Abstract
The study aimed to determine the antioxidant activities and phenolic compounds of Bambangan (Mangifera pajang), a type of wild fruit belongs to the family of Anacardiaceae during fermentation at room (28 °C) and elevated temperature (35 °C). The antioxidant capacity was estimated based on 2,2-diphenyl-1-picyrlhydrazyl (DPPH) scavenging activity, ferric-ion-reducing power (FRAP), 2,2´-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation assay and oxygen-radical absorbing capacity (ORAC). A reversed phase high performance liquid chromatography (HPLC) was used to identify the phenolic compounds. Samples of bambangan fermented at 35 °C achieved the highest FRAP (141.42 mM Fe(II)/g extract) and ABTS values (5.00 mmol TE/g) within the first six days as compared to the samples fermented at room temperature (28 °C), which required 10 days to achieve the highest FRAP and ABTS values. No significant difference was found (p > 0.05) on the antioxidant activity of the samples that were kept at prolonged fermentation and storage. The total phenolic content (TPC) increased throughout the fermentation with the highest value of 44.69 0.01 mg GAE/g. Gallic acid, chlorogenic acid, vanillin, -coumaric acid and rutin are the major phenolic compounds identified in the fermented product. The results suggested that the antioxidant capacity of bambangan is affected by the fermentation temperature and the fermented product could be a source of antioxidants.
Keywords: Bambangan, Underutilized fruit, Fermentation, Antioxidant, Phenolic composition
Introduction
Superoxide anion radicals, hydroxyl radicals and hydrogen peroxide are potentially harmful reactive oxygen species that are generated by the metabolic processes of environment pollution and UV radiation (Hoyos-Arbeláez et al. 2017). These reactive oxygen species are unstable, highly reactive and may cause oxidative damage by oxidizing some cellular macromolecules such as proteins, carbohydrates, lipids and nucleic acid (Liu et al. 2018). Oxidative damage may result in various chronic diseases such as cardiovascular diseases, diabetes and cancers while natural antioxidants have been shown to potentially mitigate or eliminate oxidative damage caused by the reactive species and protect cells from lesion or death (Adwas et al. 2019). Some natural occurring antioxidants in foods such as carotenoids, tocopherols, ascorbates, lipoic acids and polyphenols are reported giving specific protection against oxidative deterioration and promoting better health (Xu et al. 2017).
Molecules containing a structure of benzene ring substituted by at least one hydroxyl group are known as phenolic compounds. They are important biological active compounds in plant origin foods that have recently received considerable attention as potential protective factors against cancers and heart diseases due to their antioxidant potencies (Filannino et al. 2015). Many recent studies have reported that phenolic compounds possess other biological activities such as anti-inflammatory, antiulcer, antispasmodic, antiviral, antidiarrheal, and antitumoral properties (Chen et al. 2016; Nile et al. 2017). Thus, identification of these compounds provides vital information related to antioxidant function, food quality, and potential health benefits of the foods. In addition, some are natural pigments and aromatic volatiles that greatly influence the sensory quality of foods (Wiczkowski et al. 2015; Wouters et al. 2013). Fruits and vegetables are the good sources of dietary polyphenols, which are also the bioactive phytochemicals with strong antioxidant activity that could act as body’s natural defense mechanisms to oxidative damages (Hur et al. 2014).
Malaysia is famous for its rich diversity of tropical fruits. Some of these fruits including bambangan are less popular, grown in wild, seasonal and with limited commercial utilization (Jahurul et al. 2018). Bambangan (Mangifera pajang) is a type of seasonal fruit belongs to the family of Anarcardiaceae (same group with mango), which is found abundantly in Borneo Island, especially Sabah. It is oval in shape with thick brownish skin, three times larger than commercial mango (Mangifera indica), while the pulp is fibrous, juicy, yellow in color and having a strong aromatic flavor. Bambangan can be consumed in fresh or as a fermented product (pickle). Fermented bambangan is a popular indigenous food, which is very popular within the community of Kadazan Dusun, the largest ethnic group in Sabah. Based on the empirical process of bambangan fermentation, fresh bambangan pulp, grated seed and salt are mixed and left to ferment for 7–10 days under ambient temperature in a tightly closed container. This traditional lactic acid fermentation is similar to other vegetable fermentations such as olives, cabbages and cucumbers (Alexandraki et al. 2014; Cvetković et al. 2015) which is mainly depend on the naturally occurrence microorganisms in the raw materials and processing environment. Hence, the greatest challenge in this fermentation process is to get a product with consistent and uniform characteristics.
Phenolic profiles may alter upon fermentation due to the metabolism of microorganisms and endogenous enzymes. Quantitative changes on phenolic composition during fermentation probably are due to the degradation of glycoside linkages that cause different bioavailability of the bioactive compounds as compared to the unfermented counterpart (Jaiswal and Abu-Ghannam, 2013). Knockaert et al. (2014) reported acid and enzymatic hydrolysis of polymerized phenolic compounds could cause release of simple phenolic compounds, which are easily metabolized by microorganisms. Previous study has shown that microorganisms started to modify plant constituents during fermentation (Bhanja Dey et al. 2016). However, Hur et al. (2014) indicated that fermentation induced structural breakdown of red cabbage cell walls to which phenolic constituents were bound, thus liberated copious amount of bioactive compounds with potential antioxidant activity. Similarly, elevated temperatures between 35–37 °C used in the fermentation of vegetables and beans have been shown to increase fermentation rate, enhancing phenolic composition and antioxidant activity (Rochín-Medina et al., 2018; Gao et al., 2019).
Several past studies have reported bambangan as a rich source of antioxidants with high level of phenolics and flavonoids (Jahurul et al. 2018; Ling et al. 2020). However, there is still lack of information on the antioxidant properties and phenolic compounds of fermented bambangan including the changes of these compounds during storage. Refrigerated storage at 4 °C for fermented fruit beverages showed minimal loss of antioxidant properties (Choo et al., 2018; Mantzourani et al., 2018b). Therefore, the present study aims to determine the effect of fermentation and extended storage on the antioxidant activities and phenolic compounds of bambangan fruit.
Materials and methods
Materials
2,2-diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-tripyridyl-s- triazine (TPTZ), 2,2’-azobis(2-amidinopropane) dihydrochloride (AAPH), Folin-Ciocalteu’s phenol reagent, 2,5,7,8-tetramethychroman-2-carboxylic acid (Trolox), Fluorescein, gallic acid and ferric chloride were purchased from Sigma—Aldrich (St. Louis, MO, USA). All HPLC grade reagents (methanol and acetic acid) were obtained from Sigma—Aldrich (St. Louis, MO, USA). Pure compounds such as gallic acid, ( +)-catechin, chlorogenic acid, caffeic acid, mangiferin, vanillin, p-coumaric acid, quecertin-3-D-galactoside, rosmarinic acid, rutin, quercetin and kaempferol obtained from Sigma – Aldrich (St. Louis, MO, USA) were used as standards in the quantification of phenolic compounds performed by HPLC.
Freshly prepared mixture of bambangan fleshes (cubes), salts and grated kernel seed (5.0 kg) were obtained from a local producer in Kota Kinabalu, Sabah. Bambangan was cut into small cubes after peeled off the skin. Before grating the kernel, the outer layer of the kernel had to be removed. The ratios for ingredients in fermented bambangan were 90%: 6%: 4% (fresh bambangan pulps: grated kernel: salt content). All the ingredients were mixed thoroughly in a plastic container. The samples were placed into an icebox (4 °C) and transported to the Food Microbiology Laboratory, Universiti Malaysia Sabah. The samples were homogenized and distributed evenly into a few bottles (500 ml) and kept at room temperature (28 ) in a condition similar to that of the producer site. Another set of samples were kept at elevated temperature of 35 for fermentation. Fermented samples at room temperature were kept at the same condition for three months. However, samples fermented at elevated temperature (35 °C) were kept in 4 °C for a prolonged storage of three months. The fermentation was repeated using a new batch of bambangan mixture and kept in a same condition. All analysis was performed in triplicate.
