Abstract
Previously, we had demonstrated that a Legionella pneumophila prepilin peptidase (pilD) mutant does not produce type IV pili and shows reduced secretion of enzymatic activities. Moreover, it displays a distinct colony morphology and a dramatic reduction in intracellular growth within amoebae and macrophages, two phenotypes that are not exhibited by a pilin (pilEL) mutant. To determine whether these pilD-dependent defects were linked to type II secretion, we have constructed two new mutants of L. pneumophila strain 130b. Mutations were introduced into either lspDE, which encodes the type II outer membrane secretin and ATPase, or lspFGHIJK, which encodes the pseudopilins. Unlike the wild-type and pilEL strains, both lspDE and lspG mutants showed reduced secretion of six pilD-dependent enzymatic activities; i.e., protease, acid phosphatase, p-nitrophenol phosphorylcholine hydrolase, lipase, phospholipase A, and lysophospholipase A. However, they exhibited a colony morphology different from that of the pilD mutant, suggesting that their surfaces are distinct. The pilD, lspDE, and lspG mutants were similarly and greatly impaired for growth within Hartmannella vermiformis, indicating that the intracellular defect of the peptidase mutant in amoebae is explained by the loss of type II secretion. When assessed for infection of U937 macrophages, both lsp mutants exhibited a 10-fold reduction in intracellular multiplication and a diminished cytopathic effect. Interestingly, the pilD mutant was clearly 100-fold more defective than the type II secretion mutants in U937 cells. These results suggest the existence of a novel pilD-dependent mechanism for promoting L. pneumophila intracellular infection of human cells.
The gram-negative bacterium Legionella pneumophila is the agent of Legionnaires' disease, a potentially fatal pneumonia (14, 66). In nature, the organism replicates within protozoan hosts and biofilms found in aquatic environments (8, 16, 24). Following inhalation of aerosolized droplets, L. pneumophila invades and multiplies within alveolar macrophages (1, 57, 60, 64, 66). Various factors that are associated with L. pneumophila infection of protozoa and macrophages have been reported. These include major outer membrane proteins (29, 37), Mip (18, 27), flagella (49), type IV pili (58), a catalase-peroxidase (12), growth phase (17), iron acquisition (63), and the Dot-Icm putative type IV secretion apparatus (52, 55, 64).
Our previous studies have shown that a prepilin peptidase gene, pilD, is essential for Legionella growth in amoebae and human macrophages (38, 39). Moreover, a pilD mutant is dramatically reduced in virulence, following intratracheal inoculation of guinea pigs (38). By virtue of their ability to process pilin and the so-called pseudopilins, prepilin peptidases are implicated both in the formation of type IV pili and in protein secretion (41, 45, 46, 48, 59). One set of pseudopilins is involved in the assembly of the pili, and another one is involved in the genesis of a functional type II protein secretion system. Accordingly, the L. pneumophila pilD mutant does not produce type IV pili and lacks a number of secreted proteins and enzymatic activities in its culture supernatants (6, 28, 38). Interestingly, the pilD mutant also displays an altered colony morphology that is not associated with simple loss of pili (38). Since a mutant containing an insertion in the type IV pilin structural gene (pilEL) is not defective for growth within amoebae and macrophages (58), we postulated the existence of a type II secretion system in L. pneumophila and argued that some secreted proteins might be virulence factors necessary for intracellular replication (38, 39). Prior to that study (38), pilD and type II secretion had not been implicated in bacterial intracellular infection. Importantly, a subsequent study demonstrated the presence of genes, lspFGHIJK (for Legionella secretion pathway), encoding some conserved components of type II secretion systems in L. pneumophila (32).
Through mutational analysis, the present study provides a definite link between L. pneumophila type II secretion and colony morphology, all known pilD-dependent enzymatic activities, and multiple forms of intracellular infection. This genetic approach had two key aspects. First, we mutated two loci (lspDE and lspFGHIJK) since it is believed that bacterial PilD proteins do not influence all portions of the type II system equally (26, 45, 46, 50, 53). In one locus, lspD is predicted to encode the outer membrane secretin, and lspE is predicted to encode the ATPase of the system (26, 50, 53). There are no data to suggest that type II secretins or ATPase are directly cleaved by PilD. The other locus, lspFGHIJK, is predicted to encode the pseudopilins, which, in other bacteria, are directly processed by PilD (45, 46, 53). Second, we introduced these mutations into the virulent strain 130b. As a result, the lsp mutants could be directly compared to the 130b mutants deficient in pilD and pilEL, permitting clear distinctions between the relative roles of pilD, pilin, and type II protein secretion.
MATERIALS AND METHODS
Bacterial strains and media.
L. pneumophila serogroup 1 strain 130b (Wadsworth) (ATCC BAA-74) and its derivatives NU243 and BS100, which contain stable insertions of a kanamycin resistance (Kmr) gene in pilD and pilEL, respectively, were described previously (22, 38, 58). Legionellae were cultured at 37°C in buffered yeast extract (BYE) broth or on buffered charcoal yeast extract (BCYE) agar (21). Growth in liquid medium was assessed by measuring the optical density of the culture at 660 nm. Escherichia coli strains HB101 (13) and NovaBlue (Novagen, Madison, Wis.), hosts for recombinant plasmids, were grown at 37°C on Luria-Bertani agar (9). The following antibiotics were added to the media at the indicated final concentrations (micrograms per milliliter): ampicillin, 100; chloramphenicol, 3 for L. pneumophila and 30 for E. coli; and kanamycin, 25 for L. pneumophila and 50 for E. coli.
