Introduction
This brief report is to alert the public health community to the confirmed presence of Δ8-THC-O acetate in commercially available vaping products and the potential risk of pulmonary toxicity from vaping THC-O. The impetus for this study was the presence of the aryl acetate moiety in THC-O. Mass spectrometry (MS) evidence and activation energy (AE) calculations indicating that vaping of THC-O is likely to generate ketene, a suspected cause of lung injury, are provided.
An epidemic of severe lung injury associated with the use of electronic cigarettes (e-cigarettes) occurred in 2019 and is thought to be caused primarily by the inhalation of vitamin E acetate (VEA). [1] This epidemic resulted in 2807 hospitalizations and 68 deaths in the USA and the illness was termed E-cigarette or vaping product use–associated lung injury (EVALI).
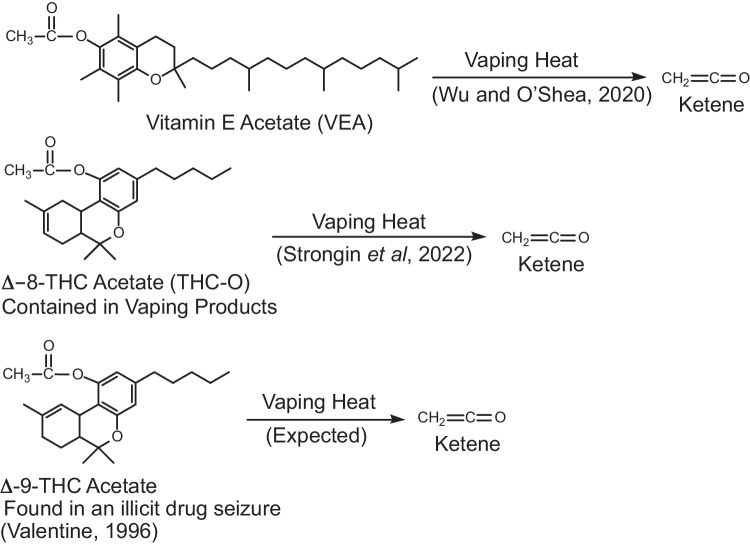
VEA (α-tocopherol acetate) is widely and safely used in food and cosmetic products. It is a synthetic derivative of vitamin E for which the acetate moiety serves to increase the stability of the natural product in commercial commodities. However, when heated during vaping, VEA undergoes thermal decomposition releasing ketene, which is a highly potent lung toxicant (Fig. 1) [2, 3]. The chemical reactivity and toxicology of ketene are broadly equated to that of phosgene, both acting as acylating agents with a delayed onset of pulmonary toxicity.
Fig. 1.
Potential sources of ketene formation from commercial vaping products.
THC-O-acetate, also called “THC-O,” is a synthetic cannabinoid that is purported to be more potent than Δ9-tetrahydrocannabinol, the primary psychoactive constituent of marijuana. In 2018, Congress passed the Agricultural Improvement Act (2018 Farm Bill), which removed hemp products containing less than 0.3% Δ9-THC and their derivatives from federal scheduling regulations [4]. The non-psychoactive cannabidiol (CBD) can be legally extracted from hemp and is currently used in numerous health and wellness products. Yet it is known that CBD can be chemically converted to Δ8-THC, which in turn can be acetylated with acetic anhydride to form Δ8-THC-O-acetate [5]. The latter chemical transformation step is the same to that used to convert vitamin E into VEA. Because Δ8-THC-O is synthetically derived from legal hemp extracts, it is currently (September 2022) available from a number of online vendors. In the same manner as VEA, it too risks generating ketene during vaping. Additionally, Δ9-THC-O has been reported in an illicit drug seizure, and the possibility exists that it could also be currently marketed (Fig. 1) [6].
Methods and Results
To confirm the presence of Δ8-THC-O-acetate in one such commercial source, a THC-O cartridge labeled Blue Dream was purchased online from Extract Labs. Gas chromatography-mass spectrometry (GC–MS) analysis of the oil contents revealed a major component (84%) with a mass spectrum consistent with that expected for THC-O-acetate. Further confirmation was obtained through saponification of the oil with methanolic sodium hydroxide to remove the acetate group. GC–MS analysis of the product revealed a chromatographic peak with the same retention time as an authentic sample of Δ8-THC and an excellent spectral match. No Δ9-THC was detected, confirming the purity of Δ8-THC-O in this commercial product.
