Highlights
-
•
A 35-year-old man reported seizures worsening and postictal psychosis after Radiofrequency-Thermocoagulation (RF-TC).
-
•
Cannabidiol treatment improved seizure frequency/intensity, resolving the postictal psychosis.
-
•
We recommend clinicians consider the rare potential of RF-TC to exacerbate seizures in patients with epilepsy.
Abbreviations: RF-TC, Radiofrequency thermocoagulation; PwP, Patients with polymicrogyria; EEG, electroencephalography; SEEG, stereo-electroencephalography; EZ, Epileptogenic Zone; FDG-PET, Fluorodeoxyglucose-Positron Emission Tomography; CBD, Cannabidiol
Keywords: Epilepsy, Stereo-EEG, Radiofrequency thermocoagulation, Post-ictal psychosis, Cannabidiol
Abstract
Radiofrequency thermocoagulation (RF-TC) is a wide-used procedure for drug-resistant epilepsy. The technique is considered safe with an overall risk of 1.1% of permanent complications, mainly focal neurological deficits. We report the case of a patient with drug-resistant epilepsy who complained of immediate seizure worsening and an unexpected event seven months following RF-TC.
A 35-year-old male with drug-resistant epilepsy from the age of 18 years underwent stereoelectroencephalography (SEEG) implantation for a right peri-silvian polymicrogyria. He was excluded from surgery due to extent of the epileptogenic zone and the risk of visual field deficits. RF-TC was attempted to ablate the most epileptogenic zone identified by SEEG. After RF-TC, the patient reported an increase in seizure severity/frequency and experienced episodes of postictal psychosis. Off-label cannabidiol treatment led to improved seizure control and resolution of postictal psychosis.
Patients with polymicrogyria (PwP) may present with a disruption of normal anatomy and the co-existence between epileptogenic zone and eloquent cortex within the malformation.
RF-TC should be considered in PwP when they are excluded from surgery for prognostic and palliative purposes. However, given the complex interplay between pathological and electrophysiological networks in these patients, the remote possibility of clinical exacerbation after RF-TC should also be taken into account.
Introduction
A major challenge for modern epileptology is the treatment of drug-resistant focal epilepsies, a condition that affects approximately 30–40 % of people with epilepsy [1]. Epilepsy surgery, including curative resective surgery and other palliative options, is an effective and suitable treatment for these patients [1]. It has been reported that epilepsy surgery leads to seizure control in 65 % of cases (range 13.5 %–92.5 %) depending on the underlying etiology, resection site and surgical procedure [2]. However worsening of seizure control after surgery varies from 0.8 % to 11 % among different surgical series [3]. Invasive stereo-electroencephalographic (SEEG) recording may be necessary to define the epileptogenic zone (EZ) and the best surgical plan for these patients, especially in cases of inconsistent anatomical and electroclinical data, in MRI-negative patients, in extensive cortical malformations, and in cases of suspected overlap between EZ and eloquent cortex [4]. In addition to its diagnostic utility, intracranial electrodes implanted for SEEG monitoring can be exploited to produce specific stereotactic lesions, with the aim of destroying the EZ or disconnecting the epileptogenic network [5]. Among the several lesioning methods proposed, radiofrequency thermocoagulation (RF-TC) is the most popular and widely used technique [6]. It consists of delivering a high-power current between two adjacent SEEG contacts to increase the temperature of brain tissue to 78-82°, producing an ovoid lesion about 6 mm long and 3.5 mm wide [7]. The results of RF-TC in terms of seizure control are highly variable, ranging from 6 % to 63 % depending on the experience of different centers, etiology and follow-up period considered [5], [8]. According to a recent meta-analysis, 23 % of patients were seizure-free after RF-TC, while those who reported a > 50 % reduction in seizure frequency at 1-year follow-up were 58 % [9]. In terms of complications, RF-TC is considered the safest procedure for drug-resistant epilepsy with a 1.1 % risk of permanent deficit and 2.4 % risk of transient deficits [6]. Even in patients in whom resective surgery is contraindicated because of overlap between EZ and eloquent cortex or in the presence of a complex EZ, RF-TC is still recommended by some authors as a palliative option, alternative to open surgery [5], [10], [11].
