Abstract
Low ferrous iron bioavailability presents a challenge for food fortification programmes. In this study, jelly foods were fortified with spray-dried chitosan microparticles that had been loaded with ferrous gluconate (FeG) and folic acid (FA) to alleviate iron deficiency anaemia and FA deficiency anaemia, respectively. The presence of FA and ascorbic acid (AA) increased the in vitro iron bioavailability of the FeG-AA-FA microparticles up to sixfold. Furthermore, the iron bioavailability of the fortified jelly foods increased more than 5 folds compared to that of the FeG–AA–FA microparticles. The use of lower temperature during the preparation of fortified jelly foods is recommended to avoid the microparticles’ decomposition and a Maillard browning reaction. These findings can help food technologists and product developers select formulations with higher ferrous bioavailability to reduce the prevalence of anaemia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05599-7.
Keywords: Ferrous gluconate, Ascorbic acid, Folic acid, Microencapsulation, Fortification
Introduction
Currently, anaemia constitutes one of the greatest nutritional public health problems in the world (Barragán-Ibañez et al., 2016). The condition’s major cause is iron deficiency, which entails many negative consequences, particularly among women and children (Akhtar et al., 2011; Barragán-Ibañez et al., 2016). Iron deficiency’s prevalence remains high in poor and developing countries (Akhtar et al., 2011). Anaemia and iron deficiency can also cause serious economic problems that interfere with national development. Given these problems, the World Health Organization included iron deficiency among its six global nutrition targets for 2025 to reduce the prevalence of anaemia among reproductive-age women (Blanco-Rojo and Vaquero, 2018). Food fortification is the most common, cost-effective, sustainable and efficacious strategy to prevent micronutrient malnutrition, particularly iron deficiency (Akhtar et al., 2011).
Iron’s poor bioavailability might result from the presence of divalent metal competitors, the transformation of ferrous iron into ferric iron and iron’s interaction with inhibitors (casein, phytate and tannin), reducing the gastrointestinal tract’s iron absorption (Handayani et al., 2020). Moreover, previous studies have reported that anaemia is also linked to folic acid (FA) deficiencies and that a feasible solution is to combine ferrous gluconate (FeG) and FA to eliminate iron and folic acid deficiency anaemia among adolescent girls (Oy et al., 2019). Ascorbic acid (AA) has also been used as an iron absorption enhancer and stabiliser (Blanco-Rojo and Vaquero, 2018). The use of multiple micronutrient fortifications has been shown to be effective (Tripathi and Platel, 2011).
Iron, AA, and FA are unstable in certain environments (under certain UV light, temperatures, pH levels and oxygen levels), and they should be protected via encapsulation before the fortification process (Gupta et al., 2015). Our previous study indicated that spray-drying is a preferable microencapsulation method to minimise the oxidation of FeG (Handayani et al., 2020). In this study, FeG, AA and FA were encapsulated in spray-dried chitosan-tripolyphosphate (TPP) microparticles. Chitosan and TPP were used as encapsulating and cross-linking agents, respectively, due to their excellent biodegradable, biocompatible and non-toxic properties (Webber et al., 2018). Then, the spray-dried microparticles were dispersed into jelly foods since these foods are a favourite among various groups of people and, therefore, an ideal vehicle (Morita, 2003). The ingredients jelly foods should be selected to ensure that no components inhibit iron absorption and – if possible – to improve iron’s bioavailability, making such foods an appropriate vehicle for iron fortification.
Some previous studies have researched iron microencapsulation using various methods (Gupta et al., 2015; Gutiérrez et al., 2016; Mulia et al., 2019). The encapsulation of AA and FA has also been examined in previous works (Desai and Park, 2005; Pérez-Masiá et al., 2015; Zhong et al., 2019). However, few studies have examined the triple-loaded encapsulation of FeG-AA-FA and its application in fortifying jelly foods. Therefore, the current study aimed to produce FeG-AA-FA microparticles as intermediate products and fortified jelly foods as final products. The spray-dried microparticles’ and fortified jelly foods’ physicochemical properties, iron-release profiles and in vitro ferrous bioavailability were also examined. The results of this study could help food technologists and product developers produce fortified jelly foods with higher iron bioavailability.
Material and methods
Material
The main materials used in this study, FeG, AA, FA, and chitosan were provided by Sigma Aldrich, Merck, Dalian Chemical & Export Group Co. Ltd., China, and CV. Chimultiguna Cirebon-Indonesia, respectively. The acetic acid, TPP and chemicals used for simulated gastrointestinal fluid and iron analysis were purchased from Merck. The ingredients for this study’s Jelly foods, such as gelatine (synthesised from cow bone with a bloom strength of 150) and low-calorie sweeteners (crystal corn sugar with a glycemic index of 22), were supplied by Global Capsule Ltd. Bangladesh and PT. Padhita-Jakarta, respectively.
Preparation of chitosan-TPP microparticles
A feed solution was prepared by mixing FeG (1 gr), AA and FA into acetic acid solution of 2.5% (v/v) 400 mL. The amounts of AA and FA were adjusted with the various ratio of Fe:AA:FA (1:0:0; 1:0.5:0; 1:1.5:0; 1:1:0; 1:1.5:0; 1:2:0; 1:0.5:0.25; 1:0.5:0.5; 1:0.5:0.75; 1:0.5:1; 1:2:0.25; 1:2:0.5; 1:2:0.75; 1:2:1). Chitosan of 0.5% (w/v) was added to this mixture and stirred at 1,000 rpm for 10 min. The cross-linking agent, TPP 1% (w/v), was added drop by drop and stirred using an IKA T18 Ultraturax at 15,000 rpm for 10 min. The amount of TPP was 5% of the mixture’s total volume. In this study, the total volume for each experiment was 400 ml. The resulting solution was fed into a spray-dryer (Mini Buchi B-290) at an inlet temperature of 160 °C, a vacuum pressure of 100% and a pump flow of 10%. Feed solutions with varying ratios of Fe:AA:FA were also formulated. The yield of dried microparticles was calculated using Eq. 1 (Webber et al., 2018).