Samples preparation
The first sample was taken on the same day the freshly prepared bambangan mixture was distributed into smaller bottles, representing the unfermented sample at day 0. Samples were withdrawn at each interval time of 2, 4, 6, 8, 10, and 14 days during the spontaneous fermentation that were kept at room temperature (28 ) and elevated temperature (35 ) for analysis. Subsequent samplings were carried out on monthly basis (after 14 days) for the extended fermentation and storage up to 3 months. Eighty grams of fermented bambangan were used for each of the interval sampling for physicochemical analysis and antioxidant activities determination.
Titratable acidity
The collected samples (10 g) were transferred into stomacher bag and homogenized with 90 ml distilled water. The pH was measured by digital pH meter (Oyster-10, Extech Instruments, USA). Subsequently, titratable acidity was determined by addition of approximately 0.3 ml of 0.1% phenolphthalein as pH indicator and titrated with 0.1 M NaOH (Wiczkowski et al. 2015). The test was performed in triplicate and the results were averaged. The amount of acid produced during fermentation was calculated by using the formula below:
where VNaOH is volume of NaOH required to neutralize the acid.
Extract preparation
Samples were extracted using 80% methanol according to the method developed by Sarwar et al. (2012). Firstly, 20 g of blended fermented bambangan was macerated in 200 ml of 80% methanol for 24 h at room temperature in an orbital shaker set at 200 rpm. The extract was vacuum filtered and the solvent was evaporated by rotary evaporator (Heildoph, Germany) at 45 and the extract was freeze-dried (Labconco, Freezone 4.5, USA). The freeze-dried extract was kept at -20 prior to the determination of antioxidant activities and phenolic contents.
Determination of antioxidant activity
2,2-diphenyl-2-picrylhydrazyl (DPPH) assay
The DPPH assay was carried out according to the method described by Wang et al. (2014). DPPH solution (6 M) in methanol was prepared and 2.7 ml of the solution was mixed with 300 l of extract. The solution was mixed vigorously, left in dark for one hour at room temperature and measured at 517 nm by using UV spectrophotometer (Lamda 35, Perkin Elmer, USA). Methanol was used as a blank, while 80% methanol with DPPH solution was used as the negative control. The butylated hydroxyanisole (BHA) was used as the positive control for comparison. The inhibitory percentage of the extract was calculated according to the following equation:
The concentration of extract providing 50% of radical scavenging activity (EC50) was calculated from the linear regression of radical scavenging activity (RSA) against the extract concentration. A synthetic antioxidant Butylated hydroxyanisole (BHA) was used as reference standard for comparison.
Ferric reducing antioxidant power (FRAP) assay
For FRAP assay, the antioxidant capacity of the extracts was determined by the method developed by Xiao et al. (2015). Freshly prepared FRAP reagent was warmed at 37 °C in a water bath and the blank reading was taken at 593 nm using UV spectrophotometer (Lamda 35, Perkin Elmer, USA). One hundred microliter of sample extract was added with 300 l of distilled water and 3 ml FRAP reagent. The absorbance was measured after 30 min left in the dark. The absorbance of the reaction mixture was measured against initial blank reading and compared with the standard curve prepared by using several concentrations ranging from 0 to 2.4 mM of Fe (II) concentration. Results were expressed as M Fe (II) equivalents/g dried weight (DW) extract.
2,2’-azinobis (3—ethylbenzothiazoline—6—sulfonic acid) (ABTS) radical cation assay
The method for determination of ABTS radical cation assay was carried out according to the method described by Re et al., (1999) and adopted by Kong et al. (2013). ABTS salt with the concentration of 7 mM was dissolved in distilled water and mixed with 2.45 mM potassium persulfate. The reaction mixture was incubated at room temperature for 12 – 16 h in dark to generate free radicals. Thirty microliter of sample extract was mixed with 3 ml of diluted ABTS solution until the absorbance reached 0.70 0.02. The absorbance was read at 734 nm after 6 min of reaction. Distilled water was used as the blank. Percentage of antioxidant capacity was calculated according to the formula below:
where was the absorbance of ABTS radical cation without sample or standard; was the absorbance of ABTS radical cation with sample or standard.
Oxygen-radical absorbing capacity (ORAC) assay
ORAC assay was used to measure the antioxidant scavenging activity against peroxyl radical generated by thermal decomposition of 2,2’-Azobis(2-amidinopropane) dihydrochloride (AAPH) (Liu et al. 2016). AAPH solution, fluorescein and Trolox were prepared in a phosphate buffer (75 mmol/l, pH 7.4). The total mixture would be 20 l of AAPH, 170 l Fluorescein and 10 l of sample. Firstly, fluorescein and sample were mixed and incubated at 37 °C for 20 min in a microplate reader (Fluoroskan Ascent Microplate Fluorometer) and AAPH was added. After 30 s of AAPH added, the readings were taken at every cycle of 1 min in shaking mode using microplate reader. Ten microlitre of phosphate buffer without extract was used as blank. The antioxidant activity is expressed in micromole Trolox equivalents per gram of dry weight (DW) extract.
Determination of total phenolic content
The total phenolic content in the extract was determined by using Folin-Ciocalteu assay reported by Deʇirmencioʇlu et al. (2016) with slight modifications. The sample extract (500 l) was mixed with 2.50 ml of freshly prepared Folin – Ciocalteu reagent, which had been previously diluted with distilled water (1:10 v/v). The mixture was incubated under room temperature for four minutes prior to its use. Then, 2 ml of 75 g/l sodium carbonate was added into the mixture before incubated at room temperature for 120 min. The absorbance of the mixture was measured at 760 nm by using UV spectrophotometer (Lamda 35, Perkin Elmer, USA).
Quantification of the phenolic content was based on the standard curve established (range of 0.05 – 0.30 mg/ml) and the content was determined as microgram of gallic acid equivalent (GAE) per g sample.
Determination of phenolic compounds using high performance liquid chromatography (HPLC–DAD)
Phenolic compound standards were prepared by dissolving them with methanol into 10–100 μg/g. The standards used included gallic acid, ( +)-catechin, chlorogenic acid, -coumaric acid, ferulic acid, vanillin, rutin, kaempferol, quercetin and mangiferin. On the other hand, the extracts were dissolved by methanol and filtered through 0.22 μm nylon membrane filter (Whatman, UK).
High Performance Liquid Chromatography (HPLC) was used to determine the phenolic compounds present in the sample extract according to the method described by Hassan et al., (2011) with slight modifications. Twenty microlitre of extract was injected automatically into Agilent 1200 series HPLC system equipped with a diode array detector (DAD). A 150 4.6 mm, 4 m particle size reversed phase column (Phenomenex, USA) was used in this analysis. The column thermostat had been set at 25 °C. The mobile phase comprises of mixture of water/acetic acid (99.5:0.5 v/v) for solvent A and 100% of methanol for solvent B at a flow rate of 0.6 ml/min and measured at wavelength of 265 nm with a gradient elution program within 35 min of run time. The gradient elution began at 100% phase A, linearly decrease to 10% in 30 min. In the next 5 min, phase A increased to 100%. Quantification of phenolic compounds was determined by comparing the retention times and absorbance recorded in the chromatograms with the reference standards from the calibration curves constructed with the known standards. The amount of phenolic compounds was calculated and expressed as microgram per gram of dry weight ( g/g DW).
Statistical analysis
Statistical Package for Social Science (SPSS) version 21.0 was used for data analysis. All of the triplicate results were expressed as mean ± standard deviation (SD). A significant difference between the variables was determined by one-way analysis of variance (ANOVA) with post hoc Tukey’s test at p 0.05.
Results and discussion
pH and acidity during fermentation of bambangan
Changes of pH and acidity (lactic acid) during fermentation of bambangan at room temperature (28 °C) and 35 °C are shown in Table 1. The pH values of the bambangan that was placed at room temperature decreased slightly faster (p > 0.05) than the fruit that was kept at elevated temperature especially for the first 6 days. The results obtained are not in agreement with Kiai and Hafidi (2014) who reported that the total acidity tend to increase at higher temperature of fermentation. Reduction in pH value is often the main contributing factor affecting the stability and shelf life of the fermented foods.
