Abstract
Bottle gourd (Lagenaria siceraria) is widely used in India for its medicinal properties. Sometimes its consumption as a fruit or juice can lead to serious health issues due to the presence of toxic cucurbitacins (Cuc) like Cuc B at high concentration. In the present study, a molecularly imprinted polymer (MIP) based method was developed to quantify Cuc B in commercial bottle gourd juice. Cuc B quantification was also done in peel and pulp of fresh bottle gourd fruit. Developed MIP was superior to NIP (non-imprinted polymer) in terms of equilibrium binding, solid-phase extraction, selectivity, and loading capacity of Cuc B. Scatchard plot analysis, adsorption kinetics, and surface area study further strengthen the fact that prepared imprinted polymer was better than NIP for Cuc extraction. TLC and high-resolution LC–MS based analysis revealed that MIP selectively extracts Cuc B from all the studied samples. It was further observed that gamma irradiation and β-glucosidase treatment did not have any significant (p > 0.05) effect on Cuc concentration. The quantity of Cuc in 100 g of juice, peel, and pulp was 2.81 ± 0.26, 3.37 ± 0.31, and 2.39 ± 0.21 mg, respectively. Analytical method validation indicates that the MIP based method was highly linear, precise, and accurate to rapidly quantify toxic Cuc B in bottle gourd consequently assuring its quality.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05600-3.
Keywords: Molecularly imprinted polymer, Method development, Rapid quantification, Cucurbitacin, Lagenaria siceraria, Food quality assurance
Introduction
Cucurbitacins (Cucs) are a group of tetracyclic triterpenes commonly found in the Cucurbitaceae plant family and are generally bitter in taste. They are named as Cuc A, B, C up to S. Bottle gourd (Lagenaria siceraria) has Cucs B, D, G, and H (Rehm et al. 1957). Cucs B, C, D, E, and I were found to be lethal (Kaushik et al. 2015). Thus, bottle gourd has two types of toxic Cucs namely B and D. Cuc B is the principal Cuc and Cuc D is produced from Cuc B through enzymatic reactions (Wang et al. 2017a, b). Toxic Cucs are naturally present at very low concentrations in plants. Nevertheless, under stressful conditions like arid environment or cross-pollination with wild variety Cucs concentration in the plant can increase up to a dangerous level. Even a small quantity of fruits or juice containing more than 130 ppm of such Cucs is harmful (KhatIb and Borawake 2014). The range of toxicity of Cucs is between 1 and 12.5 mg/kg body weight of mice. The toxicity associated with consumption of foods high in Cucs is sometimes referred to as "toxic squash syndrome". In India, multiple deaths have also been reported in recent times due to the consumption of bitter bottle gourd juice.
Fruits of the Cucurbitaceae family contain very high amounts of Cuc than the plant itself (Gry et al. 2006). Cucs are commonly present as glycosides such as 2-β-O-glucosides in growing plants. These glycosides are often hydrolyzed by highly active β-glycosidases to give aglycone. Food processing techniques, such as juice preparation or gamma irradiation, can lead to plant cell damage thus releasing naturally present β-glycosidase in processed food which in turn liberates Cucs from glycosidic bonds. It was also reported that aglycone Cuc is more toxic than glycosidic Cuc (Kaya and Melzig 2008). Thus, it is important to quantify the harmful Cuc present in bottle gourd fruit and its fruit juice. Bottle gourd fruits have cardioprotective, diuretic, and nutritive properties according to practitioners of Ayurveda (Ho et al. 2014). Thus, consumption of bottle gourd juice is very common in India. This results in the widespread availability of packed bottle gourd juice in the market as a health supplement without a proper description of its chemical constituents which is essential for determining its safety, quality, and prevention of Cuc toxicity in consumers.
There are various methods reported in the literature based on HPLC, TLC, and spectrophotometer to quantify Cuc in plants (Online Resource 1). However, these methods are time-consuming and required tedious sample preparation procedures. Another approach for the extraction of analytes from biological samples is based on the use of molecularly imprinted polymers (MIPs) (Claude et al. 2008). They are generally produced by polymerizing a mixture of functional monomer, crosslinker or co-monomer, and radical initiator in the presence of a template along with a porogenic solvent. The template gets imprinted within the polymeric matrix. MIP selectively interacts with such a template in the imprinted cavities through non-covalent interactions (electrostatic, hydrogen, π–π, and Van-der-Waals bonds). To the best of our knowledge, there is no report available on the development of MIP for Cucs. In the present study, a novel method was developed by synthesizing MIP to rapidly determine Cuc concentration in commercial commodities and bottle gourd fruit, peel, and pulp. The effect of irradiation and β-glycosidase on Cuc concentration was also determined.
Materials and methods
Preparation of MIP
Methacrylic acid (MAA, Sigma-Aldrich, USA), ethylene glycol dimethacrylate (EDMA, Sigma-Aldrich, USA) was distilled under vacuum and azobisisobutyronitrile (AIBN, Sigma-Aldrich, USA) was recrystallized from methanol before use. Cuc B (TCI Chemicals, Japan) imprinted polymer was synthesized in a glass tube as follows: 0.296 mmol Cuc B (template), 1.6 mmol MAA (functional monomer), 8 mmol EDMA (cross-linker) dissolved in 2.55 mL CHCl3 (porogenic solvent, 99.9% GC-HS, Sisco Research Laboratories, India). The obtained solution was stirred and sonicated for 5 min then 0.11 mmol of AIBN (initiator) was added. Further N2 was purged through the mixture for 10 min in an ice bath then the tube was sealed and polymerization was done by subjecting the solution to a dose of 5 kGy using a 60Co gamma irradiator having a dose rate of 6.1 kGy/h (GC-5000, BRIT, India) at room temperature. Further, grinding, wet-sieving, washing, elimination, and drying was performed as per the procedure described by Lopez et al. (2011). Non-imprinted polymer (NIP) was also prepared by the above process but without the template.