Complications of RF-TC usually consist of focal neurological deficits. However, the effect of the focal lesion produced by RF-TC on the reorganization of large epileptogenic networks and widely distributed brain functions, such as cognition or memory are less likely.
We describe the case of a patient with drug-resistant focal epilepsy secondary to right perisylvian polymicrogyria who after RF-TC reported worsening seizure control and recurrent post-ictal psychotic episodes.
Case description
A 35-year-old man with no relevant family or personal history was referred to our center with drug-resistant focal epilepsy. He developed seizures, aged 18 years, characterized by intermittent subjective sensations of eye pulsations, cephalic aura, visual misperceptions of colours and shapes followed by interruption of ongoing action, altered awareness (estrangement from surroundings), fixation of gaze, and occasional orbito-oral automatisms and myoclonia. Confusion and sometimes dysarthria were reported in the postictal phase. The episodes re-occurred mainly during daytime with a stereotyped semiology, becoming multiple per month over the years despite several antiepileptic medications attempted.
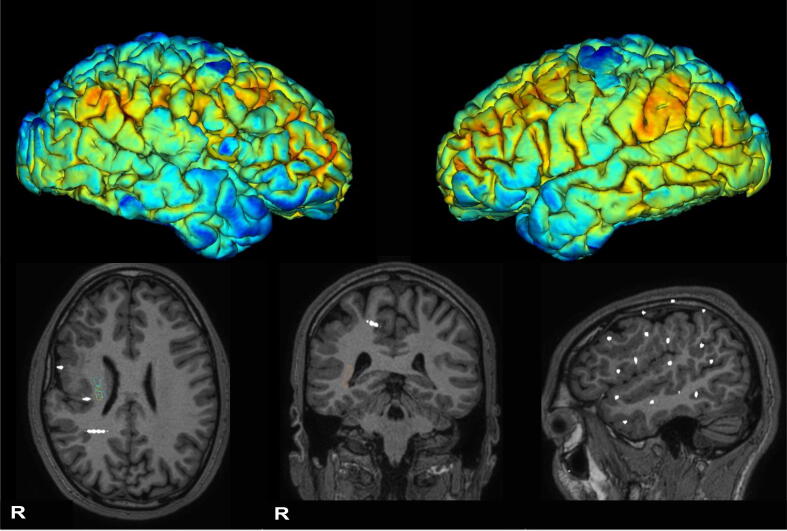
The patient underwent pre-surgical evaluation at 31 years of age. Long-term video-EEG monitoring showed right-temporal interictal epileptiform discharges and allowed the recording of three stereotyped episodes, in two cases associated with a right-temporal discharge preceding clinical onset. MRI Brain revealed extensive right perisylvian polymicrogyria and an FDG-PET scan showed concordant right perisylvian hypometabolism (Fig. 1). The neuro-psychological evaluation was unremarkable. Following a multidisciplinary meeting, it was decided to perform a right unilateral wide SEEG implant to explore the malformed cortex and surrounding areas. The exploration aimed to better delineate the real epileptogenic area within the malformation and its relationship to functional areas, and possibly to plan resective surgery or at least perform RF-TC (Fig. 1, Fig. 2a). The SEEG recording showed diffusely slow background activity, except for the perirolandic electrodes (M, N, F), which showed physiological activity.
Fig. 1.
