Abstract
Background
Daily intake of 57 g Jarlsberg cheese has been shown to increase the total serum osteocalcin (tOC). Is this a general cheese effect or specific for Jarlsberg containing vitamin K2 and 1,4-dihydroxy-2naphtoic acid (DHNA)?
Methods
66 healthy female volunteers (HV) were recruited. By skewed randomisation (3:2), 41 HV were allocated to daily intake of 57 g Jarlsberg (J-group) and 25–50 g Camembert (C-group) in 6 weeks. After 6 weeks the C-group was switched to Jarlsberg. The study duration was 12 weeks with clinical investigations every 6 weeks. The main variables were procollagen type 1 N-terminal propeptide (PINP), tOC, carboxylated osteocalcin (cOC) and the osteocalcin ratio (RO) defined as the ratio between cOC and undercarboxylated osteocalcin (ucOC). Serum cross-linked C-telopeptide type I collagen (CTX), vitamin K2, lipids and clinical chemistry were used as secondary variables.
Results
PINP, tOC, cOC, RO and vitamin K2 increased significantly (p<0.01) after 6 weeks in the J-group. PINP remained unchanged in the C-group. The other variables decreased slightly in the C-group but increased significantly (p≤0.05) after switching to Jarlsberg. No CTX-changes detected in neither of the groups.
Serum lipids increased slightly in both groups. Switching to Jarlsberg, total cholesterol and low-density lipoprotein-cholesterol were significantly reduced (p≤0.05). Glycated haemoglobin (HbA1c), Ca++ and Mg++ were significantly reduced in the J-group, but unchanged in the C-group. Switching to Jarlsberg, HbA1c and Ca++ decreased significantly.
Conclusion
The effect of daily Jarlsberg intake on increased s-osteocalcin level is not a general cheese effect. Jarlsberg contain vitamin K2 and DHNA which increases PINP, tOC, cOC and RO and decreases Ca++, Mg++ and HbA1c. These effects reflect increased bone anabolism and a possible reduced risk of adverse metabolic outcomes.
Trial registration number
Keywords: nutrient deficiencies, musculo-skeletal health, nutritional treatment, nutrition assessment, malnutrition
WHAT IS ALREADY KNOWN ON THIS TOPIC
Jarlsberg cheese contains long-chained vitamin K2 and 1,4-Dihydroxy-naphthoic acid, and the daily optimal efficiency dose of 57 g/day increases the osteocalcin level.
WHAT THIS STUDY ADDS
The present study was performed to verify the increase in osteocalcin, the impact on bone turnover markers (BTM) and to verify that the effect is not a general cheese effect.
Daily Jarlsberg cheese consumption has a positive effect on osteocalcin, other BTM’s, glycated haemoglobin and lipids.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
These results indicates that Jarlsberg cheese might have prophylactic effects on osteopenia and metabolic diseases. Further research may reveal the possibility of recommending Jarlsberg cheese as prophylactical treatment or even an addition to antiresorptive treatment in osteoporosis.
Introduction
Vitamin K is important for bone health, and low intake of vitamin K has been associated with increased risk of fractures.1 Supplementation with vitamin K2, vitamin D and calcium is a recommended first-line treatment of osteoporosis.2 Vitamin K2 is essential for carboxylation or activation of Gla proteins. Osteocalcin is 1 of the 17 Gla proteins in humans, and carboxylated osteocalcin has a key role in bone formation and maintenance. The ratio between circulating carboxylated and undercarboxylated osteocalcin reflects the extrahepatic vitamin K status.
Vitamin K2 or menaquinone (MK) has many variants.3 The short-chained MK-4 can be formed from vitamin K1 in humans and is found in animal products such as liver. The long-chained vitamin K2 vitamers like MK-7, MK-8, MK-9 and MK-9(4H) are of bacterial origin, and occur in certain fermented foods such as cheese.
These variants have greater extrahepatic activity than MK-4 and vitamin K1; possibly due to more efficient uptake and much longer serum half-life.4 Prospective cohort studies have demonstrated health benefits that can be attributed to the intake of vitamin K2, but not K1. In the Western diet cheese is the major source of long-chained vitamin K2.5
MK-9 is the most important K2 vitamer in cheese, but the amount varies considerably.6 Some cheeses also contain MK-9(4H) and Jarlsberg cheese is particularly rich in both MK-9 and MK-9(4H).7 This cheese is made with lactic acid bacteria producing MK-8 and MK-9 and Propionibacterium freudenreichii producing MK-9(4H).
A previous dose-response study of daily Jarlsberg with healthy premenopausal women was performed with a three-level between patient response surface pathway design.8 9 The maximum efficacy dose (MED) of Jarlsberg in order to obtain increased vitamin K2 status, measured as osteocalcin carboxylation and total serum osteocalcin (tOC) level was estimated to 57 g/day.10 These results were confirmed in a recent clinical study on the same study population.11 The MED of 57 g/day Jarlsberg cheese was verified to increase the vitamin K2 status and tOC level.11 The maintenance dose for this study population was estimated to 45 g/day of Jarlsberg and verified as sufficient to keep the obtained tOC level. Osteocalcin is used as a serum marker of osteoblastic bone formation indicating that Jarlsberg stimulates bone formation.12 Bone markers like procollagen type 1 N-terminal propeptide (PINP) and serum cross-linked C-telopeptide type I collagen (CTX) were not recorded.13 14
Although cheese has been associated with vitamin K2-related health benefits, the effect of cheese consumption on bone health has, to the best of our knowledge, never been investigated in human controlled clinical trials. Because of its high vitamin K2 content, Jarlsberg appears to be a good candidate for such studies.
1,4-dihydroxy-2-naphthoic acid (DHNA) has been shown to have anti-osteoporotic effects and increase bone mineral density in ovariectomised mice.15 Jarlsberg contains Proprionebacterium freudenreichii, which produces MK-9-(4H), but also secretes DHNA. This might explain the increase in tOC as well as the carboxylation of osteocalcin (cOC) and it has been suggested that DHNA could be used in treatment of postmenopausal osteoporosis.15 If the raise of tOC and cOC levels is not a general cheese effect, but a Jarlsberg effect, further studies on Jarlsberg as prophylaxis are even more relevant. The aim of this study was to compare the effect of daily Jarlsberg cheese intake with a cheese without vitamin K2, but similar fat and protein content, on PINP, tOC, cOC, RO and glucose metabolism by glycated haemoglobin (HbA1c).
