Abstract
Background and Objective
The prevalence of dementia is increasing, while new opportunities for diagnosing, treating and possibly preventing Alzheimer’s disease and other dementia disorders are placing focus on the need for accurate estimates of costs in dementia. Considerable methodological heterogeneity creates challenges for synthesising the existing literature. This study aimed to estimate the costs for persons with dementia in Europe, disaggregated into cost components and informative patient subgroups.
Methods
We conducted an updated literature review searching PubMed, Embase and Web of Science for studies published from 2008 to July 2021 reporting empirically based cost estimates for persons with dementia in European countries. We excluded highly selective or otherwise biased reports, and used a random-effects meta-analysis to produce estimates of mean costs of care across five European regions.
Results
Based on 113 studies from 17 European countries, the estimated mean costs for all patients by region were highest in the British Isles (73,712 EUR), followed by the Nordics (43,767 EUR), Southern (35,866 EUR), Western (38,249 EUR), and Eastern Europe and Baltics (7938 EUR). Costs increased with disease severity, and the distribution of costs over informal and formal care followed a North-South gradient with Southern Europe being most reliant on informal care.
Conclusions
To our knowledge, this study represents the most extensive meta-analysis of the cost for persons with dementia in Europe to date. Though there is considerable heterogeneity across studies, much of this is explained by identifiable factors. Further standardisation of methodology for capturing resource utilisation data may further improve comparability of future studies. The cost estimates presented here may be of value for cost-of-illness studies and economic evaluations of novel diagnostic technologies and therapies for Alzheimer’s disease.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-022-01212-z.
Key Points
| This study is the most comprehensive review and meta-analysis to date of the costs in persons with dementia in Europe. Costs are presented disaggregated by type of resource use, disease severity, care setting and region, based on 113 studies capturing patient-level cost data for over 300,000 persons with dementia. |
| Mean annual costs vary substantially between regions, from almost 8000 EUR in Eastern Europe and the Baltics, to over 70,000 EUR in the British Isles, and were considerably higher in institutionalised patients and those with more severe disease. |
| These data can be utilised in future economic modelling, for example of the cost effectiveness of disease-modifying interventions. |
Introduction
Rationale
Dementia is a syndrome characterised by a decline in cognition and loss of functional abilities, commonly caused by neurodegenerative disorders including Alzheimer’s disease (AD). As a result of the combination of population growth and increasing life expectancy, the global prevalence of dementia is expected to rise from 55 million cases in 2019 to 139 million cases in 2050 [1]. In Europe, the number of cases is expected to rise by almost 80% over the same period, from 14.1 to 25 million cases. The high level of care needed for persons with dementia (PWD) causes significant stress to loved ones, caregivers and healthcare systems. Thus, the rapidly growing prevalence of dementia spells an enormous burden to health and social care systems.
There are important differences between countries in how dementia care is organised, funded and delivered. A key difference lies in the balance between formal (i.e. paid care from healthcare professionals or organisations) and informal care (i.e. unpaid care through a patient’s social network). Evidence points to a cultural and institutional gradient from North to South Europe, with informal care being more prominent in Southern European countries [2]. This has consequences for costs and limits the transferability of economic studies between countries and regions.
In order to adequately allocate resources to and within dementia care, and to assess the potential value and cost effectiveness of new technologies to diagnose, treat and prevent dementia disorders, it is imperative to understand the economic impact of dementia across Europe. Specifically, the costs of care in different stages of dementia severity are a critical input for health economic evaluations. Currently, often a single source of cost data is used in economic models, rather than a comprehensive assessment of all potentially available sources of cost data [3].
A number of systematic reviews have previously examined the cost in PWD. Quentin et al. [5] identified 28 studies (14 European) published before 2008, noting an increase in both formal and informal costs by disease severity. Furthermore, since the publication of our own review of the costs of dementia in 2009 [4], the number of dementia cost-of-illness publications has increased substantially. Schaller and colleagues [6] reviewed 27 studies published in 2003–12, including 15 from Europe, and identified institutionalisation and informal care as important cost drivers, but also a high variability in methodology across studies. Marešová et al. [7] reviewed studies published in 2007–2017, and included eight studies (of which six European) in a meta-analysis of total costs by disease severity. Cantarero-Prieto and colleagues found 26 studies published in 2010–2018, and estimated the total costs of care in Europe based on results from ten studies [8].
Previous reviews have consistently found that methodological differences across studies make it difficult to combine or even compare results across studies, and none has attempted to produce meta-analytic estimates of care costs based on the full range of published studies. Few multi-national studies use consistent measurements and data collection procedures across countries [2, 9–15]. Furthermore, although efforts have been made to standardise the assessments of care costs [16, 17], there is no universal standard for categorising resource use, making across study comparisons challenging. Therefore, a comprehensive meta-analysis accounting for methodological heterogeneity is needed to synthesise the current literature.
Objectives
The purpose of this study was to compile the results from cost-of-dementia studies in Europe to obtain meta-analytic estimates of the annual medical, non-medical and informal care cost per PWD. Additionally, we aimed to stratify costs into informative categories, such as region, disease severity and care setting.
Methods
Eligibility Criteria
In addition to the 16 studies identified in our previous review, we conducted a literature search for studies reporting cost estimates for PWD in European countries published in 2008 or later. Studies presenting data for PWD with any underlying cause: AD vascular dementia, dementia with Lewy bodies or dementia with unknown aetiology were included. To be included in the analysis, studies were required to report data on estimates for at least one cost item of interest (direct medical costs, non-medical costs and/or informal care costs). Studies were required to be based on empirical data in a defined study population and with a reported sample size. We included studies where (1) the source of the data was described, (2) the study population was identified and (3) the cost estimates were clearly presented so that the type of cost, currency and year of costing could be identified.
We excluded economic models and other analyses that were based on cost data reported elsewhere as well as hypothetical cost calculation studies. Studies presenting aggregated data across multiple European regions were excluded. We did not exclude studies based on language, nor did we exclude studies that reported cost data for other populations (e.g. healthy elderly individuals), provided costs for PWD were reported separately.
Quality Assessment
We qualitatively assessed studies for the presence of methodological issues that would compromise the validity of cost estimates. We excluded studies that selected only patients utilising a specific resource (e.g. hospitalisations) without considering the population at risk, or that considered only costs related to a specific symptom or complication (e.g. behavioural disturbances). Additionally, we excluded studies that focused only on the last years of life (i.e. decedent studies), and studies that only included costs for diagnosis or only out-of-pocket payments. Such partial or selective studies could not be easily incorporated in the meta-analysis to contribute towards the estimate of costs of care.
Search Strategy
The literature search was conducted in the databases PubMed, Embase and Web of Science. We began with broad search criteria including the concepts ‘Cost’, ‘Economic’, ‘Dementia’ and ‘Alzheimer’, and iteratively optimised the strategy to give a manageable number of hits to review. We found that restricting some search terms to title fields greatly improved specificity while not compromising the sensitivity of the search, as the search identified studies quoted in previous reviews. We did not limit the search to European countries, as we found that many studies did not explicitly state the geographic setting in searchable fields. The final search term string was as follows: (cost[Title] OR costs[Title] OR economic[Title] OR economics[Title]) AND (Alzheimer OR dementia). The same search string was used in all databases, and the literature search was completed in July 2021.
Selection and Data Extraction Process
Screening of search hits and extraction of key study characteristics (study design, study population, year of data collection, country, resource use measurements, year of costing and currency) was conducted by two independent reviewers (LJ, OF), after which results were tabulated and discrepancies reconciled through consensus. Data on individual cost items were then extracted by a single reviewer (LJ). For each cost estimate, the following data were extracted: study, country, population, setting (community or institution), sex, disease stage, resource use item, mean or median cost, standard deviation, confidence interval or interquartile range and sample size. Costs were then aggregated by study and cross-checked against the original publications to identify and correct any errors in data extraction. Methods and results are reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [18].
Data Harmonisation and Cost Estimation
Cost data were classified into three major categories: direct medical costs, direct non-medical costs and informal care costs. Direct medical care costs include inpatient care, outpatient care (encompassing specialist as well as primary care, visits to nurses and other healthcare professionals) and pharmaceuticals. Direct nonmedical care includes residential care (nursing home, group living or other institution) and community care (all other non-medical care services such as home help, day care and delivered meals). Informal care costs were separated into costs related to caregiver productivity loss, and costs related to lost caregiver leisure time. The reason for this distinction is that different opportunity costs are often assigned to lost work time and lost leisure time, respectively, for caregivers. All cost data were extracted verbatim as reported in publications, and later recoded to match the categories outlined above. Thus, costs that were reclassified according to our categories are in some cases reported differently than in the original publications. All costs were converted to Euros (EUR) 2021 by first converting from local currency to EUR using historic exchange rates from the year of costing for each study.1 Historic cost data in EUR were then inflated to 2021 EUR through the European Union harmonised index of consumer prices.2
The majority of studies that reported costs by disease severity used the Mini-Mental State Examination (MMSE) to stratify patients (n = 35) [19]. The scores were stratified into mild dementia (MMSE ≥ 20), moderate dementia (MMSE 10–20) and severe dementia (MMSE < 10). If the cut-off values used in the individual studies did not match these intervals precisely, the closest interval was chosen for each subgroup. Eleven studies reported costs stratified by the Clinical Dementia Rating Scale (n = 9) [20] or the Global Deterioration Scale [21] (n = 2), for which we used the corresponding levels for mild, moderate and severe dementia, respectively.
Seven studies reported costs stratified by the Dependence Scale [22] (n = 3) or other measures of activities of daily living dependency (n = 4), while two studies reported costs by the degree of behavioural disturbances (the Cohen Mansfield Agitation Inventory [23] or the Neuropsychiatric Inventory [24]). For these studies, the reported subgroups were mapped into mild, moderate and severe dementia based on the mean reported MMSE scores or distributions across Clinical Dementia Rating (CDR) levels for each subgroup. Just over half of the studies did not report costs for specific levels of disease severity (n = 58).
