Abstract
In this study, antioxidant (DPPH and metal chelating), DNA cleavage, biofilm, and antimicrobial properties of extracted phenol from the walnut green husk (WGH) and its different concentrate and permeate samples were evaluated. For maximum phenolic compound extraction from the WGH first, the effects of solvent type (deionized water, methanol, n-hexane, acetone, and ethanol), solvent temperature (25–75 °C), and extraction time (0.5–24 h) were optimized. Then to concentrate phenolic compounds a pressure-driven membrane process was used with four different membrane types. The phenol contents of the concentrate samples were found to be microfiltration (MF) concentrate 4400 mg/L, ultrafiltration (UF) concentrate 4175 mg/L, nanofiltration (NF) concentrate 8155 mg/L, and reverse osmosis (RO) concentrate 8100 mg/L. LC-MSMS was used to determine the quantification of phenolic compounds in permeate and concentrate streams. In addition, all of the concentrate samples with high phenol content showed a high antioxidant activity as 100% with MF concentrate, UF concentrate, NF concentrated and RO concentrated. Likewise, concentrate samples were found to have very high antibiofilm activity as 82.86% for NF concentrate againts S. aureus, 85.80% for NF concentrate against P. aureginosa, 80.95% for RO concentrate against S. aureus, and 83.61% for RO-concentrate against P. aureginosa. When the antimicrobial activity of the extracted phenol from WGH and its different concentrate and permeate samples were evaluated by micro dilution and disk diffusion methods, it was found that the ability of the concentrate samples to inhibit bacterial growth was much higher than permeate ones. In addition, extracted phenol from WGH and its different concentrate and permeate samples showed significant DNA nuclease activity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05588-w.
Keywords: Walnut green husk, Phenolic compounds extraction, Membrane process, Antioxidant, Biofilm, Antimicrobial, Cell viability, DNA cleavage
Introduction
Walnuts (Juglans regia L.) are mostly grown in un-reclaimed and poor soil and harvested as covered with green organic husk (Oliveira et al. 2008a). According to FAO, world walnut production is 3.32 million tons (FAO, 2020). The green husks of the harvested walnuts are peeled and dried so that there is no quality loss in walnuts. The green husks make up a considerable percentage of the fruit by weight (∼ 20%) (Jahanban-Esfahlan et al. 2020). Different parts of the walnut, such as its green husk, leaves, shells, and seeds, find industrial use in areas such as pharmaceutical and cosmetics (Ramezani et al. 2020). The extraction steps, which can be applied in unlimited ways, are very effective on the purity and obtain components from plants (Naczk and Shaidi 2004). Components extracted from flowers, leaves, fruits, and stems of some other plants have been used in the treatment of various diseases such as malaria in history (Aminov 2010). The interest of researchers on this subject is increasing day by day due to the rapid increase in population, decrease in resources, and unexpected harms of chemical products. Herbal by-products can be used as antimicrobial agents. The interest in the use of natural antimicrobial substances has increased because consumers do not prefer to use chemical preservatives (Kadiroğlu and Ekici 2018). It is known that the green husk of walnut is rich in polyphenols, flavones, and other active components (Li and Deng 2020). Several studies have been conducted to determine the phenolic composition, antibacterial and antioxidant properties of walnut green husks. In a study, the total phenol content, as well as antioxidant and antimicrobial activities, in aqueous extracts of five different cultivars of walnut (Juglans regia L.) was investigated and it was reported that total phenols content was determined as 32.61 to 74.08 mg/g of GAE. (Oliveira et al. 2008b). In another study, methanol and petroleum ether were used to extract phenolic compounds from walnut (Juglans regia L.) seed, green husk, and leaf. It was reported that methanol extracts have better antioxidant action in comparison to petroleum ether extracts (Carvalho et al. 2010). Fernández-Agulló et al. (2013) investigated the effect of solvent (water, methanol, ethanol, and 50% aqueous solutions of methanol and ethanol) on extraction yields and bioactive properties. Results showed that water had the highest extraction yield (44.11%), and samples extracted with water/ethanol (1:1) had the highest bioactive potential (84.46 mg GAE/g extract). Juglone is an organic compound that is used as a dye for fabrics and inks, as well as a coloring agent in foods and cosmetics (Ramezani et al. 2020). Caballero et al. (2019) investigated the impact of three concentration technologies such as speed vacuum, vacuum oven, and rotavapor over the concentration yield of juglone from the extract of walnut in ethanol and methanol. The concentration of juglone obtained with ethanol for three technologies has been reported 2.94, 23.21, and 58.07 ppm respectively. Membrane separation is a filtration technique that involves the passing of solute molecules through an inorganic or organic selectively permeable membrane. When it used to recover valuable compounds from extracts, concentrates the properties of bioactive compounds and increases the final commercial value of them. The use of walnuts and their by-products such as green husk as a natural antioxidant and antimicrobial agent should be increased therefore these wastes turn into environmentally friendly raw materials.
In this study, we investigated the recovery of phenolic compounds from the walnut green husk (WGH) which is an agricultural waste produced by walnut in high amounts after harvest. Some variables such as solvent type, solvent temperature, and extraction time were optimized for maximum phenol extraction from walnut green husks. Then, a pressure-driven membrane process was used to concentrate phenol from the husks. To the best of our knowledge, our study is the first which is using a membrane technology for concentrating extracted phenolic compounds from walnut green husks. Furthermore, antimicrobial activity, DPPH scavenging activity, cleavage of pBR322 DNA activity, biofilm inhibition activity, and metal chelating activity were tested to investigate the antimicrobial and antioxidant properties of the recovered phenol.
Materials and methods
Materials
Deionized water, methanol, acetone, n-hexane, and ethanol were used as solvents to extract phenol from WGH. MCE, UP150, NF270, and BW30 membranes were used as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO), respectively. The properties of the membranes are given in Table S1.
Peeling process description
To peel WGH, there are different processes all over the world (Karimi et al. 2008). In this study, WGH were kindly provided by a local producer located in Mersin, Turkey which has using mechanical peeling method without water. WGH were stored at + 4 °C in a refrigerator until use.
Phenol extraction from walnut green husk
For the characterization of the WGH content, the phenolic compounds in the green husk were extracted with various solvents. First, the husk was dried at 50 °C for 1 day, and the grain size was ground to less than 600 µm using a laboratory-type grinder. To recover the phenolic compounds from the WGH, extraction experiments were carried out with 5 different solvents such as n-hexane, acetone, ethanol, methanol and water. The solvent type, temperature, and extraction time were optimized for the extraction of the maximum concentration of phenolic compounds. To determine the best solvent type, 5 g of dried WGH was ground (Fig. S1) and 50 mL of solvent was added at different times (0.5, 1, 2, 4, 8, 24 h) and different temperatures (25, 50, 75 °C). Then, the solution was filtered through filter paper. Before starting the membrane experiments, 50 mL of water was added to each of the filtrates and the solvents were removed at 75 °C and phenolic compounds were transferred to the water phase. Total phenols, antioxidants, and total flavonoids were analyzed in the samples.