Table 1.
pH and acidity of bambangan during process of fermentation and storage
| Fermentation time (day) | Room Temperature, (28 °C) | 35 °C | ||
|---|---|---|---|---|
| pH | Acidity (%) | pH | Acidity (%) | |
| 0 | 4.20a ± 0.05 | 0.59f ± 0.10 | 3.96a ± 0.09 | 0.78e ± 0.13 |
| 2 | 3.67b ± 0.12 | 0.77e ± 0.13 | 3.82b ± 0.05 | ±0.16 |
| 4 | 3.64b ± 0.08 | 0.82de ± 0.08 | 3.67c ± 0.12 | ±0.20 |
| 6 | 3.46c ± 0.05 | 0.84de ± 0.05 | 3.58c ± 0.16 | ±0.10 |
| 8 | 3.42c ± 0.10 | 0.89d ± 0.11 | 3.52c ± 0.13 | ±0.21 |
| 10 | 3.42c ± 0.11 | 0.88d ± 0.05 | 3.46d ± 0.05 | ±0.12 |
| 12 | 3.44c ± 0.08 | 0.95c ± 0.10 | 3.42d ± 0.08 | ±0.11 |
| 14 | 3.44c ± 0.13 | 0.97c ± 0.06 | 3.44d ± 0.09 | ±0.33 |
| Storage time (day) | Room Temperature, (28 °C) | 4 °C | ||
|---|---|---|---|---|
| 30 | 3.26d ± 0.15 | 1.29b ± 0.19 | 3.44c ± 0.13 | ± 0.25 |
| 60 | 3.24d ± 0.09 | 1.37a ± 0.27 | 3.44c ± 0.10 | ± 0.19 |
| 90 | 3.24d ± 0.11 | 1.37a ± 0.14 | 3.43c ± 0.06 | ± 0.34 |
aDifferent superscript letters on the mean values within the same column indicate significant differences (p 0.05)
The acidity of the bambangan fermented at room (28 °C) and elevated temperatures (35 °C) was found gradually increased from the day of preparation up to 10th day but remained constant thereafter. However, the change of acidity during fermentation is not in relation to the pH of the fermented product at both temperatures. Lactic acid bacteria are able to produce various organic acids, primarily lactic acid as its fermentation product. In addition, lactic acid possesses higher pKa value (acid dissociation constant) compare to other corresponding organic acids such as acetic acid and propionic acid (Filannino et al. 2015). In this context, it was suspected that sample fermented at room temperature was acidified largely by lactic acid, thus less acidity was detected than sample fermented at 35 °C despite of sharing the same final pH value. This is in accordance to the report by Wang et al., (2019) who found temperature could greatly affect the synthesis of lactic acid by lactobacilli. The main reason for the differences might be due to the variations in composition and domination of microflora naturally occurring in bambangan fermentation. Polymerase chain reaction-denaturing gradient gel electrophoresis analysis from other studies had shown that fermentation temperatures could affect the type of dominating lactic acid bacteria and their corresponding organic acids produced (Liu et al., 2017). Previous study on the fermentation of bambangan found that it was mainly fermented by Lactobacillus plantarum and Lactobacillus brevis (Ng et al. 2015). Therefore, it is possible that the number of heterofermentative Lactobacillus brevis present in bambangan fermented at 37 °C was higher than the sample fermented at room temperature.
No significant change was detected on the pH and acidity of fermented bambangan regardless of they were kept in refrigerated condition or room temperature. This indicates that the fermentation had completed and most microbial activity was retarded at low pH (3.2–3.4). Therefore, the physicochemical quality of fermented bambangan kept at refrigerated temperature would not be preserved better than the fermented bambangan stored at room temperature, as their pH had been reduced to minimum prior to storage. Similar finding was reported in sauerkraut fermentation, which their pH remained stable after 5 days of fermentation at room temperature (Gagné et al. 2015).
Antioxidant capacity during fermentation of Bambangan
During the first 10 days of spontaneous fermentation at room temperature, the FRAP and ABTS values of the extracts had increased from 50.72 ± 0.43 mM Fe(II)/g and 1.15 ± 0.21 mmol TE/g to 149.32 ± 2.03 mM Fe(II)/g and 6.09 ± 0.51 mmol TE/g respectively (Table 2). However, both of these antioxidant activities decreased (p < 0.05) in the subsequent fermentation times. Nevertheless, they required only 6 days to achieve the highest values of 141.42 ± 5.06 mM Fe(II)/g and 5.00 ± 0.45 mmol TE/g respectively, when fermentation was carried out at 35 °C (Table 3). This indicates that bambangan fermented at 35 °C was able to achieve the highest antioxidant capacity in a shorter period, which explains the enzymes produced by some microorganisms at the optimum growth temperature have been found to enhance the mass-transfer and the progress of substrate hydrolysis (Qu et al. 2020). Microbial enzymes, such as glucosidase, amylase, cellulase, phytase, xylanase, tannase, esterase, invertase or lipase produced during fermentation can hydrolyze glucosides, and break down plant cell walls or starch. These enzymes play significant role in disintegrating the plant cell wall matrix and consequently facilitating the antioxidative compounds extraction (Hur et al. 2014). Li et al. (2021) reported that the increasing antioxidative activity of plant-based foods using fermentation might be caused by the depolymerization and structural changes of high molecular weight phenolic compounds.
Table 2.
Antioxidant activity of bambangan during fermentation and storage at room temperature, 28 °C
| Fermentation Time (day) | FRAP value (mM Fe (ll)/g) |
ABTS (Trolox equivalent antioxidant capacity, mmol TE/g) |
DPPH radical scavenging activity (EC50, mg/g of extracts) | ORAC ( mol TE/g) |
Total Phenolic Content (mg GAE/g) |
|---|---|---|---|---|---|
| 0 | 50.72h ± 0.43 | 1.15h ± 0.21 | 0.78d ± 0.05 | 38.76a ± 1.43 | 15.24d ± 0.74 |
| 2 | 52.90g ± 1.03 | 3.01g ± 0.18 | 0.36b ± 0.05 | 41.22a ± 2.84 | 14.38d ± 0.95 |
| 4 | 59.77f ± 0.58 | 4.42f ± 0.15 | 0.44c ± 0.10 | 41.99a ± 1.48 | 17.02d ± 1.06 |
| 6 | 83.49e ± 2.05 | 4.67e ± 0.07 | 0.38b ± 0.08 | 39.29a ± 1.39 | 14.13d ± 0.44 |
| 8 | 119.35b ± 3.07 | 4.93d ± 0.09 | 0.31a ± 0.05 | 41.18a ± 1.44 | 25.84c ± 2.59 |
| 10 | 149.32a ± 2.03 | 6.09c ± 0.51 | 0.30a ± 0.03 | 40.73a ± 2.12 | 25.68c ± 1.47 |
| 12 | 116.70c ± 4.02 | 4.94d ± 0.06 | 0.30a ± 0.06 | 41.64a ± 2.28 | 34.09b ± 2.84 |
| 14 | 107.21d ± 2.01 | 5.08d ± 0.15 | 0.30a ± 0.03 | 42.89a ± 3.31 | 44.69a ± 4.84 |
| Storage time (day) | |||||
|---|---|---|---|---|---|
| 30 | 139.98c ± 4.03 | 6.61c ± 0.30 | 0.29a ± 0.08 | 42.27a ± 1.95 | 41.74ab ± 1.91 |
| 60 | 184.30b ± 5.03 | 6.30c ± 0.21 | 0.22a ± 0.05 | 42.30a ± 1.14 | 39.65ab ± 2.36 |
| 90 | 227.63a ± 7.04 | 10.66b ± 0.13 | 0.26a ± 0.07 | 40.24a ± 2.62 | 40.21ab ± 1.71 |
| BHA | 198.89a ± 8.60 | 10.39a ± 3.40 | 0.24a ± 0.04 | ||
aValues are mean SD of duplicates samples. Different superscripts on the means in the same column indicate significant differences (p < 0.05)
Table 3.