| 1 |
Iron fortification of jelly foods
The fortified jelly foods (GM) were made by mixing gelatine into 50 mL of cool water to bloom. The compositions of gelatine used were 10%, 20%, 30% and 40% (w/v). Low-calorie sweeteners (10% w/v) and the already swelled gelatine were then heated until a higher viscosity was achieved. Next, spray-dried microparticles (400 mg) were spread out on the cooled jelly solutions. The resultant solution was moulded, cooled and prepared for analysis. Further, the unfortified jelly foods (G) were also prepared using a slightly modified process. The FeG-AA-FA microparticles were not added to the jelly-based solutions.
Determination of total iron, Encapsulation Efficiency (EE) and iron loading
Dried microparticles of 5 mg were dissolved into 25 mL of 2.5% (v/v) acetic acid. This solution was heated at 80 °C and stirred at 250 rpm for 2 h. Next, the solution was passed through Whatman filter paper. The solution’s total iron content was determined using the phenanthroline method reported in previous research, with some modifications (Gutiérrez et al., 2016). The encapsulation efficiency (EE) and iron loading values were defined as total iron (Fe2+ and Fe3+); thus, hydroxylamine hydrochloride was added to reduce the Fe3+ into Fe2+. The solution’s absorbance was recorded using a Thermo Scientific Genesys 10 S UV–Vis spectrophotometer at 510 nm. The solution’s EE and iron loading values were determined using Eqs. 2 and 3, respectively (Krisanti et al., 2019).
| 2 |
| 3 |
Determination of water content
Water content analysis was conducted using the oven drying method at 135 °C for 2 h (AOAC 2005; method 930.15). An oven (Red Line Binder 53) was used to analyse. The water content of microparticles were determined using Eq. 4.
| 4 |
Field-emission scanning electron microscopy and transmission electron microscopy
The spray-dried microparticles’ morphology was assessed using both field-emission scanning electron microscopy (FESEM; FEI Inspect F50 at 20.0 kV of excitation voltage) and transmission electron microscopy (TEM; TECNAITM G2 Spirit Twin).
Particle size analysis
The dried microparticles’ size distribution was measured using a laser particle sizer LLPA-C10. The average particle size was shown as a volume-weighted mean. Moreover, the particle size distribution was determined using the SPAN factor, which was calculated using Eq. 5 (Jiang et al., 2017).
| 5 |
where D10, D50 and D90 are the particle sizes in a given percentage of particles smaller than a specific size.
Colour analysis
The spray-dried microparticles’ colour characteristics were determined using a digital colorimeter (Toyo Seiki Seisaku-sho Ltd., Tokyo Japan) that set up to a D65 light source and a 10 degrees angle. The sensor diameter was 20 mm in diameter and the analysis was conducted in three different positions of each sample. The values are expressed in means ± standard deviation of three replicates. These colour parameters were expressed using the values a, b and L, which indicated redness or greenness, yellowness or blueness, and lightness, respectively (Zhong et al., 2019). Differences in lightness (ΔL*) and colour (ΔE*) were determined using Eqs. 6 and 7, respectively (Habeych et al., 2016).
| 6 |
| 7 |
FTIR analysis
Thermo Scientific (Diamond Nicolet IS 5) FTIR apparatus was used to obtain the IR spectra of chitosan that had been double loaded with FeG and AA. The spectra were recorded at 500–4,000 cm−1 at a resolution of 32 cm−1 by 64 scans.
Thermogravimetry analysis
The thermogravimetric curves of FeG, AA, FA, and FeG–AA–FA microparticles in varying ratio of Fe:AA:FA were provided by using a Shimadzu DTG-60AH tool. In this study, a 10 mg of sample was used. The range temperature of 23–800 °C, with a heating rate of 10 °C min−1 under 50 mL min−1 of nitrogen’s flow were applied to observe the change in weight of sample.
Protein analysis
The protein content of this study’s jelly foods was determined using Kjedahl method (SNI 01 2354 42,006) by calculating the products' total nitrogen (N). Protein content can be calculated using Eq. 8.
| 8 |
where W is weight (g) of sample, a is volume (ml) of 0.1 N H2SO4 used in blank titration, b is volume (ml) of 0.1 N H2SO4 used in sample titration, 14 and 6.25 represent atomic weight of nitrogen and the protein-nitrogen conversion factor, respectively.
Texture analysis
A texture analyser (the CT3 Texture analyser by Brookfield) was used to determine the jelly foods textural properties (hardness, cohesiveness, springiness, and gumminess). The jelly foods samples were compacted using cylinder probe with the diameter of a 30 mm. The analysis was conducted at a 7 mm target value and 1 mm/s of test speed.
