Abstract
O-GlcNAcylation is a post-translational modification of protein in response to genetic variations or environmental factors, which is controlled by two highly conserved enzymes, i.e. O-GlcNAc transferase (OGT) and protein O-GlcNAcase (OGA). Protein O-GlcNAcylation mainly occurs in the cytoplasm, nucleus, and mitochondrion, and it is ubiquitously implicated in the development of cardiovascular disease (CVD). Alterations of O-GlcNAcylation could cause massive metabolic imbalance and affect cardiovascular function, but the role of O-GlcNAcylation in CVD remains controversial. That is, acutely increased O-GlcNAcylation is an adaptive heart response, which temporarily protects cardiac function. While it is harmful to cardiomyocytes if O-GlcNAcylation levels remain high in chronic conditions or in the long run. The underlying mechanisms include regulation of transcription, energy metabolism, and other signal transduction reactions induced by O-GlcNAcylation. In this review, we will focus on the interactions between protein O-GlcNAcylation and CVD, and discuss the potential molecular mechanisms that may be able to pave a new avenue for the treatment of cardiovascular events.
Keywords: O-GlcNAcylation, glycomics, glycosylation, cardiovascular disease
Introduction
Cardiovascular disease (CVD) is the leading cause of human death and disability worldwide. From 1999 to 2019, the number of CVD cases nearly doubled from 271 million to 523 million, and the deaths increased from 12.1 million to 18.6 million [1]. Although advanced biotechnologies have been used to decipher pathogenesis concerning CVD, they are insufficient to fully assess and intervene cardiovascular events. Therefore, there is an urgent need to apply burgeoning techniques to identify novel and supplementary etiologies and pathologies to perform risk prediction in future cardiovascular events. Among so many research fields, glycomics is one such reliable subject to pose innovative proposals and exploit new treatment targets. O-linked β-N-acetylglucosamine (O-GlcNAc) glycosylation, named O-GlcNAcylation, was widespread in the cytoplasm, nucleus, and mitochondrion, which was first identified on mouse lymphocytes surface by Carmen-Rosa Torres and Gerald Hart in 1983 [2–4]. More recently, protein O-GlcNAcylation has been found to be ubiquitously involved in the progression of cardiovascular dysfunction. However, most of the available existence exhibits an inconsistent role of O-GlcNAcylation in CVD, whether it is beneficial or detrimental depends on the unique disease environment (Fig. 1).
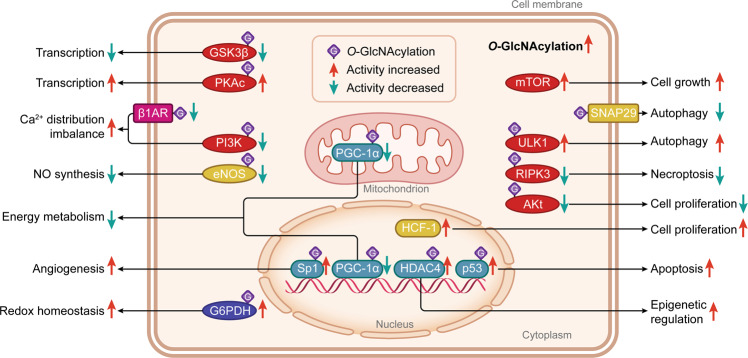
Fig. 1. The types, cellular distribution, and functions of proteins that could be O-GlcNAcylated in cells.
O-GlcNAcylated proteins, mainly located in the cytoplasm, nucleus, mitochondrion, and cell membrane, include the protein kinases (in red color), transcription factors and coactivators (in green color), metabolic proteins (in blue color), membrane receptors (in pink color), and other signaling molecules (in yellow color). They are responsible for essential activities with regard to epigenetic regulation, gene transcription, energy metabolism, cell cycle behavior, vascular function, Ca2+ distribution, NO production and redox homeostasis. Most of these proteins can be O-GlcNAcylated (in purple squares with the letter ‘G’) and their activities and functions can be changed directly. Differently, mTOR and SNAP29 are directly triggered by the higher intracellular O-GlcNAcylation levels. Proteins with enhanced activity after O-GlcNAcylation modification are indicated by red arrows, and proteins with decreased activity after O-GlcNAcylation modification are shown with green arrows. GSK-3β Glycogen synthase kinase-3β, PKAc PKA catalytic subunit, β1AR β1-adrenoceptor, PI3K Phosphoinositide 3-kinase, eNOS Endothelial nitric oxide synthase, G6PDH Glucose 6-phosphatedehydrogenase, PGC-1α Peroxisome proliferator-activated receptor-γ coactivator-1α, Sp1 Specificity protein 1, HDAC4 histone deacetylase 4, mTOR Mechanistic/mammalian target of rapamycin, SNAP29 Synaptosomal-associated protein 29, ULK1 Unc-51-like autophagy activating kinase 1, RIPK3 Receptor-interacting protein kinase 3, HCF-1 Host cell factor-1.
Hexosamine biosynthetic pathway (HBP) represents one accessory glucose metabolic branch in cells and governs the production of uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), which is the substrate of protein O-GlcNAcylation [5]. According to a recent study, the proportion of glucose entering HBP flux in isolated hearts accounts for only 0.003%–0.006% of glycolysis, yet its effect should not be dismissed [6]. The first HBP step is controlled via L-glutamine: fructose-6-phosphate amidotransferase (GFAT), a rate-limiting enzyme, which converts fructose-6-phosphate into glucosamine-6-phosphate using glutamine, and then glucosamine 6-phosphate is transformed into N-acetylglucosamine-6-phosphate, N-acetylglucosamine-1-phosphate, and finally forming UDP-GlcNAc requiring acetyl-CoA and uridine triphosphate (UTP) [7]. This conversion mostly emerges in the endoplasmic reticulum (ER) and Golgi apparatus in an extremely dynamic mode, and this modification also exists on plentiful cytosolic and nuclear proteins [8]. GFAT1 and GFAT2 are two GFAT protein subtypes, encoded by the Gfpt1 and Gfpt2 genes, respectively [9, 10]. During these days, it was announced that the localization of the two isoforms was different. GFAT1 was specifically rich in cardiomyocytes and fibroblasts, but GFAT2 was only present in fibroblasts [11], which was contrary to initial reports that mammalian hearts were an excellent source of GFAT2 rather than GFAT1 [12–15]. Most notably, there is a growing consensus that GFAT can be regulated by phosphorylation and this will be further clarified in the following sections [16].
Unlike protein phosphorylation regulated by numerous protein kinases and phosphates, the O-GlcNAc biosynthesis is separately controlled by two enzymes, i.e. O-GlcNAc transferase (OGT) and protein O-GlcNAcase (OGA), upon forming the UDP-GlcNAc group [17]. OGT connects a single N-acetylglucosamine (GlcNAc) from the UDP-GlcNAc group to serine (Ser) or threonine (Thr) residues of target proteins, correspondingly, OGA catalyzes the removal of GlcNAc moiety from amino residues [18–21]. OGT, mainly gathered in the part of the cytoplasm, mitochondrion, and nucleus, is encoded by the Ogt gene on the X chromosome Xq13.1 [22, 23]. Three splice variants are generated via alternative splicing including nucleocytoplasmic OGT (ncOGT), mitochondrial OGT (mOGT), and short OGT (sOGT) (Fig. 2a) [22]. Each of the three multidomain OGT isoforms is constituted by nuclear localization signal (NLS) domain, intervening sequence domain (Int-D), two C-terminal catalytic domains (C-cat), and an N-terminal catalytic domain (N-cat) comprising tetratricopeptide repeats (TPRs) (Fig. 2a) [24]. Although other domains are similar, the length of TPRs is distinct (ncOGT, 12.5 TPRs; mOGT, 9.5 TPRs; sOGT, 2.5 TPRs), and the TPRs manipulate the glycosite selection and the substrate specificity of OGT [25, 26]. A new finding consolidated this view that iterative truncation of OGT TPRs domain converted the OGT substrate selection, subcellular localization, and the glycosylation site although retaining the OGT activity [27]. Besides, Watson et al. demonstrated that only 12% of constitutive cardiomyocyte-specific OGT knockout mice survived to weaning age (4 weeks old) and these survived mice showed heart failure-like changes by affecting ER homeostasis [28]. This indicates an essential biological function of OGT in cardiac development and vascular formation. Differently, the human Oga gene targets the position 10q24.1-q24.3 of the chromosome [29]. Human OGA also has different splice variants, being consisted of nucleocytoplasmic OGA (ncOGA, 130 kDa) located in the cytoplasm or nucleus and short isoform OGA (sOGA, 70 kDa) residing in the nucleus, cytoplasm (especially ER), and lipid droplets (Fig. 2a) [29, 30]. ncOGA protein has various domains, including an N-terminal catalytic domain, a C-terminal pseudo histone acetyltransferase (pseudo-HAT) domain, two stalk domains, and two low complexity (LC) regions, but sOGA lacks the HAT domain (Fig. 2a) [30]. OGT and OGA can interact and coordinate with each other to control protein O-GlcNAcylation levels in a rapid and transient way, so that the organism can agilely cope with outside or internal stimuli, whilst the elaborate molecular and cellular mechanisms remain incompletely understood.