Upper part: median PET cortical metabolism superimposed on 3D Freesurfer pial model shows abnormal right perisylvian gyration and hypometabolism compared to the left hemisphere (color palette coldtohot: blue, lower metabolism, red, higher metabolism). Bottom part: T1-weighted anatomical MRI superimposed with SEEG electrodes disclosed diffuse perisylvian polymicrogyria involving the right temporal, frontal and parietal cortex. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
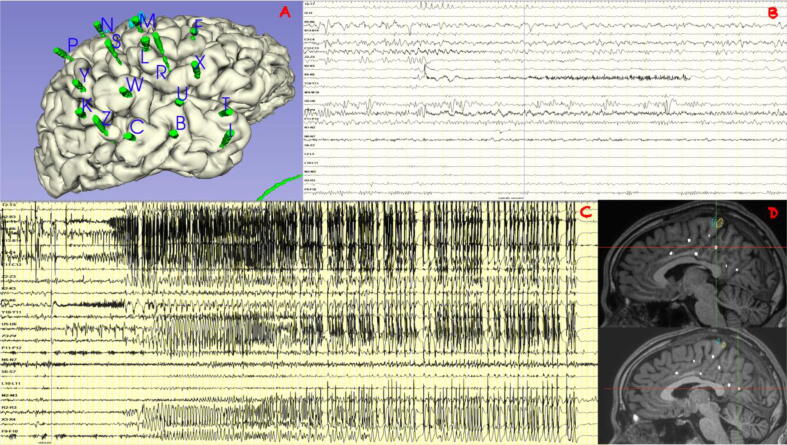
Fig. 2.
A: implantation scheme on the 3D model. B: 30-seconds SEEG trace shows low-voltage fast activity over K 5–6 and P 3–4 preceding typical subjective sensation warning. C 120-second SEEG trace shows low voltage fast activity over K electrodes with a fast propagation over mesial temporal structures (B, C); after 2 s all temporal lobe and perisylvian area are involved in the discharge. D: MRI position of P internal contact (upper) and K internal contact (lower) where RF-TC were performed. SEEG contacts position. B2-3: para-hippocampal gyrus; B5-6: hippocampus head; B13-14: middle temporal gyrus; C3-4:hippocampus body; C11-12 = posterior middle temporal gyrus; Z2-3: fusiform gyrus; K2-3 5–6: lower precuneus; Y10-11: inferior parietal gyrus; U5-6: superior temporal gyrus; P3-4: higher precuneus; P11-12: inferior parietal gyrus; N6-7: post-central gyrus; S6-7: parietal operculum; L10-11: pre-central gyrus; M2-3: supplementary motor area; R2-3: posterior short insular gyrus; X3-4: frontal operculum; F9-10: middle frontal gyrus.
High amplitude repetitive interictal spikes were documented in the temporal lobe and lateral temporo-parietal junction. During monitoring, the patient reported typical subjective sensations associated with short-duration low-voltage discharges recorded independently from multiple areas, encompassing the temporal pole, opercular region, and precuneus (Fig. 2b). The stimulations also elicited subjective sensations similar to seizure onset independently from the same regions, but did not elicit any typical seizures. A typical spontaneous seizure was recorded associated with rapid low-voltage activity on the K electrode exploring the precuneus, with rapid propagation over the mesial temporal structures and, after a few seconds, over the temporal lobe and perisylvian areas (Fig. 2c). Based on the early involvement of the precuneus (explored by the K and P electrodes) and the stimulation results eliciting some initial subjective sensations from this region, RF-TCs were performed on K 1–4 and 5–6, P 2–5 (Fig. 2d). RF-TCs were performed to ablate the most epileptogenic area and, eventually, to perform a second step surgery. The surgery appeared complicated because of the extent of the epileptogenic area, the high risk of visual deficits, and the probable risk of memory impairment, as the right mesial temporal structures were anatomically normal and showed moderate electrophysiological involvement.
After RF-TC, the patient reported an increase in the frequency/intensity of seizures (2/week, with the appearance of daily clusters) and a change in semiology: seizures were preceded by a cephalic flashing sensation in the head, followed by behavioural arrest, oro-alimentary automatisms, rightward version of the head, occasionally evolving in diffuse stiffness and falls. The post-ictal phase was also modified, characterized by confusion and speech difficulties lasting about an hour. He also presented two focal to bilateral tonic-clonic seizures.