Material and methods
Population and sample
The study population consisted of healthy Norwegian women (healthy volunteer (HV)) of premenopausal age. Pregnant women and women suffering from known gastrointestinal disorder, abnormal liver or kidney function, lactose intolerance or known milk product allergy, diabetes mellitus or verified cancer were excluded. Women under systemic treatment with corticosteroids or immunosuppressive drugs the last 3 weeks or participating in another clinical trial the last 6 weeks before start of the study were not included.
Sixty-eight HVs were recruited by eight general practitioners from Viken County in Norway. Two HVs dropped out from the study for personal reasons during the first week. The study sample consisted of 66 HVs with a mean age of 33.6 years (range: 19.4–51.9 years) and body mass index of 24.3 kg/m2 (range: 15.0–35.4). By randomisation, 41 HVs were allocated to daily intake of Jarlsberg cheese (J-group) and 25 to Camembert cheese (C-group). The two groups were found comparable on all recorded demographic data, concomitant diseases and treatment (table 1). All ongoing treatments were kept unchanged during the study. Vitamin D deficiency was initially recorded in nine participants, and supplements were provided to obtain normal range.
Table 1.
Demographics, vital signs, social habits and concomitant diseases or claims
| Variable | Jarlsberg (n=41) | Camembert (n=25) | |
| Age (years) | Mean (SD) | 34.2 (9.9) | 32.6 (7.0) |
| Min–max | 19.6–51.9 | 19.4–42.6 | |
| Weight (kg) | Mean (SD) | 66.8 (9.8) | 69.3 (13.4) |
| Min–max | 37.0–87.1 | 48.0–102.0 | |
| Height (cm) | Mean (SD) | 167 (6) | 167 (6) |
| Min–max | 156–185 | 152–178 | |
| Body mass index | Mean (SD) | 23.8 (3.0) | 25.0 (4.8) |
| Min–max | 15.0–29.8 | 17.9–35.4 | |
| Systolic blood pressure | Mean (SD) | 115 (13) | 120 (18) |
| Min–max | 92–154 | 93–169 | |
| Diastolic blood pressure | Mean (SD) | 72 (10) | 77 (10) |
| Min–max | 54–101 | 56–98 | |
| Pulse rate | Mean (SD) | 67 (13) | 74 (12) |
| Min–max | 40–120 | 46–97 | |
| Social status | Married/cohabitant | 26 | 15 |
| Divorced | 2 | 3 | |
| Single | 13 | 7 | |
| Smoking | Ex/no | 3/38 | 1/24 |
| Ethnic | Asian/Caucasian | 4/37 | 1/24 |
| Concomitant disease under treatment during the study | D-vitamin deficiency | 6 | 3 |
| Iron deficiency | 1 | 0 | |
| Allergy | 3 | 3 | |
| Asthma | 1 | 1 | |
| Atopic eczema | 2 | 0 | |
| Hypothyroidism | 2 | 1 | |
| Fibromyalgia | 0 | 1 | |
| Contraceptives | 12 | 2 | |
| Hypertension | 6 | 0 | |
| Tachycardia | 1 | 0 | |
| Dyspepsia | 1 | 1 |
Study design and allocation
The study was performed as an open, randomised multicentre trial with semi crossover design.16 The participants were randomised for either 6 weeks of Jarlsberg or 6 weeks of Camembert cheese. The participants allocated to Camembert were then switched to Jarlsberg for an additional 6 weeks. The results obtained during the first 6 weeks and the 6 weeks after switch in cheese are reported. The study subjects were skewed randomly allocated (2:3) in two treatment groups; 41 in the J-group and 27 in the C-group. The allocation of participants to treatment groups was performed by the data manager prior to the start of the study by stratified randomisation with a fixed block size of 10.17 Two HVs withdrew, reducing the C-group to 25. The participants were asked not to change their usual diet during the study except for avoiding other cheese than the cheese provided in the study. No diet registration was performed, but the participants daily registered their intake of either Jarlsberg or Camembert during the study. The cheese compliance in the study was 95% and 92% in the J-group and the C-group, respectively. The compliance in the C-group after switching to Jarlsberg was 93%.
Clinical procedure
The clinical part of the study was performed from March 2020 to August 2020. Clinical status and blood sampling were performed initially and every 6 weeks. In addition to common laboratory variables, the blood samples were used for measurements of osteocalcin; PINP; CTX; lipids and the vitamin K2 vitamers MK-7, MK-8, MK-9 and MK-9(4H).
Clinical intervention
The J-group received 57 g/day or six slices Jarlsberg, and the C-group 50 g/day of TINE Camembert cheese for 6 weeks. Participants of the J-group received 100 g packages containing cheese slices of 10 g. The C-group received 150 g packages, divided in three equal parts. The corresponding energy and fat intake per day is 680 kJ and 14.0 g in the C-group, and 729 kJ and 15.4 g in the J-group. Both the Jarlsberg and Camembert are produced by TINE SA and cheese from three production batches was used.
The average vitamin K per 100 g of Jarlsberg cheese contained 3.0 µg vitamin K1, 5.2 µg MK-4 and 1.5 µg MK-7, 6.7 µg MK-8, 23.9 µg MK-9 and 40.5 µg MK-9(4H). TINE Camembert cheese does not contain vitamin K.
Vitamin K extraction and osteocalcin analysis
Vitamin K in cheese and blood samples was analysed as described previously.10 11 The cOC and the undercarboxylated osteocalcin (ucOC) were measured in plasma by immunoassays kits (Takara Bio, Ōtsu, Japan) by Vitas AS. The tOC is defined as the sum of cOC and ucOC.
Central variables
The three main variables were PINP, tOC and the osteocalcin ratio Ro=cOC/ ucOC. The supporting variables were sum of vitamin K2 and the variants MK-7, MK-8, MK-9 and MK-9(4H). Additionally CTX, Glycated haemoglobin; the lipids; haematological and clinical chemistry were used as secondary variables.
Randomisation
The participants were skewed allocated (2:3) to the J-group and C-group by block randomisation with fixed block size of 10.17
Statistical analysis
A significance level of 0.05; a power of 90% and a clinical relevant difference between groups of one time SD, a minimum of 23 participants are required in each group. The assumed continuously distributed variables were expressed by mean values with 95% CI.18As an index of dispersion, SD were given. Categorised variables were given in contingency tables.19All tests were performed two-tailed with a significance level of 0.05. Analysis of covariance with baseline measurement as covariate was used for comparison of groups.20
Changes within groups were tested by using a paired two-tailed t-test.18
Approvals
The study was performed in accordance with the research protocol; ‘Effect of Jarlsberg cheese compared with cheese without vitamin K2 (Camembert) regarding increased Osteocalcin level in healthy women’, (ClinicalTrial.gov).
