Abstract
A narrative review regarding percutaneous vertebroplasty (PVP) for osteoporotic vertebral fracture (OVF) is provided herein, addressing the epidemic of OVF in Japan, the latest response to the criticism of PVP for OVFs, the indications and potential risks of PVP for OVFs, and a future perspective for PVP. Each year in Japan, approximately 32,000 patients aged 55 years or older suffer from chronic low back pain for several months to several years due to a compression fracture. PVP is one of the surgical treatments for an OVF, and it is less invasive compared to the traditional open surgery. PVP is suitable for OVF patients who have difficulty walking as assessed by the modified Yokoyama’s activities of daily living (ADL) scoring system, and for patients with Kummell's disease diagnosed by CT and MRI examinations. Serious adverse events related to PVP occur in 1.1–3.3% of the cases, but direct deaths from PVP are extremely rare at less than 1%. Recent studies demonstrated that OVF patients treated with PVP are less likely to die after the treatment than non-surgically treated patients, which conflicts with the Cochran reviews’ conclusion not supporting PVP for OVFs. Novel robotic systems and procedure-support devices are being developed, providing a next step toward fully automated PVP procedures.
Keywords: Osteoporotic vertebral fractures, Percutaneous vertebroplasty, Review, Complication, Perspective
Introduction
Percutaneous vertebroplasty (PVP) is a treatment for patients with one or more symptomatic vertebral fractures caused by a bone tumor, osteoporosis, or trauma. In a PVP, bone biopsy needles are inserted into the fractured vertebra with the patient under local anesthesia; bone cement made of polymethyl methacrylate (PMMA) is injected through the needles, and then symptoms such as walking difficulty or back pain are immediately alleviated [1, 2]. A PVP procedure requires only 2 h of treatment time and 2 h of postoperative bed rest; it can be performed through a 5-mm skin incision for the insertion of each bone biopsy needle, it has a low frequency of serious adverse events, it can be performed without special preoperative preparation or intensive postoperative care, and the only absolute contraindications are an uncontrollable infection and bleeding tendency [2]. The nature of PVP as a minimally invasive procedure with a low rate of complications allows patients to return home after treatment without hospitalization [3] and makes it possible to treat patients over 90 years old and to guarantee therapeutic efficacy [4, 5].
The present narrative review focuses on the following topics: the incidence of osteoporotic vertebral fracture (OVF) in Japan, a response to the controversy regarding the use of PVP for OVFs, assessment and practical indicators to determine PVP indications for OVF patients, PVP complications, features and applications of BKP, and future PVP topics.
Incidence of osteoporotic compression fractures in Japan
OVFs are common among the elderly. Based on Japan's 2020 population census, the estimated number of OVF patients per year nationwide is 720,000 for those aged 55 years and older and 607,000 for those aged 65 years and older (Appendix 1) [6, 7]. However, about two-thirds of OVF patients are asymptomatic and do not necessarily require intensive treatment. Conversely, about one-third of OVF cases are painful and sometimes require bed rest in a hospital [8]. Patients with an 11th thoracic, 12th thoracic, or 1st lumbar vertebral fracture in the acute phase often require prolonged bed rest because they suffer from severe pain when transitioning from lying to sitting or vice versa. Such long-term bed rest may cause secondary complications; e.g., decreased ambulation, constipation, urinary tract infection, aspiration pneumonia, deep vein thrombosis, and decreased ability to live independently [9].
Nonunion vertebral fractures called ‘Kummell’s disease’ can develop in the chronic phase, and their rate has been reported to reach 13.5% after 6 months from the fracture onset [10]. Based on the above data, it is estimated that each year in Japan, 32,000 patients aged 55 years and over and 27,000 patients aged 65 years and over suffer for several months to several years from chronic low back pain due to pseudoarthrosis after a compression fracture.
For OVF patients, the current treatment policy for asymptomatic patients is based on a no-treatment follow-up or the initiation of treatment for osteoporosis, and for symptomatic patients conservative therapy is recommended [11]. Surgical treatment is used only in cases of nerve compression due to a vertebral fracture or in cases of poor recovery in response to conservative therapy [12]. Considering that OVF is more common in the vulnerable elderly with multiple pre-existing and comorbidities, OVF patients are often not indicated for surgery. It is thus natural that minimally invasive PVP treatment is attracting attention as an intermediate treatment that bridges the gap between conservative treatment and surgical treatment (Table 1).
Table 1.
Incidence of complications related to PVP
| Complication | Incidence |
|---|---|
| Adverse events | |
| Serious adverse events related to PVP [3, 48, 49] | 1.1–3.3% |
| Direct deaths from PVP [87] | 0.0018–0.002% |
| Bone cement leakage [3, 24, 48, 49, 51] | 34–91.3% |
| Symptomatic bone cement leakage [79] | 1.08% |
| Venous cement leakage detected by CT [62, 91, 92] | 12–26% |
| Pulmonary cement embolism | |
| Detection by chest radiograph [62] | 1.0–6.8% |
| Detection by chest CT [62] | 2.1–26% |
| Symptomatic pulmonary embolism [69] | 0.40% |
| Respiratory disorders other than cement embolism [49, 80] | 0.2–0.4% |
| Cardiac cement embolism [70] | 3.90% |
| Symptomatic cardiac embolism [70] | 0.33% |
| Cardiac dysfunction other than cement embolism | |
| Transient hypotension due to suspected vagal reflex [49] | 1.0% |
| Myocardial infarction [69] | 0.05% |
| Paraplegia/Paraparesis | |
| Paraplegia [59–61, 89, 90, 94] | < 1% |
| Paraparesis [59] | 0.75% |
| Nerve root symptoms [59] | 0.75% |
| Vessel damage and bleeding [53, 54, 57–61] | < 1% |
| Paraparesis due to intracanal subdural hematoma [59] | 0.5% |
| Infection after PVP [69, 72, 74, 75] | 0.1–0.46% |
| Allergy to PMMA [42–44] | < 1% |
| Puncture in the spinal canal [56] | 0.8–4.0% |
| Vertebral refracture after PVP | |
| Refracture in adjacent or remote vertebra [3, 24, 48, 49, 69] | 4.9–19% |
| Refracture in treated vertebra [81–83] | 0.56–3.2% |
| Fractures other than vertebral fractures related to PVP [3, 23, 80] | 1.1–2.6% |
| Cerebral infarction [84] | one case report |
| Fat embolism [85, 86] | two case reports |
The current PVP controversy and latest responses
The use of PVP began in 1987 with the treatment of vertebral hemangiomas by bone cement injection [1]. Due to the PVP characteristics of high safety, high efficacy, and low invasiveness, many patients with OVF have been treated with PVP in the United States since the late 1990s, and the number of research papers concerning PVP surged until 2010. However, it was announced in 2009 that there was no significant difference in therapeutic effects between PVP and a placebo treatment after 6 months [13, 14], and two Cochrane Reviews concluded that PVP treatment for OVFs was not supported [13].
However, it should be noted that the previous studies and reviews not supporting the effectiveness of PVP made a fundamental mistake in drawing these conclusions. Both pain as a sensory phenomenon and the quality of life (QOL) as an emotional perception, which are the main outcomes in the previous randomized control studies, can be evaluated only by self-assessment and are often difficult for both patients and inspectors to quantify accurately. There has been no boycott against PVP around the world; this may be because PVP clearly provides immediate pain relief and a practical improvement in patients' quality of life, along with a very low frequency of adverse events even for elderly patients.
As a latest response to the PVP controversy, its survival benefit addresses the core of the controversy. Significant mortality data should be at the forefront of the conversation regarding the efficacy of vertebral augmentation [15]. Hinde and colleagues recently published a meta-analysis of more than 2 million patients, including 16 studies in the US and 6 studies in other countries. The meta-analysis demonstrated that OVF patients treated with PVP or balloon kyphoplasty (BKP) had a 22% lower chance of dying up to 10 years after the treatment compared to those who received non-surgical treatment [16]. In another article analyzing BKP and PVP utilization for vertebral compression fracture patients in the US Medicare data set from 2005 to 2014, BKP and PVP had a 19% and 7% lower propensity-adjusted 10-year mortality risk than non-surgical treatment, respectively [17, 18]. These survival benefit findings counteract the criticism regarding the efficacy of vertebral augmentation that arose after the 2009 ‘sham’ control studies and the Cochran reviews' lack of support for performing PVP for patients with OVFs [15, 17, 19].