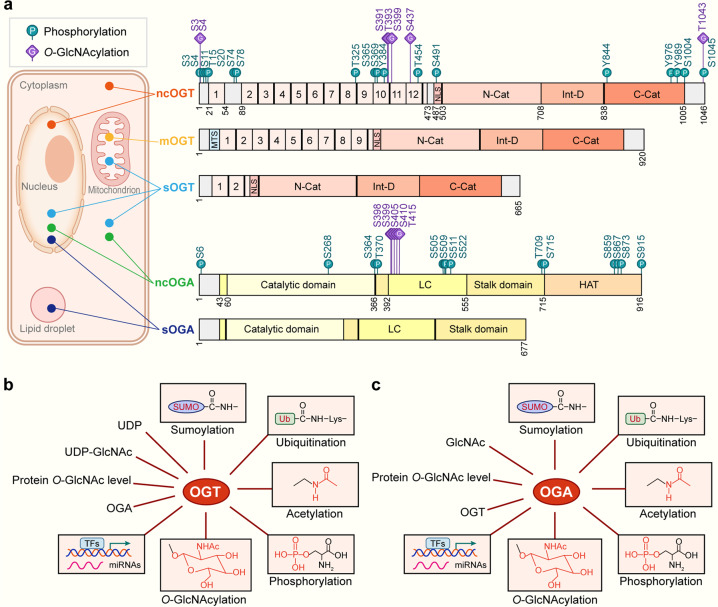
Fig. 2. The molecular structure, localization and regulation of OGT and OGA.
a Structure and localization of OGT and OGA isoforms. All the identified phosphorylation sites are shown as green circles with the letter ‘P’, and O-GlcNAcylation sites are shown as purple squares with the letter ‘G’. The N-terminal region of three OGT isoforms is consisted of TPRs and an NLS. The catalytic region is composed of an N-cat domain, an Int-D, and a C-cat domain. Additionally, mOGT has a typical MTS. These two OGA splicing variants are constituted by a catalytic domain in the N-terminal region and two stalk domains (including N-terminal and C-terminal) and two LC regions, but they differ in a pseudo-HAT domain. The localization of ncOGT and ncOGA mainly concentrates in the cytoplasm and nucleus. mOGT gathers in the mitochondrion, and sOGT is present in the cytoplasm, nucleus, and mitochondrion. sOGA is centered in the nucleus and lipid droplet. b Regulation of OGT. OGT is modulated at several aspects containing HBP substrate availability such as UDP and UDP-GlcNAc. The intracellular protein O-GlcNAc level and the counterpart magnitude of OGA can regulate OGT in a feedback style. OGT can be regulated through TFs and miRNAs at the transcriptional level. Post-translational modifications e.g., O-GlcNAcylation, phosphorylation, acetylation, ubiquitination, and sumoylation can also regulate OGT. c Regulation of OGA. OGA is regulated by various factors containing feedback inhibition by intracellular GlcNAc level. The intracellular protein O-GlcNAc level and the counterpart magnitude of OGT can regulate OGA. OGA can be regulated through TFs and miRNAs at the transcriptional level. Post-translational modifications by O-GlcNAcylation, phosphorylation, acetylation, ubiquitination, and sumoylation can also regulate OGA. OGT O-GlcNAc transferase, OGA Protein O-GlcNAcase, UDP-GlcNAc Uridine diphosphate N-acetylglucosamine, TPRs Tetratricopeptide repeats, NLS Nuclear localization signal, N-cat N-terminal catalytic domain, Int-D Intervening sequence, C-cat C-terminal catalytic domain, MTS Mitochondrial targeting sequence, LC Low complexity, pseudo-HAT Pseudohistone acetyl transferase, TFs Transcription factors, miRNAs MicroRNAs, ncOGT Nucleocytoplasmic OGT, mOGT Mitochondrial OGT, sOGT Short OGT, ncOGA Nucleocytoplasmic OGA, sOGA Short isoform OGA. The databases https://glygen.org/, https://www.uniprot.org/, and https://research.bioinformatics.udel.edu/iptmnet/ are used to search for known modification sites.
It is widely accepted that both the concentration and activity of OGT and OGA can be directly modulated, which exquisitely mediates the complex and dynamic O-GlcNAc modification procedure (Fig. 2b, c). Firstly, substrate concentration, intracellular protein O-GlcNAcylation density, and the counterpart magnitude of OGT or OGA all influence specific enzyme gene transcription (Fig. 2b, c). Intracellular UDP and UDP-GlcNAc levels were reported to inhibit OGT such as the potential OGT inhibitor Alloxan, an analogue of UDP-GlcNAc and UDP [31]. GlcNAc is probably being used to inhibit OGA like OGA inhibitor streptozotocin (STZ), an analogue of GlcNAc [32]. Lin et al. discovered that the intracellular O-GlcNAcylation fluctuations contributed to the alterations of OGT and OGA expression, in a feedback mode [33]. Moreover, OGA was proved to promote Ogt transcription through cooperation with transcription factor CCAAT/enhancer-binding protein (C/EBP), elucidating the mutual regulation effect of OGT and OGA [34]. Secondly, gene transcriptional regulation and post-transcriptional modification (e.g., miRNAs) also are factors determining OGT and OGA expression (Fig. 2b, c). For example, one research group validated that E2F transcription factor 1 (E2F1) negatively regulated OGT and OGA expression by connecting to the putative promoter regions of Ogt and Mega5 genes (the coding gene for OGA protein) in vitro [35]. Quite differently, Dassanayaka et al. confirmed an opposing conclusion that E2F1 deletion brought no changes in O-GlcNAcylation level [36]. Of unique note, miRNA-200a/200b possesses homology with the 3’-untranslated region (3’-UTR) of OGT mRNA, which can interfere with the transcription and expression level of OGT, thus reducing O-GlcNAcylation concentrations [37]. Another investigation by Wang et al. discovered that overexpressed miRNA-24 in the heart of type 2 diabetes mellitus (T2DM) mice can also competently decrease the levels of target protein OGT with a relatively smaller reduction in O-GlcNAcylation level [38]. In addition to OGT, OGA can also be post-transcriptional regulated via miRNAs [39]. Thirdly, the two enzymes are subject to post-translational modifications (PTMs) such as phosphorylation and O-GlcNAcylation, which perturbs enzyme activity, subcellular localization, structural stability and degradation rate of OGT and OGA (Fig. 2b, c). Bullen et al. reported that AMPK phosphorylated Thr-444 on OGT, which further changed its activity and nuclear localization and affected O-GlcNAcylation of numerous proteins [40]. Another investigation by Latorre-Muro et al. confirmed that OGT can be phosphorylated through activation of the protein kinase R (PKR)-like ER kinase (PERK) that enhanced OGT activity [41]. Besides, other PTMs such as acetylation, ubiquitination, and sumoylation were also demonstrated to regulate OGT and OGA (Fig. 2b, c) [42, 43]. For example, OGT was found to serve as the target of ubiquitin ligase that mediated ubiquitination-induced degradation of OGT [44]. Although a multitude of modified sites on OGT and OGA have been identified (Fig. 2a), very little is known with their functions referring to OGT and OGA. Considering the importance of O-GlcNAc turnover, more investigations regarding regulations of PTMs are anticipated, providing mechanisms on how OGT and OGA are regulated. Here, we will introduce new findings between O-GlcNAcylation and cardiovascular events and illustrate the contribution of protein O-GlcNAcylation to CVD as well as presenting current understandings of O-GlcNAc cycling (Fig. 1).
Roles Of O-Glcnacylation in the cardiovascular system and diseases
Heart failure and cardiac hypertrophy
Heart failure (HF), also known as cardiac insufficiency, is the final outcome of many heart diseases. Meanwhile, the compensatory cardiac hypertrophy may be evolved into HF under persistent stress, and protein O-GlcNAcylation signaling has been reported to be correlated with both cardiac hypertrophy and HF [13]. As exemplified by the O-GlcNAcylation level of several proteins such as c-Myc, troponin I, and troponin T, is much higher in cardiac hypertrophy and leads to poor cardiac remodeling [45–48]. Similarly, Lunde et al. clearly delineated that protein O-GlcNAcylation level was higher in HF left ventricular tissue [13]. Besides, the protein concentrations of HBP-related enzymes (OGT, OGA, GFAT2) together with their mRNA levels were all increased under pressure load [13]. Though the most cases are implying that the increased O-GlcNAcylation levels are associated with the development of HF and cardiac hypertrophy, the underlying mechanisms for this phenomenon are undefined.
Interestingly enough, in recent years, much more investigations indicated the potential role of O-GlcNAcylation in HF by adjusting mitochondrial energy metabolism, cardiomyocyte growth, autophagy, and gene expression. For example, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), a transcription coactivator interfering with mitochondrial biosynthesis and energy homeostasis, is substantially expressed in cardiac muscle [49]. The PGC activity is also found to be determined by protein O-GlcNAcylation [50]. Nevertheless, a relative decrease of the PGC-1α level was observed in cardiac hypertrophy, and one possible mechanism was that PGC-1α could be directly O-GlcNAcylated and its activity and expression were subsequently inhibited, which eventually suppressed the fatty acid oxidation/mitochondrial genes expression and caused metabolism dysregulation (Fig. 1) [51]. Another recent observation also illustrated that dilated cardiomyopathy and HF were associated with increased protein O-GlcNAcylation levels in OGT transgenic mice, where OGT overexpression caused ventricular arrhythmia and premature death independent of pathological stress, but OGA overexpression weakened the O-GlcNAcylation-induced destructive effects and stable myocardial function could be maintained [52]. Significantly, this observation also substantiated the fact that mitochondrial metabolic genes were dampened with lower activity, coupled with a lower expression of mitochondrial complex I in OGT-induced high O-GlcNAcylation context, suggesting that protein O-GlcNAcylation may lead to adverse myocardial remodeling in the progression of HF and cardiomyopathy by causing mitochondrial function impairment [52]. Moreover, compromised cell cycling is a common trait in the heart in response to HF, which is reported to have relevance with protein O-GlcNAcylation. In the case of pathological cardiac hypertrophy and HF, increased HBP and protein O-GlcNAcylation in cardiomyocytes were reported to induce cardiomyocyte growth and pathological cardiac remodeling via activating the mechanistic/mammalian target of rapamycin (mTOR) (Fig. 1) [53]. mTOR is a protein kinase responsible for cardiomyocyte growth [54], and many other investigations have confirmed that mTOR signaling is linked to cardiac hypertrophic growth [55]. In addition, cardiomyocyte autophagy bears the benefit to confront various stress stimuli in cardiovascular events. Strikingly, an investigation has shown protein O-GlcNAcylation in cardiomyocytes is an indispensable part of initiating autophagy, implying O-GlcNAcylation plays a protective role in cardiac HF and may provide new treatment insights for HF events [56]. This was performed by activating unc-51-like autophagy activating kinase 1 (ULK1), a crucial autophagy initiation regulator, and O-GlcNAcylation of ULK1 upregulated its activity and initiated autophagy in cardiomyocytes (Fig. 1) [56].
