Abstract
Atherosclerotic cardiovascular disease risk (ASCVD) is an ongoing epidemic, and lipid abnormalities are its primordial cause. Most individuals suffering a first ASCVD event are previously asymptomatic and often do not receive preventative therapies. The cornerstone of primary prevention has been the identification of individuals at risk through risk calculators based on clinical and laboratory traditional risk factors plus risk enhancers. However, it is well accepted that a clinical risk calculator misclassifies a significant proportion of individuals leading to the prescription of a lipid-lowering medication with very little yield or a missed opportunity for lipid-lowering agents with a potentially preventable event. The development of coronary artery calcium scoring (CAC) and CT coronary angiography (CCTA) provide complementary tools to directly visualize coronary plaque and other risk-modifying imaging components that can potentially provide individualized lipid management.
Understanding patient selection for CAC or potentially CCTA and the risk implications of the different parameters provided, such as CAC score, coronary stenosis, plaque characteristics and burden, epicardial adipose tissue, and pericoronary adipose tissue, have grown more complex as technologies evolve. These parameters directly affect the shared decision with patients to start or withhold lipid-lowering therapies, to adjust statin intensity or LDL cholesterol goals. Emerging lipid lowering studies with non-invasive imaging as a guide to patient selection and treatment efficacy, plus the evolution of lipid lowering therapies from statins to a diverse armament of newer high-cost agents have pushed these two fields forward with a complex interaction. This review will discuss existing risk estimators, and non-invasive imaging techniques for subclinical coronary atherosclerosis, traditionally studied using CAC and more recently CCTA with qualitative and quantitative measurements. We will also explore the current data, gaps of knowledge and future directions on the use of these techniques in the risk-stratification and guidance of lipid management.
Keywords: Plaque burden, Lipids, Plaque characterization, CAC, CCTA
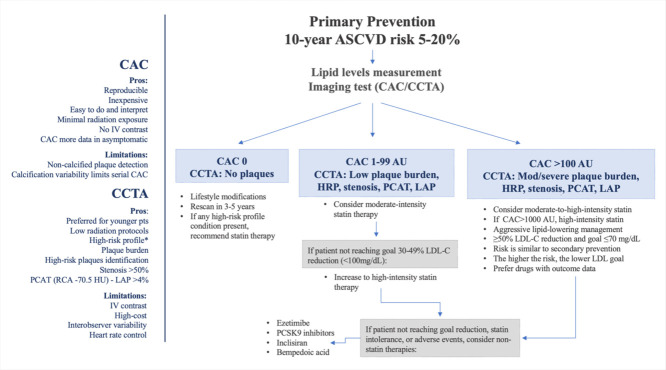
Approach to testing and treatment selection in patients with 10-year ASCVD risk between 5% and 20%. *High-risk profile: patients with diabetes, smoking history, or family history of premature ASCVD, often with considerable non-calcified plaque. AU: Agatston units, CAC: coronary artery calcium score, CCTA: coronary computed tomography angiography, HRP: high-risk plaques, IV: intravenous, LAP: low attenuation plaque, LDL-C: low-density lipoprotein cholesterol, PCSK9: proprotein convertase subtilisin/kexin type 9. PCAT: pericoronary adipose tissue attenuation, RCA: right coronary artery
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) is an ongoing epidemic, and atherogenic lipoproteins are its root cause. Most first cardiac events occur in asymptomatic individuals, and therefore waiting for symptoms to develop is not a feasible strategy. Risk scores have been developed from the Framingham Risk Score to the Pooled Cohort Equation (PCE) to estimate the likelihood of a person developing ASCVD over a defined period. However, they have shown significant limitations, including over or underperforming in ethnic minorities, the lack of acknowledgment of dynamic changes in risk factors, and the over-reliance on age. The development of coronary artery calcium scoring (CAC) and later CT coronary angiography (CCTA) surged as potential complementary tools to visualize coronary plaque and provide the basis for individualized management directly. The major scope of this review is the evaluation of such risk in asymptomatic individuals using CAC and more recently CCTA to improve risk prediction and potentially guide lipid-lowering medical therapy.
2. Risk estimators in asymptomatic individuals
European [1] and American [2] guidelines recommend using mathematical tools to predict ASCVD risk in asymptomatic patients. ASCVD risk calculators are derived from data pooled from multiple cohort studies; due to their inherent limitations, they must be interpreted in light of specific circumstances for individual patients.
The American College of Cardiology and American Heart Association (ACC/AHA) have endorsed the PCE for primary clinical risk assessment since 2013 [2]. The PCE is a race- and sex-specific traditional risk calculator derived from the data of 5 large US cohort studies. It should be used mainly for non-Hispanic African American and White adults aged 40 to 79 with low-density lipoprotein cholesterol (LDL-C) 70–190 mg/dL and only for individuals without prior ASCVD. It estimates the 10-year risk of developing a first hard ASCVD event (e.g., nonfatal MI, coronary heart disease, fatal or nonfatal stroke) and stratifies individuals into 4 absolute risk categories: low (<5%), borderline (5–7.4%), intermediate (7.5–19.9%), and high (≥20%). However, total ASCVD risk is a continuum, and the elements included in the equation are dynamic and should not be interpreted in a restricted way. The risk category cut-offs are mainly based on practical considerations concerning the net benefit of preventive therapies but with limited predictive performance in the individual patient.
The PCE has been shown to overestimate [3] or underestimate [4] ASCVD risk for certain subgroups. Thus, the 2019 ACC/AHA Guideline on the Primary Prevention of CV Disease advocates for the use of use additional risk-enhancing factors (family history [FH] of premature ASCVD, chronic kidney disease, chronic inflammatory conditions, etc.) to guide decisions about preventive interventions for borderline- or intermediate-risk adults. However, the value of preventive therapy may remain uncertain in this group, and some patients may be reluctant to take medical therapy without evidence of increased ASCVD risk. At the same time, most ASCVD events occur in patients who would have otherwise been considered intermediate or low-risk [5].
CAC and CCTA have emerged as tools to improve risk prediction and potentially guide therapy in the individual patient. Given the low cost of statins, a treat-all versus test-guided approach has been considered. Pletcher et al. demonstrated that CAC testing in intermediate-risk patients is cost-effective, especially when statins are costly or when there is significant statin dysutility [6]. In asymptomatic patients without prior ASCVD, with CAC=0 and intermediate risk, statin therapy has a proven low benefit (with certain exceptions such as diabetics and patients with LDL-C>190 mg/dL). The EISNER trial demonstrated that compared with no scanning, randomization to CAC scanning was associated with improved coronary artery disease (CAD) risk factor control without increasing downstream medical testing [7]. In the ROBINSCA trial [8], CAC scoring significantly reduced the number of individuals indicated for preventive treatment compared to the SCORE model. Additionally, testing provides the benefit of a treat-to-goal approach and guidance on adding non-statin lipid-lowering therapies. Recently, in the DANCAVAS trial [9], screening with CAC, among other non-invasive methods, was performed in men 65 to 74 years of age living in 15 Danish municipalities. The authors demonstrated that after more than 5 years, the invitation to undergo comprehensive cardiovascular screening did not significantly reduce the incidence of death from any cause. However, the results of subgroup analyses suggested the possibility of a greater benefit of screening among participants in the younger age group (65–69 years of age).
Polygenic risk scores (PRS) are a newly attractive tool for quantifying inherited risk. One advantage of genetic risk scores is that they can be measured early in life (even at birth), and they remain constant over a lifetime [10]. In older adult populations, these scores add relatively little to risk prediction; by that stage of life, lifetime exposure to risk factors predominates, and such cumulative risk may be best captured by a direct measure of subclinical disease burden (e.g., CAC). However, at young ages, genetic risk scores appear to convey unique data—distinct from family history—that could be used to drive downstream behavior. For example, PRS may inform when the first CAC scan should be obtained, thereby potentially improving the efficiency of CAC for early detection of conversion to CAC >0 [11].
3. Coronary artery calcium
We have known about the presence of vascular calcification for centuries. Before the development of the light microscope, the pathological diagnosis was made based on macroscopic observation with the naked eye.
High circulating lipids, specifically APOB-containing lipoproteins, including LDL-C, have been implicated as an important modifiable risk factor for coronary atherosclerosis. Coronary artery calcium has been promulgated as a specific marker of atherosclerosis since the 1940s. The extent of coronary artery calcification reflects the lifetime effect of all known and unknown factors that cause CAD. Lifelong exposure to LDL-C has been demonstrated to simultaneously promote vascular calcification and skeletal bone dimerization, explaining the paradoxical coexistence of osteoporosis and atherosclerosis in many patients [9].
Even though triglycerides are independently associated with CV events, their role in the process of vascular calcification remains unclear. In the MESA study, individuals with TG >400mg/dL were excluded, and therefore findings should be analyzed with caution. However, high TG levels (≥175 mg/dL) were not associated with CAC in the intermediate CV risk population included in the study [12]. Recently, a Taiwanese study of 3586 patients showed that higher level of TG and lower level of HDL-C were significantly associated with the risk of CAC>100, particularly in patients younger than 65 years and with normal-weight [13].
3.1. Detection and quantification of CAC
The potential value of CAC in predicting future coronary events was first acknowledged in the 70s and 80s using chest radiography and fluoroscopy [14,15]. It was not until 1990 that CAC could be precisely detected and quantified with the introduction of cardiac gated electron-beam computed tomography (EBCT) [16]. Later, multidetector computed tomographic (MDCT) scanners replaced EBCT and were documented to provide CAC quantification that correlated well with the EBCT [17]. Nowadays, thanks to the increasingly larger numbers of detectors and faster gantry speeds in current MDCT scanners, even nongated studies can provide accurate semi-quantitative or quantitative measurements [18].
The Agatston score remains the reference standard and the most commonly used CAC score in clinical practice; it is a summed score of calcified coronary lesions, accounting for both the total area and the maximal density of coronary calcification [16]. A CT attenuation threshold of 130 Hounsfield units (HU) is used for calcium detection [19]. The traditional CAC risk categories are: 0=very low risk, 1–99=mildly increased, 100–299=moderately increased, 300–1000=moderate to severely increased, and >1000=severely increased [16]. Additionally, CAC percentiles based on age, gender, and ethnicity, are also routinely reported, given that individuals in the ≥75th percentile are considered of increased risk [20]. The calcium area and density values may have wide inter-observer variability within a CAC score category, with implications for coronary heart disease (CHD) development and the probability of culprit lesion events. It has been shown recently that for a given CAC score, high CAC density relative to plaque area (called “discordant”) confers lower long-term ASCVD risk, likely serving as an imaging marker of biological resilience for lesion vulnerability [21].
Non-gated studies provide already existing valuable data and can be used to calculate semiquantitative (ordinal scores) or quantitative CAC (Agatston scoring), with a high correlation with ECG-gated CT studies and CVD outcomes [22]. At a minimum, a visual assessment analysis should be performed as follows: 0=none with very low risk, 1=mild with mildly increased risk, 2=moderate with moderately increased risk, and 3=severe with moderately to severely increased risk, and the corresponding 2018 Coronary Artery Calcium Data and Reporting System (CAC-DRS) categories range from 0-3 [20].
Given its wealth of evidence regarding risk stratification, low cost, and wide availability, CAC plays a central role in determining long-term ASCVD risk and mortality [23,24] in asymptomatic patients. It has been shown to be stronger in risk prediction over hs-CRP, carotid intima-media thickness, and other biomarkers [5], as well as clinical risk scores.
3.2. Power of Zero to de-escalate risk
In 2009, the landmark studies by Blaha et al.[25] and Sarwar et al. [26] provided the first glimpse into the potential value of the absence of CAC for “de-risking” otherwise at-risk individuals. This concept was coined as the “power of zero” and is firmly supported by the fact that the absence of CAC is the strongest negative risk marker in clinical practice, identifying patients at very-low 10-year risk. Consequently, recent AHA/ACC guidelines (2018 & 2019) recommended CAC scoring to aid in the decision-making regarding statin in borderline- or intermediate-risk patients for whom the indication to recommend therapy is unclear.