Antioxidant activity of bambangan during fermentation process at 35 °C and storage at 4 °C
| Fermentation Time (day) | Elevated Temperature (35 °C) | ||||
|---|---|---|---|---|---|
| FRAP value (mM Fe (ll)/g) |
ABTS (Trolox equivalent antioxidant capacity, mmol TE/g) |
DPPH radical scavenging activity (EC50, mg/g of extracts) | ORAC ( mol TE/g) |
Total Phenolic Content (mg GAE/g) | |
| 0 | 57.98f ± 3.27 | 1.68d ± 0.11 | 0.77d ± 0.07 | 40.78a ± 0.79 | 10.12 g ± 1.00 |
| 2 | 82.72e ± 4.31 | 4.27bc ± 0.25 | 0.61c ± 0.04 | 41.03a ± 0.74 | 17.24e ± 1.65 |
| 4 | 95.44cde ± 6.60 | 4.56bc ± 0.07 | 0.49bc ± 0.06 | 43.46a ± 1.63 | 19.63b ± 1.31 |
| 6 | 141.42b ± 7.16 | 5.00b ± 0.35 | 0.36ab ± 0.09 | 39.24a ± 1.85 | 26.68a ± 4.15 |
| 8 | 114.44c ± 7.57 | 4.78bc ± 0.14 | 0.33a ± 0.05 | 41.51a ± 1.30 | 15.57f ± 1.63 |
| 10 | 99.89cde ± 1.17 | 3.81bc ± 0.13 | 0.30a ± 0.08 | 43.92a ± 2.72 | 19.58c ± 1.40 |
| 12 | 88.17de ± 0.83 | 3.66bc ± 0.11 | 0.33a ± 0.05 | 42.33a ± 1.02 | 18.57d ± 1.92 |
| 14 | 63.46f ± 1.46 | 3.32c ± 0.30 | 0.30a ± 0.06 | 42.59a ± 2.21 | 17.59e ± 1.35 |
| Storage time (day) | Refrigerated storage (4 °C) | ||||
|---|---|---|---|---|---|
| 30 | 110.08cd ± 4.36 | 4.08bc ± 0.07 | 0.34a ± 0.06 | 41.60a ± 3.15 | 16.40f ± 1.24 |
| 60 | 109.70cd ± 4.86 | 3.72bc ± 0.20 | 0.34a ± 0.05 | 41.10a ± 2.22 | 16.54f ± 0.68 |
| 90 | 105.82cd ± 5.07 | 3.58bc ± 0.17 | 0.22a ± 0.13 | 41.50a ± 1.40 | 16.41f ± 0.49 |
| BHA | 198.89a ± 9.52 | 10.39a ± 1.23 | 0.24a ± 0.16 | ||
aValues are mean SD of duplicates samples. Different superscripts on the means in the same column indicate significant differences (p < 0.05)
In contrast, the EC50 of DPPH for the fermented bambangan decreased significantly (p < 0.05) from the initial value of 0.78 mg/g to 0.30 0.02 mg/g of extract at day 14th in both room temperature (28 ) and 35 . The lower of the EC50 value indicates the higher the ability of the sample to scavenge DPPH radicals. According to Molaveisi et al. (2019), the radical scavenging ability of phenols increased following the increase in temperature due to the accelerated chemical reactions by the faster moving atoms. Consequently, the liberation and synthesis of bioactive compounds are greatly improved due to the increased degradation of the plant cell walls (Xu et al. 2017).
Oxygen-radical absorbing capacity (ORAC) values showed no significant difference throughout the fermentation of bambangan at room and elevated temperature (35 °C). It could be explained by the differences in the detection mechanisms, as ORAC is based on hydrogen atom transfer, which measures antioxidant inhibition by peroxyl radical induced oxidation and thus reflects classical radical chain breaking antioxidant activity; while DPPH, ABTS and FRAP assays are based on single electron transfer (Schaich et al. 2015). Thus, ORAC assay is limited to the measurement of hydrophilic chain breaking capacity against peroxyl radicals but ignoring lipophilic antioxidants that are important against lipid oxidation (Shalaby and Shanab, 2013). Therefore, the results obtained in the present study indicate that lipohilic antioxidants are most likely dominated the fermentation probably due to the present of carotenoids in the fruit.
The total phenolic content of bambangan fermented at room temperature recorded the highest value at 44.69 ± 4.84 mg GAE/g after 14 days. The result is sixfold higher than the fresh bambangan fruit (7.06 0.34 mg GAE/g) as reported by Mirfat et al. (2016). It shows the spontaneous fermentation could have induced the formation of the phenolics, leading to a higher antioxidative potential (Zou et al. 2017). It is apparent that the phenolic content of the bambangan sample determined prior to the fermentation (15.24 mg GAE/g) is much higher in this study probably due to the difference in the maturity of fruits used in the fermentation process. The increase of phenolic content during lactic acid fermentation may result from the depolymerisation or hydrolysis of bound phenolic compounds (Hur et al. 2014). Despite the tremendous higher increment of total phenolic content was noticed for samples fermented at elevated temperature in the early stage, as this can be seen from the bambangan fermented at 35 °C that achieved the highest phenolic content on day-6 (26.68 mg GAE/g) as compared to 14.13 mg GAE/g for sample fermented at room temperature (Table 2). However, the final phenolic content was lower in bambangan fermented at 35 °C than those fermented at room temperature (Table 3), since a decreasing trend of phenolics is noticeable after day-6 for the higher fermentation temperature apposing fermentation held at the room temperature. Similarly, Qu et al. (2020) had found fermentation temperature at 28 °C significantly increases polyphenols in bush tea as compared to the levels obtained at 35 °C. It could be explained that some microbial enzymes (namely cinnamoyl ester hydrolases) are capable of cleaving hydroxycinnamyl esters, and release phenolic acids from the cell walls to become the substrates of phenolic acid decarboxylase (PAD) enzyme and convert the former compounds into their vinyl phenol derivatives (Kitaoka et al. 2021). Therefore, most of the phenolic compounds in bambangan fermented at 35 °C were suspected still entrapped by the cell walls. This indicates that the optimal temperature control during fermentation plays an important role in facilitating antioxidative activity of the product, which could be influenced by the type of microorganisms present in the substrates (Queiroz Santos et al., 2018; Rochín-Medina et al., 2018).
During prolonged fermentation and storage (30–90 days) of bambangan, no significant change was observed in most of the antioxidant activities and total phenolic content, regardless of the temperature used. The result is in agreement with Mantzourani et al. (2018a) and Tabaszewska et al. (2018), which indicated that storage of fermented foods did not affect their corresponding total phenolic content and antioxidant activity. It is hypothesized that the changes in antioxidant activities of the fermented food are closely associated to the microbial activity as the growth stabilized after 14 days of fermentation before slightly decreased during extended storage. Further investigation into the metabolite changes during fermentation would lead to a better understanding of the observed properties of fermented bambangan.