In Japan, in contrast, the application of PVP for OVFs has not become widespread even though the PVP procedure has been covered in part by the country’s national health insurance program since 2012. The reasons for this are as follows: First, the bone cement is surprisingly indicated only for neoplastic vertebral fractures but not for osteoporotic vertebral fractures. Strategic ideas aiming for the Japanese regulatory approval may be needed such as a new medical device kit for the bone cement along with bone biopsy needles like the BKP medical device kit. Second, the indication criteria for PVP are not well known. It is easier to understand if the vertebral fracture is considered separately for the acute vertebral fracture and Kummell’s disease, which is described later. Third, there are not many experts who can perform PVP. Appropriate training meetings for PVP procedures should be encouraged, together with full PVP insurance coverage. In light of Japan’s ‘super-aging’ society, the above problems will be hopefully solved in order to prevent the poor prognoses of elderly who need long-term care due to vertebral fractures.
Assessment of the pathological state of OVF patients
Once elderly people incur an OVF, they usually undergo the medical assessment of their back pain and QOL. Pain assessments are generally performed by using a numerical rating scale (NRS) or a visual analog scale (VAS). An NRS is used to estimate a patient's pain by having the patient choose a number in the range 0–10 where 0 indicates no pain and 10 indicates the maximum possible pain. A VAS is a 10-cm line with the words “no pain” on one end and “worst pain ever” on the other, and the patient is instructed to show a point on the line that represents the patient’s perception of his or her current condition [20]. Investigations of the QOL of patients with vertebral fracture have used the following: a Medical Outcome-Short Form (SF-36) [21], the Oswestry Disability Index (ODI) [22], the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO-41) [23], and Roland-Morris Disability Questionnaire (RMDQ) [3, 24].
However, as mentioned above, pain and quality of life are highly dependent on a patient's subjective perceptions. In the medical community, it is known that the satisfaction a patient feels after receiving a treatment that is valuable plus a good relationship with the treating medical staff are effective in providing the pain relief and a better QOL for the patient. In contrast, the circumstances that caused a patient's OVF, treatment-related bad feelings, anxiety about the future, and/or worsening relationships around the patient can contribute to sustained pain and even a worsening of his or her quality of life [25, 26]. Self-assessment indices should be used only as a reference when deciding on the indications for PVP and patient monitoring after PVP.
Indications and contraindications of PVP based on the characteristics of OVF
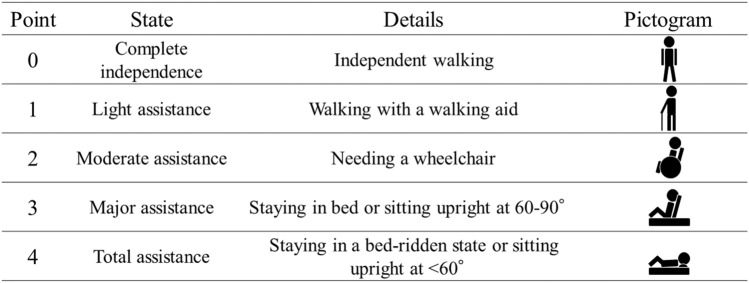
While both pain and QOL are subjective no matter what measurement system is adopted, the modified Yokoyama's activities of daily living (ADL) scoring system, which is an ADL measurement, is noteworthy [27, 28]. This system is as follows (Fig. 1): 0 points = complete independence, independent walking; 1 point = light assistance is needed, walking with a walking aid; 2 points = moderate assistance, needing a wheelchair for locomotion; 3 points = major assistance, mostly staying in bed or sitting upright at 60°–90°; 4 points = total assistance, mostly staying in a bed-ridden state or sitting upright at less than 60°. This scoring system has several advantages, including ease of use, simple and objective estimates by any medical staff, and a direct relationship between the mobility scores and the physical condition of patients with vertebral fractures. Clinical problems are also easy to predict with this system. For example, patients who score 3 or 4 points usually stay in bed, urinate/defecate in a bedridden state, require frequent medical staff assistance, and are at increased risk of secondary illness and long-term hospitalization. Patients with 2 points are normally in a wheelchair, urinate and defecate in a bathroom, undergo advanced rehabilitation for walking but still need assistance, and go on limited outings. Patients with 0 points or 1 point can walk, return home, and expand their activities [27].
Fig. 1.
The modified Yokoyama’s ADL scoring system is a numerical value from 0 to 4 that objectively and simply expresses mobility in bedridden, sitting, transferring, and walking after an OVF
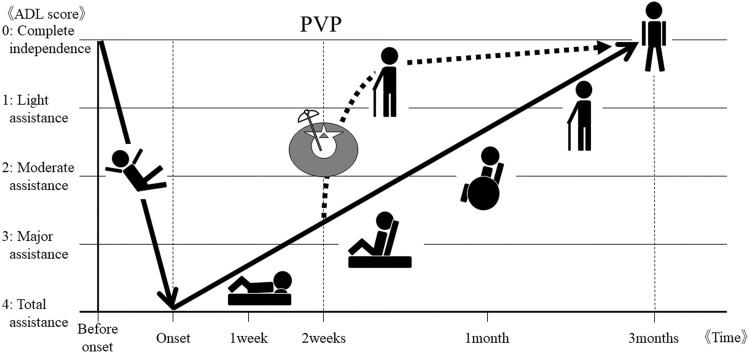
Figure 2 illustrates the temporal mobility change after an OVF due to a fall, which is common situation for the occurrence of an OVF. The vertical axis is the modified Yokoyama's ADL scoring system and the horizontal axis is the time course. After the patient's mobility becomes extremely low due to the vertebral fracture, in most cases, it may gradually recover in approximately 3 months with conservative treatment (as shown by the solid black line in Fig. 2) [29]. If the patient undergoes PVP, he or she is expected to have a rapid recovery (the dotted black line). PVP is positioned as a first-line treatment to achieve early recovery and early out-of-bed status, especially for such patients who are old enough for there to be concern about weakness, aspiration pneumonia, delirium, and/or cognitive decline due to prolonged bed rest. Thus, the modified Yokoyama's ADL scoring system is an excellent indicator for objectively determining PVP indications and for assessing post-PVP effects.
Fig. 2.
A hypothesis regarding the relationship between vertebral fractures and PVP. When an OVF significantly reduces a patient's QOL, most patients gradually recover with conservative therapy in 3 months, as shown by the solid black line. Patients are expected to recover rapidly with PVP intervention (black dotted line). A PVP can help a patient achieve early recovery and early bed leaving, especially for patients who are at risk of cognitive decline due to debilitation or to aspiration pneumonia or delirium due to long bed rest
On the other hand, there are also cases in which low back pain persists (i.e., Kummell's disease) in which the healing of trivial vertebral fractures or bone bruises is prolonged and nonunionized [30]. Kummell's disease is thought to be caused by the avascular osteonecrosis of the vertebral body, but the detailed pathogeny is unknown [31]. Kummell's disease is objectively diagnosed by imaging examinations that prove gas or fluid retention in the cleft inside the vertebral body [32]. Patients with Kummell's disease may be treated by non-surgical interventions including bed rest, lumbar traction, a brace, analgesics and anti-osteoporosis drugs, all of which may not be sufficient and may be ineffective. Moreover, these interventions might pose the risk of a delayed spinal collapse causing paralysis [33, 34]. Thus, patients with Kummell's disease without neurological symptoms related to spinal cord compression may be good candidates for PVP, whether or not they are able to walk [35–37]. PVP has been shown to be able to immediately relieve low back pain in Kummell's disease, achieve satisfactory clinical efficacy, partially restore vertebral height, and correct kyphosis [34, 38, 39].
Ambulatory OVF patients with no Kummell's disease have shown limited benefits of PVP and should be treated conservatively. However, Venmans et al. reported that while 40% of conservatively treated patients had sufficient pain relief during the first 3 months post-fracture onset, the other 60% still had pain at the last follow-up at 12 months. Patients who have low back pain for more than 3 months due to an unhealed vertebral fracture can thus be considered candidates for PVP [29].
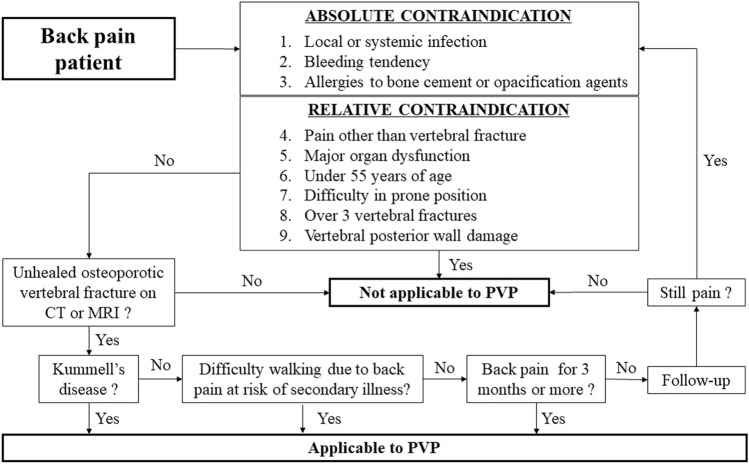
Clinicians should also be aware of the exclusion criteria for PVP as proposed in the guidelines for PVP for osteoporotic vertebral fracture [40, 41]; (1) uncontrollable local or systemic infections, (2) uncontrollable bleeding tendency, (3) allergies to bone cement or opacification agents, (4) back pain from a condition other than a vertebral fracture, (5) major organ dysfunction, (6) under 55 years of age, (7) difficulty in the prone position, (8) 4 or more vertebral fractures, and (9) vertebral posterior wall damage. (1)–(3) and (4)–(9) are considered to be absolute and relative contraindications to PVP, respectively.