Phenolic compounds recovery using the membranes
The performance of the commercially available membranes were tested for phenolic compounds recovery using a dead-end filtration system (Sterlitech, HP4750 Stirred Cell). First pure water was filtrated for 30 min at the different operating pressures then dead-end filtration system was filled with 100 mL aqueous solution which was rich-phenolic compounds. The rejection of phenolic compounds was calculated using Eq. (1).
| 1 |
where Cf and Cp are phenol concentrations in feed and permeate samples, respectively.
Analyses
Total phenol analysis
The total phenolic content of extracted and concentrated samples were spectrophotometrically measured at approximately 760 nm, using the Folin-Ciocalteu method which is based on the redox reaction of phenolic compounds (Singleton et al. 1999). Gallic acid was used as a standard to construct the standard curve in this assay.
LC-MSMS
Quantification of the selected phenols was performed via LC– MS/MS in negative ionisation mode on a 6410 triple quadrupole mass spectrometer from Agilent Technologies (Palo Alto, CA, United States) equipped with an electrospray ionisation (ESI) interface. Nitrogen was used as desolvation- and collision gas. The detailed decsription can be found in elswhere (Jaitz et al. 2010).
DPPH scavenging activity
The antioxidant activities of the extracted phenol from WGH and its different concentrate and permeate samples were examined using a 2, 2-Diphenyl-2-picryl-hydrazyl (DPPH) free radical scavenging assay. This technique is one of several commonly used methods to evaluate the capacity of radical scavenging activity. The test is widely used in antioxidant screening because it is simple, fast, and clear (Daravath, Rambabu, Shankar, & Nile, 2019). The extracted phenol from WGH and its different concentrate and permeate samples were evaluated for in vitro antioxidant activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical. (Ağırtaş et al. 2015). The DPPH scavenging effect was found by calculating the percentage of color degradation of the DPPH in the equation below (2).
| 2 |
Metal chelating activity
The ferrous iron chelating activity of the extracted phenol from WGH and its different concentrate and permeate samples were made by adding minor changes to the reference cited in the source (Dinis, Madeira, and Almeida 1994). Percent inhibition of iron-ferrozine complex formation, Eq. (3).
| 3 |
Cleavage of pBR322 DNA activity
DNA cleavage studies for extracted phenol from WGH and its different concentrate and permeate samples were investigated using pBR322 DNA by gel electrophoresis technique using 1% agarose. Reaction mixtures (20 μL) containing pBR322 DNA together with extracted phenol from WGH and its different concentrate and permeate samples were prepared and then allowed to incubate at 37 ℃ for 1 h in the dark. After the appropriate incubation time, the samples were loaded onto the agarose gel. The samples were subjected to electrophoresis at 80 V for 1 h. Evaluation was done with a UV illuminator using the Bio-Rad Gel documentation system.
Antimicrobial activity of the extracted phenol from WGH and its different concentrate and permeate samples by microdilution method
Antimicrobial activity studies of the extracted phenol from WGH and its different concentrate and permeate samples were investigated with minor modifications to the present reference (Elbeshehy, Elazzazy and Aggelis 2015). Micro-dilution assay was employed to test the antimicrobial property of the extracted phenol from WGH and its different concentrate and permeate samples. The tested microbial strains were Enterococcus faecalis (ATCC 29,212), Staphylococcus aureus (ATCC 25,923), Enterococcus hirae (ATCC 10,541), Escherichia coli (ATCC 25,922), Pseudomonas aeruginosa (ATCC 27,853), Legionella pneumophila subsp. pneumophila (ATCC 33,152), Candida parapisilosis (ATCC 22,019), and Candida tropicalis (ATCC 750).The extracted phenol from WGH and its different concentrate and permeate samples solutions were first prepared in microplates wells and a serial two-fold dilutions (1:2) at different concentrations. Then, the microbial strains which were prepared 0.5 McFarland Scale were inoculated to the microplate-wells and incubated at 37 ℃ for one day. Later, antimicrobial activity was evaluated with minimum inhibition concentration (MIC) values defined as the lowest concentration that inhibits microbial growth.
Antimicrobial activity of the extracted phenol from WGH and its different concentrate and permeate samples by disc diffusion method
The antimicrobial activity of extracted phenol from WGH and its different concentrate and permeate samples were evaluated using the disc diffusion method described in the previous reference, but with minor revisions (Chandramohan et al. 2016). The tested microbial strains were Gr ( +), Gr (–), and fungal strains mentioned above. Microbial cultures were adjusted to 0.5 McFarland turbidity standards to determine antimicrobial activity. Empty antimicrobial test discs were placed on nutrient agar plates with a sterile loop and then soaked with extracted phenol from WGH and its different concentrate and permeate samples. Then the plates were incubated at 37 °C for 24 h. Antimicrobial activity was determined by measuring the inhibition zone around each paper disc with the aid of a digital caliper.
Biofilm inhibition activity of the extracted phenol from WGH and its different concentrate and permeate samples
Biofilm inhibition activity was measured using a crystal violet-based staining procedure. S. aureus and P. aeruginosa were used as test microorganisms for biofilm formation inhibition of extracted phenol from WGH and its different concentrate and permeate samples. Extracted phenol from WGH and its different concentrate and permeate samples solutions were added into the wells which contain Nutrient Broth and then incubated at 37 °C for 72 h. When the incubation period was over, the supernatants were removed. The remaining biofilm biomasses were washed with twice distilled water and air dried. After drying, crystal violet was added to each well and incubated for 30 min at room temperature. After 30 min of incubation, the unbound dye was washed off with distilled water and the wells were air-dried. Finally, optical density at 595 nm was measured using a spectrophotometer (Thermo Scientific). The determined results were calculated according to the biofilm inhibition Eq. (4).
