Abstract
Background
Breast-conserving surgery with radiotherapy is one of standard treatments for early breast cancer. However, it is regarded as an option to treat elderly patients with small hormone receptor-positive breast cancer with breast-conserving surgery and hormone therapy without radiotherapy. We conducted two sequential prospective studies to examine the feasibility of breast-conserving surgery without radiotherapy since 2002 and present the results.
Patients and methods
Primary female breast cancer patients who fulfilled the strict eligibility criteria were prospectively enrolled in two sequential studies named WORTH 1 and 2. The surgical materials were sliced in 5-mm intervals and all slices were examined microscopically. Postoperative radiotherapy was not allowed, but tamoxifen or anastrozole was administered for 5 years. Ipsilateral breast tumor recurrence (IBTR)-free survival was the primary outcome.
Results
The data of the two studies were combined (N = 321). The median follow-up period for IBTR was 94 months (4–192 months). Only three patients were treated with adjuvant chemotherapy. The 5- and 10-year IBTR-free rates were 97.0% and 90.5%, respectively. The age at operation and PR status affected IBTR rates independently. When we calculated IBTR-free rates of patients who were 65 years of age or older at the time of surgery and had PR-positive tumors, the 5- and 10-year IBTR rates were both 98.4%.
Conclusions
Our “5-mm-thick slice and 5-mm free-margin” method may be effective to select patients who can be treated by breast-conserving surgery and hormone therapy without radiotherapy.
Keywords: Breast cancer, Breast-conserving treatment, Radiotherapy, Surgical margins, Prospective study
Introduction
Breast-conserving surgery with radiotherapy is one of the standard treatments for early breast cancer. There were no differences in disease-free and overall survival between breast-conserving treatment with radiotherapy and mastectomy in randomized trials and meta-analysis [1–8]. Radiotherapy was demonstrated to markedly reduce ipsilateral breast tumor recurrence (IBTR) in many randomized trials and meta-analysis [7, 9–12]. However, radiotherapy is time-consuming and costly, and can cause severe treatment-related adverse events [13].
Randomized trials have been conducted to identify the population not requiring radiotherapy after breast-conserving surgery [14]. Accordingly, it has been regarded as an option to treat elderly patients with small hormone receptor-positive breast cancer by breast-conserving surgery and hormone therapy without radiotherapy. However, the 10-year IBTR rate in patients treated without radiotherapy was around 10% in the randomized trials. As such, a good method to reduce the IBTR rate without radiotherapy is needed. We therefore conducted two sequential prospective studies to examine the feasibility of breast-conserving surgery without radiotherapy using our 5-mm interval slice and 5-mm margin-free method since 2002.
Patients and methods
Primary female breast cancer patients who fulfilled the following eligibility criteria were prospectively enrolled in two sequential studies named WORTH 1 and 2: (1) a tumor of 3 cm or less by palpation, (2) pathologically node negative by axillary dissection or sentinel node biopsy and M0, (3) no treatment before surgery, (4) 50 years of age or older at the time of surgery and postmenopausal, (5) no tumor cells within 5 mm from the margins histologically, (6) no lymphatic invasion around the primary cancer, (7) estrogen receptor-positive judged by each institution, and (8) within 8 weeks after definitive surgery. The surgical mammary gland materials must have been sliced in 5-mm intervals and all the slices must have been examined microscopically. The status of hormone receptors and HER2 was judged at each institution. We regarded HER2 in immunohistochemistory (IHC) 3 + , or 2 + and FISH amplification as positive. But we included HER2 IHC 2 + without the information of FISH in negative in Table 1. Postoperative radiotherapy was prohibited, but adjuvant chemotherapy was allowed. The patients were administered tamoxifen or anastrozole in WORTH 1 and anastrozole in WORTH 2 as postoperative adjuvant endocrine therapy for 5 years. The exclusion criteria were: (1) suspected multifocality of the tumor, (2) history of breast cancer or bilateral breast cancer, or (3) psychological disease.
Table 1.