Others identified protein O-GlcNAcylation as a critical element in regulating gene expression, especially for hypertrophy-related genes and inflammatory cytokine genes. This represented one mechanism underlying its contribution to HF. The study by Chen et al. affirmed that the protein O-GlcNAcylation elevation induced cardiomyocyte hypertrophy-like changes in primary cultured mouse cardiomyocytes and thickened the left ventricular wall and finally induced cardiac hypertrophy by activation of protein kinase A (PKA) [57]. It can be explained that PKA catalytic subunit, PKAc, was directly O-GlcNAcylated and its kinase activity was enhanced (Fig. 1) [58], which augmented the synthesis of downstream myocardial cell proteins such as atrial natriuretic peptide (ANP) and β-myosin heavy chain (β-MHC) and resulted in cardiac hypertrophy and cardiac dysfunction [57]. Remarkably, transcription factors are important O-GlcNAcylation target proteins. Nakagawa et al. showed that O-GlcNAcylation prevented intermittent hypoxia (IH)-induced cardiac remodeling, emphasizing that O-GlcNAcylation also exerted a beneficial effect via modulating the transcription of inflammatory cytokine genes [59]. For example, the nuclear factor of activated T cells (NFAT) and nuclear factor kappa-B (NF-κB), two inflammation-related transcription factors, can be activated or inhibited by different PTMs such as phosphorylation and O-GlcNAcylation. The phosphorylated NFAT exists in the cytoplasm rather than nuclear and thus it is unable to trigger nuclear target hypertrophy-related genes, while the phosphorylation modification can activate NF-κB and induce gene transcription. In an IH mouse model, the elevated O-GlcNAcylation of glycogen synthase kinase-3β (GSK-3β) upregulated the GSK-3β-mediated phosphorylation of NFAT, which was followed by the lower expression of target hypertrophy-related genes (Fig. 1) [59]. On the other hand, NF-κB was directly O-GlcNAcylated and its phosphorylation was inhibited, which concurrently alleviated the transcriptional activity for inflammatory cytokine genes [59]. In summary, these studies demonstrate a continuously growing correlation between protein O-GlcNAc modification and functional consequences of cardiovascular events. The versatile roles of protein O-GlcNAcylation in HF highlight the significance of maintaining O-GlcNAcylation homeostasis.
Diabetic heart disease
Previous diabetic-related studies showed that a wide variety of diabetic heart diseases were affected through altering protein O-GlcNAcylation, such as diabetic cardiomyopathy and diabetes-induced HF. Si et al. have established an association among protein O-GlcNAcylation and cell apoptosis and coronary microvascular disease (CMD) [60]. The p53 transcription factor is responsible for regulating cell apoptosis [61]. However, in coronary ECs, the O-GlcNAcylation of p53 can inhibit the Thr-155 phosphorylation thereby shunning p53 ubiquitination degradation, and the accumulated p53 competently induced CMD and cardiac contractility damage, all of which can be reversed by down-regulating p53 O-GlcNAcylation (Fig. 1) [60, 62]. Inhibiting p53 O-GlcNAcylation is, therefore, a feasible therapy scheme for controlling CMD. Likewise, protein O-GlcNAcylation was reported to worsen diabetic myocardial injury via disturbing autophagy response. In the autophagy reaction, synaptosomal-associated protein 29 (SNAP29)-syntaxin-17 (STX17)-vesicle-associated membrane protein 8 (VAMP8) complex is involved in membrane fusion between autophagosome and lysosome [63]. SNAP29 can be modified by O-GlcNAcylation [64]. O-GlcNAcylation of SNAP29 concomitantly inhibited the formation of SNAP29-STX17-VAMP8 complex and blocked the autophagy-mediated degradation and impaired cardiac diastolic function (Fig. 1) [65]. Targeting the O-GlcNAcylation level in SNAP29 may be an executable approach for ameliorating cardiac function.
Some other studies also confirmed that protein O-GlcNAcylation may accelerate diabetic cardiomyopathy and diabetes-induced HF by affecting intracellular Ca2+ distribution. One recent analysis bore out that the O-GlcNAc modification of β1-adrenoceptor (β1AR) may contribute to decreased β1AR activity and repressive cAMP-PKA-phospholamban (PLB) pathway in diabetic cardiomyopathy, which further produced inhibitive SERCA2a and augmented Ca2+ influx and aggravated myocardial cell death in the end, and β1AR was a momentous constituent of activating cAMP-PKA-PLB signaling pathway (Fig. 1) [66]. Additional clues pointed out that diabetic cardiac Ca2+ homeostasis can also be instantly regulated via OGT and OGA. In non-diabetic mice, OGT impaired left ventricular diastolic function and generated poor cardiac remodeling, while OGA reversed the above maladaptive cardiac remodeling via changing the Ca2+-related signaling pathway: phosphoinositide 3-kinase (PI3K)-Akt-SERCA2a pathway [67]. In this experiment, OGT-induced high O-GlcNAcylation suppressed PI3K-Akt-SERCA2a signaling and disrupted Ca2+ location and prompted diabetes-induced HF, which was interrelated with lower PI3K activity itself and activated PI3K inactivator PTEN (Fig. 1) [67]. Of special note, protein O-GlcNAcylation has also been demonstrated to regulate epigenetics and possess the ability to protect diabetic hearts. As an example, the increased O-GlcNAcylation in the position of histone deacetylase 4 (HDAC4) Ser-642, a crucial epigenetic regulator involved in cardiac growth, contributed to the generation of HDAC4-NT, which was proved to protect the failing heart previously, and thus prevented diabetic hearts from HF in type 1 diabetes mellitus and T2DM mouse models (Fig. 1) [68, 69]. Collectively, these data drop a hint that targeting the reconstruction balance of protein O-GlcNAcylation is significant for diabetes-related disease signaling transduction.
Ischemia reperfusion (I/R) injury
I/R injury is liable to cause myocardial infarction, arrhythmia, cardiac hypertrophy, and HF [70, 71]. Some studies have found that I/R injury is tightly related to protein O-GlcNAcylation, as evidenced by O-GlcNAcylation-mediated changes in redox state, cellular life activities, and clinical treatment effects. Supportively, Ou et al. discovered that hypoxic acclimation-induced O-GlcNAcylation efficaciously alleviated I/R injury via activating glucose 6-phosphatedehydrogenase (G6PDH) and enhancing redox homeostasis, which established the principal role of O-GlcNAcylation in hypoxic acclimation-initiated antioxidative and cardioprotective effects (Fig. 1) [72]. In addition, it has been concluded that receptor-interacting protein kinase 1 (RIPK1)/receptor-interacting protein kinase 3 (RIPK3)/mixed lineage kinase domain-like (MLKL) complex holds prime roles in necrosis [73]. However, the higher O-GlcNAcylation of RIPK3 induced by sevoflurane may inhibit its cross-linking with MLKL, which restricted the RIPK3/MLKL-mediated necroptosis and protected myocardial I/R injury, emphasizing the role of O-GlcNAcylation against I/R injury (Fig. 1) [74]. Nowadays, glucose-insulin-potassium (GIK) therapy is a common treatment strategy for I/R injury, and it is speculated that GIK-induced O-GlcNAcylation is one potential mechanism elucidating this cardiac protective benefit [75, 76]. Normally, insulin plays a beneficial role by promoting Akt phosphorylation and inducing Akt-mediated cell pro-survival signaling pathways [77]. Yet in obese people who had much hyper O-GlcNAcylation, the hyper O-GlcNAcylation of Akt competitively inhibited insulin-induced Akt phosphorylation, and the impaired Akt activation, in turn, attenuated cell proliferation and insulin’s cardio-protection against I/R injury, which helped to reveal the reduced efficacy of GIK therapy in obese people (Fig. 1) [78]. The existing concept indicates a contradictory role of protein O-GlcNAcylation that the moderately increased O-GlcNAcylation can prevent I/R-heart injury, but the hyper O-GlcNAcylation would mitigate the beneficial effect of GIK.
Pulmonary artery hypertension (PAH)
More intriguingly, protein O-GlcNAcylation has been reported to participate in the progression of PAH, which maintains diverse effects including promoting cell proliferation, angiogenesis, aggravating right ventricular dysfunction (RVD), and inhibiting nitric oxide (NO) biosynthesis. In the case of idiopathic pulmonary artery hypertension (IPAH), energy metabolism supply shifting from fatty acids to glucose is a representative nature for highly proliferative IPAH cells, and the excessive demand for intracellular glucose subsequently upregulates the HBP flow and protein O-GlcNAcylation level [79]. Specifically, the activated OGT/O-GlcNAc axis was reported to drive IPAH pulmonary artery smooth muscle cell (PASMC) proliferation and deteriorate IPAH, through hydrolytic activation of the cell cycle regulator, host cell factor-1 (HCF-1) (Fig. 1) [80]. In mammalian cells, the normal cell mitosis will also be destroyed if disturbing the HCF-1 expression due to the essential role of HCF-1 in enhancing cell proliferation and cell cycle regulation [81]. Furthermore, the association of O-GlcNAcylation with neovascularization has also been reported in IPAH via stimulating transcription factor specificity protein 1 (Sp1)-mediated VEGF expression and angiogenesis [82]. O-GlcNAcylation was affirmed to increase the stability of Sp1, which further promoted the expression of VEGF and its ligands in IPAH PAECs and enhanced angiogenesis by changing vascular sprouting (Fig. 1) [83]. In addition, RVD is a progressive clinical pathological phenotype and the most fatal factor in PAH, and energy metabolism conversion is a sufficient contributor to RVD. Some interesting studies indicate that protein O-GlcNAcylation is involved in ventricular mitochondrial derangement and energy deficiency [84, 85]. For example, Prisco et al. found that excessive O-GlcNAcylation levels of mitochondrial proteins accelerated metabolic derangements and led to PAH-RVD, which can be counteracted by decreasing protein O-GlcNAcylation [86]. Their subsequent research confirmed that, in PAH, the hyper O-GlcNAcylation production induced through activating with no lysine kinase 1 (WNK1)/Akt signal transduction was linked to the mitochondrial metabolism disorder. Correspondingly, inhibiting WNK1 corrected mitochondrial dysfunction and enhanced PAH right ventricular contraction and diastolic function accompanied with intracellular O-GlcNAcylation reduction [87]. It is equally important that, the content of NO, a cardinal pulmonary vessel diastolic agent, is considerably lower in PAH patients, which partially leads to increased pulmonary vascular resistance [88–91]. Prior studies have supported a link between elevated O-GlcNAc modification and endothelial nitric oxide synthase (eNOS) which is responsible for NO synthesis [92, 93]. Aulak et al. further confirmed that, during PAH, the heightened flux of eNOS Ser-615 O-GlcNAcylation emulously inhibited eNOS Ser-1177 phosphorylation and thus suppressing eNOS activity, and the eNOS O-GlcNAcylation also hindered the dimerization of eNOS with lower stability, all of which down-regulated the generation of NO (Fig. 1) [94]. Identifying the O-GlcNAcylation sites and related signaling pathways in PAH warrants our further studies with the hope to find and develop new drugs.