In a 2016 study from MESA, CAC zero was better than 12 other negative risk markers, including the absence of family history, healthy lifestyle, hsCRP>2 mg/L, low N-terminal pro-brain natriuretic peptide, and absence of carotid plaque in reducing an individual patient's post-test risk of developing clinical CVD [27] (Table 1). Along the same line are the results from the BioImage study, where 86% of the study participants qualified for statin therapy for primary prevention due to an ASCVD risk of ≥7.5%. In this population, CAC=0, as well as CAC <10, were the strongest negative risk factors for the development of CVD over a median follow-up of 2.7 years [28].
Table 1.
Main studies reporting prognostic role of CAC among asymptomatic patients.
| Authors | Publication Year | Population Number | Endpoints | Follow-up | Main results |
|---|---|---|---|---|---|
| Prospective Army Coronary Calcium Project [29] | 2005 | 2000 | CAD: SCD, MI, UA | 3.0 ± 1.4 years |
CAC was associated with an increase in coronary event risk by a factor of 12 (p = 0.002). |
| Rotterdam Study [30] | 2005 | 1795 | Hard CAD: MI, CAD mortality | 3.3 ± 0.8 years |
Relative risk of coronary events for CAC 101–400, 401–1000, and >1000 (compared with scores of 0–100) were 3.1, 4.6, and 8.3, respectively. |
| Cooper Clinic Cohort [31] | 2005 | 10,476 | Hard CAD: CHD death, nonfatal MI | 3.5 years |
Age-adjusted rates (per 1000 person-years) of hard events for CAC 0 and incremental sex-specific thirds of detectable CAC were 0.4, 1.5, 4.8, and 8.7, respectively. |
| St. Francis Heart Study [32] | 2005 | 4903 | Coronary death, nonfatal MI, revascularization procedures, stroke, and vascular surgery | 4.3 years |
Subjects with ASCVD events had higher baseline CAC scores than those without events. Relative risk for all ASCVD events of CAC ≥100 was 11.1 compared to CAC <100. |
| Budoff et al. [33] | 2007 | 25,253 | All-cause mortality | 6±3 years |
CACS provides independent incremental information in addition to traditional risk factors in the prediction of all-cause mortality. |
| MESA [34] | 2008 | 6814 | Major CAD events: MI, CAD death; coronary events: major CAD events | 3.9 years |
Adjusted risk of a coronary event increased by 7.73 when CAC 101–300 and 9.67 when CAC >300 regardless of ethnicity. |
| Heinz Nixdorf Recall [35] | 2010 | 4129 | Hard CV events: nonfatal MI, coronary death | 5.1±0.3 years | Reclassifying intermediate-risk subjects with CAC <100 to the low-risk category and CAC >400 to high-risk yielded a reclassification improvement of 21.7% and 30.6% for the FRS, respectively. |
| CARDIA [36] | 2017 | 3043 | CAD events: MI, ACS, CAD death, revascularization | 12.5 years |
In adults 32 to 46 years, those with any CAC had a 5-fold increase in CHD events and a 3-fold increase in CVD events. |
| CAC Consortium [37] | 2020 | 66,636 | CVD and CHD mortality | 12.5 years | CAC was the most reliable predictor for long-term mortality. |
| Impact of statins treatment by CAC | |||||
| Mitchel et al. [38] | 2018 | 13,622 | MI, stroke CVD | 9.4 years | ARR and RRR proportional to CAC, 8.5% ARR when CAC >100 (NNT = 12) |
| Zhou et al. [39] | 2020 | 6301 | ASCVD events | 14.2 years | ARR and RRR proportional to CAC, 10% ARR when CAC >400 (NNT = 10) |
ACS: acute coronary syndrome, ARR: absolute risk reduction, ASCVD: atherosclerotic cardiovascular disease, CAC: coronary artery calcium, CAD: coronary artery disease, CHD: coronary heart disease, CV: cardiovascular, CVD: cardiovascular disease, MI: myocardial infarction, NNT: number needed to treat, RRR: relative risk reduction, UA: unstable angina.
The value of CAC=0 to re-classify ASCVD risk in those at borderline and intermediate ASCVD risk, the group for whom its use has been deemed most appropriate in the 2018 AHA/ACC Cholesterol Guideline, was initially shown in a study of the MESA population. Of participants eligible for statins, a total of 1316 (44%) had CAC=0 at baseline and were reclassified to lower risk with a resultant observed 10-year ASCVD event rate of 4.2 per 1,000 person-years [40].
Furthermore, recent studies have demonstrated the prognostic significance of CAC scoring in low-risk individuals with a family history of CHD, a group identified for potential CAC scoring in the new Society of Cardiovascular Computed Tomography (SCCT) guidelines. In this population, CAC=0 maintains its strong prognostic capacity [41].
3.3. CAC in higher risk groups
A 2020 study from the CAC Consortium characterized individuals with CAC scores >1000. They found that individuals with CAC>1000 have a higher area and density of calcification than the other CAC groups. In addition, those with CAC scores >1000 are at an almost 2-fold higher risk of CVD mortality than those with CAC scores of 400 to 999 [42]. Notably, CAC scores ranging from 775 to 900 for a general at-risk primary prevention population and CAC scores of 300–375 in persons with diabetes were associated with an ASCVD mortality risk equivalent to the overall FOURIER trial population of patients with clinical cardiovascular disease [43]. The threshold for CAC scoring to mimic secondary prevention risk varies according to risk factors, and the population studied. Although there are no published randomized trials of PCSK9i in primary prevention populations free of familial hypercholesterolemia, current ACC/AHA and ESC/European Atherosclerosis Society guidelines already recommend consideration of this therapy in some asymptomatic populations without FH. Using CAC in this population could enhance the identification of potential candidates likely to derive the smallest and largest absolute benefit from aggressive LDL-C lowering with PCSK9i improving cost-effectiveness [44].
Likewise, CAC is able to improve current risk stratification and therapy allocation paradigms among individuals with hypertriglyceridemia without clinical ASCVD. In a pooled analysis of 2345 individuals from MESA, CARDIA, Dallas Heart Study, and Heinz Nixdorf Recall study cohorts with hypertriglyceridemia and no clinical ASCVD, the role of CAC in further risk stratification was evaluated. Among participants eligible for icosapent-ethyl (EPA), 38% had CAC >100, their event rates were markedly higher (15.9% vs. 7.2%), and the NNT for 5 years was 2.2-fold lower (29 vs. 64) than those of the 25% eligible participants with CAC=0 [45].
Considering the exposed above, guidelines from medical societies, notably the American Association of Clinical Endocrinologists and the European Society of Cardiology, have begun recommending very low LDL-C goals (<55 mg/dl) in those who are at “extreme or very high risk”.
3.4. CAC in special groups (Diabetes, LDL-C >190mg/dL, and young)
Patients with diabetes are at particular risk for developing ASCVD; however, this risk has been recognized as heterogeneous. CAC has been proposed to accurately classify a diabetic individual's ASCVD risk compared to traditional ASCVD risk factors. Notably, up to 40% of patients with diabetes have CAC=0, and even though they portend a higher risk than non-diabetes individuals, they still have a lower risk than the different categories of CAC-positive patients. This could be useful for selecting a lower-intensity statin therapy or seeking less restrictive LDL-C targets in this population [27]. In a trial including participants from MESA, Malik et al. demonstrated that the addition of CAC scoring to global risk assessment was associated with significantly improved risk classification in those with metabolic syndrome (MetS) and diabetes [46]. Similarly, Wong et al. evaluated sex differences in this population and revealed that women had higher total and CVD death rates than men when CAC >100. CAC predicted CHD, CVD, and all-cause mortality in patients with diabetes [47]. Likewise, other authors indicated that the mortality risk increased significantly for diabetic individuals even with a baseline CAC=0 after 5 years [48]. Hence, in these patients rescanning should be considered for a shorter period. Guidelines recommend, in adults aged 40 to 75 years with diabetes, the initiation of statin therapy before a clinical ASCVD assessment, but further risk assessment is required for other decision-making, like statin intensity and the addition of higher-cost nonstatin lipid-lowering therapies [49].
Patients with LDL-C ≥190 mg/dL have been traditionally excluded from risk calculators and considered high-risk. Recently, it has been suggested that this represents a heterogenous risk group. CAC scoring has been shown to stratify CVD risk in asymptomatic patients with LDL-C effectively ≥190 mg/dL [50]. In a recent study, individuals with CAC=0 had a lower risk for future CV events (incidence rate per 1000 person-years = 4.7; 10-year risk = 3.7%; risk/year = 0.4%) [50]. These results do not indicate that subjects with CAC=0 and severe hypercholesterolemia should not be treated with statins, especially younger patients, given their lifetime risks. In this scenario, imaging can help guide the use of more expensive therapies, such as PCSK9 inhibitors, for those who still have elevated LDL-C levels after subsequent follow-up.
Moreover, in a retrospective study of 811 patients aged ≥40 years with LDL-C ≥190 mg/dL evaluated with non-gated chest CT, visual CAC and thoracic aorta calcification (TAC), quantified using simple “ordinal scores” were found to be independently associated with all-cause mortality [51].
Although universal screening for CAC in young adults has not been endorsed, we suggest certain “CAC benefit groups” in which ASCVD risk may be sufficiently high to warrant an assessment of CAC assessment from a shared-decision discussion. These groups include the following: 1) those with diagnosed familial hypercholesterolemia; 2) those with a family history of premature ASCVD; 3) those with multiple risk factors; and 4) those aged 40–45 years who are identified as borderline-intermediate risk by the current PCE. Identifying the presence and degree of CAC in adults ≤45 years in these elevated risk groups provides the opportunity to initiate early preventive efforts with greater potential to change the natural history of ASCVD than if started later in life. The exact age for initial testing has however not been studied and more data is needed but is usually considered in the 30s to early 40s. Considering that in a large cohort of U.S. adults aged 30–45 years without symptomatic ASCVD, the probability of CAC >0 varied by age, sex, and race ranging from 7% to 26% [52].
3.5. CAC progression & the re-scan interval
In patients whose development or progression of CAC would support intensification or alteration in preventive management, it may be appropriate to consider repeat CAC scanning. The interval is still a matter of debate, but initially, data supported repeat CAC scanning at an interval of 5 years for patients with CAC=0 and a 3–5 year interval for patients with CAC>0 [23].
In PESA, a large, middle-aged, and asymptomatic population (40–54 years employees of Santander Bank) was evaluated using comprehensive serial multimodality imaging of multiple vascular territories. At baseline, 63% of the asymptomatic participants showed evidence of atherosclerosis, defined as the presence of atherosclerotic plaque in any of the screened territories by ultrasound (carotids, iliofemoral, aorta) or evidence of CAC in the coronary tree. CAC was detected in only 18% of the cohort (CAC 1-99 in 14%, 100–399 in 3%, and ≥ 400 in 0.7%), in line with previous data from the CARDIA and MESA studies. Importantly, at the 3-year follow-up visit, >40% of the healthy middle-aged PESA cohort showed evidence of disease progression [53]. In a recently published work using the MESA cohort, it was suggested that the so-called “warranty period” of a CAC=0 could be as long as 6–7 years for the low-risk population and as short as 3 years for high-risk and diabetic individuals. Family history of CHD and smoking affected the estimation for both men and women. The conversion from CAC=0 to a CAC score of >10 took an average of 5 to 8 years (depending on ASCVD risk category and age), and the conversion to CAC >100 would take at least 9 years for high-risk individuals [54].
An important limitation in assessing CAC progression has been the measurement variability in detecting real changes in patients with CAC>0. A study by Budoff et al. showed in 4609 asymptomatic individuals that CAC progression added incremental value in predicting all-cause mortality over baseline score, demographics, and CV risk factors [55]. On the contrary, the analysis of CAC progression did not add any benefit to risk prediction models for coronary and cardiovascular events prediction when compared to 10 published algorithms using HNR data. Nevertheless, the best coronary disease prognosis was found for participants with “double zero,” meaning CAC=0 at baseline and the CT scan 5 years later [56]. Therefore, a repeat scan after 5 years seems to be of additional value, except for those who already have a double-zero CAC scan or have already been classified at high risk because of CAC ≥400.