Results
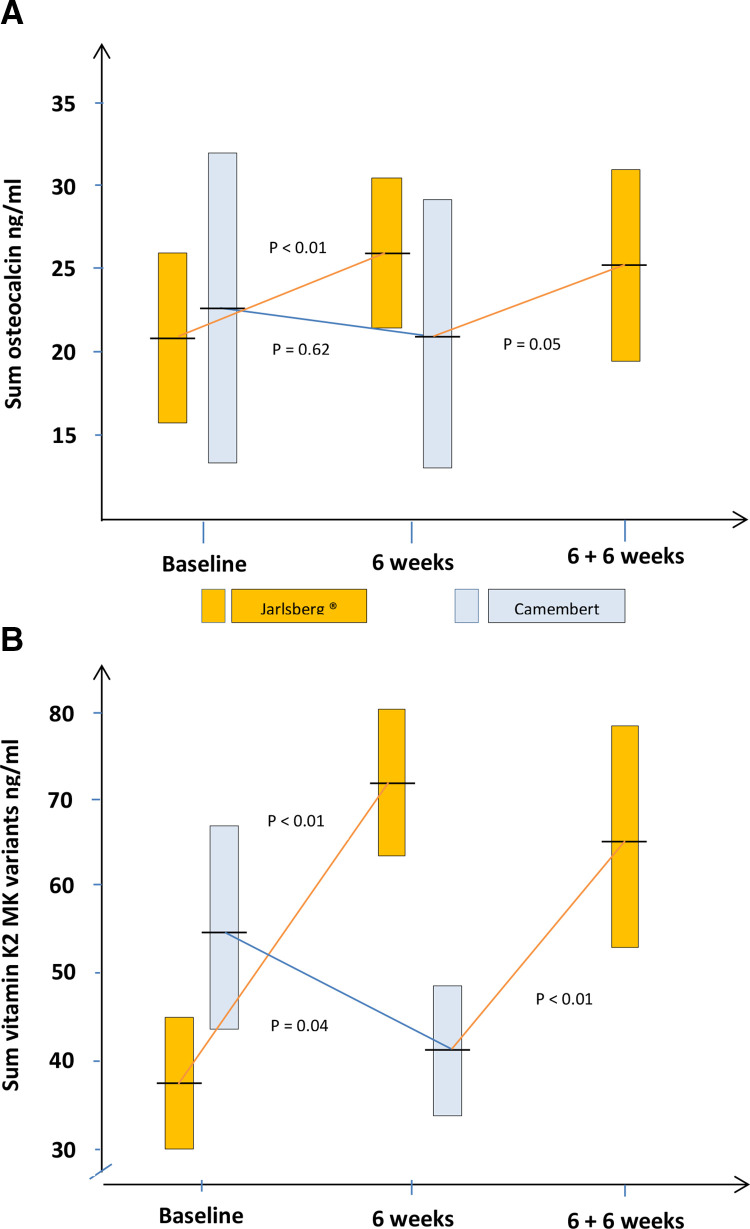
The tOC level increased significantly (p<0.01) from 21.4 ng/mL (95% CI: 16.6 to 26.2) to 25.8 ng/mL (95% CI: 21.0 to 30.6) after 6 weeks in the J-group (figure 1A). In the C-group this level was slightly reduced from 23.3 ng/mL (95% CI: 13.7 to 32.8) to 21.3 ng/mL (95% CI: 13.3 to 29.3). The difference in tOC increase between groups was 6.4 ng/mL (95% CI: 13.7 to 32.8) and significantly (p<0.01) in favour of Jarlsberg cheese. After switching to daily intake of Jarlsberg in the C-group, the tOC level increased significantly (p=0.05) from 21.3 ng/mL to 25.1 ng/mL (95% CI: 18.8 to 31.4) (figure 1A). A similar pattern was detected for cOC and the Ro (table 2).
Figure 1.
Development within- and comparison between cheese groups on (A) total serum osteocalcin and (B) Sum vitamin K2 vitamers. The yellow boxes show 95% confidence intervals of the mean in the Jarlsberg treated group and the blue similarly in the Camembert group. The mean values are given as horizontal lines crossing the boxes.
Table 2.
Comparison of Jarlsberg and Camembert cheese in development of osteocalcin variables and bone markers and the vitamin K2 vitamers during 6 weeks of daily cheese intake. The results are expressed by mean values with SD in bracket and 95% CIs
| Variables | Baseline | Week 6 | Week 6—baseline | P values between groups | |||
| Jarlsberg (n=41) | Camembert (n=25) |
Jarlsberg (n=41) |
Camembert (n=25) |
Jarlsberg (n=41) |
Camembert (n=25) |
||
| cOC=carboxylated osteocalcin (ng/mL) |
11.2 (9.0) | 16.1 (18.3) | 15.3 (9.5) | 14.9 (15.0) | 4.1 (6.8) | −1.2 (9.7) | p<0.01 |
| 8.4 to 14.1 | 8.6 to 23.7 | 12.3 to 18.3 | 8.7 to 21.0 | 2.0 to 6.2 | −5.3 to 2.8 | ||
| ucOC=undercarboxylated osteocalcin (ng/mL) | 10.2 (10.4) | 7.2 (5.4) | 10.3 (12.9) | 6.6 (5.0) | 0.2 (6.0) | −0.6 (1.9) | p=0.47 |
| 6.9 to 13.5 | 5.0 to 9.4 | 6.3 to 14.4 | 4.5 to 8.6 | −1.7 to 2.1 | −1.4 to 0.2 | ||
| RO =carboxylated/ undercarboxylated |
1.47 (0.94) | 1.86 (1.14) | 2.19 (1.31) | 2.09 (1.34) | 0.72 (0.90) | 0.23 (1.09) | p=0.03 |
| 1.17 to 1.76 | 1.39 to 2.33 | 1.78 to 2.60 | 1.54 to 2.64 | 0.44 to 1.01 | −0.26 to 0.65 | ||
| PINP=procollagen (ng/mL) | 61.1 (27.5) | 61.4 (24.1)* | 69.1 (34.9) | 58.4 (21.7) | 8.1 (13.9) | −1.3 (11.4)* | p<0.01 |
| 52.4 to 69.7 | 51.0 to 71.8 | 58.1 to 80.2 | 49.4 to 67.4 | 3.7 to 12.4 | −6.2 to 3.6 | ||
| CTX=collagen I (ng/mL) | 0.29 (0.13) | 0.27 (0.17)* | 0.27 (0.15) | 0.28 (0.15) | −0.02 (0.13) | 0.02 (0.15)* | p=0.27 |
| 0.25 to 0.33 | 0.19 to 0.34 | 0.22 to 0.32 | 0.22 to 0.34 | −0.06 to 0.02 | −0.04 to 0.08 | ||
| MK-7 (ng/mL) |
0.12 (0.07) | 0.21 (0.17) | 0.15 (0.06) | 0.18 (0.08) | 0.04 (0.07) | −0.03 (0.15) | p=0.02 |
| 0.09 to 0.14 | 0.14 to 0.28 | 0.14 to 0.17 | 0.15 to 0.22 | 0.02 to 0.06 | −0.09 to 0.04 | ||
| MK-8 (ng/mL) |
0.09 (0.07) | 0.12 (0.08) | 0.22 (0.11) | 0.07 (0.06) | 0.12 (0.10) | −0.05 (0.06) | p<0.01 |
| 0.07 to 0.12 | 0.09 to 0.15 | 0.18 to 0.25 | 0.04 to 0.09 | 0.09 to 0.16 | −0.08 to 0.03 | ||
| MK-9 (ng/mL) |
0.09 (0.09) | 0.11 (0.07) | 0.21 (0.09) | 0.05 (0.04) | 0.12 (0.11) | −0.06 (0.07) | p<0.01 |
| 0.06 to 0.12 | 0.08 to 0.14 | 0.18 to 0.24 | 0.04 to 0.07 | 0.09 to 0.16 | −0.09 to −0.03 | ||
| MK-9(4H) (ng/mL) |