| 4 |
Results and discussion
The effect of solvent type for phenolic compounds extraction
The effect of solvent type was tested to extract maximum total phenol from the walnut green husk. Five different solvent types such as n-hexane, acetone, ethanol, methanol, and water were tested and the results are shown in Fig. S2. Accordingly, the phenol concentration after hexane extraction had a mean value of 0.30 ± 0.025 mg/g with a standard deviation of 0.044. The 95% confidence interval for the mean was 0.19–0.41 mg/g. The extraction by acetone resulted in a mean phenol concentration of 0.65 ± 0.03 mg/g with a 0.05 standard deviation. Also, the confidence interval (95%) ranged from 0.53 to 0.77 mg/g. Ethanol positively affected the extraction process (mean phenol concentration of 0.80 ± 0.05). The standard deviation for the phenol extracted by ethanol was 0.09. The lower and the upper bounds for the 95% confidence for the mean were 0.58 and 1.02, respectively. The concentration of phenol extracted by methanol was 3.50 ± 0.36, with a standard deviation of 0.62 and a confidence interval of 1.95 to 5.05. The results showed that water supplied the maximum phenol extraction (11.75 ± 0.26 mg/g) from the green husk with a standard deviation of 0.46. The lower bound was 10.61, and the upper bound reached 12.89. Table S2 shows the statistical analysis for the solvent type effects on the phenol extraction. The observed differences in phenol extraction could be attributed to differences in solvent strength and polarity, which alters the solubility of bioactive compounds in the hull matrix (Akinmoladun et al. 2022). Higher solvent polarity ensures higher total phenol extraction yields, so we obtained a higher total phenol extraction yield when water was used as a solvent, which is consistent with other studies (Goli et al. 2005; Ozay et al. 2021).
The effect of solvent temperature for phenol extraction
Temperature is another important factor in extraction process. The effect of solvent temperature was investigated to extract maximum total phenol from the green husk. Three different solvent temperatures (25, 50, 75 °C) were tested and the results are shown in Fig. S3.
The results showed that the phenol extraction at 25 °C was 11.79 ± 0.47 mg/g with a standard deviation of 0.81. The confidence interval at 95% of the mean was 9.77–13.81. The increases in the temperature from 50 °C to 75 °C increased the phenol extraction concentration from 15.5 ± 0.29 mg/g to 17.05 ± 0.45 mg/g. The standard deviations for the phenol extraction at 50 °C and 75 °C were 0.50 and 0.79, respectively (Table S3). The Pearson correlation test was performed to confirm the correlation between the temperature and the extracted phenol concentration. Table S4 shows that the correlation is positively significant at the 0.01 level (2- tailed). Paired Samples T-Test was employed in temperature significance determination. Table S5 shows that the average difference for the concentrations at different temperature and 50 °C and 75 °C were statistically significant at α = 0.05. The test also presented a significance between the mean concentrations at 25 °C and 75 °C at α = 0.01. Heating which is applied during the extraction process mellow the plant texture and decrease the phenol–protein and phenol–polysaccharide interactions, thus more phenolic compounds may transfer into the solvent (Mokrani and Madani 2016). But after a point, higher temperatures may reduce extraction yield because they cause oxidation and degradation of the desired compounds (Silva et al. 2007). Pinelo et al. (2005) investigated the effect of solvent temperature (values ranging from 25 to 50 °C) on the total phenolic content of extracts from grape pomace and concluded that the antiradical activity of phenolic extracts is maximized at 50 °C.
The effect of extraction time for phenol extraction
The effect of extraction time was investigated to extract maximum total phenol from the walnut green husk. Different extraction times were tested and the results are shown in Fig. S4. One of the most efficient parameters is the extraction time. The results showed that phenol extraction increased from 11.5 ± 0.29 mg/g (std. deviation 0.20) to 19.0 ± 0.29 mg/g (std. deviation 0.50) when extraction time increased from 0.5 to 4 h. At the end of the 24 h, there was an insignificant increase to 19.5 ± 0.55 mg/g. Longer extraction times do not have the same effect on the extraction yield of phenolic compounds. Paired Samples T-Test was employed to study the significance of the time for the extracted phenol concentration. According to Table S6, the difference between the means for the first pair (mean 0.5 h – mean 4 h) was − 7.5, which means that the mean concentration after 4 h is higher than the mean concentration after 0.5 h by 7.5 mg/g. The mean difference between the first pair was found to be statistically significant at α = 0.01. In contrast, the second pair was not statistically significant (p = 0.199), and the average extracted concentration after 24 h was higher than the 4 h concentration of just 0.5 mg/g. The statistical results confirmed the obtained results. In a study, effect of different parameters such as temperature, solvent ratio or time (80–120 min) on the extraction of phenolic compounds from plant leaves has investigated (Che Sulaiman et al. 2017). It was discovered that the extraction yield was highest at time of 80 min when the water to ethanol ratio was (80:20).
Membrane rejection performance
To achieve ideal process design parameters and maximum yield of phenolic compounds, it is critical to select efficient extraction and separation technologies. Dead-end filtration system was used to concentrate the phenolic compounds from an aqueous solution. A wide range of commercially available membranes was screened from MF to RO. MFCE, UP150 NF270, and BW30 were operated at 1, 5, 10, and 20 bar, respectively. Figure 1 shows total phenol concentration in permeate and concentrates at membranes were used. The results depicted that the BW30 membrane enhanced the maximum phenol recovery. Total phenol concentration increased up to 8300 ± 225 mg/L in the concentrate phase. These findings can be explained by the fact that the nominal MWCO of BW30 membrane is higher than the other membranes. MCE, UP150, and NF270 membranes supplied 4400 ± 160, 4500 ± 190, and 8155 ± 220 mg/L total phenol recovery in concentrate phase while 865 ± 110, 787,5 ± 90 and 358 ± 35 mg/L in permeate phase, respectively. Similar results were reported by Machado et al. (2015) who studied extraction of antioxidant compounds from a typical fruit and concentrate it with UF and NF membranes. It was reported that NF membranes showed better efficiency. Moreover, Arend et al. (2017) concentrated phenolic compounds of strawberry extract by nanofiltration process and reported a recovery of anthocyanin in the retentate around 95%.
Fig. 1.
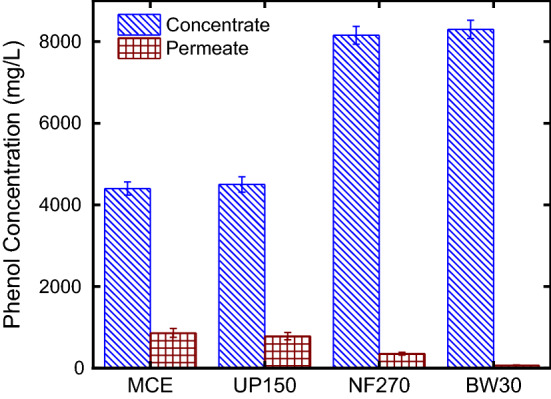
Membrane rejection performance for membranes
LC-MSMS results
Table 1 shows the quantification of phenolic compounds in permeate and concentrate of the membrane process. The results depicted that the feed stream was rich for cafeic acid, cinnamic acid, ferrulic acid, gallic acid, coumaric acid, syringic acid, vanilic acid, and quercetin dihydrate. These phenolic compounds were concentrated by BW30 membrane.