3.6. Limitations and new perspectives
Although CAC has many strengths (it can be performed rapidly, in a reproducible fashion with minimal radiation exposure, without the need for intravenous contrast, and at a low cost), it is not without limitations. Its use for ruling-out CAD has been challenged in the past. In MESA, up to a third of total events occurred in patients with a CAC zero prior to the performance of a subsequent scan at 3 to 5 years [54]. In the CAC consortium cohort, 0.09% of CV deaths occurred in participants without evidence of coronary calcium in a 12-year follow-up period [57]. In the recent Miami Heart Study (MiHEART), 2359 middle-aged and predominantly low-risk (74% in ASCVD risk<5%) asymptomatic individuals were evaluated with CAC score and CCTA. The prevalence of any coronary plaque on CCTA was 16% among those with CAC=0. Furthermore, in this group, 0.8% had stenosis ≥ 50%, 0.1% stenosis ≥70%, and 2.3% high-risk plaque features [58]. It could be argued then that using CAC similar to risk scores can underestimate the youngest individuals with the most to gain from preventive therapies [59], as the presence of isolated non-calcified plaque is more frequent in that population. Finally, the absolute event rates in these studies are likely modified by high baseline use of statin therapy and significant statin drop-in in most studies. For these reason, even that CAC can play a central role in patient selection for lipid lowering therapies, its utility for potentially monitoring progression/regression of disease while on therapy may be limited.
Another potential limitation of CAC is the non-rare presence of subtle calcified plaque below the Agatston score threshold of 130 HU, which, even when minimal, is associated with increased adverse CV outcomes. Although modern imaging technology has advanced, the Agatston scoring method has remained unchanged since its introduction, and consideration of a change in CAC scoring methodology has been recommended. Lately, it has been proposed that smaller and less dense calcified plaques below the conventional detection threshold can be identified by eliminating the size threshold and reducing the HU threshold to ≥120 [60].
4. Coronary computed tomography angiography
CCTA is currently the modality of choice to exclude disease in symptomatic patients with low to intermediate-risk pretest probability (15–50%) for obstructive CAD. Compared to functional ischemia tests, CCTA allows the detection of non-obstructive coronary plaques and the reliable ruling-out of left main disease. In recent large randomized clinical trials, such as the PROMISE [61] and SCOT-HEART [62], the use of CCTA was associated with a lower risk for myocardial infarction than conventional pharmacological management, mostly due to the intensification of preventive therapies. Importantly, in the SCOT-HEART trial, the findings were also present in patients with non-cardiac chest pain, raising the possibility of its value in plaque detection to guide preventative therapies initiation in asymptomatic individuals [62].
Given low radiation with newer scanners (down to 1 mSv with some scanners and safety protocols), the possibility of performing CCTA over CAC has gained momentum in patients in whom CCTA has been previously not commonly used. These include selected asymptomatic high-risk or younger individuals, especially those with a higher likelihood of having a large amount of non-calcified plaque. The presence of predominantly non-calcified plaque is more prevalent in young (age< 45–50 years) and female individuals [63]. Moreover, the burden of CAC and the relationship to non-calcified plaque may differ between ethnicities. Other groups of patients who have risk factors such as diabetes, HIV, smoking, inflammatory conditions (e.g., systemic lupus erythematosus, rheumatoid arthritis, or psoriasis), familial hypercholesterolemia, or those working in high-hazard occupations or a strong family history of premature ASCVD, may also present underlying CAD without CAC [64] and may benefit from CCTA screening instead of CAC. Testing such asymptomatic individuals should be performed in the context of shared decision-making if there is uncertainty regarding initiation and intensity of lipid lowering therapies (Table 2).
Table 2.
Main studies reporting prognostic role of CCTA among asymptomatic patients.
| Authors | Publication Year | Population | Endpoints | Follow-up | Main results |
|---|---|---|---|---|---|
| CONFIRM [65] | 2012 | 7590 | Death Non-cardiac MI |
2 years | CCTA predicts mortality and non-fatal MI in asymptomatic patients with moderately high CAC, but not for lower or higher CAC. |
| Min et al. [66] | 2014 | 27,125 | Cardiac death, revascularization, ACS | 2.4 ± 1.1 years |
For asymptomatic diabetic individuals, CCTA measures of CAD severity confer incremental risk prediction. |
| Kang et al. [67] | 2016 | 591 | Cardiac deaths, Nonfatal MI, UA, late coronary revascularization | 6 years | Results suggested long-term prognostic value of CCTA for asymptomatic DM |
| Halon et al. [68] | 2019 | 630 | ACS | 9.2 years | In asymptomatic DM pts, CCTA plaque volume, %low-density content and mild calcification predicted late plaque events. |
| Impact of statins treatment by CCTA | |||||
| Chow et al. [69] | 2015 | 10,418 | All-cause mortality | 2.2 years | ARR and RRR proportional to nonobstructive plaque burden, 0.57% ARR with nonobstructive plaque. |
| Øvrehus et al. [70] | 2014 | 33,552 | All-cause mortality, MI | 3.5 years | ARR proportional to CAC and nonobstructive plaque burden, RRR similar across groups, ARR 7.8% when CAC >400 (NNT = 13) |
ACS: acute coronary syndrome, ARR: absolute risk reduction, CAC: coronary artery calcium, CCTA: coronary computed tomography angiography, CAD: coronary artery disease, DM: type 2 diabetes mellitus, MI: myocardial infarction, NNT: number needed to treat, RRR: relative risk reduction, UA: unstable angina.
4.1. Visual assessment of coronary plaque burden
Beyond stenosis and clinical variables, recent data suggests that the overall amount of coronary plaque by CCTA is a lead predictor of incident CHD events [71]. Indeed, the ability to detect calcified and non-calcified plaque is a unique attribute of CCTA.
Standard plaque burden estimation on CCTA is based on a visual or semi-quantitative assessment of coronary segments using the Segment Involvement Score (SIS), Segment Stenosis Score (SSS), and CT-adapted Leaman score (CT-Leaman) [72]. The SIS provides independent prognostic information above and beyond the presence of obstructive CAD [73].
The SCAPIS trial, conducted in middle-aged individuals without known CHD (50.6% women), demonstrated any CCTA-detected atherosclerosis in 42.1% and any significant stenosis (≥50%) in 5.2%. Atherosclerosis was more prevalent in older individuals and increasingly detected with higher CAC scores [74]. Similarly, the MiHeart study included asymptomatic individuals with independent predictors plaque: older age, male sex, tobacco use, diabetes, overweight, and obesity. Overall, 49% of participants had coronary plaque [58]. Larger studies, such as the SCOT-HEART 2 trial (NCT03920176), are currently underway to provide evidence on whether a CCTA-based strategy for asymptomatic patients with at least one risk factor is of benefit in clinical practice. It hopes not only to answer the question of whether CCTA is better than risk scoring but also how to apply an imaging-based screening tool.
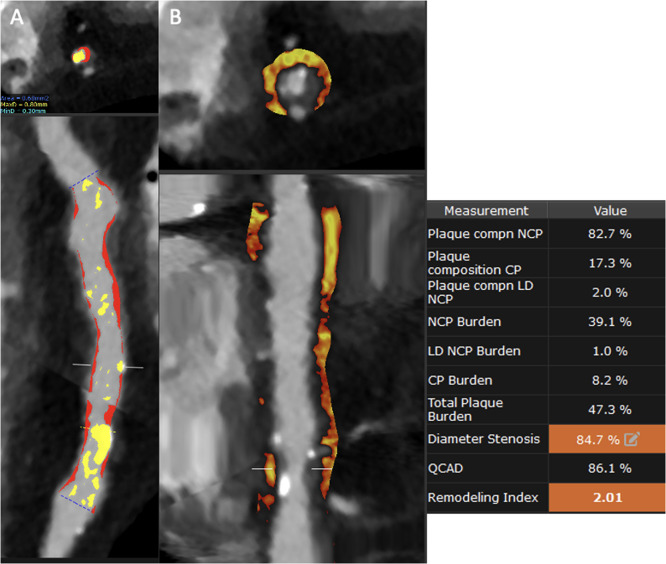
Epicardial adipose tissue (EAT) and pericoronary adipose tissue (PCAT) are inflammation features evaluated by CCTA that have proven to be associated with coronary atherosclerosis and CV events [75]. Tzolos et al. demonstrated that PCAT attenuation of the RCA was predictive of myocardial infarction (HR: 1.55; p=0.017, per 1 SD increment) with an optimum threshold of −70.5 HU (HR: 2.45; p=0.01) [76] (Fig. 1).
Fig. 1.
A. Curved MPR with Autoplaque analysis demonstrating right coronary artery ectasia with an obstructive lesion in the distal segment. The yellow pattern describes the calcified components of the plaque, and the red one indicates the noncalcified. A 2% is composed of low attenuated plaque (<30HU) with a remodeling index of 2.01, indicating high-risk plaque. B. Straight MPR PCAT analysis with AutoPlaque demonstrating an attenuation higher than -70 HU.
4.2. Qualitative high-risk features of coronary plaque
Similar to invasive studies, analysis of coronary plaque by CCTA for risk assessment may be divided into two different approaches: (1) qualitative assessment with identification of “high-risk” plaque features and (2) quantitative measurement of total coronary plaque volume and burden [72].
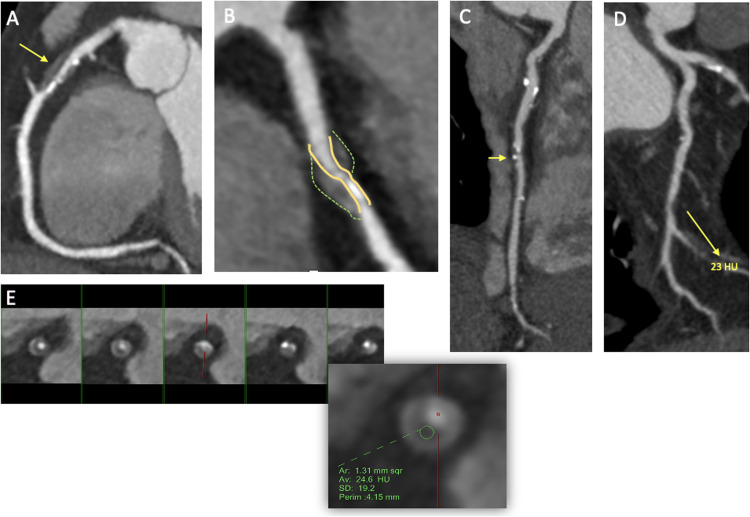
Qualitative analysis typically focuses on detecting stenosis and estimating overall plaque volume and composition. More recently, this has included identifying specific plaque findings associated with high-risk plaques (HRP). These characteristics include four specific imaging findings, namely the presence of low-attenuation plaque (LAP), outward or positive remodeling (PR) of the coronary wall, small "spotty" plaque calcification, and the napkin-ring sign (NRS) (Fig. 2). Currently, the presence of two or more of these features is required to define a HRP [77].
Fig. 2.
A-B. Curved MPR with a severe mixed plaque at the proximal LAD (arrow) exhibiting positive remodeling and spotty calcification. C. Curved MPR showing a mid-LAD plaque with spotty calcification (arrow). D. Curved MPR with a severe noncalcified plaque at the distal LCx (arrow) with low attenuation plaque (<30HU). E. Cross-sectional view at different levels of the LAD demonstrating a napkin-ring sign.