0.08 (0.06) | 0.10 (0.07) | 0.14 (0.07) | 0.10 (0.05) | 0.06 (0.05) | 0.00 (0.05) | p<0.01 |
| 0.06 to 0.09 | 0.06 to 0.09 | 0.12 to 0.16 | 0.08 to 0.12 | 0.05 to 0.08 | −0.02 to 0.02 | ||
| 1.15 to 1.26 | 1.11 to 1.26 | 1.18 to 1.29 | 1.10 to 1.22 | −0.02 to 0.09 | −0.08 to 0.03 | ||
*Two baseline blood samples in the Camembert group were lost and reduced the sample to 23 for estimation and comparison of the development in PINP and CTX.
cOC, carboxylation osteocalcin; CTX, C-telopeptide type I collagen; MK, menaquinone; PINP, procollagen type 1 N-terminal propeptide; Ro, osteocalcin ratio; ucOC, undercarboxylated osteocalcin.
Both variables increased significantly in the J-group, but remained unchanged in the C-group. The increase in cOC and Ro was significant different in the two groups (p<0.01; p=0.04). The level of ucOC was nearly unchanged in both groups. After switching to Jarlsberg in the C-group, both the cOC and the Ro increased significantly (p<0.01; p=0.05) whereas the ucOC were unaffected (table 3).
Table 3.
Development in osteocalcin, bone markers and the vitamin MK variants after switching from Camembert to 6 weeks daily Jarlsberg cheese intake. The results are expressed by mean values with SD in bracket and 95% CIs
| Variables | Baseline Jarlsberg (n=25) |
6 weeks on Jarlsberg (n=25) |
Week 6—baseline (n=25) |
P values within variables |
| Carboxylated osteocalcin (ng/mL) |
14.9 (15.0) | 19.0 (12.2) | 4.2 (8.5) | p=0.02 |
| 8.7 to 21.0 | 14.0 to 24.1 | 0.7 to 7.7 | ||
| Undercarboxylated osteocalcin (ng/mL) |
6.6 (5.0) | 6.1 (3.4) | −0.5 (2.6) | p=0.32 |
| 4.5 to 8.6 | 4.6 to 7.5 | −1.6 to 0.6 | ||
| Ratio: carboxylated/undercarboxylated | 2.09 (1.34) | 3.1 (1.28) | 0.96 (1.06) | p<0.01 |
| 1.54 to 2.64 | 2.53 to 3.58 | 0.53 to 1.40 | ||
| PINP: procollagen (ng/mL) | 58.4 (21.7 | 61.1 (24.1) | 2.7 (9.6) | p=0.17 |
| 49.4 to 67.4 | 51.2 to 71.1 | −1.2 to 6.7 | ||
| CTX: (ng/mL) |
0.28 (0.15) | 0.25 (0.12) | −0.03 (0.10) | p=0.07 |
| 0.22 to 0.34 | 0.20 to 0.30 | −0.07 to 0.01 | ||
| MK-7 (ng/mL) |
0.18 (0.08) | 0.17 (0.10) | −0.02 (0.07) | p=0.30 |
| 0.15 to 0.22 | 0.13 to 0.21 | −0.05 to 0.02 | ||
| MK-8 (ng/mL) |
0.07 (0.06) | 0.17 (0.10) | 0.10 (0.08) | p<0.01 |
| 0.04 to 0.09 | 0.13 to 0.21 | 0.07 to 0.14 | ||
| MK-9 (ng/mL) |
0.05 (0.04) | 0.19 (0.12) | 0.14 (0.11) | p<0.01 |
| 0.04 to 0.07 | 0.15 to 0.24 | 0.10 to 0.19 | ||
| MK-9(4H) (ng/mL) |
0.10 (0.05) | 0.13 (0.06) | 0.02 (0.04) | p=0.01 |
| 0.08 to 0.12 | 0.10 to 0.15 | 0.01 to 0.04 |
CTX, C-telopeptide type I collagen; MK, menaquinone; PINP, procollagen type 1 N-terminal propeptide.
PINP increased significantly (p<0.01) in the J-group, but remained unchanged in the C-group (table 2). The mean difference between the groups was 9.4 ng/mL (95% CI: 1.3 to 9.3) and significant (p=0.01) in favour of Jarlsberg. CTX was unaffected in both groups during the study. After switching to Jarlsberg in the C-group, PINP increased, but not significantly (table 3). However, PINP decreased in 7 of the 25 participants; a proportion significantly (p≤0.05) below 50%. Four of these seven HVs had a remarkably large PINP reduction, resulting in the lack of significance on the mean level. CTX was unaffected after switching in cheese.