As the aryl acetate moieties found in both THC-O-acetate and VEA are structurally similar, it could be anticipated that their thermally induced chemical reactivity would also be alike (Fig. 1). The presence of this key acetate functional group in THC-O should be of concern as it too would be expected to generate ketene when heated above its decomposition temperature.
In our MS analysis of the GC peak for Δ8-THC, the tell-tale fragmentation pattern showing the liberation of ketene was clearly evident by the ketene molecular weight (42 amu) loss from the molecular ion of 356 amu. This fragmentation pattern is consistent with previously published mass spectra for Δ8-THC-O-acetate and for that of VEA [3, 7]. As negative control, our analyzed sample of Δ8-THC did not show a fragment loss of 42 from the parent ion.
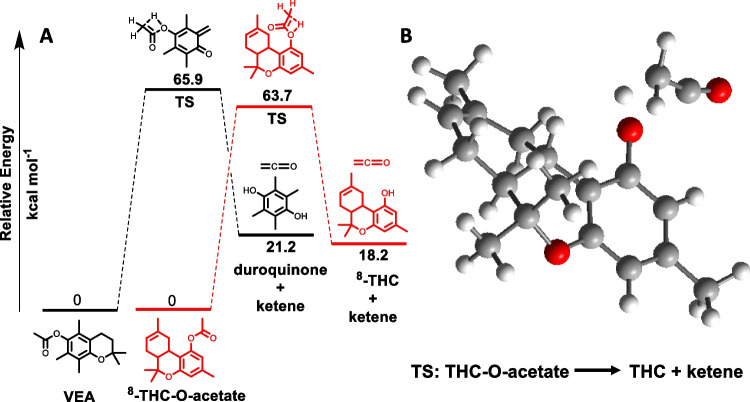
Next, density-functional theory (DFT) calculations were utilized to determine the AE for ketene release from THC-O and the results compared with previously reported data for VEA. One identified pathway of ketene formation from aryl acetates is via a four-membered transition state (TS) with a concerted [1, 3] hydrogen shift from methyl to oxygen [8]. Using this unimolecular decomposition pathway, the AE barrier for ketene elimination from Δ8-THC-O-acetate was calculated as 63.7 kcal mol−1 (Fig. 2, panel A). To put this value in context, the previously reported AE for ketene elimination from VEA was higher at 65.9 kcal mol−1 (calculated using the same DFT basis set following first pyrolysis of the chroman ring), suggesting a strong likelihood of vaping-induced ketene formation. [3] The optimized geometry of the four-membered cyclic TS for the conversion of Δ8-THC-O-acetate to THC and ketene showed similarities to that of VEA and was unimpeded by the fused bicyclic ring substituent of THC (Fig. 2, panel B). Using the same calculation protocols, an AE of 62.7 kcal mol−1 for Δ9-THC-acetate was obtained, indicating its use should also be of concern. These results corroborate the recently experimentally obtained results that Δ8-THC-O-acetate is capable of thermally generating ketene under real-world vaping conditions (Fig. 1) [9].
Fig. 2.
Calculated energy profiles for the pyrolysis of acetate substrates via concerted [1, 3] hydrogen shift mechanism using gas-phase M06-2X/6–311G (d,p) basis set. A Relative free energies (kcal mol−1) for starting materials, transition states, and products formed for the pyrolysis of VEA3 (black) and Δ8-THC-O-acetate (red) leading to the elimination of ketene. B Optimized geometry for the TS corresponding to the pyrolysis of Δ8-THC-O-acetate, leading to the elimination of ketene. Simplified structures used for THC calculations without C4H9 alkyl group.
Discussion
In this report we alert the public health community to the confirmed presence of THC-O in commercially available vaping products and the potential risk of pulmonary toxicity from vaping THC-O. Depending on the dose and duration of use, the toxicity might be acute or chronic. Clinical toxicity from vaping THC-O has not to the best of our knowledge been reported. However, we suggest that the use of THC-O be considered by health care providers when evaluating lung injury in people who have vaped cannabis products.