Generation of lspDE and lspG mutants.
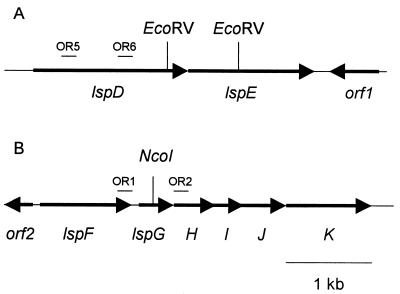
To isolate cloned lsp genes for mutagenesis, L. pneumophila 130b genomic libraries (7, 34) were screened by colony hybridization using digoxigenin-labeled probes (Boehringer Mannheim, Indianapolis, Ind.). The two DNA fragments that served as probes were amplified from 130b genomic DNA by PCR using primers OR5 (5′-TTGATTCTGTCTGGTCGAGC) and OR6 (5′-ATCAAGGACTACTACGGAGG) for lspD and primers OR1 (5′-TCAGACATGATGGAACGCTC) and OR2 (5′-CTTGTTGTTGAGCCAGGCTT) for lspG (see Fig. 1). As the next step towards generating a mutation in lspD and lspE, a 3.6-kb PstI fragment containing lspD and lspE sequences was subcloned into pUC119 (62). Then, a 0.8-kb EcoRV fragment containing part of lspD and lspE was deleted and replaced by a Kmr cassette from plasmid pVK3 (63), generating pUE4Kan. Following BamHI and SphI digestion, the insert of pUE4Kan was subcloned into sacB-containing pBOC20 (47), giving pOE4Kan. This final plasmid was electroporated into strain 130b, and lspDE mutants were isolated by allelic exchange, as described previously (38). To disrupt the lspG gene, a 3.8-kb EcoRI-HindIII fragment containing lspG was subcloned into pBluescript II KS(+) (Stratagene, La Jolla, Calif.). The resulting plasmid was digested with NcoI, which cuts 289 bp after the lspG start codon; treated with Klenow fragment; and ligated to the Kmr cassette from pVK3, giving pBFK4Kan. Using BamHI and SalI digestions, the insert of pBFK4Kan was then cloned into pBOC20, resulting in plasmid pOFK4Kan. Following pOFK4Kan electroporation, the lspG insertion mutation was introduced by allelic exchange in the genome of L. pneumophila 130b (38). The identity of the lspDE and lspG mutants was confirmed by Southern blot analysis (data not shown). Standard techniques were used for DNA isolation, cloning and sequencing (9, 35).
FIG. 1.
Schematic of L. pneumophila strain 130b open reading frames (indicated by arrows) in the lspDE (A) and lspFGHIJK (B) loci. For construction of the lspDE mutant, an internal 0.8-kb EcoRV fragment was deleted and replaced by a Kmr cassette (A). For the lspG mutant, the Kmr cassette was inserted in the NcoI site in the lspG open reading frame (B). Note that there is no NcoI site in lspH from L. pneumophila strain 130b, unlike in L. pneumophila strain Philadelphia-1 (32). The binding locations of the primers OR5, OR6, OR1, and OR2 used for amplification of the lsp probes are indicated by the small bars. Lower right bar, scale.
Analysis of supernatants and cell lysates.
L. pneumophila supernatants and cell lysates were prepared from cultures in late exponential phase (6). They were tested for protease activity as determined by azocasein hydrolysis, for acid phosphatase activity as determined by the release of p-nitrophenol (p-NP) from p-NP phosphate at pH 5.0, for the ability to release p-NP from p-NP phosphorylcholine (p-NPPC), for lipase activity as determined by 1-monopalmitoyl glycerol hydrolysis, and for phospholipase A activity as determined by phosphatidylcholine hydrolysis (6). Lipolytic activities were also determined by p-NP palmitate and p-NP caprylate hydrolysis (6). To analyze the presence of the secreted lysophospholipase A activity (28), 100 μl of supernatant was incubated at 37°C for 5 h with 100 μl lysophosphatidyl choline palmitoyl (6.8 mg per ml; Sigma catalog no. L-5254) in 20 mM Tris (pH 8)–0.5% Triton X-100–3 mM sodium azide. Free fatty acid levels were then determined by the NEFA-C kit (Wako Chemicals, Neuss, Germany). One unit of acid phosphatase and p-NPPC hydrolase activity is defined as that which yields 1 nmol of p-NP per min per ml of supernatant. One unit of lipolytic enzyme activity is defined as that which yields 1 nmol of free fatty acid per min per ml of supernatant.
Intracellular infection of Hartmannella amoebae and human U937 cells by L. pneumophila.
To assess the ability of L. pneumophila to grow within a protozoan host, Hartmannella vermiformis was infected as previously described (38). As has been done by a number of investigators, human U937 cells served as the model for L. pneumophila intracellular infection of macrophages (25, 31, 42, 51, 58, 65). Briefly, wells containing amoebae or U937 cells were infected with comparable numbers of CFU of wild-type and mutant strains, and at various time points the number of bacteria per monolayer was determined by plating (38). To measure the cytopathic effect of L. pneumophila strains on U937 cells, the ability of the infected monolayers to reduce alamar blue (Biosource International, Vacaville, Calif.) was determined (5, 58). Briefly, at different time points, the monolayers were washed twice with RPMI 1640 (Mediatech, Herndon, Va.) and then incubated with RPMI 1640 containing alamar blue at 37°C for 3 h. The reduction of the dye was then measured by fluorescence; i.e., 540-nm excitation and 590-nm emission wavelengths.