The sum of vitamin K2 vitamers increased significantly (p<0.01) from 0.38 ng/mL (95% CI: 0.30 to 0.45) to 0.72 ng/mL (95% CI: 0.64 to 0.81) after 6 weeks in the J-group (figure 1A). A significant reduction (p=0.04) was detected in the C-group. The development in sum of vitamin K2 was significant in favour of the J-group (p<0.01). After switching to Jarlsberg in the C-group, the sum of vitamin K2 increased significantly (p<0.01) from 0.41 ng/mL (95% CI: 0.34 to 0.48) to 0.65 ng/mL (95% CI: 0.53 to 0.79) (figure 1B). All the vitamin K2 vitamers MK-7, MK-8, MK-9 and MK-9(4H) increased significantly (p<0.01) after 6 weeks in the J-group but decreased in the C-group (0.02≤p≤0.21) (table 2). The increases in all MK-variants were significant in favour of Jarlsberg cheese. After switching from Camembert to Jarlsberg, MK-8, MK-9 and MK-9(4H) increased significantly (p<0.01) whereas MK-7 was nearly unchanged (table 3).
Mean HbA1c decreased significantly (p<0.01) after 6 weeks in the J-group (table 4). In the C-group this level was significantly increased (p=0.05) The mean difference in HbA1c development between groups was significantly (p<0.01) in favour of Jarlsberg cheese. After switching to daily intake of Jarlsberg in the C-group, mean HbA1c decreased significantly (p<0.01) (table 5).
Table 4.
Comparison of Jarlsberg and Camembert cheese in development of biochemical variables during 6 weeks of daily cheese intake. The results are expressed by mean values with SD in bracket and 95% CIs
| Variables | Baseline | Week 6 | Week 6—baseline | P values between groups | |||
| Jarlsberg (n=41) | Camembert (n=25) |
Jarlsberg (n=41) |
Camembert (n=25) |
Jarlsberg (n=41) |
Camembert (n=25) |
||
| Triglyceride (mmol/L) |
1.0 (0.6) | 1.2 (0.6) | 1.1 (0.5) | 1.4 (0.8) | 0.1 (0.5) | 0.2 (0.6) | p=0.41 |
| 0.9 to 1.1 | 1.0 to 1.5 | 0.9 to 1.3 | 1.1 to 1.7 | 0.0 to 0.3 | −0.1 to 0.4 | ||
| LDL-cholesterol (mmol/L) |
2.7 (0.7) | 3.2 (0.8) | 2.9 (0.7) | 3.4 (0.9) | 0.2 (0.4) | 0.2 (0.6) | p=0.67 |
| 2.5 to 2.9 | 2.9 to 3.6 | 2.6 to 3.2 | 3.1 to 3.8 | 0.0 to 0.4 | 0.0 to 0.4 | ||
| HDL-cholesterol (mmol/L) |
1.7 (0.4) | 1.6 (0.4) | 1.7 (0.4) | 1.6 (0.3) | 0.0 (0.2) | 0.0 (0.2) | p=0.99 |
| 1.6 to 1.8 | 1.4 to 1.7 | 1.6 to 1.8 | 1.5 to 1.8 | −0.1 to 0.1 | −0.1 to 0.1 | ||
| Total cholesterol (mmol/L) |
4.6 (0.7) | 4.9 (0.6) | 4.8 (0.7) | 5.1 (0.7) | 0.2 (0.4) | 0.2 (0.5) | p=0.52 |
| 4.4 to 4.8 | 4.6 to 5.1 | 4.6 to 5.0 | 4.8 to 5.4 | 0.1 to 0.4 | 0.0 to 0.5 | ||
| LDL/HDL | 1.7 (0.6) | 2.2 (1.0) | 1.85 (0.62) | 2.3 (0.9) | 0.1 (0.3) | 0.1 (0.4) | p=0.94 |
| 1.5 to 1.9 | 1.8 to 2.6 | 1.6 to 2.0 | 1.9 to 2.7 | 0.0 to 0.2 | −0.1 to 0.2 | ||
| Creatinine (µmol/L) |
60.9 (10.0) | 64.1 (7.8) | 59.9 (8.7) | 61.2 (8.9) | −1.0 (6.5) | −2.9 (4.0) | p=0.43 |
| 57.8 to 64.0 | 60.9 to 67.3 | 57.1 to 62.6 | 57.5 to 64.9 | −3.1 to 1.0 | −4.5 to −1.2 | ||
| Urea (mmol/L) |
5.15 (1.25) | 5.67 (1.61) | 5.78 (1.06) | 5.39 (1.62) | 0.63 (0.95) | −0.28 (1.25) | p<0.01 |
| 4.76 to 5.55 | 5.01 to 6.34 | 5.45 to 6.12 | 4.72 to 6.06 | 0.33 to 0.93 | −0.80 to 0.23 | ||
| Glycated haemoglobin (mmol/L) |
34.2 (2.8) | 33.1 (2.4) | 33.3 (3.1) | 33.8 (3.1) | −1.0 (1.5) | 0.7 (1.5) | p<0.01 |
| 33.3 to 35.1 | 32.1 to 34.1 | 32.4 to 34.3 | 32.6 to 35.1 | −1.4 to −0.5 | 0.0 to 1.4 | ||
| Calcium (Ca+)
(mmol/L) |
2.34 (0.09) | 2.31 (0.09) | 2.31 (0.08) | 2.33 (0.09) | −0.03 (0.08) | 0.02 (0.08) | p=0.05 |
| 2.31 to 2.37 | 2.28 to 2.35 | 2.29 to 2.34 | 2.29 to 2.37 | −0.06 to 0.00 | −0.01 to 0.05 | ||
| Magnesium (Mg+) (mmol/L) | 0.86 (0.06) | 0.86 (0.05) | 0.83 (0.07) | 0.85 (0.05) | −0.03 (0.06) | 0.00 (0.04) | p=0.05 |
| 0.81 to 0.85 | 0.83 to 0.88 | 0.81 to 0.85 | 0.83 to 0.87 | −0.05 to −0.01 | −0.02 to 0.01 | ||
| Phosphate (mmol/L) |
1.20 (0.16) | 1.18 (0.18) | 1.24 (0.18) | 1.16 (0.14) | 0.04 (0.15) | −0.02 (0.14) | p=0.06 |
| 1.15 to 1.26 | 1.11 to 1.26 | 1.18 to 1.29 | 1.10 to 1.22 | −0.02 to 0.09 | −0.08 to 0.03 | ||
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 5.
