible but does not patronise the reader” g “Sentences should be written in the active voice, rather than the passive voice” g |
|
“Written in easy-to-understand language rather than complex words (see Universal Patient Language)”k “Written in plain English”k “Use the active voice rather than the passive voice (for example, “Dr Smith’s team report several improvements” rather than “Several improvements were reported by Dr Smith’s team”)”k “Phrase sentences in neutral language, remaining as objective as possible”k “Use person-centred language rather than focusing on the condition/illness or disability”k “Keep statements factual and avoid providing opinions or speculation on the study’s findings and significance. It is of primary importance that the PLS not be misleading”k “Avoid complex grammatical structures”k | |||
| Jargon, acronyms and abbreviations | Use of jargon, acronyms or abbreviations | 19 (70.4) |
“Jargon should be avoided other than where necessary; in which case it should be defined in full the first time it is used”l “Abbreviations should be avoided”l |
| “Avoid jargon, use every day English terms to convey your message. If you need to use technical terminology or abbreviations, please explain the term when introduced”m | |||
| Resources | Resources for writing a plain language summary from an external source | 19 (70.4) | “For further information on how to write about biomedical and health research in plain English, please read the INVOLVE Plain English Summaries (http://www.invo.org.uk/resource-centre/plain-english-summaries/) resource from the National Institute for Health Research”o |
| “A few examples of online resources include the following: https://www.elsevier.com/connect/authors-update/in-a-nutshell-how-to-write-a-lay-summary; https://hbg.cochrane.org/sites/hbg.cochrane.org/files/public/uploads/Writing%20Plain%20Language%20Summaries.docx; https://www.agu.org/Share-and-Advocate/Share/Community/Plain-language-summary”p |
aEuropean Urology Oncology
bNeurology and Therapy
cEuropean Urology
dPain and Therapy
eJournal of Cardiac Failure
fRheumatology and Therapy
gCNS Drugs
hTherapeutic Advances in Gastroenterology
iCancer
jExpert Review of Respiratory Medicine
kExpert Review of Gastroenterology and Hepatology
lAmerican Journal of Clinical Dermatology
mTherapeutic Advances in Musculoskeletal Disease
nCochrane Database of Systematic Reviews
oExpert Review of Respiratory Medicine
pOsteoarthritis and Cartilage
Fig. 1.
Selection of sources of evidence. PLS plain language summary. *Incites Journal of Citation Reports 2020 [17]
Elements in Author Instructions for Plain Language Summaries
See Table 3 for a description of elements in the author instructions for PLSs along with selected examples to illustrate the diversity of findings.
Audience
The intended or potential audience of the PLS was noted in all 27 journals. The audience was expressed as lay, patient, caregiver, consumer, or public in 22 journals, and expressed as non-medical/non-academic in 14 journals. Other nominated audiences included non-specialist researchers (five journals), clinicians (six journals) and stakeholders, such as decision makers, journalists, and funding bodies (seven journals).
Word Count/Length
We found a reference to the word count or length of the PLS in all 27 journals. It was expressed as a numerical value (e.g., up to ‘X’ words) in 20 journals and as a suggested length (e.g., two to three short sentences or a short paragraph) in seven journals. In two journals, the word limit was up to 100 words, in 14 journals it was up to 250 words, in three journals it was up to 300 words and in one journal it was up to 850 words.
Structure
We found 26/27 journals referred to the structure of the PLS, with some making more than one suggestion relating to the structure. In 25 journals, the suggested structure was short sentences, a single paragraph in 11 journals, bullet points in six journals, and headings or suggested questions to answer in four journals. For example, “The structure of a lay summary should answer the main questions of ‘who/what/where/when/how many/why?’” (JACC: Cardiovascular Imaging).
Content
Specific information about the content of the PLS was found in all 27 journals. The amount of detail varied between journals, with 14 providing a low level of detail and 13 providing a high level of detail. A low level of detail was defined as a basic one-sentence instruction, e.g. “Summarising the main message of the article” (Journal of Hepatology). A high level of detail was defined as multiple points, questions or details to include. For example, the journal Therapeutic Advances in Musculoskeletal Disease included the following: “Define the who, what, why, when, where and how of the research. Provide answers to the following questions: why was this study done, what did the researchers do? What did the researchers find and what do the findings mean? Ensure that your conclusion/take home message is clear. The PLS should be a true reflection of the research presented written in an engaging and accessible way, without exaggeration. Both merits and limitations should be discussed”. In three journals, content was prompted by suggested questions to answer, such as “Why was the study done?”, “What did the researchers do and find?”, “What do the results mean?” and “What is the objective influence on the wider field?” (Postgraduate Medicine).