For studies that reported standard errors (SEs), standard deviations (SDs) were calculated from . For studies reporting only 95% confidence intervals (CIs), SDs were obtained by [25]. We assumed statistical independence between cost categories, thus the variance of the sum of the cost components was estimated by the sum of the variance of the components.
Countries were grouped into regions according to a modification of the United Nations classification of world regions [26]. Northern Europe was split into the British Isles (UK, Ireland) and the Nordic countries (Sweden, Norway, Denmark, Finland). The Nordic countries were reported separately as many studies originated from these countries that were also relatively similar in care system structure. The Baltic countries were grouped with Eastern Europe, as levels of Gross Domestic Product (GPD) and care spending are closer than with Northern Europe (Table 1).
Table 1.
Number of included studies and sample size by region, population and study methodology
| Categories | No. of studies | Sample size |
|---|---|---|
| All studies | 113 | 3,187,26 |
| British Isles | 36 | 15,311 |
| Ireland | 4 | 577 |
| UK | 32 | 14,244 |
| Multiple countries | 1 | 490 |
| Eastern Europe and Baltics | 5 | 61,390 |
| Czechia | 1 | 119 |
| Estonia | 2 | 495 |
| Hungary | 2 | 60,776 |
| Nordics | 23 | 182,164 |
| Denmark | 5 | 106,375 |
| Finland | 6 | 72,443 |
| Norway | 3 | 350 |
| Sweden | 11 | 2726 |
| Multiple countriesa | 1 | 270 |
| Southern Europe | 26 | 12,035 |
| Italy | 5 | 1215 |
| Portugal | 3 | 225 |
| Spain | 18 | 9933 |
| Multiple countriesa | 1 | 662 |
| Western Europe | 39 | 48,036 |
| Austria | 1 | 1341 |
| Belgium | 2 | 741 |
| France | 9 | 2691 |
| Germany | 21 | 41,282 |
| The Netherlands | 11 | 1491 |
| Multiple countriesa | 1 | 490 |
| Care setting | ||
| Allb | 41 | 286,287 |
| Community | 67 | 24,718 |
| Institution | 20 | 7931 |
| Disease severity | ||
| Allb | 58 | 286,806 |
| Mild | 44 | 10,394 |
| Moderate | 53 | 14,570 |
| Severe | 39 | 6986 |
| Diagnosis | ||
| Dementiac | 71 | 179,468 |
| AD | 41 | 137,185 |
| DLB | 1 | 194 |
| VaD | 2 | 2089 |
| Study methodology | ||
| Cross-sectional | 33 | 13,234 |
| Prospective | 33 | 13,108 |
| Retrospective | 18 | 287,102 |
| Clinical trial | 29 | 5492 |
aStudies reporting aggregated data for multiple countries within a region
bAll refers to studies reporting aggregated data only
cAetiological diagnosis not specified
AD Alzheimer’s disease, DLB dementia with Lewy bodies, VaD vascular dementia
Study designs were classified as the following categories: retrospective database analyses when based on secondary analysis of existing data, prospective observational studies when involving longitudinal data collection in a non-interventional setting, clinical trials when including randomisation, surveys when based on data collected through postal or online surveys and otherwise as cross-sectional observation studies when collecting data at a single timepoint.
Imputations
As some studies presented only aggregations of cost components (e.g. total direct medical cost), a complete break-down by cost component was computed by applying the distribution over cost components from other studies within the same region for the same care setting and disease severity. Where no other studies were available in the same group, consecutive broader groupings were utilised until all studies had been matched with breakdown over cost components. Missing standard deviations were imputed based on the mean coefficient of variation for the same cost component across all studies. We assumed that the total sample size distributed equally across subgroups for two studies [27, 28], which did not report the sample size by subgroup.
Meta-analysis
Meta-analytic estimates of mean costs were generated separately for each cost component within each region, care setting and level of disease severity. In total, 420 separate meta-analyses were conducted: by seven cost components, five regions, three care settings (community, institution, all) and four disease severity categories (mild, moderate, severe, all). Results from these meta-analyses were then combined into final cost estimates and aggregated into total medical, non-medical and informal care costs. Statistical precision of meta-analytical estimates was based on actual sample size, excluding imputations. Studies were pooled using a random-effects model with inverse variance weighting. The random-effects model was chosen (over a fixed-effect model) as we expected considerable methodological heterogeneity across studies. For each meta-analysis, we report the studies included, sample size, per-study mean costs and the meta-analytic cost estimate with 95% CI. Between-study heterogeneity was assessed with the chi-squared test and I2 statistic [25].
To explore the effect of relevant covariates on cost estimates, separate linear meta-regression models were estimated for each cost component with the following covariates: study method, diagnosis (all dementia vs AD, AD vascular dementia, dementia with Lewy bodies), region, disease severity, care setting and time period (before 2005, 2005–2009, 2010–2014, 2015 and later). All analyses were conducted in R Studio 1.4.1717 using the meta package [29].
Results
Study Selection
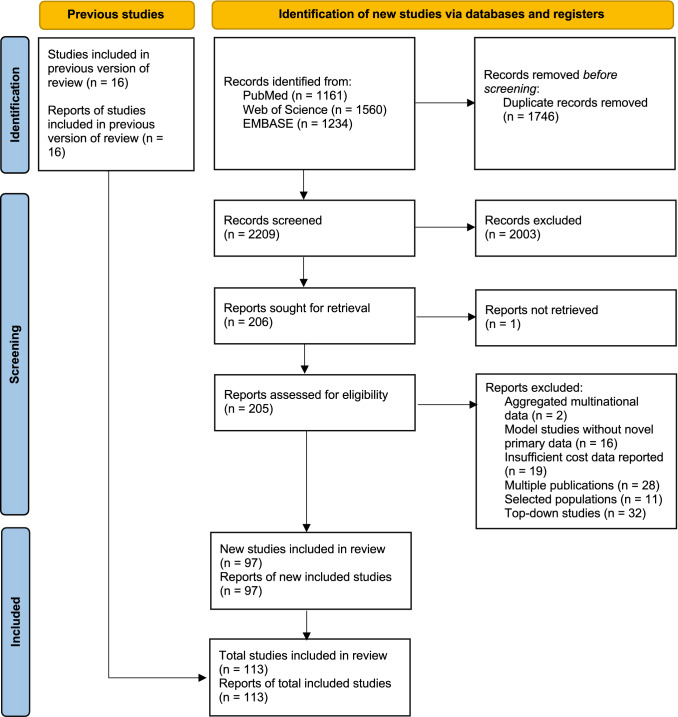
Figure 1 presents the PRISMA flow chart for the literature search. The initial search contained 2209 hits after the removal of duplicates. After review of the title and abstract, 205 studies were accessed and assessed for eligibility. The most common reasons for exclusion were studies based on top-down methodology (n = 32), duplicate reports of the same study (n = 28) and insufficiently reported cost data (n = 28). The final selection for analysis included 113 studies, which includes the 16 studies published before 2008 from our previous review.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flow chart
Study Characteristics
Table 1 presents an overview of characteristics of the studies included in the meta-analysis. In total, 113 studies were included, capturing data for 318,936 patients from 17 European countries [2, 9–15, 27, 28, 30–131]. Most studies were relatively small, with a median sample size per study of 233 patients. Excluding retrospective studies, the median sample size was 198 (range 22–1678). Participants had an average age of 79 years (range 47–101 years). Western Europe was covered by 34.5% of publications (n = 39), followed by the British Isles (n = 36), Southern Europe (n = 26), the Nordics (n = 23), and Eastern Europe and the Baltics (n = 5). The UK, Germany and Spain were covered by the most publications (32, 21 and 18 studies, respectively), while the largest numbers of patients were captured by retrospective studies in Denmark, Finland, Hungary and Germany (Table 1). Primarily, studies included patients with unspecified dementia (n = 71) or patients with AD (n = 41), while a small number of studies captured other dementia disorders: vascular dementia (n = 2) and dementia with Lewy bodies (n = 1). Cross-sectional and prospective observational designs were most common, each represented by 33 studies, while 18 retrospective database studies and 29 clinical trials were included.
Studies were most frequently conducted in community-dwelling patients (n = 67), while 20 studies reported data specifically for institutionalised patients and 41 studies enrolled patients both from the community and institutions and did not report costs separately by care setting. Fifty-five studies reported cost data by disease severity for 32,130 patients. Only two studies presented cost data separately by sex. A detailed summary of each included study is provided in the Electronic Supplementary Material.
In Table 2, we show a summary of the number of studies providing evidence on different cost components by geographic region and care setting. The study counts presented in the table were produced before any imputations were carried out. The distribution of studies is highly uneven, with many studies providing evidence on costs in community care, particularly in Western Europe and the British Isles. Eastern Europe and the Baltics have comparatively fewer institutional care studies and only a handful of studies report costs.
Table 2.