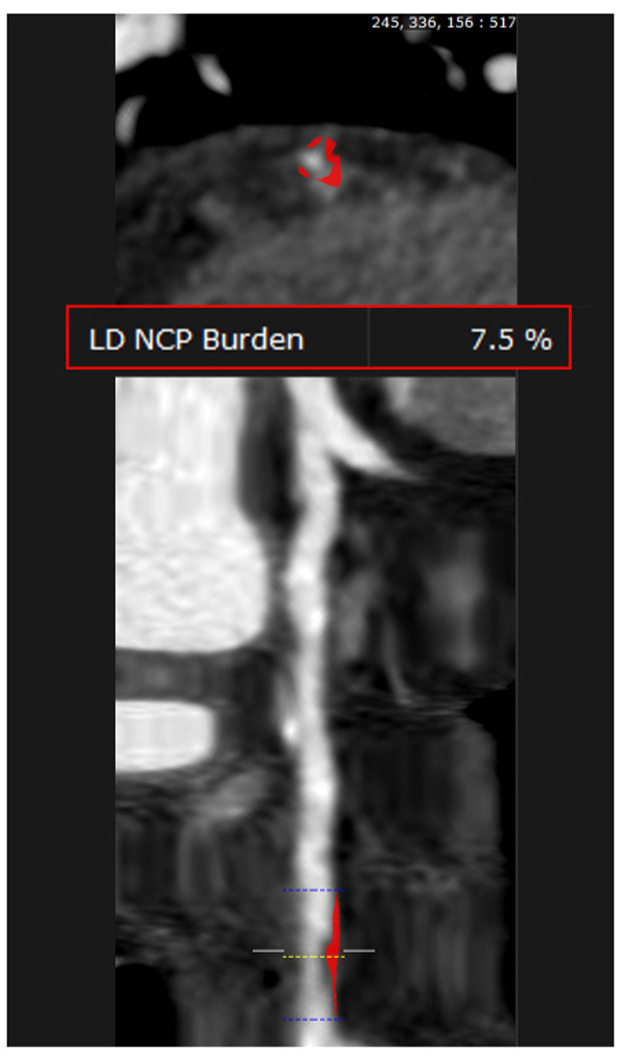
Low-attenuation plaque identifies the presence of a large necrotic core, one of the hallmarks of vulnerable, rupture-prone coronary plaques in pathology studies [78]. Motoyama et al. were the first to suggest using the currently widely used threshold of <30 HU for LAP [79] (Fig. 3). However, other studies have used various, typically higher HU cut-offs to differentiate LAP from other non-calcified (fibrous or fibro-fatty) plaque. These cut-offs range from 30 to 75 HU. Based on this definition, LAP is more often observed in patients with ACS than those with stable angina and has been associated with an eight-fold increased risk for ACS in patients with acute chest pain undergoing CCTA [80].
Fig. 3.
Straight MPR with AutoPlaque analysis demonstrating noncalcified stenosis of the left anterior descending artery with a 7.5% low attenuation plaque burden (<30HU).
Positive remodeling is the expansion of the external elastic membrane area and the compensatory enlargement of the vessel wall at the site of atherosclerotic lesions. This vascular remodeling dissociates plaque size from the degree of luminal stenosis during the early stages of plaque growth [81]. The remodeling in the vascular wall is defined as a remodeling index >1.1 (i.e., the ratio of the smallest vessel cross-sectional area of the lesion to the proximal reference luminal area). In the ROMICAT-II study, this high-risk feature was associated with an 11-fold increased risk for ACS [80].
Spotty calcification is the initial stage of a calcified plaque occurring due to active local inflammation. On CCTA, SC has a density greater than 130 HU and a diameter of <3 mm surrounded by non-calcified components [82]. The use of SC as a marker for the vulnerable plaque is, however, limited by its high prevalence and low specificity in predicting future events [83].
The most notorious HRP feature is the NRS, which is currently considered the CCTA hallmark of the vulnerable atherosclerotic plaque [84]. Studies employing CCTA and intracoronary imaging have shown that the NRS is predominantly found in high-risk lesions such as in thin cap fibroatheroma (44% vs. only 4% in non-thin cap fibroatheroma). This finding is characterized by a plaque core with low attenuation surrounded by a rim-like area of higher attenuation, potentially representing thin-cap fibroatheroma [85].
Combining HRP features with data on stenosis severity maximizes the prognostic value of CCTA-derived information. In one of the most significant to-date studies on the prognostic value of HRP, they were proven to be an independent predictor of MACE [86]. The risk reclassification provided by HRP features seems to be greatest in the lower-risk groups, such as younger patients, women, and those with non-obstructive CAD.
In 2016, vulnerable plaque (LAP, PR, spotty calcification, or the NRS) was incorporated into the SCCT CAD-RADS [77]. The “V” modifier was used to communicate the presence of vulnerable plaque as part of CAD-RADS and prompted more aggressive management or investigation to be considered. Recently, Cury et al. published a modified and actualized version named CAD-RADS 2.0, where the V modifier was replaced by the letters HRP referring to high-risk plaques. This new version also includes a grading scale for plaque burden (P1-P4), a modifier for the presence or absence of ischemia in CT-FFR, and a modifier for exception, indicating other causes of non-atherosclerotic coronary abnormalities, such as coronary fistulae, dissection or anomalous origin, among others [87].
These HRP features should be considered to start or expand aggressive preventative medical therapies and not in isolation to pursue down-stream testing such as invasive coronary angiography.
4.3. Semi-quantitative and quantitative measurement of coronary plaque burden
For a semi-quantitative assessment and reporting of plaque burden, several visual scores were developed. The SIS offers a simple method of determining the extent of atherosclerosis throughout the coronary tree with an increased risk of subsequent cardiac events with a higher segment-involved score [88]. Others, such as the CT-Leaman score, also consider the severity of stenosis and the type of atherosclerotic plaque [89]. These semi-quantitative scores are currently quicker to perform than quantitative assessments, but they only provide an estimate of the disease burden.
Quantitative analysis of individual coronary plaque has now been described using a variety of software approaches. They allow the measurement of calcified, noncalcified, low-attenuation plaque and total plaque volume. In a post-hoc analysis of a cohort of 1769 patients with stable chest pain undergoing CCTA in the multicenter SCOT-HEART trial, LAP burden was the strongest predictor of fatal or nonfatal myocardial infarction [90]. Patients with LAP burden greater than 4% were nearly five times more likely to have a subsequent myocardial infarction. In a further analysis of patients undergoing CCTA in the SCOT-HEART trial [76], PCAT-RCA of ≥-70.5 HU added to LAP burden of >4% provided an improved prediction of future myocardial infarction (HR: 11.7; p< 0.0001). It still remains unknown if these particular features can predict events in asymptomatic individuals and whether they can be used to select intensity of preventative treatments such as lipid lowering therapies.
For intermediate stenoses diagnosed on invasive angiography, performing Fractional Flow Reserve (FFR) measurements is recommended to assess their functional relevance [85]. However, FFR-CT (HeartFlow, Redwood City, California) is a technology whereby patient-specific blood flow models are constructed from CCTA images and used to estimate FFR noninvasively without requiring an additional scan [91]. In a meta-analysis, the AUCs of CTA-derived FFR at the patient and vessel level were 0.90 and 0.91, respectively, in identifying obstructive stenosis as identified by invasive FFR [92]. Also, in another meta-analysis, Noorgard et al. demonstrated the prognostic value of this tool. Still, it should be noted that this was not shown in the respective individual trials included [93]. Therefore, recently published American multi-society guidelines for the Evaluation and Diagnosis of Chest Pain stated that for intermediate-high risk patients with stable chest pain and known coronary stenosis of 40–90% in a proximal or middle coronary segment on CCTA, FFR-CT can be useful for diagnosis of vessel-specific ischemia and to guide decision-making regarding the use of coronary revascularization (IIaB) [94]. There is however almost no data on the use of FFR-CT in asymptomatic patients and for now its potential use is only speculative.
FFR-CT analysis can also evaluate the coronary artery lumen volume to left ventricle myocardial mass ratio (V/M). This value represents a measure of the ability of the epicardial coronary arteries to supply blood in relation to myocardial demand and could be used as an additional metric of coronary circulatory function. Patients with low V/M ratios following coronary vasodilation mediated by nitrates have been found more likely to have FFR values ≤0.80 than patients with high V/M, regardless of the presence or absence of focal stenosis. Importantly, while subjects with a low V/M were found to have significantly greater atherosclerosis than patients with high V/M, the ratio is considered an independent predictor of FFR ≤0.80 [95]. These intriguing findings motivate further research on the role of V/M and FFR-CT in other patient populations, such as asymptomatic patients with borderline obstructive plaques but its role over plaque burden and characteristics remains to be determined.
4.4. Limitations and new perspectives
Although the presence of high-risk plaque features has been widely recognized in the prediction of clinical events, its positive predictive value for ASCVD events is still limited. Longitudinal imaging studies have recently demonstrated that plaques with at least one adverse feature are, in fact, relatively common and appear dynamic, with the ability to revert to a more stable phenotype spontaneously or in response to medications, such as statin therapy [96]. Further CCTA randomized trials are needed to investigate the pathophysiology of the rapid progression of high-risk coronary plaques leading to ASCVD events, which will offer clinical utility in the management of asymptomatic patients.
There are several barriers to progressing from a risk score-based approach to one that incorporates CCTA. Exposure to radiation requires some consideration, along with the use of contrast and medications, such as beta-blockers and nitrates, to optimize image quality brings a small risk of adverse reactions. Finally, the use of CCTA has the potential for further downstream testing in patients felt to have a ‘severe’ burden of disease or have clinically important incidental findings [97]. This would require careful consideration and may be the subject of future research.
The SCOT-HEART [98] and PROMISE [99] trials were sufficiently large to assess the impact of CCTA on hard clinical outcomes in symptomatic individuals. In asymptomatic patients, there has only been one randomized trial assessing the use of CCTA in a primary prevention setting. The FACTOR-64 study recruited asymptomatic patients with diabetes and failed to demonstrate a clinical outcome benefit [100].
In addition, CCTA, potentially provides information to assess progression or regression of coronary atherosclerosis as response to medical therapy and its been recently used as an endpoint in clinical trials. In this particular feature, it is superior to CAC providing unique information as listed above. However, further trials are needed to standardize and evaluate the clinical implications of these findings as response to medical therapy.
Consequently, we still need more prospective randomized trials in asymptomatic populations like the ongoing SCOT-HEART 2 to evaluate the clinical impact of a CCTA-based strategy in hard outcomes.
5. Lipid management according to CAC/CCTA results
Even that the added value of risk stratification by coronary atherosclerosis imaging has been described above, this assessment can do little for our patients without the appropriate preventative interventions. The studied effects of most lipid therapies (as shown on Table 3) on lipid parameters and cardiovascular risk are derived from studies in symptomatic patients. Until newer data is available, this secondary prevention effect size can be considered to guide therapeutic selection for patients with high-risk subclinical coronary atherosclerosis.
Table 3.
Summary of Lipid Lowering Therapies: dosing, side effects, lipids, and cardiovascular outcomes reduction.