Purpose
Most journals (24/27) referred to the purpose of the PLS. As with content, the level of detail for purpose varied between journals. We found a low level of detail (one to three sentences) in 12 journals and a high level of detail (at least two to three paragraphs) in 12 journals. The stated purpose varied from broad descriptions such as to “Describe your findings” (European Urology Oncology) to those focusing on the importance of the plain language summary as a tool for researchers to communicate their findings to a wider audience e.g., “an effective tool to summarise your paper, extending the reach and impact that the paper can have, and making it accessible to a wider audience.” (Rheumatology and Therapy).
Wording
We found reference to specific wording or writing style of the PLS in 25/27 journals. Using plain English or simple language was referred to in 23 journal instructions. Sixteen journals recommended the use of active rather than passive voice and four suggested writing in the first person. Person-centred language (rather than a focus on illness or disability) was mentioned in seven journals and positive language was mentioned in four. Language that considered a lay non-expert audience was recommended, with 10 journals suggesting the writer of the PLS should use “language that does not patronise the reader”. Five journals suggested the reading level of the audience or the readability of the PLS should be considered, and one suggested the use of a readability tool. Using neutral or objective language, or factual statements rather than opinions, was recommended by seven journals. Three journals suggested the use of “interesting” or “engaging” language. Examples included “The plain language summary should be distinct from the abstract and should be written in an accessible, interesting way without spinning or exaggerating the story” (Journal of Asthma and Allergy) and “written in an engaging and accessible way, without exaggeration” (Therapeutic Advances in Gastroenterology). One journal made a note about “findings that readers might find upsetting, controversial or disappointing. When this is the case, we encourage you to follow the guidance about handling findings sensitively in the Dissemination checklist, item 15.” (Cochrane Database of Systematic Reviews).
Jargon, Acronyms and Abbreviations
A total of 19/27 journals recommended jargon should be avoided or explained when it is necessary to be used. Sixteen journals suggested abbreviations be avoided or explained before they are used. However, no journals suggested acronyms should be explained or avoided.
Resources
We found a reference to resources in the PLS writing instructions of 19/27 journals. Resources included short articles with tips on how to write a PLS and example PLSs. We located 14 resources written by the journal itself and seven from third parties, such as the PLS guidance resource published by the NIHR [2].
Discussion
This is the first study to report a detailed analysis of the author instructions for writing PLSs provided by leading biomedical and health journals. Despite reviewing 534 journals across 10 non-communicable disease areas and 11 journal categories, we found only 27 (5.1%) contained written instructions for PLSs. Author instructions were highly heterogeneous between journals. There was inconsistency regarding the word count (e.g., between 100 and 850 words), structure (e.g., paragraphs or bullet points), and varying levels of detail for other elements in the instructions. Although only one journal recommended consumer involvement in the development of PLSs, many recommended authors to consult those who are not an expert in their field to review their summary prior to submission.
Our findings build on and extend existing research in the area. Most previous research on the author instructions for writing PLSs have reported findings for elements such as word count/PLS length or structure and labels for PLSs. Some report findings similar to the current study, however previous studies have lacked the scope of included health and medical journals and the systematic approach taken with this review. Haughton and Machin [9], for example, assessed the author instructions for PLSs from the websites of 31 journal publishers, representing ~ 7630 journals in the fields of biology, economics, and medicine. The authors found word count/PLS length ranged from 30 to 500 words. Shailes’ [12] review of plain language summaries from biomedical journals and scientific organisations reported PLSs with a word count/PLS length ranging from one sentence to 1000 words [12]. A small study conducted by Narayanan et al. [10] reviewing 30 journals that contained plain language summaries also had a range from one sentence to 1000 words. Word count/PLS length is the only element of PLS author instructions to be reviewed in detail by previous studies, except for Haughton and Machin [7], who recorded the percentage of PLSs that were structured and unstructured. The results of these studies were like that of our review, which identified a range of 25–30 words to a maximum of 300 words, except for Cochrane, which was a maximum of 850 words.