Nucleotide sequence accession number.
The nucleotide sequences for the lspDE locus and the lspF gene are deposited in the National Center for Biotechnology Information GenBank under accession number AF330136 and AF330137, respectively.
RESULTS AND DISCUSSION
Identification and mutation of lspDE and lspFGHIJK in L. pneumophila.
A portion of the lspDE locus was identified by a BLAST search (4) of the developing L. pneumophila Philadelphia-1 genome database (http://www.genome3.cpmc.columbia.edu/∼legion). To identify the Legionella secretin, we applied three criteria to our database search. First, the search was performed using, as query sequences, protein sequences of three type II secretion secretins; i.e., Klebsiella pneumoniae PulD, Pseudomonas aeruginosa XcpQ, and Erwinia chrysanthemi OutD (2, 20, 40). Second, only the sequences that gave the highest scores with all three secretins were analyzed further. Third, the selected open reading frame had to be more similar to secretins involved in type II secretion than those involved in type IV pilus assembly. The lspFGHIJK locus of strain Philadelphia-1 had been previously identified, and complete published sequence data were available for the last five genes, lspGHIJK (32). L. pneumophila 130b genomic libraries were screened by colony hybridization for lspD and lspG. The screen for lspD yielded a 4.5-kb fragment of Legionella DNA. Sequence analysis identified two complete open reading frames, in an operon arrangement (Fig. 1A), that encoded proteins with 32% identity and 53% similarity with secretin OutD from E. chrysanthemi and 58% identity and 75% similarity with type II ATPase XcpR from P. aeruginosa, respectively (11, 19) (Fig. 1). Additionally, a reading frame with no homology was found downstream of, but in the opposite direction from, lspE (Fig. 1A). PCR and sequence analysis of the clones obtained with the lspG probe confirmed the presence of lspGHIJK in strain 130b and demonstrated the existence of a complete L. pneumophila lspF gene, which is predicted to encode an inner membrane protein with 39% identity and 57% similarity with OutS from Pseudomonas alcaligenes (30). Upstream of lspF was an incomplete open reading frame, which showed homology to a glutamine synthetase from Shewanella violacea (36) (Fig. 1B). According to the restriction maps of our 130b clones, as well as the Philadelphia-1 genome database, the lspDE and lspFGHIJK loci are predicted to be separated by at least 2.7 kb in L. pneumophila, a situation unlike that of all previously identified type II secretion genes (44).
To mutate lspD and lspE, a portion of both genes was deleted and replaced with a Kmr cassette (Fig. 1A). To inactivate the lspFGHIJK locus, the Kmr cassette was introduced in lspG (Fig. 1B). Three lspDE mutants and two lspG mutants were obtained independently. All lspDE mutants behaved the same in all assays described below, and a similar result was obtained with the lspG mutants, strongly suggesting that the alterations in phenotype observed were due to the loss of lsp function and not to spontaneous second-site mutations. Thus, for simplicity, findings will only be presented for one lspDE mutant (i.e., NU258) and one lspG mutant (i.e., NU259).
Colony morphology of the L. pneumophila lspDE and lspG mutants.
Since the pilD mutant NU243 shows a different colony morphology than the wild-type strain 130b and the pilEL mutant BS100 when grown on BCYE agar (38), we examined colony morphologies of NU258 and NU259. After 3 days on the agar plates, the lsp mutant colonies, like those of the pilD mutant, exhibited a flatter shape and a darker gray color than did the wild-type and pilEL mutant colonies (data not shown), indicating that the type II secretion pathway is indeed responsible for the previously noted change in morphology. As was previously observed for the pilD mutant (38), a fivefold reduction in recoverable NU258 and NU259 was observed when bacteria were examined after 72 h of growth on BCYE agar. The altered colony phenotype, which has been rarely observed in other systems, could be due to the absence of secreted or surface-exposed substances in the pilD and lsp mutants. Indeed, Vibrio cholerae type II secretion mutants can no longer switch from a smooth to rugose colony morphology (3), a phenotype that normally correlates with the secretion of exopolysaccharides (67). Moreover, V. cholerae mutants lacking the prepilin peptidase VcpD or the type II secretion system have aberrant outer membrane protein profiles (41, 54).
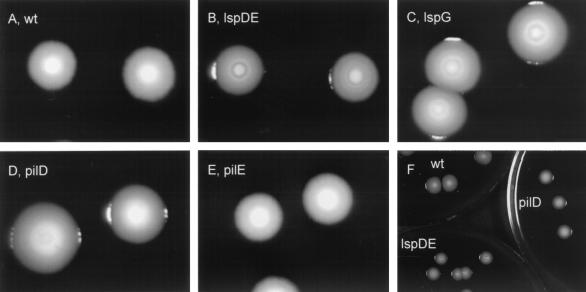
After 6 or more days of incubation, NU258 and NU259 colonies, unlike 130b colonies, displayed concentric circles (Fig. 2A to C). In contrast, NU243 colonies possessed a third type of morphology; i.e., they exhibited a cavity in their center, reminiscent of a collapsed dome (Fig. 2D). The morphology, browner color, and rougher edges of the pilEL colonies were always similar to that of the wild type (Fig. 2A and E). These data suggest that the secreted and/or surface profiles of the pilD and lsp mutants are not always identical and that this difference cannot be explained by differences in type IV pilus assembly. It seems unlikely that this difference is only due to loss of PilD, since prepilin peptidases have been localized in the inner membrane (10, 53). To our knowledge, differences in colony morphology between pilD and type II secretion mutants have not been observed before.