Characteristics of the patients, and their tumors and treatments
| WORTH 1 (n = 123) | WORTH 2 (n = 198) | Total (n = 321) | |
|---|---|---|---|
| Median age at operation | 65 (range 51–84) | 66 (range 50–84) | 65 (range 50–84) |
| Median tumor size by palpation | 1.6 cm (range 0–3.0 cm) | 1.4 cm (range 0–4.0 cm) | 1.5 cm (range 0–4.0 cm) |
| Personal history of cancer | |||
| Yes | 5 (4.2%) | 19 (9.6%) | 24 (7.5%) |
| No | 118 (95.9%) | 179 (90.4%) | 297 (92.5%) |
| Axillary surgeries | |||
| Sentinel node biopsy | 75 (61.0%) | 183 (92.4%) | 258 (80.4%) |
| Axillary dissection | 44 (35.8%) | 8 (4.0%) | 52 (16.2%) |
| None | 3 (2.4%) | 7 (3.5%) | 10 (3.1%) |
| Unknown | 1 (0.8%) | 0 | 1 (0.3%) |
| Progesterone receptor status | |||
| Positive | 86 (69.9%) | 171 (86.4%) | 257 (80.1%) |
| Negative | 37 (30.1%) | 27 (13.6%) | 64 (19.9%) |
| HER2 status | |||
| Positive | 2 (1.6%) | 1 (0.5%) | 3 (0.9%) |
| Negative (IHC 2 + but FISH was not done) | 105 (85.4%) (5) | 190 (96.0%) (3) | 295 (91.9%) (8) |
| Unknown | 16 (13.0%) | 7 (3.5%) | 23 (7.2%) |
| Histological types | |||
| Invasive ductal cancer | 102 (82.9%) | 174 (87.9%) | 276 (86.0%) |
| Ductal carcinoma in situ | 10 (8.1%) | 11 (5.6%) | 21 (6.5%) |
| Others | 11 (8.9%) | 13 (6.6%) | 24 (7.5%) |
| Adjuvant hormonal agents | |||
| Tamoxifen | 86 (69.9%) | 15 (7.6%) | 101 (31.5%) |
| Aromatase inhibitors | 34 (27.6%) | 178 (89.9%) | 212 (66.0%) |
| Others | 2 (toremifene) (1.6%) | 0 | 2 (0.6%) |
| Unknown | 1 (0.8%) | 4 (2.0%) | 5 (1.6%) |
| None | 0 | 1 (0.5%) | 1 (0.3%) |
| Adjuvant chemotherapy | |||
| Yes | 0 | 3 (1.5%) | 3 (0.9%) |
| No | 122 (99.2%) | 192 (97.0%) | 314 (97.8%) |
| Unknown | 1 (0.8%) | 3 (1.5%) | 4 (1.2%) |
IBTR-free survival and distant relapse-free survival (DRFS) were recorded as the interval from initial definitive surgery until IBTR or distant relapse, respectively. Only IBTR that occurred as the first event was regarded as events of IBTR-free survival. IBTR that developed at the same time as other relapses or within 2 months of the time of other relapses was regarded as an event of IBTR-free survival. Patients who did not develop IBTR or distant relapse were censored at the time of last follow-up or death as the IBTR-free rate.
Survival rates were calculated using the Kaplan–Mayer method. Statistical analyses were conducted using the log-rank test for univariate analyses or proportional hazards model for multivariate analysis. Only variables that were significant in the univariate analyses were included in the multivariate analysis. P values of < 0.05 were considered significant.
The primary end point was IBTR-free survival, and secondary end points were DRFS and overall survival.
As safety monitoring, interim monitoring by Bayesian approach was carried out. As stopping criteria based on the posterior distribution, WORTH 1 was planned to stop if the posterior probability exceeding 1% in the annual IBTR rate exceeded 95%. WORTH 2 adopted a criterion of 0.5% in the annual IBRT rate. The determination of the sample size for WORTH 1 can be shown with an accuracy such that the upper limit of the 95% confidence interval of the estimated IBRT rate is about 10% when the 5-year IBRT rate is assumed to be 5%. In WORTH 2, assuming that the 5-year IBRT rate is 2.5%, the accuracy that the upper limit is within about 5% is obtained. The sample size for WORTH 1 was set 120 and that for WORTH 2 was 200.
The protocols were approved by the ethics committee at each participating institution.
Written informed consent was received from all participants.