Besides word count, previous research has also assessed other elements of plain language summary writing instructions. Following on from the work of Shailes [12], Fitzgibbon et al. [18] reviewed a cross-section of 11 journals that produce PLSs from the list compiled and updated by the journal eLife [12]. Contrary to our review, in which most (29.6%) PLS were not required by journals, Fitzgibbon et al. [18] found they were mandatory in 8/11 (72.7%) journals. In this way, our study was unique in highlighting the high proportion of journals that consider PLSs to be optional (70%) in those journals that provide author instructions for PLSs. Haughton and Machin [9], Shailes [12], and Fitzgibbon et al. [18] also report labels used by journals for PLSs such as author summary [9, 18], editor’s summary [9], key points [9], highlights [12], lay abstracts [12, 18] and author summaries [12]. Fitzgibbon et al. [18] note that the use of varying labels to designate a PLS reduces the chance they will be located by search engines. They suggest the adoption of a universal label such as PLS to increase their accessibility. Although we found fewer labels in our study, this is likely on account of our strict inclusion criteria, which meant that the intended audience had to be prespecified as a non-expert lay public.
Unlike other studies in this area, the Patients Participate! Project undertook a study to determine whether crowdsourcing of PLSs contributes to improved understanding of health information by the public [19]. One outcome of this project was the production of a “working-level guide for academics on producing lay summaries” [20]. This guide [11] included elements of PLSs that we located in our review, such as consideration for the intended audience, structure, avoidance of technical language and the questions relating to content, such as who, what, where, when, why, how? [11]. In this guide, Duke [11] observed variation in the author instructions for writing PLSs, which is consistent with our findings. Although this guide is freely available to download and use, it is unclear to what extent it is used by authors. It was not recommended as a resource by any journals in our review.
While our study evaluated author instructions for writing PLSs, other work has evaluated the content of the summaries themselves. For example, Narayanan et al. [10] found 22/30 PLSs from journals contained jargon or technical language. This is a notable finding, as our review showed that most (70.4%) journals provided guidance on the avoidance of jargon. This suggests that either authors are not following the instructions from journals when writing PLSs, or journals are not checking PLSs for compliance to their instructions. It is also possible that there is confusion or debate about what is considered jargon, leaving it open to interpretation by authors. Investigation into these factors is an area for future research that could assist in the development of PLS author instructions that are practical and useful.
Another vital step in the development of consistent instructions for writing PLSs is consumer involvement. Our review found only one (3.7%) journal recommended consumer involvement in the co-design of PLSs and 11 (40.7%) suggested involvement in reviewing the PLS. Unfortunately, we did not find any data from this review that helps to explain the low proportion of journals recommending consumer involvement. Clearly there is interest from consumers in PLS co-design, as evidenced by the recent update of the Cochrane guidelines for their systematic reviews. There is also support for PLS co-design from the perspective of researchers, as illustrated by Barnfield et al. [22], who detail the authors’ experience working with consumers to develop PLSs for some studies in their Cognitive Function after Stroke (COGFAST) project. In this project, the research team suggest making iterative changes to their drafts based on consumer feedback. The authors suggest that “collaboration with members of the public to revise and refine content and layout of lay summaries is more likely to achieve the desired outcome of readability and comprehension” [22]. As awareness of the benefits of consumer involvement in PLS co-design increases, it is expected that this practice will be more widely adopted by researchers and recommended by journals.
As PLSs become a more common feature of journals, less emphasis could be placed on reinforcing their purpose and value in author instructions. This would allow an increased focus on elements poorly represented or presented with varying levels of detail in some journals, such as the type of content to include and recommendations for the use of tools that support plain language and ease of reading, and the involvement of consumers in PLS co-design.
Strengths and Limitations
There are both strengths and limitations to this review. The review was limited to journals that specified that the audience for the PLS was a non-expert lay public. This excluded many journals that published summaries with labels such as ‘Highlights’ and ‘Key Points’, most of which did not specify their intended audience. Other reviews of author instructions for writing PLSs took a different approach, including journals for which the intended audience was not explicitly stated as being non-expert. Although our approach limited our review to 27 included journals, we conducted a thorough analysis of the author instructions, reporting in detail on six characteristics of PLSs, such as the label used and consumer involvement, as well as eight elements such as word count/PLS length, content and structure.
Some journals only provided services or resources for producing PLSs that were outside the scope of our inclusion criteria, therefore they were not included in the review. For example, some journal publishers such as Wiley provide an outsourcing option for PLSs. We did not include these in our study as we did not have access to the instructions/guidelines writers followed when compiling these PLSs. Had they been included, our data would have been incomplete. Likewise, some journals provided links to additional resources to assist authors, e.g., the plain English summaries resource published by the NIHR [2]. We only included resources produced directly by the journal, not third parties
A final limitation is the fact that several journals were from the same publishing group and we noted a high level of homogeneity in the PLS author instructions between these journals, e.g., in their purpose and recommendation to avoid jargon. Some slight differences did however occur, highlighting the value of not assuming that journals from the same publishing group have identical author instructions.