Identification phenolic compounds during Bambangan fermentation
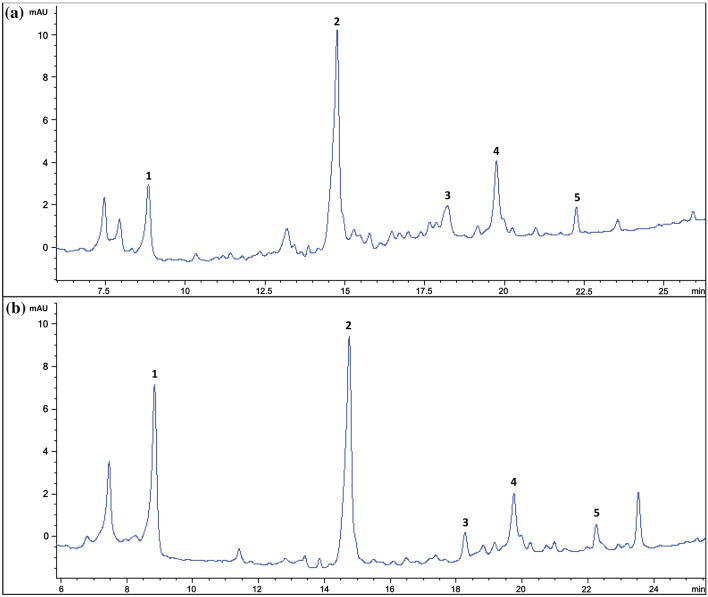
The profile of phenolic compounds in the fermented bambangan during fermentation was determined. Figure 1 shows the HPLC–DAD chromatograms of the phenolic extract of the bambangan fermented at room temperature (28 °C) and 35 °C, respectively. Five main phenolic compounds were identified, namely gallic acid, chlorogenic acid, vanillin, ρ-coumaric acid and rutin. Among the phenolic compounds, chlorogenic acid is the major phenolic acid present throughout the fermentation regardless of the temperature. This is in accordance to the previous findings that the level of chlorogenic acid was significantly higher in fruits (Shao et al. 2014). Surprisingly, mangiferin is not detected in the fermented bambangan although it is a natural polyphenol widely found in the peels of the fruit of its near relative mangoes (Mangifera indica) (Pinsirodom et al. 2018). In the making of fermented bambangan, the skin has been removed and only the flesh of the fruit was used. This is one of the main reasons the mangiferin could not be detected in the fermented samples. In addition, several studies have reported that the mangiferin could be degraded into simple phenolic acids under several natural and induced conditions. These include bacterial degradation, thermal degradation, oxidative degradation and emulsion conditions (Bock and Ternes, 2010; Jutiviboonsuk and Leeprechanon, 2019; Khurana et al., 2017; Padh et al., 2017).
Fig. 1.
HPLC chromatograms of extract from Bambangan fermented at a room temperature, (28 °C) on day-12 and b 35 °C on day-4 which displayed the most diverse phenolic compounds throughout the fermentation process. Identified phenolic compounds are 1-Gallic acid, 2-Chlorogenic acid, 3-Vanillin, 4-ρ-coumaric acid and 5-Rutin
Table 4 shows the evolutions of phenolic compounds during spontaneous bambangan fermentation and storage at room temperature. The chlorogenic acid was initially present at 10.57 mg/g and 7.30 mg/g in bambangan fermented at 28 °C and 35 °C respectively, but their concentrations increased gradually to achieve the highest at 12.74 mg/g within 4 days and 22.95 mg/g within 6 days of fermentation before decreased dramatically thereafter (Table 5). However, vanillin was not detected until the late fermentation (day-12) in bambangan that was kept at room temperature. The increment could be due to the formation of chlorogenic acid from other phenolic compounds, as Shin et al. (2015) had showed that caffeic acid and quinic acid are able to form chlorogenic acid during fermentation. This finding seemed corresponding well with the antioxidant assays and total phenolic content determined in this study that achieved the highest values on day 6th of fermentation (141.42 mM Fe (II)/g; 5.00 mmol TE/g; EC50 0.36 mg/g of extracts; 26.68 mg GAE/g). However, when the fermentation is prolonged (after 12 days), the concentration of the phenolic compounds declined gradually, probably due to the hydrolysis of chlorogenic acid by esterase, an enzyme that was synthesized by the lactic acid bacteria during fermentation (Pérez-Martín et al. 2013).
Table 4.
Evolution of phenolic compounds during bambangan fermentation and storage at room temperature, 28 °C
| Phenolic compounds identified (mg/g DW) | |||||
|---|---|---|---|---|---|
| Fermentation time (day) | Gallic acid | Chlorogenic acid | Vanillin | -coumaric acid | Rutin |
| 0 | 0.20d ± 0.05 | 10.57def ± 0.08 | ND | 0.21ab ± 0.06 | 0.11b ± 0.03 |
| 2 | 0.55d ± 0.10 | 8.00fg ± 0.13 | ND | 0.25ab ± 0.11 | 0.10b ± 0.05 |
| 4 | 0.74d ± 0.04 | 12.74cde ± 0.09 | ND | 0.27ab ± 0.07 | 0.83a ± 0.22 |
| 6 | 0.46d ± 0.07 | 7.33f ± 0.19 | ND | 0.19b ± 0.06 | 0.72a ± 0.03 |
| 8 | 0.40d ± 0.09 | 11.97de ± 1.13 | ND | 0.30ab ± 0.09 | 0.13b ± 0.03 |
| 10 | 1.00d ± 0.33 | 15.40bc ± 0.33 | ND | 0.34ab ± 0.04 | 0.11b ± 0.06 |
| 12 | 0.75d ± 0.23 | 22.19a ± 2.31 | 0.20bc ± 0.09 | 0.40ab ± 0.09 | 0.12b ± 0.02 |
| 14 | 0.74d ± 0.35 | 12.75cde ± 1.51 | 0.20bc ± 0.04 | 0.19b ± 0.08 | ND |
| Storage time (day) | |||||
|---|---|---|---|---|---|
| 30 | 3.46c ± 0.33 | 10.01efg ± 1.21 | 0.38b ± 0.08 | 0.22ab ± 0.02 | ND |
| 60 | 13.44a ± 1.58 | 13.62cd ± 1.93 | 1.05a ± 0.10 | 0.37ab ± 0.06 | ND |
| 90 | 5.87b ± 0.94 | 16.87b ± 1.05 | 1.06a ± 0.23 | 0.42a ± 0.11 | ND |
aValues are mean SD of duplicates samples. Different superscripts on the means in the same column indicate significant differences (p < 0.05)
‘ND’ indicate Not Detected
Table 5.
Evolution of phenolic compounds during Bambangan fermentation process at 35 °C and storage (4 °C)
| Phenolic compounds identified (mg/g DW) | |||||
|---|---|---|---|---|---|
| Fermentation time (day) | Gallic acid | Chlorogenic acid | Vanillin | -coumaric acid | Rutin |
| 0 | 0.25e ± 0.05 | 7.30cd ± 0.73 | 0.12b ± 0.04 | 0.21b ± 0.03 | 0.11a ± 0.15 |
| 2 | 0.25e ± 0.06 | 7.32cd ± 1.36 | 0.13b ± 0.06 | 0.21b ± 0.06 | 0.18a ± 0.08 |
| 4 | 1.87bcd ± 0.36 | 6.88d ± 0.67 | 0.13b ± 0.05 | 0.33b ± 0.11 | 0.14a ± 0.05 |
| 6 | 1.02de ± 0.04 | 22.95a ± 2.12 | 0.16ab ± 0.06 | 0.14b ± 0.06 | ND |
| 8 | 1.52cd ± 0.21 | 9.58cd ± 1.87 | 0.18ab ± 0.07 | 0.25b ± 0.07 | ND |
| 10 | 1.69bcd ± 0.09 | 10.44cd ± 0.66 | 0.19ab ± 0.03 | 1.98a ± 0.25 | ND |
| 12 | 2.41b ± 0.12 | 15.51b ± 1.62 | 0.15ab ± 0.05 | 0.19b ± 0.09 | ND |
| 14 | 3.22a ± 0.24 | 11.14cd ± 0.31 | 0.34a ± 0.06 | 0.16b ± 0.03 | ND |
| Storage time (day) | Refrigerated (4 °C) | ||||
|---|---|---|---|---|---|
| 30 | 3.63a ± 0.48 | 11.39c ± 0.56 | 0.18ab ± 0.08 | 0.17b ± 0.08 | ND |
| 60 | 1.93bc ± 0.72 | 7.27 cd ± 2.09 | 0.26ab ± 0.07 | 0.19b ± 0.11 | ND |
| 90 | 1.40 cd ± 0.42 | 9.81 cd ± 2.63 | 0.27ab ± 0.14 | 0.29b ± 0.08 | ND |
aValues are mean SD of duplicates samples. Different superscripts on the means in the same column indicate significant differences (p < 0.05)
‘ND’ indicate Not Detected
Gallic acid was present relatively low with the initial concentration of 0.20 and 0.25 mg/g DW respectively. However, the values increased gradually throughout the fermentation at both temperature, from 0.20 to 0.74 mg/g (28 °C) and 0.25 to 3.22 mg/g (35 °C). This could be due to the presence of microbial enzyme, tannase, which could hydrolyze the ester bonds of tannin in the substrates to produce gallic acid by the conversion of Lactobacillus plantarum or Lactobacillus brevis through fermentation (Bravo-Ferrada et al. 2016; Fritsch et al. 2016; Nemec et al. 2017). The amount of gallic acid determined in the bambangan fermented at 35 °C was relatively higher than the sample fermented at room temperature. It is speculated that higher fermentation temperature favors the production of gallic acid due to the increase microbial activity at their optimum growth temperature (Yao et al. 2014).