In vitro iron release
An iron-release study was conducted using simulated gastric fluid (SGF pH 1.2) and simulated intestinal fluid (SIF pH 7.4). The temperature during this analysis was set and controlled to 37 °C, according to body temperature in common. The ratio of Fe:AA = 1:0.0 is also used as controlled variable, in this experiment. Dried microparticles (20 mg) and this study’s jelly foods were dispersed into 100 mL of SGF solution for 2 h. An SIF solution (100 mL) was added to this mixture, and iron release was assessed for the next 6 h. Samples were taken and passed through a 0.45 µm filter (Aijiren nylon filter syringe) at a certain time. When these samples were collected, fresh SGF and SIF were added to the solution. The samples were analysed using a UV–vis spectrophotometer at 510 nm to calculate their cumulative total iron release as presented in Eq. 9.
| 9 |
In vitro iron bioavailability
The invitro iron bioavailability was analysed using the simulated saliva-gastro-intestinal fluid described in previous studies, with slight modifications (Gupta et al., 2015; Singh and Prasad, 2018). Iron’s bioavailability can be determined by its solubility, and only soluble iron can be absorbed (Kumar et al., 2020). Therefore, the iron bioavailability of microparticles and jelly foods was determined in terms of their soluble iron (ferrous iron). The enzymes’ composition and concentration in the saliva-gastrointestinal fluid were carefully matched to the value reported by Gupta et al. (2015), and the final execution was conducted per Singh and Prasad (2018) description. The saliva solution (10 mL, pH 6.5) and α-amylase (700 mg/L of saliva solution) were added to each flask containing a sample, which was incubated at 37 °C and 100 rpm for five min. Then, gastric fluid (15 mL) with pepsin (2 g/L of gastric fluid) from a porcine stomach was added to each flask. The samples’ pH was adjusted to 1.2 via HCl and incubated at 37 °C and 100 rpm for 1 h. Intestinal fluid (25 mL) with porcine pancreatin (6 g/L of intestinal fluid) was then added and incubated at 37 °C and 100 rpm for 3 h after the pH (7.4) was neutralised. The resultant solutions were centrifuged at 3,500 rpm for 60 min. A supernatant was collected to determine ferrous bioavailability using the phenanthroline method. In this analysis, a reducing agent (hydroxylamine hydrochloride) was not added into these solutions since only ferrous iron (soluble iron) was to be determined. The solutions’ ferrous bioavailability was calculated using Eq. 10 (Singh and Prasad, 2018).
| 10 |
Statistical analysis
The data was presented by means ± standard deviation of three replicates. The statistical analysis used one-way analysis of variance (one-way ANOVA) with Dunnett's multiple comparison test and p < 0.05 was approved as significant.
Results and discussion
Preparation of chitosan-TPP microparticles loaded with FeG, AA and FA
Yield, water content, EE, and iron loading
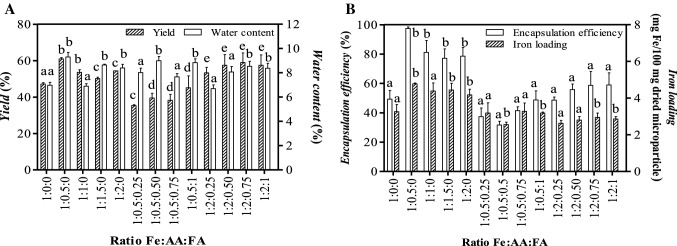
The characteristics of the chitosan-TPP spray-dried microparticles loaded with FeG-AA-FA (yield, water content, EE, and iron loading) are shown in Fig. 1A, B. The microparticles’ yields were in the range of 46.4–65.9%. This narrow range occurred because the same proportion of chitosan was used across the formulations. Our results were similar to those of Desai and Park (2005) and Jiang et al. (2017) but higher than the results of Mulia et al. (2019). The low yields of the chitosan-TPP microparticles in the current study could indicate highly adhesive particles that adhered to the wall of the spray-dryer (Jiang et al., 2017).
Fig. 1.
Characteristics of FeG–AA–FA microparticles prepared by a spray dryer, as A yield–water content and B EE–iron loading. The values are means ± standard deviation of three replicates. a−e Samples represented with different letters are significantly different (P < 0.05)
The microparticles in the current study had a water content of 6.7–9.3%, which was consistent with a previous report on chitosan-based spray-dried mucoadhesive microparticles (Jiang et al., 2017). Under our experiment’s spray-dryer conditions, water content did not vary much. It depends on a spray-dryer’s operational conditions, such as its inlet temperature and compressed air flow (Desai and Park, 2005).
The result shows that the EE of the current study’s dried microparticles was 36.0–98.9%. This result indicates that the 0.5% (w/v) chitosan used in this study sufficiently entrapped FeG. The amount of iron encapsulated in the FeG-AA microparticles exceeded the corresponding amounts of FeG and FeG-AA-FA microparticles (Fig. 1B). Thus, FeG, AA and FA could have competed due to their different solubilities in water. The system consisted of 2.5% (v/v) acetic acid and was mostly water. FeG, AA and FA’s solubilities in water were 36.6 g/L, 330 g/L and 1.6 mg/L, respectively. Chitosan entrapped FA and FeG more easily than AA because of its lower solubility. This result is consistent with a previous report (Desai & Park, 2005a) and higher than some other studies’ results (Mulia et al., 2019; Webber et al., 2018).
Figure 1B shows that 2.5–4.8 mg of iron was contained in 100 mg of spray-dried microparticles. These values exceeded the corresponding values in previous research (Krisanti et al., 2019; Mulia et al., 2019). Figure 1B depicts that the presence of AA and FeG can enhance iron loading, whereas the presence of AA, FA and FeG can reduce iron loading. This difference is probably due to the number of iron encapsulated and FeG, AA and FA’s different solubilities, as described above. Furthermore, the highest EE and iron loading values were obtained by chitosan microparticles loaded with FeG-AA at an Fe:AA ratio of 1:0.5.