Registration of WORTH 1 was not required in 2002 when it was started. WORTH 2 was registered in UMIN-CTR, numbered UMIN000000534, on December 10, 2006.
Results
The number of patients who participated in the two trials was 123 and 198 in WORTH 1 and WORTH 2, respectively. The characteristics of patients, and their tumors and treatments are shown in Table 1. Almost all patients were HER2 negative and others in histological types in Table 1 had invasive cancers other than invasive ductal carcinoma.
WORTH 1 study
The patient enrollment for WORTH 1 was conducted between October 2002 and March 2005. In total, 123 patients participated in WORTH 1. The median age at the time of surgery was 65 years (range 51–84). The median tumor size by palpation was 1.6 cm (range 0–3.0 cm). The median follow-up period for IBTR was 102 months (range 18–192 months). The hormonal agents used were tamoxifen in 86 patients, anastrozole in 34, toremifene in 2, and unknown in 1. Adjuvant chemotherapy was not administered to all patients, but one lacked information about adjuvant chemotherapy.
The 5- and 10-year IBTR-free rates were 94.8% and 87.7%, respectively. The 5- and 10-year overall survival rates were 98.3% and 95.1%, respectively, and 5- and 10-year distant DRFS rates were 98.3% and 94.9%, respectively.
We examined the effects of age at the time of surgery, tumor size by palpation, and progesterone receptor (PR) status on the IBTR rates, but none significantly affected the IRTR rates, although older ages, smaller tumor sizes, and PR positivity slightly reduced IRTR rates.
WORTH 2 study
One hundred and ninety-eight patients were enrolled in WORTH 2 between December 2006 and November 2011. The median age at the time of surgery was 66 years (range 50–84) and ages were unknown in two patients. The median tumor size by palpation was 1.4 cm (range 0–4.0 cm) and the tumor sizes were not known in 18. The median follow-up period for IBTR was 88 months (range 4–145 months). Anastrozole was used in 167 patients, exemestane in 11, tamoxifen in 15, unknown in 4, and no hormonal agent was used in 1. One hundred and ninety-two patients did not receive adjuvant chemotherapy, whereas adjuvant chemotherapy was administered to three and three lacked information.
The 5- and 8-year IBTR-free rates were 98.4% and 92.9%, respectively. The 5- and 8-year overall survival rates were 98.9% and 96.2%, respectively, and 5- and 8-year distant DRFS rates were 100% and 97.6%, respectively.
We analyzed the effects of age at the time of surgery, tumor size by palpation, and PR status on IBTR-free rates. Older patients developed IBTR significantly less frequently than younger patients (5-year IBTR-free rates: 97.6% for 64 years or younger vs. 99.0% for 65 or older, P = 0.044). There was no difference in IBTR between the large and small tumors (5-year IBTR-free rates: 97.4% for 1.3 cm or smaller vs. 98.9% for 1.4 cm or larger, P = 0.698). PR positivity significantly increased the IBTR-free rate (5-year IBTR-free rates: 99.4% for PR positive vs. 91.4% for PR negative, P = 0.0003).
Results of the combined analyses
The combined data of WORTH 1 and 2 are presented hereafter. The median age at the time of surgery was 65 years (range 50–84). The median tumor size by palpation was 1.5 cm (0–4.0 cm). The median follow-up period for IBTR was 94 months (4–192 months). Only three patients received adjuvant chemotherapy.
The 5- and 10-year IBTR-free rates were 97.0% and 90.5%, respectively (Fig. 1). The 5- and 10-year overall survival rates were 98.7% and 95.1%, respectively (Fig. 2), and 5- and 10-year distant DRFS rates were 99.3% and 96.3%, respectively (Fig. 3).
Fig. 1.