FIG. 2.
Colony morphology of L. pneumophila strains. Wild-type (wt) 130b (A), lspDE mutant NU258 (B), lspG mutant NU259 (C), pilD mutant NU243 (D), and pilEL mutant BS100 (E) were grown on BCYE agar at 37°C for 3 days and subsequently at room temperature for 9 days. Note that it may be difficult to see the cavity of the NU243 colonies in panel B. Although it might appear from the images in panels A to E that the strains' colonies had different sizes, this was not the case; e.g., panel F presents an image of three plates containing either 130b (upper left), NU243 (right) or NU258 (lower left).
Analysis of culture supernatants from L. pneumophila lspDE and lspG mutants.
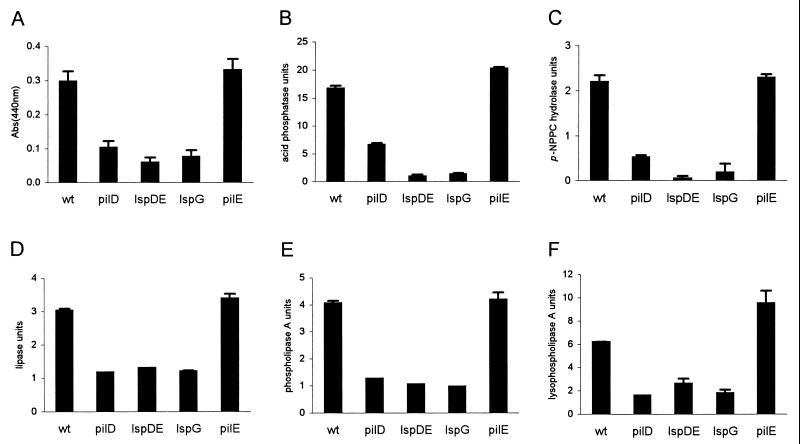
Previous analysis of the supernatant of the L. pneumophila prepilin peptidase mutant showed the absence of protease, acid phosphatase, p-NPPC hydrolase, lipase, phospholipase A, and lysophospholipase A activities (6, 28, 38). Aside from the zinc-metalloprotease (32), none of these activities had been formally linked to the Legionella type II secretion pathway. Thus, mutants NU258 and NU259 were grown in BYE to late exponential phase, the time at which differences between the pilD mutant and wild type are maximal, and then filtered supernatants and cell lysates were assayed as described previously (6). The protease, acid phosphatase, and the p-NPPC hydrolase activities were reduced in the supernatants of the pilD, lspDE, and lspG mutants compared to the wild type and the pilEL mutant (Fig. 3A to C). As expected for secretion mutants, these activities accumulated in the cell lysates (data not shown). Using 1-monopalmitoyl glycerol, phosphatidyl choline, and lysophosphatidyl choline as substrates, we also observed that the lipase, phospholipase A, and lysophospholipase A activities, respectively, were reduced in the supernatants of the lspDE- and lspG-deficient strains, as they are in the pilD, but not the pilEL mutant (Fig. 3D to F). The hydrolysis of p-NP palmitate and p-NP caprylate, artificial substrates for lipolytic enzymes, was also reduced in the supernatants of NU258 and NU259 (data not shown). When we compared acid phosphatase activity between the supernatants of the lsp mutants and the wild type at early logarithmic and at stationary phase, we confirmed that the reduction in enzymatic activity was maximal at late exponential phase (data not shown), as they had been before for the pilD mutant (6). In the course of performing these studies, we observed that the lsp mutants grew from exponential to stationary phases in a manner that was comparable to the wild type (data not shown). In summary, in L. pneumophila, the secretion of protease, acid phosphatase, p-NPPC hydrolase, lipase, phospholipase A, and lysophospholipase A activities is dependent on the type II secretion system. To our knowledge, this is the first time an acid phosphatase and a phospholipase A have been formally linked to type II secretion.
FIG. 3.
Secreted enzymatic activities of L. pneumophila strains. Culture supernatants of wild-type (wt) 130b, pilD mutant NU243, lspDE mutant NU258, lspG mutant NU259, and pilEL mutant BS100 were tested for protease (A), acid phosphatase (B), p-NPPC hydrolase (C), lipase (D), phospholipase A (E), and lysophospholipase A (F) activities. These data represent the mean and standard deviation (error bars) for duplicate cultures. For all, the differences between the wild type and the pilD and lsp mutants were significant (P < 0.01 [Student's t test]). Comparable results were obtained on at least two other occasions (data not shown).
Intracellular infection by L. pneumophila lsp mutants.
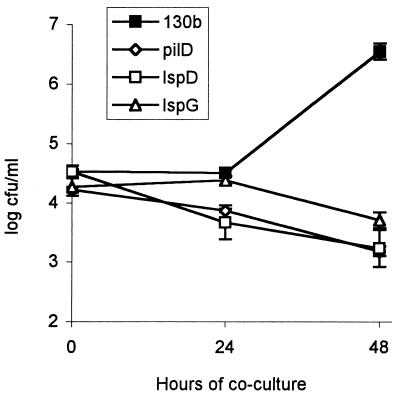
To assess the contribution of type II secretion versus PilD to intracellular replication within a natural Legionella host, NU258, NU259, and NU243 were assessed for their relative ability to infect the amoeba H. vermiformis (Fig. 4). In four different experiments, the pilD and lsp mutants were similarly and dramatically impaired for intracellular growth; i.e., by 48 h postinfection, there was a ca. 1,000-fold reduction in CFU compared to the wild type. Since the pilin mutant strain BS100 did not have any defect in intracellular growth (data not shown) (38, 58), this result shows that type II secretion is important for intra-amoebal growth. A similar conclusion was obtained previously within the Acanthamoeba castellanii model using Philadelphia-1 and an lspG mutant derivative (32). It is now clear, from our findings, that the intracellular growth defect of the pilD mutant in amoebae can be explained nearly, if not completely, by the loss of the type II secretion system.