Field-emission scanning and transmission electron microscopy analysis
The morphology and shape of this study’s chitosan-TPP microparticles loaded with FeG, AA and FA are illustrated in Fig. 2A–I. Microparticles are generally spherical, with buckle structures. SEM analysis showed a wrinkled surface for the FeG and FA microparticles (Fig. 2A and C) and a smooth surface for the AA microparticles (Fig. 2B). These wrinkled surfaces could have occurred due to the use of a low chitosan concentration (0.5% w/v) in this study (Desai & Park, 2005a). A previous study reported that the use of 1% w/v chitosan and 5–10 ml of 1% w/v TPP could induce a smoother surface (Desai and Park, 2005). The FeG-AA and FeG-AA-FA microparticles (Fig. 2D–I) had smoother surfaces than the FeG and FA microparticles and more wrinkled surfaces than the AA microparticles indicated that the increase in AA and FA generated a smoother surface for spray-dried microparticles. The observed differences in surface morphology could also have been affected by the type of core materials encapsulated. This result aligned with the results of previous studies on chitosan-TPP microparticles that had been loaded with FeG (Mulia et al., 2019), AA (Desai and Park, 2005), FA (Pérez-Masiá et al., 2015), and other active compounds (Webber et al., 2018).
Fig. 2.
SEM micrograph of chitosan–TPP microparticles in varying mass ratios of Fe:AA:FA A 1:0:0, B 0:1:0, C 0:0:1, D 1:0.5:0, E 1:1:0, F 1:0.5:0.25, G 1:0.5:1, H 1:2:0.5, I 1:2:1, and TEM micrograph of chitosan–TPP microparticles of varying mass ratios of Fe:AA:FA J 1:0:0, K 1:0.5:0 and L 1:0.5:1
The TEM micrographs of the current study’s FeG, FeG-AA and FeG-AA-FA microparticles are presented in Fig. 2J–L. Both micrographs showed that FeG was well encapsulated and contained the chitosan-TPP microparticles. The black dots in Fig. 2J–L indicate the presence of FeG; however, the number of black dots in the FeG-AA-FA microparticles (Fig. 2L) was less than the corresponding number in the FeG microparticles (Fig. 2J) and FeG-AA microparticles (Fig. 2L). This difference could correlate with the FeG-AA-FA microparticles’ lower iron loading, as Fig. 1B shows.
Particle size distribution
The microparticles’ size distributions are shown in Supp. File 1. The mean diameters of the FeG–AA–FA microparticles in varying ratios of Fe:AA:FA (1:0:0, 1:0.5:0, 1:0.5:0.25, 1:0.5:1, 1:2:0.25 and 1:2:1) stood at 17.7, 19.8, 20.5, 42.5, 25.6 and 29.6 µm, respectively. As Fig. 2 shows, the addition of AA, FA and FeG as core materials increased the microparticles’ mean diameters. The diameter of the FeG-AA and FeG-AA-FA microparticles seemed to exceed the diameter of the FeG microparticles at the same magnification. Furthermore, these observed differences were smaller than the results obtained by Liu et al. (2011) and greater than the results obtained by Jiang et al. (2017). The SPAN factors of the FeG–AA–FA microparticles in varying Fe:AA:FA ratios (1:0:0, 1:0.5:0, 1:0.5:0.25, 1:0.5:1, 1:2:0.25 and 1:2:1) stood at 1.3, 1.3, 1.6, 3.0, 2.2 and 2.3, respectively. Thus, spray-dried microparticles seemed to have a narrow particle size distribution.
Colour
The colour changes in this study’s spray-dried microparticles were analysed at varying ratios of Fe:AA:FA (Supp. File 2). FeG spray-dried microparticles are generally greenish white, which indicates that the microencapsulation method may minimise the iron auto-oxidation that can occur when iron is added directly to food (Habeych et al., 2016). The double-loaded FeG-AA microparticles expressed colour changes from greenish-yellowish white to brown. Moreover, an increased AA ratio generated a dark brown colour for spray-dried microparticles. Thus, the microparticles with a Fe:AA:FA ratio of 1:2:0 achieved the darkest brown colour. This result also correlated with decreases in ΔL* (Supp. File 2A). The ΔE* values increased as the microparticles’ obvious colour differences were shown in AA’s presence. This result could have been caused by the Maillard browning reaction, as reported previously (Umemura and Kawai, 2007).
However, FeG–AA–FA microparticles in varying Fe:AA:FA ratios (1:0.5:0.25, 1:0.5:0.50, 1:0.5:0.75 and 1:0.5:1.0) expressed colour changes from light brown to yellowish-brown. Since FA is yellow, a higher amount of FA would lighten microparticles’ colour. This change was indicated by an increase in ΔL* and ΔE* values, as shown in Figure Supp. File 2B. However, the microparticles with varying Fe:AA:FA ratios (1:2:0.25, 1:2:0.50, 1:2:0.75 and 1:2:1.0) darkened more than the FeG-AA microparticles. Thus, ΔL* values decreased and ΔE* values increased (Figure Supp. File 2C). The addition of FA did not cause significant colour changes since the FA amount was less than the amount of AA. Therefore, AA’s presence may play a significant role in producing a darker colour through the Maillard browning reaction.
FTIR spectra
The FTIR spectra of FeG–AA microparticles at varying Fe:AA ratios are shown in Fig. 3A. The chitosan spectrum was observed at 1,558 cm−1, corresponding with N–H bending (Kulig et al., 2016). The recorded peaks for AA’s functional groups were shown, including CH2 stretching (1,396 cm−1) and C–O–C stretching (1,056 cm−1). Furthermore, AA’s presence in the microparticles decreased in the N–H peak and C–O–C peak, potentially due to a certain reaction consuming both chitosan and AA. This result aligns well with previous research (Zhong et al., 2019). Figure 3A also presents the functional groups of AA, including C–O–C stretching (1,077 cm−1), C=C stretching (1,675 cm-1) and C=O stretching (1,750 cm−1) (Kulig et al., 2016; Zhong et al., 2019). This result shows that AA’s C=O peak disappeared in the FeG-AA microparticles, again demonstrating a particular reaction involving AA (Zhong et al., 2019).
Fig. 3.