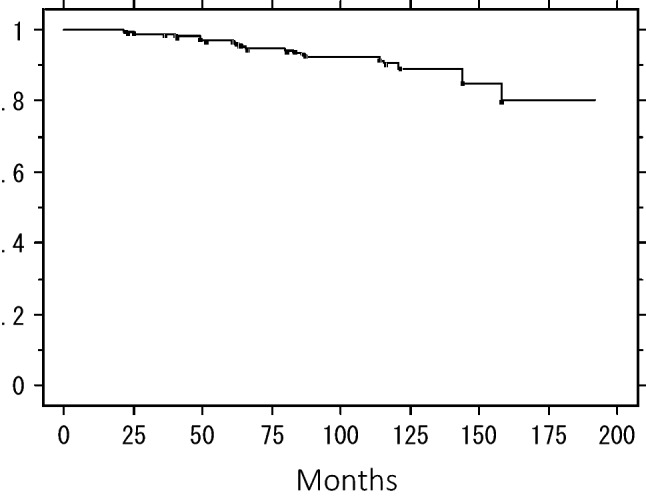
Ipsilateral breast tumor recurrence-free rate in the combined patients
Fig. 2.
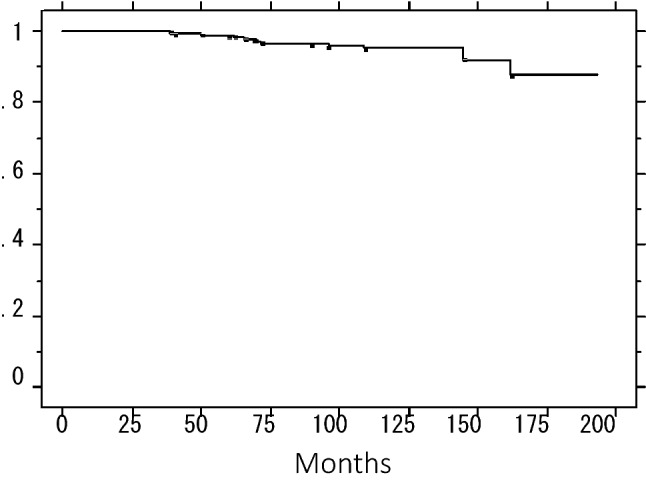
Overall survival of the combined patients
Fig. 3.
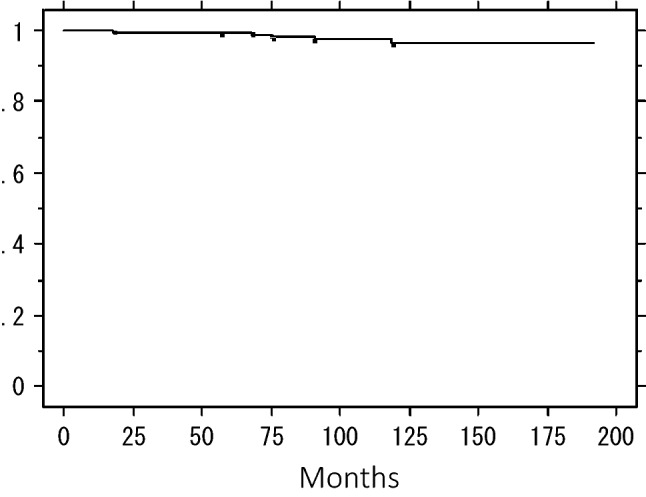
Distant disease-free survival of the combined patients
We analyzed the effects of age at the time of surgery, tumor size by palpation, PR status, and hormonal agents on IBTR-free rates. Older patients developed IBTR significantly less frequently (5-year IBTR-free rates: 95.8% for 64 or younger vs. 98.1% for 65 or older, P = 0.025) (Fig. 4). There was no significant difference in the IBTR-free rate between the patients with large and small tumors (5-year IBTR-free rates: 96.9% for 1.4 cm or smaller vs. 96.8% for 1.5 cm or larger, P = 0.125). PR positivity significantly increased the IBTR-free rate (5-year IBTR-free rates: 98.3% for PR-positive vs. 91.5% for PR-negative, P = 0.006) (Fig. 5). Hormonal agents had no effect (5-year IBTR-free rates: 95.9% for tamoxifen or toremifene vs. 97.4% for aromatase inhibitors, P = 0.435). Both the tumor size and PR status affected IBTR-free rates independently (64 years or younger, P = 0.024, hazard ratio 2.90: 95% confidence interval 1.15–7.31) (PR-negative, P = 0.007, hazard ratio 3.10: 95% confidence interval 1.37–6.99). When we calculated the IBTR-free rates of patients aged 65 years or older at the time of surgery with PR-positive tumors (N = 136) in the combined population in WORTH 1 and 2, the 5- and 10-year rates were both 98.4%.