Number of studies providing data on different cost components, by region and care setting
| Care setting | British Isles | Eastern Europe and Baltics | Nordics | Southern Europe | Western Europe | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Community | Institution | All | Community | Institution | All | Community | Institution | All | Community | Institution | All | Community | Institution | |
| Direct medical costs | 5 | 6 | 4 | 1 | 2 | 1 | 5 | 2 | 2 | 4 | 6 | 3 | 6 | 21 | 6 |
| Inpatient care | 9 | 15 | 5 | 3 | 2 | 1 | 9 | 4 | 2 | 4 | 8 | 3 | 7 | 20 | 6 |
| Outpatient care | 6 | 13 | 4 | 1 | 1 | 0 | 6 | 3 | 1 | 3 | 5 | 2 | 6 | 18 | 5 |
| Pharmaceuticals | 7 | 6 | 3 | 2 | 1 | 0 | 6 | 1 | 1 | 4 | 6 | 4 | 6 | 17 | 5 |
| Direct non-medical costs | 4 | 9 | 3 | 1 | 1 | 1 | 7 | 4 | 2 | 4 | 10 | 4 | 2 | 18 | 2 |
| Institutional care | 4 | 8 | 4 | 1 | 1 | 1 | 6 | 5 | 2 | 3 | 6 | 3 | 4 | 15 | 3 |
| Community care | 6 | 16 | 4 | 1 | 2 | 1 | 7 | 4 | 2 | 4 | 9 | 3 | 3 | 18 | 4 |
| Total direct costs | 5 | 12 | 4 | 2 | 1 | 1 | 5 | 5 | 2 | 5 | 5 | 3 | 5 | 18 | 2 |
| Informal care costs | 3 | 11 | 1 | 1 | 2 | 1 | 6 | 4 | 1 | 6 | 11 | 1 | 2 | 21 | 2 |
| Caregiver work loss | 2 | 2 | 1 | 0 | 1 | 0 | 2 | 3 | 1 | 4 | 3 | 2 | 0 | 6 | 1 |
| Caregiver time | 1 | 1 | 0 | 0 | 1 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 4 | 0 |
| Total costs | 3 | 4 | 0 | 0 | 0 | 0 | 3 | 6 | 3 | 2 | 1 | 0 | 0 | 6 | 1 |
No imputations or aggregations were carried out before counts were produced
Meta-analytic Cost Estimates
Detailed results from the individual meta-analyses for each cost component and for each subgroup are presented in the ESM. The median number of studies included in each analysis was 5 (range 1–28). There was moderate heterogeneity across studies, indicated by a mean I2 statistic of 55%. I2 was similar across disease severity levels, but lower in the institutional care setting (33%) compared with the community setting (72%). This can be expected as costs in the institutional setting are dominated by the cost of residential care, which is relatively uniformly assessed across studies. Heterogeneity was lowest in Southern Europe (39%) and highest in Eastern Europe (73%).
Table 3 presents estimated mean costs by region, based on data averaged across care settings and disease severity levels. The mean annual cost per PWD ranged from 7938 EUR (Eastern Europe and Baltics) to 73,712 EUR (British Isles); the Nordics, Southern and Western Europe fell between these values with 43,767 EUR, 35,866 EUR and 38,249 EUR, respectively. The higher cost in the British Isles region was mainly driven by a higher cost of caregiver leisure time loss, which in turn was not caused by a single outlying study but rather several studies reporting high costs for informal care using different methodologies [39, 77, 91, 111].
Table 3.
Annual mean costs per person with dementia, by region and cost component (95% confidence interval), Euros
| British Isles | Eastern Europe and Baltics | Nordics | Southern Europe | Western Europe | |
|---|---|---|---|---|---|
| Direct medical costs | 5630 (4336–6924) | 1218 (249–2186) | 5439 (4002–6877) | 3292 (2106–4478) | 6189 (5234–7145) |
| n = 10 | n = 3 | n = 13 | n = 7 | n = 12 | |
| Inpatient care | 3509 (2345–4674) | 309 (152–466) | 3212 (1993–4431) | 537 (113–962) | 2448 (1788–3108) |
| n = 9 | n = 3 | n = 12 | n = 6 | n = 12 | |
| Outpatient care | 962 (601–1323) | 329 (18–641) | 985 (416–1554) | 734 (359–1110) | 2386 (1778–2994) |
| n = 9 | n = 3 | n = 10 | n = 6 | n = 12 | |
| Pharmaceuticals | 1159 (725–1593) | 579 (0–1483) | 1242 (735–1749) | 2020 (978–3062) | 1356 (1027–1684) |
| n = 9 | n = 3 | n = 11 | n = 7 | n = 12 | |
| Direct non-medical costs | 15530 (7758–23,303) | 3169 (0–7508) | 26,595 (19,483–33,708) | 6926 (3131–10,722) | 16,008 (12,060–19,955) |
| n = 8 | n = 3 | n = 11 | n = 7 | n = 10 | |
| Institutional care | 9511 (2282–16,739) | 2242 (0–6495) | 21631 (14,800–28,462) | 2617 (0–5279) | 9154 (6066–12,243) |
| n = 7 | n = 3 | n = 11 | n = 7 | n = 9 | |
| Community care | 6020 (3163–8877) | 926 (64–1789) | 4964 (2984–6945) | 4310 (1605–7015) | 6853 (4394–9313) |
| n = 7 | n = 3 | n = 11 | n = 7 | n = 8 | |
| Total direct costs | 21,160 (13,281–29,040) | 4386 (0–8833) | 32035 (24,778–39,291) | 10218 (6242–14,195) | 22,197 (18,135–26,259) |
| n = 10 | n = 3 | n = 14 | n = 8 | n = 12 | |
| Informal care costs | 52,552 (0–114,583) | 3551 (2432–4671) | 11,733 (95–23,370) | 25,647 (6046–45,249) | 16,052 (6763–25,340) |
| n = 5 | n = 2 | n = 8 | n = 8 | n = 6 | |
| Caregiver work loss | 1740 (122–3358) | 581 (0–1619) | 861 (453–1270) | 915 (106–1725) | 1061 (429–1694) |
| n = 5 | n = 2 | n = 8 | n = 8 | n = 5 | |
| Caregiver time | 50812 (0–112,822) | 2970 (2548–3392) | 10,871 (0–22,501) | 24732 (5147–44,317) | 14,991 (5724–24,257) |
| n = 5 | n = 2 | n = 8 | n = 6 | n = 6 | |
| Total costs | 73712 (11,182–136,242) | 7938 (3352–12,523) | 43,767 (30,053–57,481) | 35,866 (15,865–55,867) | 38,249 (28,111–48,386) |
| n = 10 | n = 3 | n = 15 | n = 10 | n = 12 |
Mean costs were averaged across care settings and disease severity levels
n number of studies contributing to each estimate
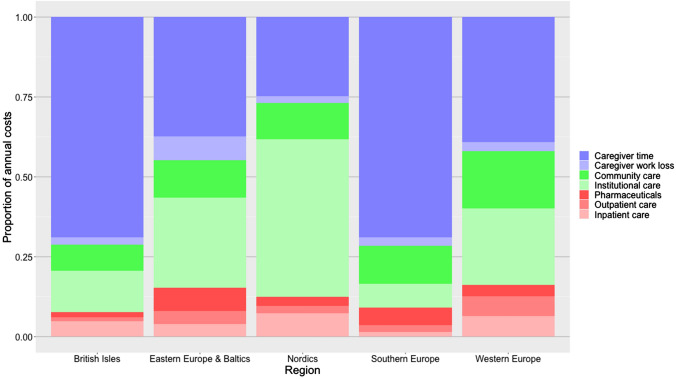
The distribution of costs over cost components (direct medical costs, direct non-medical costs and informal care costs) varied substantially between regions (Fig. 2). There is a North–South gradient with higher use of formal care services in the Nordics and greater reliance on informal care in Southern Europe, with other regions falling between these extremes while British Isles had a similar cost distribution to Southern Europe.
Fig. 2.
Distributions of dementia cost over components, by region
Table 4 presents costs by disease severity and care setting in each region. The studies included in meta-analytical estimates vary between cells in the table, thus the estimated cost across all severity levels is not necessarily equal to the average of the cost estimates for each severity level. In line with the literature, institutionalised patients generally have higher overall costs than community-dwelling patients, driven by higher direct non-medical costs [2]. Estimated total annual costs of care increased with higher disease severity in all regions, though CIs around estimates are wide. When examined separately within each care setting, the relationship between disease severity and cost is clear in the community setting but less consistent for institutionalised patients. In some regions, costs for institutionalised patients with mild dementia are lower than in community-dwelling patients with mild dementia, but this is based on a small sample size as most institutionalised patients have moderate-to-severe dementia. A major part of the increase in costs in more severe disease is driven by a higher rate of institutionalisation.
Table 4.
Mean annual care costs by care setting, disease severity and region (95% confidence interval), Euros
| Setting | Severity | British Isles | Eastern Europe and Baltics | Nordics | Southern Europe | Western Europe |
|---|---|---|---|---|---|---|
| Community | Mild | 30,098 (13,517–46,679) | 3360 (0–8068) | 24,928 (19,699–30,157) | 21,452 (19,066–23,837) | 28,642 (20,697–36,587) |
| n = 6 | n = 2 | n = 5 | n = 6 | n = 11 | ||
| Community | Moderate | 37,008 (23,190–50,827) | 5205 (0–10675) | 28,444 (19,519–37,369) | 26,610 (22,075–31,145) | 46,220 (30,341–62,098) |
| n = 9 | n = 3 | n = 6 | n = 9 | n = 14 | ||
| Community | Severe | 51,567 (22,142–80,993) | 6081 (0–12286) | 32,732 (24,316–41,148) | 31,495 (26,190–36,799) | 37,626 (27,258–47,993) |
| n = 5 | n = 2 | n = 5 | n = 6 | n = 7 | ||
| Community | All | 36,776 (20,025–53,526) | 5398 (0–11058) | 27,740 (20,250–35,230) | 25,020 (15,659–34,380) | 38,208 (31,103–45,313) |
| n = 17 | n = 2 | n = 8 | n = 14 | n = 25 | ||
| Institution | Mild | 59,764 (0–158,891) | 18,928 (0–40433) | 75,098 (30,863–119,333) | 43,006 (0–99,771) | 43,096 (5673–80,520) |
| n = 2 | n = 1 | n = 2 | n = 2 | n = 3 | ||
| Institution | Moderate | 48,183 (43,610–52,756) | 19,446 (5726–33,166) | 61,687 (36,991–86,384) | 51,065 (36,401–65,728) | 54,327 (22,916–85,737) |
| n = 4 | n = 2 | n = 5 | n = 4 | n = 4 | ||
| Institution | Severe | 47,655 (40,007–55,302) | 19,123 (9271–28,974) | 61,805 (35,596–88,015) | 60,272 (35,031–85,513) | 51,432 (19,159–83,705) |
| n = 3 | n = 1 | n = 4 | n = 4 | n = 3 | ||
| Institution | All | 36,029 (2447–69,611) | 19,309 (11,805–26,812) | 77,225 (61,180–93,270) | 36,706 (18,368–55,044) | 49,846 (30,217–69,475) |
| n = 3 | n = 1 | n = 3 | n = 5 | n = 6 | ||
| All | Mild | 19,909 (14,977–24,841) | 7616 (1929–13,304) | 20,876 (16,690–25,061) | 20,420 (10,479–30,361) | 31,984 (18,254–45,714) |
| n = 5 | n = 2 | n = 7 | n = 8 | n = 6 | ||
| All | Moderate | 34,223 (25,263–43,183) | 9670 (4904–14,435) | 37,540 (30,391–44,690) | 40,953 (9996–71,909) | 47,934 (29,324–66,544) |
| n = 9 | n = 3 | n = 10 | n = 10 | n = 7 | ||
| All | Severe | 61,958 (10,603–113,312) | 11,236 (3797–18,675) | 58,198 (45,291–71,105) | 61,906 (14,167–109,645) | 56,104 (30,761–81,447) |
| n = 6 | n = 2 | n = 9 | n = 8 | n = 6 | ||
| All | All | 73,712 (11,182–136,242) | 7938 (3352–12,523) | 43,767 (30,053–57,481) | 35,866 (15,865–55,867) | 38,249 (28,111–48,386) |
| n = 10 | n = 3 | n = 15 | n = 10 | n = 12 |
N number of studies contributing to each estimate
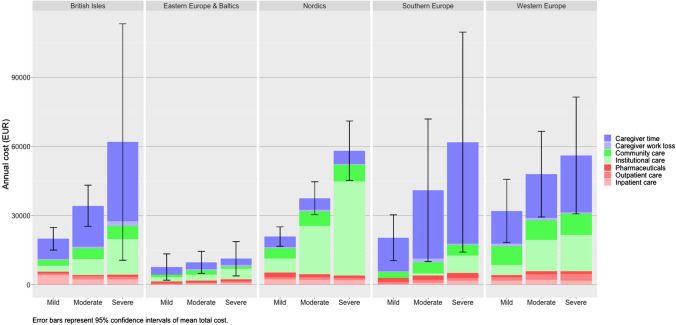
Figure 3 presents costs by disease severity and region, disaggregated by cost component. The relationship between costs and disease severity is evident in direct non-medical costs and costs of informal care, while direct medical costs do not clearly increase with disease severity.