A FTIR spectra of AA and chitosan–TPP microparticle loaded with FeG–AA and B the possible mechanism of Maillard reaction in chitosan—TPP microparticles double loaded with FeG and AA
Meanwhile, Fig. 3B shows the possible mechanism of the Maillard reaction. Some parts of these mechanisms have been reported in a prior study (Zhong et al., 2019). AA can ionise into ascorbate ions, initiating the formation of dehydroascorbic acid (DHA). Ascorbate ions are also excellent reducing agents, converting ferric iron into ferrous iron (Blanco-Rojo and Vaquero, 2018). Moreover, DHA is the oxidised form of AA that can act as an anti-cancer agent (Toohey, 2008). Both AA and DHA are notable compounds in many dietary components (Deutsch, 2000). However, DHA is more unstable and reactive in solution than AA; thus DHA can be converted into degradation products (Deutsch, 2000; Zhong, Tan, & Langrish, 2019). The amine groups (–NH2) of chitosan may react with the carbonyl groups (C=O) of AA’s product, inducing the Maillard browning reaction.
Thermal stability
Thermo-gravimetry analysis (TGA) was conducted to investigate microparticles’ thermal stability loaded with FeG, AA and FA (Supp. File 3). Generally, all microparticles have similar patterns of thermosgram profiles up to 200 °C (Supp. File 3) This tendency may be due to the same concentration of chitosan (0.5% w/v) used. However, the FeG-AA-FA microparticles at a Fe:AA:FA ratio of 1:0.5:0.5 showed a different pattern at over 200 °C. The chitosan-TPP microparticles underwent the first stage of chitosan decomposition at 100 °C because of their initial water reduction of 6.4–10.9%. Supp. File 3 also shows a weight loss of 36.9–51.2% at 100–280 °C. Further weight loss of 11.9–14.7% occurred at 280–380 °C due to the chitosan’s deacetylation and depolymerisation (Webber et al., 2018). These results align well with a previous study that reported chitosan initially decomposes due to water reduction of up to 10% at 100 °C, undergoing weight loss of 35% at 100–280 °C and deacetylation-depolymerisation at 280–380 °C (Webber et al., 2018).
Chitosan-TPP microparticles loaded with FeG, AA and FA are intermediate products added to food through a fortification process. Therefore, TGA analysis is important to determine the appropriate fortification method. Following a thermal stability profile, microparticles should be added to an already cooled system (under 100 °C) in order to prevent microparticles’ initial decomposition.
Iron release and ferrous bioavailability profile
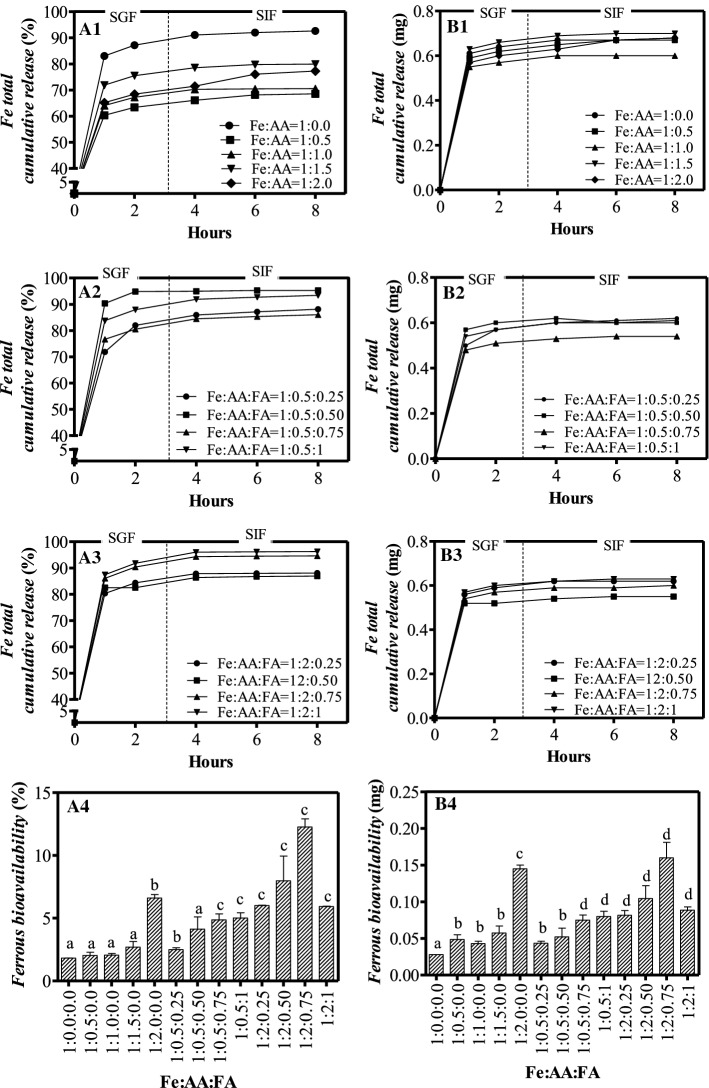
Figure 4A shows the total cumulative iron-release profile of the FeG–AA–FA microparticles at varying Fe:AA:FA ratios. All formulations showed a rapid iron release during the first 2 h, and approximately 63.4–87.2% of iron was released in the SGF solution. However, the chitosan-TPP cross-link in this study could extend iron release compared to the findings of previous studies (Jiang et al., 2017).
Fig. 4.
A1-A3 Fe total cumulative release (%), B1-B3 Fe total actual release (mg), A4 Ferrous bioavailability (%) and B4 Ferrous bioavailability (mg) of chitosan–TPP microparticles in varying ratios of Fe:AA:FA. The values of ferrous bioavailability are means ± standard deviation of three replicates and.a−d samples represented with different letters are significantly different (P < 0.05)
Although Fig. 4A1–A3 show different iron-release percentages, the actual amount of iron released (mg) was insignificant across all variations (Fig. 4B1–B3). This insignificance probably resulted from the same composition of chitosan used across the study’s encapsulations. The actual number of iron released from the FeG-AA-FA microparticles was less than the corresponding number from the FeG and FeG-AA microparticles. It may have been due to the FeG-AA-FA microparticles’ lower iron loading.