| Category | Dosing | Potential Side effects | Usual Lipid reduction | CVD outcomes reduction |
|---|---|---|---|---|
|
Statins Atorvastatin Rosuvastatin Simvastatin Pravastatin Pitavastatin Lovastatin Fluvastatin |
High intensity: Atorva 40–80 and Rosuva 20–40 mg Low/Moderate intensity: Atorva 10–20 mg; Rosu 5–10mg; Simva 10–40mg; Fluva 20–80 mg; Lova 20–80 mg, Pitava 1–4 mg, Prava 10–80 mg |
|
High intensity: >50% Moderate intensity: 30–49% Low intensity: <30% |
Primary prevention JUPITER (Rosu) [101] HR: 0.56 (0.46–0.69) WOSCOPS (Simva) [102] HR: 0.69 (0.57–0.83) CARDS (Atorva) [103] HR: 0.65 (0.30 to 1.55) |
|
Cholesterol absorption inhibitors Ezetimibe |
10 mg daily |
|
LDL-C reduction of 15–20% as monotherapy. Combined with statin, there is a 25% extra reduction. |
Primary prevention EWTOPIA 75 [104] HR: 0.66 (0.50–0.86) SHARP [105] HR: 0.83 (0.74–0.94) |
|
PCSK9 Inhibitors Evolocumab Alirocumab |
Alirocumab: dosed 75–150mg q 2 weeks or 300 mg q 4 weeks Evolocumab 140 mg q 2 weeks or 420mg q 4 weeks |
|
LDL-C reduction of 40–65% when added on to statin and/or ezetimibe Mean LDL-C decrease is 30% with evolocumab in patients with HoFH |
Secondary prevention FOURIER [106] HR: 0.85 (0.79–0.92) ODYSSEY [107] HR: 0.85 (0.78–0.93) |
|
Bile Acid Sequestrants Colesevelam Cholestyramine Colestipol |
Colesevelam: 3.75gr daily or 1.87 gr twice a day Cholestyramine: 8–16 g/day orally Colestipol: 2–16 g/day orally |
|
Typically lowers LDL-C by 15–20% |
Primary prevention LRC (Cholestyramine) [108] HR: 0.83 (0.09 to 1.37) |
| Bempedoic Acid | 180 mg oral daily |
|
Lowers LDL-C by -19% In combination with Ezetimibe by -38% |
No outcomes trial CLEAR – Safety trial [109] |
| Inclisiran | 284 mg SQ q 3months |
|
Lowers LDL-C by -50% | No outcomes trial ORION-9 – LDL reduction [110] |
|
n-3 Fatty Acids Omega-3-acid ethyl esters (Lovaza) Icosapent ethyl (Vascepa) |
Lovaza 4 g/day Vascepa 4 g/day |
|
Neutral effect on LDL-C lowering TG lowering of 20–30% |
Secondary prevention REDUCE-IT (Icosapent ethyl) [111] HR: 0.75 (0.68–0.83) ORIGIN (Ethyl esters) [112] HR: 0.98 (0.87–1.10) |
|
Fibrates Fenofibrate Gemfibrozil |
Fenofibrate 50–160 mg/day Gemfibrozil 600 mg twice daily |
|
TG lowering of 40–50% Lowers LDL-C by 30% |
Secondary prevention ACCORD [113] HR: 0.92 (0.79–1.08) |
| Niacin | 500 to 2000 mg/day |
|
TG lowering of 15–25% LDL-C 10–20%; |
Secondary prevention Systematic Review [114] RR 0.99; 95% CI 0.88–1.12) |
CVD: cardiovascular disease. HoFH: Homozygous familial hypercholesterolemia, HR: hazard ratio, LDL-C: low-density lipoprotein cholesterol, RR: relative risk.
The relationship between lipid disorders and non-invasive coronary plaque imaging has been previously established. A cross-sectional analysis of the MESA showed that participants with combined hyperlipidemia, simple hypercholesterolemia, and dyslipidemia of MetS had a statistically significant probability of having a multivessel CAC (2 or more) as compared to the normolipidemia reference group, even after adjusting for the demographic and cardiac risk factors [115]. Additionally, in patients with optimal LDL-C levels, remnant cholesterol (total cholesterol minus LDL-C minus high-density lipoprotein cholesterol) levels are associated with a significant coronary atherosclerotic burden as assessed by CCTA [116]. On the other hand, isolated hypertriglyceridemia was not associated with CAC extent [115,116]. There also seems to be an association between the time course and duration of elevated lipid levels and CAC. Measurements of lipid levels done early (15–30 years before the CAC study), followed by long-term averaged measurements, are strongly associated with CAC in a middle-aged to elderly adult population (mean age 63 years). However, contemporary lipid measures were associated with elevated CAC only in younger adults (mean age 42–43 years) [117,118]. It has been proposed that this lack of association between the presence of CAC and a more recent measurement of lipid values in older people could be explained by other risk factors and changes throughout life [117].
As mentioned above, CAC is helpful in tailoring the decision to start or withhold statin therapy and the selection of lipid-lowering therapy intensity [119]. For a given CAC score, diffuse distribution of CAC suggests a higher risk than more localized CAC. The presence of left main coronary calcification, especially when >25% of the total score is in the left main, also suggests a higher risk. The National Lipid Association, in a consensus document, underpins the importance of CAC regarding lipid management [119].
Similarly, in a more recent expert consensus, the ACC provides a treatment pathway according to the CAC results (Central Illustration). For those with intermediate risk and CAC scores of 0, in the absence of high-risk profile features, it is reasonable to defer statin therapy with a plan for CAC reassessment in 3–5 years [120]. For those with a CAC score of 1-99 AU, moderate-intensity statin therapy is reasonable. Based on data from MESA and consistent with the recommendations of the 2018 Cholesterol Guideline, a CAC score >100 is a clear indication to engage in a clinician-patient discussion about the initiation of statin therapy [121]. For those with a CAC score of >300 per MESA or >400 per Heinz Nixdorf Recall Study, with greater than the 75th percentile for their age/sex/race group, initiation or titration to high-intensity statin therapy may be considered [120,122]. In the higher risk groups, non-statin therapies should be considered if the LDL-C level remains ≥70 mg/dl and/or less than 50% LDL-C reduction from baseline is achieved on maximally tolerated statin therapy [120]. Of note, when CAC=0 or CCTA is without evidence of plaque, lifestyle modifications, including dietary changes, can play a central role without the immediate need for medications [121].
Serial CAC measurement in patients already treated with statin therapy has limited utility [123]. Although statins are associated with slower progression of overall coronary atherosclerosis volume and reduction of high-risk plaque features, they increase plaque density and thus increase the CAC score.
It has been proven that a CAC score >100 is associated with ≥7.5% 10-year ASCVD risk, the guideline-based threshold of statin benefit in primary prevention. Individuals with a CAC score ≥300 are associated with proportionately higher ASCVD risk than those with scores >100, suggesting a benefit from greater LDL-C lowering. Increases in Agatston CAC scores caused by statins are generally modest, and very elevated CAC scores (eg, >400 or >1000) should still be interpreted as indicative of extensive atherosclerosis and prompt aggressive preventive therapies. When the goals of therapy in the clinician-patient discussion have been achieved, it is reasonable to continue to monitor adherence to lifestyle modifications, medication, and LDL-C response to therapy [120].
When starting lipid lowering therapies it is essential to understand the effects on lipid parameters and cardiovascular risk. In both primary and secondary prevention, it is established that for every 40 mg/dL LDL-C reduction in patients treated with statins, there is a 23% reduction of events in 5 years (Table 3) [124]. Furthermore, multiple trials have reported no systemic increase in adverse events in those achieving LDL-C <50 mg/dL [125], which may be appropriate for patients with high risk imaging findings. Many studies have confirmed that the lower the LDL-C, the lower the ASCVD risk and, with no evidence of any clinically significant harm, no matter how low the achieved LDL-C level is [126,127].
The FOURIER [106] and ODYSSEY trials [107] demonstrated in symptomatic patients with established ASCVD that even for patients with LDL-C below 70–100 mg/dl, further reduction in LDL-C improved outcomes in several clinical scenarios. It seems logical that the same would be even more effective before clinically advanced atherosclerosis has developed. FOURIER and ODYSSEY clarified that no matter how low LDL-C, even below 20 mg/dl, there was no greater incidence of adverse events than from placebo. Mendelian randomization studies have also very convincingly showed that the lower the LDL-C, the less atherosclerosis and the fewer the resulting ASCVD-related events [128]. Moreover, when these changes are earlier in life and atherosclerosis stages, smaller LDL-C changes can lead to larger ASCVD risk reduction.
In a subgroup of the MESA cohort (n =4085), the 10-year number needed to treat (NNT) with statins to prevent an ASCVD event for CAC 0, 1 to 100, and >100 were 87, 37, and 19, respectively. These results are based on an average relative risk reduction of 30% for statin therapy in the referenced primary prevention trials [129]. Furthermore, in a large (n=13,644) relatively lower-risk cohort, CAC >100 was consistently associated with a greater reduction in the hazard for MACE with statin therapy relative to CAC <100. On the contrary, in the 10-year NNT analysis, statins had no significant effect among patients with CAC=0. Patients with a CAC of 1 to 100 had a trend toward benefit (NNT=100; p=0.095), whereas patients with a CAC >100 derived significant benefit with a NNT of only 12 (p < 0.0001) [38].
Mortensen et al. investigated the association between CAD severity assessed by CCTA in patients without prior CVD and ASCVD events, with low event rates (4.4 per 1000 person-years) among individuals with no CAD and CAC=0. In contrast, event rates were substantially higher in patients with 3-vessel disease (39.8 per 1000 person-years). Consequently, the NNT to prevent 1 ASCVD event in 6 years by treating LDL-C to targets varied greatly from 233 (ESC) and 110 (ACC/AHA) for patients with no CAD to 8-9 for patients with 3-vessel disease (both ACC/AHA and ESC) [130].
Despite being on statin therapy, many patients still have a residual risk for CV events, mostly integrated by residual LDL-C, triglycerides, lipoprotein(a) [Lp(a)], and inflammation. Omega-3 fatty acids have been known to reduce TG levels, but until recently, it remained unclear if this translated to a reduction in CV events. The direct effect of EPA on reducing CV events was established by the REDUCE-IT trial [111]. The results of this trial, in turn, inspired the EVAPORATE trial [131]. The authors indicated that patients treated with EPA 4 g/day had a reduction in LAP volume compared with placebo at 18 months, as measured by CCTA. In the setting of a small sample size, there was no difference in TG levels; however, this research study served as a hypothesis generator and propelled the potential use for plaque measurements by CCTA as an endpoint in clinical trials. Similarly, but using invasive imaging, the HUYGENS trial demonstrated that the combination of statin and evolocumab after a non–ST-segment elevation myocardial infarction produces favorable changes in coronary atherosclerosis consistent with stabilization and regression [132]. Even though this trial is out of the scope of this literature review since it was done with symptomatic patients, it demonstrates the benefits of lipid-lowering therapies in plaque morphology. Further studies assessing the effects of PCSK9 inhibitor on plaque measured by CCTA are needed. A primary prevention trial with PCSK9 is currently in place (VESALIUS) to evaluate the impact of evolocumab on MACE in high-risk patients without prior MI or stroke (NCT03872401).
5.1. Statin therapy considerations
Statins represent the backbone of lipid lowering therapies. Individual statin selection should be based on patients’ risk, LDL-C goal and various conditions determined by patients' comorbidities. Risk of diabetes, chronic kidney disease (CKD) and statin-associated muscle symptoms (SAMS) represent some factors to considerate. In the JUPITER trial, the incidence of diabetes was 25% higher in the rosuvastatin group, and was associated with advanced age, increased fasting blood glucose, and metabolic syndrome. The risk of new-onset diabetes must be weighed against the risk of MACE and mortality [133]. In patients with CKD, a post hoc analysis demonstrated that the glomerular filtration rate remained stable with atorvastatin but significantly decreased in the rosuvastatin group (p=0.036). Atorvastatin but not rosuvastatin, has proven renoprotective effects in the CKD population [133].
Overall, statins as a class are associated with an increased risk of diabetes and hepatic transaminase elevations, with no statistically detectable effect on myalgia, myopathy, rhabdomyolysis, and cancer. Across the totality of the evidence, higher dose of statins result in higher odds of experiencing transaminase elevations, creatine kinase (CK) elevations, and discontinuations because of adverse events [134]. In head-to-head comparison and network meta-analyses, there were no differences among individual statins. Although rare, adverse events associated with statin therapy range from mild to moderate and seem to increase with treatment intensity [134]. Even though side effects are rare, CAC or CCTA can detect patients where the benefit is higher with a more advantageous risk/benefit ratio.
Statin intolerance is defined as one or more adverse effects associated with statin therapy, which resolves or improves with dose reduction or discontinuation. They can be classified as a complete inability to tolerate any dose of a statin or partial intolerance, with an inability to tolerate the dose necessary to achieve the patient-specific therapeutic objective [135]. To classify a patient as having statin intolerance, a minimum of two statins should have been attempted, including at least one at the lowest approved daily dosage [136]. An exception are cases of rare severe adverse events such as statin-induced necrotizing autoimmune myopathy, in which case patients should not be rechallenged with any statin.
The most frequently reported patient complaints in the clinical setting are SAMS. In most cases, they occur without CK elevation [135]. In the JUPITER trial, the incidence of myalgia in the statin- and placebo-treated groups were 16% and 15.4%, respectively [101]. In the Heart Protection Study, after 5 years, the incidence of SAMS was 32.9% and 33.1% in the statin- and placebo-treated groups, respectively [137]. Based on the placebo arms of these trials, SAMS are highly prevalent in general.