Seven months after RF-TC (May-2019), the patient presented with a two-day episode of structured delirium characterized by the belief that his neighbor had been raped and injured. This belief led him to call the police and break down the door. The night before the onset of the episode, the patient had a cluster of seizures. The patient was then taken to the emergency room, where an EEG showed mild background asymmetry, slower in the right hemisphere with right hemispheric contoured theta, consistent with post-ictal findings. No seizures were reported during hospitalization. Despite trying many antiseizure medications, the seizures continued several times a week and he had three more psychotic episodes in the following years (April-2020, October-2020, June-2021). The patient was included in a recently published cohort of patients with post-ictal psychosis (PIP) [12].
At the age of 34, (October-2021) the patient was given off-label treatment with cannabidiol (CBD) up to 500 mg/day (8.3 mg/kg/day), based on preliminary evidence suggesting improvement of seizures in patients with polymicrogyria [13], [14]. This led to an immediate and dramatic reduction in seizure frequency (two short seizures per month) and resolution of seizure clusters, falls, post-ictal confusion, and psychotic episodes at 12-month follow-up. His cognitive and work performance improved, as did his quality of life, as documented by the QOLIE-31 questionnaire, with an overall score of 51.9/100 compared with 38.4/100 in the pre-treatment period.
Discussion
Patients with polymicrogyria (PwP) often develop a drug-resistant epilepsy, making them suitable candidates for epilepsy surgery [7]. Surgery in these patients is usually guided by SEEG exploration; yet, despite its use, some previous series have shown that surgery is not feasible in about 31 % of cases because of the risk of functional deficit and extensive/bilateral EZ, as exemplified by our patient [15]. When the surgical option is discarded, the probability of achieving seizure freedom in these patients is very low [7], [16]. We decided to exclude this patient from surgery given the lack of a confined epileptogenic area, the partial overlap with eloquent cortex, the high risk of visual deficits and the probable risk of memory impairment. Consequently, as there were no conditions for resective surgery without major risks, we decided to perform RF-TC with the aim of improving seizure control, following the indication of performing RF-TC even in non-eligible cases given the safety profile and to the efficacy of the procedure [5], [10], [17]. RF-TC was also supported by our in-house experience regarding two patients with extensive polymicrogyria and partial overlap with the eloquent cortex. In these patients the use of RF-TC, limited to the few contacts showing the greatest epileptogenic activity, led to an improvement in seizure control more than expected.
In contrast, this patient presented an unfavorable reorganization of the epileptogenic network, with an even more striking worsening of seizure frequency/intensity. To our knowledge, seizure worsening after RF-TC is reported in only one other case in a small surgical series [18]. Most surgical series report, as negative outcome, the absence of benefit from RF-TC without further details [19]. The patient developed post-ictal psychotic episodes a few months after RF-TC. This type of event has not been reported in the literature after RF-TC. The nature of the psychotic episodes in our patient could not be ascertained because of lack of continuous EEG recording during the attack. However, at least in one occasion, the psychosis was preceded by a seizure cluster and the EEG performed not long after the end of this episode was compatible with a postictal tracing. This leads us to interpret the psychotic episodes as PIP. PIP is defined as the onset of psychotic symptoms typically following a cluster of seizures, which may last from a few days to weeks in the absence of documented ictal activity [12], [20]. Psychiatric complications related to the invasive procedure have been poorly considered in the surgical series. However, some authors have documented the occurrence of psychiatric symptoms 6 h after cortical stimulation, especially in older patients with a longer duration of illness, a previous history of PIP, and nondominant temporal lobe epilepsy [20]. Psychiatric symptoms may be caused by altered brain network dynamics induced by cortical stimulation, particularly when the temporal and limbic systems are involved [20]. Similarly, the effect of RF-TC is usually considered focal and, consequently, the reported complications are mostly focal deficits. It is also necessary to consider the influence of a focal lesion on large epileptogenic networks and widely distributed brain functions, especially in PwP, in which a complex epileptogenic area and an eloquent cortex coexist in the malformation, and the normal anatomy may be disrupted [15]. This concept has been nicely explored by a recent RF-TC study which demonstrated that focal narrow ablation can impact the function of anatomically normal distant cortical areas through percolation of slow waves up to 60 mm from the focal lesion, thus providing empirical evidence of network alteration due to disruption of a specific node and an electrophysiological basis of diaschisis [21]. In line with all these observations, the RF-TC localized to the precuneus performed in our patient might have affected distant temporal or frontal areas, including possibly the limbic system, which was already involved in the epileptogenic network as demonstrated by the SEEG recording. It could be speculated that this unpredictable and unfavorable reorganization of the epileptogenic network, secondary to ablation of a specific node, may have worsened seizure control, inducing PIP following prolonged seizure clusters.