The ρ-coumaric acid concentration increased gradually during bambangan fermentation at both temperatures. However, bambangan fermented at 35 °C was found to achieve significant higher ρ-coumaric acid in a slightly shorter time as compared to the bambangan fermented at 28 °C with the values of 1.98 mg/g (10 days) and 0.40 mg/g (12 days), respectively. The result shows fermented bambangan is a better source of ρ-coumaric acid than rice bran as reported previously (Pang et al. 2018). Similar result was reported by Okcu et al. (2016) on fermented Bokbunja (Rubus coreanum Miq), a type of medicinal fruit where its p-coumaric acid increased due to the enzymatic hydrolysis of hydroxycinnamic-ester (fertaric) during the fermentation. Hence, the increase of -coumaric acid during bambangan fermentation is probably due to the ability of Lactobacillus plantarum metabolized gallic acid to ρ-coumaric acid during fermentation though lactic acid bacteria are found to have phenolic acid decarboxylase activity (Salgado et al. 2012).
Bambangan possess low level of vanillin, which is only detectable almost at the end of fermentation if the spontaneous process was carried out at room temperature. The vanillin might be synthesized through microbial conversion (β-oxidation) of ferulic acid (presents as a free phenolic acid) by ferulic acid esterase (Kaur et al. 2013). Ferulic acid esterase released by the microorganisms could hydrolyze sugar-phenolic ester linkages present in the cell wall and assimilate ferulic acid as a sole carbon source, which is the most promising process for vanillin production via biotransformation (Kumar and Pruthi 2014; Yang et al. 2013). Furthermore, the rutin concentration was present during bambangan fermentation at both temperatures. However, bambangan fermented at room temperature was found to achieve much higher rutin in a shorter time as compared to the bambangan fermented at 35 °C with the values of 0.83 mg/g and 0.14 mg/g at day 4, respectively.
During storage of fermented bambangan that was kept at room temperature (28 ) for 3 months, the chlorogenic acid, vanillin and -coumaric acid increased with the concentration of 16.87, 1.06 and 0.42 mg/g respectively. Gallic acid increased to 13.44 mg/g on day-60. However, rutin was not found after the fermented fruit was stored. A longer duration of storage at room temperature is also known as a prolonged fermentation, which leads to the increased sensitivity of the phenolic compounds towards oxidative reactions. However, the loss of rutin could be explained by the deglycosylation of rutin to quecertin-3-O-glucoside by -L-rhamnosidase and thereafter degraded by -D-glucosidase to form leucocyanidin, due to the microbial activities that remained in the fermented sample during storage at room temperature (Cecchi et al. 2013).
For the fermented bambangan that was kept at 4 , the vanillin and -coumaric were slightly increased from 0.18 to 0.27 mg/g and 0.17 to 0.29 mg/g throughout the 90 days of storage. On the other hand, the chlorogenic acid decreased from 11.39 to 9.81 mg/g DW, whereas that of gallic acid decreased from 3.63 to 1.40 mg/g DW after the fermented bambangan was kept for 90 days. The decrease of chlorogenic acid during storage probably due to its degradation into more simple phenolic acids such as caffeic acid and quinic acid, which later decarboxylated into their vinyl derivatives (Cecchi et al. 2013).
Conclusion
Despite of the similarity between the bambangan fermented spontaneously at 28 °C and 35 °C in their pH and acidity values, different fermentation temperatures have resulted in distinct antioxidant activity. Bambangan fermented at room temperature seemed to hold a higher antioxidant activity and phenolic contents than its corresponding counterparts. However, storage temperature has negligible influence on the antioxidant activity and total phenolic content of the fermented products. In addition, the antioxidant activities of the fermented bambangan were contributed mainly by phenolic compounds such as gallic acid, chlorogenic acid, vanillin, -coumaric acid and rutin. Thus, bambangan produced from spontaneous fermentation at room temperature could be a potential source of natural dietary antioxidants especially within the remote community with limited facility of food processing.
Acknowledgements
This work was financial supported by Seaweed Research Unit, Universiti Malaysia Sabah (Project Number: GPRL 006). The authors also gratefully acknowledge Mr. Chin from Penampang, Kota Kinabalu, Sabah, for providing samples of fermented bambangan used in the study.
Chemicals
- DPPH
2,2-Diphenyl-2-picrylhydrazyl
- TPTZ
2,4,6-Tripyridyl-s- triazine
- AAPH
2,2’-Azobis(2-amidinopropane) dihydrochloride
- Trolox
2,5,7,8-Tetramethychroman-2-carboxylic acid
- BHA
Butylated hydroxy anisole
Instrumental techniques
- EC50
Effective concentration providing 50% radical scavenging activity
- RSA
Radical scavenging activity
- ORAC
Oxygen-radical absorbing capacity
- HPLC
High performance liquid chromatography
- DAD
Diode array detector
- UV
Ultraviolet
- DW
Dry weight
- GAE
Gallic acid equivalent
- SPSS
Statistical Package for Social Science
- ANOVA
One-way analysis of variance
- SD
Standard deviation
Author contributions
STC carried out the experiments, analyzed data and preparing the draft; BSP analyzed data and corrected the draft; FYC supervised the work and edited the manuscript.