Less commonly, statin therapy has been associated with myopathy occurring in ∼1/10,000 patients per year. A rare muscle-related side effect is rhabdomyolysis occurring in ∼1/100,000 patients per year of treatment [134]. When adverse effects are present, clinicians should use the approach of reassessing, rediscussing (net clinical benefit), and rechallenge when appropriate [121]. The presence or absence of CAC or plaque in CCTA can aid in the discontinuation of a problematic statin for the individual patient versus the need to find a tolerated regimen.
5.2. Non-statin lipid-lowering therapies considerations
Based on the high ASCVD risk in individuals with CAC >100, if maximally tolerated statin and ezetimibe therapy results in inadequate lowering of LDL-C, with <50% LDL-C reduction or LDL-C ≥70 mg/dL, the addition of a PCSK9 inhibitors may be considered [120]. Doubling the dose of statin results in only approximately 6% further decrease in LDL-C levels. The addition of ezetimibe to statin therapy typically provides an additional 15% to 25% reduction in LDL-C. Much greater additive reductions occur by adding a PCSK9 inhibitor to statins. The maximum percentage change will occur 4 to 6 weeks after starting a statin or combined therapy. At this time, drug efficacy or initial adherence to therapy should be evaluated. Periodic remeasurements will make it possible to confirm adherence to therapy [138].
Ezetimibe is indicated in treating disorders of elevated cholesterol levels, including LDL-C and ApoB, as monotherapy or in combination with statins [138]. A meta-analysis of eight randomized placebo-controlled trials that included over 2700 subjects showed that monotherapy with ezetimibe 10 mg daily in hypercholesterolemic subjects for a minimum of 12 weeks was associated with a significant 18.5% reduction in LDL-C as compared to placebo [139].
In the wait for clinical outcomes trials, particularly for patients with possible non-compliance or difficulty with administration, the newer agent targeting PCSK9, Inclisiran, has demonstrated an LDL-C reduction of approximately 50% [110]. Similarly, Bempedoic acid demonstrated a decreased LDL-C by 21.4%, non-HDL-C by 17.9%, and apolipoprotein B by 15%. These drugs can be possible additions for those patients that wish to avoid frequent subcutaneous injections and/or statin-intolerant patients [140].
6. Progression/regression of plaque burden after treatment
When evaluating plaque progression, it is essential to consider the influence of remnant cholesterol (RC) on CAC progression as demonstrated by Hao et al. [141]. In a sample of 6544 ASCVD–free individuals from the CARDIA and MESA studies, 42.5% of patients had a high CAC score from baseline. After multivariable adjustment for demographics and CV risk factors, a 1-mg/dL increase in RC levels was associated with a 1.3% higher risk of CAC progression. In addition to RC, elevated TG levels were associated with an increased risk of CAC progression. Similarly, Won et al. revealed the TG glucose index was significantly associated with CAC progression in individuals with baseline CAC score ≤100 [142].
Zeb et al. [143] conducted a retrospective study on patients without known CVD and two consecutive CCTA. They demonstrated that the total plaque volume and the progression of non-calcified plaque were significantly reduced among statin users compared to non-statin users. However, there was a non-statistically significant increase in calcified plaque volume between treated and non-treated participants. In 2015, Lo et al. [144] reported the results of a randomized, double-blind clinical trial done in HIV patients with subclinical evidence of CVD defined by the presence of one or more plaques on CCTA and its relationship with statin treatment. In a group of patients in which LDL-C levels were not elevated as per the inclusion criteria of the trial (LDL-C <130 mg/dl), treatment with statins resulted in a reduction of coronary non-calcified plaque volume by 19% in 1 year, compared with an increase of 20% in the placebo group. In addition, Atorvastatin reduced HRP features.
Although small, nonrandomized studies initially suggested that statin therapy may slow the progression of coronary calcification [145,146], prospective, randomized studies disagree with this statement, showing no effect of any statin dose on the progression of coronary calcification [147,148]. More recently, in a cohort of 1255 subjects of the PARADIGM study, Lee et al. [149] analyzed the effect of statins treatment on plaque characteristics and found that statins were associated with slower progression of overall coronary atherosclerosis volume and reduction of HRP features but with increased plaque calcification. These findings go hand in hand with the procalcifying effect of statins, besides their known reduction of CV events.
In addition to the assessment of protective therapies such as statins, imaging of subclinical coronary atherosclerosis (in particular CCTA) can be used to monitor the potential deleterious effect of medical therapies on coronary plaque. In 2017, Budoff et al. [150] published a study in which 138 men >65 years old with symptomatic hypogonadism were treated with testosterone gel for 1 year and reported a statistically significant increase in coronary artery noncalcified plaque volume between two different scans (a baseline and a 12 month follow up CCTA), independent of statin use.
Similarly, a study that included patients with angiographically proven evidence of CVD found a relationship between higher levels (>70mg/dl) of Lp-(a) and accelerated progression of coronary LAP in comparison with the group of Lp-(a) < 70 mg/dl. This could, to some extent, explain the known increased risk of myocardial infarction in patients with elevated Lp-(a). In contrast, there was no change in total plaque volume or calcific and noncalcific plaque volumes between the high- and low-Lp(a) groups [151].
7. Conclusion
In summary, CAC and CCTA are highly reproducible tests that directly assess the total burden of coronary plaque and improve risk stratification of asymptomatic individuals over purely clinical risk estimators through the integration of lifetime exposure to the upstream modifiable risk factors, genetic determinants, and therapies. CCTA can provide additional information over and above the presence of calcium, including plaque burden, high-risk phenotypes, and progression/regression of the disease in response to lipid lowering therapies; hence this still remains to be proved in randomized clinical trials.
No funding or grant was received for the conception or implementation of this manuscript.
Disclosures
MB - Grants: NIH, FDA, AHA, Amgen, Novo Nordisk, Bayer, Advisory Board: Amgen, Novartis, Novo Nordisk, Bayer, Roche, 89Bio, Kaleido, Inozyme, Agepha, Consulting: Emocha health, Kowa
MJB – Grants: NIH, Novo Nordisk, Novartis, Speakers Bureau – Esperion, Amgen.
SV – Received research support from the Department of Veterans Affairs, NIH, Tahir, and Jooma Family, and an Honorarium from the American College of Cardiology (Associate Editor for Innovations, acc.org).
DSB – Received software royalties from Cedars-Sinai Medical Center. Grant received from Dr. Miriam and Sheldon G. Adelson Medical Research Foundation
LSB – Received consulting Honoraria from Amgen, Regeneron, and Phillips and Grant support from Amgen.
CRediT authorship contribution statement
Pamela Piña: Conceptualization, Writing – review & editing. Daniel Lorenzatti: Conceptualization, Writing – review & editing. Rita Paula: Writing – review & editing. Jonathan Daich: Writing – review & editing. Aldo L Schenone: Writing – review & editing. Carlos Gongora: Writing – review & editing. Mario J Garcia: Writing – review & editing. Michael J Blaha: Writing – review & editing. Matthew J Budoff: Writing – review & editing. Daniel S Berman: Writing – review & editing. Salim S Virani: Writing – review & editing. Leandro Slipczuk: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 2.Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfson J, Vock DM, Bandyopadhyay S, et al. Use and customization of risk scores for predicting cardiovascular events using electronic health record data. J Am Heart Assoc. 2017;6(4) doi: 10.1161/JAHA.116.003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311(14):1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeboah J, Young R, McClelland RL, et al. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67(2):139–147. doi: 10.1016/j.jacc.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276–284. doi: 10.1161/CIRCOUTCOMES.113.000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57(15):1622–1632. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Aalst CM, Denissen SJAM, Vonder M, et al. Screening for cardiovascular disease risk using traditional risk factor assessment or coronary artery calcium scoring: the ROBINSCA trial. Eur Heart J Cardiovasc Imaging. 2020;21(11):1216–1224. doi: 10.1093/ehjci/jeaa168. [DOI] [PubMed] [Google Scholar]
- 9.Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24(2):e6–e10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- 10.Natarajan P, Young R, Stitziel NO, et al. Polygenic risk score identifies subgroup with higher burden of atherosclerosis and greater relative benefit from statin therapy in the primary prevention setting. Circulation. 2017;135(22):2091–2101. doi: 10.1161/CIRCULATIONAHA.116.024436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cainzos-Achirica M, Mortensen MB, Blaha MJ. Exploring the intersection between genetic risk scores and coronary artery calcium- mutually exclusive or complementary? J Cardiovasc Comput Tomogr. 2020;14(2):206–207. doi: 10.1016/j.jcct.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Patel J, Pallazola VA, Dudum R, et al. Assessment of coronary artery calcium scoring to guide statin therapy allocation according to risk-enhancing factors: the multi-ethnic study of atherosclerosis. JAMA Cardiol. 2021;6(10):1161–1170. doi: 10.1001/jamacardio.2021.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JS, Chiang HY, Wang YC, et al. Dyslipidemia and coronary artery calcium: from association to development of a risk-prediction nomogram. Nutr Metab Cardiovasc Dis. 2022;32(8):1944–1954. doi: 10.1016/j.numecd.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Bartel AG, Chen JT, Peter RH, Behar VS, Kong Y, Lester RG. The significance of coronary calcification detected by fluoroscopy. A report of 360 patients. Circulation. 1974;49(6):1247–1253. doi: 10.1161/01.cir.49.6.1247. [DOI] [PubMed] [Google Scholar]
- 15.Margolis JR, Chen JT, Kong Y, Peter RH, Behar VS, Kisslo JA. The diagnostic and prognostic significance of coronary artery calcification. A report of 800 cases. Radiology. 1980;137(3):609–616. doi: 10.1148/radiology.137.3.7444045. [DOI] [PubMed] [Google Scholar]
- 16.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 17.Daniell AL, Wong ND, Friedman JD, et al. Concordance of coronary artery calcium estimates between MDCT and electron beam tomography. AJR Am J Roentgenol. 2005;185(6):1542–1545. doi: 10.2214/AJR.04.0333. [DOI] [PubMed] [Google Scholar]
- 18.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434–447. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blaha MJ, Mortensen MB, Kianoush S, Tota-Maharaj R, Cainzos-Achirica M. Coronary artery calcium scoring: is it time for a change in methodology? JACC Cardiovasc Imaging. 2017;10(8):923–937. doi: 10.1016/j.jcmg.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the society of cardiovascular computed tomography (SCCT) J Cardiovasc Comput Tomogr. 2018;12(3):185–191. doi: 10.1016/j.jcct.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA J Am Med Assoc. 2014;311(3):271–278. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budoff MJ, Nasir K, Kinney GL, et al. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: comparison of ungated and gated examinations in patients from the COPD Gene cohort. J Cardiovasc Comput Tomogr. 2011;5(2):113–118. doi: 10.1016/j.jcct.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of cardiovascular computed tomography. J Cardiovasc Comput Tomogr. 2017;11(2):157–168. doi: 10.1016/j.jcct.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Hecht HS, Cronin P, Blaha MJ, et al. SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the society of cardiovascular computed tomography and society of thoracic radiology. J Cardiovasc Comput Tomogr. 2017;11(1):74–84. doi: 10.1016/j.jcct.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Sarwar A, Shaw L, Shapiro M, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–688. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 27.Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the multi-ethnic study of atherosclerosis (MESA) Circulation. 2016;133(9):849–858. doi: 10.1161/CIRCULATIONAHA.115.018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muntendam P, McCall C, Sanz J, Falk E, Fuster V. High-risk plaque initiative. The BioImage study: novel approaches to risk assessment in the primary prevention of atherosclerotic cardiovascular disease–study design and objectives. Am Heart J. 2010;160(1):49–57.e1. doi: 10.1016/j.ahj.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O'Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807–814. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 30.Vliegenthart R, Oudkerk M, Hofman A, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112(4):572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 31.LaMonte MJ, FitzGerald SJ, Church TS, et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162(5):421–429. doi: 10.1093/aje/kwi228. [DOI] [PubMed] [Google Scholar]