PVP for a patient with infectious spondylitis or an uncontrollable systemic infection should be avoided because the removal of bone cement is difficult when the activation of an infection occurs. PVP is one of the nonvascular procedures and involves less bleeding compared to other vascular-approach procedures. However, a bleeding tendency should be controlled, as there is no guarantee that vessels will never be accidentally punctured during the procedure. Allergy to bone cement or opacification agents is reported to be very rare [42–44], and is an absolute contraindication to PVP.
PVP is not effective for back pain other than vertebral fractures such as nerve compression pain, because PVP is effective by stabilizing the vertebral body with bone cement injected into the cleft of the fracture [45, 46]. However, nerve compression pain that coexists with vertebral fracture pain might be indicated for PVP because nerve compression caused by spinal instability may be reduced by PVP. Major organ dysfunctions are relatively contraindicated. In particular, patients with a substantial amount of pleural effusion can find it difficult to tolerate the prone position for the approximately 2-h period that is necessary to perform PVP. Patients under the age of 55 years are also relatively unsuitable for PVP indications because the bone cement is permanently placed and difficult to remove. Difficulty in the use of prone position is thus one of the relative contraindications for PVP. The use of the lateral decubitus position for PVP is possible [47], but technically difficult. The PVP for patients with four or more spinal fractures may be accomplished in two or more sessions, because the safety of the in vivo use of two or more packs of bone cement preparation is not guaranteed. In cases of a vertebral fracture with posterior wall damage, close attention should be paid to the possibility of bone cement leakage into the spinal canal. In patients with a history of thoracic or lumbar spine surgery, difficulty in obtaining visibility on fluoroscopy during the procedure may be encountered.
Figure 3 is a flowchart based on the above information. First, the presence of an unhealed OVF on CT or MRI should be confirmed in a patient with low back pain, and the patient's condition should not meet any of the PVP exclusion criteria. The patient may be eligible for PVP if he or she has Kummell's disease, difficulty walking due to back pain at risk of secondary illness, or back pain that lasts more than 3 months.
Fig. 3.
Clinical reasoning flowchart of PVP indications for low back pain. Symptomatic OVF patients without an uncontrollable local or systemic infection, uncontrollable bleeding tendency, allergies to bone cement or opacification agents, major organ dysfunction, under 55 years of age, with difficulty in being in the prone position, more than 3 vertebral fractures, or vertebral posterior wall damage may be eligible for PVP if they have Kummell's disease, difficulty walking due back pain at risk of secondary illness, or back pain that lasts more than 3 months
Potential risk of PVP procedure for osteoporotic vertebral fractures
It must be acknowledged that like essentially every medical procedure, PVP is not perfectly safe [3, 24, 48–52]. Adverse events of PVP include damage to the spinal cord and thoracoabdominal organs or bleeding due to inappropriate puncture needle insertion [53–61], compression or damage to the spinal cord or nerve roots, venous thrombosis, or cardiopulmonary cement embolization due to leakage of bone cement outside the vertebral body [3, 24, 42–44, 48, 49, 51, 55, 59, 62–70], sudden reduction in blood pressure and shock symptoms due to allergic reactions [42–44], infection due to a non-sterile operation [69, 71–75], vertebral refractures or fractures other than in a vertebra [3, 23, 24, 48, 49, 69, 76–83], and the other rare but serious complications [49, 69, 80, 84–86]. This section describes notable complications in conjunction with their incidence even if they occur very rarely.
Incidence of all adverse events
Symptomatic adverse events related to PVP occur in 1.1–3.9% of cases [3, 41, 48, 49], but the patients have almost always recovered. Direct deaths from PVP are extremely rare at 0.0018–0.002% [87]. However, OVF patients who do or do not undergo PVP are at a 1.2–5% risk of death from another disease within 1 year after fracture onset [3, 24, 48, 49, 51, 52]. The 10-year risk of death for OVF patients was reported to be surprisingly high at 85.1% [17]. It should be noted that even though PVP is a low-risk treatment, OVF patients are at high risk of dying from diseases other than osteoporosis.
Bone cement leakage
Bone cement leakage has been observed in 34–91.3% of vertebral bodies [3, 24, 48, 49, 51]. However, most of these cases are asymptomatic. Lee et al. reported in their meta-analysis that symptomatic cement leakage occurs in 1.08% [79]. Complications associated with bone cement leakage include pulmonary cement embolism [62, 69, 88], cardiac cement embolism [63, 70], bilateral lower limb paraplegia due to intravertebral canal cement leakage [55, 59, 89, 90], and a cement-embolic cerebral infarction [84], which are described below.
Pulmonary cement embolism (PCE)
The reported incidence of pulmonary cement embolism (PCE) in PVP cases was 1–6.8% as shown by chest radiographs and 2.1–26% revealed by chest computed tomography (CT) [62]. However, a PCE is almost always asymptomatic; symptoms associated with a PCE were observed only in 0.4% of PVP cases [69]. Patients with a PCE can be divided into four groups; those with a (1) asymptomatic peripheral embolism, (2) symptomatic peripheral embolism, (3) asymptomatic central embolism, and (4) symptomatic central embolism (central embolism includes the main pulmonary trunk and / or right or left main pulmonary artery, and an embolism beyond that is considered a peripheral pulmonary embolism). For group 1 patients, no treatment is recommended. For groups 2 and 3 patients with a symptomatic peripheral or asymptomatic central embolism, it is recommended that standard treatment guidelines for the treatment of thrombotic pulmonary embolism should be followed. For group 4 patients with a symptomatic central embolism, surgical treatment by embolectomy has been proposed [62].
Respiratory disorders other than PCE
The exact frequency or respiratory disorders other than PCE is at 0.2–0.4% [49, 80]. Kobayashi et al. reported the case of a PVP patient with a transient decrease in oxygen saturation during treatment due to a suspected sedative overdose [49]. Two respiratory arrest cases have also been reported [80]. There were no deaths as a result of PVP in either report.
Cardiac cement embolism
Venous cement leakage of patients treated with PVP occurs in 12–26% detected by CT [62, 91, 92], and a cardiac cement embolism may further migrate to the right ventricle and pulmonary artery [63]. The reported incidence of intracardiac cement embolism after PV was 72/1854 sessions (3.9%), of these, only 5 cases (0.33%) were symptomatic [70]. The most common symptoms appear to be chest pain and dyspnea. There is a case report of PMMA leaking from a vein moving to the heart and piercing the heart, but the patient recovered after emergency surgery [93].
Cardiac dysfunction other than cement embolism
Transient hypotension due to suspected vagal reflex was reported in five of 485 patients (1.0%) during PVP [49]. The rate of myocardial infarction was one in 1,938 (0.05%) PVP cases [69]. There were no deaths as a result of PVP in either of these reports.
Neurological damage related to PVP
Paraparesis and nerve root symptoms after PVP are each estimated to occur in approximately 0.75% of cases, respectively [59]. Paraplegia or paraparesis is reported to be caused by spinal canal stenosis due to bone cement leakage, a migration of bone cement to anterior spinal artery, an intracanal hematoma associated with needle puncture, or an unknown cause. To prevent these complications, penetration of the inside of the pedicle should be avoided, and the viscosity of the PMMA must be monitored. Immediate spinal canal decompression surgery is recommended when paraplegia is due to a space-occupying lesion in the spinal canal [59–61, 89, 90, 94].
Vessel damage and bleeding
There are reports about incorrect punctures of the thoracoabdominal aorta and lumbar artery [53, 54, 57–59]. Such punctures can also cause intracanal bleeding (extradural, subdural, subarachnoid hemorrhage), which can lead to transient lower limb paralysis or sequelae [59–61]. Paraparesis due to the intracanal spinal epidural hematoma were reported to be 0.5% [59].
Post-PVP infections
Infectious complications after PVP are extremely rare at 0.1–0.46% [69, 72, 74, 75]. However, in PVP patients with significant neurological symptoms, severe instability due to an infected fracture, or resistance to antibiotics, prompt surgical treatment should be considered. Risks of infection are suspected to be associated with a history of bacteremia, urinary tract infection, or pulmonary tuberculosis prior to PVP [72, 74, 75]. At least for the prevention of perioperative infections, prophylactic antibiotics during a PVP procedure are recommended [95].