Data availability
Data are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare there is no conflict of interest in this work and publication.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adwas AA, Elsayed ASI, Azab AE, Quwaydir FA. Oxidative stress and antioxidant mechanisms in human body. J Appl Biotechnol Bioeng. 2019;6(1):43–47. [Google Scholar]
- Alexandraki V, Georgalaki M, Papadimitriou K, Anastasiou R, Zoumpopoulou G, Chatzipavlidis I, et al. Determination of triterpenic acids in natural and alkaline-treated Greek table olives throughout the fermentation process. LWT Food Sci Technol. 2014;58(2):609–613. doi: 10.1016/j.lwt.2014.04.005. [DOI] [Google Scholar]
- Bhanja Dey T, Chakraborty S, Jain KK, Sharma A, Kuhad RC. Antioxidant phenolics and their microbial production by submerged and solid-state fermentation process: A review. Trends Food Sci Technol. 2016;53:60–74. doi: 10.1016/j.tifs.2016.04.007. [DOI] [Google Scholar]
- Bock C, Ternes W. The phenolic acids from bacterial degradation of the mangiferin aglycone are quantified in the feces of pigs after oral ingestion of an extract of Cyclopia genistoides (honeybush tea) Nutr Res. 2010;30(5):348–357. doi: 10.1016/j.nutres.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Bravo-Ferrada BM, Hollmann A, Brizuela NL, Hens DV, Tymczyszyn E, Semorile L. Growth and consumption of l-malic acid in wine-like medium by acclimated and non-acclimated cultures of Patagonian Oenococcus oeni strains. Folia Microbiol. 2016;61(5):365–373. doi: 10.1007/s12223-016-0446-y. [DOI] [PubMed] [Google Scholar]
- Cecchi L, Migliorini M, Cherubini C, Giusti M, Zanoni B, Innocenti M, Mulinacci N. Phenolic profiles, oil amount and sugar content during olive ripening of three typical Tuscan cultivars to detect the best harvesting time for oil production. Food Res Intern. 2013;54(2):1876–1884. doi: 10.1016/j.foodres.2013.04.033. [DOI] [Google Scholar]
- Chen C, Zhang Y, Gao Y, Xu Q, Ju X, Wang L. Identification and anti-tumour activities of phenolic compounds isolated from defatted adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) seed meal. J Funct Foods. 2016;26:394–405. doi: 10.1016/j.jff.2016.08.016. [DOI] [Google Scholar]
- Choo KY, Kho C, Ong YY, Thoo YY, Lim RLH, Tan CP, Ho CW. Studies on the storage stability of fermented red dragon fruit (Hylocereus polyrhizus) drink. Food Sci Biotechnol. 2018;27:1411–1417. doi: 10.1007/s10068-018-0367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetković BR, Pezo LL, Tasić T, Šarić L, Kevrešan Ž, Mastilović J. The optimisation of traditional fermentation process of white cabbage (in relation to biogenic amines and polyamines content and microbiological profile) Food Chem. 2015;168:471–477. doi: 10.1016/j.foodchem.2014.07.068. [DOI] [PubMed] [Google Scholar]
- Deʇirmencioʇlu N, Gürbüz O, Herken EN, Yildiz AY. The impact of drying techniques on phenolic compound, total phenolic content and antioxidant capacity of oat flour tarhana. Food Chem. 2016;194:587–594. doi: 10.1016/j.foodchem.2015.08.065. [DOI] [PubMed] [Google Scholar]
- Filannino P, Bai Y, Di Cagno R, Gobbetti M, Gänzle MG. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015;46:272–279. doi: 10.1016/j.fm.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Fritsch C, Heinrich V, Vogel RF, Toelstede S. Phenolic acid degradation potential and growth behavior of lactic acid bacteria in sunflower substrates. Food Microbiol. 2016;57:178–186. doi: 10.1016/j.fm.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Gagné MJ, Barrette J, Savard T, Brassard J. Evaluation of survival of murine norovirus-1 during sauerkraut fermentation and storage under standard and low-sodium conditions. Food Microbiol. 2015;52:119–123. doi: 10.1016/j.fm.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Gao H, Wen JJ, Hu JL, Nie QX, Chen HH, Nie SP, Xiong T, Xie MY. Momordica charantia juice with Lactobacillus plantarum fermentation: chemical composition, antioxidant properties and aroma profile. Food Biosci. 2019;29:62–72. doi: 10.1016/j.fbio.2019.03.007. [DOI] [Google Scholar]
- Hassan FA, Ismail A, Abdulhamid A, Azlan A. Identification and quantification of phenolic compounds in bambangan (Mangifera pajang Kort.) peels and their free radical scavenging activity. J Agric Food Chem. 2011;59(17):9102–9111. doi: 10.1021/jf201270n. [DOI] [PubMed] [Google Scholar]
- Hoyos-Arbeláez J, Vázquez M, Contreras-Calderón J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: a review. Food Chem. 2017;221:1371–1381. doi: 10.1016/j.foodchem.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Hur SJ, Lee SY, Kim YC, Choi I, Kim GB. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014;160:346–356. doi: 10.1016/j.foodchem.2014.03.112. [DOI] [PubMed] [Google Scholar]
- Jahurul MHA, Leykey B, Sharifudin MS, et al. Optimization of fat yield of bambangan (Mangifera pajang) kernel using response surface methodology and its antioxidant activities. Food Measure. 2018;12:1427–1438. doi: 10.1007/s11694-018-9758-8. [DOI] [Google Scholar]
- Jaiswal AK, Abu-Ghannam N. Kinetic studies for the preparation of probiotic cabbage juice: Impact on phytochemicals and bioactivity. Ind Crops Prod. 2013;50:212–218. doi: 10.1016/j.indcrop.2013.07.028. [DOI] [Google Scholar]
- Jutiviboonsuk Aranya, Leeprechanon Wilaipan. Stability of Mangiferin in Lotion and its Antioxidant Activity. Key Eng Mater. 2019;819:79–84. doi: 10.4028/www.scientific.net/KEM.819.79. [DOI] [Google Scholar]
- Kaur B, Chakraborty D, Kumar B. Phenolic Biotransformations during conversion of ferulic acid to vanillin by lactic acid bacteria. Biomed Res Int. 2013;2013:e590359. doi: 10.1155/2013/590359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana RK, Bansal AK, Beg S, Burrow AJ, Katare OP, Singh KK, Singh B. Enhancing biopharmaceutical attributes of phospholipid complex-loaded nanostructured lipidic carriers of mangiferin: systematic development, characterization and evaluation. Int J of Pharm. 2017;518(1-2):289–306. doi: 10.1016/j.ijpharm.2016.12.044. [DOI] [PubMed] [Google Scholar]
- Kiai H, Hafidi A. Chemical composition changes in four green olive cultivars during spontaneous fermentation. LWT - Food Sci Technol. 2014;57(2):663–670. doi: 10.1016/j.lwt.2014.02.011. [DOI] [Google Scholar]
- Kitaoka N, Nomura T, Ogita S, Kato Y. Bioproduction of 4-vinylphenol and 4-vinylguaiacol β-primeverosides using transformed bamboo cells expressing bacterial phenolic acid decarboxylase. Appl Biochem Biotechnol. 2021 doi: 10.1007/s12010-021-03522-y. [DOI] [PubMed] [Google Scholar]
- Knockaert D, Raes K, Struijs K, Wille C, Van Camp J. Influence of microbial conversion and change in pH on iron-gallic acid complexation during lactobacillus fermentation. LWT Food Sci Technol. 2014;55(1):335–340. doi: 10.1016/j.lwt.2013.08.007. [DOI] [Google Scholar]
- Kong KW, Khoo HE, Prasad NK, Chew LY, Amin I. Total phenolics and antioxidant activities of pouteria campechiana fruit parts. Sains Malaysiana. 2013;42(2):123–127. [Google Scholar]
- Kumar N, Pruthi V. Potential applications of ferulic acid from natural sources. Biotechnol Rep. 2014;4(1):86–93. doi: 10.1016/j.btre.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Jiang T, Liu N, Wu C, Xu H, Lei H. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021;339(1):127859. doi: 10.1016/j.foodchem.2020.127859. [DOI] [PubMed] [Google Scholar]
- Ling JKU, Chan YS, Nandong J. Extraction of antioxidant compounds from the wastes of Mangifera pajang fruit: a comparative study using aqueous ethanol and deep eutectic solvent. SN Appl Sci. 2020;2(8):1365. doi: 10.1007/s42452-020-3153-x. [DOI] [Google Scholar]
- Liu S, Chang X, Liu X, Shen Z. Effects of pretreatments on anthocyanin composition, phenolics contents and antioxidant capacities during fermentation of hawthorn (Crataegus pinnatifida) drink. Food Chem. 2016;212:87–95. doi: 10.1016/j.foodchem.2016.05.146. [DOI] [PubMed] [Google Scholar]
- Liu A, Li X, Pu B, Ao X, Zhou K, He L, Chen S, Liu S. Use of psychrotolerant lactic acid bacteria (Lactobacillus spp. and Leuconostoc spp.) isolated from chinese traditional paocai for the quality improvement of paocai products. J Agric Food Chem. 2017;65(12):2580–2587. doi: 10.1021/acs.jafc.7b00050. [DOI] [PubMed] [Google Scholar]