Fig. 3.
Mean annual costs per person with dementia, by disease severity, and region
Only two studies assessed costs separately for male and female individuals: Ruiz-Adame Reina et al. [103] found that there was an increased proportion of direct, indirect and out-of-pocket spending for male compared with female individuals for direct medical (56.5% on male individuals), indirect (53.1% on male individuals) and out-of-pocket spending (56.7% on male individuals), while Schwarzkopf et al. [107] only looked at direct medical costs and found a light increase in spending on female individuals (48.9% on male individuals).
Meta-regression
Results from the meta-regression are shown in Table 5. I2 statistics close to 100% indicate that, as can be expected, heterogeneity between cost estimates was extremely high when pooling data across regions, care settings and severity levels. The amount of heterogeneity explained by the regression model (indicated by the R2 statistics) was substantial, with a range from 22% for drug costs to 67% for residential care.
Table 5.
Meta-regression of mean annual costs, by cost component, Euros
| Direct medical costs | Direct non-medical costs | Informal care cost | |||||
|---|---|---|---|---|---|---|---|
| Inpatient | Outpatient | Drugs | Residential care | Home care | Productivity loss | Caregiver time | |
| Intercept | 2310** | 1139** | 732** | 9501** | 5550** | 461** | 24,957** |
| Study methodology | |||||||
| Cross-sectional (reference) | |||||||
| Prospective | 220 | − 255 | − 110 | 1659 | 881 | − 35 | − 6703** |
| Retrospective | 1225** | − 197 | 90 | − 5946** | − 550 | 1982** | 38,811** |
| Clinical trial | 551 | − 549** | 91 | 1618 | − 233 | 357** | 9904** |
| Diagnosis | |||||||
| Dementia (reference) | |||||||
| Alzheimer’s disease | − 284 | − 131 | − 5 | − 311 | − 2031** | 163* | 2731 |
| Vascular dementia | 264 | − 89 | 109 | 1194 | − 2945 | 749 | 1257 |
| Lewy body dementia | 516 | NA | NA | NA | NA | NA | NA |
| Region | |||||||
| British Isles (reference) | |||||||
| Eastern Europe and Baltics | − 1563** | − 1002** | − 310 | − 7450** | − 4299** | − 141 | − 14,387** |
| Nordics | 91 | − 1 | 411** | 5649** | 330 | 8 | − 10,539** |
| Southern Europe | − 652** | − 159 | 702** | − 2880 | − 648 | 157 | − 2712 |
| Western Europe | − 36 | 773** | 508** | 41 | 1503* | 137 | − 5576* |
| Disease severity | |||||||
| All (reference) | |||||||
| Mild | − 175 | − 100 | 42 | − 3480** | − 350 | − 140 | − 4925* |
| Moderate | − 56 | 178 | 52 | 360 | 400 | 40 | − 389 |
| Severe | − 301 | 87 | 60 | 4374** | 1259* | − 80 | 1725 |
| Care setting | |||||||
| All (reference) | |||||||
| Community | − 37 | − 13 | − 202* | − 10,272** | 1219* | 84 | − 84 |
| Institution | − 635** | 600** | − 484** | 26,312** | − 5542** | − 532** | − 12,304** |
| Time period | |||||||
| < 2005 (reference) | |||||||
| 2005–2009 | − 125 | 430* | 379** | 4255** | 135 | 65 | − 1637 |
| 2010–2014 | − 307 | 288 | 100 | 515 | 153 | 134 | 1235 |
| 2015– | − 327 | − 3 | 269 | − 1079 | − 3362** | − 25 | − 6420 |
| Number of cost estimates | 389 | 355 | 356 | 343 | 344 | 297 | 263 |
| I2 (%) | 98.37 | 99.22 | 99.62 | 100 | 100 | 100 | 100 |
| R2 (%) | 26.79 | 33.04 | 21.88 | 66.73 | 37.85 | 57.56 | 39.4 |
Each column in the table corresponds to a separate regression model. Coefficient estimates in Euros indicating the effect of the variable on cost components. For example, annual inpatient costs for patients with a diagnosis of Alzheimer’s disease are 284 Euros lower than the reference category (all-cause dementia)
NA=not applicable, *p < 0.05; **p < 0.01
There were some important differences in cost estimates depending on study methodology. Compared with cross-sectional studies (reference category), retrospective designs gave somewhat higher costs for inpatient care. This could be due to a longer follow-up time and more accurate recall in retrospective studies, increasing the likelihood of accurately capturing rare hospitalisation events. The cost for caregiver time varied substantially across methodologies; the high cost in retrospective study designs is driven by a single study reporting informal care data from an administrative database [33]. Randomised trials also reported higher costs for informal care compared with cross-sectional or prospective observational designs, potentially because of a greater use of comprehensive assessment scales for informal care.
Few studies reported cost data for other diagnoses than AD, and there were no systematic differences depending on diagnosis (AD, AD vascular dementia, dementia with Lewy bodies or dementia without specific aetiological diagnosis). Cost differences by disease severity were statistically significant only for residential care and informal care costs, but with a trend toward higher cost in more severe disease for other cost components except inpatient care. Institutionalised patients had higher costs for accommodation and outpatient medical care, but lower costs for all other services including informal care. There was no clear time trend of costs, though the latest time period had numerically lower costs across all components, except for drug costs, compared with the earliest period.
Discussion
Findings in Context
In this study, we estimate the costs of dementia in Europe by synthesising evidence from a large body of literature mainly published over the past two decades. Though there is extensive diversity across the 113 studies included in the analysis, our study demonstrates the feasibility of producing meta-analytic estimates of costs of care regions and informative subgroups.
Annual Costs of Care
The mean total annual cost estimates presented here are largely consistent with previous estimates; a recent assessment of the global costs of dementia conducted by the World Health Organization reported a mean annual cost per PWD in Europe of US$31,114 [1]. Marešová et al. [7] estimated the costs in mild, moderate and severe dementia to 16,659, 22,677 and 33,726 EUR per year, respectively, based on a meta-analysis of eight mainly European studies. Cantarero-Prieto and colleagues reported a mean annual cost care in Europe of 32,507 EUR based on ten studies [8], while Schaller et al. estimated mean annual costs to $31,896 based on an international sample of 11 studies [6].
Few studies thus far have collected cost data across multiple European countries using uniform data collection methodology, allowing direct comparison of resource utilisation and costs across countries. Published after the conclusion of our analysis, Meijer et al. [132] analyzed survey data from 11 European countries to assess out-of-pocket health and social care and unpaid formal care. They found that informal care made up 50–90% of the total cost of dementia and estimated the total cost per individual to be between 2689 EUR in Estonia to 15,468 EUR in Germany per year. Their lower estimates compared with the present study could potentially be attributed to under-sampling patients with severe dementia and overestimating the proportion of out-of-pocket spending to total spending [133].
Distribution Over Cost Components
Dementia care costs across Europe were dominated by informal care and direct non-medical costs (i.e. residential care and home care). There was a distinct contrast between regions in terms of the balance of informal care and direct non-medical care. The Nordics and Western Europe had higher direct non-medical costs compared with informal care, while Southern Europe had the lowest direct non-medical costs. The variability in cost across a North-to-South gradient has been noted in the literature and is likely owing to differences in cultural expectations of ageing and familial duty, as well as the availability and coverage of formal care services [134]. Differences in valuations of informal care may also play an important role, and should be the topic of further study as there is currently no established consensus regarding the appropriateness of alternative methods for valuing informal caregiving time.
In the World Health Organization report, annual direct medical costs were estimated to US$3624, direct non-medical costs US$13,128 and informal care costs US$14,393 [1]. These results are of similar magnitude and distribution over cost categories as the estimates reported here, if averaged across European countries.