Allergy to PMMA
The exact frequency of allergy to PMMA is unknown, as it is rarely reported or summarized; the incidence is probably less than 1%. PMMA is commonly used for medical products such as dialysis membranes, intraocular lenses, dental fillers, and more. PMMA allergic reactions are thus extremely rare. However, it should be noted that cases of an acute or delayed allergic reaction to PMMA (including death) have been reported [42–44].
Puncture into the spinal canal
The rate of a puncture-needle penetration into the spinal canal during PVP is 0.8–4.0% [56]. All of the reported patients with a puncture-needle penetration into the spinal canal were asymptomatic with conservative treatment of extended postoperative resting time and follow-up by imaging examinations. Even if an experienced PVP expert administers the needle punctures, he or she cannot accomplish zero penetration into the spinal canal. CT images should be obtained immediately after the puncture(s), and the path of the puncture needle should be checked; if the needle penetrates the spinal canal, the leakage of PMMA into the spinal canal must be prevented, and a careful follow-up after PVP for secondary complications such as cerebrospinal fluid leakage and intracanal bleeding should be conducted.
Refracture in adjacent or remote levels of the vertebra after PVP
A vertebral refracture occurs in 4.9–19% of patients after PVP. However, the frequency of vertebral refracture after PVP is equal to that without PVP. Vertebral refractures after PVP can also occur in adjacent as well as distant vertebral bodies [3, 24, 48, 49, 69], but, empirically, patients who are re-treated for recurrence of compression fractures a few days after PVP have often new compression fractures of adjacent vertebral bodies directly above or below the PVP-treated vertebral body [96]. Prophylactic PVP as an optional treatment is preferably performed on normal upper and lower vertebral bodies adjacent PVP-treated vertebra [97, 98].
Refracture of PVP-treated vertebra
In rare cases at 0.56–3.2%, PVP-treated vertebral bodies may re-collapse and symptoms may recur [81–83]. In particular, lumpy cement may move within the vertebral body [99], resulting in incomplete compression fracture healing. In such cases, PVP should be re-executed and the movable cement should be fixed to the vertebral body with additional bone cement, or a corpectomy in conjunction with posterior fixation and internal fixation should be performed. In order To prevent such cement movement, it is desirable to perform PVP as well as a pediculoplasty in which cement is placed along the pathway of the needle within the vertebral arch as an anchor. Experimentally, there was a case in which the anchor broke and started to move the cement during the patient's activities of daily living when the pediculoplasty was performed with a 13-gauge needle. Therefore, for post-PVP patients with Kummell's disease, in which lumpy cementation can be expected, a pediculoplasty with an 11-gauge needle is considered desirable.
Fractures other than vertebral fractures related to PVP
In rare cases at 1.1–2.6% [3, 23, 80], fractures other than compression fractures occurring during PVP including rib fractures [41, 80, 100], humerus fractures [3], and transverse process fractures [80] have been reported. Careful PVP treatment for patients with osteoporosis is essential. As a pitfall, the new symptoms that appear after PVP may actually be due to preexisting fractures elsewhere. Some patients with severe back pain may not be aware of relatively mild pain in another part of the body. A careful preoperative assessment and a review of pre- and postoperative imaging examination may be informative.
Cerebral infarction
A cerebral infarction was reported in a 71-year-old woman who had a stroke ~ 30 min after the completion of PVP. Bone cement migrated into the venous system, resulting in a left middle cerebral artery embolus. The patient was presumed to have a right-to-left shunt from either a patent foramen ovale or a pulmonary arteriovenous malformation [84].
Fat embolism
A fatal case with fat embolism and a cerebral embolism case with fat embolism were reported. Fat embolism is considered rare, but clinicians should be aware of it [85, 86].
Features and applications of BKP
Whereas PVP aims to stabilize vertebral fractures by percutaneously injecting bone cement into the vertebral body, BKP not only stabilizes the vertebral body but also restore body height, which may be thought as an advanced method of PVP [101]. BKP, which was first clinically applied to patients with OVFs by Lieberman et al., restores vertebral body height by inflating a balloon inserted into the vertebral body to form a cavity and injecting bone cement into the vertebral body [102]. As a secondary advantage, BKP has a low frequency of cement leakage. This is because BKP allows low-pressure cement injection into the cavity, whereas PVP requires high-pressure cement injection into the vertebral body with residual trabecular structure [103]. In meta-analyses of clinical studies, BKP indeed restored higher vertebral body height with less frequent cement leakage compared to PVP. However, cement leakage usually did not cause clinical symptoms, and radiographic differences of vertebral height restoration did not significantly affect clinical outcomes. That is, both BKP and PVP were effective in reducing pain, improving ODI, and controlling major complications, without any significant difference between the two [104, 105]. Also, regarding the hypothesis that recovery of kyphosis promotes recovery of respiratory function, both BKP and PVP improved respiratory function in patients with chronic obstructive pulmonary disease (COPD) with no significant difference between the two although BKP had higher vertebral height recovery than PVP [106]. We should reconfirm the fact that PVP has a considerable effect of vertebral body height restoration, where BKP is significantly superior to PVP [107]. On the other hand, regarding the hypothesis that BKP's bone cement forms a solid lump which reduces the elasticity of the bone and induces refracture of the adjacent vertebral body [76], there was no significant difference of the vertebral refracture rate between the two [108].
The cost of BKP is 3 to 4 times that of PVP [108, 109]. BKP requires medical equipment such as an inflatable bone tamp in addition to bone biopsy needles and bone cement, and BKP is usually performed under general anesthesia and requires hospitalization for at least one night [101]. On the other hand, PVP is usually performed with local anesthesia, requires only bone biopsy needle and bone cement, and outpatient treatment is also possible [3].
Heini et al. proposed the indication of BKP as follows [101]; (1) patients with osteoporotic vertebral fractures whose height loss is related to a spinal stenosis and height restoration can relieve the symptoms, (2) patients with traumatic fractures where the endplate relocation should be attempted, or (3) patients with bone tumor for whom the cavity formation might help with difficulty of tumorous lesions.
Future advancement of PVP
Novel robotic systems and procedure-support devices are being developed, providing a next step in fully automated PVP procedures. Neumann et al. developed a robotic system that can be remotely controlled to inject bone cement, along with a cold passive exchanger that slows cement hardening [110]. A navigation system in which a C-arm tracker, patient tracker, and puncture-needle tracker coordinate spinal imaging information to assist in accurate punctures in PVP was described by Xu et al. [111]. Wang et al. proposed a method to guide the puncture in BKP that uses a system consisting of a reference tracker, robotic arm, and monitor [112]. As a unique attempt, a 3D-printer was used to create a puncture guide adapter for PVP with two sockets for needle insertion [113].
As an advanced computing system applied to PVP, Uchiyama et al. developed a 'spinal needling intervention practice using ray-summation imaging' (SNIPURS) to assist radiology trainees to learn needle puncturing [114]. Trainees freely orient the workstation-generated spinal ray-summation image to view the appearance of the spine in any direction by using a computer mouse, and they can then determine the on-end puncture point in the selected direction. As the post-processing, the virtual needle is visualized with an excavation tool, and the path of the puncture needle is evaluated on the workstation. Trainees undergo a total of 48 vertebral needle targeting simulations and complete basic PVP procedures. The above-described equipment and methods are promising, and further developments are expected.
Summary
In Japan, each year roughly 32,000 patients aged 55 years or older are estimated to suffer from low back pain for several months to several years due to pseudoarthrosis after experiencing a compression fracture. PVP is a treatment that can provide immediate pain relief and mobility improvement from difficulty in sitting, transfer, and walking due to vertebral fractures. PVP is also suitable for patients without neurological deficit but with Kummell's disease, which is diagnosed by imaging examinations that confirm gas or fluid retention inside the vertebral body. Serious adverse events related to PVP occur in 1.1–3.3% of cases, and direct deaths from PVP are extremely rare (less than1%). Recent reports demonstrated that OVF patients treated with PVP or balloon kyphoplasty had a lower chance of dying within 10 years post-fracture onset than those who received non-surgical treatment. This is in opposition to the Cochrane Reviews' conclusion that vertebral augmentation for osteoporotic vertebral fractures is not supported. Both BKP and PVP are effective in improving the pain and QOL caused by OVF with minimal complications. Advanced robotic systems, procedure-support devices, and computing training systems are promising, and further PVP developments are expected.
Acknowledgements
This work was supported in part by Grant-in-Aid for Scientific Research of Japan Society for the Promotion of Science (21K07636).
