Visual Abstract
Abstract
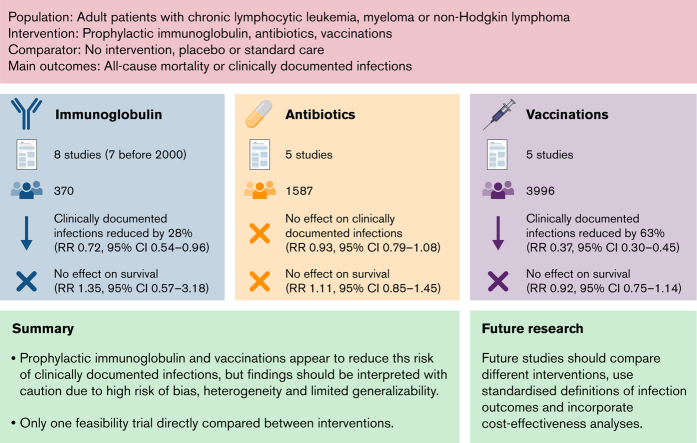
Acquired hypogammaglobulinemia is common in chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), and multiple myeloma (MM). No previous systematic reviews (SRs) have compared different approaches to infection prevention. We sought to assess the efficacy and safety of prophylactic immunoglobulin, antibiotics, and vaccination in these patients. We performed an SR and meta-analysis of randomized controlled trials (RCTs) evaluating the efficacy and safety of prophylactic immunoglobulin, antibiotics, and vaccination in adult patients with hematological malignancies commonly associated with acquired hypogammaglobulinemia, specifically, CLL, NHL, and MM. We searched PubMed (MEDLINE), EMBASE, and Cochrane Registry up to 9 January 2021. Results for dichotomous data were expressed as relative risk (RR) with 95% confidence interval (CI) and pooled in a random-effects model. This review was registered with PROSPERO CRD42017070825. From 10 576 studies screened, there were 21 completed RCTs and 1 ongoing. Of these, 8 evaluated prophylactic immunoglobulin (n = 370; 7 published before 2000), 5 evaluated prophylactic antibiotics (n = 1587), 7 evaluated vaccination (n = 3996), and 1 compared immunoglobulin to antibiotics (n = 60). Prophylactic immunoglobulin reduced the risk of clinically documented infection (CDI) by 28% (n = 2 trials; RR, 0.72; 95% CI, 0.54-0.96), and vaccination reduced the risk by 63% (RR, 0.37; 95% CI, 0.30-0.45). Prophylactic antibiotics did not reduce the risk. No intervention reduced all-cause mortality. Prophylactic immunoglobulin and antibiotics increased the risk of adverse events. Findings should be interpreted with caution, given the high risk of bias in many studies. There is a clear need for high-quality contemporary trials to establish the effectiveness of different approaches to preventing infection.
Introduction
Acquired hypogammaglobulinemia is common in people with B-cell malignancies, such as chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), and multiple myeloma (MM). These conditions are associated with a multifactorial immune deficiency attributable to the underlying disease, its treatment, or both. Strategies to reduce infections are important for quality of life and mortality and are rated highly by patient and public involvement groups and in research prioritization exercises.1 There is a need to understand how to prevent infection, given increasing concerns about emerging antimicrobial resistance and pandemics. Interventions commonly used to prevent infection include prophylactic immunoglobulin, antibiotics, and vaccination, but the relative effects of these approaches is unclear, and assessment is difficult, given the considerable variation in clinical practice.2
The most recent systematic review (SR) evaluating immunoglobulin prophylaxis in hematological malignancies identified 9 randomized controlled trials (RCTs) performed in the 1980s and 1990s. They demonstrated a significant reduction in the number of major infections, antibiotic use, and hospitalization, with no difference in all-cause mortality.3
Oral antibiotics are also used, but there are increasing concerns about evolving resistance with inappropriate or excessive use. A previous SR evaluating antibiotic prophylaxis in patients with neutropenia showed reduced all-cause mortality and fewer microbiologically documented infections (MDIs), and clinically documented infections (CDIs),4 but most studies were from the 1980s and 1990s and evaluated participants with acute leukemia. This review predates current therapeutic and supportive care strategies, and there have been shifts in pathogen prevalence and antibiotic susceptibilities over time.5 In patients with MM, a more recent SR found that short-term antibiotic prophylaxis reduced infections within 3 months of commencement, with borderline statistical significance.6 There was no reduction in all-cause mortality.
A 2011 SR on vaccination in hematological malignancies found that varicella zoster virus (VZV) vaccination did not significantly reduce herpes zoster infections, but influenza vaccination significantly reduced lower respiratory tract infections.7
Targeted therapies that deplete B lymphocytes or plasma cells, including anti-CD20 and anti-CD38 antibodies, Bruton tyrosine kinase inhibitors, BCL2 inhibitors, and chimeric antigen receptor T cells have improved disease-specific survival, but also contribute to hypogammaglobulinemia.8 Therefore, prevalence of hypogammaglobulinemia is expected to increase. In addition, the demand for and cost of immunoglobulin are considerable and continue to increase.9 Given the challenges for blood services to meet this demand and the concerns regarding antimicrobial resistance, urgent attention is needed to address the utility of prophylactic immunoglobulin and alternatives. Although some international guidelines recommend a trial of antibiotics before immunoglobulin, no previous SRs have compared the efficacy of different approaches.10 Our objectives were to assess the effect of prophylactic immunoglobulin, antibiotics, and vaccination on infection, mortality, and safety in patients with CLL, NHL, and MM.
Methods
Search strategy and selection criteria
We performed an SR and meta-analysis according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The protocol was registered at PROSPERO CRD42017070825.11
We searched the following databases on 9 January 2021 to identify RCTs published in English with no restriction on study years: PubMed (MEDLINE), EMBASE, and Cochrane Registry of Controlled Trials. The search strategy is shown in supplemental Table 1. Other study designs (eg, observational and case reports) were excluded. We used methods recommended by the Cochrane Handbook for Systematic Reviews of Interventions.12
We included adult patients (≥18 years of age) with hematological malignancies commonly associated with secondary hypogammaglobulinemia: CLL, NHL, and MM. We excluded patients who had undergone allogeneic stem cell transplantation.
We included prophylactic IV immunoglobulin (IVIg) or subcutaneous immunoglobulin (SCIg), prophylactic antibiotics including but not limited to the following, used alone or in combination: quinolones (eg, ciprofloxacin, ofloxacin, and norfloxacin), Gram-positive prophylaxis (penicillin and roxithromycin), trimethoprim-sulfamethoxazole and doxycycline, and vaccination.
The primary outcomes were all-cause mortality and patients with CDIs (symptoms or signs of infection requiring oral/intravenous treatment). Secondary outcomes were patients with MDI, infection-related mortality, number of CDIs and MDIs, patients with ≥1 serious infection, patients ≥3 infections, patients with ≥3 serious infections, number of hospitalizations for infection, intensive care admissions for infection, adverse events, and adverse events leading to discontinuation of treatment.