Binding measurement, FTIR, SEM, and solid-phase extraction
The calibration curve was produced by analyzing Cuc B standard solutions in CHCl3 (1–100 μg/mL). The absorbance maxima were reported at 245 nm using a spectrophotometer (U-2910, UV–Vis Spectrophotometry, Hitachi, Japan). Equilibrium binding experiment of MIP and NIP was also analyzed. Imprinted and non-imprinted polymers in increasing amount (0–140 mg) separately and carefully mixed with 10 mL of CHCl3 solution of Cuc B (5 μg/mL) at room temperature for 6 h under continuous stirring. The obtained mixture was filtered through a 0.45 μm syringe filter (Genetix Biotech, India), and free Cuc B in the filtrate was measured by spectrophotometer. Imprint parameter (IP) was obtained as per the procedure detailed by Claude et al. (2008). IP corresponds to the ratio of the number of specific interactions to non-specific interactions. Spectra of MIP and NIP were scanned in the range of 4000–700 cm−1 on a FTIR (FT/IR 4100, Jasco) spectrometer. ATR assembly was used for obtaining spectra with 40 scans for each sample. The surface morphology of MIP was analyzed by FEG–SEM. The scanning electron micrographs were taken with a JSM-7600F instrument (Joel, Japan). Solid-phase extraction (SPE) experiment was performed by packing 100 mg of polymer in 6 mL extraction cartridges. All cartridges preconditioned with 5 mL of methanol followed by 5 mL of CHCl3. Cuc B solution of 1 mL (25 μg/mL) was loaded on MIP and NIP cartridges followed by washing with 5 mL of CHCl3 to remove impurities which were retained by polymer due to non-specific interactions. Elution was done using 5 mL of methanol. All fractions were collected and dried by rotary evaporator (Rotavapor® R-300, Buchi, Switzerland) followed by re-dissolving each fraction in 1 mL CHCl3 before quantification of Cuc B by spectrophotometer.
Capacity, selectivity, and Scatchard analysis
The capacity of 100 mg of MIP and NIP cartridges was measured by loading 1 mL of Cuc B solutions at different concentrations (5–100 μg/mL). Eluted fractions were collected, dried, and re-dissolved as per the above detailed procedure, and further quantification was performed by spectrophotometer. The selectivity of developed MIP was determined by measuring the SPE recovery of Cuc I, stigmasterol, and betulinic acid. Standards were purchased from Sigma-Aldrich, USA. Standards (25 μg/mL) were loaded in a preconditioned cartridge of MIP and NIP. Preconditioning, loading, washing, elution, drying, and quantification was performed as per the procedure detailed above. Scatchard analysis gives an approximate model generally used to estimate the binding parameters of polymers. 10 mg of MIP and NIP incubated with 5 mL of different concentrations of Cuc B (up to 50 μg/mL) for 2 h. Further processing of sample and quantification was done as per procedure already been discussed above. Binding at equilibrium experiments was transformed into a linear equation by Scatchard equation (Zeng et al. 2015):
Qmax = Maximum binding capacity.
Kd = Equilibrium dissociation constant.
Adsorption kinetics, surface area, and swelling studies
Adsorption kinetics was determined by adding 100 mg of MIP/NIP into 10 mL of Cuc B std. solution (5 μg/mL CHCl3) and at different time intervals concentration of Cuc B was measured as per the method described above. The surface area of the prepared polymers was determined by using Brunauer, Emmett, Teller (BET) micromeritics ASAP 2004 (Micromeritics, Norcross, GA, USA) as previously described by Rosengren et al. (2013). The swelling ratio (Sr) was calculated according to the following equation (Rosengren et al. 2013):
V0 = volume of dry polymer.
Vsw = volume of polymer soaked in excess of solvent (CHCl3, methanol, and water) for 24 h.
Sample preparation
Bottle gourd fruits used in this investigation were purchased from the local market. Procured fruits were washed in running tap water followed by peeling and cutting. Samples were finally divided into peel and pulp. Commercially available bottle gourd juice purchased from BioGreen Healthcare, Mumbai, India. Pulp, peel, and juice exposed to gamma radiation processing at room temperature. Samples were subjected to a dose of 0.5, 1, 2.5, and 5 kGy. Irradiated and non-irradiated pulp weighing 150 g blended (Philips HL7756/00 750 W Mixer Grinder, India) for 5 min with 75 mL double distilled water and 150 g of peel blended with 150 mL double distilled water.
Enzymatic treatment
Non-irradiated blends (peel and pulp) and commercially available juice were incubated with pectinase enzyme (Pectinase from Aspergillus Niger, Sigma, USA) (500 μL/100 g sample) separately for 1, 2, and 4 h at 37 °C, 150 RPM at pH between 5 and 6. The control sample was without any addition of enzyme and incubation period. Incubation followed by filtration of all samples through a double layered muslin cloth and finally filtrate was collected.
Cucurbitacin extraction
All samples were extracted with chloroform and the solvent to sample ratio was 2:1. Extraction was done in triplicate using a separating funnel. Further, 5 mL of a chloroform extract (bottom layer) was loaded on a preconditioned MIP and NIP cartridge. Washing and elution were performed with 10 mL of chloroform and methanol, respectively. Finally, the drying of all fractions and quantification was done as per the procedure detailed above. Re-dissolved fractions were further analyzed by analytical TLC using a solvent system composed of CHCl3/MeOH = 9.5/0.5. Sample volume loaded on TLC plates (TLC silica gel 60, Merck, Germany) was 20 μL along with 20 μg of standard Cuc B. TLC plate after the run sprayed with 10% (v/v) sulfuric acid in methanol and visualized by heating the plate in an oven at 120 °C for 10 min. TLC bands were scrapped and dissolved in 5 mL chloroform followed by HRLC-MS analysis.