Ferrous bioavailability values are shown in Fig. 4A4 and B4. The ferrous bioavailability of the FeG-AA and FeG-AA-FA microparticles exceeded the corresponding bioavailability of the FeG microparticles due to AA and FA’s role as enhancers. The addition of AA and FA could lead to a twofold to fourfold increase in ferrous bioavailability (Fig. 4A4 and B4). The microparticles that obtained the highest iron bioavailability had a Fe:AA:FA ratio of 1:2:0.75. In this study, AA played important roles through two possible mechanisms: (i) reducing ferric iron to ferrous iron (Fig. 4B) and (ii) interacting with FeG to form other complex compounds (ferrous ascorbate). Both products are more soluble in gastrointestinal fluids, enhancing their iron bioavailability (Blanco-Rojo and Vaquero, 2018). Furthermore, iron tends to remain ferrous in more acidic solutions. In the current study, FA’s addition may have led solutions to become more acidic, such that their iron tended to be in ferrous form. This result is consistent with the findings of previous studies, which reported that the inclusion of 10 mg of AA could increase green gram’s iron bioavailability from 1.8 to 6.5% and wholemeal bread’s iron bioavailability from 2.4 to 8.8% (Singh and Prasad, 2018). Another study reported that the addition of FA to flour at a level of 140 µg/100 g could increase iron absorption from 0.3 mg/100 mg of flour to 0.4 mg/100 mg of flour (Tripathi and Platel, 2011).
Fortification of iron microparticles in jelly foods
FeG-AA-FA microparticles at a Fe:AA:FA ratio of 1:2:0.75 were used as fortifiers since this composition offers optimal iron bioavailability values. The microparticles were added to an already cooled (± 50 °C) gelatine solution in order to prevent their initial decomposition at 100 °C (Supp. File 3). This Jelly foods were produced by moulding and cooling a jelly solution.
Nutrition facts
Fortified jelly-based foods of varying gelatine concentrations were produced, and the average weight of one jelly foods was 4.40 g. The jellys’ nutritional protein was evaluated to determine their iron and protein content. Their iron levels at gelatine compositions of 10%, 20%, 30% and 40% (w/v) were 25.8, 25.8, 21.2 and 20.8 mg Fe/100 g of jelly foods, respectively. These results show that iron levels lowered because a higher amount of gelatine can increase such jelly foods’ total weight. The recommended daily intake of iron is 18–20 mg/day (Barragán-Ibañez et al., 2016). Generally, people’s regular dietary habits include consuming iron – for example, in nuts, spinach, broccoli and meat. Therefore, people generally require an additional iron intake of 0.9–1.1 mg, for which they can consume one piece of jelly-based foods per day. This study also calculated our jelly’s AA and FA content. These values were 41.68–51.58 mg and 15.63–19.34 mg per 100 g of fortified jelly-based foods, respectively.
The jelly foods’ protein levels were measured by calculating protein through total nitrogen (N). The jellies’ protein content was 7.4%, 14.8%, 21.3% and 24.4% for gelatine compositions of 10%, 20%, 30% and 40%, respectively. Accordingly, a higher gelatine composition could increase protein levels through higher N. The presence of N from chitosan may also be detected as protein.
Scanning electron microscope analysis
Figure 5 shows that variations in gelatine concentrations induced appearance differences between this study’s fortified jelly foods. A clear yellow colour was observed in these jelly foods using 10% (w/v) gelatine, clearly indicating microparticles’ presence. The more gelatine was used, the more a jelly-based foods would turn a cloudy yellow colour; thus, in these cases, the added microparticles could not be seen clearly.
Fig. 5.
The appearance and SEM of unfortified jelly foods (G) and fortified jelly foods (GM) in varying concentrations of gelatine
Figure 5 also presents the microstructures of this study’s unfortified and fortified jelly foods. This SEM image showed that the lower-gelatine-concentration, unfortified jelly foods (G-10%, G-20%) had thin, homogenous surfaces. Meanwhile, the fortified jelly foods (GM-10%, GM-20%) had protruding surfaces due to the presence of FeG-AA-FA microparticles. In contrast, the higher-gelatine-concentration, unfortified jelly foods (G-30%, G-40%) had a very dense and rough structure while the fortified jelly foods (GM-30%, G-40%) had flatter surfaces than the other formulas (GM-10%, GM-20%). The presence of FeG-AA-FA microparticles could not be observed well due to the thick, dense surface of the jelly foods.
Texture analysis
The texture analysis profiles of our fortified jelly foods were investigated at various gelatine concentrations. The hardness values of GM-10%, GM-20%, GM-25% and GM-40% were 42.4, 132.0, 188.8 and 268.0 g, respectively. Meanwhile, the gumminess values of GM-10%, GM-20%, GM-30% and GM-40% were 39.4, 128.0, 183.1 and 259.9 g, respectively. These results are caused by the higher gelatine concentrations used. In this study, gelatine was utilized as a gelling agent, and higher amounts of gelatine may produce higher-viscosity jelly solutions, giving jelly foods a harder texture after cooling. These findings are similar to the results of a previous study which reported that the addition of gelatine could enhance gel strength (Otálora et al., 2019). The use of gelatine 10% provided the softest texture of jelly food which might be liked by consumers; however, this texture caused the jelly food difficult to package. In contrast, the composition of 40% gelatine gave the hardest texture which might not be like by consumers; but this texture led the jelly food easy to package. Therefore, the use of 20–30% gelatine might meet the consumers taste and easy for packaging process. Moreover, this study’s jelly foods exhibited no differences in cohesiveness (0.9–1.0) or springiness (4.9 mm) properties despite increased gelatine amounts.