Data analysis
Two authors (from A.K., J.W., K.L.C., P.C., R.W., and Z.K.M.) independently screened all abstracts and full-text articles. A third author resolved any discrepancies. Two authors (from J.W., K.L.C., R.W., and Z.K.M.) independently assessed all included studies for risk of bias (ROB) using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions.12 Discrepancies were resolved by discussion or adjudication by a third reviewer. Assessment criteria included study design, conduct, and analysis (including random sequence generation, allocation concealment, blinding of participants, physician, and outcome assessors, number lost to follow-up, and whether all outcomes were reported) on a 3-point scale: (−) low, (?) unclear, or (+) high ROB.
Two authors (from J.W., K.L.C., and Z.K.M) independently performed data extraction using a standardized form. Discrepancies were resolved through discussion or adjudication by a third reviewer. Where necessary, corresponding authors were contacted for clarification.
Data extracted included author, citation, study objective, number of sites, clinical setting, population, study design, dates of recruitment, sample size, number of participants, inclusion and exclusion criteria, interventions, primary and secondary outcomes, length of follow-up, co-interventions, compliance, loss to follow-up, results, and statistical analysis.
Studies were grouped by type of intervention. Results for dichotomous data were expressed as relative risk (RR) with 95% confidence interval (CI) and pooled using a random-effects model in RevMan 5.3 software. We assessed heterogeneity of treatment effects using a Chi2 test with a significance level at P < 0.1. We will use the I2 statistic to quantify possible heterogeneity (>30% to signify moderate heterogeneity and >75% to signify considerable heterogeneity).
For continuous outcomes, we planned to record the mean, standard deviation, and number of participants in treatment and control groups. However, most studies did not provide the standard deviation in each group.
We planned to perform exploratory subgroup analyses of our primary outcomes by publication year (before or after 2000), indication for intervention (hypogammaglobulinemia with or without a history of recurrent infections), and degree of hypogammaglobulinemia.
We performed a sensitivity analysis for our primary outcomes to explore the effects of excluding studies with a high ROB (studies with a low ROB or some concerns vs studies with high ROB).
Results
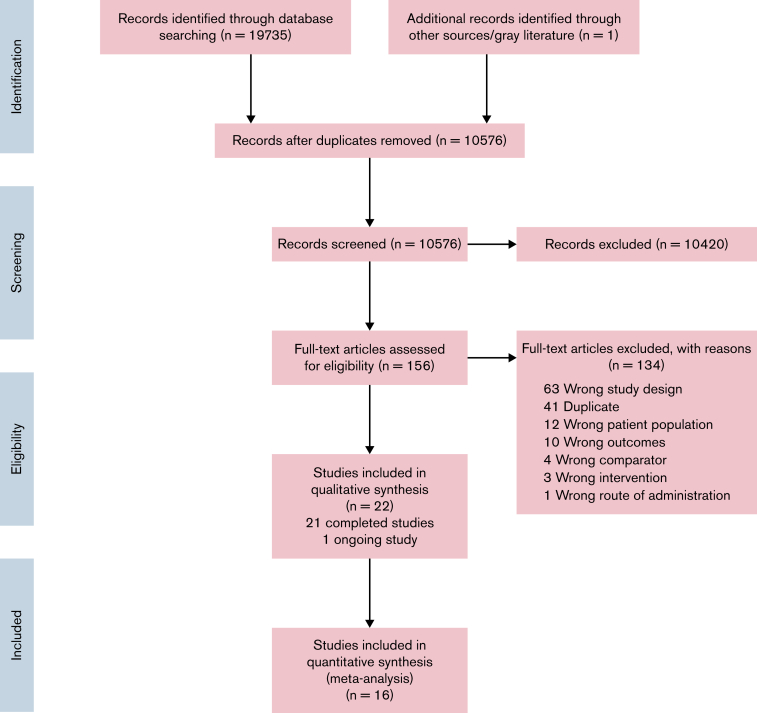
The search strategy identified 10 576 studies (Figure 1). We excluded 134 studies at full-text review stage (reasons in Figure 1). Twenty-one completed (n = 5953 participants) studies and 1 ongoing study were included (Table 1).
Figure 1.
PRISMA flow diagram for study selection.
Table 1.
Characteristics of the included studies
| Number | Study | Study design, years conducted | Types of intervention | Dose and schedule of interventions | Types and number of participants (n), multicenter or single center | Outcomes |
|---|---|---|---|---|---|---|
| 1 | Boughton et al13 | RCT, not stated | Prophylactic immunoglobulin compared with placebo | IVIg 18 g (sandoglobulin) vs placebo (0.6 g albumin) every 3 wk for 12 mo | CLL, serum IgG <5.5 g /L and 2 or more documented infections in the preceding 12 mo; N = 42, multicenter | Patients with ≥1 CDI, patients with ≥1 MDI, number of MDIs, patients with ≥3 infections, patients with ≥3 serious infections, number of CDIs (overall but not by treatment arm), adverse effects, adverse events leading to treatment discontinuation |
| 2 | Chapel et al14 | RCT, not stated | Prophylactic immunoglobulin compared with placebo | IVIg 0.4 g/kg (Gammagard) vs placebo (4% albumin) every 4 wk for 12 mo | MM in stable phase; N = 83, multicenter | All-cause mortality, IRM, patients with ≥1 CDI, number of CDIs, number of MDIs, number of serious infections, time to first major infection, adverse events, adverse events leading to treatment discontinuation |
| 3 | Cooperative CLL16 | RCT, not stated | Prophylactic immunoglobulin compared with placebo | IVIg 0.4 g/kg (Gammagard) vs placebo (0.9% sodium chloride) every 4 wk for 12 mo | CLL, serum IgG level < 50% of lower limit of normal or a history of serious infections; N = 84, multicenter | All-cause mortality, patients with ≥1 infections, patients with ≥3 infections, patients with ≥1 serious infections, patients with ≥1 MDI, number of MDIs, number of CDIs, number of serious infections, adverse events, adverse events leading to treatment discontinuation |
| 4 | Dagnew et al26 | RCT, 2013-2015 | Vaccination compared with placebo | Adjuvanted recombinant zoster vaccine (2 doses) vs placebo 1-2 mo apart | CLL, lymphoma, MM, receiving or had just finished immunosuppressive cancer treatments; N = 562, multicenter | All-cause mortality, patients with ≥1 CDI, patients with ≥1 MDI, number of CDI, number of MDIs, adverse events |
| 5 | Drayson et al21 | RCT, 2012-2016 | Prophylactic antibiotics compared with placebo | Levofloxacin 500 mg daily vs placebo for 12 wk | MM, newly diagnosed with planned antimyeloma treatment; N = 977, multicenter | All-cause mortality, IRM, patients with ≥1 CDI, number of CDIs, number of MDIs, number of hospitalizations, number of ICU admissions, adverse events |
| 6 | Gamm et al15 Chapel et al14 |
RCT, not stated | Prophylactic immunoglobulin comparing differing doses | IVIg 0.5 g/kg vs IVIg 0.25 g/kg (Gammagard) every 4 wk for 12 mo | CLL and lymphoma, serum IgG below lower limit of normal or a recent history of serious infections; N = 36, multicenter | All-cause mortality, IRM, patients with ≥1 CDI, number of CDIs, number of MDIs, number of serious infections, adverse events |
| 7 | Gregersen et al22 | RCT, not stated | Prophylactic antibiotics compared with placebo | Clarithromycin 500 mg BD vs placebo for 3 mo | MM, newly diagnosed and receiving VCD induction chemotherapy; N = 58, multicenter | All-cause mortality, patients with ≥1 CDI, patients with ≥1 MDI, patients with ≥1 serious infection, adverse events |