In comparison to multi-national studies with a standardised data collection, our results of the cost breakdown between informal care and formal care closely match the estimates published in the ICTUS study [2]. Our finding of high informal care costs in the UK and Ireland have also been observed in other studies. In the GERAS study [14], the UK had substantial higher caregiver time as well as more caregivers missing work days compared with Germany and France.
Costs by Setting and Disease Severity
There was a higher cost associated with patients living in nursing homes compared with those who were community dwelling. However, this result was contingent on the methodology used to value informal care, highlighting the importance of standardised practice for evaluating informal care costs.
Although the CIs around our estimates were wide, costs increased with disease severity, in particular informal care and direct non-medical costs. This finding has been widely reported by many individual studies as well as two large reviews with a broad geographic scope [5, 6]. Schaller et al. reported mean annual costs of US$22,113 in mild dementia, US$42,930 in moderate dementia and US$51,659 in severe dementia [6]. This corresponds to an increase of 94% in moderate versus mild disease, and 134% in severe versus mild disease. This is consistent in magnitude with our estimates, though we see a steeper gradient in some regions such as Southern Europe (101% and 203%, respectively) and a less steep gradient in Eastern Europe (27%, 48%) and Western Europe (50%, 75%). Further, we found that mean costs were more similar across regions when broken down by disease severity compared with pooled estimates, namely, if also disaggregated by care setting.
Strengths and Limitations
In contrast to most previous reviews of costs of dementia, we pool all available studies providing estimates of individual cost elements for specified patient populations in terms of region, care setting and disease severity. By disaggregating cost data and patient populations, we can explain much of the disparities across studies, and residual heterogeneity is managed through the application of a random-effects meta-analysis. This arguably provides a systematic and transparent approach for summarising all available cost data and producing estimates of mean costs of care for specific regions and patient populations, which can later be utilised for example in cost-of-illness and cost-effectiveness modelling studies. Accurate estimates of costs of care by disease stage are critically important, for example economic evaluations of novel disease-modifying therapies in AD [135].
In contrast to our previous reviews, we elected not to adjust costs for differences in purchasing power across countries, but used standard exchange rates to convert costs into EUR. There were several reasons: all our analyses grouped countries by regions of similar purchasing power, which reduces the necessity for further adjustment. Additionally, the main objective of the study was to provide relevant estimates of costs of dementia care within each European region, rather than providing comparable estimates across regions.
The chosen approach also comes with limitations and is based on assumptions that are difficult to fully underpin with data. Computation of SDs and CIs relies on assumptions of independence of cost components. This is unlikely to fully hold, but at the same time it is not obvious which sign these correlations might take. Consequently, the presented measures of dispersion should be interpreted with caution. Additionally, the imputation of disaggregated cost components based on cost distributions from other studies relies on the assumption of comparability across similar studies.
There are also sources of heterogeneity that are not considered in our analysis. We did not attempt to disaggregate costs into resource use and unit costs or to disentangle differences in resource utilisation versus valuation of resources. This is of special relevance for informal care, where different valuation principles (e.g. replacement cost vs opportunity cost methods) can lead to disparate cost estimates. Harmonising the measurement and valuation of informal care would potentially decrease heterogeneity across studies and produce more comparable, though not necessarily more ‘correct’, cost estimates. Further, with a random-effects model, meta-analytic estimates are more likely to be influenced by small studies reporting outlying cost estimates, compared with fixed-effects models.
The reclassification of costs into our defined cost categories was unproblematic in most cases; however, for some resource types, in particular nursing services, there was inconsistency across publications whether to classify these as direct medical cost or a direct non-medical (community care) cost. Though we attempted as far as possible to apply a consistent approach across studies, there may be some residual inconsistency between estimates in the breakdown of costs between direct medical and direct non-medical costs.
Additionally, the estimates in our study are less precise compared with what would been achieved with more stringent inclusion and exclusion criteria. The inclusion of studies with large variations in methodology has likely inflated heterogeneity, which is illustrated by the impact of study methodology seen in the results of the meta-regression. However, we were also able to observe the complementary strengths of different methodologies, for example the greater sensitivity of large retrospective database studies to measure costs of rare but costly events such as hospitalisations, and the greater level of detail on informal care recorded in prospective clinical trials. Furthermore, applying strict criteria for quality and homogeneity across studies can lead to exclusion of the majority of reports and make the resulting analysis less conclusive [7]. In the literature search, some search terms were limited to the title field. This may have resulted in potentially relevant studies not being identified, and thus the search may not have been completely exhaustive.
We did not attempt to attribute causality to dementia costs, i.e. what we estimate is costs in PWD, rather than costs specifically due to dementia. A number of the studies included in this review also provided estimates of attributable costs, usually by calculating the difference between costs in PWD to costs in a control group of elderly individuals without dementia. The distinction between costs in dementia and costs due to dementia is relevant for cost-of-illness studies, but perhaps less critical for applications where the focus lies on the gradient of costs across disease stages, as is usually the case in economic evaluations that might leverage these cost estimates.
Future Research
As informal care constitutes a major component of dementia care costs, it is imperative that future studies measure and valuate informal care in an accurate and transparent manner. The results of the informal care analysis varied based on the method used, which highlights the need for standardisation of capturing informal care costs in future studies. The Resource Utilization in Dementia instrument was the most commonly used scale in the included studies, and has been designed to standardise the measurement of informal care through self-reporting from caregivers [16]. Similarly, to better understand regional differences, there is a need for multi-national studies that use a standardised data collection protocol across each of the sampled countries. The ICTUS [2] and GERAS [14] studies are good examples; however, in particular, high-quality studies of the impact of dementia in Central and Eastern European countries are lacking. Initiatives to enhance consistency and availability of research data across European registries and cohorts currently focus on clinical and biological data but should also be extended to include a harmonised collection of socioeconomic and resource utilisation data.
Over 60% of PWD are estimated to have three or more comorbidities [136], but the extent to which comorbidities might moderate the cost of care is unclear. So far, investigations have been limited by sample size, selected recruitment and limited data collection on co-morbidities. There is also a paucity of studies reporting costs in patients with dementia of other aetiologies than AD.
Finally, with the increasing opportunities for early diagnosis of AD, such as clinically relevant blood-based biomarkers [137], accurate characterisation is needed of the burden of early AD stages (i.e. subjective cognitive decline through mild cognitive impairment and mild dementia). This includes the impact on work capacity in subjects participating in the work force.
Conclusions
To our knowledge, this study represents the most extensive meta-analysis of the cost of dementia in Europe to date. Though there is considerable heterogeneity across studies, much of this is explained by identifiable factors. By disaggregating costs and patient subgroups, we attempt to fully utilise the existing literature to produce estimates of mean costs of care by region, care setting and disease severity. These estimates may prove useful in the evaluations of future health technologies, such as precision diagnostics and disease-modifying therapies for AD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This is an European Union Joint Programme—Neurodegenerative Disease Research (JPND) project. The project is supported through the following funding organisation under the aegis of JPND—http://www.jpnd.eu: the Swedish Research Council for Health, Working Life and Welfare (FORTE).
Funding
Open access funding provided by Karolinska Institute.
Declarations
Funding
The study was funded by a research grant from the Swedish Research Council for Health, Working Life and Welfare (FORTE), 2018-01887.
Author's contributions
Study conception and design: LJ, AW. Literature review and data abstraction: LJ, OF, AT. Data management and analysis: LJ, AT. Draft manuscript preparation: AT, LJ. All authors reviewed the results, contributed to and approved the final version of the manuscript.
Conflict of interest
Linus Jönsson was previously employed by H. Lundbeck, but this work was unrelated to the employment. He is a minority shareholder in H. Lundbeck and has received license fees for the data collection instrument (Resource Utilization in Dementia). Anders Wimo declares that none of the potential list of conflicts of interest listed has had any impact on the conduct of this study. Oskar Frisell and Ashley Tate have no conflicts of interest that are directly relevant to the content of this study.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The dataset is available from the corresponding author on reasonable request.
Code availability
The R code for data processing and analysis is available from the author on reasonable request.
Footnotes
Downloaded from Eurostat https://ec.europa.eu/eurostat/web/exchange-and-interest-rates/data/database, table ert_bil_eur_a, accessed 14 August 2021.
Downloaded from https://ec.europa.eu/eurostat/web/hicp, accessed 14 August 2021.