Development in lipid and biochemical variables after switching from Camembert to 6 weeks daily intake of Jarlsberg cheese. The results are expressed by mean values with SD in bracket and 95% CIs
| Variables | Baseline Jarlsberg (n=25) |
6 weeks on Jarlsberg (n=25) |
Week 6—baseline (n=25) |
P values within variables |
| Triglyceride (mmol/L) |
1.4 (0.8) | 1.3 (0.6) | −0.1 (0.7) | p=0.32 |
| 1.1 to 1.7 | 1.0 to 1.5 | −0.4 to 0.1 | ||
| LDL-cholesterol (mmol/L) |
3.4 (0.9) | 3.1 (0.8) | −0.3 (0.5) | p<0.01 |
| 3.1 to 3.8 | 2.8 to 3.5 | −0.5 to −0.1 | ||
| HDL-cholesterol (mmol/L) |
1.6 (0.3) | 1.5 (0.3) | −0.1 (0.2) | p=0.05 |
| 1.5 to 1.8 | 1.4 to 1.7 | −0.2 to 0.0 | ||
| Total cholesterol (mmol/L) |
5.1 (0.7) | 4.7 (0.6) | −0.4 (0.4) | p<0.01 |
| 4.8 to 5.4 | 4.5 to 5.0 | −0.6 to −0.2 | ||
| LDL/HDL cholesterol | 2.3 (0.9) | 2.2 (0.9) | −0.1 (0.3) | p=0.20 |
| 1.9 to 2.7 | 1.8 to 2.6 | −0.2 to 0.1 | ||
| Creatinine (µmol/L) |
61.2 (8.9) | 61.3 (6.2) | 0.2 (6.4) | p=0.90 |
| 57.5 to 64.9 | 58.8 to 63.9 | −2.5 to 2.8 | ||
| Urea (mmol/L) |
5.39 (1.62) | 5.42 (1.63) | 0.0 (1.5) | p=0.92 |
| 4.72 to 6.06 | 4.76 to 6.08 | −0.6 to 0.7 | ||
| Glycated haemoglobin (mmol/L) |
33.8 (3.1) | 32.8 (2.4) | −1.0 (1.2) | p<0.01 |
| 32.6 to 35.1 | 31.9 to 33.8 | −1.5 to −0.5 | ||
| Calcium (Ca++)
(mmol/L) |
2.33 (0.09) | 2.29 (0.07) | −0.04 (0.08) | p=0.03 |
| 2.29 to 2.37 | 2.26 to 2.32 | −0.07 to 0.00 | ||
| Magnesium (Mg++) (mmol/L) |
0.85 (0.05) | 0.86 (0.07) | 0.00 (0.06) | p=0.72 |
| 0.83 to 0.87 | 0.83 to 0.88 | −0.02 to 0.03 | ||
| Phosphate (mmol/L) |
1.16 (0.14) | 1.20 (0.19) | 0.04 (0.15) | p=0.19 |
| 1.10 to 1.22 | 1.12 to 1.28 | −0.02 to 0.10 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Triglycerides, low-density lipoprotein (LDL)-cholesterol, total cholesterol and the cholesterol ratio LDL/high-density lipoprotein (HDL) increased slightly, but significantly (p≤0.05) in both groups during the first 6 weeks of cheese intake (table 4). No significant difference was detected between groups. After switching cheese in the C-group, total cholesterol and LDL-cholesterol were significantly reduced (p≤0.05). A slight but not significant reduction was detected in the triglycerides and the cholesterol ratio (table 5).
Ca++ and Mg++ were significantly reduced in the J-group (p≤0.05) but remained unchanged in the C-group (table 4). The differences between the groups were found significant (p≤0.05). After switching cheese in the C-group, Ca++ were significantly reduced (p≤0.05), but Mg++ was found unchanged (table 5).
Phosphate increased in the J-group, but reduced in the C-group. None of the changes were significant, but the difference between groups was borderline significant (table 4). When switching to Jarlsberg in the C-group, the phosphate increased, but not significantly (table 5).
Urea increased significantly (p<0.01) in the J-group and decreased non-significantly in the C-group (table 4). The difference between groups was found significant (p<0.01). No change in urea was detected in the C-group after the switch to Jarlsberg (table 5). Creatinine was reduced significantly (p<0.01) in the C-group, but non-significantly in the J-group (table 4). The difference between groups was not significant. No change in creatinine was detected in the C-group after the switch to Jarlsberg cheese (table 5).
Discussion
In the J-group, the tOC and cOC increased significantly confirming the results in the two previous dose-response-studies.10 11 In the C-group the tOC was slightly, but not significantly reduced after the first 6 weeks. Switching from Camembert cheese to Jarlsberg was led to a significant increase in tOC and cOC. This supports that Jarlsberg has a significant stimulatory effect on serum osteocalcin. A Mediterranean diet enriched with virgin olive oil has been associated with increased tOC in elderly men.21 The present study is probably the first time dietary intervention has been able to demonstrate a significant increase in the tOC level in a randomised controlled trial.
Measurable changes in Bone Mass Density (BMD) require a minimum of 1 year observation. Like tOC, PINP is an osteoblast-derived bone formation marker.13 CTX is a marker of osteoclast activity and used to assess the level of bone resorption.14 PINP and CTX are used as bone turnover biomarkers and may predict changes in BMD within 1–3 months.22 The present study included healthy premenopausal women, but detected a significant increase in PINP combined with unchanged CTX level the J-group; reflecting stimulated osteoblast activity and unaltered bone resorption. This indicates that daily use of 57 g Jarlsberg during a period of at least 6 weeks may increase BMD. The decreases in Ca ++ and Mg ++ caused by consumption of Jarlsberg probably also reflect uptake from the blood for net bone formation. Daily intake of Camembert did not show these effects.
The vitamin K2 vitamers MK-7, MK-8, MK-9 and MK-9(4H) all increased significantly during the 6 weeks in the Jarlsberg group. Significant reductions were recorded in the C-group. After switching to Jarlsberg, the increase in vitamin K2 in the C-group showed an almost parallel pattern to the increase in the J-group. There was a high degree of covariation between the rise of tOC and vitamin K2. The calculated correlation between these two variables is significant, but substantially weaker than the graphical illustration may indicate, and was obvious in the previous studies.10 11 In the dose-response study, the level of vitamin K2 increased with increasing cheese dose whereas tOC and cOC showed a maximum at 57 g cheese a day and falling trends above this dose.10 A similar pattern was also detected in the dose de-escalation study.11 This should not be the case if vitamin K2 was the only factor which increases tOC. Thus the increase in tOC cannot solely be ascribed to the vitamin K2 content of Jarlsberg.