| 8 | Griffiths et al17 | RCT Crossover, 1984-1987 | Prophylactic immunoglobulin compared with placebo | IVIg 0.4 g/kg (Gammagard) vs placebo (saline) every 3 wk for months, then crossover for another 12 mo | CLL, serum IgG level <3.5 g/L or a history of serious infections; N=12, single center | Number of CDIs, adverse events |
| 9 | Hata et al27 | RCT, 1997-2000 | Vaccination compared with placebo | Heat-inactivated, live attenuated varicella vaccine vs placebo given within 30 d before transplantation and 30, 60, and 90 d after transplantation | Lymphoma, autologous stem cell transplant; N = 119, multicenter | All-cause mortality, patients with ≥1 CDI, number of CDIs, adverse events (treatment group only) |
| 10 | McQuilten et al35 | RCT, 2017-2020 | Prophylactic antibiotics compared with prophylactic immunoglobulin | IVIg 0.4 g/kg every 4 wk or SCIg 0.1 g/kg every week vs oral antibiotics (trimethoprim-sulfamethoxazole 160 mg/800 mg daily) for 12 mo | CLL, MM, lymphoma, other hematological malignancy, serum IgG level <4 g/L or a history of serious infections; N = 60, multicenter | Time to first major infection, patients with ≥1 serious infection |
| 11 | Molica et al18 | RCT, crossover, not stated | Prophylactic immunoglobulin compared with no treatment | IVIg 0.3g/kg (Vena-N) every 4 wk vs no treatment for 6 mo, then switched to observation or IVIg for another 12 mo; then IVIG or no therapy for 6 more months. | CLL, serum IgG level <6 g/L or a history of serious infections; N = 42, multicenter | All-cause mortality, IRM, number of CDIs, number of MDIs |
| 12 | Musto et al19 | RCT crossover, not stated | Prophylactic immunoglobulin compared with no treatment | IVIg 0.3 g/kg (Vena-N) every 4 wk vs no treatment for 6 mo, then switched to observation or IVIg for another 12 mo; then IVIG or no therapy for 6 more months | MM, serum IgG below lower limit of normal or a recent history of serious infections; N = 25, not stated | All-cause mortality, IRM, number of CDIs, number of serious infections |
| 13 | Musto and Carotenuto28 | RCT, 1995-1996 | Vaccination compared with no treatment | Influenza vaccine (trivalent subvirion containing antigens from the component strains A/Singapore/6/86, A/Johannesburg/33/94 and B/Bijing/184/93) vs no treatment | MM undergoing chemotherapy; N = 50, not stated | IRM, patients with ≥1 CDI, number of hospitalizations, adverse events (treatment group only), adverse events leading to treatment discontinuation (treatment group only) |
| 14 | Oken et al23 1996 | RCT, not stated | Prophylactic antibiotics compared with no treatment | Oral antibiotics (trimethoprim-sulfamethoxazole 160mg/800mg bd) vs no treatment for 2 mo | MM, newly diagnosed undergoing chemotherapy; N = 54, multicenter | IRM, patients with ≥1 CDI, patients with ≥1 serious infection, number of CDIs, IRM, adverse events, adverse events leading to treatment discontinuation. number of major infections |
| 15 | Puig et al24 | RCT, 2015-2019 | Prophylactic antibiotics with standard care | Oral antibiotics (clarithromycin 500 mg bd) vs placebo until disease progression or unacceptable toxicity | MM, newly diagnosed, ≥65 y of age undergoing RD chemotherapy; N = 286, multicenter | All-cause mortality, IRM, patients with ≥1 CDI, patients with ≥1 serious infection, adverse events |
| 16 | Stadtmauer et al29 | RCT, 2009-2012 | Vaccination compared with placebo or differing doses | Three doses of 50 mg VZV glycoprotein E (gE) adjuvanted with AS01B vs 3 doses of gE adjuvanated with AS01E vs dose of saline followed by 2 doses of gE/AS01B vs 3 doses of saline at months 0, 1, 3. | CLL, MM, lymphoma, AML, had autologous stem cell transplant; N = 121, multicenter | Patients with ≥1 CDI, adverse events |
| 17 | Stadtmauer et al30 Bastidas et al39 |
RCT, 2012-2017 | Vaccination compared with placebo | Adjuvanted recombinant zoster vaccine vs placebo with the first dose given 50-70 d after transplantation and the second dose 1-2 mo thereafter. | Post autologous HSCT; N = 1846, multicenter | All-cause mortality, patients with ≥1 CDI, number of CDIs, adverse events |
| 18 | Teh et al31 | RCT, 2019-2020 | Vaccination, comparing different doses | High-dose (HD) inactivated influenza vaccine followed by standard dose (SD) vaccine (HD-SD arm) or 2 SD vaccines (SD-SD arm) 4 wk apart | MM, lymphoma, post autologous stem cell transplant; N = 68, single center | Patients with one or more CDIs, patients with ≥1 MDI, number of CDIs, number of MDIs, adverse events |
| 19 | Vacca et al20 | RCT, not stated | Prophylactic immunoglobulin compared with no treatment | SCIg 0.1 g-0.2 g/kg weekly (Hizentra) vs no treatment | MM, serum IgG level < 5 g/L; N = 46, single center | Number of CDIs, number of serious infections, incidence of hospitalization, adverse events, adverse events requiring treatment discontinuation |
| 20 | Vesole et al25 | RCT, 1998-2008 | Prophylactic antibiotics compared with no treatment | Ciprofloxacin 500 mg twice daily vs trimethoprim sulfamethoxazole (160/800 mg twice daily) vs no treatment for 2 mo | MM, receiving front-line chemotherapy; N = 212, not stated | All-cause mortality, IRM, patients with ≥1 infection, patients with ≥1 serious infection |
| 21 | Winston et al32 | RCT, 2010-2013 | Vaccination compared with placebo | Four doses of inactivated zoster vaccine or placebo, with the first dose 5-60 d before auto-HSCT, and the second, third, and fourth doses at about 30, 60, and 90 d after transplantation | MM, lymphoma, acute leukemia; autologous stem cell transplant; N = 1230, multicenter | Patients with ≥1 CDI, number of CDIs, adverse events, adverse events leading to treatment discontinuation |
HSCT, hematopoietic stem cell transplantation; ICU, intensive care unit; IRM, infection-related mortality; Rd, lenalidomide and dexamethasone.
Those RCTs were published from 1988 through 2021, including 9 before 2000. Fifteen were multicenter, 3 were single center, and the rest were unspecified.