References
- 1.World Health Organization . Global status report on the public health response to dementia. Geneva: World Health Organization; 2021. [Google Scholar]
- 2.Gustavsson A, et al. Differences in resource use and costs of dementia care between European countries: baseline data from the ICTUS study. J Nutr Health Aging. 2010;14(8):648–654. doi: 10.1007/s12603-010-0311-7. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez L, et al. Systematic review of model-based economic evaluations of treatments for Alzheimer's disease. Pharmacoeconomics. 2016;34(7):681–707. doi: 10.1007/s40273-016-0392-1. [DOI] [PubMed] [Google Scholar]
- 4.Jönsson L, Wimo A. The cost of dementia in Europe: a review of the evidence, and methodological considerations. Pharmacoeconomics. 2009;27(5):391–403. doi: 10.2165/00019053-200927050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Quentin W, et al. Cost-of-illness studies of dementia: a systematic review focusing on stage dependency of costs. Acta Psychiatr Scand. 2010;121(4):243–259. doi: 10.1111/j.1600-0447.2009.01461.x. [DOI] [PubMed] [Google Scholar]
- 6.Schaller S, et al. The main cost drivers in dementia: a systematic review. Int J Geriatr Psychiatry. 2015;30(2):111–129. doi: 10.1002/gps.4198. [DOI] [PubMed] [Google Scholar]
- 7.Marešová P, et al. Cost of treatment and care for people with Alzheimer's disease: a meta-analysis. Curr Alzheimer Res. 2019;16(14):1245–1253. doi: 10.2174/1567205017666200102144640. [DOI] [PubMed] [Google Scholar]
- 8.Cantarero-Prieto D, et al. The economic cost of dementia: a systematic review. Dementia (London). 2020;19(8):2637–2657. doi: 10.1177/1471301219837776. [DOI] [PubMed] [Google Scholar]
- 9.Afonso-Argilés FJ, et al. Emergency department and hospital admissions among people with dementia living at home or in nursing homes: results of the European RightTimePlaceCare project on their frequency, associated factors and costs. BMC Geriatr. 2020;20(1):453. doi: 10.1186/s12877-020-01835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodel R, et al. Determinants of societal costs in Alzheimer's disease: GERAS study baseline results. Alzheimers Dement. 2015;11:933–945. doi: 10.1016/j.jalz.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Gustavsson A, et al. Predictors of costs of care in Alzheimer's disease: a multinational sample of 1222 patients. Alzheimers Dement. 2011;7(3):318–327. doi: 10.1016/j.jalz.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Handels RLH, et al. Quality of life, care resource use, and costs of dementia in 8 European countries in a cross-sectional cohort of the Actifcare Study. J Alzheimers Dis. 2018;66(3):1027–1040. doi: 10.3233/JAD-180275. [DOI] [PubMed] [Google Scholar]
- 13.Jonsson L, et al. Determinants of costs of care for patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2006;21(5):449–459. doi: 10.1002/gps.1489. [DOI] [PubMed] [Google Scholar]
- 14.Reed C, et al. What drives country differences in cost of Alzheimer's disease? An explanation from resource use in the GERAS study. J Alzheimers Dis. 2017;57(3):797–812. doi: 10.3233/JAD-160449. [DOI] [PubMed] [Google Scholar]
- 15.Wubker A, et al. Costs of care for people with dementia just before and after nursing home placement: primary data from eight European countries. Eur J Health Econ. 2015;16(7):689–707. doi: 10.1007/s10198-014-0620-6. [DOI] [PubMed] [Google Scholar]
- 16.Wimo A, Jonsson L, Zbrozek A. The Resource Utilization in Dementia (RUD) instrument is valid for assessing informal care time in community-living patients with dementia. J Nutr Health Aging. 2010;14(8):685–690. doi: 10.1007/s12603-010-0316-2. [DOI] [PubMed] [Google Scholar]
- 17.Wimo A, Nordberg G. Validity and reliability of assessments of time. Comparisons of direct observations and estimates of time by the use of the resource utilization in dementia (RUD)-instrument. Arch Gerontol Geriatr. 2007;44(1):71–81. doi: 10.1016/j.archger.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arevalo-Rodriguez I, et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI) Cochrane Database Syst Rev. 2015;3:CD010783. doi: 10.1002/14651858.CD010783.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl. 1):173–176. doi: 10.1017/S1041610297004870. [DOI] [PubMed] [Google Scholar]
- 21.Reisberg B, et al. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 22.Lenderking WR, et al. Reliability, validity, and interpretation of the dependence scale in mild to moderately severe Alzheimer's disease. Am J Alzheimers Dis Other Dement. 2013;28(8):738–749. doi: 10.1177/1533317513504609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol. 1989;44(3):M77–84. doi: 10.1093/geronj/44.3.M77. [DOI] [PubMed] [Google Scholar]
- 24.Cummings JL, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308. doi: 10.1212/WNL.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). 2022 Cochrane.
- 26.World Population Prospects 2022. Documentation of country groupings. 2022. https://population.un.org/wpp/Download/Documentation/Documentation/. Accessed 28 Aug 2022.
- 27.Wolstenholme J, et al. Estimating the relationship between disease progression and cost of care in dementia. Br J Psychiatry. 2002;181:36–42. doi: 10.1192/bjp.181.1.36. [DOI] [PubMed] [Google Scholar]
- 28.Rigaud AS, et al. Patients with Alzheimer's disease living at home in France: costs and consequences of the disease. J Geriatr Psychiatry Neurol. 2003;16(3):140–145. doi: 10.1177/0891988703252558. [DOI] [PubMed] [Google Scholar]
- 29.R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2022.
- 30.Amador S, et al. Exploring resource use and associated costs in end-of-life care for older people with dementia in residential care homes. Int J Geriatr Psychiatry. 2014;29(7):758–766. doi: 10.1002/gps.4061. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee S, et al. Study of the use of antidepressants for depression in dementia: the HTA-SADD trial—a multicentre, randomised, double-blind, placebo-controlled trial of the clinical effectiveness and cost-effectiveness of sertraline and mirtazapine. Health Technol Assess. 2013;17(7):1–166. doi: 10.3310/hta17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boada M, et al. Costs of health care resources of ambulatory-care patients diagnosed with Alzheimer's disease in Spain. Med Clin (Barc) 1999;113(18):690–695. [PubMed] [Google Scholar]
- 33.Braun A, et al. Cost of care for persons with dementia: using a discrete-time Markov chain approach with administrative and clinical data from the dementia service Centres in Austria. Health Econ Rev. 2020;10(1):29. doi: 10.1186/s13561-020-00285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brilleman SL, et al. Implications of comorbidity for primary care costs in the UK: a retrospective observational study. Br J Gen Pract. 2013;63(609):e274–e282. doi: 10.3399/bjgp13X665242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruno G, et al. Costs and resource use associated with Alzheimer's disease in Italy: results from an observational study. J Prev Alzheimers Dis. 2018;5(1):55–64. doi: 10.14283/jpad.2017.31. [DOI] [PubMed] [Google Scholar]
- 36.Brüggenjürgen B, et al. Medical management, costs, and consequences of Alzheimer's disease in Germany: an analysis of health claims data. J Med Econ. 2015;18(6):466–473. doi: 10.3111/13696998.2015.1014090. [DOI] [PubMed] [Google Scholar]
- 37.Caravau H, Martín I. Direct costs of dementia in nursing homes. Front Aging Neurosci. 2015;7:146. doi: 10.3389/fnagi.2015.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavallo MC, Fattore G. The economic and social burden of Alzheimer disease on families in the Lombardy region of Italy. Alzheimer Dis Assoc Disord. 1997;11(4):184–190. [PubMed] [Google Scholar]
- 39.Charlesworth G, et al. Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial. Health Technol Assess. 2008;12(4):iii, v–ix, 1–78. [DOI] [PubMed]
- 40.Chiatti C, et al. The economic impact of moderate stage Alzheimer's disease in Italy: evidence from the UP-TECH randomized trial. Int Psychogeriatr. 2015;27(9):1563–1572. doi: 10.1017/S104161021500040X. [DOI] [PubMed] [Google Scholar]
- 41.Coduras A, et al. Prospective one-year cost-of-illness study in a cohort of patients with dementia of Alzheimer's disease type in Spain: the ECO study. J Alzheimers Dis. 2010;19(2):601–615. doi: 10.3233/JAD-2010-1258. [DOI] [PubMed] [Google Scholar]
- 42.D'Amico F, et al. Maintenance cognitive stimulation therapy: an economic evaluation within a randomized controlled trial. J Am Med Dir Assoc. 2015;16(1):63–70. doi: 10.1016/j.jamda.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 43.D'Amico F, et al. Cost-effectiveness of exercise as a therapy for behavioural and psychological symptoms of dementia within the EVIDEM-E randomised controlled trial. Int J Geriatr Psychiatry. 2016;31(6):656–665. doi: 10.1002/gps.4376. [DOI] [PubMed] [Google Scholar]
- 44.Dahlrup B, et al. Health economic analysis on a psychosocial intervention for family caregivers of persons with dementia. Dement Geriatr Cogn Disord. 2014;37(3–4):181–195. doi: 10.1159/000355365. [DOI] [PubMed] [Google Scholar]
- 45.Darbà J, Kaskens L, Lacey L. Relationship between global severity of patients with Alzheimer's disease and costs of care in Spain; results from the co-dependence study in Spain. Eur J Health Econ. 2015;16(8):895–905. doi: 10.1007/s10198-014-0642-0. [DOI] [PubMed] [Google Scholar]
- 46.Érsek K, et al. Costs of dementia in Hungary. J Nutr Health Aging. 2010;14(8):633–639. doi: 10.1007/s12603-010-0309-1. [DOI] [PubMed] [Google Scholar]
- 47.Farre M, et al. Direct and indirect costs and resource use in dementia care: a cross-sectional study in patients living at home. Int J Nurs Stud. 2016;55:39–49. doi: 10.1016/j.ijnurstu.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Farré M, et al. Costs and burden associated with loss of labor productivity in informal caregivers of people with dementia: results from Spain. J Occup Environ Med. 2018;60(5):449–456. doi: 10.1097/JOM.0000000000001229. [DOI] [PubMed] [Google Scholar]
- 49.Ferry F, et al. Economic costs and health-related quality of life associated with individual specific reminiscence: results from the InspireD Feasibility Study. Dementia (London) 2020;19(7):2166–2183. doi: 10.1177/1471301218816814. [DOI] [PubMed] [Google Scholar]
- 50.Frahm-Falkenberg S, et al. Health, social and economic consequences of dementias: a comparative national cohort study. Eur J Neurol. 2016;3(9):1400–1407. doi: 10.1111/ene.13043. [DOI] [PubMed] [Google Scholar]