Future Directions
Given that this review identified that only 5.1% of journals we screened included a PLS, there is a clear and urgent need for the academic publishing industry to include more PLSs in the first instance. There is a lack of understanding of what constitutes a useful PLS, and current instructions are not well supported by evidence [11]. As PLSs become a more common part of health research communication, the development of consistent guidelines for writing PLSs will be necessary. These guidelines should be evidence-based, which includes feedback from end-users. Since PLSs are written in accordance with the author instructions provided by journals, such instructions should include guidance to ensure the PLS is written with the audience in mind. To assist this process, the involvement of end-users is important and their perspectives on the content, layout, and structure of a PLS should be reflected in any future development of evidence-based PLS instructions. Such consumer involvement is already established, as the Cochrane Collaboration and Guidelines International Network [21, 23] both support the involvement of consumers in guideline development, and the Cochrane Collaboration recently undertook a revision of their guidelines for writing PLSs and incorporated consumer input. The Patient-Centred Outcomes Research Institute and The Cochrane Collaboration recently developed templates for writing PLSs in conjunction with end-users [21, 24]. Future research could build on these templates with a focus on the needs of the end-users of plain language summaries such as consumers.
As most journals did not require the inclusion of a PLS, it may be difficult for consumers to locate PLSs on topics of interest. To make PLSs more accessible to end-users, it would be beneficial for journal publishers to consider making them mandatory, and also publicly available on searchable databases such as PubMed.
Conclusion
The effectiveness and use of PLSs could be enhanced by the development of consistent guidelines written with the audience in mind. The guidelines should be developed in conjunction with consumers to help authors write PLSs that best cater for a non-expert lay audience. Existing templates developed in conjunction with consumers could be further developed and enhanced to cater to other end-users of PLSs, such as policy makers and health journalists.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Study conceptualisation: KG, DM and KM. Methodology: KG, DM, KM and SC. Data collection and analysis: KG and JS. Writing—original draft preparation: KG. Writing—reviewing and editing: All authors. Supervision: DM and KM. Project administration: KG.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Declarations
Funding
Not applicable.
Conflicts of interest
Karen M. Gainey, Jenna Smith, Kirsten J. McCaffery, Sharon Clifford, and Danielle M. Muscat have completed the International Committee of Medical Journal Editors (ICMJE) uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work, with the exception of Health Literacy Solutions Pty Ltd, at which Kirsten McCaffrey and Danielle Muscat are directors.
Availability of data and material
All data were collated from publicly available sources. The datasets generated and/or analysed during the current study are available from the corresponding author on request.
Ethical approval
Not required.
Dissemination to participants and related patient and public communities
A consumer representative reviewed the design and implementation of the study, and also reviewed the manuscript, including the plain language summary. The authors plan to disseminate the study findings to the general public through social media, our bi-monthly newsletter, and through partnerships with relevant organisations.
References
- 1.Wada M, Sixsmith J, Harwood G, et al. A protocol for co-creating research project lay summaries with stakeholders: guideline development for Canada’s AGE-WELL network. Res Involv Engagem. 2020. 10.1186/s40900-020-00197-3 (eCollection 2020). [DOI] [PMC free article] [PubMed]
- 2.NIHR. Plain English Summaries. https://www.nihr.ac.uk/documents/plain-english-summaries/27363. Accessed Jul 2022.