Fig. 4.
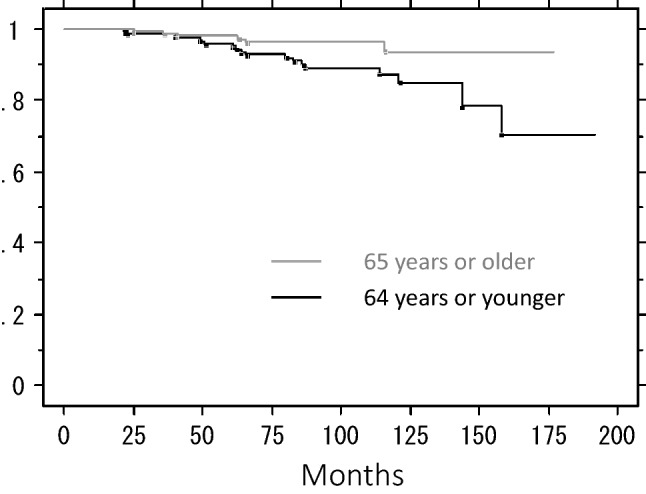
Ipsilateral breast tumor recurrence-free rate by age at the time of surgery
Fig. 5.
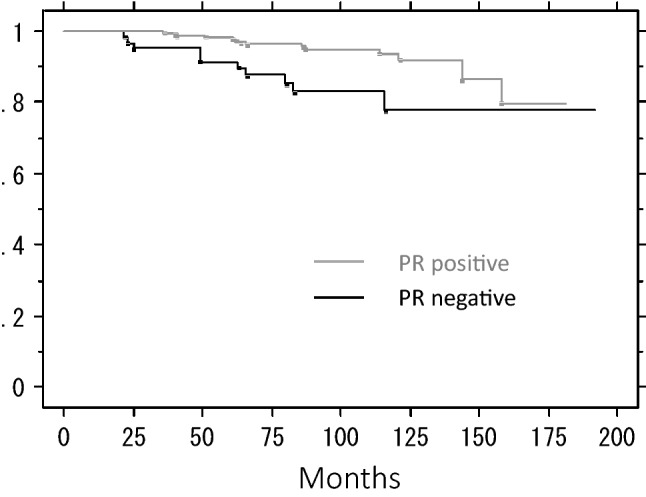
Ipsilateral breast tumor recurrence-free rate by PR status
Discussion
Breast-conserving surgery with radiotherapy to the breast is one of the standard local treatments for early breast cancer because randomized trials and meta-analyses demonstrated it to have equivalent effects to mastectomy in terms of disease-free and overall survival [1–8]. Radiotherapy is regarded as an essential component of breast-conserving treatment because it markedly reduced local recurrence in the randomized trials [7, 9–11], and meta-analysis [12] demonstrated that radiotherapy significantly reduced not only locoregional recurrence, but also distant recurrence and breast cancer deaths. However, pathologically negative node, old age, and positive hormonal receptor status are known to reduce local recurrence rates. Therefore, randomized trials comparing radiotherapy with no radiotherapy after breast-conserving surgery have been conducted for elderly patients with node-negative and estrogen receptor-positive breast cancer treated using adjuvant hormonal agents [14]. Radiotherapy reduced the risk of IBTR, but it did not impact distant recurrence or overall survival of early breast cancer treated by breast-conserving surgery and tamoxifen in elderly women (70 years or older) in CALGB 9343. The IBTR rate was 9% at 10 years in the no radiotherapy group. McCormick et al. reported that 88% of women aged 70 years and older with stage I, estrogen receptor-positive breast cancer received radiotherapy after breast-conserving surgery, even after the National Comprehensive Cancer Network (NCCN) guidelines for elderly breast cancer patients stated the omission of radiotherapy after breast-conserving surgery in women meeting the CALGB 9343 entry criteria who were prescribed hormonal agents in 2009 [15]. Two other groups also reported low IBTR rates in 65-year-old or older breast cancer patients [16, 17]. One study is a randomized trial of PRIME II. This trial randomly assigned women with hormone receptor-positive and node-negative breast cancer of 3 cm or less and negative excision margins (1 mm or more) into either whole-breast radiotherapy or no radiotherapy. At median follow-up of 5 years, actuarial ipsilateral breast cancer recurrence was 1.3% in women allocated whole-breast radiotherapy and 4.1% in those assigned no radiotherapy. The other study demonstrated a very low rate of IBTR after breast-conserving surgery without radiotherapy [17]. This was a single arm study. They reported an IBTR rate of 1.2% at 5 years in 601 participants with hormonal agents. They used the criteria of T1 tumor, node-negative, ER-positive, and Elston–Ellis histological grade 1 or 2. However, they used sector resection of the tumor, the area of which was larger than that of local excision that we used.