Possible mechanisms
Investigations over the past years have discovered changes of HBP flux and protein O-GlcNAcylation concentration in CVD conditions, highlighting a pivotal role of protein O-GlcNAcylation in regulating cardiovascular events. In fact, these O-GlcNAcylated proteins, such as transcription factors, receptors, protein kinases, and other functional proteins, are engaged in diverse life activities, including gene transcription, signal transduction, cell cycle, and energy metabolism (Table 1). Therefore, extrapolating that protein O-GlcNAcylation participates in CVD via changing distinct signaling responses is rational. The potential mechanisms include, but are not limited to, variable electricity signaling cascades, energy metabolism, cell cycle, stress response, and vascular function, which are summarized below.
Table 1.
List of selected O-GlcNAcylated proteins and their function types.
| Protein name | Classification | Disease type | Ref. |
|---|---|---|---|
| PGC-1α | Coactivator | Hypertrophy | [51] |
| mTOR | Kinase | Hypertrophy, HF | [53] |
| ULK1 | Kinase | HF | [56] |
| PKAc | Kinase | Hypertrophy | [57] |
| GSK-3β | Kinase | Hypertrophy | [59] |
| p53 | Transcription factor | Diabetic CMD | [60] |
| SNAP29 | Autophagy related protein | Diabetic myocardial injury | [65] |
| β1AR | Receptor | Diabetic hypertrophy | [66] |
| PI3K | Kinase | Diabetic cardiomyopathy | [67] |
| HDAC4 | Transcription factor | HF | [69] |
| G6PDH | Dehydrogenase | I/R injury | [72] |
| RIPK3 | Kinase | I/R injury | [74] |
| Akt | Kinase | I/R injury | [78] |
| HCF-1 | Cytokine | IPAH | [80] |
| Sp1 | Transcription factor | IPAH | [83] |
| eNOS | Synthetase | IPAH | [94] |
Cardiac electromyographic signal
The cardiomyocyte bioelectrical signal is implicated in diverse steps in cardiac movement, assuring the achievement of cardiac contractile and diastolic movements in a regular manner. Protein O-GlcNAcylation was documented to change ion channels performance as well as the cardiomyocyte potentials, thus transforming depolarization or repolarization tendency and ultimately causing abnormal cardiac contractility and diastolic function. The effect of protein O-GlcNAcylation on Ca2+ channels is much more prominent. It was confirmed that OGT knockout-induced O-GlcNAcylation reduction directly diminished cardiomyocyte L-type Ca2+ channel activity and the intracellular Ca2+ leak and cell contractility [95]. In addition, another test by Hegyi et al. proved that diabetic hyperglycemia-induced CaMKIIδ-S280 O-GlcNAcylation augmented inward rectifier K+ current amplitude and transient outward K+ current recovery [96]. Interestingly, it is well-known that altered K+ current is responsible for the cardiac repolarization insult and this is a common pathogenic insight for cardiovascular events [97, 98]. Consequently, these results imply the fundamental role of different bioelectrical signals on CVD physiology in response to dynamic protein O-GlcNAcylation cycling.
Energy metabolism
Accumulating data show that protein O-GlcNAcylation is associated with cardiac metabolic derangement. A few glucose metabolic-related enzymes and fatty acid oxidation-related enzymes can directly undergo O-GlcNAc modification, for example G6PDH [72, 99]. In addition, as a nutrient sensor, HBP flux can swiftly capture fluctuations from energy turnover and nutrient content, and normal work of HBP subjects to ample substrates supplement, such as acetyl-CoA, glucose, glutamine, and UTP, all of which are requisites for synthetizing UDP-GlcNAc [11]. Accordingly, this conduces to lay a foundation for the proposal of a relationship between protein O-GlcNAcylation and cardiac energy metabolism. Most remarkably, the mitochondrion is the major workplace responsible for energy production and metabolic conversion, therefore, it is reasonable to link O-GlcNAcylation with mitochondrion and cardiac energy metabolism. Prisco et al. revealed that mitochondrial proteins can be O-GlcNAcylated, which may induce mitochondrial dysfunction in PAH-RVD [86]. Further findings illustrated the direct effect of O-GlcNAcylation on mitochondrion function, in murine embryonic fibroblasts, that genetic deletion induced global hyper-O-GlcNAcylation irritated mitochondrial fission and decreased the activity of electron transport chain complexes, partly via activating dynamin-related protein 1 (Drp1) signal [100]. Drp1 belongs to dynamics proteins mediating mitochondrial fission and the O-GlcNAc modification of which potently heightens its fission activity, which further confirms the inseparable relationship between protein O-GlcNAcylation and metabolism dysfunction [100]. In conclusion, these studies stably demonstrate the cardial effect of protein O-GlcNAcylation on mitochondrion and cardiac energy metabolism.
Cell cycle and vascular function
Current studies have confirmed that elevated O-GlcNAcylation and O-GlcNAcylated vascular proteins are involved in the modulation of vascular reactivity dysfunction. For example, in VSMCs, ET-1, an important peptide that mediates vasoconstriction, can augment the proportion of O-GlcNAcylated vascular proteins [101]. ET-1-induced O-GlcNAcylation was proved to enhance the activity of RhoA/Rho-kinase pathway plus the expression of RhoA/Rho-kinase-related proteins, which increased the thoracic aortic vasoconstriction reactivity [101]. Another finding forcefully supported the links between protein O-GlcNAcylation and vascular reactivity, during chronic IH-induced vascular dysfunction, the chronic increases in vascular protein O-GlcNAcylation promoted the contractility impairment of rat aortic and mesenteric artery segments, via increasing mitogen-activated protein kinase (MAPK) P38 phosphorylation [102]. Whereas, the same study also demonstrated that sharp increases in protein O-GlcNAcylation concentration showed vascular protection role versus acute IH insults [102]. Evidently, these studies demonstrate the heterogeneous effects of O-GlcNAcylation on blood vessel reactivity, which may be determined by disease settings or research models.
Additionally, Cui et al. proved that in mice and human umbilical vein ECs, cholinergic drugs, pyridostigmine and acetylcholine (ACh), inhibited high glucose-induced O-GlcNAcylation and apoptosis and improved endothelial dysfunction, by activating AChR-AMPK signaling [103]. Moreover, many other HBP cycling enzymes were reported to have correlations with VSMCs and vascular homeostasis. OGT was found to activate the expression of angiopoietin-1 in cardiomyocytes, which in turn drove myocardium cell growth and coronary arterial development [104]. Meanwhile, losing OGT down-regulated angiopoietin-1, which disrupted cardiac remodeling and promoted early postnatal death [104]. Notably, as one substrate of AMPK, GFAT1 itself can be phosphorylated via AMPK and its activity was inhibited. Zibrova et al. further confirmed that the low activated GFAT1 inhibited HBP flux and decreased the production of O-GlcNAcylation, which finally generated the promotion effect of VEGF-induced angiogenesis on primary human ECs, yet the reaction mechanism was not clear [105]. These data highlight the vital function of O-GlcNAcylation in affecting different cells and the vascular system and they are regulated through diverse signaling transduction, importantly, future systematic and comprehensive studies are still needed to determine whether it can be positioned as therapeutic targets.
ER stress/unfolded protein reaction
Usually, unfolded proteins are transported from the cytoplasm into ER lumen followed by suitable folding and the formation of correct protein spatial structure. ER homeostasis may be perturbed by both cellular stress and pathological stimuli, which is detrimental to protein folding function and results in the accumulation of unfolded and misfolded proteins in ER lumen, and that is widely known as ER stress [106]. Unfolded protein reaction (UPR), upon ER stress, emerges and initiates many other signal transduction responses to restore ER homeostasis, especially three representative UPR branches containing inositol-requiring enzyme-1 (IRE1), protein kinase RNA-like ER kinase (PERK) and activating transcription factor 6 (ATF6) [106]. Interestingly, the activated IRE1 could act as endonuclease and subsequently cleaves 26 bases from the encoding region of X-box binding protein-1 (Xbp1) mRNA to form Xbp1, a highly active transcription factor [107]. This IRE1/Xbp1 branch has been corroborated to have a close correlation with HBP in the cardiovascular system. For example, when Xbp1 was activated by UPR in the I/R injury model, Xbp1 can further facilitate the transcription ability of GFAT1 promoter and upregulate the HBP/O-GlcNAc axis, and the UPR-Xbp1-O-GlcNAc exhibited a protective role of heart against I/R injury [16]. Beyond the cardiovascular system, IRE1/Xbp1/O-GlcNAc axis was also demonstrated to ameliorate the ischemic stroke outcome [108]. Moreover, what is becoming increasingly evident is that ER stress-induced UPR promotes cell survival in the short term, but prolonged UPR activation expedites cell death [109]. One research was congruent with this point that, the speed of O-GlcNAcylation upregulation was acute and fast in ischemia/hypoxia, which prevented ER stress-induced cardiomyocyte death via reducing ER stress-induced UPR activation. It revealed that O-GlcNAcylation may affect cardiovascular events via ER stress-induced UPR. Contrary to the above statements, in neuronal cells, Van der Harg et al. stated that UPR activation could decrease protein O-GlcNAcylation and inhibit mitochondrial respiration via stimulating the IRE1 pathway [110]. Taken together, combined with the previous discussion, all these findings interpret that ER stress/UPR plays a regulatory role in HBP/O-GlcNAcylation balance and they are interrelated and interacted with each other, although to date, the exact mechanism is confused. Nevertheless, these studies may provide an illuminating perspective into new pathogenic mechanisms for the occurrence of CVD.