FIG. 4.
Intracellular infection of amoebae with L. pneumophila strains. Wells containing H. vermiformis were infected at a multiplicity of infection of 0.1 with 130b (black squares), NU243 (white diamonds), NU258 (white squares), or NU259 (white triangles). Bacterial CFU per well were determined at 0, 24, and 48 h after inoculation. Each datum point represents the mean and standard deviation (error bars) of three wells. Significant differences in recovery between 130b and its mutant derivatives were evident at 48 h (P < 0.01 [Student's t test]). These differences were observed in three additional experiments (data not shown).
To assess the importance of type II secretion in macrophage infection, we determined the relative ability of NU258 and NU259 to infect U937 cells, a human macrophage-like cell line (Fig. 5). In four independent experiments, the lsp mutant strains showed a 5- to 10-fold reduction in CFU compared to the wild type (Fig. 5A). In the same experiments, the pilD mutant exhibited a 100- to 1,000-fold reduction in CFU (Fig. 5A), as had been seen before (6, 38). The reduced recovery of both types of lsp mutants correlated with a reduced cytopathic effect (Fig. 5B). These results indicate that the type II secretion pathway has a role, albeit a modest one, in L. pneumophila intracellular infection within U937 macrophages. It was reported previously that an L. pneumophila Philadelphia-1 lspG mutant did not have a reduced cytopathic effect on HL-60 macrophages, and it was concluded that the Legionella type II secretion system has no role in macrophage infection (32). No CFU determinations were reported in that study. The discrepancy between the two results could be explained by differences in the L. pneumophila strains, cytopathicity assays, or macrophage cell lines used in the studies. We favor the last hypothesis, since preliminary results indicate that the pilD mutant only showed a 10-fold reduction in CFU within HL-60 cells (data not shown), in contrast to the 100- to 1,000-fold reduction seen within U937 macrophages (6, 38).
FIG. 5.
Macrophage infection by L. pneumophila strains. (A) U937 cells were infected at a multiplicity of infection of 0.1 with 130b (black squares), NU243 (white diamonds), NU258 (white squares), or NU259 (white triangles). Bacterial CFU per monolayer were determined at 0, 24, and 48 h after inoculation. Each datum point represents the mean and standard deviation (error bars) of three wells. Significant differences in recovery between 130b and its lsp mutant derivatives were evident at 48 h (P < 0.001 [Student's t test]) and were observed in three additional experiments (data not shown). (B) Replicate U937 cell monolayers (n = 6) were either not infected or infected at a multiplicity of infection of 1 with wild-type 130b (black bars), pilD mutant NU243 (white bars), lspDE mutant NU258 (horizontally striped bars), or lspG mutant NU259 (wavy bars). After 16 and 42 h of incubation, the viability of the host cells was measured by their ability to reduce alamar blue. Datum points represent the mean and standard deviation (error bars) of the percentage of dye reduction by infected cells compared to uninfected cells. Significant differences in cytopathic effect between 130b and its mutant derivatives were evident at 42 h (P < 0.001 [Student's t test]). Similar results were obtained in two additional experiments, which used either a multiplicity of infection of 1 or 0.1 (data not shown).
Since the Legionella Lsp system is indeed important for optimal infection of both amoebae and macrophages, consideration should be given to defining which of the type II exoproteins are promoting intracellular growth. Mutants lacking the zinc-metalloprotease or the major acid phosphatase activity do not exhibit intracellular growth defects in protozoa and phagocytes (5, 43, 61), and a similar observation was made with mutants deficient in secretion of p-NPPC hydrolase (6). Hence, other secreted proteins, such as the lipolytic enzymes and yet unknown proteins, remain to be studied for their role in intracellular infection. Clearly, type II secretion mutants are more defective in H. vermiformis than in U937 cells, and therefore type II exoproteins may play a larger role in amoebae infection than in macrophages. There have been other cases where a particular L. pneumophila mutant was more defective in protozoans than in human monocyte cell lines (15, 23, 33, 56; M. Robey, W. A. O'Connell, and N. P. Cianciotto, submitted for publication). Although the importance of type II secretion appears to be relatively modest in macrophage cell lines, it needs to be emphasized that the system may still be quite significant in disease progression. For example, it may promote L. pneumophila extracellular survival in the lung or facilitate tissue destruction. The fact that the pilD mutant was more defective in the guinea pig lung than in U937 cells suggests the importance of extramacrophage growth and/or survival (38).
Perhaps the most intriguing and particularly novel result of our study was the observed difference between the lsp and pilD mutants for growth and cytopathicity in macrophages (Fig. 5). The growth defect of the prepilin peptidase mutant was consistently 100-fold more than those for both types of lsp mutants (Fig. 5A). It is unlikely that this difference is explained by the absence of the type IV pilus in the pilD mutant, since the pilEL pilin mutant shows no defect in growth within U937 cells (data not shown) (38, 58). Taken together, these data indicate that the L. pneumophila PilD protein influences an additional pathway that has particular relevance for infection in macrophages. Given that the pilD and lsp mutants differed significantly in colony morphology (Fig. 2), it is conceivable that secreted and/or surface determinants are part of this virulence system. To our knowledge, the existence of a third PilD-dependent pathway has not been hypothesized before based upon data from any other bacterium. Thus, continued analysis of L. pneumophila pilD, lsp, and pilus mutants not only should expand our understanding of Legionnaires' disease but also may provide new paradigms for protein secretion systems.