- 32.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events. J Am Coll Cardiol. 2005;46(1):158–165. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 33.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification. J Am Coll Cardiol. 2007;49(18):1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 34.Detrano R, Bild DE, Liu K, Bluemke DA, Watson K. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008:10. doi: 10.1056/NEJMoa072100. Published online. [DOI] [PubMed] [Google Scholar]
- 35.Erbel R, Möhlenkamp S, Moebus S, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56(17):1397–1406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 36.Carr JJ, Jacobs DR, Terry JG, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol. 2017;2(4):391–399. doi: 10.1001/jamacardio.2016.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grandhi GR, Mirbolouk M, Dardari ZA, et al. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD mortality: the CAC consortium. JACC Cardiovasc Imaging. 2020;13(5):1175–1186. doi: 10.1016/j.jcmg.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell JD, Fergestrom N, Gage BF, et al. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol. 2018;72(25):3233–3242. doi: 10.1016/j.jacc.2018.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Z, Ong KL, Breslin M, Allison MA, Curtis AJ, Nelson MR. Association of statin use with cardiovascular outcomes by coronary calcium: MESA. JACC Cardiovasc Imaging. 2020;13(4):1094–1096. doi: 10.1016/j.jcmg.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American college of cardiology/American heart association cholesterol management guidelines: MESA (Multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2015;66(15):1657–1668. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 41.Dudum R, Dzaye O, Mirbolouk M, et al. Coronary artery calcium scoring in low risk patients with family history of coronary heart disease: validation of the SCCT guideline approach in the coronary artery calcium consortium. J Cardiovasc Comput Tomogr. 2019;13(3):21–25. doi: 10.1016/j.jcct.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC ≥1000. JACC Cardiovasc Imaging. 2020;13(1_Part_1):83–93. doi: 10.1016/j.jcmg.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dzaye O, Razavi AC, Michos ED, et al. Coronary artery calcium scores indicating secondary prevention level risk: findings from the CAC consortium and FOURIER trial. Atherosclerosis. 2022;347:70–76. doi: 10.1016/j.atherosclerosis.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cainzos-Achirica M, Quispe R, Mszar R, et al. Coronary artery calcium score to refine the use of PCSK9i in asymptomatic individuals: a multicohort study. J Am Heart Assoc. 2022 doi: 10.1161/JAHA.122.025737. Published online August 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cainzos-Achirica M, Quispe R, Dudum R, et al. CAC for risk stratification among individuals with hypertriglyceridemia free of clinical atherosclerotic cardiovascular disease. JACC Cardiovasc Imaging. 2022;15(4):641–651. doi: 10.1016/j.jcmg.2021.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Malik S, Zhao Y, Budoff M, et al. Coronary artery calcium score for long-term risk classification in individuals with type 2 diabetes and metabolic syndrome from the multi-ethnic study of atherosclerosis. JAMA Cardiol. 2017;2(12):1332–1340. doi: 10.1001/jamacardio.2017.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong ND, Cordola Hsu AR, Rozanski A, et al. Sex differences in coronary artery calcium and mortality from coronary heart disease, cardiovascular disease, and all causes in adults with diabetes: the coronary calcium consortium. Diabetes Care. 2020;43(10):2597–2606. doi: 10.2337/dc20-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valenti V, Hartaigh BÓ, Cho I, et al. Absence of coronary artery calcium identifies asymptomatic diabetic individuals at low near-term but not long-term risk of mortality: a 15-year follow-up study of 9715 patients. Circ Cardiovasc Imaging. 2016;9(2) doi: 10.1161/CIRCIMAGING.115.003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Budoff MJ, Raggi P, Beller GA, et al. Noninvasive cardiovascular risk assessment of the asymptomatic diabetic patient: the imaging council of the American college of cardiology. JACC Cardiovasc Imaging. 2016;9(2):176–192. doi: 10.1016/j.jcmg.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandesara PB, Mehta A, O'Neal WT, et al. Clinical significance of zero coronary artery calcium in individuals with LDL cholesterol ≥190 mg/dL: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2020;292:224–229. doi: 10.1016/j.atherosclerosis.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Castagna F, Miles J, Arce J, et al. Visual coronary and aortic calcium scoring on chest computed tomography predict mortality in patients with low-density lipoprotein-cholesterol ≥190 mg/dL. Circ Cardiovasc Imaging. 2022;15(6) doi: 10.1161/CIRCIMAGING.122.014135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Javaid A, Dardari ZA, Mitchell JD, et al. Distribution of coronary artery calcium by age, sex, and race among patients 30-45 years old. J Am Coll Cardiol. 2022;79(19):1873–1886. doi: 10.1016/j.jacc.2022.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ibanez B, Fernández-Ortiz A, Fernández-Friera L, García-Lunar I, Andrés V, Fuster V. Progression of Early Subclinical Atherosclerosis (PESA) study: JACC focus seminar 7/8. J Am Coll Cardiol. 2021;78(2):156–179. doi: 10.1016/j.jacc.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Dzaye O, Dardari ZA, Cainzos-Achirica M, et al. Warranty period of a calcium score of zero: comprehensive analysis from MESA. JACC Cardiovasc Imaging. 2021;14(5):990–1002. doi: 10.1016/j.jcmg.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3(12):1229–1236. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 56.Lehmann N, Erbel R, Mahabadi AA, et al. Value of progression of coronary artery calcification for risk prediction of coronary and cardiovascular events: result of the HNR study (Heinz Nixdorf Recall) Circulation. 2018;137(7):665–679. doi: 10.1161/CIRCULATIONAHA.116.027034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miedema MD, Dardari ZA, Nasir K, et al. Association of coronary artery calcium with long-term, cause-specific mortality among young adults. JAMA Netw Open. 2019;2(7) doi: 10.1001/jamanetworkopen.2019.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nasir K, Cainzos-Achirica M, Valero-Elizondo J, et al. Coronary atherosclerosis in an asymptomatic U.S. population. JACC Cardiovasc Imaging. 2022 doi: 10.1016/j.jcmg.2022.03.010. Published online MayS1936878X22001735. [DOI] [PubMed] [Google Scholar]
- 59.Meah MN, Tzolos E, Wang KL, et al. Plaque burden and 1-year outcomes in acute chest pain. JACC Cardiovasc Imaging. 2022;0(0) doi: 10.1016/j.jcmg.2022.04.024. [DOI] [PubMed] [Google Scholar]
- 60.Tzolos E, Han D, Klein E, et al. Detection of small coronary calcifications in patients with Agatston coronary artery calcium score of zero. J Cardiovasc Comput Tomogr. 2022;16(2):150–154. doi: 10.1016/j.jcct.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Hoffmann U, Ferencik M, Udelson JE, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) Circulation. 2017;135(24):2320–2332. doi: 10.1161/CIRCULATIONAHA.116.024360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet Lond Engl. 2015;385(9985):2383–2391. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 63.Mortensen MB, Gaur S, Frimmer A, et al. Association of age with the diagnostic value of coronary artery calcium score for ruling out coronary stenosis in symptomatic patients. JAMA Cardiol. 2022;7(1):36–44. doi: 10.1001/jamacardio.2021.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8(5):342–358. doi: 10.1016/j.jcct.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 65.Cho I, Chang HJ, Sung JM, et al. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM registry (Coronary CT angiography evaluation for clinical outcomes: an international multicenter registry) Circulation. 2012;126(3):304–313. doi: 10.1161/CIRCULATIONAHA.111.081380. [DOI] [PubMed] [Google Scholar]
- 66.Min JK, Labounty TM, Gomez MJ, et al. Incremental prognostic value of coronary computed tomographic angiography over coronary artery calcium score for risk prediction of major adverse cardiac events in asymptomatic diabetic individuals. Atherosclerosis. 2014;232(2):298–304. doi: 10.1016/j.atherosclerosis.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang SH, Park GM, Lee SW, et al. Long-term prognostic value of coronary CT angiography in asymptomatic type 2 DIABETES Mellitus. JACC Cardiovasc Imaging. 2016;9(11):1292–1300. doi: 10.1016/j.jcmg.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 68.Halon DA, Lavi I, Barnett-Griness O, et al. Plaque morphology as predictor of late plaque events in patients with asymptomatic type 2 diabetes: a long-term observational study. JACC Cardiovasc Imaging. 2019;12(7 Pt 2):1353–1363. doi: 10.1016/j.jcmg.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 69.Chow BJW, Small G, Yam Y, et al. Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease. Arterioscler Thromb Vasc Biol. 2015;35(4):981–989. doi: 10.1161/ATVBAHA.114.304351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Øvrehus KA, Diederichsen A, Grove EL, et al. Reduction of myocardial infarction and all-cause mortality associated to statins in patients without obstructive CAD. JACC Cardiovasc Imaging. 2021;14(12):2400–2410. doi: 10.1016/j.jcmg.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 71.Mortensen MB, Dzaye O, Steffensen FH, et al. Impact of plaque burden versus stenosis on ischemic events in patients with coronary atherosclerosis. J Am Coll Cardiol. 2020;76(24):2803–2813. doi: 10.1016/j.jacc.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 72.Williams MC, Earls JP, Hecht H. Quantitative assessment of atherosclerotic plaque, recent progress and current limitations. J Cardiovasc Comput Tomogr. 2022;16(2):124–137. doi: 10.1016/j.jcct.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7(2):282–291. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 74.Bergström G, Persson M, Adiels M, et al. Prevalence of subclinical coronary artery atherosclerosis in the general population. Circulation. 2021;144(12):916–929. doi: 10.1161/CIRCULATIONAHA.121.055340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brandt V, Decker J, Schoepf UJ, et al. Additive value of epicardial adipose tissue quantification to coronary CT angiography–derived plaque characterization and CT fractional flow reserve for the prediction of lesion-specific ischemia. Eur Radiol. 2022;32(6):4243–4252. doi: 10.1007/s00330-021-08481-w. [DOI] [PubMed] [Google Scholar]
- 76.Tzolos E, Williams MC, McElhinney P, et al. Pericoronary adipose tissue attenuation, low-attenuation plaque burden, and 5-year risk of myocardial infarction. JACC Cardiovasc Imaging. 2022;15(6):1078–1088. doi: 10.1016/j.jcmg.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) coronary artery disease - reporting and data system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American college of cardiology. J Cardiovasc Comput Tomogr. 2016;10(4):269–281. doi: 10.1016/j.jcct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 78.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the unstable plaque. Prog Cardiovasc Dis. 2002;44(5):349–356. doi: 10.1053/pcad.2002.122475. [DOI] [PubMed] [Google Scholar]
- 79.Motoyama S, Kondo T, Anno H, et al. Atherosclerotic plaque characterization by 0.5-mm-slice multislice computed tomographic imaging. Circ J Off J Jpn Circ Soc. 2007;71(3):363–366. doi: 10.1253/circj.71.363. [DOI] [PubMed] [Google Scholar]
- 80.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64(7):684–692. doi: 10.1016/j.jacc.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schoenhagen P, Ziada KM, Vince DG, Nissen SE, Tuzcu EM. Arterial remodeling and coronary artery disease: the concept of “dilated” versus “obstructive” coronary atherosclerosis. J Am Coll Cardiol. 2001;38(2):297–306. doi: 10.1016/s0735-1097(01)01374-2. [DOI] [PubMed] [Google Scholar]
- 82.Shaw LJ, Blankstein R, Bax JJ, et al. Society of cardiovascular computed tomography /North American society of cardiovascular imaging - expert consensus document on coronary ct imaging of atherosclerotic plaque. J Cardiovasc Comput Tomogr. 2021;15(2):93–109. doi: 10.1016/j.jcct.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 83.van Veelen A, van der Sangen NMR, Henriques JPS, Claessen BEPM. Identification and treatment of the vulnerable coronary plaque. Rev Cardiovasc Med. 2022;23(1):1. doi: 10.31083/j.rcm2301039. [DOI] [PubMed] [Google Scholar]
- 84.Antonopoulos AS, Angelopoulos A, Tsioufis K, Antoniades C, Tousoulis D. Cardiovascular risk stratification by coronary computed tomography angiography imaging: current state-of-the-art. Eur J Prev Cardiol. 2022;29(4):608–624. doi: 10.1093/eurjpc/zwab067. [DOI] [PubMed] [Google Scholar]