Table 1.
The quantification of phenolic compounds in permeate and concentrate streams (mg/L)
| Phenolic compounds | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 4-Hydroxybenzoic acid | 0.299 | 0.152 | 0.356 | 0.131 | 0.452 | 0.117 | 0.622 | 0.105 | 0.652 |
| Cafeic acid | 5.629 | 3.991 | 5.676 | 2.565 | 5.815 | 0.839 | 9.034 | 0.766 | 10.913 |
| Ferulic acid | 2.195 | 1.097 | 2.222 | 0.836 | 2.965 | 0.634 | 3.470 | 0.285 | 5.866 |
| Gallic acid | 21.914 | 16.217 | 21.966 | 15.362 | 22.063 | 13.012 | 32.632 | 11.889 | 41.622 |
| Genistisie acid | 0.666 | 0.352 | 0.717 | 0.328 | 0.775 | 0.310 | 0.977 | 0.255 | 1.160 |
| Coumaric acid | 1.111 | 0.440 | 1.382 | 0.421 | 1.414 | 0.160 | 2.403 | 0.031 | 3.649 |
| Quercetin Dihydrate | 5.776 | 3.641 | 6.790 | 2.437 | 8.021 | 0.775 | 10.242 | 0.394 | 12.707 |
| Syringic acid | 3.159 | 2.109 | 3.627 | 1.781 | 3.982 | 1.620 | 5.737 | 1.526 | 8.307 |
| Vanilic acid | 6.752 | 3.198 | 7.462 | 2.301 | 9.044 | 1.413 | 15.338 | 1.226 | 19.773 |
1: extracted phenol; 2: MF permeate; 3: MF concentrate 4: UF permeate; 5: UF concentrate 6: NF permeate; 7: NF concentrate; 8: RO permeate; 9: RO concentrate
DPPH scavenging activity
DPPH free radical scavenging assay is also one of the most tested in vitro antioxidant activity methods for natural and chemical synthetic compounds. The method is also inexpensive, easy and effective. From that point of view, DPPH free radical scavenging activity of the extracted phenol from WGH and its different concentrate and permeate samples was tested in our study. The antioxidant activity of the extracted phenol from WGH and its different concentrate and permeate samples is shown in Fig. 2. MF, UF, NF, and RO from the concentrate samples had the highest concentrations of DPPH activity, as well as the highest phenol content. The DPPH scavenging activity of extracted phenol, MF, UF, NF, and RO concentrate samples obtained from waste WGH was also found to be 100%. The DPPH scavenging activities of the permeate samples of UF, NF, MF, and RO were 98.56%, 97.73%, 92.95%, and 78.29%, respectively. Agullo et al. (2013) investigated the effect of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. They showed that the antioxidant activities of walnut green husk extracts changed at different concentrations. It was found that walnut green husk extract obtained from ethanol and methanol achieved the highest DPPH free radicals scavenging activity as 50% (Agullo et al. 2013). Mehdizadeh et al. (2020) investigated the antioxidant activity of Cu nanoparticles fixed on cellulosic WGH material and it was found that all samples showed antioxidant effects, but significant changes were observed according to the concentrations difference. It was observed that antioxidant activity increased depending on the increase in phenol content or concentration. These radical scavenging activities are vital to prevent the opposite role of free radicals in certain diseases. It should be noted that the antioxidant activity of the phenol content is also very important, just as it is important whether the compounds are concentrate or permeate. In light of our results, we can conclude that especially concentrate WGH extracts samples offer a good radical scavenging effect.
Fig. 2.
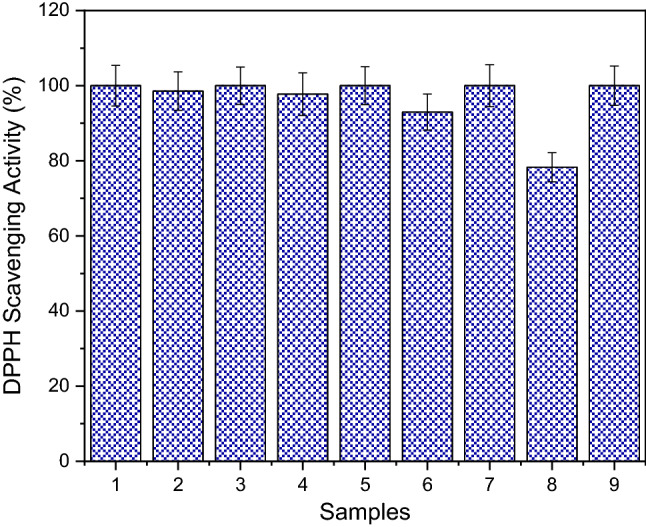
DPPH scavenging ability. (1: extracted phenol; 2: MF permeate; 3: MF concentrate 4: UF permeate; 5: UF concentrate 6: NF permeate; 7: NF concentrate; 8: RO permeate; 9: RO concentrate)
Metal chelating activity
Iron is an essential trace element for the human body, and its deficiency in the body causes iron deficiency anemia, poor cognitive development, increased maternal mortality, and low energy levels (Kong et al. 2021). On the other hand, iron and other metal ions can be caused cellular damage at high concentrations. Therefore, metal chelating agents are becoming more and more important every day. This study aimed to determine the iron chelating properties of concentrate and permeate samples obtained from walnut green husks. The metal chelating ability of extracted phenol from WGH and its different concentrate and permeate samples was measured by the formation of ferrosine complex of iron ion and the ability of extracted phenol from WGH and its different concentrate and permeate samples to chelate Fe2+ ion is shown in Fig. 3. The chelating activities of the extracted phenol from WGH and its different concentrate and permeate samples were found to be concentration dependent and the concentrate samples showed better activity than the permeate ones. Iron ion chelating activity was 100% for extracted phenol, UF, NF, MF, and RO concentrate samples. Iron ion chelating activity was determined as 74.96% for extracted phenol RO permeate, 90.08% for extracted phenol NF permeate, 95.21% for extracted phenol UF permeate, and 96.42% for extracted phenol MF permeate samples. The results of this study showed that all test samples had an effective capacity for iron binding, revealing their antioxidant potential. For example, various foods obtained from vegetables and animal such as whey, anchovies, chickpeas, date seeds, walnuts have shown iron chelating properties (Zarei et al. 2016; Lv et al. 2017). Phenolic compounds with chelating effects on metal ions may be one of the antioxidant mechanisms and may be somewhat beneficial to protect against oxidative damage. Therefore, it was concluded that walnut green husk may be promising antioxidants for functional food ingredients and/or pharmaceuticals.
Fig. 3.