High-resolution liquid chromatography mass-spectrophotometer (HRLC-MS) analysis
The HRLC-MS analysis was performed by using a 1290 Infinity UHPLC System (Agilent Technologies, USA) with mass as a detector, equipped with a binary pump (G4220B), autosampler (G4226A), thermostatted column compartment (G1316C), 1260 Infinity Nano HPLC with Chipcube (Microfluidic column), Hypersil gold 3 micron 100 × 2.1 mm, and 6550 iFunnel Q-TOFs (G6550A). The injection volume of each sample was 5 μL. The separation attained with the mobile phase consists of 0.1% formic acid in water (solvent A) and 0.1% of formic acid in 90% aqueous acetonitrile (solvent B). Gradient employed went from an initial 95:5 (A:B) to 100% solvent B in 20 min with a flow rate of 0.3 mL/min. This was held for 5 min with the same flow rate. Later the gradient went from 100% solvent B at 25 min to back to 95:5 (A:B) at 26 min using the same flow rate. Afterward, the flow rate changed to 0.2 mL/min and the run was terminated at 30 min. The system was operated at 20 °C. The detection was performed after electrospray ionization in positive ionization mode. The source temperature was maintained at 200 °C and the spray voltage at 5.5 kV with the temperature was set at 450 °C. Source parameters like gas flow rate was 13 L/min, nebulizer was 35 psig, and sheath gas flow was 11 L/min.
Analytical method validation
The validity of the developed method was determined by packing 200 mg of polymer in a cartridge. Precision and accuracy were calculated by using 1 mL of standard solutions of Cuc B 5 (low), 25 (average), and 50 (high) μg/mL. Relative standard deviation (RSD) is a measurement of precision and relative mean error (RME) is a measurement of accuracy. RSD and RME were calculated as per the procedure described by Wang et al. (2001). Recovery was calculated by employing three sets of samples namely: 1 mL of Cuc B standard solutions of 5, 10, and 20 μg/mL, 10 mL of commercial juice, and standards added to the juice. All three sets of samples were subjected to the MIP based extraction procedure as detailed above with slight change including solvents used in preconditioning, washing, and elution was doubled in volume. Absolute recovery and relative recovery was also calculated in the present study (Wang et al. 2001). Limit of detection was calculated as the lowest concentration of standard Cuc B solution for which both RSD and RME were less than 20% (Denver et al. 2019). All quantitative determinations were subjected to statistical analysis by employing DSAASTAT ver. 1.101 by Andrea Onofri. Three samples were taken for each treatment and all samples were further analyzed in triplicates. Analysis of variance and multiple comparisons of means calculated by Duncan's multiple range test.
Results and discussion
Evaluation and binding experiment, FTIR, and SEM
In the present study, gamma irradiation was used for the preparation of imprinted polymers. It was reported that radiation induced smaller cavity size in the imprinted matrix and this lead to a denser cross-linked structure by limiting the mesh size (Söylemez and Güven 2018). This may enable MIP prepared through gamma irradiation to have higher analyte uptake. Furthermore, imprinted polymers prepared through thermal polymerization required long incubation hours as compared to γ-irradiation induced polymerization (Wu et al. 2016). MAA was selected as a functional monomer to non-covalently (mainly hydrogen bonding) interact with Cuc B since this monomer has already been successfully used with another triterpene (Claude et al. 2008). The ratio of the template/monomer/cross-linker = 1/5.4/27 was found optimal for the isolation of Cuc B. CHCl3 dissolves all ingredients of MIP including Cuc B and induced porosity by phase separation between porogen and polymer during polymerization. Additionally, CHCl3 is an aprotic solvent that favors hydrogen bonds between MAA and Cuc B. Nuclei of growing polymeric chains were fused to give the inter-linked polymer matrix, and this results in pores formation. These pores were occupied by the porogenic solvent. Such a macroporous structure helps in mass transfer during the releasing and rebinding of the template (Ye and Mosbach 2001). An increase in porogen volume encourages the formation of large pores diameter in MIP thus induce better accessibility of the template into the imprinted cavities but particle size decreases (Farrington et al. 2006). Therefore, for the formation of particles having > 20 μm in diameter, the volume of porogen must be carefully chosen to avoid the loss of particles through the frits of SPE cartridge (Pichon 2007). Different ratio of monomer to porogenic solvent (0.1–1.8 mmol/mL) was studied and the optimum ratio was found to be 0.64.
Equilibrium binding experiment was done in the presence of CHCl3 because binding of templates to MIP cavities is specific in the presence of porogen (Zhu et al. 2002). Figure 1a demonstrates the equilibrium binding isotherms of MIP and NIP against Cuc B concentration. B/T (%) is the ratio of the amount of template bound at equilibrium to the amount of template introduced initially in the vial. It was observed that the binding capacity of the MIP was always significantly (p > 0.05) higher than that of NIP. Similar findings were previously reported by Sun et al. (2017). At 80 mg of polymer 75% of Cuc B was bound to MIP compared to 15% to NIP and at 100 mg 91% binding occurred to MIP as compared to 19% binding to NIP, highlighting the specificity of MIP towards template. Binding percentage became constant at 100 mg or higher synthesized polymer concentration. FTIR spectra of MIP, NIP, and MIP bound with Cuc B are shown in Fig. 1b. Bands at 1725 cm–1 and 1635 cm–1 were attributed to stretching vibrations of C=O and C=C of MAA/EGDMA, respectively, indicating successful polymerization. Band located at 2928 cm−1 corresponds to C–-H stretching which belongs to Cuc B. Cuc B adsorbed MIP had CH stretching at 2965 cm−1 while the CH stretching in the spectrum of MIP and NIP was at 2985 cm−1. The surface morphology of MIP was studied by SEM (Fig. 1c). It had rough surface and pores were scattered throughout the MIP. Observed pores facilitate in carrying Cuc B to the binding sites thus favoring its adsorption on polymer.