Our first study, WORTH 1, was started to identify a population with breast cancer who had a low risk of IBTR after breast-conserving surgery without radiotherapy shortly after the first reports of the study of Fyles et al. [18] and the CALGB 9343 study [19] in 2001. We included the “5-mm-thick slice and 5-mm free-margin” method to reduce the IBTR rate, and “no lymphatic invasion around the tumor”, which was a possible factor to avoid inflammatory-type IBTR, in the eligibility criteria. After WORTH 1 was started, the IBTR rate was low. Therefore, we started the second study, WORTH 2, with the same eligibility criteria as WORTH 1 to ensure low IBTR rates.
The 5-year IBTR-free rates were 94.8%, 98.4%, and 97.0% in WORTH 1, WORTH 2, and in combination, respectively. The rate was lower in WORTH 1 than in WORTH 2 even though we used the same eligibility criteria. One reason for the difference is the hormonal agents used. The most frequently used agent was tamoxifen in WORTH 1, but it was anastrozole in WORTH 2. Aromatase inhibitors, including anastrozole, are more effective at reducing hormone receptor-positive breast cancer recurrence [20]. The second possible reason was the difference in PR-negative rates. Thirty-seven of 123 patients were PR negative in WORTH 1, whereas 27 of 198 were PR negative in WORTH 2 (30.1% vs. 13.6%, P = 0.0003 by chi-square test). The last possible factor was MRI use before the surgery. Recently, we often use MRI to make sure that multifocality or multicentricity is not present before the surgery in Japan, but we did not collect the data of MRI use.
The “5-mm interval slice and 5-mm margin-free” method were used in both WORTH 1 and 2. This method had been used to judge pathologically negative margins in Japan. This method is labor- and time-consuming, but effective in reducing the IBTR rate because the combined 5-year IBTR-free rate was 97.0%, which was high.
Two factors, age at the time of surgery and PR status, were identified as independent prognostic factors for the IBTR rate in this combined analysis. The IBTR-free rate of patients aged 65 years or older at the time of surgery with PR-positive tumors was 98.4% at both 5 and 10 years. Wickberg et al. reported that luminal A tumors have a lower IBTR rate than luminal B tumors after breast-conserving surgery with or without radiotherapy [21]. Based on this favorable rate, we concluded that patients aged 65 years or older with both estrogen and progesterone receptor-positive breast cancer of 3 cm or smaller by palpation, who underwent breast-conserving surgery, had histologically negative margins judged by the “5-mm interval slice and 5-mm margin-free” method and no lymphatic invasion around the tumor do not need radiotherapy.
The strong points of our studies were the relatively large sample size and prospective design, but there were some limitations. First, our studies were not randomized. Therefore, we cannot measure the effects of radiotherapy. However, this was not the purpose of our studies. Second, it is unclear whether our “5-mm interval slice and 5-mm margin-free” method is effective for reducing the IBTR rate. ASCO recommends the criteria of no tumor cells on inked margins for histologically negative margins [22]. However, ASCO guidelines only consider patients who will receive radiotherapy. Our “5-mm interval slice and 5-mm margin-free” method may be useful to select patients who do not need radiotherapy. The previously mentioned study reported a low IBTR rate of 1.2% at 5 years [17]. They enrolled patients with PR-positive breast cancer, comprising 89.1% of the cohort and used sector resection of the breast. The area of sector resection is larger than that of local excision which we used. So, we believe that our “5-mm interval slice and 5-mm margin-free” method was effective to reduce IBTR in local excision. Third, the IBTR rate of WORTH2 was lower than that of WORTH1. The reasons for this were thought to be the higher rates of PR positivity and use of aromatase inhibitors in WORTH2, but there might have been a higher rate of the use of MRI before surgery in WORTH2. MRI might have detected the multiple cancers in the breast. We did not collect the data on MRI use. Therefore, this might have been one of the reasons. Lastly, the percentages of ER and/or PR-positive tumor cells in the tumors were not obtainedd. However, patients with higher percentages of these receptors may have been enrolled in the studies.