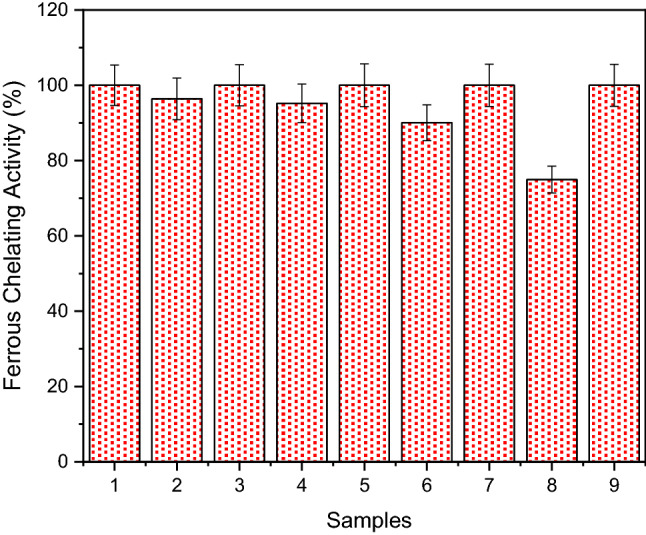
Ferrous chelating ability. (1: extracted phenol; 2: MF permeate; 3: MF concentrate 4: UF permeate; 5: UF concentrate 6: NF permeate; 7: NF concentrate; 8: RO permeate; 9: RO concentrate)
DNA cleavage ability
DNA interactions of natural or synthetic compounds are very important. The most important reasons for this are that DNA is one of the most important target molecules in anticancer and antimicrobial studies. Therefore, DNA cleavage activities of the of extracted phenol from WGH and its different concentrate and permeate samples were tested. In our study, DNA fragmentation activity of extracted phenol from WGH and its different concentrate and permeate samples was determined by agarose gel electrophoresis. As seen in Fig. 4, concentrate samples of waste WGH showed excellent DNA nuclease activity. Extracted phenol from WGH and all tested concentrate samples were found to completely degrade DNA. While a double strand break (from Form I to Form II) was observed in plasmid DNA with WGH permeate samples of MF, UF, and NF, and DNA cleavage did not occur with WGH permeate sample of RO. Therefore, the DNA cleavage activities of newly synthesized and natural substances in the search for alternative products is very important in terms of shedding light on future research. In our study, striking and important results have emerged for DNA cleavage ability with waste WGH concentrate samples.
Fig. 4.
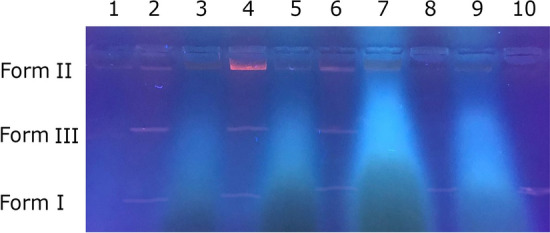
DNA Cleavage activity of extracted phenol from WGH and its different concentrate and permeate samples. Lane 1: pBR 322 DNA + extracted phenol; Lane 2: pBR 322 DNA + MF permeate of WGH; Lane 3: pBR 322 DNA + MF concentrate of WGH; Lane 4: pBR 322 DNA + UF permeate of WGH; Lane 5: pBR 322 DNA + UF concentrate of WGH; Lane 6: pBR 322 DNA + NF permeate of WGH; Lane 7: pBR 322 DNA + NF concentrate of WGH; Lane 8: pBR 322 DNA + RO permeate of WGH; Lane 9: pBR 322 DNA + RO concentrate of WGH; Lane 10: pBR 322 DNA
Antibacterial activity of extracted phenol from WGH and its different concentrate and permeate samples by using micro dilution method
In this study, antimicrobial activity was evaluated by the micro dilution method. At the end of 24 h, antimicrobial activity was evaluated as the lowest concentration inhibiting microbial growth. The results are shown in detail in Table 2. The MIC of sample 1 ranged from 8 to 256 mg/L. In our study, it was observed that concentrate samples showed better antimicrobial activity and they were more effective on Gram-negative microorganisms. The MIC values efficiency of test samples were in the order of 7 > 1 = 9 > 3 = 5 > 2 = 4 > 6 = 8 for E. coli and 9 > 3 = 5 = 7 > 1 > 4 > 2 = 6 = 8. On the other hand, MIC values of 7 were determined as 8 mg/L E. coli, 16 mg/L for P. aeruginosa, 32 mg/L for E. hirae, and L. pneumophila, 64 mg/L for E. fecalis, S. aureus and C. parapisilosis, and 128 mg/L for C. tropicalis. MIC values of 9 were also found as 16 mg/L E. coli, 32 mg/L for L. pneumophila, P. aeruginosa, S. aureus, and E. fecalis, 64 mg/L for E. hirae, and C. parapisilosis, and 128 mg/L for C. tropicalis. According to these results, E. coli was determined to be the most sensitive microorganism to the extracted phenol from WGH and its different concentrate and permeate samples and 8 was also exhibited the most ineffective antimicrobial activity against the test microorganisms. Dolatabadi et al. (2018) reported that methanol extract from walnut leaves was found to have good antibacterial activity against P. aeruginosa and it was found that the methanol extract showed a mean MIC value of 16 mg/mL and strong activity in preventing biofilm formation. In a different study, it was found that the permeate extract of J. regia did not show a significant antibacterial effect on S. mutans, unlike the ethanol extract. They also found that the ethanol extract of J. regia was effective against all tested bacteria, such as S. aureus, S. sanguis, and S. salivarius (Zakavi et al. 2013). Darvishi et al. (2019) investigated the antibacterial properties of zinc oxide nanoparticles synthesized using Juglans regia L. extract on E. coli, P. aeruginosa, and Acinetobacter baumannii resistant strains that cause wound and burn infections. It was observed that synthesized ZnO nanoparticles demonstrated antimicrobial activity and the anti-bacterial assay supported that A. baumannii was more sensitive than P. aeruginosa. The new extracted phenol from WGH and its different concentrate and permeate samples can be performed as an antimicrobial agent in the medicine industry after further studies.
Table 2.