- Liu Z, Ren Z, Zhang J, Chuang C-C, Kandaswamy E, Zhou T, Zuo L. Role of ROS and nutritional antioxidants in human diseases. Front Physiol. 2018;9:477. doi: 10.3389/fphys.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzourani I, Kazakos S, Terpou A, Alexopoulos A, Bezirtzoglou E, Bekatorou A, Plessas S. potential of the probiotic Lactobacillus plantarum ATCC 14917 strain to produce functional fermented pomegranate juice. Foods. 2018;8(1):4. doi: 10.3390/foods8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzourani I, Kazakos S, Terpou A, Mallouchos A, Kimbaris A, Alexopoulos A, Bezirtzoglou E, Plessas S. Assessment of volatile compounds evolution, antioxidant activity, and total phenolics content during cold storage of pomegranate beverage fermented by Lactobacillus paracasei K5. Fermentation. 2018;4(4):95. doi: 10.3390/fermentation4040095. [DOI] [Google Scholar]
- Mirfat AHS, Salma I, Razali M. Natural antioxidant properties of selected wild Mangifera species in Malaysia. J Trop Agric Food Sci. 2016;44(1):63–72. [Google Scholar]
- Molaveisi M, Beigbabaei A, Akbari E, Noghabi MS, Mohamadi M. Kinetics of temperature effect on antioxidant activity, phenolic compounds and color of Iranian jujube honey. Heliyon. 2019;5(1):e01129. doi: 10.1016/j.heliyon.2019.e01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemec MJ, Kim H, Marciante AB, Barnes RC, Hendrick ED, Bisson WH, et al. Polyphenolics from mango (Mangifera indica L.) suppress breast cancer ductal carcinoma in situ proliferation through activation of AMPK pathway and suppression of mTOR in athymic nude mice. J Nutr Biochem. 2017;41:12–19. doi: 10.1016/j.jnutbio.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Ng SY, Koon SS, Padam BS, Chye FY. Evaluation of probiotic potential of lactic acid bacteria isolated from traditional Malaysian fermented Bambangan (Mangifera pajang) CYTA J Food. 2015;13(4):563–572. [Google Scholar]
- Nile SH, Nile AS, Keum YS. Total phenolics, antioxidant, antitumor, and enzyme inhibitory activity of Indian medicinal and aromatic plants extracted with different extraction methods. Biotech. 2017;7(1):76. doi: 10.1007/s13205-017-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okcu G, Ayhan K, Gunes Altuntas E, Vural N, Poyrazoglu ES. Determination of phenolic acid decarboxylase produced by lactic acid bacteria isolated from shalgam (şalgam) juice using green analytical chemistry method. LWT Food Sci Technol. 2016;66:615–621. doi: 10.1016/j.lwt.2015.10.072. [DOI] [Google Scholar]
- Padh H, Parmar S, Patel B. Stability indicating HPTLC method for estimation of mangiferin in bulk and dosage form. Int J Pharma Bio Sci. 2017;7(3):71–77. [Google Scholar]
- Pang Y, Ahmed S, Xu Y, Beta T, Zhu Z, Shao Y, Bao J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018;240:212–221. doi: 10.1016/j.foodchem.2017.07.095. [DOI] [PubMed] [Google Scholar]
- Pérez-Martín F, Seseña S, Izquierdo PM, Palop ML. Esterase activity of lactic acid bacteria isolated from malolactic fermentation of red wines. Int J Food Microbiol. 2013;163(2–3):153–158. doi: 10.1016/j.ijfoodmicro.2013.02.024. [DOI] [PubMed] [Google Scholar]
- Pinsirodom P, Taprap R, Parinyapatthanaboot T. Antioxidant activity and phenolic acid composition in different parts of selected cultivars of mangoes in Thailand. Int Food Res J. 2018;25(4):1435–1443. [Google Scholar]
- Qu F, Zeng W, Tong X, Feng W, Chen Y, Ni D. The new insight into the influence of fermentation temperature on quality and bioactivities of black tea. LWT. 2020;117:108646. doi: 10.1016/j.lwt.2019.108646. [DOI] [Google Scholar]
- Queiroz Santos VA, Nascimento CG, Schmidt CAP, Mantovani D, Dekker RFH, da Cunha MAA. Solid-state fermentation of soybean okara: Isoflavones biotransformation, antioxidant activity and enhancement of nutritional quality. LWT. 2018;92:509–515. doi: 10.1016/j.lwt.2018.02.067. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rochín-Medina JJ, Ramírez K, Rangel-Peraza JG, et al. Increase of content and bioactivity of total phenolic compounds from spent coffee grounds through solid state fermentation by Bacillus clausii. J Food Sci Technol. 2018;55:915–923. doi: 10.1007/s13197-017-2998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado JM, Rodríguez-Solana R, Curiel JA, de las Rivas B, Muñoz R, Domínguez JM. Production of vinyl derivatives from alkaline hydrolysates of corn cobs by recombinant Escherichia coli containing the phenolic acid decarboxylase from Lactobacillus plantarum CECT 748T. Bioresour Technol. 2012;117:274–285. doi: 10.1016/j.biortech.2012.04.051. [DOI] [PubMed] [Google Scholar]
- Sarwar S, Anwar F, Raziq S, Nadeem M, Zreen Z, Cecil F. Antioxidant characteristics of different solvent extracts from almond (Prunus dulcis L.) shell. J Med Plants Res. 2012;6:3311–3316. [Google Scholar]
- Schaich KM, Tian X, Xie J. Hurdles and pitfalls in measuring antioxidant efficacy: a critical evaluation of ABTS, DPPH, and ORAC assays. J Funct Foods. 2015;14:111–125. doi: 10.1016/j.jff.2015.01.043. [DOI] [Google Scholar]
- Shalaby E, Shanab SMM. Antioxidant compounds, assays of determination and mode of action. Afr J Pharma Phamacol. 2013;7(10):528–539. doi: 10.5897/AJPP2013.3474. [DOI] [Google Scholar]
- Shao P, Zhang J, Fang Z, Sun P. Complexing of chlorogenic acid with β-cyclodextrins: Inclusion effects, antioxidative properties and potential application in grape juice. Food Hydrocoll. 2014;41:132–139. doi: 10.1016/j.foodhyd.2014.04.003. [DOI] [Google Scholar]
- Shin HS, Satsu H, Bae MJ, Zhao Z, Ogiwara H, Totsuka M, Shimizu M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015;168:167–175. doi: 10.1016/j.foodchem.2014.06.100. [DOI] [PubMed] [Google Scholar]
- Tabaszewska M, Gabor A, Jaworska G, Drożdż I. Effect of fermentation and storage on the nutritional value and contents of biologically—active compounds in lacto-fermented white asparagus (Asparagus officinalis L.) LWT. 2018;92:67–72. doi: 10.1016/j.lwt.2018.02.003. [DOI] [Google Scholar]
- Wang CY, Wu SJ, Shyu YT. Antioxidant properties of certain cereals as affected by food-grade bacteria fermentation. J Biosci Bioeng. 2014;117(4):449–456. doi: 10.1016/j.jbiosc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Wang Y, He L, Xing Y, Zhou W, Pian R, Yang F, Chen X, Zhang Q. Bacterial diversity and fermentation quality of Moringa oleifera leaves silage prepared with lactic acid bacteria inoculants and stored at different temperatures. Bioresour Technol. 2019;284:349–358. doi: 10.1016/j.biortech.2019.03.139. [DOI] [PubMed] [Google Scholar]
- Wiczkowski W, Szawara-Nowak D, Topolska J. Changes in the content and composition of anthocyanins in red cabbage and its antioxidant capacity during fermentation, storage and stewing. Food Chem. 2015;167:115–123. doi: 10.1016/j.foodchem.2014.06.087. [DOI] [PubMed] [Google Scholar]
- Wouters D, Bernaert N, Conjaerts W, Van Droogenbroeck B, De Loose M, De Vuyst L. Species diversity, community dynamics, and metabolite kinetics of spontaneous leek fermentations. Food Microbiol. 2013;33(2):185–196. doi: 10.1016/j.fm.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Wang L, Rui X, Li W, Chen X, Jiang M, Dong M. Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1–6. J Funct Foods. 2015;12:33–44. doi: 10.1016/j.jff.2014.10.033. [DOI] [Google Scholar]
- Xu D, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, Zhang J. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int J Mol Sci. 2017;18(1):96. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Tang H, Ni J, Wu Q, Hua D, Tao F, Xu P, Janssen PJ. Characterization of two streptomyces enzymes that convert ferulic acid to vanillin. PLoS ONE. 2013;8(6):e67339. doi: 10.1371/journal.pone.0067339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Guo GS, Ren GH, Liu YH. Production, characterization and applications of tannase. J Mol Catal B Enzym. 2014;101:137–147. doi: 10.1016/j.molcatb.2013.11.018. [DOI] [Google Scholar]
- Zou B, Wu J, Yu Y, et al. Evolution of the antioxidant capacity and phenolic contents of persimmon during fermentation. Food Sci Biotechnol. 2017;26:563–571. doi: 10.1007/s10068-017-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author on reasonable request.
Not applicable.