ACKNOWLEDGMENTS
We thank Yousef Abu Kwaik for kindly providing strain BS100.
This work was supported by National Institutes of Health grant AI43987 awarded to N.P.C.
REFERENCES
- 1.Abu Kwaik Y. Fatal attraction of mammalian cells to Legionella pneumophila. Mol Microbiol. 1998;30:689–695. doi: 10.1046/j.1365-2958.1998.01092.x. [DOI] [PubMed] [Google Scholar]
- 2.Akrim M, Bally M, Ball G, Tommassen J, Teerink H, Filloux A, Lazdunski A. Xcp-mediated protein secretion in Pseudomonas aeruginosa: identification of two additional genes and evidence for regulation of xcp gene expression. Mol Microbiol. 1993;10:431–443. doi: 10.1111/j.1365-2958.1993.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 3.Ali A, Johnson J A, Franco A A, Metzger D J, Connell T D, Morris J G, Jr, Sozhamannan S. Mutations in the extracellular protein secretion pathway genes (eps) interfere with rugose polysaccharide production in and motility of Vibrio cholerae. Infect Immun. 2000;68:1967–1974. doi: 10.1128/iai.68.4.1967-1974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Aragon V, Kurtz S, Cianciotto N P. The Legionella pneumophila major acid phosphatase and its role in intracellular infection. Infect Immun. 2001;69:177–185. doi: 10.1128/IAI.69.1.177-185.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aragon V, Kurtz S, Flieger A, Neumeister B, Cianciotto N P. Secreted enzymatic activities of wild-type and pilD-deficient Legionella pneumophila. Infect Immun. 2000;68:1855–1863. doi: 10.1128/iai.68.4.1855-1863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arroyo J, Hurley M C, Wolf M, McClain M S, Eisenstein B I, Engleberg N C. Shuttle mutagenesis of Legionella pneumophila: identification of a gene associated with host cell cytopathicity. Infect Immun. 1994;62:4075–4080. doi: 10.1128/iai.62.9.4075-4080.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atlas R M. Legionella: from environmental habitats to disease pathology, detection and control. Environ Microbiol. 1999;1:283–293. doi: 10.1046/j.1462-2920.1999.00046.x. [DOI] [PubMed] [Google Scholar]
- 9.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley; 1989. [Google Scholar]
- 10.Bally M, Ball G, Badere A, Lazdunski A. Protein secretion in Pseudomonas aeruginosa: the xcpA gene encodes an integral inner membrane protein homologous to Klebsiella pneumoniae secretion function protein PulO. J Bacteriol. 1991;173:479–486. doi: 10.1128/jb.173.2.479-486.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bally M, Filloux A, Akrim M, Ball G, Lazdunski A, Tommassen J. Protein secretion in Pseudomonas aeruginosa: characterization of seven xcp genes and processing of secretory apparatus components by prepilin peptidase. Mol Microbiol. 1992;6:1121–1131. doi: 10.1111/j.1365-2958.1992.tb01550.x. [DOI] [PubMed] [Google Scholar]
- 12.Bandyopadhyay P, Steinman H M. Legionella pneumophila catalase-peroxidases: cloning of the katB gene and studies of KatB function. J Bacteriol. 1998;180:5369–5374. doi: 10.1128/jb.180.20.5369-5374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 14.Brand B C, Hacker J. The biology of Legionella infection. In: Kaufmann S H E, editor. Host response to intracellular pathogens. R. G. Austin, Tex: Landes; 1996. pp. 291–312. [Google Scholar]
- 15.Brieland J, McClain M, LeGendre M, Engleberg C. Intrapulmonary Hartmannella vermiformis: a potential niche for Legionella pneumophila replication in a murine model of legionellosis. Infect Immun. 1997;65:4892–4896. doi: 10.1128/iai.65.11.4892-4896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown M R, Barker J. Unexplored reservoirs of pathogenic bacteria: protozoa and biofilms. Trends Microbiol. 1999;7:46–50. doi: 10.1016/s0966-842x(98)01425-5. [DOI] [PubMed] [Google Scholar]
- 17.Byrne B, Swanson M S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cianciotto N P, Eisenstein B I, Mody C H, Engleberg N C. A mutation in the mip gene results in an attenuation of Legionella pneumophila virulence. J Infect Dis. 1990;162:121–126. doi: 10.1093/infdis/162.1.121. [DOI] [PubMed] [Google Scholar]
- 19.Condemine G, Dorel C, Hugouvieux-Cotte-Pattat N, Robert-Baudouy J. Some of the out genes involved in the secretion of pectate lyases in Erwinia chrysanthemi are regulated by kdgR. Mol Microbiol. 1992;6:3199–3211. doi: 10.1111/j.1365-2958.1992.tb01775.x. [DOI] [PubMed] [Google Scholar]
- 20.d'Enfert C, Reyss I, Wandersman C, Pugsley A P. Protein secretion by gram-negative bacteria. Characterization of two membrane proteins required for pullulanase secretion by Escherichia coli K-12. J Biol Chem. 1989;264:17462–17468. [PubMed] [Google Scholar]
- 21.Edelstein P H. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 1981;14:298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engleberg N C, Drutz D J, Eisenstein B I. Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect Immun. 1984;44:222–227. doi: 10.1128/iai.44.2.222-227.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fettes P S, Susa M, Hacker J, Marre R. Characterization of the Legionella pneumophila gene ligA. Int J Med Microbiol. 2000;290:239–250. doi: 10.1016/S1438-4221(00)80121-6. [DOI] [PubMed] [Google Scholar]