Appendix
The 10-year cumulative incidence of compression fractures in men and women with absence of vertebral fractures at the initial survey is 2.2% and 2.1% for 40–49 years of age, 4.9% and 4.5% for 50–59 years, 6.1% and 14% for 60–69 years, and 10.8% and 22.2% for 70–79 years, respectively [6]. From these findings, the average annual incidence is calculated to be 0.222% and 0.212% for 40–49 years, 0.501% and 0.459% for 50–59 years, 0.522% and 1.497% for 60–69 years, and 1.136% and 2.479% for 70–79 years, respectively. Applying this to Japan's 2020 population census [7] with assumption that the one-year incidence of aged 80 and over is same as 70–79 years, the incidence of compression fractures aged 55 and over was 720,000, and the incidence of compression fractures aged 65 and over was 607,000. While many of these patients are asymptomatic, 33% of them develop symptomatic compression fractures [8]. Accordingly, the incidence of symptomatic compression fractures aged 55 and over was 240,000, and those aged 65 and over was 203,000. 13.5% of these patients develop nonunion and chronic low back pain after 6 months [10]. The nonunion rate after compression fractures over 55 years was 32,000, and those aged 65 and over was 27,000 in Japan.
Author contributions
TN data analysis, paper writing. KY paper correction. RK data collection. JM data collection.
Declarations
Conflict of interest
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Ethical statement
This review does not apply to studies involving human subjects and/or animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tomoyuki Noguchi, Email: tnogucci@gmail.com.
Koji Yamashita, Email: yamakou@radiol.med.kyushu-u.ac.jp.
Ryotaro Kamei, Email: fortuna.caeca1489@gmail.com.
Junki Maehara, Email: ddprogress@hotmail.co.jp.
References
- 1.Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33(2):166–168. [PubMed] [Google Scholar]
- 2.Mathis JM, Deramond H, Belkoff SM. Percutaneous vertebroplasty and kyphoplasty. New York: Springer; 2006. [Google Scholar]
- 3.Clark W, Bird P, Gonski P, Diamond TH, Smerdely P, McNeil HP, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10052):1408–1416. doi: 10.1016/S0140-6736(16)31341-1. [DOI] [PubMed] [Google Scholar]
- 4.DePalma MJ, Ketchum JM, Frankel BM, Frey ME. Percutaneous vertebroplasty for osteoporotic vertebral compression fractures in the nonagenarians: a prospective study evaluating pain reduction and new symptomatic fracture rate. Spine (Phila Pa 1976) 2011;36(4):277–282. doi: 10.1097/BRS.0b013e3181cf8a37. [DOI] [PubMed] [Google Scholar]
- 5.Kamei S, Noguchi T, Shida Y, Okafuji T, Yokoyama K, Uchiyama F, et al. The safety and efficacy of percutaneous vertebroplasty for patients over 90 years old. Jpn J Radiol. 2019;37(2):178–185. doi: 10.1007/s11604-018-0797-1. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura N, Kinoshita H, Oka H, Muraki S, Mabuchi A, Kawaguchi H, et al. Cumulative incidence and changes in the prevalence of vertebral fractures in a rural Japanese community: a 10-year follow-up of the Miyama cohort. Arch Osteoporos. 2006;1(1–2):43–49. doi: 10.1007/s11657-006-0007-0. [DOI] [Google Scholar]
- 7.Statistics Bureau of Japan. Population by sex, age (single years) and all nationality or Japanese, and average age and median age by sex and all nationality or Japanese—Japan, Prefectures, 21 Major Cities, Ku-area of Tokyo and Shi with population of 500,000 or more (00200521) 2020.
- 8.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 9.Parry SM, Puthucheary ZA. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem Physiol Med. 2015;4:16. doi: 10.1186/s13728-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsujio T, Nakamura H, Terai H, Hoshino M, Namikawa T, Matsumura A, et al. Characteristic radiographic or magnetic resonance images of fresh osteoporotic vertebral fractures predicting potential risk for nonunion: a prospective multicenter study. Spine (Phila Pa 1976) 2011;36(15):1229–1235. doi: 10.1097/BRS.0b013e3181f29e8d. [DOI] [PubMed] [Google Scholar]
- 11.Nishida K, Kusumegi A, Sakamoto Y. Treating osteoporotic compression fracture: from conservative treatment to BKP. Jpn J Neurosurg. 2016;25(9):718–729. doi: 10.7887/jcns.25.718. [DOI] [Google Scholar]
- 12.Ito M, Harada A, Nakano T, Kuratsu S, Deguchi M, Sueyoshi Y, et al. Retrospective multicenter study of surgical treatments for osteoporotic vertebral fractures. J Orthop Sci. 2010;15(3):289–293. doi: 10.1007/s00776-010-1455-3. [DOI] [PubMed] [Google Scholar]
- 13.Buchbinder R, Johnston RV, Rischin KJ, Homik J, Jones CA, Golmohammadi K, et al. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database Syst Rev. 2018;11(11):CD006349. doi: 10.1002/14651858.CD006349.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569–579. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jennings JW. Vertebral augmentation is more than just pain palliation, it is about improved mortality. Radiology. 2020;295(1):104–105. doi: 10.1148/radiol.2020192806. [DOI] [PubMed] [Google Scholar]
- 16.Hinde K, Maingard J, Hirsch JA, Phan K, Asadi H, Chandra RV. Mortality outcomes of vertebral augmentation (vertebroplasty and/or balloon kyphoplasty) for osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Radiology. 2020;295(1):96–103. doi: 10.1148/radiol.2020191294. [DOI] [PubMed] [Google Scholar]
- 17.Ong KL, Beall DP, Frohbergh M, Lau E, Hirsch JA. Were VCF patients at higher risk of mortality following the 2009 publication of the vertebroplasty "sham" trials? Osteoporos Int. 2018;29(2):375–383. doi: 10.1007/s00198-017-4281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edidin AA, Ong KL, Lau E, Kurtz SM. Life expectancy following diagnosis of a vertebral compression fracture. Osteoporos Int. 2013;24(2):451–458. doi: 10.1007/s00198-012-1965-2. [DOI] [PubMed] [Google Scholar]
- 19.Ong KL, Beall DP, Frohbergh M, Lau E, Hirsch JA. Reply to "At what price decreased mortality risk?". Osteoporos Int. 2018;29(8):1929–1930. doi: 10.1007/s00198-018-4551-4. [DOI] [PubMed] [Google Scholar]
- 20.Crichton N. Visual analogue scale (VAS) J Clin Nurs. 2001;10(5):706–716. [Google Scholar]
- 21.Rousing R, Andersen MO, Jespersen SM, Thomsen K, Lauritsen J. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures: three-months follow-up in a clinical randomized study. Spine (Phila Pa 1976) 2009;34(13):1349–1354. doi: 10.1097/BRS.0b013e3181a4e628. [DOI] [PubMed] [Google Scholar]
- 22.Son S, Lee SG, Kim WK, Park CW, Yoo CJ. Early vertebroplasty versus delayed vertebroplasty for acute osteoporotic compression fracture: are the results of the two surgical strategies the same? J Korean Neurosurg Soc. 2014;56(3):211–217. doi: 10.3340/jkns.2014.56.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557–568. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 24.Klazen CA, Lohle PN, de Vries J, Jansen FH, Tielbeek AV, Blonk MC, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet. 2010;376(9746):1085–1092. doi: 10.1016/S0140-6736(10)60954-3. [DOI] [PubMed] [Google Scholar]
- 25.Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. Lancet Neurol. 2004;3(11):679–684. doi: 10.1016/S1474-4422(04)00908-1. [DOI] [PubMed] [Google Scholar]
- 26.Varelmann D, Pancaro C, Cappiello EC, Camann WR. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110(3):868–870. doi: 10.1213/ANE.0b013e3181cc5727. [DOI] [PubMed] [Google Scholar]
- 27.Shida Y, Noguchi T, Okafuji T, Murakami K, Iraha T, Yokoyama K, et al. Percutaneous vertebroplasty for acute osteoporotic vertebral fracture contributes to restoration of ambulation. Interv Radiol. 2017;2:1–5. doi: 10.22575/interventionalradiology.2017-0005. [DOI] [Google Scholar]