The crosstalk between O-GlcNAcylation and phosphorylation
As the O-GlcNAc moiety can be attached to protein Ser or Thr sites, two identified phosphorylation locations, it is possible that protein O-GlcNAcylation can regulate cardiac function via changing phosphorylation-related pathways. It is widely accepted that the connection between protein O-GlcNAcylation and phosphorylation is not simply competitive but also reciprocal. Firstly, O-GlcNAcylation is akin to phosphorylation such as the coincident modification sites mainly in protein Ser or Thr residues. Next, both of them can be precisely regulated by different enzymes, e.g., OGT, OGA, phosphatases and kinases, but the number of phosphatases and kinases involved in the process of phosphorylation is much larger than OGT and OGA used for O-GlcNAcylation regulation. According to the available evidence, there are mainly three modes of interactions between protein O-GlcNAcylation and protein phosphorylation on substrates [111]. Firstly, O-GlcNAc and phosphate can competently and alternately occupy the same protein Ser or Thr site, which means there is only one O-GlcNAcylation or phosphorylation modification in a specific amino acid site (Fig. 3a). Secondly, different protein positions can be competently and alternately modified via O-GlcNAc or phosphate proximally, adjacently or distantly (Fig. 3b). Thirdly, protein O-GlcNAcylation and phosphorylation can coexist at different sites of protein substrate (Fig. 3c) [111]. But certain given sites can be modified either by O-GlcNAc or phosphate at one site alone, or both modifications occur at different sites simultaneously. In addition, each modification can directly regulate the activity of the other modification’s cycling enzymes. That is to say, OGT and OGA can be phosphorylated and kinases are able to bear O-GlcNAcylation as well, which is sufficient to transform their efficacy and localization and substrate selection in an opposite or concerted pattern. One canonical example has corroborated that AMPK reduces the production of O-GlcNAcylation through phosphorylation-induced inhibition of GFAT [112]. Hence, intersections between O-GlcNAcylation and phosphorylation are complicated and diverse, although we have conquered a lot about sites of the two modifications, the clearer and more precise mechanisms targeting the crosstalk are still needed.
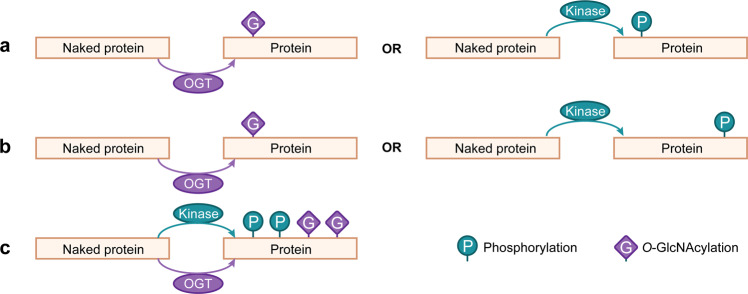
Fig. 3. The crosstalk between protein phosphorylation and O-GlcNAcylation.
a Protein substrate can be either O-GlcNAcylated or phosphorylated at the same amino acid residue in a substitutable and competitive way. b Protein substrate is either phosphorylated at given sites or O-GlcNAcylated at other different sites, which is both reciprocal and alternative. c Protein substrate is simultaneously under O-GlcNAcylation and phosphorylation at different locations.
Perspective in clinical practice
At present, a multitude of reports with regard to molecules, cell culture and animal models have elucidated a tight relationship between protein O-GlcNAcylation and CVD. However, in the context of different diseases or even in the same disease, the O-GlcNAcylation-induced effects have obvious heterogeneities. Remarkably, one pivotal question that whether O-GlcNAcylation is a protective or destructive factor in the occurrence and development of cardiovascular events is inconclusive and controversial. Although the long-period exposure to O-GlcNAcylation had deleterious effects in most cases, O-GlcNAcylation was also documented to execute protective impact. A widely appreciated view partially shows that the acute increase of O-GlcNAcylation performs a cardioprotective role such as in I/R injury, while chronic and long-term growth is detrimental to the normal heart function. As being proved both in early and established hypertrophy, the upregulated O-GlcNAcylation level of PKAc promoted protective cardiac compensation [113]. All these incompatible effects further consolidate the dynamic and intricate feature of protein O-GlcNAcylation and the potential as a target for the treatment of cardiovascular events, but unfortunately, there are currently no reports that drugs targeting O-GlcNAcylation for CVD treatment have been developed.
However, many drugs used in experiments effectively change the intracellular O-GlcNAcylation abundance mostly through increasing or inhibiting the activity of enzymes like OGT, OGA, GFAT or altering the nutrient flux, which has the accessibility to be turned into drugs. Here are common used experimental agents, including OGT inhibitors such as Alloxan, GFAT inhibitors such as O-diazoacetyl-L-serine (azaserine), OGA inhibitors such as PUGNAc (O-(2-acetamido-2-deoxy-D-glucopyranosyliden) amino-N-phenylcarbamate), and others such as glutamine and glucosamine [114]. These drugs have been studied in research laboratories, but not clinically. Therefore, their clinical effectiveness and safety warrant our further studies in treating CVD.
Otherwise, O-GlcNAcylation is also reported to have correlations with NT-proBNP, a major indicator for diagnosis and evaluation of risk stratification in HF [115]. One research has found that proBNP itself is the O-GlcNAc modification target, which has 9 O-GlcNAcylation sites in plasma samples of severe HF patients [116]. Given that the 71st amino acid is located closely to the enzymatic hydrolysis site [117, 118], so the O-GlcNAcylation at this site can inhibit the enzymatic hydrolysis of proBNP, which reduces the production of BNP and NT-proBNP in peripheral circulation [119, 120]. The O-GlcNAcylation degree is shown to be negatively correlated with the concentration of NT-proBNP. This was backed by another research, that in patients with acutely decompensated heart failure (ADHF), the decreased O-GlcNAcylation of proBNP resulted in hyper NT-proBNP [120]. The increased NT-proBNP in ADHF may be a response to myocardial cell function damage [121–124]. Taken together, O-GlcNAcylation is intimately associated with NT-proBNP, and the level of O-GlcNAcylation may be an outstanding measurement for elevating HF.
Of note, protein O-GlcNAcylation also has links with the effect of operational treatment. One recent review summarized that protein O-GlcNAcylation may be an important factor in inducing coronary artery bypass graft failure in coronary heart disease patients with T2DM, and the high O-GlcNAcylation environment in T2DM changed the function of saphenous VSMCs and ECs, which resulted in the reconstruction of saphenous vein and induced graft failure [125, 126]. This shows the great prospect of protein O-GlcNAcylation in clinical application and we have good reason to believe that protein O-GlcNAcylation can be used to explain more characterizations related to the cardiovascular system in the future.
Conclusion
O-GlcNAcylation is a ubiquitous post-translational modification that plays a critical role in modulating cardiovascular function. The level of O-GlcNAcylation is demonstrated to have either positive or negative effects. The acute increase in the level of cardiac O-GlcNAcylation plays beneficial roles, conversely, sustained elevation of cardiac protein O-GlcNAcylation may exert adverse effects inducing severe cardiac dysfunction. By modification of proteins directly or at the transcription level, protein O-GlcNAcylation can further disturb electricity signaling cascades, energy metabolism, cell cycle, stress response and vascular function. The fast-growing analytical tools are being carried out to extrapolate O-GlcNAcylation-induced molecular and physiological alterations of cardiovascular function. Therefore, an enhanced understanding of the mechanisms that O-GlcNAcylation exerted on all of these cardiac signaling pathways will create novel perspectives into the role of O-GlcNAcylation, which may facilitate the generation of therapy approaches for cardiovascular diseases.
Acknowledgements
We thank Professor Ji-wang Chen (from Cardiovascular Research Center, University of Illinois at Chicago) for carefully proofreading this manuscript. This work was supported by the CAMS Innovation Fund for Medical Sciences (No. 2021-I2M-1-018), and Ningxia Hui Autonomous Region Key Research and Development Project (No. 2019BFG02027).
Author contributions
HFW and YXW conceived and prepared the draft of the manuscript. YPZ and YPW revised the manuscript. YY prepared the figures and table. ZCJ and ZJZ supervised the project.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Hui-fang Wang, Yi-xuan Wang.
These authors jointly supervised this work: Zhi-cheng Jing and Ze-jian Zhang.
Contributor Information
Ze-jian Zhang, Email: zejianzhang2018@163.com.
Zhi-cheng Jing, Email: jingzhicheng@vip.163.com.