Eight trials evaluated prophylactic immunoglobulin.13, 14, 15, 16, 17, 18, 19, 20 Seven studies compared immunoglobulin with placebo (saline/albumin) or no intervention.13,14,16, 17, 18, 19, 20 Seven evaluated IVIg and 1 evaluated SCIg. Three studies had a crossover trial design.17, 18, 19 One17 compared IVIg to saline and switched arms after 12 months to complete the 24-month follow-up, and the other 2 compared IVIg to no intervention, switched arms after 6 months, then switched back after 12 months to complete the 24-month follow-up.18,19 One study compared the use of different doses of IVIg (0.25 g/kg vs 0.5 g/kg, 4 weekly for 12 months).15 Three studies evaluated patients with MM,14,19,20 4 evaluated those with CLL, 13,16, 17, 18 and 1 evaluated those with CLL or lymphoma.15
Five MM studies compared prophylactic antibiotics with placebo or no intervention.21, 22, 23, 24, 25 Two studies22,24 compared chemotherapy regimens with or without clarithromycin, to evaluate the antimyeloma effect of clarithromycin. One study used levofloxacin,21 1 used ciprofloxacin and trimethoprim-sulfamethoxazole,25 and 1 used trimethoprim-sulfamethoxazole.23 Duration of antibiotic treatment was variable, ranging from 2 to 15 months (median). In 1 study,21 low-dose trimethoprim-sulfamethoxazole was permitted as a prophylaxis against Pneumocystis pneumonia (dosed according to local practice).21,22
Seven studies evaluated vaccination.26, 27, 28, 29, 30, 31, 32 Four evaluated VZV vaccines after autologous stem cell transplant (ASCT).27,29,30,32 One compared VZV vaccine to placebo within 6 months after treatment.26 Of these studies, 3 evaluated the use of a more recently available adjuvanted recombinant zoster vaccine (Shingrix), which can be administered to patients with hematological malignancies, unlike the live attenuated zoster vaccine, which is generally contraindicated in immunocompromised people.26,29,30 The other 2 studies evaluated the use of heat-inactivated or γ-irradiated VZV vaccine.27,32 Two studies evaluated influenza vaccination in patients with MM on chemotherapy28 or patients after ASCT.31
One study directly compared antibiotics (trimethoprim-sulfamethoxazole) to immunoglobulin (IVIg/SCIg) in patients with hematological malignancies.11
One ongoing study was identified that is evaluating immunoglobulin vs placebo as primary infection prophylaxis in CLL (PROSID).33
ROB
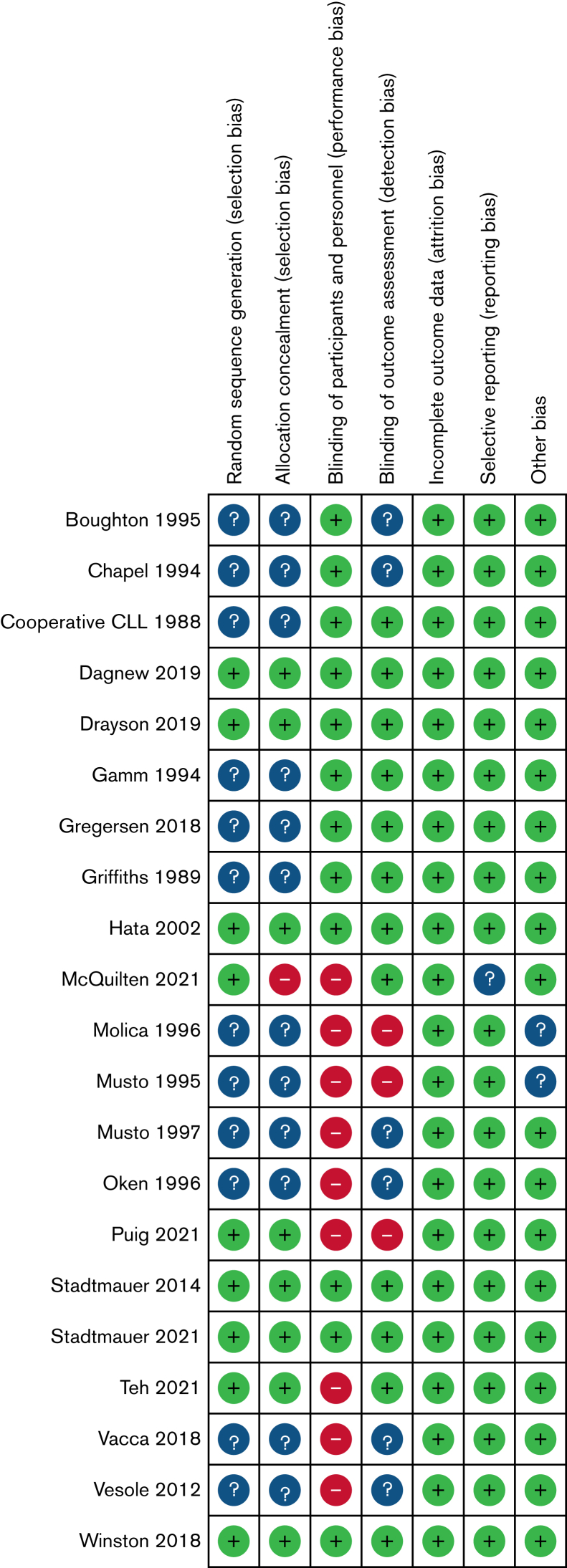
The ROB assessment is shown in Figure 2. Generation of a randomization sequence was adequate in 9 studies (+), but unclear in the remaining 12 (?). Allocation concealment was adequate in 8 studies (+), high in 1 (−), and unclear in the remaining 12 (?). Blinding of participants and personnel was adequate in 12 studies (+) and high (not blinded) in 9 (−). Blinding of outcome assessment was adequate in 12 studies (+), not blinded in 3 (−), and unclear in the remaining 6(?). Incomplete outcome data were adequate in 21 studies (+). Selective reporting was adequate in 20 studies (+) and unclear in 1(?).
Figure 2.
ROB assessments for included studies.
Effects of interventions
Prophylactic immunoglobulin vs standard care
All-cause mortality
Two trials (n = 164) reported this outcome (supplemental Figure 1). There was no difference in the risk of all-cause mortality between prophylactic immunoglobulin and standard care (placebo vs no intervention) (RR, 1.35; 95% CI, 0.57-3.18). There was no statistical evidence of heterogeneity (P = .62; I 2 = 0%).
Infection-related mortality
One trial (n = 83) reported this outcome (supplemental Figure 2). Prophylactic immunoglobulin did not significantly reduce the risk of infection-related mortality (RR, 0.33; 95% CI, 0.01-7.77).