The minimum inhibition concentration (MIC) of test microorganisms
| MIC values of samples (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Microorganisms | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| E. coli | 16 | 128 | 32 | 128 | 32 | 256 | 8 | 256 | 16 |
| P. aeruginosa | 64 | 256 | 64 | 256 | 64 | 256 | 16 | 512 | 32 |
| L. pneumophila | 128 | 128 | 64 | 128 | 64 | 512 | 32 | 512 | 32 |
| E. hirae | 128 | 256 | 128 | 256 | 64 | 512 | 32 | 512 | 64 |
| E. fecalis | 128 | 512 | 64 | 256 | 64 | 512 | 64 | 512 | 32 |
| S. aureus | 128 | 256 | 64 | 256 | 64 | 512 | 64 | 512 | 32 |
| C. parapisilosis | 256 | 512 | 128 | 516 | 128 | 256 | 64 | 512 | 64 |
| C. tropicalis | 256 | 256 | 128 | 256 | 128 | 512 | 128 | 512 | 128 |
1: extracted phenol; 2: MF permeate; 3: MF concentrate 4: UF permeate; 5: UF concentrate 6: NF permeate; 7: NF concentrate; 8: RO permeate; 9: RO concentrate
Antibacterial activity of extracted phenol from WGH and its different concentrate samples by using disc diffusion
In this study, extracted phenol from WGH and its different concentrate samples were also tested by the disc diffusion method for their antibacterial activity against Gram-positive and Gram-negative bacteria. It was also tested for their antifungal activity against two fungal species. The results obtained are tabulated in Table S7. When the antimicrobial activity of extracted phenol from WGH and its different concentrate samples were examined by the disc diffusion method, it was seen that NF concentrate and RO concentrate showed much better antimicrobial activity. The biofilm properties of samples NF concentrate and RO concentrate were also found to be the highest in parallel with their antimicrobial activities as mentioned below. The highest antimicrobial activity was measured against E. hire with a zone diameter of 16 mm, 17 mm, and 19 mm for the MF concentrate, UF concentrate, and NF concentrate, respectively. The highest antimicrobial activity was measured against P. aeruginosa and E. hirae with a zone diameter of 16 mm for the RO concentrate. The lowest antimicrobial activity was measured against E. faecalis with UF concentrate among the concentrate samples. Agullo et al. (2013) investigated aqueous extracts of walnut green husk for their antimicrobial properties against various Gram ( +) bacteria and Gram ( −) bacteria. While the extracts showed antimicrobial activity for all Gram positive bacteria tested, they did not show antimicrobial activity against Gram negative bacteria. It was concluded that the compounds found in the juicy walnut green husk extract were probably unable to pass through the cell membrane. Gram-negative bacteria have an outer membrane for the transfer of molecules. The transfer of molecules is provided through this outer membrane. The antimicrobial effects of microorganisms are related to the size and shape of the compounds, their ability to reach the field of action, as well as their penetration into the outer membrane (Kavak et al. 2010). Gomes et al. (2018) investigated the combination of Juglans regia and different phenolic plant extracts with other drugs its effectiveness on S. aureus. Antimicrobial susceptibility tests were performed for Eucalyptus globulus, Juglans regia, and Foeniculum vulgare extracts, which are the most prominent antibacterial potential. Regarding the results obtained in susceptibility tests, it was found that the bactericidal effect of J. regia extracts caused complete growth inhibition in S. aureus strains. These results also highlight that natural antimicrobial agents can be used as disinfectants in surface cleaning and disinfection to control some diseases and pathogenic microorganisms responsible for dairy industry contaminations.
Biofilm inhibition of the extracted phenol from WGH and its different concentrate and permeate samples
Biofilm formation makes some bacterial strains resistant to antibiotics and many conventional disinfectants, while some bacterial strains survive by forming a complex biofilm matrix (Lu et al. 2021). Biofilm is a very important resistance mechanism for bacteria as it can help various bacteria to withstand antimicrobial agents and immunological defense systems as well (Lee et al. 2019). In addition, it is known that there is a positive relationship between biofilm formation and some bacteria that provide resistance to antibiotics since biofilm can create a suitable environment for horizontal gene transfer (Shrestha et al. 2019; Sobisch et al. 2019). For such reasons, it is important to search for more effective biofilm inhibitors including the use of plant origin. In our study, the effect of extracted phenol from WGH and its different concentrate and permeate samples on biofilm inhibition was tested against P. aeruginosa and S. aureus. Biofilm inhibition results are shown in Fig. 5. The extracted phenol from WGH, concentrate, and permeate samples showed higher biofilm inhibition on P. aeruginosa than on S. aureus. Concentrate samples also showed better biofilm inhibition properties than extracted phenol from WGH and all permeate samples. The biofilm inhibitions of 1, 2, 3, 4, 5, 6, 7, 8, and 9 for P. aeruginosa than on S. aureus were found as 58.07% and 62.51%, 47.05% and 48.97%, 68.53% and 75.25%, 40.75% and 44.67%, 63.35% and 64.98%, 37.19% and 41.08%, 82.86% and 85.80%, 19.43% and 27.07%, and 80.95% and 83.61%, respectively. The highest the rate of biofilm inhibition was achieved with 7 as 82.86% for S. aureus and 85.8% for P. aeruginosa. Dolatabadi et al. (2018) indicated that they investigated the evaluation of the anti-biofilm effect of Juglans regia L. leaf extracts on P. aeruginosa. The results of inhibition of biofilm formation by extracts indicated that the addition of Juglans regia L. extracts to microtiter plates successfully affected the biofilm formation of P. aeruginosa. Comparison of biofilm formation levels between various cultures in microtiter plates showed that biofilm formation for the experimental group (containing Juglans regia L. extract) was 60% less than for the control (no Juglans regia L. extract). Both aqueous and methanol extracts appeared to potently inhibit biofilm formation in a dose-dependent manner. The methanol extract was found to be more potent than the aqueous extract, and on the contrary, it reduced biofilm formation by 60% with an average of 16 mg/mL of 32 mg/mL (Dolatabadi, Moghadam). Alam et al. (2020) informed that they authenticated the biofilm inhibition of different extracts of selected medicinal plant species viz. Clematis viticella, Clematis grata and Berginia ciliate. They found that B. ciliata 1% of methanolic extract demonstrated more than 80% biofilm formation inhibition against P. aeruginosa. It can be concluded that extracted phenol of WGH, UF, NF, MF, and RO concentrate samples could be utilized as a powerful alternate to decrease the infection severity by biofilm inhibition in medical applications.
Fig. 5.