- 85.Eckert J, Schmidt M, Magedanz A, Voigtländer T, Schmermund A. Coronary CT angiography in managing atherosclerosis. Int J Mol Sci. 2015;16(2):3740–3756. doi: 10.3390/ijms16023740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferencik M, Mayrhofer T, Bittner DO, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol. 2018;3(2):144–152. doi: 10.1001/jamacardio.2017.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cury RC, Blankstein R, Leipsic J, et al. CAD-RADSTM 2.0 - 2022 Coronary Artery Disease – Reporting and Data System an expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR) and the North America society of cardiovascular imaging (NASCI) J Cardiovasc Comput Tomogr. 2022 doi: 10.1016/j.jcct.2022.07.002. Published online July. [DOI] [PubMed] [Google Scholar]
- 88.Ayoub C, Erthal F, Abdelsalam MA, et al. Prognostic value of segment involvement score compared to other measures of coronary atherosclerosis by computed tomography: a systematic review and meta-analysis. J Cardiovasc Comput Tomogr. 2017;11(4):258–267. doi: 10.1016/j.jcct.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Andreini D, Pontone G, Mushtaq S, et al. Long-term prognostic impact of CT-Leaman score in patients with non-obstructive CAD: Results from the COronary CT Angiography EvaluatioN For Clinical Outcomes InteRnational Multicenter (CONFIRM) study. Int J Cardiol. 2017;231:18–25. doi: 10.1016/j.ijcard.2016.12.137. [DOI] [PubMed] [Google Scholar]
- 90.Williams MC, Kwiecinski J, Doris M, et al. Low-Attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial (Scottish computed tomography of the HEART) Circulation. 2020;141(18):1452–1462. doi: 10.1161/CIRCULATIONAHA.119.044720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xaplanteris P, Fournier S, Pijls NHJ, et al. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;379(3):250–259. doi: 10.1056/NEJMoa1803538. [DOI] [PubMed] [Google Scholar]
- 92.Zhuang B, Wang S, Zhao S, Lu M. Computed tomography angiography-derived fractional flow reserve (CT-FFR) for the detection of myocardial ischemia with invasive fractional flow reserve as reference: systematic review and meta-analysis. Eur Radiol. 2020;30(2):712–725. doi: 10.1007/s00330-019-06470-8. [DOI] [PubMed] [Google Scholar]
- 93.Nørgaard BL, Terkelsen CJ, Mathiassen ON, et al. Coronary CT angiographic and flow reserve-guided management of patients with stable ischemic heart disease. J Am Coll Cardiol. 2018;72(18):2123–2134. doi: 10.1016/j.jacc.2018.07.043. [DOI] [PubMed] [Google Scholar]
- 94.Gulati M, Levy PD, Mukherjee D, et al. AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2021;144(22):e368–e454. doi: 10.1161/CIR.0000000000001030. [DOI] [PubMed] [Google Scholar]
- 95.Taylor CA, Gaur S, Leipsic J, et al. Effect of the ratio of coronary arterial lumen volume to left ventricle myocardial mass derived from coronary CT angiography on fractional flow reserve. J Cardiovasc Comput Tomogr. 2017;11(6):429–436. doi: 10.1016/j.jcct.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 96.Kini AS, Baber U, Kovacic JC, et al. Changes in plaque lipid content after short-term intensive versus standard statin therapy: the YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy) J Am Coll Cardiol. 2013;62(1):21–29. doi: 10.1016/j.jacc.2013.03.058. [DOI] [PubMed] [Google Scholar]
- 97.Meah MN, Maurovich-Horvat P, Williams MC, Newby DE. Debates in cardiac CT: coronary CT angiography is the best test in asymptomatic patients. J Cardiovasc Comput Tomogr. 2022;16(4):290–293. doi: 10.1016/j.jcct.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 98.Investigators SCOT-HEART, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924–933. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 99.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291–1300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muhlestein JB, Lappé DL, Lima JAC, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA. 2014;312(21):2234–2243. doi: 10.1001/jama.2014.15825. [DOI] [PubMed] [Google Scholar]
- 101.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 102.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 103.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet Lond Engl. 2004;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 104.Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or Older (EWTOPIA 75) Circulation. 2019 doi: 10.1161/CIRCULATIONAHA.118.039415. Published online September 17. [DOI] [PubMed] [Google Scholar]
- 105.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 107.Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 108.The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251(3):351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 109.Nicholls S, Lincoff AM, Bays HE, et al. Rationale and design of the CLEAR-outcomes trial: evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. Am Heart J. 2021;235:104–112. doi: 10.1016/j.ahj.2020.10.060. [DOI] [PubMed] [Google Scholar]
- 110.Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 111.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 112.n–3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–318. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- 113.Effects of combination lipid therapy in type 2 diabetes Mellitus. N Engl J Med. 2010;362(17):1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garg A, Sharma A, Krishnamoorthy P, et al. Role of niacin in current clinical practice: a systematic review. Am J Med. 2017;130(2):173–187. doi: 10.1016/j.amjmed.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 115.Abd alamir M, Goyfman M, Chaus A, et al. The correlation of dyslipidemia with the extent of coronary artery disease in the multiethnic study of atherosclerosis. J Lipids. 2018 doi: 10.1155/2018/5607349. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin A, Nerlekar N, Rajagopalan A, et al. Remnant cholesterol and coronary atherosclerotic plaque burden assessed by computed tomography coronary angiography. Atherosclerosis. 2019;284:24–30. doi: 10.1016/j.atherosclerosis.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 117.Allison MA, Pavlinac P, Wright CM. The differential associations between HDL, non-HDL and total cholesterols and atherosclerotic calcium deposits in multiple vascular beds. Atherosclerosis. 2007;194(2):e87–e94. doi: 10.1016/j.atherosclerosis.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 118.Loria CM, Liu K, Lewis CE, et al. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007;49(20):2013–2020. doi: 10.1016/j.jacc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 119.Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33–60. doi: 10.1016/j.jacl.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 120.Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2022;80(14):1366–1418. doi: 10.1016/j.jacc.2022.07.006. Writing Committee. [DOI] [PubMed] [Google Scholar]
- 121.Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mahabadi AA, Möhlenkamp S, Moebus S, et al. The Heinz Nixdorf Recall study and its potential impact on the adoption of atherosclerosis imaging in European primary prevention guidelines. Curr Atheroscler Rep. 2011;13(5):367–372. doi: 10.1007/s11883-011-0199-7. [DOI] [PubMed] [Google Scholar]
- 123.Osei AD, Mirbolouk M, Berman D, et al. Prognostic value of coronary artery calcium score, area, and density among individuals on statin therapy vs. non-users: the coronary artery calcium consortium. Atherosclerosis. 2021;316:79–83. doi: 10.1016/j.atherosclerosis.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Makover ME, Shapiro MD, Toth PP. There is urgent need to treat atherosclerotic cardiovascular disease risk earlier, more intensively, and with greater precision. A review of current practice and recommendations for improved effectiveness. Am J Prev Cardiol. 2022 doi: 10.1016/j.ajpc.2022.100371. Published online August 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin. J Am Coll Cardiol. 2011;57(16):1666–1675. doi: 10.1016/j.jacc.2010.09.082. [DOI] [PubMed] [Google Scholar]
- 126.Cholesterol Treatment Trialists’ (CTT) Collaboration. Fulcher J, O'Connell R, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet Lond Engl. 2015;385(9976):1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 127.Olsson AG, Angelin B, Assmann G, et al. Can LDL cholesterol be too low? Possible risks of extremely low levels. J Intern Med. 2017;281(6):534–553. doi: 10.1111/joim.12614. [DOI] [PubMed] [Google Scholar]
- 128.Ference BA, Yoo W, Alesh I, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60(25):2631–2639. doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 129.Mortensen MB, Falk E, Li D, et al. Statin trials, cardiovascular events, and coronary artery calcification. JACC Cardiovasc Imaging. 2018;11(2):221–230. doi: 10.1016/j.jcmg.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mortensen MB, Steffensen FH, Bøtker HE, et al. CAD severity on cardiac CTA identifies patients with most benefit of treating LDL-cholesterol to ACC/AHA and ESC/EAS targets. JACC Cardiovasc Imaging. 2020;13(9):1961–1972. doi: 10.1016/j.jcmg.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 131.Budoff M, Brent Muhlestein J, Le VT, May HT, Roy S, Nelson JR. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200–499 mg/dL) on statin therapy: rationale and design of the EVAPORATE study. Clin Cardiol. 2018;41(1):13–19. doi: 10.1002/clc.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nicholls SJ, Kataoka Y, Nissen SE, et al. Effect of evolocumab on coronary plaque phenotype and burden in statin-treated patients following myocardial infarction. JACC Cardiovasc Imaging. 2022;15(7):1308–1321. doi: 10.1016/j.jcmg.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 133.Borges R de P, Degobi NAH, Bertoluci MC. Choosing statins: a review to guide clinical practice. Arch Endocrinol Metab. 2020 doi: 10.20945/2359-3997000000306. Published online November 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins. Circ Cardiovasc Qual Outcomes. 2013;6(4):390–399. doi: 10.1161/CIRCOUTCOMES.111.000071. [DOI] [PubMed] [Google Scholar]
- 135.Cheeley MK, Saseen JJ, Agarwala A, et al. NLA scientific statement on statin intolerance: a new definition and key considerations for ASCVD risk reduction in the statin intolerant patient. J Clin Lipidol. 2022:S1933–S2874. doi: 10.1016/j.jacl.2022.05.068. Published online June 9(22)00167-2. [DOI] [PubMed] [Google Scholar]
- 136.Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4(3):206–213. doi: 10.1001/jamacardio.2018.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebocontrolled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 138.Phan BAP, Dayspring TD, Toth PP. Ezetimibe therapy: mechanism of action and clinical update. Vasc Health Risk Manag. 2012;8:415–427. doi: 10.2147/VHRM.S33664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265(5):568–580. doi: 10.1111/j.1365-2796.2008.02062.x. [DOI] [PubMed] [Google Scholar]
- 140.Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7) doi: 10.1161/JAHA.118.011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hao QY, Gao JW, Yuan ZM, et al. Remnant cholesterol and the risk of coronary artery calcium progression: insights from the CARDIA and MESA study. Circ Cardiovasc Imaging. 2022;15(7) doi: 10.1161/CIRCIMAGING.122.014116. [DOI] [PubMed] [Google Scholar]
- 142.Won KB, Park EJ, Han D, et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol. 2020;19(1):34. doi: 10.1186/s12933-020-01008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zeb I, Li D, Nasir K, et al. Effect of statin treatment on coronary plaque progression - a serial coronary CT angiography study. Atherosclerosis. 2013;231(2):198–204. doi: 10.1016/j.atherosclerosis.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 144.Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2(2):e52–e63. doi: 10.1016/S2352-3018(14)00032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Budoff MJ, Lane KL, Bakhsheshi H, et al. Rates of progression of coronary calcium by electron beam tomography. Am J Cardiol. 2000;86(1):8–11. doi: 10.1016/s0002-9149(00)00820-1. [DOI] [PubMed] [Google Scholar]
- 146.Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998;339(27):1972–1978. doi: 10.1056/NEJM199812313392703. [DOI] [PubMed] [Google Scholar]
- 147.Raggi P, Davidson M, Callister TQ, et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women. Circulation. 2005;112(4):563–571. doi: 10.1161/CIRCULATIONAHA.104.512681. [DOI] [PubMed] [Google Scholar]
- 148.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis heart study randomized clinical trial. J Am Coll Cardiol. 2005;46(1):166–172. doi: 10.1016/j.jacc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 149.Lee JM, Choi KH, Koo BK, et al. Prognostic implications of plaque characteristics and stenosis severity in patients with coronary artery disease. J Am Coll Cardiol. 2019;73(19):2413–2424. doi: 10.1016/j.jacc.2019.02.060. [DOI] [PubMed] [Google Scholar]
- 150.Budoff MJ, Ellenberg SS, Lewis CE, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317(7):708–716. doi: 10.1001/jama.2016.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kaiser Y, Daghem M, Tzolos E, et al. Association of lipoprotein(a) with atherosclerotic plaque progression. J Am Coll Cardiol. 2022;79(3):223–233. doi: 10.1016/j.jacc.2021.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]