References
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–17. doi: 10.1016/S0021-9258(17)43295-9. [DOI] [PubMed] [Google Scholar]
- 3.Butkinaree C, Park K, Hart GW. O-linked β-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta. 2010;1800:96–106. doi: 10.1016/j.bbagen.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanover JA, Krause MW, Love DC. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim Biophys Acta. 2010;1800:80–95. doi: 10.1016/j.bbagen.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran DH, Wang ZV. Glucose metabolism in cardiac hypertrophy and heart fail- ure. J Am Heart Assoc. 2019;8:e012673. doi: 10.1161/JAHA.119.012673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson AK, Bouchard B, Zhu WZ, Chatham JC, Des Rosiers C. First characterization of glucose flux through the hexosamine biosynthesis pathway (HBP) in ex vivo mouse heart. J Biol Chem. 2020;295:2018–33. doi: 10.1074/jbc.RA119.010565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, et al. Modulation of dynamin-related protein 1 (DRP1) function by increased O- linked-β-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem. 2012;287:30024–34. doi: 10.1074/jbc.M112.390682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–62. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oki T, Yamazaki K, Kuromitsu J, Okada M, Tanaka I. cDNA cloning and mapping of a novel subtype of glutamine:fructose-6-phosphate amidotransferase (GFAT2) in human and mouse. Genomics. 1999;57:227–34. doi: 10.1006/geno.1999.5785. [DOI] [PubMed] [Google Scholar]
- 10.McKnight GL, Mudri SL, Mathewes SL, Traxinger RR, Marshall S, Sheppard PO, et al. Molecular cloning, cDNA sequence, and bacterial expression of human glutamine:fructose-6-phosphate amidotransferase. J Biol Chem. 1992;267:25208–12. doi: 10.1016/S0021-9258(19)74026-5. [DOI] [PubMed] [Google Scholar]
- 11.Nabeebaccus AA, Verma S, Zoccarato A, Emanuelli G, Santos CX, Streckfuss- Bömeke K, et al. Cardiomyocyte protein O-GlcNAcylation is regulated by GFAT1 not GFAT2. Biochem Biophys Res Commun. 2021;583:121–27. doi: 10.1016/j.bbrc.2021.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belke DD. Swim-exercised mice show a decreased level of protein O-GlcNAcylation and expression of O-GlcNAc transferase in heart. J Appl Physiol (1985) 2011;111:157–62. doi: 10.1152/japplphysiol.00147.2011. [DOI] [PubMed] [Google Scholar]
- 13.Lunde IG, Aronsen JM, Kvaløy H, Qvigstad E, Sjaastad I, Tønnessen T, et al. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol Genomics. 2012;44:162–72. doi: 10.1152/physiolgenomics.00016.2011. [DOI] [PubMed] [Google Scholar]
- 14.Fricovsky ES, Suarez J, Ihm SH, Scott BT, Suarez-Ramirez JA, Banerjee I, et al. Excess protein O-GlcNAcylation and the progression of diabetic cardiomyo- pathy. Am J Physiol Regul Integr Comp Physiol. 2012;303:R689–99. doi: 10.1152/ajpregu.00548.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovas-cular system. Circ Res. 2010;107:171–85. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL, Morales CR, et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine bio- synthetic pathway. Cell. 2014;156:1179–92. doi: 10.1016/j.cell.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng YH, Okolo CA, Erickson JR, Baldi JC, Jones PP. Protein O-GlcNAcylation in the heart. Acta Physiol (Oxf) 2021;233:e13696. doi: 10.1111/apha.13696. [DOI] [PubMed] [Google Scholar]
- 18.Lima VV, Giachini FR, Hardy DM, Webb RC, Tostes RC. O-GlcNAcylation: a novel pathway contributing to the effects of endothelin in the vasculature. Am J Physiol-Regulatory, Integr Comp Physiol. 2011;300:R236–R50. doi: 10.1152/ajpregu.00230.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–22. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 20.Lubas WA, Hanover JA. Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity. J Biol Chem. 2000;275:10983–8. doi: 10.1074/jbc.275.15.10983. [DOI] [PubMed] [Google Scholar]
- 21.Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, et al. Dynamic O- glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem. 2002;277:1755–61. doi: 10.1074/jbc.M109656200. [DOI] [PubMed] [Google Scholar]
- 22.Hanover JA, Yu S, Lubas WB, Shin SH, Ragano-Caracciola M, Kochran J, et al. Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch Biochem Biophys. 2003;409:287–97. doi: 10.1016/S0003-9861(02)00578-7. [DOI] [PubMed] [Google Scholar]
- 23.Chatham JC, Zhang J, Wende AR. Role of O-linked N-acetylglucosamine protein modification in cellular pathophysiology. Physiol Rev. 2021;101:427–93. doi: 10.1152/physrev.00043.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma X, Liu P, Yan H, Sun H, Liu X, Zhou F, et al. Substrate specificity provides insights into the sugar donor recognition mechanism of O-GlcNAc transferase (OGT) PLoS One. 2013;8:e63452. doi: 10.1371/journal.pone.0063452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazarus BD, Roos MD, Hanover JA. Mutational analysis of the catalytic domain of O-linked N-acetylglucosaminyl transferase. J Biol Chem. 2005;280:35537–44. doi: 10.1074/jbc.M504948200. [DOI] [PubMed] [Google Scholar]
- 26.Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature. 2011;469:564–7. doi: 10.1038/nature09638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez DH, Yang B, D’Souza AK, Shen D, Woo CM. Truncation of the TPR domain of OGT alters substrate and glycosite selection. Anal Bioanal Chem. 2021;413:7385–99. doi: 10.1007/s00216-021-03731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson LJ, Long BW, DeMartino AM, Brittian KR, Readnower RD, Brainard RE, et al. Cardiomyocyte Ogt is essential for postnatal viability. Am J Physiol Heart Circ Physiol. 2014;306:H142–53. doi: 10.1152/ajpheart.00438.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comtesse N, Maldener E, Meese E. Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a beta-N-acetylglucosaminidase. Biochem Bio- phys Res Commun. 2001;283:634–40. doi: 10.1006/bbrc.2001.4815. [DOI] [PubMed] [Google Scholar]
- 30.Keembiyehetty CN, Krzeslak A, Love DC, Hanover JA. A lipid-droplet-targeted O- GlcNAcase isoform is a key regulator of the proteasome. J Cell Sci. 2011;124:2851–60. doi: 10.1242/jcs.083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma J, Wu C, Hart GW. Analytical and biochemical perspectives of protein O- GlcNAcylation. Chem Rev. 2021;121:1513–81. doi: 10.1021/acs.chemrev.0c00884. [DOI] [PubMed] [Google Scholar]
- 32.Junod A, Lambert AE, Orci L, Pictet R, Gonet AE, Renold AE. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967;126:201–5. doi: 10.3181/00379727-126-32401. [DOI] [PubMed] [Google Scholar]
- 33.Lin CH, Liao CC, Chen MY, Chou TY. Feedback regulation of O-GlcNAc trans- ferase through translation control to maintain intracellular O-GlcNAc home- ostasis. Int J Mol Sci. 2021;22:3463. doi: 10.3390/ijms22073463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian K, Wang S, Fu M, Zhou J, Singh JP, Li MD, et al. Transcriptional regulation of O-GlcNAc homeostasis is disrupted in pancreatic cancer. J Biol Chem. 2018;293:13989–4000. doi: 10.1074/jbc.RA118.004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muthusamy S, Hong KU, Dassanayaka S, Hamid T, Jones SP. E2F1 transcription factor regulates O-linked N-acetylglucosamine (O-GlcNAc) transferase and O- GlcNAcase expression. J Biol Chem. 2015;290:31013–24. doi: 10.1074/jbc.M115.677534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dassanayaka S, Brittian KR, Jurkovic A, Higgins LA, Audam TN, Long BW, et al. E2f1 deletion attenuates infarct-induced ventricular remodeling without affecting O-GlcNAcylation. Basic Res Cardiol. 2019;114:28. doi: 10.1007/s00395-019-0737-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo WY, Yang WK, Peng CT, Pai WY, Wang HJ. MicroRNA-200a/200b modulate high glucose-induced endothelial inflammation by targeting O-linked N-acetylglucosamine transferase expression. Front Physiol. 2018;9:786. doi: 10.3389/fphys.2018.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D, Hu X, Lee SH, Chen F, Jiang K, Tu Z, et al. Diabetes exacerbates myocardial ischemia/reperfusion injury by down-regulation of microRNA and up-regulation of O-GlcNAcylation. JACC Basic Transl Sci. 2018;3:350–62. doi: 10.1016/j.jacbts.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang M, Xu B, Li X, Shang Y, Chu Y, Wang W, et al. O-GlcNAcylation promotes colorectal cancer metastasis via the miR-101-O-GlcNAc/EZH2 regulatory feed- back circuit. Oncogene. 2019;38:301–16. doi: 10.1038/s41388-018-0435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bullen JW, Balsbaugh JL, Chanda D, Shabanowitz J, Hunt DF, Neumann D, et al. Cross-talk between two essential nutrient-sensitive enzymes: O-GlcNAc trans- ferase (OGT) and AMP-activated protein kinase (AMPK) J Biol Chem. 2014;289:10592–606. doi: 10.1074/jbc.M113.523068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Latorre-Muro P, O’Malley KE, Bennett CF, Perry EA, Balsa E, Tavares CDJ, et al. A cold-stress-inducible PERK/OGT axis controls TOM70-assisted mitochondrial protein import and cristae formation. Cell Metab. 2021;33:598–614.e7. doi: 10.1016/j.cmet.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dias WB, Cheung WD, Wang Z, Hart GW. Regulation of calcium/calmodulin- dependent kinase IV by O-GlcNAc modification. J Biol Chem. 2009;284:21327–37. doi: 10.1074/jbc.M109.007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, et al. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ speci- ficity and subcellular patterns. Cell Rep. 2012;2:419–31. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng K, Liu R, Jia C, Wang Y, Jeong GH, Zhou L, et al. Regulation of O-linked N-acetyl glucosamine transferase (OGT) through E6 stimulation of the ubiquitin ligase activity of E6AP. Int J Mol Sci. 2021;22:10286. doi: 10.3390/ijms221910286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou TY, Hart GW, Dang CV. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem. 1995;270:18961–5. doi: 10.1074/jbc.270.32.18961. [DOI] [PubMed] [Google Scholar]