- 51.François C, et al. Cost effectiveness of memantine in moderately severe to severe Alzheimer's disease: a Markov model in Finland. Clin Drug Investig. 2004;24(7):373–384. doi: 10.2165/00044011-200424070-00001. [DOI] [PubMed] [Google Scholar]
- 52.Garcia-Garcia RR, Calleja-Hernandez MAMA. Drug cost optimization of cholinesterase inhibitor therapy in advanced dementia: results of a prospective study of discontinuation. Trop J Pharm Res. 2021;20(8):1711–1714. doi: 10.4314/tjpr.v20i8.23. [DOI] [Google Scholar]
- 53.Gervès C, Chauvin P, Bellanger MM. Evaluation of full costs of care for patients with Alzheimer's disease in France: the predominant role of informal care. Health Policy. 2014;116(1):114–122. doi: 10.1016/j.healthpol.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Gillespie P, et al. The effects of dependence and function on costs of care for Alzheimer's disease and mild cognitive impairment in Ireland. Int J Geriatr Psychiatry. 2013;28(3):256–264. doi: 10.1002/gps.3819. [DOI] [PubMed] [Google Scholar]
- 55.Gola AB, et al. Healthcare utilization and monetary costs associated with agitation in UK care home residents with advanced dementia: a prospective cohort study. Int Psychogeriatr. 2020;32(3):359–370. doi: 10.1017/S1041610219002059. [DOI] [PubMed] [Google Scholar]
- 56.Graff MJ, et al. Community occupational therapy for older patients with dementia and their care givers: cost effectiveness study. BMJ. 2008;336(7636):134–138. doi: 10.1136/bmj.39408.481898.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gustavsson A, et al. Disease progression and costs of care in Alzheimer's disease patients treated with donepezil: a longitudinal naturalistic cohort. Eur J Health Econ. 2012;13(5):561–568. doi: 10.1007/s10198-011-0334-y. [DOI] [PubMed] [Google Scholar]
- 58.Handels RL, et al. Determinants of care costs of patients with dementia or cognitive impairment. Alzheimer Dis Assoc Disord. 2013;27(1):30–36. doi: 10.1097/WAD.0b013e318242da1d. [DOI] [PubMed] [Google Scholar]
- 59.Happich M, et al. Excess costs associated with possible misdiagnosis of Alzheimer's disease among patients with vascular dementia in a UK CPRD population. J Alzheimers Dis. 2016;53(1):171–183. doi: 10.3233/JAD-150685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henderson C, et al. Use and costs of services and unpaid care for people with mild-to-moderate dementia: baseline results from the IDEAL cohort study. Alzheimers Dement (N Y) 2019;5:685–696. doi: 10.1016/j.trci.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holmerova I, et al. Costs of dementia in the Czech Republic. Eur J Health Econ. 2017;18(8):979–986. doi: 10.1007/s10198-016-0842-x. [DOI] [PubMed] [Google Scholar]
- 62.Howard R, et al. The effectiveness and cost-effectiveness of assistive technology and telecare for independent living in dementia: a randomised controlled trial. Age Ageing. 2021;50(3):882–890. doi: 10.1093/ageing/afaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jakobsen M, et al. Costs of informal care for people suffering from dementia: evidence from a Danish survey. Dement Geriatr Cogn Dis Extra. 2011;1(1):418–428. doi: 10.1159/000333812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jetsonen V, et al. Total cost of care increases significantly from early to mild Alzheimer's disease: 5-year ALSOVA follow-up. Age Ageing. 2021;50:2116–2122. doi: 10.1093/ageing/afab144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joling KJ, et al. Predictors of societal costs in dementia patients and their informal caregivers: a two-year prospective cohort study. Am J Geriatr Psychiatry. 2015;23(11):1193–1203. doi: 10.1016/j.jagp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 66.Jones RW, et al. Dependence in Alzheimer's disease and service use costs, quality of life, and caregiver burden: the DADE study. Alzheimers Dement. 2015;11(3):280–290. doi: 10.1016/j.jalz.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Jönsson L, et al. The cost-effectiveness of donepezil therapy in Swedish patients with Alzheimer's disease: a Markov model. Clin Ther. 1999;21(7):1230–1240. doi: 10.1016/S0149-2918(00)80025-9. [DOI] [PubMed] [Google Scholar]
- 68.Khan I, et al. Does structured exercise improve cognitive impairment in people with mild to moderate dementia? A cost-effectiveness analysis from a confirmatory randomised controlled trial: the Dementia and Physical Activity (DAPA) trial. Pharmacoecon Open. 2019;3(2):215–227. doi: 10.1007/s41669-018-0097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiencke P, et al. Direct costs of Alzheimer's disease in Germany. Eur J Health Econ. 2011;12(6):533–539. doi: 10.1007/s10198-010-0267-x. [DOI] [PubMed] [Google Scholar]
- 70.Knapp M, et al. Predictors of care home and hospital admissions and their costs for older people with Alzheimer's disease: findings from a large London case register. BMJ Open. 2016;6(11):e013591. doi: 10.1136/bmjopen-2016-013591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knapp M, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer's disease (the DOMINO-AD trial) Int J Geriatr Psychiatry. 2017;32(12):1205–1216. doi: 10.1002/gps.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Konig HH, et al. The costs of dementia from the societal perspective: is care provided in the community really cheaper than nursing home care? J Am Med Dir Assoc. 2014;15(2):117–126. doi: 10.1016/j.jamda.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Kronborg Andersen C, et al. The cost of dementia in Denmark: the Odense Study. Dement Geriatr Cogn Disord. 1999;10(4):295–304. doi: 10.1159/000017135. [DOI] [PubMed] [Google Scholar]
- 74.Leicht H, et al. Net costs of dementia by disease stage. Acta Psychiatr Scand. 2011;124(5):384–395. doi: 10.1111/j.1600-0447.2011.01741.x. [DOI] [PubMed] [Google Scholar]
- 75.Lenox-Smith A, et al. Resource utilisation, costs and clinical outcomes in non-institutionalised patients with Alzheimer's disease: 18-month UK results from the GERAS observational study. BMC Geriatr. 2016;16(1):195. doi: 10.1186/s12877-016-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Livingston G, et al. Long-term clinical and cost-effectiveness of psychological intervention for family carers of people with dementia: a single-blind, randomised, controlled trial. Lancet Psychiatry. 2014;1(7):539–548. doi: 10.1016/S2215-0366(14)00073-X. [DOI] [PubMed] [Google Scholar]
- 77.Livingston G, et al. A dependency model for patients with Alzheimer's disease: its validation and relationship to the costs of care: the LASER-AD Study. Curr Med Res Opin. 2004;20(7):1007–1016. doi: 10.1185/030079904125003980. [DOI] [PubMed] [Google Scholar]
- 78.Longo F, et al. Investigating the economic case of a service to support carers of people with dementia: a cross-sectional survey-based feasibility study in England. Health Soc Care Community. 2019;27(5):e734–e743. doi: 10.1111/hsc.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopez-Bastida J, et al. Social-economic costs and quality of life of Alzheimer disease in the Canary Islands, Spain. Neurology. 2006;67(12):2186–2191. doi: 10.1212/01.wnl.0000249311.80411.93. [DOI] [PubMed] [Google Scholar]
- 80.Luz Pires C, et al. Costs of informal caregiving in dementia. Acta Med Port. 2020;33(9):559–567. doi: 10.20344/amp.11922. [DOI] [PubMed] [Google Scholar]
- 81.MacNeil Vroomen J, et al. The cost-effectiveness of two forms of case management compared to a control group for persons with dementia and their informal caregivers from a societal perspective. PLoS One. 2016;11(9):e0160908. doi: 10.1371/journal.pone.0160908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meeuwsen E, et al. Cost-effectiveness of one year dementia follow-up care by memory clinics or general practitioners: economic evaluation of a randomised controlled trial. PLoS One. 2013;8(11):e79797. doi: 10.1371/journal.pone.0079797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Menn P, et al. Dementia care in the general practice setting: a cluster randomized trial on the effectiveness and cost impact of three management strategies. Value Health. 2012;15(6):851–859. doi: 10.1016/j.jval.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 84.Mesterton J, et al. Cross sectional observational study on the societal costs of Alzheimer's disease. Curr Alzheimer Res. 2010;7(4):358–367. doi: 10.2174/156720510791162430. [DOI] [PubMed] [Google Scholar]
- 85.Michalowsky B, et al. Medication cost of persons with dementia in primary care in Germany. J Alzheimers Dis. 2014;42(3):949–958. doi: 10.3233/JAD-140804. [DOI] [PubMed] [Google Scholar]
- 86.Michalowsky B, et al. Healthcare utilization and costs in primary care patients with dementia: baseline results of the DelpHi-trial. Eur J Health Econ. 2018;19(1):87–102. doi: 10.1007/s10198-017-0869-7. [DOI] [PubMed] [Google Scholar]
- 87.Morris S, et al. Monetary costs of agitation in older adults with Alzheimer's disease in the UK: prospective cohort study. BMJ Open. 2015;5(3):e007382. doi: 10.1136/bmjopen-2014-007382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mostardt S, et al. Efficacy and cost effectiveness of case management in patients with dementia. Z Gerontol Geriatr. 2012;5(7):642–646. doi: 10.1007/s00391-012-0298-2. [DOI] [PubMed] [Google Scholar]
- 89.Mueller C, et al. Hospitalization in people with dementia with Lewy bodies: frequency, duration, and cost implications. Alzheimers Dement (Amst) 2018;10:143–152. doi: 10.1016/j.dadm.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neubert L, et al. Excess costs of dementia in old age (85+) in Germany: results from the AgeCoDe-AgeQualiDe study. J Econ Ageing. 2021;20:100346. doi: 10.1016/j.jeoa.2021.100346. [DOI] [Google Scholar]
- 91.O'Shea E, Monaghan C. An economic analysis of a community-based model for dementia care in Ireland: a balance of care approach. Int Psychogeriatr. 2017;29(7):1175–1184. doi: 10.1017/S1041610217000400. [DOI] [PubMed] [Google Scholar]
- 92.Oberfrank F, Donka-Verebes É, Boncz I. Health insurance cost of Alzheimer dementia in Hungary: a cost of illness study. Value Health. 2014;17(7):A768. doi: 10.1016/j.jval.2014.08.298. [DOI] [PubMed] [Google Scholar]
- 93.Olazaran J, et al. Costs and quality of life in community-dwelling patients with Alzheimer's disease in Spain: results from the GERAS II observational study. Int Psychogeriatr. 2017;29(12):2081–2093. doi: 10.1017/S1041610217001211. [DOI] [PubMed] [Google Scholar]