CDI
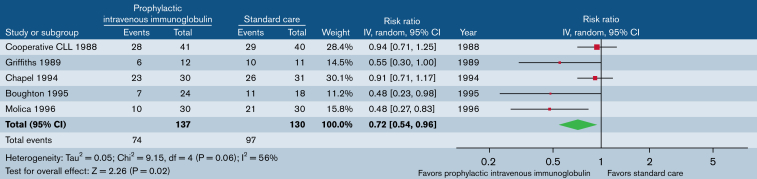
Five trials (n = 267) reported the patients who had at least 1 CDI (Figure 3). Prophylactic immunoglobulin reduced the risk of CDI by 28% (RR, 0.7; 95% CI, 0.54-0.96). There was statistical evidence of moderate heterogeneity (P = .06; I2 = 56%). Two trials (n = 99) reported the proportion of patients who had at least 3 CDIs (supplemental Figure 3). Immunoglobulin prophylaxis significantly reduced the risk of at least 3 CDIs by 57% (RR, 0.42; 95% CI, 0.21-0.84). There was no statistical evidence of heterogeneity (P = .36; I2 = 0%). One trial (n = 42) reported the proportion of patients who had at least 3 serious infections (supplemental Figure 4), with significant reduction (66%; RR, 0.34; 95% CI, 0.14-0.81).
Figure 3.
Prophylactic immunoglobulin vs standard care. Outcome: patients with ≥1 CDI.
MDI
Two trials (n = 99) reported patients with at least 1 MDI (supplemental Figure 5). Immunoglobulin prophylaxis did not significantly reduce the risk of MDI (RR, 0.92; 95% CI, 0.41-2.08). There was statistical evidence of moderate heterogeneity (P = .12; I2 = 58%). Further details are available in the (supplemental Information).
Adverse events
Three trials (n = 205) reported adverse events (supplemental Figure 6). Immunoglobulin prophylaxis significantly increased the risk of adverse events (RR, 2.23; 95% CI, 1.67-2.99). There was no statistical evidence of heterogeneity (P = .40; I2 = 0%).
Side-effects included fever, chills, headache, hypotension, and rash, mostly occurring early.
Three studies reported adverse events resulting in treatment discontinuation (supplemental Figure 7). There was no significant difference (RR, 4.80; 95% CI, 0.57-40.30) and no statistical evidence of heterogeneity (P = .53; I2 = 0%).
Crossover studies evaluating prophylactic immunoglobulin
Three studies had a crossover study design and were not included in the meta-analysis, as data for first randomization were not available.17, 18, 19 Importantly, no washout period was reported, and therefore a carry-over effect cannot be excluded. More details are provided in the supplemental Information.
Prophylactic immunoglobulin comparing different doses
One small study evaluating CLL and NHL compared different doses of prophylactic immunoglobulin.15,34 Results are provided narratively in the supplemental Information. Although no difference was observed in the rate of infection between 0.5 and 0.25 g/kg every 4 weeks, the data are insufficient to demonstrate equivalence.
Subgroup analysis
Subgroup analyses could not be performed because of insufficient data (only 1 study was published after 2000) and missing information.
Sensitivity analysis
We summarized the sensitivity analyses in Table 2. After removing studies with a high ROB, the effect estimate was similar for the outcome of all-cause mortality, but it should be noted that the outcome of patients with ≥1 CDI became statistically nonsignificant.
Table 2.
Sensitivity analyses for the primary outcomes
| Intervention | Outcome | Main analysis | ROB: excluding studies at high ROB |
|---|---|---|---|
| Prophylactic immunoglobulin | All-cause mortality | RR 1.35 (95% CI, 0.57-3.18); including 164 participants from 2 studies | RR 1.35 (95% CI, 0.57-3.18); including 164 participants from 2 studies |
| Prophylactic immunoglobulin | Patients with ≥1 CDI | RR 0.72 (95% CI, 0.54-0.96); including 267 participants from 5 studies | RR 0.80 (95% CI, 0.62-1.04); including 207 participants from 4 studies |
| Prophylactic antibiotic | All-cause mortality | RR 1.11 (95% CI, 0.85-1.45); including 1533 participants from 4 studies | RR 1.03 (95% CI, 0.68-1.56); including 1035 participants from 2 studies |
| Prophylactic antibiotic | Patients with ≥1 CDI | RR 0.93 (95% CI, 0.79-1.08); including 1576 participants from 5 studies | RR 0.87 (95% CI, 0.76-1.00); including 1035 participants from 2 studies |
| VZV vaccination | All-cause mortality | RR 0.92 (95% CI, 0.75-1.14); including 3738 participants from 4 studies | RR 0.92 (95% CI, 0.75-1.14); including 3738 participants from 4 studies |
| VZV vaccination | Patients with ≥1 CDI | RR 0.36 (95% CI, 0.29-0.44); including 3515 participants from 5 studies | RR 0.36 (95% CI, 0.29-0.44); including 3515 participants from 5 studies |
Ongoing studies
One ongoing study was identified that evaluated IVIg vs placebo in patients with CLL as the primary infection prophylaxis (PROSID).33 The estimated completion date is September 2023.
Prophylactic antibiotics vs standard care
All-cause mortality
Four trials (n = 1533) reported this outcome (supplemental Figure 8). Prophylactic antibiotics did not significantly reduce the risk of all-cause mortality standard care (placebo or no intervention) (RR, 1.11; 95% CI, 0.85-1.45). There was no statistical evidence of heterogeneity (P = .89; I2 = 0%).
Infection-related mortality
Four trials (n = 1518) reported this outcome (supplemental Figure 9). There was no difference in risk of infection-related mortality (RR, 1.15; 95% CI, 0.59-2.25). There was no statistical evidence of heterogeneity (P = .28; I2 = 22%).
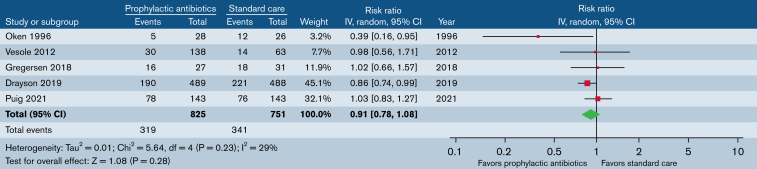
CDI
Five trials (n = 1576) reported patients who had at least 1 CDI (Figure 4). Prophylactic antibiotics did not significantly reduce the risk of CDIs (RR, 0.93; 95% CI, 0.79-1.08). There was no statistical evidence of heterogeneity (P = .27; I2 = 23%). When limited to studies evaluating antibiotics for the purpose of infection prevention only (excluding trials evaluating the antimyeloma effect of clarithromycin), our findings were similar.
Figure 4.
Prophylactic antibiotics vs standard care. Outcome: patients with ≥1 CDI.
Four trials (n = 599) reported the proportion of patients who had at least 1 serious infection (supplemental Figure 10). There was no significant difference in ≥1 serious infection (RR, 0.84; 95% CI, 0.34-2.09). There was statistical evidence of moderate heterogeneity (P = .02; I2 = 69%).
MDI
One trial (n = 58) reported patients who had at least 1 MDI (supplemental Figure 11) and there was no significant reduction (RR, 5.7; 95% CI, 0.71-46.14).