- 3.Nunn E, Pinfield S. Lay summaries of open-access journal articles: engaging with the general public on medical research. Learn Publ. 2014;27(3):173–184. doi: 10.1087/20140303. [DOI] [Google Scholar]
- 4.Pushparajah DS, Manning E, Michaels E, et al. Value of developing plain language summaries of scientific and clinical articles: a survey of patients and physicians. Ther Innov Regul Sci. 2018;52(4):474–481. doi: 10.1177/2168479017738723. [DOI] [PubMed] [Google Scholar]
- 5.Kirkpatrick E, Gaisford W, Williams E, et al. Understanding Plain English summaries. A comparison of two approaches to improve the quality of Plain English summaries in research reports. Res Involv Engagem. 2017;3:17. 10.1186/s40900-017-0064-0 (eCollection 2017) [DOI] [PMC free article] [PubMed]
- 6.Rakedzon T, Segev E, Chapnik N, et al. Automatic jargon identifier for scientific engaging with the public and science communication educators. PLoS ONE. 2017;12(8):e0181742. doi: 10.1371/journal.pone.0181742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho FC, Elkins MR, Franco MR, et al. Are plain-language summaries included in published reports of evidence about physiotherapy interventions? Analysis of 4421 randomised trials, systematic reviews and guidelines on the Physiotherapy Evidence Database (PEDro) Physiotherapy. 2019;105(3):354–361. doi: 10.1016/j.physio.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Statement on Consumer and Community Involvement in Health and Medical Research, National Health and Medical Research Council. 2016. Consumers Health Forum of Australia. Accessed Aug 2022. https://www.nhmrc.gov.au/about-us/publications/statement-consumer-and-community-involvement-health-and-medical-research
- 9.Haughton M, Machin D. The prevalence and characteristics of lay summaries of published journal articles. Original abstracts from the 2017 European Meeting of the ISMPP. Curr Med Res Opin. 2017;33(Suppl 1):S23–30. doi: 10.1080/03007995.2017.1296269. [DOI] [Google Scholar]
- 10.Narayanan R, Ganpathy P, Pitre S, et al. Patient lay summaries in biomedical journals: what and how much is currently available? Original Abstract from the 14th Annual Meeting of the ISMPP. Curr Med Res Opin. 2018;34(Suppl 1):S9–28. doi: 10.1080/03007995.2018.1440994. [DOI] [Google Scholar]
- 11.Duke M. How to write a lay summary. DCC How-to-Guides. 2012. Edinburgh: Digital Curation Centre. Accessed April 2022. https://www.dcc.ac.uk/guidance/how-guides/write-lay-summary
- 12.Shailes S. Something for everyone. eLife. 2017;6:e25411. 10.7554/eLife.25411. [DOI] [PMC free article] [PubMed]
- 13.Aromataris E, Munn Z, eds. JBI manual for evidence synthesis. JBI; 2020. Accessed May 2020. https://jbi-global-wiki.refined.site/space/MANUAL.
- 14.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews [PRISMA-ScR]: checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 15.Gainey KM, Kamper SJ, Traeger AC, 2019. What instructions are available to health researchers for writing lay summaries? A pilot scoping review. Open Sci Framew. [DOI]
- 16.Institute for Health Metrics and Evaluation. GBD Compare. https://vizhub.healthdata.org/gbd-compare/. Accessed Jul 2020
- 17.Incites Journal Citation Reports 2020. https://jcr2.clarivate.com/JCRHomePageAction.action? Accessed Aug 2021
- 18.FitzGibbon H, King K, Piano P, et al. Where are biomedical research plain-language summaries? Health Sci Rep. 2020 doi: 10.1002/hsr2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duke M. "Lay summaries for research articles: a citizen science approach to bridge the gap in access". In: Scale, openness and trust: new avenues for electronic publishing in the age of infinite collections and citizen science: scale, openness and trust, 1–7. ELPUB. Valetta, Malta: IOS Press; 2015. https://elpub.architexturez.net/doc/oai-elpub-id-12015-01-duke-elpub2015-paper-38. 10.3233/978-1-61499-562-3-1
- 20.Patients Participate! Deliverables (Weblog). http://blogs.ukoln.ac.uk/patientsparticipate/deliverables/index.html. Accessed Jan 2022
- 21.Cumpston M, Lasserson T, Chandler J, Page MJ. Chapter III: reporting the review. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane; 2022. Accessed May 2022. http://www.training.cochrane.org/handbook.
- 22.Barnfield S, Pitts AC, Kalaria R, Allan L, Tullo E. “Is all this stuff about neurons necessary?” The development of lay summaries to disseminate findings from the Newcastle Cognitive Function after Stroke (COGFAST) study. Res Involve Engag. 2017. https://researchinvolvement.biomedcentral.com/articles/10.1186/s40900-017-0066-y. 10.1186/s40900-017-0066-y. [DOI] [PMC free article] [PubMed]
- 23.Anne S, Rosenfeld R. Role of consumers in guideline development process. Otolaryngol Head Neck Surg. 2018;159(2):211–212. doi: 10.1177/0194599818767617. [DOI] [PubMed] [Google Scholar]
- 24.Maurer M, Siegel JE, Firminger KB, et al. Lessons learned from developing plain language summaries of research studies. Health Lit Res Pract. 2021;5(2):e155–e161. doi: 10.3928/24748307-20210524-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gainey KM, Kamper SJ, Traeger AC, 2019. What instructions are available to health researchers for writing lay summaries? A pilot scoping review. Open Sci Framew. [DOI]