- 46.Zhong W, Mao S, Tobis S, Angelis E, Jordan MC, Roos KP, et al. Hypertrophic growth in cardiac myocytes is mediated by Myc through a cyclin D2-dependent pathway. EMBO J. 2006;25:3869–79. doi: 10.1038/sj.emboj.7601252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramirez-Correa GA, Jin W, Wang Z, Zhong X, Gao WD, Dias WB, et al. O-linked GlcNAc modification of cardiac myofilament proteins: A novel regulator of myocardial contractile function. Circ Res. 2008;103:1354–8. doi: 10.1161/CIRCRESAHA.108.184978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dubois-Deruy E, Belliard A, Mulder P, Bouvet M, Smet-Nocca C, Janel S, et al. Interplay between troponin T phosphorylation and O-N-acetylglucosaminylation in ischaemic heart failure. Cardiovasc Res. 2015;107:56–65. doi: 10.1093/cvr/cvv136. [DOI] [PubMed] [Google Scholar]
- 49.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–71. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, Hunt DF, et al. A PGC-1alpha-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J Biol Chem. 2009;284:5148–57. doi: 10.1074/jbc.M808890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brainard RE, Facundo HT. Cardiac hypertrophy drives PGC-1α suppression associated with enhanced O-glycosylation. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166080. doi: 10.1016/j.bbadis.2021.166080. [DOI] [PubMed] [Google Scholar]
- 52.Umapathi P, Mesubi OO, Banerjee PS, Abrol N, Wang Q, Luczak ED, et al. Excessive O-GlcNAcylation causes heart failure and sudden death. Circulation. 2021;143:1687–703. doi: 10.1161/CIRCULATIONAHA.120.051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran DH, May HI, Li Q, Luo X, Huang J, Zhang G, et al. Chronic activation of hexosamine biosynthesis in the heart triggers pathological cardiac remodeling. Nat Commun. 2020;11:1771. doi: 10.1038/s41467-020-15640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jewell JL, Guan KL. Nutrient signaling to mTOR and cell growth. Trends Biochem Sci. 2013;38:233–42. doi: 10.1016/j.tibs.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014;114:549–64. doi: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu H, Wen L, Mu Y. O-GlcNAcylation is essential for autophagy in cardiomyo- cytes. Oxid Med Cell Longev. 2020;2020:5602396. doi: 10.1155/2020/5602396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X, Zhang L, He H, Sun Y, Shen Q, Shi L. Increased O-GlcNAcylation induces myocardial hypertrophy. Vitr Cell Dev Biol Anim. 2020;56:735–43. doi: 10.1007/s11626-020-00503-z. [DOI] [PubMed] [Google Scholar]
- 58.Xie S, Jin N, Gu J, Shi J, Sun J, Chu D, et al. O-GlcNAcylation of protein kinase A catalytic subunits enhances its activity: a mechanism linked to learning and memory deficits in Alzheimer’s disease. Aging Cell. 2016;15:455–64. doi: 10.1111/acel.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakagawa T, Furukawa Y, Hayashi T, Nomura A, Yokoe S, Moriwaki K, et al. Augmented O-GlcNAcylation attenuates intermittent hypoxia-induced cardiac remodeling through the suppression of NFAT and NF-κB activities in mice. Hypertens Res. 2019;42:1858–71. doi: 10.1038/s41440-019-0311-x. [DOI] [PubMed] [Google Scholar]
- 60.Si R, Zhang Q, Tsuji-Hosokawa A, Watanabe M, Willson C, Lai N, et al. Over- expression of p53 due to excess protein O-GlcNAcylation is associated with coronary microvascular disease in type 2 diabetes. Cardiovasc Res. 2020;116:1186–98. doi: 10.1093/cvr/cvz216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018;25:104–13. doi: 10.1038/cdd.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8:1074–83. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- 63.Diao J, Liu R, Rong Y, Zhao M, Zhang J, Lai Y, et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature. 2015;520:563–6. doi: 10.1038/nature14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo B, Liang Q, Li L, Hu Z, Wu F, Zhang P, et al. O-GlcNAc-modification of SNAP-29 regulates autophagosome maturation. Nat Cell Biol. 2014;16:1215–26. doi: 10.1038/ncb3066. [DOI] [PubMed] [Google Scholar]
- 65.Huang L, Yuan P, Yu P, Kong Q, Xu Z, Yan X, et al. O-GlcNAc-modified SNAP29 inhibits autophagy-mediated degradation via the disturbed SNAP29-STX17- VAMP8 complex and exacerbates myocardial injury in type I diabetic rats. Int J Mol Med. 2018;42:3278–90. doi: 10.3892/ijmm.2018.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao H, Hu Y, Zhu X, Yao N, Gu J, Wang Y, et al. O-GlcNAc transferase affects the signal transduction of β1 adrenoceptor in adult rat cardiomyocytes by increasing the O-GlcNAcylation of β1 adrenoceptor. Biochem Biophys Res Commun. 2020;528:71–7. doi: 10.1016/j.bbrc.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 67.Prakoso D, Lim SY, Erickson JR, Wallace RS, Lees JG, Tate M, et al. Fine-tuning the cardiac O-GlcNAcylation regulatory enzymes governs the functional and struc- tural phenotype of the diabetic heart. Cardiovasc Res. 2022;118:212–25. doi: 10.1093/cvr/cvab043. [DOI] [PubMed] [Google Scholar]
- 68.Kronlage M, Dewenter M, Grosso J, Fleming T, Oehl U, Lehmann LH, et al. O- GlcNAcylation of histone deacetylase 4 protects the diabetic heart from failure. Circulation. 2019;140:580–94. doi: 10.1161/CIRCULATIONAHA.117.031942. [DOI] [PubMed] [Google Scholar]
- 69.Lehmann LH, Jebessa ZH, Kreusser MM, Horsch A, He T, Kronlage M, et al. A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat Med. 2018;24:62–72. doi: 10.1038/nm.4452. [DOI] [PubMed] [Google Scholar]
- 70.Elgendy IY, Mahtta D, Pepine CJ. Medical therapy for heart failure caused by ischemic heart disease. Circ Res. 2019;124:1520–35. doi: 10.1161/CIRCRESAHA.118.313568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ou W, Liang Y, Qin Y, Wu W, Xie M, Zhang Y, et al. Hypoxic acclimation improves cardiac redox homeostasis and protects heart against ischemia-reperfusion injury through upregulation of O-GlcNAcylation. Redox Biol. 2021;43:101994. doi: 10.1016/j.redox.2021.101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Mole- cular mechanisms of cell death: recommendations of the Nomenclature Com- mittee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Yu P, Hua F, Hu Y, Xiao F, Liu Q, et al. Sevoflurane postconditioning reduces myocardial ischemia reperfusion injury-induced necroptosis by up- regulation of OGT-mediated O-GlcNAcylated RIPK3. Aging (Albany NY) 2020;12:25452–68. doi: 10.18632/aging.104146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chun WJ, Nah DY, Bae JH, Chung JW, Lee H, Moon IS. Glucose-insulin-potassium solution protects ventricular myocytes of neonatal rat in an in vitro coverslip ischemia/reperfusion model. Korean Circ J. 2015;45:234–41. doi: 10.4070/kcj.2015.45.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Howell NJ, Ashrafian H, Drury NE, Ranasinghe AM, Contractor H, Isackson H, et al. Glucose-insulin-potassium reduces the incidence of low cardiac output episodes after aortic valve replacement for aortic stenosis in patients with left ventricular hypertrophy: results from the Hypertrophy, Insulin, Glucose, and Electrolytes (HINGE) trial. Circulation. 2011;123:170–7. doi: 10.1161/CIRCULATIONAHA.110.945170. [DOI] [PubMed] [Google Scholar]
- 77.Whelan SA, Dias WB, Thiruneelakantapillai L, Lane MD, Hart GW. Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-linked beta-N-acetylglucosamine in 3T3-L1 adipocytes. J Biol Chem. 2010;285:5204–11. doi: 10.1074/jbc.M109.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jin L, Gao F, Jiang T, Liu B, Li C, Qin X, et al. Hyper-O-GlcNAcylation impairs insulin response against reperfusion-induced myocardial injury and arrhythmias in obesity. Biochem Biophys Res Commun. 2021;558:126–33. doi: 10.1016/j.bbrc.2021.04.066. [DOI] [PubMed] [Google Scholar]
- 79.Diaz-Ruiz R, Rigoulet M, Devin A. The Warburg and Crabtree effects: On the origin of cancer cell energy metabolism and of yeast glucose repression. Bio- chim Biophys Acta. 2011;1807:568–76. doi: 10.1016/j.bbabio.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 80.Barnes JW, Tian L, Heresi GA, Farver CF, Asosingh K, Comhair SA, et al. O-linked β-N-acetylglucosamine transferase directs cell proliferation in idiopathic pul- monary arterial hypertension. Circulation. 2015;131:1260–8. doi: 10.1161/CIRCULATIONAHA.114.013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Popay TM, Wang J, Adams CM, Howard GC, Codreanu SG, Sherrod SD, et al. MYC regulates ribosome biogenesis and mitochondrial gene expression programs through its interaction with host cell factor-1. Elife. 2021;10:e60191. doi: 10.7554/eLife.60191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Donovan K, Alekseev O, Qi X, Cho W, Azizkhan-Clifford J. O-GlcNAc modification of transcription factor Sp1 mediates hyperglycemia-induced VEGF-A upregula- tion in retinal cells. Invest Ophthalmol Vis Sci. 2014;55:7862–73. doi: 10.1167/iovs.14-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barnes JW, Tian L, Krick S, Helton ES, Denson RS, Comhair SAA, et al. O-GlcNAc transferase regulates angiogenesis in idiopathic pulmonary arterial hyperten- sion. Int J Mol Sci. 2019;20:6299. doi: 10.3390/ijms20246299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shimizu Y, Nicholson CK, Polavarapu R, Pantner Y, Husain A, Naqvi N, et al. Role of DJ-1 in modulating glycative stress in heart failure. J Am Heart Assoc. 2020;9:e014691. doi: 10.1161/JAHA.119.014691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Billia F, Hauck L, Grothe D, Konecny F, Rao V, Kim RH, et al. Parkinson-susceptibility gene DJ-1/PARK7 protects the murine heart from oxidative damage in vivo. Proc Natl Acad Sci USA. 2013;110:6085–90. doi: 10.1073/pnas.1303444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prisco SZ, Rose L, Potus F, Tian L, Wu D, Hartweck L, et al. Excess protein O- GlcNAcylation links metabolic derangements to right ventricular dysfunction in pulmonary arterial hypertension. Int J Mol Sci. 2020;21:7278. doi: 10.3390/ijms21197278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prisco SZ, Eklund M, Raveendran R, Thenappan T, Prins KW. With No Lysine Kinase 1 promotes metabolic derangements and RV dysfunction in pulmonary arterial hypertension. JACC Basic Transl Sci. 2021;6:834–50. doi: 10.1016/j.jacbts.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, et al. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med. 1998;158:917–23. doi: 10.1164/ajrccm.158.3.9802066. [DOI] [PubMed] [Google Scholar]