Fig. 1.
a Binding of cucurbitacin B to MIP and NIP (Both polymers in different amount (0–140 mg) added to 10 mL of Cuc B (5 μg/mL) at RT for 6 h) b FTIR spectra c FEG–SEM image of MIP
SPE, capacity, and selectivity determination
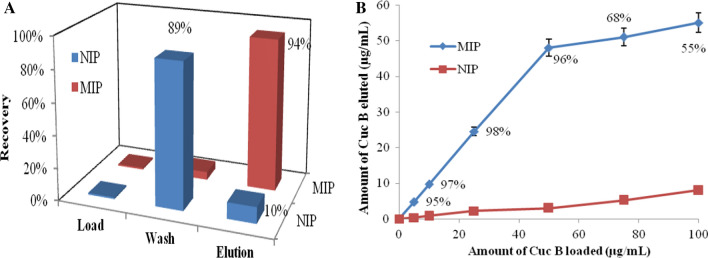
Imprint parameter of 100 and 120 mg of the polymer was 3.8 and 3.4, respectively thus further experiments were performed employing 100 mg of polymer. MIP affinity can be higher when the template binds to polymer in the non-equilibrium state as compared to the equilibrium state (Wei and Mizaikoff 2007). SPE of Cuc B from MIP and NIP is illustrated in Fig. 2a. It was observed that during washing 89% and 5% of analyte was washed away by CHCl3 from NIP and MIP cartridge, respectively. Thus chloroform successfully removed Cuc from NIP column. During elution, Cuc B recovery was 94% for MIP and only 10% for NIP. Being protic and polar solvent, methanol effectively disrupts specific non-covalent interaction by breaking hydrogen bonds between template and MIP.
Fig. 2.
a Solid-phase extraction of cucurbitacin B from MIP and NIP (SD is ≤ 5% of mean) (1 mL of Cuc B solution (25 μg/mL) was loaded on 100 mg of preconditioned extraction cartridges). b cartridge capacity of MIP and NIP. MIP elution recoveries (%) are reported on the graph (SD is ≤ 5% of mean) (100 mg of cartridges was loaded with 1 mL of different concentrations (5–100 μg/mL) of Cuc B)
Figure 2b demonstrates that the recovery of Cuc B from the MIP cartridge was always higher than 95% when the amount of template was ≤ 50 μg. However, for the same amount of analyte, less than 10% were retained by NIP during washing. MIP cartridge saturated when higher than 50 μg of Cuc B loaded with a drastic reduction in recovery percentage and a minor increase in total analyte retention was observed. The observed improvement in retention of the template at higher concentration was due to non-specific interactions that were identical for MIP and NIP as all the accessible specific cavities of the MIP were saturated (Bakhtiar et al. 2019). The loading capacity of the developed MIP cartridge was 0.5 μg of template per mg of MIP.
Selectivity of MIP was measured by comparing the recovery of Cuc B, Cuc I, stigmasterol, and betulinic acid. All standards are plant based tetracyclic triterpenoid except betulinic acid which is a pentacyclic triterpenoid (Fig. 3a). Selected compounds have the potential to act as an interfering compound while binding on imprinted cavities because their chemical structure is analogous to that of Cuc B. Cuc I is toxic and naturally found in Cucumis sativus, Bryonia alba, etc. Excellent selectivity for Cuc B with elution recovery for MIP was 94% and 10% for NIP was observed (Fig. 3b). However, for Cuc I recovery from MIP cartridge was 39% and for NIP 9%. The lowest selectivity was for betulinic acid followed by stigmasterol with a recovery of 9% and 13% from MIP. Besides Cuc B, all compounds were effectively removed from the polymer during washing. Functional groups and its orientation in MIP cavities are key for the selective recognition of analyte and because of this, the developed MIP was highly selective for Cuc B in comparison with other triterpenoids (Madikizela and Chimuka 2016).
Fig. 3.
a Chemical structure of Cuc B, Cuc I, stigmasterol, and betulinic acid. b Elution recoveries of Cuc B, Cuc I, stigmasterol, and betulinic acid from MIP and NIP (SD is ≤ 5% of mean) (25 mg of standards in 1 mL of chloroform were loaded in a preconditioned extraction cartridges). c Scatchard plots for the MIP isotherm (10 mg of MIP incubated with 5 mL of different concentration of Cuc B (up to 50 μg/mL) for 2 h). d Scatchard plots for the NIP isotherm (10 mg of NIP incubated with 5 mL of different concentration of Cuc B (up to 50 μg/mL) for 2 h)
Scatchard analysis, adsorption kinetics, surface area, and swelling ratio
MIP isotherm generated a straight line for the Scatchard plot which indicated that polymer has one type of binding site (Fig. 3c). On the other hand NIP isotherm was curved thus it did not have any specific binding sites (Fig. 3d). The linear regression equation for MIP was:
and for NIP it was:
From slope (− 1/Kd) and intercept (Qmax/Kd) of the fitted lines Kd and Qmax value was determined:
Qmax is indicative of adsorption capacity thus it can be concluded that MIP adsorption performance is better than NIP. Kd value denotes the overall affinity therefore MIP had a high affinity toward the target analyte than NIP (Zeng et al. 2015). The rate of the adsorption of Cuc B by the MIP and NIP was measured as a function of time. The result of this kinetics study is shown in Fig. 4. The adsorption process could be divided into two phases: rapid adsorption in the first 5 min and slow adsorption thereafter. The rate of adsorption was higher in MIP as compared to NIP. Both polymers gradually reached equilibrium at 60 min. The surface area of MIP and NIP was found to be 151 m2 g−1 and 119 m2 g−1. Larger surface area of MIP was due to the presence of cavities on imprinted polymer. Cuc binding would be directly proportional to the total surface area of the polymers. The swelling ratio of MIP when soaked in CHCl3, methanol, and water was 1.91, 1.34, and 1.21, respectively. NIP when soaked for 24 h in CHCl3, methanol, and water swelling ratio was 1.83, 1.29, and 1.22, respectively. It was observed that the use of CHCl3 as a porogen had a high effect on the swelling ratio. Overall low swelling capacity of synthesized polymers demonstrated a high degree of crosslinking.