- 28.Yokoyama K, Kawanishi M, Yamada M, Tanaka H, Ito Y, Hirano M, et al. Validity of intervertebral bone cement infusion for painful vertebral compression fractures based on the presence of vertebral mobility. AJNR Am J Neuroradiol. 2013;34(1):228–232. doi: 10.3174/ajnr.A3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venmans A, Klazen CA, Lohle PN, Mali WP, van Rooij WJ. Natural history of pain in patients with conservatively treated osteoporotic vertebral compression fractures: results from VERTOS II. AJNR Am J Neuroradiol. 2012;33(3):519–521. doi: 10.3174/ajnr.A2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kummell H. Die rareficirende ostitis der wirbelkorper. Verh Ges Dtsch Naturf Aerzte. 1891;64:282–285. [Google Scholar]
- 31.Matzaroglou C, Georgiou CS, Panagopoulos A, Assimakopoulos K, Wilke HJ, Habermann B, et al. Kummell's disease: clarifying the mechanisms and patients' inclusion criteria. Open Orthop J. 2014;8:288–297. doi: 10.2174/1874325001408010288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malghem J, Maldague B, Labaisse MA, Dooms G, Duprez T, Devogelaer JP, et al. Intravertebral vacuum cleft: changes in content after supine positioning. Radiology. 1993;187(2):483–487. doi: 10.1148/radiology.187.2.8475295. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Liang CZ, Chen QX. Kummell's disease, an uncommon and complicated spinal disorder: a review. J Int Med Res. 2012;40(2):406–414. doi: 10.1177/147323001204000202. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B, Chen G, Yang X, Fan T, Chen Z. Percutaneous kyphoplasty versus percutaneous vertebroplasty for neurologically intact osteoporotic Kummell's disease: a systematic review and meta-analysis. Glob Spine J. 2022;12(2):308–322. doi: 10.1177/2192568220984129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu RS, Kan SL, Ning GZ, Chen LX, Cao ZG, Jiang ZH, et al. Which is the best treatment of osteoporotic vertebral compression fractures: balloon kyphoplasty, percutaneous vertebroplasty, or non-surgical treatment? A Bayesian network meta-analysis. Osteoporos Int. 2019;30(2):287–298. doi: 10.1007/s00198-018-4804-2. [DOI] [PubMed] [Google Scholar]
- 36.Adamska O, Modzelewski K, Stolarczyk A, Kseniuk J. Is Kummell's disease a misdiagnosed and/or an underreported complication of osteoporotic vertebral compression fractures? A pattern of the condition and available treatment modalities. J Clin Med. 2021;10(12):2584. doi: 10.3390/jcm10122584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang JZ, Bei MJ, Shu DP, Sun CJ, Chen JB, Xiao YP. Comparison of the clinical outcomes of percutaneous vertebroplasty vs kyphoplasty for the treatment of osteoporotic Kummell's disease: a prospective cohort study. BMC Musculoskelet Disord. 2020;21(1):238. doi: 10.1186/s12891-020-03271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang X, Chang H, Xu H, Chen X, Wang H, Song Y. Comparison of outcomes between percutaneous vertebroplasty and percutaneous kyphoplasty for the treatment of Kummell's disease: a meta-analysis. Clin Spine Surg. 2022;35(6):276–286. doi: 10.1097/BSD.0000000000001269. [DOI] [PubMed] [Google Scholar]
- 39.Cabrera JP, Camino-Willhuber G, Guiroy A, Carazzo CA, Gagliardi M, Joaquim AF. Vertebral augmentation plus short-segment fixation versus vertebral augmentation alone in Kummell's disease: a systematic review and meta-analysis. Neurosurg Rev. 2022;45(2):1009–1018. doi: 10.1007/s10143-021-01661-8. [DOI] [PubMed] [Google Scholar]
- 40.Kato S, Kawakami N, Togawa D, Kawanishi M, Takahashi T, Arai Y, et al. Guidelines for safe percutaneous vertebroplasty (PVP) for osteoporotic vertebral fractures. The Japanese Society for Spine Surgery and Related Research (JSSR) tNSoJN, and the Japanese Society of Interventional Radiology (JSIR), editor. website of Japanese Society Interventional Radiology(JSIR) (http://www.jsir.or.jp/about/guide_line/pvp/)2013.
- 41.Tsoumakidou G, Too CW, Koch G, Caudrelier J, Cazzato RL, Garnon J, et al. CIRSE guidelines on percutaneous vertebral augmentation. Cardiovasc Intervent Radiol. 2017;40(3):331–342. doi: 10.1007/s00270-017-1574-8. [DOI] [PubMed] [Google Scholar]
- 42.Kirby BS, Doyle A, Gilula LA. Acute bronchospasm due to exposure to polymethylmethacrylate vapors during percutaneous vertebroplasty. AJR Am J Roentgenol. 2003;180(2):543–544. doi: 10.2214/ajr.180.2.1800543. [DOI] [PubMed] [Google Scholar]
- 43.Mahadevia AA, Weiland D, Kvamme P, Murphy KP, Srinivas A, Wyse G. Polymethylmethacrylate contact dermatitis after vertebroplasty. J Vasc Interv Radiol. 2007;18(4):585. doi: 10.1016/j.jvir.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 44.Yoo KY, Jeong SW, Yoon W, Lee J. Acute respiratory distress syndrome associated with pulmonary cement embolism following percutaneous vertebroplasty with polymethylmethacrylate. Spine (Phila Pa 1976) 2004;29(14):E294–E297. doi: 10.1097/01.BRS.0000131211.87594.B0. [DOI] [PubMed] [Google Scholar]
- 45.Noguchi T, Ueshima E, Komemushi A, Sugawara S, Nishio M, Haraguchi T, et al. 2019 Guidelines for Percutaneous Vertebroplasty (PVP) for Spinal Metastases Version 1.00 (JSIR) tJSoIR, editor. JSIR website (http://www.jsir.or.jp/about/guide_line/pvp/)2019.
- 46.Shaibani A, Ali S, Bhatt H. Vertebroplasty and kyphoplasty for the palliation of pain. Semin Intervent Radiol. 2007;24(4):409–418. doi: 10.1055/s-2007-992329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noguchi T, Shida Y, Okafuji T, Kamei S, Yokoyama K, Uchiyama F, et al. Safety and efficacy of percutaneous vertebroplasty in lateral decubitus position: a retrospective evaluation. Interv Radiol. 2018;3(3):115–120. doi: 10.22575/interventionalradiology.2018-0006. [DOI] [Google Scholar]
- 48.Firanescu CE, de Vries J, Lodder P, Venmans A, Schoemaker MC, Smeets AJ, et al. Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): randomised sham controlled clinical trial. BMJ. 2018;361:k1551. doi: 10.1136/bmj.k1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi N, Noguchi T, Kobayashi D, Saito H, Shimoyama K, Tajima T, et al. Safety and efficacy of percutaneous vertebroplasty for osteoporotic vertebral compression fractures: a multicenter retrospective study in Japan. Interv Radiol. 2021;6(2):21–28. doi: 10.22575/interventionalradiology.2020-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baerlocher MO, Saad WE, Dariushnia S, Barr JD, McGraw JK, Nikolic B, et al. Quality improvement guidelines for percutaneous vertebroplasty. J Vasc Interv Radiol. 2014;25(2):165–170. doi: 10.1016/j.jvir.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Hansen EJ, Simony A, Carreon LY, Rousing R, Tropp HT, Andersen MØ. Vertebroplasty vs SHAM for treating osteoporotic vertebral compression fractures: a double blind RCT. Integr J Orthop Traumatol. 2019;2(4):1–6. [Google Scholar]
- 52.Barr JD, Jensen ME, Hirsch JA, McGraw JK, Barr RM, Brook AL, et al. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the Society of Interventional Radiology (SIR), American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS), American College of Radiology (ACR), American Society of Neuroradiology (ASNR), American Society of Spine Radiology (ASSR), Canadian Interventional Radiology Association (CIRA), and the Society of NeuroInterventional Surgery (SNIS) J Vasc Interv Radiol. 2014;25(2):171–181. doi: 10.1016/j.jvir.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Giordano AV, Arrigoni F, Bruno F, Carducci S, Varrassi M, Zugaro L, et al. Interventional radiology management of a ruptured lumbar artery pseudoaneurysm after cryoablation and vertebroplasty of a lumbar metastasis. Cardiovasc Intervent Radiol. 2017;40(5):776–779. doi: 10.1007/s00270-016-1551-7. [DOI] [PubMed] [Google Scholar]
- 54.Puri AS, Colen RR, Reddy AS, Groff MW, DiNobile D, Killoran T, et al. Lumbar artery pseudoaneurysm after percutaneous vertebroplasty: a unique vascular complication. J Neurosurg Spine. 2011;14(2):296–299. doi: 10.3171/2010.10.SPINE1082. [DOI] [PubMed] [Google Scholar]