- 94.Panca M, et al. Healthcare resource utilisation and costs of agitation in people with dementia living in care homes in England: the Managing Agitation and Raising QUality of LifE in Dementia (MARQUE) study. PLoS One. 2019;14(2):e0211953. doi: 10.1371/journal.pone.0211953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paraponaris A, Davin B. Economics of the iceberg: informal care provided to French elderly with dementia. Value Health. 2015;18(4):368–375. doi: 10.1016/j.jval.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 96.Peña-Longobardo LM, Oliva-Moreno J. Economic valuation and determinants of informal care to people with Alzheimer's disease. Eur J Health Econ. 2015;16(5):507–515. doi: 10.1007/s10198-014-0604-6. [DOI] [PubMed] [Google Scholar]
- 97.Rapp T, et al. Resource use and cost of Alzheimer's disease in France: 18-month results from the GERAS observational study. Value Health. 2018;21(3):295–303. doi: 10.1016/j.jval.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 98.Rapp T, et al. Exploring the relationship between Alzheimer's disease severity and longitudinal costs. Value Health. 2012;15(3):412–419. doi: 10.1016/j.jval.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 99.Reese JP, et al. Cost and care of patients with Alzheimer's disease: clinical predictors in German health care settings. J Alzheimers Dis. 2011;27(4):723–736. doi: 10.3233/JAD-2011-110539. [DOI] [PubMed] [Google Scholar]
- 100.Romeo R, et al. The cost of care homes for people with dementia in England: a modelling approach. Int J Geriatr Psychiatry. 2017;32(12):1466–1475. doi: 10.1002/gps.4637. [DOI] [PubMed] [Google Scholar]
- 101.Rosenvall A, et al. Potential cost savings for selected non-pharmacological treatment strategies for patients with Alzheimers disease in Finland. J Rehabil Med. 2020;52:jrm00106. doi: 10.2340/16501977-2722. [DOI] [PubMed] [Google Scholar]
- 102.Rubinsztein JS, et al. A memory clinic v. traditional community mental health team service: comparison of costs and quality. BJPsych Bull. 2015;39(1):6–11. doi: 10.1192/pb.bp.113.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruiz-Adame Reina M, Correa M, Burton K. The opportunity costs of caring for people with dementia in Southern Spain. Gac Sanit. 2019;33(1):17–23. doi: 10.1016/j.gaceta.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 104.Schulenberg J, Schulenberg I, Horn R, et al. Cost of treatment and cost of care for Alzheimer’s disease in Germany. In: Wimo A, et al., editors. The health economics of dementia. Chichester: Wiley; 1998. pp. 217–230. [Google Scholar]
- 105.Schwarzkopf L, et al. Costs of care for dementia patients in community setting: an analysis for mild and moderate disease stage. Value Health. 2011;14(6):827–835. doi: 10.1016/j.jval.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 106.Schwarzkopf L, et al. Are community-living and institutionalized dementia patients cared for differently? Evidence on service utilization and costs of care from German insurance claims data. BMC Health Serv Res. 2013;13:2. doi: 10.1186/1472-6963-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schwarzkopf L, et al. Excess costs of dementia disorders and the role of age and gender: an analysis of German health and long-term care insurance claims data. BMC Health Serv Res. 2012;12:165. doi: 10.1186/1472-6963-12-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scuvee-Moreau J, Kurz X, Dresse A. The economic impact of dementia in Belgium: results of the National Dementia Economic Study (NADES) Acta Neurol Belg. 2002;102(3):104–113. [PubMed] [Google Scholar]
- 109.Sopina E, et al. Long-term medical costs of Alzheimer's disease: matched cohort analysis. Eur J Health Econ. 2019;20(3):333–342. doi: 10.1007/s10198-018-1004-0. [DOI] [PubMed] [Google Scholar]
- 110.Soto-Gordoa M, et al. The cost of applying the dependency law to Alzheimer disease. Gac Sanit. 2014;28(5):389–392. doi: 10.1016/j.gaceta.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 111.Souêtre E, Thwaites RM, Yeardley HL. Economic impact of Alzheimer's disease in the United Kingdom. Cost of care and disease severity for non-institutionalised patients with Alzheimer's disease. Br J Psychiatry. 1999;174:51–55. doi: 10.1192/bjp.174.1.51. [DOI] [PubMed] [Google Scholar]
- 112.Souêtre EJ, et al. Economic analysis of Alzheimer's disease in outpatients: impact of symptom severity. Int Psychogeriatr. 1995;7(1):115–122. doi: 10.1017/S1041610295001906. [DOI] [PubMed] [Google Scholar]
- 113.Steinbeisser K, et al. Cost-effectiveness of a non-pharmacological treatment vs. "care as usual" in day care centers for community-dwelling older people with cognitive impairment: results from the German randomized controlled DeTaMAKS-trial. Eur J Health Econ. 2020;21(6):825–844. doi: 10.1007/s10198-020-01175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Søgaard R, et al. Early psychosocial intervention in Alzheimer's disease: cost utility evaluation alongside the Danish Alzheimer's Intervention Study (DAISY) BMJ Open. 2014;4(1):e004105. doi: 10.1136/bmjopen-2013-004105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taipale H, et al. Hospital care and drug costs from five years before until two years after the diagnosis of Alzheimer's disease in a Finnish nationwide cohort. Scand J Public Health. 2016;44(2):150–158. doi: 10.1177/1403494815614705. [DOI] [PubMed] [Google Scholar]
- 116.Trabucchi M. An economic perspective on Alzheimer's disease. J Geriatr Psychiatry Neurol. 1999;12(1):29–38. doi: 10.1177/089198879901200107. [DOI] [PubMed] [Google Scholar]
- 117.Turró-Garriga O, et al. Pharmaceutical consumption and cost in patients with dementia: a longitudinal study by the Registry of Dementias of Girona (ReDeGi) in Catalonia (Spain) Arch Gerontol Geriatr. 2015;60(3):448–452. doi: 10.1016/j.archger.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 118.Turró-Garriga O, et al. Consequences of anosognosia on the cost of caregivers' care in Alzheimer's disease. J Alzheimers Dis. 2016;54(4):1551–1560. doi: 10.3233/JAD-160419. [DOI] [PubMed] [Google Scholar]
- 119.Turró-Garriga O, et al. Annual economic cost of informal care in Alzheimer's disease. Rev Neurol. 2010;1(4):201–207. [PubMed] [Google Scholar]
- 120.Turró-Garriga O, et al. Caregivers' sense of coherence: implications on direct and indirect costs of dementia care. J Alzheimers Dis. 2020;78(1):117–126. doi: 10.3233/JAD-200350. [DOI] [PubMed] [Google Scholar]
- 121.van de Ven G, et al. The economics of dementia-care mapping in nursing homes: a cluster-randomised controlled trial. PLoS One. 2014;9(1):e86662. doi: 10.1371/journal.pone.0086662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Santen J, et al. Cost-effectiveness of exergaming compared to regular day-care activities in dementia: results of a randomised controlled trial in The Netherlands. Health Soc Community. 2022;30:e1794–e1804. doi: 10.1111/hsc.13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vandepitte S, et al. Factors associated with costs of care in community-dwelling persons with dementia from a third party payer and societal perspective: a cross-sectional study. BMC Geriatr. 2020;20(1):18. doi: 10.1186/s12877-020-1414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vossius C, et al. The use and costs of formal care in newly diagnosed dementia: a three-year prospective follow-up study. Am J Geriatr Psychiatry. 2014;22(4):381–388. doi: 10.1016/j.jagp.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 125.Vossius C, et al. Cost analysis of day care centres in Norway. PLoS One. 2019;14(8):e0219568. doi: 10.1371/journal.pone.0219568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Walsh S, et al. Factors influencing the cost of care and admission to long-term care for people with dementia in Ireland. Aging Ment Health. 2021;25(3):512–520. doi: 10.1080/13607863.2019.1699901. [DOI] [PubMed] [Google Scholar]
- 127.Wimo A, Johansson L, Jönsson L. Prevalence study of societal costs for dementia 2000–2005. More demented people—but somewhat reduced costs per person. Lakartidningen. 2009;106(18–19):1277–1282. [PubMed] [Google Scholar]
- 128.Wimo A, Winblad B. Societal burden and economics of vascular dementia: preliminary results from a Swedish-population-based study. Int Psychogeriatr. 2003;15(Suppl. 1):251–256. doi: 10.1017/S1041610203009281. [DOI] [PubMed] [Google Scholar]
- 129.Wohlgemuth A, et al. Drug-related problems increase healthcare costs for people living with dementia. J Alzheimers Dis. 2020;73(2):791–799. doi: 10.3233/JAD-190819. [DOI] [PubMed] [Google Scholar]
- 130.Woods RT, et al. REMCARE: reminiscence groups for people with dementia and their family caregivers—effectiveness and cost-effectiveness pragmatic multicentre randomised trial. Health Technol Assess. 2012;16(48):v–xv, 1–116. [DOI] [PubMed]
- 131.Åkerborg Ö, et al. Cost of dementia and its correlation with dependence. J Aging Health. 2016;28(8):1448–1454. doi: 10.1177/0898264315624899. [DOI] [PubMed] [Google Scholar]
- 132.Meijer E, et al. Economic costs of dementia in 11 countries in Europe: estimates from nationally representative cohorts of a panel study. Lancet Reg Health Eur. 2022;20:100445. doi: 10.1016/j.lanepe.2022.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jönsson L. The personal economic burden of dementia in Europe. Lancet Reg Health Eur. 2022;20:100472. doi: 10.1016/j.lanepe.2022.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stephan A, et al. Barriers and facilitators to the access to and use of formal dementia care: findings of a focus group study with people with dementia, informal carers and health and social care professionals in eight European countries. BMC Geriatr. 2018;18(1):1–16. doi: 10.1186/s12877-018-0816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mattingly TJ, 2nd, McQueen RB, Lin PJ. Contextual considerations and recommendations for estimating the value of Alzheimer's disease therapies. Pharmacoeconomics. 2021;39(10):1101–1107. doi: 10.1007/s40273-021-01079-6. [DOI] [PubMed] [Google Scholar]
- 136.Doraiswamy PM, et al. Prevalence and impact of medical comorbidity in Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2002;57(3):M173–M177. doi: 10.1093/gerona/57.3.M173. [DOI] [PubMed] [Google Scholar]
- 137.Hansson O, et al. The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement. 2022 doi: 10.1002/alz.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.