Fig. 4.

Adsorption rate of Cuc B by MIP and NIP (100 mg of polymers added in 10 mL of 5 μg/mL of Cuc B solution)
Extraction of Cuc B from bottle gourd
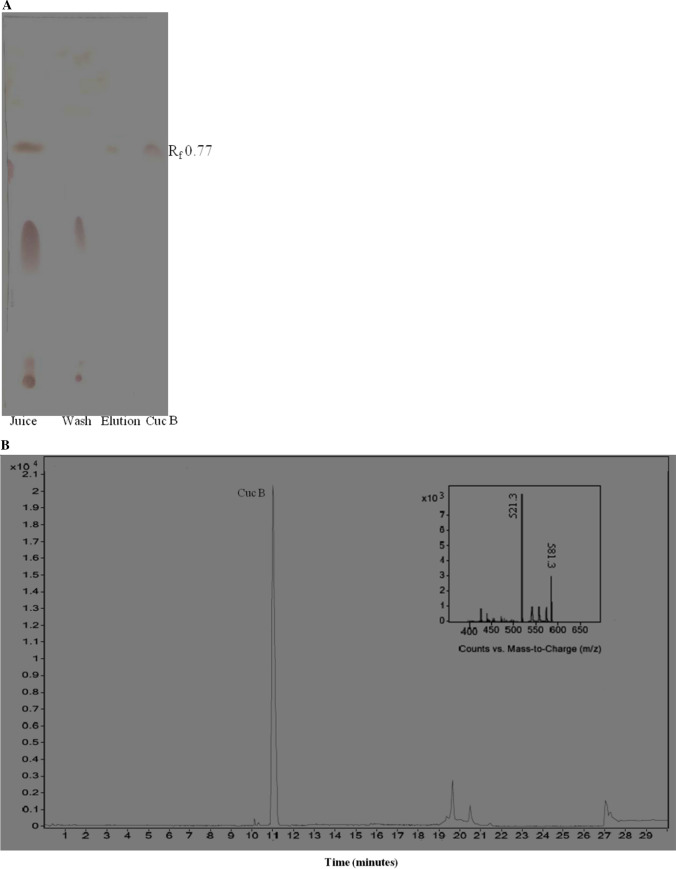
TLC profile of control commercial juice with its washed and eluted fraction from MIP cartridge along with the Cuc B standard is shown in Fig. 5a. It was observed that Cuc B had an Rf value of 0.77 with absorbance maxima at 245 nm. TLC profiles of juice and washed fraction were similar except for one band with an Rf value of 0.77 which was missing from the washed faction. Absent spot reappeared in eluted fraction and Rf value of this band corresponded to Cuc B. This demonstrated that MIP selectively bound with Cuc B from bottle gourd juice and it was eluted with methanol whereas impurities were washed away by CHCl3. The eluted fraction band was scrapped and extracted with chloroform followed by HRLC-MS analysis. Similar results were obtained for juice and peel samples.
Fig. 5.
a TLC profile of Cuc B standard and control bottle gourd juice with it’s washed and eluted MIP fractions (chloroform/methanol = 9.5/0.5). b HPLC chromatogram of TLC spot of the eluted fraction of bottle gourd juice. Mass spectrum of peak at 11.031 min is inset (0.1% formic acid in water (solvent A) & 0.1% of formic acid in 90% aq. acetonitrile (solvent B). 95:5 (A:B) to 100% solvent B in 20 min, flow rate 0.3 mL/min. After 5 min gradient went from 100% solvent B at to 95:5 (A:B) in 1 min. afterward, flow rate was 0.2 mL/min and run terminated at 30 min)
HPLC chromatogram of the TLC spot of the eluted fraction is shown in Fig. 5b with the most prominent peak was observed at 11.031 min. The mass spectrum of this peak showed that the mass transitions from m/z 581.3031 ((M + Na) +) → m/z 521.3443 (Fig. 5b inset) where M is the molecular mass of Cuc B i.e. 558.712 g/mol. Similar observed changes in mass were also reported by Wang et al. (2017a, b) and Xiao et al. (2018) for Cuc B. Thus based on the mass spectrum, it was established that the eluted fraction spot was due to the presence of Cuc B. Likewise, eluted fractions of bottle gourd pulp and peel also showed similar mass transition thus confirming the presence of Cuc B in all samples. Thus it can be concluded that in the present study, Cuc B was successfully extracted from all samples through developed MIP.