Iron release and iron bioavailability profiles
In the jelly-based food production, jelly foods solutions comprised a gelatine solution and chitosan microparticles with pH values of 4.7–4.9. In this pH range, gelatine and chitosan are negatively and positively charged, respectively, such that a polyelectrolyte complex (PEC) can be formed (Voron’ko et al., 2016). The PEC might significantly influence the release of iron in the gastrointestinal fluid.
Figure 6A shows the total cumulative iron releases of chitosan-TPP microparticles and this study’s fortified jelly foods. The FeG-AA-FA microparticles had a burst iron-release profile in the first 2 h; moreover, 95.5% of iron was released in SGF while only 3.4% of iron was released in SIF. However, the total iron-release profiles slightly differed across this study’s jelly foods. Thus, the iron released from fortified jelly foods depends on gelatine’s properties and composition, as well as chitosan. Figure 6A also shows that using 10% (w/v) gelatine in jelly foods could slightly extend the iron release in SGF and successfully increase the amount of iron released in SIF, achieving 85.9–89.3% of iron released in SGF and 5.1–9.7% of iron released in SIF. Moreover, gelatine could act as a second layer of chitosan-TPP microparticles, inhibiting the release of iron. However, higher gelatine concentrations (20–40% w/v) provided a burst iron profile. The gelatine of the PEC could have swollen since it absorbed the simulated digested fluid; thus, the chitosan microparticles’ pore size increased, leading to the rapid release of iron.
Fig. 6.
A The Fe total cumulative release and B ferrous bioavailability of FeG–AA–FA microparticles (M) and fortified jelly foods (GM) in varying concentrations of gelatine. The values of ferrous bioavailability are means ± standard deviation of three replicates and.a−b samples represented with different letters are significantly different (P < 0.05)
Figure 6B shows the ferrous bioavailability of this study’s FeG-AA-FA microparticles and fortified jelly foods. Ferrous bioavailability can be determined via iron’s solubility and valence state, and only soluble iron can be absorbed (Kumar et al., 2020). Soluble iron can take either a ferrous form or the form of complex compounds (Kumar et al., 2020). In this study, jelly foods’ iron bioavailability increased more than fivefold compared to FeG-AA-FA microparticles. Again, this result indicated that the foods’ gelatine successfully protected their iron content; thus, these jelly foods released more soluble ferrous iron than the microparticles themselves.
Conclusions
This study produced chitosan-TPP microparticles that were loaded with FeG, AA and FA using a spray-dryer. It also investigated the fortification of jelly foods using FeG-AA-FA microparticles. The presence of AA, FA and FeG were found to enhance chitosan-TPP microparticles’ ferrous bioavailability. Furthermore, fortified jelly foods’ ferrous bioavailability increased more than fivefold compared to FeG-AA-FA microparticles. The use of a lower temperature during the preparation of fortified jelly foods is recommended to avoid the microparticles’ decomposition and a Maillard browning reaction. These findings can help food technologists and product developers select suitable formulations to offer higher iron bioavailability, thus successfully fortifying jelly-based foods’ could reduce the prevalence of anaemia.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr. Umi Fahmida for her valuable comments and suggestions for this article.
Abbreviations
- FeG
Ferrous gluconate
- FA
Folic acid
- AA
Ascorbic acid
- M
FeG, AA, and FA-loaded chitosan microparticles
- G
Unfortified jelly foods
- GM
Fortified jelly foods
Author contributions
Conceptualization (NAH, KM, SK, EAK); methodology (NAH); validation (NAH, KM, SK, EAK); formal analysis (NAH); investigation (NAH); resources (KM, SK, EAK); data curation (NAH); writing—original draft preparation (NAH); writing—review and editing (NAH, KM, SK, EAK); visualization (NAH); supervision (KM, SK, EAK); funding acquisition (SK).
Funding
This study was financially supported by Ministry of Research and Technology/National Agency for Research and Innovation through PDD Research Scheme contract number 1852/PKS/R/UI/2019.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sutrasno Kartohardjono, Email: sutrasno@che.ui.ac.id.
Elsa Anisa Krisanti, Email: elsakm@che.ui.ac.id.