Conclusions
Our “5-mm-thick slice and 5-mm margin-free” method may be effective for selecting patients who can be treated by breast-conserving surgery and hormone therapy without radiotherapy. Patients who fulfill our eligibility criteria for WORTH 1 and 2 and HER2 negative, and aged 65 years or older at the time of surgery with PR-positive tumors, may not need radiotherapy if they receive hormonal agents.
Acknowledgements
We appreciate the programming of these studies by the late Prof. Yasuo Ohashi and we thank Dr. Natsumi Yamashita for writing the statistical part in this paper. We thank the late Prof. Hiroshi Yagata, the late Dr. Shigeru Murakami, and the late Dr. Yuji Tokunaga for the entry of patients into these studies, and Ms. Yumiko Nomura for helping in data collection.
Author contributions
OS: protocol writing, data analyses, case contribution, NR, MN, A-TS, SK, YH, IT, NT, HH, IY, TY, IH: case contribution. OY: statistical support.
Funding
WORTH1 and WORTH2 were supported by a Grant-in-Aid for research of cancer treatment from the Ministry of Health, Labour and Welfare of Japan No. 13–9 and No. 21–7-4, respectively.
Data availability
Please e-mail Ohsumi S.
Code availability
StatView 5, SAS Institute Inc.
Declarations
Conflict of interest
Ohsumi S.: fees for non-CME Services Received Directly from Commercial Interest or their Agents (e.g., speakers' bureaus); AstraZeneca. Nishimura R.: none. Masuda N.: Fees for Non-CME Services Received Directly from Commercial Interest or their Agents (e.g., speakers' bureaus); Chugai, AstraZeneca, Pfizer, Eli-Lilly, Eisai, Takeda. Contracted Research; Chugai, AstraZeneca, Kyowa-Kirin, MSD, Novartis, Pfizer, EliLilly, Eisai, DaiichiSankyo. Akashi-Tanaka S.: fees for Non-CME Services Received Directly from Commercial Interest or their Agents (e.g., speakers' bureaus); AstraZeneca. Suemasu K.: none. Yamauchi H.: contracted Research; AstraZeneca. Tokunaga E.: fees for Non-CME Services Received Directly from Commercial Interest or their Agents (e.g., speakers' bureaus); AstraZeneca. Ikeda T.: none. Nishi T.: none. Hayashi H.: none. Iino Y.: none. Takatsuka Y.: none. Ohashi Y.: none, Inaji H.: none.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was received from all individual participants included in the study.