Adverse events
Four trials (n = 398) reported adverse events (supplemental Figure 12). Prophylactic antibiotics significantly increased the risk of adverse events (RR, 1.75; 95% CI, 1.01-3.03). There was statistical evidence of moderate heterogeneity (P = .06; I2 = 60%).
Clarithromycin was found to be associated with serious adverse events in 2 studies. One trial was terminated early because of increased sepsis, attributed to the reduced metabolism of bortezomib.22 In another study, clarithromycin led to delayed clearance of steroids.24 When these 2 trials were excluded, our findings remained similar.
Reported adverse events in other studies were diverse, including gastrointestinal disturbances, rash, musculoskeletal pains, and tendonitis (with levofloxacin).
Subgroup analysis
Including only studies published after 2000, our primary outcomes remained the same (supplemental Figure 13). Other subgroup analyses could not be performed because of missing information.
Sensitivity analysis
We summarized the effects of sensitivity analyses in Table 2. Reported effects for patients with CDI and all-cause mortality were robust when removing studies with high ROB.
Vaccination vs standard care
All-cause mortality
Four trials (n = 3738) assessing VZV vaccination reported this outcome (supplemental Figure 14). There was no difference in risk of all-cause mortality between VZV vaccination and standard care (placebo or no intervention) (RR, 0.92; 95% CI, 0.75-1.14). There was no statistical evidence of heterogeneity (P = .84; I2 = 0%).
Infection-related mortality
One trial reported this outcome (supplemental Figure 15).28 There was no difference in risk of infection-related mortality between influenza vaccination and standard care (RR, 0.20; 95% CI, 0.01-3.97).
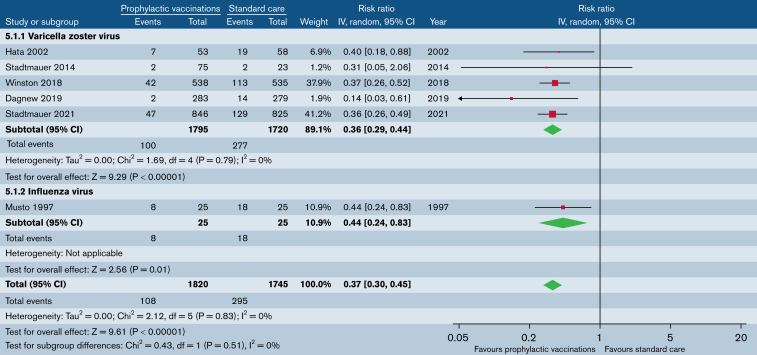
CDI
Five trials (n = 3515) that evaluated VZV vaccination reported on patients with clinically documented VZV infection (Figure 5). VZV vaccination significantly reduced the risk of clinically documented VZV infection (RR, 0.36; 95% CI, 0.29-0.44). There was no statistical evidence of heterogeneity (P = .79; I2 = 0%). In reviewing Stadtmauer et al,30 we excluded patients with nonhematological malignancies from the meta-analysis.
Figure 5.
Prophylactic vaccination vs standard care. Outcome: patients with ≥1 CDI.
One trial (n = 50) that evaluated influenza vaccination reported on patients with at least 1 CDI (Figure 5).28 Influenza vaccination significantly reduced the risk of clinically documented influenza infection (RR, 0.44; 95% CI, 0.24-0.83).
Overall, vaccination reduced the risk of CDI (RR, 0.37; 95% CI, 0.30-0.45; Figure 5). There was no heterogeneity (P = .83; I2 = 0%).
MDI
One trial (n = 562) that evaluated VZV vaccination reported patients with at least 1 microbiologically documented VZV infection (supplemental Figure 16).26 VZV vaccination significantly reduced the risk of microbiologically documented VZV infection (RR, 0.18; 95% CI, 0.04-0.80).
Adverse events
Six trials reported adverse events (supplemental Figure 17). Two of those trials reported adverse events in the treatment arm only and were not included in the meta-analysis.27,28 In the remaining 4 trials, VZV vaccination did not significantly increase the risk of adverse events (RR, 1.93; 95% CI, 0.99-3.76). There was statistical evidence of heterogeneity (P < .00001; I2 = 93%).
In the studies evaluating VZV vaccination, adverse events were predominantly mild and included nausea, fever, and injection-site reactions.32
Comparison of different vaccination doses
Two trials compared different doses of VZV29 and influenza vaccination.31 There was no difference in CDIs or adverse events (supplemental Figures 18-20).
Subgroup analysis
Subgroup analysis could not be performed because of missing information.
Sensitivity analysis
The reported effects of VZV vaccination were robust when studies with high ROB were removed (Table 2). We could not perform a sensitivity analysis for influenza vaccination (1 trial).
Prophylactic antibiotics vs immunoglobulin
One study included 60 hematology patients who were randomly assigned to receive prophylactic immunoglobulin or trimethoprim/sulfamethoxazole for 12 months.35 As feasibility was the primary outcome, this study was not powered for efficacy outcomes. Findings are available from a conference abstract only.
Discussion
In this review of the efficacy and safety of different interventions to prevent infection in adult patients with CLL, NHL, and MM, we identified 21 completed trials: 8 evaluated immunoglobulin, 5 evaluated antibiotics, 7 evaluated vaccination, and 1 compared antibiotics to immunoglobulin. We identified some general themes that limited our interpretation. (1) Many trials were published >15 years ago, precluding generalizability to current treatments. (2) The standard care arms were variable in the studies that evaluated immunoglobulin, as illustrated by the use of various placebo types, and there was a lack of description of other interventions used to prevent infection across all studies. (3) Definitions for outcomes were heterogenous, including reporting of the number and severity of infections, which limited the pooling of results for the meta-analysis. (4) We judged the ROB to be high for 9 RCTs, and many trials were small and thus insufficiently powered for clinical outcomes. (5) Only 1 ongoing study evaluating prophylactic immunoglobulin was identified, suggesting that this area is not one of active research, despite the importance of infection management.
Of the 8 studies that evaluated prophylactic immunoglobulin, 7 were published before 2000. Since the previous SR published in 2008,3 only 1 completed RCT and 1 ongoing RCT evaluating immunoglobulin were identified, highlighting the paucity of supportive care data in the current era. Our findings are similar to those in the previous review, showing that prophylactic immunoglobulin reduced the risk of CDIs, but did not reduce the risk of mortality and increased the risk of adverse events. Our confidence in these findings are very low, given the ROB assessments and the low number of patients in many of the included trials, which were not powered to assess differences in clinical outcomes such as mortality.
Cost-effectiveness and quality of life were not prospectively compared, but 1 study evaluated cost-effectiveness using physician assessment of quality of life.36 The increase in adverse events accompanying reduced infection rates indicates the requirement for future studies to include patient-reported outcomes, so that clinicians can better understand how to balance provision of infection prevention with adverse effects by focusing on outcomes that matter most to patients.