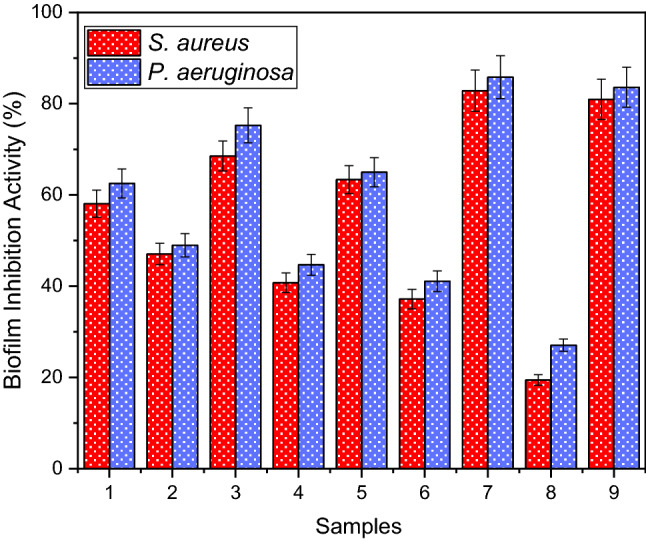
Biofilm inhibition extracted phenol from WGH and its different permeate and concentrate samples. (1: extracted phenol; 2: MF permeate; 3: MF concentrate 4: UF permeate; 5: UF concentrate 6: NF permeate; 7: NF concentrate; 8: RO permeate; 9: RO concentrate)
Conclusions
In this study, various biological activities of extracted phenol from WGH and its different concentrate and permeate samples such as antioxidant, DNA cleavage, antimicrobial, and biofilm inhibition were evaluated. As a result, it has been seen that WGH can be evaluated as a good source of antioxidants and antimicrobial agents. LC-MSMS results showed that gallic acid, vanilic acid, and cafeic acid were the main phenolic compounds in WGH extracts. While the antioxidant properties were found 100% in the concentrate samples, it was observed that the antioxidant activity decreased with the decrease in the phenol content in the permeate forms. The phenol content of MF permeate was 865 mg/L and the DPPH scavenging and metal chelating activity were 95.6% and 96.42%, respectively and the phenol amount of RO permeate was determined 70 mg/L, and the DPPH scavenging and metal chelating activity were 78.29% and 74.96%, respectively. It was found that except RO permeate of WGH extract, all test samples demonstrated perfect DNA cleavage activities. In line with other findings, concentrate samples also showed much better antimicrobial activity when compared with permeate samples. Likewise, it was found that concentrate samples displayed better anti-biofilm activity than permeate samples, and also test samples showed better biofilm inhibition activity on P. aeruginosa than on S. aureus.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- DPPH
2, 2-Diphenyl-1-picrylhydrazyl
- MF
Microfiltration
- MIC
Minimum inhibition concentration
- NF
Nanofiltration
- RO
Reverse osmosis
- UF
Ultrafiltration
- WGH
Walnut green husk
Authors contributions
HA and ND contributed to the study conception and design. Material preparation and analysis were performed by YO, SO, OC, and GT. YO and EOK wrote the manuscript with support from ND and SO, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ağırtaş M, Karataş C, Ozdemir S. Synthesis of some metallophthalocyanineswith dimethyl 5-(phenoxy)-isophthalate substituents and evaluation of theirantioxidant-antibacterial activities. Spectrochim Acta Part A Mol Biomolspectrosc. 2015;135:20–24. doi: 10.1016/j.saa.2014.06.139. [DOI] [PubMed] [Google Scholar]
- Agullo A, Pereira E, Freire M, Valentão P, Andrade P, Alvarez J, et al. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind Crops Products. 2013;42:126–132. doi: 10.1016/j.indcrop.2012.05.021. [DOI] [Google Scholar]
- Akinmoladun AC, Falaiye E, Ojo OB, Adeoti A, Amoo ZA, Olaleye MT. Effect of extraction technique, solvent polarity, and plant matrix on the antioxidant properties of Chrysophyllum albidum G Don. (African Star Apple) Bull Natl Res Centre. 2022 doi: 10.1186/s42269-022-00718-y. [DOI] [Google Scholar]
- Aminov R. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol. 2010;8(1):134. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend GD, Adorno WT, Rezzadori K, Di Luccio M, Chaves VC, Reginatto FH, Petrus JCC. Concentration of phenolic compounds from strawberry (Fragaria X ananassa Duch) juice by nanofiltration membrane. J Food Eng. 2017;201:36–41. doi: 10.1016/J.JFOODENG.2017.01.014. [DOI] [Google Scholar]
- Caballero E, Soto C, Jara J. Thermal stability data of juglone from extracts of walnut (Juglans regia) green husk, and technologies used to concentrate juglone. Data Brief. 2019;25:104081. doi: 10.1016/J.DIB.2019.104081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jerónimo C, Silva BM. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol. 2010;48(1):441–447. doi: 10.1016/J.FCT.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Chandramohan Y, Padmanaban V, Bethunaickan R, Tripathy S, Swaminathan S, Ranganathan UD (2019) In vitro interaction profiles of the new antitubercular drugs bedaquiline and delamanid with moxifloxacin against clinical Mycobacterium tuberculosis isolates. J Glob Antimicrob Resist 19:348–353. 10.1016/j.jgar.2019.06.013 [DOI] [PubMed]
- Che Sulaiman IS, Basri M, Fard Masoumi HR, Chee WJ, Ashari SE, Ismail M. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem Cent J. 2017;11(1):1–11. doi: 10.1186/s13065-017-0285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvishi E, Kahrizi D, Arkan E (2019) Comparison of different properties of zinc oxide nanoparticles synthesized by the green (using Juglans regia L. leaf extract) and chemical methods. J Mol Liq 286:110831. 10.1016/j.molliq.2019.04.108
- Dolatabadi S, Moghadam HN, Mahdavi-Ourtakand M (2018) Evaluating the anti -biofilm and antibacterial effects of Juglans regia L. extracts against clinical isolates of Pseudomonas aeruginosa. Microb Pathog 118:285–289. 10.1016/j.micpath.2018.03.055 [DOI] [PubMed]
- FAO (2020) Statistical Yearbook. Agricultural production. https://www.fao.org/home/en
- Fernández-Agulló A, Pereira E, Freire MS, Valentão P, Andrade PB, González-álvarez J, Pereira JA. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind Crops Products. 2013;42(1):126–132. doi: 10.1016/J.INDCROP.2012.05.021. [DOI] [Google Scholar]
- Goli AH, Barzegar M, Sahari MA. Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chem. 2005;92(3):521–525. doi: 10.1016/j.foodchem.2004.08.020. [DOI] [Google Scholar]
- Gomes F, Martins N, Barros L, Rodrigues ME, Oliveira M, Beatriz PP, Henriques M, Ferreira ICFR (2018) Plant phenolic extracts as an effective strategy to control Staphylococcus aureus the dairy industry pathogen. Ind Crops Prod 112:515–520. 10.1016/j.indcrop.2017.12.027