Consent for publication
Authors are responsible for correctness of the statements provided in the manuscript. All individual participants agreed on the data being published in an academic article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Veronesi U, Saccozzi R, Vecchio MD, Banfi A, Clemente C, De Lena M, et al. Comparison of radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancer of the breast. N Engl J Med. 1981;305:6–11. doi: 10.1056/NEJM198107023050102. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Bauer M, Margolese R, Poisson R, Pilch Y, Redmond C, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–673. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 3.Sarrazin D, Le MG, Arriagada R, Contesso G, Fontaine F, Spielmann M, et al. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol. 1989;14:177–184. doi: 10.1016/0167-8140(89)90165-5. [DOI] [PubMed] [Google Scholar]
- 4.Lichter AS, Lippman ME, Danforth DN, d'Angelo T, Steinberg SM, deMoss E, et al. Mastectomy versus breast conserving therapy in the treatment of stage I and II carcinoma of the breast: a randomized trial at the National Cancer Institute. J Clin Oncol. 1992;10:976–983. doi: 10.1200/JCO.1992.10.6.976. [DOI] [PubMed] [Google Scholar]
- 5.van Dongen JA, Bartelink H, Fentiman IS, Lerut T, Mignolet F, Olthuis G, et al. Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer. EORTC 10801 trial. J Natl Cancer Inst Monogr 1992; 11: 15–8. [PubMed]
- 6.Blichert-Toft M, Rose C, Andersen JA, Overgaard M, Axelsson CK, Andersen KW, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. J Natl Cancer Inst Monogr. 1992;11:19–25. [PubMed] [Google Scholar]
- 7.Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995;333:1456–1461. doi: 10.1056/NEJM199511303332203. [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists' Collaborative Group. Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med 1995; 333:1444–55. [DOI] [PubMed]
- 9.Veronesi U, Luini A, Vecchio ID, Greco M, Galimberti V, Merson M, et al. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med. 1994;328:1587–1591. doi: 10.1056/NEJM199306033282202. [DOI] [PubMed] [Google Scholar]
- 10.Clark RM, Whelan T, Levine M, Roberts R, Willan A, McCulloch P, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node negative breast cancer: An update. J Natl Cancer Inst. 1996;88:1659–1664. doi: 10.1093/jnci/88.22.1659. [DOI] [PubMed] [Google Scholar]
- 11.Forrest AP, Stewart HJ, Everington D, Prescott RJ, McArdle CS, Harnett AN, et al. Randomized controlled trial of conservation therapy: 6-year analysis of Scottish trial. Scottish Cancer Trials Breast Group Lancet. 1996;348:708–713. doi: 10.1016/s0140-6736(96)02133-2. [DOI] [PubMed] [Google Scholar]
- 12.Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011; 378:1707–16. [DOI] [PMC free article] [PubMed]
- 13.Takigawa N, Segawa Y, Saeki T, Kataoka M, Ida M, Kishino D, et al. Bronchiolitis obliterans organizing pneumonia syndrome in breast-conserving therapy for early breast cancer: radiation-induced lung toxicity. Int J Radiat Oncol Biol Phys. 2000;48:751–755. doi: 10.1016/S0360-3016(00)00654-4. [DOI] [PubMed] [Google Scholar]
- 14.Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31:2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormick B, Ottesen RA, Hughes ME, Javid SH, Khan SA, Mortimer J, et al. Impact of guideline changes on use or omission of radiation in the elderly with early breast cancer: practice patterns at national comprehensive cancer network institutions. J Am Coll Surg. 2014;219:796–802. doi: 10.1016/j.jamcollsurg.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM; PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015; 16: 266–73. [DOI] [PubMed]
- 17.Wickberg Å, Liljegren G, Killander F, Lindman H, Bjöhle J, Carlberg M, et al. Omitting radiotherapy in women ≥ 65 years with low-risk early breast cancer after breast-conserving surgery and adjuvant endocrine therapy is safe. Eur J Surg Oncol. 2018;44:951–956. doi: 10.1016/j.ejso.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Fyles A, McCready D, Manchul L, et al. Preliminary results of a randomized study of tamoxifen +/- breast radiation in T1/2 N0 disease in women over 50 years of age. Proc ASCO. 2001;20:24a. [Google Scholar]
- 19.Hughes KS, Schnaper L, Berry D, et al. Comparison of lumpectomy plus tamoxifen with and without radiotherapy (RT) in women 70 years of age or older who have clinical stage I, estrogen receptor positive (ER +) breast carcinoma. Proc ASCO. 2001;20:24a. [Google Scholar]
- 20.The ATAC (Arimidex, Tamoxifen Alone or in Combination) Trialists’ Group. Anastrozole alone or in combinatiuon with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer. Results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 2003; 98:1802–10. [DOI] [PubMed]
- 21.Wickberg Å, Magnuson A, Holmberg L, Adami HO, Liljegren G. Influence of the subtype on local recurrence risk of breast cancer with or without radiation therapy. Breast. 2018;42:54–60. doi: 10.1016/j.breast.2018.08.097. [DOI] [PubMed] [Google Scholar]
- 22.Buchholz TA, Somerfield MR, Griggs JJ, El-Eid S, Hammond ME, Lyman GH, et al. Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol. 2014;32:1502–1506. doi: 10.1200/JCO.2014.55.1572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please e-mail Ohsumi S.
StatView 5, SAS Institute Inc.