Quantification of Cuc
Cuc B concentration in bottle gourd peel was 3.37 ± 0.31 mg/100 g which was significantly (p > 0.05) highest among all the studied samples followed by juice with a concentration of 2.81 ± 0.26 mg/100 g and pulp had significantly (p > 0.05) lowest concentration with a value of 2.39 ± 0.21 mg/100 g. On the other hand, Kumbhalkar et al. (2015) observed that Cuc content in fruits of Lagenaria siceraria was 0.062% (w/w). Cuc concentration depends on various factors including fruit maturity, stress like heat and water, etc. This might be the reason for the difference in the present and reported Cuc concentration in Lagenaria siceraria. In another report, the highest level of Cuc was found in the fruit pulp of Citrullus colocynthis instead of its peel (Darwish-Sayed et al. 1974). This might be due to different species of Cucurbitaceae family analyzed in the present and previous studies.
Effect of irradiation and enzymatic treatment on Cuc content
There was no significant (p > 0.05) effect of γ-irradiation on the Cuc concentration of juice, peel, and pulp which was in the range of 2.79–3.01, 3.35–3.51, and 2.36–2.51 mg/100 g, respectively (Table 1a). There are reports available on the effect of irradiation on enhancing the mucilage yield and productivity of L. siceraria, however, there is no literature available regarding the impact of radiation processing on the Cuc quantity of bottle gourd (Abbas et al. 2017; Shah et al. 2010). Nevertheless, present data are in agreement with the report published by Tripathi et al. (2016) where gamma irradiation had no significant effect on the Cuc glycosides content of pumpkin (Cucurbita pepo). Gamma irradiation induced degradation of triterpenes was previously reported by Campos et al. (1997) where it was found that Friedelin content did not change even at 100 kGy but friedelan-3-ol started degrading at 10 kGy. However, in the present study maximum dose given to samples was only 5 kGy, whereas, fresh fruits and vegetables usually subject to even less than 2 kGy of irradiation for shelf life extension (Perera et al. 2019). Thus further experiments were performed on non-irradiated samples only.
Table 1.
(a) Effect of gamma irradiation on cucurbitacin concentration of bottle gourd juice, peel, and pulp (b) effect of enzymatic treatment on cucurbitacin concentration of bottle gourd juice, peel, and pulp (c) precision, accuracy, and recovery data
| mg/100 g | |||
|---|---|---|---|
| Juice | Peel | Pulp | |
| (a) Dose (kGy) | |||
| 0 | 2.81 ± 0.26a | 3.37 ± 0.31a | 2.39 ± 0.21a |
| 0.5 | 2.97 ± 0.18a | 3.41 ± 0.28a | 2.48 ± 0.17a |
| 1 | 3.01 ± 0.22a | 3.51 ± 0.24a | 2.51 ± 0.19a |
| 2.5 | 2.82 ± 0.23a | 3.41 ± 0.27a | 2.38 ± 0.16a |
| 5 | 2.79 ± 0.19a | 3.35 ± 0.26a | 2.36 ± 0.17a |
| (b) Incubation period (h) | |||
| 0 | 2.81 ± 0.26a | 3.37 ± 0.31a | 2.39 ± 0.21a |
| 1 | 2.78 ± 0.27a | 3.33 ± 0.22a | 2.35 ± 0.15a |
| 2 | 2.95 ± 0.28a | 3.47 ± 0.24a | 2.54 ± 0.14a |
| 4 | 3.04 ± 0.21a | 3.62 ± 0.21a | 2.61 ± 0.13a |
| 5 μg/mL | 25 μg/mL | 50 μg/mL | |
|---|---|---|---|
| (c) | |||
| RSD (%) | 8.15 | 5.89 | 1.11 |
| RME (%) | 9.32 | − 3.56 | 2.74 |
| Absolute recovery (%)* | 91.09 ± 5.23 | 92.81 ± 6.03 | 95.33 ± 2.66 |
| Relative recovery (%)* | 109.5 ± 4.6 | 107.1 ± 6.1 | 106.2 ± 3.8 |
Any two means in same column followed by same letter are not significantly (p > 0.05) different
*Data is presented as mean ± SD
Pectinase has β-glucosidase activity thus its treatment can release Cuc from the glycosidic bond. Enzymatic treatment including 0–4 h of incubation also had no significant (p > 0.05) effect on the Cuc concentration of all the studied samples. Cuc concentration of juice, peel, and pulp was in the range of 2.78–3.04, 3.33–3.62, and 2.35–2.61 mg/100 g, respectively (Table 1b). This observation was possible because species of Lagenaria possess high elaterase activities which also include β-glucosidase activity thus Cuc glycosides were found only in very small amounts in the fruits of Lagenaria siceraria (Rehm et al. 1957). In another study by Kaya and Melzig (2008), it was demonstrated that Cuc I and its glycosides were 0.0031% and 0.0006% (w/v), respectively present in Gratiola officinalis.
Method validation
In the present study, the standard curve of Cuc B was highly linear between concentrations ranges from 1 to 100 μg/mL. The equation of the calibration curve was y = 0.011x + 0.049 and the value of R2 is 0.999 (Online Resource 2). Denver et al. (Denver et al. 2019) reported that a method is suitable if RSD and RME are ≤ 15%, however, in this study both were less than 10% (Table 1c). Relative and absolute recoveries were higher than 90% indicating very less loss of Cuc B during MIP based extraction process. Thus it can be concluded that the developed method was highly linear, precise, and accurate for samples having toxic Cuc B. Limit of detection of the present method was 5 g/mL.
Overall proposed method is fast and doesn’t require extensive sample preparation as compared to already reported methods (Online Resource 1). Key features of the developed MIP based SPE cartridge that assured fast quantification was its high affinity towards Cuc B thus low run time of sample through the cartridge was required. Furthermore, removal of the template from MIP was also easy, and obtained elute can be directly subjected to quantification by spectrophotometer without any further processing. Recently, Xiao et al. (2018) developed a new method to determine Cuc B in rat plasma using UPLC-MS/MS. The lower limit of quantification was 0.05 ng/mL for the reported method which was significantly lower than the present methodology. However, for food quality control proposed method is highly suitable owing to its simple sample preparation procedure, no requirement of sophisticated instrumental analysis, and less time required for quantification.