Finally considering the antipsychotic effect of cannabidiol reported in literature [22] and the response in our patient, we might suggest a possible role of cannabidiol in the management of perioperative psychiatric symptoms.
Conclusion
RF-TC is a safe and effective procedure in epilepsy surgery that should be considered in some patients with epilepsy, especially those who are excluded from surgery for prognostic and palliative reasons. We report a complex case of post-ictal psychosis after RF-TC, not preivously described in the literature, and seizure worsening, that to our knowledge was reported in one other case. We recommend clinicians take into account the remote possibility of clinical aggravation and seizure exacerbation after RF-TC.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
Author contributions: LF, RM, VM and FB contributed to conception and design of the study; all authors contributed to the acquisition and/ or analysis of data; LF, RM, LM and LdV drafted the manuscript; LF and MM prepared the figure; FB supervised the study. We thank Dr Sakaria Ali University College London Hospital for reviewing the English content of the manuscript.
Funding: The study was supported by the Italian Ministry of Health (RF-2019-12370564 to Francesca Bisulli).
Ethical approval: All investigations were carried out according to the Declaration of Helsinki. The study was approved by Central Emilia Wide Area Ethical Committee of the Emilia-Romagna Region on 18/12/2019 (n. 19125). Written informed consent was obtain from the patient for his participation in the study.
References
- 1.Rugg-Gunn F, Miserocchi A, McEvoy A. Epilepsy surgery. Pract Neurol. 2019 practneurol-2019-002192. https://doi.org/10.1136/practneurol-2019-002192.
- 2.West S., Nolan S.J., Newton R. Surgery for epilepsy: A systematic review of current evidence. Epileptic Disord. 2016;18(2):113–121. doi: 10.1684/epd.2016.0825. [DOI] [PubMed] [Google Scholar]
- 3.Sarkis R.A., Jehi L., Bingaman W., Najm I.M. Seizure worsening and its predictors after epilepsy surgery. Epilepsia. 2012;53 doi: 10.1111/j.1528-1167.2012.03642. [DOI] [PubMed] [Google Scholar]
- 4.Isnard J., Taussig D., Bartolomei F., Bourdillon P., Catenoix H., Chassoux F., et al. French guidelines on stereoelectroencephalography (SEEG) Neurophysiol Clin. 2018;48(1):5–13. doi: 10.1016/j.neucli.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Cossu M., Cardinale F., Casaceli G., et al. Stereo-EEG–guided radiofrequency thermocoagulations. Epilepsia. 2017;58 doi: 10.1111/epi.13687. [DOI] [PubMed] [Google Scholar]
- 6.Bourdillon P., Devaux B., Job-Chapron A.-S., Isnard J. SEEG-guided radiofrequency thermocoagulation. Neurophysiol Clin. 2018;48(1):59–64. doi: 10.1016/j.neucli.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Cossu M., Pelliccia V., Gozzo F., Casaceli G., Francione S., Nobili L., et al. Surgical treatment of polymicrogyria-related epilepsy. Epilepsia. 2016;57(12):2001–2010. doi: 10.1111/epi.13589. [DOI] [PubMed] [Google Scholar]
- 8.Bourdillon P., Isnard J., Catenoix H., Montavont A., Rheims S., Ryvlin P., et al. Stereo electroencephalography-guided radiofrequency thermocoagulation (SEEG-guided RF-TC) in drug-resistant focal epilepsy: Results from a 10-year experience. Epilepsia. 2017;58(1):85–93. doi: 10.1111/epi.13616. [DOI] [PubMed] [Google Scholar]