In vitro, vitamin K2 has been shown to stimulate osteoblast differentiation and mineralisation, and some human studies have found a BMD increase in postmenopausal women taking 45 mg daily of vitamin K2.23 24 The vitamin K2 in Jarlsberg is formed by lactic acid bacteria and propionic acid bacteria. These vitamin K2 vitamers are different from those K2-tablets tested in clinical trials. Propionibacterium freudenreichii has been found to produce DHNA; an intermediate in the vitamin K2 biosynthesis.25 It has been suggested that DHNA could be an alternative or at least an additional treatment of postmenopausal osteoporosis.16 In ovariectomised mice, this compound was shown to stimulate the production of osteocalcin, while vitamin K2 could not.16 DHNA is the possible key to the tOC stimulatory effect of Jarlsberg. DHNA is formed as an intermediate in the biosynthesis of vitamin K2 in all organisms that produce the vitamin. However, to our knowledge propionic acid bacteria are the only bacteria reported to produce DHNA in substantial excess.
These results may come out additionally as important facts for future pharmaceutical interventions.
The participants were asked to maintain their usual diet during the study except for any cheese provided in the study. The cheese compliances were found satisfactory, but the daily diet or possible change in diet was not recorded. This might reduce the value of the obtained results to some extent. However, the participants were properly followed-up during the study and a systematic significant change in the diet it is not likely.
Some studies on Asian women have reported that high doses of vitamin K2 gave an increase in BMD, but this has not been detected in Europeans.24 The bone anabolic effects of daily Jarlsberg intake is more possibly caused by its content of DHNA with stronger osteoblast stimulation compared with vitamin K2.16
A possible explanation is that the increased tOC is mainly caused by DHNA and carboxylated by the long-chained vitamin K2 in Jarlsberg. In this process, calcium and additionally magnesium is absorbed from serum and increases BMD. Further studies should be performed to investigate if daily intake of Jarlsberg cheese could affect bone mass and structure in humans. Such studies could be performed on borderline, untreated osteoporotic patients or on endurance athletes. Several of such athletes suffer from decreased BMD due to energy deficiency; reduced resistance to infections and lack of nutritional factors.26
During the 6 weeks daily intake of 57 g Jarlsberg, the mean HbA1c was significantly reduced with 3% and increased with 2% in the C-group. Switching from Camembert cheese to Jarlsberg led to a significant 3% decrease in HbA1c. The inverse association of tOC with blood glucose, HbA1c, the risk of type 2 diabetes and metabolic syndrome is previously reported.27 28 The mechanisms underlying these effects in humans are unknown, but the role of osteocalcin as a hormone controlling energy metabolism has been suggested.29 The obtained reduction of HbA1c during daily Jarlsberg intake indicates that these associations are maintained even in the healthy population, and suggests that daily intake of Jarlsberg can be an aid in controlling major lifestyle-related diseases.
In the previous dose-response study of Jarlsberg a significantly reduction of blood lipids was observed.10 All the results obtained in this dose-response study were verified in the de-escalation study except for the reduction in blood lipids. This may possibly be due to temporary changes in dietary habits and physical activity caused by measures to limit the spread of SARS-CoV2.30 In the present study, such factors might explain the lack of blood lipid reduction during the first 6 weeks. After switching to Jarlsberg in the C-group, a significant reduction of the lipids was observed. This is in line with the inverse association between tOC and body fat reported in prospective studies.23The present study detected an increase in s-phosphate connected with daily Jarlsberg intake which might indicate that this cheese is not suitable for nutrition in end stage renal disease.
Conclusion
The effect of daily Jarlsberg intake on increased s-osteocalcin level is not a general cheese effect. Jarlsberg containing vitamin K2 and DHNA increases tOC, cOC, RO and PINP and decreases s-Ca++, s-Mg++ and HbA1c. These effects reflect increased bone anabolism and a possible reduced risk of adverse metabolic outcomes.
bmjnph-2022-000424supp001.pdf (582KB, pdf)
Footnotes
Contributors: All the authors participated in construction of the trial protocol, manuscript writing and approved the manuscript. The author acting as guarantor is HEL. TINE SA provided Jarlsberg and Camembert cheese, along with financial support, but did not play any role in the design, implementation, analysis, interpretation or manuscript writing. MSc Vivy Liang Larsen, Meddoc was in charge of Data Management and BSc Natharat Thiendilokkul, Meddoc in charge of all clinical monitoring. Vitas Laboratory performed all the osteocalcin analyses.
Funding: Norwegian Research Council; project number 310059, TINE SA, and Meddoc Research Unit funded this project.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed by Joerg Saupe, Germany.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data is saved in a SAS database at Meddoc Research.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Norwegian Regional Ethical Committee South-East, project nr.84845. Participants gave informed consent to participate in the study before taking part.