Conclusion
In the present study, a method was developed to quantify toxic Cuc B in commercial bottle gourd juice and bottle gourd fruit peel and pulp. A comparative analysis of both polymers was done.
Equilibrium binding experiment showed that the binding capacity of developed MIP was superior to NIP at all the studied concentrations of Cuc B. Based on IP 100 mg of both the polymers was selected for further characterization.
SPE of Cuc from MIP and NIP demonstrated that 5% and 89%, respectively of analyte was removed by CHCl3 during washing due to non-specific interaction. The remaining analyte was recovered during elution using protic and polar solvent i.e. methanol.
The loading capacity of developed MIP was 0.5 μg of template per mg of MIP. Present MIP was highly selective to Cuc B as compared to other triterpenoids including Cuc I, stigmasterol, and betulinic acid.
Scatchard analysis revealed that MIP had better binding capacity and low equilibrium dissociation constant as compared to NIP.
MIP had higher rate of adsorption and surface area than NIP.
On all parameters, MIP performed better than NIP. Thereafter affectivity of the developed cartridge was tested against different bottle gourd samples. TLC analysis showed that MIP successfully extracts Cuc from all samples and the presence of Cuc B in the eluted fraction was further confirmed by HRLC-MS. Cuc concentration in juice, peel, and pulp was 0.0028%, 0.0038%, 0.0024%, (w/w) respectively. Gamma irradiation had no significant effect on triterpene concentration in samples at all the studied doses up to 5 kGy. Further, it was also observed that enzymatic treatment did not affect Cuc quantity due to the conversion of Cuc glycoside to aglycone by naturally present elaterase in Lagenaria siceraria. Based on the recovery of Cuc as well as precision and accuracy of the developed MIP it can be concluded that the present method is highly suitable to quantify toxic Cuc B in bottle gourd, therefore, assuring its quality. The developed quantification technique was rapid since the time required to quantify Cuc concentration in packed bottle gourd juice was around 45 min only.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors' contribution
CKS: conceptualization, experimentation, writing—original draft. SKG: Supervision. BS: Writing—review and editing.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas M, Arshad M, Nisar N, Nisar J, Ghaffar A, Nazir A, Tahir MA, Iqbal M. Muscilage characterization, biochemical and enzymatic activities of laser irradiated Lagenaria siceraria seedlings. J Photochem Photobiol B. 2017;173:344–352. doi: 10.1016/j.jphotobiol.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Bakhtiar S, Bhawani SA, Shafqat SR. Synthesis and characterization of molecular imprinting polymer for the removal of 2-phenylphenol from spiked blood serum and river water. Chem Biol Technol Agric. 2019;6(1):15. doi: 10.1186/s40538-019-0152-5. [DOI] [Google Scholar]
- Campos P, Vilegas JHY, Lancas FM. Behavior of triterpenes from Maytenus aquifolium Martius (“espinheira santa”) upon X-and gamma-rays irradiation. J Radioanal Nucl Ch. 1997;224(1–2):99–102. doi: 10.1007/BF02034619. [DOI] [Google Scholar]
- Claude B, Morin P, Lafosse M, Belmont AS, Haupt K. Selective solid-phase extraction of a triterpene acid from a plant extract by molecularly imprinted polymer. Talanta. 2008;75(2):344–350. doi: 10.1016/j.talanta.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Darwish-Sayed M, Balbaa SI, Afifi MS. The glycosidal content of the different organs of Citrullus colocynthis. Planta Med. 1974;26(07):293–298. doi: 10.1055/s-0028-1099390. [DOI] [PubMed] [Google Scholar]
- Denver N, Khan S, Stasinopoulos I, Church C, Homer NZ, MacLean MR, Andrew R. Derivatization enhances analysis of estrogens and their bioactive metabolites in human plasma by liquid chromatography tandem mass spectrometry. Anal Chim Acta. 2019;1054:84–94. doi: 10.1016/j.aca.2018.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington K, Magner E, Regan F. Predicting the performance of molecularly imprinted polymers: selective extraction of caffeine by molecularly imprinted solid phase extraction. Anal Chim Acta. 2006;566(1):60–68. doi: 10.1016/j.aca.2006.02.057. [DOI] [Google Scholar]
- Gry J, Søborg I, Andersson HC (2006) Cucurbitacins in plant food. Nordic Council of Ministers Chapter 5 TemaNord 10.6027/tn2006-556
- Ho CH, Ho MG, Ho SP, Ho HH. Bitter bottle gourd (Lagenaria siceraria) toxicity. Int J Emerg Med. 2014;46(6):772–775. doi: 10.1016/j.jemermed.2013.08.106. [DOI] [PubMed] [Google Scholar]
- Kaushik U, Aeri V, Mir SR. Cucurbitacins—an insight into medicinal leads from nature. Pharmacogn Rev. 2015;9(17):12–18. doi: 10.4103/0973-7847.156314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya GI, Melzig MF. Quantitative determination of cucurbitacin E and cucurbitacin I in homoeopathic mother tincture of Gratiola officinalis L. by HPLC. Die Pharmazie- Int J Pharm Sci. 2008;63(12):851–853. [PubMed] [Google Scholar]