- Jahanban-Esfahlan A, Jahanban-Esfahlan R, Tabibiazar M, Roufegarinejad L, Amarowicz R. Recent advances in the use of walnut (Juglans regia L.) shell as a valuable plant-based bio-sorbent for the removal of hazardous materials. Res Adv. 2020;10:7026. doi: 10.1039/c9ra10084a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaitz L, Siegl K, Eder R, Rak G, Abranko L, Koellensperger G, Hann S (2010) LC–MS/MS analysis of phenols for classification of red wine according to geographic origin grape variety and vintage. Food Chem 122(1):366–372. 10.1016/j.foodchem.2010.02.053
- Kadiroğlu P, Ekici H. Yeşil ceviz kabuklarının biyoaktif özelliklerinin FT-IR spektroskopi yöntemiyle tahmin edilmesi. Akademik Gıda. 2018;16(1):20–26. doi: 10.24323/AKADEMIK-GIDA.415643. [DOI] [Google Scholar]
- Karimi N, Minaei S, Hasani D, Eyvani A. Parameters involved in walnut peeling process. J Agric Machinery Sci. 2008;4(4):389–393. [Google Scholar]
- Kavak DD, Altıok E, Bayraktar O, Ülkü S (2010) Pistacia terebinthus extract: As a potential antioxidant antimicrobial and possible β-glucuronidase inhibitor. J Mol Catal B Enzym 64(3–4):167–171. 10.1016/j.molcatb.2010.01.029
- Kong X, Bao S, Song W, Hua Y, Zhang C, Chen Y, Li X (2021) Contributions of ethanol fractionation on the properties of vegetable protein hydrolysates and differences in the characteristics of metal (Ca Zn Fe)-chelating peptides. LWT 146:111482. 10.1016/j.lwt.2021.111482
- Lee JH, Kim YG, Khadke SK, Yamano A, Woo JT, Lee J (2019) Antimicrobial and antibiofilm activities of prenylated flavanones from Macaranga tanarius. Phytomedicine 63:153033. 10.1016/j.phymed.2019.153033 [DOI] [PubMed]
- Li X, Deng S. Synergistic inhibition effect of walnut green husk extract and potassium iodide on the corrosion of cold rolled steel in trichloroacetic acid solution. J Market Res. 2020;9(6):15604–15620. doi: 10.1016/J.JMRT.2020.11.018. [DOI] [Google Scholar]
- Lu C, Liu H, Shangguan W, Chen S, Zhong Q (2021) Antibiofilm activities of the cinnamon extract against Vibrio parahaemolyticus and Escherichia coli. Arch Microbiol 203(1):125–135. 10.1007/s00203-020-02008-5 [DOI] [PubMed]
- Lv Y, Wei K, Meng X, Huang Y, Zhang T, Li Z (2017) Separation and identification of iron-chelating peptides from defatted walnut flake by nanoLC-ESI–MS/MS and de novo sequencing. Process Biochem 59:223–228. 10.1016/j.procbio.2017.05.010
- Machado MTC, Mello BCBS, Hubinger MD. Evaluation of pequi (Caryocar Brasiliense Camb.) aqueous extract quality processed by membranes. Food Bioprod Process. 2015;95:304–312. doi: 10.1016/J.FBP.2014.10.013. [DOI] [Google Scholar]
- Mehdizadeh T, Zamani A, Froushani SMA (2020) Preparation of Cu nanoparticles fixed on cellulosic walnut shell material and investigation of its antibacterial antioxidant and anticancer effects. Heliyon 6(3):e03528. 10.1016/j.heliyon.2020.e03528 [DOI] [PMC free article] [PubMed]
- Mokrani A, Madani K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep Purif Technol. 2016;162:68–76. doi: 10.1016/J.SEPPUR.2016.01.043. [DOI] [Google Scholar]
- Naczk M, Shaidi F. Extraction and analysis of phenolics in food. J Chromatogr A. 2004;1504:95–111. doi: 10.1016/S0021-9673(04)01409-8. [DOI] [PubMed] [Google Scholar]
- Nile SH, Nile A, Liu J, Kim DH, Kai G (2019) Exploitation of apple pomace towards extraction of triterpenic acids antioxidant potential cytotoxic effects and inhibition of clinically important enzymes. Food Chem Toxicol 131:110563. 10.1016/j.fct.2019.110563 [DOI] [PubMed]
- Oliveira I, Sousa A, Ferreira ICFR, Bento A, Estevinho L, Pereira JA. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol. 2008;46(7):2326–2331. doi: 10.1016/J.FCT.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Ozay Y, Ozdemir S, Gonca S, Canli O, Dizge N. Phenolic compounds recovery from pistachio hull using pressure-driven membrane process and a cleaner production of biopesticide. Environ Technol Innov. 2021;24:101993. doi: 10.1016/J.ETI.2021.101993. [DOI] [Google Scholar]
- Pinelo M, Rubilar M, Jerez M, Sineiro J, Núñez MJ. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J Agric Food Chem. 2005;53(6):2111–2117. doi: 10.1021/jf0488110. [DOI] [PubMed] [Google Scholar]
- Ramezani N, Raji F, Rezakazemi M, Younas M. Juglone extraction from walnut (Juglans regia L.) green husk by supercritical CO2: Process optimization using Taguchi method. J Environ Chem Eng. 2020;8(3):103776. doi: 10.1016/J.JECE.2020.103776. [DOI] [Google Scholar]
- Shrestha R, Khanal S, Poudel P, Khadayat K, Ghaju S, Bhandari A, Lekhak S, Pant ND, Sharma M, Marasini BP (2019) Extended spectrum β-lactamase producing uropathogenic Escherichia coli and the correlation of biofilm with antibiotics resistance in Nepal. Ann Clin Microbiol Antimicrob 18(1):42. 10.1186/s12941-019-0340-y [DOI] [PMC free article] [PubMed]
- Silva EM, Souza JNS, Rogez H, Rees JF, Larondelle Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2007;101(3):1012–1018. doi: 10.1016/J.FOODCHEM.2006.02.055. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Sobisch LY, Rogowski KM, Fuchs J, Schmieder W, Vaishampayan A, Oles P, Novikova N, Grohmann E (2019) Biofilm Forming Antibiotic Resistant Gram-Positive Pathogens Isolated From Surfaces on the International Space Station. Front Microbiol 10:543. 10.3389/fmicb.2019.00543 [DOI] [PMC free article] [PubMed]
- Zakavi F, Hagh L, Daraeighadikolaei A, Farajzadeh Sheikh A, Leilavi Shooshtari Z (2013)Antibacterial effectof Juglans regiabark against oral pathologic bacteria. Int J Dent [DOI] [PMC free article] [PubMed]
- Zarei M, Ghanbari R, Tajabadi N, Abdul-Hamid A, Bakar F. Generation, fractionation, and characterization of iron-chelating protein hydrolysate from palm kernel cake proteins. J Food Sci. 2016;81:C341–C347. doi: 10.1111/1750-3841.13200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