- 9.Bourdillon P., Cucherat M., Isnard J., Ostrowsky-Coste K., Catenoix H., Guénot M., et al. Stereo-electroencephalography-guided radiofrequency thermocoagulation in patients with focal epilepsy: A systematic review and meta-analysis. Epilepsia. 2018;59(12):2296–2304. doi: 10.1111/epi.14584. [DOI] [PubMed] [Google Scholar]
- 10.Catenoix H., Mauguiere F., Guenot M., Ryvlin P., Bissery A., Sindou M., et al. SEEG-guided thermocoagulations: A palliative treatment of nonoperable partial epilepsies. Neurology. 2008;71(21):1719–1726. doi: 10.1212/01.wnl.0000335166.20451.88. [DOI] [PubMed] [Google Scholar]
- 11.Bourdillon P, Isnard J, Catenoix H, et al. Stereo-electro-encephalography-guided radiofrequency thermocoagulation: from in vitro and in vivo data to technical guidelines. World Neurosurg 94:73-79. https://doi.org/10.1016/j.wneu.2016.06.095. [DOI] [PubMed]
- 12.Braatz V., Martins Custodio H., Leu C., Agrò L., Wang B., Calafato S., et al. Postictal psychosis in epilepsy: A clinicogenetic study. Ann Neurol. 2021;90(3):464–476. doi: 10.1002/ana.26174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caraballo R., Demirdjian G., Reyes G., Huaman M., Gutierrez R. Effectiveness of cannabidiol in a prospective cohort of children with drug-resistant epileptic encephalopathy in Argentina. Seizure. 2020;80:75–80. doi: 10.1016/j.seizure.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Pane C., Saccà F. The use of medical grade cannabis in Italy for drug-resistant epilepsy: a case series. Neurol Sci. 2020;41(3):695–698. doi: 10.1007/s10072-019-04162-1. [DOI] [PubMed] [Google Scholar]
- 15.Maillard L., Ramantani G. Epilepsy surgery for polymicrogyria: a challenge to be undertaken. Epileptic Disord. 2018;20:319–338. doi: 10.1684/epd.2018.1004. [DOI] [PubMed] [Google Scholar]
- 16.Licchetta L., Teglia A., Di Mauro C., Toni F., Vignatelli L., Belotti L., et al. Focal epilepsy due to malformations of cortical development: Long-term outcome and prognosis predictors. J Neurol Sci. 2021;429:117708. [Google Scholar]
- 17.Catenoix H., Bourdillon P., Guénot M., Isnard J. The combination of stereo-EEG and radiofrequency ablation. Epilepsy Res. 2018;142:117–120. doi: 10.1016/j.eplepsyres.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z., Zhao Q., Tian Z., Zhang J., Xiao X., Lin H., et al. Efficacy and safety of a new robot-assisted stereotactic system for radiofrequency thermocoagulation in patients with temporal lobe epilepsy. Exp Ther Med. 2014;7(6):1728–1732. doi: 10.3892/etm.2014.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirza F.A., Hall J.A. Radiofrequency thermocoagulation in refractory focal epilepsy: the Montreal Neurological Institute experience. Can J Neurol Sci. 2021;48(5):626–639. doi: 10.1017/cjn.2020.263. [DOI] [PubMed] [Google Scholar]
- 20.Conde-Blanco E., Reyes-Leiva D., Pintor L., Donaire A., Manzanares I., Rumia J., et al. Psychotic symptoms in drug resistant epilepsy patients after cortical stimulation. Epilepsy Res. 2021;173:106630. doi: 10.1016/j.eplepsyres.2021.106630. [DOI] [PubMed] [Google Scholar]
- 21.Russo S., Pigorini A., Mikulan E., Sarasso S., Rubino A., Zauli F.M., et al. Focal lesions induce large-scale percolation of sleep-like intracerebral activity in awake humans. Neuroimage. 2021;234:117964. doi: 10.1016/j.neuroimage.2021.117964. [DOI] [PubMed] [Google Scholar]
- 22.Devinsky O., Cilio M.R., Cross H., Fernandez-Ruiz J., French J., Hill C., et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. doi: 10.1111/epi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]