References
- 1. Moore AE, Kim E, Dulnoan D, et al. Serum vitamin K1 (phylloquinone) is associated with fracture risk and hip strength in post-menopausal osteoporosis: A cross-sectional study. Bone 2020;141:115630. 10.1016/j.bone.2020.115630 [DOI] [PubMed] [Google Scholar]
- 2. Orimo H, Nakamura T, Hosoi T, et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis--executive summary. Arch Osteoporos 2012;7:3–20. 10.1007/s11657-012-0109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gröber U, Reichrath J, Holick MF, et al. Vitamin K: an old vitamin in a new perspective. Dermatoendocrinol 2014;6:e968490. 10.4161/19381972.2014.968490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sato T, Schurgers LJ, Uenishi K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr J 2012;11:93. 10.1186/1475-2891-11-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nimptsch K, Rohrmann S, Kaaks R, et al. Dietary vitamin K intake in relation to cancer incidence and mortality: results from the Heidelberg cohort of the European prospective investigation into cancer and nutrition (EPIC-Heidelberg). Am J Clin Nutr 2010;91:1348–58. 10.3945/ajcn.2009.28691 [DOI] [PubMed] [Google Scholar]
- 6. Manoury E, Jourdon K, Boyaval P, et al. Quantitative measurement of vitamin K2 (menaquinones) in various fermented dairy products using a reliable high-performance liquid chromatography method. J Dairy Sci 2013;96:1335–46. 10.3168/jds.2012-5494 [DOI] [PubMed] [Google Scholar]
- 7. Hojo K, Watanabe R, Mori T, et al. Quantitative measurement of tetrahydromenaquinone-9 in cheese fermented by propionibacteria. J Dairy Sci 2007;90:4078–83. 10.3168/jds.2006-892 [DOI] [PubMed] [Google Scholar]
- 8. Dewi S, Kristiansen V, Lindr-Jensen S, et al. Between- and within-patient N-level response surface pathway design in dose-finding studies. Open Access J Clin Trials 2014;6:63–74. 10.2147/OAJCT.S57955 [DOI] [Google Scholar]
- 9. Larsen S, Holand T, Bjørnæs K, et al. Randomized two dimensional between patient response surface pathway design with two interventional and one response variable in estimating minimum efficacy dose. Int J Clin Trials 2018;6:75–83. 10.18203/2349-3259.ijct20193210 [DOI] [Google Scholar]
- 10. Lundberg HE, Holand T, Holo H, et al. Increased serum osteocalcin levels and vitamin K status by daily cheese intake. Int J Clin Trials 2020;7:55–65. 10.18203/2349-3259.ijct20201712 [DOI] [Google Scholar]
- 11. Lundberg HE, Holo H, Holand T, et al. Determination of maintenance Jarlsberg® cheese dose to keep the obtained serum osteocalcin level; a response surface pathway designed de-escalation dose study with individual starting values. Int J Clin Trials 2021;8:174–83. 10.18203/2349-3259.ijct20212841 [DOI] [Google Scholar]
- 12. Zoch ML, Clemens TL, Riddle RC. New insights into the biology of osteocalcin. Bone 2016;82:42–9. 10.1016/j.bone.2015.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krege JH, Lane NE, Harris JM, et al. Pinp as a biological response marker during teriparatide treatment for osteoporosis. Osteoporos Int 2014;25:2159–71. 10.1007/s00198-014-2646-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee CYS, Suzuki JB. Ctx biochemical marker of bone metabolism. is it a reliable predictor of Bisphosphonate-associated osteonecrosis of the jaws after surgery? Part II: a prospective clinical study. Implant Dent 2010;19:29–38. 10.1097/ID.0b013e3181cec8bc [DOI] [PubMed] [Google Scholar]
- 15. Kita K, Yamachika E, Matsubara M, et al. Anti-Osteoporosis effects of 1,4-dihydroxy-2-naphthoic acid in ovariectomized mice with increasing of bone density. J Oral Maxillofac Surg Med Pathol 2016;28:66–72. 10.1016/j.ajoms.2015.07.001 [DOI] [Google Scholar]
- 16. Carlsen KH, Larsen S, Ørstavik I. Acute bronchiolitis in infancy. The relationship to later recurrent obstructive airways disease. Eur J Respir Dis 1987;70:86–92. [PubMed] [Google Scholar]
- 17. Pocock ST. Clinical trials; a practical approach. Chichester: John Wiley & Sons, 1989. [Google Scholar]
- 18. Altman DG. Practical statistics for medical research. London: Chapman & Hall, 1991. [Google Scholar]
- 19. Agresti A. Categorical data analysis. New Jersey: John Wiley & Sons, 2002. [Google Scholar]
- 20. Kleinbaum DG, Kupper LL, Muller KE, et al. Applied regression analysis and other multivariable methods. CA: Duxbury Press Belmont, 1988. [Google Scholar]
- 21. Fernández-Real JM, Bulló M, Moreno-Navarrete JM, et al. A Mediterranean diet enriched with olive oil is associated with higher serum total osteocalcin levels in elderly men at high cardiovascular risk. J Clin Endocrinol Metab 2012;97:3792–8. 10.1210/jc.2012-2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flatman R, Férard G, Dybkaer R. Understanding the ‘Silver Book’ — An important reference for standardised nomenclature in clinical laboratory sciences. Clinica Chimica Acta 2017;467:4–7. 10.1016/j.cca.2016.06.035 [DOI] [PubMed] [Google Scholar]
- 23. Villa JKD, Diaz MAN, Pizziolo VR, et al. Effect of vitamin K in bone metabolism and vascular calcification: a review of mechanisms of action and evidences. Crit Rev Food Sci Nutr 2017;57:3959–70. 10.1080/10408398.2016.1211616 [DOI] [PubMed] [Google Scholar]
- 24. Rønn SH, Harsløf T, Oei L, et al. The effect of vitamin MK-7 on bone mineral density and microarchitecture in postmenopausal women with osteopenia, a 3-year randomized, placebo-controlled clinical trial. Osteoporos Int 2021;32:185–91. 10.1007/s00198-020-05638-z [DOI] [PubMed] [Google Scholar]
- 25. Isawa K, Hojo K, Yoda N, et al. Isolation and identification of a new bifidogenic growth stimulator produced by Propionibacterium freudenreichii ET-3. Biosci Biotechnol Biochem 2002;66:679–81. 10.1271/bbb.66.679 [DOI] [PubMed] [Google Scholar]
- 26. Hutson MJ, O'Donnell E, Brooke-Wavell K, et al. Effects of low energy availability on bone health in endurance athletes and high-impact exercise as a potential countermeasure: a narrative review. Sports Med 2021;51:391–403. 10.1007/s40279-020-01396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kunutsor SK, Apekey TA, Laukkanen JA. Association of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes: systematic review and meta-analysis of observational evidence. Eur J Epidemiol 2015;30:599–614. 10.1007/s10654-015-0058-x [DOI] [PubMed] [Google Scholar]
- 28. Mohammad Rahimi GR, Niyazi A, Alaee S. The effect of exercise training on osteocalcin, adipocytokines, and insulin resistance: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 2021;32:213–24. 10.1007/s00198-020-05592-w [DOI] [PubMed] [Google Scholar]
- 29. Pi M, Nishimoto SK, Darryl Quarles L. Explaining divergent observations regarding Osteocalcin/GPRC6A endocrine signaling. Endocrinology 2021;162:bqab011. 10.1210/endocr/bqab011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Di Renzo L, Gualtieri P, Pivari F, et al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med 2020;18:229–44. 10.1186/s12967-020-02399-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjnph-2022-000424supp001.pdf (582KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data is saved in a SAS database at Meddoc Research.