References
- Akhtar S, Anjum FM, Anjum MA. Micronutrient fortification of wheat flour: recent development and strategies. Food Res Int. 2011;44:652–659. doi: 10.1016/j.foodres.2010.12.033. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 18. Arlington: Association of Official Analytical Chemists; 2005. [Google Scholar]
- Barragán-ibañeZ G, Santoyo-sánchez A, Ramos-Peñafiel C. Iron deficiency anaemia. Revista Méd Del Hospital General De México. 2016;79:88–97. doi: 10.1016/j.hgmx.2015.06.008. [DOI] [Google Scholar]
- Blanco-rojo R, Vaquero M. Iron bioavailability from food fortification to precision nutrition. A review. Innov Food Sci Emerging Technol. 2018;51:126–138. doi: 10.1016/j.ifset.2018.04.015. [DOI] [Google Scholar]
- Desai K, Park H. Encapsulation of vitamin C in tripolyphosphate cross-linked chitosan microspheres by spray drying. J Microencapsul. 2005;22:179–192. doi: 10.1080/02652040400026533. [DOI] [PubMed] [Google Scholar]
- Deutsch JC. Dehydroascorbic acid. J Chromatogr A. 2000;881:299–307. doi: 10.1016/S0021-9673(00)00166-7. [DOI] [PubMed] [Google Scholar]
- Gupta C, Chawla P, Arora S, Tomar S, Singh A. Iron microencapsulation with blend of gum arabic, maltodextrin and modified starch using modified solvent evaporation method–milk fortification. Food Hydrocolloids. 2015;43:622–628. doi: 10.1016/j.foodhyd.2014.07.021. [DOI] [Google Scholar]
- Gutiérrez G, Matos M, Barrero P, Pando D, Iglesias O, Pazos C. Iron-entrapped niosomes and their potential application for yogurt fortification. LWT-Food Sci Technol. 2016;74:550–556. doi: 10.1016/j.lwt.2016.08.025. [DOI] [Google Scholar]
- Habeych E, Van Kogelenberg V, Sagalowicz L, Michel M, Galaffu N. Strategies to limit colour changes when fortifying food products with iron. Food Res Int. 2016;88:122–128. doi: 10.1016/j.foodres.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Handayani NA, Krisanti EA, Kartohardjono S, Mulia K. Cyclic voltammetry and oxidation rate studies of ferrous gluconate complex solutions for preparation of chitosan-tripolyphosphate microparticles. J Chem. 2020;2092:1–8. doi: 10.1155/2020/3417204. [DOI] [Google Scholar]
- Jiang W-Z, Cai Y, Li H-Y. Chitosan-based spray-dried mucoadhesive microspheres for sustained oromucosal drug delivery. Powder Technol. 2017;312:124–132. doi: 10.1016/j.powtec.2017.02.021. [DOI] [Google Scholar]
- Krisanti EA, Naziha GM, Amany NS, Mulia K, Handayani NA. Effect of biopolymers composition on release profile of iron (II) fumarate from chitosan-alginate microparticles. IOP Conf Ser: Mater. Sci. Eng. 2019;509:012100. doi: 10.1088/1757-899X/509/1/012100. [DOI] [Google Scholar]
- Kulig D, Zimoch-Korzycka A, Jarmoluk A, Marycz K. Study on alginate–chitosan complex formed with different polymers ratio. Polymers. 2016;8:167. doi: 10.3390/polym8050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Anukiruthika T, Dutta S, Kashyap A, Moses JA, Anandharamakrishnan C. Iron deficiency anemia: A comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends Food Sci Technol. 2020;99:58–75. doi: 10.1016/j.tifs.2020.02.021. [DOI] [Google Scholar]
- Liu W, Wu WD, Selomulya C, Chen XD. Uniform chitosan microparticles prepared by a novel spray-drying technique. Int J Chem Engi. 2011;26:1–7. [Google Scholar]
- Morita T. Development of deglutition aid jelly for oral administration. Yakugaku Zasshi: Journal of the Pharmaceutical Society of Japan. 2003;123:665–671. doi: 10.1248/yakushi.123.665. [DOI] [PubMed] [Google Scholar]
- Mulia K, Putri T, Krisanti EA, Handayani NA. Preparation and evaluation of chitosan biopolymers encapsulated iron gluconate using spray drying method. AIP Conf Proc. 2019;2054:030005. doi: 10.1063/1.5096709. [DOI] [Google Scholar]
- Otálora MC, de Jesús Barbosa H, Perilla JE, Osorio C, Nazareno MA. Encapsulated betalains (Opuntia ficus-indica) as natural colorants. Case Study: Gummy Candies LWT. 2019;103:222–227. [Google Scholar]
- Oy S, Witjaksono F, Mustafa A, Setyobudi SI, Fahmida U. Problem nutrients in adolescent girls with anemia versus nonanemic adolescent girls and the optimized food-based recommendations to meet adequacy of these nutrients in adolescent school girls in East Java, Indonesia. Food Nutr Bull. 2019;40:295–307. doi: 10.1177/0379572119851326. [DOI] [PubMed] [Google Scholar]
- Pérez-masiá R, López-nicolás R, Periago MJ, Ros G, Lagaron JM, López-rubio A. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chem. 2015;168:124–133. doi: 10.1016/j.foodchem.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Singh P, Prasad S. Determination of ascorbic acid and its influence on the bioavailability of iron, zinc and calcium in Fijian food samples. Microchem J. 2018;139:119–124. doi: 10.1016/j.microc.2018.02.019. [DOI] [Google Scholar]
- Toohey JI. Dehydroascorbic acid as an anti-cancer agent. Cancer Lett. 2008;263:164–169. doi: 10.1016/j.canlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Tripathi B, Platel K. Iron fortification of finger millet (Eleucine coracana) flour with EDTA and folic acid as co-fortificants. Food Chem. 2011;126:537–542. doi: 10.1016/j.foodchem.2010.11.039. [DOI] [Google Scholar]
- Umemura K, Kawai S. Modification of chitosan by the Maillard reaction using cellulose model compounds. Carbohyd Polym. 2007;68:242–248. doi: 10.1016/j.carbpol.2006.12.014. [DOI] [Google Scholar]
- Voron’ko NG, Derkach SR, Kuchina YA, Sokolan NI. The chitosan–gelatin (bio) polyelectrolyte complexes formation in an acidic medium. Carbohydrate Polym. 2016;138:265–272. doi: 10.1016/j.carbpol.2015.11.059. [DOI] [PubMed] [Google Scholar]
- Webber V, De Siqueira Ferreira D, Barreto PLM, Weiss-Angeli V, Vanderlinde R. Preparation and characterization of microparticles of β-cyclodextrin/glutathione and chitosan/glutathione obtained by spray-drying. Food Res Int. 2018;105:432–439. doi: 10.1016/j.foodres.2017.11.035. [DOI] [PubMed] [Google Scholar]
- Zhong C, Tan S, Langrish T. Redness generation via Maillard reactions of whey protein isolate (WPI) and ascorbic acid (vitamin C) in spray-dried powders. J Food Eng. 2019;244:11–20. doi: 10.1016/j.jfoodeng.2018.09.020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.
Not applicable.