- 55.Baumann C, Fuchs H, Kiwit J, Westphalen K, Hierholzer J. Complications in percutaneous vertebroplasty associated with puncture or cement leakage. Cardiovasc Intervent Radiol. 2007;30(2):161–168. doi: 10.1007/s00270-006-0133-5. [DOI] [PubMed] [Google Scholar]
- 56.Noguchi T, Yamashita K, Shida Y, Okafuji T, Kamei R, Maehara J, et al. Accuracy of vertebral puncture in percutaneous vertebroplasty. Jpn J Radiol. 2022;40(4):419–429. doi: 10.1007/s11604-021-01216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biafora SJ, Mardjetko SM, Butler JP, McCarthy PL, Gleason TF. Arterial injury following percutaneous vertebral augmentation: a case report. Spine (Phila Pa 1976) 2006;31(3):E84–E87. doi: 10.1097/01.brs.0000197596.88416.02. [DOI] [PubMed] [Google Scholar]
- 58.Umeda A, Saeki N, Matsumoto C, Nakao M, Kawamoto M. Abdominal aortic injury during vertebroplasty. Spine (Phila Pa 1976) 2015;40(7):E439–E441. doi: 10.1097/BRS.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 59.Cosar M, Sasani M, Oktenoglu T, Kaner T, Ercelen O, Kose KC, et al. The major complications of transpedicular vertebroplasty. J Neurosurg Spine. 2009;11(5):607–613. doi: 10.3171/2009.4.SPINE08466. [DOI] [PubMed] [Google Scholar]
- 60.Yaltirik K, Ashour AM, Reis CR, Ozdogan S, Atalay B. Vertebral augmentation by kyphoplasty and vertebroplasty: 8 years experience outcomes and complications. J Craniovertebr Junction Spine. 2016;7(3):153–160. doi: 10.4103/0974-8237.188413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim JB, Park JS, Kim E. Nonaneurysmal subarachnoid hemorrhage: rare complication of vertebroplasty. J Korean Neurosurg Soc. 2009;45(6):386–389. doi: 10.3340/jkns.2009.45.6.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ignacio JMF, Ignacio KHD. Pulmonary embolism from cement augmentation of the vertebral body. Asian Spine J. 2018;12(2):380–387. doi: 10.4184/asj.2018.12.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatzantonis C, Czyz M, Pyzik R, Boszczyk BM. Intracardiac bone cement embolism as a complication of vertebroplasty: management strategy. Eur Spine J. 2017;26(12):3199–3205. doi: 10.1007/s00586-016-4695-x. [DOI] [PubMed] [Google Scholar]
- 64.Lee BJ, Lee SR, Yoo TY. Paraplegia as a complication of percutaneous vertebroplasty with polymethylmethacrylate: a case report. Spine (Phila Pa 1976) 2002;27(19):E419–E422. doi: 10.1097/00007632-200210010-00022. [DOI] [PubMed] [Google Scholar]
- 65.Morghen I, Borrelli M, Saletti A, Zoppellari R. Percutaneous vertebroplasty and spinal cord compression: a case report. J Radiol Case Rep. 2009;3(3):17–20. doi: 10.3941/jrcr.v3i3.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sidhu GS, Kepler CK, Savage KE, Eachus B, Albert TJ, Vaccaro AR. Neurological deficit due to cement extravasation following a vertebral augmentation procedure. J Neurosurg Spine. 2013;19(1):61–70. doi: 10.3171/2013.4.SPINE12978. [DOI] [PubMed] [Google Scholar]
- 67.Omidi-Kashani F, Ebrahimzadeh M, Peivandy M. Late onset sciatalgia as a rare complication of percutaneous vertebroplasty; a case report. Cases J. 2009;2:7960. doi: 10.4076/1757-1626-2-7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kelekis AD, Martin JB, Somon T, Wetzel SG, Dietrich PY, Ruefenacht DA. Radicular pain after vertebroplasty: compression or irritation of the nerve root? Initial experience with the "cooling system". Spine (Phila Pa 1976) 2003;28(14):E265–E269. doi: 10.1097/01.BRS.0000076835.53947.F4. [DOI] [PubMed] [Google Scholar]
- 69.Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2008;8(3):488–497. doi: 10.1016/j.spinee.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Fadili Hassani S, Cormier E, Shotar E, Drir M, Spano JP, Morardet L, et al. Intracardiac cement embolism during percutaneous vertebroplasty: incidence, risk factors and clinical management. Eur Radiol. 2019;29(2):663–673. doi: 10.1007/s00330-018-5647-0. [DOI] [PubMed] [Google Scholar]
- 71.Ivo R, Sobottke R, Seifert H, Ortmann M, Eysel P. Tuberculous spondylitis and paravertebral abscess formation after kyphoplasty: a case report. Spine (Phila Pa 1976) 2010;35(12):E559–E563. doi: 10.1097/BRS.0b013e3181ce1aab. [DOI] [PubMed] [Google Scholar]
- 72.Abdelrahman H, Siam AE, Shawky A, Ezzati A, Boehm H. Infection after vertebroplasty or kyphoplasty. A series of nine cases and review of literature. Spine J. 2013;13(12):1809–1817. doi: 10.1016/j.spinee.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 73.Kang JH, Kim HS, Kim SW. Tuberculous spondylitis after percutaneous vertebroplasty: misdiagnosis or complication? Korean J Spine. 2013;10(2):97–100. doi: 10.14245/kjs.2013.10.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park JW, Park SM, Lee HJ, Lee CK, Chang BS, Kim H. Infection following percutaneous vertebral augmentation with polymethylmethacrylate. Arch Osteoporos. 2018;13(1):47. doi: 10.1007/s11657-018-0468-y. [DOI] [PubMed] [Google Scholar]
- 75.Liao JC, Lai PL, Chen LH, Niu CC. Surgical outcomes of infectious spondylitis after vertebroplasty, and comparisons between pyogenic and tuberculosis. BMC Infect Dis. 2018;18(1):555. doi: 10.1186/s12879-018-3486-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frankel BM, Monroe T, Wang C. Percutaneous vertebral augmentation: an elevation in adjacent-level fracture risk in kyphoplasty as compared with vertebroplasty. Spine J. 2007;7(5):575–582. doi: 10.1016/j.spinee.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 77.Nagoshi N, Fukuda K, Shioda M, Machida M. Anterior spinal fixation for recollapse of cemented vertebrae after percutaneous vertebroplasty. BMJ case reports. 2016;2016. [DOI] [PMC free article] [PubMed]
- 78.Hierholzer J, Fuchs H, Westphalen K, Baumann C, Slotosch C, Schulz R. Incidence of symptomatic vertebral fractures in patients after percutaneous vertebroplasty. Cardiovasc Intervent Radiol. 2008;31(6):1178–1183. doi: 10.1007/s00270-008-9376-7. [DOI] [PubMed] [Google Scholar]
- 79.Lee MJ, Dumonski M, Cahill P, Stanley T, Park D, Singh K. Percutaneous treatment of vertebral compression fractures: a meta-analysis of complications. Spine (Phila Pa 1976) 2009;34(11):1228–1232. doi: 10.1097/BRS.0b013e3181a3c742. [DOI] [PubMed] [Google Scholar]
- 80.Koch CA, Layton KF, Kallmes DF. Outcomes of patients receiving long-term corticosteroid therapy who undergo percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2007;28(3):563–566. [PMC free article] [PubMed] [Google Scholar]
- 81.Chen LH, Hsieh MK, Liao JC, Lai PL, Niu CC, Fu TS, et al. Repeated percutaneous vertebroplasty for refracture of cemented vertebrae. Arch Orthop Trauma Surg. 2011;131(7):927–933. doi: 10.1007/s00402-010-1236-7. [DOI] [PubMed] [Google Scholar]
- 82.Heo DH, Chin DK, Yoon YS, Kuh SU. Recollapse of previous vertebral compression fracture after percutaneous vertebroplasty. Osteoporos Int. 2009;20(3):473–480. doi: 10.1007/s00198-008-0682-3. [DOI] [PubMed] [Google Scholar]
- 83.Lin WC, Lee YC, Lee CH, Kuo YL, Cheng YF, Lui CC, et al. Refractures in cemented vertebrae after percutaneous vertebroplasty: a retrospective analysis. Eur Spine J. 2008;17(4):592–599. doi: 10.1007/s00586-007-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marden FA, Putman CM. Cement-embolic stroke associated with vertebroplasty. AJNR Am J Neuroradiol. 2008;29(10):1986–1988. doi: 10.3174/ajnr.A1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Syed MI, Jan S, Patel NA, Shaikh A, Marsh RA, Stewart RV. Fatal fat embolism after vertebroplasty: identification of the high-risk patient. AJNR Am J Neuroradiol. 2006;27(2):343–345. [PMC free article] [PubMed] [Google Scholar]
- 86.Ahmadzai H, Campbell S, Archis C, Clark WA. Fat embolism syndrome following percutaneous vertebroplasty: a case report. Spine J. 2014;14(4):e1–5. doi: 10.1016/j.spinee.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 87.Nussbaum DA, Gailloud P, Murphy K. A review of complications associated with vertebroplasty and kyphoplasty as reported to the Food and Drug Administration medical device related web site. J Vasc Interv Radiol. 2004;15(11):1185–1192. doi: 10.1097/01.RVI.0000144757.14780.E0. [DOI] [PubMed] [Google Scholar]