- 89.Cremona G, Higenbottam T, Borland C, Mist B. Mixed expired nitric oxide in primary pulmonary hypertension in relation to lung diffusion capacity. Qjm. 1994;87:547–51. [PubMed] [Google Scholar]
- 90.Girgis RE, Champion HC, Diette GB, Johns RA, Permutt S, Sylvester JT. Decreased exhaled nitric oxide in pulmonary arterial hypertension: Response to bosentan therapy. Am J Respir Crit Care Med. 2005;172:352–7. doi: 10.1164/rccm.200412-1684OC. [DOI] [PubMed] [Google Scholar]
- 91.Förstermann U, Sessa WC. Nitric oxide synthases: Regulation and function. Eur Heart J. 2012;33:829–37. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–58. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heiss EH, Dirsch VM. Regulation of eNOS enzyme activity by posttranslational modification. Curr Pharm Des. 2014;20:3503–13. doi: 10.2174/13816128113196660745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aulak KS, Barnes JW, Tian L, Mellor NE, Haque MM, Willard B, et al. Specific O-GlcNAc modification at Ser-615 modulates eNOS function. Redox Biol. 2020;36:101625. doi: 10.1016/j.redox.2020.101625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ednie AR, Bennett ES. Intracellular O-linked glycosylation directly regulates cardiomyocyte L-type Ca2+ channel activity and excitation-contraction cou- pling. Basic Res Cardiol. 2020;115:59. doi: 10.1007/s00395-020-00820-0. [DOI] [PubMed] [Google Scholar]
- 96.Hegyi B, Borst JM, Bailey LRJ, Shen EY, Lucena AJ, Navedo MF, et al. Hypergly- cemia regulates cardiac K+ channels via O-GlcNAc-CaMKII and NOX2-ROS-PKC pathways. Basic Res Cardiol. 2020;115:71. doi: 10.1007/s00395-020-00834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ravens U, Cerbai E. Role of potassium currents in cardiac arrhythmias. Europace. 2008;10:1133–7. doi: 10.1093/europace/eun193. [DOI] [PubMed] [Google Scholar]
- 98.Schmitt N, Grunnet M, Olesen SP. Cardiac potassium channel subtypes: New roles in repolarization and arrhythmia. Physiol Rev. 2014;94:609–53. doi: 10.1152/physrev.00022.2013. [DOI] [PubMed] [Google Scholar]
- 99.Bacigalupa ZA, Bhadiadra CH, Reginato MJ. O-GlcNAcylation: Key regulator of glycolytic pathways. J Bioenerg Biomembr. 2018;50:189–98. doi: 10.1007/s10863-018-9742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Akinbiyi EO, Abramowitz LK, Bauer BL, Stoll MSK, Hoppel CL, Hsiao CP, et al. Blocked O-GlcNAc cycling alters mitochondrial morphology, function, and mass. Sci Rep. 2021;11:22106. doi: 10.1038/s41598-021-01512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lima VV, Giachini FR, Carneiro FS, Carvalho MH, Fortes ZB, Webb RC, et al. O- GlcNAcylation contributes to the vascular effects of ET-1 via activation of the RhoA/Rho-kinase pathway. Cardiovasc Res. 2011;89:614–22. doi: 10.1093/cvr/cvq338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guo X, Deng Y, Zhan L, Shang J, Liu H. O‑GlcNAcylation contributes to inter- mittent hypoxia‑associated vascular dysfunction via modulation of MAPKs but not CaMKII pathways. Mol Med Rep. 2021;24:744. doi: 10.3892/mmr.2021.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cui YL, Xue RQ, Xi H, Ming Z, Yu XJ, Liu LZ, et al. Cholinergic drugs ameliorate endothelial dysfunction by decreasing O-GlcNAcylation via M3 AChR-AMPK-ER stress signaling. Life Sci. 2019;222:1–12. doi: 10.1016/j.lfs.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 104.Mu Y, Yu H, Wu T, Zhang J, Evans SM, Chen J. O-linked β-N-acetylglucosamine transferase plays an essential role in heart development through regulating angiopoietin-1. PLoS Genet. 2020;16:e1008730. doi: 10.1371/journal.pgen.1008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zibrova D, Vandermoere F, Göransson O, Peggie M, Mariño KV, Knierim A, et al. GFAT1 phosphorylation by AMPK promotes VEGF-induced angiogenesis. Bio- chem J. 2017;474:983–1001. doi: 10.1042/BCJ20160980. [DOI] [PubMed] [Google Scholar]
- 106.Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21:421–38. doi: 10.1038/s41580-020-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 108.Jiang M, Yu S, Yu Z, Sheng H, Li Y, Liu S, et al. XBP1 (X-Box-Binding Protein-1)- dependent O-GlcNAcylation is neuroprotective in ischemic stroke in young mice and its impairment in aged mice is rescued by Thiamet-G. Stroke. 2017;48:1646–54. doi: 10.1161/STROKEAHA.117.016579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reti- culum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van der Harg JM, van Heest JC, Bangel FN, Patiwael S, van Weering JR, Scheper W. The UPR reduces glucose metabolism via IRE1 signaling. Biochim Biophys Acta Mol Cell Res. 2017;1864:655–65. doi: 10.1016/j.bbamcr.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 111.Zeidan Q, Hart GW. The intersections between O-GlcNAcylation and phos- phorylation: implications for multiple signaling pathways. J Cell Sci. 2010;123:13–22. doi: 10.1242/jcs.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gélinas R, Mailleux F, Dontaine J, Bultot L, Demeulder B, Ginion A, et al. AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat Commun. 2018;9:374. doi: 10.1038/s41467-017-02795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu WZ, El-Nachef D, Yang X, Ledee D, Olson AK. O-GlcNAc transferase pro- motes compensated cardiac function and protein kinase A O-GlcNAcylation during early and established pathological hypertrophy from pressure overload. J Am Heart Assoc. 2019;8:e011260. doi: 10.1161/JAHA.118.011260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ferron M, Denis M, Persello A, Rathagirishnan R, Lauzier B. Protein O-GlcNAcylation in cardiac pathologies: past, present, future. Front Endocrinol (Lausanne) 2018;9:819. doi: 10.3389/fendo.2018.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nishikimi T, Kuwahara K, Nakao K. Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. J Cardiol. 2011;57:131–40. doi: 10.1016/j.jjcc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 116.Halfinger B, Hammerer-Lercher A, Amplatz B, Sarg B, Kremser L, Lindner HH. Unraveling the molecular complexity of O-glycosylated endogenous (N-Terminal) pro-B-type natriuretic peptide forms in blood plasma of patients with severe heart failure. Clin Chem. 2017;63:359–68. doi: 10.1373/clinchem.2016.265397. [DOI] [PubMed] [Google Scholar]
- 117.Schellenberger U, O’Rear J, Guzzetta A, Jue RA, Protter AA, Pollitt NS. The pre- cursor to B-type natriuretic peptide is an O-linked glycoprotein. Arch Biochem Biophys. 2006;451:160–6. doi: 10.1016/j.abb.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 118.Seferian KR, Tamm NN, Semenov AG, Tolstaya AA, Koshkina EV, Krasnoselsky MI, et al. Immunodetection of glycosylated NT-proBNP circulating in human blood. Clin Chem. 2008;54:866–73. doi: 10.1373/clinchem.2007.100040. [DOI] [PubMed] [Google Scholar]
- 119.Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, Serebryanaya DV, et al. Processing of pro-B-type natriuretic peptide: Furin and corin as can- didate convertases. Clin Chem. 2010;56:1166–76. doi: 10.1373/clinchem.2010.143883. [DOI] [PubMed] [Google Scholar]
- 120.Vodovar N, Séronde MF, Laribi S, Gayat E, Lassus J, Boukef R, et al. Post- translational modifications enhance NT-proBNP and BNP production in acute decompensated heart failure. Eur Heart J. 2014;35:3434–41. doi: 10.1093/eurheartj/ehu314. [DOI] [PubMed] [Google Scholar]
- 121.Christ-Crain M, Breidthardt T, Stolz D, Zobrist K, Bingisser R, Miedinger D, et al. Use of B-type natriuretic peptide in the risk stratification of community-acquired pneumonia. J Intern Med. 2008;264:166–76. doi: 10.1111/j.1365-2796.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 122.Jensen J, Ma LP, Fu ML, Svaninger D, Lundberg PA, Hammarsten O. Inflamma- tion increases NT-proBNP and the NT-proBNP/BNP ratio. Clin Res Cardiol. 2010;99:445–52. doi: 10.1007/s00392-010-0140-z. [DOI] [PubMed] [Google Scholar]
- 123.Pirracchio R, Deye N, Lukaszewicz AC, Mebazaa A, Cholley B, Matéo J, et al. Impaired plasma B-type natriuretic peptide clearance in human septic shock. Crit Care Med. 2008;36:2542–6. doi: 10.1097/CCM.0b013e318183f067. [DOI] [PubMed] [Google Scholar]
- 124.Yetkin O, Hacievliyagil SS, Gunen H. Assessment of B-type natriuretic peptide in patients with pneumonia. Int J Clin Pract. 2008;62:488–91. doi: 10.1111/j.1742-1241.2007.01305.x. [DOI] [PubMed] [Google Scholar]
- 125.Bolanle IO, Riches-Suman K, Loubani M, Williamson R, Palmer TM. Revascular- isation of type 2 diabetics with coronary artery disease: Insights and therapeutic targeting of O-GlcNAcylation. Nutr Metab Cardiovasc Dis. 2021;31:1349–56. doi: 10.1016/j.numecd.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 126.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogen- esis, predisposition, and prevention. Circulation. 1998;97:916–31. doi: 10.1161/01.CIR.97.9.916. [DOI] [PubMed] [Google Scholar]