- 24.Fields B S. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 25.Fields B S, Barbaree J M, Sanden G N, Morrill W E. Virulence of a Legionella anisa strain associated with Pontiac fever: an evaluation using protozoan, cell culture, and guinea pig models. Infect Immun. 1990;58:3139–3142. doi: 10.1128/iai.58.9.3139-3142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filloux A, Michel G, Bally M. GSP-dependent protein secretion in gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol Rev. 1998;22:177–198. doi: 10.1111/j.1574-6976.1998.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 27.Fischer G, Bang H, Ludwig B, Mann K, Hacker J. Mip protein of Legionella pneumophila exhibits peptidyl-prolyl-cis/trans isomerase (PPlase) activity. Mol Microbiol. 1992;6:1375–1383. doi: 10.1111/j.1365-2958.1992.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 28.Flieger, A., S. Gong, M. Faigle, S. Stevanovic, N. Cianciotto, and B. Neumeister. Novel lysophospholipase A secreted by Legionella pneumophila. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 29.Gabay J E, Blake M, Niles W D, Horwitz M A. Purification of Legionella pneumophila major outer membrane protein and demonstration that it is a porin. J Bacteriol. 1985;162:85–91. doi: 10.1128/jb.162.1.85-91.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerritse G, Ure R, Bizoullier F, Quax W J. The phenotype enhancement method identifies the Xcp outer membrane secretion machinery from Pseudomonas alcaligenes as a bottleneck for lipase production. J Biotechnol. 1998;64:23–38. doi: 10.1016/s0168-1656(98)00101-1. [DOI] [PubMed] [Google Scholar]
- 31.Hacker J, Ott M, Ludwig B, Rdest U. Intracellular survival and expression of virulence determinants of Legionella pneumophila. Infection. 1991;19:S198–S201. doi: 10.1007/BF01644033. [DOI] [PubMed] [Google Scholar]
- 32.Hales L M, Shuman H A. Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect Immun. 1999;67:3662–3666. doi: 10.1128/iai.67.7.3662-3666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hales L M, Shuman H A. The Legionella pneumophila rpoS gene is required for growth within Acanthamoeba castellanii. J Bacteriol. 1999;181:4879–4889. doi: 10.1128/jb.181.16.4879-4889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hickey E K, Cianciotto N P. Cloning and sequencing of the Legionella pneumophila fur gene. Gene. 1994;143:117–121. doi: 10.1016/0378-1119(94)90615-7. [DOI] [PubMed] [Google Scholar]
- 35.Hickey E K, Cianciotto N P. An iron- and fur-repressed Legionella pneumophila gene that promotes intracellular infection and encodes a protein with similarity to the Escherichia coli aerobactin synthetases. Infect Immun. 1997;65:133–143. doi: 10.1128/iai.65.1.133-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikegami A, Nakasone K, Kato C, Nakamura Y, Yoshikawa I, Usami R, Horikoshi K. Glutamine synthetase gene expression at elevated hydrostatic pressure in a deep-sea piezophilic Shewanella violacea. FEMS Microbiol Lett. 2000;192:91–95. doi: 10.1111/j.1574-6968.2000.tb09364.x. [DOI] [PubMed] [Google Scholar]
- 37.Krinos C, High A S, Rodgers F G. Role of the 25 kDa major outer membrane protein of Legionella pneumophila in attachment to U-937 cells and its potential as a virulence factor for chick embryos. J Appl Microbiol. 1999;86:237–244. doi: 10.1046/j.1365-2672.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 38.Liles M R, Edelstein P H, Cianciotto N P. The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol Microbiol. 1999;31:959–970. doi: 10.1046/j.1365-2958.1999.01239.x. [DOI] [PubMed] [Google Scholar]
- 39.Liles M R, Viswanathan V K, Cianciotto N P. Identification and temperature regulation of Legionella pneumophila genes involved in type IV pilus biogenesis and type II protein secretion. Infect Immun. 1998;66:1776–1782. doi: 10.1128/iai.66.4.1776-1782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindeberg M, Collmer A. Analysis of eight out genes in a cluster required for pectic enzyme secretion by Erwinia chrysanthemi: sequence comparison with secretion genes from other gram-negative bacteria. J Bacteriol. 1992;174:7385–7397. doi: 10.1128/jb.174.22.7385-7397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marsh J W, Taylor R K. Identification of the Vibrio cholerae type 4 prepilin peptidase required for cholera toxin secretion and pilus formation. Mol Microbiol. 1998;29:1481–1492. doi: 10.1046/j.1365-2958.1998.01031.x. [DOI] [PubMed] [Google Scholar]
- 42.Matthews M, Roy C R. Identification and subcellular localization of the Legionella pneumophila IcmX protein: a factor essential for establishment of a replicative organelle in eukaryotic host cells. Infect Immun. 2000;68:3971–3982. doi: 10.1128/iai.68.7.3971-3982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moffat J F, Edelstein P H, Regula D P, Jr, Cirillo J D, Tompkins L S. Effects of an isogenic Zn-metalloprotease-deficient mutant of Legionella pneumophila in a guinea-pig pneumonia model. Mol Microbiol. 1994;12:693–705. doi: 10.1111/j.1365-2958.1994.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 44.Nunn D. Bacterial type II protein export and pilus biogenesis: more than just homologies? Trends Cell Biol. 1999;9:402–408. doi: 10.1016/s0962-8924(99)01634-7. [DOI] [PubMed] [Google Scholar]