- 88.Choe DH, Marom EM, Ahrar K, Truong MT, Madewell JE. Pulmonary embolism of polymethyl methacrylate during percutaneous vertebroplasty and kyphoplasty. AJR Am J Roentgenol. 2004;183(4):1097–1102. doi: 10.2214/ajr.183.4.1831097. [DOI] [PubMed] [Google Scholar]
- 89.Baek IH, Park HY, Kim KW, Jang TY, Lee JS. Paraplegia due to intradural cement leakage after vertebroplasty: a case report and literature review. BMC Musculoskelet Disord. 2021;22(1):741. doi: 10.1186/s12891-021-04625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsai YD, Liliang PC, Chen HJ, Lu K, Liang CL, Wang KW. Anterior spinal artery syndrome following vertebroplasty: a case report. Spine (Phila Pa 1976) 2010;35(4):E134–E136. doi: 10.1097/BRS.0b013e3181b52221. [DOI] [PubMed] [Google Scholar]
- 91.Martin DJ, Rad AE, Kallmes DF. Prevalence of extravertebral cement leakage after vertebroplasty: procedural documentation versus CT detection. Acta Radiol. 2012;53(5):569–572. doi: 10.1258/ar.2012.120222. [DOI] [PubMed] [Google Scholar]
- 92.Gao T, Chen ZY, Li T, Lin X, Hu HG, Yuan DC, et al. Correlation analysis of the puncture-side bone cement/vertebral body volume ratio and bone cement leakage in the paravertebral vein in vertebroplasty. BMC Musculoskelet Disord. 2022;23(1):184. doi: 10.1186/s12891-022-05135-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y, Liu X, Liu H. Cardiac perforation caused by cement embolism after percutaneous vertebroplasty: a report of two cases. Orthop Surg. 2022;14(2):456–460. doi: 10.1111/os.13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Birkenmaier C, Seitz S, Wegener B, Glaser C, Ruge MI, von Liebe A, et al. Acute paraplegia after vertebroplasty caused by epidural haemorrhage. A case report. J Bone Jt Surg Am. 2007;89(8):1827–1831. doi: 10.2106/JBJS.F.01612. [DOI] [PubMed] [Google Scholar]
- 95.Chehab MA, Thakor AS, Tulin-Silver S, Connolly BL, Cahill AM, Ward TJ, et al. Adult and pediatric antibiotic prophylaxis during vascular and IR procedures: a Society of Interventional Radiology Practice Parameter Update Endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Association for Interventional Radiology. J Vasc Interv Radiol. 2018;29(11):1483–501.e2. doi: 10.1016/j.jvir.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 96.Sun HB, Shan JL, Tang H. Percutaneous vertebral augmentation for osteoporotic vertebral compression fractures will increase the number of subsequent fractures at adjacent vertebral levels: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25(16):5176–5188. doi: 10.26355/eurrev_202108_26531. [DOI] [PubMed] [Google Scholar]
- 97.Yen CH, Teng MM, Yuan WH, Sun YC, Chang CY. Preventive vertebroplasty for adjacent vertebral bodies: a good solution to reduce adjacent vertebral fracture after percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2012;33(5):826–832. doi: 10.3174/ajnr.A2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kobayashi N, Numaguchi Y, Fuwa S, Uemura A, Matsusako M, Okajima Y, et al. Prophylactic vertebroplasty: cement injection into non-fractured vertebral bodies during percutaneous vertebroplasty. Acad Radiol. 2009;16(2):136–143. doi: 10.1016/j.acra.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 99.Mueller M, Daniels-Wredenhagen M, Besch L, Decher C, Seekamp A. Postoperative aseptic osteonecrosis in a case of kyphoplasty. Eur Spine J. 2009;18(Suppl 2):213–216. doi: 10.1007/s00586-008-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Layton KF, Thielen KR, Koch CA, Luetmer PH, Lane JI, Wald JT, et al. Vertebroplasty, first 1000 levels of a single center: evaluation of the outcomes and complications. AJNR Am J Neuroradiol. 2007;28(4):683–689. [PMC free article] [PubMed] [Google Scholar]
- 101.Heini PF, Orler R, Boszczyck B. Percutaneous vertebroplasty and kyphoplasty. New York: Springer; 2006. 8. Balloon Kyphoplasty and Lordoplasty; pp. 112–133. [Google Scholar]
- 102.Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of "kyphoplasty" in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila Pa 1976) 2001;26(14):1631–1638. doi: 10.1097/00007632-200107150-00026. [DOI] [PubMed] [Google Scholar]
- 103.Phillips FM, Todd Wetzel F, Lieberman I, Campbell-Hupp M. An in vivo comparison of the potential for extravertebral cement leak after vertebroplasty and kyphoplasty. Spine (Phila Pa 1976) 2002;27(19):2173–2178. doi: 10.1097/00007632-200210010-00018. [DOI] [PubMed] [Google Scholar]
- 104.Liang L, Chen X, Jiang W, Li X, Chen J, Wu L, et al. Balloon kyphoplasty or percutaneous vertebroplasty for osteoporotic vertebral compression fracture? An updated systematic review and meta-analysis. Ann Saudi Med. 2016;36(3):165–174. doi: 10.5144/0256-4947.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang B, Zhao CP, Song LX, Zhu L. Balloon kyphoplasty versus percutaneous vertebroplasty for osteoporotic vertebral compression fracture: a meta-analysis and systematic review. J Orthop Surg Res. 2018;13(1):264. doi: 10.1186/s13018-018-0952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu X, Tang X, Tan M, Yi P, Yang F. Is Balloon kyphoplasty a better treatment than percutaneous vertebroplasty for chronic obstructive pulmonary disease (COPD) patients with osteoporotic vertebral compression fractures (OVCFs)? J Orthop Sci. 2018;23(1):39–44. doi: 10.1016/j.jos.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 107.Liu JT, Li CS, Chang CS, Liao WJ. Long-term follow-up study of osteoporotic vertebral compression fracture treated using balloon kyphoplasty and vertebroplasty. J Neurosurg Spine. 2015;23(1):94–98. doi: 10.3171/2014.11.SPINE14579. [DOI] [PubMed] [Google Scholar]
- 108.Cheng J, Muheremu A, Zeng X, Liu L, Liu Y, Chen Y. Percutaneous vertebroplasty vs balloon kyphoplasty in the treatment of newly onset osteoporotic vertebral compression fractures: a retrospective cohort study. Medicine. 2019;98(10):e14793. doi: 10.1097/MD.0000000000014793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gan M, Zou J, Song D, Zhu X, Wang G, Yang H. Is balloon kyphoplasty better than percutaneous vertebroplasty for osteoporotic vertebral biconcave-shaped fractures? Acta Radiol. 2014;55(8):985–991. doi: 10.1177/0284185113511603. [DOI] [PubMed] [Google Scholar]
- 110.Neumann N, Meylheuc L, Barbe L, Garnon J, Koch G, Gangi A, et al. Robot-Assisted bone cement injection. IEEE Trans. Bio-Med. Eng. 2022;69(1):138–47. doi: 10.1109/TBME.2021.3088347. [DOI] [PubMed] [Google Scholar]
- 111.Xu HT, Zheng S, Kang MY, Yu T, Zhao JW. A novel computer navigation model guided unilateral percutaneous vertebroplasty for vertebral compression fracture: a case report. Medicine. 2020;99(44):e22468. doi: 10.1097/MD.0000000000022468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang B, Cao J, Chang J, Yin G, Cai W, Li Q, et al. Effectiveness of Tirobot-assisted vertebroplasty in treating thoracolumbar osteoporotic compression fracture. J Orthop Surg Res. 2021;16(1):65. doi: 10.1186/s13018-021-02211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu PL, Lin JS, Meng H, Su N, Yang Y, Fei Q. A novel "three-dimensional-printed individual guide template-assisted percutaneous vertebroplasty" for osteoporotic vertebral compression fracture: a prospective, controlled study. J Orthop Surg Res. 2021;16(1):326. doi: 10.1186/s13018-021-02471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Uchiyama F, Noguchi T, Kamei S, Yamashita K, Shida Y, Okafuji T, et al. The usefulness of vertebral needle targeting simulation training system using ray-summation imaging: experimental study. Jpn J Radiol. 2022 [Online ahead of print]. [DOI] [PMC free article] [PubMed]