The discovery that two marketed products (this report and that of Munger et al. [9]) containing acetate esters produce ketene under vaping conditions serves to warn that products generally recognized as safe (GRAS) by oral administration may produce toxic, chemically reactive substances when vaped or smoked. A limitation of this study is that generation of ketene during experimental conditions does not guarantee production during actual use. Among individual users, actual vaping conditions vary considerably (temperature, presence of absence of catalytic sites in the devices), and milder conditions might not produce significant amounts of ketene. Another limitation is that only a few commercial products have been confirmed to contain THC-O. Surveillance of various products on the market is encouraged.
The possibility exists that other esters, particularly those derived from phenolic compounds such as THC and vitamin E, might likewise produce ketene or substituted ketenes if contained in vaping products. In cases of lung injury resulting from vaping novel products, this possibility should be considered. Of specific relevance to this report are the aryl ester functional groups of THC compounds. It is plausible that the emerging use of aryl esters may be associated with their perceived enhanced physical properties for vaping (e.g., viscosity, boiling point, solubility) over their phenolic precursors, but this comes with the unanticipated risk of producing highly toxic substances as they thermally degrade. In conclusion, the use of experimental mass spectrometry and theoretical DFT calculations can act as an early predictor of potential dangers prior to more extensive experimental studies.
Sources of Funding
None.
Declarations
Conflicts of Interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lozier MJ, Wallace B, Anderson K, Ellington S, Jones CM, Rose D, Baldwin G, King BA, Briss P, Mikosz CA, Lung Injury Response Epidemiology/Surveillance Task Force Update: Demographic, product, and substance-use characteristics of hospitalized patients in a nationwide outbreak of E-cigarette, or vaping, product use-associated lung injuries - United States, December 2019. MMWR Morb Mortal Wkly Rep. 2019;68(49):1142–1148. doi: 10.15585/mmwr.mm6849e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attfield KR, Chen W, Cummings KJ, Jacob P, 3rd, O’Shea DF, Wagner J, Wang P, Fowles J. Potential of ethenone (ketene) to contribute to electronic cigarette, or vaping, product use-associated lung injury. Am J Respir Crit Care Med. 2020;202(8):1187–1189. doi: 10.1164/rccm.202003-0654LE. [DOI] [PubMed] [Google Scholar]
- 3.Wu D, O’Shea DF. Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. Proc Natl Acad Sci USA. 2020;117:6349–6355. doi: 10.1073/pnas.1920925117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babalonis S, Raup-Konsavage WM, Akpunonu PD, Balla A, Vrana KE. Δ8-THC: legal status, widespread availability, and safety concerns. Cannabis Cannabinoid Res. 2021;6(5):362–365. doi: 10.1089/can.2021.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golombek P, Müller M, Barthlott I, Sproll C, Lachenmeier DW. Conversion of cannabidiol (CBD) into psychotropic cannabinoids including tetrahydrocannabinol (THC): a controversy in the scientific literature. Toxics. 2020;8(2):41. doi: 10.3390/toxics8020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valentine MD. Δ-9-Tetrahydrocannabinol acetate from acetylation of cannabis oil. Sci Justice. 1996;36(3):195–197. doi: 10.1016/S1355-0306(96)72595-9. [DOI] [Google Scholar]
- 7.Inayama S, Sawa A, Hosoya E. Mass spectrometry of oxidation products of Δ1 and Δ6-tetrahydrocannabinols. Chem Pharm Bull. 1976;24:2209–2218. doi: 10.1248/cpb.24.2209. [DOI] [PubMed] [Google Scholar]
- 8.Ghibaudi E, Colussi AJ. Very low pressure pyrolysis of phenyl acetate. Int J Chem Kinet. 1984;16:1575–1583. doi: 10.1002/kin.550161211. [DOI] [Google Scholar]
- 9.Munger KR, Jensen RP, Strongin RM. Vaping cannabinoid acetates leads to ketene formation. Chem Res Toxicol. 2022;35:1202–1205. doi: 10.1021/acs.chemrestox.2c00170. [DOI] [PubMed] [Google Scholar]