- 45.Nunn D N, Lory S. Cleavage, methylation, and localization of the Pseudomonas aeruginosa export proteins XcpT, -U, -V, and -W. J Bacteriol. 1993;175:4375–4382. doi: 10.1128/jb.175.14.4375-4382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nunn D N, Lory S. Components of the protein-excretion apparatus of Pseudomonas aeruginosa are processed by the type IV prepilin peptidase. Proc Natl Acad Sci USA. 1992;89:47–51. doi: 10.1073/pnas.89.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Connell W A, Hickey E K, Cianciotto N P. A Legionella pneumophila gene that promotes hemin binding. Infect Immun. 1996;64:842–848. doi: 10.1128/iai.64.3.842-848.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pepe C M, Eklund M W, Strom M S. Cloning of an Aeromonas hydrophila type IV pilus biogenesis gene cluster: complementation of pilus assembly functions and characterization of a type IV leader peptidase/N-methyltransferase required for extracellular protein secretion. Mol Microbiol. 1996;19:857–869. doi: 10.1046/j.1365-2958.1996.431958.x. [DOI] [PubMed] [Google Scholar]
- 49.Pruckler J M, Benson R F, Moyenuddin M, Martin W T, Fields B S. Association of flagellum expression and intracellular growth of Legionella pneumophila. Infect Immun. 1995;63:4928–4932. doi: 10.1128/iai.63.12.4928-4932.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pugsley A P, Francetic O, Possot O M, Sauvonnet N, Hardie K R. Recent progress and future directions in studies of the main terminal branch of the general secretory pathway in Gram-negative bacteria—a review. Gene. 1997;192:13–19. doi: 10.1016/s0378-1119(96)00803-7. [DOI] [PubMed] [Google Scholar]
- 51.Rodgers F G, Gibson F C D. Opsonin-independent adherence and intracellular development of Legionella within U-937 cells. Can J Microbiol. 1993;39:718–722. doi: 10.1139/m93-103. [DOI] [PubMed] [Google Scholar]
- 52.Roy C R, Berger K H, Isberg R R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 53.Russel M. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J Mol Biol. 1998;279:485–499. doi: 10.1006/jmbi.1998.1791. [DOI] [PubMed] [Google Scholar]
- 54.Sandkvist M, Michel L O, Hough L P, Morales V M, Bagdasarian M, Koomey M, DiRita V J. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J Bacteriol. 1997;179:6994–7003. doi: 10.1128/jb.179.22.6994-7003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segal G, Shuman H A. How is the intracellular fate of the Legionella pneumophila phagosome determined? Trends Microbiol. 1998;6:253–255. doi: 10.1016/s0966-842x(98)01308-0. [DOI] [PubMed] [Google Scholar]
- 56.Segal G, Shuman H A. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect Immun. 1999;67:2117–2124. doi: 10.1128/iai.67.5.2117-2124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shuman H A, Purcell M, Segal G, Hales L, Wiater L A. Intracellular multiplication of Legionella pneumophila: human pathogen or accidental tourist? Curr Top Microbiol Immunol. 1998;225:99–112. doi: 10.1007/978-3-642-80451-9_6. [DOI] [PubMed] [Google Scholar]
- 58.Stone B J, Abu Kwaik Y. Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect Immun. 1998;66:1768–1775. doi: 10.1128/iai.66.4.1768-1775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strom M S, Nunn D, Lory S. Multiple roles of the pilus biogenesis protein pilD: involvement of pilD in excretion of enzymes from Pseudomonas aeruginosa. J Bacteriol. 1991;173:1175–1180. doi: 10.1128/jb.173.3.1175-1180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swanson M S, Hammer B K. Legionella pneumophila pathogenesis: a fateful journey from amoebae to macrophages. Annu Rev Microbiol. 2000;54:567–613. doi: 10.1146/annurev.micro.54.1.567. [DOI] [PubMed] [Google Scholar]
- 61.Szeto L, Shuman H A. The Legionella pneumophila major secretory protein, a protease, is not required for intracellular growth or cell killing. Infect Immun. 1990;58:2585–2592. doi: 10.1128/iai.58.8.2585-2592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viera J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 63.Viswanathan V K, Edelstein P H, Pope C D, Cianciotto N P. The Legionella pneumophila iraAB locus is required for iron assimilation, intracellular infection, and virulence. Infect Immun. 2000;68:1069–1079. doi: 10.1128/iai.68.3.1069-1079.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogel J P, Isberg R R. Cell biology of Legionella pneumophila. Curr Opin Microbiol. 1999;2:30–34. doi: 10.1016/s1369-5274(99)80005-8. [DOI] [PubMed] [Google Scholar]
- 65.Wiater L A, Dunn K, Maxfield F R, Shuman H A. Early events in phagosome establishment are required for intracellular survival of Legionella. Infect Immun. 1998;66:4450–4460. doi: 10.1128/iai.66.9.4450-4460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winn W C., Jr Legionnaires' disease: historical perspective. Clin Microbiol Rev. 1988;1:60–81. doi: 10.1128/cmr.1.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yildiz F H, Schoolnik G K. Vibrio cholerae O1 E1 Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc Natl Acad Sci USA. 1999;96:4028–4033. doi: 10.1073/pnas.96.7.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]