- KhatIb KI, Borawake KS. Bottle Gourd (Lagenaria Siceraria) toxicity: a “Bitter” diagnostic dilemma. J Clin Diagn Res. 2014;8(12):MD05–MD07. doi: 10.7860/JCDR/2014/10826.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumbhalkar B, Tamhankar S, Upadhye A. Development of a high-performance thin-layer chromatographic method for quantification of Cucurbitacin B in bottle gourd (Lagenaria siceraria) for quality control. JPC-J Planar Chromat. 2015;28(4):294–299. doi: 10.1556/1006.2015.28.4.5. [DOI] [Google Scholar]
- Lopez C, Claude B, Morin P, Max JP, Pena R, Ribet JP. Synthesis and study of a molecularly imprinted polymer for the specific extraction of indole alkaloids from Catharanthus roseus extracts. Anal Chim Acta. 2011;683(2):198–205. doi: 10.1016/j.aca.2010.09.051. [DOI] [PubMed] [Google Scholar]
- Madikizela LM, Chimuka L. Synthesis, adsorption and selectivity studies of a polymer imprinted with naproxen, ibuprofen and diclofenac. J Environ Chem Eng. 2016;4(4):4029–4037. doi: 10.1016/j.jece.2016.09.012. [DOI] [Google Scholar]
- Perera CO, Perera AD. Technology of processing of horticultural crops. In: Kutz M, editor. Handbook of farm, dairy and food machinery engineering. 3. New York: Academic Press; 2019. pp. 300–353. [Google Scholar]
- Pichon V. Selective sample treatment using molecularly imprinted polymers. J Chromatogr A. 2007;1152(1–2):41–53. doi: 10.1016/j.chroma.2007.02.109. [DOI] [PubMed] [Google Scholar]
- Rehm S, Enslin PR, Meeuse ADJ, Wessels JH. Bitter principles of the Cucurbitaceae VII—the distribution of bitter principles in this plant family. J Sci Food Agric. 1957;8(12):679–686. doi: 10.1002/jsfa.2740081203. [DOI] [Google Scholar]
- Rosengren AM, Karlsson BC, Nicholls IA. Consequences of morphology on molecularly imprinted polymer-ligand recognition. Int J Mol Sci. 2013;14(1):1207–1217. doi: 10.3390/ijms14011207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BN, Seth AK, Nayak BS. Microwave assisted isolation of mucilage from the fruits of Lagenaria siceraria. Der Pharmacia Lett. 2010;2(2):202–205. [Google Scholar]
- Söylemez MA, Güven O. Radiation synthesis of molecularly imprinted hydroxyethylmethacrylate-based matrices for glucose recognition. Hacettepe J Biol Chem. 2018;46(1):53–60. doi: 10.15671/HJBC.2018.214. [DOI] [Google Scholar]
- Sun C, Wang J, Huang J, Yao D, Wang CZ, Zhang L, Hou S, Chen L, Yuan CS. The multi-template molecularly imprinted polymer based on SBA-15 for selective separation and determination of panax notoginseng saponins simultaneously in biological samples. Polymers. 2017;9(12):653. doi: 10.3390/polym9120653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi J, Variyar AP, Mishra PK, Variyar PS. Impact of radiation processing on the stability of cucurbitacin glycosides in ready-to-cook (RTC) pumpkin during storage. LWT-Food Sci Technol. 2016;73:239–242. doi: 10.1016/j.lwt.2016.06.023. [DOI] [Google Scholar]
- Wang S, Tang L, Guo Y, Yan F, Chen F. Determination of Momordicoside A in bitter melon by high-performance liquid chromatography after solid-phase extraction. Chromatographia. 2001;53(7–8):372–374. doi: 10.1007/BF02491069. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhu W, Gao M, Wu C, Yang C, Yang J, Wu G, Yang B, Kuang H. Simultaneous determination of cucurbitacin B and cucurbitacin E in rat plasma by UHPLC-MS/MS: a pharmacokinetics study after oral administration of cucurbitacin tablets. J Chromatogr B. 2017;1065–1066:63–69. doi: 10.1016/j.jchromb.2017.09.024. [DOI] [PubMed] [Google Scholar]
- Wang X, Tanaka M, Peixoto HS, Wink M. Cucurbitacins: elucidation of their interactions with the cytoskeleton. PeerJ. 2017;5:e3357. doi: 10.7717/peerj.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Mizaikoff B. Binding site characteristics of 17β-estradiol imprinted polymers. Biosens Bioelectron. 2007;23(2):201–209. doi: 10.1016/j.bios.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Wu X, Wang X, Lu W, Wang X, Li J, You H, Xiong L, Chen L. Water-compatible temperature and magnetic dual-responsive molecularly imprinted polymers for recognition and extraction of bisphenol A. J Chromatogr A. 2016;1435(2016):30–38. doi: 10.1016/j.chroma.2016.01.040. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Zhao Q, Wu Q, Chang J, Xue H, Liu C, Liu X. A new sensitive UPLC-MS/MS method for the determination of cucurbitacin B in rat plasma: application to an absolute bioavailability study. RSC Adv. 2018;8(54):30978–30985. doi: 10.1039/C8RA05941A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Mosbach K. Molecularly imprinted microspheres as antibody binding mimics. React Funct Polym. 2001;48(1–3):149–157. doi: 10.1016/S1381-5148(01)00050-5. [DOI] [Google Scholar]
- Zeng S, She Y, Jiao B, Liu G, Wang J, Su X, Ma X, Jin M, Jin F, Wang S. Molecularly imprinted polymer for selective extraction and simultaneous determination of four tropane alkaloids from Przewalskia tangutica Maxim. fruit extracts using LC-MS/MS. RSC Adv. 2015;5(115):94997–95006. doi: 10.1039/C5RA18608K. [DOI] [Google Scholar]
- Zhu QZ, Haupt K, Knopp D, Niessner R. Molecularly imprinted polymer for metsulfuron-methyl and its binding characteristics for sulfonylurea herbicides. Anal Chim Acta. 2002;468(2):217–227. doi: 10.1016/S0003-2670(01)01437-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.