Five studies evaluated antibiotics in patients with MM, with no studies identified in CLL or NHL. Since the previous SR, 2 new studies have been identified. In patients with MM, prophylactic antibiotics did not significantly reduce the risk of mortality or CDIs, but increased the risk of adverse events. A major concern regarding antibiotic prophylaxis is the emergence of resistant organisms.37 Of the included studies, only 1 reported this outcome and found that prophylactic levofloxacin did not increase carriage of resistant organisms within its 12-month follow-up.21
Seven studies evaluated VZV and influenza vaccinations. Since the previous review,3 we have identified 5 new trials. Vaccination did not reduce mortality, but significantly reduced risk of clinically documented VZV and influenza infections. Although our search strategy did not directly address COVID-19 vaccines, we did not locate any completed RCTs in hematology patients, in accordance with a recent SR that identified multiple prospective studies on COVID-19 vaccination, but no completed or ongoing RCTs.38 We are aware, however, that future RCTs evaluating COVID-19 booster vaccination are planned.
Although some international guidelines recommend a trial of antibiotics before commencement of prophylactic immunoglobulin, evidence is lacking to support this recommendation. Only 1 feasibility RCT was identified that directly compared these interventions.
There are some limitations. Most of the included studies evaluating immunoglobulin predate modern B-cell– and plasma cell–targeted therapies, thus limiting the applicability of our findings to current practice. There was significant variability in the reporting of infection outcomes, limiting the pooling of studies in the meta-analysis. Many of the included trials were small and insufficiently powered for mortality outcomes. In addition, none of the trials provided information about other interventions administered. However, to our knowledge, this is the first SR to evaluate different types of interventions. Our review provides a comprehensive evaluation of current evidence to support the use of prophylactic immunoglobulin, prophylactic antibiotics, and vaccination.
In summary, prophylactic immunoglobulin and vaccination, but not prophylactic antibiotics, may reduce risk of CDI in patients with CLL, NHL, and MM; however, these findings should be interpreted with caution because of the low number of patients, high ROB in the included studies, and lack of contemporary data applicable to the current standard of care for such patients. Given the variation in international guidelines, rising global demand, cost of immunoglobulin, and concerns regarding antimicrobial resistance, more evidence is needed to inform infection prevention strategies. Future studies should compare different interventions, specifically immunoglobulin vs antibiotics, should use standardized definitions of infection outcomes to enable comparison across studies, and should report on cost-effectiveness analyses and impacts on patient-reported outcome measures.
Conflict-of-interest disclosure: P.C. is on the Medical Advisory Board for Aegros and has received conference travel funds from CSL-Behring. R.W. has received speaker fees from Janssen, AbbVie, and BeiGene. Monash University has received funding from CSL Behring for unrelated projects.
Acknowledgments
The authors thank Lorena Romero, research and training librarian at the Ian Potter Library (Alfred Health), for assistance with developing the search strategy.
This work was supported by a project grant from the Commonwealth of Australia represented by and acting through the National Blood Authority (ABN 87361602478). The funder of the study had no role in study design; data collection, analysis, and interpretation; or writing of the report. K.L.C. is a PhD candidate at Monash University, and this work has been submitted in partial fulfilment of the degree requirements. K.L.C. is supported by scholarships from Monash University, the Hematology Society of Australia and New Zealand (HSANZ), and the Leukemia Foundation. R.W. is supported by a fellowship from the Health Research Council of New Zealand (19/139). Z.K.M. and E.M.W. are supported by National Health and Medical Research Council (NHMRC) Investigator and NHMRC Synergy grants.
Authorship
Contribution: A.K., C.O.M., E.M.W., P.C., R.W., S.S., and Z.K.M. conceived the research; A.K., J.W., K.L.C., P.C., R.W., and Z.M. performed the literature search, data screening, and data analysis; J.W., K.L.C., and Z.M. drafted the manuscript; and all authors reviewed, edited, and approved the final version of the manuscript.
Footnotes
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Nygaard A, Halvorsrud L, Linnerud S, Grov EK, Bergland A. The James Lind Alliance process approach: scoping review. BMJ Open. 2019;9(8) doi: 10.1136/bmjopen-2018-027473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong J, Wood EM, Crispin P, Weinkove R, McQuilten ZK, Australasian Leukaemia and Lymphoma Group (ALLG) Supportive Care Group Managing hypogammaglobulinaemia secondary to haematological malignancies in Australia and New Zealand: a clinician survey. Intern Med J. 2019;49(3):358–363. doi: 10.1111/imj.14082. [DOI] [PubMed] [Google Scholar]
- 3.Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in hematological malignancies and hematopoietic stem cell transplantation. Cochrane Database Syst Rev. 2008;4:CD006501. doi: 10.1002/14651858.CD006501.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gafter-Gvili A, Fraser A, Paul M, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev. 2012;1(1):CD004386. doi: 10.1002/14651858.CD004386.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Paul M, Gafter-Gvili A, Goldberg E, Yahav D. Infections in hematogical cancer patients: the contribution of systematic reviews and meta-analyses. Acta Haematol. 2011;125(1-2):80–90. doi: 10.1159/000318900. [DOI] [PubMed] [Google Scholar]
- 6.Mohyuddin GR, Aziz M, McClune B, Abdallah AO, Qazilbash M. Antibiotic prophylaxis for patients with newly diagnosed multiple myeloma: systematic review and meta-analysis. Eur J Haematol. 2020;104(5):420–426. doi: 10.1111/ejh.13374. [DOI] [PubMed] [Google Scholar]
- 7.Cheuk DK, Chiang AK, Lee TL, Chan GC, Ha SY. Vaccines for prophylaxis of viral infections in patients with hematological malignancies. Cochrane Database Syst Rev. 2011;(3):CD006505. doi: 10.1002/14651858.CD006505.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Wyndham A, Vogan A, Newton S, Schubert C. Immunoglobulin for acquired hypogammaglobulinaemia secondary to haematological malignancies, or post-haemopoietic stem cell transplantation. 2019. https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww1.health.gov.au%2Finternet%2Fmsac%2Fpublishing.nsf%2FContent%2F9C7DCF1C2DD56CBECA25801000123C32%2F%24File%2FMSAC%2520SBA%2520or%2520CA%2520Assessment%2520Report%2520Template%2520-Therapeutic.docx&wdOrigin=BROWSELINK MSAC Assessment Report. Available at:
- 9.Prevot J, Jolles S. Global immunoglobulin supply: steaming towards the iceberg? Curr Opin Allergy Clin Immunol. 2020;20(6):557–564. doi: 10.1097/ACI.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Department of Health D Clinical guidelines for immunoglobulin use. Gov.UK. https://www.gov.uk/government/publications/clinical-guidelines-for-immunoglobulin-use-second-edition-update Available at:
- 11.McQuilten Z, Morrissey O, Stanworth S, Weinkove R, Wong J, Waters N. Interventions to reduce infections in patients with hypogammaglobulinaemia secondary to haematologic malignancies. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=70825 Available at:
- 12.Higgins JPT, Thomas J, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; Hoboken, NJ: 2019. [Google Scholar]
- 13.Boughton BJ, Jackson N, Lim S, Smith N, Clinical Trial Multicenter Study Randomized Controlled Trial Randomized trial of intravenous immunoglobulin prophylaxis for patients with chronic lymphocytic leukaemia and secondary hypogammaglobulinaemia. Clin Lab Haematol. 1995;17(1):75–80. doi: 10.1111/j.1365-2257.1995.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 14.Chapel HM, Lee M, Hargreaves R, Pamphilon DH, Prentice AG, The UK Group for Immunoglobulin Replacement Therapy in Multiple Myeloma Randomised trial of intravenous immunoglobulin as prophylaxis against infection in plateau-phase multiple myeloma. Lancet. 1994;343(8905):1059–1063. doi: 10.1016/s0140-6736(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 15.Gamm H, Huber C, Chapel H, Lee M, Ries F, Dicato MA, Clinical Trial Randomized Controlled Trial Intravenous immune globulin in chronic lymphocytic leukaemia. Clin Exp Immunol. 1994;97(suppl 1):17–20. [PMC free article] [PubMed] [Google Scholar]
- 16.Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic L. Gale RP, Chapel HM, Bunch C, et al. Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia. N Engl J Med. 1988;319(14):902–907. doi: 10.1056/NEJM198810063191403. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths H, Brennan V, Lea J, Bunch C, Lee M, Chapel H. Crossover study of immunoglobulin replacement therapy in patients with low-grade B-cell tumors. Blood. 1989;73(2):366–368. [PubMed] [Google Scholar]
- 18.Molica S, Musto P, Chiurazzi F, et al. Prophylaxis against infections with low-dose intravenous immunoglobulins (IVIG) in chronic lymphocytic leukemia. Results of a crossover study. Haematologica. 1996;81(2):121–126. [PubMed] [Google Scholar]
- 19.Musto P, Brugiatelli M, Carotenuto M, Clinical Trial Letter Randomized Controlled Trial Prophylaxis against infections with intravenous immunoglobulins in multiple myeloma. Br J Haematol. 1995;89(4):945–946. doi: 10.1111/j.1365-2141.1995.tb08447.x. [DOI] [PubMed] [Google Scholar]
- 20.Vacca A, Melaccio A, Sportelli A, Solimando AG, Dammacco F, Ria R. Subcutaneous immunoglobulins in patients with multiple myeloma and secondary hypogammaglobulinemia: a randomized trial. Clin Immunol. 2018;191:110–115. doi: 10.1016/j.clim.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Drayson MT, Bowcock S, Planche T, et al. Levofloxacin prophylaxis in patients with newly diagnosed myeloma (TEAMM): a multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. Lancet Oncol. 2019;20(12):1760–1772. doi: 10.1016/S1470-2045(19)30506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregersen H, Do T, Kristensen IB, et al. A randomized placebo-controlled phase II study of clarithromycin or placebo combined with VCD induction therapy prior to high-dose melphalan with stem cell support in patients with newly diagnosed multiple myeloma. Exp Hematol Oncol. 2018;7(1):18. doi: 10.1186/s40164-018-0110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oken MM, Pomeroy C, Weisdorf D, Bennett JM. Prophylactic antibiotics for the prevention of early infection in multiple myeloma. Am J Med. 1996;100(6):624–628. doi: 10.1016/s0002-9343(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 24.Puig N, Hernández MT, Rosiñol L, et al. Lenalidomide and dexamethasone with or without clarithromycin in patients with multiple myeloma ineligible for autologous transplant: a randomized trial. Blood Cancer J. 2021;11(5):101. doi: 10.1038/s41408-021-00490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vesole DH, Oken MM, Heckler C, et al. University of Rochester Cancer Center and the Eastern Cooperative Oncology Group. Oral antibiotic prophylaxis of early infection in multiple myeloma: a URCC/ECOG randomized phase III study. Leukemia. 2012;26(12):2517–2520. doi: 10.1038/leu.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988–1000. doi: 10.1016/S1473-3099(19)30163-X. [DOI] [PubMed] [Google Scholar]
- 27.Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347(1):26–34. doi: 10.1056/NEJMoa013441. [DOI] [PubMed] [Google Scholar]
- 28.Musto P, Carotenuto M, Clinical Trial Letter Randomized Controlled Trial Vaccination against influenza in multiple myeloma. Br J Haematol. 1997;97(2):505–506. [PubMed] [Google Scholar]
- 29.Stadtmauer EA, Sullivan KM, Marty FM, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood. 2014;124(19):2921–2929. doi: 10.1182/blood-2014-04-573048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stadtmauer EA, Sullivan KM, El Idrissi M, et al. Adjuvanted recombinant zoster vaccine in adult autologous stem cell transplant recipients: polyfunctional immune responses and lessons for clinical practice. Hum Vaccin Immunother. 2021;17(11):4144–4154. doi: 10.1080/21645515.2021.1953346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teh BW, Leung VKY, Mordant FL, et al. A randomised trial of two 2-dose influenza vaccination strategies for patients following autologous haematopoietic stem cell transplantation. Clin Infect Dis. 2020;73(11):e4268–e4277. doi: 10.1093/cid/ciaa1711. [DOI] [PubMed] [Google Scholar]
- 32.Winston DJ, Mullane KM, Cornely OA, et al. Inactivated varicella zoster vaccine in autologous haemopoietic stem-cell transplant recipients: an international, multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10135):2116–2127. doi: 10.1016/S0140-6736(18)30631-7. [DOI] [PubMed] [Google Scholar]
- 33.Cornely O, Mato A, Robak T, Mellinghoff S, Lavrova T. The Prosid Study: evaluating efficacy and safety of intravenous immunoglobulin (IVIG) 10% in primary infection prophylaxis in patients with chronic lymphocytic leukemia- study design [abstract] Blood. 2020;136(suppl 1):20–21. [Google Scholar]
- 34.Chapel H, Dicato M, Gamm H, et al. Immunoglobulin replacement in patients with chronic lymphocytic leukaemia: a comparison of two dose regimes. Br J Haematol. 1994;88(1):209–212. doi: 10.1111/j.1365-2141.1994.tb05002.x. [DOI] [PubMed] [Google Scholar]
- 35.McQuilten ZWR, Crispin P, et al. EHA2021 Virtual Congress Abstract Book. HemaSphere. 2021;5:e566. [Google Scholar]
- 36.Weeks JC, Tierney MR, Weinstein MC. Cost effectiveness of prophylactic intravenous immune globulin in chronic lymphocytic leukemia. N Engl J Med. 1991;325(2):81–86. doi: 10.1056/NEJM199107113250202. [DOI] [PubMed] [Google Scholar]
- 37.Gafter-Gvili A, Paul M, Fraser A, Leibovici L. Effect of quinolone prophylaxis in afebrile neutropenic patients on microbial resistance: systematic review and meta-analysis. J Antimicrob Chemother. 2007;59(1):5–22. doi: 10.1093/jac/dkl425. [DOI] [PubMed] [Google Scholar]
- 38.Lee ARYB, Wong SY, Chai LYA, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: a systematic review and meta-analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bastidas A, de la Serna J, El Idrissi M, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322(2):123–133. doi: 10.1001/jama.2019.9053. [published correction appears in JAMA. 2019;322(8)785] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.